Biology of Oligonychus yothersi (McGregor) (Acari: Tetranychidae) on three host plants
Punina, Erika  1
; Vásquez, Carlos
1
; Vásquez, Carlos  2
; Colmenarez, Yelitza C.
2
; Colmenarez, Yelitza C.  3
; Villa-Murillo, Adriana
3
; Villa-Murillo, Adriana  4
; Núñez, Patricio
4
; Núñez, Patricio  5
and Santana, Rita
5
and Santana, Rita  6
6
1Faculty of Agricultural Sciences, Technical University of Ambato. Cevallos, province of Tungurahua, Ecuador.
2✉ Faculty of Agricultural Sciences, Technical University of Ambato. Cevallos, province of Tungurahua, Ecuador.
3CABI-UNESP-FEPAF, Rúa José Barbosa de Barros, 1780, Botucatú, SP, 18610-307, Brazil.
4Universidad Viña del Mar, Escuela de Ciencias, Viña del Mar, Chile.
5Faculty of Agricultural Sciences, Technical University of Ambato. Cevallos, province of Tungurahua, Ecuador.
6Universidad Nacional de Trujillo. Av. Juna Pablo II S/n. Ciudad Universitaria, Trujillo, Perú.
2025 - Volume: 65 Issue: 4 pages: 1032-1040
https://doi.org/10.24349/nwgg-a8oyOriginal research
Keywords
Abstract
Introduction
The Tetranychidae family, commonly known as spider mites, includes approximately 1,250 species associated with around 3,877 plant species, however, only about 100 of these mite species can cause significant losses in economically important agricultural and ornamental crops (Migeon and Dorkeld 2024). The most significant agricultural pest species belong to the genera Tetranychus, Eutetranychus, Panonychus, and Oligonychus (Devi et al. 2019).
The coffee red spider mite, Oligonychus yothersi (McGregor), is a significant pest in coffee cultivation, it feeds on the upper leaf surface, causing bronzing, reduced photosynthetic efficiency, and potentially premature leaf abscission, with severe infestations reducing photosynthetic capacity by up to 70% (Góngora et al. 2023). However, due to its polyphagous nature and widespread distribution in the Neotropical region, it also feeds on over 70 plant species, including avocado (Persea americana), tea (Camellia sinensis), mango (Mangifera indica), cassava (Manihot esculenta), and ornamental plants (Bolland et al. 1998; Sacoto and Santana 2003; Matioli et al. 2010; Giraldo et al. 2011). It has also been reported in the United States (California, Florida, and Hawaii), China, and Iran (Migeon and Dorkeld 2024).
In Colombia, O. yothersi is a significant pest in coffee crops, causing up to a 30% reduction in photosynthetic activity, premature leaf drop (Bustillo 2008). Additionally, it has also emerged as an important pest in avocado cultivation (Cango et al. 2015). Reyes-Bello and Mesa-Cobo (2011) studied the biology of the mite on mature avocado leaves cv. Lorena, demonstrating its potential as a pest under favourable environmental and host suitability.
Although O. yothersi had been recorded in coffee crops and cherry trees (Prunus salicifolia Kunth) in the Pichincha province, Ecuador, no further report had been made until the pest was found feeding on capuli trees (Prunus serotina Ehrh.) in Cevallos and Pelileo municipalities (Vásquez and Dávila 2018).
In Ecuador, ornamental plant production is one of the most important horticultural activities, reaching about 9,316 hectares of flowers in 2019, of which 77.39% corresponds to Hypericum, Gypsophila, Aster, and rose cultivation. The latter represents 62.09% of the total flowers cultivated, with a national production of 3,346 million cut stems (Rivero Guerra 2021). Moreover, the highland regions of Ecuador contribute 21.7% of the current national demand for apples, 40.2% for pears, 55% for peaches, and 92% for plums (Viera et al. 2020), therefore, these are considered crops of economic importance.
Given the wide range of host plant species for O. yothersi, there is a potential risk that various crops could be attacked by the mite species in the Ecuadorian highlands. Therefore, the objective of this study was to evaluate the effect of the host plant on the biology and life tables of O. yothersi on three economically important crops (rose, apple, and peach) cultivated in Tungurahua province.
Materials and methods
Field collection, identification and mite rearing
Mites were collected from capuli trees (P. serotina) at the Querochaca Experimental Farm of the Faculty of Agricultural Sciences at the Technical University of Ambato, Cevallos, Ecuador (1°21′59″S; 78°36′29″W, 2884 meters above sea level). Leaves showing symptoms of tetranychid feeding and colony presence were taken, placed on towel paper, and stored in plastic bags before being transported to the Entomology Laboratory. Mites were reared on capuli seedlings for three months (several generations) before the beginning of experiments.
In the laboratory, leaf samples were examined under a stereoscopic microscope, and male and female specimens were mounted on slides for microscopic observation using Hoyer's medium and dried in an oven at 40 °C for 3-4 days. The genus was identified using the taxonomic key by Gutierrez (1985), and the species was identified by comparing the morphology of the aedeagus (Ochoa et al. 1994).
Biology of Oligonychus yothersi
An age cohort was obtained following the procedure used by Helle et al. (1985), which consisted of rearing units set up in Petri dishes (diameters: 9 cm). Each rearing unit consisted of a leaf disk (3 cm diameter) obtained from the middle region of a mature leaf of the plant to be tested (peach, apple and rose), placed with the lower surface up onto a polyurethane layer maintained continuously wet by the daily addition of distilled water. Thirty replicates for each treatment were used. An O. yothersi adult female was transferred to each unit. At 12-h interval, leaf disks were examined to determine when oviposition took place. The adult female and the excess eggs in each unit were discarded, leaving only one egg per leaf disk.
The units continued to be examined at 12-h intervals to determine the duration of each developmental stage (egg incubation, mobile immature, and quiescent stages) and also the survival rate. Each 3–4 days, leaf disks were replaced by new disks to ensure a physiologically adequate rearing substrate throughout the work.
Furthermore, the preoviposition, oviposition, and postoviposition periods, total fecundity, daily oviposition rate, and female longevity were determined for each host plant. For this purpose, a single newly emerged female mite was paired with one adult male mite on a 3-cm diameter leaf disc from each plant species. Each leaf disc was placed individually under controlled conditions as previously described. A total of 30 replicates were conducted for each treatment.
The experiment was conducted using a Completely Randomized Design. To evaluate effect of host plant on life cycle span a one-way Analysis of Variance (ANOVA) was performed. Prior to conducting the ANOVA, the assumptions of normality and homogeneity of variances were assessed by using the Shapiro–Wilk test Levene's test, respectively. The variables pre-oviposition period, oviposition period, and fecundity were transformed using y=√(x+0.5) to satisfy the assumptions of normality and homogeneity of variance. Upon finding significant differences, the means of each treatment were compared using Tukey's multiple comparison test (p>0.01). Statistical analyses were conducted using Statistix software for Windows version 10.0.
The life table of O. yothersi was constructed based on daily age-specific survival rates and age-specific fecundity, following the methodology described by Birch (1948). The calculated parameters included: the net reproductive rate (R0=∑lx*mx), which represents the average number of daughters produced per female over her lifetime; the intrinsic rate of increase (rm= Σe-r(x+1) (lx mx)=1), defined as the maximum exponential growth rate of the population, expressed in females per female per day; the finite rate of increase (λ=erm), indicating the population's growth multiplier per female per unit of time; and the mean generation time (T = (ln R0)/rm), which is the average time between the birth of individuals and the birth of their offspring. Bootstraping tests were performed to determine differences between plant species. Using the original samples (host plants), new pseudo-samples were generated to create subgroups of similar size to the original samples, and statistical differences were then calculated. The means and standard errors of the population parameters between plant species were analyzed by bootstrapping tests via 1,000 bootstraps with replacement (Ullah et al., 2020) using the R language (version 4.1.2) with the tidyverse, rsample, and boot libraries
Results
The life cycle of O. yothersi showed significant differences depending on the host plant species. Oligonychus yothersi developed faster on peach leaves, while on apple and rose leaves, the development time was 4.3% and 5.9% slower, respectively, with no significant differences between them (Table 1). No differences were observed in the duration of the larval and protonymph stages, which lasted between 1.86 and 2.06 days and 1.13 and 1.06 days on apple and peach leaf discs, respectively. Conversely, the egg and deutonymph stages, as well as the immobile stages (protochrysalis, deutochrysalis, and teliochrysalis), showed differences depending on the host plant.
Download as Means within a row followed by the same letter are not significantly different (Tukey’s test: P <0,01).
Peach (n= 30)
Apple (n= 30)
Rose (n= 30)
F; df and p-value
Egg
7.93 ± 0.258 b
8.66 ± 0.488 a
8.26 ± 0.458 ab
F=11.8; df= 2; p= 0.0001
Larva
2.06 ± 0.258
1.86 ± 0.352
1.86 ± 0.352
F=1.91; df= 2; p=0.1609
Protochrysalis
1.06 ± 0.258 b
1.33 ± 0.488 ab
1.73 ± 0.458 a
F=9.85; df= 2; p=0.0003
Protonymph
1.06 ± 0.258
1.13 ± 0.352
1.06 ± 0.258
F=0.26; df= 2; p= 0.7728
Deutochrysalis
1.80 ± 0.414 a
1.73 ± 0.458 a
1.13 ± 0.352 b
F=12.02; df= 2; p= 0.0001
Deutonymph
1.33 ± 0.488 c
2.00 ± 0.000 b
2.933 ± 0.258 a
F=95.37; df= 2; p= 0.0000
Teliochrysalis
1.80 ± 0.414 a
1.06 ± 0.258 b
1.06 ± 0.258 b
F= 26.47; df= 2; p= 0.0000
Total (egg-adult)
17.06 ± 0.457 b
17.80 ± 0.560 a
18.07 ± 0.258 a
F= 16.11; df= 2; p= 0.0000
The preoviposition, oviposition, and postoviposition periods in O. yothersi females varied with the host plant species. The preoviposition period was significantly shorter in females reared on peach leaves, while on apple and rose, it was 62.4% and 77.66% longer, respectively (Table 2). The longest oviposition and postoviposition periods were observed in females reared on peach leaves, being 25.3% and 52.9% longer than on apple and 60.7% and 75.0% longer than on rose, respectively (Table 2).
Download as Means within a row followed by the same letter are not significantly different (Tukey’s test: P <0,01).
Peach (n=30)
Apple (n=28)
Rose (n=24)
F; df and p-value
Preoviposition (days)
1.00 ± 0.000a
2.66 ± 0.488b
2.60 ± 0.737b
F= 63.51; df= 2; p=0.0000
Oviposition
19.00 ± 0.926a
14.20 ± 6.394a
7.46 ± 5.680b
F=13.74; df= 2; p= 0.0001
Postoviposition
4.80 ± 1.900a
2.26 ± 1.624b
1.20 ± 2.426b
F=9.52; df= 2; p= 0.0004
Longevity
24.80 ± 2.110a
19.07 ± 7.84a
11.47 ± 7.68b
F=17.91; df= 2; p= 0.0001
Fecundity (number of eggs)
69.46 ± 12.047a
27.40 ± 15.305b
14.86 ± 16.431b
F= 48.57; df= 2; p= 0.0001
The fecundity of O. yothersi females was 60.6% and 78.6% higher on peach compared to the fecundity observed on apple and rose, respectively. Regarding longevity, females lived 1.3 times longer when reared on peach leaves compared to apple leaves and 2.2 times longer compared to rose (Table 2).
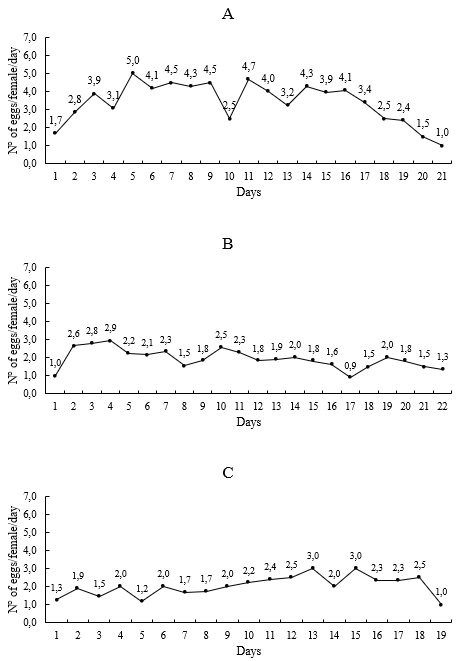


Additionally, when the daily oviposition rate was analysed, oviposition on peach was sustained until day 21, with average values between 1.7 and 5.0 during the first five days when the maximum daily oviposition value (5 eggs/female) was reached. Subsequently, it began to decline, fluctuating between 2.5 and 4.7 eggs/female/day from day 6 to day 17, after which it tended to decrease to values of 1 egg/female/day (Figure 1A). On apple and rose leaves, the oviposition curve was lower compared to peach, with the maximum number of eggs being 2.9 and 3 eggs/female/day, respectively (Figure 1B-C).
As shown in Figure 2, the survival rate (lx) of O. yothersi peaked during the first six days on all host plants, then gradually declined to zero between days 19 and 20. The daily production of female offspring per female (mx) increased from day 16 on peach, and from day 18 on apple and rose.
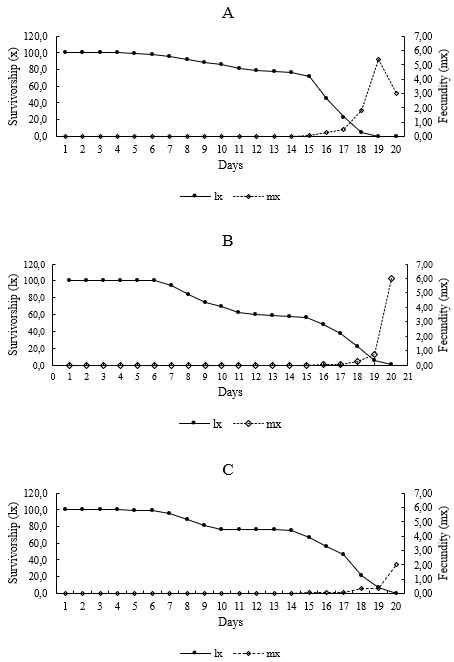


Several life table parameters showed significant differences among the host plant species evaluated (p = 0.001) (Table 3). According to the results, peach provided more favourable conditions for the development and reproduction of O. yothersi, as indicated by the highest net reproductive rate (39.44), compared to apple (15.03) and rose (20.54). In contrast, the longest generation time was recorded on apple leaves (18.58 days), followed by rose (17.34 days), while the shortest was on peach leaves (16.90 days). No significant differences were observed in the intrinsic rate of increase or the finite rate of population growth among the three host plant species.
Download as
Host Plant
Net Reproductive Rate (Ro)
Mean Generation Time (T) (days)
Intrinsic Rate of Increase (rm)
Finite Rate of Growth (λ)
Peach
39.44 a
16.90 a
0.04 a
1.01 a
Apple
15.03 c
18.58 c
0.02 a
1.01 a
Rose
20.54 b
17.34 b
0.03 a
1.01 a
Discussion
Variations due to host plant effect were observed in life time of O. yothersi. According to Beckerman et al. (2006) environmental conditions in which the progenitors grew, including the host plant can account for this differences. Observed results differed from those obtained by Alves et al. (2004), who reported an average duration of the life cycle of O. yothersi of 14 days when reared on Ilex paraguensis under laboratory conditions at 25 °C. Similarly, Reyes-Bello and Mesa-Cobo (2011) showed a duration of 14.34 days on avocado cultivars as a host plant at 26 °C and 56% RH. The observed differences could be due to the effects of both the host plant and temperature, as these factors can affect the duration of the biological cycle (Chaaban et al., 2012; Mitra et al., 2021; Praslička and Huszár, 2018). According to Rebaudo and Rabhi (2018), although temperature is the primary determinant factor for the development, survival, reproduction, and dispersion of arthropods, other factors, such as photoperiod, food, relative humidity, population density, and their interactions, also have significant influence.
Host plant species can affect the development and survival of polyphagous herbivorous arthropods due to their chemical (nutritional quality, allelochemicals) or physical (trichomes, tissue hardness) characteristics, thus for a plant to be considered a suitable host for an herbivorous arthropod, it must support its development (Kalaitzaki et al. 2023). Thus, shorter development times and lower mortality rates in an arthropod indicate the suitability of a plant as a host (Awmack and Leather, 2002). On the other hand, mite survival is linked to the thickness of the leaf parenchyma, as well as the thickness, hardness, and pubescence of the leaf (Paredes 2017), which could influence the development of O. yothersi. Both morphological and chemical traits of plants that serve as defence mechanisms impact the fitness of mites. These traits and mechanisms depend on the genetic makeup of the plant, the species of pest involved, and the interactions between them (Pazmiño et al. 2018; Vásquez et al. 2018).
In regard to the reproductive parameters, previous studies showed a preoviposition period ranging from 1.07 to 1.09 days on avocado leaves for the species (Reyes-Bello and Mesa-Cobo, 2011; Rioja et al., 2019). Additionally, fecundity ranged from 79.93 eggs/female on avocado variety Hass to 14.43 eggs/female on avocado cv. Lorena with minimums of 0 and maximums of 74 eggs, while longevity ranged from 24.23 days to 8.35 days on these avocado cultivars (Reyes-Bello and Mesa-Cobo, 2011; Rioja et al., 2019).
Studies with other Oligonychus species demonstrate the effect of the host plant on reproductive parameters. For instance, both fecundity and longevity of O. afrasiaticus females were significantly higher when reared on date palm cv. Bartamoda (27.8 eggs/female and 14.18 days) compared to the cultivar Zaghlol (15.94 eggs/female and 10.0 days) (Elhalawany et al. 2020).
It is well known that the host plant species possess different mechanisms of defence that can differentially affect the biological characteristics of agricultural pests, such as population growth, reproductive potential, and the severity of pest damage (Gerson and Aronowitz, 1980; Złotkowska et al., 2024). Thus, mite oviposition is a criterion used to measure the adaptability of their populations to a plant, which depends on the induction or suppression of plant defences (Sarmento et al. 2011; Dias et al. 2023). Therefore, adult females must select oviposition sites that provide high-quality resources that confer benefits for the development of their offspring (Yin et al. 2022).
Although this study did not evaluate the leaf characteristics, previous studies demonstrate that both the chemical and morphological features of the leaf can affect the biological attributes of the pest. On one hand, the presence of trichomes can provide a microenvironment, in terms of temperature, humidity, and air movement, that is more suitable for mite feeding and oviposition (Reddall et al. 2011). On the other hand, plants have a wide variety of secondary metabolites that play an important role in plant defence against herbivores (Yamane et al. 2010; Ali et al. 2024).
Conclusion
Biological parameters suggest that peach leaves offer better conditions for the development of O. yothersi, potentially serving as a source of infestation and dispersion, given their common use as commercial cultivars for peach production in the Andean region of Ecuador. Therefore, some strategies may become necessary to control O. yothersi in peach crops.
Acknowledgments
The authors would like to thank the Dirección de Investigación y Desarrollo (DIDE), Technical University of Ambato, for partially financing.
Declaration of interest statement
No potential conflict of interest was reported by the author(s).
References
- Ali J, Tonğa A, Islam T, Mir S, Mukarram M, Konôpková AS, Chen R. 2024. Defense strategies and associated phytohormonal regulation in Brassica plants in response to chewing and sap-sucking insects. Front Plant Sci., 15:1376917. https://doi.org/10.3389/fpls.2024.1376917
- Alves LFA, Spongoski S, da S. Vieira FN, de Moraes G. 2004. Biologia e danos de Oligonychus yothersi (Mcgregor) (Acari\,: Tetranychidae) em Ilex paraguariensis. Arq Inst Biol (Sao Paulo), 71(2):211-214. https://doi.org/10.1590/1808-1657v71p2112004
- Awmack CS, Leather SR. 2002. Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol., 47(1):817-844. https://doi.org/10.1146/annurev.ento.47.091201.145300
- Beckerman AP, Benton TG, Lapsley CT, Koesters N. 2006. How effective are maternal effects at having effects? Proc R Soc B Biol Sci., 273(1585):485-493. 3315. https://doi.org/10.1098/rspb.2005.3315
- Birch LC. 1948. The Intrinsic Rate of Natural Increase of an Insect Population. J Anim Ecol., 17(1):15-26. https://doi.org/10.2307/1605
- Bolland HR, Gutierrez J, Flechtmann CHW. 1998. World Catalogue of the Spider Mite Family (Acari: Tetranychidae). Leiden, Tlle Netherlands: Koninklijke Brill NV.
- Bustillo AE. 2008. La arañita roja, Oligonychus yothersi; Insectos chupadores en los cafetales; Cochinillas harinosas en cafetales colombianos. In: Bustillo AE, editor. Los insectos y su manejo en la caficultura colombiana. Cenicafe.
- Cango MN, Cabrejo CEV, Quispe RAQ, Cornejo RMB, Castro EGV. 2015. Distribución poblacional de la arañita roja Oligonychus sp. (Acari: Tetranychidae), sobre árboles del palto (Persea americana Miller) en Lima, Perú. In: VIII Congreso Mundial de la Palta. Lima, Perú. p. 198-202.
- Chaaban S, Chermiti B, Kreiter S. 2012. Effects of host plants on distribution, abundance, developmental time and life table parameters of Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae). Pap Avulsos Zool., 52(10):121-133. https://doi.org/10.1590/S0031-10492012001000001
- Devi M, Challa N, Mahesh G. 2019. Important mite pests of temperate and sub- tropical crops: A review. J Entomol Zool Stud., 7:1378-1384.
- Dias CR, Cardoso AC, Kant MR, Mencalha J, Bernardo AMG, da Silveira MCAC, Sarmento RA, Venzon M, Pallini A, Janssen A. 2023. Plant defences and spider-mite web affect host plant choice and performance of the whitefly Bemisia tabaci. J Pest Sci (2004)., 96(2):499-508. https://doi.org/10.1007/s10340-022-01516-1
- Elhalawany A, Ahmad N, Amer A. 2020. Biological aspects of date palm dust mite, Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae) on fronds of three Date palm cultivars. Egypt Acad J Biol Sci A, Entomol., 13(1):89-98.
- Gerson U, Aronowitz A. 1980. Feeding of the Carmine Spider Mite on Seven Host Plant Species. Entomol Exp Appl., 28(2):109-115. https://doi.org/10.1111/j.1570-7458.1980.tb02995.x
- Giraldo M, Galindo L, Benavides P. 2011. La arañita roja del Café. Av Tec Cenicafé.(403):8.
- Góngora CE, Gil ZN, Constantino LM, Benavides P. 2023. Sustainable strategies for the control of pests in coffee crops. Agronomy, 13:2940. https://doi.org/10.3390/agronomy13122940
- Gutierrez J. 1985. Systematics. In: Helle W, Sabelis M. (Eds). Spider Mites: their biology, natural enemies and control. Amsterdam: Elsevier Science. p. 75-90.
- Helle W, Sabelis MW, Spider E. 1985. Rearing techniques. In: Hellen W, Sabelis MW. (Eds). Rearing techniques Spider Mites. Their Biology, Natural Enemies and Control. Volume 1A. Amsterdam: Amsterdam: Elsevier. p. 331-335.
- Kalaitzaki A, Amara A, Dervisoglou S, Perdikis D, Τzοbanoglou D, Koufakis I, Tsagkarakis Α. 2023. Effect of host plant species and temperature on the development and survival of the plant bug Closterotomus trivialis (Costa) (Hemiptera: Miridae). Phytoparasitica, 51(1):19-28. https://doi.org/10.1007/s12600-022-01030-1
- Matioli A, Gomes J, Sato M. 2010. Euseius citrifolius and Galendromus annectens (Acari: Phytoseiidae) preying upon Oligonychus santoantoniensis (Acari: Tetranychidae) on Lagerstroemia indica L. In: de Moraes GJ, Caastzilho R, Flechtmann CHW, editors. XIII International Congress of Acarology. Recife: Taylor & Francis. p. 148.
- Migeon A, Dorkeld F. 2024. Spider Mites Web: a comprehensive database for the Tetranychidae. Spider Mite Web. http://www1.montpellier.inra.fr/CBGP/spmweb.
- Mitra S, Acharya S, Ghosh S. 2021. Implication of five host plants on the life history trait of Tetranychus urticae (Acari: Tetranychidae). Biologia (Bratisl), 76(2):517-524. https://doi.org/10.2478/s11756-020-00547-2
- Ochoa R, Aguilar H, Vargas C. 1994. Phytophagous mites of Central America: An illustrated guide. Turrialba, Costa Rica: CATIE.
- Paredes SN. 2017. Ciclo biológico de Oligonychus coffeae (Acari: Tetranychidae) en aliso (Alnus acuminata) y café (Coffea arabica) y el uso de extractos etanólicos complementarios para su control [Undergraduate thesis]. Ambato: Universidad Técnica de Ambato.
- Pazmiño P, Lema G, Mendoza D, Velástegui G, Vásquez C. 2018. Parámetros biológicos de Tetranychus urticae Koch (Acari: Tetranychidae) alimentados sobre dos cultivares de fresa en Ecuador. Bioagro., 30(3):229-234.
- Praslička J, Huszár J. 2018. Influence of temperature and host plants on the development and fecundity of the spider mite Tetranychus urticae (Acarina: Tetranychidae). Plant Prot Sci. 40(No. 4):141-144. https://doi.org/10.17221/465-PPS
- Rebaudo F, Rabhi VB. 2018. Modeling temperature-dependent development rate and phenology in insects: review of major developments, challenges, and future directions. Entomol Exp Appl., 166(8):607-617. https://doi.org/10.1111/eea.12693
- Reddall AA, Sadras VO, Wilson LJ, Gregg PC. 2011. Contradictions in host plant resistance to pests: Spider mite (Tetranychus urticae Koch) behaviour undermines the potential resistance of smooth-leaved cotton (Gossypium hirsutum L.). Pest Manag Sci., 67(3):360-369. https://doi.org/10.1002/ps.2075
- Reyes-Bello J, Mesa-Cobo N. 2011. Biología de Oligonychus yothersi (McGregor) (Acari: Tetranychidae) sobre aguacate Persea americana Mill. cv. Lorena (Lauraceae). Caldasia, 33(1):211-220.
- Rioja T, Tello V, Zarzar M, Cardemil A, Ceballos R. 2019. Avocado `Hass' leaf age affects life table parameters of Oligonychus yothersi (Mcgregor) (Acari: Tetranychidae) under laboratory conditions. Chil J Agric Res., 79(4):557-564. https://doi.org/10.4067/S0718-58392019000400557
- Rivero Guerra AO. 2021. Caracterización de los viveros de Pelileo, Tunguragua, Ecuador. Rev Investig e Innovación Agropecu y Recur Nat., 8(1):62-82. https://doi.org/10.53287/tpjc7586ek97g
- Sacoto V, Santana H. 2003. Proyecto de produccion de almidón de yuca en la provincia De Los Rios para su exportacion [Undergraduate thesis]. Guayaquil: Escuela Superior Politécnica del Litoral.
- Sarmento RA, Lemos F, Bleeker PM, Schuurink RC, Pallini A, Oliveira MGA, Lima ER, Kant M, Sabelis MW, Janssen A. 2011. A herbivore that manipulates plant defence. Ecol Lett., 14(3):229-236. https://doi.org/10.1111/j.1461-0248.2010.01575.x
- Ullah MS, Kobayashi Y, Gotoh T. 2022. Development and reproductive capacity of the Miyake spider mite Eotetranychus kankitus (Acari: Tetranychidae) at different temperatures. Insects, 13(10):910. https://doi.org/10.3390/insects13100910
- Vásquez C, Dávila M. 2018. Some plant mites (Acari: Tetranychidae: Stigmaeidae) from Province of Tungurahua in Ecuador. Rev Chil Entomol., 44(3):339-345.
- Vásquez C, Pérez M, Dávila M, Mangui J, Telenchana N. 2018. Biological parameters of Tetranychus urticae Koch (Acari: Tetranychidae) on strawberry cultivars in Ecuador. Rev Chil Entomol., 44(3):271-278.
- Viera W, Merino J, Martínez A, Viera A, Rueda S, Ron L. 2020. Determinant aspects of the deciduous fruit production in the province of Tungurahua, Ecuador. Manglar, 17(3):239-246. https://doi.org/10.17268/manglar.2020.035
- Yamane H, Konno K, Sabelis M, Takabayashi J, Sassa T, Oikawa H. 2010. Chemical defence and toxins of plants. In: Liu H-W, Mander L, editors. Comprehensive Natural Products II: Chemistry and Biology. Vol. 4. Elsevier Science Publishers. p. 339-385. https://doi.org/10.1016/B978-008045382-8.00099-X
- Yin W, Hoffmann AA, Bai C, Ma C Sen. 2022. A conservative oviposition preference in spider mites for complex habitats as a preventive strategy for reducing predation risk. Entomol Gen., 42(3):389-401. https://doi.org/10.1127/entomologia/2021/1282
- Złotkowska E, Wlazło A, Kiełkiewicz M, Misztal K, Dziosa P, Soja K, Barczak-Brzyżek A, Filipecki M. 2024. Automated imaging coupled with AI-powered analysis accelerates the assessment of plant resistance to Tetranychus urticae. Sci Rep., 14(1):1-16. https://doi.org/10.1038/s41598-024-58249-7



2025-03-02
Date accepted:
2025-10-03
Date published:
2025-10-13
Edited by:
Migeon, Alain

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Punina, Erika; Vásquez, Carlos; Colmenarez, Yelitza C.; Villa-Murillo, Adriana; Núñez, Patricio and Santana, Rita
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



