New and little-known species of soil mites of the family Oppiidae (Acari: Oribatida) from Korea
Bayartogtokh, Badamdorj  1
and Bae, Yang-Seop
1
and Bae, Yang-Seop  2
2
1✉ Department of Biology, School of Arts and Sciences, National University of Mongolia, Ulaanbaatar 14201, Mongolia.
2College of Life Sciences and Bioengineering, Incheon National University, Incheon 22012, Republic of Korea.
2025 - Volume: 65 Issue: 4 pages: 1264-1284
https://doi.org/10.24349/kt8v-mbl6ZooBank LSID: 31B2F823-ED5F-42C3-BBA0-373DDD4E0144
Original research
Keywords
Abstract
Introduction
The Oppiidae Sellnick, 1937 is the largest family of oribatid mites, which comprises 13 subfamilies, 177 genera and more than 1,150 species, and their number is constantly increasing (Subías 2004, online version 2024). They are widespread, occurring on all biogeographical regions, inhabiting nearly all terrestrial habitats worldwide as well as some arboreal and semiaquatic environments (e.g., Weigmann 2006; Bayartogtokh 2010; Murvanidze and Mumladze 2016; Corpuz-Raros and Ermilov 2020). Although there are a great number of tropical species, Oppiidae are also very diverse in the temperate regions (Subías et al. 2012, online annual update).
Their small body size (body length mostly ~160 μm or little more), high diversity and relatively complex structure make the systematics of Oppiidae difficult. Therefore, the family is still insufficiently known in terms of species diversity in most regions of the world, including Korea, where only 27 species have hitherto been recorded (Choi 1997, 1998, 1999; Choi and Jung 1998; Bayartogtokh and Bae 2022a, b, 2024). This species number may be far less than one third of the potential species richness of this family in Korea. Therefore, it is highly desirable to discover more unknown species, and at the same time, to redefine the hitherto little-known species more precisely in order to promote the systematics of Oppiidae.
Among the materials collected from southern and central Korea, the authors found four species belonging to the genera Rhinoppia Balogh, 1983, Goyoppia Balogh, 1983, and Graptoppia Balogh, 1983. Rhinoppia is a member of the subfamily Medioppiinae, which comprises two subgenera (Rhinoppia Balogh, 1983 and Paramedioppia Mahunka & Mahunka-Papp, 2000) and 49 species (including two subspecies), which collectively have a semicosmopolitan distribution, except the Antarctic and Oriental regions. Previously, only one species, Rhinoppia properecta Bayartogtokh & Bae, 2022 has been recorded in Korea (Bayartogtokh and Bae 2022b). Another genus, Goyoppia is a member of the subfamily Oppiinae, and it comprises only three known species, which are distributed in the Palaearctic, Oriental and Afrotropical regions (Subías et al. 2012, online annual update). Only one species, Goyoppia sagami (Aoki, 1984) has been recorded in Korea (Choi 1986). The third genus, Graptoppia comprises three subgenera (Graptoppia Balogh, 1983, Apograptoppia Subías & Rodríguez, 1985, Stenoppia Balogh, 1983) and 25 species, distributed in the Palaearctic and Pantropical regions. Only two species, Graptoppia (Stenoppia) crista Ohkubo, 1996 and G. (S.) italica (Bernini, 1973) have been recorded from Korea (Bayartogtokh and Bae 2024).
The main aim of the present work is to propose two new species of Rhinoppia and Goyoppia, and to provide supplementary description on the morphology of other two little-known species of Graptoppia, with appropriate illustrations, and data on their distribution and habitat ecology.
Materials and methods
Study area
A portion of the material was collected from Heuksando, a mountainous island in the Yellow Sea, located southwest of the Korean peninsula, within the administrative boundaries of Sinan County, Jeollanam Province. The entire area of Heuksando, along with the nearby islands, has been designated as a World Biosphere Reserve. Geological analysis shows that the majority of islands are composed of Cretaceous tuff and volcaniclastic sedimentary rock. The dominant tree species on this island are evergreen trees, namely Castanopsis sieboldii, Machilus thunbergii, and Camellia japonica, characteristic species of Korea's warm temperate evergreen broad-leaved forest zone (Hong 2015). Along the shores of the island, Pinus thunbergii effectively blocks wind blowing from the sea (Hong et al. 2011).
Another part of the study material was collected from Wirye Park, Seongnam-shi, Gyeonggi Province, located in the central part of Korea. This park functions as an ecological island surrounded by urban constructions of Wirye New Town. The environment is excellent as most of the areas of this town are created by lifting the green belt. Its mixed forests consist of tree species such as Quercus acutissima, Q. mongolica, Q. variabilis, Acer pseudosieboldianum, A. mono, Fagus crenata, Pinus densiflora etc. A waterside park is created along Jangjicheon Stream, which originates from Cheongnyangsan Mountain near the Wirye Seongnam area. The collection locality and habitat data for each species are given in the respective ''material examined'' subsections.
Specimens and sampling
Samples were collected from the soil and litter of forests composed by various trees and shrubs, at layers of 0–10 cm. Soil mites were extracted with modified Berlese-Tullgren's funnels over the course of 5–6 days in the laboratory. All types and specimens examined are preserved in 80% ethanol with a drop of glycerol, and deposited in the collection of the National Institute of Biological Resources, Incheon, Korea.
Observation and documentation
Specimens were cleared in lactic acid and mounted in lactic acid on temporary cavity slides for measurement and illustration. A differential interference contrast microscope was used for investigation of the morphology of specimens in transmitted light. Line drawings were made using a camera lucida attached to the compound microscope. Micrographs were taken using a digital camera attached to the microscope with multiple shots.
Body lengths were measured in lateral view, from the tip of the rostrum to the posterior edge of the ventral plate. Notogastral width refers to the maximum width of the notogaster. Lengths of body setae were measured in lateral aspect. All measurement values are given in micrometres, and average measurement values are given in parentheses after the range.
Terminology and abbreviations
The morphological terminology used in this paper follows that of F. Grandjean: see Travé and Vachon (1975) for references, Norton and Behan-Pelletier (2009), and Ermilov (2016, 2023). Formulas for leg setation are given in parentheses according to the sequence trochanter–femur–genu–tibia–tarsus (famulus included). Formulas for leg solenidia are given according to the sequence genu–tibia–tarsus.
The following abbreviations are used. Prodorsum: ro, le, in, bs, ex – rostral, lamellar, interlamellar, bothridial, and exobothridial setae, respectively; bo – bothridium; ibt – interbothridial tubercle; pbt – postbothridial tubercle; lateral prodorsal ridge – lpr. Notogaster: c, la, lm, lp, h1–h3, p1–p3 – setae; ia, im, ip, ih, ips – lyrifissures; gla – opisthonotal gland opening. Gnathosoma: a, m, h – subcapitular setae, cha, chb – cheliceral setae, Tg – Trägårdh's organ, or – adoral seta; d, l, v, cm, ul, su, vt, sup, inf, lt – palp setae; ω – palp solenidion. Epimeral and anogenital regions: PdI – pedotectum I; 1a–1c, 2a, 3a–3c, 4a–4c – epimeral setae; dis – discidium; g1–g5, ag, an1, an2, ad1–ad3 – genital, aggenital, anal, and adanal setae, respectively; iad – adanal lyrifissure; po – preanal organ. Legs: ω, φ, σ – solenidia; ɛ – famulus; d, l, v, bv, ev, ft, tc, it, p, u, a, s, pv, pl – setae.
Results
This work deals with two new species as well as two little-known species of Oppiidae, which are reported for the first time from Korea. The descriptions of new species are presented, along with appropriate illustrations, and supplementary descriptions of known species based on Korean material and compared with existing descriptions. We briefly discuss aspects of the distribution and habitat ecology of each species.
Family Oppiidae Sellnick, 1937
Genus Rhinoppia Balogh, 1983
Type species Oppia nasuta Moritz, 1965
Rhinoppia chuleuijungi sp. nov.
ZOOBANK: 518BE3ED-3A1D-4C31-A944-A9392744D7D4 ![]()
(Figures 1–4)
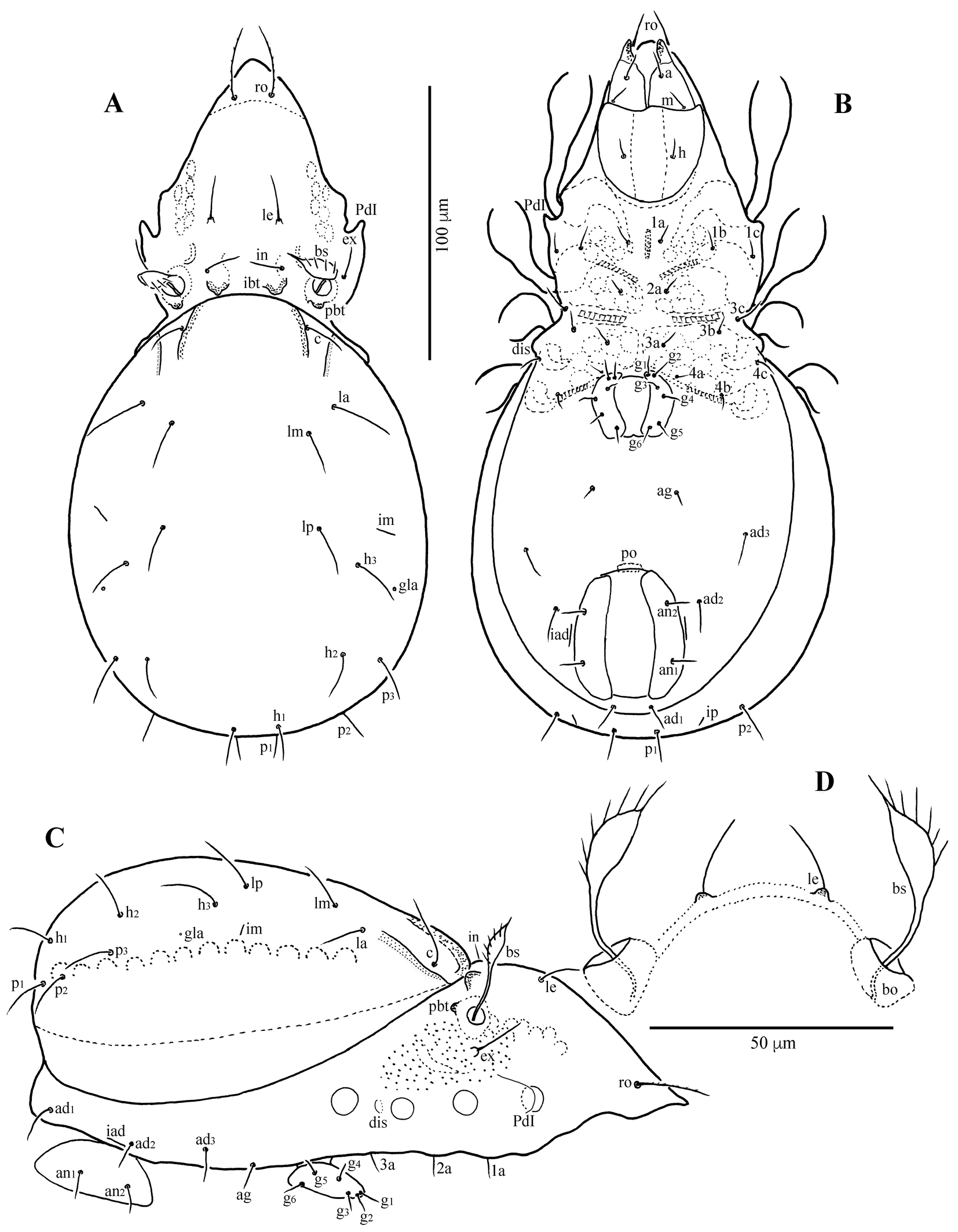


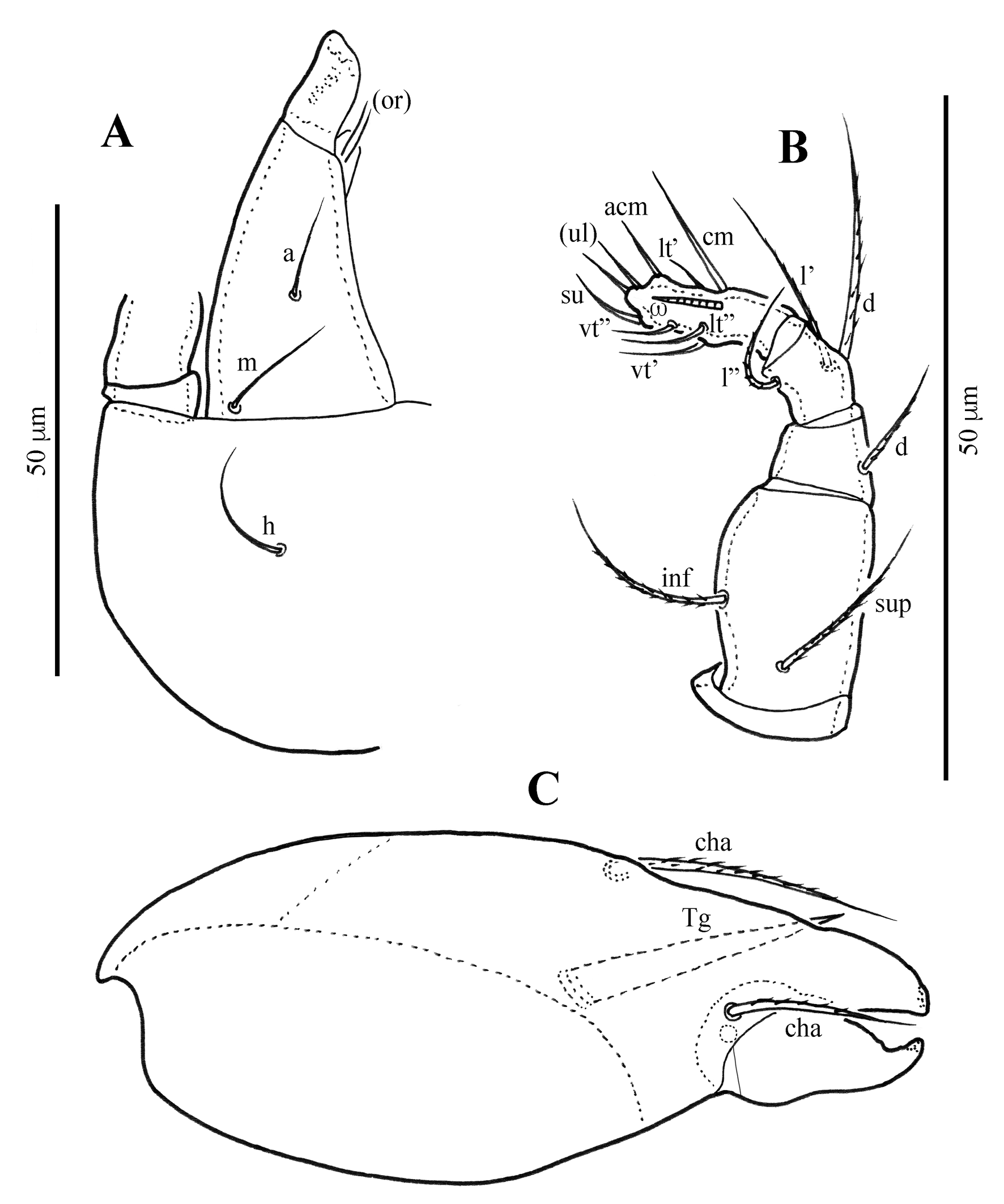


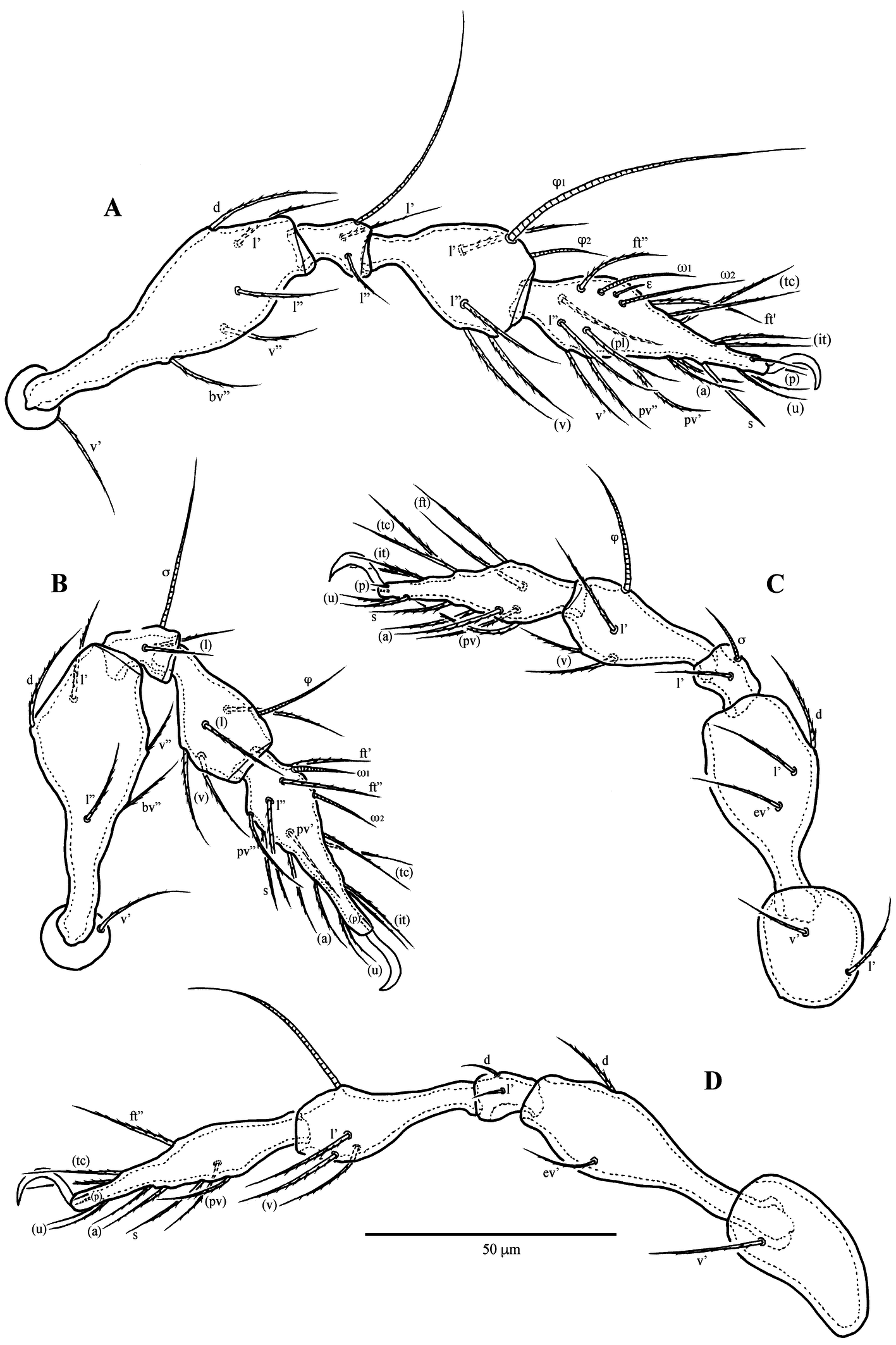


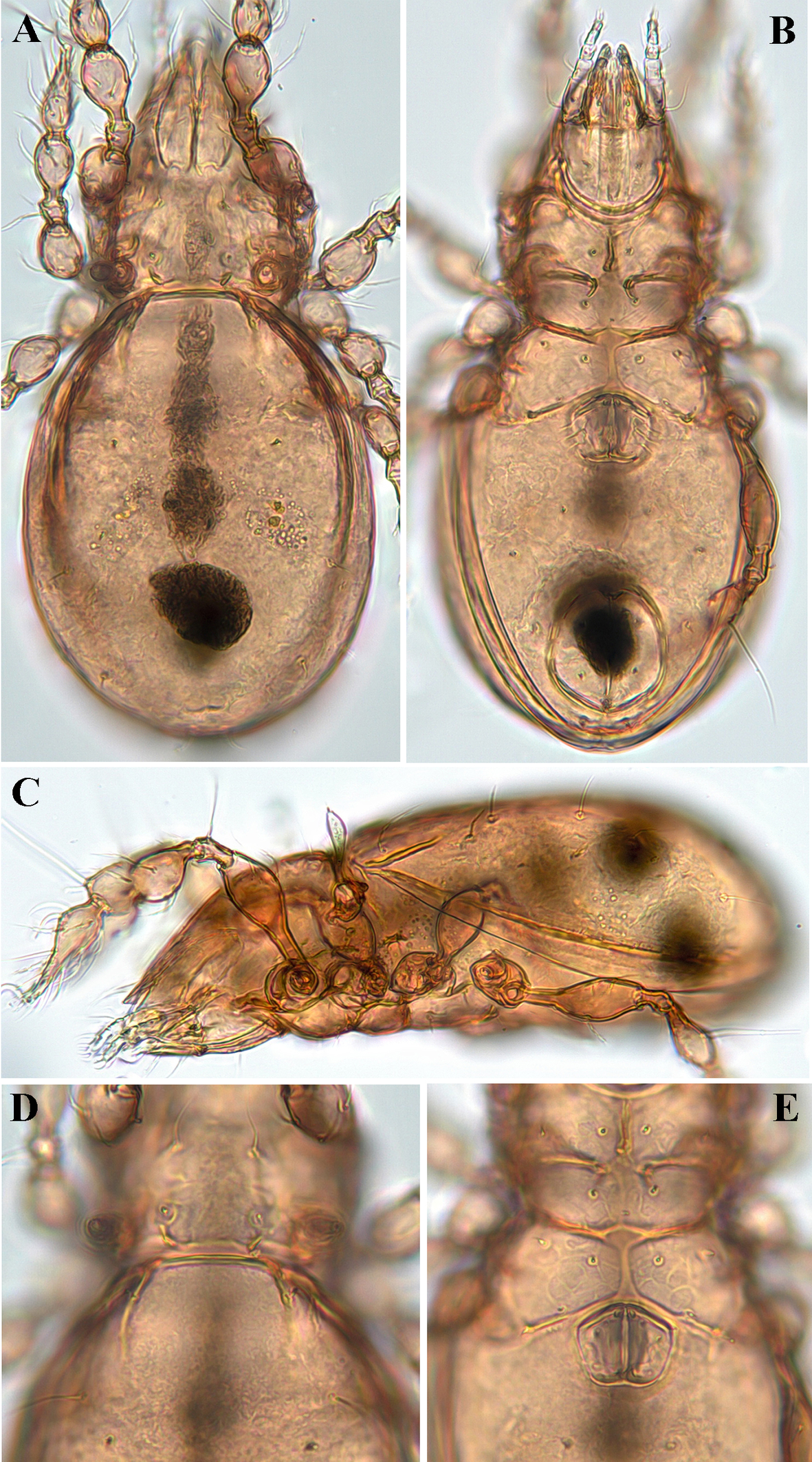


Diagnosis
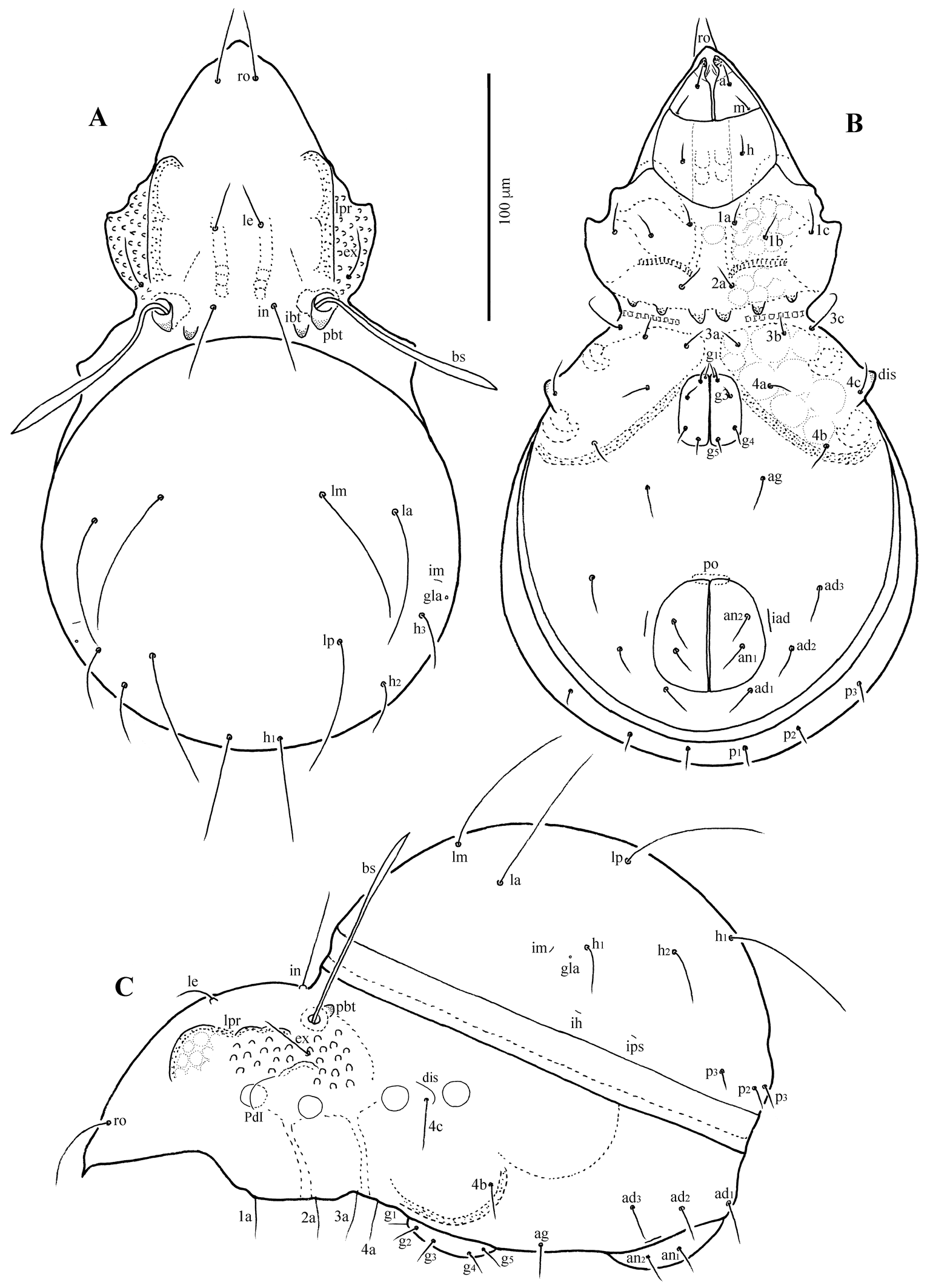
Body length 232–253 μm, width 116–131 μm. Rostrum slightly narrowed anteriorly, but entire, without incision or dentation; rostral seta slightly curving inward, minutely barbed; lamellar seta medium long, thin, smooth, inserted on distinct tubercle; interlamellar seta shorter than lamellar seta, thin, smooth. Bothridial seta lanceolate, with 6-7 long cilia. Interbothridial and postbothridial tubercles well developed; interlamellar region smooth; prodorsum with six to eight pairs of muscle sigillae laterally to lamellar seta, and many granular tubercles in exobothridial region. Humeral crista well developed, extending posteriorly, beyond insertion of seta c; ten pairs of notogastral setae medium long, thin, smooth. Epimeral and anogenital setae smooth; six pairs of genital setae. Discidium small, not projected, but rounded distally.
Description
Measurements — Body length: 232 μm (holotype: male), 240–253 (245) μm (five paratypes: two females and three males); width of notogaster: 128 μm (holotype), 116–131 (124) μm (five paratypes); length of notogaster 153 μm (holotype), 160–179 (171) μm (five paratypes). There was no difference in body size between females and males.
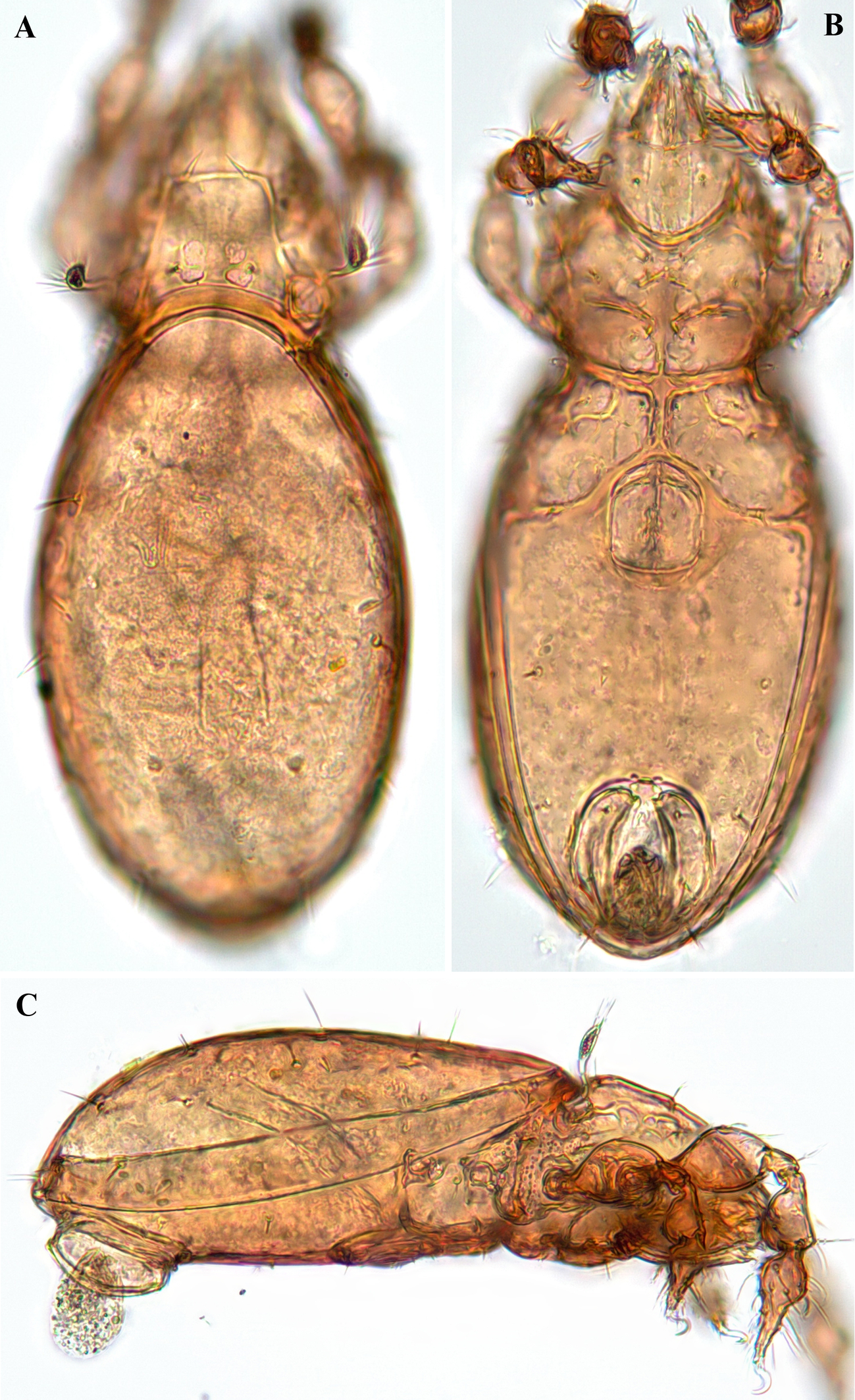
Integument — Body colour yellowish brown; body surface nearly smooth or finely granulated; exobothridial region, i.e., between bothridium and leg acetabula with many granular tubercles.
Prodorsum (Figs 1A, C, D, 4A, C, D) — Rostrum narrowly rounded anteriorly in dorsal view, but entire, without incision or dentation; conspicuously projecting in lateral view. Rostral seta (ro ~30 μm) inserted dorsolaterally, slightly curving inward, minutely barbed. Lamellar seta (le ~25 μm) thin, smooth, inserted on distinct tubercle. Interlamellar seta (in ~22 μm) thin, smooth. Exobothridial seta (ex) nearly as long as in, inserted laterally to bothridium. Bothridial seta (bs) lanceolate, with 6–7 long cilia, ~45 μm in length. Lamellar line not developed; interlamellar region nearly smooth; prodorsum with six to eight pairs of muscle sigillae laterally of lamellar seta. Interbothridial (ibt) and postbothridial (pbt) tubercles well developed, rounded in shape.
Notogaster (Figs 1A, C, 4A, C, D) — Almost rounded, slightly longer than wide, anterior margin widely rounded; with numerous muscle sigillae marginally. Humeral crista well developed, both medial and lateral crista extending posteriorly, beyond insertion of seta c; ten pairs of notogastral setae 20–24 μm long, thin, smooth. Lyrifissures im and ip clearly developed, other lyrifissures not evident. Opisthonotal gland opening (gla) located posterolateral to seta h3.
Gnathosoma (Figs 1B, 2, 4B) — Subcapitulum slightly wider than long; subcapitular setae a, m and h setiform, smooth, 11–14 μm. Palp and chelicera typical for family, no distinct tooth dorsally; palpal setation: 0–2–1–3–9(+ω); cheliceral setae setiform, finely barbed; cha longer than chb.
Epimeral region (Figs 1B, 4B, E) — Epimeral fields with muscle sigillae; apodemes apo.2, apo.sj and apo.4 well developed; epimeral setal formula: 3–1–3–3, all setae thin, smooth, 10–13 μm. Discidium small, not projected, but rounded distally.
Anogenital region (Figs 1B, 4B, E) — All anogenital setae thin, smooth: six pairs of genital setae (10–13 μm), setae g1 and g2 inserted close to anterior border of genital plate; aggenital seta (ag ~10 μm) short. Anal setae (an ~15 μm) slightly shorter than adanal setae (ad ~17 μm); ad1 inserted posterior, ad2 lateral, ad3 anterolateral to anal aperture. Lyrifissure iad inserted adjacent and parallel to anal aperture.
Legs (Fig 3) — Structure and setation of legs typical for family; leg I ~260 μm, leg II ~212 μm, leg III ~252 μm, leg IV ~364 μm. Formula of leg setation: I (1–5–2–4–20), II (1–5–2–4–16), III (2–3–1–3–15), IV (1–2–2–3–12); formula of solenidia: I (1–2–2), II (1–1–2), III (1–1–0), IV (0–1–0). Homology of setae and solenidia indicated in Table 1.
Download as Note: Roman letters refer to normal setae, Greek letters to solenidia, ɛ to famulus; single prime (’) designates setae on the anterior and double prime (") setae on the posterior side of a given leg segments; parentheses refer to a pair of setae.
Legs
Tr
Fe
Ge
Ti
Ta
I
v’
d,* (l), bv“, v”*
(l),* *σ
(l),* (v), *φ1, φ2
(ft), (tc), (it), (p), (u), (a), s, (pv), v’,* (pl), l", *ɛ, ω1, ω2
II
v’
d,* (l), bv“, v”*
(l),* *σ
(l),* (v), *φ
(ft),* (tc), (it), (p), (u), (a), s, (pv), l", ω1, *ω2
III
l’,* v’*
d,* l’, ev’*
l’,* *σ
l’,* (v)*, φ
(ft),* (tc), (it), (p), (u), (a), s, (pv)*
IV
v’
d,* ev’*
d,* l’*
l’,* (v)*, φ
ft",* (tc), (p), (u), (a), s, (pv)*
Material examined
Holotype (male) and five paratypes (three males and two females): Heuksan-myeon, Heuksando Island, Sinan-gun, Jeollanam-do, Korea, mixed forest, soil and litter under dead trees, Pinus thunbergii, 27 July 2023, Coll. S.J. Yoon (NIBR accession number: PFZUIV0000000061).
Distribution and habitat ecology
Currently, this species is known only from the type locality, Heuksando, which is a volcanic island with rocky mountains covered by forests composed by such evergreen trees as Itajii chinkapin (Castanopsis sieboldii), Red machilus (Machilus thunbergii), Common camellia (Camellia japonica), and Black pine (Pinus thunbergii).
Etymology
This species is dedicated to our friend and colleague, Dr. Chuleui Jung, professor at the Andong National University, Andong, Korea, for his extensive contribution to the ecological study of soil mites of Korea.
Remarks
According to the initial diagnosis of Rhinoppia by Balogh (1983) and subsequent definitions by Subías and Rodríguez (1986), Subías and Balogh (1989), Mahunka and Mahunka-Papp (2000), Subías and Shtanchaeva (2023), our new species belongs to the nominal subgenus as it has unilaterally ciliate bothridial seta (bilaterally ciliate in the subgenus Paramedioppia).
Among the 42 species of Rhinoppia (Rhinoppia), R. exobothridialis Toluk & Ayyildiz, 2009, R. heterotricha (Ivan & Vasiliu, 1997), R. hygrophila (Mahunka, 1987), R. incognita (Vasiliu & Ivan, 2011), R. obsoleta (Paoli, 1908), R. parapectinata (Ryabinin, 1987), and R. zaballosi Subías & Shtanchaeva, 2011 (all species except the semicosmopolitan R. obsoleta are exclusively found in the Palaearctic region), resemble the new species in the similar structure of bothridial and notogastral setae, and evenly rounded rostrum. The other known species of this subgenus are distinguished from this new species by their either distally thickened head with short barbs or thinner, pectinated bothridial setae; either relatively long or barbed notogastral setae, and either pointed or incised rostrum.
Rhinoppia exobothridialis, which is known from Turkey (see Toluk and Ayyildiz 2009), differs from the new species by the distinctly longer bothridial seta with short, clavate head (vs. medium long, lanceolate in new species); very long, thick and barbed exobothridial seta (vs. short, thin, smooth in new species); much shorter lamellar and interlamellar setae (vs. relatively long in new species); presence of six pairs of genital setae (vs. five pairs in new species); much larger body size (body length 352–388 vs. 232–253 μm).
Rhinoppia heterotricha, which is known from the eastern Mediterranean (see Toluk and Ayyildiz 2009) is distinguishable from the new species by the presence of a well-developed medial tubercle between interbothridial tubercles (vs. absent in new species); relatively thick, but short interlamellar seta (vs. thin, longer in new species); blunt triangular shape of interbothridial tubercle (vs. rounded, tubercle-like in new species); elongate and slender head of bothridial seta (vs. relatively short, more swollen head of bothridial seta in new species); barbed epimeral setae 1c, 3b, 3c, 4b, 4c (vs. smooth in new species); and six pairs of genital setae (vs. five pairs in new species); much larger body size (body length 313 vs. 232–253 μm).
Rhinoppia hygrophila, which is known from the central and eastern Europe (see Mahunka 1987) is different from the new species by the distinctly longer, fusiform bothridial seta with short cilia (vs. medium long, lanceolate, with long cilia in new species); posterior margin of apo.4 with series of small tubercles (vs. absent in new species); presence of six pairs of genital setae (vs. five pairs in new species); much larger body size (body length 320–368 vs. 232–253 μm).
Another European species, R. incognita (see Valiliu and Ivan 2011) can be distinguished from the new species by the distinctly longer, sickle-like bothridial seta (vs. medium long, lanceolate in new species); relatively long and thick interlamellar seta (vs. thin, shorter in new species); long notogastral setae c, la and lm, which are at least twice longer than other setae (in new species, all notogastral setae subequal in size); distally bifurcate epimeral setae 1b, 1c, 3b, 3c, 4b, and 4c (in new species, all epimeral setae simple, not bifurcate); much larger body size (body length 425–440 vs. 232–253 μm).
The semicosmopolitan species, R. obsoleta, proposed by Paoli (1908) and studied by Willmann (1931), Schweizer (1956), Woas (1986), Miko (2006) can be distinguished from the new species by the much slender, fusiform bothridial seta with long pectinations (vs. lanceolate, more swollen seta bs in new species); relatively thick, short, barbed interlamellar seta (vs. thin, longer in new species); much larger body size (body length 285–360 vs. 232–253 μm). The subspecies R. obsoleta curtiramosa described by Subías and Shtanchaeva (2011) also can be distinguished from the new species by the bothridial seta, which bears short cilia, and much shorter lamellar seta. Although the body size for this subspecies was not provided in the original description, Subías and Arillo (2001) described it as 285–360 mm, so it also seems to be larger than our newly proposed species.
The Russian Far East species, R. parapectinata (see Ryabinin 1987) can be distinguished from the new species by the elongated body, with length being 2.1 times greater than its width (vs. relatively stout in new species, body length × width ratio: 1.8); slender bothridial seta with fusiform head (vs. lanceolate, more swollen seta bs in new species); very short lamellar, interlamellar, and notogastral setae (vs. longer in new species); presence of a pair of short costular ridges laterally to each interlamellar seta (vs. absent in new species); much longer body size (body length 300 vs. 232–253 μm).
Another Mediterranean species, R. zaballosi (see Subías and Shtanchaeva 2011) can be distinguished from the new species by the medially strongly curved rostral setae (vs. slightly curved in new species); bothridial seta with 10 long, recurved cilia (vs. 6–7 straight cilia in new species); barbed exobothridial seta (vs. smooth in new species); poorly developed humeral crista (vs. well developed in new species); genital setae g1 and g2 inserted in longitudinal row (vs. in new species, g1 and g2 inserted transversally close to anterior border of genital plate); relatively short, stocky body size (body length 185–220 vs. 232–253 μm).
Genus Goyoppia Balogh, 1983
Type species Oppia sexpilosa Balogh, 1961
Goyoppia heuksandoensis sp. nov.
ZOOBANK: 2617D075-244E-4C4D-ACE5-FE14FD52CE05 ![]()
(Figures 5, 6)





Diagnosis
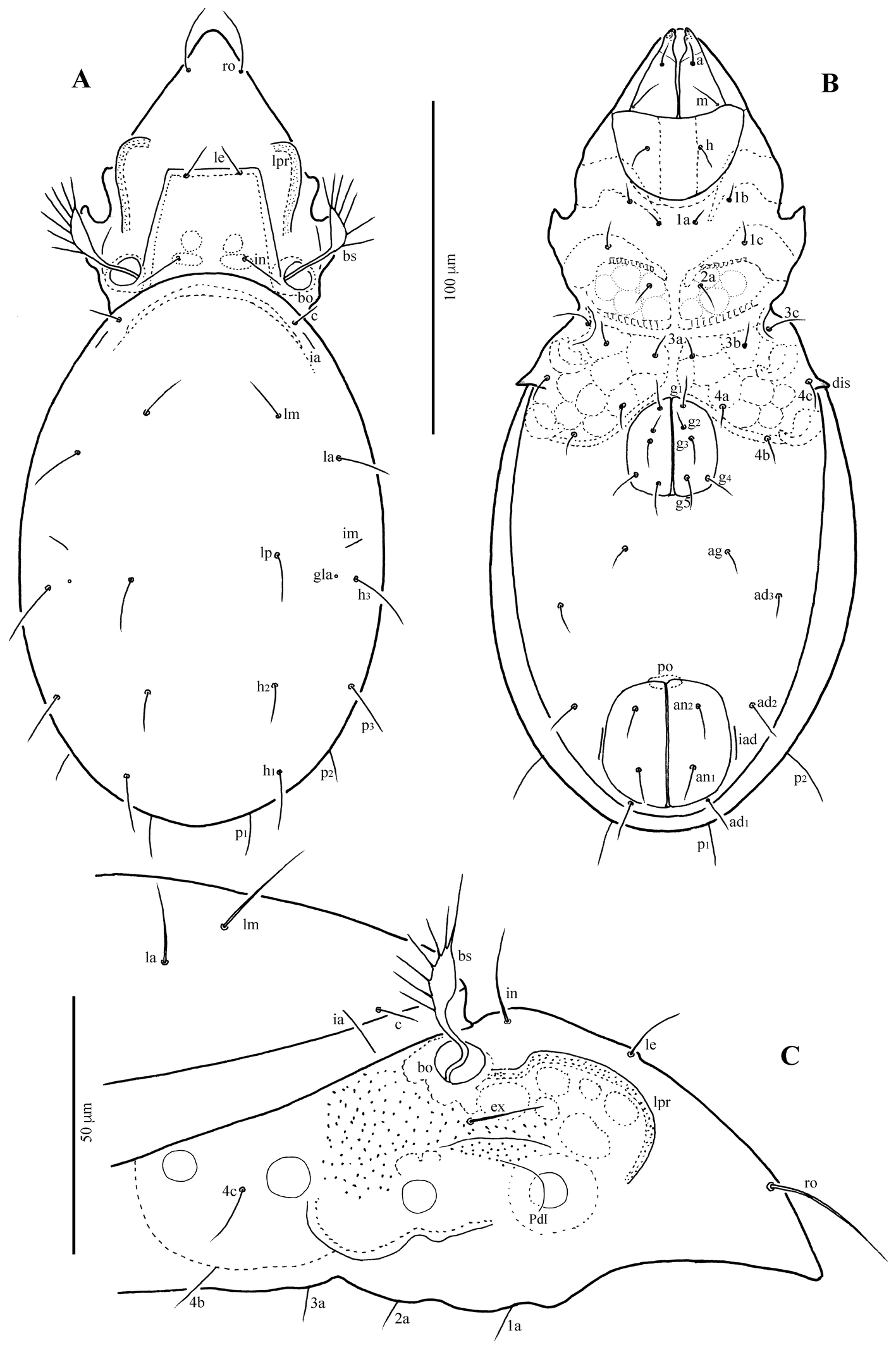
Body length 287–311 μm, width 172–189 μm. Rostrum narrowed anteriorly, but entire, without incision or dentation; rostral seta thin, smooth, nearly straight, slightly converging distally; lamellar seta medium long, thin, smooth, directed anteromedially; interlamellar seta thin, smooth, longer than lamellar seta, directed posterolaterally. Bothridial seta long, with slightly developed, elongate lanceolate, smooth head having pointed tip. Interbothridial region with three pairs of muscle sigillae, and a pair of faint ridges anteromedially to each bothridium. Interbothridial and postbothridial tubercles well developed; lateral prodorsal ridge strongly developed; exobothridial region densely tuberculate. Nine pairs of notogastral setae thin, smooth: c absent, la, lm, lp, and h1 very long, h2 and h3 medium long, p1–p3 short. Sejugal apodeme with three pairs of large tubercles; epimeral and anogenital setae thin, smooth; five pairs of genital setae. Discidium conspicuously projected, but rounded distally.
Description
Measurements — Body length: 287–311 μm; width of notogaster: 172–189 μm; length of notogaster 189–218 μm.
Integument — Body yellowish brown, nearly smooth or finely granulated; exobothridial region densely tuberculate.
Prodorsum (Figs 5A, C, 6A, C) — Rostrum narrowly rounded anteriorly in dorsal view, but entire, without incision or dentation; conspicuously projecting and pointed distally in lateral view. Rostral seta (ro ~30 μm) thin, smooth, inserted dorsolaterally, nearly straight, slightly converging distally. Lamellar seta (le ~18 μm) thin, smooth, directed anteromedially. Interlamellar seta (in ~30 μm) thin, smooth, directed posterolaterally. Exobothridial seta (ex ~20 μm) thin, smooth, inserted anterolaterally to bothridium. Bothridial seta (bs ~83 μm) long, with slightly developed, elongate lanceolate, smooth head having pointed tip. Interbothridial region with three pairs of muscle sigillae, and a pair of short, faint ridges anteromedially to each bothridium. Interbothridial and postbothridial tubercles well developed; lateral prodorsal ridge strongly developed, distally rounded medially.
Notogaster (Figs 5A, C, 6A, C) — Rounded, very slightly longer than wide (length × width ratio: 1.1 × 1), anterior margin broadly rounded. Nine pairs of notogastral setae thin, smooth; seta c absent; la, lm, lp, and h1 very long (42–62 μm); h2 and h3 medium long (23–25 μm); p1–p3 short (8–12 μm). Lyrifissure im, ih, and ips clearly developed, ia and ip not evident. Opisthonotal gland opening (gla) located anterolateral to seta h3.
Gnathosoma (Figs 5B, 6B) — Subcapitulum slightly wider than long; subcapitular setae a, m and h setiform, smooth, 8–12 μm. Palpal setation: 0–2–1–3–9(+ω); cheliceral setae setiform, finely barbed; cha longer than chb.
Epimeral region (Figs 5B, 6B) — Epimeral fields with numerous muscle sigillae; apodemes apo.2, apo.sj and apo.4 well developed; anterior margin of sejugal apodeme with three pairs of large tubercles; epimeral setal formula: 3–1–3–3; all setae thin, smooth, 3c ~18 μm, other setae 9–12 μm long. Discidium conspicuously projected, not sharply pointed, but rounded distally.
Anogenital region (Figs 5B, 6B) — All anogenital setae thin, smooth: five pairs of genital setae (6–8 μm), aggenital seta (ag ~13 μm) short. Anal and adanal setae 12–14 μm; ad1 inserted posterior, ad2 lateral, ad3 anterolateral to anal aperture. Lyrifissure iad inserted adjacent and parallel to anal aperture.
Legs — Formula of leg setation: I (1–5–2–4–20), II (1–5–2–4–16), III (2–3–1–3–15), IV (1–2–2–3–12); formula of solenidia: I (1–2–2), II (1–1–2), III (1–1–0), IV (0–1–0). Homology of setae and solenidia same as in Table 1.
Material examined
Holotype (female) and one paratype (male): Heuksan-myeon, Heuksando Island, Sinan-gun, Jeollanam-do, Korea, mixed forest, soil and litter under dead trees, Pinus thunbergii, 27 July 2023, Coll. S.J. Yoon (NIBR deposition number: PFZUIV0000000058).
Distribution and habitat ecology
Currently, this species is known only from the type locality, Heuksando.
Etymology
The species name ''heuksandoensis'' refers to Heuksando, a volcanic island in the Yellow Sea, located southwest of the Korean peninsula, where this species was discovered.
Remarks
The Palaearctic species, G. sagami described by Aoki (1984) and studied by Choi (1986) can be distinguished from the new species by the distinctly barbed bothridial seta (vs. smooth in new species); absence of interbothridial tubercle (vs. strongly developed in new species); relatively short interlamellar seta, which is shorter than mutual distance between alveoli of setal pair (vs. in new species, seta in longer than their mutual distance); relatively short notogastral seta lm, which is conspicuously shorter than lp (vs. in new species, lm subequal to or slightly longer than lp); relatively long notogastral seta h1, which is longer than or subequal to la and lp (vs. in new species, h1 shorter than la and lp).
Another Palaearctic species, G. longissima can be distinguished from the new species by the thinner, bothridial seta with long distal tip (vs. bs thicker and with short distal tip in new species); much longer notogastral setae la and lp, which are more than twice longer than lm and h1 (in new species, la and lp subequal to or slightly shorter than lm, but slightly longer than h1); relatively narrow, elongated body (body width 135–156 vs. 172–189 μm).
The third known species, Goyoppia sexpilosa, which is known from Madagascar, can be distinguished from the new species by the absence or minute seta in (vs. seta in long new species); absence of interbothridial tubercle (well developed in new species); very short notogastral setae lm, h2 and h3 (in new species, lm very long, h2 and h3 medium long); presence of alveolus of the notogastral seta c (vs. absent in new species); poorly developed lateral prodorsal ridge (vs. strongly developed in new species).
Genus Graptoppia Balogh, 1983
Type species Graptoppia paraanalis Subías & Rodríguez, 1985
Graptoppia nukusia (Shtanchaeva, 1984)
(Figures 7, 8)
Oppia nukusia Shtanchaeva, 1984 (in Shtanchaeva & Koshchanova, 1984), p. 1107, fig. a–b.
Graptoppia nukusia: Subías & Balogh, 1989, p. 378.




Measurements — Body length 223–226 μm, width of notogaster 104–111 μm, length of notogaster 156–165 μm.
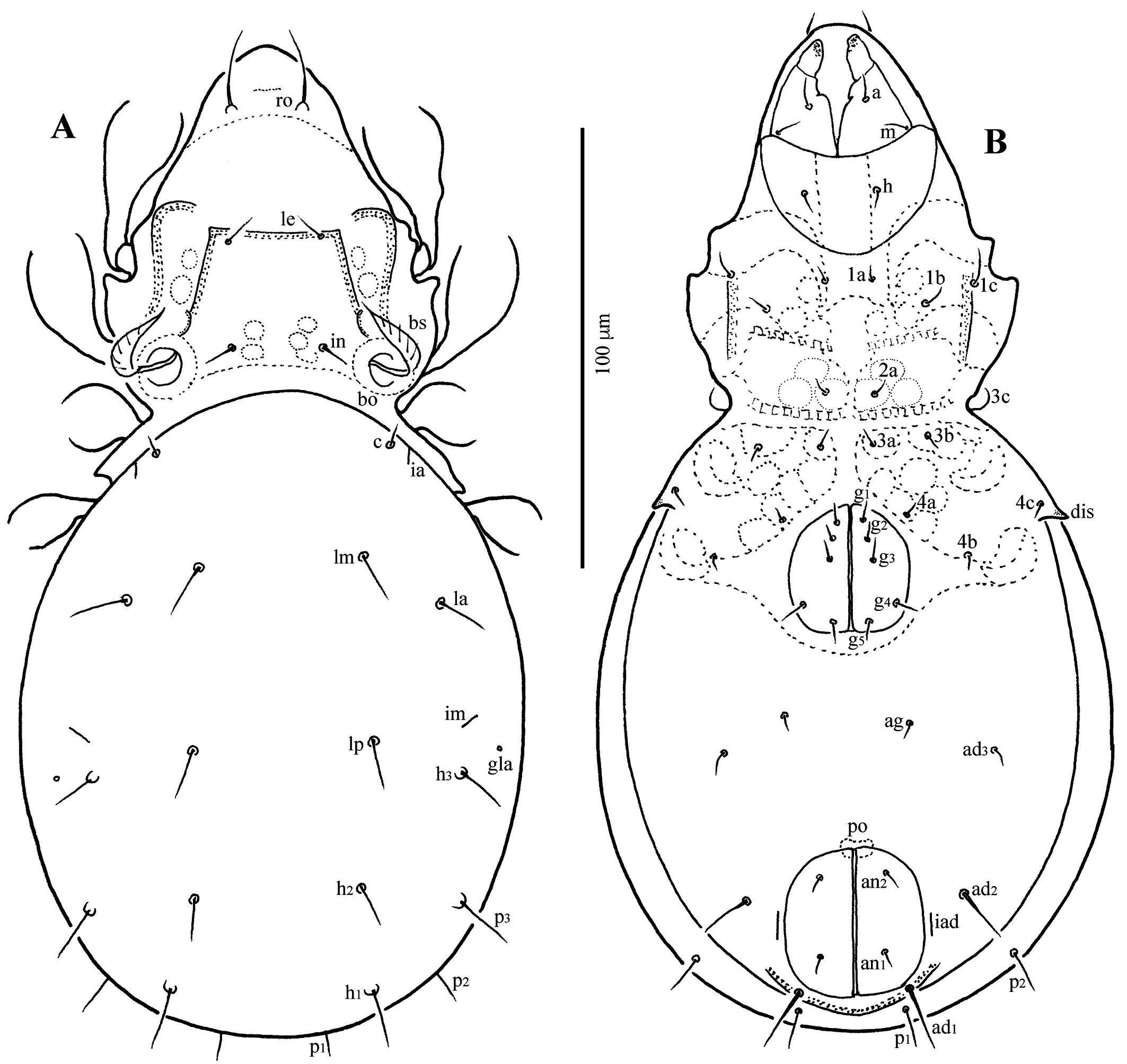
Supplementary description — Body colour light brown; body surface microsculpturing granulate. Lateral part of body between bothridium and leg acetabula densely granulate. Rostrum rounded; rostral seta ~28 μm, thin, smooth, curved inward. Lamellar costula and translamellar ridge well developed, lineate, forming trapezoid structure. Lamellar seta ~14 μm, thin, smooth, inserted on translamellar ridge, medially to lamellar costula. Interlamellar seta ~19 μm, thin, smooth. Bothridium with large opening directed upward. Bothridial seta ~35 μm, lanceolate, pectinate with seven long pectinations. Lateral prodorsal ridge well developed, strongly curved downward in lateral view. Interbothridial region contains two pairs of round to oval muscle sigillae. Notogaster elongate oval, 1.5 × as long as wide, anterior margin rounded, crista not evident. Ten pairs of notogastral setae thin, smooth: c well developed (~8 μm), but much shorter than other setae (15–21 μm); seta la located posterolaterally to lm. Opisthonotal gland opening and lyrifissures ia, im, ih, and ips clearly developed; ip not evident; im located anterior to h3; gla located posteromedially to im (Figs 7A, C, 8A, C). Subcapitulum wider than long; subcapitular setae a, m and h setiform, smooth. Palpal setation: 0–2–1–3–9(+ω); cheliceral setae setiform, barbed; cha longer than chb. Epimeres with muscle sigillae; epimeral setae thin, smooth, 3c longer than other setae; setal formula: 3–1–3–3. Pedotectum I well developed, pedotectum II indistinct; discidium triangular, projecting distally. All anogenital setae thin, smooth; five pairs of genital setae; adanal setae ad1 inserted posterior, ad2 lateral, ad3 anterolateral to anal aperture. Lyrifissure iad inserted longitudinally (paranal) lateral to anal aperture (Figs 7B, 8B). Leg claws smooth. Formula of leg setation: I (1–5–2–4–20), II (1–5–2–4–16), III (2–3–1–3–15), IV (1–2–2–3–12); formula of solenidia: I (1–2–2), II (1–1–2), III (1–1–0), IV (0–1–0).
Material examined
Five females: Heuksan-myeon, Heuksando Island, Sinan-gun, Jeollanam-do, Korea, mixed forest, soil and litter under dead trees, Pinus thunbergii, 27 July 2023, Coll. S.J. Yoon (NIBR accession number: PFZUIV0000000006).
Distribution and habitat ecology
This species was previously only known from Central Asia, i.e., Karakalpaki region of Uzbekistan, found from soil-litter under grape trees (Shtanchaeva and Koshchanova 1984), and is reported here for the first time from the eastern Palaearctic region.
Remarks
The characteristics of the present material correspond well with those of the type material studied by Shtanchaeva and Koshchanova (1984). The main characters, such as well-developed lamellar costula, translamellar and lateral prodorsal ridges, prodorsal and notogastral setae, are identical. The only difference we noticed was in the body size, which is smaller in specimens from Karakalpaki (205 × 93 vs. 223–226 × 104–111 μm). We suspect that Shtanchaeva may have misrepresented the average body size of this species, because in addition to the body length and width, the author also provided the length and width of the proterosoma and hysterosoma, and the sum of their lengths being much larger than the body length informed. Furthermore, the author provided the epimeral setal formula as 2–1–2–2, but her drawing (fig. b) clearly indicates that she omitted the setae 1c, 3c, and 4c. Excepting for these points, the features of our material correspond well to those given in the original description.
Graptoppia tanaitica Karppinen & Poltavskaja, 1990
(Figures 9, 10)
Graptoppia tanaitica Karppinen & Poltavskaja, 1990, p. 104, fig. 2.


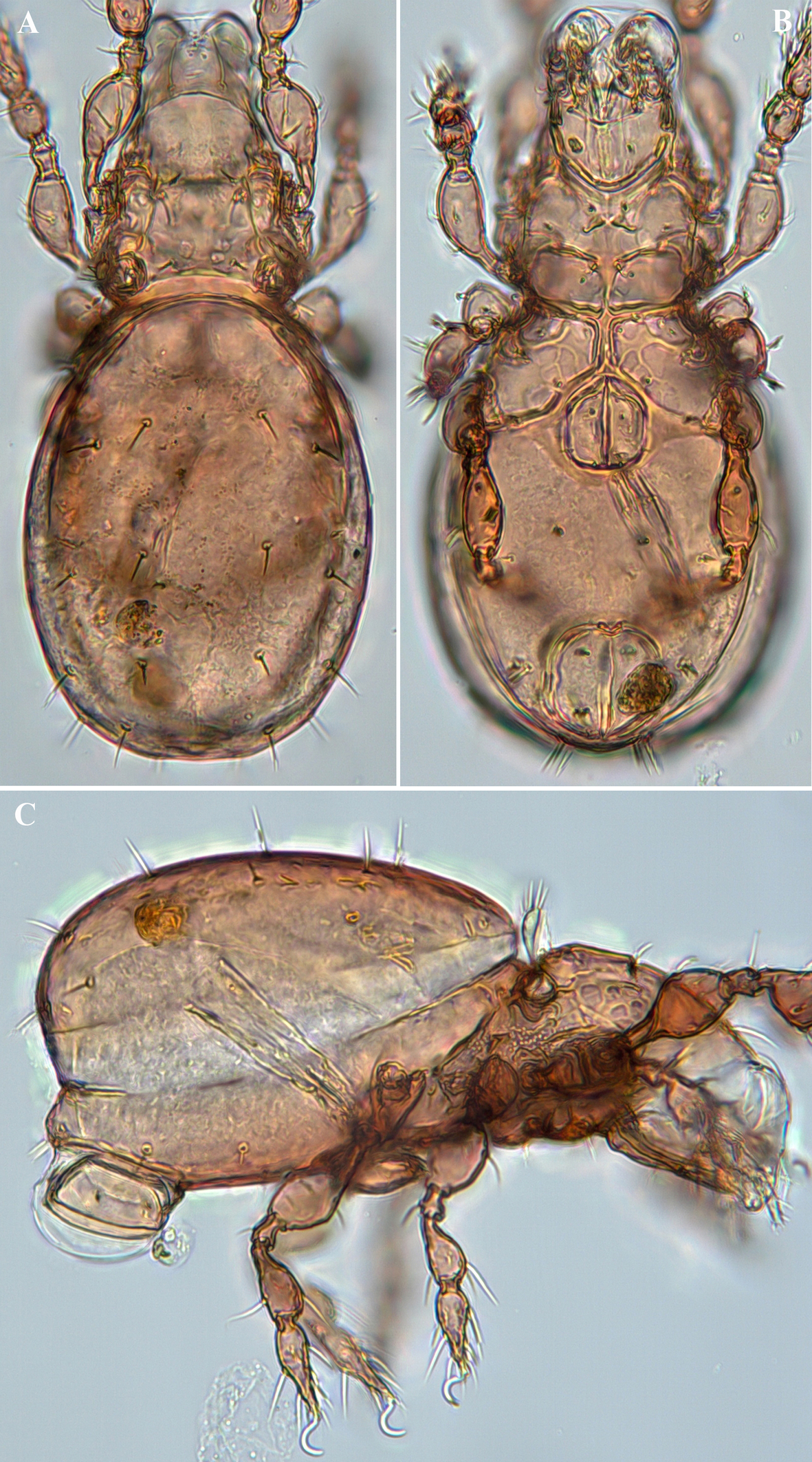


Measurements — Body length 225–239 μm, width of notogaster 113–126 μm, length of notogaster 147–172 μm.
Supplementary description — Body colour light brown; body surface microsculpturing granulate. Lateral part of body between bothridium and leg acetabula I–III densely tuberculate. Rostrum rounded; rostral seta ~19 μm, thin, smooth, slightly curved inward. Lamellar costula and translamellar ridge well developed, lineate, forming trapezoid structure; translamellar ridge faintly concave. Lamellar seta ~7 μm, thin, smooth, inserted on translamellar ridge, medially to lamellar costula. Interlamellar seta ~7 μm, thin, smooth. Bothridium with large opening directed posterolaterad. Bothridial seta ~29 μm, lanceolate, pectinate with 10–12 long pectinations. Lateral prodorsal ridge well developed, strongly curved downward in lateral view. Interbothridial region contains two pairs of muscle sigillae. Notogaster oval, 1.3 × as long as wide, anterior margin widely rounded, crista not evident. Ten pairs of notogastral setae thin, smooth: c clearly developed (~4 μm), but much shorter than other setae (10–13 μm); seta la located posterolaterally to lm; all dorsal setae inserted on large tubercle. Opisthonotal gland opening and lyrifissures ia, im, ih, and ips clearly developed; ip not evident; im located anterior to h3; gla located posterolaterally to im (Figs 9A, 10A, C). Subcapitulum wider than long; subcapitular setae a, m and h setiform, smooth. Palpal setation: 0–2–1–3–9(+ω); cheliceral setae setiform, barbed; cha longer than chb. Epimeres with muscle sigillae; longitudinal ridge present lateral to apodeme 2, i.e., posterior to seta 1c; epimeral setae thin, smooth, 3c longer than other setae; setal formula: 3–1–3–3. Pedotectum I well developed, pedotectum II indistinct; discidium triangular, projecting distally. All anogenital setae thin, smooth; five pairs of genital setae; adanal setae ad1 and ad2 much longer (12–14 μm) than other anogenital setae (~3–6 μm); ad1 inserted posterior, ad2 lateral, ad3 anterolateral to anal aperture. Lyrifissure iad inserted longitudinally (paranal) lateral to anal aperture. Postanal process strongly developed, nearly semicircular (Figs 9B, 10B). Leg claws smooth. Formula of leg setation: I (1–5–2–4–20), II (1–5–2–4–16), III (2–3–1–3–15), IV (1–2–2–3–12); formula of solenidia: I (1–2–2), II (1–1–2), III (1–1–0), IV (0–1–0).
Material examined
Ten females: Wirye Park, Seongnam-shi, Gyeonggi Province, Korea, from a mixed forest, soil and litter under broad leaved trees, such as oak, maple, and beech, Coll. Y.S. Bae, 10 July 2023 (NIBR accession number: PFZUIV0000000005).
Distribution and habitat ecology
This species was previously known only from its type locality, i.e., Rostov district of Russia in southeastern Europe, where it was found from soils of the grassland dominated by Festuca spp. as well as agricultural land (Karppinen and Poltavskaja 1990; Krivolutsky et al. 1995; Lebedeva and Poltavskaya 2013), and is reported herein for the first time from the eastern Palaearctic region.
Remarks
The characteristics of the present material correspond well with those of the type material studied by Karppinen and Poltavskaja (1990). The main characters, such as lamellar costula, translamellar (slightly concaved) and lateral prodorsal ridges, prodorsal and notogastral setae (especially clearly developed seta c), are identical. The only difference we noticed is that the adanal seta ad3 was inserted at the same level as the aggenital seta in south European specimens, while in Korean specimens, ad3 is positioned posterolaterally to ag. Moreover, the authors did not show a clearly developed postanal process in the original description of this species, but we consider these differences as intraspecific variations of different populations.
Acknowledgments
We would like to thank all members of the Laboratory of Animal Diversity, Division of Life Sciences, Incheon National University, Korea for their kind assistance during the laboratory research. Thanks also are due to two anonymous reviewers for their valuable suggestions and comments. This research was supported by the National Institute of Biological Resources, Ministry of Environment, Korea (NIBR202304202, 202402103). Additional support by the National University of Mongolia (P2024-4833) is highly appreciated.
References
- Aoki J. 1984. New and unrecorded oribatid mites from Kanagawa, Central Japan (I). Bulletin of the Institute of Environmental Science and Technology, Yokohama National University, 11: 107-118.
- Balogh J. 1960. Oribates (Acari) nouveaux de Madagascar (1ère serie). Mémoirs de L'Institut Scientifique de Madagascar, Série A, 14: 7-37.
- Balogh J. 1983. A partial revision of the Oppiidae Grandjean, 1954 (Acari: Oribatei). Acta Zoologica Academiae Scientiarum Hungaricae, 29: 1-79.
- Bayartogtokh B. 2010. Oribatid mites of Mongolia (Acari: Oribatida). Moscow: KMK Scientific Press. pp. 372. (in Russian)
- Bayartogtokh B., Bae Y-S. 2022a. New and little known species of soil mites of the family Oppiidae (Acari: Oribatida) from Korea. International Journal of Acarology, 48: 241-255. https://doi.org/10.1080/01647954.2022.2058086
- Bayartogtokh B., Bae, Y-S. 2022b. New findings of oribatid mites of the genera Rhinoppia and Suctobelbella (Acari: Oribatida) from Korea. Systematic and Applied Acarology, 27: 1356-1387. https://doi.org/10.11158/saa.27.7.5
- Bayartogtokh B., Bae, Y-S. 2024. New and little-known species of soil mites of the families Oppiidae and Suctobelbidae (Acari: Oribatida) from Korea. International Journal of Acarology, 50: 18-38. https://doi.org/10.1080/01647954.2023.2290070
- Choi S.S. 1986. The oribatid mites (Acari: Cryptostigmata) of Korea (5). On new and unrecorded species of the family Oppiidae. Acta Arachnologica, 34: 61-67. https://doi.org/10.2476/asjaa.34.61
- Choi S.S. 1997. Checklist of oribatid mites of Korea. Korean Arachnology, 13: 83-104. (in Korean)
- Choi S.S. 1998. A faunal list of oribatid mites from Mt. Jiri. Korean Journal of Soil Zoology, 3: 42-49. (in Korean)
- Choi S.S. 1999. Oribatida mites from Mt. Mai. Journal of Life Science and Natural Research. 23:1-19. (in Korean)
- Choi S.S., Jung S-H. 1998. A faunal list of oribatid mites (Acari: Oribatida) from Cheju Island. Korean Journal of Soil Zoology, 3: 106-110. (in Korean)
- Corpuz-Raros L., Ermilov S.G. 2020. Catalogue of oribatid mites (Acari: Oribatida) from Continental Southeast Asia. Zootaxa, 4893: 001-216. https://doi.org/10.11646/zootaxa.4893.1.1
- Ermilov S.G. 2016 New Oppiidae (Acari, Oribatida) from Chile. Acarologia, 56: 505-516. https://doi.org/10.1051/acarologia/20164142
- Ermilov S.G. 2023. Contribution to the taxonomy of the oribatid mite genus Graptoppia Balogh 1983 (Acari, Oribatida, Oppiidae). Zoologichesky Zhurnal, 102: 744-750. https://doi.org/10.31857/S0044513423060041
- Hong S-K. 2015. Socio-economic foundation by biocultural resources management: Suggestion for UNESCO Shinan Dadohae Biosphere Reserve, Korea. Journal of Marine and Island Cultures. 4: 81-88. https://doi.org/10.1016/j.imic.2015.11.001
- Hong S-K., Wu J., Kim J-E., Nakagoshi N. 2011. Landscape Ecology in Asian Cultures. Tokyo: Springer. pp. 334. https://doi.org/10.1007/978-4-431-87799-8
- Ivan O., Vasiliu N. 1997. New species of the family Oppiidae Grandjean, 1954 (Acari: Oribatida). Travaux du Muséum National d'Histoire Naturelle "Grigore Antipa″, 39: 7-29.
- Karppinen E., Poltavskaja M.P. 1990. Some new oribatids (Acarina, Oribatei) from the Rostov-Don region, Soviet Union (USSR). Entomologica Fennica, 1: 103-106. https://doi.org/10.33338/ef.83362
- Krivolutsky D.A., Lebrun P., Kunst M., Akimov I.A., Bayartogtokh B., Vasiliu N., Golosova L.D., Grishina L.D., Karppinen E., Kramnoy V.J., Laskova L.M., Luxton M., Marshall V., Matveenko A.A., Netuzhilin I.A., Norton R.A., Sitnikova L.G., Smrž J., Starý J., Tarba Z.M., Shaldybina E.S. & Eitminavichuite I. 1995. Oribatid mites: morphology, development, phylogeny, ecology, methods of study and characteristics of the model species Nothrus palustris C.L. Koch, 1839. Moscow: Nauka Publishers. pp. 224. (in Russian)
- Lebedeva N.V., Poltavskaya M.P. 2013. Oribatid mites (Acari, Oribatida) of plain area of the Southern European Russia. Zootaxa, 3709: 101-133. https://doi.org/10.11646/zootaxa.3709.2.1
- Mahunka S. 1987. A survey of the oribatids of Kiskunság National Park (Acari: Oribatida). In: Mahunka S. (Ed.). Fauna of Kiskunság National Park, Vol. 2. Budapest: Akadémiai Kiadó. p. 346-397.
- Miko L. 2006. Damaeidae. In: Weigmann G. (Ed.). Hornmilben (Oribatida). Die Tierwelt Deutschlands. 76 Teil. Keltern: Goecke & Evers. p. 179-207.
- Murvanidze M., Mumladze L. 2016. Annotated checklist of Georgian oribatid mites. Zootaxa, 4089: 1-81. https://doi.org/10.11646/zootaxa.4089.1.1
- Norton R.A., Behan-Pelletier V.M. 2009. Suborder Oribatida. Chapter 15. In: Krantz G.W., Walter D.E. (Eds). A manual of acarology. Lubbock: Texas Tech University Press, p. 430-564.
- Paoli G. 1908. Monografia del genere Dameosoma Berl. e generi affini. Redia, 5: 31-91.
- Ryabinin N.A. 1987. New oribatid mites of the genus Oppia C.L. Koch, 1836 from the USSR fauna. Izvestiya Sibirskogo Otdeleniya Akademii Nauk USSR, Series of Biological Sciences, 20: 104-106. (in Russian)
- Schweizer J. 1956. Die Landmilben des Schweizerischen Nationalparkes. 3. Teil, Sarcoptiformes Reuter 1909. Ergebnisse der wissenschaftlichen Untersuchungen des schweizerischen Nationalparks, Neue Folge, Liestal, 5: 213-377.
- Shtanchaeva U.Ya., Koshchanova R.E. 1984. New species of Oribatei from Karakalpakia. Zoologichesky Zhurnal, 63: 1107-1109. (In Russian)
- Subías L.S. 2004. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (1758-2002). Graellsia, 60: 3-305. https://doi.org/10.3989/graellsia.2004.v60.iExtra.218
- Subías L.S. 2024. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes, Oribatida) del mundo. (19a actualización, annual online update). Available from: http://bba.bioucm.es/cont/docs/RO_1.pdf
- Subías L.S., Arillo, A. 2001. Acari, Oribatei, Gymnonota II. Oppioidea. In: Ramos A. (Ed.). Fauna Iberica, Vol. 15. Madrid, Museo de Ciencias Naturales, p. 22-48.
- Subias L.S., Balogh P. 1989. Identification keys to the genera of Oppiidae Grandjean, 1951 (Acari: Oribatei). Acta Zoologica Academiae Scientiarum Hungaricae, 35: 355-412.
- Subías L.S., Shtanchaeva U.Ya. 2011. Un nuevo subgénero, seis nuevas especies y dos nuevas subespecies del género Rhinoppia Balogh, 1983 (Acari, Oribatida, Oppiidae, Medioppiinae) de la Península Ibérica y de Marruecos. Boletín de la Sociedad Española de Historia Natural Sección Biológica, 105: 1-10.
- Subías L.S., Shtanchaeva U.Ya. 2023. Claves de familias, géneros y subgéneros de ácaros oribátidos del mundo (Acari, Oribatida). Monografías electrónicas Sociedad Entomológica Aragonesa, 13. pp. 290. Available from: http://sea-entomologia.org/MeSEA13_2023.pdf
- Subías L.S., Shtanchaeva U.Ya., Arillo, A. 2012. Listado de los ácaros oribátidos (Acariformes, Oribatida) de las diferentes regiones biogeográficas del mundo. Monografías electrónicas Sociedad Entomológica Aragonesa, 4. pp. 819. Available from: http://sea-entomologia.org/Publicaciones/MonografiaElectronica4/ACARI_ORIBATIDA_MESEA4.pdf
- Toluk A., Ayyildiz N. 2009. Three new species of Oppiidae from Turkey (Acari: Oribatida). Zootaxa, 1988: 33-47. https://doi.org/10.11646/zootaxa.1988.1.3
- Travé J., Vachon M. 1975. François Grandjean, 1882-1975 (Notice biographique et bibliographique). Acarologia, 17: 1-19.
- Vasiliu N.A., Ivan O. 2011. New Oppiid species (Acari, Oribatida, Oppiidae) from Romanian caves. Travaux de l'Institut de Speologie Emile Racovitza, 50: 3-14.
- Weigmann G. 2006. Hornmilben (Oribatida). Die Tierwelt Deutschlands. 76 Teil. Keltern: Goecke & Evers. pp. 520.
- Wen Z. 1987. Two new oribatid mites from China (Acarina: Oppiidae and Galumnidae). Acta Zootaxonomica Sinica, 12: 61-65. (in Chinese)
- Willmann C. 1931. Moosmilben oder Oribatiden (Cryptostigmata). In: Dahl F. (Ed.). Die Tierwelt Deutschlands, Bd. 22, Vol. 5, Jena: Gustav Fischer. p.79-200.
- Woas S. 1986. Beitrag zur Revision der Oppioidea sensu Balogh, 1972 (Acari, Oribatei). Andrias, Karlsruhe, 5: 21-224.



2025-10-01
Date accepted:
2025-12-05
Date published:
2025-12-15
Edited by:
Pfingstl, Tobias

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Bayartogtokh, Badamdorj and Bae, Yang-Seop
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



