Two new feather mite species of the genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae: Pterodectinae) from Red-tailed Laughingthrush Trochalopteron milnei (Passeriformes: Leiothrichidae) in China
Constantinescu, Ioana Cristina  1
; Chișamera, Gabriel Bogdan
1
; Chișamera, Gabriel Bogdan  2
; Motoc, Rozalia
2
; Motoc, Rozalia  3
; Gustafsson, Daniel R.
3
; Gustafsson, Daniel R.  4
; Zou, Fasheng
4
; Zou, Fasheng  5
; Wang, Zhengzhen
5
; Wang, Zhengzhen  6
; Grossi, Alexandra A.
6
; Grossi, Alexandra A.  7
; Zhou, Wenyi
7
; Zhou, Wenyi  8
and Adam, Costică
8
and Adam, Costică  9
9
1✉ "Grigore Antipa" National Museum of Natural History, Sos. Kiseleff no.1, 011341 Bucharest, Romania.
2Institute of Biology – Bucharest, Romanian Academy, Splaiul Independenței 296, 060031 Bucharest, Romania.
3"Grigore Antipa" National Museum of Natural History, Sos. Kiseleff no.1, 011341 Bucharest, Romania.
4Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Library of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, 105 Xingang West Road, Haizhu District, Guangzhou, 510260, Guangdong Province, China.
5Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Library of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, 105 Xingang West Road, Haizhu District, Guangzhou, 510260, Guangdong Province, China.
6Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Library of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, 105 Xingang West Road, Haizhu District, Guangzhou, 510260, Guangdong Province, China.
7Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Library of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, 105 Xingang West Road, Haizhu District, Guangzhou, 510260, Guangdong Province, China.
8Department of Health and Environmental Sciences, Xi’an Jiaotong-Liverpool University, Suzhou, Jiangsu, China & Department of Biology, University of Florida, Gainesville, Florida, USA & Florida Museum of Natural History, University of Florida, Gainesville, Florida, USA.
9"Grigore Antipa" National Museum of Natural History, Sos. Kiseleff no.1, 011341 Bucharest, Romania.
2025 - Volume: 65 Issue: 4 pages: 1225-1245
https://doi.org/10.24349/1xp0-t80kZooBank LSID: D192F942-F08D-41D5-8A9A-90136D708E05
Original research
Keywords
Abstract
Introduction
Feather mites (Astigmata: Analgoidea and Pterolichoidea) are common and diverse ectosymbionts of birds that spend their entire lives on their hosts. The genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae) belongs to the Proterothrix generic group in the subfamily Pterodectinae. This group currently comprises eight genera of putatively early derivative pterodectines with setae ps3 anterior to the adanal suckers in males and seta wa situated noticeably anterior to setae ra and la on tarsi I and II in both sexes (Mironov 2009, Mironov & Proctor 2009, Hernandes & Valim 2014, Mironov & OConnor 2017). The genus Proterothrix clearly differs from other genera of this group in having the following combinations of characters: in both sexes, solenidion σ of genu III is present and shorter than this segment, and idiosomal setae d2 are present; in males, the genital arch is well developed, the genital papillae are close to each other and situated anterior to or at the level of this arch, and the opisthosomal lobes are devoid of membranous projections. To date, the genus has included 41 species, and 38 of them are arranged in the four morphology-based species groups: megacaula (3 species), schizothyra (5 species), wolffi (20 species) and paradoxornis (10 species). Representatives of the schizothyra group are restricted to kingfishers (Coraciiformes: Alcedinidae), species of the megacaula group are only known from several hosts of the passerine family Muscicapidae, those of the paradoxornis group are known from hosts of the four sylvioidean families (Alcippeidae, Leiothrichidae, Pellorneidae and Paradoxornithidae), and mites of the wolffi group are known from various passerine families (Muscicapidae, Rhipiduridae, Paradisaeidae, Monarchidae, Dicruridae, Acanthizidae and Eurylaimidae) and from woodpeckers (Piciformes: Picidae) (Gaud 1952, 1962, 1968, 1979, Gaud & Atyeo 1996, Mironov et al. 2008, 2010, 2012, Mironov & Proctor 2009, Mironov & Tolstenkov 2013, Mironov & Galloway 2021, Constantinescu et al. 2014, 2017a, 2017b, 2018, 2019, 2021, 2023, 2024a, 2024b, Han & Min 2019, He et al. 2024).
Materials and methods
The material used in the present paper was collected from the Red-tailed Laughingthrush, Trochalopteron milnei David, 1874, in Yunnan Province, China, in a study of ectoparasites of birds. The bird was captured with the help of ornithological nets, identified and visually checked for the presence of mites; after collecting of mites, it was released back into the wild. Mite specimens were placed in tubes with 95% ethanol, and later, in the laboratory, were cleared in lactic acid and mounted on microscope slides in Hoyer's medium. Drawings were made using an Olympus CX21 microscope equipped with a camera lucida drawing device.
The nomenclature of body setation follows that of Griffiths et al. (1990) with the coxal setae modifications made by Norton (1998), and that of the legs follows Grandjean (1939). The description of the new species of Proterothrix is given according to the current format used for species of pterodectine mites (Mironov & Fain 2003, Mironov 2006, Valim & Hernandes 2006, Mironov et al. 2008), and the measuring techniques of particular structures used herein were described by Mironov and Proctor (2009).
For scanning electron microscope study (SEM), feather mites were cleaned in an ultrasonic cleaner (Evo Sonic) for 10–30 seconds to remove debris and then fixed in 2.5% glutaraldehyde in sodium cacodylate buffer (0.1M, pH 7.4) in a refrigerator at 4°for 4h. The specimens were dehydrated in an ascending alcohol series (30%, 50%, 70%, 80%, 95%, and 100% ethanol; 10 min. each) and then in hexamethyldisilazane (HMDS) for 1h (Han & Min 2019, Han et al. 2019). After being air-dried, the samples were mounted on aluminum stubs covered with conductive double-sided adhesive carbon tabs. The samples were sputter-coated with gold for 30 seconds. The feather mites were analyzed and photographed using a Phenom Pro scanning electron microscope (Phenom-World, Thermo Fisher Scientific, The Netherlands), at 10kV acceleration voltage.
The bird specimens were identified according to Arlott (2017), but the taxonomy of the birds follows Clements et al. (2024), which differs in species limits used. We give the full set of measurements for a holotype (male) and range of measurements for all corresponding paratypes. All measurements are in micrometers (μm).
The type specimens of the new species are deposited in the Institute of Zoology, Guangdong Academy of Science, Guangzhou, China (IZGAS, previously the Guangdong Institute of Applied Biological Resources, GIABR) and in the Acarological Collection of the ''Grigore Antipa″National Museum of Natural History, Bucharest, Romania (MGAB). Specimens with the collection index GD-ACARI are in the IZGAS, and those with the accession collection index ANA are in MGAB.
Taxonomy
Family Proctophyllodidae Trouessart and Mégnin, 1884
Subfamily Pterodectinae Park and Atyeo, 1971
Genus Proterothrix Gaud, 1968
Proterothrix papilio Constantinescu n. sp.
ZOOBANK: 8A8B1E54-6638-4521-8A4D-00E0E34D9EAC ![]()
(Figures 1–4, 9, 10)
Type material
From Trochalopteron milnei David, 1874 (Passeriformes: Leiothrichidae): male holotype (ANA2162), 7 male (ANA2163–2169) and 6 female (ANA2170–2175) paratypes, in MGAB; 2 male (GD-ACARI-59–60) and 2 female (GD-ACARI-61–62) paratypes, in IZGAS, China, Yunnan Province, Dehong Prefecture, Tongbiguan, Nanduhe, 24°38′25.0″N, 97°38′45.0″E, 11 December 2023, coll. D.R. Gustafsson, Z. Wang, A.A. Grossi & W. Zhou, bird ID: J4913.
Description
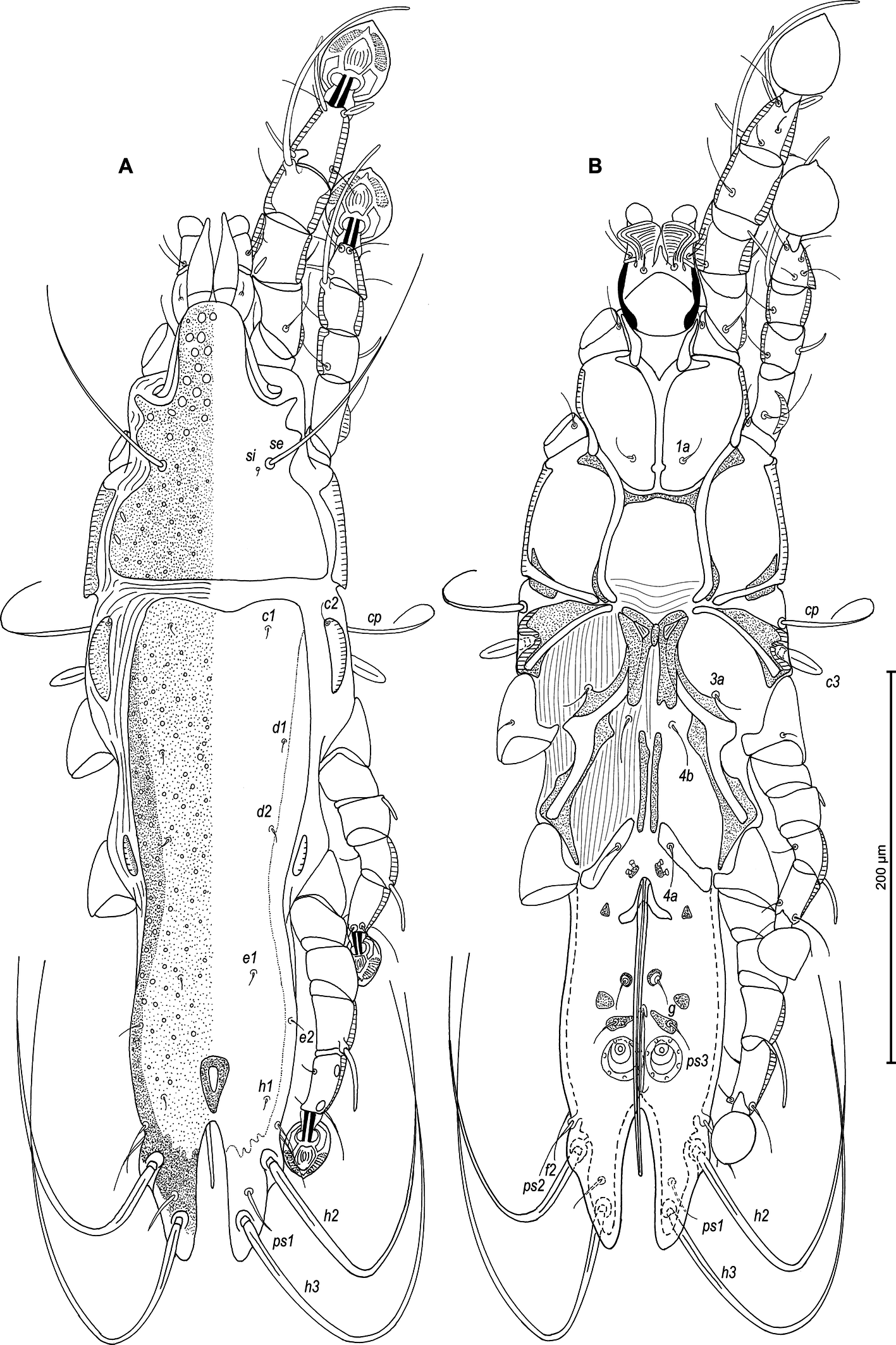


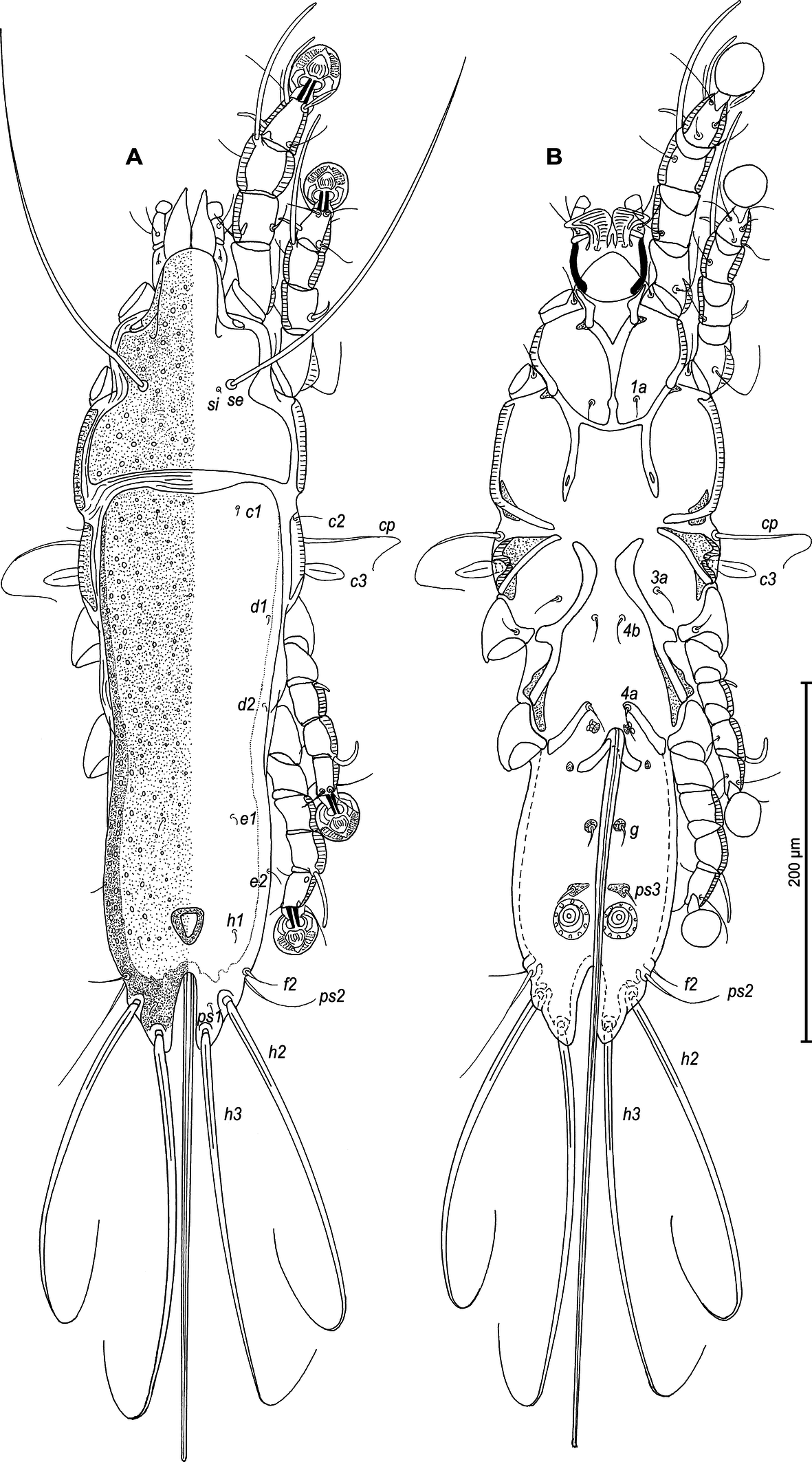
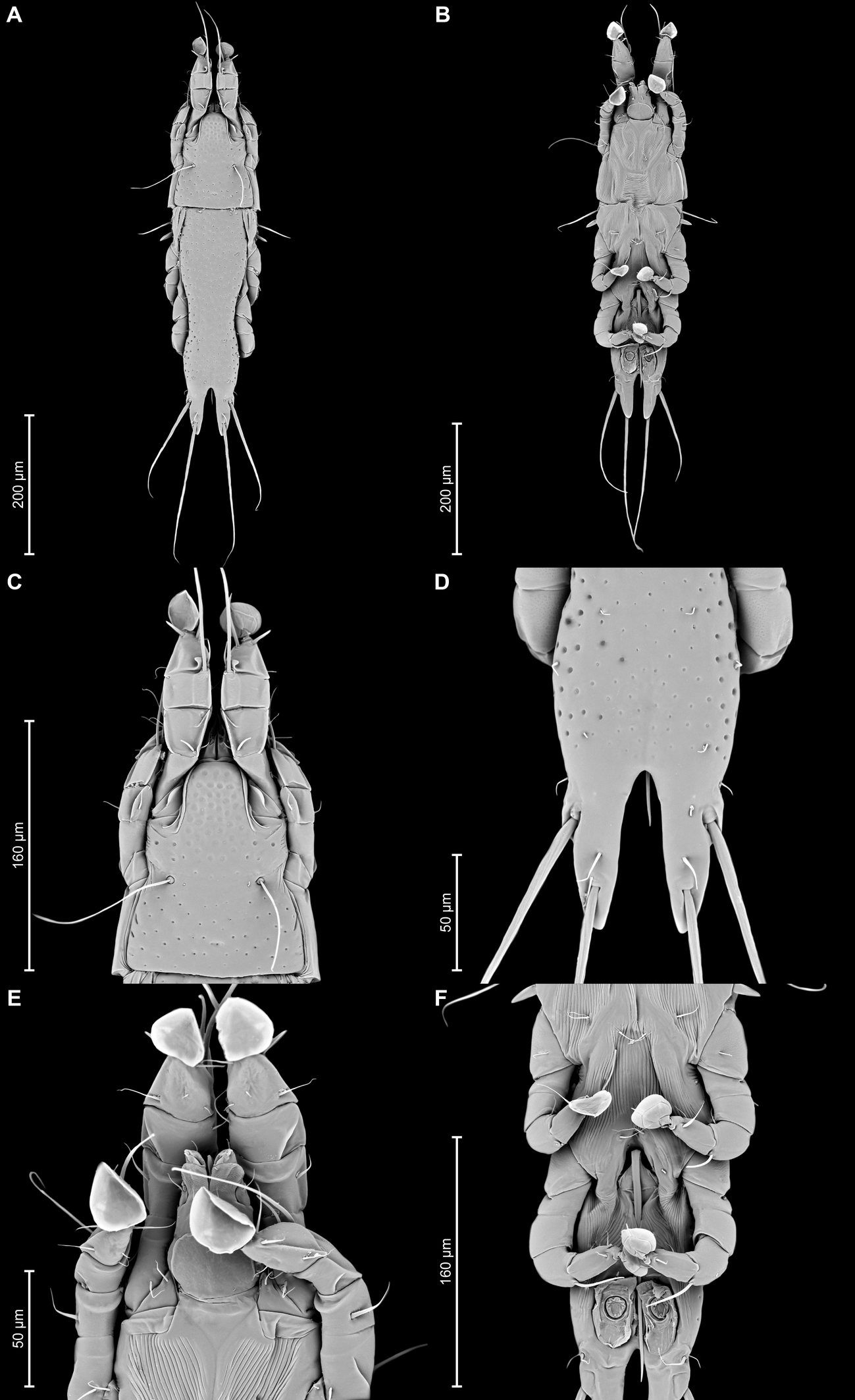
Male — (holotype, range for 4 paratypes in parentheses) (Figures 1A, B, 2A–E, 9A–F): Length of idiosoma 510 (490–515), width 148 (138–145), length of hysterosoma 360 (340–365). Pseudorutellar lobes without acute lateral extensions. Prodorsal shield entire, anterolateral extensions acute, lateral margins shallowly concave, posterior margin straight, posterior angles round, length of shield along midline 143 (138–145), width at posterior margin 120 (113–120), surface with numerous small circular lacunae, which noticeably larger in anterior part of this shield (Figure 1A, 9C). Scapular setae se separated by 60 (55–60). Scapular shields narrow. Humeral shields wide, not fused ventrally with outer sclerotized areas of epimerites III. Setae cp situated ventrally, on striated tegument. Setae c2 filiform, on anterior end of humeral shield. Subhumeral setae c3 lanceolate, 27 (25–28) × 8 (8–10). Hysteronotal shield with anterior margin slightly concave, anterior angles round, distance from anterior margin to bases of setae h3 335 (320–340), greatest width in anterior part 100 (95–100), surface except for lobar area with small circular lacunae. Lateral hysteronotal sclerites present, situated at level of trochanters IV. Opisthosomal lobes elongated, attenuate to apex, posterior end of apices rounded, lateral margins of lobes with rounded ledges bearing setae h2, setae h3 situated near lobar apices. Supranal concavity elongate shaped as inverted teardrop, with margins clearly outlined. Terminal cleft narrow, parallel-sided, with angular anterior end, 75 (73–78) in length. Setae f2 slightly anterior to setae ps2. Setae h1 at level of supranal concavity, distant from lateral margins of opisthosoma. Setae ps1 spiculiform, 25 (20–28) long, situated approximately at midlength between setae h2 and h3, equidistant from outer and inner lobar margins; setae ps2 setiform, 30 (25–33) long. Setae h3 represented by macrosetae, slightly longer than macrosetae h2, 275 (225–255) × 10 (8–10), 190 (175–200) × 8(6–7), respectively. Distances between dorsal setae: c2–d2 120 (115–130), d2–e2 113 (93–105), e2–h3 103 (98–100), d1–d2 63 (63–68), e1–e2 43 (38–39), h1–ps2 25 (20–28), h2–h2 65 (63–68), h3–h3 35 (25–38), ps2–ps2 70 (68–75).
Epimerites I fused into a Y with long sternum, its posterior end connected with middle parts of epimerites II by transverse sclerotized extensions. Epimerites II long, with posterior ends free. Epimerites IIIa elongated, extending to sejugal area, their medial parts with long extensions directed backward, their anterior ends connected to each other by median sclerotized plate (transventral sclerite) resembling a butterfly. Pregenital apodemes represented by a pair of stick-shaped sclerites. Epimerites IVa well developed, with setae 4a on inner ends, not connected with pregenital sclerites. Coxal fields I, II without wide sclerotized areas. Coxal fields I closed, coxal fields II and III almost closed, with narrow gaps between tips of epimerites (Fig 1B). Genital arch 25 (20–23) long, 30 (25–30) wide. Aedeagus long whip-shaped, extending to midlength of terminal cleft, length of aedeagus from its anterior bend to tip 143 (134–144). Genital papillae anterior to genital arch apex, on each side on small ovate sclerite. Paragenital sclerites at tips of genital arch minute, triangular. Genital setae g on small circular genital sclerites, situated approximately equidistant from genital arch and adanal suckers. Opisthoventral shields absent. Adanal shields well developed, represented by two pairs of small sclerites: anterior sclerites roughly triangular, posterior sclerites teardrop-shaped and bearing bases of setae ps3. Setae ps2 thickened basally, 30 (25–33) in length. Anal suckers 20 (20–23) in diameter; corolla indented, with 7 denticles. Distances between ventral setaes: 3a–4b 30 (25–28), 4b–4a 68 (60–68), 4a–g 70 (63–70), g–ps3 23 (23–28), ps3–ps3 32 (25–33), ps3–h3 99 (93–97).
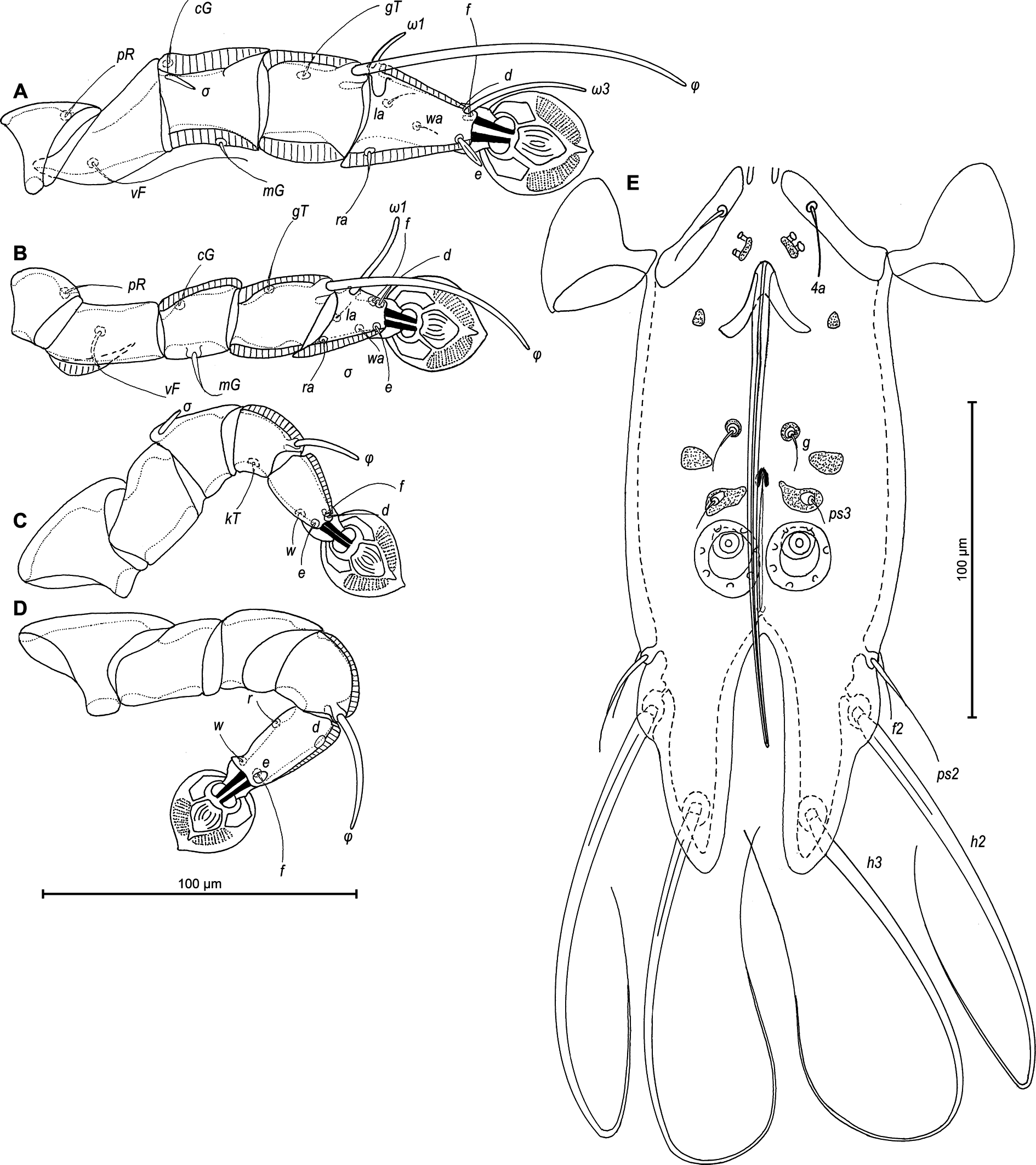


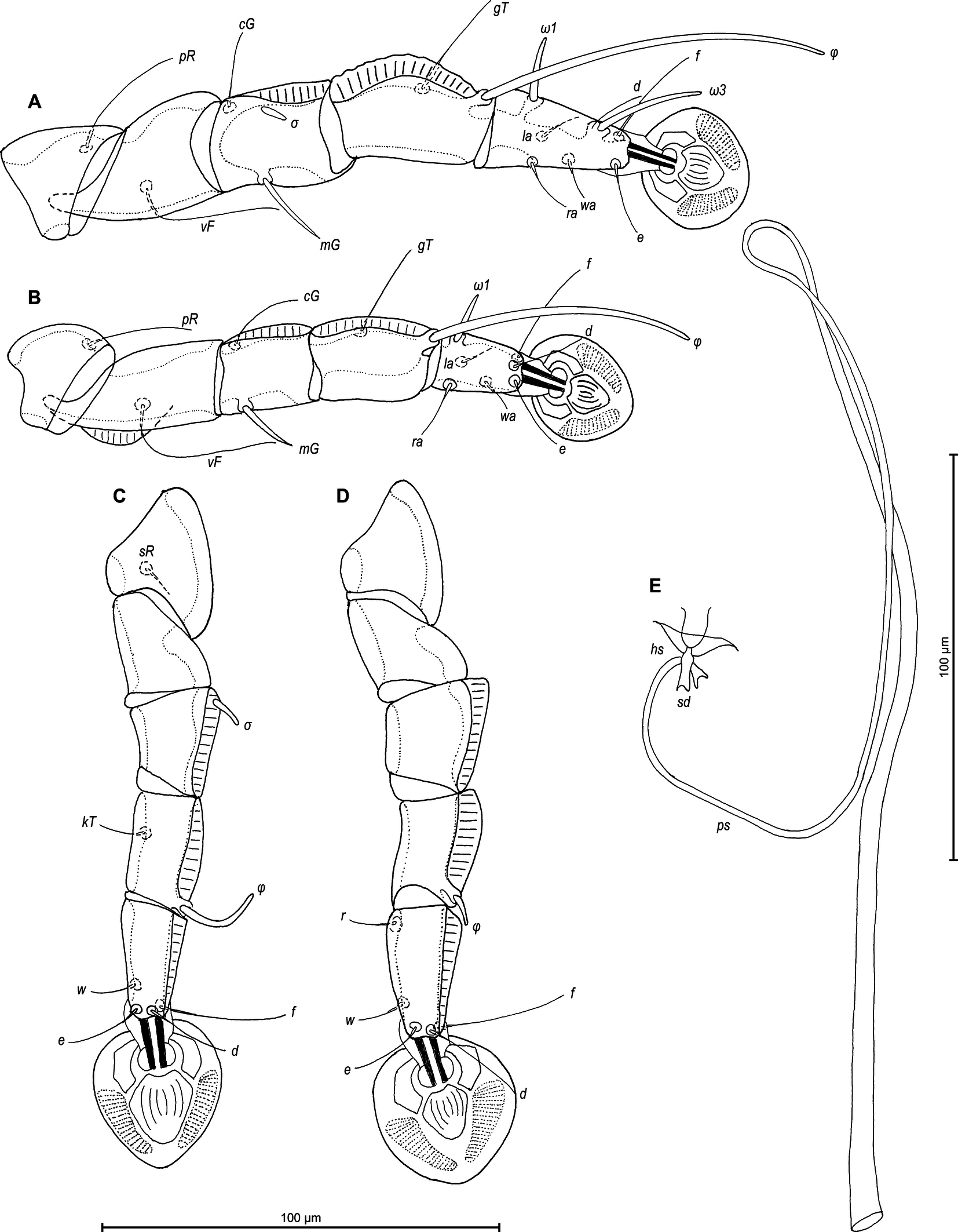
Legs I longer and thicker than legs II; femur II with ventral crest (Figure 2 A, B). Genu, tibia and tarsus I with dorsal and lateral longitudinal crests, tarsus I with thumb-like dorsal process (Figure 2A). Genu II with dorsal longitudinal crest, tibia II with dorsal and lateral longitudinal crests, tarsus II with lateral longitudinal crest (Figure 2B). Seta e of tarsus I lanceolate, setae mG of genu I setiform, seta mG of genu II spiculiform with acute apex. Setae d of tarsi II, III similar in length to corresponding setae f. Tarsus IV with a small apicoventral extension bearing setae w, 30 (30–35) long, setae d, e button-like, situated in basal and apical parts of segment, respectively (Figure 2D). Length of solenidia: ω1I 15 (15–18), ω1II 17 (18–20), φI 100 (93–100), φII 70 (63–70), φIII 27 (25–30), φIV 38 (32–38).


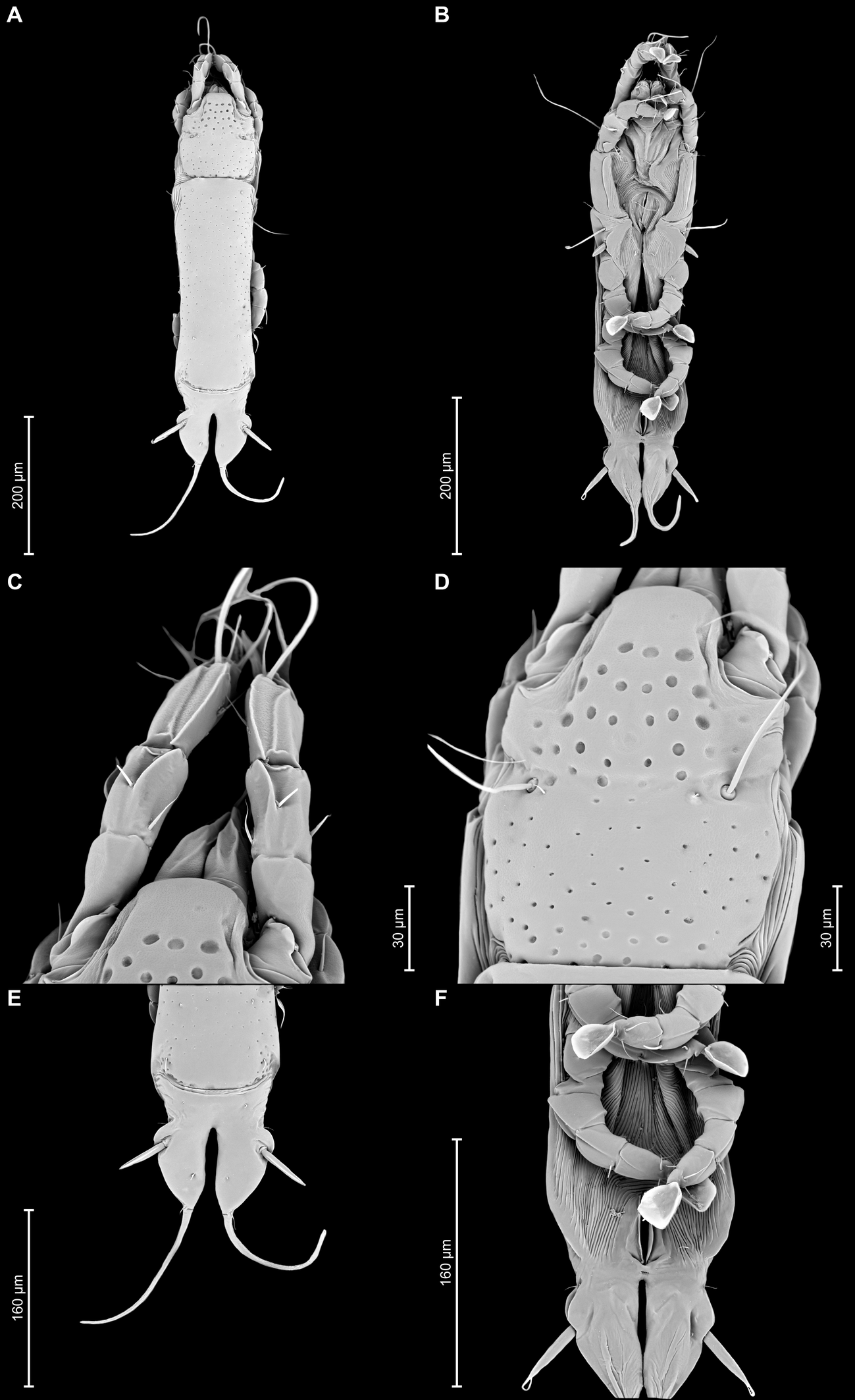
Female — (range for 5 paratypes) (Figures 3A, B, 4A–E, 10A–F): Length of idiosoma 590–620, width 160–190, and length of hysterosoma 440–460. Pseudorutellar lobes without acute lateral extensions. Prodorsal shield entire, anterolateral extensions acute, lateral margins shallowly concave, posterior margin straight, posterolateral margins widely rounded, length of shield along midline 150–168, largest width 113–125, surface with numerous small circular lacunae, which noticeably larger in anterior part of the shield (Figure 3A, 10D). Scapular setae se separated by 73–800. Scapular shields narrow. Humeral shields wide, not fused ventrally with outer sclerotized areas of epimerites III. Setae cp situated ventrally, on striated tegument. Setae c2 filiform, on anterior end of humeral shield. Subhumeral setae c3 lanceolate, 27–30 × 10–13.
Anterior hysteronotal shield: almost rectangular, anterior margin straight, posterior margin slightly convex, with a a pair of narrow incision near posterior angles, greatest length 310–330, greatest width in anterior part 118–125, surface with small circular lacunae. Length of lobar region 125–130, width at level of h2 setae 118–125. Anterior and lobar pieces of hysteronotal shield separated dorsally by narrow transverse band but remain connected ventrolaterally. Terminal cleft narrow, with lateral margins almost touching and slightly divergent posteriorly, length 75–88. Supranal concavity circular, well outlined. Setae h1 on lobar shield, at level of anterior margin of supranal concavity; surface of lobar shield without ornamentation. Setae h2 spindle-shaped, with long terminal filament, 78–88 × 10–13. Setae ps1 closer to inner margin of opisthosomal lobes, approximately at midlevel between h2 and h3. Setae h3 20–28 long, about 1/3 the length of terminal cleft. Distances between dorsal setae: c2–d2 140–145, d2–e2 128–135, e2–h2 80–93, h2–h3 60–68, d1–d2 73–88, e1–e2 53–60, h1–h2 40–48, h2–ps1 35–38, h1–h1 50–60, h2–h2 80–83.
Epimerites I fused as a Y with very short stem, fused part with short lateral extensions. Coxal fields I, II without sclerotized areas, epimerites IVa absent (Figure 3B). Translobar apodemes of opisthosomal lobes present, fused to each other anterior to terminal cleft. Epigynum horseshoe-shaped, greatest width 78–88. Copulatory opening situated ventrally at anterior margin of fused translobar apodemes. Distal half of primary spermaduct gradually thickened to copulatory opening, secondary spermaducts short (Figure 4E). Distance between pseudanal setae: ps2–ps2 63–75, ps3–ps3 23–33, ps2–ps3 30–40.


Legs I slightly longer and thicker than legs II. Femur II with ventral crest, genu and tibia I, II with dorsal crests, genu, tibia and tarsus III, IV with dorsal crests. Setae mG of genu I and II spiculiform. Setae d of tarsi II, III much shorter than corresponding setae f. Length of solenidia: ω1I 15–18, ω1II 13–18, φI 70–83, φII 63–73, φIII 18–25, φIV 8–10 (Fig. 4A–D).
Etymology
The specific name is derived from papilio (L., butterfly) and refers to the shape of sclerotized plate connecting epimerites IIIa in males.
Remarks
Proterothrix papilio n. sp. belongs to the paradoxornis species group in having, in males, seta e of tarsus I lanceolate and the aedeagus long whip-shaped. Among the species of this group which has included to date 10 species, P. papilio n. sp. appears the closest to P. sarahbushae Mironov and Proctor, 2009 from Paradoxornis verreauxi (Sharpe, 1883) (Passeriformes: Paradoxornithidae). In males of both species, the structure of the propodosomal epimerites I, II are very similar (epimerites I are fused into a Y with long sternum, with its posterior end connected with middle parts of epimerites II by transverse sclerotized extensions and epimerites II are long), and the lateral hysteronotal sclerites are present. Proterothrix papilio n. sp. is easily differentiated from P. sarahbushae in the following features of males: the pregenital sclerite is represented by a pair of longitudinal sclerites and they are not connected with epimerites IVa, coxal fields II are almost closed, the genital papillae are situated anterior to the level of the genital arch apex, the aedeagus is 134-144 long, and genual setae mGII are spiculiform. In males of P. sarahbushae, there is one stick-like pregenital sclerite connected posteriorly with epimerites IVa, coxal fields II are closed, the genital papillae are situated posterior to the level of the genital arch apex, the aedeagus is 188-205 long, and setae mGII are lanceolate. In females of both species, the anterior and lobar parts of the hysteronotal shield are connected ventro-laterally by narrow bands. In females of P. papilio n. sp., setae h2 have a terminal filament, setae h1 are situated at the level of supranal concavity, and genual setae mGI, mGII are thick spiculiform. In females of P. sarahbushae, setae h2 lack the terminal filament, setae h1 are distinctly anterior to the level of supranal concavity, and setae mGI, mGII are lanceolate or shaped as thick spines.
Proterothrix nanduhensis Constantinescu n. sp.
ZOOBANK: 06DEB5DC-3DDB-45BF-9091-BB5EDCADE9B9 ![]()
(Figures 5–8, 11,12)
Type material
From Trochalopteron milnei David, 1874 (Passeriformes: Leiothrichidae): male holotype (ANA2176), 2 male (ANA2177, ANA2178) and 4 female (ANA2179–2182) paratypes, in MGAB; 1 male (GD-ACARI-63) and 2 female (GD-ACARI-64, GD-ACARI-65) paratypes, in IZGAS, China, Yunnan Province, Dehong Prefecture, Tongbiguan, Nanduhe, 24°38′25.0″N, 97°38′45.0″E, 11 December 2023, coll. D.R. Gustafsson, Z. Wang, A.A. Grossi & W. Zhou, bird ID: J4913.
Description


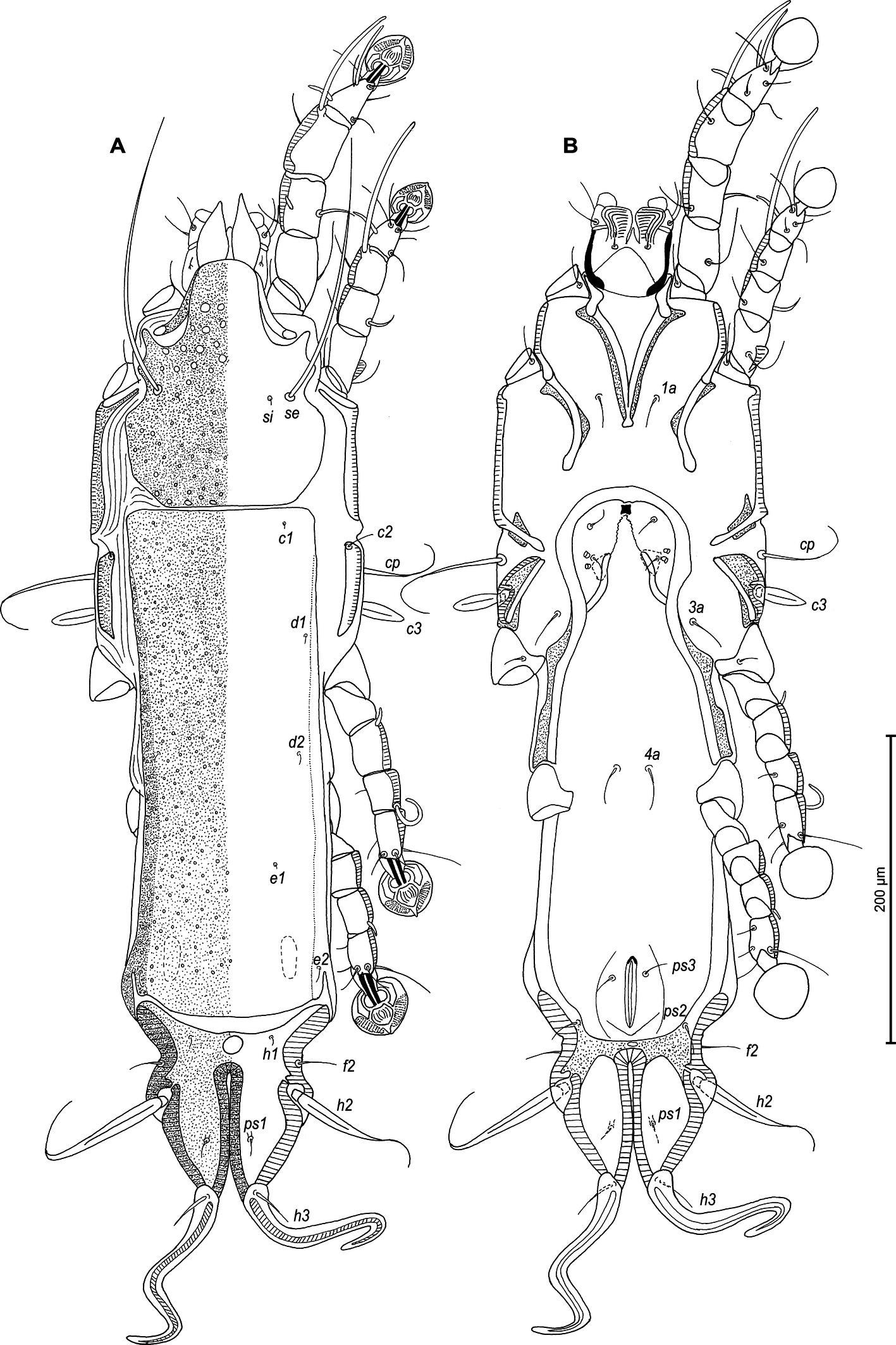
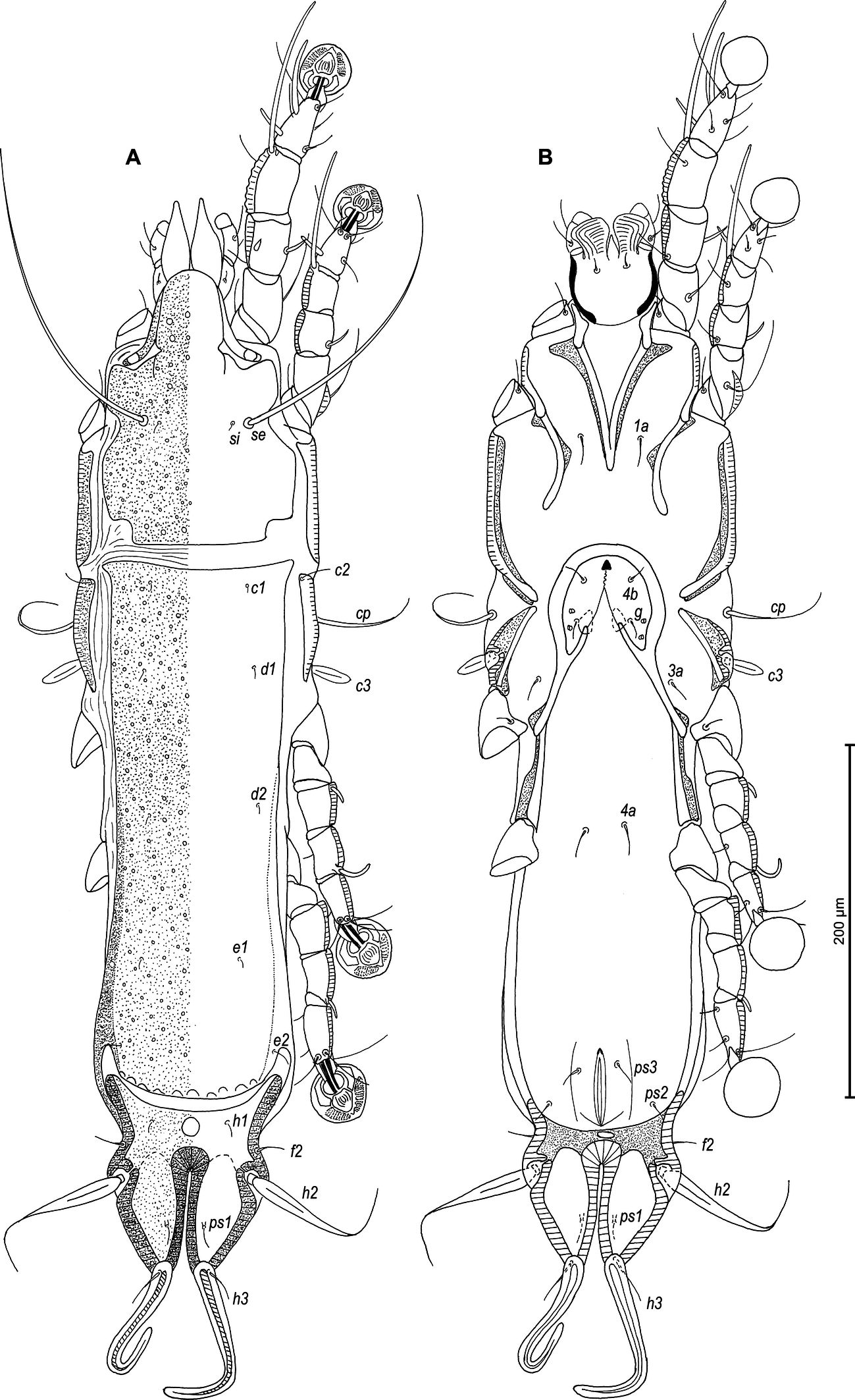
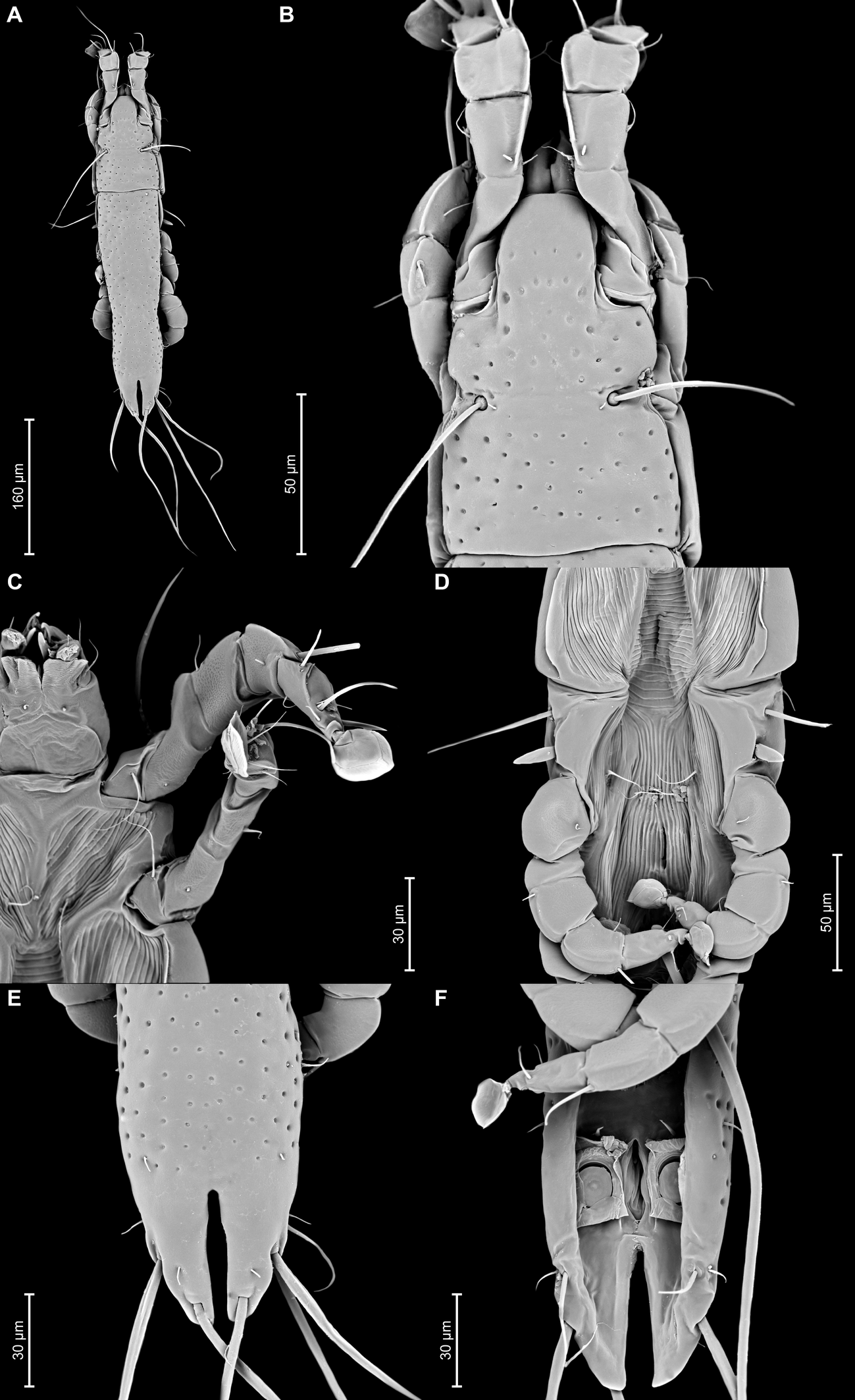
Male — (holotype, range for 3 paratypes in parentheses) (Figures 5A, B, 6A–E, 11A–F): Length of idiosoma 440 (420–430), width 130 (125–128), length of hysterosoma 310 (300–305). Pseudorutellar lobes without acute lateral extensions. Prodorsal shield entire, anterolateral extensions rounded, lateral margins concave, without incisions around scapular setae, posterior margin concave, posterior corners angular, length 125 (125–138), width 108 (98–105), surface with small circular lacunae (Figure 5A). Scapular setae se separated by 48 (48–53). Scapular shields narrow. Humeral shields narrow, not fused ventrally with epimerites III. Setae cp situated ventrally on soft tegument. Setae c2 filiform, situated dorsally on anterior ends of humeral shields. Subhumeral setae c3 lanceolate, 27 (25–27) × 8 (7–8). Hysteronotal shield with anterior margin slightly concave, anterior angles rounded, length from anterior margin to bases of setae h3 285 (280–290), greatest width in anterior part 88 (80–85), surface with small circular lacunae. Lateral hysteronotal sclerites absent. Opisthosomal lobes elongated, attenuate to apex, posterior margin of apices rounded, setae h3 situated near lobar apices. Terminal cleft small U-shaped, 43 (43–45) in length, margins of terminal cleft without membrane. Supranal concavity triangular, clearly outlined. Setae f2 and ps2 almost at the same level. Setae h1 at midlevel of supranal concavity, distant from lateral margins of opisthosoma. Setae ps1 minute filiform, about 7 (7–8) in length, situated slightly posterior to level of setae h2, approximately equidistant from outer and inner lobar margins. Setae ps2 setiform, slightly thickened basally, length 40 (45–50). Setae h3 represented by macrosetae, slightly longer than macrosetae h2, 250 (230–240) × 7 (7–8), 182 (183–190) × 7 (7–8), respectively. Distances between dorsal setae: c2–d2 100 (93–108), d2–e2 103 (95–103), e2–h3 88 (83–90), d1–d2 50 (48–50), e1–e2 45 (35–38), h1–ps2 23 (25–30), h2–h2 50 (45–53), h3–h3 25 (20–23), ps2–ps2 65 (60–65).
Epimerites I fused into a Y with long stem, fused part connected with epimerites II by transverse bands. Epimerites II long, with posterior ends free. Rudimentary sclerites rEpIIa absent. Epimerites IIIa moderately elongated, extending to level of inner tips of epimerites III. Coxal field I closed, coxal fields II, III open. Epimerites IVa present, long, flanking genital apparatus laterally, with setae 4a on anterior ends (Figure 5B). Genital arch 25 (22–27) long, 33 (33–38) wide. Aedeagus long whip-shaped, extending beyond lobar apices by almost half its length, length of aedeagus from anterior bend to tip 355 (345–355). Genital papillae situated anterior to level of genital arch, on each side on small ovate sclerite. Paragenital sclerites at tips of genital arch minute, roughly ovate. Genital setae g on small circular genital sclerites, situated approximately equidistant from genital arch and adanal suckers. Opisthoventral shields absent. Adanal shields represented by one pair of sclerites shaped as triangular plates, with setae ps3 on outer part. Adanal suckers 22 (20–22) in diameter; corolla multidentate, with 10 small denticles. Setae ps2 thickened in basal part. Distances between ventral setae: 3a–4b 25(25–28), 4b–4a 55 (48–50), 4a–g 62 (60–63), g–ps3 37 (33–38), ps3–ps3 28 (25–28), ps3–h3 73 (70–80).
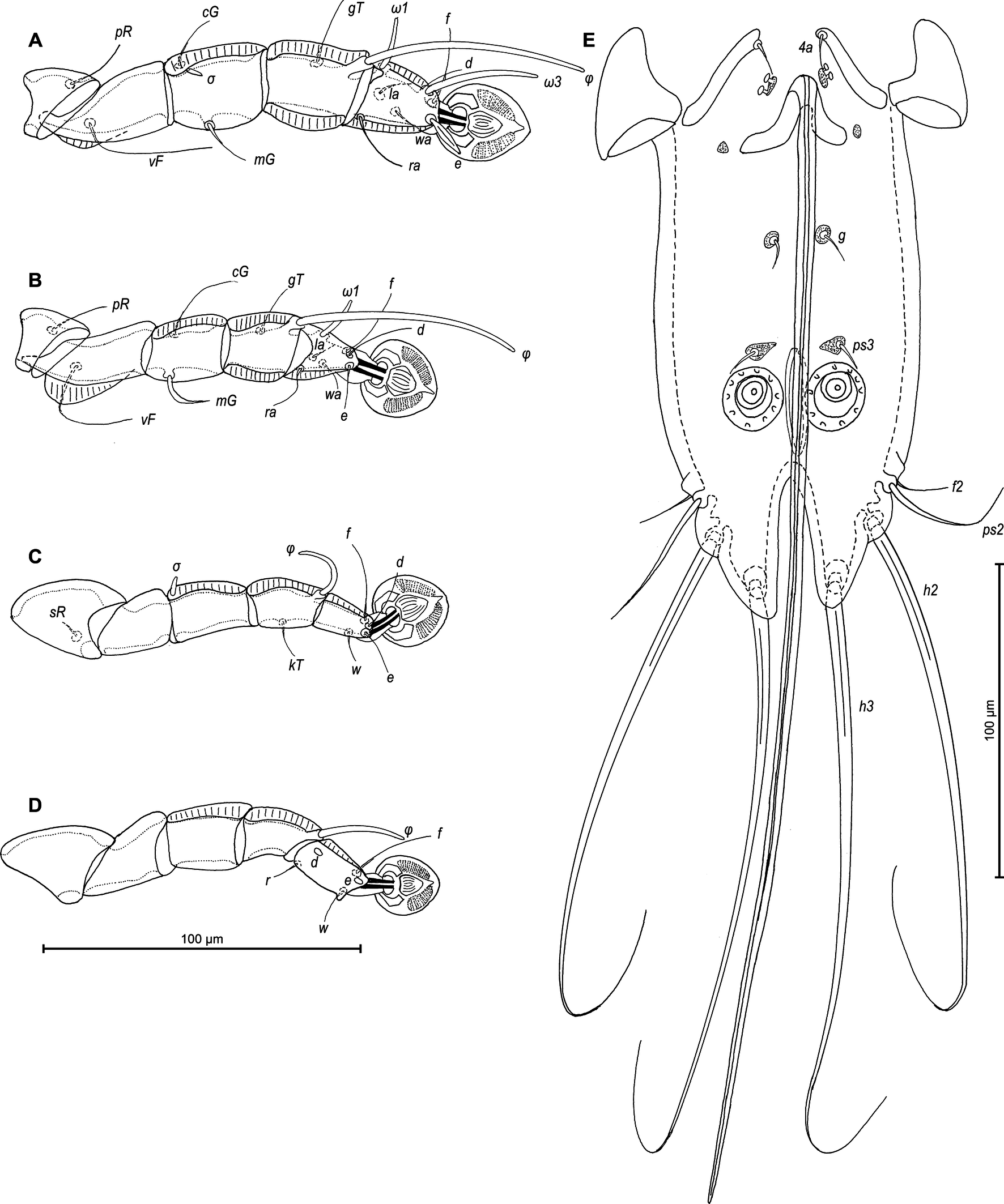


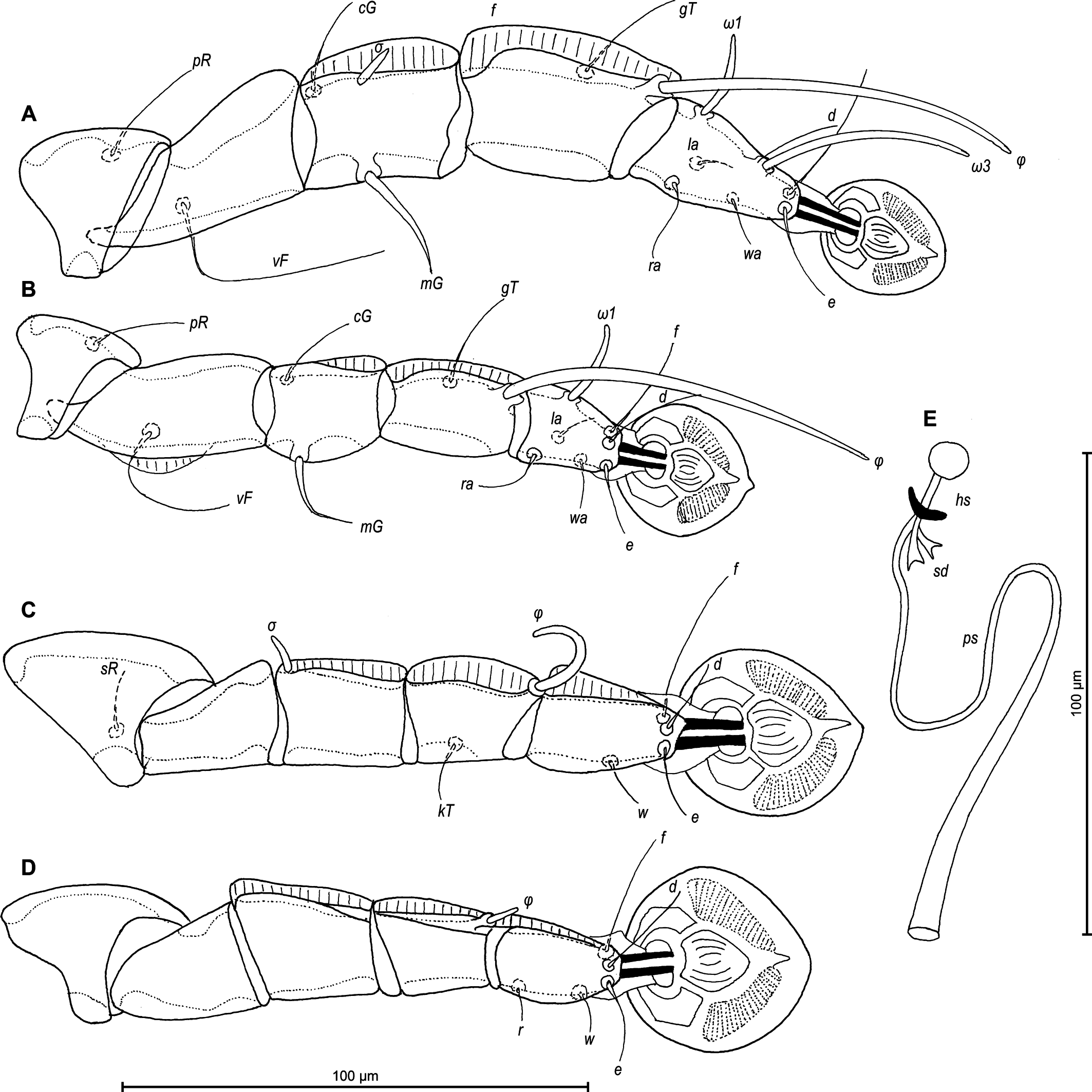
Legs I slightly longer and thicker than legs II. Tarsus I with small thumb-like dorsal process anterior to base of solenidion ω1. All legs with processes (longitudinal sclerotized crests): femora I and II with ventral crests, tibia and tarsus I, tibia II with dorsal and lateral crests; genu I–IV, tibia and tarsus III and IV, each with dorsal crests; tarsus II with lateral crest (Figure 6A–D). Seta e of tarsus I lanceolate, setae mGI setiform, setae mGII curved spiculiform. Setae d of tarsi II, III shorter than corresponding setae f. Tarsus IV 30 (25–30) long, with setae d, e button-like, situated in basal and apical parts of segment, respectively, with a small apicoventral extension bearing setae w (Figure 6D). Length of solenidia: ω1I 15 (10–12), ω1II 13 (12–13), φI 70 (70–90), φII 58 (53–58), φIII 25 (18–22), φIV 33 (28–37).


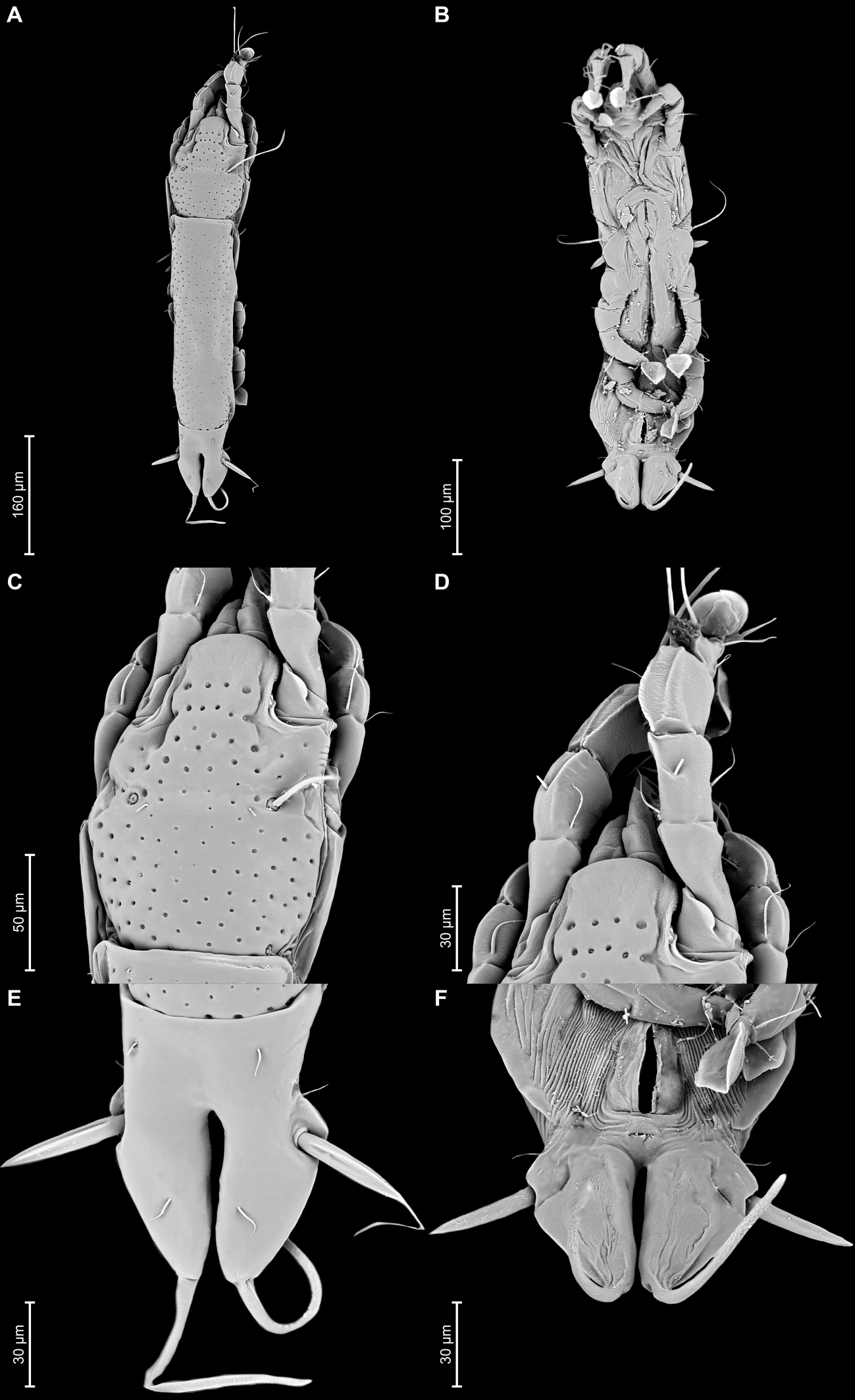
Female — (range for 5 paratypes) (Figures 7A, B, 8A–E, 12A–F): Length of idiosoma 560–580, width 140–150, and length of hysterosoma 400–415. Pseudorutellar lobes without acute lateral extensions. Prodorsal shield entire, strongly attenuate posteriorly, anterolateral extensions with rounded tips, lateral margins with small round incisions in anterior quarter (at level of trochanters I) and shallowly concave at level of scapular setae, posterior margin straight, posterolateral parts with rectangular incisions, length 155–163, width 113–123, surface with small circular lacunae (Figure 7A, 12C). Scapular setae se separated by 65–70. Scapular shields narrow. Humeral shields well developed, separated from outer sclerotization of epimerites III. Setae cp situated ventrally, on soft tegument. Setae c2 filiform, situated dorsally on anterior ends of humeral shields. Subhumeral setae c3 lanceolate, 27–28 × 8–10. Anterior and lobar parts of hysteronotal shield separated dorsally by narrow transverse band but remain connected ventrolaterally. Anterior hysteronotal shield: roughly rectangular, anterior margin slightly concave, anterior angles acute, extended laterally, posterior margin strongly convex, with small angular incisions near posterior angles, greatest length 300–325, greatest width in anterior part 108–120, surface with small circular lacunae. Length of lobar region 110–113, width at level of setae h2 90–105. Terminal cleft narrow slit-like, with almost touching lateral margins, slightly divergent posteriorly, 58–63 long. Supranal concavity well developed, circular. Setae h1 on lobar shield at level of supranal concavity; surface of lobar shield without ornamentation. Setae h2 spindle-shaped, with terminal filament, 67–88 × 10–13. Setae ps1 on inner margin of opisthosomal lobes, setae h3 20–30 long, about 1/3 the length of terminal cleft. Dorsal measurements: c2–d2 110–128, d2–e2 130–138, e2–h2 65–75, h2–h3 58–68, d1–d2 63–75, e1–e2 53–63, h1–h2 25–33, h2–ps1 35–45, h1–h1 45–50, h2–h2 70–83.


Epimerites I fused as a short-stemmed Y, sternum short, without lateral extensions. Coxal fields I, II without large sclerotized areas, epimerites IVa absent (Figure 7B). Translobar apodemes of opisthosomal lobes present, fused to each other anterior to terminal cleft. Epigynum horseshoe-shaped, greatest width 63–75. Copulatory opening situated ventrally at anterior margin of fused translobar apodemes. Distal half of primary spermaduct gradually thickened to copulatory opening, secondary spermaducts short (Figure 8E). Distance between pseudanal setae: ps2–ps2 65–75, ps3–ps3 23–33, ps2–ps3 33–38.
Legs I slightly longer and thicker than legs II, all legs with processes: femur II with wide ventral crest; genu, tibia of legs I, II and genu, tibia, tarsus of legs II, IV, each with dorsal longitudinal crest (tibia I with a particularly shaped crest, finely indented). Setae d of tarsi II–IV about 2/3 as corresponding setae f. Setae mGI and mGII spiculiform. Length of solenidia: ω1I 15–20, ω1II 13–18, φI 73–83, φII 58–63, φIII 18–25, φIV 8–10. (Figures 8A–D).








Etymology
The specific name nanduhensis refers to the type locality (Nanduhe).
Remarks
Proterothrix nanduhensis n. sp. belongs to the paradoxornis species group in having, in males, seta e of tarsus I lanceolate and the aedeagus long whip-shaped. Among the species of this complex, P. nanduhensis n. sp. appears the closest to P. longicaula Mironov and Proctor, 2009 from Paradoxornis gularis Gray, 1845 (Passeriformes: Paradoxornithidae). Males of both species have the aedeagus long and whip-shaped, extending beyond lobar apices by almost half its length, the opisthosomal lobes elongated, attenuate to apex, with posterior ends rounded and coxal fields II, III open. Males of P. nanduhensis n. sp. easily differ from P. longicaula in the following features: the prodorsal shield has rounded anterolateral extensions, coxal fields I are closed, the adanal shields are present, the opisthoventral shields are absent and setae h1 are situated at the midlevel of the supranal concavity. In males of P. longicaula, the prodorsal shield has acute anterolateral extensions, coxal fields I are almost closed, the adanal shields are absent, the opisthoventral shields are present and setae h1 are situated slightly posterior to supranal concavity. In females of both species, the anterior and lobar parts of hysteronotal shield are connected ventrolaterally, and tibia I has finely indented longitudinal dorsal crest. Females of P. nanduhensis n. sp. are easily distinguishable from P. longicaula by the following features: the prodorsal shield has the lateral margins with small rounded incisions in anterior quarter (at level of trochanters I), the hysteronotal shield has an acute anterior angle extended laterally and the setae h2 are with terminal filaments. In females of P. longicaula, the lateral margins of prodorsal shield in the anterior quarter are almost straight and lack incisions, the anterior angles of the hysteronotal shield are roughly rectangular and not extended laterally, and setae h2 are without terminal filaments.
Acknowledgments
The present study was carried out in strict accordance with the Regulation for the Administration of Laboratory Animals (Decree No. 2, State Science and Technology Commission of the People's Republic of China, 14 November 1988). We obtained approval for the study from the Guangdong Institute of Zoology's (formerly Guangdong Institute of Applied Biological Resources) Administrative panel on Laboratory Animal Care. This study was supported by grant GIABR-GJRC201701 from the Introduction of Full-Time High-Level Talent Fund of the Institute of Zoology, Guangdong Academy of Sciences, grant 2019QN01N968 from the Pearl River Talent Recruitment Program of Guangdong Province, grant QN20200130012 from the Foreign Young Talent Plan, and grant 31961123003 from the National Natural Science Foundation of China. Gabriel Bogdan Chișamera was funded by project no. RO1567-IBB09/2025 from the Institute of Biology Bucharest of Romanian Academy.
References
- Arlott N. 2017. Birds of South-East Asia. London, William Collins. 448 pp.
- Clements J.F., Rasmussen P.C., Schulenberg T.S., Iliff M.J., Fredericks T.A., Gerbracht J.A., Lepage D., Spencer A., Billerman S.M., Sullivan B.L., Smith M., Wood C.L. 2024. The eBird/Clements checklist of Birds of the World: v2024. Downloaded from https://www.birds.cornell.edu/clementschecklist/download/. Accessed: September 2025.
- Constantinescu I.C., Chişamera G., Mukhim D.K.B., Adam C. 2014. Three new species of feather mite of the genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae: Pterodectinae) from passerines in Meghalaya, North East India. Syst. Parasitol., 89: 45-58. https://doi.org/10.1007/s11230-014-9508-1
- Constantinescu I.C., Cobzaru I., Geamana N.A., Mukhim D.K.B., Adam C. 2017a. Two new species of feather mites (Acarina: Psoroptidia) from the blue-throated blue flycatcher, Cyornis rubeculoides (Passeriformes: Muscicapidae). J. Nat. Hist., 51: 277-297. https://doi.org/10.1080/00222933.2017.1280194
- Constantinescu I.C., Popa O.P., Popa L.O., Cobzaru I., Mukhim D.K.B., Adam C. 2017b. A new feather mite species of the genus Proterothrix Gaud, 1968 (Acarina, Proctophyllodidae) from the Large Niltava, Niltava grandis (Passeriformes, Muscicapidae) - an integrative description. ZooKeys, 661: 1-14. https://doi.org/10.3897/zookeys.661.11793
- Constantinescu I.C., Chişamera G.B., Adam C. 2018. Redescription of six feather mite species of the genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae: Pterodectinae) from the ''Édouard Louis Trouessart'' Collection. Zootaxa, 4486: 451-479. https://doi.org/10.11646/zootaxa.4486.4.3
- Constantinescu I.C., Chişamera G.B., Petrescu A., Adam C. 2019. Two new species of feather mites of the subfamily Pterodectinae (Analgoidea: Proctophyllodidae) from Indonesia. Acarologia, 59 (2): 196-210. https://doi.org/10.24349/acarologia/20194324
- Constantinescu I.C., Chişamera G.B., Motoc R., Gustafsson D.R., Zou F., Chu X., Adam C. 2021. Two new species of feather mites (Acarina: Psoroptidia) from the Huet's fulvetta, Alcippe hueti (Passeriformes: Leiothrichidae), in China. Syst. Appl. Acarol., 26(1): 146-165. https://doi.org/10.11158/saa.26.1.9
- Constantinescu I.C., Adam C., Chişamera G., Gavril V., Motoc R., Mukhim D.K.B., Cobzaru I. 2023. Two new species of feather mites (Acari: Psoroptidia) from the Rusty-capped Fulvetta, Schoeniparus dubius (Passeriformes: Pellorneidae) in India. Syst. Appl. Acarol., 28: 269-288. https://doi.org/10.11158/saa.28.2.10
- Constantinescu I.C., Chişamera G.B., Motoc R., Adam C. 2024a. Three new feather mite species of the genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae: Pterodectinae) from birds of paradise (Passeriformes: Paradisaeidae). Acarologia, 64(2): 661-682. https://doi.org/10.24349/bx89-qaob
- Constantinescu I.C., Adam C., Chişamera G.B., Gavril V., Motoc R., Mukhim D.K.B., Dobre D.S., Cobzaru I. 2024b. Two new species of feather mites (Acarina: Psoroptidia) from Old World flycatchers (Passeriformes: Muscicapidae) in India. Acarologia, 64(4): 1213-1231. https://doi.org/10.24349/eb9q-pjy9
- Gaud J. 1952. Sarcoptides plumicoles des oiseaux de Madagascar. Mém. LʼInstit. Scient. Madagascar, 7: 81-107.
- Gaud J. 1962. Sarcoptiformes plumicoles (Analgesoidea) parasites dʼoiseaux de IʼIle Rennell. The Nat. Hist. Rennell Isl., Brit. Solomon Is., 4: 31-51.
- Gaud J. 1968. Sarcoptiformes plumicoles (Analgoidea) parasites dʼoiseaux de IʼIle Rennell. The Nat. Hist. Rennell Isl., Brit. Solomon Is., 5: 121-151.
- Gaud J. 1979 Sarcoptiformes plumicoles des oiseaux Coraciiformes dʼAfrique. II. Parasites des Alcedinidae. Rev. Zool. Afr., 93: 245-266.
- Gaud J., Atyeo W.T. 1996. Feather mites of the world (Acarina, Astigmata): the supraspecific taxa. Ann. Mus. roy. Afr. centr., 277, 1-191 (Part I, text), 1-436 (Part II, illustrations).
- Grandjean F. 1939. La chaetotaxie des pattes chez les Acaridae. Bull. Soc. Zool. France, 64: 50-60.
- Griffith D.A., Atyeo W.T., Norton R.A., Lynch C.A. 1990. The idiosomal chaetotaxy of astigmatid mites. J. Zool., 220: 1-32. https://doi.org/10.1111/j.1469-7998.1990.tb04291.x
- Han Y.D., Min G.S. 2019. Three feather mites (Acari: Sarcoptiformes: Astigmata) isolated from Tringa glareola in South Korea. J. Species Res., 8(2): 215-224.
- Han Y.D., Mironov S.V., Min G.S. 2019. Two new feather mites (Acari: Analgoidea) isolated from the grey-headed woodpecker, Picus canus (Piciformes: Picidae) in Korea. Syst. Appl. Acarol., 24(11): 2167-2183. https://doi.org/10.11158/saa.24.11.9
- He S.X., Sun L.H., Liu H., Yuan Y.C., Wang Z.Y. 2024. Three new feather mites of the genus Proterothrix Gaud (Astigmata: Proctophyllodidae) from passerines (Aves: Passeriformes) in China. Acarologia 64(3): 843-864. https://doi.org/10.24349/y1xh-2eq2
- Hernandes F.A., Valim M.P. 2014. On the identity of two species of Proctophyllodidae (Acari: Astigmata: Analgoidea) described by Herbert F. Berla in Brazil, with a description of Lamellodectes gen. nov. and a new species. Zootaxa, 3794: 179-200. https://doi.org/10.11646/zootaxa.3794.1.8
- doi:10.11646/zootaxa.3794.1.8 https://doi.org/10.11646/zootaxa.3794.1.8
- Mironov S.V. 2006. Feather mites of the genus Montesauria Oudemans (Astigmata: Proctophyllodidae) associated with starlings (Passeriformes: Sturnidae) in the Indo- Malayan region, with notes on systematic of the genus. Acarina, 14: 21-40.
- Mironov S.V. 2009. Phylogeny of feather mites of the subfamily Pterodectinae (Astigmata: Proctophyllodidae) and their host associations with passerines (Aves: Passeriformes). Proc. Zool. Inst. Russ. Acad. Sci., 313 (2): 97-118. https://doi.org/10.31610/trudyzin/2009.313.2.97
- Mironov S.V., Fain A. 2003. New species of feather mite subfamily Pterodectinae (Astigmata: Proctophyllodidae) from African passerines (Aves: Passeriformes). Bull. Annls Soc. Roy. Belge Ent., 139: 75-91.
- Mironov S.V., Galloway T.D. 2021. Feather mites of the subfamily Pterodectinae (Acariformes: Proctophyllodidae) from passerines and kingfishers in Canada. Zootaxa, 5016 (1): 1-55. https://doi.org/10.11646/zootaxa.5016.1.1
- Mironov S.V., OConnor B.M. 2017. A new feather mite of the genus Neodectes Park and Atyeo 1971 (Acari: Proctophyllodidae) from New Zealand wrens (Passeriformes: Acanthisittidae). Acta Parasitol., 62: 171-177. https://doi.org/10.1515/ap-2017-0020
- Mironov S.V., Proctor H.C. 2009. Feather mites of the genus Proterothrix Gaud (Astigmata: Proctophyllodidae) from parrotbills (Passeriformes: Paradoxornithidae) in China. J. Parasitol., 95: 1093-1107. https://doi.org/10.1645/GE-1961.1
- Mironov S.V., Tolstenkov O.O. 2013. Three new feather mites of the subfamily Pterodectinae (Acari: Proctophyllodidae) from passerines (Aves: Passeriformes) in Vietnam. Proc. Zool. Inst. Russ. Acad. Sci., 317: 11-29. https://doi.org/10.31610/trudyzin/2013.317.1.11
- Mironov S.V., Diao W., Zhang Y., Zhang C., Yan, Z. 2008. A new feather mite species of the genus Proterothrix Gaud (Astigmata, Proctophyllodidae) from Ficedula zanthopygia (Hay) (Passeriformes: Muscicapidae) in China. Acarina, 16: 31-38.
- Mironov S.V., Literak I., Čapek M., Koubek P. 2010. New species of the feather mite subfamily Pterodectinae (Astigmata, Proctophyllodidae) from passerines in Senegal. Acta Parasitol., 55: 399-413. https://doi.org/10.2478/s11686-010-0051-1
- Mironov S.V., Literak I., Hung M.N., Čapek M. 2012. New feather mites of the subfamily Pterodectinae (Acari: Proctophyllodidae) from passerines and woodpeckers (Aves: Passeriformes and Piciformes) in Vietnam. Zootaxa, 3440: 1-49. https://doi.org/10.11646/zootaxa.3440.1.1
- Norton A.R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarol., 22: 559-594. https://doi.org/10.1023/A:1006135509248
- Valim M.P., Hernandes F.A. 2006. Redescription of four species of the feather mite genus Pterodectes Robin, 1877 (Acari: Proctophyllodidae: Pterodectinae) described by Herbert F. Berla. Acarina, 14: 41-55.



2025-09-25
Date accepted:
2025-12-02
Date published:
2025-12-08
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Constantinescu, Ioana Cristina; Chișamera, Gabriel Bogdan; Motoc, Rozalia; Gustafsson, Daniel R.; Zou, Fasheng; Wang, Zhengzhen; Grossi, Alexandra A.; Zhou, Wenyi and Adam, Costică
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



