New Records of Tarsonemid Mites (Acari: Trombidiformes) for Cuba
de la Torre Santana, Pedro Enrique  1
1
1✉ Laboratorio Central de Cuarentena Vegetal. Ayuntamiento #231, entre San Pedro y Lombillo, Plaza de la Revolución, La Habana, Cuba.
2025 - Volume: 65 Issue: 4 pages: 1082-1091
https://doi.org/10.24349/xu18-1k5iZooBank LSID: F3A395B8-E18D-47A7-B46D-C2AD6E9CDB21
Original research
Keywords
Abstract
Introduction
The Tarsonemidae is a large family of mites present in many different habitats worldwide. Their feeding habits are greatly diverse: many are fungivores, algivores and herbivores, and others are predators of other mites, parasites of insects and possibly symbionts of insects. Some phytophagous tarsonemids are important pests on agricultural crops. Tarsonemid mites are thus of great interest to those who study their ecology and evolution, as well as to those applied workers who study ways to control them on economic plants (Lin & Zhang, 2002)
To date, 11 species of Tarsonemidae are known in Cuba: Acarapis woodi (Rennie), Ceratotarsonemus scitus De Leon, Fungitarsonemus peregrinosus Attiah, Polyphagotarsonemus latus (Banks), Steneotarsonemus bancrofti (Michael), S. furcatus De Leon, S. konoi Smiley and Emmanouel, S. lobosus Torre, S. spinki Smiley, Tarsonemus mycelyophagus (Hussey) and Ununguitarsonemus beameri (Beer) (Torre, 2016; Torre & Cuervo, 2019). However, this family is poorly studied in Cuba in many topics, including its taxonomy. The objective of this communication is to report new records of tarsonemids species for the Cuban fauna.
Materials and methods
A total of 633 slide mounts belonging to the Tarsonemidae family from the collection of the Central Laboratory for Plant Quarantine (CPQL) of Cuba were examined. A ZEISS model Axioscop 40 phase contrast microscope and a Zeiss P8x drawing eyepiece were used to observe the slides.
The terminology follows that of Lindquist (1986), except for the dgs and vgs setae of the gnathosomes (Suski 1967; Magowski et al., 1998). For all structures, measurements are given in micrometers (μm). The length and proportion of the legs include the segments from the femurs to the apex of the tarsus. Leg chaetotaxy is expressed by formulas of the number of setae (and solenidia in parentheses, when present) on each leg. The number of setae on each segment is separated from each other by a ''-''. Data on specimens were taken from the collection record including the catalogue number (CPQL N°).
Results and discussion
Eight new species not previously reported in the country were identified and are listed below.
Daidalotarsonemus annonae Sousa, Lofego & Gondim Jr., 2014
Daidalotarsonemus annonae Sousa, Lofego & Gondim Jr., 2014, 430
(Fig.1-3, 4 A-B)
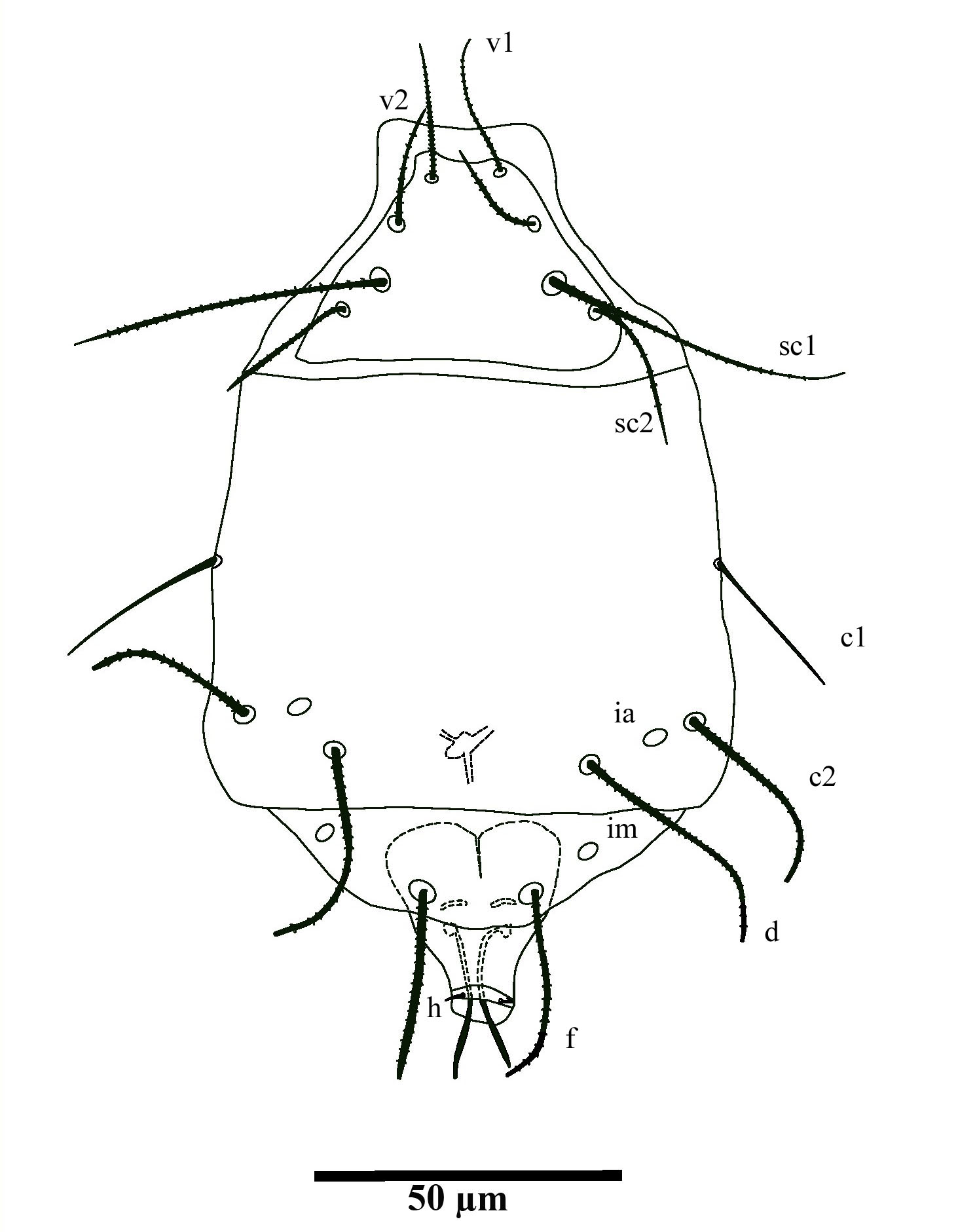


Material examined — 1 female and 1 male, leaves of Citrus sp., El rodeo, Santa Clara, 16 Nov.2007, collector Pedro de la Torre, CPQL N°2671-2673.
Previous distribution — Brazil, USA (Florida), (Rezende et al., 2024).
Remarks — This species has been found on other hosts such as Trichilia casaretti C.DC. (Euphorbiaceae), Calliandra americana (Fabaceae), Clerodendron japonica (Lamiaceae), Hibiscus rosa-sinensis L. (Malvaceae), Quercus virginiana Mill. (Fagaceae), Chrysobalanus icaco L. (Chrysobalanaceae) and Viburnum obovatum (Caprifoliaceae), (Rezende et al., 2024). These authors mention that larva and male of this species are unknown; therefore the male is described below. The absence of φ1 is confirmed in the female, but the male has the complete cluster (φ1, φ2 and k).
Description of male (One specimen)
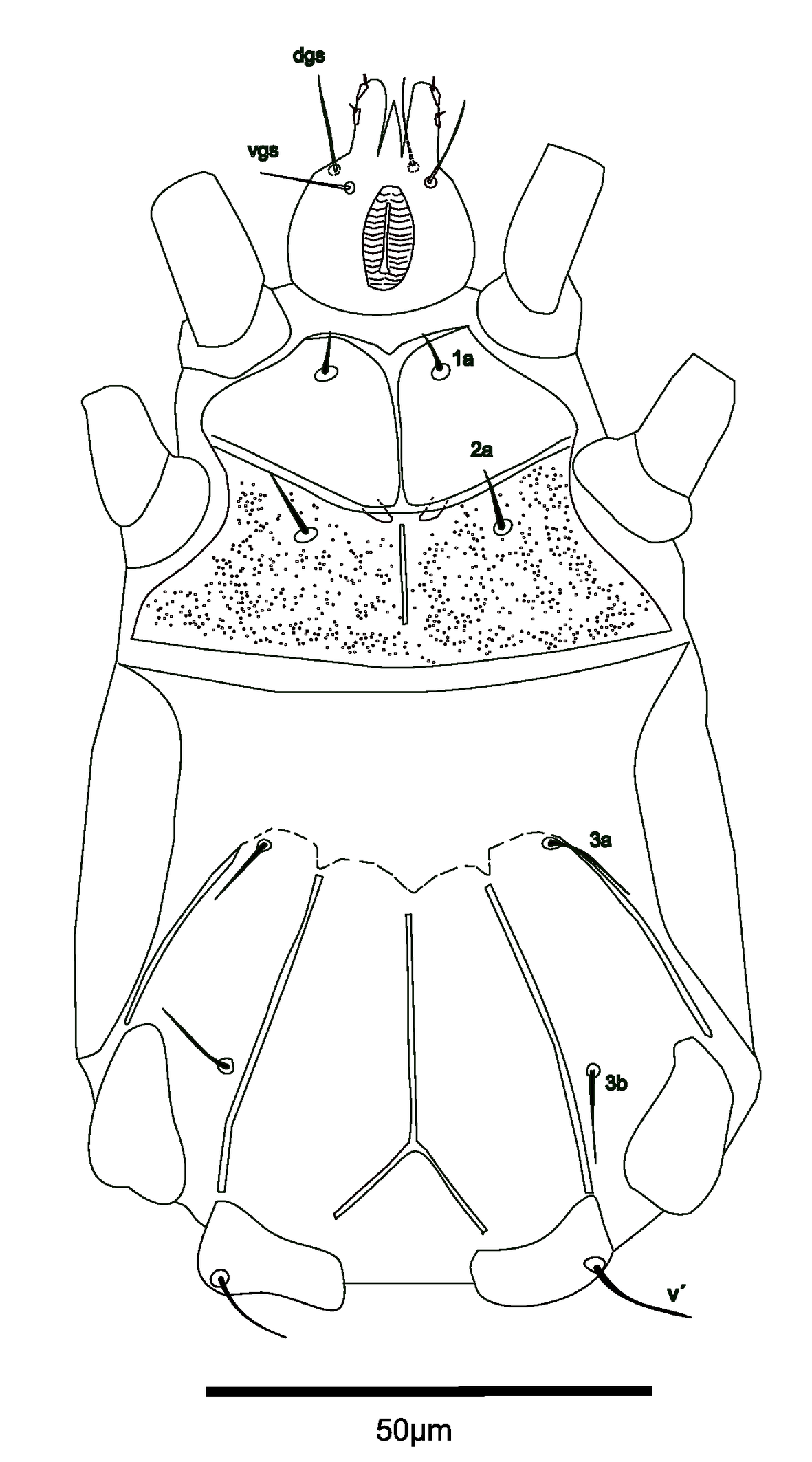


Gnathosoma — semicircular in ventral view; length 19, maximum width 21. Setae dgs (13) and vgs (6) smooth; Palps moderately long (7). Pharynx fusiform, 14 long and 6 wide.
Idiosoma dorsum — length 122, maximum width 75. Prodorsal shield trapezoidal. Length of dorsal setae: v1 (25), v2 (19), sc1 (49), sc2 (20), c1 (29) c2 (20), d (33), f (31), h (4). All setae are serrate except smooth c2 and h; setae c1, d and f blunt-tipped. Distances between dorsal setae: v1–v1 (11), sc1–sc1 (25), sc2–sc2 (37), v1–sc2 (25), c1–c1 (68), c2–c2 (70), c1–c2 (39), d–d, (38), f–f (16) h-h (5). Setae sc2 lateral and slightly posterior to sc1; setae c1 closer to d than to c2.
Idiosoma venter — setae 1a (5) posterior to apodemes 1; setae 2a (10) located slightly posterior to apodemes 2; setae 3a (10) located near anterior end of apodemes 3 and setae 3b (12) located on the posteromedial margins of apodemes 4. Apodeme 1 is fused with the anterior end of the prosternal apodeme; apodeme 2 fused to prosternal apodeme; coxal field II punctate. Prosternal apodeme visible between coxisternal plates I and II but not joined to sejugal apodeme. Sejugal apodeme conspicuous. Lines of fusion between coxae III and IV with ventral area of idiosoma mostly conspicuous; connection between apodemes 3, 4 and postesternals apodeme diffuse.
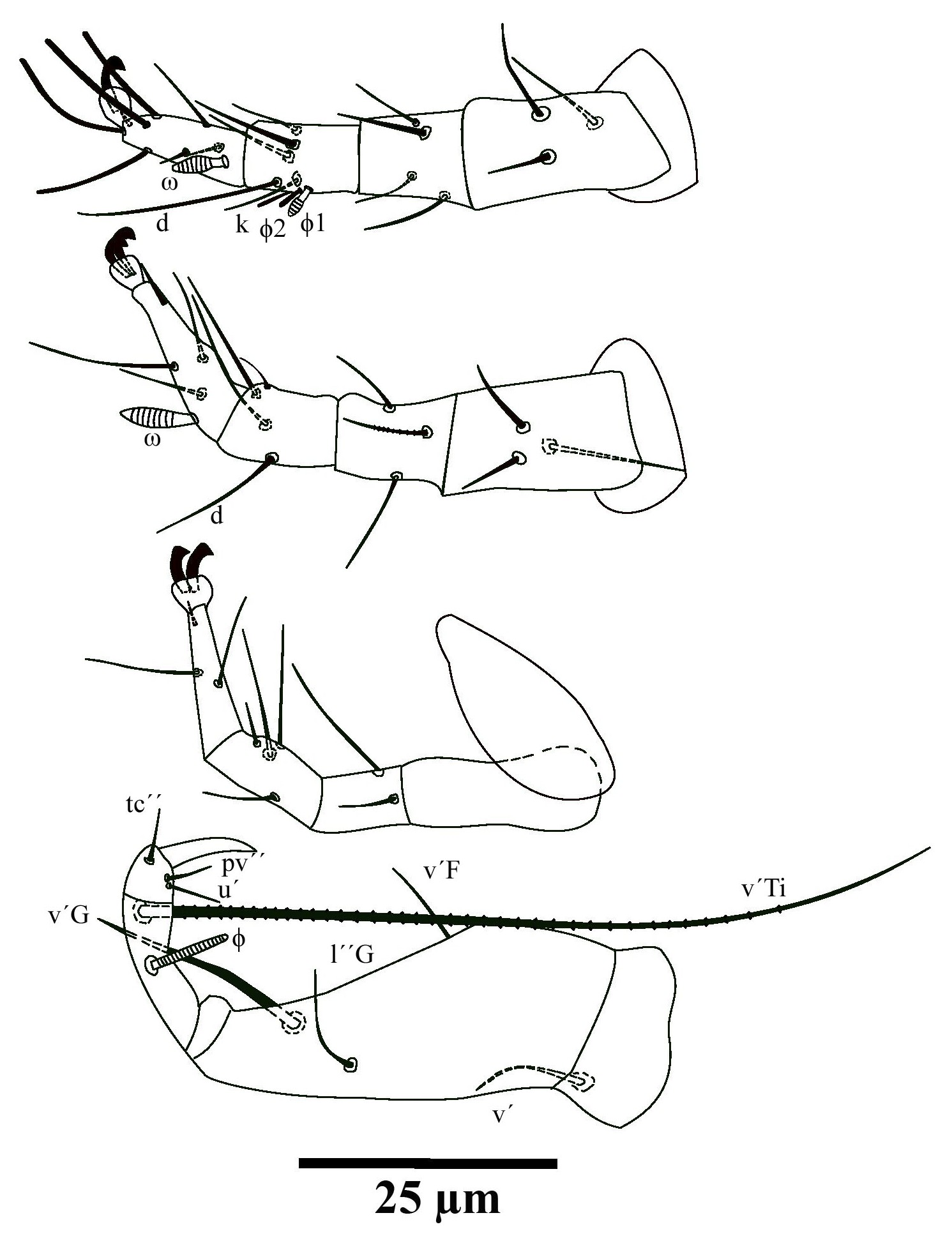


Legs — Lengths: leg I 50, leg II 51, leg III 50, leg IV 56. Number of setae on femur, genu, tibia and tarsus, respectively: leg I: 3-4-6(2)+7(1), leg II: 3-3-4-4(1), leg III: 0-2-4-3. Solenidion ω of tarsus I (4), robust, wider medially. Sensory cluster of tibia I composed of φ1 (2), φ2 (2) and famulus k (4), both inserted at approximately the same level. Solenidion ω of tarsus II (5) inserted proximally and robust, wider medially. Trochanter IV wider than long, seta v′ (12), smooth. Femurogenu IV 39 long and 15 wide at level of v′ F; setae v′ F (6), smooth. Setae v′ G (22) and l′′G (10), smooth. Tibia IV 17 long; solenidion φ (3); seta v′ Ti 75, serrate. Tarsus IV short, with three smooth setae of the following lengths: tc′′ 4, pv′′ 5 and u′ 5. Claw well developed.
Key for Daidalotarsonemus males (based on Rezende et al., 2024)
1. Tarsal solenidion II larger than 5 µm
...... 2
Tarsal solenidion II smaller than or equal to 5 µm
...... 4
2. Tibiotarsal sensory cluster I complete (φ1, φ2, k)
...... 3
Tibiotarsal sensory cluster I incomplete
...... vandevriei
3. Setae sc1 similar in size to sc2
...... euonymus
Seta sc1 longer than sc2
...... ternifoliae
4. Tibia of leg IV longer than v′G
...... deleoni
Tibia of leg IV shorter than or equal to v′G
...... 5
5. Dorsal seta f shorter than the others of the hysterosoma (c1, c2 and d)
...... 6
Dorsal seta f longer than or equal to the others of the hysterosoma
...... 7
6. Setae v1 similar to sc2; v′Ti twice the length of tibia IV; v′G four times the length of v′F in leg IV
...... azofeifai
Setae v1 longer than sc2; v′Ti similar to length of tibia IV; v′G 2 times the length of v′F in leg IV
...... oliveirai
7. Apodemes 2 fused with prosternal
...... 8
Apodemes 2 do not fused with prosternal
...... 9
8. Setae v2 smaller than sc2; v′Ti twice longer than tibia IV
...... serissae
Setae v2 similar to sc2; v′Ti four times longer than tibia IV
...... annonae
9. Tibia I with two tactile setae, k and ϕ 1
...... leonardi
Tibia I with four tactile setae, k and ϕ 1
...... tessellatus
Fungitarsonemus lodici (De Leon, 1956)
Hemitarsonemus lodici De Leon, 1956, 110
(Fig. 4 C-D)
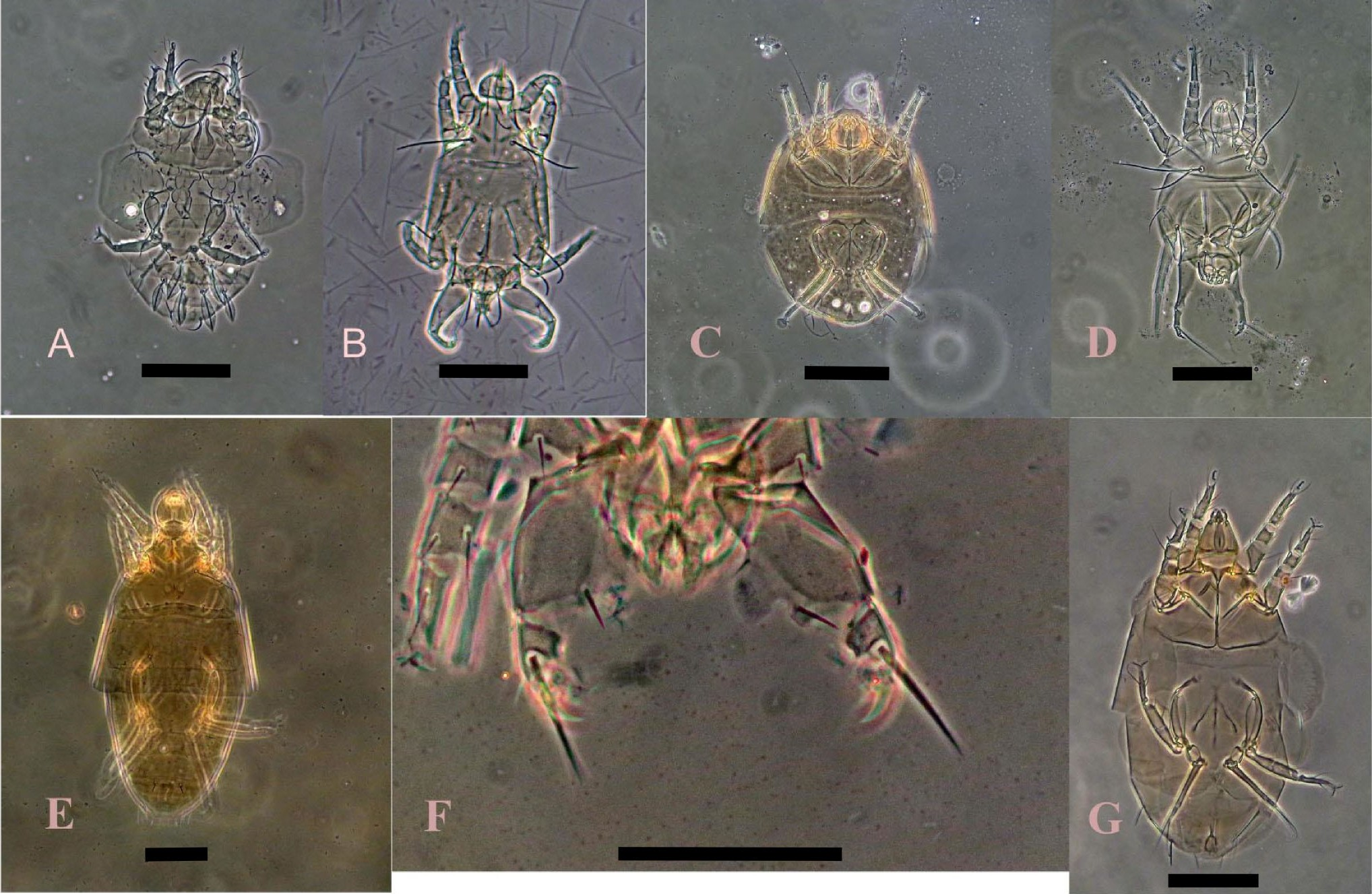


Material examined — Three females, leaves of Trichilia hirta L., Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 10 Feb.2001, col. Pedro de la Torre, CPQL No. 2325, 2326; Three females, leaves of Conocarpus erecta L., Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 9 Mar.2010, col. Pedro de la Torre, CPQL No. 2330, 2331; six females and one male, Gymnanthes lucida Sw , Cojímar, Havana, 24 Mar.2009, col. Pedro de la Torre, CPQL No. 2332, 2333, 2334; five females, leaves of Trichilia hirta L., Cojímar, Havana, 20 Feb. 2020, col. Pedro de la Torre, CPQL No. 2346, 2347, 2349.
Previous distribution — China, Costa Rica, USA (Florida) (Lin & Zhang, 2002)
Remarks — Diagnostic characters are in agreement with those in the literature. It has been collected on the following hosts: Psychotria undata Jacq., Pithecolobium guadalupense (Pers.) Chapm, Dipholis salicifolia (L.) and Citharexsylum fruticosum (L.) (De Leon, 1956); Macadamia ternifolia Muel (Ochoa et al., 1991b); and on various hosts in China (Lin et al., 1995). Other species of the genus awaiting identification were found in the collection. They were observed errant on leaves carrying debris on their bodies.
Steneotarsonemus comosus Ochoa, 1991
Steneotarsonemus comosus Ochoa et al., 1991,52.
(Fig. 4 E-F)
Material examined — 26 females, five males, Ananas comosus (L.) Merr. var. MD2, Ceballos Agroindustrial Company, Ciego de Ávila, 4 May. 2017, collector: Pedro de la Torre, CPQL No. 1921-1930; two females, one male, Ananas comosus (L.) Merr. var. MD2, Ciego de Ávila, 28 Jun. 2017, collector: Luis Raúl Machado, CPQL No. 2019,2020.
Previous distribution — Costa Rica, (Ochoa, 1991b); Rep. Dominican, (Perez-Gelabert, 2020)
Remarks — The labels on the collections' slides erroneously identified the specimens as S. ananas (Tyron). However, their identity was verified byIt was previously identified as by the differences in the setae c1, c2, 3a, 3b and leg IV of the male according to Aguilar-Piedra et al., (2021). It is recognized in the field by locating the mite at the base of the tender leaves of the crown. It occurs from the formation of the flower to the already developed fruit. It has been associated with the symptom known as multiple crown which, in interrelation with high temperatures, can reach 10% of affected plants for the first cutting and in some cases up to 50% for the second. This symptom can be confused with a varietal or physiological problem (Ochoa et al., 1991a).
It was found associated with fungal attack in the crop, so it can become an important pest in Cuba. This species is likely to cause fungal entry pathways just like S. ananas (Ochoa et al., 1991a).
Tarsonemus bilobatus Suski, 1965
Tarsonemus bilobatus Suski, 1965, 539
Tarsonemus hungaricus Schaarschmidt, 1967, 59: 389
Lupotarsonemus bilobatus: Suski & Schaarschmidt, 1971, 119
Tarsonemus fuzhouensis Lin .& Zhang, 1982, 137
(Fig. 4 G)
Material examined — 1 female, 1 male, Oryza sativa L., Villa Clara, 1 Dec. 1997, collector Zuleika Martinez, CPQL No. 1456-1457; 5 females, 4 males, 2 larvae, Oryza sativa L., Rio Cauto, Granma, 24 Mar. 2008, collector Pedro de la Torre, CPQL No. 1461-1464; 1 female, Pennisetum purpureum Schuman, San Jose de las Lajas, Mayabeque, 26 Oct. 2004, collector: Pedro de la Torre, CPQL No. 1458; 1 female, 3 larvae, Panicum maximun Jacq., La Lisa, Havana, collector: 21 Jan. 2005, Pedro de la Torre, CPQL No. 1459-1460; 9 females, 1 male, 1 larva, Phylla nodiflora (L) Greene, Reparto Eléctrico, Havana, 17 Jul. 2006, collector: Pedro de la Torre, CPQL No. 1704-1714; eigh females, leaf litter, Reparto Eléctrico, Havana, 17 Jul. 2006, collector: Pedro de la Torre, CPQL No. 1715-1721; 10 females, Sorghum vulgare Pers., Güines, Mayabeque, 09 Nov. 2006, collector Mayra Ramos, CPQL No. 1722; one female, Xanthium chinense Mill. (Asteraceae), Reparto Eléctrico, Havana, 28 Sep. 2003, collector: Pedro de la Torre, CPQL No. 1761; two females, Eleucine indica (L.) Gaertn., Reparto Eléctrico, Havana, 08 Nov. 2005, collector: Pedro de la Torre, CPQL No. 1762-1763; seven females, four males, Coletotrichium; Lasiodiplodia theobromae, Laboratory culture (CPQL), 29 Oct. 2009, collector: Pedro de la Torre, CPQL No. 1824-1828; six females, three males, Chrysoporthe cubensis (Bruner) Gryzenhout & M.J. Wingf, Laboratory culture (CPQL), 29 Oct. 2009, collector: Pedro de la Torre, CPQL No. 1829-1833, 1835; one female, Bambusa vulgaris Schrad., 17 Nov. 2016, Presa Ejército Rebelde, Havana, collector: Pedro de la Torre, CPQL No. 1942; three females, Ananas comosus G. Maza var. MD2, Ciego de Ávila, 28 Jun. 2017, collector: Luis Raúl Machado, CPQL No. 1847-1848. one female, Ananas comosus G. Maza var. MD2, Ciego de Ávila, 28 Jun. 2017, collector: Justino Martínez, CPQL No. 2027.
Previous distribution — Belarus, Brazil, China, Costa Rica, Egypt, Hungary, Iran, Italy, Japan, Korea, Poland, Ukraine (Gheblealivand et al., 2018).
Remarks — The observed female specimens have the setae tc″ of leg IV greater than the combination of the length of leg IV and not as expressed by Kaliszewski, (1993).
Tarsonemus fusarii Cooreman, 1941
Tarsonemus fusarii Cooreman, 1941,1
(Fig. 5 A-B)
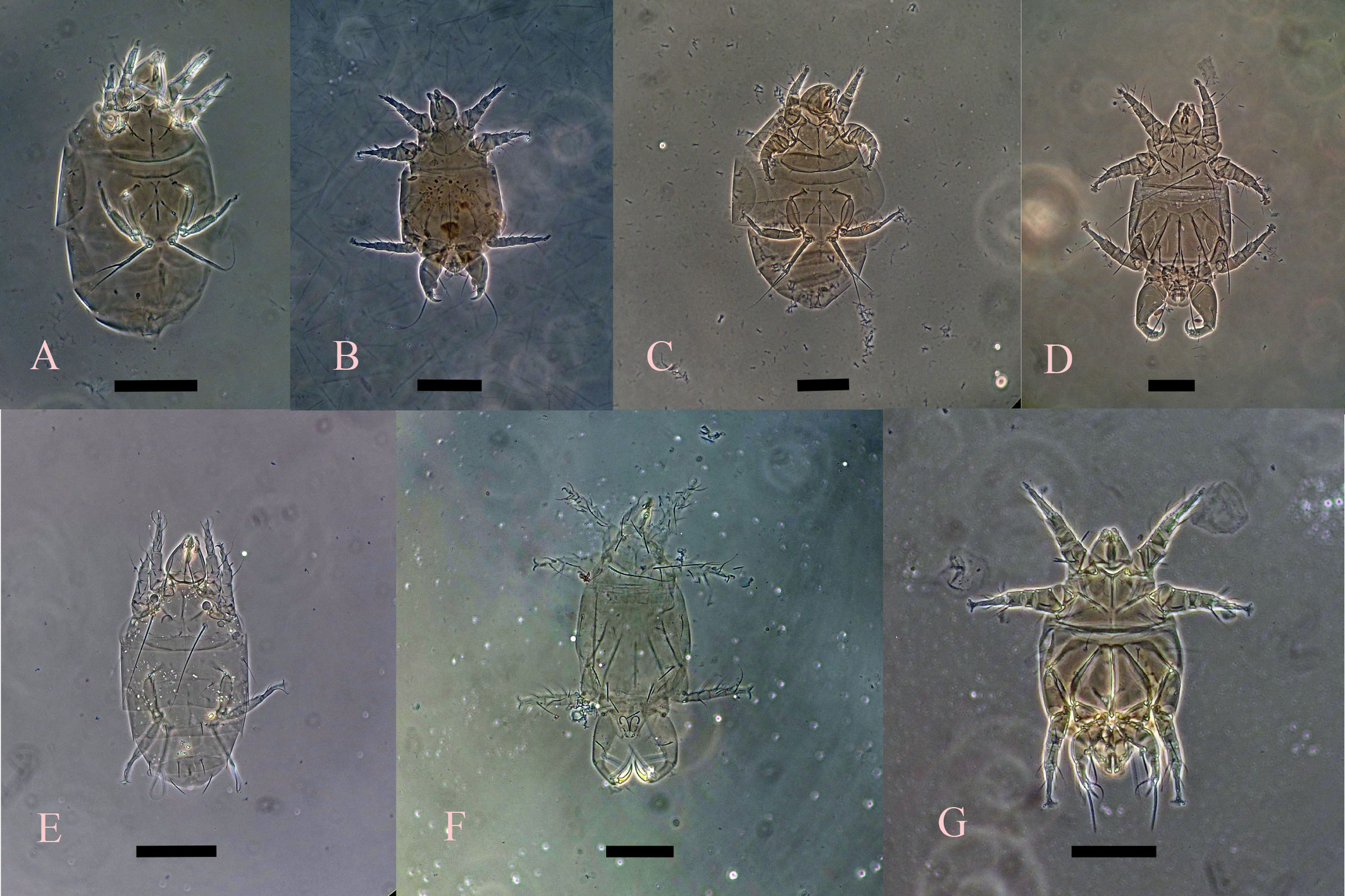


Material examined — four males, four females, fungal culture in the laboratory of the Tropical Fruit Growing Institute, Havana, 4 Jan. 2011, collector: Israel Cabrera, CPQL No. 1843-1849; 11 females, four males, dust in the CPQL laboratory, 4 Jul. 2016, collector: Pedro de la Torre, CPQL No. 2190-2193; six females, sugar, CAI Melanio Hernández, Taguasco, Santi Spíritus, 12 Dec. 2016, collector: Tomás J. Gómez, CPQL No. 1943-1946; four females, organic matter, Puerto de Cienfuegos, 26 May. 2017, collector: Pedro de la Torre, CPQL No. 2026.
Previous distribution — Germany, Belgium, China, Costa Rica, USA, India, Japan, Poland, Russia, Ukraine and UK (Lin & Zhang, 2002), Iran (Gheblealivand et al., 2018)
Remarks — Diagnostic characters are consistent with literature. It is a common contaminant in the laboratory.
Tarsonemus smithi Ewing, 1939
Tarsonemus smithi Ewing, 1939, 18
Tarsonemus femoralis Ewing, 1939, 38
Tarsonemus biungulatus Ewing, 1939, 50
(Fig. 5 C-D)
Material examined — One male, Waltheria indica L., Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 1 Mar. 2002, collector: Pedro de la Torre, CPQL No. 1554; two females, five males, Heliotropium sp., Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 26 Mar. 2002, collector: Pedro de la Torre, CPQL No. 1370, 1371, 1555-1558; one female, two males, Amaranthus spinosus L. Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 26 Mar. 2002, collector: Pedro de la Torre, CPQL No. 1373, 1553.
Previous distribution — China, Korea, USA, Italy, Japan, Poland, Portugal, Ukraine (Lin & Zhang, 2002).
Remarks — Diagnostic characters agree with those in the literature. Found associated with the inflorescences of plants
Tarsonemus waitei Banks, 1912
Tarsonemus waitei Banks, 1912, 98
Tarsonemus selifer Ewing, 1939, 19
Tarsonemus pauperoseatus Suski, 1967, 267
(Fig. 5 E-F)
Material examined — three females, Waltheria indica L., San Martín cave, Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 10 Feb. 2001, collector: Pedro de la Torre, CPQL No. 1465-1466; four females, five males, Poaceae, Punta de Maisí, Guantánamo, 22 Jun. 2003, collector: Pedro de la Torre, CPQL No. 1467, 1479-1483; one female, Sesuvium portulacastrum L., Playa, Havana, 14 Ago. 2003, collector: Pedro de la Torre, CPQL No. 1468; one female, Poaceae, Rincon de Guanabo, Havana, 23 Jan. 2005, collector: Pedro de la Torre, CPQL No. 1470; one female, Cynodon dactylon (L.) Pers., Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 27 Feb. 2006, collector: Pedro de la Torre, CPQL No. 1471; one female, Bidens pilosa; L., Isla de la Juventud, 29 Jun. 2005, collector: Pedro de la Torre, CPQL No. 1472; three females, Waltheria indica L., Boca de Jaruco, Santa Cruz del Norte, Mayabeque, 13 Mar. 2001, collector: Pedro de la Torre, CPQL No. 1473-1474; two females, one male, Poaceae, Punta de Maisí, Guantánamo, 1 May. 2002, collector: Pedro de la Torre, CPQL No. 1475-1477; one female, one male, Vitis vinífera L., Sancti Spíritus, 1 Oct. 2002, collector: Marisel Santos, CPQL No. 1478; four females, Waltheria indica L., Santa Cruz del Norte, Mayabeque, 9 Mar. 2010, collector: Pedro de la Torre, CPQL No. 1857-1858.
Previous distribution — Brazil, China, Congo, Korea, Costa Rica, USA, Egypt, Japan, New Zealand, Poland, Portugal, Russia, Ukraine, (Lin & Zhang, 2002), Iran, (Gheblealivand et al., 2018)
Remarks — Diagnostic characters agree with the literature. Found associated with the inflorescences of plants
Xenotarsonemus cadeae Cromroy, 1958
Xenotarsonemus cadeae Cromroy, 1958, 115
(Fig. 5 G)
Material examined — three females, six males, Thelypteris sp., Río Santa Cruz, Pinar del Río, 26 Jul. 2001, collector: Pedro de la Torre, CPQL N°2680, 2682-2684, 2689,2690; one male, Eichornia azurea (Kunth) Solms, Presa Ejército Rebelde, Havana,17 Nov. 2016, collector: Pedro de la Torre, CPQL No. 2691.
Previous distribution — Puerto Rico (Lin & Zhang, 2002)
Remarks — Diagnostic characters agree with the literature. Dimensions of the main body structures of both sexes are provided due to the limited data available in the literature on this species:
Females (2 specimens measured): Idiosoma length 147 (141-153); idiosoma width 98 (93-103); v1 21 (21); sc2 28 (26-30); c1 13 (13-14); c2 22 (22-23); d 13 (12-14); e 11 (11-12); f 13 (13); h 15 (15); 1a 8 (8); 2a 10 (10); 3a 11 (9-13); 3b 9 (8-10); ps 6 (6); ω tarsus I 8 (8); ω tarsus II 5 (5-6); setae tc″ tarsus IV 35 (35); Φ1 Tibia I 3 (3); Φ 2 Tibia I 3 (3); k Tibia I 5 (5); leg IV: setae v′ti 28 (27-29); setae v′F 9 (9); setae v′G , 14 (14-15).
Males (7 specimens measured): length idiosoma 110 (100-116); idiosoma width 80 (76-86); v1 20 (19-21); v2 11 (10-12); sc1 28 (25-30); sc2 28 (26-32); c1 20 (15-22); c2 23 (21-26); d 34 (31-37); e 11 (11-12); h 1 (1-2); 1a 5 (4-6); 2a 6 (5-7); 3a 6 (14-20); 3b 15 (14-16); ω tarsus I 8 (6-10); ω tarsus II 8 (8-10); Φ1 Tibia I 3 (3-4); Φ 2 Tibia I 5 (4-6); k Tibia I 4 (4-5); leg IV: setae v′ti 31 (30-32); setae v′F 8 (7-8); setae v′G 23 ; (22-26); setae l» 7 (6-8); Φ Tibia 11 (10-15).
In the CPQL Acarology collection there are more specimens of Tarsonemidae awaiting correct identification. Some species may be new to science, so much more effort is needed in collecting and taxonomic work. Mainly the genera Fungitarsonemus and Tarsonemus need to be studied due to their wide biodiversity in the country. In addition, it would allow a better management strategy for possible pests such as in the case of S. comosus in pineapple. It is likely that the distribution of the species mentioned in this work is even wider in Central America and the Caribbean than known today. With this publication, the fauna of Tarsonemidae in Cuba rises to 19 species. This is a starting point for further taxonomic studies of this interesting family
Acknowledgements
The author is grateful to José M. Rezende, Institute of Biosciences, Humanities and Exact Sciences (IBILCE), Brazil for providing photos of Fungitarsonemus lodici; To Dr. Alexander Khaustov of Tyumen State University, Tyumen, Russia; Dr. Antonio Carlos Lofego of the Institute of Biosciences, Humanities and Exact Sciences (IBILCE), Brazil and to Dr. Ronald Ochoa Systematic Entomology Laboratory, USDA, USA for the literature provided.
References
- Aguilar-Piedra H., Solano-Guevara A.M., Seeman O.D., Ochoa R. 2021. Steneotarsonemus ananas (Acari Tarsonemidae) a complementary description from Australian pineapple and a new pest on Neoregelia spp. (Bromeliaceae) in Costa Rica. Acarología 61 (4): 802-823. https://doi.org/10.24349/7u12-OKqx
- De Leon D. 1956. Four new Acarina in the family Tarsonemidae. The Fla. Entomologist, 39(3): 105-112. https://doi.org/10.2307/3492424
- Gheblealivand S.S., Haddad I.-N., Magowski W.Ł K., Manzari S.2018.The genus Tarsonemus Canestrini and Fanzago, 1876 (Acari: Heterostigmatina: Tarsonemidae) in East Azerbaijan, Iran, with a description of T. lenticulatus sp. nov. and re-description of T. annotatus Livshits, Mitrofanov and Sharonov, 1979. Zootaxa 4446 (1): 013-038 https://doi.org/10.11646/zootaxa.4446.1.2
- Kaliszewski M. 1993. Key to Palearctic of the Genus Tarsonemus. Acari, Tarsonemidae. Uniwersytet im. Adama Mickiewicza w Poznantu, Seria Zoologia Nr 14, 1-204.
- Lin J.-Z., Zhang Y.-X., Liu H.-G., Pen W.-F., Chen Y.-M. 1995. The list of tarsonemid mites in Fujian province, China (Acari: Acarifornles). J. Fujian Aca. of Agri. Sci., 10(1):44-47.
- Lin J.Z., Zhang Z.Q. 2002. Tarsonemidae of the World (Acari: Prostigmata): Key to Genera, Geographical Distribution, Systematic Catalogue and Annotated Bibliography. Sys. and Appl. Acar. Soc., England, 440 pp.
- Lindquist E. E. 1986. The World Genera of Tarsonemidae (Acari: Heterostigmata): A morphological, Phylogenetic, and Systematic Revision, With a Reclassification of Family, Group Taxa in the Heterostigmata. Entomol. Soc. of Can. Memoirs. pp 517. https://doi.org/10.4039/entm118136fv
- Magowski W.L., Di Palma A. Khaustov A.A. 1998. Ununguitarsonemus rarus (Acari: Tarsonemidae), a new species of mite associated with bark beetle from Crimea, Ukraine. Entomologica. 32:139-151.
- Ochoa R., Aguilar H. Vargas C. 1991a. Acaros fitofagos de America Central: Guía Ilustrada. CATIE, Costa Rica. pp.251
- Ochoa R., Smiley R. L., Saunders J.L. 1991b. The Family Tarsonemidae in Costa Rica (Acari: Heterostigmata). Internat. J. Acarol. 17(1): 41-86. https://doi.org/10.1080/01647959108683885
- Perez-Gelabert D. E. 2020. Checklist, Bibliography and Quantitative Data of the Arthropods of Hispaniola. Zootaxa 4749 (1): 001-668 https://doi.org/10.11646/zootaxa.4749.1.1
- Rezende J. M., Bauchan G., Lin J-Z., Ochoa R., Lofego A.C. 2024.Review of the genus Daidalotarsonemus De Leon (Acari: Prostigmata. Tarsonemidae) Zootaxa 5426. 1-170. https://doi.org/10.11646/zootaxa.5426.1.1
- Suski Z.W. 1967. Tarsonemid mites on apple trees in Poland. IX. Tarsonemus pauperoseatus n. sp. (Acarina, Heterostigmata). Bull. of the Polish Aca. of Scis. 15:267-272.
- Torre P.E. de la. 2016. Datos preliminares sobre la diversidad de la Familia Tarsonemidae (Acari: Heterostigmata: Tarsonemoidea) en Cuba. In: Rueda-Ramírez D., Carrillo D, Moraes G.J., Florez A.E., Combita-Heredia J.O. (Eds.). Proceedings II Congreso Latinoamericano de Acarología. Vol. 2. Soc. Latin. de Acarol., Colombia. pp 73.
- Torre P.E. de la, Cuervo N. 2019. Actualización de la lista de ácaros (Arachnida: Acari) de Cuba. Rev. Ibérica de Aracnol. 34: 102-118



2025-04-01
Date accepted:
2025-10-29
Date published:
2025-11-05
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 de la Torre Santana, Pedro Enrique
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



