Redescription of Bryobia pritchardi Rimando, 1962 (Acari: Tetranychidae), with an ontogeny of chaetotaxy
Li, Juan1 ; Yi, Tian-Ci2 ; Guo, Jian-Jun3 and Jin, Dao-Chao4
1Institute of Entomology, Guizhou University, Guiyang, Guizhou, 550025, P.R. China & Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Mountainous Region. Scientific Observing and Experimental Station of Crop Pest in Guiyang, Ministry of Agriculture, P. R. China, Guiyang 550025, P. R. China.
2Institute of Entomology, Guizhou University, Guiyang, Guizhou, 550025, P.R. China & Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Mountainous Region. Scientific Observing and Experimental Station of Crop Pest in Guiyang, Ministry of Agriculture, P. R. China, Guiyang 550025, P. R. China.
3Institute of Entomology, Guizhou University, Guiyang, Guizhou, 550025, P.R. China & Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Mountainous Region. Scientific Observing and Experimental Station of Crop Pest in Guiyang, Ministry of Agriculture, P. R. China, Guiyang 550025, P. R. China.
4✉ Institute of Entomology, Guizhou University, Guiyang, Guizhou, 550025, P.R. China & Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Mountainous Region. Scientific Observing and Experimental Station of Crop Pest in Guiyang, Ministry of Agriculture, P. R. China, Guiyang 550025, P. R. China.
2019 - Volume: 59 Issue: 1 pages: 73-110
https://doi.org/10.24349/acarologia/20194312ZooBank LSID: 29BF586F-406C-4406-8A15-9300F1F35468
Research article
Keywords
Abstract
Introduction
The subfamily Bryobiinae usually identified by their plesiotypic features, such as: the empodium bearing tenent hairs; the lack of silk production; the position of the duplex setae at the apex of the tarsus; declivate tip of the tarsus; three pairs of pseudanal setae usually present; and four pairs of propodosomal setae often present (Lindquist 1985). Numerous Bryobiinae taxa add more whorls of leg setae during ontogeny, a feature seen in no other Tetranychoidea (Lindquist 1985).
Bryobia is placed within the tribe Bryobiine, which is defined by its uncinate claws. The genus Bryobia is the largest genus of Bryobiinae and contains several species of significant economic importance in many parts of world (Hatzinikolis & Emmanouel 1991), it is distinctive by the following features: (1) Four pairs of propodosomal setae present, the anterior two pairs of which (v1, v2) are usually located on prominent projections over the gnathosoma; (2) the usual 12 pairs of dorsal hysterosomal setae; and (3) empodia II-IV with more than one pair of tenent hairs (Gonzalez 1977).
A total of 137 species of Bryobia are described, 19 of them from China (Migeon & Dorkeld 2006–2017; Hong et al. 2010; Hu & Chen 1993; He & Tan 1993; Wang & Cui 1991; Wang 1985, 1981; Wang & Zhang 1984; Ma & Yuan 1984, 1981, 1980). Beyond these descriptions, knowledge of the Bryobia mite fauna in China is relatively poor, but Bryobia pritchardi Rimando 1962 is common nationwide and in the east-Asian region. Bryobia pritchardi, was erected by Rimando in 1962 from Paederia foetida (Rubiaceae) in the Philippines; Ehara (1969) and Tseng (1990) collected it in Taiwan, from Paederia scandens; Wang (1981) and Ma et al. (1984) reported it from Hainan Island, China, collected from P. foetida (as P. scandens, a junior synonym) and Acalypha hainanensis (Euphorbiaceae); Ehara (1999) reported it in Japan (Shikoku and Kyushu islands) from P. scandens and Cucumis melo (Cucurbitaceae). Bryobia pritchardi is redescribed herein based on all immature stages collected from Drymaria cordata (Caryophyllaceae) in Hainan province, China. We also examined the type specimens of B. pritchardi, in order to compare the types and our specimens.
Recently great importance has been attached to understanding the patterns of setal additions throughout ontogenetic development of the Tetranychoidea (Khanjani et al. 2018; Seeman et al. 2017; Li et al. 2018, 2017; Xu et al. 2017; Yi et al. 2017, 2013; De Castro et al. 2015; Beard & Ochoa 2010; Beard & Walter 2010). There are few scholars who have payed attention to the ontogenetic patterns for species of Bryobia. Immature stages for some other species have been described, such as Auger & Migeon (2014), who noted the development of the prodorsal lobes during ontogeny and also provided leg setal counts. However, since the important work of Lindquist (1985), authors have provided full characteristic notations for a Bryobia sp. Until the important task of comparative homologization of leg setae is accomplished for the families of Tetranychoidea as a group, a great deal of data of potential importance to the systematics and classification of these groups will remain masked. We herein examine setal variations of female leg I based on 25 specimens, and also discuss other intraspecific morphological variation.
Material and methods
The mite specimens were mounted in Hoyer's medium on slides and examined using a Nikon DS–Ri2 microscope with differential interference contrast. Line drawings were prepared with the aid of a drawing tube attached to the microscope. All measurements were taken with the software Leica Application Suite V 4.4 and are given in micrometers (μm). Setae were measured from the centre of the setal base to the tip of the seta; distances between setae were measured from the centre of one setal base to that of another. Legs were measured from the base of the trochanter to the distal end of tarsus (excluding pretarsus). Terminology follows that of Lindquist (1985).
Results
Family Tetranychidae Donnadieu, 1875
\subsubsubsection*{Subfamily Bryobiinae Berlese, 1913
Tribe Bryobiini Reck, 1952
Genus Bryobia Koch, 1836}
Bryobia pritchardi Rimando, 1962: 9, Figs 3–4; Ehara 1969; 83, Figs 1–3; Wang 1981: 33, Fig. 19; Ma et al. 1984:103, Fig.8 (16); Tseng 1990: 15, Figs 28–32.
Bryobia pritchardi Rimando, 1962 (Figs. 1–28)
Material examined
Twenty-five females (slides NO. 1601090301–1601090325), seven males (1601090326–1601090332), one deutonymph (1601090333), eight protonymphs (1601090334–1601090341) and ten larvae (1601090342–1601090352), collected from Drymaria cordata in Hainan Xinlong Tropical Botanical Garden, Hainan province, P. R. China, 106°16′39″E, 19°54′12″N, elevation 71 m, on 9 Jan. 2016, by Juan Li. The specimens studied are deposited in Institute of Entomology, Guizhou University, P. R. China (GUGC). Holotype female, ex. Paederia foetida, from Lipa City, Batangas, Philippines, coll. L.C. Rimando, currently on loan to the Smithsonian in Washington D. C.
Diagnosis
With the generic characters and:
Female — Body subovate. Prodorsal setae each on four well-developed anterior lobes, of which inner row shares more or less triangular broad base and well separated distally, with obvious incision; dorsal transverse line reaching to the tops of the outer lobes and crossing or reaching up to the incision of inner lobes. The line joining tips of second pair of propodosomal seta (v2) located on the outer lobes generally passes slightly under the bases of the first pair (v1). Dorsal setae spatulate to orbicular, rough and serrate. Stylophore longer than wide. Peritreme anastomosed distally in hook-liked enlargement with a shallow incision. Ventral setae smooth and filiform with the exception of lanceolate and serrate 1c and 2b; sacculus elongate, constricted medially and bulbous distally. Leg segment setal formula as follows: trochanters 1–1–1–1; femora 16\textasciitilde{}17–10–5–5; genua 8–5–6–6; tibiae 15 (1φ)–9–9–9; tarsi 19 (5ω+2dup)–17 (0ω+1dup)–15 (0ω)–14 (1ω).
Male — Prodorsal setae on weakly developed anterior lobes: inner lobes with large fused base, incision between median lobes wide and shallow; outer lobes on weak projectons. Leg segment setal formula as follows: trochanters 1–1–1–1; femora 16\textasciitilde{}17–10–5–5; genua 8–5–6–6; tibiae 15 (1φ)–9–9–9; tarsi 18(9ω+2dup)–16(0ω+1dup)–14 (1ω)–14 (1ω). Empodia I–IV same as that of females; shaft of aedeagus abruptly constricted towards its distal part, ending in needle-like round tip.
Redescription
Female (n=10)
Dorsum (Fig. 1; 5D; 24B–D; 25A, E; 26D; 28C) — Length of idiosoma 564–713 (including gnathosoma), 468–615 (excluding gnathosoma). Body oval, except for posterior edge of hysterosoma somewhat ``W'' shaped (Figs. 3E; 25B). Body striae fingerprint-like, mostly transverse, striae in depressed areas denser; integument also with several large irregular folds (Figs. 1A; 24B–D; 25A; 26D). Prodorsal setae on well-developed anterior lobes (Figs. 5D; 25E). Inner lobes sharing more or less triangular broad base and well separated distally, with obvious incision (incision length 18–21). Outer prodorsal lobes teat-like, 22–31 in length, not extending beyond base of inner lobes, incision between outer and median lobes deep and wide. Setae v1 14–18, v2 15–22, both spatulate and serrated (Figs. 1B, 5D). Dorsal setae spatulate to orbicular rough, serrate, with small bulge-like bases, subequal in length (Figs. 1B; 1C; 28C). Dorsocentral setae (c1, d1 and e1) short. Setal lengths: sc1 10–17, sc2 8–18, c1 7–13, c2 8–12, c3 10–13, d1 9–12, d2 9–13, d3 9–12, e1 9–13, e2 10–12, e3 9–12, f1 10–14, f2 12–15, h1 10–14. Distances between setal bases: v1–v1 13–26, v2–v2 100–128, c1–c1 63–75, d1–d1 65–89, e1–e1 60–83, c1–d1 95–123, d1–e1 80–97. Sacral setae (f1 and f2) contiguous and posited posteriorly.



Venter (Figs. 2; 3B, D; 24A; 26C) — Ventral setae smooth and filiform with the exception of lanceolate and serrated 1c and 2b. Anterior intercoxal region without striae (irregular folds occasionally present); lateral striae on hysterosoma U-shaped, sparse. Area immediately anterior to genital flap with irregular longitudinal striae, pregenital setae (ag) 21–28 long. Genital setae (g1-2) 30–37 long. Pseudanal setae (ps1-3) 28–31 long. Ventral opisthonotal setae (h2-3) 19–24 long (Figs. 24A; 26C). Spermatheca with funnel-shaped sacculus (Figs. 3B, 3D). Length of other ventral setae: 1a 31–34, 1b 38–49, 1c 27–38, 2b 12–28, 3a 24–35, 3b 12–18, 4a 24–32, 4b 10–15. Distances between setae: 1a–1a 60–70, 3a–3a 81–91, 4a–4a 63–113, ag–ag 61–75, g1–g1 26–37, g2–g2 67–103.



Gnathosoma (Figs. 3A, C; 25C–D; 28A–B) — Stylophore longer than wide. Peritreme anastomosed and enlarged distally with a shallow incision. Tracheal system variation as shown in Fig. 4D.






All tactile setae, except for setae dPFe, on palp slender and bare. Setae dPFe orbicular and serrate, long 14–17. Seta l′PGe 15–22 in length. Palptibia with a bidentate `claw' and three setae. Seta l′PTi long 5–7, located near base of tibial claw. seta dPTi 12–15, about twice as long as seta l″PTi 5–7. Palptarsus elongated, with three tactile setae (a, b, c), three eupathidia and one solenidion (Fig. 3C). Eupathidia (ul′ζ, ul″ζ) and solenidion (ω) slightly shorter than terminal sensillum (suζ), (Figs. 25C–D; 28A–B): ω 5–7, ul′ζ 3–5, ul″ζ 3–5, suζ 7–9, a 9–11, b 5–8 , c 3–4.
Spermatheca (Figs. 3B, D) — Sacculus elongate, constricted medially and bulbous distally.
Legs (Figs. 6–7) — Empodia I–IV bearing two rows of ventrally directed hairs. True claws I pad-like with three pairs of tenant hairs (Fig. 5F), claw II–IV uncinate with several pairs of hairs (Fig. 5G).



Tarsus I with two pairs of duplex setae distal and adjacent, proximal solenidion ω′ 55–64, distal solenidia ω″ 62–82, ω′1 34–44, ω′2 26–35, ω″2 28–40, ω′3 23–42, ω″3 23–35 in length; tarsus II with one pair of duplex setae: ω′ 29–40 in length. Tarsi III–IV without duplex setae and tarsus III without solenidia, tarsus IV with one solenidion ω′1 13–20.
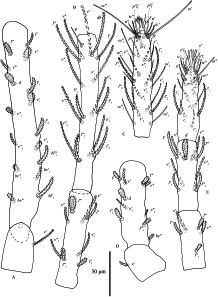





All leg setae barbed (Figs. 6–7). Number of phaneres on legs I–IV (number of solenidia and eupathidia respectively shown in parentheses):
Trochanter – Femur – Genu – Tibia – Tarsus
leg I – 1 – 16-17 – 8 – 15 (1) – 18 (7ω+3)
leg II – 1 – 10 – 5 — 9 – 15 (1ω+3)
leg III – 1 – 5 – 6 – 9 – 15 (0ω+0)
leg IV – 1 – 5 – 6 – 9 – 14 (1ω+0)
One solenidion φ 17–24 on tibia I. Tibiae II–IV without solenidia. Lengths of legs (measured from trochanter to tarsus): leg I 609–667, leg II 342–379, leg III 326–411, leg IV 425–455. Lengths of leg segments: trochanter I 40–52, femur I 204–228, genu I 90–106, tibia I 151–170, tarsus I 109–120; trochanter II 35–42, femur II 91–105, genu II 63–70, tibia II 72–81, tarsus II 76–84; trochanter III 39–54, femur III 80–109, genu III 58–74, tibia III 73–90, tarsus III 83–98; trochanter IV 41–54, femur IV 115–137, genu IV 70–88, tibia IV 88–100, tarsus IV 95–109.
Leg setation as shown in Figures 6–7. We examined 25 type specimens: no variation was found on the genu, tibia and trochanter. Anomalies on tarsus I: 2 specimens lack seta v′4; 2 specimens lack seta l″2, 1 specimen lacks solenidion ω′2, 1 specimen without ω″3, 1 specimen lacks v″2, v″3 and v′4, 1 specimen lacks setae ω′2 and l″2; 1 specimen lacks setae ω′3 and v′4. Anomalies on femur I: the setae of femur I vary in number and position: femur I with 15\textasciitilde{}17 setae, including nine ``steady'' setae (d, v′–v″, l′–l″, bv″, bv′1, bl′1–bl″1) shown on deutonymph femur I, remaining setae (l′1–l″1, l′2–l″2, v′1–v″1, v′2–v″2) usually varying in number and position (Fig. 23; Table 3).
Male (n=7)
Dorsum (Fig. 8) — Length of idiosoma 390–502 (including gnathosoma), 308–410 (excluding gnathosoma). Prodorsum with 4 pairs of setae and with weakly developed anterior lobes: inner lobes with large fused base forming cone-shaped projection, incision between median lobes wide and shallow (Fig. 5E). Distance between first (v1) and second (v2) pair of propodosomal setae 20–34 and 58–97, respectively. Seta v2 17–25 about three times as long as the seta v1 7–12. Dorsal body setae spatulate to orbicular, rough, serrate, inserted on small bulge-like structures, subequal in length: sc1 10–15, sc2 10–15, c1 8–13, c2 9–15, c3 7–11, d1 7–15, d2 7–15, d3 8–14, e1 6–11, e2 8–14, e3 7–14, f1 8–12, f2 8–11, h1 9–13. Distances between setal bases: v1–v1 20–34, v2–v2 58–97, c1–c1 33–52, d1–d1 28–44, e1–e1 34–59, c1–d1 45–67, d1–e1 49–67. Sacral setae (f1 and f2) in marginal position.



Venter (Fig. 9) — All ventral setae smooth, including 1c and 2b. Striate sparse, irregularly transverse when present; area immediately anterior with few irregular striae, setae ag 14–22; genital and pseudanal setae (ps1-5), subequal in length 9–13, much shorter than those of female (Fig. 10B; 27A–C); ventral opisthosomal setae (h2-3) 5–8 long. Almost all ventral setae much shorter than those of female: 1a 29–35, 1b 33–42, 1c 31–35, 2b 17–26, 3a 19–27, 3b 13–20, 4a 23–29, 4b 16–27. Distances between setae: 1a–1a 39–55, 3a–3a 53–59, 4a–4a 37–48, ag–ag 17–29.



Gnathosoma (Figs. 10A, C) — All tactile setae on palp slender, bare except setae dPFe orbicular, serrate, length 14–17. Subcapitular seta m smooth, length 19–29, distance m–m 18–27. Seta l′PGe 20–24 in length. Palptibia with bidentate `claw', similar to female. Seta l′PTi length 14–23, seta dPTi length 18–27, about twice as long as seta l″PTi, length 7–12. Palptarsus elongated, with three tactile setae (a, b, c), three eupathidia and one solenidion (Fig. 10C). Solenidion ω length 13–18, slightly thinner and longer than female's. Eupathidia (ul′ζ, ul″ζ) slightly shorter than terminal sensillum (suζ): ul′ζ 5–9, ul″ζ 6–8, suζ 8–15, a 10–15, b 6–11, c 12–22.
Aedeagus (Figs. 10D–E) — Shaft abruptly constricted towards distal part, ending in needle-like round tip.



Legs (Figs. 11–12) — Empodia I–IV similar to female (Figs. 10F–G).






All leg setae barbed (Figs. 11–12). Number of phaneres on legs I–IV (number of solenidia and eupathidia respectively shown in parentheses):
Trochanter – Femur – Genu – Tibia – Tarsus
leg I – 1 – 16-17 – 8 – 15 (1) – 17 (11ω+3)
leg II – 1 – 11 – 5 – 9 – 14 (1ω+3)
leg III – 1 – 6 – 6 – 9 – 14 (1ω+0)
leg IV – 1 – 6 – 6 – 9 – 14(1ω+0)
Tarsus I: two pairs of duplex setae distal and adjacent, proximal solenidion ω′ 54–67, distal solenidia ω″ 61–74, ω″1 35–57, ω′1 43–63, ω′2 38–48, ω″2 34–57, ω′3 53–67, ω″3 42–63, ω′4 31–42, ω″4 42–60, ω′5 40–59 in length; tarsus II with one pair of duplex setae: ω′37–45 in length; tarsi III–IV without duplex setae and tarsus III without solenidia, tarsus IV with one solenidion ω′ long 17–22.
One solenidion φ 30–36 on tibia I. Tibiae II–IV without solenidia. Leg I length 624–692 (measured from trochanter to tarsus), longer than body, length of other legs: leg II 254–384, leg III 333–362, leg IV 384–417. Length of leg segments: trochanter I 42–50, femur I 205–230, genu I 96–114, tibia I 164–180, tarsus I 108–127; trochanter II 35–39, femur II 92–123, genu II 60–68, tibia II 71–81, tarsus II 70–86; trochanter III 33–46, femur III 75–99, genu III 52–65, tibia III 65–84, tarsus III 70–86; trochanter IV 39–45, femur IV 84–114, genu IV 70–76, tibia IV 86–96, tarsus IV 92–100*.
Deutonymph (n=1)
One specimen measured: length of idiosoma 542 (including gnathosoma), 475 (excluding gnathosoma).
Dorsum (Fig. 13) — Prodorsal lobes and dorsal striae similar in shape to female (Fig. 5C), outer lobes do not reach level of inner lobe incision. Dorsal body setae inserted on tubercles (stronger in posterior area), small, orbicular to obovate. Setae v1 and v2 longest dorsal setae, subequal in length. Distance between first (v1) and second (v2) pair of propodosomal setae insertions 15 and 70, respectively.



Dorsocentral setae c1, d1 and e1 short, and setae f1 and f2 in marginal position. Lengths of dorsal setae: v1 11, v2 12, sc1 10, sc2 9, c1 6, c2 9, c3 8, d1 6, d2 9, d3 9, e1 7, e2 10, e3 9, f1 10, f2 7, h1 8. Distances between setal bases: c1–c1 46, d1–d1 30, e1–e1 29, c1–d1 105, d1–e1 48.
Venter (Fig. 14) — All ventral setae smooth with the exception of 1c, 2b and 3b which are lanceolate, serrate, and seta 1b is barbed: 1a 27, 1b 20, 1c 9, 2b 13, 3a 17, 3b 9, 4a 12, 4b 8, ag 7, g1, 10, ps1 7, ps2 8, ps3 7, h2 10, h3 9. Distances between setal bases: 1a–1a 49, 3a–3a 63, 4a–4a 59, g1–g132, ag–ag 47.



Gnathosoma (Fig. 22C) — Subcapitular seta m smooth (Fig. 14), long 22, subequal to distance m–m 25; seta dPFe 18 serrate and columned. Seta l″PGe 19, dPTi 15, l′PTi 12, l″PTi 10. Palp tarsus with clavate spinneret, suζ 7 in length with nipple-like tip. Single solenidion, ω 5 in length, two eupathidia (ul′ζ and ul″ζ) subequal, length 5, three normal setae: seta a 12, b 6, c 4.
Legs (Fig. 15) — True claws and empodia similar to that of female. All leg setae barbed (Fig. 15). Number of phaneres on legs I–IV (number of solenidia and eupathidia respectively shown in parentheses):



Trochanter – Femur – Genu – Tibia – Tarsus
leg I – 1 – 9 – 4 – 9 (1) – 13 (3ω+3)
leg II 1 – 3 – 4 – 5 – 9 (1ω+3)
leg III 1 – 2 – 2 – 5 – 10 (1ω+0)
leg IV 1 – 2 – 2 – 5 – 10 (0ω+0)
Tarsus I: two pairs of duplex setae distal and adjacent, proximal solenidion ω′ 41, distal solenidia ω″ 55, ω″1 17; tarsus II with one pair of duplex setae: ω″ 17 in length; tarsi III–IV without duplex setae, tarsus III with one solenidion ω″ 17. Tarsus III with solenidion well-separated from tactile setae, proximal.
One solenidion φ 13 on tibia I. Tibiae II–IV without solenidia. Length of leg I 317 (measured from trochanter to tarsus), shorter than body, length of other legs: leg II 224, leg III 223, leg IV 241. Length of leg segments: trochanter I 25, femur I 97, genu I 54, tibia I 76, tarsus I 64; trochanter II 21, femur II 58, genu II 43, tibia II 52, tarsus II 52; trochanter III 27, femur III 55, genu III 39, tibia III 46, tarsus III 56; trochanter IV 25, femur IV 64, genu IV 43, tibia IV 51, tarsus IV 59.
Protonymph (n=5)
Dorsum (Fig. 16) — Length of idiosoma 416–453 (including gnathosoma), 338–394 (excluding gnathosoma). Dorsal striae similar in shape to female. Propodosomal lobes (Fig. 5B) shorter than those of deutonymph; rest of dorsum resembles that of deutonymph. Prodorsal lobes weakly developed, tubercle like, v1 10–15, spatulate and serrate, shorter than v2 29–42, spatulate and serrate with spiky appearance. Dorsocentral setae (c1, d1 and e1) short. Setal lengths: sc1 7–10, sc2 7–9, c1 5–7, c2 5–11, c3 7–8, d1 4–7, d2 5–8, d3 6–8, e1 5–7, e2 7–11, e3 6–7, f1 6–8, f2 6–8, h1 6–9. Distances between setal bases: v1–v1 12–15, v2–v2 44–55, c1–c1 26–43, d1–d1 26–41, e1–e1 27–35, c1–d1 58–68, d1–e1 26–69, h1–h1 19–37.



Venter (Fig. 17) — Ventral setae shorter than those of deutonymph. All ventral setae smooth with the exception of 1c, 2b and 3b lanceolate and serrate: 1a 14–20, 1b 19–22, 1c 10–13, 2b 6–10, 3a 11–16, 3b 7–8, ag 6–9, ps1 7–10, ps2 6–8, ps3 6–8, h2 7–11, h3 10–10. Distances between setal bases: 1a–1a 36–42, 3a–3a 37–65, ag–ag 27–52.



Gnathosoma (Figs. 22A, D) — Subcapitular seta m 20–24 smooth, slightly longer than distance m–m 16–20; seta dPFe 12–16 serrate and columned, seta l′PGe length 13–16, dPTi 11–12, l′PTi 8–11, l″PTi 5–7. Palp tarsus with clavate spinneret, suζ 8–9. Single solenidion, ω 6–7, two eupathidia (ul′ζ and ul″ζ) subequal in length 3–5, three normal setae: a 8–11, b 5–6, c 3–6.
Legs (Fig. 18) — True claws and empodia resembles those of female. All leg setae barbed (Fig. 18). Number of phaneres on legs I–IV (number of solenidia and eupathidia respectively shown in parentheses):



Trochanter – Femur – Genu – Tibia – Tarsus
leg I – 0 – 3 – 4 – 5 (1) – 9 (2ω+3)
leg II – 0 – 3 – 4 – 5 – 7 (1ω+3)
leg III – 0 – 2 – 2 – 5 – 8 (0ω+0)
leg IV – 0 – 2 – 2 – 5 – 6 (0ω+0)
Tarsus I: two pairs of duplex setae distal and adjacent, ω′ 28–33, ω″ 44–53 in length; tarsus II with one pair of duplex setae: ω″ 19–20 in length; tarsi III–IV without solenidia.
One solenidion φ 10–11 on tibia I. Tibiae II–IV without solenidia. Length of leg I 207–223 (measured from trochanter to tarsus), shorter than body, length of other legs: leg II 138–177, leg III 158–164, leg IV 158–171. Length of leg segments: trochanter I 21–24, femur I 53–65, genu I 37–39, tibia I 41–48, tarsus I 49–55; trochanter II 17–21, femur II 35–45, genu II 21–35, tibia II 28–32, tarsus II 39–41; trochanter III 21–24, femur III 32–38, genu III 25–28, tibia III 33–38, tarsus III 39–45; trochanter IV 22–25, femur IV 37–44, genu IV 26–28, tibia IV 29–35, tarsus IV 45–46*.
Larva (n=6)
Dorsum (Fig. 19) — Length of idiosoma 301–361 (including gnathosoma), 249–338 (excluding gnathosoma). Dorsal striae irregular, not finger-print like, sparse on opisthonotum. Prodorsal lobes absent, v1 very short, seta v2 18–23 about three times as long as seta v1 6–7 (Fig. 5A). Other dorsal setae resemble that of protonymph. Dorsocentral setae (c1, d1 and e1) short. Setal lengths: sc1 8–16, sc2 8–10, c1 5–7, c2 6–9, c3 9–11, d1 5–5, d2 7–8, d3 5–5, e1 5–6, e2 6–6, e3 6–10, f1 9–15, f2 9–13, h1 9–12. Distances between setal bases: v1–v1 12–21, v2–v2 43–51, c1–c1 29–39, d1–d1 15–23, e1–e1 14–20, c1–d1 40–64, d1–e1 39–47.



Venter (Fig. 20) — Ventral setae shorter than those of protonymph: 1a 14–17, 1b 12–20, 3a 16–19, ps1 7–8, ps2 6–8, ps3 7–8, h2 10–13, h3 10–12. Distances between setal bases: 1a–1a 33–39, 3a–3a 49–67.



Gnathosoma (Figs. 22B, E) — Seta dPFe serrate and columned, 10–12 in length, Seta l″PGe 9–12, dPTi 11–12, l′PTi 8–11, l″PTi 5–6. Palp tarsus, suζ 6–7. Single solenidion, ω 6–8, two eupathidia (ul′ζ and ul″ζ) subequal in length 4–5, three normal setae: a 8–11, b 5–6, c 4.
Legs (Fig. 21) — All leg setae barbed. Number of phaneres on legs I–IV (number of solenidia and eupathidia respectively shown in parentheses):






Trochanter – Femur – Genu – Tibia – Tarsus
leg I – 0 – 3 – 4 – 5 (1) – 5 (1ω+3)
leg II – 0 – 3 – 4 – 5 – 6 (1ω+2)
leg III – 0 – 2 – 2 – 5 – 6 (0ω+0)
Tarsus I: one pair of duplex setae, ω″ 35–53 in length; tarsus II with one pair of duplex setae: ω″ 16–23; tarsi III–IV without solenidia.
One solenidion φ on tibia I, length 9–14. Tibiae II–IV without solenidia. Length of leg I 160–177 (measured from trochanter to tarsus), shorter than body, length of other legs: leg II 129–136), leg III 137–153. Length of leg segments: trochanter I 18–23, femur I 40–48, genu I 24–28, tibia I 33–35, tarsus I 45–49; trochanter II 17–22, femur II 29–34, genu II 18–25, tibia II 22–27, tarsus II 35–37; trochanter III 15–26, femur III 30–36, genu III 21–25, tibia III 26–29, tarsus III 38–43.






Leg
Co
Tr
Fe
Ge
Ti
Ta
I
L
1b
-
d, v′, bv″
(l), (v)
db,
(l), (v), φ
(pζ), (ft), (u), (pv), ω″
P
1c
-
-
-
-
(tc), ω′, l′1, v′1
D
-
v′
v″, l′, , (bv1), (bl1)
-
(l1), (v1)
l″1, v″1, (v2), ω″1
F
-
-
l″, l′1, l′2, (v1), (v2)
(l1), (v1)
(v2), (v3), (l2)
(ω2), (v3), (l2), (ω3), v′4
M
-
-
l″, (l1), (v1), (l2), v′2
(l1), (v1)
(v2), (v3), (l2)
ω′1, (ω2), (ω3), (l2), (v3), (ω4), ω′5
II
L
-
-
d, v′, bv″
(l), (v)
d, (l), (v)
(pζ), (ft), (u), (pv), ω″
P
-
-
-
-
-
(tc)
D
2b
v′
-
-
-
(v1)
F
-
-
v″, (l), (v1), (l1)
l″1
(l1), (v1)
(v2), (v3), (l1)
M
-
v″, (l), (v1), l″1, (l2)
l″1
(l1), (v1)
(v2), v″3, (l1)
III
L
-
-
d, ev′
v′, l′
d, (l), (v)
(pv), (ft), (u)
P
3b
-
-
-
-
(tc)
D
-
v′
-
-
-
(v1)
F
-
-
(v), l′1
d, v″, (l1)
(l1), (v1)
(v2), v″3, (l1)
M
-
-
(v), l″, l′1
d, v″, (l1)
(l1), (v1)
(v2), (l1)
IV
P
4a,4b
-
d, ev′
v′, l′
d, (l) ,(v)
(pv), (u), ( ft)
D
-
v′
-
-
-
(tc), (v1)
F
-
-
v′, l′, l′1
d, (l1), v″
(v1), (l1)
ω′, (v2), (l1)
M
-
-
(v), l′, l′1
d, (l1), v″
(v1), (l1)
ω′, (v2), (l1)
†: Setae are indicated
in the stage in which they first appear. L: larva, P: protonymph D: deutonymph, F: female, M: male. The number of female
tarsus I and femur I are 16, 18 (7ω+3), respectively.

Remarks
Comparison with the type specimens showed a perfect match in the setal number of adult female legs I–IV as follows: trochanters 1–1–1–1; femora 16–10–5–5; genua 8–5–6–6; tibiae 15 (1φ)–9–9–9; tarsi 19 (5ω+2dup)–17 (0ω+1dup)–15 (0ω)–14 (1ω). The type specimens also match in dorsal setae, which are spatulate to orbicular, rough, serrate, with small bulge-like bases (Figure 25B). Additionally, they share similar prodorsal lobes, which are well-developed, the inner lobes sharing a more or less triangular broad base, and are well separated distally with an obvious incision (Figure 25E).






Bryobia pritchardi is most similar to B. praetiosa Koch, 1836, with both species characterized by: the shape of the dorsal setae; having strong anterior propodosomal projections; and empodia II-IV about as long as the claw, with two rows of ventrally directed tenent hairs. B. pritchardi is diagnosed by empodia I, which bears two rows of three ventrally directed tenent hairs, and true claws I have 3 pairs of tenant hairs. In B. praetiosa, empodia I has only a single pair of tenent hairs, and true claw I is pad-like and has more than three pairs of tenant hairs (Pritchard & Baker 1955; Ehara 1956).
We also note the recent work of Fashing (2016), who demonstrated that the prodorsal lobes, a critical feature for species and even genus level differentiation, is prone to intraspecific variation. However, here we found no variation within our populations, but we do draw attention to the differences between life stages and sexes (Fig. 5).
The addition of leg setae on adult spider mites is potentially more variable in the Bryobiinae than in the Tetranychinae because of the richer setation in the former, especially on the tarsus and femur of adults. On occasion, in the genus Bryobia, the tactile setae on femur and tarsus I may vary in number within the same species, having fewer or more than the typical pattern (Tables 2, 3). From 25 specimens of B. pritchardi, we found that the typical pattern in this species for tarsus I of the female is 28 setae, including seven solenidia (Table 1). Ten setae (pζ′–pζ″, ft′–ft″, u′–u″, pv′ζ–pv″, tc′–tc″) are ``constant setae'' and are typically added earlier in ontogeny, whereas the remaining setae, which tend to be added later in ontogeny (7 solenidia and 11 tactile setae), are mutative, which is very similar to the results found by Seeman et al. (2017) for Eotetranychus, and also Wauthy's (1998) work on Tetranychus.
Specimens
NR
NL
Specimens
NR
NL
n1
28
27 - v′4
n14
28
28
n2
28
29 + ω′1
n15
27 -
v′4
28
n3
28
27 - ω′2
n16
28
28
n4
28
28
n17
28
27 - ω″3
n5
28
28
n18
28
28
n6
28
28
n19
28
28
n7
28
28
n20
28
26 - ω′3, v′4
n8
26 - ω′2, l″2
27- v′4
n21
28
28
n9
28
28
n22
28
28
n10
28
28
n23
28
28
n11
28
28
n24
28
27 - l″2
n12
28
28
n25
27 - l″2
27 - l″2
n13
25 -
v″2, v″3, v′4
28
Notes: “NR” present the number of
right leg tarsus I female; “NL” present the number of left leg tarsus I female (right
leg and left leg from one specimen); “28” indicate tarsus I female with 28 setae (including
seven solenidia (\textit{ω′}–\textit{ω″}, \textit{ω″}1, \textit{ω′}2–\textit{ω″}2, \textit{ω′}3–\textit{ω″}3), 21 tactile setae (\textit{pζ′}–\textit{pζ″}, \textit{ft′}–\textit{ft″}, \textit{u′}–\textit{u″}, \textit{pv′ζ}–\textit{pv″}, \textit{tc′}–\textit{tc″}, \textit{l′}1–\textit{l″}1, \textit{l′}2–\textit{l″}2, \textit{v′}1–\textit{v″}1, \textit{v′}2–\textit{v″}2, \textit{v′}3–\textit{v″}3, \textit{v′}4), which is
the typical pattern in tarsus I.

Most additions or losses were expressed asymmetrically, with only one specimen (n=25, Table 2) with a symmetrical setal loss. The asymmetrical variation in the other nine specimens was the loss of seta v′4 (n=2), loss of seta l″2 (n=2), loss of solenidion ω′2 or ω″3 (n=2), addition of solenidion ω′1 (n=1); loss of setae v″2, v″3, v′4 (n=1); loss of ω′2, l″2 (n=1); and loss of ω′3, v′4 (n=1) (Table 2).
Femur I is also variable (Fig. 23; Table 3) with two main types of variation: (1) 50% specimens (n=13) present the symmetry on femur I (one type): including ten specimens are type ``16 (femur I setae number of left)–16 (femur I setae number of right)''; two with ``17–17''; one with ``15–15''; (2) 50% specimens (n=12) show asymmetry on femur I (one type): eight specimens with ``17–16''; three with ``15–16''; one with ``15–17'' (Table 3).
Discussion
Ontogenetic development of podosoma
The ontogenetic changes of B. pritchardi are listed in Table 1.
The setation and ontogeny of idiosomal setae is typical for the Bryobiinae (Lindquist 1985). All dorsal setae and pseudanal setae are present throughout all stages. Ventral setae (1c, 2c and 3b) are added in the protonymph, 2b, 4a and 4b are added in the deutonymph. Aggenital setae ag are added in the protonymph; the first pair of genital setae g1 are added in the deutonymph. The second pair g2 are added in the adults, with males grouping their genital setae closely as a series of five pairs on the genitoanal valves (Lindquist 1985; Figs. 27A–B).
Specimens
R
L
Specimens
R
L
n1
16
16
n14
17
16
n2
16
16
n15
16
17
n3
16
16
n16
17
16
n4
16
16
n17
17
16
n5
16
16
n18
17
16
n6
16
16
n19
17
16
n7
16
16
n20
17
16
n8
16
16
n21
16
17
n9
16
16
n22
15
16
n10
15
16
n23
15
16
n11
17
17
n24
15
16
n12
17
17
n25
15
17
n13
15
15

Ontogenetic development of leg setae
Leg I: Compared with the basic pattern described by Lindquist (1985), there are variations of leg setae found on the tarsus and femur, while almost no variation was found on the tibia, genu and trochanter.
On the larva, the number of setae from the tarsus to trochanter is 8 (1ω)–5 (1ω)–4–3–0. There are eight setae (pv′–pv″, pζ′–pζ″, ft′–ft″, u′–u″) and one solenidion (ω″) on the tarsus, five setae (db, v′–v″, l′–l″) and one solenidion (φ) on tibia I, four setae (v′–v″,l′–l″) on the genua, and three setae (d, v′, bv″) on the femora. Larval setae show the basic pattern described by Lindquist (1985). On the protonymph, the setal counts are 12(2ω)–5(1ω)–4–3–0, with four setae (tc′–tc″, l1′, v1′) and one solenidion ω′ usually added on the tarsus. On the deutonymph, the setal counts from tarsus to trochanter are 16 (3ω)–9 (1ω)–4–9–1: there are nine setae on femur I, compared with the basic pattern described by Lindquist (1985), femur I lacks seta l″ but seta bv1′ is present.
On adults of B. pritchardi, the number of setae from tarsus to trochanter are 20 (7ω)–15(1ω)–8–16–1. The additions on the tarsus are much richer than in the Tetranychinae: five setae l′2–l″2, v′3–v″3, v′4 and four solenidia ω′2–ω″2, ω′3–ω″3 are added on tarsus I. Compared to the species examined by Lindquist (1985), setae v′4–v″4 are absent on tibia I. On the femora, the complement of setae on adult female is more variable, with setae l″, l″1, v′1, l″2 suppressed on B. pritchardi.
Leg II: The setae basically show the basic pattern as described by Lindquist (1985) from larva to deutonymph, but there are variable changes in adult females of B. pritchardi (Tables 2, 3).
On the larva, the number of setae for B. pritchardi, from tarsus to trochanter are 8 (1ω)–5–4–3–0; setae tc′–tc″ are added to the tarsus on the protonymph; on the deutonymph, v′1–v″1 are added on the tarsus, while three setae (l′–l″, v″) are suppressed on femur compared with Lindquist (1985). The setal counts in females of B. pritchardi are 18 (1ω)–9–5–10–1. The basic pattern described by Lindquist is 16 (1ω)–9–6–10–1. Compared to Lindquist (1985), setae v′3–v″3 are present on the tarsus, seta l′1 are lacking on the genu, and setae v″, l′–l″ are added on femur for the adult female of B. prtichardi.
Leg III: The setae from larva to protonymph in two species basically show the basic pattern as described by Lindquist (1985): 6–5–2–2–0 (in larva), 8–5–2–2–0 (in protonymph). On the deutonymph, 10–5–2–2–1 is the setae counts in B. pritchardi: the tarsus without solenidia ( ω″ is suppressed); and setae d, v′, respectively, are absent on the genu and femur. As with adult females, setae v″3 and v′ respectively are added on the tarsus and femur.
Leg IV: The complement of setae of two species from larva to deutonymph is less variable. The number of protonymphal setae in this species from tarsus to trochanters are 6–5–2–2–0, showing the basic pattern, as does the deutonymph (10–5–2–2–1), except that setae d suppressed on the genua. On adult females, setae l″ (instead of v″) are present on the genua, and l′ (instead of v″) are present on femora.






Acknowledgements
We express our thanks to Prof. Owen Seeman (Queensland Museum, Australia) for providing some literature; and to the reviewers for their insightful comments on the manuscript. We also thank Alain Migeon, who gave valuable comments. We sincerely thank Dr. Leonila Corpuz-Raros (University of the Philippines), and Dr. Ronald Ochoa (Smithsonian Museum of Natural History, Washington DC) who helped borrow the holotype from Philippines. This study was supported by the National Natural Science Foundation of China, No. 31472034 and No. 31750002; the China Postdoctoral Science Foundation, No. 2015M582576, the Science and the Program Foundation for Talents of Guizhou University, No. [2010] 015, the Innovation Team Program for Systematic and Applied Acarology, No. [2014] 33 and the Project of Monitoring and Control of crop pests, disease outbreaks from China Agricultural Department (2017), and the International cooperation base for insect evolutionary biology and pest control ([2016]5802). Science and Technology Plan Project of Guizhou Province, No: Qian Ke He [2018] 5781. The Graduate Education Innovation Project of Guizhou Province, NO: Qian Jiao He YJSCXJH (2018) 065.
References
Baker E. W., Tuttle D. M. 1994. A guide to the spider mites (Tetranychidae) of the United States. West Bloomfield, USA, Indira Publishing House, 347pp.
Beard J., Ochoa R. 2010. Ontogenetic modification in the Tuckerellidae (Acari: Tetranychoidea). International Journal of Acarology, 36 (2): 169–173. doi:10.1080/01647950903555459 ![]()
Beard J., Walter D. E. 2010. New spider mite genus (Prostigmata: Tetranychidae) from Australia & New Zealand, with a discussion of Yezonychus Ehara. Zootaxa, 2578: 1–24. doi:10.11646/zootaxa.2578.1.1 ![]()
De Castro E. B., Ramos F. A. M., Feres R. J. F., Ochoa R. 2015. A new species of Tenuipalpus Donnadieu (Acari: Tenuipalpidae) from Brazil, with ontogeny of chaetotaxy. Systematic & Applied Acarology, 20 (3): 339–356. https://doi.org/10.11158/saa.20.3.11
Ehara S 1956. Some spider mites from Northern Japan. Zoological Institute, Hokkaido University, 12: 244–258
Ehara S. 1969. The tetranychoid mites of Taiwan (Acarina : Prostigmata). The Journal of the Faculty of Education, Tottori University, Natural Science, 20: 79–103.
Ehara S. 1999. Revision of the spider mite family Tetranychidae of Japan (Acari, Prostigmata). Species Diversity, 4: 63–141. doi:10.12782/specdiv.4.63 ![]()
Fashing N. J., Ueckermann E. A., Fashing P. J., Nguyen N., Back A. M. & Allison L. A. 2016. Bryobia abyssiniae (Prostigmata: Tetranychidae), a new species from the highlands of Ethiopia. International Journal of Acarology, 42 (7), 366–376. doi:10.1080/01647954.2016.1194891 ![]()
Gonzalez R. H. 1977. The tetranychoid mites of Chile. I. The subfamily Bryobiinae (Acari: Tetranychidae). Acarologia, 19(4), 633–653.
Hatzinikolis E. N., Emmanouel N.G. 1991. A revision of the genus Bryobia in Greece (Acari: Tetranychidae). Entomologia Hellenica, 9: 21–34. doi:10.12681/eh.13989 ![]()
He F. D., Tan R. C. 1993. A new species of Bryobia from Xinjiang, China (Acarina: Tetranychidae). Entomotaxonomia, 15 (1): 74–76 [in Chinese, with English abstract].
Hong X. Y., Zhang Z. Q., Li G.Q. 2010. Tetranychidae of China: a review of progress, with a checklist. Xin Jie-Liu Centenary: Progress in Chinese Acarology. Zoosymposia, 4: 133–157.
Hu S. Q., Chen X. W. 1993. Two new record species of Tetranychidae from Jiangxi (Acarina: Tetranychidae). Journal of Nanchang University (Natural Science), 17 (3), 34–36 [in Chinese, with English abstract].
Khanjani M., Seeman O. D. 2018. The spider mites of the genus Oligonychus Berlese (Acari: Tetranychidae) from Iran. Systematic and Applied Acarology, 23 (2): 223–287. doi:10.11158/saa.23.2.4 ![]()
Li, J., Yi, T. C., Guo, J. J. & Jin, D. C. 2018. Ontogenetic development and redescription of Oligonychus pratensis (Banks, 1912) (Acari: Tetranychidae). Zootaxa, 4486 (3), 349–375. doi:10.11646/zootaxa.4486.3.7 ![]()
Li J., Jin D. C., Yi T. C. 2017. Ontogenetic development and redescription of Oligonychus metasequoiae (Acari: Tetranychidae). Systematic and Applied Acarology, 22 (9): 1495–1520. doi:10.11158/saa.22.9.14 ![]()
Lindquist E. E. 1985. 1.1.1 External anatomy. In: Helle, W. & Sabelis, M.W. (Eds.), Spider mites. Their biology, natural enemies and control. Vol. A. Amsterdam, Elsevier. pp. 3–28.
Ma E. P., Yuan Y. L. 1980. New species and new records of tetranychid mites from China I. (Acarina: Tetranychidae). Acta Zootaxonomica Sinica, 5 (1): 42–45.
Ma E. P., Yuan Y. L. 1981. New species and new records of tetranychid mites from China. II. (Acarina: Tetranychidae). Zoological Research, 3: 013.
Ma E. P., Yuan Y. L, Qian Y. H. 1984. Spider mites. In: Jiangxi University (ed.). Agricultural Mites of China (Zhong Guo Nong Ye Man Lei). Shanghai Science and Technology Press, Shanghai, pp. 88–164.
Migeon A., Dorkeld F. 2006-2017. Spider Mites Web: a comprehensive database for Tetranychidae [Internet]. Montpellier: INRA/CBGP. Available from: http://www1.montpellier.inra.fr/CBGP/spmweb/ ![]() .
.
Pritchard A. E., Baker E. W. 1955. A revision of the spider mite family Tetranychidae. Memoirs Series, San Francisco, Pacific Coast Entomological Society, 2, 472 pp. doi:10.5962/bhl.title.150852 ![]()
Rimando L. C. 1962. The tetranychoid mites of the Philippines. University of the Philippines College of Agriculture Technical Bulletin, 11: 1–52.
Seeman O. D., Beard J. J, Zhang L. N. 2017. A new Australian species of Eotetranychus (Acari: Tetranychidae) from buck spinifex Triodia mitchelli (Poaceae), intraspecific variation in Eotetranychus, and the synonymy of Platytetranychus with Eotetranychus. Zootaxa, 4324 (3): 491–517. doi:10.11646/zootaxa.4324.3.5 ![]()
Tseng Y. H. 1990. A monograph of the mite family Tetranychidae (Acarina: Trombidiformes) from Taiwan. Taiwan Museum Special Publication Series, 9, 1–224.
Wauthy, G., Noti, M. I., Leponce, M. & Bauchau, V. 1998. Taxy and variations of leg setae and solenidia in Tetranychus urticae (Acari, Prostigmata). Acarologia, 39 (3): 233–255.
Wang H. F., Cui Y. Q. 1991. Three new species of Tetranychidae from Hengduan mountains, China. Acta Zootaxonomica Sinica, 16 (3): 302–312 [in Chinese, with English abstract].
Wang H. F., Zhang W. G. 1984. Description of a new species of the genus Bryobia from Xinjiang (Acarina: Tetranychidae). Acta Zootaxonomica Sinica, 9 (1): 41–43 [in Chinese, with English abstract].
Wang H. F. 1981. Acariformes: Tetranychoidea. Economic insect fauna of China. Fasc. 23. Science Press. Beijing, China. 150pp.
Wang H. F. 1985. Notes on Bryobia from China with four new species and a new record. Acta Zootaxonomica Sinica, 28 (3): 330–340 [in Chinese, with English abstract].
Xu Y., Fan Q. H., Zhang F. P., Huang, J. 2017. Morphological ontogeny in Aegyptobia exarata Livchitz & Mitrofanov (Acari: Tenuipalpidae). Systematic and Applied Acarology, 22 (7): 968–979. doi:10.11158/saa.22.7.6 ![]()
Yi T. C., Fan Q. H., Zhang, Z. Q. 2013. Ontogenetic development and redescription of Tribolonychus collyerae (Acari: Tetranychidae). Zootaxa, 3721 (4): 301–333. doi:10.11646/zootaxa.3721.4.1 ![]()
Yi T. C., Liu M., Guo J. J., Jin D. C. 2017. Ontogenetic development and redescription of Eotetranychus xuzhouensis (Wang and Ma 1987) comb. nov. (Acari: Tetranychidae). Systematic and Applied Acarology, 22 (1): 58–73. doi:10.11158/saa.22.1.7 ![]()



2018-09-15
Date accepted:
2019-01-29
Date published:
2019-02-04
Edited by:
Migeon, Alain

This work is licensed under a Creative Commons Attribution 4.0 International License
2019 Li, Juan; Yi, Tian-Ci; Guo, Jian-Jun and Jin, Dao-Chao
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



