A new feather mite species of the genus Ingrassia (Acariformes: Xolalgidae) from the Jacksnipe Lymnocryptes minimus (Charadriiformes: Scolopacidae) in Faroe Islands
Mironov, Sergey V  1
and Haarder, Simon
1
and Haarder, Simon  2
2
1✉ Zoological Institute, Russian Academy of Sciences, Universitetskaya embankment 1, 199034, Saint Petersburg, Russia.
2Independent researcher, Ornebjergvej 43, 4760 Vordingborg, Denmark.
2025 - Volume: 65 Issue: 4 pages: 1018-1031
https://doi.org/10.24349/xegi-38o4ZooBank LSID: AF644823-CDB7-4BE1-AC63-72288DBC7D4E
Original research
Keywords
Abstract
Introduction
The feather mite genus Ingrassia Oudemans, 1905 (Analgoidea: Xolalgidae), currently including 30 species, and is the most specious genus in the subfamily Ingrassiinae (Oudemans 1905; Gaud 1972a; Gaud and Atyeo 1981; Chirov and Mironov 1990; Dabert and Ehrnsberger 1991; Vasyukova and Mironov 1991; Dabert 2000; Mironov and Palma 2006; Mironov and Proctor 2008; Stefan et al. 2013; Han et al. 2021; Mironov et al. 2025). Representatives of this genus are mostly small feather mites with the body size of adults 300-400 μm and with weakly sclerotized cuticular covers. In the plumage of avian hosts, mites of this genus, as for all xolalgids, inhabit the downy feathers and body covert feathers, where the barbs are not hooked together forming dense vanes.
Of 16 genera recognized in the subfamily Ingrassiinae (Gaud and Atyeo 1981, 1996; Mironov and Galloway 2002; Dabert et al. 2007), the genus Ingrassia and three more genera, Leptosphyra Hull, 1934, Tectingrassia Gaud, 1972 and Vingrassia Vasyukova & Mironov, 1991, constitute the Ingrassia generic group (Mironov et al. 2025). Representatives of this group are associated with aquatic bird orders. Only mites of the genus Ingrassia are distributed on hosts from five different orders, Charadriiformes, Phaethontiformes, Podicipediformes, Procellariiformes and Sphenisciformes (Table 1), while other genera are restricted to particular bird orders. Mites of the genera Leptosphyra and Tectingrassia occur on waders, or shorebirds (Charadriiformes: Charadrii), and representatives of the genus Vingrassia—on ducks and geese (Anseriformes). Key to species of these genera have been constructed only for species associated with charadriiform birds from Africa and Eurasia (Gaud 1972a; Chirov and Mironov 1990; Vasyukova and Mironov 1991); descriptions of species from other bird orders are scattered across various taxonomic publications (Vitzthum 1921; Černý 1967; Mironov and Proctor 2008; Stefan et al. 2013).
Mironov et al. (2025) recently provided the main diagnostic characteristics, world checklist and type hosts for all species of the Ingrassia generic group. This paper also noticed that the relatively weak differential features of genera and the pattern of host associations give evidence that the Ingrassia generic group certainly needs a revision of diagnostic characters and taxonomic borders of recognized genera (Gaud 1972a; Gaud and Atyeo 1981; Chirov and Mironov 1990). Phylogenetic study of xolalgids based on morphological features supported the monophyly of the group within the subfamily Ingrassiinae, but showed that the genus Ingrassia appears to be paraphyletic (Mironov 2005).
In the present work, we describe a new Ingrassia species found on the Jacksnipe, Lymnocryptes minimus (Brünnich, 1764), from Faroe Islands, Denmark. Additionally, we provide the list of host associations of the genus Ingrassia and compare the feather mite fauna of selected species ofsnipes (tribe Scolopacini).
Material and methods
Mite specimens used in this study were collected by SH from three individuals of the Jacksnipes from Faroe Islands, Denmark. Dead birds were collected by Jens-Kjeld Jensen (Nólsoy, Faroe Islands) and kept in his freezer until sampling of mites and other ectoparasites. Some birds were killed by cats while the cause of death of others was unknown. Wing and body covert feathers were examined individually with a dissection microscope, and recovered mites were placed in tubes with 70% ethanol. Collected mite specimens were mounted on microscope slides in Hoyer's medium according to the standard technique used for small mites (Krantz and Walter 2009).
The species description follows the format used for feather mites of the subfamily Ingrassiinae (Mironov and Palma 2006; Mironov and Proctor 2008; Stefan et al. 2013; Mironov et al. 2017, 2025; Han et al. 2021). General morphological terms, idiosomal and leg chaetotaxies follow Gaud and Atyeo (1981, 1996) with minor corrections for coxal setae by Norton (1998). All the measurements are in micrometers (μm). Primary drawings and measurements were made using a Leica light microscope DM2500 (Leica Microsystems, Inc.) with DIC illumination and camera lucida; and final digital drawings were prepared with a Wacom Cintiq 22 graphics tablet (Wacom Co., Ltd). Systematics and scientific names of birds follow the IOC World Bird List, v 15.1 (Gill et al. 2025). Type material is deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia (ZISP) and in the Department of Ecology and Zoology of the Universidade Federal de Santa Catarina, Florianópolis, Brazil (ECZ–UFSC); additional unmounted specimens are kept in ethanol in the private collection of the junior author and will eventually be deposited in the Acari collection at the National History Museum of Denmark, Copenhagen, Denmark (NHMD).
Systematics
Family Xolagidae Dubinin, 1953
Subfamily Ingrassiinae Gaud & Atyeo, 1981
Genus Ingrassia Oudemans, 1905
Type species: Megninia veligera Oudemans, 1904 by original designation.
Ingrassia lymnocrypti sp. n.
ZOOBANK: 164B46AC-D207-4D2B-967D-286389110B94 ![]()
(Figures 1–4)
Type material
Male holotype (ZISP 23875), 5 male and 6 female paratypes (ZISP 23876–23886) from Lymnocryptes minimus (Brünnich, 1764) (Charadriiformes: Scolpacidae). Denmark, Faroe Islands, Torshavn, 13 September 2019, coll. S. Haarder; other paratypes: 5 male and 5 female paratypes (ZISP 23887–23896), same host, Denmark, Faroe Islands, Stremoy, 31 December 2001, coll. S. Haarder; 6 male and 3 female paratypes (ZISP 23897–23905), same host, Denmark, Faroe Islands, Torshavn, 14 February 2005, coll. S. Haarder.
Type depository
Holotype, 13 male and 11 female paratypes—ZISP, remaining paratypes—ECZ-UFSC.
Description
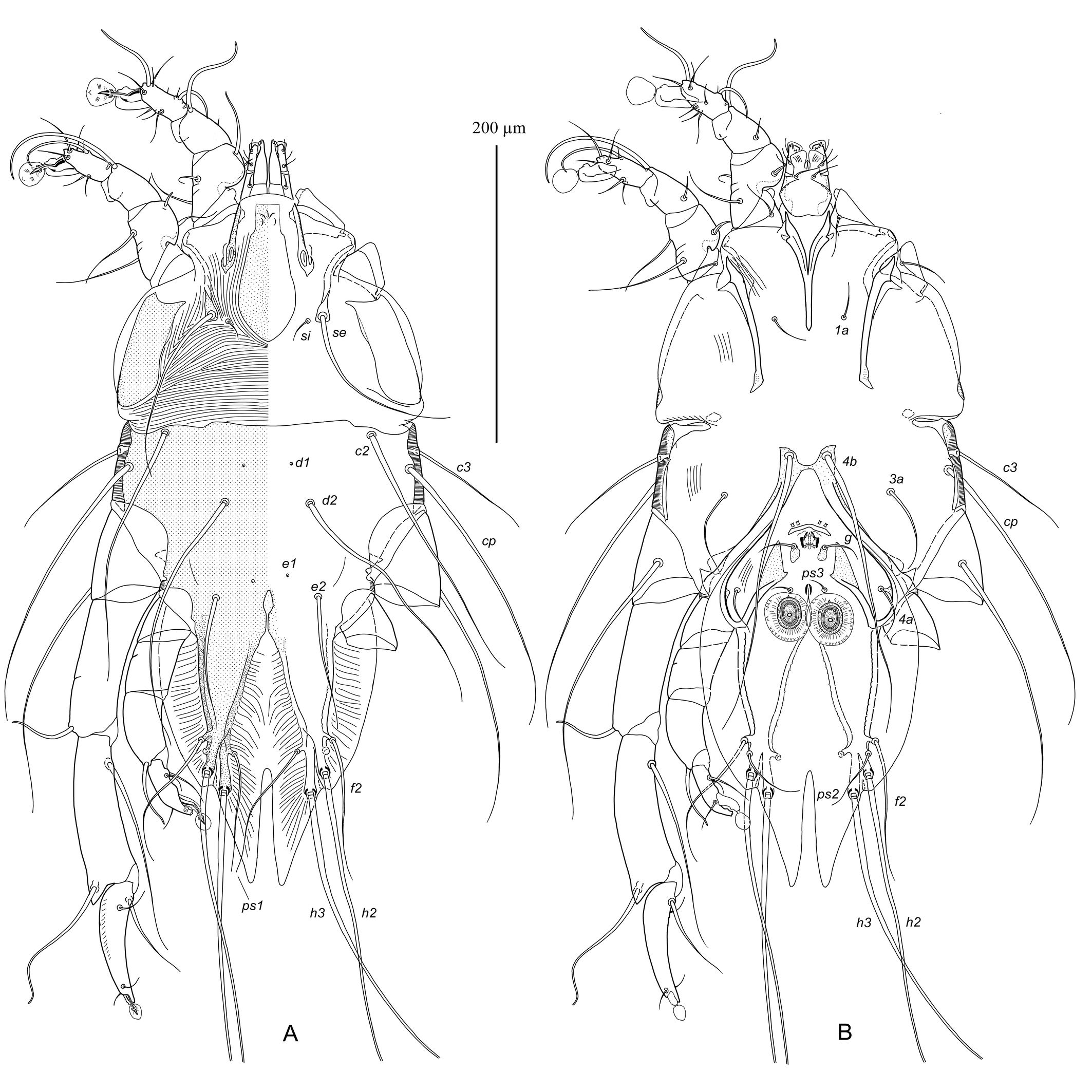


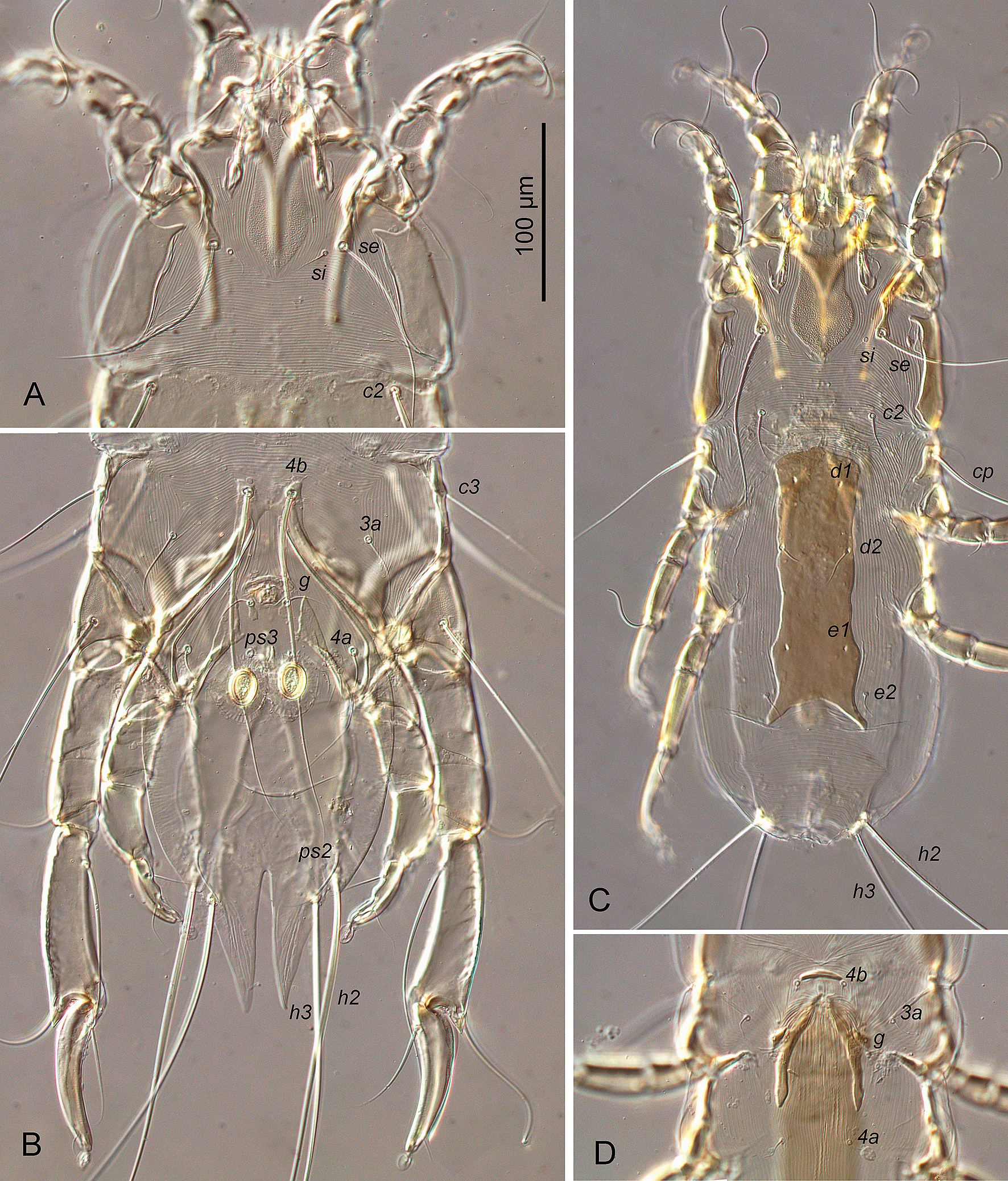
Male — (holotype, range for 10 paratypes in parentheses), (Figures 1, 3A-E, 4A, B). Idiosoma length (from anterior end to bases of setae h3) 410 (395–440), greatest width 200 (200–240), length of hysterosoma 259 (250–280). Prodorsal shield: longitudinal plate gradually enlarging from anterior end to level of scapular setae; narrowed anterior end rectangular, with 3-5 erratically orientated minute ridges; posterior part extending beyond scapular setae angular or semi-ovate, length 90 (88–100), greatest width 38 (35–40) (Figures 1A, 4A). Scapular setae se situated on teardrop-shaped sclerites base of setae se separated by 73 (70–80); setae si on striated tegument slightly posterior to level of setae se. Scapular shields: wide; inner margins of shield oblique, widely rounded posteriorly, without suprategumental extensions. Distance between prodorsal and hysteronotal shields along midline 20 (18–23). Hysteronotal shield: anterior margin slightly concave, length of shield from anterior margin to bases of setae h3 255 (255–285), surface without ornamentation, traces of fusion with humeral shields indistinct. Setae c3 filiform, longer than trochanter III. Setae c2, d2, e2 represented by macrosetae; all extending approximately to midlength of terminal cleft. Setae d1 and e1 posterior to levels of setae c2 and d2, respectively. Supranal concavity narrowly ovate, connected posteriorly with terminal cleft by short and narrow grove. Opisthosomal lobes long, approximately 3–3.5 times longer than wide at base. Terminal cleft long triangular, with anterior end extending to level of trochanters IV, length of the cleft from anterior end to bases of setae h3 110 (110–125), greatest width anterior to setae ps1 50 (50–58). Terminal extensions on interlobar membrane long and narrowly triangular, with pointed apices, length of extensions from bases of setae h3 to apices 60 (55–70); incision between extensions narrow, almost parallel-sided, with rounded anterior end extending to level of setae h2, and 78 (75–88) long. Setae h3 posterior to level of setae h2 and situated on apices of opisthosomal lobes. Setae ps1 and ps2 at the same transverse level anterior to setae h2, setae f2 anterior to both pairs of pseudanal setae. Setae f2 and ps1 extending to apices of terminal extensions, setae ps2 barely extending to midlength of these extensions. Distances between dorsal setae: c2:d2 50 (50–55), d2:e2 60 (60–68), e2:h3 135 (130–155), c2:c2 140 (135–165), h2:h2 78 (75–95), h3:h3 57 (53–63), ps1:ps1 40 (40–48). Lengths of dorsal setae: c2 175 (170–205), c3 85 (85–110), d2 170 (160–185), e2 95 (90–100), f2 90 (90–120), ps1 85 (85–105).
Epimerites I fused in a Y, with stem (sternum) approximately half as long as total length of epimerites I. Epimerites III rudimentary. Inner ends of epimerites IIIa with sclerotized fields connected by narrow sclerotized bridge. Epimerites IVa long, encircling anterolaterally adanal area, inner ends of these epimerites with large triangular sclerotized fields flanking laterally postgenital area (Figures 1B, 4B). Coxal fields IV open anteriorly, central part of coxal fields IV not sclerotized. Genital apparatus small, 10 (10–11) × 12 (12–13); aedeagus minute, barely extending from genital arch. Epiandrum (pregenital apodeme) shaped as low bow, 7 (7–9) long, 28 (28–32) wide. Genital shields represented by small longitudinal plates, with setae g on anterior ends. Adanal shields absent, setae ps3 on soft tegument anterior to adanal suckers. Adanal suckers longitudinally ovate, with small denticle on anterior margin, 24 (24–28) in greater diameter; surrounding membrane with radial striation. Setae 1a short filiform, not extending to level of tips of epimerites II. Setae 3a filiform, barely extending to level of posterior margin of trochanters IV. Setae 4a filiform, situated on striated tegument or on small sclerotized area of coxal field IV. Setae 4b situated on inner ends of epimerites IIIa, represented by macrosetae, and extending beyond midlength of opisthosomal lobes. Setae ps3 and 4a approximately at same transverse level. Distances between ventral setae: 4b:3a 25 (25–35), 4b:g 60 (58–65), g:ps3 28 (25–32), ps3:h3 140 (135–155), 4b:4b 25 (25–28), g:g 20 (20–24), ps3:ps3 23 (23–25). Lengths of ventral setae: 1a 25 (25–35), 3a 60 (58–62), 4a 35 (35–50), 4b 195 (190–210), g 28 (25–30), ps2 50 (48–58).
Tarsi I, II, each with a small nearly rectangular apicodorsal projection (Figure 3A, B). Setae s of tarsus II spiculiform with short filiform apex, remaining ventral setae of tarsi I, II filiform. Tibiae I, II, each with rounded ventral processus; setae gT of tibiae I, II spiculifom. Seta mG of genu I spiniform with filiform apex, seta mG of genu II long filiform. Setae cGI, cGII spiniform basally, with filiform apex. Femoragenu II with dorsal retrograde spine-shaped projection. Tibia III with large angular extension bearing solenidion φ (Figure 3C). Tarsus III 85 (83–98) long, curved, and with small finger-like apical projection. Tarsus IV 43 (38–48) long, with slightly convex inner margin, with slightly curved finger-like apical projection bearing setae d and e; seta w thickened and extending to ambulacral disc; modified setae d, e button-like, with barely distinct central nipple (Figure 3D). Legs IV excluding pretarsus 150 (145–155) long, with distal half of tarsus extending beyond lobar apices (level of setae h3), but not reaching terminal extensions of interlobar membrane. Lengths of solenidia: ω1I 33 (30–35), ω1II 45 (44–48), σI 42 (40–44), σII 8 (7–8), σIII 45 (43–50), φIV 44 (42–50). Ambulacral discs of tarsi III, IV approximately half as long as those on tarsi I, II and more ovate in shape.
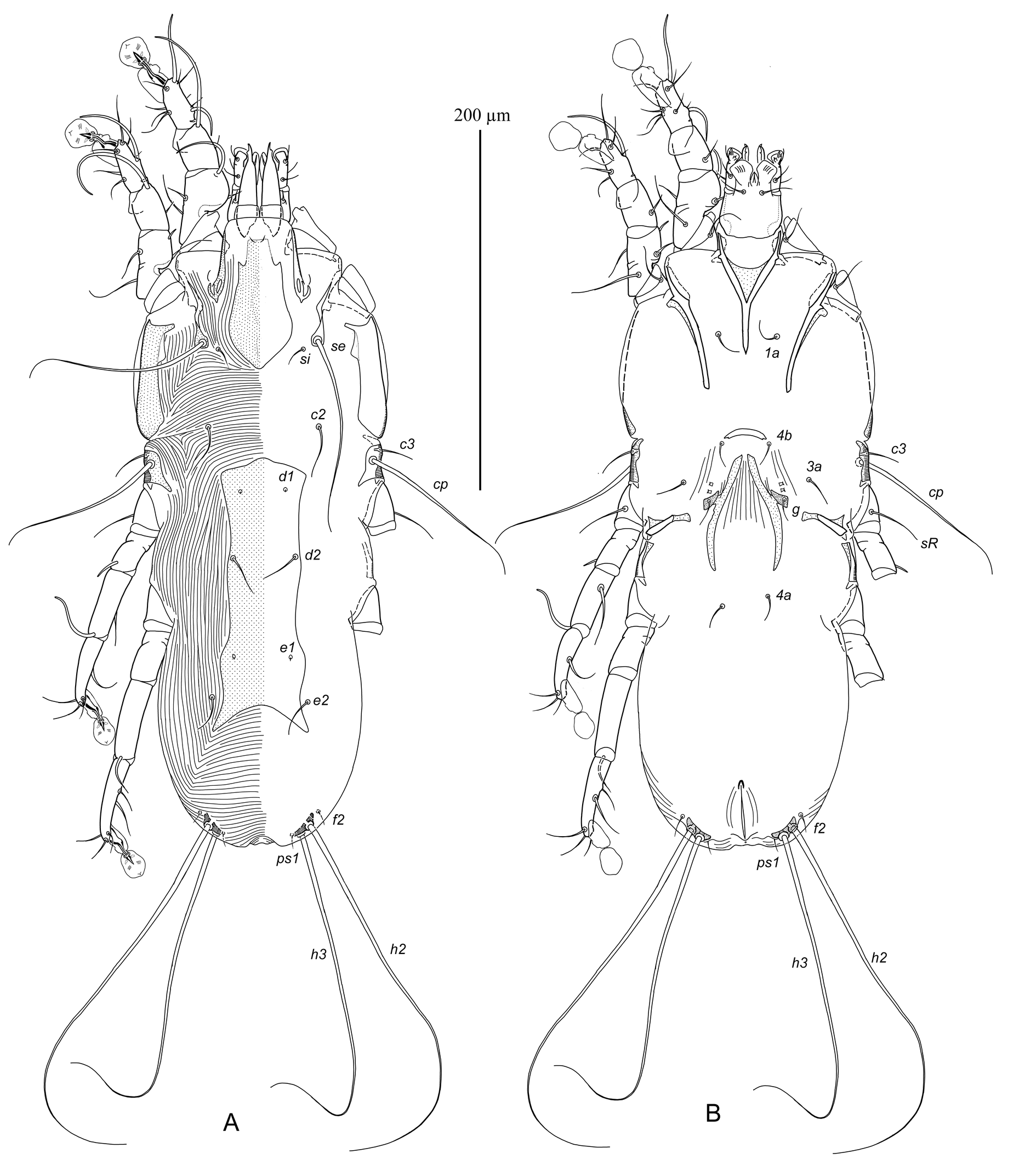


Female — (range for 10 paratypes) (Figures 2, 3F, G; 4C, D). Idiosoma, length × width, 340–375 × 135–150; length of hysterosoma 215–250. Prodorsal shield: generally shaped as in the male, except lateral margins of anterior one third with distinct ledge and anterior end with 2-3 erratically orientated minute ridges; length 75–80, greatest width 36–40. Scapular setae se situated on teardrop-shaped sclerites, bases of setae se separated by 66–70; setae si on striated tegument slightly posterior to level of setae se. Scapular shields: wide; inner margins straight, rounded posteriorly, with suprategumental angular extension at level of anterior one third. Distance between prodorsal and hysteronotal shields along midline 50–54. Hysteronotal shield: shaped as narrow longitudinal plate in median area of hysterosoma, stretching from humeral shields to midlength of opisthosoma; anterior margin convex; lateral margins slightly sinuous; posterior margin deeply concave; posterior corners elongated and pointed, extending beyond level of setae e2; greatest length 155–165, width of anterior part 48–55, width at posterior margin 55–60 (Figures 2A, 4C). Setae c3 filiform, shorter than trochanters III. Setae c2, d2, e2 filiform, equal to or slightly longer than trochanters III; setae c2 and e2 on striated tegument, setae d2 on hysteronotal shield near lateral margins. Microsetae d1 and e1 on hysteronotal shield, posterior to levels of setae c2 and d2, respectively. Macrosetae h3 about 3/4 the length of macrosetae h2. Distances between dorsal setae: c2:d2 75–80, d2:e2 82–90, e2:h3 70–75, c2:d2 62–65, h2:h2 58–64, h3:h3 48–52. Lengths of dorsal setae: c2 25–38, c3 15–20, d2 18–30, e2 17–20.
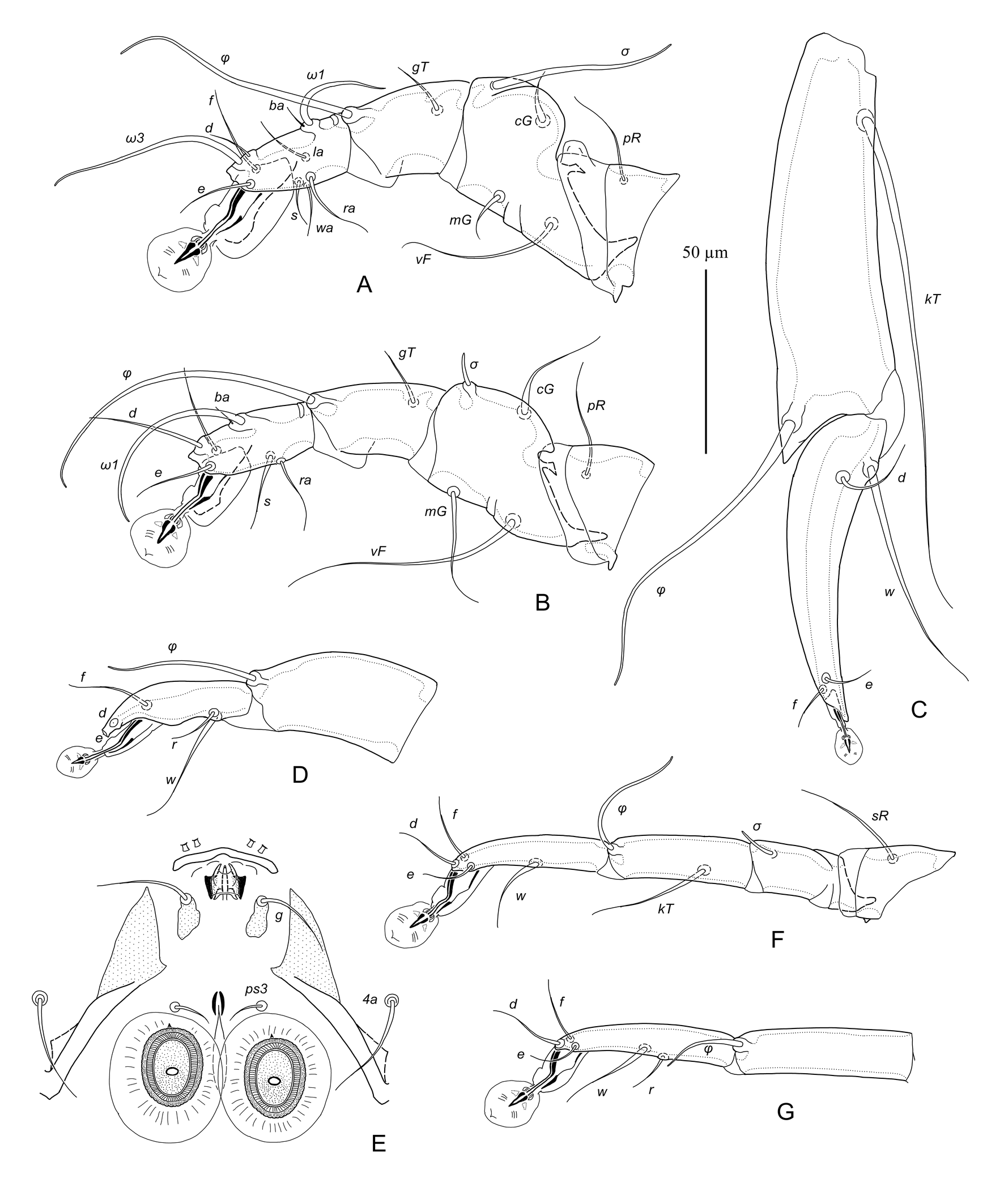


Epimerites I fused into a Y, with stem half as long as total length of epimerites I. Epigynum (pregenital apodeme) shaped as slightly curved transverse sclerite between bases of setae 4b, 25–28 wide. Apodemes of oviporus long, with posterior ends extending to midlength of epimerites IV (Figures 2B, 4D). Setae 1a, 3a, 4a, 4b and g short filiform. Setae g situated posterior to level of setae 3a. Distance between setae 4a approximately equal to that between 4b. Copulatory opening situated terminally; spermatheca indistinct. Distances between ventral setae: 4b:3a 20–24, 4b:g 30–34, g:4a 52–58, 4a:4a 28–34. Lengths of ventral setae: 1a 15–18, 3a 18–24, 4a 12–17, 4b and g 10–12.
Legs I, II as in the male, except femoragenu II without retrograde spine-like extension. Legs IV with pretarsus barely extending beyond posterior margin of opisthosoma (Figure 2A, B). Tarsi III, IV without distinct apicodorsal spine, 80–85 and 88–95 long, respectively (Figure 3F, G). Trochanteral setae sRIII 28–36 long, approximately equal to length of femoragenu III. Setae w of tarsi III, IV thickened basally, with short filiform apices. Lengths of solenidia: ω1I 28–30, ω1II 40–45, σI 38–42, σII 7–8, σIII 10–12, φIII 33–35, φIV 18–25.


Remarks
The new species Ingrassia lymnocrypti sp. n. is most close to I. limnodromi Vasyukova & Mironov, 1986 from the long-billed dowitcher, Limnodromus scolopaceus (Say) (Vasyukova and Mironov 1986: 11, figs 5–7, 1991: 71, figs 46, 47, 56a). These species share the following combination of characters: in both sexes, the prodorsal shield is gradually enlarged to the level of scapular setae, with posterior ends extending beyond these setae angular or semi-ovate; in males, setae c2 , d2 and e2 are represented by the macrosetae, the anterior ends of epimerites IIIa are connected with transverse bridge, tibia III with apicodorsal angular projection; in females, the hysteronotal shield is shaped as a longitudinal plate occupying the median area of hysterosoma, with deeply concave posterior margin, elongated long and pointed posterior corners extending beyond the level of setae e2. Ingrassia lymnocrypti sp. n. differs from the latter species in the following clear features: in males, the anterior margin of the hysteronotal shield is almost straight, the anterior ends of epimerites IVa bear triangular sclerotized fields, the terminal extensions of interlobar membrane are long and extend to the articulation of tibia and tarsus III, and legs IV reach only the midlenth of terminal extensions; in females, setae c2 are filiform (25–35 long), the anterior end of the hysteronotal shield extends to the level of humeral shields, setae e2 are situated off the lateral margins of the hysteronotal shield, and the oviporus lacks additional lateral sclerites. In males of I. limnodromi, the anterior margin of the hysteronotal shield is convex, the anterior ends of epimerites IVa lack sclerotized fields, the terminal extensions of interlobar membrane extend to the proximal one third of tibia III, and legs IV with entire tarsus extend beyond the apices of terminal extensions; in females, setae c2 are represented by macrosetae (100–120 long), the anterior end of the hysteronotal shield extends far anterior to the humeral shields, setae e2 are situated on the lateral margins of the hysteronotal shield, and the oviporus has a pair of longitudinal additional sclerites bearing bases of setae 4b (Vasyukova and Mironov, 1986: 11, figs. 5–7).
Etymology
The specific name is derived from the generic name of the type host and is a noun in the genitive case.
Discussion
Download as Remarks: *—type host, if a mite species is known from several avian species, (?)—questionable host association.
Mite species
Host
Host family
Host order
Locality
Reference
I. aequinoctialis (Trouessart, 1889)
Phaethon aethereus Linnaeus*
Phaethontidae
Phaethontiformes
Tropical seas
Trouessart 1899
"
P. lepturus Daudin
Phaethontidae
Phaethontiformes
Tropical seas
Trouessart 1899
"
P. rubricauda Boddaert
Phaethontidae
Phaethontiformes
Tropical seas, Pacific Ocean (Johnston Island)
Trouessart 1899; Mironov et al. 2025
I. americana Dabert & Ehrnsberger, 1991
Tringa melanoleuca (Gmelin)
Scolopacidae
Charadriiformes
Unknown (North America?)
Dabert and Ehrnsberger 1991
I. antarctica (Gaud, 1952)
Pelecanoides georgicus Murphy & Harper
Procellariidae
Procellariiformes
France: Kerguelen Islands
Gaud, 1952
I. aphrizae Dabert & Ehrnsberger,1991
Calidris virgata (Gmelin)
Scolopacidae
Charadriiformes
Unknown (North America?)
Dabert and Ehrnsberger 1991
I. arenariae (Gaud, 1958)
Arenaria interpres (Linnaeus)
Scolopacidae
Charadriiformes
Russia (European pt.), Kirghizia, Morocco,
Gaud 1958; Chirov 1979; Vasyukova and Mironov 1990
I. calonectris Stefan, Gomez-Diaz & Mironov, 2013
Calonectris borealis Cory
Procellariidae
Procellariiformes
Portugal (Azores Archipelago)
Stefan et al. 2013
I. centrotibia Gaud,1972
Actitis hypoleucos (Linnaeus) (?)
Scolopacidae
Charadriiformes
Cameroon
Gaud 1972a
"
Anarhynchus alexandrinus (Linnaeus)
Charadriidae
Charadriiformes
Cameroon
Gaud 1972a
"
Charadrius dubius Scopoli
Charadriidae
Charadriiformes
Morocco
Gaud 1972a
"
Charadrius hiaticula Linnaeus
Charadriidae
Charadriiformes
Russia (Euopean pt.), Morocco, Cameroon
Gaud 1972a; Vasyukova and Mironov 1990
"
Himantopus himantopus (Linnaeus)*
Recurvirostridae
Charadriiformes
Morocco
Gaud 1972a
"
Phalaropus lobatus (Linnaeus) (?)
Scolopacidae
Charadriiformes
Kirghizia
Chirov 1979
I. chionis Han, Mironov, Kim & Min,2021
Chionis albus (Gmelin)
Chionidae
Charadriiformes
Antarctica (King George Island)
Han et al. 2021
I. colymbi Gaud, 1974
Tachybaptus ruficollis (Pallas)
Podicipedidae
Podicipediformes
Morocco
Gaud 1974
I. dubinini Černý, 1967
Ardenna tenuirostris (Temminck)***
Procellariidae
Procellariiformes
Russia (Wrangel Island)
Dubinin 1949; Černý 1967
"
Hydrobates pelagicus (Linnaeus) (?)
Hydrobatidae
Procellariiformes
Russia (Sea of Okhotsk)
Dubinin 1949; Černý 1967
I. eudyptula Mironov & Proctor,2008
Eudyptula minor (Forster)
Spheniscidae
Sphenisciformes
Australia (Fraser Island)
Mironov and Proctor 2008
I. fissitarsa (Gaud, 1958)
Gallinago gallinago (Linnaeus)
Scolopacidae
Charadriiformes
Russia (European pt.), Morocco
Gaud 1958; Mironov 1981
I. falcifera (Gaud, 1953)
Hydrobates castro (Harcourt)
Hydrobatidae
Procellariiformes
Senegal
Gaud 1953
I. forcipata (Haller, 1882)
Actitis macularia (Linnaeus)*
Scolopacidae
Charadriiformes
Canada
Haller 1882
"
Actitis hypoleucos (Linnaeus)
Scolopacidae
Charadriiformes
Kirghizia
Chirov 1979; Vasyukova and Mironov 1990, 1991
"
Tringa glareola Linnaeus (?)
Scolopacidae
Charadriiformes
Cameroon, Namibia
Gaud 1972a
"
Tringa nebularia (Gunnerus)(?)
Scolopacidae
Charadriiformes
Cameroon
Gaud 1972a
"
Tringa stagnatilis (Bechstein) (?)
Scolopacidae
Charadriiformes
Cameroon
Gaud 1972a
"
Tringa totanus (Linnaeus) (?)
Scolopacidae
Charadriiformes
Cameroon
Hull 1934; Gaud 1972a
I. limnodromi Vasyukova & Mironov,1986
Limnodromus scolopaceus (Say)
Scolopacidae
Charadriiformes
Russia (Yakutia)
Vasyukova and Mironov 1986, 1991
I. limosae Gaud,1972
Limosa limosa (Linneaeus)*
Scolopacidae
Charadriiformes
Morocco
Gaud 1972a
"
Limosa lapponica (Linnaeus)
Scolopacidae
Charadriiformes
Russia (Yakutia)
Vasyukova and Mironov 1991
I. lymnocrypti sp. n.
Lymnocryptes minimus (Brünnich)
Scolopacidae
Charadriiformes
Denmark (Faroe Islands)
Present work
I. micronota Stefan, Gomez-Diaz & Mironov, 2013
Bulweria bulwerii (Jardine & Selby)
Procellariidae
Procellariiformes
Cape Verde (Ilheu Grande)
Stefan et al. 2013
I. oceanica (Vitzthum, 1921)
Hydrobates pelagicus (Linnaeus)
Hydrobatidae
Procellariiformes
Germany (Helgoland)
Vitzthum 1921
I. oceanodromae Černý, 1967
Hydrobates leucorhous (Vieillot)
Hydrobatidae
Procellariiformes
Denmark (Faroe Islands)
Černý 1967
I. phalaropi Gaud,1972
Phalaropus fulicarius (Linneaus)
Scolopacidae
Charadriiformes
Morocco
Gaud 1972a
"
Phalaropus lobatus (Linnaeus)
Scolopacidae
Charadriiformes
Russia (European pt., W. Siberia)
Vasyukova and Mironov 1990
I. philomachi Gaud,1972
Calidris pugnax (Linnaeus)
Charadriidae
Charadriiformes
Russia (European pt., Yakutia), Kirghizia, Cameroon
Gaud 1972a; Chirov 1979; Vasyukova and Mironov 1990, 1991
I. platyspina Mironov & Palma, 2006
Prosobonia parvirostris (Peale)
Scolopacidae
Charadriiformes
French Polynesia (Tuamotu Island)
Mironov and Palma 2006
I. semifissa Gaud, 1972
Glareola pratincola (Linnaeus)
Glareolidae
Charadriiformes
Zambia
Gaud 1972a
I. slonskiana Dabert, 2000
Tringa ochropus Linneaus
Scolopacidae
Charadriiformes
Poland
Dabert 2000
I. spinata (Gaud & Mouchet, 1959)
Glareola nuchalis Gray
Glareolidae
Charadriiformes
Cameroon
Gaud and Mouchet 1959
I. strictior (Berlese, 1898)
Calidris pugnax (Linnaeus)
Scolopacidae
Charadriiformes
Italy
Berlese 1898
I. tridactyla (Gaud & Mouchet, 1959)
Actophilornis africanus (Gmelin)
Jacanidae
Charadriiformes
Cameroon
Gaud and Mouchet 1959
I. tringae (Vitzthum, 1922)
Calidris minuta (Leisler)
Scolopacidae
Charadriiformes
Bulgaria, Russia (European pt., Yakutia), Zambie, South Africa
Vitzthum 1922; Gaud 1972a; Vasyukova and Mironov 1990, 1991
I. veligera (Oudemans, 1904)
Tringa flavipes (Gmelin)*
Scolopacidae
Charadriiformes
Guyana
Oudemans 1904
"
Tringa glareola Linnaeus
Scolopacidae
Charadriiformes
Russia (European pt., Yakutia), Kirghizia
Chirov 1979; Vasyukova and Mironov 1990, 1991
"
Tringa nebularia (Gunnerus)
Scolopacidae
Charadriiformes
Russia (European pt., Yakutia)
Vasyukova and Mironov 1991
"
Tringa stagnatilis (Bechstein)
Scolopacidae
Charadriiformes
Poland, Russia (European pt.)
Vasyukova and Mironov 1990; Dabert, 2000
"
Tringa totanus (Linnaeus)
Scolopacidae
Charadriiformes
Kirghizia
Chirov 1979
I. xiphidiopteri Gaud, 1972
Vanellus albiceps Gould
Charadriidae
Charadriiformes
Cameroon
Gaud 1972a
The recently published world checklist of the Ingrassia generic group presented valid species and only a type host for each mite species (Mironov et al. 2025). In the present work, we have compiled the list of currently known species of the genus Ingrassia supplemented with all reported hosts and localities (Table 1). Based on the number of charadriiform species of the suborders Charadrii and Scolopaci known to date as hosts of Ingrassia (Gaud 1972a; Vasyukova and Mironov 1990, 1991), it is possible to evaluate that the fauna of this genus on shorebirds has been studied more or less well; while that on procellariiforms is obviously known quite superficially (Stefan et al. 2013). On tropicbirds (Phaethontiformes: Phaethontidae), I. aequinoctialis is the only species known from all three species of the genus Phaethon comprising this order (Mironov et al. 2025). On grebes (Podicipediformes), the only I. colymbi from the little grebe Tachybaptus ruficollis is currently known (Gaud 1974), although Ingrassia species could probably be expected on other grebe species of this order. The record of I. eudyptula on a penguin Eudyptula minor (Sphenisciformes) is still enigmatic, and this finding could represent the result of natural horizontal transfer from a procellariform host (Mironov and Proctor 2008).
Most of currently known Ingrassia species are monoxenous ectoparasites. Much lesser number of species are stenoxenous, i.e. associated with various species of the same genus (Ingrassia aequinoctialis, I. limosae, I. phalaropi, and I. veligera). Associations of I. forcipata with hosts from different genera and I. centrotibia with hosts from different families look highly doubtful. Perhaps, the reports of too different hosts for these mite species could be caused by contamination or misidentification of similar species.
The species I. centrotibia was originally found on the black-winged stilt, Himantopus himantopus (Recurvirostridae), and plovers of the genera Anarhynchus and Charadrius (Charadriidae) (Gaud 1972a). Therefore, the records of this mite on some scolopacids (Table 1), the common sandpiper Actitis hypoleucos and the red-necked phalarope Phalaropus lobatus having their own specific Ingrassia species, seem highly likely misidentifications. Additionally, although plovers and stilts are closely related families, the reports of I. centrotibia from H. himantopus and plovers of the genera Anarhynchus and Charadrius allow suggesting that even on these hosts this mite could potentially be a complex of closely related species.
Download as
Host species
Alloptidae
Avenzoariidae
Xolalgidae
Pterolichidae
Syringobiidae
Proctophyllodidae
References
Tringini
Tringa nebularia
Alloptes atelesthetus Gaud, 1972
Avenzoaria totani (Canestrini, 1878); Bregetovia obtusolobata Dubinin, 1951
Ingrassia veligera (Oudemans, 1904)
Montchadskiana ulitae Dubinin, 1951
Plutarchusia chelopus (Oudemans, 1904);
—
Dubinin 1951, 1956; Gaud 1972; Vasyukova and Mironov 1990, 1991; Mironov 1992; Dabert and Ehrnsberger 1999; Dabert 2003
Syringobia parachelopus Vasyukova & Mironov, 1986
Scolopacini
Limnodromus scolopaceus
Alloptes limnodromi Vasyukova & Mironov, 1991
Avenzoaria macrorhamphi Dubinin, 1956
Ingrassia limnodromi Vasyukova & Mironov, 1986
—
Phyllochaeta gracilis Vasyukova & Mironov, 1986;
—
Dubini 1956; Vasyukova and Mironov 1986, 1990, 1991; Dabert 2003
Ph. latior Vasyukova & Mironov, 1986
Lymnocryptes minimus
Alloptes lymnocrypti Mironov, 1981
Rafalskiata rackae (Gaud, 1972)
Ingrassia lymnocrypti sp. n.
—
—
—
Gaud 1972b; Mironov 1981; Mironov and Dabert 1997
Gallinago gallinago
—
Capelloptes flagellicaulus (Trouessart & Neumann, 1888)
Ingrassia fissitarsa (Gaud, 1958)
—
—
—
Dubinin 1951; Gaud 1958, 1972a
Scolopax rusticola
—
—
—
—
—
Proctophyllodes scolopacinus (Koch, 1842)
Atyeo and Braasch 1966
Ingrassia forcipata was originally described from the spotted sandpiper Actitis macularia by Haller (1882) and further found on the common sandpiper A. hypoleucos (Vasyukova and Mironov 1990). Reports of this mite species from shanks and tattlers (Scolopacidae: Tringa) by Gaud (1972a) rise doubts, because from most of Eurasian and North American species of the genus Tringa was also reported I. veligera, which is knows exclusively from tattlers and shanks (Oudemans 1904; Vasyukova and Mironov 1991; Dabert 2000). Indeed, the species I. forcipata and I. veligera are very similar in general appearance (Vasyukova and Mironov 1991: fig. 49 vs. fig. 50); therefore, it is possible to suggest that specimens from tattlers from Africa and Europe were misidentified as ''I. forcipata″ by Gaud (1972a) and Mironov (1981).
With the description of a new Ingrassia species, the feather mite fauna of the jacksnipe now includes three species inhabiting its plumage: Alloptes lymnocrypti Mironov, 1981 (Alloptidae), Ingrassia lymnocrypti (Xolalgidae), and Rafalskiata rackae (Gaud, 1972) (Avenzoariidae). It is interesting to compare its fauna with those of close species from the tribe Scolopacini, like snipes, woodcocks and dowitchers, and other scolopacids. According to fundamental studies of feather mite fauna of Charadriiformes (e.g. Dubinin 1951, 1956; Gaud and Till 1961; Gaud 1972a; Vasyukova and Mironov 1986, 1990, 1991; Dabert and Ehrnsberger 1999; Dabert 2003), the set of mite species on most of typical scolopacids, such as sandpipers, tattlers and shanks (tribes Tringini and Arenariini), is rich and commonly includes at least one species from each of the following families: Alloptidae, Avenzoariidae, Xolalgidae (Analgoidea), Pterolichidae and Syringobiidae (Pterolichoidea). The set of mite species associated with well-explored host species of the tribe Scolopacini, most of which are common game birds, is shown in Table 2. The data for the common greenshank, Tringa nebularia (Tringini), having a typical set of feather mites for tringines, are presented for comparison. It is easy to see that the feather mite fauna on birds of the tribe Scolopacini is reduced compared to those on typical waders, because all these hosts have lost representatives the family Pterolichidae and almost all have lost mites of the family Syringobiidae. Of these four species of scolopacines, two bird species, the jacksnipe and long-billed dowitcher, Limnodromus scolopaceus, harbor mite species from three analgoidean families, Alloptidae, Avenzoariidae, and Xolalgidae. Additionally, the long-billed dowitcher retains quill-inhabiting feather mites of the family Syringobiidae (Pterolichoidea). The common snipe, Gallinago gallinago, has lost representatives of the family Alloptidae and harbors only mites of the families Avenzoariidae and Xolalgidae. Finally, the Eurasian woodcock, Scolopax rusticola, has entirely lost the primary feather mite fauna being specific to charadriiforms and has accepted a new feather mite fauna obviously received from some passerines—Proctophyllodes scolopacinus (Koch, 1842) (Proctophyllodidae). Mites of the family Proctophyllodidae are predominately distributed on passerines, and P. scolopacinus being specific to the woodcocks (Scolopax) is the closest to P. corvorum Vitzthum, 1922 associated with various corvids of the genus Corvus (Atyeo and Braasch 1966). The feather mite fauna of the woodcock is a rare and bright example of horizontal transfer of ectoparasites between hosts of different avian orders that gives a wide field of speculation, how the ancestor of woodcocks could accept a feather mite species from corvids. In general, the considered avian hosts of the tribe Scolopacini demonstrate a model of gradual loss of the primary feather mite fauna of Scolopacidae and the appearance of a new fauna by the horizontal transfer.
Acknowledgements
The authors wish to thank Jens-Kjeld Jensen (Nólsoy, Faroe Islands) for providing access to the studied bird skins. The taxonomic part of the study was supported by the Ministry of Science and Higher Education of the Russian Federation (project No. 125013001089-0) for SM.
References
- Atyeo W.T., Braasch N.L. 1966. The feather mite genus Proctophyllodes (Sarcoptiformes: Proctophyllodidae). Bull. Univ. Nebraska State Mus., 5: 1-354.
- Berlese A. 1898. Megninia centropodos var. strictior. Acari, Myriopoda et Scorpiones hucusque in Italia reperta, Padova, Fascicolo 87, No 6.
- Černý V. 1967. Trois espèces nouvelles des Acariens plumicoles (Analgoidea) parasites des Procellariiformes. Folia Parasitol., 14: 87-91.
- Chirov P.A. 1979. Mites of the superfamily Analgoidea, which live on birds of Kirgizia. Entomologicheskie issledovaniia v Kirgizii, Akademiia nauk Kirgizskoi SSR, 49-54. [In Russian]
- Chirov P.A., Mironov S.V. 1990. Feather mites of the subfamily Ingrassiinae of limicolines and ducks. Izvestiya Akademii nauk Kirgizkoi SSR, Khimiko-Teknologicheskie i Biologicheskie Nauki, 3: 74-83. [In Russian]
- Dabert J. 2000. Feather mites (Acari: Astigmata) of water birds of the Slońsk nature reserve with description of a new species. Biol. Bull. Poznań, 37: 303-316.
- Dabert J. 2003. The feather mite family Syringobiidae Trouessart, 1896 (Acari, Astigmata, Pterolichoidea). I. Systematics of the family and description of new taxa. Acta Parasitol., 48 (Supplement): S1-S184.
- Dabert J., Badek A., Skoracki M. 2007. New feather mite species (Acari, Astigmata) from the Sulphur-crested Cockatoo Cacatua galerita and Yellow-crested Cockatoo C. sulphurea (Psittaciformes, Cacatuidae). Acta Parasitol., 52(3): 250-267. https://doi.org/10.2478/s11686-007-0034-z
- Dabert J., Ehrnsberger R. 1991. Zwei neue Federmilben-Arten aus der Gattung Ingrassia Oudemans, 1905 (Analgoidea; Xolalgidae; Ingrassiinae). Osnabrücker Naturwissenshaftliche Mitteilungen, 17: 127-142.
- Dabert J., Ehrnsberger R. 1999. Systematics of the feather mite genus Montchadskiana Dubinin, 1951 (Pterolichoidea, Pterolichidae, Magimeliinae) with description of five new species. Acta Zool. Cracov., 42 (2): 219-249.
- Dubinin V.B. 1949. Feather mite fauna of birds of the order Procellariiformes and its features. Parazitologicheskii Sbornik, 11: 201-228. [In Russian]
- Dubinin V.B. 1951. Feather mites of birds of the Baraba Steppe. Report I. Feather mites of waterfowl and wading birds of the orders of rails, grebes, palmipedes, anserines, herons, gulls, and limicoles. Parazitologicheskii Sbornik, 13: 120-256. [In Russian]
- Dubinin V.B. 1956. Feather Mites (Analgesoidea). Part 3. Family Pterolichidae. Fauna of the USSR, Arachnida, Vol. 6, Issue 7. Publisher: Akademia Nauk SSSR, Moscow-Leningrad, 813 pp. [In Russian]
- Gaud J. 1952. Acariens plumicoles (Analgesidae) de quelques oiseaux des Îles Kerguelen (Récolte P. Paulian). Mém. Inst. Sci. Madagascar, Sér. A, 7: 161-166.
- Gaud J. 1953. Sarcoptides plumicoles des oiseaux d'Afrique occidentale et centrale. Ann. Parasitol. Hum. Comp., 28: 193-226. https://doi.org/10.1051/parasite/1953283193
- Gaud J. 1958. Acariens plumicoles (Analgesoidea) parasites des oiseaux du Maroc. II. Analgidae. Bull. Soc. Sci. Natur. Phys. Maroc, 38: 27-49.
- Gaud J. 1972a. Acariens Sarcoptiformes plumicoles (Analgoidea) parasites sur les oiseaux Charadriiformes d'Afrique. Mus. Roy. Afr. Centr., Ann., Sér. in-8°, Sci. Zool., 193: 1-116.
- Gaud J. 1972b. Les Sarcoptiformes plumicoles (Analgoidea) parasites des Scolopacinae, avec la description d'une espèce nouvelle: Avenzoaria rackae n. sp. Entomologische Mitteilungen aus dem Zoologischen Museum, Hamburg, 4: 241-247.
- Gaud J. (1973) 1974. Quelques espèces nouvelles de Sarcoptiformes plumicoles (Analgidae et Dermoglyphidae) parasites d'oiseaux d'Europe. Acarologia, 15: 727-758.
- Gaud J., Atyeo W.T. 1981. La famille Xolalgidae Dubinin, nouveau statut (Sarcoptiformes plumicoles, Analgoidea). I. Sous-famille Ingrassiinae, n. sub. fam. Acarologia, 22: 63-79.
- Gaud J., Atyeo W.T. 1996. Feather mites of the World (Acarina, Astigmata): the supraspecific taxa. Mus. Roy. Afr. Centr., Ann., Sci. Zool., 277 (Pt. 1): 1-193 (text) and (Pt. 2): 1-436 (illustrations).
- Gaud J., Mouchet J. 1959. Acariens plumicoles (Analgesoidea) parasites des oiseaux du Cameroun. II. Analgesidae. Ann. Parasitol. Hum. Comp., 34: 149-208. https://doi.org/10.1051/parasite/1959341149
- Gaud J., Till W.M. 1961. Suborder Sarcoptiformes. In: Zumpt F. (Ed.). The Arthropod Parasites of Vertebrates in Africa South of the Sahara (Ethiopian Region). Vol. I. Chelicerata. Publications of the South African Institute of Medical Research, No 1 (Vol. IX), Johannesburg, pp. 180-352.
- Gill F., Donsker D., Rasmussen P. (Eds.). 2025. IOC World Bird List (v 15.1). Available from http://www.worldbirdnames.org/ (accessed 25 June 2025). https://doi.org/10.14344/IOC.ML.15.1.
- Haller G. 1882. Zur Kenntniss der Dermaleichiden. Archiv für Naturgeschichte, 48: 47-79.
- Han Y.-D., Mironov S.V., Kim J.-H., Min, G.-S. 2021. Feather mites (Acariformes: Astigmata) from marine birds of the Barton Peninsula (King George Island, Antarctica), with descriptions of two new species. Zookeys, 1061: 109-130. https://doi.org/10.3897/zookeys.1061.71212
- Hull J.E. 1934. Concerning British Analgidae (Feather-mites). Transact. Northern Naturalists Union, 1: 200-206.
- Krantz G., Walter D. (Eds.). 2009. A Manual of Acarology, 3rd Edition. Texas Tech University Press, Lubbock, Texas, 807 pp.
- Mironov S.V. 1981. Feather mites (Acarina, Sarcoptiformes, Analgoidea) of the birds of the family Charadriidae from the Curonian Spit. In: Balashov Yu.S. (Ed.). Morphological Peculiarities of Mites and Arachnids. Trudy Zoologicheskogo Instituta AN SSSR, 106: 66-75. [In Russian]
- Mironov S.V. 1992. The review of species of the feather mite genus Bregetovia in the USSR's fauna (Analgoidea: Avenzoariidae). Parazitologicheskii Sbornik, 37: 126-150. [In Russian]
- Mironov S.V. (2004) 2005. Phylogeny of the feather mite family Xolalgidae (Astigmata: Analgoidea) and revolutionary trends with non-passerine birds. Phytophaga, 14: 433-449.
- Mironov S.V., Dabert J. 1997. Taxonomic notes on the feather mite subfamily Avenzoariinae with establishing of two new genera (Acarina: Analgoidea: Avenzoariidae). Genus, 8 (1): 75-79.
- Mironov S. V., Galloway T.D. 2002. Four new species of feather mites (Acari: Analgoidea). Canadian Entomol., 134: 605-618. https://doi.org/10.4039/Ent134605-5
- Mironov S.V., Palma R.L. 2006. Two new feather mite species (Acari: Analgoidea) from the Tuamotu sandpiper Aechmorhynchus parvirostris (Charadriiformes: Scolopacidae). Tuhinga - Records of the Museum of New Zealand Te Papa Tongarewa, 17: 49-59.
- Mironov S.V., Proctor H.C. 2008. The probable association of feather mites of the genus Ingrassia Oudemans, 1905 (Analgoidea: Xolalgidae) with the blue penguin Eudyptula minor (Aves: Sphenisciformes) in Australia. J. Parasitol., 94: 1243-1248. https://doi.org/10.1645/GE-1579.1
- Mironov S.V., Ehrnsberger R., Dabert J. 2017. Feather mites of the genera Dubininia and Cacatualges (Acari: Xolalgidae) associated with parrots (Aves: Psittaciformes) of the Old World. Zootaxa, 4272 (4): 451-490. https://doi.org/10.11646/zootaxa.4272.4.1
- Mironov S.V., Stormer H., Proctor H.C. 2025. Redescription of the feather mite Ingrassia aequinoctialis (Trouessart, 1899) (Acariformes: Xolalgidae) with notes on systematics and host associations of the Ingrassia genus-group. Acarologia, 65(2): 482-496. https://doi.org/10.24349/f5zx-leve
- Norton R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarology, 22: 559-594. https://doi.org/10.1023/A:1006135509248
- Oudemans A.C. 1904. Acarologische Aanteekeningen XIV. Entomol. Bericht., 20: 190-195. https://doi.org/10.5962/bhl.part.1123
- Oudemans A.C. 1905. Acarologische Aanteekeningen XVII. Entomol. Bericht., 23: 222-226. https://doi.org/10.5962/bhl.part.1126
- Stefan L.M., Gomez-Diaz, E., Mironov S.V. 2013. Three new species of the feather mite subfamily Ingrassiinae (Acariformes: Xolalgidae) from shearwaters and petrels (Procellariiformes: Procellariidae). Zootaxa, 3682 (1): 105-120. https://doi.org/10.11646/zootaxa.3682.1.4
- Trouessart E.L. (1898) 1899. Diagnoses préliminaires d'espèces nouvelles d'Acariens plumicoles. Additions et corrections à la sous-famille des Analgésinés. Bull. Soc. Étud. Sci. Angers, 28: 1-62.
- Vasyukova T.T., Mironov S.V. 1986. New species of feather mites of Siberia. Publisher: Nauka, Siberian Dept., Novosibirsk, 72 pp. [In Russian]
- Vasyukova T.T., Mironov S.V. 1990. Fauna and ecology of feather mites of Anseriformes and Charadriiformes of Yakutia. Publisher: Yakutskii Nauchnyi Tsentr, Sibirskoye Otdelenie Akademii Nauk SSSR, Yakutsk, 96 pp. [In Russian]
- Vasyukova T.T., Mironov S.V. 1991. Feather mites of Anseriformes and Charadriiformes of Yakutia. Systematics. Publisher: Nauka, Siberian Dept., Novosibirsk, 201 pp. [In Russian]
- Vitzthum H. 1921. Acarologische Beobachtungen. (5. Reihe). Archiv für Naturgeschichte, 87A (4): 1-77.
- Vitzthum H. 1922. Acarologische Beobachtungen. Zoologische Jahrbücher, Abteilung für Systematik, Ökologie und Geographie der Tiere, 44 (6): 517-564.



2025-07-26
Date accepted:
2025-09-19
Date published:
2025-10-09
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Mironov, Sergey V and Haarder, Simon
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



