Bridging the knowledge gap: advances in the distribution of Tenuipalpidae mites in Central and Eastern Europe
Zhovnerchuk, Olha V.  1
; Ochoa, Ronald
1
; Ochoa, Ronald  2
; Glik, Tobias E.
2
; Glik, Tobias E.  3
; Tassi, Aline D.
3
; Tassi, Aline D.  4
and De Giosa, Marcello
4
and De Giosa, Marcello  5
5
1Institute of Zoology, Slovak Academy of Sciences (SAS), Dubravska cesta 9, Bratislava, 84506, Slovakia & I.I. Schmalhausen Institute of Zoology, National Academy of Sciences (NAS) of Ukraine, vul. B. Khmelnytskogo 15, Kyiv, 01054 Ukraine.
2Systematic Entomology Laboratory, ARS-USDA, BARC-West, Beltsville, MD, 20705 USA.
3USDA Animal and Plant Health Inspection Service, Plant Protection and Quarantine, Romulus, MI, 48174, USA.
4University of Florida, Department of Entomology and Nematology, Tropical Research and Education Center, Homestead, FL, 33031, USA.
5✉ University of Florida, Department of Entomology and Nematology, Tropical Research and Education Center, Homestead, FL, 33031, USA.
2025 - Volume: 65 Issue: 4 pages: 1199-1212
https://doi.org/10.24349/yunh-z34kOriginal research
Keywords
Abstract
Introduction
The Tenuipalpidae family Berlese comprises over 1,100 described species of plant-feeding mites with a global distribution (Castro et al. 2025). These mites are of increasing concern in agriculture and ornamental horticulture due to their ability to damage plant tissues and to vector plant viruses (de Lillo et al. 2021; Ramos-Gonzalez et al. 2023). Understanding their diversity, distribution, and host range is fundamental for plant protection and biosecurity. In this context, faunistic surveys play a critical role in uncovering mite biodiversity, detecting invasive species, and guiding pest risk assessments.
Comprehensive faunistic surveys based on systematic sampling, accurate identifications, and host plant documentation are essential for establishing baseline data in regions where the acarofauna remains poorly known. Surveys address major knowledge gaps in mite distribution, which is critical for effective pest surveillance and biosecurity, especially in regions where agricultural trade is expanding and baseline faunistic data are limited, such as Central and Eastern Europe. Although some records originate from taxonomic revisions or port-of-entry interceptions rather than targeted field surveys, they highlight the necessity of reliable identifications for quarantine decision-making and regulatory enforcement.
Tenuipalpid systematics remain complex, with many species exhibiting cryptic diversity and unresolved species complexes, requiring expert morphological and molecular approaches (Navia et al. 2013; Beard et al. 2015). New faunistic records are important in this context, as they help clarify species distributions, inform ecological studies, and support regulatory frameworks for pest management (Hoy 2011).
The online databases on the Tenuipalpidae family by Castro et al. (2025), compile faunistic data from the literature, and bring evidences of geographic gaps in knowledge, particularly across Central and Eastern Europe. Several countries, including Slovakia and the Czech Republic, have no documented records of Tenuipalpidae, while others, including Bulgaria (4 records from 1876–1949), Hungary (22 records from 1867–2018), Ukraine (32 records from 1876–1983), and the Netherlands (9 records from 1867–1972), lack recent survey efforts and remain underrepresented in regional syntheses (Kontschán and Ripka 2017; Zhovnerchuk et al. 2021).
This study presents new faunistic data from Slovakia and Ukraine, derived from systematic field sampling of diverse host plants. It also incorporates previously unpublished interception records from the United States to document tenuipalpid species associated with plant imports from Bulgaria, Hungary, and the Netherlands. By combining targeted fieldwork with archival and interception data, this study contributes to a broader understanding of the tenuipalpid fauna in Central and Eastern Europe. It also highlights the importance of faunistic surveys as a foundational tool for acarological research and highlights the critical role of agricultural trade interceptions in biosecurity decision-making.
Materials and methods
Samplings in Slovakia and Ukraine
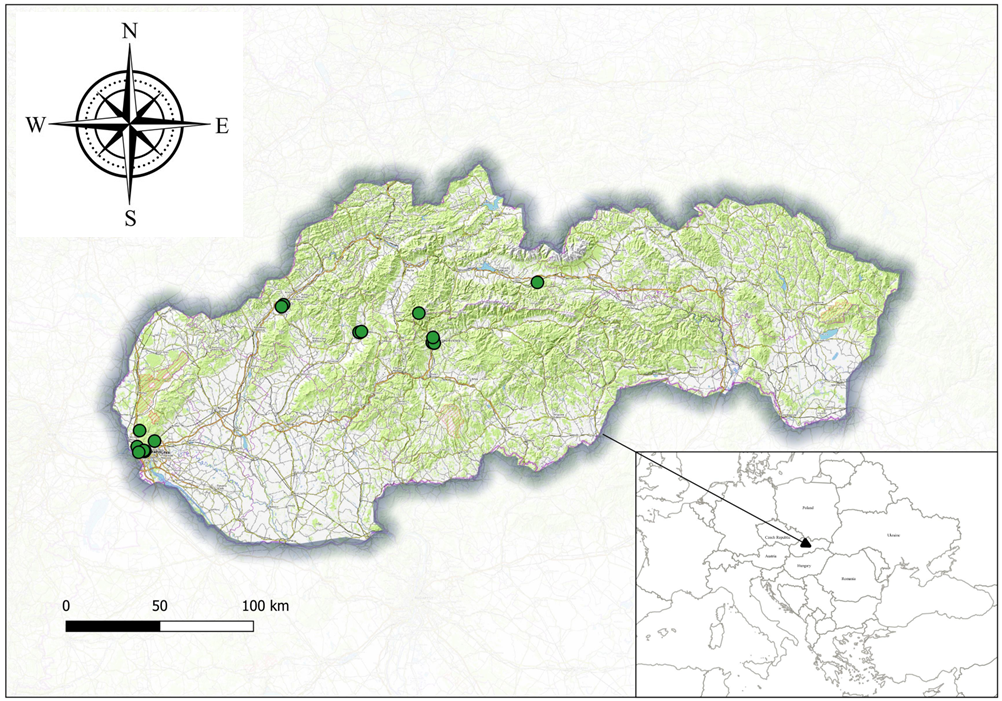


Samplings were conducted across Slovakia and Ukraine's urban and natural ecosystems by the first author (Table 1; Figure 1). In Slovakia, mite samples were collected between 2023 and 2024, primarily from April to October, with additional sampling in February 2024. Surveys were carried out across 10 different cities and two national parks, examining a total of 85 samples from over 30 species of host plants. In Ukraine, sampling took place from July to October 2024 in two botanical gardens in Kyiv. A total of 26 samples were collected from 20 species of host plants, predominantly introduced conifers.
Download as
Country
Sampling locality
GPS coordinates
Slovakia
Bratislava
Park Andreja Hlinku
48°9’23.2’‘N 17°9’27.7’’E
48°9’22.6’‘N 17°9’20.2’’E
48°9’22.9’‘N 17°9’9.8’’E
Sad Janka Kráľa
48°8’5.2’‘N 17°6’41.1’’E
Magurska Street
48°09’59.32’‘N 17°5’37.87’’E
Ružinovska Street
48°9’22.6’‘N 17°9’5.8’’E
Sibirska Street
48°9’59.3’‘N 17°7’26.6’’E
Tbilisska Street
48°12’54.2’‘N 17°9’36.6’’E
Karlova Ves
48°9’27.4’‘N 17°3’2.9’’E
Rusovce park
48°3’13.5’‘N 17°9’7.2’’E
48°44’5.9’‘N 19°9’7.6’’E
Banská Bystrica
City Park* *
48°44’13.7’‘N 19°8’17.5’’E
Ovocna Street* *
48°45’43.4’‘N 19°8’31.5’’E
Na Karlove Street
48°45’20.3’‘N 19°8’40.4’’E
Gabčikovo
Na
47°53’38.1’‘N 17°35’27.4’’E
Komarno
Na
47°45’34.8’‘N 18°7’35.6’’E
Tesáre - Nové
Na
48°37’10.7’‘N 18°4’16.3’’E
48°37’6.2’‘N 18°4’18.1’’E
48°37’8.2’‘N 18°4’17.5’’E
48°37’9.6’‘N 18°4’14.0’’E
Trenčin
Park Milana Rastislava Štefánika
48°53’46.8’‘N 18°2’51.6’’E
Na
Legionarska Street* *
48°53’11.9’‘N 18°2’2.7’’E
Prievidza
Bojnicka Street
48°46’31.1’‘N 18°36’32.6’’E
48°46’35.7’‘N 18°36’14.6’’E
48°46’37.1’‘N 18°36’14.4’’E
48°46’35.7’‘N 18°36’14.7’’E
48°46’50.3’‘N 18°37’16.8’’E* *
Patince
Najjužnejší bod Slovenska
47°44’5.2’‘N 18°17’20.8’’E
Nitra
Na
48°18’51.9’‘N 18°5’40.9’’E
48°20’44.1’‘N 18°5’26.2’’E
48°19’5.8’‘N 18°5’15.7’’E
Štúrovo
Na
47°47’52.9’‘N 18°43’30.6’’E
Veľka Fatra National Park
Kráľova studňa
48°52’37.2’‘N 19°2’4.4’’E
Low Tatras (Nízke Tatry) National Park
Vychodna (Liptovský Mikuláš)
49°2’10.7’‘N 19°53’41.4’’E
Ukraine
Kyiv
O.V. Fomin Botanical Garden
50°26’38.2’‘N 30°30’9.7’’E
National botanical garden
50°24’55.6’‘N 30°33’46.7’’E
Mite extraction, preservation, and identification
Tenuipalpid specimens were collected by shaking branches of the plant samples over a black PVC film and then transferred into an Eppendorf tube with 70% ethanol. The collected specimens were mounted on microscope slides using Hoyer's medium. The mounted slides were examined using Leica DMR microscopes with differential interference contrast (DIC). Mite identifications were conducted by M. De Giosa, A. D. Tassi and R. Ochoa at Systematic Entomology Laboratory, ARS-USDA, Beltsville, MD using available key literature and the original species descriptions (Donnadieu 1875; Canestrini and Fanzago 1876; Haller 1877; Ewing 1917; McGregor 1949; Reck 1951; Baker and Tuttle 1987; Meyer and Van Dis 1993; Khanjani and Gotoh 2008; Ueckermann and Ripka 2016; Negm et al. 2020). Plant species identification follows World Flora Online (WFO) Plant List classification (2025). Mounted slides are deposited at the Institute of Zoology, Bratislava, Slovakia (SAS) and Council for Agricultural Research and Economics - Research Centre for Plant Protection and Certification, via Lanciola 12a del Riccio, Firenze, Italy (CREA).
Detailed information on number of specimens identified, sampling locality, plant species, and month and year of the collection are provided under ''Specimens examined'' section. The ''Remarks'' section includes comments on new host plant associations and on the literature review.
Literature review for Slovakia
A comprehensive literature review was conducted to identify all known records of Tenuipalpidae from Slovakia. Sources included published peer-reviewed articles, local literature, database records (see Castro et al. 2025), conference and symposium abstracts, and books. This review was essential for establishing a baseline prior to fieldwork and for comparing new records to existing data in neighboring countries.
Interceptions from Bulgaria, Hungary and Netherlands
Interceptions of tenuipalpid mites were investigated by using reports from the United States Department of Agriculture, United States Animal and Plant Health Inspection Service, and Plant Protection and Quarantine (USDA-APHIS-PPQ), covering data from 1996 onward. Archived slides of intercepted specimens originating from Bulgaria, Hungary, and the Netherlands were reviewed and re-identified by M. De Giosa, A. D. Tassi, and R. Ochoa. The reports were tabulated to include the inspection location (port of entry), country of origin, inspected host or commodity, year of interception, final genus or species determination, and the name of the acarologist responsible for the identification.
Results
First reports from Slovakia
Genus Cenopalpus Pritchard et Baker
Cenopalpus cumanicus Ueckermann et Ripka
Specimens examined — 2♀♀, Botanicka Zahrada UK (Bratislava), 48°9′23.2″N 17°9′27.7″E, 152m, ex. Chamaecyparis sp. (Cupressaceae) October 2023 (1 slide, SAS).
Remarks — Cenopalpus cumanicus was described from Hungary on Populus × canescens (Aiton) Sm. and Populus alba L. (Salicaceae) (Ueckermann and Ripka 2016). In this study, Cupressaceae is reported as a new host plant family record, while Chamaecyparis is a new host plant genus record. Previously, this Cenopalpus species had only been reported from Hungary. No morphological differences were observed between the collected specimens and the original description.
Cenopalpus lineola (Canestrini et Fanzago)
Specimens examined — 11♀♀, 1♂, 4 deutonymphs, Park Andreja Hlinku (Bratislava), 48°9′23.2″N 17°9′27.7″E, 135 m, ex. Pinus sylvestris L. (Pinaceae), July 2023 (1 slide, CREA); 8♀♀, 1♂, 4 deutonymph, 1 protonymph, Park Milana Rastislava Štefánika (Trenčin), 48°53′46.8″N 18°2′51.6″E, 215 m, ex. P. sylvestris, August 2023 (2 slides, SAS); 1♀, 1♂, Bojnicka Street (Prievidza), 48°46′31.1″N 18°36′32.6″E, 263 m, ex. P. sylvestris, August 2023 (1 slide, CREA); 6♀♀, Nitra, 48°18′51.9″N 18°5′40.9″E, 141 m, ex. Thujia occidentalis L. (Cupressaceae), April 2024 (2 slides, SAS); 5♀♀, Nitra, 48°20′44.1″N 18°5′26.2″E, 293 m, ex. P. sylvestris, April 2024 (1 slide, CREA).
Remarks — Cenopalpus lineola was described from Italy on pine (Canestrini and Fanzago 1876). This mite species is widely distributed across Africa, Asia, and Europe, primarily on plant species of the family Pinaceae, followed by Compositae, Cupressaceae, Ebenaceae, Lamiaceae, Oleaceae, Primulaceae, Rosaceae, Salicaceae, and Taxaceae (Castro et al. 2025). Thujia occidentalis is a new host plant species record. No morphological differences were observed between the collected specimens and the original description.
Cenopalpus pulcher (Canestrini et Fanzago)
Specimens examined —. 1♀, Bojnicka Street (Prievidza), 48°46′35.7″N 18°36′14.6″E, 263 m, ex. Piceae sp. (Pinaceae), August 2023 (1 slide, SAS); 20♀♀, 1 deutonymph, Vychodna (Liptovský Mikuláš), 49°2′10.7″N 19°53′41.4″E, 781 m, ex. Salix sp. (Salicaceae), September 2023 (2 slides, CREA and SAS); 4♀, Sad Janka Kráľa (Bratislava), 48°8′5.2″N 17°6′41.1″E, 137 m, ex. Piceae sp., September 2023 (1 slide, SAS); 3♀♀, 1 deutonymph, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Chamaecyparis lawsoniana (A.Murray bis) Parl., October 2023 (1 slide, SAS); 4♀♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Crataegus sp. (Rosaceae), October 2023 (1 slide, SAS); 18♀♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Chamaecyparis pisifera (Siebold and Zucc.) Endl., October 2023 (3 slides, 1 in CREA and 2 SAS); 1♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Viburnum rhytidophyllum Hemsl. (Adoxaceae), October 2023(1 slide, SAS); 3 ♀♀, 1 ♂, Gabčikovo, 47°53′38.1″N 17°35′27.4″E, 120 m, ex. Populus sp. (Salicaceae), August 2024(1 slide, CREA); 1♀, Najjužnejší bod Slovenska (Patince), 47°44′5.2″N 18°17′20.8″E, 120 m, ex. Crataegus sp., August 2024 (1 slide, SAS); 4♀♀, Najjužnejší bod Slovenska (Patince), 47°44′5.2″N 18°17′20.8″E, 120 m, Poaeceae, August 2024(2 slides, SAS).
Remarks — Cenopalpus pulcher was described from Italy on hedge plant (Canestrini and Fanzago 1876). Among Cenopalpus species, it is the most widely distributed worldwide, with records from Argentina and Oregon (Bajwa et al. 2001; De Giosa et al. 2024). Cenopalpus pulcher seems to be a polyphagous species (Jeppson et al. 1975), feeding on more than 19 plant families (Castro et al. 2025). In this study, Adoxaceae and Poaceae are new host plant family records, while C. pisifera and V. rhytidophyllum are newly recorded host species. Given that this species is primarily linked to Rosaceae hosts (Castro et al. 2025), and no nymphs were found on conifers, we hypothesize that these collections might represent dispersion specimens rather than true hosts. This warrants further investigation, particularly through molecular analyses, to confirm whether conifers serve as feeding hosts, simply temporary shelters for dispersing mites, or if a complex of species is present. Although no morphological differences were observed between the collected specimens and the original description, we encourage the redescription of C. pulcher types. The original description is missing morphometric and ontogenetic analyses, as well as molecular information.
Genus Pentamerismus McGregor
Pentamerismus arbutusae Baker et Tuttle
Specimens examined — 7♀♀, Botanicka Zahrada UK (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Juniperus sp. (Cupressaceae), February 2024 (2 slides, CREA); 2♀♀, Botanicka Zahrada UK (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. C. lawsoniana June 2024 (1 slide, SAS); 3♀♀, Sad Janka Kráľa (Bratislava), 48°8′5.3″N 17°6′41.1″E, 137 m, ex. Pinus sp., February 2024 (1 slide, SAS); 2♀♀, 1 protonymph, Gabčikovo, 47°53′38.1″N 17°35′27.4″E, 120m, ex. Platycladus orientalis (L.) Franco (Cupressaceae), August 2024 (1 slide, SAS); 1♀, Najjužnejší bod Slovenska (Patince), 47°44′5.2″N 18°17′20.8″E, 104 m, ex. Chamaecyparis sp., August 2024 (1 slide, SAS); 4♀♀,1 deutonymph, Komarno, 47°45′34.8″N 18°7′35.6″E, 115 m, ex. C. lawoniana (A.Murray bis) Parl., August 2024 (1 slide, CREA).
Remarks — Pentamerismus arbutusae has previously been documented only in Mexico on Arbutus sp. (Ericaceae) (Baker and Tuttle 1987). This study represents the first European record of the species. Cupressaceae is a new host plant family record, while Juniperus sp., C. lawsoniana, P. orientalis, and Pinus sp. are new host plant species record. No morphological differences were observed between the adult specimens and the original description.
Pentamerismus canadensis McGregor
Specimens examined — 1♀, Magurska Street (Bratislava), 48°09′59.32″N 17°5′37.87″E, ex. Pinus nigra J.F.Arnold (Pinaceae), April 2023 (1 slide, SAS); 22♀♀, Ružinovska Street (Bratislava), 48°9′22.6″N 17°9′5.8″E, 135 m, ex. Juniperus sp., July 2023 (2 slides, SAS); 4♀♀, Park Andreja Hlinku (Bratislava), 48°9′22.6″N 17°9′20.2″E, 135 m, ex. P. nigra, July 2023 (1 slide, CREA); 2♀♀, Park Andreja Hlinku (Bratislava), 48°9′22.9″N 17°9′9.8″E, 135 m, ex. Juniperus sp., July 2023 (1 slide, CREA); 2♀♀, Legionarska Street (Trenčin), 48°53′11.9″N 18°2′2.7″E, 210 m, ex. Juniperus sp., August 2023 (1 slide, CREA); Kráľova studňa (Veľká Fatra National Park), 48°52′37.2″N 19°2′4.4″E, 1258 m, ex. Piceae sp., August 2023; 5♀♀, park under the SNP Museum (Banská Bystrica), 48°44′5.9″N 19°9′7.6″E, 342 m, ex. Juniperus sp., August 2023 (2 slides, SAS); 4♀♀, 2 deutonymphs, park under the SNP Museum (Banská Bystrica), 48°44′5.9″N 19°9′7.6″E, 342 m, ex. Thuja sp., August 2023 (1 slide, SAS); 1♀, Ovocna Street (Banská Bystrica), 48°45′43.4″N 19°8′31.5″E, 378 m, ex. Alnus sp. (Betulaceae), August 2023 (1 slide, SAS); 10♀♀, Bojnicka Street (Prievidza), 48°46′37.1″N 18°36′14.4″E, 263 m, ex. Juniperus sp., August 2023 (2 slides, CREA); 6♀♀, Karlova Ves (Bratislava), 48°9′27.4″N 17°3′2.9″E, 202 m, ex. Juniperus sp., September 2023 (2 slides, CREA and SAS); 1♀, Rusovce, 48°3′13.5″N 17°9′7.2″E, 150 m, ex. Taxus baccata L. (Taxaceae), May 2024 (1 slide, CREA); 2♀♀, 1 deutonymph, Sibirska, Street (Bratislava), 48°9′59.3″N 17°7′26.6″E, 146 m, ex. P. orientalis, May 2024 (1 slide, CREA); 5♀♀, Tesáre - Nové Mlyny, 48°37′10.7″N 18°4′16.3″E, 224 m, ex. Juniperus virginiana L., May 2024 (2 slides, CREA); 5♀♀, Tesáre - Nové Mlyny, 48°37′10.7″N 18°4′16.3″E, 224 m, ex. Juniperus sabina L., May 2024 (2 slides, CREA and SAS); 7♀♀, Tesáre - Nové Mlyny, 48°37′6.2″N 18°4′18.1″E, 223 m, ex. Juniperus sp., May 2024 (1 slide, SAS); 3♀♀, Tesáre - Nové Mlyny, 48°37′8.2″N 18°4′17.5″E, 223 m, ex. Juniperus sp., May 2024 (1 slide, CREA); 8♀♀, Tesáre - Nové Mlyny, 48°37′9.6″N 18°4′14.0″E, 222 m, P. orientalis, May 2024 (1 slide, CREA).
Remarks — Pentamerismus canadensis has previously been documented in Asia and North America (McGregor 1949; Pritchard and Baker 1958; Khosrowshahi and Arbabi 1997; Lehman 1982). This study represents the first European record of the species. Betulaceae is a new host plant family record, while J. virginiana, J. sabina, P. nigra and T. baccata are new host plant species records. No morphological differences were observed between the collected specimens and the original description.
Pentamerismus collinus Meyer et Van Dis
Specimens examined — 13♀♀, Karlova Ves (Bratislava), 48°9′27.4″N 17°3′2.9″E, 202 m, ex. Taxus sp., September 2023 (2 slides, CREA); 7♀♀, 2 deutonymphs City Park (Banská Bystrica), 48°44′13.7″N 19°8′17.5″E, 350 m, ex. Juniperus sp., August 2023 (3 slides, SAS); 3♀♀, Prievidza, 48°46′50.3″N 18°37′16.8″E, 266 m, ex. Juniperus sp., August 2023 (1 slide, SAS).
Remarks — Pentamerismus collinus has been reported only in South Africa on Passerina falcifolia C.H. Wright (Thymelaeaceae) (Meyer and Van Dis 1993). This study represents the first European record of the species. Cupressaceae and Taxaceae are new host plant family records. No morphological differences were observed between the collected specimens and the original description.
Pentamerismus coronatus (Canestrini et Fanzago)
Specimens examined — 7♀♀, Botanicka Zahrada UK (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Juniperus sp., February 2024 (2 slides, CREA and SAS); 8♀♀, 1 deutonymph, Štúrovo, 47°47′52.9″N 18°43′30.6″E, 106 m, ex. Juniperus sp., August 2024 (2 slides, SAS); 9♀♀, Štúrovo, 47°47′52.9″N 18°43′30.6″E, 106 m, Pinus sp., August 2024 (1 slide, CREA).
Remarks — Pentamerismus coronatus was first described from Italy on Cupressaceae (Canestrini and Fanzago 1876) and has since been reported only from Greece, also on host from the same family (Hatzinikolis 1987). Juniperus sp. is a new host plant species record. The original description (in Italian) is too general and lacks the detail required by current taxonomic standards, providing only information on dorsal body color and a general description of setae. A redescription of the type series, including nymphs, is needed.
Pentamerismus erythreus (Ewing)
Specimens examined — 9♀♀, Bojnicka Street (Prievidza), 48°46′35.7″N 18°36′14.7″E, 263 m, ex. Thuja sp., August 2023 (2 slides, CREA); 4♀♀, Prievidza, 48°46′50.3″N 18°37′16.8″E, 266 m, ex. Thuja sp., August 2023 (1 slide, SAS); 3♀♀, Karlova Ves (Bratislava), 48°9′27.4″N 17°3′2.9″E, 202 m, ex. Thuja sp., September 2023 (1 slide, SAS); 3♀♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Tsuga sp. (Pinaceae), October 2023 (1 slide, CREA); 5♀♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. J. sabina October 2023 (1 slide, CREA); 4♀♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Larix decidua (L.) Mill. (Pinaceae), October 2023 (1 slide, SAS); 1♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Abies nordmanniana (Steven) Spach (Pinaceae), October 2023 (1 slide, SAS); 7♀♀, Botanicka Zahrada UK (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Thuja sp., February 2024 (1 slide, CREA).
Remarks — Pentamerismus erythreus has previously been documented in Asia and North America, primarily on plant species of the family Cupressaceae, although it was first described on Pinaceae (Castro et al. 2025). This study represents the first European record of the species. Abies nordmanniana is a new host plant species record. A comparison with the type specimens or the original description was not possible, as the type depository is unknown and the original description includes only a setal drawing. New collections from the type locality and a redescription following current taxonomic standards are needed.
Pentamerismus juniperi (Reck)
Specimens examined — 1♀, Na Karlove Street (Banská Bystrica), 48°45′20.3″N 19°8′40.4″E, 377 m, ex. Chamaecyparis sp. (Cupressaceae), August 2023 (1 slide, SAS); 4♀ Nitra, 48°19′5.8″N 18°5′15.7″E, 167 m, ex. Juniperus sp., April 2024 (2 slides, CREA).
Remarks — Pentamerismus juniperi has been reported in several Asian and European countries (Castro et al. 2025), including the neighboring countries Hungary and Ukraine. This species is typically associated with host plants of the family Cupressaceae (Castro et al. 2025), though it has been reported only once on Pinaceae (Kontschán 2014). Chamaecyparis sp. is a new host plant genus record. The types need to be redescribed, as the original description lacks morphometric and ontogenetic information, and the drawings omit the dorso-ventral reticulation.
Pentamerismus oregonensis McGregor
Specimens examined — 6♀♀, Nitra, 48°19′5.8″N 18°5′15.7″E, 167 m, ex. Taxus canadensis Marshall (Taxaceae), April 2024 (1 slide, SAS).
Remarks — Pentamerismus oregonensis is widely distributed across Asia, Europe and the Americas countries (Castro et al. 2025), including the neighboring countries Hungary and Ukraine. This mite species is mostly associated with Cupressaceae (Castro et al. 2025). Taxus canadensis is a new host plant species record. No morphological differences were observed between the collected specimens and the original description.
Pentamerismus taxi (Haller)
Specimens examined — 6♀♀, Tbilisska Street (Bratislava), 48°12′54.2″N 17°9′36.6″E, 152 m, ex. Taxus sp., September 2023 (2 slides, CREA and SAS); 11♀♀, Sad Janka Kráľa (Bratislava), 48°8′5.2″N 17°6′41.1″E, 137 m, ex. Taxus sp., September 2023 (2 slides, SAS); 1♀, Botanical Garden of the Comenius University (Bratislava), 48°8′50.7″N 17°4′23.4″E, 152 m, ex. Crataegus sp., October 2023 (1 slide, SAS); 7♀♀, Sad Janka Kráľa (Bratislava), 48°8′5.3″N 17°6′41.1″E, 137 m, ex. Taxus sp., February 2024 (1 slide, SAS); 3♀♀, Nitra, 48°19′5.8″N 18°5′15.7″E, 167 m, ex. Taxus sp., April 2024 (1 slide, SAS); 13♀♀, Nitra, 48°18′51.9″N 18°5′40.9″E, 141 m, ex. Taxus sp., April 2024 (2 slides, CREA and SAS); 5♀♀, Najjužnejší bod Slovenska (Patince), 47°44′5.2″N 18°17′20.8″E, 104 m, ex. Prunus sp. (Rosaceae), August 2024 (1 slide, CREA); 7♀♀, 2 deutonymphs, Komarno, 47°45′34.8″N 18°7′35.6″E, 115 m, Platycladus sp., August 2024 (1 slide, SAS).
Remarks — Pentamerismus taxi is widely distributed across Asia, Europe and North America (Castro et al. 2025). Platycladus sp. is a new host plant species record. A new collection from the type locality is needed, as the type depository is unknown and the original description does not meet current taxonomic standards.
Genus Tenuipalpus Donnadieu
Tenuipalpus kobachidzei Reck
Tenuipalpus kobachidzei was first described from Georgia on Clematis vitalba L. (Ranunculaceae) and has only been reported from Ukraine on the same host. This mite species was collected on Thymus sp. (Lamiaceae) in Slovakia (Kalúz and Majzlan 2007). The Lamiaceae family represents a new host plant record. Similar to P. juniperi, T. kobachidzei, described by the same author in the same paper as P. juniperi, does not meet current taxonomic standards, including morphometric and ontogenetic details, and the drawings lack dorso-ventral reticulation.
New records of Tenuipalpidae from Ukraine
Genus Pentamerismus
Pentamerismus arbutusae
Specimens examined — 5♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, Quercus robur L. (Fagaceae), October 2024 (1 slide, SAS).
Remarks — Ukraine represents the second European country where this mite has been reported. Fagaceae is a new host plant families record, while Q. robur is a new host plant species record.
Pentamerismus canadensis
Specimens examined — 7♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, C. pisifera, September 2024 (2 slides, SAS); 4♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, ex. Pinus (Pinaceae), September 2024 (1 slide, CREA); 1♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, Pinus pumila (Parl.) Regel (Pinaceae), September 2024 (1 slide, SAS); 20♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 160 m, ex. Juniperus sp., October 2024 (2 slides, CREA).
Remarks — Ukraine represents the second European country where this mite has been reported. Pinus pumila is a new host plant species record.
Pentamerismus collinus
Specimens examined — 3♀♀, National botanical garden (Kyiv), 50°24′55.6″N 30°33′46.6″E, 160 m, ex. P. orientalis, July 7, 2024 (1 slide, SAS).
Remarks — Ukraine represents the second European country where this mite has been reported. Platycladus orientalis is a new host plant species record.
Pentamerismus coronatus
Specimens examined — 8♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, ex. Pseudotsuga menziesii (Mirb.) Franco (Pinaceae), October 2024 (1 slide, SAS).
Remarks — Ukraine represents the fourth European country where this mite has been reported, after Italy, Greece, and Slovakia. Pinaceae is a new host plant family record while P. menziesii is a new host plant species record.
Pentamerismus erythreus
Specimens examined — 5♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, ex. Pinus sp., October 2024 (1 slide, CREA); 7♀♀, Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, ex. T. occidentalis, October 2024 (2S slides, SAS); 6♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, ex. Picea asperata Mast. (Pinaceae), October 2024 (1 slide, CREA); 4♀♀, 1 deutonymph O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, ex. Juniperus sp., October 2024 (1 slide, SAS); O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N 30°30′9.7″E, 150 m, ex. Abies pinsapo Boiss., October 2024.
Remarks — Ukraine represents the second European country where this mite has been reported. Abies pinsapo and P. asperata are new host plant species records.
Pentamerismus taxi
Specimens examined — 4♀♀, National Botanical Garden (Kyiv), 50°24′55.6″N, 30°33′46.7″E, 160 m, ex. Prunus tomentosa Thunb., July 2024 (1 slide, SAS); 4♀♀, 2 deutonymphs, 2 protonymphs, National Botanical Garden (Kyiv), 50°24′55.6″N, 30°33′46.7″E, 160 m, ex. T. occidentalis, July 2024 (1 slide, SAS); 7♀♀, National Botanical Garden (Kyiv), 50°26′38.2″N, 30°30′9.7″E, 160 m, ex. Abies koreana E.H.Wilson, September 2024 (1 slide, CREA); 11♀♀, O.V. Fomin Botanical Garden (Kyiv), 50°26′38.2″N, 30°30′9.7″E, 150 m, ex. T. baccata, September 2024 (1 slide, SAS).
Remarks — Pentamerismus taxi has already been reported several times from Ukraine (Livshitz and Mitrofanov 1967; Bondareva et al. 2017; Bondareva and Chumak 2018). The family Rosaceae represents a new host family record, while A. koreana, P. tomentosa, T. baccata, and T. occidentalis are new host plant species records.
New records of Tenuipalpidae from Bulgaria
Genus Brevipalpus Donnadieu
Brevipalpus lewisi McGregor
Specimens examined — Brevipalpus lewisi was intercepted on propagative material of Vitis vinifera L. (Vitaceae) at San Francisco International Airport in March 2017.
Remarks — This mite has been reported from Africa, Asia, Europe and North America on different host plant families (Castro et al. 2025). Brevipalpus lewisi is the first genus record for Bulgaria.
New records of Tenuipalpidae from Hungary
Genus Brevipalpus
Brevipalpus essigi Baker
Specimens examined — Brevipalpus essigi was intercepted on cuttings of Sorbus pseudolatifolia K.P. Popov (Rosaceae) at John F. Kennedy International Airport in August 1996.
Remarks — Brevipalpus essigi was previously reported in Europe only from Greece (Hatzinikolis 1968). Sorbus pseudolatifolia is a new host family and species association for this mite.
Genus Tenuipalpus
Tenuipalpus sarcophilus
Specimens examined — Tenuipalpus sarcophilus Welbourn and Beard was intercepted on Conophytum sp. (Aizoaceae), Haworthia sp. (Asphodelaceae), Lenophyllum sp. (Crussulaceae), Stapelia sp. (Apocynaceae) at San Francisco International Airport in July 2022.
Remarks — This represents the second European record for this species, which it has been previously reported in Italy (De Giosa et al. 2021). The plant genera Conophytum, Haworthia, Lenophyllum, Stapelia and families Aizoaceae and Asphodelaceae are new host associations for T. sarcophilus. Despite T. sarcophilus preference for feeding on succulents, these new host plant family associations may be incidental. Succulent plants and related genera are often cultivated together (Eggli 2002), potentially leading to accidental infestations. The cultivation practices and management of certain ornamentals demand careful attention, as they may inadvertently aid in the dispersion of this mite (Hoy 2011).
New records from the Netherlands
Genus Brevipalpus
Brevipalpus obovatus Donnadieu
Specimens examined — Brevipalpus obovatus was intercepted on Hibiscus sp. plant (Malvaceae) at Seattle-Tacoma International Airport in May 2006.
Remarks — Brevipalpus obovatus is widely distributed Africa, Asia, Europe and the Americas and and feeds on a broad range of host plant families. In Europe, it has previously been reported from eight countries (Castro et al. 2025). This species is vector of solanum violifolium ringspot virus and cestrum ringspot virus (de Lillo et al. 2021)
Genus Pentamerismus
Pentamerismus erythreus
Specimens examined — Pentamerismus erythreus was intercepted on cuttings of C. lawsoniana at John F. Kennedy International Airport in February 2001.
Remarks — Pentamerismus erythreus is the first Pentamerismus species reported from the country. However, further confirmation of its presence through surveys is needed. The Netherlands is the largest exporter of agricultural commodities in Europe (Government of the Netherlands, 2025). Consequently, uncertainty remains regarding whether C. lawsoniana is naturally occurring in the Netherlands or if it arrived as an agricultural commodity from another country, passing through Dutch ports en route to other destinations (Qiang et al. 2020).
Discussion
This study presents the first comprehensive faunistic survey of tenuipalpid mites in Slovakia and expands recent findings from Ukraine, with additional records from Bulgaria, Hungary, and the Netherlands based on interception data. Through extensive field sampling and meticulous morphological identifications, we uncovered 13 species across three genera Cenopalpus, Pentamerismus, and Tenuipalpus. The majority of species were associated with coniferous hosts, particularly within Cupressaceae and Pinaceae, which are widespread in urban and ornamental landscapes. Notably, several Pentamerismus species, including P. arbutusae, P. canadensis, P. collinus, and P. erythreus were recorded for the first time in Europe. These detections extend their known distributions from North America, Africa, or Asia and emphasize the value of targeted faunistic surveys in revealing overlooked or recently introduced species (McGregor 1949; Knowlton and Ma 1950; Pritchard and Baker 1952; Pritchard and Baker 1958; Baker and Tuttle 1964; Prasad 1970; Thewke and Enns 1970; Baker and Tuttle 1972; Lehman 1982; Hennessey et al. 1986; Baker and Tuttle 1987; Evans et al. 1993; Meyer and Van Dis 1993; Khosrowshahi and Arbabi 1997; Sağlam and Çobanoğlu 2010). Our findings also include numerous new host plant associations, suggesting broader ecological adaptability than previously documented for some taxa. The faunistic survey in Slovakia revealed twelve species currently known from the country, many of which had not been previously recorded in Central Europe. In Ukraine, complementary sampling conducted in botanical gardens led to the discovery of new national records and host associations, including multiple Pentamerismus species previously unreported from the country (Zhovnerchuk et al. 2021). These findings confirm the presence and establishment of several species with potential economic relevance, especially in ornamental landscapes and nursery plant systems.
Beyond regional biodiversity documentation, our results have important biosecurity implications. Several of the recorded species, such as P. erythreus and T. sarcophilus, are known to cause damage to ornamental plants through leaf discoloration and necrosis, compromising their aesthetic value (Lehman 1982; Welbourn et al. 2017). These symptoms are particularly problematic for aesthetic plant quality and may result in commercial losses. The Netherlands, a major exporter of ornamental plants (Dutch Horticulture 2025), has been implicated in the unintentional distribution of T. sarcophilus, highlighting the need for routine surveillance in production and export facilities. In addition to detecting new records, this study underscores the importance of re-evaluating historical identifications. The Brevipalpus phoenicis species complex, for example, includes multiple cryptic species with distinct biological traits, including virus transmission capabilities (de Lillo et al. 2021; Ramos-Gonzalez et al. 2023). Older regional records, including those from Hungary (Kerényiné Nemestóthy and Vályi 1978) and Ukraine (Chumak 2003), may have misidentified B. phoenicis, potentially overlooking important risks associated with virus transmission. The slide depository of the Ukrainian collection reported by Livschitz and Mitrofanov remains unknown, despite efforts by the first author to locate it. This highlights the need for new faunistic surveys to ensure accurate pest risk assessments.
In summary, our work illustrates the essential role of faunistic surveys and interception analysis in detecting new mite introductions, updating species distributions, and identifying previously unknown host associations. Accurate species identification, particularly of economically relevant taxa, is foundational to effective quarantine, pest management, and conservation strategies across Europe.
Acknowledgments
The authors thank Dr. Denise Navia and Andrew Ulsamer (SEL-USDA) for the revision and helpful suggestions on the manuscript. This study was funded by the EU NextGenerationEU through the Recovery and Resilience Plan for Slovakia under the project No. 09I03-03-V01-00022. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA; USDA is an equal opportunity provider and employer.
References
- Bajwa W.I., Krantz G.W., Kogan M. 2001. Discovery of Cenopalpus pulcher (C. & F.) (Acari: Tenuipalpidae) in the New World. Proc. Entomol. Soc. Wash., 103(3): 754-756.
- Baker E.W., Tuttle D.M. 1964. The false spider mite of Arizona (Acarina: Tenuipalpidae). Bull. - Univ. Ariz., Agric. Exp. Stn., 163: 1-80.
- Baker E.W., Tuttle D.M. 1972. New species and further notes on the Tetranychoidea mostly from Southwestern United States (Acarina: Tetranychidae and Tenuipalpidae). Smithson. Contrib. Zool., 116: 1-36. https://doi.org/10.5479/si.00810282.116
- Baker E.W., Tuttle D.M. 1987. The false spider mites of Mexico (Tenuipalpidae: Acari). Tech. Bull. U.S. Dep. Agric. ARS., 1706: 1-236.
- Beard J.J., Ochoa R., Braswell W.E., Bauchan G.R. 2015. Brevipalpus phoenicis (Geijskes) species complex (Acari: Tenuipalpidae) - a closer look. Zootaxa, 3944: 1-67. doi:10.11646/zootaxa.3944.1.1 https://doi.org/10.11646/zootaxa.3944.1.1
- Bondareva L.M., Chumak P.Y., Bondariev S.I. 2017. Revealing the sustainable population of Pentamerismus taxi (Acari, Tenuipalpidae) outside the zone of its natural habitation in Ukraine. Vestnik Zoologii, 51(5): 435-438. https://doi.org/10.1515/vzoo-2017-0052
- Bondareva L.M., Chumak P.Y. 2018. Pentamerismus taxi (Haller, 1877) (Acari: Tenuipalpidae): A new pest in the conditions of Kyiv. Russ. J. Biol. Invasions, 9: 9-12. https://doi.org/10.1134/S2075111718010034
- Canestrini G., Fanzago F. 1876. Nuovi acari Italiani. Atti Academia Cientifico Veneto, Trentino, Istriana, Pádua, Italy, 5:130-142.
- Castro E.B., Mesa N.C., Feres R.J.F., Moraes GJde, Ochoa R., Beard J.J., Demitem P.R. 2025. Tenuipalpidae Database. Available from: http://www.tenuipalpidae.ibilce.unesp.br (accessed February 2025).
- Chumak P.Y. 2003. On the Arachnida living in greenhouses of botanical gardens in Ukraine. Bulletin of Taras Shevchenko National University of Kyiv. Series Introduction and preservation of plantdiversity, 6: 47-48.
- De Giosa M., Tassi A.D., McDonald E.M., Ochoa R. (2021a). First record of Cenopalpus officinalis Papaioannou-Souliotis (Tenuipalpidae) for Israel, Italy and Mexico and a redescription. Acarologia, 61(4): 978-995. https://doi.org/10.24349/opjx-3ucc
- De Giosa M., Bassini-Silva R., de Lillo E., McDonald E.M., Ochoa R. 2021b. Italian Acarine species intercepted in the United States. Int. J. Acarol., 47(8): 689-694. https://doi.org/10.1080/01647954.2021.1990407
- De Giosa M., Ochoa R., Castro E.B., Simoni S., Glik T., Tassi A.D., de Lillo E. 2024. Updated Italian checklist of Tenuipalpidae with description of a new species and new worldwide records of the genus Cenopalpus (Pritchard et Baker). Int. J. Acarol., 50(3): 237-286. https://doi.org/10.1080/01647954.2024.2318364
- de Lillo E., Freitas-Astúa J., Watanabe Kitajima E., Ramos-González P.L., Simoni S., Tassi A.D., Valenzano D. 2021. Phytophagous mites transmitting plant viruses: update and perspectives. Entomol. Gen., 41(5): 439-462. https://doi.org/10.1127/entomologia/2021/1283
- Donnadieu A.L., Donnadieu A.L. 1875. Recherches pour servir à l′histoire des Tétranyques. Georg. Thèses. Faculté des Sciences de Lyon-Francia, 1-131.
- Duch Horticulture 2025. https://www.dutchhorticulture.nl/en (accessed December 2024).
- Eggli, U. (Ed.). (2002). Illustrated handbook of succulent plants: dicotyledons. Springer Science and Business Media.
- Evans G., Cromroy H.L., Ochoa R. 1993. The Tenuipalpidae of Honduras (Tenuipalpidae: Acari). Fla. Entomol., 76 (1): 126-155. https://doi.org/10.2307/3496021
- Ewing H.E. 1917. New Acarina. Part 2, Descriptions of new species and varieties from Iowa, Missouri, Illinois, Indiana, and Ohio. Bulletin of the AMNH; v. 37, article 2.
- Government of the Netherlands 2025. https://www.government.nl/ (accessed January 2025)
- Haller G. 1877. Mitteilungen. Schweizerische Zeitschrift für Forstwesen, Zurich, 2, 85-89.
- Hatzinikolis E.N. 1986. The genus Brevipalpus in Greece (Acari: Tenuipalpidae). Entomol. Hell., 4: 37-48. https://doi.org/10.12681/eh.13931
- Hatzinikolis Ε.Ν. 1987. Α revision of tenuipalpid mites of Greece (Acari: Tenuipalpidae). Entomol. Hell., 5, 47-60. https://doi.org/10.12681/eh.13948
- Hennessey M.K., Sephan D.L., Farrier M.H. 1986. Spider mites and the false mites (Acari: Tetranychidae) recorded or expected to occur in North Carolina. Brimleyana, 12, 19-27.
- Hoy M.A. 2011. Agricultural acarology: introduction to integrated mite management (Vol. 7). CRC press. 1-410.
- Jeppson L.R., Keifer H.H., Baker E.W. 1975. Mites injurious to economic plants. University of California Press, Berkeley, 614 pp. https://doi.org/10.1525/9780520335431
- Kalúz S., Majzlan O. 2007. Roztoče (Acari) PR Ostrov Kopáč. Príroda ostrova Kopáč. Fytoterapia OZ, Bratislava, 53-66.
- Kerényiné Nemestóthy, K. & Vályi, Á. (1978). Áltakácsatkák (Tenuipalpidae) kártétele dísznövényeken. (Damage of tenuipalpid mites on ornamental plants.). Növényvédelem, 14(8): 342-348.
- Khanjani M., Gotoh T. 2008. False spider mites of the genus Pentamerismus McGregor (Acari: Tenuipalpidae) from Iran. Zootaxa, 1768(1): 52-60. https://doi.org/10.11646/zootaxa.1768.1.3
- Khosrowshahi M., Arbabi M. 1997. The Tenuipalpidae (Acari) of Iran with introduction of new species for the world fauna and Iran. Ministry of Agriculture, Agriculture Research, Education and Extension Organization Plant Pests and Diseases Research Institute, Tehran, 1-19.
- Knowlton G.F., Ma S.C. 1950. Some Utah mites - 1949. J. Kans. Entomol. Soc., 23(2): 74-76.
- Kontschán J. 2014. Contribution to the Tetranychidae and Tenuipalpidae Fauna of Hungary (Acari: Prostigmata). Acta Phytopathol. Entomol. Hung., 49(2): 261-269. https://doi.org/10.1556/APhyt.49.2014.2.12
- Kontschán J., Ripka G. 2017. Checklist of the Hungarian spider mites and flat mites (Acari: Tetranychidae and Tenuipalpidae). Syst. Appl. Acarol., 22(8): 1199-1225. https://doi.org/10.11158/saa.22.8.6
- Lehman R.D. 1982. Mites (Acari) of Pennsylvania conifers. Transactions of the American Entomological Society, 181-286.
- Livshitz I.Z., Mitrofanov V.I. 1967. Materials to the cognition of the Acariformes: Tenuipalpidaes fauna. Proceedings Nikitsky Botanic Garden, 39:1-72.
- McGregor E.A. 1949. Nearctic mites of the family Pseudoleptidae. Mem. South. Calif. Acad. Sci., 3(2): 1-45. https://doi.org/10.5962/bhl.title.146948
- Meyer M.K.P., Van Dis J.C.S. 1993. Notes on some species of the related genera Aegyptobia Sayed, Phytoptipalpus Trägärdh and Pentamerismus McGregor, with descriptions of eight new species (Acari: Tenuipalpidae). Genus, 4: 309-357.
- Navia D., Mendonça R.S., Ferragut F., Miranda L.C., Trincado R.C., Michaux J., Navajas M. 2013. Cryptic diversity in Brevipalpus mites (Tenuipalpidae). -Zool. Scr., 42, 406-426. https://doi.org/10.1111/zsc.12013
- Negm M.W., Ueckermann E.A., Gotoh T. 2020. A new species of Cenopalpus Pritchard & Baker (Acari: Tenuipalpidae) from Japan, with ontogeny of chaetotaxy and a key to the world species. PeerJ, 8: e9081. https://doi.org/10.7717/peerj.9081
- Prasad V. 1970. Some tetranychoid mites of Michigan. Mich. Entomol., 3: 24-31. https://doi.org/10.22543/0090-0222.1118
- Pritchard A.E., Baker E.W. 1952. The false spider mites of California (Acarina: Phytoptipalpidae). University of California Publicationsin Entomology, 9(1): 1-94.
- Pritchard A.E., Baker E.W. 1958. The false spider mites (Acarina: Tenuipalpidae). University of California Publications in Entomology, 14(3): 175-274.
- Qiang W., Niu S., Liu A., Kastner T., Bie Q., Wang X., Cheng, S. 2020. Trends in global virtual land trade in relation to agricultural products. Land use policy, 92: 104439. https://doi.org/10.1016/j.landusepol.2019.104439
- Ramos-González P.L., Dias Arena G., Tassi A.D., Chabi-Jesus C., Watanabe Kitajima E., Freitas-Astúa J. 2023. Kitaviruses: A window to atypical plant viruses causing nonsystemic diseases. Ann. Rev. Phytopathol., 61: 97-118. https://doi.org/10.1146/annurev-phyto-021622-121351
- Reck G. 1951. Kleshchirodov Tenuipalpus, Brevipalpus e Brevipalpoides (Trichadenidae, Acarina) po materalam iz grunzii. Trudy Instituta Zoologii Akademiya Nauk Gruz SSR, 10: 289-297.
- Saccaggi D.L., Ueckermann E.A. 2024. The problem of taxonomic uncertainty in biosecurity: South African mite interceptions as an example. Acarologia, 64(2): 363-369. https://doi.org/10.24349/top1-r59v
- Sağlam H.D., Çobanoğlu S. 2010. Determination of Tenuipalpidae (Acari: Prostigmata) species in parks and ornamental plants of Ankara, Turkey. Turk. J. Entomol., 34(1): 37-52.
- Thewke S.E., Enns W.R. 1970. The spider-mite complex (Acarina: Tetranychoidea) in Missouri. University of Missouri, Missouri Agricultural Experimental Station, Contribution No. 5969, and No. 7294, Entomology Research Division, USDA, pp. 1-106.
- Ueckermann E. A., Ripka G. 2016. Three new species and a new record of tenuipalpid mites (Acari: Tenuipalpidae) from Hungary. J. Nat. Hist., 50(15-16): 989-1015. https://doi.org/10.1080/00222933.2015.1091104
- Welbourn W.C., Beard J.J., Bauchan G.R., Ochoa R. 2017. Description of a new species of Tenuipalpus (Acari: Trombidiformes) from succulent plants in Florida, USA, and a redescription of T. crassulus Baker and Tuttle. Int. J. Acarol., 43(2): 112-136. https://doi.org/10.1080/01647954.2016.1255253
- WFO 2025. World Flora Online. Published on the Internand; http://www.worldfloraonline.org. (accessed May 2024)
- Zhovnerchuk O.V., Bondareva L.M., Chumak P.Y. 2021. An annotated checklist of Tenuipalpidae (Acari: Trombidiformes) of Ukraine. Ukrainska Entomofaunistyka, 12(2): 31-35.



2024-07-20
Date accepted:
2025-11-24
Date published:
2025-11-28
Edited by:
Navia, Denise

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Zhovnerchuk, Olha V.; Ochoa, Ronald; Glik, Tobias E.; Tassi, Aline D. and De Giosa, Marcello
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



