Selected Acari groups in the Czarne Doły nature reserve as a model group for studying the impact of an umbrella species
Błoszyk, Jerzy  1
; Niedbała, Wojciech
1
; Niedbała, Wojciech  2
; Wendzonka, Jacek
2
; Wendzonka, Jacek  3
; Rutkowski, Tomasz
3
; Rutkowski, Tomasz  4
; Kulczak, Marta
4
; Kulczak, Marta  5
and Napierała, Agnieszka
5
and Napierała, Agnieszka  6
6
1✉ Department of General Zoology, Faculty of Biology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 6, Poznań 61-614, Poland.
2Department of Animal Taxonomy and Ecology, Faculty of Biology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 6, Poznań 61-614, Poland.
3Natural History Collections, Faculty of Biology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 6, 61-614 Poznań, Poland.
4Department of General Zoology, Faculty of Biology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 6, Poznań 61-614, Poland.
5Natural History Collections, Faculty of Biology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 6, 61-614 Poznań, Poland.
6Department of General Zoology, Faculty of Biology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 6, Poznań 61-614, Poland.
2025 - Volume: 65 Issue: 3 pages: 947-961
https://doi.org/10.24349/hn9c-fxf8Original research
Keywords
Abstract
Introduction
Soil fauna, especially mites (Acari), serve an important role in soil formation processes and the circulation of matter in forest ecosystems (Pande and Berthet 1973; Behan-Pelletier 1999; Illig et al. 2005; Krantz and Walter 2009). These organisms, due to an ecological requirements of particular groups or species, are excellent bioindicators of environmental changes (Gulvik 2007; Ivan and Vasiliu 2009; Andrievskii and Syso 2012; Meehan et al. 2019; Napierała and Błoszyk 2021). The first reaction to unfavorable changes in the soil quality is often a decrease in the abundance of individual species in the mite communities (Napierała 2008; Napierała et al. 2014; Błoszyk et al. 2022). However, despite the enormous ecological importance of these tiny arthropods and the various threats caused by human activity (Sullivan and Ozman-Sullivan 2021), they are not embraced by any form of legal protection. They can only be protected indirectly by preserving their habitats in areas that are legally protected for other natural values, such as national parks, nature reserves or habitats of some legally protected species which may serve as umbrella species (Gulvik 2007; Roland et al. 2015; Marić et al. 2018; Napierała et al. 2018; Seniczak et al. 2021a; Niedbała et al. 2024; Coletta et al. 2025).
The term an umbrella species was first used by Frankel and Solué (1981) to show the fact that certain actions aimed at preserving some species can also serve as a protection for other species, which are not the main focus of the undertaken protection measures. Due to the fact that umbrella species are usually quite closely related to a specific biotope and can serve as characteristic species of ecosystems requiring protection, this notion was quite popular in many publications on biological conservation (Ehrlich and Murphy 1987; Peterson 1988). Initially it was used only as a theoretical concept, but since the 90's, many researchers proposed to check usefulness of the umbrella taxa in practice of nature conservation (Launer and Murphy 1994; Berger 1997; Lambeck 1997, 1999; New 1997; Fleury et al. 1998; Caro and O'Doherty 1999; Fleishman et al. 2000, 2001).
In this study we aim to check the usefulness of the European pond turtle (Emys orbicularis (L.)) as an umbrella species for some groups of soil mites. The European pond turtle is a rare species in Europe listed in Annex II of the Habitat Directive (European Union 1992), which means that European Union member countries are obliged to create a special areas of conservation for protection of this species. The European pond turtle is legally protected in Poland since 1935 (Dz.U. z 1935 r. nr 80, poz. 498) and requires an active protection (Dz.U. z 2016 r. poz. 2183).
In order to protect the habitats of the European pond turtle, a faunistic nature reserve called Czarne Doły was established in December 2022 in Wielkopolska (Greater Poland) (Dz. Urz. z 2022 r. poz. 9710). This opportunity made that the authors decided to consider the reserve area as a research proving ground, which could be used to assess the impact of legal protection on the natural environment in this area (Napierała et al. 2014; Napierała and Błoszyk 2021; Błoszyk et al. 2022). At the same time, the authors intended to test the hypothesis assuming that the European pond turtle can be an umbrella species for soil mites. Wilcox (1984) assumed that the role of the umbrella species in relation to other species in the environment can be fulfilled by species whose habitat requirements are ''at least as comprehensive as that of the rest of the community''. This in turn raises the question whether the conservation actions undertaken to protect the European pond turtle will favour the increase in biodiversity of soil mites, and whether the pond turtle can serve as an umbrella species for these arachnids. To demonstrate the impact of legal protection and reduction of human interference into the environment within the established nature reserve on the soil mites, it is necessary to conduct observations over a longer period of time. The fact is that long-term monitoring research studies focusing on soil fauna are rare (Briones et al. 1997; Błoszyk 1999; McGeoch et al. 2006; Konestabo et al. 2007; Bokhorst et al. 2008; Napierała 2008; Napierała et al. 2009; Błoszyk et al. 2022) and none of them was conducted since the area under survey began to be legally protected. The study presented in the current article initiates this type of monitoring research in Czarne Doły nature reserve and, at the same time, is the first study of the acarofauna occurring in this area. By analysing the material from soil and dead wood samples collected after few and several months since the reserve was established in selected ground plots within the area of the reserve, the authors were able to characterise the structure of the communities of selected groups of mites (Prostigmata: Labidostommidae, Oribatida: ptyctimous mites and the families Nothridae and Camisiidae, Mesostigmata: Uropodina and Sejina). The results presented here will serve as a reference point for future studies which will be regularly conducted and focused on the changes occurring in the communities of mites from overmentioned groups in the area of the established nature reserve. It is expected that over the time of protection, not only condition of the soil environment will improve but also the number of unstable habitats (such as dead wood), which are frequently inhabited by mites, will also increase in this area, which will positively affect the alpha-biodiversity of the reserve (Błoszyk et al. 2021; Napierała and Błoszyk 2021). If the fact of establishing a nature reserve for the protection of the pond turtle positively affects the analysed communities of mites, it can be assumed that the pond turtle may serves as the umbrella species for these arthropods.
Material and methods
Study area
The study area for this research project is the faunistic nature reserve Czarne Doły, which is located in the municipality of Osieczna near the city of Leszno (Wielkopolska, Greater Poland), where the habitat of the European pond turtle is protected (51°56′00″N 16°40′46″E) (Figure 1A). The reserve covers an area of 135.33 ha, which includes a forest, meadow and field area (transformed into a pasture) partially adjacent to Drzeczkowskie Lake (part of the artificial Wonieść reservoir), of which over 90 hectares constitutes a forest ravine within the boundaries of Karczma Borowa forest district. The forest areas within the reserve consists mostly of commercial pine forest (about 50-80 years) with small patches of different tree stands left. These parts form a mosaic of different types of tree stands, including fragments with oaks, hornbeam, beech and larch, alder, depending on the degree of habitat humidity. A number of small water bodies, located both in the forest and open areas, are important elements of the reserve, as well as wet meadows, which form in some places of the old reservoirs. In the near distance there is the village of Drzeczkowo and the nature reserve Ostoja Żółwia Błotnego. The entire protected area is within the boundaries of the special habitat protection area Natura 2000 Western Krzywińskie Lake District PLH300014 and the special bird protection area Wonieść Reservoir PLB300005. It is also located within the Krzywińsko-Osiecki Protected Landscape Area along with the afforestation area of the Gen. Dezydery Chłapowski and the Osieczna-Góra forest complex (Dz. Urz. z 2022 r. poz. 9710).
Data collection
The samples for the analysis were collected twice from plots delineated within the reserve area (Figures 1B and 2). First part of material (20 soil samples) was collected on August 2023 and next part – on September, 2024. During the second phase of research, a series of 65 samples from soil and various types of dead wood were collected. All of 85 samples includes 35 litter sievings (volume about 0.8 l), 18 unsieved samples from soil collected in meadows (11 samples) and reed beds (7), and 32 unsieved samples (volume about 0.8 l) from different types of dead wood. Samples from dead wood, such as: lying logs (20 samples), stumps (4 samples), trees decayed at the base (4 samples), and broken branches (4 samples) were collected in all types of tree stands. The examined decayed wood, were usually well-decomposed and humid. The samples came from the following tree species: birch (5), oak (11), pine (3), black alder (2), aspen (1), hazel (2), ash (2), and larch (1) (because of degree of decay it was not possible to identify a tree species for all samples). Samples from litter were collected from different types of forests, such as tree stands with: hornbeam (12), beech (2), oak (2), undefined deciduous forest (4), pine (5), larch (6) and shrubs (4). The number of collected samples was proportional to the area of each habitat within the reserve.



The collected samples were extracted in Tullgren funnels for 3-4 days (depending on the moisture of the sample). The extracted specimens were preserved in 75% ethyl alcohol. The mites from the extracted material were sorted out with an Olympus SZX16 stereoscopic microscope. The species identification was performed with Olympus BX51 and 53 microscopes with Nomarski contrast. The found mites from the Uropodina suborder and the Nothridae family, as well as other Prostigmata, were identified by Błoszyk with the keys published in the works of Karg (1989) and Olszanowski (1996). The ptyctimous mites were identified by Niedbała, based on his own studies (2008). The aforementioned groups of mites have been the subject of long-standing research in Poland, therefore there are the most extensive information on their biology, ecology, and distribution. That is why, we considered them to be the suitable model groups for this type of study. All identified specimens, as well as other extracted specimens of invertebrates from various groups not used in this analysis, have been deposited in the Natural History Collections at the Faculty of Biology of Adam Mickiewicz University in Poznań. The collection of material will be repeated in 2026, with particular emphasis laid on unstable habitats, and then the ground plots will be regularly monitored every five years.



Data analysis methods
The structure of the analyzed mite communities found in the area of Czarne Doły nature reserve was expressed as relative abundance (dominance – D), and the frequency of occurrence (F). (Błoszyk 1999). The data used for analyses have been stored in the computer database Analizator 2.0 in the Natural History Collections (Faculty of Biology, AMU in Poznań).
In order to assess the natural value of the area of the reserve and it's changes occurring in the Uropodina communities over the time, the Maturity Index (MI) has been used in the study (Napierała and Błoszyk 2021). The MI was calculated on the basis of the formula proposed by Ruf (1998) and N′Dri et al. (2018):
\[M I=\frac{\sum_{i=1}^S K i}{\sum_{i=1}^S K i+\sum_{i=1}^S r i}\]
with S – number of species, K – K-value ranging from 1 to 3, r – r-value ranging from 1 to 3 for the species i.
The MI can be used for monitoring of forest ecosystems and its value is higher in less disturbed areas (Ruf 1998; N′Dri et al. 2018). The index is based on the occurrence in the community the species with K and r-selected life-history traits ranging from 1 to 3. In this study the MI has been calculated only for Uropodina because only for this group r-to-K reproductive strategies, have been established so far (Napierała and Błoszyk 2021). The assignment of particular Uropodina species to the r/K scale was carried out according to such features as the ecological indices (dominance and frequency), ecological tolerance based on habitat, population growth rate, occurrence of larvae and the presence and intensity of phoresy. The procedure has been described in details in the publication of Napierała and Błoszyk (2021).
The similarity of the Uropodina species composition for mites in communities found in different nature reserves in Poland was calculated by means of the Marczewski–Steinhaus species similarity index: S = c/(a + b − c), where c is the number of species present in both compared communities, and a and b stand for the total number of species in each community. The full joining analysis, which uses the most distant neighbors, was used to prepare the dendrogram (Magurran 2004).
Results
Community of soil mites of Czarne Doły nature reserve
In 85 samples collected in 2023 and 2024, a total of 4985 specimens of mites were collected (Tables 1, 2), of which 3968 were assigned to 40 species (Table 1). The remaining 1017 specimens were identified only to the family level (Table 2). Among 40 identified mite species from orders Prostigmata (families Labidostommidae and Crypthognathidae), Oribatida (families Nothridae, Camisiidae, Mesoplophoridae, Euphthiracaridae, Phthiracaridae, Steganacaridae) and Mesostigmata (suborders Uropodina and Sejina) 14 were found only in the second phase of the study (in 2024) (Table 1).
Download as
Species
N
D%
F%
Ave±SD
M
R
H
D
B
O
P
L
S
Dw
Prostigmata
Labidostomma luteum Kramer, 1889
19
0.5
7.5
3.17±2.32
+
+
+
Cryptognathus lagena Kramer, 1878*
16
0.4
3.8
5.33±1.53
+
Oribatida
Phthiracarus globosus (C. L. Koch, 1841)
391
9.9
50.0
9.72±9.17
+
+
+
+
+
+
+
+
+
+
Phthiracarus longulus (C. L. Koch, 1841)
286
7.2
45.0
7.94±9.85
+
+
+
+
+
+
+
+
+
+
Phthiracarus crinitus (C. L. Koch, 1841)*
262
6.6
27.5
11.91±18.03
+
+
+
+
+
+
+
Acrotritia duplicata (Grandjean, 1953)
250
6.3
27.3
11.90±15.75
+
+
+
+
+
+
+
+
+
+
Acrotritia ardua (C. L. Koch, 1841)
229
5.8
35.0
8.18±12.02
+
+
+
+
+
+
+
+
Atropacarus (Atropacarus) striculus (C. L. Koch, 1836)
195
4.9
26.3
9.29±15.55
+
+
+
+
+
+
+
+
Heminothrus peltifer (C. L. Koch, 1839)
164
4.1
25.0
8.20±14.82
+
+
+
+
+
+
+
+
Phthiracarus laevigatus (C. L. Koch, 1841)
145
3.7
35.0
5.18±5.52
+
+
+
+
+
+
+
+
Euphthiracarus cribrarius (Berlese, 1904)*
101
2.6
7.5
16.83±21.18
+
+
+
Phthiracarus nitens (Nicolet, 1855)
101
2.6
31.3
4.04±3.55
+
+
+
+
+
+
+
+
Heminothrus targionii (Berlese, 1885)
84
2.1
25.0
4.20±6.34
+
+
+
+
+
+
Nothrus silvestris Nicolet, 1855
52
1.3
8.8
7.43±12.29
+
+
+
Mesoplophora (Parplophora) pulchra (Sellnick, 1928)*
51
1.3
10.0
6.37±7.52
+
+
+
Atropacarus (Atropacarus) csiszarae (Balogh et Mahunka, 1979)*
41
1.0
11.3
4.56±5.10
+
+
+
+
+
+
+
Nothrus palustris C. L. Koch, 1839
12
0.3
3.8
4.00±5.20
+
+
Phthiracarus bryobius Jacot, 1930
11
0.3
1.3
11.00
+
Phthiracarus boresetosus (Jacot, 1930)*
6
0.2
2.5
3.00±2.83
+
Microtritia minima (Berlese, 1904)*
6
0.2
1.3
6.00
+
Phthiracarus ferrugineus (C. L. Koch, 1841)
2
0.05
1.3
2.00
+
Steganacarus (Steganacarus) magnus (Nicolet, 1855)*
2
0.1
2.5
1.00
+
Camisia spinifer (C. L. Koch, 1835)
1
0.1
1.3
1.00
+
Mesostigmata
Oodinychus ovalis (C. L. Koch, 1839)
615
15.6
58.8
13.08±19.25
+
+
+
+
+
+
+
+
+
+
Oodinychus karawaiewi (Berlese, 1904)
440
11.1
25.0
22.00±23.30
+
+
+
+
+
+
+
+
Olodiscus minima (Kramer, 1882)
154
3.9
41.3
4.67±4.88
+
+
+
+
+
+
+
+
+
+
Pulchellaobovella pulchella (Berlese, 1904)*
88
2.2
8.8
12.57±17.42
+
Trachytes aegrota (C. L. Koch, 1841)
65
1.6
13.8
5.91±13.66
+
+
+
+
Dinychus inermis (C. L. Koch, 1841)
44
1.11
8.75
6.29±5.62
+
+
Sejus togatus C. L. Koch, 1836*
32
0.81
12.50
3.20±3.12
+
+
+
+
Nenteria breviunguiculata (Willmann, 1949)
22
0.6
1.2
22.00
+
Dinychus perforatus Kramer, 1882
19
0.5
3.8
6.33±9.24
+
+
+
Dinychus carinatus (Berlese, 1903)***
17
0.5
5.0
4.25±1.71
+
Uroobovella obovata (Canestrini, Berlese, 1884)*
17
0.4
3.8
5.67±4.04
+
Dinychus woelkei (Hirschmann et Zirngiebl-Nicol, 1969)*
7
0.4
3.8
2.33±2.31
+
+
Uropoda orbicularis (O. F. Müller, 1776)
7
0.2
6.3
1.40±0.55
+
+
Urodiaspis tecta (Kramer, 1876)
5
0.2
2.5
2.50±0.71
+
+
Dinychus septentrionalis (Trägårdh, 1943)
5
0.1
1.3
5.00
+
Uroobovella sp.*
3
0.1
1.3
3.00
+
Discourella modesta (Leonardi, 1889)
1
0.1
1.3
1.00
+
Total
3.968
100.00
23
13
17
15
10
13
14
9
22
29
The Prostigmata order was represented by two identified species, namely L. luteum and C. lagena (Table 1) and other species belonging to six families (Table 2). The participation of overmentioned species in the analyzed community was very low (< 1%), as was their frequency in the samples (Table 1). Among representatives of the order Oribatida, 21 species were identified in the collected samples (16 species of ptyctimous mites and 5 species of Crotonioidea). The most numerous in this group and the most frequently encountered species was P. globosus. The second most numerous and frequent species was P. longulus. The remaining Oribatida species were moderately numerous or rare, with A. ardua, P. laevigatus, and P. nitens appearing in over 30% of the collected samples, thus being quite common. The identified Crotonioidea species were quite rare in the collected material, but some of them, such as H. peltifer and H. targionii, were moderately common as they appeared in 25% of the collected samples.
Mesostigmata were represented by 17 identified species from suborders Uropodina and Sejina (Table 1) as well as by representatives of four families (Table 3). Among the 17 species representing mentioned suborders, the most numerous were O. ovalis and O. karawaiewi. The former is also the most frequently encountered species in the area of reserve (Table 1). The second most frequent species from suborder Uropodina in the reserve is O. minima. Moderately frequent, but not very numerous, is the representative of the suborder Sejina, namely S. togatus. A similar frequency in the samples is shown by T. aegrota, which, however, occurs in relatively low number in the reserve. The most numerous among the studied Mesostigmata and Prostigmata families in the reserve were mites from the family Zerconidae, which accounted for over 70% of all included mites (Table 2). The other families were relatively few in number (group share >10%).
Download as
Order
Family
N
D%
F%
Ave± SD
M
Zerconidae
730
71.8
36.3
25.17±60.83
P
Stigmaeidae
78
7.7
20.0
4.87±6.17
P
Rhagididae
49
4.8
36.3
1.69±1.28
M
Ixodidae
48
4.7
23.8
2.53±4.14
P
Bdellidae
47
4.6
23.8
2.47±2.12
P
Trombididae
27
2.6
16.3
2.08±1.26
P
Cunaxidae
20
2.0
15.0
1.67±1.07
M
Epicriidae
10
0.1
3.8
3.33±2.08
M
Eviphidae
5
0.5
5.0
1.250±0.50
P
Cheyletidae
3
0.3
1.3
3.000
Total
1.017
100.00
The analysis of occurrence of particular species in studied habitats revealed that the most species (29) have been found in different types of dead wood (Table 1). Next two habitats with the biggest number of identified species were meadows (23) and shrubs (22). The least number of species (10) has been found in tree stand with beech. Only five species identified from the collected material were found in all (10) habitats under scrutiny. These are ptyctimous mites such as: P. globosus, P. longulus, A. duplicata and uropodid mites: O. ovalis and O. minima. There is also group of species, such as: A. ardua, A. (A.) striculus, H. peltifer, P. laevigatus, P. nitens and O. karawaiewi which occurs in almost all (8 from 10) habitats. Over ¼ of species for example: C. lagena, P. boresetosus and D. modesta have been found only in one type of habitat (Table 1).
Maturity Index for Czarne Doły nature reserve
Due to the analysis of the life strategies for individual species of Uropodina mites which has been conducted by Napierała and Błoszyk (2021), it was possible to use the MI index to assess the condition of the soil environment in the area of Czarne Doły nature reserve. Among the Uropodina species found in Czarne Doły, most of them, except for D. intermis, D. woelkei, U. obovata and D. septentrionalis, were species with the r-type strategy (Napierała and Błoszyk 2021). Thus, the MI index for this area is low and is 0.21 (Table 3).
Download as
Strategy*
Value
O. ovalis
r1
1
O. karawaiewi
r1
1
O. minima
r1
1
P. pulchella
r2
2
T. aegrota
r1
1
D. inermis
K1
1
N. breviunguiculata
r3
3
T. aegrota
r1
1
D. perforatus
r1
1
D. carinatus
r2
2
U. obovata
K1
1
D. woelkei
K1
1
U. tecta
r1
1
D. modesta
r3
3
U. orbicularis
r2
2
D. septentrionalis
K2
2
Total of K-species
4
Total of r-species
12
The acarofauna of Uropodina in the Czarne Doły reserve in comparison with several other well-studied reserves in Poland
To compare the similarity of Uropodina communities in the Czarne Doły reserve with other well-studied nature reserves in Poland, data from selected reserves in the Greater Poland Voivodeship were used. These reserves are: Jakubowo and Las Grądowy nad Mogilnicą, Huby Grzebieniskie, Bytyńskie Brzęki, Brzęki przy Starej Gajówce, as well as the oldest reserve in Poland and one of the oldest in Europe, Cisy Staropolskie im. Leona Wyczółkowskiego in Wierzchlas, located in the Tuchola Forest (Kuyavian-Pomeranian province). This reserve has the richest Uropodina communities composed of 48 species (Rutkowski 2024) and was used as a reference object in this comparison.
The highest similarity (at the level of 65%) in species composition of Uropodina communities was found between the closely located reserves of Las Grądowy and Jakubowo, as well as the reserves Huby Grzebieniskie and Bytyńskie Brzęki (Figure 3). The mite community of the Czarne Doły reserve was most similar in species composition to the community found in Las Grądowy nad Mogilnicą and Huby Grzebieniskie reserves. However, this similarity was relatively low, at about 43%. The lowest similarity in species composition (26%) was found between the Czarne Doły reserve and the Brzęki przy Starej Gajówce reserve. Low similarity in species composition (33%) was also observed in relation to the richest reserve in terms of Uropodina species - Cisy Staropolskie im. Leona Wyczółkowskiego reserve.
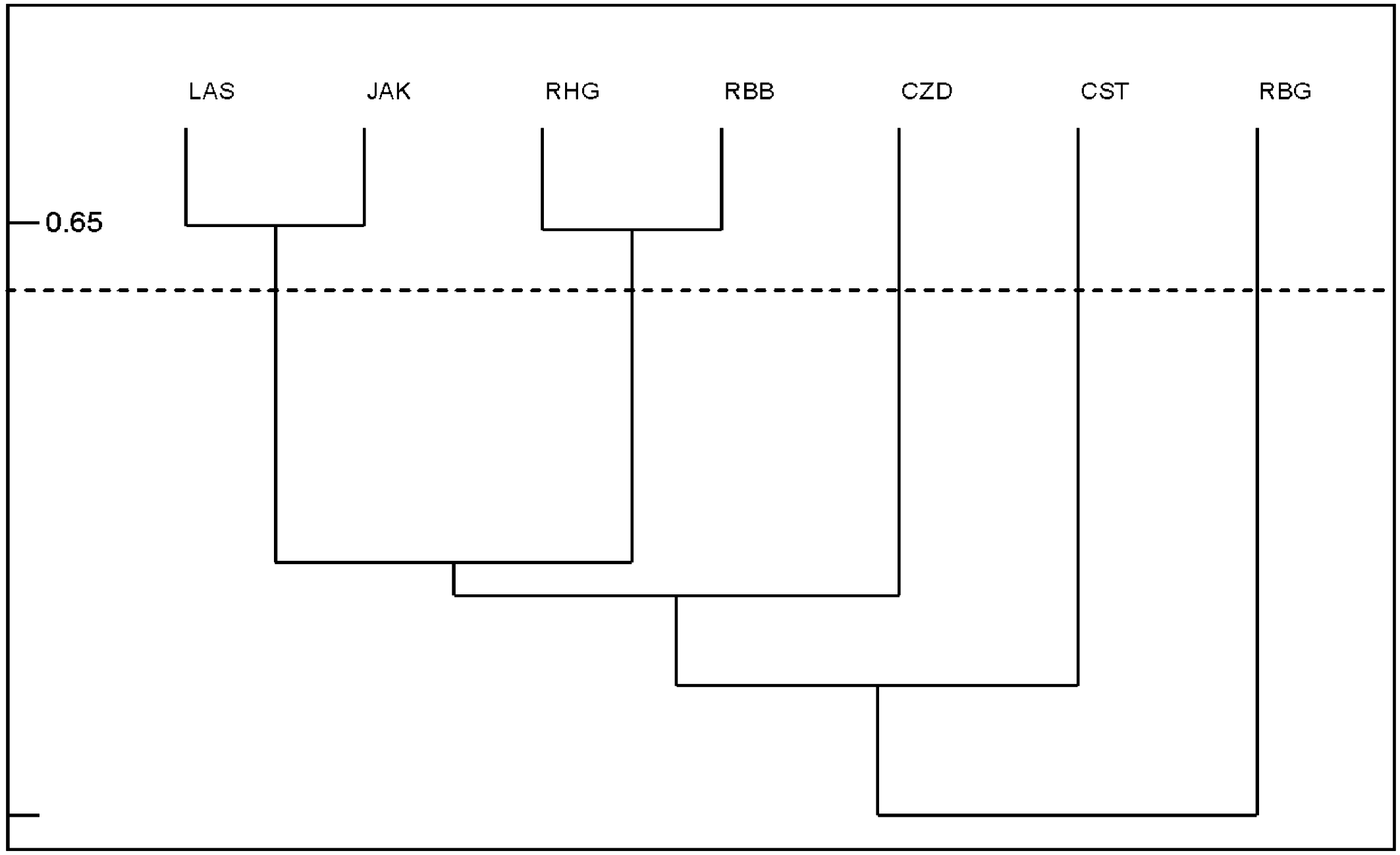


Discussion
The obtained results of the research into soil mites conducted in 2023 and 2024 in Czarne Doły nature reserve illustrate the current state of the acarofauna in the reserve shortly after its establishment. The species composition and structure of the community provide baseline data for future studies on the impact of the reserve's protected status on the functioning of these communities in future. The area of the reserve is the third site in Poland of D. septentrionalis which is a rare Uropodina species, found in the wet meadow in the center of the reserve. So far, this species has been found in Poland only in dead wood in the Białowieża Primeval Forest (Napierała et al. 2020) and in Cisy Staropolskie nature reserve also in dead wood and in litter of riparian forest (Rutkowski 2024). Besides this, most species of Uropodina found there, were common species known from various types of habitats (Błoszyk 1999). The high percentage of the synanthropic Uropodina species O. karawaiewi in this area (it has been found in ¼ of collected samples in almost all the studied habitats, see Table 1) is also noteworthy because this species is often found in areas affected by human activity and is a good indicator of strong anthropogenic pressure i.e. the more the environment resembles a natural state, the more limited the occurrence of it. That is why it is commonly found in parks and commercial forests (Błoszyk et al. 2006). Due to the fact that establishing the reserve will limit the impact of various types of anthropopressure, such as tree cutting down and tree stand maintenance activities, it will undoubtedly activate natural restoration processes that will affect the soil fauna. It can be hypothesized that one of the first expected changes that should be observed in the mite community will be a decrease in the number and frequency of O. karawaiewi, which will lead to the entire loss of it, especially in the southern part of the reserve, where middle-aged planted pine trees prevail. A similar effect was observed in Cisy Staropolskie reserve of Leona Wyczółkowski in Wierzchlas, after closing a section of the tourist trail (manuscript in progress).
The examined mite community also contained a high frequency (\textgreater40%) of O. minima and D. inermis, which both prefer humid habitats (Błoszyk 1999; Kaczmarek et al. 2013; Mašán 2001). The first species has been found in all studied habitats, which is not surprising, because it is an eurytopic species which occurs in different types of habitats in Poland and Europe (Błoszyk 1999; Kurek et al. 2020; Mašán 2001; Zduniak et al. 2019). D. intermis has been found in Czarne Doły reserve in the most humid habitats, such as meadows and reeds (Table 1). Previous studies revealed that the species is associated with litter of alder forests, humid meadows and peat bogs (Błoszyk 1999; Kaczmarek et al. 2013; Mašán 2001). In the case of this species, as well as the aforementioned D. septentrionalis, one can expect a further increase in the percentage of these species in the community with time because their habitat requirements regarding humidity overlap with the habitat preferences of the pond turtle and the undertaken conservation actions for this species. The actions aimed at reducing the anthropogenic pressure should also have a positive impact on the population of L. luteum from the Labidostommidae family (Acari: Prostigmata) which has been found in only three habitats in studied reserve (Table 1). This species is a good bioindicator of low anthropogenic pressure on the soil of forest ecosystems, as it is practically non-existent in intensively exploited commercial forests (Błoszyk 1980; Zduniak et al. 2019). That is why one can expect a gradual increase in the population size of this species, although probably over a slightly longer period of time.
Many previous observations show that the soil fauna in nature reserves is usually richer and more diverse than in commercial forests (Gwiazdowicz and Kmita 2004; Napierała 2008; Napierała et al. 2009; Skorupski et al. 2009; Marquardt and Kaczmarek 2010; Manu et al. 2021; Pollierer et al. 2021; Seniczak et al. 2021a,b; Langridge et al. 2023; Junggebauer et al. 2024). Thus, the species richness of mite community in the discussed reserve was compared with the fauna of other nature reserves. In general, mites from the suborder Uropodina found in Czarne Doły nature reserve form a community of similar species composition to those that can be found both in the commercial forests of Wielkopolska (Greater Poland) and partly in nature reserves in Poland (Błoszyk 1999; Skorupski 2001; Napierała 2008; Napierała et al. 2009; Gwiazdowicz and Kmita 2004; Gwiazdowicz et al. 2011). Among the seven nature reserves compared in terms of Uropodina species composition, the community in the area of Czarne Doły reserve was most similar to the communities of the four nature reserves with hornbeam tree stand in Wielkopolska (Figure 3), such as: Jakubowo, Las Grądowy nad Mogilnicą, Huby Grzebieniskie and Bytyńskie Brzęki. Jakubowo and Las Grądowy nad Mogilnicą reserves (both established in 1959) protect over 100 years old oak-hornbeam forest and oak-hornbeam tree stand with beech (M. P. z 1959 r. Nr 78, poz. 414). The communities in these reserves have been studied since over 40 years and consists – respectively – of 17 and 14 species of Uropodina (Błoszyk 1999; Napierała et al. 2014; Błoszyk et al. 2021). Huby Grzebieniskie and Bytyńskie Brzęki reserves were established also in 1959, in order to protect also the oak-hornbeam forests but with admixture of pine and larch trees (in case of the second reserve) (M. P. z 1959 r. Nr 78, poz. 414). The communities of Uropodina in these reserves are composed of 18 (Bytyńskie Brzęki) and 15 (Huby Grzebieniskie) species (Błoszyk et al. 2015). In all these four reserves not only samples from soil and litter but also from different type of dead wood have been collected (Napierała et al. 2014; Błoszyk et al. 2015, Błoszyk et al. 2021). One may conclude, that the observed similarity of Uropodina species composition in four above described reserves and in Czarne Doły may results from existence of the mosaic of small parts of various tree stands (see Table 1) in the area of Czarne Doły reserve. All these small parts of tree stands, enables existence of similar number of Uropodina species in this newly established reserve as in the more uniform and older reserves in Wielkopolska.
Comparing the ecological value of Czarne Doły reserve to other sites based on the MI index (Table 3), it should be noted that this reserve is poorer in this respect than the communities found in the oldest nature reserves, such as Cisy Staropolskie im. Leona Wyczółkowskiego in Wierzchlas (established in the 1950s), where the MI value is 0.32. The MI value for Czarne Doły reserve, which is 0.21, is also lower than for Jakubowo nature reserve (MI=0.27), but it is equal to the value for Las Grądowy nad Mogilnicą reserve (Napierała and Błoszyk 2021). The MI value for Czarne Doły reserve is rather low because only four species with the K life startegy were found in this area (Napierała and Błoszyk 2021).
In case of oribatid mites, the preliminary studies have shown that their fauna is mostly composed of species that are eurytopic, i.e. numerous, and those which commonly occur in different habitats all over the area of Poland (Niedbała 2008). The most numerous and frequent oribatid mites found in Czarne Doły reserve belong to ptyctimous mites from genera Phthiracarus and Acrotritia (Table 1). These oribatids are known as frequent and numerous in various types of different habitats, mainly deciduous and coniferous forests (Niedbała 2008; Seniczak et al. 2021b). The representatives of other examined families of oribatid mites, i.e. Camisiidae and Nothridae, which were found here, are also common and eurytopic species. They have been found so far in various types of habitats such as coniferous and mixed forests, brushwoods, parks as well as open habitats (Olszanowski 1996). In the area of Czarne Doły reserve representatives of genus Heminothrus were the most frequent and occurred in ¼ of collected samples from most of the studied habitats (Table 1). Thus, fauna of oribatid mites found in this area is not specific but definitely common, composed of species which are known also from environments under anthropogenic pressure (Olszanowski 1996; Niedbała 2008), without species with a distinguished preference for specific environmental conditions. Furthermore, due to the significantly higher degree of eurytopy in the discussed groups of oribatid mites (Olszanowski 1996; Niedbała 2008), the predicted changes in the species composition of these groups will certainly have a smaller range and slower pace than in the case of Uropodina, which are more sensitive to changes in the environment (Napierała 2008; Napierała and Błoszyk 2021). Moreover, an increase in the number of unstable microhabitats, which will be analysed in the future, will probably be less important because the current observations have revealed that the differences in the species composition of ptyctimous mites inhabiting the soil of a given area and in the merocenoses occurring there are still small (Napierała et al. 2020).
One can also assume that changes in the natural environment of Czarne Doły nature reserve resulting from the legal protection, will contribute to the increase in the number and species diversity of mites from various mite groups. At the same time, the course of these changes may vary in each monitoring plot for different species, depending on their habitat preferences. There is no doubt that regular monitoring of these changes will enable the collection of very valuable data, especially about the ecology of the particular species. The data will also be very useful in nature conservation planning. Research repeated in the following year (2024) showed an 35% increase in the biodiversity of mite communities present in the area of the reserve, which was related both to the increase in the number of samples and the inclusion of a new microhabitat, i.e., dead wood. The community studied in 2024 included a second species from the order Prostigmata (C. lagena), seven new species of Oribatida, and six species from order Mesostigmata, among which there were species typical for dead wood, such as P. pulchella, D. carinatus, and D. woelkei (Błoszyk, 1999; Mašán 2001).
Conclusion
Wetlands are extremely valuable for nature conservation, and at the same time, they are often sensitive and threatened ecosystems. This is often reflected in the recommendation of their protection formulated under the Ramsar Convention (UNESCO 1971). Although Czarne Doły nature reserve was established to protect the population of the pond turtle, the new legal status will also protect all other organisms in this area. The pilot studies on soil fauna presented here have shown that in the area of occurrence of a protected vertebrate species, in this case the pond turtle, there are also valuable and rare species of soil mites, i.e. L. luteum and D. septentrionalis, with similar habitat requirements, which are currently neither well-studied species, nor legally protected. What these species – the pond turtle and the mite species – have in common are their similar habitat requirements and the fact that they are threatened with habitat destruction due to ongoing global climate changes. In this respect, we may assume that the pond turtle may serve as an umbrella species for the soil fauna present in this reserve. Moreover, the results of preliminary studies conducted in this nature reserve, may constitute a good starting point of long-term, unique and valuable monitoring studies focusing on changes occurring in the soil environment. The idea of regular monitoring of soil, litter and various types of unstable microhabitats conducted in the reserve should show what impact the legal protection of the area will have on the mesofauna in the future. These observations in the long run may be a premise to exclude small fragments from use in forest grown for commercial purposes, even without the need to establish reserves, mainly by allowing for the undisturbed operation of natural processes occurring in the forest ecosystem.
Acknowledgements
We would like to thank the Regional Directorate for Environmental Protection in Poznań (permit No. WPN-I.6205.30.2024.MM.2) and the Polish Society for Nature Conservation ''Salamandra'' for all the help they provided us. This work was supported by International Union for Conservation of Nature - IUCN (Grant number SMA-G00-GG-0000000779).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrievskii V.S., Syso A.I. 2012. The effect of different types of anthropogenic changes in soils on communities of oribatids in urban ecosystems. Cont. Probl. of Ecol., 5: 574-579. https://doi.org/10.1134/S1995425512060030
- Behan-Pelletier V.M. 1999. Oribatid mite biodiversity in agroecosystems: Role for bioindication. Agric. Ecosyst. Environ., 74: 411-423. https://doi.org/10.1016/S0167-8809(99)00046-8
- Beier P. 1993. Determining minimum habitat areas and habitat corridors for cougars. Conserv. Biol., 7: 94-108. https://doi.org/10.1046/j.1523-1739.1993.07010094.x
- Berger J. 1997. Population constraints associated with the use of black rhinos as an umbrella species for desert herbivores. Conserv. Biol., 11: 69-78. https://doi.org/10.1046/j.1523-1739.1997.95481.x
- Błoszyk J. 1980. Badania nad rodziną Nicoletiellidae (Acari, Prostigmata) w Polsce. Prace Komisji Biol. PTPN., 54: 53-85.Błoszyk J. 1999. Geograficzne i ekologiczne zróżnicowanie zgrupowań roztoczy z kohoryty Uropodina (Acari: Mesostigmata) w Polsce: Uropodina lasów grądowych (Carpinion betuli). Poznań: Kontekst. pp. 245.
- Błoszyk J., Krysiak D., Napierała A., Dylewska M. 2006. Can soil fauna undergo synantropisation. In: Gabryś G., Ignatowicz S. (Eds). Advances in Polish Acarology. Warszawa: SGGW. p. 26-37.
- Błoszyk J., Markowicz M., Labijak B., Skwierczyński F., Napierała A. 2015. Microgeographic diversity of Uropodina (Acari: Mesostigmata) communities in dead wood and tree hollows. Redia, 98: 3-12.
- Błoszyk J., Napierała A., Kulczak M., Zacharyasiewicz M. 2022. Changes in forest stand and stability of Uropodine mites communities (Acari: Parasitiformes) in Jakubowo nature reserve in the light of long-term research. Forests, 13(8): 1219. https://doi.org/10.3390/f13081219
- Błoszyk J., Rutkowski T., Napierała A., Konwerski S., Zacharyasiewicz M. 2021. Dead wood as an element enriching biodiversity of forest ecosystems: a case study based on mites from the suborder Uropodina (Acari: Parasitiformes). Diversity, 13(10): 476. https://doi.org/10.3390/d13100476
- Bokhorst S., Huiskes A., Convey P., Bodegom P.M., Aerts R. 2008. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biol. Biochem., 40: 1547-1556. https://doi.org/10.1016/j.soilbio.2008.01.017
- Briones M.J.I., Ineson P., Piearce T.G. 1997. Effects of climate change on soil fauna. Responses of enchytraeids, diptera larvae, and tardigrades in a transplant experiment. Appl. Soil Ecol., 6: 117-134. https://doi.org/10.1016/S0929-1393(97)00004-8
- Caro T.M., O'Doherty G. 1999. On the use of surrogate species in conservation biology. Conserv. Biol., 13: 805-814. https://doi.org/10.1046/j.1523-1739.1999.98338.x
- Coletta M., Monticelli M., D'Alessandro A., et al. 2025. Managing soil to support soil biodiversity in protected areas agroecosystems: a comparison between arable lands, olive groves, and vineyards in the Conero Park (Italy). Environ. Monit. Assess., 197: 200. https://doi.org/10.1007/s10661-025-13658-7
- Dz.U. z 1935 r. nr 80, poz. 498. Rozporządzenie Ministra Wyznań Religijnych i Oświecenia Publicznego z dnia 16 października 1935 r. wydane w porozumieniu z Ministrem Rolnictwa i Reform Rolnych o uznaniu żółwia za gatunek chroniony [Internet]. [16 October 1935]. Warszawa: Sejm RP; [accessed 22 Dec 2024]. Available from: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU19350800498
- Dz.U. z 2016 r. poz. 2183. Rozporządzenie Ministra Środowiska z dnia 16 grudnia 2016 r. w sprawie ochrony gatunkowej zwierząt. [Internet]. [16 December 2016]. Warszawa: Sejm RP; [accessed 24 April 2024]. Available from: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160002183
- Dz.Urz. z 2022 r. poz. 9710. Zarządzenie w sprawie uznania rezerwatu przyrody ''Czarne Doły'' [Internet]. Poznań: General Directorate for Environmental Protection; [accessed 20 December 2023]. Available from: https://crfop.gdos.gov.pl/CRFOP/widok/viewrezerwatprzyrody.jsf?fop=PL.ZIPOP.1393.RP.1617
- Ehrlich P.R., Murphy D.D. 1987. Monitoring populations on remnants of native vegetation. In: Saunders D.A., Arnold G.W., Burbidge A.A., Hopkins A.J.M. (Eds). Nature conservation: the role of remnants of native vegetation. Sydney: Surrey Beatty & Sons. pp. 201-210.
- European Union. 1992. Council Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora [Internet]. [21 May 1992]. Official Journal of the European Communities; [accessed 22 Dec 2024]. Available from: http://data.europa.eu/eli/dir/1992/43/oj
- Fleishman E., Blair R.B., Murphy D.D. 2001. Empirical validation of a method for umbrella species selection. Ecol. Appl., 11: 1489-1501. https://doi.org/10.1890/1051-0761(2001)011%5B1489:EVOAMF%5D2.0.CO;2
- Fleishman E., Murphy D.D., Brussard P.F. 2000. A new method for selection of umbrella species for conservation planning. Ecol. Appl., 10: 569-579. https://doi.org/10.1890/1051-0761(2000)010%5B0569:ANMFSO%5D2.0.CO;2
- Fleury S.A., Mock P.J., O'Leary J.F. 1998. Is the California gnatcatcher a good umbrella species? West. Birds, 29: 453-467.
- Frankel O.H., Soulé M.E. 1981. Conservation and evolution. Cambridge: Cambridge University Press. pp. 327.
- Gulvik M.E. 2007. Mites (Acari) as indicators of soil biodiversity and land use monitoring: a review. Pol. J. Ecol. 55: 415-440
- Gwiazdowicz D.J., Kamczyc J., Rakowski R. 2011. Mesostigmatid mites in four classes of wood decay. Exp Appl Acarol 55: 155-16. https://doi.org/10.1007/s10493-011-9458-0
- Gwiazdowicz D. J., Kmita M. 2004. Mites (Acari, Mesostigmata) from selected microhabitats of the Ujscie warty national park. Acta Sci. Pol. Silv. Colendar. Rat. Ind. Lignar. 3(2): 49-55.
- Illig, J., Langel, R., Norton, R.A., Scheu, S., Maraun, M. 2005. Where are the decomposers? Uncovering the soil food web of a tropical montane rain forest in southern Ecuador using stable isotopes (15N). J. Trop. Ecol. 21, 589-593. https://doi.org/10.1017/S0266467405002646
- Ivan O., Vasiliu N. A. 2009. Oribatid mites (Acari, Oribatida) - bioindicators of forest pollution with heavy metals and fluorine. Annal. of For. Res. 52: 11-18.
- Junggebauer A., Gericke N. M., Krakau L. K., Bluhm S. L., Maraun M., Pollierer, M. M., Scheu S. 2024. Effects of forest gap formation and deadwood enrichment on oribatid mites (Acari: Oribatida) vary between regions. For. Ecol. Manag. 565: 122015. https://doi.org/10.1016/j.foreco.2024.122015
- Kaczmarek S., Marquardt T., Faleńczyk-Koziróg K., Marcysiak K. 2013. Diversity of soil mite communities (Acari) within habitats seasonally flooded by the Vistula River (Ostromecko, Poland). Biol. Lett., 49: 97-105. https://doi.org/10.2478/v10120-012-0006-3
- Karg W. 1989. Acari (Acarina) Milben, Unterordnung Parasitiformes (Anactinochaeta). Uropodina Kramer, Schildkrötenmilben. In: Senglaub K., Hannemann H.J., Schumann H. (Eds). Die Tierwelt Deutschlands und der Angrenzenden Meeresteile. Jena: Gustav Fischer Verlag. Vol. 67, pp. 203.
- Konestabo H.S., Michelsen A., Holmstrup M. 2007. Responses of springtail and mite populations to prolonged periods of soil freeze-thaw cycles in a sub-arctic ecosystem. Appl. Soil Ecol., 36: 136-146. https://doi.org/10.1016/j.apsoil.2007.01.003
- Krantz G.W., Walter D.E. 2009. A Manual of Acarology. Lubbock: Texas Tech University Press. pp. 816..
- Kurek P., Nowakowski K., Rutkowski T., Ważna A., Cichocki J., Zacharyasiewicz M., Błoszyk J. 2020. Underground diversity: Uropodina mites (Acari: Mesostigmata) from European badger (Meles meles) nests. Exp. and App. Acarol., 82: 503-513. https://doi.org/10.1007/s10493-020-00563-6
- Lambeck R.J. 1997. Focal species: a multi-species umbrella for nature conservation. Conserv. Biol., 11: 849-856. https://doi.org/10.1046/j.1523-1739.1997.96319.x
- Lambeck R.J. 1999. Landscape planning for biodiversity conservation in agricultural regions: a case study from the wheatbelt of Western Australia. Canberra: Biodiversity Technical Publication.
- Langridge J., Delabye S., Gilg O., Paillet Y., Reyjol Y., Sordello R., Touroult J., Gosselin, F. 2023. Biodiversity responses to forest management abandonment in boreal and temperate forest ecosystems: A meta-analysis reveals an interactive effect of time since abandonment and climate. Biol. Conserv., 287: 110296. https://doi.org/10.1016/j.biocon.2023.110296
- Launer A.E., Murphy D.D. 1994. Umbrella species and the conservation of habitat fragments: a case of a threatened butterfly and a vanishing grassland ecosystem. Biol. Conserv., 69: 145-153. https://doi.org/10.1016/0006-3207(94)90054-X
- Magurran A.E. 2004. Measuring biological diversity. Oxford: Blackwell Publishing. pp. 256.
- Manu M., Băncilă R.I., Bîrsan C.C., Mountford O., Onete M. 2021. Soil mite communities (Acari: Mesostigmata) as indicators of urban ecosystems in Bucharest, Romania. Sci. Rep. 11: 3794. https://doi.org/10.1038/s41598-021-83417-4
- Marić I., Međo I., Jovanović S., Petanović R., Marčić D. 2018. Spider mites (Acari: Tetranychidae) in protected natural areas of Serbia. Syst. Appl. Acarol., 23(10): 2033-2053 https://doi.org/10.11158/saa.23.10.12
- Marquardt T., Kaczmarek S. 2010. New and rare species of the Gamasida (Acari) in the Polish fauna, recorded in ′Bagno Stawek′ Reserve (Tuchola Forest, northern Poland). Biol. Lett., 46(1): 37-42. https://doi.org/10.2478/v10120-009-0012-2
- Mašán P. 2001. Roztoce kohorty Uropodina (Acari, Mesostigmata) Slovenska. Annot. Zool. Bot., 223: 1-320.
- McGeoch M.A., Le Roux P.C., Hugo E., Chown S.L. 2006. Species and community responses to short-term climate manipulation: microarthropods in the sub-Antarctic. Austral. Ecol., 31: 719-731. https://doi.org/10.1111/j.1442-9993.2006.01614.x
- Meehan M.L., Song Z., Lumley L.M., Cobb T.P., Proctor H. 2019. Soil mites as bioindicators of disturbance in the boreal forest in northern Alberta, Canada: testing taxonomic sufficiency at multiple taxonomic levels. Ecol. Indic., 102: 349-365. https://doi.org/10.1016/j.ecolind.2019.02.043
- M.P. z 1959 r. Nr 78, poz. 414. Zarządzenie Ministra Leśnictwa i Przemysłu Drzewnego z dnia 2 lipca 1959 r. w sprawie uznania za rezerwat przyrody [Internet]. Poznań: General Directorate for Environmental Protection; [accessed 17 August 2025]. Available from: https://crfop.gdos.gov.pl/CRFOP/widok/viewrezerwatprzyrody.jsf?fop=PL.ZIPOP.1393.RP.862
- N′Dri J.K., Pokou P.K., Séka F.A., N′Da R.A.G., Lagerlöf J. 2018. Edaphic characteristics and environmental impact of rubber tree plantations on soil mite (Acari) communities. Acarologia, 58: 951-962. https://doi.org/10.24349/acarologia/20184300
- Napierała A. 2008. Struktura zgrupowań i rozkład przestrzenny Uropodina (Acari: Mesostigmata) w wybranych kompleksach leśnych Wielkopolski. PhD thesis. Poznań: Adam Mickiewicz University.
- Napierała A., Błoszyk J. 2021. The maturity index for Uropodina (Acari: Mesostigmata) communities as an indicator of human-caused disturbance in selected forest complexes of Poland. Exp. Appl. Acarol., 83: 475-491. https://doi.org/10.1007/s10493-021-00607-5
- Napierała A., Błoszyk J., Bruin J. 2009. Communities of uropodine mites (Acari: Mesostigmata) in selected oak-hornbeam forests of the Wielkopolska region (Poland). Exp. Appl. Acarol., 49: 291-303. https://doi.org/10.1007/s10493-009-9262-2
- Napierała A., Konwerski S., Gutowski J.M., Błoszyk J. 2020. Species diversity of Uropodina communities (Acari: Parasitiformes) in soil and selected microhabitats in the Białowieża Primeval Forest. In: Błoszyk J., Napierała A. (Eds). Mites (Acari) of the Białowieża Primeval Forest. Poznań: Kontekst. pp. 11-60.
- Napierała A., Książkiewicz-Parulska Z., Błoszyk J. 2018. A Red List of mites from the suborder Uropodina (Acari: Parasitiformes) in Poland. Exp. Appl. Acarol. 75: 467-490. https://doi.org/10.1007/s10493-018-0284-5
- Napierała A., Labijak B., Skwierczyński F., Konwerski S., Błoszyk J. 2014. Influence of habitat type and natural disturbances on uropodine mite communities (Acari: Mesostigmata: Uropodina) in oak hornbeam forests in Central Europe. Int. J. Acarol., 41: 41-52. https://doi.org/10.1080/01647954.2014.985713
- New T.R. 1997. Are Lepidoptera an effective "umbrella group» for biodiversity conservation? J. Insect Conserv., 1: 5-12. https://doi.org/10.1023/A:1018433406701
- Niedbała W. 2008. Ptyctimous mites (Acari, Oribatida) of Poland. Fauna Poloniae, Vol. 3. Warsaw: Museum and Institute of Zoology at the Polish Academy of Science. pp. 242.
- Niedbała W., Napierała A., Wendzonka J., Lubińska K., Kulczak M., Błoszyk J. 2024. Species Diversity and Spatial Distribution of Some Oribatid Mites in Bory Tucholskie National Park (N Poland). Diversity 16(11): 678. https://doi.org/10.3390/d16110678
- Olszanowski Z. 1996. A monograph of the Nothridae and Camisiidae of Poland (Acari: Oribatida: Crotonioidea). Genus Int. J. Invertebr. Taxon. Suppl., 201.
- Pande Y.D., Berthet P. 1973. Studies on the food and feeding habits of soil Oribatei in a Black Pine plantation. Oecologia, 12: 413-426. https://doi.org/10.1007/BF00345051
- Peterson R.O. 1988. The pit or the pendulum: issues in large carnivore management in natural ecosystems. In: Agee J.K., Johnson D.R. (Eds). Ecosystem management for parks and wilderness. Seattle: University of Washington Press. pp. 105-117.
- Pollierer M.M., Klarner B., Ott D. Digel Ch., Ehnes R.B., Eitzinger B., Erdmann G., Brose U., Maraun M. Scheu S. 2021. Diversity and functional structure of soil animal communities suggest soil animal food webs to be buffered against changes in forest land use. Oecologia 196: 195-209. https://doi.org/10.1007/s00442-021-04910-1
- Roland E., Kościelska A., Massalska J., Koperwas K., Gabryś G. 2015. New data on Trombidia (Acari: Prostigmata: Parasitengona) in protected areas in Lubuskie Province (Western Poland). Zesz. Nauk. Acta Biol. Univ. Szczec. 22. https://doi.org/10.18276/ab.2015.22-16
- Ruf A. 1998. A maturity index for predatory soil mites (Mesostigmata: Gamasina) as an indicator of environmental impacts of pollution on forest soils. Appl. Soil Ecol., 9: 447-452. https://doi.org/10.1016/S0929-1393(98)00103-6
- Rutkowski T. 2024. Unikatowe zgrupowanie roztoczy z podrzędu Uropodina (Acari: Mesostigmata) rezerwatu Cisy Staropolskie im. Leona Wyczółkowskiego w Wierzchlesie. PhD Thesis. Zielona Góra: Uniwersytet Zielonogórski. 167 pp.
- Seniczak A., Seniczak S., Starý J., Kaczmarek S., Jordal B.H., Kowalski J., Bolger T. 2021a. High diversity of mites (Acari: Oribatida, Mesostigmata) supports the high conservation value of a broadleaf forest in Eastern Norway. Forests 12(8): 1098. https://doi.org/10.3390/f12081098
- Seniczak A., Niedbała W., Iturrondobeitia J.C. Seniczak S., Roth S., Jordal B. H. 2021b. Type of broadleaf forest matters most for ptyctimous mite communities (Acari, Oribatida) in Norway. Biodivers. Conserv. 30: 2929-2953. https://doi.org/10.1007/s10531-021-02228-1
- Skorupski, M. 2001. Mites (Acari) from the order Gamasida in the Wielkopolski National Park. Fragm. Faun., 44(1): 129-167. https://doi.org/10.3161/00159301FF2001.44.1.129
- Skorupski M., Butkiewicz G., Wierzbicka A. 2009. The first reaction of soil mite fauna (Acari, Mesostigmata) caused by conversion of Norway spruce stand in the Szklarska Poręba Forest District. J. For. Sci., 55(5): 234-243 https://doi.org/10.17221/19/2009-JFS
- Sullivan G.T., Ozman-Sullivan S. 2021. Alarming evidence of widespread mite extinctions in the shadows of plant, insect and vertebrate extinctions. Austral. Ecol., 46: 163-176. https://doi.org/10.1111/aec.12932
- UNESCO. Convention on Wetlands of International Importance, especially as Waterfowl Habitat [Internet]. [2 February 1971]. Paris: UNESCO; [accessed 24 April 2024]. Available from: https://www.unesco.org/en/legal-affairs/convention-wetlands-international-importance-especially-waterfowl-habitat?hub=348
- Wilcox B.A. 1984. In situ conservation of genetic resources: determinants of minimum area requirements. In: McNeely J.A., Miller K.R. (Eds). National parks, conservation and development: the role of protected areas in sustaining society. Washington, D.C.: Smithsonian Institution Press. pp. 639-647.
- Zduniak M., Błoszyk J., Nowak M., Napierała A. 2019. Soil mites (Acari) of natural areas of a former military training field in Olsztyn (Poland). Eur. J. Biol. Res., 9: 245-258. https://doi.org/10.5281/zenodo.3534449



2025-01-23
Date accepted:
2025-09-05
Date published:
2025-09-23
Edited by:
Minor, Maria

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Błoszyk, Jerzy; Niedbała, Wojciech; Wendzonka, Jacek; Rutkowski, Tomasz; Kulczak, Marta and Napierała, Agnieszka
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



