Phytoseiid mites (Mesostigmata: Phytoseiidae) from Fuerteventura (Canary Islands): A taxonomic and biogeographical approach
Ferragut, Francisco  1
1
1✉ Instituto Agroforestal Mediterráneo. Universidad Politécnica de Valencia. Camino de Vera, s/n. 46022 Valencia, Spain.
2025 - Volume: 65 Issue: 3 pages: 962-997
https://doi.org/10.24349/33af-ivt4ZooBank LSID: 9201E22D-95EA-4759-B6C0-B2D84784B15E
Original research
Keywords
Abstract
Introduction
Oceanic islands like the Canaries are considered important natural laboratories for the study of evolution and life´s diversity and have been used as ecological models for understanding how their geological and environmental history has determined the existing biotas (Whittaker et al. 2023). The Canaries were built over oceanic crust hotspots on the African Plate and have never been connected with the African continent. The plants and animals that survive there arrived from the continent or near islands by dispersal or have evolved and differentiate as new species within the archipelago.
The phytoseiid mites (Mesostigmata, Phytoseiidae) of the Canary Islands have been studied since the late 1980´s, and today 29 species are known from the entire archipelago, including eight Canarian endemisms (Evans, 1952; Ferragut et al. 1988; Ferragut and Peña-Estévez, 2003, 2007; Moraza and Peña-Estévez, 2005, 2006; Moraza et al. 2005; Pande et al. 1989). All these reports refer to collections made in the central islands, Gran Canaria, Tenerife and La Gomera, while the mite species from the eastern (Fuerteventura and Lanzarote) and western (La Palma and El Hierro) islands of the archipelago remains largely unexplored.
In this study, I examine the phytoseiid fauna of Fuerteventura, the easternmost island of the archipelago, separated from the African coast by only 96 km and characterized by a semi-desertic climate that only allows the development of a sparse cover of shrubs and herbaceous plants with few trees but with a high level of plant endemism. This research has led to the discovery of a new phytoseiid species, Neoseiulella extraseta sp. nov. In addition, two species are recorded for the first time in the Canaries. The study also includes a detailed morphological description of all the collected immature stages, and provides a tentative biogeographical analysis of the mite fauna.
Material and methods
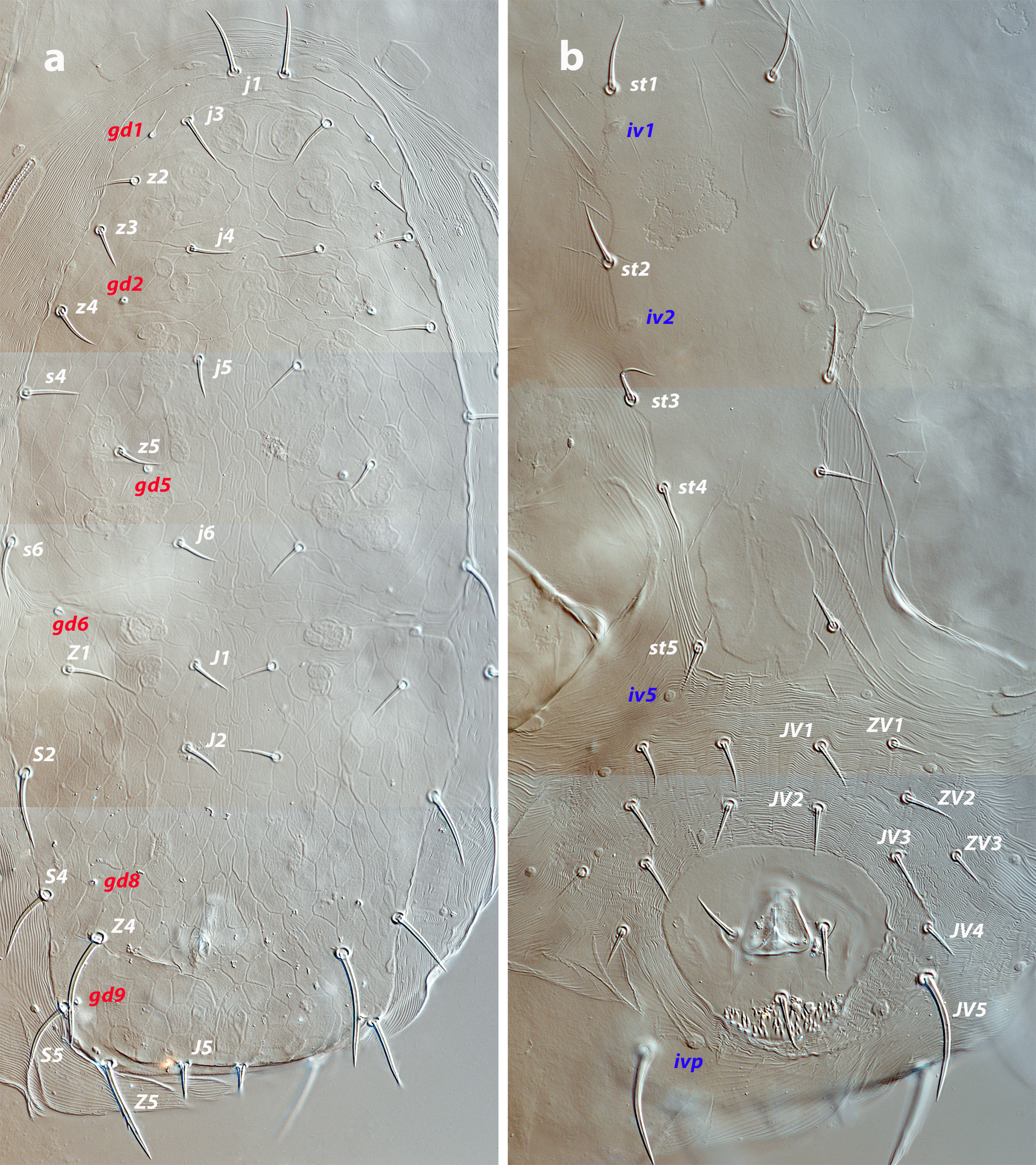
The mites were collected from 25 to 30 May 2024 in Fuerteventura and the nearby islet of Lobos. Only natural and native vegetation was surveyed. Mites were extracted from plants by examining the leaves under a stereomicroscope or beating parts of the plant on a fine sieve. Specimens were mounted and clarified in Heinze-PVA medium and stored in an oven at 50 °C until total clarification. After clarification, the phytoseiids were examined using differential interference contrast (DIC) in a compound microscope Nikon Eclipse Ni-U (Nikon Corporation, Tokyo, Japan). Illustrations were performed on a graphic tablet using Concepts software (https://concepts.app ![]() ). Images were captured with a digital camera Nikon DS-Fi3 (Nikon Corporation, Tokyo, Japan) and edited in Lightroom and Photoshop CC (© Adobe Systems Inc.). Measurements of morphological traits were obtained with the software NIS-Elements D 3.1 and are given in the text in micrometres (μm). In the description of the new species, values of measurements in the female holotype and the male allotype are represented in bold, followed by the ranges of all individuals studied (in parentheses). Measurements in the immatures of the new species and adults of already known species correspond to the range of all specimens examined. World distribution of each species is based on Demite et al. (2025).
). Images were captured with a digital camera Nikon DS-Fi3 (Nikon Corporation, Tokyo, Japan) and edited in Lightroom and Photoshop CC (© Adobe Systems Inc.). Measurements of morphological traits were obtained with the software NIS-Elements D 3.1 and are given in the text in micrometres (μm). In the description of the new species, values of measurements in the female holotype and the male allotype are represented in bold, followed by the ranges of all individuals studied (in parentheses). Measurements in the immatures of the new species and adults of already known species correspond to the range of all specimens examined. World distribution of each species is based on Demite et al. (2025).
The generic concepts are those proposed by Chant and McMurtry (1994, 2007). Idiosomal setal nomenclature follows that of Lindquist and Evans (1965) as adapted by Rowell et al. (1978) for the dorsal surface and Chant and Yoshida-Shaul (1991) for the ventral surface. Leg chaetotaxy follows Evans (1963) and Rowell and Chant (1979). Terminology for the morphology of the spermatodactyl is that of Beard (2001). Nomenclature for idiosomal solenostomes and poroids follows Athias-Henriot system (Athias-Henriot, 1975) recently modified by Moraza (2025). I adopted the modifications proposed by Moraza (2025) for being more practical and intuitive without losing the ontogenetic value of the notation system. The modifications introduced in the text affect the designation of the peritrematal gland pore gd3 which is now gp, the peritrematal poroid id3, now ip, and the opisthonotal poroids is1, idm1, idm5, idm6 and idx, renamed as idl1, id3, idl5, idm5 and idm3, respectively. The remaining idiosomal pores and poroids maintain the same nomenclature.
Results
Subfamily Typhlodrominae Wainstein, 1962
Tribe Typhlodromini Wainstein, 1962
Genus Neoseiulella Muma, 1961
Neoseiulella extraseta Ferragut sp. nov.
ZOOBANK: D5A739E3-E76F-4368-9299-8DB82D8AE4BB ![]()
Type material and depository
Female holotype, seven female paratypes, one deutonymph on Caroxylon tetrandrum (Forsskål) Akhani & Roalson (Amaranthaceae) and two females on Arthrocaulon macrostachyum (Moricand) Piirainen & Kadereit (Amaranthaceae); Isla Lobos, 28°44′21″N, 13°49′21″W, 3 m a.s.l.; 28 May 2024. Three females on unidentified Amaranthaceae; El Cotillo, Aljibe de la Cueva beach, 28°39′53″N, 14°00′41″W, 18 m a.s.l.; 27 May 2024. Fourteen females, five males, one deutonymph, one protonymph on Traganum moquinii Webb ex Moquin-Tandon (Amaranthaceae) and one female on Cakile maritima Scopoli subsp. maritima (Brassicaceae); Dunas de Corralejo, 28°40′13″N, 13°50′00″W, 12 m a.s.l.; 27 May 2024. Female holotype, eleven paratype females, male allotype and one male paratype, and one deutonymph paratype were deposited in the Museo de Ciencias Naturales de Tenerife, Santa Cruz de Tenerife, Canary Islands. The remaining specimens are in the Acari collection, Instituto Agroforestal Mediterráneo, Universidad Politécnica de Valencia.
Diagnosis
Female dorsal shield thoroughly reticulated; midline cells between setae j4–J2 elongate, oriented axially; cells between setae J2–Z4 diverging from the midline and obliquely oriented; most of cells covered by longitudinal striae. Twenty pairs of short setae, the common dorsal setal pattern for Neoseiulella plus the pair J1, occurring on all the developmental stages and sexes (larvae were not examined); setae j1 longer than j3, setae J2 shorter than J5. Six pairs of dorsal solenostomes, lacking gland pore gd4. Peritremes ending anteriorly between setae j1–j3. Sternal shield with two pairs of setae. Ventrianal shield subpentagonal, transversally striated, with four pairs of preanal setae and without preanal pores. Spermathecal calyx bowl or dome-shaped, 3–5 deep. Fixed digit of chelicera with two subapical teeth; mobile digit edentate. Genua of legs II–IV with seven setae each; one macroseta on tarsus IV. Male dorsal shield elongate, similar in shape to that of female, not capturing the setal pairs r3 and R1, which remain on the soft cuticle. Dorsal ornamentation as in female. Peritrematal shields merged to the dorsal shield at the level of j3. Ventrianal shield with seven pairs of preanal setae. Spermatodactyl L-shaped, with an inconspicuous heel and a short foot.
Description
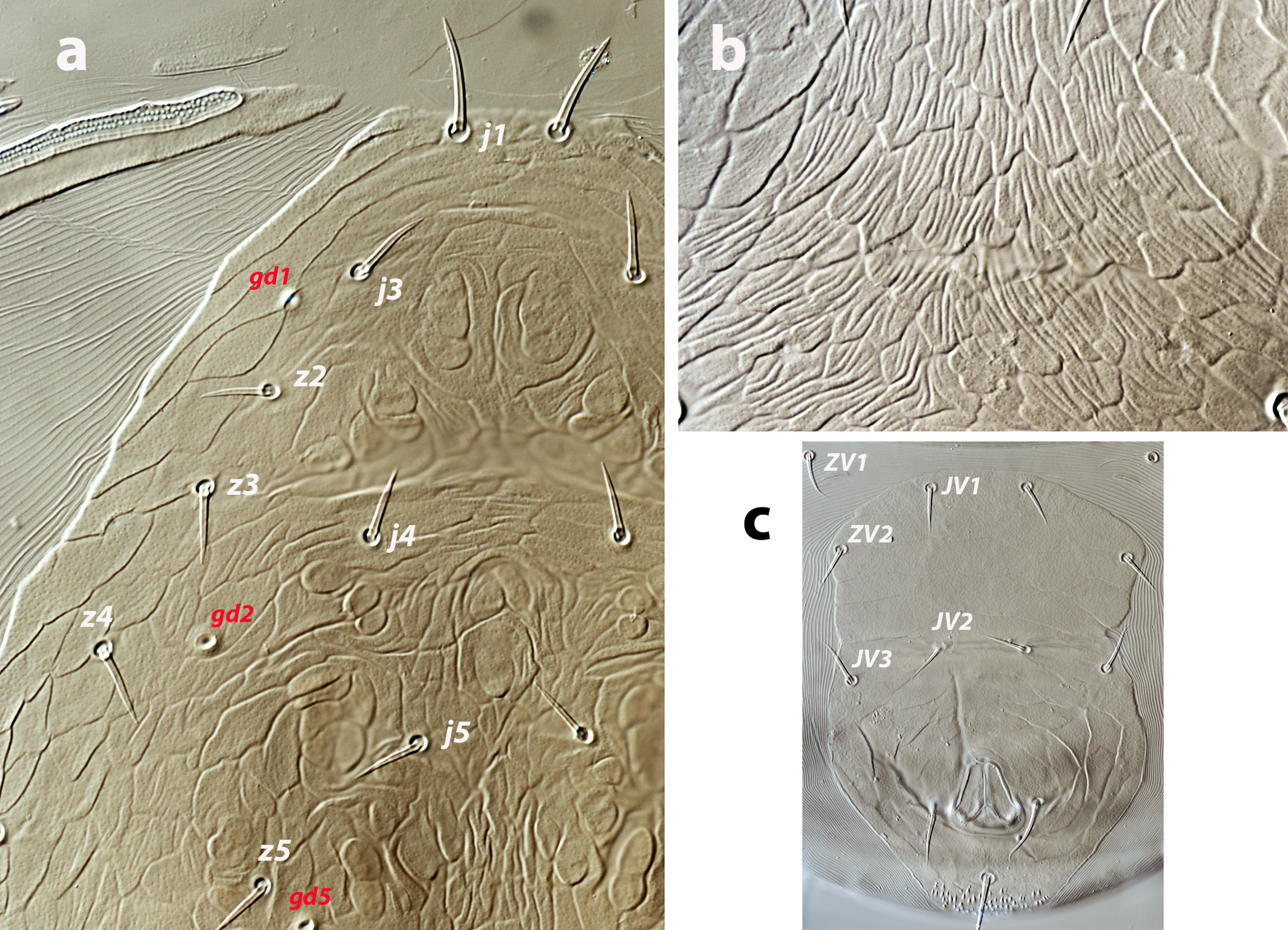
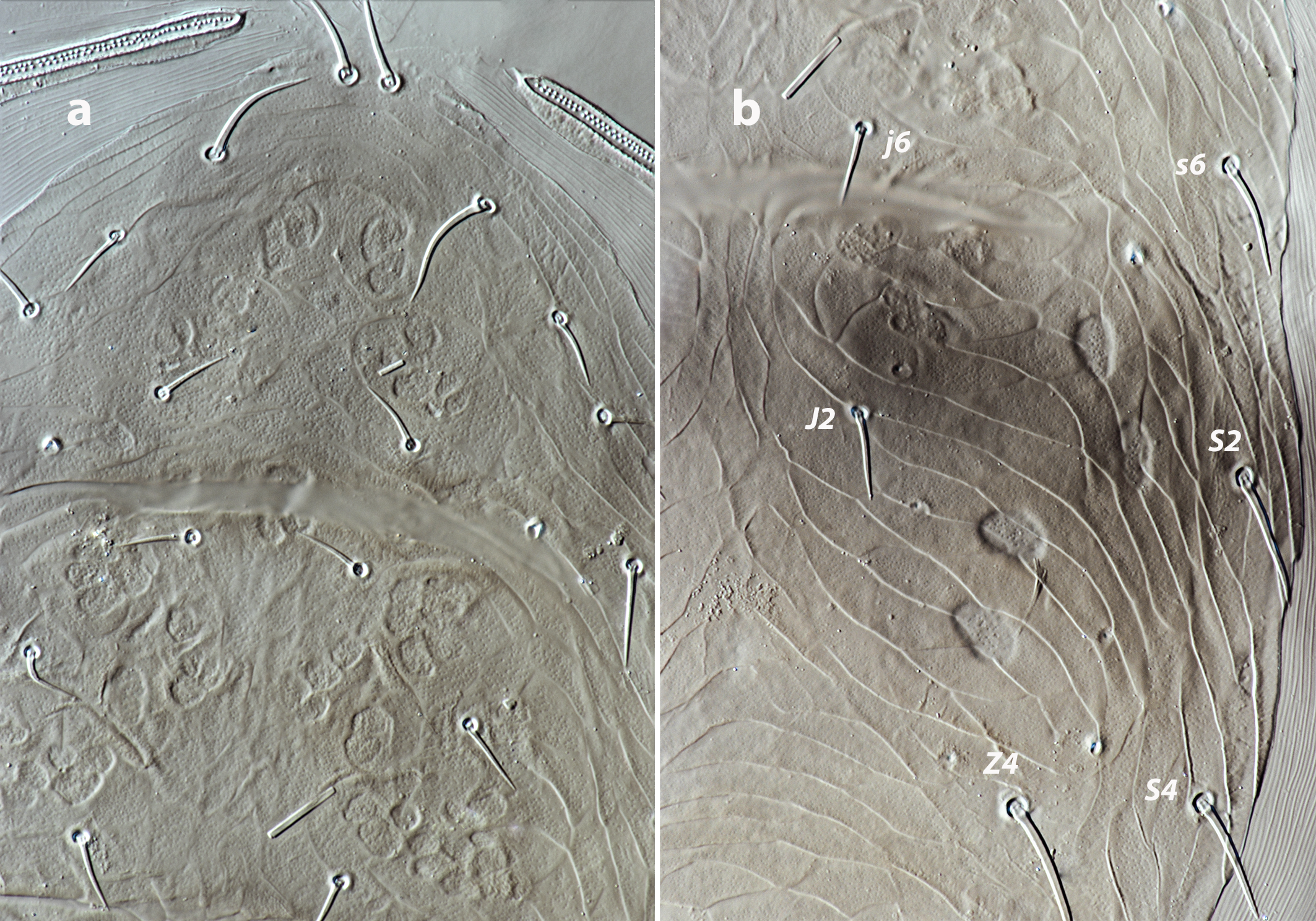
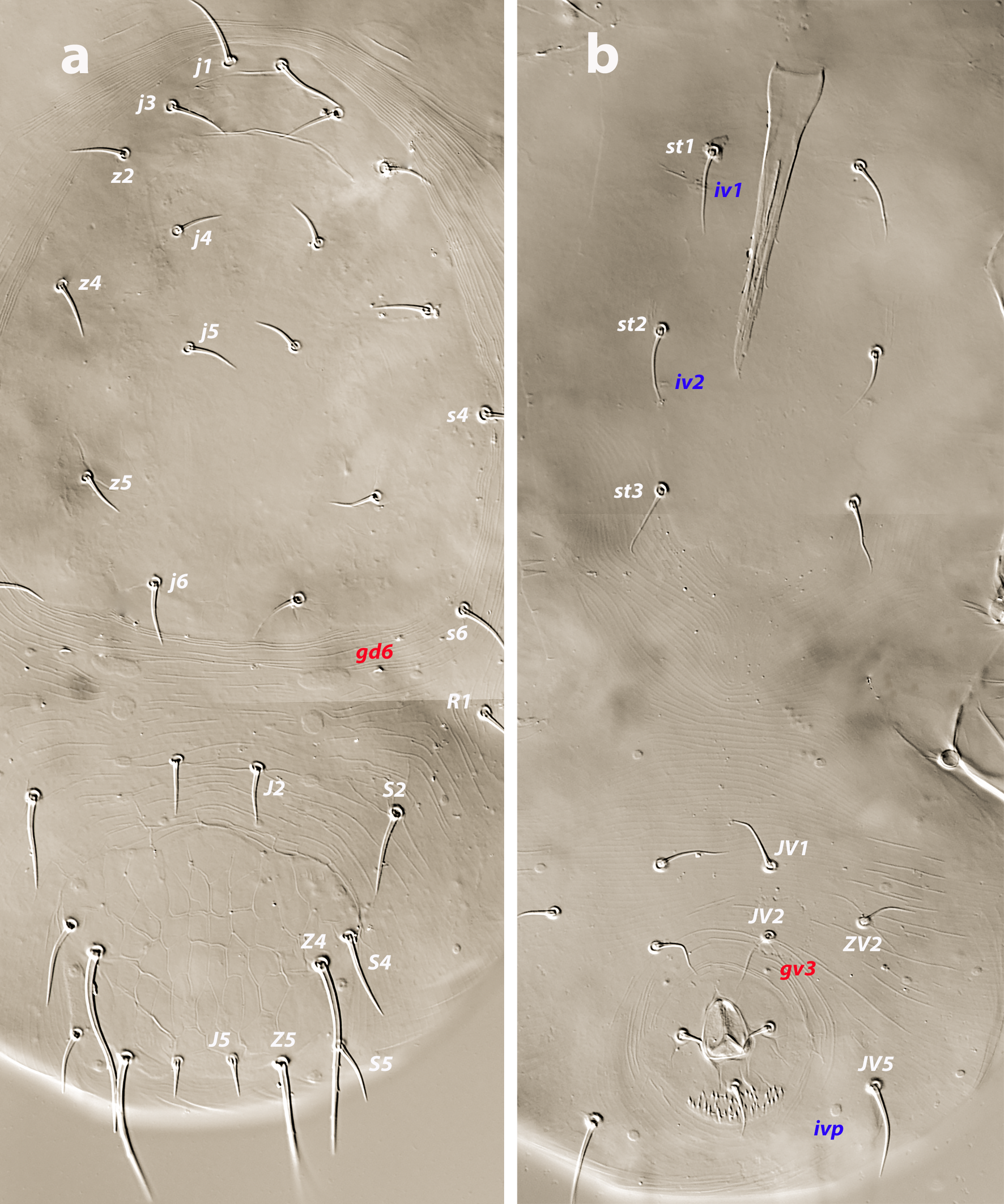
Female (twenty-eight females examined, ten females measured; Figures 1 a–e, 5 a–c)
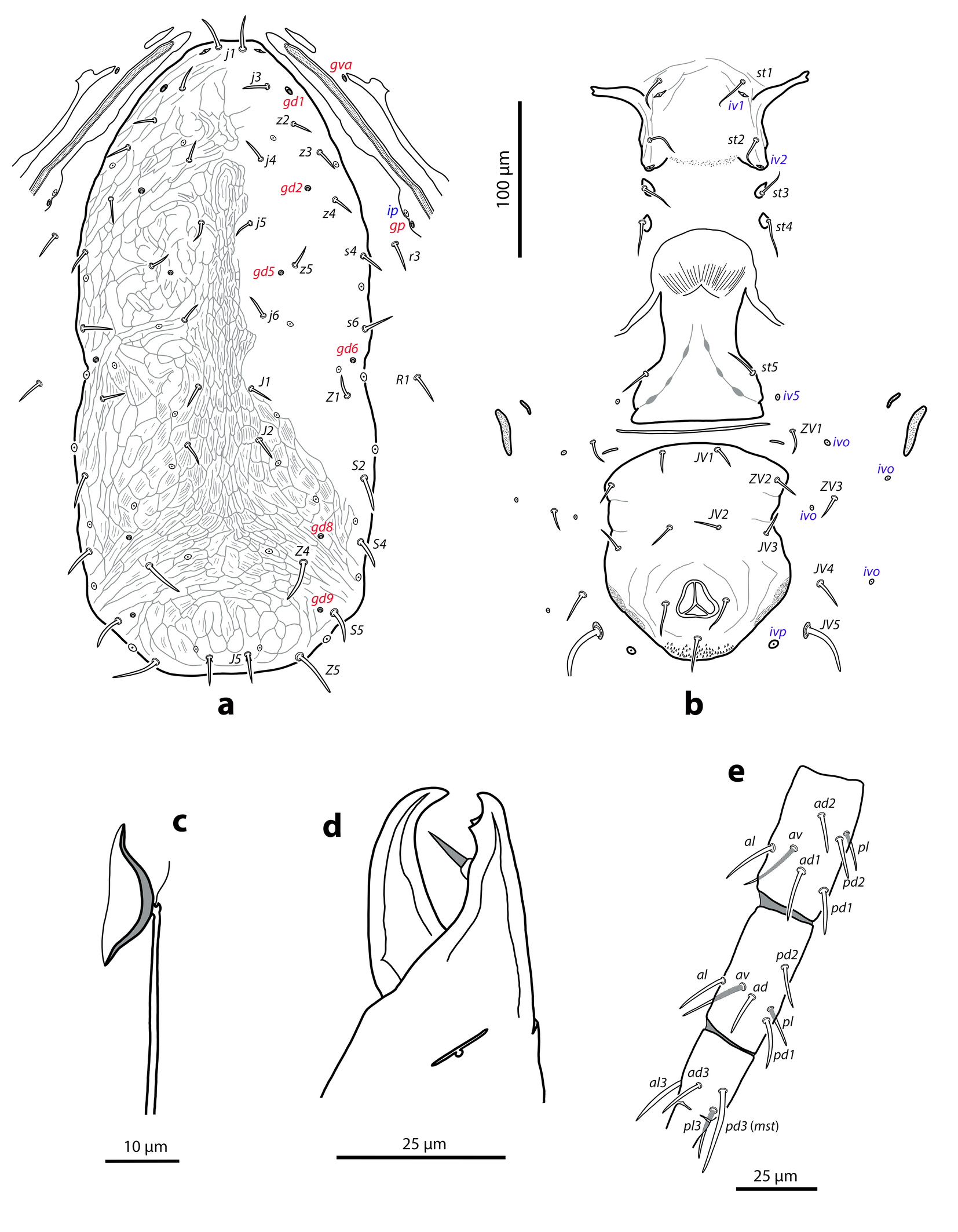


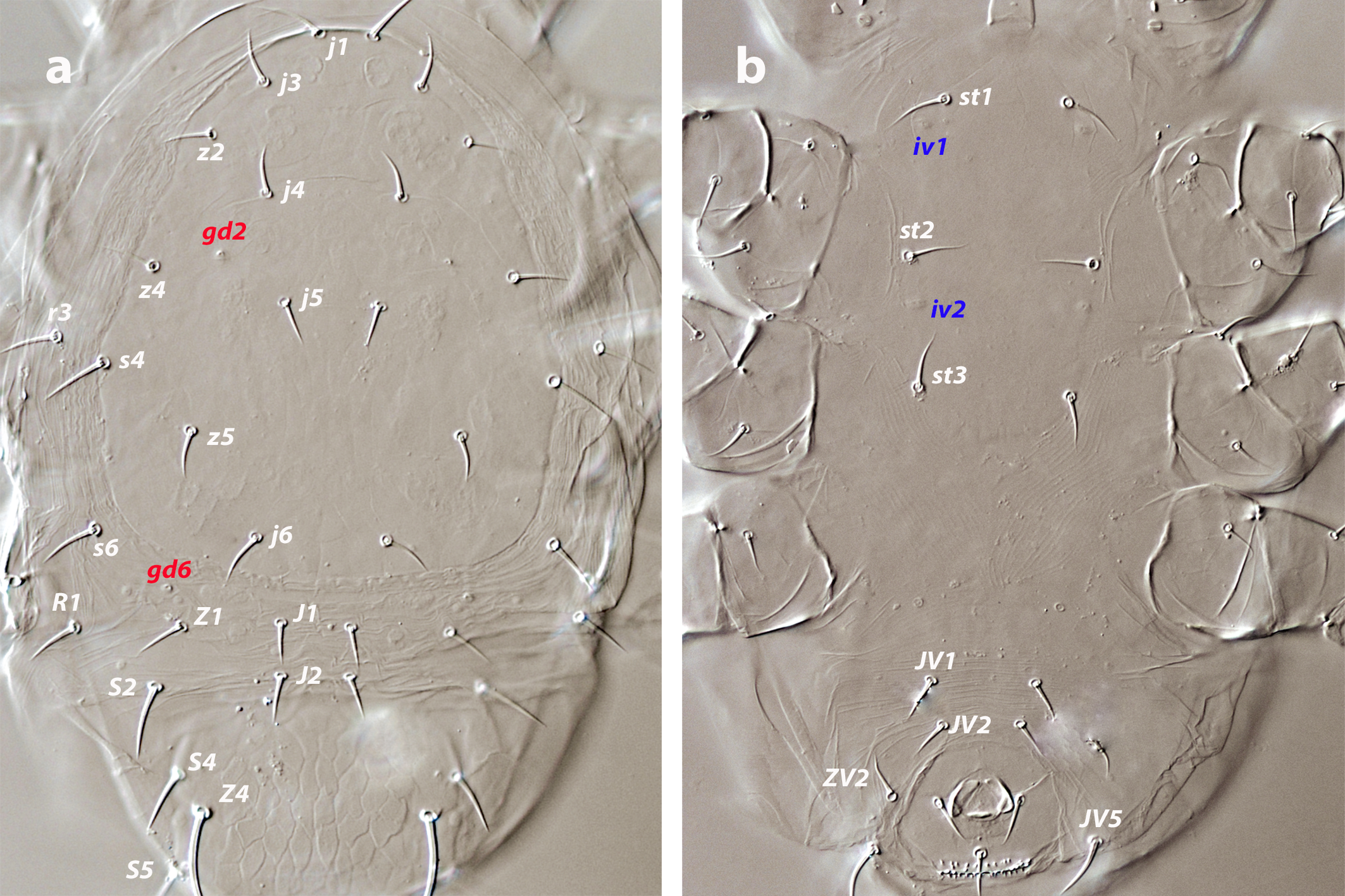
Dorsal and lateral idiosoma — (Figure 1a). Dorsal shield slender and elongate, 417 (377–420) long, 196 (175–196) wide, ratio L/W 2.1 (2.1–2.2). The lateral waist that separates podonotum from opisthonotum little pronounced. Surface evenly ornamented, consisting in lateral striae and closed cells, large cells pentagonal or elongate between the laterals and the midline, and smaller cells elongate, forming a peculiar axial pattern in the midline, between j4 and J2; posterior to that point the cells are oriented transversally to both sides of the shield until the insertions of setae Z4. Most of cells, mostly on the opisthosoma, are furrowed by longitudinal striae (Figure 5 b–c). Twenty pairs of short, smooth, and pointed setae, except Z4, Z5 blunt, and Z5, J5 barbed. Lengths of j1 22 (20–22), j3 16 (14–17), j4 12 (12–14), j5 11 (11–14), j6 13 (13–14), J1 14 (12–16), J2 13 (13–15), J5 15 (15–18), z2 16 (12–16), z3 15 (13–16), z4 13 (12–17), z5 12 (12–15), Z1 13 (13–16), Z4 28 (24–30), Z5 30 (27–35), s4 16 (14–19), s6 20 (18–22), S2 21(19–24), S4 21 (17–23), S5 22 (19–27). Sublateral setae r3 19 (19–21), R1 22 (20–25). Six pairs of dorsal solenostomes, with gland pore gd4 absent; pore opening gd1 slit-like, the remaining openings circular; pore gd1 lateral or slightly posterolateral to sockets of setae j3; pore gd8 anteroantiaxial to setae Z4; gd9 anteroparaxial to S5. The complete set of sixteen poroids present. Peritrematal shield not fused anteriorly to dorsal shield; peritremes 202 (169–214) long, reaching the level of poroid id1, between setae j1–j3; internal groove with two rows of microvilli. Peritrematal shield with poroids ip and solenostome gp on the margin of a shield widening.
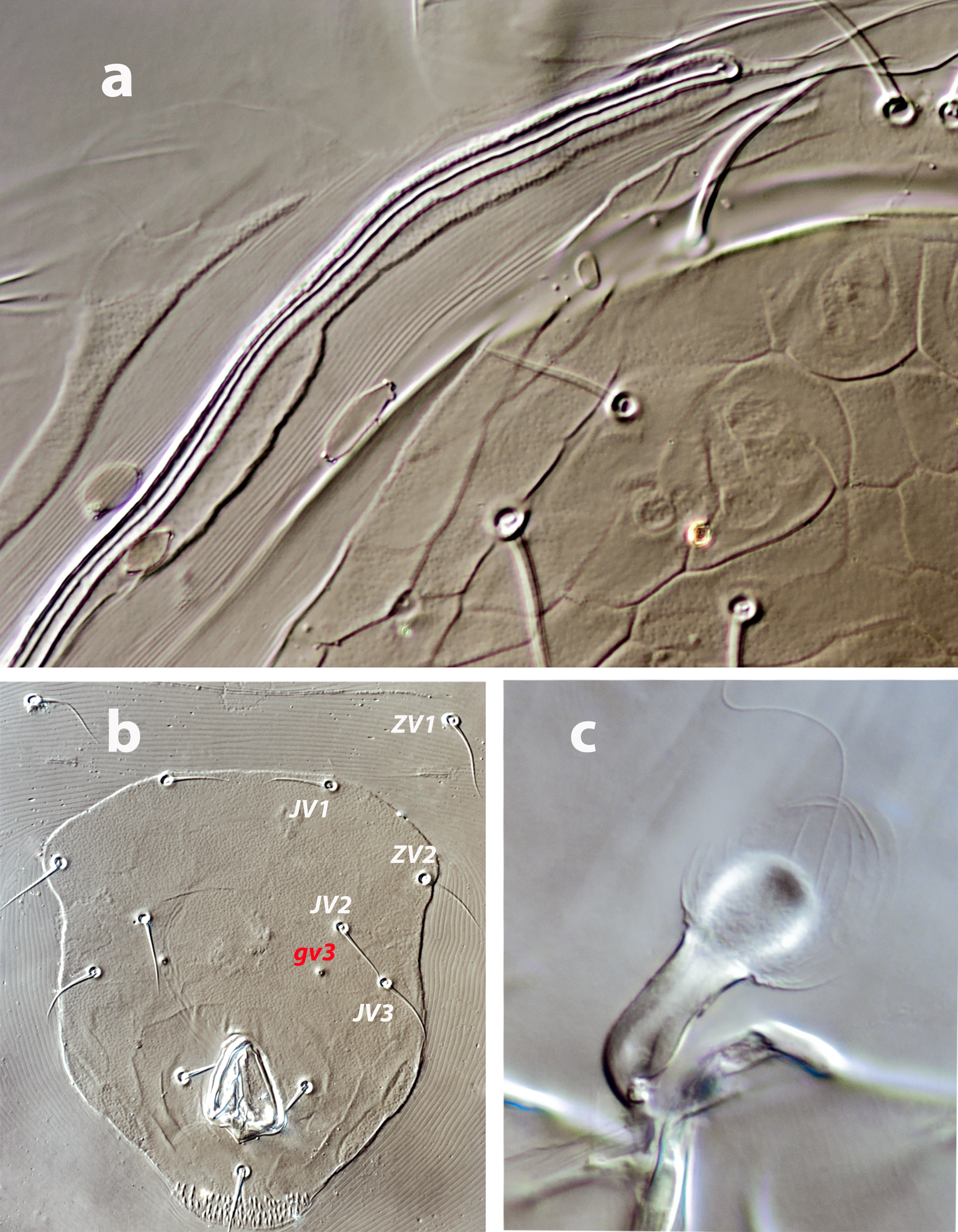
Ventral idiosoma — (Figure 1b). Tritosternum 97 (96–100) long, with two plumose laciniae 63 (51–63) long. Sternal shield with two pairs of setae, st1–st2, and two pairs of poroids iv1–iv2; anterior margin concave, posterior margin poorly defined, apparently straight; setae st3–st4 on piriform platelets, poroids iv3 not found; distance st1–st3 83 (70–83), st2–st2 63 (57–64). Epigynal shield 131 (118–136) long, distance st5–st5 60 (55–65). Ventrianal shield subpentagonal, anterior margin convex between the insertions of ZV2, laterals concave between insertions of ZV2–JV3; 141 (126–143) long, 101 (87–103) wide at level of ZV2, 90 (81–91) wide at level of anus. Shield surface with a few transverse striae on the anterior part, striae more pronounced around the anus; bearing the preanals JV1, JV2, JV3, ZV2, without preanal pores, setae JV2 anterior to the insertions of JV3. Four pairs of setae, ZV1, ZV3, JV4, JV5, and five pairs of poroids, four ivo and ivp, around the ventrianal shield. Posterior metapodal plate 32 (32–39) long. Setae JV5 pointed, 35 (30–39).
Spermathecal apparatus — (Figure 1c). Major duct tubular, of a uniform thickness; atrium simple; calyx short, bowl or dome-shaped, 3–5 deep, 17–20 wide.
Gnathosoma — (Figure 1d). Base of gnathosoma 56 (48–58) long, 91 (86–96) wide. Tectum subtriangular, anterior angle rounded, poorly defined. Hypostomal setae h1 26 (26–28), h2 20 (19–22), h3 24 (19–24), pc 25 (24–28). Length of first cheliceral segment 30 (29–32), of second segment 100 (93–102). Fixed digit of chelicera 35 (34–39) long, with two subapical teeth; mobile digit 32 (31–36) long, without teeth. Palp 121 (117–127) long; palp chaetotaxy (from trochanter to tarsus) 2-5-6-14-15; all setae smooth and simple except for femoral seta al thickened and distally spatulate and genual setae al1–al2 thickened and distally knobbed and blunt, respectively; palp apotele 2-tined.
Legs — (Figure 1e). Lengths of leg I 326 (313–335), leg II 275 (261–278), leg III 271 (251–271), leg IV 367 (347–367). Ventral coxal glands of leg I hypertrophied forming a large and paired opening (about 12 long) in the form of eight. Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 6-5-5-5; femora 12-10-6-6; genua 10-7-7-7; tibiae 10-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanters to tibiae as follows. Leg I: trochanter 1 0/1, 1/2 1; femur 2 3/1, 2/2 2; genu 2 2/1, 2/1 2; tibia 2 2/1, 2/1 2. Leg II: trochanter 1 0/1, 0/2 1; femur 2 3/1, 2/1 1; genu 2 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/0 1. One moderately long macroseta on tarsus IV (pd3), smooth and blunt apically, slightly curved at the base, 27 (25–31).
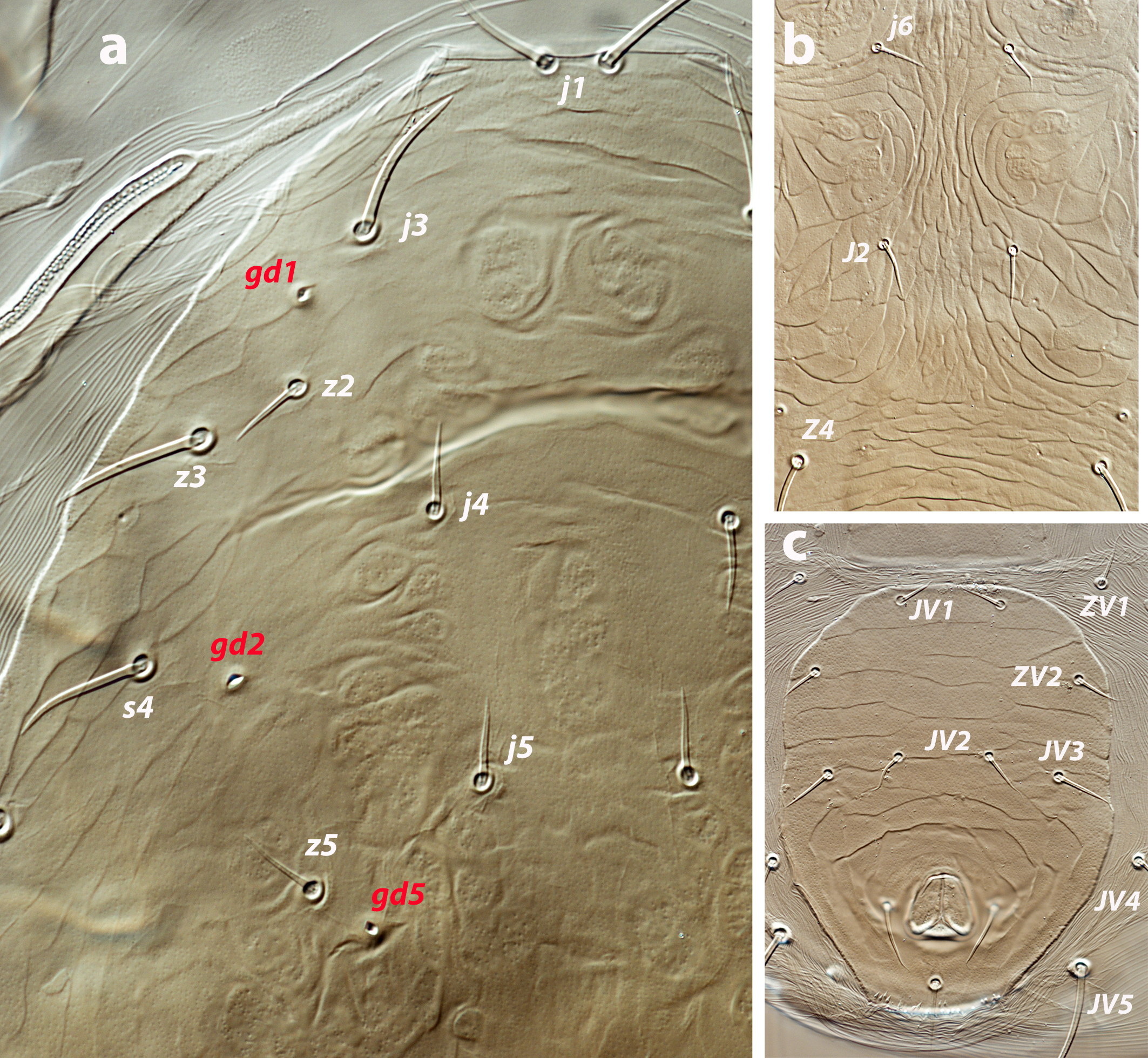
Male (five examined and measured; Figures 2 a–c).
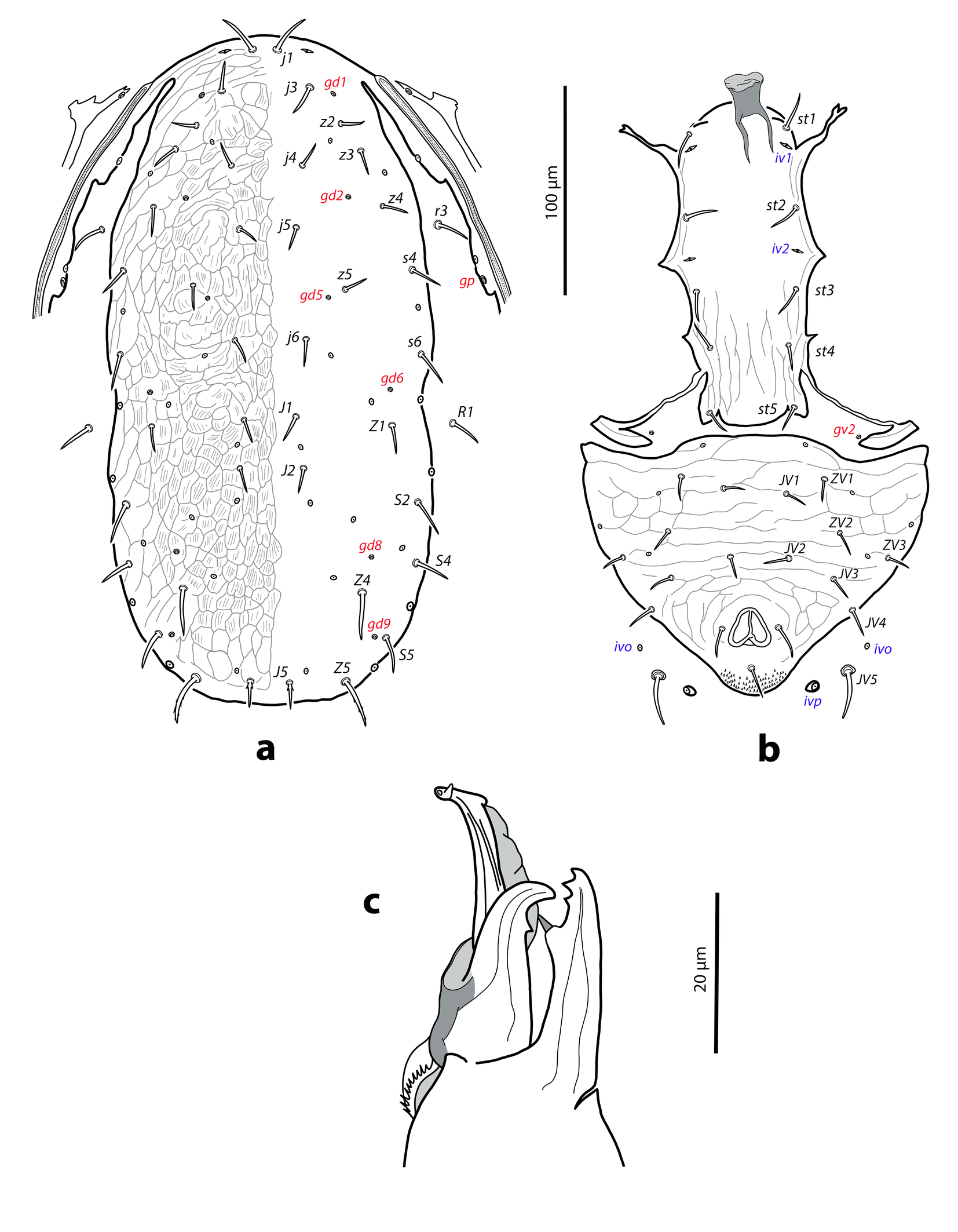


Dorsal and lateral idiosoma — (Figure 2a). Dorsal shield elongate, ratio L/W 2.1–2.2, similar to that of female by the narrowing of the shield at the level of r3 and R1, both setal pairs not captured by the dorsal shield; 327 (320–331) long, 158 (148–158) wide. Surface thoroughly reticulated; most of cells multistriated, following the female ornamental pattern. Peritrematal shield attached to the dorsal shield in unusual anterior position, at the level of j3. Twenty pairs of short, smooth and pointed dorsal setae excepting Z5 and J5 barbed. Lengths of j1 21 (20–21), j3 15 (15–16), j4 12 (10–13), j5 11 (11–12), j6, J1, J2 13 (12–13), J5 15 (13–15), z2 12, z3 13 (12–14), z4 15 (13–15), z5 11 (11–12), Z1 14 (13–15), Z4 23 (23–24), Z5 25 (24–25), s4 15 (15–16), s6 18 (16–18), S2 20 (18–21), S4 17 (16–18), S5 19 (18–19). Sublateral setae r3 16 (16–19), R1 18 (17–18). Dorsal adenotaxy and poroidotaxy as in the female; gland opening gp on the peritrematal shield. Peritremes arriving anteriorly to the level of solenostome gd1, between setal insertions of j3–z2; length 151 (148–152).
Ventral idiosoma — (Figure 2b). Sternogenital shield with five pairs of setae (st1–st5) and three pairs of poroids (iv1–iv3), smooth, except on the posterior region covered by longitudinal faint striae; posterior margin of the shield lobate; posterolateral margins partially attached to the exopodal plates; distance st1–st5 135 (130–136), st3–st3 47 (46–49). Exopodal plates not fused with the ventrianal in the males examined; gland pore gv2 on the soft cuticle. Ventrianal shield subtriangular attached to the peritrematal shield, anterior margin convex; 125 (121–127) long, 160 (150–163) wide. Surface evenly striate, transversal striae on the anterior part, concentric striae around the anal opening; some transversal striae can be connected forming closed cells. Seven pairs of preanal setae (JV1, JV2, JV3, JV4, ZV1, ZV2, ZV3) and four pairs of poroids (iv5, three ivo) on the shield. Soft integument around the shield bearing the pair JV5, 24 (23–25) long, and poroids ivo and ivp.
Chelicerae and spermatodactyl — (Figure 2c). Fixed digit of chelicera 27 (27–28) long, with two teeth distal to pilus dentilis; movable digit 25 (24–26), with one strong tooth. Spermatodactyl L-shaped, with a short foot and heel not developed; shaft 19 (19–21), foot 5 (4–6) long; one lateral and pointed projection facing upwards, near the distal end of foot. Vellum present.
Legs — Leg chaetotaxy similar to female; macroseta on tarsus IV smooth and blunt, 26 (24–28) long.
Deutonymph (two examined and measured; Figure 3 a–b).


Dorsal idiosoma — (Figure 3a). Dorsal shield 298–333 long, 140–154 wide; lateral margins partially incised at the level of gland pore gd6. Shield ornamentation similar to that of female, though less accentuated. Most of cells on the podosomal midline and throughout the opisthosoma multi-striated. Twenty pairs of smooth and pointed dorsal setae, except Z5, J5 barbed. Lengths of j1 21–23, j3 17, j4 13–14, j5 12, j6 13–14, J1 12–13, J2 13–14, J5 13, z2, z3 14, z4 14–15, z5 12–14, Z1 13–14, Z4 37–38, Z5 33–36, s4 16–18, s6 17–19, S2 24–25, S4 22–25, S5 25–27. Sublateral setae r3 18, R1 18–19. Six pairs of solenostomes, without gland pore gd4, and sixteen pairs of poroids. Peritremes 145–157 long, reaching anteriorly the level of setae z2.
Ventral idiosoma — (Figure 3b). Base of tritosternum 12–13 wide, total length 94–97, laciniae 49–51. Sternogenital shield well-defined, elongate and smooth, with four pairs of setae (st1–st4) and two pairs of poroids (iv1–iv2); setae st5 on the soft cuticle; distance st1–st5 150–156, st3–st3 52–54. Opisthogastric region with eight pairs of setae (ZV1, ZV2, ZV3, JV1, JV2, JV3, JV4, JV5) and five pairs of poroids (four ivo and ivp) surrounding the anal plate. Setae JV5 smooth and pointed, 35–37.
Gnathosoma — Gnathosomal base 69–74 long, 78–83 wide. Deutosternum with seven rows of paired and lateral denticles. Hypostome with four pairs of setae, h1 24–26, h2 19–20, h3 19–22, pc 23. First cheliceral segment 25–26, second segment 82–86. Fixed digit of chelicera 30–32 long, with two teeth, movable digit 26–29, without teeth; cheliceral seta present, very short and subtle, about 2 m. Palp 113–116 long; palp chaetotaxy (from trochanter to tarsus) 2-5-6-14-15; femoral seta al thickened and expanded apically, genual setae al1 pointed and longer than al2, which is blunt; apotele 2-tined.
Legs — Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 6-5-5-5; femora 12-10-6-6; genua 10-7-7-7; tibiae 10-7-7-6; basitarsi 0-4-4-4. Setal formulas as follows. Leg I: trochanter 1 0/1, 1/2 1; femur 2 3/1, 2/2 2; genu 2 2/1, 2/1 2; tibia 2 2/1, 2/1 2. Leg II: trochanter 1 0/1, 0/2 1; femur 2 3/1, 2/1 1; genu 2 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/0 1. Macroseta on tarsus IV (pd3) pointed, 30–33. Pore-like structures resembling gland openings on the distal margin of femur, genu and tibia, and on the proximal margin on tarsus of legs I–IV.
Protonymph (one examined and measured; Figure 4 a–b)


Dorsal idiosoma — (Figure 4a). Podonotal shield 147 long, 120 wide. Surface smooth, with nine pairs of setae (j1, j3, j4, j5, j6, z2, z4, z5, s4), poroids id5, id2, id4, and solenostomes gd1 and gd2. Setae r3 and s6 on the soft integument. Mesonotum between the podonotal and pygidial shields bearing the setal pairs J1, J2, Z1, S2, and R1, the gland pore gd6 and the poroids idl1, idl2, and idm1. Pygidial shield 59 long, 74 wide, reticulate, composed by elongated cells, pentagonal or hexagonal. Five pairs of setae (J5, S4, S5, Z4, Z5), four pairs of poroids (idm4, idm5, idl4, idl5) and gland openings gd8, gd9, on the shield. Setae Z5 barbed, J5 basally barbed. Lengths of j1 19, j3 15, j4 13, j5 12, j6 14, J1 11, J2 12, J5 11, z2 13, z4 16, z5 12, Z1 13, Z4 19, Z5 36, s4 19, s6 16, S2 19, S4 17, S5 19, r3 16, R1 15. Peritremes very short, 34 long.
Ventral idiosoma — (Figure 4b). Base of tritosternum 12 wide, total length 76, laciniae 39. Margins of sternal shield poorly defined, with three pairs of setae, st1–st3, and two pairs of poroids, iv1, iv2. Setal nubs of st5 discernible. Setae JV1, JV2, ZV2, JV5, and poroids ivo and ivp around the anal plate. Setae JV5 blunt, 19 long.
Gnathosoma — Base of gnathosoma 48 long, 68 wide. Deutosternal groove with seven rows of denticles. Setae h1 17, h2 16, h3 19, pc 18. First cheliceral segment 19, second segment 64. Fixed digit 26, with two subapical teeth; mobile digit 23, without teeth. Palp 91 long; palp chaetotaxy (from trochanter to genu) 1-4-5; femoral setae al slightly expanded apically, setae al on genu blunt; apotele 2-tined.
Legs — Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 4-4-4-4; femora 10-7-5-4; genua 8-6-6-5; tibiae 8-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanter to tibia as follows. Leg I: trochanter 1 0/1, 0/1 1; femur 2 2/1, 2/1 2; genu 1 2/1, 2/1 1; tibia 1 2/1, 2/1 1. Leg II: trochanter 1 0/1, 0/1 1; femur 1 2/1, 2/0 1; genu 1 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 0/1, 0/1 1; femur 1 2/1, 1/0 0; genu 1 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/1 0; femur 1 2/0, 1/0 0; genu 1 2/0, 2/0 0; tibia 1 1/1, 2/0 1. Macroseta on tarsus IV 29 long.
Larva. Not found
Etymology
The specific name extraseta is derived from the Latin terms ''extra» meaning above or beyond the common or ordinary and ''seta″, in reference to the additional pair J1 occurring in this species, which represents a novel dorsal setal pattern in the genus Neoseiulella.
Comparative diagnosis and remarks
Neoseiulella extraseta sp. nov. features the new dorsal setal pattern 12A:10B in the genus by the addition of setae J1 on the opisthosoma. The occurrence of this setal pair has been previously mentioned by some authors in another member of Neoseiulella. Rivnay and Swirski (1980) described N. montforti (Rivnay & Swirski) from Israel mentioning and depicting a member of the pair J1 on the dorsal shield of one specimen. Denmark and Rather (1984) examined a female paratype and reported that the specimen had the two members of setae J1. Later, Chant and Yoshida–Shaul (1989) agree with the original description by studying an additional paratype and illustrate a single seta J1 considering that this character is ''an aberration» in the normal dorsal chaetotaxy. By contrast, all the specimens of N. extraseta sp. nov. examined in this study plus an abundant set of specimens collected in the nearby island of Lanzarote (unpublished data) have the complete pair J1, indicating that this setal pair is stable for the species. Furthermore, N. montforti differs from the new species by additional features, such as the presence of preanal solenostomes on the ventrianal shield, the absence of gland pores gd5 and the presence of gd4, the cup-shaped calyx of the spermatheca, and the longer dorsal setae with ratios J2/J5 equal to 2.8 instead of 0.9 in N. extraseta sp. nov. In the original description of N. montforti it is said that the ventrianal shield lacks solenostomes, but the drawing that accompanies it (Fig. 15 in Rivnay and Swirski, 1980) shows a pair of small punctiform solenostomes. Likewise, the later redescriptions of the species that examined type material (Chant and Yoshida-Shaul, 1989; Denmark and Rather, 1984, 1996; Kanouh et al. 2012) confirm the presence of these pores and include them in their illustrations. The existence of this morphological character is also indicated in the revision of the phytoseiids of Israel (Swirski et al. 1998). Table 1 lists the Neoseiulella species related to N. extraseta sp. nov., all of them native to the Mediterranean basin and the Macaronesian islands. Neoseiulella extraseta sp. nov. is the only one with a flattened spermathecal calyx, and the only one, alongside N. elongata Ferragut & Peña-Estévez, in having short dorsal setae; however, the latter taxon only has four pairs of dorsal solenostomes, the dorsal ornamentation is distinct, and the peritremes are shorter, with the internal groove striate. The new species also differs from the others by the greater length of setae j1 and J5 in relation to setae j3 and J2, respectively. Differences between the new species and N. litoralis (Swirski & Amitai) are discussed below. Neoseiulella paralias Stathakis, Kapaxidi & Papadoulis (not included in the table) also resembles the new species, but it can easily be differentiated by having three macrosetae on leg IV, cup-shaped calyx of the spermatheca, and six pairs of preanal setae on the male ventrianal shield (Stathakis et al. 2016).
Download as
N. extraseta sp. nov.
N. carmeli (Rivnay & Swirski) 1
N. elongata Ferragut & Peña-Estévez 2
N. kazaki Döker 3
N. litoralis (Swirski & Amitai) 4
N. perforata (Athias–Henriot) 5
N. splendida Ferragut & Peña-Estévez 6
Calyx spermatheca
shallow, bowl-shaped, 3–5 deep
cup or bell-shaped, 12 deep
cup or bell-shaped, 12 deep
cup or bell-shaped, 10–11 deep
cup or bell-shaped, 10–12 deep
cup or bell-shaped, 11 deep
cup or bell-shaped, 12 deep
Setae genu II
7
6
7
8
7
8
7
Dorsal solenostomes (pairs)
6 (without gd4)
6 (without gd5)
4 (without gd1, gd4, gd5)
4 (without gd4, gd5, gd8)
6 (without gd4)
6 (without gd4)
6 (without gd4)
Sternal setae (pairs)
2
3
2
2
2
2
2
Ratio j1/j3
1.4
0.8
1.3
0.6
0.9
1
0.9
Ratio J2/J5
0.9
2.3
1.5
2.2
1.3
1.3
1.1
Length S2
19–24
35–40
18–21
41–42
35–38
27–30
42–49
Length Z5
27–35
51–56
26–36
50–52
42–66
50–53
70–80
Male preanal setae (pairs)
7 pairs
–
–
–
6 pairs
6 pairs
7 pairs
Distribution
Canary Islands
Israel
Canary Islands
Türkiye
Mediterranean, Macaronesia
West Mediterranean
Canary Islands
Neoseiulella extraseta sp. nov. exhibits some very original morphological traits in both, females and males. The female and male dorsal ornamentation is made of striated cells resembling scales, which form characteristic patterns comprised of cells of different size and shape arranged in a characteristic way on the shield. This suggests that a detailed description of the dorsal sculpture may provide useful insights as well as additional characters for species diagnosis in the genus. The female peritrematal plate is not fused anteriorly with the dorsal shield, often it is fully separated or at most attached to the dorsal shield by a bridge of soft integument. This feature has never been mentioned in the literature for Neoseiulella species, but can also be observed in at least N. litoralis (see Figures 5a and 6a).


The dorsal aspect of the male is eye-catching as the lateral sides of the dorsal shield are roughly parallel, with the podonotal region about as wide as the opisthonotal rather than wider than the opisthonotal, as is the norm in the males of Phytoseiidae. Because of this narrowing, the setal pairs r3 and R1, commonly found on the shield, are in the males of this species placed on the soft integument, like in the females. Furthermore, the peritrematal shield is not merged with the dorsal plate at the level of setae r3 as usual in other species, but in a very anterior position, near the insertion of setae j3, as in females.
In the ontogenetic development, dorsal setae z3, dorsal solenostomes gd1 and gd5, and ventral setae st5 (visible as incipient setal nubs), JV3, JV4, ZV1, ZV3 are added to the deutonymph. Additionally, some leg setae are incorporated in the moult to deutonymphal stage. On leg I, setae ad1 and pv2 of trochanter; ad3 and pv2 of femur; al2, pl2 of both genu and tibia are added. On leg II seta pv2 of trochanter; setae al2, ad3, pv1 of femur; al2 of genu are incorporated. On leg III, ad1 of trochanter, pl1 of femur, and av1 of genu are added. On leg IV, pv2 of trochanter; av1, pl1 of both femur and genu first appeared in the deutonymph. No differences in leg chaetotaxy were observed between the deutonymphal and adult stages.
Neoseiulella litoralis (Swirski & Amitai, 1984)
Typhloctonus litoralis Swirski & Amitai, 1984: 73.
Neoseiulella (Typhloctona) litoralis Denmark & Rather, 1996: 71.
Neoseiulella litoralis Swirski & Amitai, 1997: 37.
Specimens examined and depository
Ten females, three males, four deutonymphs, one protonymph on Ononis hesperia (Maire) H. Förther & Podlech (Fabaceae) and two females on Cakile maritima (Brassicaceae); Dunas de Corralejo, 28°40′13″N, 13°50′00″W, 12 m a.s.l.; 27 May 2024. Three females on Suaeda vera Forsskål ex J.F. Gmelin (Amaranthaceae) and three females on Suaeda vermiculata Forsskål ex J.F. Gmelin (Amaranthaceae); Isla Lobos, 28°45′13″N, 13°49′35″W, 15 m a.s.l.; 28 May 2024. One female on Suaeda vermiculata (Amaranthaceae); Betancuria, mirador Morro Velosa, 28°26′68″N, 14°02′59″W, 663 m a.s.l.; 29 May 2024. Twelve females, six males, two deutonymphs, two protonymphs on Caroxylon tetrandrum (Forsskål) Akhani & Roalson (Amaranthaceae); Morro Jable. El Saladar, 28°03′03″N, 14°19′34″W, 2 m a.s.l.; 30 May 2024.
Thirteen females, one male, and three deutonymphs were deposited in the Museo de Ciencias Naturales de Tenerife, Santa Cruz de Tenerife, Canary Islands. The remaining specimens are in the Acari collection, Instituto Agroforestal Mediterráneo, Universidad Politécnica de Valencia.
Geographical distribution
Mediterranean and Macaronesian species, distributed from Israel in the east to the African Atlantic islands of Cape Verde in the west. Usually found on plants near the coast. This is the first report in the Canaries.
Diagnosis
Female dorsal shield suboval, thoroughly reticulate; cells on the dorsal midline between setae j5–J2 narrow, elongate and oriented axially, which posteriorly become larger and divergent heading towards solenostomes gd8 and insertions of Z4; cells smooth, not striated. Nineteen pairs of dorsal setae; setae j1 similar in length to j3; z2 short, near setae z3 and in some females distinctly paraxial to the line j3–z3; setae z3 twice as long as z2; setae J2 longer than J5. Six pairs of dorsal solenostomes, gland pore gd4 absent. Peritremes ending between j3–z2; peritrematal shield not fused anteriorly with dorsal shield. Sternal shield with two pairs of setae. Ventrianal shield transversally striate, with four pairs of preanal setae and without preanal pores; margin anterior to setae ZV2 broadly convex. Spermathecal calyx cup-shaped, 9–10 deep. Fixed digit of chelicera with three subapical teeth, only two visible in profile; movable digit with 0–1 tooth. Genua of legs II–IV with seven setae each; one macroseta on leg IV. Male peritrematal shield fused to the dorsal shield at the level of j3; setae r3 on the soft cuticle, R1 on the dorsal shield. Ventrianal shield with six pairs of preanal setae, without solenostomes. Spermatodactyl L-shaped; heel rounded and reduced in size; foot short; lateral projection short, pointed, at the end of foot.
Complementary description
Female (Thirty-one females examined, ten females measured; Figures 6 a–c)


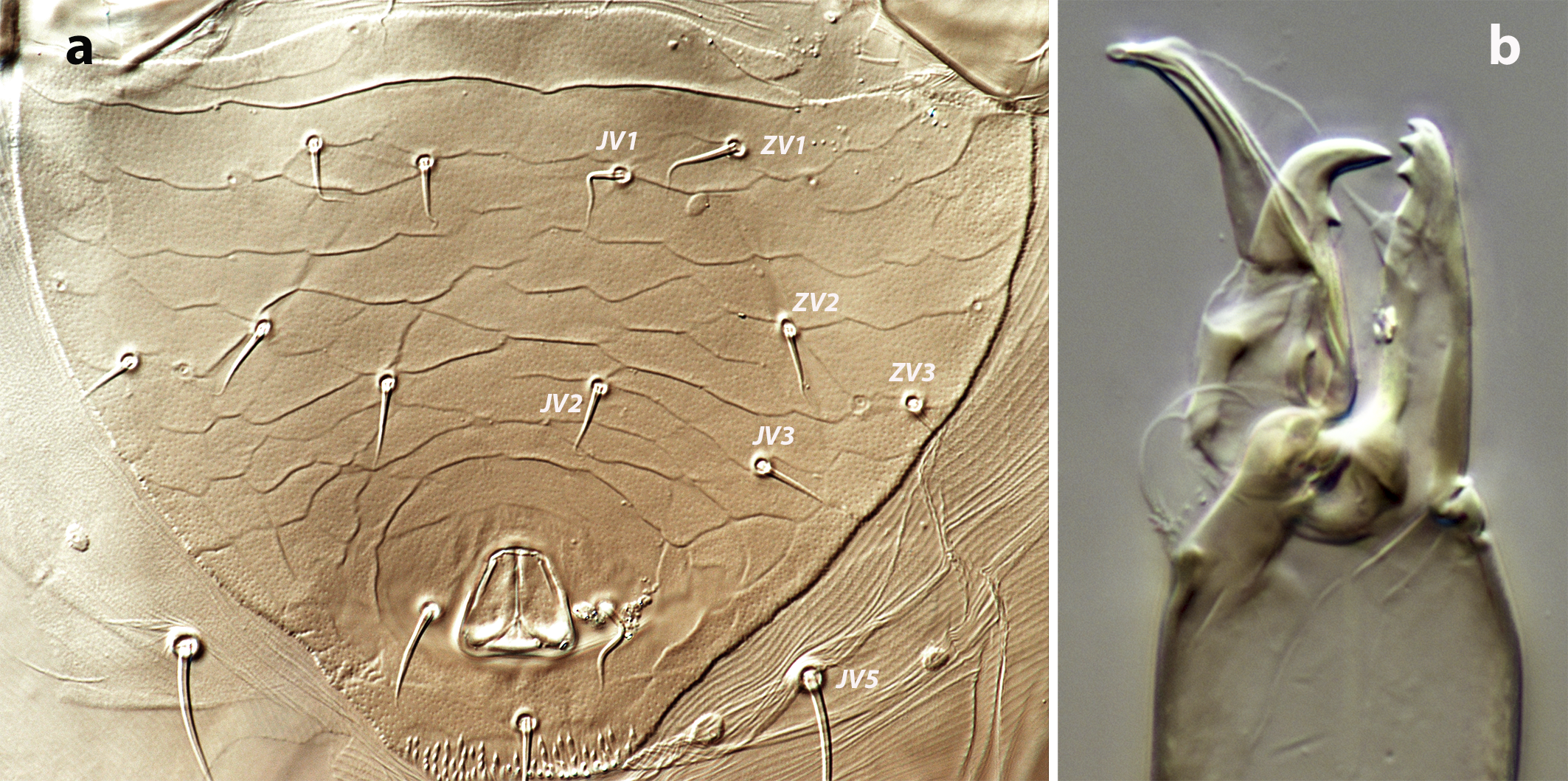
Dorsal and lateral idiosoma — (Figures 6 a–b). Dorsal shield suboval, lateral margins with a wide concave waist between setae s6 and poroids idl2; 347–398 long, 155–189 wide, ratio L/W = 2.1–2.3; surface reticulate, except on the anterolateral margins, which are striate-reticulate and the region between the insertions of Z4, with transversal striae which can form irregular cells; ornamental pattern with midline cells (j5–beyond J2) narrow, elongate, and axially oriented, posterior cells becoming larger and diverging towards gland pores gd8; cells smooth, without striae. Nineteen pairs of dorsal setae, all smooth and pointed except Z5 barbed and blunt and J5 basally barbed. Lengths of j1 20–25, j3 19–25, j4, j5 12–14, j6 15–18, J2 16–21, J5 13–15, z2 10–13, z3 19–24, z4 17–21, z5 12–14, Z1 15–19, Z4 32–45, Z5 42–57, s4 21–26, s6 21–30, S2 27–40, S4 21–28, S5 21–27. Sublateral setae r3 19–24, R1 20–25. Six pairs of dorsal solenostomes, gd4 absent; all the gland openings conspicuous and circular. Sixteen pairs of dorsal poroids. Peritremes arriving anteriorly at the level of pore gd1, between insertions of j3 and z2; 138–165 long. Peritrematal shield usually extends anteriorly through a narrow and sclerotized strip, but without merging with the dorsal shield.
Ventral idiosoma — (Figure 6c). Tritosternum 80–85 long, with two plumose laciniae 43–46 long. Sternal shield with two pairs of setae and two pairs of poroids, poroids iv2 just on the posterior margin; lateral margins straight, posterior margin concave and irregular; distance st1–st3 66–73, st2–st2 54–61. Setae st3 and st4 on platelets. Epigynal shield narrower than the ventrianal; 112–131 long, distance st5–st5 51–70. Ventrianal shield transversally striate, with striae bending around the anal opening (Figure 6c); 119–139 long, 89–102 wide at level of ZV2, 76–88 wide at level of anus; anterior margin of the shield sharply convex, laterals straight; with four pairs of preanal setae and without preanal pores. Opisthogastric region around the ventrianal plate with setal pairs ZV1, ZV3, JV4, JV5, and poroids ivo (four pairs) and ivp. Setae JV5 smooth, on small platelets, 36–45 long. Posterior metapodal shield bacillar, with laterals straight, 29–42 long.
Spermatheca — Major duct thin and tubular, 25–27 long; atrium simple; calyx cup-shaped, with thin walls; 9–10 deep, 14–16 wide.
Gnathosoma — Gnathosomal base 40–43 long, 83–88 wide. Tectum subtriangular, anterior angle rounded. Hypostomal setae h1 22–25, h2 19–20, h3 23-26, pc 24–28. First cheliceral segment 23–28, second segment 80–88 long. Fixed digit on chelicerae 27–36 long, with three subapical teeth, almost parallel, commonly only two visible in lateral view; movable digit 26–34 long, usually with one tooth, but some females without teeth. Palp 111–116 long: anterolateral setae (al) on palpfemur thicker than the others on the segment, but scarcely spatulated distally; on the palpgenu setae al1 longer than al2, setae al1 pointed, al2 blunt.
Legs — Ventral coxal glands of leg I hypertrophied with an opening in the form of eight (about 10 long). Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 6-5-5-5; femora 12-10-6-6; genua 10-7-7-7; tibiae 10-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanters to tibiae as follows. Leg I: trochanter 1 0/1, 1/2 1; femur 2 3/1, 2/2 2; genu 2 2/1, 2/1 2; tibia 2 2/1, 2/1 2. Leg II: trochanter 1 0/1, 0/2 1; femur 2 3/1, 2/1 1; genu 2 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/0 1. One macroseta on tarsus IV (pd3), smooth and blunt, 32–36 long.
Male (nine examined, five measured; Figure 7 a–b)


Dorsal and lateral idiosoma — Dorsal shield suboval, 302–330 long, 147–175 wide; weakly reticulate, ornamental pattern resembling that of female but with midline cells broader and more irregular in shape. Twenty pairs of smooth and acuminate dorsal setae, except Z4, Z5, blunt, and J5 basally barbed. Lengths of j1 19–22, j3 19–21, j4 11–13, j5 10–13, j6 13–14, J2 13–16, J5 12–14, z2 10–12, z3 17–21, z4 17–19, z5 11–14, Z1 12–16, Z4 30–40, Z5 37–43, s4 19–21, s6 21–26, S2 23–31, S4 16–21, S5 13–21, r3 17–18, R1 17–19. Setae r3 always on the soft integument, R1 on the shield. Six pairs of dorsal solenostomes (gd4 absent), openings with the same morphology as on females. Peritremes ending at the level of gland pores gd1 and gva, between setae j3–z2; 128–132 long. Peritrematal shield merged with dorsal shield at the level of j3 insertions or even between j1–j3.
Ventral idiosoma — (7a). Sternogenital shield mostly smooth, with a few lateral striae; posterior margin convex, lobate; distance st1–st5 121–137, st3–st3 47–61. Exopodal plates not attached to the ventrianal shield. Ventrianal shield subtriangular, broadly convex anteriorly, covered by transversal striae, 122–130 long, 152–179 wide; with six pairs of preanal setae (JV1, JV2, JV3, ZV1, ZV2, ZV3) and without preanal solenostomes. Setae JV5 24–29, blunt and smooth, on small platelets.
Chelicera and spermatodactyl — (Figure 7b). Cheliceral fixed digit 24–28 long, with two subapical teeth, the distal tooth larger than the proximal; movable digit with a strong tooth, 22–24 long. Spermatodactyl L-shaped; shaft 17–18 long; heel rounded, slightly developed; foot short, 5–6; lateral projection near the toe, acute, directed upwards; vellum present.
Legs — Ventral coxal gland on leg I similar to that of female. Leg chaetotaxy as in females. One macroseta on tarsus IV (pd3), smooth and blunt, 28–34 long.
Deutonymph (six examined and measured; Figure 8 a–b)



Dorsal and lateral idiosoma — (Figure 8a). Dorsal shield 291–308 long, 124–142 wide, lateral margins widely invaginated at the level of gland pores gd6 and poroids idl1, both cuticular organs on the posterior margin of invagination. Surface of the shield weakly reticulate, cells more marked on the opisthonotum. Nineteen pairs of smooth and pointed dorsal setae, except for Z5, J5, barbed. Lengths of j1 19–21, j3 18–20, j4, j5 11–14, j6 14–16, J2 13–16, J5 12–13, z2 12–15, z3 15–18, z4 14–17, z5 12–14, Z1 14–19, Z4 39–46, Z5 42–45, s4 20–23, s6 21–24, S2 25–31, S4 19–25, S5 21–25. Sublateral setae r3 17–19, R1 16–21. Six pairs of dorsal solenostomes, without gland pore gd4, and sixteen pairs of poroids. Peritremes 130–135 long, reaching the level of setae z2.
Ventral idiosoma — (Figure 8b). Sternogenital shield poorly sclerotized, with irregular margins, anterior margin deeply concave; setae st1–st4 on the shield, st5 on the soft cuticle; distance st1–st5 137–144, st3–st3 50–58. Eight pairs of setae (ZV1, ZV2, ZV3, JV1, JV2, JV3, JV4, JV5) and five pairs of poroids (four ivo and ivp) around the anal plate. Setae JV5 smooth and blunt, 31–36.
Gnathosoma — Hypostomal setae h1 21–23, h2 15–17, h3 19–21, pc 21–24. Fixed digit of chelicera 25–27, with two subapical teeth; movable digit 23–25, with one small tooth. Palp 93–101 long; tip of seta al on femur slightly expanded, al1 on genu acuminate, al2 blunt.
Legs — Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 6-5-5-5; femora 12-10-6-6; genua 10-7-7-7; tibiae 10-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanter to tibia as follows. Leg I: trochanter 1 1/1, 0/2 1; femur 2 3/1, 2/2 2; genu 2 2/1, 2/1 2; tibia 2 2/1, 2/1 2. Leg II: trochanter 1 0/1, 0/2 1; femur 2 3/1, 2/1 1; genu 2 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/0 1. Macroseta on tarsus IV (pd3), distally blunt, 33–42.
Protonymph (three examined and measured; Figure 9 a–b)



Dorsal and lateral idiosoma —- (Figure 9a). Podonotal shield 135–149 long, 113–125 wide, with nine pairs of setae (j1, j3, j4, j5, j6, z2, z4, z5, s4), four pairs of poroids (id5, id2, id4, id6) and solenostomes gd1 and gd2; gland opening gd1 minute, on the shield margin, at the level of the insertions of setae j3. Surface of the shield with faint striae. Setae r3 and s6 on the soft cuticle. Mesonotum with five pairs of sclerotized scutella, setal pairs J2, Z1, S2, R1, solenostomes gd6, and poroids id3, idl1, idl2, idm1, idm2. Pygidial shield reticulate, anterior margin lobate, 59–65 long, 77–89 wide, bearing the setae S4, S5, Z4, Z5, J5, the gland pores gd8, gd9, and the poroids idm3, idm4, idm5, idl3, idl4, idl5. All the dorsal setae smooth and pointed, except Z4, Z5 barbed and J5 basally barbed. Lengths of j1 17–21, j3 15–16, j4 10–12, j5 9–12, j6 13, J2 11–12, J5 9, z2 11–12, z4 15–16, z5 10–11, Z1 12–14, Z4 37–49, Z5 32–38, s4 19–22, s6 16–20, S2 21–25, S4 16, S5 14–18, r3 11–14, R1 9–12. Peritremes 27–33 long, extending to between s4–s6.
Ventral idiosoma — (Figure 9b). Sternogenital shield poorly sclerotized, bearing the setal pairs st1–st3 and the poroids iv1, iv2; anterior margin concave, posterior margin subtriangular; distance st1–st3 69–81, distance st2–st2 48–57. Setal nubs of st5 and setal pairs JV1, JV2, ZV2, JV5 on the integument anterior to the anal plate. Setae JV5 smooth and blunt, 16–19 long.
Gnathosoma — Cheliceral fixed digit 13–25 long, with two subapical teeth; movable digit 19–22, without dentition. Setae h1 15–17, h2 13–14, h3 17-18, pc 18-20. Palp 78–95 long; anterolateral seta on femur distally expanded, on genu thicken and pointed.
Legs — Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 4-4-4-4; femora 10-7-5-4; genua 8-6-6-5; tibiae 8-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanter to tibia as follows. Leg I: trochanter 1 0/1, 0/1 1; femur 2 2/1, 2/1 2; genu 1 2/1, 2/1 1; tibia 1 2/1, 2/1 1. Leg II: trochanter 1 0/1, 0/1 1; femur 1 2/1, 2/0 1; genu 1 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 0/1, 0/1 1; femur 1 2/1, 1/0 0; genu 1 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/1 0; femur 1 1/0, 1/0 1; genu 1 2/0, 2/0 0; tibia 1 1/1, 2/0 1. Macroseta on tarsus IV (pd3), blunt, 28–37 long.
Larva. Not found
Remarks
The most distinctive feature in the females is the shape of the ventrianal shield. This shield is broad, and the margin of the anterior third, above the insertions of setae ZV2, is remarkably rounded, without anterior corners (Figure 6c). On the dorsal shield, setae z2 are short, about half as long as j3 and z3, placed near z3, and with their insertions paraxial to the line j3–z3 (Figure 6a). Furthermore, as pointed out by Ferragut and Baumann (2021), the peritrematal shield is not fused with the dorsal shield but simply extends forward over a narrow bridge (Figure 6a). The number of teeth on the movable cheliceral digit is variable, from zero to one tooth depending on the source and depending on the females, as observed in the sample of Fuerteventura. The male dorsal shield never contains the setal pair r3, which is located on the interscutal integument, and the peritremal shield is attached to the dorsal shield between setae j1–j3. The number of male preanal setae varies according to the literature. Although it is considered that the males have six pairs of setae as originally described by Swirski and Amitai (1984), the corresponding figure in the original description shows six setae on the left side of the shield and five on the right side (figure 7, page 75). Later, Denmark and Rather (1996) examined one paratype male and concluded (and depicted) that it has only five pairs of preanal setae. Subsequent redescriptions (Kanouh et al. 2012; Stathakis et al. 2016) emphasized that the males have six pairs of setae, but Ferragut and Baumann (2021) observed seven pairs on the three males collected in Cape Verde archipelago, with the addition of setae JV4.
This species is common and widespread in the coastal regions of Fuerteventura and Lobos Island and it can coexist on the same plants with N. extraseta sp. nov. Females of N. litoralis can be easily differentiated from those of the new species by: 1) the cup-like calyx of the spermatheca, 2) the absence of dorsal setal pair J1, 3) the dorsal ornamentation, made of smooth cells, never lineate or striate, and 4) the longer dorsal setae (about 10–66% longer).
The ontogenetic development of dorsal and ventral setation and adenotactic pattern, although incomplete by the absence of larvae, seems identical to that of N. extraseta sp. nov., with setae z3, dorsal solenostomes gd1, gd5, and ventral setae st5, JV3, JV4, ZV1, ZV3 incorporated to the deutonymph. Likewise, development of leg chaetotaxy seems similar to that of the new species. On leg I, setae ad1 and pv2 of trochanter; ad3 and pv2 of femur; al2, pl2 of both genu and tibia are added. On leg II seta pv2 of trochanter; setae al2, ad3, pv1 of femur; al2 of genu are incorporated. On leg III, ad1 of trochanter, pl1 of femur, and av1 of genu are added. On leg IV, pv2 of trochanter; av1, pl1 of both femur and genu are added to the deutonymphal complement. There were no differences in leg chaetotaxy between the deutonymphal and adult life stages.
Genus Typhlodromus Scheuten, 1857
Subgenus Anthoseius De Leon, 1959
Typhlodromus (Anthoseius) charactus Ueckermann
Typhlodromus charactus Ueckermann, 1996: 21.
Typhlodromus (Anthoseius) charactus – Moraes et al., 2004: 318.
Amblydromella charactus – Denmark & Welbourn, 2002: (although these authors incorporate most of species of the subgenus Anthoseius within the genus Amblydromella Muma, they never explicitly mentioned this species. Had they done, they should have named it Amblydromella [Lindquistoseia] characta).
Specimens examined
Three females on Heliotropium ramosissimum (Lehmann) Sieber ex de Candolle (Boraginaceae); Tindaya, Barranco de Esquinzo, 28°37′47″N, 14°00′18″W, 59 m a.s.l.; 26 May 2024.
Geographical distribution
Previously known from Yemen. The new record in Fuerteventura proves it has a disjunct distribution.
Diagnosis (female)
Dorsal shield deeply incised laterally at the level of sublateral setae R1; shield striate-reticulate on the anterior and lateral margins, almost smooth between setae j4 and j6, longitudinally striate between j6–J2, with some large cells, transversal striae on the posterior part. Eighteen pairs of short, smooth, and pointed dorsal setae; all shorter than 20 μm. Five pairs of dorsal solenostomes (gd2, gd4, gd6, gd8, gd9). Peritremes extending to between setae j1–j3. Sternal shield with two pairs of setae, setae st3 on the soft cuticle; posterior margin of the shield lobate. Ventrianal shield longer than wide; anterior margins straight or slightly convex, laterals concave; surface on the anterior half smooth, on the posterior half striate-reticulate; three pairs of preanal setae (JV1, JV2, ZV2) and preanal pores present, posteroantiaxial to JV2. Major duct of spermatheca broad, atrium fissured and conical, calyx short cup-shaped. Fixed digit of chelicera with three subapical teeth, dentition of movable digit not discernible (four teeth in the original description). Genua of legs II–IV with eight, seven, and eight setae, respectively. Without macrosetae on leg IV.
Complementary description
Female (Three females examined and measured; Figures 10 a–c)
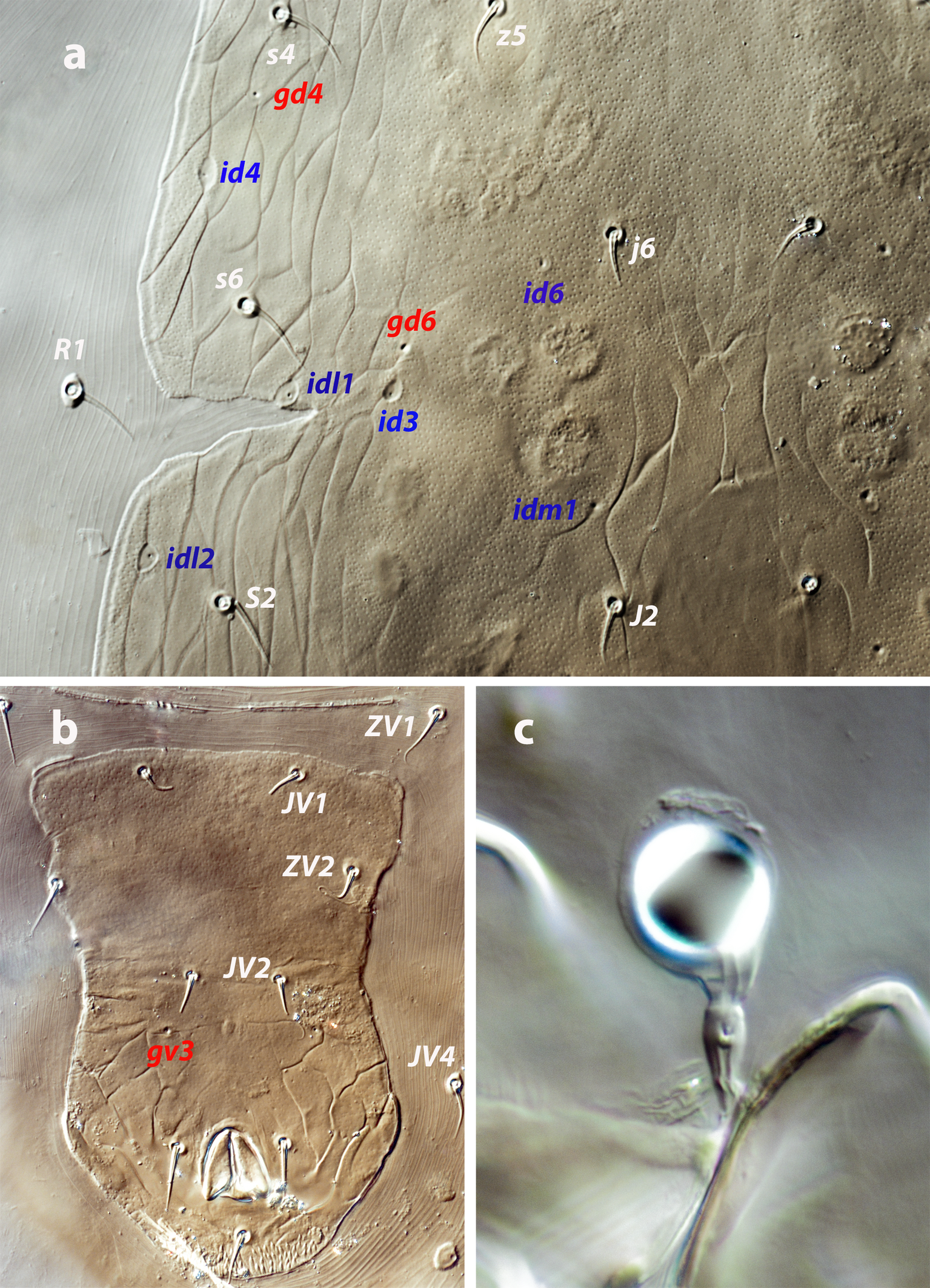


Dorsal and lateral idiosoma — (Figures 10a). Dorsal shield piriform, 359–378 long, wider at the level of opisthosoma (S4, 221–226) than at the level of podosoma (179–192); with a characteristic lateral incision near the insertion of setae R1, deep and extending beyond poroid idl1 or even poroid id3. Dorsal ornamentation consisting of elongate and narrow cells covering the margins of the shield; midline between setae j4–j6 smooth, between j6–J2 reticulate, with large cells; posterior region between the insertions of Z4 transversally striate. Eighteen pairs of short, smooth, and pointed dorsal setae, only J5 barbed basally. Lengths of j1 11–12, j3 14–15, j4 9–13, j5 9–11, j6 8–13, J2 9–13, J5 9–11, z2 13–14, z3 13–15, z4 13–16, z5 10–11, Z4 12–14, Z5 16–17, s4 14–18, s6 16–19, S2 16–18, S4, S5 14–16. Sublateral setae r3 and R1 14–16, placed on small platelets. Five pairs of dorsal gland pores, gd2, gd4, gd6, gd8, gd9; gland pore gd8 anteroantiaxial to Z4, pore gd9 paraxial and aligned with the insertions of S5; sixteen pairs of poroids on the shield. Peritremes reaching the level of j1 or between j1–j3, 202–205 long; peritrematal shield narrow, attached to the dorsal shield above the poroid id1.
Ventral idiosoma — (Figure 10b). Tritosternum relatively short (69–72), plumose throughout except at the base, divided into two laciniae, 34–36 long. Sternal shield smooth, with a few lateral striae; anterior margin concave, posterior margin trilobate, posterolateral lobes penetrating deeply into the shield, up to the level of setae st2, lateral margins divergent; poroid iv2 just on the posterior margin of lateral lobes. Shield with two pair of setae (st1–st2) and poroids iv1, iv2. Setae st3 placed directly on the soft integument; st4 on piriform metasternal plates, with poroid iv3 in anterior position. Distance st1–st3 68–79, st2–st2 62–66. Epigynal shield narrower than the ventrianal, 111–124 long, distance st5–st5 59–66. Ventrianal shield elongate, almost vase-shaped (Figure 10b); anterior margin slightly convex, laterals between ZV2 and the anal opening deeply concave; 122–137 long, 88–95 wide at the level of ZV2, 74–80 at the level of anus; ratio L/W = 1.4–¬1.6. Anterior half of the shield almost smooth, posterior half irregularly striate, some striae forming closed cells. Three pairs of setae JV1, JV2, ZV2, and solenostomes gv3 on the shield; setae JV2 in posterior position, transversally aligned with the greatest shield narrowing; preanal pores punctiform, posteroantiaxial to the sockets of JV2. Setae ZV1, ZV3, JV4, JV5, four pairs of poroids ivo and one pair ivp surrounding the shield. Setae JV5 smooth and pointed, 17–19 long, on platelets. Metapodal shields bacillar; posterior metapodal 25–33 long.
Spermatheca — (Figure 10c). Major duct short, vacuolated near the atrium; atrium well developed, almost as long as calyx; calyx short, subconical, 4–6 deep, 8–9 wide at the junction with the vesicle.
Gnathosoma — Base of gnathosoma 27–30) long, 72–76 wide. Tectum dome shaped. Deutosternal groove with seven rows of two denticles. Hypostomal setae h1 19–20, h2 14–15, h3 16, pc 18–19. Length of first cheliceral segment 14–15, of second segment 69–76. Fixed digit of chelicera 24–28 long, with three subapical teeth; movable digit 19–14 long, dentition not visible. Palp 92–96 long; femoral seta al thickened and distally enlarged, genual setae al1–al2 thickened and distally blunt; apotele 2-tined.
Legs — Lengths of leg I 250–262, leg II 220–224, leg III 206–213, leg IV 264–269. Distal margin of coxae on all the legs dentate, with 8–10 acute spine-like teeth. All the setae on the legs short and pointed. Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 6-5-5-5; femora 12-10-6-6; genua 10-8-7-8; tibiae 10-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanter to tibia as follows. Leg I: trochanter 1 1/1, 0/2 1; femur 2 3/1, 2/2 2; genu 2 2/1, 2/1 2; tibia 2 2/1, 2/1 2. Leg II: trochanter 1 0/1, 0/2 1; femur 2 3/1, 2/1 1; genu 2 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 2; tibia 1 1/1, 2/0 1. Without leg macrosetae.
Remarks
Typhlodromus (A.) charactus is an atypical member of the subgenus Anthoseius in that the lateral margins of the female dorsal shield are incised in such a way that it resembles that shield in the phytoseiid deutonymphal stage. Unfortunately, no males or immatures of this or other related species are known. This feature is shared by three species, T. (A.) charactus, T. (A) kutabus Schicha & Corpuz-Raros from the Philippines, and T. (A.) eremicus Ueckermann from Northern Cape Province in South Africa. The three taxa share, in addition, an elongate ventrianal shield wider at the level of anterior corners than at the level of anus, a subconical calyx and enlarged atrium in the spermatheca, and only three pairs of preanal setae. Despite the limited number of species, there is much confusion about their taxonomical identity. Ueckermann (in Meyer and Ueckermann, 1989) described T. (A.) eremicus from the Kalahari Gemsbok National Park (now incorporated into the Kgalagadi Transfrontier Park). The description was rather complete, indicating that genua II and IV have eight setae. Later, Schicha and Corpuz-Raros (1992) described superficially T. (A.) kutabus, with an account of the most relevant characters and some measures but without references to the leg chaetotaxy, although their illustrations are clear and the genu IV bears eight setae. Nevertheless, there are no comments on this work to the previously described T. (A.) eremicus. Finally, Ueckermann (1996) described T. (A.) charactus. The morphological description and pictures are pretty informative, but again he did not include either T. (A.) eremicus and T. (A.) kutabus in the comparison to justify the status of the new species.
According to the information appearing in the mentioned papers, T. (A.) eremicus seems to be distinct by the shortness of the peritremes, which extend to between the lateral setae z2–z3, and the size of the spermathecal calyx, at least twice the length of this structure in the other two species. Ueckermann et al. (2008) indicated that there are five pairs of dorsal solenostomes, but they did not specify which ones, and their Figure 17A only shows the gland pores gd2, gd4, and gd9. Typhlodromus (A.) kutabus and T. (A.) charactus are similar; the former differs by having three instead of two sternal setae, and the posterior margin of sternal shield is concave rather than lobate. Even though there is no indication of the dorsal adenotaxy for any of the two species, the respective drawings seem to show five pairs of pores in T. (A.) kutabus (without openings gd1 and gd5) and six pairs in T. (A.) charactus (without gd5), but this interpretation is subjective and it is based on the confidence that the drawings accurately reflect mite morphology.
Taking into account all these uncertainties, I opted to identify provisionally the specimens collected as T. (A.) charactus for the following reasons: 1) some differential features in this species are coincident with the material collected in Fuerteventura; e.g., the number of pairs of sternal setae and the posterior trilobate margin of the sternal shield, and 2) the biogeographical proximity of this species compared to the Philippine T. (A.) kutabus. Further detailed examination of the respective types and genetic studies would be necessary to finally elucidate the taxonomic validity of these taxa and their phylogenetic relationships.
Typhlodromus (Anthoseius) maspalomensis Ferragut & Peña-Estévez, 2003
Typhlodromus (Anthoseius) maspalomensis Ferragut & Peña-Estévez, 2003: 158.
Specimens examined
Thirteen females, four males on Tamarix canariensis Willdenow (Tamaricaceae); Puerto del Rosario, 28°30′02″N, 13°50′53″W, 5 m a.s.l.; 26 May 2024. One female on Dittrichia viscosa (L.) Greuter (Asteraceae) and one female on Suaeda vermiculata (Amaranthaceae); Betancuria, mirador Morro Velosa, 28°26′68″N, 14°02′59″W, 663 m a.s.l.; 29 May 2024. Three females, one male on Tamarix canariensis (Tamaricaceae) and one male on Salsola divaricata Masson ex Link s. l. (Amaranthaceae); ravine near Vega del Rio Palmas, 28°23′25″N, 14°05′41″W, 213 m a.s.l.; 29 May 2024.
Geographical distribution
Canary Islands, Greece, and Morocco. It also occurs in the Iberian Peninsula (unpublished data).
Diagnosis
Female dorsal shield mostly reticulate, with the midline on the opisthonotum smooth. Dorsal setae smooth and pointed; setae j3 longer than j1; S5 longer than S2 and S4. Five pairs of dorsal gland pores, gd2, gd4, gd6, gd8, gd9; gd8 anteroantiaxial to Z4, gd9 distant from S5. Peritremes extending to level between j1–j3. Sternal shield with two pairs of setae and posterior margin trilobate. Ventrianal shield subpentagonal, with four pairs of setae JV1, JV2, JV3, ZV2, and without preanal pores. Spermathecal calyx bell-shaped, with a short neck between the base of calyx and the atrium. Cheliceral fixed digit with two–three subapical teeth, movable digit with one tooth. Genua of legs II–IV with seven setae. Three macrosetae on leg IV. Male dorsal shield with six pairs of solenostomes; gland pore gp on the shield, marginal to the insertions of s4. Ventrianal shield with four pairs of setae. Spermatodactyl almost straight, a bit curved, not being able to distinguish where the foot begins; distal toe bifurcate; vellum present.
Complementary description
Female (eighteen examined, eight measured. Figures 11 a–b)


Dorsal and lateral idiosoma — (Figures 11 a–b). Dorsal shield with lateral waist slightly developed. Margins of the shield striate-reticulate, inner region of podonotum without reticulation, with fine furrows and crevices; opisthonotum striate-reticulate, cells very long, obliquely oriented; very noticeable muscle marks throughout the shield, many of them scattered between podonotal setae j3–j4 and around setae z5 (Figure 11a), and four large marks between gland pore gd6 and insertions of Z4 (Figure 11b). Eighteen pairs of smooth and acute dorsal setae, except Z5 barely barbed on its distal half and J5 basally barbed. Lengths of j1 18–19, j3 22–24, j4 13–14, j5 12–15, j6 14–17, J2 14–19, J5 6–8, z2 13–16, z3 and z4 17–18, z5 13–16, Z4 27–29, Z5 41–43, s4 17–19, s6 20–23, S2 22–26, S4 21–25, S5 24–29. Sublateral setae r3 17–19, R1 16–17, placed on small platelets. Five pairs of dorsal gland pores, gd2, gd4, gd6, gd8, gd9; gland pore gd8 transversally aligned with poroid idm4, anteroantiaxial to the insertions of Z4; gd9 separated from and anteroparaxial to S5. Fifteen pairs of poroids on the shield; poroid idl2 placed on the lateral integument, near the level of S2. Peritremes ending between the setae j1–j3, detached from the dorsal shield, 170–207 long; internal groove with two continuous rows of microvilli.
Ventral idiosoma — Tritosternum 76–80 long, with two laciniae 55–57 long. Sternal shield subquadrate, with two pairs of setae and two pairs of poroids; posterior margin lobate; distance st1–st3 62–68, st2–st2 54–58; setae st3 and st4 on sclerotized platelets, that of st3 larger. Epigynal shield 104–117 long, distance st5–st5 62–66. Ventrianal shield pentagonal; 99–109 long, 87–94 wide at the level of ZV2, 64–67 wide at the level of anus. Anterior margin between insertions of JV1 straight, laterals between ZV2 and anus strongly concave, anterior corners between JV1–ZV2 sometimes eroded. Shield surface smooth, with four pairs of setae and without preanal solenostomes (Figure 16c). Setae ZV1, ZV3, JV4, JV5 and five pairs of poroids surrounding the ventrianal shield; setae JV5 33–36. Posterior metapodal shield 23–28 long, elliptical.
Spermatheca — Major duct short, proximal part near the sperm induction pore wide, apparently membranous, distal part near the atrium tubular, narrow and sclerotized; atrium nodular, a short neck between atrium and calyx; calyx bell-shaped, relatively small, 7–9 deep, 10–12 wide close to the vesicle.
Gnathosoma — Base of gnathosoma 26–30 long, 69–74 wide. Deutosternal groove with seven rows of paired and acute denticles. Hypostomal setae h1 22–24, h2 18–19, h3 20–22, pc 20–24. Length of first cheliceral segment 16–20, of second segment 76–83. Fixed digit of chelicera 26–29 long, with two or three subapical teeth; movable digit 22–25 long, with one tooth. Palp 98–105 long; femoral seta al thickened and distally spatulate, 14–15 long, genual setae al1–al2 thicken and blunt, similar in size, 8–9 long; 2-tined apotele.
Legs — Length of leg I 250–261, leg II 218–226, leg III 220–229, leg IV 293–299. Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 6-5-5-5; femora 12-10-6-6; genua 10-8-7-8; tibiae 10-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanter to tibia as follows. Leg I: trochanter 1 0/1, 1/2 1; femur 2 3/1, 2/2 2; genu 2 2/1, 2/1 2; tibia 2 2/1, 2/1 2. Leg II: trochanter 1 0/1, 0/2 1; femur 2 3/1, 2/1 1; genu 2 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/0 1. Three macroseta on leg IV; those of genu and tibia blunt or very scarcely knobbed, 24–27 and 23–28 long, respectively; that on basitarsus knobbed, 45–49 long.
Male (six examined and measured)
Dorsal and lateral idiosoma — Dorsal shield reticulate, except on the midline of the podonotum, which is smooth; ornamentation on the opisthonotum as in female, but cells are smaller. Shield inversely oval, wider at the level of s4 (169–180) than at level of S4 (135–148), 252–290 long. Lengths of j1 16–18, j3 22–23, j4 11–13, j5 11–12, j6 11–15, J2 13–14, J5 6–7, z2 13–14, z3 13–15, z4 15–17, z5 12–14, Z4 25–27, Z5 36–37, s4 16–17, s6 17–18, S2 18–20, S4 17–20, S5 22–24, r3 15–19, R1 13–14. Six pairs of dorsal solenostomes, gd2, gd4, gd6, gd8, gd9, gp; gland pore gp very near the shield margin, hypertrophied, opening fundibuliform, placed in line with the insertions of setae s4. Poroid idl2 off the shield, as in females. Peritremes extending forward to setae z2 or between j3–z2, 135–140 long; peritrematal shield fused with the dorsal shield at the level of r3 insertions.
Ventral idiosoma — Sternogenital shield with five pairs of setae (st1–st5) and three pairs of poroids (iv1–iv3); distances st1–st5 107–119, st3–st3 54–57; anterior margin indiscernible, posterior margin gently convex; shield surface mostly smooth, with marginal striae. Ventrianal shield strongly convex anteriorly, subtriangular, transversally striated, 93–102 long, 137–153 wide; setae JV1, JV2, JV3, ZV2 and four pairs of poroids (iv5, ivo1–3) on the shield; poroid ivo2 in marginal position, ivo3 slightly posteroparaxial to ivo2 and equidistant from ivo2 and setae ZV2. A fifth poroid ivo, poroids ivp and setae JV5 off the shield; JV5 smooth and pointed, 20–24 long.
Chelicera and spermatodactyl — Fixed digit of chelicera with two subapical teeth, 19–22 long; movable digit with one tooth, 18–21 long. Spermatodactyl almost straight, describing a smooth curve in which the foot is a continuation of the shaft; combined length shaft plus foot 29–33; foot ending in a bifid toe. Vellum present on the shaft.
Legs — Leg chaetotaxy and setal formulas like females. Leg IV with three macrosetae on genu 19–22, tibia 20–23, and basitarsus 37–39.
Remarks
Typhlodromus (A.) maspalomensis is very close to T. (A.) rhenanoides and both species may coexist on the same plants. The latter species can be easily distinguished by the following attributes: 1) calyx of spermatheca long tubular, about 20 micrometres, 2) occurrence of preanal pores, 3) only one macroseta on leg IV (at least the Canarian specimens), 4) setae Z4 long enough to reach the insertions of S5, 5) gland pore gd9 adjacent to S5, instead of clearly separated from it, and 6) male spermatodactyl L-shaped rather than slightly curved in T. (A.) maspalomensis.
As stated by previous authors (Ferragut and Peña-Estévez, 2003; Stathakis et al., 2016; Tixier et al. 2016) the species appears to have a close relationship with plants of the genus Tamarix (Tamaricaceae) on which it has been collected across its geographical range. Other host plants reported in Fuerteventura, e.g. Amaranthaceae, always were close to tamarisks.
Typhlodromus (Anthoseius) rhenanoides Athias-Henriot, 1960
Typhlodromus rhenanoides Athias-Henriot (1960): 85.
Neoseiulus rhenanoides – Schuster & Pritchard (1963): 205.
Anthoseius rhenanoides – Charlet & McMurtry (1977): 186.
Amblydromella rhenanoides – Tenorio et al. (1985): 303.
Typhlodromus (Anthoseius) rhenanoides – Ueckermann & Loots (1988): 60.
Amblydromella (Aphanoseia) rhenanoides – Denmark & Welbourn (2002): 308.
Specimens examined
Seven females and two males on unidentified Amaranthaceae, three females on Suaeda vermiculata (Amaranthaceae), one male on Cakile maritima (Brassicaceae); Dunas de Corralejo, 28°40′13″N, 13°50′00″W, 12 m a.s.l.; 27 May 2024. One female on Suaeda sp. (Amaranthaceae); Beach of Aljibe de la Cueva, El Cotillo, 28°39′53″N, 14°00′41″W, 18 m a.s.l.; 27 May 2024. Two females, two males and one deutonymph on Limonium sp. (Plumbaginaceae), two females on Suaeda vera (Amaranthaceae), two females, one male, one deutonymph on Salsola divaricata (Amaranthaceae), three females and two deutonymphs on Caroxylon tetrandrum (Amaranthaceae); Isla Lobos, 28°44′21″N, 13°49′21″W, 3 m a.s.l. Three females and one deutonymph on Suaeda vermiculata (Amaranthaceae); Isla Lobos, 28°45′13″N, 13°49′35″W, 15 m a.s.l.; 28 May 2024. Nineteen females, two males, one deutonymph, two protonymphs on Andryala pinnatifida Aiton subsp. cuneifolia (Schultz Bipontinus) M.Z. Ferreira (Asteraceae), one female on Echium bonetti Coincy (Boraginaceae); Betancuria, Mirador de Guise y Ayose, 28°26′27″N, 14°03′22″W, 694 m a.s.l.; 29 May 2024. One female on Dittrichia viscosa L. Greuter (Asteraceae); Betancuria, mirador Morro Velosa, 28°26′68″N, 14°02′59″W, 663 m a.s.l.; 29 May 2024. Three females on Echium decaisnei Webb & Berthelot subsp. purpuriensis Bramwell (Boraginaceae); Betancuria, 28°25′31″N, 14°03′24″W, 390 m a.s.l.; 29 May 2024. Four females, one male, one deutonymph, one protonymph on Salsola divaricata (Masson ex Link) Akhani (Amaranthaceae), one female on Pulicaria sp. (Asteraceae); La Pared, Pueblo del Mar, 28°12′28″N, 14°13′27″W, 69 m a.s.l.; 30 May 2024.
Geographical distribution
Mediterranean basin countries, Madeira, Canary Islands, Belgium. Also reported from California and Hawaii, although these records due to their biogeographical distance and age should be revised.
Diagnosis
Medium sized phytoseiid mite with relative short legs. Female dorsal shield evenly reticulated except on the striate anterolateral margins. Dorsal setae simple, smooth and pointed, only Z4, Z5, J5 with a few barbs. Setae j1 and j3 subequal in length; z3 equal to z4; z2 2/3 the length of z3 and z4; s4 equal to s6; Z4 reaching the S5 sockets; dimensions of Z5 variable, 47–60. Five pairs of noticeable dorsal solenostomes (gd2, gd4, gd6, gd8, gd9). Poroid idl2 detached from the shield. Peritremes ending near the level of j1; peritrematal channel not stippled, with an internal strip through all of its length. Sternal shield with two pairs of setae and posterior median lobe (inversely triangular). Ventrianal shield with four pairs of preanal setae; preanal pores small, punctiform, posteroparaxial to JV2. Calyx of spermatheca tubular, about 20 μm, atrium inserted into the base of calyx. Fixed digit of chelicera with four teeth, three subapical and smaller and one posterior to pilus dentilis and larger; movable digit with two teeth. Trochanter I with six setae, genua II–IV with seven setae. One slightly knobbed macrosetae on tarsus IV (other references mention three on leg IV, see Remarks). Male dorsal ornamentation as in female. Setae r3, R1 on the dorsal shield. Six pairs of dorsal solenostomes, gd2, gd4, gd6, gd8, gd9, gp; gland opening gp on the margin of the dorsal shield, posterior to the level of s4. Cheliceral fixed digit with three teeth distal and one strong tooth proximal to pilus dentilis; movable digit with one large tooth. Spermatodactyl L-shaped with a short foot.
Complementary description
Female (52 examined, eight measured; Figure 12 a–c)


Dorsal and lateral idiosoma — (Figure 12a). Dorsal shield slender and elongate, 317–343 long, 145–166 wide; completely reticulate, except on the anterolateral margins, mainly striated; reticulation more extensive and marked on the opisthonotum. Eighteen pairs of setae, most of them smooth and pointed, Z4 with a few barbs, Z5 scarcely barbed, J5 basally barbed. Lengths of j1 23–26, j3 22–28, j4 13–18, j5 16–18, j6 18–21, J2 20–23, J5 9–10, z2 14–16, z3 20–25, z4 20–23, z5 16–19, Z4 32–41, Z5 47–60, s4 22–27, s6 25–28, S2 28–33, S4 32–38, S5 17–20. Sublateral setae r3 23–25, R1 22–27, on small basal platelets. Five pairs of very conspicuous solenostomes, gd2, gd4, gd6, gd8, gd9; gland opening gd2 buttonhole-like, the remaining horseshoe-shaped; gd9 adjacent to the insertions of setae S5. Fifteen pairs of poroids on the shield; poroid idl2 always on the soft integument, posterior to setae R1. Peritremes extending anteriorly to almost the level of j1, 154–190 long; peritrematal groove without papillae or with just a few microvilli around the stigmata, with a continuous and internal strip (Figure 12a).
Ventral idiosoma — (Figure 12b). Tritosternum 77–84 long, split into two laciniae, 43–51 long. Sternal shield slightly sclerotized, almost smooth, with parallel sides and posterior margin with a median subtriangular lobe; bearing setae st1–st2 and poroids iv1, iv2, the latter poroid just on its posterior margin. Each seta st3–st4 on platelets, poroid iv3 free on the unsclerotized cuticle, between the metasternal platelets. Distance st1–st3 65–70, st2–st2 51–56. Epigynal shield 104–111 long, distance st5–st5 57–64. Ventrianal shield broadly pentagonal (Figure 12b); anterior margin between setae JV1 straight, laterals between ZV2–JV3 distinctly concave. Surface of the shield almost smooth, with a few and weak transversal striae; with four pairs of setae (JV1, JV2, JV3, ZV2) and preanal solenostomes, punctiform and widely separated (distance between them 34–40), placed posteroparaxial to JV2 and almost at the same level as the insertions of JV3. Elongate and narrow platelets on the soft cuticle, near the anterolateral corners of the shield. Posterior metapodal shield 22–28 long. Setae ZV1, ZV3, JV4 and JV5 around the ventrianal shield; JV5 smooth and pointed, on platelets, 43–50 long.
Spermatheca — (Figure 12c). Major duct short, narrow and cylindrical; atrium c-shaped just on the base of calyx; calyx tubular, 19–22 long, lateral walls almost parallel, flaring distally, 7–11 wide at the junction with the vesicle. The proximal portion of calyx near the atrium is less sclerotized and may appear folded.
Gnathosoma — Base of gnathosoma 40–54 long, 75–84 wide. Tectum dome-shaped, without a clear anterior angle. Deutosternal groove with seven rows of two lateral denticles. Hypostomal setae h1 23–25, h2 19–21, h3 24–25, pc 26–28. First cheliceral segment 20–25 long, second segment 81–85. Fixed digit of chelicera 27–30 long, with four teeth, three anterior and one larger and triangular (in profile) posterior to pilus dentilis; movable digit 24–26, with two teeth, the proximal being larger than the distal. Palp 113–118 long; femoral seta al 15–16 long, flattened and expanded at the tip; on the genu al1 9–10, al2 7-8 long, both thick and pointed.
Legs — Five-six caliciform glands and a large presumably gland opening on ventral side of coxa I; the opening elongate, piriform, in anteroventral position. Length of leg I 255–282, leg II 213–228, leg III 220–228, leg IV 297–305. Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 6-5-5-5; femora 12-10-6-6; genua 10-8-7-8; tibiae 10-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanter to tibia as follows. Leg I: trochanter 1 0/1, 1/2 1; femur 2 3/1, 2/2 2; genu 2 2/1, 2/1 2; tibia 2 2/1, 2/1 2. Leg II: trochanter 1 0/1, 0/2 1; femur 2 3/1, 2/1 1; genu 2 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 1 1/1, 0/2 0; femur 1 2/1, 1/0 1; genu 1 2/1, 2/0 1; tibia 1 1/1, 2/0 1. One macroseta on tarsus IV, slightly knobbed, 38–51 long.
Male (nine examined, five measured)
Dorsal idiosoma — Dorsal shield 257–264 long, wider at the podosoma (147–153) than at the opisthosoma (135–139); evenly reticulate except for the striated anterolateral margins, cells elongate and oriented axially between setae j5–j6, pentagonal and regular in size between setae j6–J2. Twenty pairs of dorsal setae morphologically similar to those of female. Lengths of j1 17–18, j3 19–22, j4 12–14, j5 11–14, j6 13–16, J2 15–17, J5 8–9, z2 12–17, z3 15–16, z4 14–20, z5 11–14, Z4 28–33, Z5 35–40, s4 16–22, s6 19–24, S2 21–26, S4 20–26, S5 14–18, r3 16–17, R1 15–18. Six pairs of dorsal solenostomes, without gland pores gd1 and gd5, but with pore gp placed just on the shield margin, a bit posterior to the level of s4; all the gland openings more developed than on the female, horseshoe-shaped, except gp, oval in shape. Peritrematal shield attached to the dorsal shield at the level of r3; peritremes 127–131 long, ending anteriorly between j3–z2; peritrematal groove with a few papillae in the proximal section, near the stigmata, the remaining lineate, without microvilli, as in females.
Ventral idiosoma — Sternogenital shield mostly smooth; distance st1–st5 108–113, st3–st3 49–54; posterior margin between setae st5 smoothly convex. Ventrianal shield subtriangular, 101–108 long, 139–143 wide; surface transversally striate, with some closed cells anterior to preanal pores; four pairs of setae (JV1, JV2, JV3, ZV2), and four pairs of poroids (iv5 and three pairs ivo) all anterior to preanal solenostomes; preanal pores gv3 punctiform and posterior to the insertions of setae JV2, distance between them 23–28. Setae JV5 smooth and pointed, 23–26 long.
Chelicera and spermatodactyl — Fixed digit of chelicera 22–25 long, with three teeth distal and one strong tooth proximal to pilus dentilis; movable digit 18–22 long, with one large tooth. Spermatodactyl L-shaped; shaft 17–19 long, a bit curved; heel rounded, little marked; foot short (about 5–6 μm), directed upwards, ending in small toe.
Legs — Same leg chaetotaxy as in female. Macroseta on tarsus IV blunt or knobbed, 31–33 long.
Deutonymph (seven examined, five measured. Figure 13 a–b)



Dorsal and lateral idiosoma — (Figure 13a). Elongate dorsal shield, 248–268 long, 110–114 wide; laterals incised between the insertions of setae s6 and S2, incision reaching the gland opening gd6. Surface mostly reticulate, cells more abundant and pronounced on the opisthonotum. Eighteen pairs of simple and pointed setae, except Z4, Z5, finely barbed and J5 basally barbed. Lengths of j1 19–20, j3 16–17, j4 10–12, j5 12–13, j6 and J2 14–16, J5 8–9, z2 11–12, z3 11–14, z4 13–14, z5 11–13, Z4 35–38, Z5 40–46, s4 17–19, s6 17–20, S2 23–24, S4 24–26, S5 15–16. Sublaterals r3 18–20, R1 16–17. Same adenotaxy (absence of gland pores gd1, gd5) and poroidotaxy (complete set of sixteen poroids) than on female. Peritremes extending to the level between j3–z2, 124–141 long; peritrematal groove with an incomplete row of microvilli.
Ventral idiosoma — (Figure 13b). Tritosternum 77–82, with two laciniae 48–51 long. Sternogenital shield with four pairs of setae and two pairs of poroids, setae st5 off the plate; distance st1–st5 124–136, st3–st3 48–50. Caudoventral soft cuticle surrounding the anal plate with eight pairs of setae (JV1, JV2, JV3, JV4, JV5, ZV1, ZV2, ZV3); pair ZV3 half as long as companions. Setae JV5 smooth and pointed, on platelets, 29–33 long.
Gnathosoma — Gnathosomal base 34–38 long, 66–70 wide. Deutosternal channel with seven rows of paired denticles. Hypostomal setae h1 21–22, h2 18, h3 19–20, pc 21–23. First cheliceral segment 21–22, second segment 70–73. Fixed digit of chelicera 21–24 long, with four teeth, three anterior and one posterior to pilus dentilis; movable digit 19–22, with two small teeth. Palp 97–102; anterolateral seta on femur spatulated, al1, al2 on genu thickened.
Legs — Coxa I with eight caliciform glands arranged into two groups of four; anteroventral opening present. Same chaetotaxy and setal formulas as the female. Only one macroseta on basitarsus IV, usually knobbed, 41–46 long.
Protonymph (three examined and measured. Figures 14 a–b)


Dorsal and lateral idiosoma — (Figure 14a). Podonotal shield smooth, weakly sclerotized, 125–136 long, 104–115 wide. Solenostomes and poroids not discernible on the shield. Nine pairs of setae on the shield; length of j1 17–18, j3 14, j4 11–12, j5 10–11, j6 14–15, z2 12–13, z4 13–14, z5 10–11, s4 17. Setae s6, r3, R1 off the shield; s6 16–17, r3 17–18, R1 12–13. Setal pairs J2 and S2, and six pairs of mesonotal scutella, one of the platelets bearing the gland pore gd6 between podonotal and pygidial shields. Setae J2 13–14, S2 21–22. Pygidial shield finely reticulated, anterior margin convex, 53–57 long, 66–68 wide. Setae S4, S5, Z4, Z5, J5, and poroids idl3, idl4, idl5, idm5 on the shield; setae Z4 and Z5 scarcely barbed, J5 basally barbed. Lengths of J5 8–9, Z4 39–44, Z5 34–36, S4 19–21, S5 14. Peritremes 31–37 long, almost reaching the level of setae s4; peritrematal channel with papillae or microvilli.
Ventral idiosoma — (Figure 14b). Tritosternum 67–68 long, laciniae 46–48. Sternogenital shield poorly sclerotized, with setae st1–st3 and poroids iv1–iv2; distance st1–st3 71–76, st2–st2 44–46. Opisthoventral soft integument with setae JV1, JV2, ZV2, and JV5 around the anal plate; solenostomes gv3 on the plate, posterior to JV2, punctiform, minute. Setae JV5 20–21 long.
Gnathosoma — Base of gnathosoma 45–47 long, 59–63 wide. Deutosternum with seven rows of denticles. Setae h1 18, h2 14–15, h3 16–18, pc 19–20. First cheliceral segment 14–16, second segment 56–59 long. Cheliceral fixed digit 20–21 long, with three teeth anterior and one tooth posterior to pilus dentilis; movable digit 17–18 long, with two small denticles. Palp 83–89 long; setae al on femur 12–13 long, distally expanded; setae al on genu 8–9, spiniform.
Legs — Three caliciform glands and one small and punctiform pore on the ventral surface of coxa I. Chaetotaxy of leg segments I–IV: coxae 2-2-2-1; trochanters 4-4-4-4; femora 10-7-5-4; genua 8-6-6-5; tibiae 8-7-7-6; basitarsi 0-4-4-4. Setal formulas from trochanter to tibia as follows. Leg I: trochanter 1 0/1, 0/1 1; femur 2 2/1, 2/1 2; genu 1 2/1, 2/1 1; tibia 1 2/1, 2/1 1. Leg II: trochanter 1 0/1, 0/1 1; femur 1 2/1, 2/0 1; genu 1 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg III: trochanter 1 0/1, 0/1 1; femur 1 2/1, 1/0 0; genu 1 2/0, 2/0 1; tibia 1 1/1, 2/1 1. Leg IV: trochanter 0 0/2, 1/1 0; femur 1 2/0, 1/0 0; genu 1 2/0, 2/0 0; tibia 1 1/1, 2/0 1. Macrosetae on genu (ad1) and tibia (ad) IV differentiate; lengths of macroseta on genu 15–17, on tibia 18–19, on tarsus 40–42.
Larva. Not found.
Remarks
Athias-Henriot (1960) pointed out that this species was the most abundant and widespread in Algeria, in both coastal and semi-arid inland landscapes. Likewise, according to Tixier et al. (2016), it appears to be very common and abundant in Morocco. Although the phytoseiid fauna in the Canaries is still incompletely known, the available information suggests that T. (A.) rhenanoides is likely the most abundant and frequent phytoseiid throughout the archipelago (unpublished data).
Since its original description it has been redescribed several times, although some important morphological features were overlooked by previous authors and the morphology of immature stages was completely unknown. There is no mention in the literature to the peculiar peritrematal groove, which is devoid of microvilli or papillae and is instead furrowed by a continuous strip from the stigmata to the end of the peritreme (Figure 12a). In some females there are papillae around the stigmatal opening and at the beginning of the channel. All the previous illustrations in the literature show a ''normal'' and stippled peritreme, but the excellent photograph by Döker et al. (2022) (their Figure 5a) reveals the lineate structure on females collected in Belgium. It would be desirable to check this character in mites from other geographical locations to confirm whether it is universal in the species or restricted to certain populations.
Another trait that raises questions is the shape of the sternal posterior margin in females. Athias-Henriot neither mentioned or illustrated this character. In Ueckermann and Loots (1988) and Moraes et al. (1999) the margin is described as straight or concave, whereas according to Papadoulis et al. (2009), Ferragut et al. (2010), Faraji et al. (2011), and Tixier et al. (2016) it is lobate. Again, this character should be mentioned in future redescriptions, and ideally revisited, in particular in specimens collected far from the supposed centre of geographical distribution, the western Mediterranean and North of Africa. Similarly, some reports indicated the absence of preanal pores (e.g., Charlet and McMurtry, 1977; Faraji et al., 2011), although the occurrence of this trait is evident in other redescriptions and it was highlighted in Athias-Henriot´s original description, where she depicted them and mentioned their remarkable separation. Finally, the number of macrosetae on leg IV is variable in the literature. The original description includes measures of three macrosetae, and this number has been reported by Schuster and Pritchard (1963), Ueckermann and Loots (1988), Faraji et al. (2011), Tixier et al. (2016), Döker et al. (2022), and it occurs in females from the Iberian Peninsula (per. observation). However, other references report a single macroseta (Papadoulis et al., 2009). These discrepancies may result more from differing criteria for identifying a macroseta than from a real morphological variation. Nevertheless, the females from Fuerteventura do not have the dorsal setae ad1 on genu and tibia IV differentiate as macrosetae. The length of those setae in the females is only 2–3 micrometres longer than the remaining setae on the segment; on the contrary, in the protonymph these setae are clearly longer. Thus, the absence of three macrosetae seems to be characteristic of the Canarian populations, as females from Gran Canaria and Tenerife Islands only have one macroseta. Accordingly, the mentioned morphological variability should be traced using molecular techniques on specimens from different locations to determine whether they represent a single species or, conversely, they hide a complex of closely related taxa.
Geo-environmental dynamism reveals patterns of island colonization
A biogeographical approach to better understand the occurrence of phytoseiid species in the Canaries must be based on the geo-environmental dynamics of the islands since their emergence from the Atlantic Ocean and on the origin and dispersal pattern of host plants on which the mites thrive. The seven main islands in the archipelago are, more or less, arranged in a linear age sequence from the continental mainland, decreasing in age with greater distance from Africa. Fuerteventura lies barely 96 km from the African littoral and it is the oldest island, estimated to have emerged above sea level between 20–24 Ma (million years ago) (Carracedo and Troll, 2021). Geologically, each oceanic island undergoes a life cycle or ontogeny with several stages: since its emergence breaking the sea surface, to further growth to great heights increasing its topographic complexity, followed by erosion and lengthy island subsidence, and eventually, both processes reducing the island to a seamount again (Whittaker et al., 2024). Hence, Fuerteventura is an ''old oceanic island'' on which the high volcanos have long since disappeared due to erosion, though these structures are still evident in the landscape. There are no high altitudes, and as a result, clouds from the ocean do not release rain, rendering the island semi-desertic, almost devoid of trees, and similar to the nearby African coast.
As this short review illustrates, Fuerteventura is under extreme environmental conditions, particularly in terms of low humidity, scarce water resources, and limited habitat complexity, and the mites living there must thrive in these harsh conditions. Unfortunately, the phytoseiid fauna on the whole archipelago and the near African coast is only partially known or completely unknown and, referring to the species´ geographic origin, I can only offer tentative explanations, aiming to provide at least the foundations for further and improved predictive models of both species richness and insular colonization patterns.
Species richness
Even though different habitats were surveyed over several days, only five species were collected. This limited diversity contrast with Fuerteventura´s land surface (about 1,670 km2) which is second only to Tenerife in the archipelago, and the expected species-area relationship, which predicts a rise in species richness with increased land surface. However, in this case, other interlinked factors have a greater predictable power, as for example the island age, elevation, climate and habitat diversity. The mean altitude of the island is only 44 m, and most of the landscape is dominated by plains and lowlands, with just one southern mountain range reaching 800 m a.s.l. This topographical simplicity is a consequence of the island age, which in turn influences the other factors. Low oceanic islands tend to have dry climates, and islands that undergo a long period of subsidence and erosion have limited habitats and microclimates for future colonizers (Leihy et al. 2018). Surely, Fuerteventura once hosted a richer mite fauna, but the geo-environmental changes over time have greatly reduced ecological opportunities and driven most of species to extinction.
Biogeographical origin of species
The origin of the species collected can be inferred from their currently known geographic ranges and the distribution of their host plants. Typhlodromus (A.) rhenanoides, T. (A.) maspalomensis and N. litoralis have wide distribution ranges that span the Mediterranean basin and are common and locally abundant in Northwest Africa and Southwest Europe. Hence, they are strong candidates for having colonized the islands via long-distance dispersal from the African continent. Phytoseiids disperse mostly by aerial means aided by their small size (Sabelis and Dicke, 1985), and the Canaries are influenced by constant northeast to southwest trade winds that enable the transport of small animals and plant propagules from the continent. Both T. (A.) rhenanoides and N. litoralis are commonly found on a wide range of host plants from different plant families, but T. (A.) maspalomensis is more plant-specific, mainly retrieved from Tamarix (Tamaricaceae) species. Three tamarisks occur in the Canaries, T. africana, T. canariensis and T. boveana, all of which also inhabit Southwest Europe, Morocco, and other Macaronesian islands (Sauebier et al. 2023). The distribution of the preferred host-plant may not only explain mite distribution, but also play a crucial role in providing ecological opportunities for colonizing mites when they arrived to new environments already furnished with suitable habitats.
Typhlodromus (A.) charactus exhibits a curious disjunct distribution. It was described from Yemen and this remains the only known record of the species. This distribution bridging the Arabic peninsula and the Canary Islands via northern Africa is congruent with the biogeographical concept of ''Rand flora″; that is, the disjunct distribution in many plant lineages between Macaronesia–Northern Africa, Southwest Asia, and East–South Africa (Sanmartín et al. 2010; Pokorni et al. 2015). Several hypotheses have been proposed to explain the evolutionary origin of this flora, but what is relevant here is that phytoseiid mites are carried by plants and it is not possible to understand mite movements accurately without considering the long-distance displacements of their host plants. A plausible explanation for their arrival in the Canaries involves a route across the huge Sahara Desert. In the current scenario, the Sahara is a barrier to the species dispersal, but its desert character is relatively recent in geological terms (it began forming 7 My ago) and the desert was once a verdant land that sustained rich and abundant live communities enabling the free movement of plants and animals across northern Africa (the desert experienced several intervals of wet and dry climates, but current aridity in the Sahara began only 4500–4000 years ago. See Williams (2023) for a readable and comprehensive account). Finally, N. extraseta sp. nov. is likely a Canarian endemic. However, based on current knowledge, we cannot safely report that. The narrow coastal strip in southwest Morocco, which harbour vegetation quite similar to that of the eastern Canary Islands, remains unexplored. This taxon could have originated in situ on the islands by diversification from a former Neoseiulella species, or could have conspecific populations thriving in the African littoral or, even, being a relictual form once present in the continent but now extinct on the mainland following island colonization.
Acknowledgements
The author was provided with a permit to collect plants by the Cabildo de Fuerteventura (Resolución 1087/2024).
References
- Athias-Henriot C. 1960. Phytoseiidae et Aceosejidae (Acarina, Gamasina) d'Algérie IV. Genre Typhlodromus Scheuten, 1857. Bull. Soc. Hist. Nat. Afr. Nord, 51 (1-3): 62-107.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini II. Le rélève organotaxique de la face dorsal adulte (Gamasides protoadéniques, Phytoseiidae). Acarologia, 17(1): 20-29.
- Beard J.J. 2001. A review of Australian Neoseiulus Hughes and Typhlodromips De Leon (Acari: Phytoseiidae: Amblyseiinae). Inv. Taxon., 15(1): 73-158. https://doi.org/10.1071/IT99017
- Carracedo J.C., Troll V.R. 2021. North-East Atlantic Islands: The Macaronesian Archipelagos. In Alderton D., Elias S.A. Editor(s). Encyclopaedia of Geology (2nd. Edition). Academic Press, Cambridge, Massachusetts, U.S.A., pp: 674-699.
- Chant D.A., McMurtry J.A. 1994. A review of the subfamilies Phytoseiinae and Typhlodrominae (Acari: Phytoseiidae). Int. J. Acarol., 20(4): 223-310. https://doi.org/10.1080/01647959408684022
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the World (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA. 219 pp.
- Chant D.A., Yoshida-Shaul E. 1989. A world review of the tiliarum species group in the genus Typhlodromus Scheuten (Acari: Phytoseiidae). Can. J. Zool., 67(4): 1006-1046. https://doi.org/10.1139/z89-144
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Int. J. Acarol., 17(3): 187-199. https://doi.org/10.1080/01647959108683906
- Charlet L.D., McMurtry J.A. 1977. Systematics and bionomics of predaceous and phytophagous mites associated with pine foliage in California. Hilgardia, 45 (7): 173-236. https://doi.org/10.3733/hilg.v45n07p173
- De Leon D. 1959. Two new genera of phytoseiid mites with a note on Proprioseius meridionalis Chant (Acarina: Phytoseiidae). Entomol. News, 70 (10): 257-262.
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2025. Phytoseiidae Database. Available from: http://www.lea.esalq.usp.br/phytoseiidae (last accessed 05 April 2025).
- Denmark H.A., Rather A.Q. 1984. Revision of the genus Typhloctonus Muma, 1961 (Acarina: Mesostigmata). Int. J. Acarol., 10: 163-177. https://doi.org/10.1080/01647958408683371
- Denmark H.A., Rather A.Q. 1996. Revision of the genus Neoseiulella Muma (Acari: Phytoseiidae). Int. J. Acarol., 22: 43-77. https://doi.org/10.1080/01647959608684080
- Denmark H.A., Welbourn W.C. 2002. Revision of the genera Amblydromella Muma and Anthoseius De Leon (Acari: Phytoseiidae). Int. J. Acarol., 28: 291-316. https://doi.org/10.1080/01647950208684308
- Döker I. 2018. Re-description of two new records and description of Neoseiulella kazaki sp. nov. (Acari: Phytoseiidae) from Turkey. Syst. Appl. Acarol. 23 (1): 113-122. https://doi.org/10.11158/saa.23.1.9
- Döker I., Vangansbeke D., Merckx J. 2022. New records of phytoseiid mites (Acari: Mesostigmata: Phytoseiidae) in Belgium with an identification key to Belgian species. Acarologia, 62 (4): 1070-1083. https://doi.org/10.24349/yshq-dgl8
- Evans G.O. 1952. On a new predatory mite of economic importance. Bull. Entomol. Res., 43: 397-401. https://doi.org/10.1017/S0007485300040566
- Evans G.O. 1963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Br. Mus. (Nat. Hist.), Zool., 10: 275-303. https://doi.org/10.5962/bhl.part.20528
- Faraji F., Roig J., Bakker F. 2011. Some new records of Phytoseiidae from southwest Europe with description of a new species from Spain (Acari: Mesostigmata). Int. J. Acarol., 37 (4): 331-346. https://doi.org/10.1080/01647954.2010.519722
- Ferragut F., Baumann J. 2021. New species and new records of phytoseiid mites (Acari: Phytoseiidae) from Cape Verde archipelago. Syst. Appl. Acarol., 26 (2): 395-426. https://doi.org/10.11158/saa.26.2.6
- Ferragut F., Peña-Estévez M.A. 2003. Phytoseiid mites of the Canary Islands (Acari: Phytoseiidae): 1. Gran Canaria Island. Int. J. Acarol., 2 (2): 149-170. https://doi.org/10.1080/01647950308683654
- Ferragut F., Peña-Estévez M.A. 2007. Phytoseiid mites of the Canary Islands (Acari, Phytoseiidae). II Tenerife and La Gomera Islands. Graellsia, 63: 349-358. https://doi.org/10.3989/graellsia.2007.v63.i2.102
- Ferragut F., Carnero A., Peña-Estévez M.A., Hernández-García M & Marzal C. 1988. El ácaro rojo Panonychus citri (McGregor), (Acari, Tetranychidae), nueva plaga de los cítricos en las islas Canarias. Actas V Congresso Internacional da Sociedade Portuguesa de Entomologia and IX Jornadas de la Asociación Española de Entomología, Granada, Spain, pp: 891-897.
- Ferragut F., Pérez-Moreno I., Iraola V., Escudero L.A. 2010. Ácaros depredadores de la familia Phytoseiidae en los cultivos españoles [in Spanish]. Ediciones Agrotécnicas, Madrid. 202 pp.
- Kanouh M., Kreiter S., Douin M., Tixier M.-S. (2012) Revision of the genus Neoseiulella Muma (Acari: Phytoseiidae). Re-description of species, synonymy assessment, biogeography, plant supports and key to adult females. Acarologia, 52: 259-348. https://doi.org/10.1051/acarologia/20122048
- Leihy R.I., Duffy G.A., Chown S.L. 2018. Species richness and turnover among indigenous and introduced plants and insects of the Southern Ocean Islands. Ecosphere, 9 (7): e02358. https://doi.org/10.1002/ecs2.2358
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina. Memoirs of the Entomological Society of Canada, 47: 1-64. https://doi.org/10.4039/entm9747fv
- Meyer M.K.P.S., Ueckermann E.A. 1989. South African Acari V. Some mites of the Kalahari Gemsbok National Park. Koedoe, 32 (1): 1-24. https://doi.org/10.4102/koedoe.v32i1.461
- Moraes G.J. de, Kreiter S., Lofego A.C. 1999. Plant mites (Acari) of the French Antilles. 3. Phytoseiidae (Gamasida). Acarologia, 40 (3): 237-264. https://www1.montpellier.inrae.fr/CBGP/acarologia/article.php?id=149
- Moraes G.J. de, McMurtry J.A., Denmark H.A., Campos C.B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa, 434: 1-494. https://doi.org/10.11646/zootaxa.434.1.1
- Moraza M.L. 2025. Glandular and non-glandular cuticular organs on the idiosoma of Gamasina mites (Acari: Mesostigmata). Persian Journal of Acarology, 14 (1): 151-171. https://doi.org/10.22073/pja.v14i1.86027
- Moraza M.L., Peña-Estévez M.A. 2005. Ácaros Mesostigmata (Acari, Mesostigmata) de habitats seleccionados de La Gomera (Islas Canarias, España). Graellsia, 61(1): 109-114. https://doi.org/10.3989/graellsia.2005.v61.i1.9
- Moraza M.L., Peña-Estévez M.A. 2006. A new species of Neoseiulella (Acari: Phytoseiidae) from the Macaronesian Region, Canary Islands. Zootaxa, 1366: 55-59. https://doi.org/10.11646/zootaxa.1366.1.3
- Moraza M.L., Peña-Estévez M.A., Ferragut F. 2005. Two new species of Neoseiulella Muma of the Canary Islands (Acari: Phytoseiidae). Int. J. Acarol., 31 (2): 107-112. https://doi.org/10.1080/01647950508683659
- Muma M.H. 1961. Subfamilies, genera, and species of Phytoseiidae (Acarina: Mesostigmata). Flor. St. Mus. Bull., 5: 267-302. https://doi.org/10.58782/flmnh.tqpo4380
- Pande Y.D., Carnero A., Hernández M. 1989. Notes on biological observations on some unrecorded species of phytophagous and predatory mites in Canary Islands. Investigación Agraria: Producción y Protección Vegetales, 4 (2): 275-281.
- Papadoulis G.T., Emmanouel N.G., Kapaxidi E.V. 2009. Phytoseiidae of Greece and Cyprus (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA, 200 pp.
- Pokorny L., Riina R., Mairal M., Meseguer A., Culshaw V., Cendoya J., Serrano M., Carbajal R., Ortiz S., Heuertz M., Sanmartín I. 2015. Living on the edge: timing of Rand Flora disjunctions congruent with ongoing aridification in Africa. Frontiers in Genetics, 6-2015. https://doi.org/10.3389/fgene.2015.00154
- Rivnay T., Swirski E. 1980. Four new species of phytoseiid mites (Acarina: Mesostigmata) from Israel. Phytoparasitica, 8: 173-187. https://doi.org/10.1007/BF03158314
- Rowell H.J., Chant D.A. 1979. Observations on the ontogeny of setae in the family Phytoseiidae (Acarina: Gamasina). Can. J. Zool., 57 (3): 670-682. https://doi.org/10.1139/z79-080
- Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. J. Zool., 110: 859-876. h https://doi.org/10.4039/Ent110859-8
- Sabelis M.W., Dicke M. 1985. Long-range dispersal and searching behaviour. In: Helle W. and Sabelis M.W. (eds), Spider mites. Their biology, natural enemies and control. Elsevier, Amsterdam, The Netherlands, pp. 141-157.
- Sanmartin I., Anderson L., Alarcón M., Ronquist, F., Aldasoro J.J. 2010. Bayesian island biogeography in a continental setting: the Rand Flora case. Biology Letters, 6: 703-707. https://doi.org/10.1098/rsbl.2010.0095
- Sauerbier H., Cabrera F., Muer T. 2023. Flora Vascular de Canarias. Publ. Turquesa, La Laguna, Tenerife. 1514 pp.
- Scheuten A. 1857. Einiges über Milben. Archiv für Naturgeschichte, 23: 104-112.
- Schicha E., Corpuz-Raros L.A. 1992. Phytoseiidae of the Philippines. Indira Publishing House, West Bloomfield, USA, 190 pp.
- Schuster R.O., Pritchard A.E. 1963. Phytoseiid mites of California. Hilgardia, 34: 191-285. https://doi.org/10.3733/hilg.v34n07p191
- Stathakis T.I., Kapaxidi E.V., Papadoulis G.T. 2016. A new species and three new records of Phytoseiidae (Acari: Mesostigmata) found on coastal and wetland vegetation in Greece. Syst. Appl. Acarol., 21(5): 567-582. https://doi.org/10.11158/saa.21.5.2
- Swirski E., Amitai, S. 1984. Notes on phytoseiid mites (Mesostigmata: Phytoseiidae) from the Mediterranean littoral zone of Israel, with a description of a new species of Typhloctonus. Isr. J. Entomol., 18: 71-82.
- Swirski E., Amitai S. 1997. Notes on phytoseiid mites (Mesostigmata: Phytoseiidae) of Mt. Carmel (Israel), with descriptions of two new species. Isr. J. Entomol., 31: 1-20.
- Swirski E., Ragusa Di Chiara S., Tsolakis H. 1998. Keys to the phytoseiid mites (Parasitiformes, Phytoseiidae) of Israel. Phytophaga, 8: 85-154.
- Tenorio J.M., Denmark H.A., Swift S.F. 1985. Catalog of Acari in the Hawaiian Islands. I. Mesostigmata (or Gamasida) (Acari). Int. J. Entomol., 27 (4): 297-309.
- Tixier MS, Allam L, Douin M, Kreiter S. 2016. Phytoseiidae (Acari: Mesostigmata) of Morocco: new records, descriptions of five new species, re-descriptions of two species, and key for identification. Zootaxa, 4067 (5): 501-551. https://doi.org/10.11646/zootaxa.4067.5.1
- Ueckermann E.A. 1996. Some Phytoseiidae of Yemen (Acari: Mesostigmata). Fauna of Saudi Arabia, 15: 20-36.
- Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomology Memoir, Department of Agriculture and Water Supply, Republic of South Africa, 168 pp.
- Ueckermann E.A., Zannou I., Moraes G.J. de, Oliveira A., Hanna R., Yaninek, J.S. 2008. Phytoseiid mites of the tribe Typhlodromini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1901: 1-122. https://doi.org/10.11646/zootaxa.1901.1.1
- Wainstein B.A. 1962. Revision du genre Typhlodromus Scheuten, 1857 et systematique de la famille des Phytoseiidae (Berlese 1916) (Acarina: Parasitiformes). Acarologia, 4: 5-30. https://www1.montpellier.inrae.fr/CBGP/acarologia/article.php?id=3956
- Williams M. 2023. When the Sahara was green. Princeton University Press, New Jersey, USA. 222 pp.
- Whittaker R.J., Fernández-Palacios J.M., Matthews T.J. 2023. Island Biogeography. Oxford University Press-Academic, UK, 496 pp. https://doi.org/10.1093/oso/9780198868569.001.0001



2025-07-25
Date accepted:
2025-09-15
Date published:
2025-09-26
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Ferragut, Francisco
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



