Five new species of water mite genus Atractides Koch, 1837 from Bhutan with new records (Acari: Hydrachnidia: Hygrobatidae)
Pešić, Vladimir  1
; Smit, Harry
1
; Smit, Harry  2
and Gurung, Mer Man
2
and Gurung, Mer Man  3
3
1✉ University of Montenegro, Cetinjski put bb, 81000 Podgorica, Montenegro.
2Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, the Netherlands.
3Department of Forest Science, College of Natural Resources, Royal University of Bhutan, Lobesa, Punakha, Bhutan.
2023 - Volume: 63 Issue: 1 pages: 148-168
https://doi.org/10.24349/e399-mzq1ZooBank LSID: 11213451-F90A-4263-AE0C-E14607263F5F
Original research
Keywords
Abstract
Introduction
Water mites of the genus Atractides Koch, 1837 have been found in all biogeographical regions except Antarctica and Australasia (Smit 2020) with the highest diversity in the Palaearctic and Oriental regions (Pešić and Smit 2011). In the Himalayan region, species of the genus Atractides are among the most frequently collected mites in running waters, as well as in springs and interstitial waters. In the latter region, the genus is represented by species belonging to three subgenera, i.e., Atractides s. s., Tympanomegapus Thor, 1923, and Polymegapus K. Viets, 1926 (Gurung et al. 2022b).
So far, no Atractides species were reported from Bhutan, a small landlocked country in the Eastern Himalayas, whose water mite fauna recently has become the object of comprehensive research (Gerecke and Smit 2022; Pešić et al. 2022a, b; Smit et al. 2022; Gurung et al. 2022a, b; Smit and Gurung 2022). As a result of the present study seven species of the genus Atractides from Bhutan were identified. In this paper, descriptions of five new species are given.
Material and methods
Water mites were collected by hand netting, sorted live in the field, and immediately preserved in Koenike fluid and/or 96% ethanol. Some specimens were dissected, and slide mounted in Faure's medium. Most material has been collected in 2021 from the tributaries of the Mangde Chhu river in central Bhutan. Morphological nomenclature follows Gerecke et al. (2016). Unless stated otherwise, all material is collected by Mer Man Gurung, and this is not repeated in the text. All material will be deposited in the National Biodiversity Center, Thimphu (NBC), a part of the paratypes and the non-type material will be lodged as a long-term loan in Naturalis Biodiversity Center, Leiden (RMNH). In the section ′Material examined', collecting site abbreviations derive from the geographical database of Mer Man Gurung.
All measurements are in µm. The following abbreviations are used: Ac-1-3 = first to third acetabulum; asl = above sea level; Cx-I-IV = first to fourth coxae; Dgl-4 = dorsoglandularia 4; dL = dorsal length; H = height; I-L-4-6 = fourth-sixth segments of first leg; L = length; lL =lateral length; mL = medial length; P-1-P-5 = palp segment 1-5; RMNH = Naturalis Biodiversity Center, Leiden; S-1 = proximal large ventral seta at I-L-5; S-2 = distal large ventral seta at I-L-5; Vgl-1 = ventroglandulare 1; Vgl-2 = ventroglandulare 2; vL = ventral length; W = width.
Systematics
Family Hygrobatidae
Genus Atractides Koch, 1837
Subgenus Atractides s. s.
Atractides (Atractides) garhwali Pešić, Kumar & Kumar, 2007
Figures 1-2






Material examined — Bhutan, MG3 Bipgang Chhu, 27.15729°N, 90.66721°E, 596 m asl, 25 Oct. 2021, 3♂, 4♀ (1♂, 1♀, dissected and slide mounted; RMNH); MG4 Takabi Chhu, 27.14782°N, 90.68833°E, 543 m asl, 26 Oct. 2021, 1♀.
Morphology — General features – Integument striated, muscle insertions unsclerotized. Mediocaudal margin Cx-I convex, apodemes of Cx-II forming an acute angle with the median line. Excretory pore smooth; Vgl-1 not fused to Vgl-2. Palp with a weak sexual dimorphism, P-2 ventral margin nearly straight, without a ventrodistal protrusion, but forming a distinct ventrodistal angle, ventral margin P-3 straight or slightly concave, P-4 slightly protruding near insertion of proximoventral seta, sword seta between ventral setae, nearer to distoventral seta (Figures 1A, D). I-L-5 with S-1 longish and bluntly pointed, S-2 thicker, proximally enlarged and pointed; I-L-6 curved, basally thickened, from the centre to the claw furrow with parallel dorsal and ventral margins (Figures 1B, E).
Male – Genital plate anterior margin convex, with a narrow border of secondary sclerotization, posterior margin deeply indented, acetabula in triangular arrangement (Figure 1C); P-4 thickened, ventral sectors 1 : 1 : 1 (Figure 1A).
Female – Genital field with acetabula in an obtuse triangle (Figure 2), P-4 slender than in male.
Measurements — Male – Idiosoma L 388. Coxal shield L 250; Cx-III W 283; Cx-I+II mL 88, Cx-I+II lL 183. Genital field L/W 86/98, ratio 0.87, L Ac-1-3: 34-35, 34-36, 30.
Palp – Total L 232; dL/H, dL/H ratio: P-1, 22/22, 1.0; P-2, 50/35, 1.42; P-3, 55/34, 1.65; P-4, 77/31, 2.45; P-5, 28/12, 2.3; L ratio P-2/P-4, 0.65.
Legs – I-L-5 dL 138, vL 91, dL/vL ratio 1.52, maximum H 42, dL/maximum H 3.26, S-1 L 77, L/W ratio 10.2, S-2 L 60, L/W ratio 5.47, distance S-1-2, 15, dL ratio S-1/2 1.27; I-L-6 dL 101, central H 16, dL/central H ratio 6.15; L I-L-5/6 ratio 1.36.
Female – Idiosoma L 703, W 555; maximum diameter Dgl-4, 27. Coxal shield L 353; Cx-III W 445; Cx-I+II mL 134, Cx-I+II lL 231. Genital field L/W 159/177, genital plates L 128-130, pregenital sclerite 70, gonopore L 127, L Ac-1-3: 48-53, 53-55, 49-50.
Palp – Total L 346; dL/H, dL/H ratio: P-1, 31/27, 1.18; P-2, 67/46, 1.46; P-3, 97/40, 2.44; P-4, 109/30, 3.68; P-5, 42/14, 3.0; L ratio P-2/P-4, 0.61.
Legs – I-L-5 dL 222, vL 144, dL/vL ratio 1.54, maximum H 58, dL/maximum H 3.84, S-1 L 114, L/W ratio 10.4, S-2 L 91, L/W ratio 5.3, distance S-1-2, 31, dL ratio S-1/2 1.26; I-L-6 dL 156, central H 19, dL/central H ratio 8.3; L I-L-5/6 ratio 1.42.
Remarks — The specimens from Bhutan generally match the description of Atractides garhwali Pešić, Kumar & Kumar, 2007, a species widely distributed in the Western Himalayas (Pešić et al. 2007, 2019). However, we cannot exclude the possibility that A. garhwali represents a species complex. To address the question of possible cryptic speciation in the latter species complex additional material should be tested with the application of molecular techniques in case data are available from the type locality of A. garhwali.
Distribution — India (Uttarakhand), Bhutan.
Atractides (Atractides) cf. panesari Pešić & Ranga Reddy, 2009
Figure 3



Material examined — Bhutan, MG9 Kartigang Chhu, 27.27896°N, 90.63088°E, 1456 m asl, 1 Nov. 2021, 1♀, dissected and slide mounted (RMNH).
Morphology — Female – Integument striated, muscle insertions unsclerotized. Posterior margin of Cx-I+II broad, apodemes of Cx-II forming an acute angle with the median line. Excretory pore smooth; Vgl-1 not fused to Vgl-2. Genital field with acetabula in a curved line (Figure 3B). P-2 ventral margin straight, ventral margin P-3 slightly concave, P-4 sword seta between ventral setae (Figure 3C). I-L-5 with S-1/-2 strongly heteromorphic and separated, S-1 long, slightly bent inwards, distally truncate, S-2 shortened, enlarged, with a blunt tip; I-L-6 long and slender, distally slightly narrowed (Figure 3D).
Measurements — Idiosoma L 540; maximum diameter Dgl-4, 23. Coxal shield L 281; Cx-III W 334; Cx-I+II mL 116, Cx-I+II lL 197. Genital field L/W 133/123, genital plates L 81, pregenital sclerite 81, gonopore L 113, L Ac-1-3: 32-34, 31, 28. Egg maximum diameter (n = 2) 100-103.
Palp – dL/H, dL/H ratio: P-1, -/-, -; P-2, 61/42, 1.46; P-3, 72/36, 2.0; P-4, 84/27, 3.06; P-5, 34/11, 3.16; L ratio P-2/P-4, 0.73.
Legs – I-L-5 dL 167, vL 92, dL/vL ratio 1.82, maximum H 63, dL/maximum H 2.64, S-1 L 124, L/W ratio 11.64, S-2 L 82, L/W ratio 5.26, distance S-1-2, 37, dL ratio S-1/2 1.50; I-L-6 dL 159, central H 16, dL/central H ratio 10.2; L I-L-5/6 ratio 1.05.
Remarks — The examined female from Bhutan matches the description of Atractides panesari Pešić & Ranga Reddy, 2009, a species originally described on the basis of a single female collected from hyporheic waters of a stream in Uttarakhand State of India (Pešić and Ranga Reddy 2009). The specimen from India (in parentheses data taken from Pešić and Ranga Reddy 2009) differs in comparatively longer S-1 (138 µm) and a larger S-1-2 distance (46 µm).
Distribution — India (Uttarakhand), Bhutan.
Atractides (Atractides) mangdensis sp. nov.
ZOOBANK: F228834C-4255-4BC3-A7B9-58CB077B4F26 ![]()
Figure 4



Type material — Holotype ♀, dissected and slide mounted (NBC), Bhutan, MG5 Dakpay Chhu, 27.14621°N, 90.69220°E, 539 m asl, 1 May 2021.
Diagnosis — Female (male unknown) – Integument striated; muscle insertions unsclerotized; excretory pore smooth; Vgl-1 not fused to Vgl-2; genital plates short and stout flanking the postgenital sclerite, gonopore comparatively short, not exceeding genital plate length; P-2 with a pointed ventrodistal protrusion.
Description — Female – Integument dorsally striated, muscle insertions unsclerotized. Coxal field slender and longish, mediocaudal margin of Cx-I tongue-shaped (Figure 4B). Genital plates short and stout flanking the postgenital sclerite, directed laterally, acetabula in triangular position (Figure 4C). Excretory pore smooth; Vgl-1 not fused to Vgl-2. P-2 ventral margin straight, ending in a pointed ventrodistal protrusion, ventral margin of P-3 slightly concave, P-4 slender, insertions of ventral setae flanked by denticles, sword seta between ventral setae, nearer to distoventral seta (Figure 4E). I-L-5 with S-1/2 slender, with knob-shaped tip, and close to each other; I-L-6 nearly straight, with strong claw (Figure 4D).
Measurements — Idiosoma L 475, W 303; maximum diameter Dgl-4, 22. Coxal shield L 253; Cx-III W 244; Cx-I+II mL 111, Cx-I+II lL 178. Genital field L/W 97/122, genital plates L 70, pregenital sclerite 48, gonopore L 63, L Ac-1-3: 28, 30, 36. Egg maximum diameter (n = 2) 97-100.
Palp – Total L 302; dL/H, dL/H ratio: P-1, 23/23, 1.0; P-2, 70/48, 1.45; P-3, 78/44, 1.78; P-4, 98/27, 3.67; P-5, 33/11, 3.0; L ratio P-2/P-4, 0.72. Gnathosoma vL 95; chelicera total L 152.
Legs – I-L-5 dL 123, vL 92, dL/vL ratio 1.33, maximum H 30, dL/maximum H 4.13, S-1 L 63, L/W ratio 10.6, S-2 L 55, L/W ratio 7.1, distance S-1-2, 7.0, dL ratio S-1/2, 1.13; I-L-6 dL 94, central H 17, dL/central H ratio 5.56; L I-L-5/6 ratio 1.31.
Male — Unknown.
Etymology — Named after the Mangde Chhu river.
Discussion — The new species belongs to the Atractides pygmaeus complex characterized by small dimensions (idiosoma L < 500), unsclerotized excretory pore, slightly modified I-L-5/6 with short and bluntly pointed S-1/2 and the presence of well pronounced denticles accompanying the ventral setae of P-4 (Gerecke 2003). In regard to unsclerotized muscle attachments, P-2 with a pointed ventrodistal protrusion and unfused Vgl-1/2 the new species is similar to A. manasi Pešić & Smit, 2018, a species described on the basis of a single female from a stream in the Tien Shan Mountains in Kyrgyzstan (Pešić and Smit 2018). The latter species differs from the new species from Bhutan by having a comparatively much longer gonopore, exceeding the genital plate length, and a comparatively shorter setae S-1/-2 (L S-1, 51, S-2, 44), shifted to each other. As mentioned in some other papers (see for example, Pešić et al. 2012; Pešić and Smit 2018), no particular sexual dimorphism is to be expected in the male of A. pygmaeus complex. Therefore, in regard to clear cut differences between examined specimens from Bhutan and Kyrgyzstan, description of the new species based on a female is justified.
Distribution — Bhutan; known only from the type locality (Figure 12C).
Atractides (Atractides) conflatus sp. nov.
ZOOBANK: 7A6E86EB-25AC-443A-A8A5-B1EF6ACFB0E2 ![]()
Figures 5-6
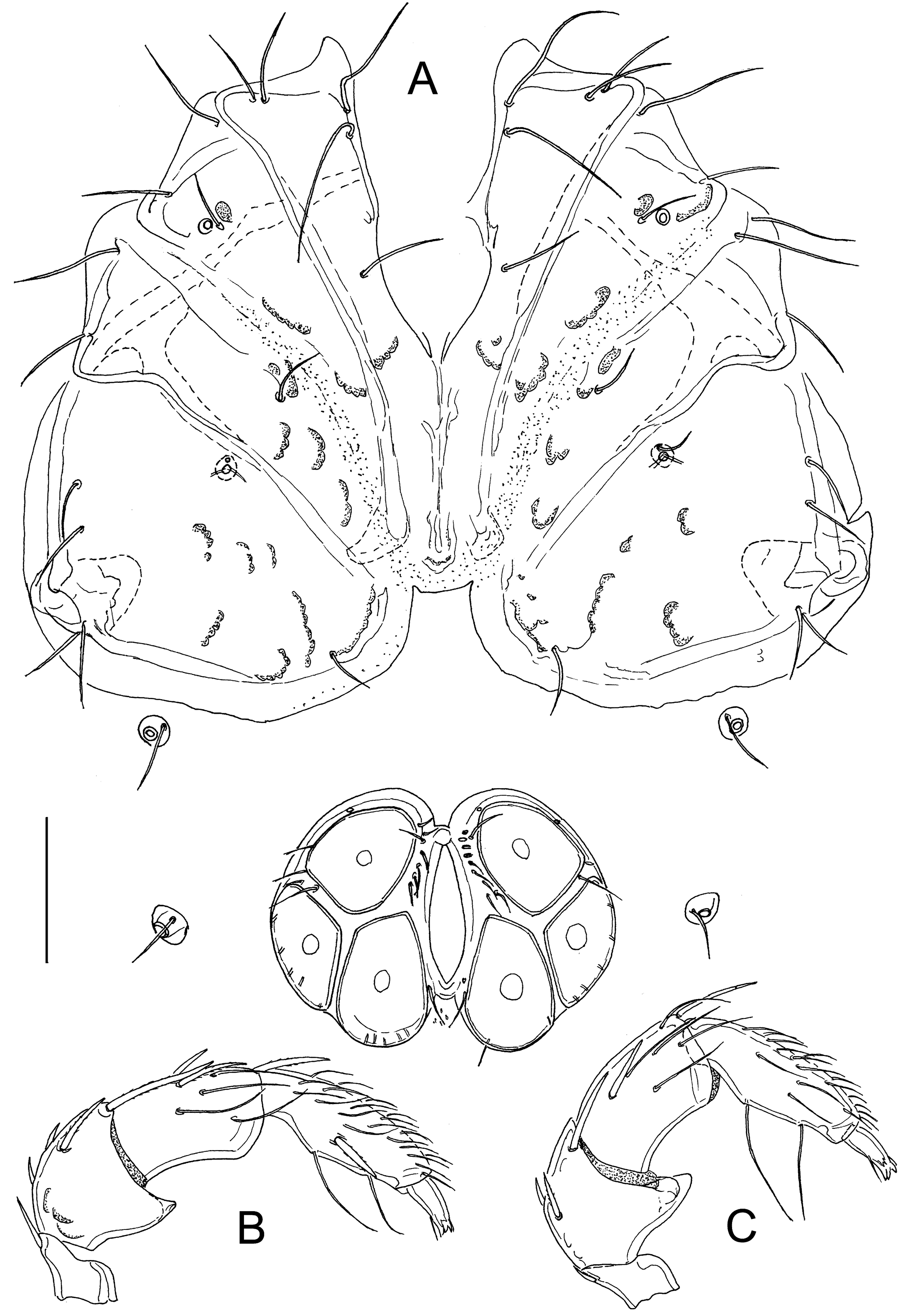




Type material — Holotype ♂, dissected and slide mounted (NBC), Bhutan, Royal Botanical Park, Lampelri, 27.50750°N, 89.75269°E, 2667 m asl, 10 Aug. 2016, leg. W. Klein. Paratypes: 2♀, same site and data as the holotype, 1♀ dissected and slide mounted (NBC).
Diagnosis — Characters of the nodipalpis species group (integument finely striated, muscle insertions unsclerotized; males with anteriorly indented genital field, P-2 with distoventral projection and ventral margin of P-4 projecting); Vgl-1 separate from Vgl-2; coxae in male fused forming a coxal shield.
Description — General features – Integument striated; dorsal and ventrocaudal idiosoma without sclerotized muscle insertions. Acetabula large, in triangular arrangement. Excretory pore smooth; Vgl-1 separate from Vgl-2. Palp with a strong sexual dimorphism in the shape of P-2 and P-4, in both sexes medial peg-like seta inserting between ventral setae. Legs: I-L-5 with dorsal and ventral margins diverging distally, S-1 and S-2 distanced with blunt tips, I-L-6 curved and slender, maximum height proximally (Figures 6D-E).
Male – Coxae fused, forming a coxal shield (Figure 5A). Genital plate anterior indented, with a narrow central notch, posterior margin deeply indented (Figure 5A); ventral margin P-2 with a strongly developed distoventral protrusion, P-3 concave, P-4 strongly thickened near proximoventral seta (Figures 5B-C).
Female – P-2 ventral margin slightly convex, P-3 dorsal margin straight, P-4 slender than in male (Figure 6C).
Measurements — Male – Idiosoma L 788, W 625; maximum diameter Dgl-4, 24. Coxal shield L 463; Cx-III W 494; Cx-I+II mL 156, Cx-I+II lL 338. Genital field L/W 178/234, ratio 0.76, L Ac-1-3: 78-81, 88-91, 94. Ejaculatory complex L 147.
Palp – Total L 441; dL/H, dL/H ratio: P-1, 44/42, 1.04; P-2, 102/86, 1.18; P-3, 111/59, 1.87; P-4, 139/50, 2.78; P-5, 45/17, 2.63; L ratio P-2/P-4, 0.73. Chelicera total L 306.
Legs – I-L-5 dL 308, vL 206, dL/vL ratio 1.49, maximum H 98, dL/maximum H 3.13, S-1 L 139, L/W ratio 8.9, S-2 L 106, L/W ratio 5.65, distance S-1-2, 30, dL ratio S-1/2, 1.31; I-L-6 dL 200, central H 27, dL/central H ratio 7.35; L I-L-5/6 ratio 1.54.
Female – Idiosoma L 925, W 688; maximum diameter Dgl-4, 22. Coxal shield L 419; Cx-III W 550; Cx-I+II mL 131, Cx-I+II lL 269. Genital field L/W 213/222, genital plates L 159, pregenital sclerite W 69, gonopore L 172, L Ac-1-3: 56-60, 72-75, 61-63.
Palp – Total L 406; dL/H, dL/H ratio: P-1, 39/33, 1.3; P-2, 84/59, 1.42; P-3, 117/45, 2.59; P-4, 127/36, 3.58; P-5, 39/17, 2.27; L ratio P-2/P-4, 0.66.
Legs – I-L-5 dL 273, vL 178, dL/vL ratio 1.54, maximum H 71, dL/maximum H 3.85, S-1 L 137, L/W ratio 11.7, S-2 L 106, L/W ratio 6.48, distance S-1-2, 37, dL ratio S-1/2, 1.29; I-L-6 dL 186, central H 22, dL/central H ratio 8.5; L I-L-5/6 ratio 1.45.
Etymology — The specific name comes from the Latin conflatus, meaning fused and referring to the shape of coxae in male.
Discussion — The new species belongs to the nodipalpis group (for diagnostic features of the group see under diagnosis of the new species). The latter group includes a number of species known from the Himalayas, i.e., A. nodipalpis acutidens Lundblad, 1969, a species originally described as a form of its stem species on the basis of a single male collected in a stream at Kambaiti Pass in Myanmar (Lundblad 1969), A. angulipalpisanus Jin, 1997, A. menglaensis Jin, 1997, A. binodipalpis Jin, 1997, and A. arcusocellus Jin, 1997, described by Jin (1997) from Yunnan province in China, and A. indicus Pešić & Smit, 2019, a species recently described from Uttarakhand State of India (Pešić et al. 2019). Males of the new species from Bhutan differ from these species in the coxae fused to a coxal shield.
Distribution — Bhutan; know from the high-order stream at an elevation of 2667 m.
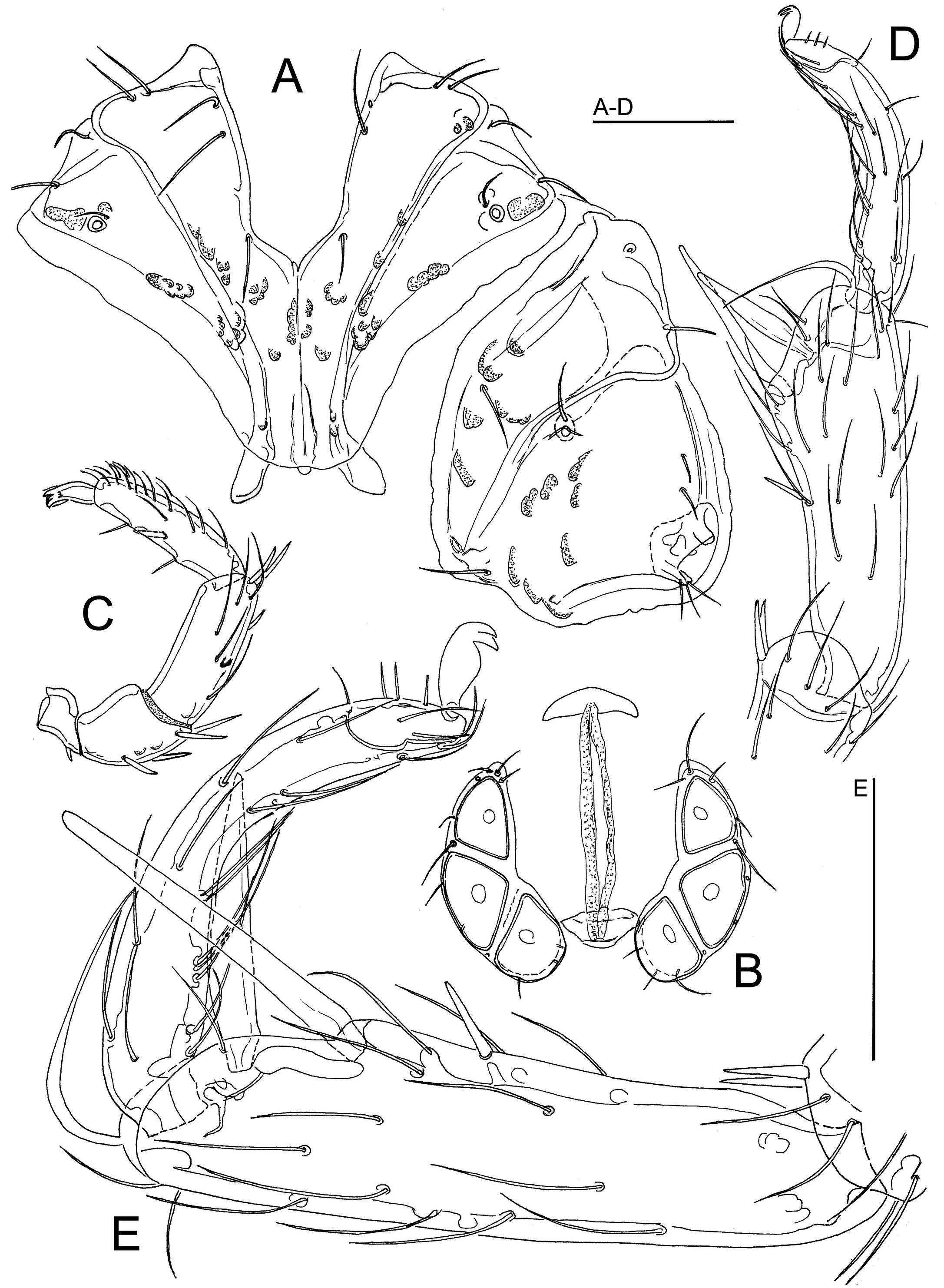
Atractides (Atractides) bhutanicus sp. nov.
ZOOBANK: F09D45C0-7937-441A-AA3D-CA3D46DE9537 ![]()
Figures 7-8






Type material — Holotype ♂, dissected and slide mounted (NBC), Bhutan, MG16 Chendebji Chhu, 27.47692°N, 90.35265°E, 2483 m asl, 8 Nov. 2021. Paratypes: 2♀, same site and data as the holotype, 1♀ dissected and slide mounted (NBC).
Other material — Bhutan, MG13 Rukhubji Chhu, 27.51174°N, 90.29711°E, 2587 m asl, 5 Nov. 2021, 1♂, 6♀.
Diagnosis — Integument finely striated, muscle insertions unsclerotized; anterior margin of male genital field slightly concave, but without an indentation; Vgl-1 separate from Vgl-2; P-2 with a distoventral projection, ventral margin of P-4 projecting.
Description — General features – Integument striated, muscle insertions unsclerotized; mediocaudal margin Cx-I convex, apodemes of Cx-II in an acute angle with the median line. Genital field with large Ac in a triangular arrangement. Excretory pore smooth; Vgl-1 not fused to Vgl-2. Palp with sexual dimorphism, P-4 sword seta slender, between ventral setae, nearer to distoventral seta. I-L-5 with seta S-1 slender and bluntly pointed, S-2 shorter and pointed, proximally enlarged; I-L-6 slender, curved, basally slightly thickened (Figure 7C).
Male – Genital plate anterior margin slightly concave, posterior margin deeply indented (Figure 7A); P-2 with a finger-like distoventral protrusion, P-3 ventral margin concave, P-4 thickened, maximum height at level of proximoventral hair (Figure 7B).
Female – P-2 ventral margin convex distally (Figure 8B).
Measurements — Male – Idiosoma L 558, W 428; maximum diameter Dgl-4 27. Coxal shield L 344; Cx-III W 375; Cx-I+II mL 131, Cx-I+II lL 250. Genital field L/W 117/136, ratio 0.86, L Ac-1-3: 52-58, 52, 44-52. Ejaculatory complex L 105.
Palp – Total L 324; dL/H, dL/H ratio: P-1, 33/31, 1.08; P-2, 72/63, 1.15; P-3, 77/48, 1.6; P-4, 106/44, 2.41; P-5, 36/17, 2.16; L ratio P-2/P-4, 0.68. Gnathosoma vL 150, chelicera total L 217.
Legs – I-L-5 dL 209, vL 137, dL/vL ratio 1.53, maximum H 63, dL/maximum H 3.31, S-1 L 102, L/W ratio 10.8, S-2 L 80, L/W ratio 5.65, distance S-1-2, 27, dL ratio S-1/2, 1.28; I-L-6 dL 140, central H 22, dL/central H ratio 6.39; L I-L-5/6 ratio 1.5.
Female – Idiosoma L 713, W 550; maximum diameter Dgl-4, 22. Coxal shield L 378; Cx-III W 447; Cx-I+II mL 112, Cx-I+II lL 253. Genital field L/W 152/167, genital plates L 125-128, pregenital sclerite 49, gonopore L 118, L Ac-1-3: 55-56, 53-55, 46-47.
Palp – Total L 412; dL/H, dL/H ratio: P-1, 37/34, 1.07; P-2, 84/57, 1.48; P-3, 116/45, 2.55; P-4, 134/34, 3.91; P-5, 41/16, 2.6; L ratio P-2/P-4, 0.63. Gnathosoma vL 141, chelicera total L 247.
Legs – I-L-5 dL 281, vL 180, dL/vL ratio 1.57, maximum H 66, dL/maximum H 4.25, S-1 L 139, L/W ratio 11.1, S-2 L 106, L/W ratio 6.2, distance S-1-2, 44, dL ratio S-1/2, 1.31; I-L-6 dL 205, central H 20, dL/central H ratio 10.1; L I-L-5/6 ratio 1.37.
Etymology — The new species is named after the country where the new species was collected.
Discussion — In regard to the striated integument and the shape of the palp in the male (P-2 with distoventral projection, ventral margin of P-4 projecting), the new species resembles species of the A. nodipalpis complex (see above, under discussion of A. conflatus sp. nov.). However, males in the latter species complex differ from the new species by the shape of genital field with anterior and posterior indentations (anterior margin slightly concave, but without medial identation in A. bhutanicus sp. nov.). As Gerecke (2003) pointed out, the development of a ventrodistal protrusion of P-2 in males probably has evolved independently in various, not closely related species groups.
Distribution — Bhutan; know from high-order streams at an elevation above 2400 m (Figure 12B).
Atractides (Atractides) indentatus sp. nov.
ZOOBANK: A07B4D67-582A-48DA-AD8C-D60DC8C3E700 ![]()
Figures 9-10
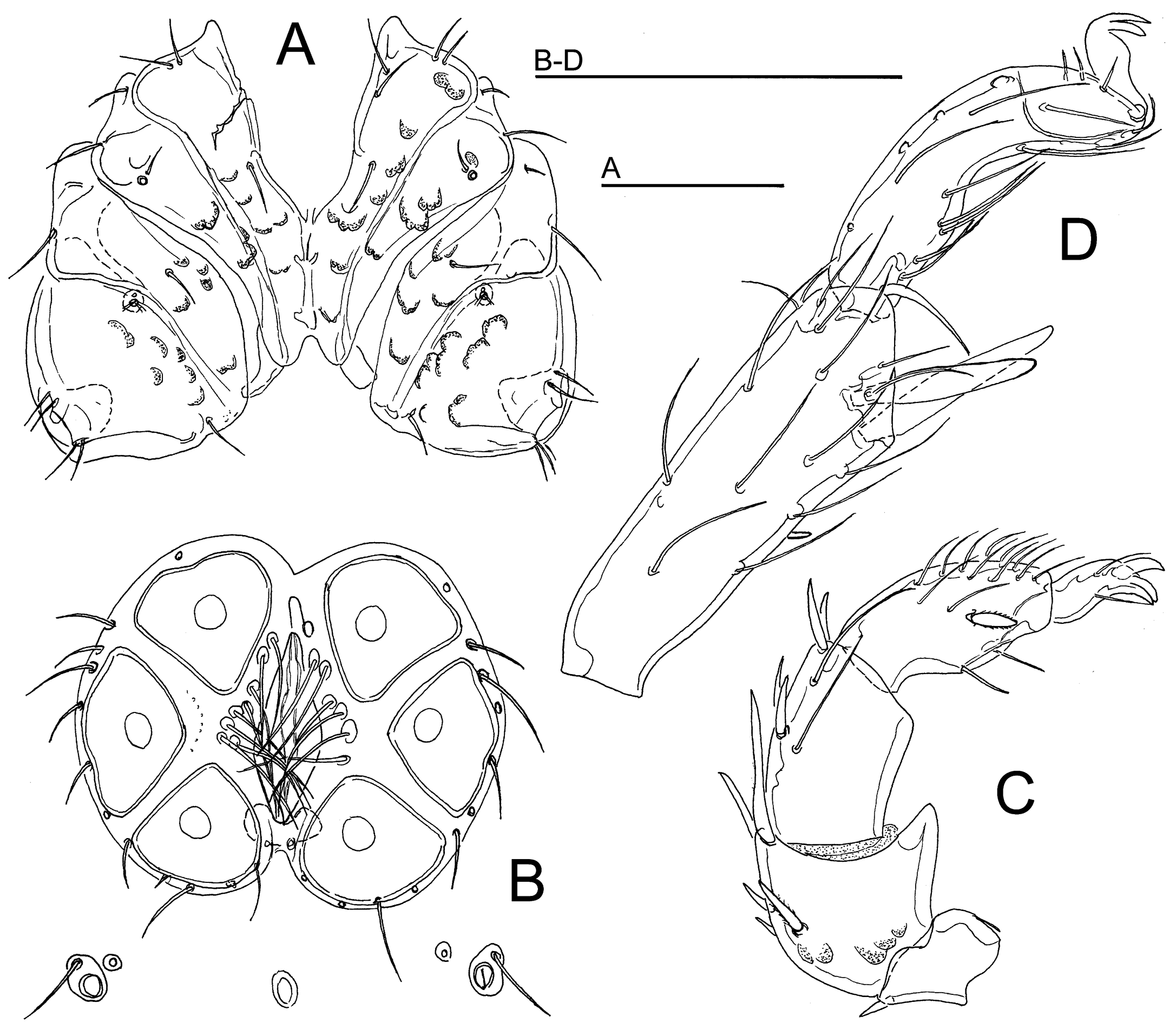





Type material — Holotype ♂, dissected and slide mounted (NBC), Bhutan, MG3 Bipgang Chhu, 27.15729°N, 90.66721°E, 596 m asl, 25 Oct. 2021. Paratypes: 4♀, same site and data as the holotype, 1♀ dissected and slide mounted (NBC).
Diagnosis — Integument finely striated, muscle insertions unsclerotized; mediocaudal margin Cx-I+II deeply indented; Vgl-1 separate from Vgl-2; strong sexual dimorphism (in male P-2 with pointed distoventral projection, P-4 shortened, P-2/P-4 ratio 0.94), anterior margin of the male genital plate with a V-shaped indentation.
Description — General features – Integument striated, muscle insertions unsclerotized; mediocaudal margin Cx-I+II deeply indented (Figures 9A, 10A), caudal apodemes of Cx-II strongly protruding and forming an acute angle. Excretory pore smooth; Vgl-1 not fused to Vgl-2. Palp with a strong sexual dimorphism, P-4 sword seta stout, nearer to distoventral setae. I-L-5 setae S-1 and S-2 nearer to each other, bluntly pointed, S-1 longer than S-2; I-L-6 stout, proximally thickened, slightly curved (Figures 9D, 10C).
Male – Anterior and posterior margin of genital plate deeply indented, anterior indentation V-shaped, P-2 ventral margin with a strongly developed pointed distal extension, P-3 slightly concave, P-4 shortened, ventral margin slightly protruding at level of ventral setae (Figure 9C).
Female – P-2 ventral margin convex distally, P-3/4 straight, P-4 slender than in male (Figure 10B).
Measurements — Male – Idiosoma L 390, W 319; maximum diameter Dgl-4, 17. Coxal shield L 241; Cx-III W 280; Cx-I+II mL 63, Cx-I+II lL 150. Genital field L/W 101/118, ratio 0.85, L Ac-1-3: 39-41, 44-47, 34-36.
Palp – Total L 228; dL/H, dL/H ratio: P-1, 23/26, 0.91; P-2, 56/45, 1.24; P-3, 56/36, 1.57; P-4, 60/31, 1.97; P-5, 33/13, 2.62; L ratio P-2/P-4, 0.94.
Legs – I-L-5 dL 142, vL 98, dL/vL ratio 1.46, maximum H 44, dL/maximum H 3.25, S-1 L 59, L/W ratio 5.3, S-2 L 53, L/W ratio 4.9, distance S-1-2, 5.0, dL ratio S-1/2, 1.12; I-L-6 dL 97, central H 20, dL/central H ratio 4.77; L I-L-5/6 ratio 1.47.
Female – Idiosoma L 594, W 447; maximum diameter Dgl-4, 20. Coxal shield L 325; Cx-III W 391; Cx-I+II mL 63, Cx-I+II lL 175. Genital field L/W 144/164, genital plates L 109, pregenital sclerite W 59, gonopore L 103, L Ac-1-3: 41-47, 52-54, 47.
Palp – Total L 295; dL/H, dL/H ratio: P-1, 28/30, 0.94; P-2, 63/44, 1.43; P-3, 78/40, 1.96; P-4, 85/27, 3.2; P-5, 41/15, 2.74; L ratio P-2/P-4, 0.73. Gnathosoma vL 72, chelicera total L 173.
Legs – I-L-5 dL 198, vL 145, dL/vL ratio 1.36, maximum H 51, dL/maximum H 3.9, S-1 L 77, L/W ratio 5.18, S-2 L 65, L/W ratio 4.41, distance S-1-2, 7.0, dL ratio S-1/2, 1.17; I-L-6 dL 125, central H 25, dL/central H ratio 5.0; L I-L-5/6 ratio 1.58.
Etymology — The specific name refers to deeply indented mediocaudal margin of Cx-I+II.
Discussion — The new species can easily be distinguished from other members of the genus Atractides known from the Himalayas and Indo-Malayan region in the combination of a striated integument, unsclerotized dorsal muscle attachments, a male genital plate with an anterior V-shaped indentation, a strong sexual dimorphism of P-2 and P-4, male with a pointed ventrodistal protrusion of P-2 and a comparatively shortened P-4, and the shape of I-L-5/6 (S-1 and -2 rather homomorphic, sword-shaped, close to each other, I-L-6 stout and distally tapering).
Atractides amplipalpis Lundblad, 1969, a species originally described on basis of a single male collected from a stream in Myanmar (Lundblad 1969) resembles the new species in the shape of the male genital field with an anterior V-shaped indentation, and setae S-1/2 close to each other, but differs in the shape of the palp (P-2 protruding convexly, but without a ventrodistal projection, P-4 longer with very long ventral setae), I-L-5 (I-L-5 dorsal and ventral margins distally strongly diverging, S-2 slender and curved), mediocaudal margin of Cx-I+II, nearly straight, not indented and excretory pore surrounded by a sclerotized ring (Lundblad 1969).
Distribution — Bhutan, known only from the type locality (Figure 12A).
Subgenus Tympanomegapus Thor, 1923
Atractides (Tympanomegapus) himalayicus sp. nov.
ZOOBANK: 50BE8FE5-D47C-4F2B-A7C8-F7E639DBEF46 ![]()
Figure 11






Type material — Holotype ♀, dissected and slide mounted (NBC), Bhutan, MG3 Bipgang Chhu, 27.15729°N, 90.66721°E, 596 m asl, 25 Oct. 2021. Paratypes: 2♀, same site and data as the holotype (NBC).
Diagnosis — Female (male unknown) – Muscle insertions unsclerotized; excretory pore sclerotized; Vgl-1 not fused to Vgl-2; P-4 sword seta thicker, not hair-like; P-5 with lateral ′cheeks'; claws without dorsal clawlets.
Description — Female – Integument striated, muscle insertions unsclerotized. Glandularia enlarged (Dgl-4, 41). Mediocaudal margin of Cx-I+II curved, apodemes of Cx-II forming an acute angle, lateral and posterior margins of Cx-III+IV with extended secondary sclerotization, distinctly projecting posteriorly at lateral edges. Acetabula in triangular arrangement, pregenital sclerite strong and curved, genital plates weakly concave medially (Figure 11C). Excretory pore sclerotized; Vgl-1 not fused to Vgl-2. Ventral margin of P-2 straight, P-3 ventral margin weakly concave, P-4 with straight ventral and equally curved dorsal margin, sword seta of P-4 pointed, thicker, but not hair-like, inserted distally from distoventral hair, P-5 with lateral ′cheeks'. (Figure 11F). I-L-5/-6 with S-1/-2 bluntly pointed; I-L-6 with a regular row of ventral setae, claws strong, without dorsal clawlets (Figure 11G).
Measurements — Idiosoma L 838, W 600; maximum diameter Dgl-4, 41. Coxal shield L 422; Cx-III W 472; Cx-I+II mL 127, Cx-I+II lL 256. Genital field L/W 166/223, genital plates L 134-139, pregenital sclerite W 92, gonopore L 128, L Ac-1-3: 59, 56-63, 58-59.
Palp – Total L 388; dL/H, dL/H ratio: P-1, 45/14, 3.21; P-2, 81/44, 1.85; P-3, 103/36, 2.83; P-4, 124/31, 3.97; P-5, 35/16, 2.24; L ratio P-2/P-4, 0.65. Gnathosoma vL 205, chelicera total L 336.
Legs – I-L-5 dL 214, vL 160, dL/vL ratio 1.34, maximum H 47, dL/maximum H 4.59, S-1 L 73, L/W ratio 9.3, S-2 L 73, L/W ratio 7.3, distance S-1-2, 10, dL ratio S-1/2, 1.0; I-L-6 dL 147, central H 27, dL/central H ratio 5.37; L I-L-5/6 ratio 1.46.
Male — Unknown.
Etymology — The species is named after the Himalayan mountain range from where the new species was collected.
Discussion — Due to the presence of a thicker, not hair-like sword seta on P-4, P-5 with lateral ''cheeks″, unfused Vgl-1 and -2, and leg claws without dorsal clawlet, the new species resembles Atractides projectus Yi & Jin, 2010 and A. anhuiensis Yi & Jin, 2010, both described from Anhui Province in China (Yi et al. 2010). The latter species from China can be distinguished easily from the new species by the presence of an unsclerotized excretory pore. Moreover, A. projectus differs in the shape of the genital field with pregenital sclerite shorter than postegenital sclerite and Cx-III-IV possessing anterior, median and posterior apodemes (Yi et al. 2010).
Atractides jukii Cook, 1967, a species originally described from Maharashtra State (Cook, 1967) and later on reported from the Himalayan region from Uttarakhand state differs from the new species from Bhutan in the presence of a hair-like sword seta on P-4 (Pešić et al. 2019). Atractides brevis Yi & Jin, 2010, another Tympanomegapus species reported from the Himalayas, known from Guizhou province in China, differs in the absence of lateral ′cheeks' on P-5, fused Vgl-1+2, and unsclerotized excretory pore (Yi et al. 2010).
Distribution — Bhutan; known only from the type locality (Figure 12A).
Acknowledgements
The fieldwork of this research was made possible by grants of the National Geographic Society (NGS-72271C-20) for a project titled ''Exploring the invertebrate diversity of the last virgin rivers of Bhutan, the Eastern Himalayas″ and a grant from the foundation Pro Acarologia Basiliensis (Basel). Vincent J. Kalkman (RMNH) coordinated the contact between Naturalis Biodiversity Center and institutions in Bhutan and assisted with the logistics. Moreover, we are indebted to Wim Klein and Oscar Vorst (RMNH) for collecting a number of water mites in Bhutan, and Mer Man Gurung would like to thank Cheten Dorji for his guidance and company during the collecting trips. We thank Joanna Mąkol (Wrocław), Hiroshi Abé (Fujisawa) and one anonymous reviewer, whose constructive comments greatly improved this work.
References
- Cook D.R. 1967. Water mites from India. Mem. Amer. Entomol. Inst., 9: 1-411.
- Gerecke R. 2003. Water mites of the genus Atractides Koch, 1837 (Acari: Parasitengona: Hygrobatidae) in the western Palaearctic region: A revision. Zool. J. Linn. Soc., 138(2-3): 141-378. https://doi.org/10.1046/j.1096-3642.06-0.00051.x
- Gerecke R., Smit H. 2022. Water mites of the genus Lebertia Neuman, 1880 from the eastern Himalayas (Acari: Hydrachnidia: Lebertiidae). Acarologia, 62(2): 302-316. https://doi.org/10.24349/esot-nc22
- Gerecke R., Gledhill T., Pešić V., Smit H. 2016. Chelicerata: Acari III. In: Gerecke R, ed. Süßwasserfauna von Mitteleuropa, Bd. 7/2-3. Springer-Verlag Berlin, Heidelberg, pp. 1-429. https://doi.org/10.1007/978-3-8274-2689-5
- Gurung M.M., Dorji C., Gurung D.B., Smit H. 2022a. Checklist of water mites (Acari: Hydrachnidia) of the Himalayan and Tien Shan Mountains. Ecol. Monten., 57: 8-23. https://doi.org/10.37828/em.2022.57.2
- Gurung M.M., Dorji C., Gurung D.B., Smit H. 2022b. Environmental factors affecting water mites (Acari: Hydrachnidia) assemblage in streams, Mangde Chhu basin, central Bhutan. J. Threat Taxa, 14(10): 21976-21991. https://doi.org/10.11609/jott.7979.14.10.21976-21991
- Lundblad O. 1969. Indische Wassermilben, hauptsächlich von Hinterindien. Ark. Zool., 22: 289-443.
- Jin D.C. 1997. Hydrachnellae-morphology systematics a primary study of Chinese fauna. Guizhou Science and Technology Publishing House, Guiyang.
- Pešić V., Ranga Reddy Y. 2009. New records of water mites (Acari: Hydrachnidia) from interstitial freshwaters of India, with descriptions of three new species. Zootaxa, 2158: 20-32. https://doi.org/10.11646/zootaxa.2158.1.2
- Pešić V., Smit H. 2011. A new species of Atractides Koch, 1837 (Acari, Hydrachnidia, Hygrobatidae) from Ethiopia, with a discussion on the biodiversity of the genus Atractides in the Afrotropical region. ZooKeys, 86: 1-10. https://doi.org/10.3897/zookeys.86.972
- Pešić V., Smit H. 2018. A checklist of the water mites of Central Asia with description of six new species (Acari, Hydrachnidia) from Kyrgyzstan. Acarologia, 58(1): 165-185. https://doi.org/10.24349/acarologia/20184236
- Pešić V., Kumar N., Kumar K. 2007. Two new species of water mites of the family Hygrobatidae (Acari: Hydrachnidia) from the Garhwal Himalayas (India). Syst. Appl. Acarol., 12: 161-166. https://doi.org/10.11158/saa.12.2.11
- Pešić V., Smit H., Gerecke R. 2012. A contribution to the knowledge of the genus Atractides Koch, 1837 (Acari: Hydrachnidia, Hygrobatidae) in France. Zootaxa, 3221: 60-68. https://doi.org/10.11646/zootaxa.3221.1.5
- Pešić V., Smit H., Bahuguna P. 2019. New records of water mites (Acari: Hydrachnidia) from the Western Himalaya with the description of four new species. Syst. Appl. Acarol., 24: 59-80. https://doi.org/10.11158/saa.24.1.5
- Pešić V., Smit H., Gurung M.M. 2022a. Neumania bhutana sp. nov. a new water mite from Bhutan (Acari, Hydrachnidia: Unionicolidae). Ecol. Monten., 54: 53-56. https://doi.org/10.37828/em.2022.54.7
- Pešić V., Smit H., Gurung M.M. 2022b. Torrenticolid water mites of Bhutan. Genera Torrenticola Piersig, 1896 and Neoatractides Lundblad, 1941 (Acari: Hydrachnidia: Torrenticolidae). Acarologia, 62(3): 821-860. https://doi.org/10.24349/xn0u-5px2
- Smit H. 2020. Water mites of the world with keys to the families, subfamilies, genera and subgenera (Acari: Hydrachnidia). Monogr. Ned. Ent. Ver., 12: 1-774.
- Smit H., Gurung M.M. 2022. Description of the first species of the water mite genus Aturus Kramer, 1875 from the Himalaya Mountains (Acari: Hydrachnidia: Aturidae). Zootaxa, 5169(5): 494-496. https://doi.org/10.11646/zootaxa.5169.5.8
- Smit H., Pešić V., Gurung M.M. 2022. The water mite genus Sperchon Kramer, 1877 in Bhutan (Acari: Hydrachnidia: Sperchontidae), with the description of three new species. Acarologia, 62(3): 754-762. https://doi.org/10.24349/pfqk-ad5d
- Yi T.C., Jin D.C., Wang X.J. 2010. Water mites of the subgenus Tympanomegapus Thor (Acari: Hygrobatidae: Atractides) from China. Int. J. Acarol., 36(5): 419-429. https://doi.org/10.1080/01647954.2010.483236



2023-01-02
Date accepted:
2023-01-25
Date published:
2023-01-30
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Pešić, Vladimir; Smit, Harry and Gurung, Mer Man
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



