Ion Flux Coordination and signaling in plant cells (INFLUX)
 Group leader: Alexis De Angeli
Group leader: Alexis De Angeli
Senior Scientist CNRS
Key words
Stomata, electrophysiology, biosensor, cell imaging, ion transport
Presentation
The aim of the group is to understand the molecular mechanisms involved in the regulation and coordination of ion fluxes across the cellular membranes of plant cells. To have coherent cellular responses to environmental stimuli the events occurring in the different cellular membranes of eukaryotic cells need to be coordinated. In plants, stomata are illustrative of the importance of the control of ion fluxes in cells. Stomata are plant specific structures forming pores at the surface of leaves controlling the gas exchanges with the atmosphere. The stomata pore aperture controls CO2 uptake and water loss by evaporation and consequently contributes to biomass production and drought tolerance in land plants. The stomata pore is delimited by two specialized cells, the guard cells, controlling its aperture. The control of the stomata pore depends on the capacity of guard cells to induce massive and controlled ion fluxes across the plasma and the vacuolar membranes to change their volume and consequently the stomata aperture.
In plant cells, similarly to other eukaryotic cells, ion transport is fundamental in signaling cascades, in regulating transmembrane potential and intracellular ion concentrations (e.g. Cl–, NO3–, malate2-, K+, Ca2+ and H+). Further, ion transport in also involved in mineral nutrition and in the maintenance of the turgor pressure. Cellular responses involving ion fluxes rely on the coordination of the transport reactions occurring in the cellular membranes.
To unravel the mechanisms underlying the coordination of the events occurring in the different plant cell membranes we use different approaches. We investigate the mechanisms of ion fluxes coordination from the biophysical properties of ion channels, to their function within the cell and their role at a whole plant level. We specifically focus on the ion channels of the ALMT (Aluminum activated Malate Transporters) family that plays an important role in stomata functioning. We investigate the molecular basis of the regulation of the ALMTs (ion selectivity, activation by malate and nucleotide block, secondary modifications) and its impact in plant cells. Because guard cells are an ideal paradigm to study flux coordination and they are important for plant adaptation to the environment we use this cell type as a model for our study.
To achieve our goal we use and develop different experimental approaches:
- electrophysiology (patch-clamp, TEVC) to understand the molecular properties (transport reactions and regulation) of ion transporters of the vacuolar and plasma membrane.
- live cell imaging coupled to genetically encoded fluorescent biosensors to visualize the dynamic of intracellular ionic concentrations (e.g. NO3–, Cl– and H+) and the in vivo activity of ion transporters.
- physiology and genetics
- mathematical models
Team members
Significant publications
Doireau R*, Jaślan J*, Cubero-Font P*, Demes-Causse E, Bertaux K, Cassan C, Pétriarcq P, De Angeli A✉ (2024) AtALMT5 mediates vacuolar fumarate import and regulates the malate/fumarate balance in Arabidopsis. New Phytol., (in press)
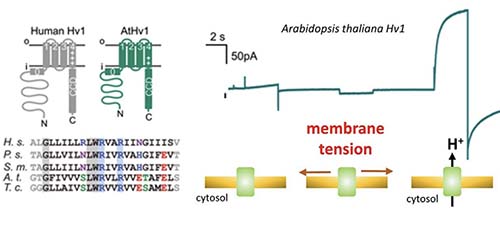
Zhao C, Webster PD, De Angeli A✉, Tombola F✉ (2023) Mechanically-primed voltage-gated proton channels from angiosperm plants. Nat. Commun., 14:7515
Jaślan J, Marten I✉, Jakobson L, Arjus T, Deeken R, Sarmiento C, De Angeli A, Brosché M, Kollist H, Hedrich R✉ (2023) ALMT-independent guard cell R-type anion currents. New Phytol., 239(6):2225-2234
Mirasole FM, Nastasi SP, Cubero-Font P, De Angeli A✉ (2023) Vacuolar control of stomatal opening revealed by 3D imaging of the guard cells. Sci. Rep.-UK, 13:7647
Hodin J, Lind C, Marmagne A, Espagne C, Bianchi MW, De Angeli A, Abou-Choucha F, Bourge M, Chardon F, Thomine S, Filleur S✉ (2023) Proton exchange by the vacuolar nitrate transporter CLCa is required for plant growth and nitrogen use efficiency. Plant Cell, 35(1):318-335
De Angeli A (2022) Pollen likes sugars: Sucrose-specific transport by AtSWEET13. P. Natl. Acad. Sci. USA, 119(46):e2216610119
Jaślan J, De Angeli A✉ (2022) Heterologous expression reveals that GABA does not directly inhibit the vacuolar anion channel AtALMT9. Plant Physiol., 189(2):469-472
Morales de los Ríos L, Corratgé-Faillie C, Raddatz N, Mendoza I, Lindahl M, De Angeli A, Lacombe B, Quintero FJ, Pardo JM✉ (2021) The Arabidopsis protein NPF6.2/NRT1.4 is a plasma membrane nitrate transporter and a target of protein kinase CIPK23. Plant Physiol. Bioch., 168:239-251
Cubero-Font P, De Angeli A✉ (2021) Connecting vacuolar and plasma membrane transport networks. New Phytol., 229(2):755-762
Wege S✉, De Angeli A (2020) Novel electrical signaling: First fast voltage-gated sodium channel identified outside of the animal kingdom. Plant Physiol., 184(4):1618-1619
Demes E, Besse L, Cubero-Font P, Satiat-Jeunemaître B, Thomine S, De Angeli A✉ (2020) Dynamic measurement of cytosolic pH and [NO3-] uncovers the role of the vacuolar transporter AtCLCa in cytosolic pH homeostasis. P. Natl. Acad. Sci. USA, 117(26):15343-15353
Eisenach C✉, Baetz U, Huckb NV, Zhang J, De Angeli A, Beckers GJM, Martinoia E (2017) ABA-induced stomatal closure involves ALMT4, a phosphorylation-dependent vacuolar anion channel of Arabidopsis. Plant Cell, 29(10):2552-2569
Eisenach C✉, De Angeli A (2017) Ion transport at the vacuole during stomatal movements. Plant Physiol., 174(2):520-530
Baetz U, Eisenach C, Tohge T, Martinoia E, De Angeli A✉ (2016) Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiol., 172(2):1167-1181
De Angeli A, Thomine S, Frachisse J-M✉ (2016) Anion channel blockage by ATP as a means for membranes to perceive the energy status of the cell. Mol. Plant, 9(3):320-322
Zhang J, Martinoia E✉, De Angeli A✉ (2014) Cytosolic nucleotides block and regulate the Arabidopsis vacuolar anion channel AtALMT9. J. Biol. Chem., 289(37):25581-25589
Wege S, De Angeli A, Droillard M-J, Kroniewicz L, Merlot S, Cornu D, Gambale F, Martinoia E, Barbier-Brygoo H, Thomine S, Leonhardt N, Filleur S✉ (2014) Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci. Signal., 7(333):ra65
Zhang J*, Baetz U*, Krügel U, Martinoia E, De Angeli A✉ (2013) Identification of a probable pore-forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiol., 163(2):830-843
De Angeli A*✉, Baetz U*, Zhang J, Chaves MM, Regalado A (2013) The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera. Planta, 238(2):283-291
De Angeli A*✉, Zhang J*, Meyer S*, Martinoia E (2013) AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun., 4:1804
Collaborations
Funding sources

ATIP Avenir project 2018 “Intracellular ion flux coordination, a novel perspective on the mechanisms regulating ion transport in A. thaliana guard cells”
Main results
Former team members