Redescriptions of Amblyseius cucurbitae Rather and A. paraaerialis Muma (Parasitiformes: Phytoseiidae) with new synonymies
Döker, Ismail  1
; Atchia, Isabelle
1
; Atchia, Isabelle  2
; Gasparro-Brewer, Jude
2
; Gasparro-Brewer, Jude  3
; Fang, Xiao-Duan
3
; Fang, Xiao-Duan  4
and Bolton, Samuel J.
4
and Bolton, Samuel J.  5
5
1✉ Cukurova University, Agricultural Faculty, Department of Plant Protection, Acarology Laboratory, 01330, Adana, Türkiye.
2Florida Department of Agriculture and Consumer Services, 32608, Gainesville, FL, USA.
3Florida Department of Agriculture and Consumer Services, 32608, Gainesville, FL, USA.
4Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, 510260, Guangdong, China.
5Florida Department of Agriculture and Consumer Services, 32608, Gainesville, FL, USA.
2025 - Volume: 65 Issue: 3 pages: 934-946
https://doi.org/10.24349/2yok-2sifZooBank LSID: 88858CD9-23F5-4DCD-9787-0FA819D1E168
Original research
Keywords
Abstract
Introduction
Phytoseiid mites, a diverse group within the superorder Parasitiformes, are widely studied due to their importance as biological control agents (McMurtry et al. 2013). However, the taxonomy of this family remains problematic, largely due to insufficient original descriptions and the lack of comprehensive redescriptions. Early descriptions often lack critical morphological detail, and new species are frequently described using limited comparisons with the known taxa. These issues have led to the repeated description of the same species under different names, particularly in geographically similar regions.
The genus Amblyseius Berlese, established in 1914, represents one of the most species-rich and globally distributed groups within Phytoseiidae, with over 400 nominal species (Demite et al. 2014; Ferragut and Navia 2015; Kreiter et al. 2021; Khaustov et al. 2023). Amblyseius species are known for their broad predatory habits, feeding on a variety of prey such as mites, thrips, whiteflies, and nematodes (McMurtry et al. 2013). Some species, including A. andersoni (Chant, 1957) and A. swirskii Athias-Henriot, 1962, have been successfully implemented in biological control and integrated pest management programs worldwide.
Amblyseius cucurbitae Rather, 1985, and A. paraaerialis Muma, 1967, were described from India approximately 40 and 60 years ago, respectively. However, both descriptions are brief, based on simplified illustrations, and lack key diagnostic features, making their reliable identification challenging. A review of the literature suggests that, likely due to these deficiencies, both species may not have been properly recognized and could have been described under different names in subsequent studies. Based on their original descriptions, several nominal species such as A. amorphalae, A. bhadrakshae, A. coffeae, A. indirae, A. kundurukkae, A. mohanasundarami, and A. velayudhani, appear morphologically similar to A. cucurbitae. Likewise, A. hainanensis, and A. brachycalyx, show strong affinities to A. paraaerialis. Given these similarities, we attempted to examine the type specimens of all these species in order to clarify their identities. While we were able to access and study the types of some species, others were unavailable; for those, detailed morphological comparisons were carried out based on the original descriptions.
The aim of this study is to redescribe A. cucurbitae and A. paraaerialis based on their type material and to assess the taxonomic status of the potentially synonymous species through direct comparison with the respective type specimens and/or a comprehensive literature review.
Material and methods
Specimens were examined using a Zeiss Axio Imager.M2 compound microscope equipped with differential interference contrast (DIC) optics and an AxioCam 820 mono camera, as well as Zeiss Axioscope 5 TL compound microscopes with phase contrast optics and Canon EOS 80D and 90D cameras. All relevant morphological features commonly used in phytoseiid mite taxonomy were measured. Measurements were taken with ZEN 2012 software (version 8.0) and are reported in micrometers (μm). The dorsal shield length was measured along the midline from anterior to posterior margins. The ventrianal shield length, including the cribrum, was measured along the midline. Leg lengths were measured from the base of the coxa to the tip of the tarsus, excluding the ambulacrum.
The classification system follows Chant and McMurtry (2007). Dorsal idiosomal setal nomenclature is based on Lindquist and Evans (1965), as modified by Rowell et al. (1978) for phytoseiids. Ventral setal nomenclature follows Chant and Yoshida-Shaul (1991). Adenotaxy, poroidotaxy, and sigillotaxy were interpreted according to Athias-Henriot (1971, 1975), Evans and Fain (1995), and Tsolakis and Ragusa (2016). Leg chaetotaxy follows Evans (1963), and macrosetal terminology (e.g., SgeIV, StiIV) follows Athias-Henriot (1957). Following Athias-Henriot (1975), the term solenostomes used in this study refers to gland openings.
A paratype of A. cucurbitae and the holotype of A. paraaerialis, examined in this study, are deposited at the Florida State Collection of Arthropods, 1911 SW 34th St, Gainesville, FL 32608, USA. A paratype of A. hainanensis is deposited at the Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Guangdong Institute of Applied Biological Resources, Guangzhou, Guangdong, China.
Results
Amblyseius cucurbitae Rather
Amblyseius cucurbitae Rather, 1985: 291.
Amblyseius (Amblyseius) indirae Gupta, 1985: 209. New synonym.
Amblyseius (Amblyseius) kundurukkae Anithalatha & Ramani, 2004: 58. New synonym.
Amblyseius (Amblyseius) mohanasundarami Anithalatha, 2005: 81. New synonym.
Amblyseius (Amblyseius) amorphalae Sadanandan & Ramani, 2006: 2267. New synonym.
Amblyseius (Amblyseius) bhadrakshae Sadanandan & Ramani, 2006: 2267. New synonym.
Amblyseius (Amblyseius) coffeae Sadanandan, 2006: 39. New synonym.
Amblyseius velayudhani Santhosh & Sadanandan, 2015: 67. New synonym.
Material examined
Paratype female, Amblyseius cucurbitae on Cucurbita pepo L. (Cucurbitaceae), 14 August 1977, Srinagar, Jammu and Kashmir, India, coll. A. Q. Rather.
Diagnosis
Idiosomal setal pattern 10A: 9B/14 JV-3: ZV (r3 and R1 off shield). Dorsal shield smooth with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, and gd9). Dorsal setae smooth, except Z5 with a few barbs and J5 with one barb. Dorsal setae Z4, Z5, and s4 greatly elongated; j1, and j3 moderately elongated; remaining setae short/minute. Peritremes long, extending bases of setae j1. Solenostome gd3 present on peritrematal shield. Sternal shield smooth with three pairs of setae (st1–3). Genital shield smooth wider than ventrianal shield. Ventrianal shield elongated, vase-shaped, smooth, with three pairs of pre-anal setae (JV1, JV2, and ZV2); one pair of large crescentic solenostomes. Spermatheca with bell-shaped calyx with nodular and forked atrium attached to calyx, neck absent. Fixed digit of chelicera with 10 teeth and movable digit with three teeth. Trochanter I with five (1 0/1 0/2 1) and genu II with seven setae (2 2/0 2/0 1). Macrosetae present on all legs. Tarsus I with two erect setae. SgeI (pd1), SgeII (pd1), SgeIII (ad1), StiIII (ad), StIII (ad3), SgeIV (ad1), StiIV (ad), and StIV (pd3) present.
Re-description
Female (n = 1) (Figures 1–3)
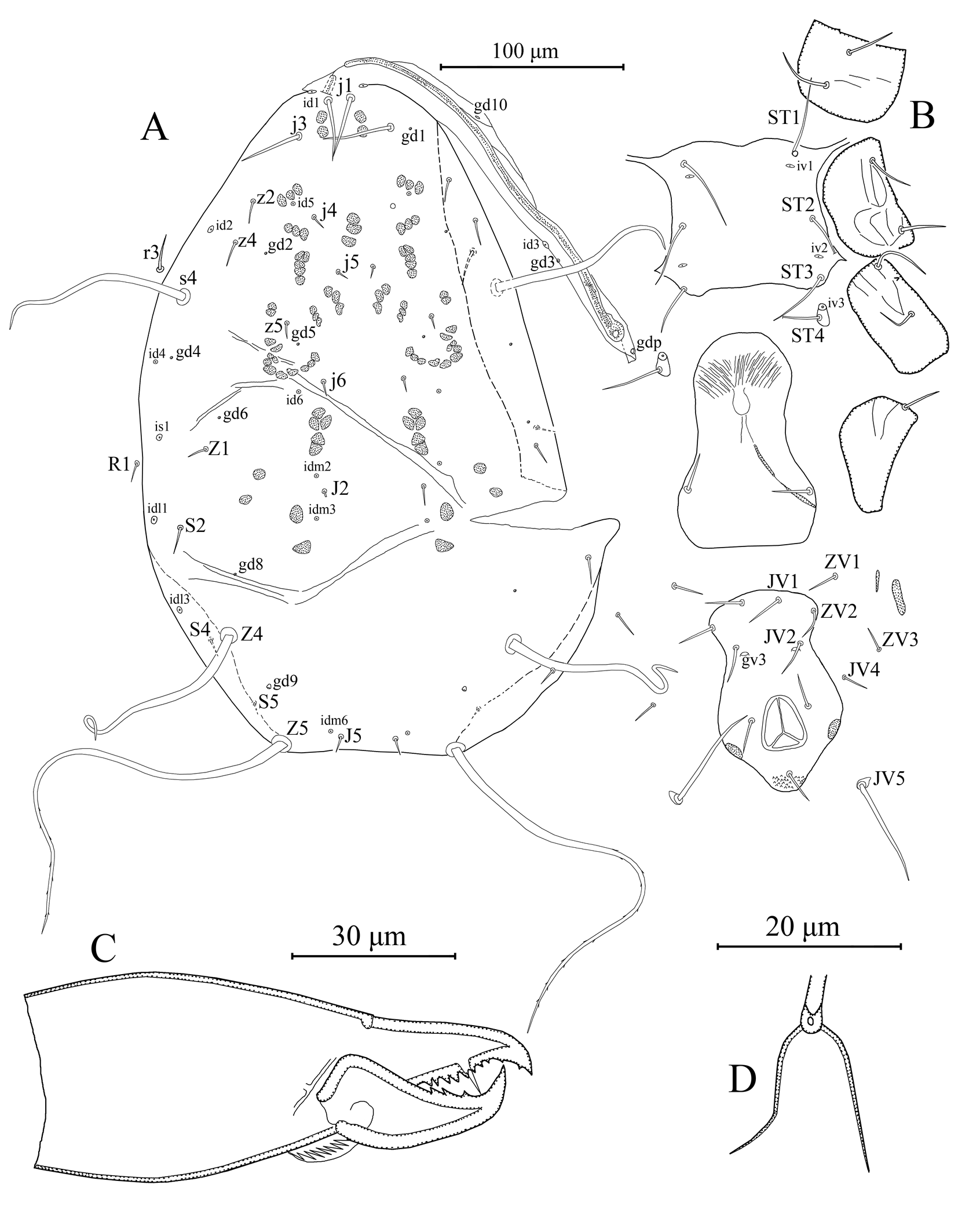


Dorsal idiosoma – (Figure 1A). Dorsal setal pattern 10A: 9B (r3 and R1 off shield). Dorsal shield sclerotized, smooth; with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, and gd9), gd9 conspicuously larger than others; 11 pairs of visible poroids (id1–id6, idm2, idm3, idm6, is1, idl1, and idl3). Muscle marks (sigilla) visible on podosoma. Length of dorsal shield 355, width at level of s4 210, width at level of S2 250. All dorsal setae smooth except Z5 with a few barbs and J5 with one barb. Measurements of dorsal setae as follows: j1 33, j3 38, j4 8, j5 8, j6 8, J2 11, J5 10, z2 13, z4 13, z5 8, Z1 12, Z4 115, Z5 238, s4 105, S2 15, S4 13, S5 8, r3 19, and R1 11.
Peritrematal shield – Peritrematal shield fused with dorsal shield at level of seta j1. Peritremes begin at the stigmatic opening with four to five lines of microvilli, continuing with two lines anteriorly; extending bases of setae j1. Solenostomes gd3 and gd10, and poroid id3 visible. Solenostome gdp, large, inserted posterior to stigmatic opening.
Ventral idiosoma – (Figure 1B). Ventral setal pattern 14: JV–3: ZV. Sternal shield sclerotized, smooth; with three pairs of setae (st1–3) and two pair of elliptical poroids (iv1 and iv2); distance between st1–st3 69, distance between setae st2 71; metasternal setae st4 and poroid iv3 on metasternal platelets. Genital shield smooth, wider than ventrianal shield, with one pair of setae st5, and three pairs of sigilla (gs1–3); 115 in length, 73 width at level of st5. Ventrianal shield vase-shaped, smooth; with three pairs of pre-anal setae (JV1, JV2, and ZV2), one pair of para-anal setae PA, unpaired post-anal seta PST, and a pair of large crescentic pre-anal solenostomes gv3 located near base of setae JV2, distance between solenostomes 28. Length of ventrianal shield 110, width at level of ZV2 60, width at level of paraanal setae 68, width at level of waist 45. Four pairs of caudoventral setae (ZV1, ZV3, JV4, and JV5) on soft cuticle surrounding ventrianal shield. Setae JV5 smooth inserted on small platelets, 60 in length.
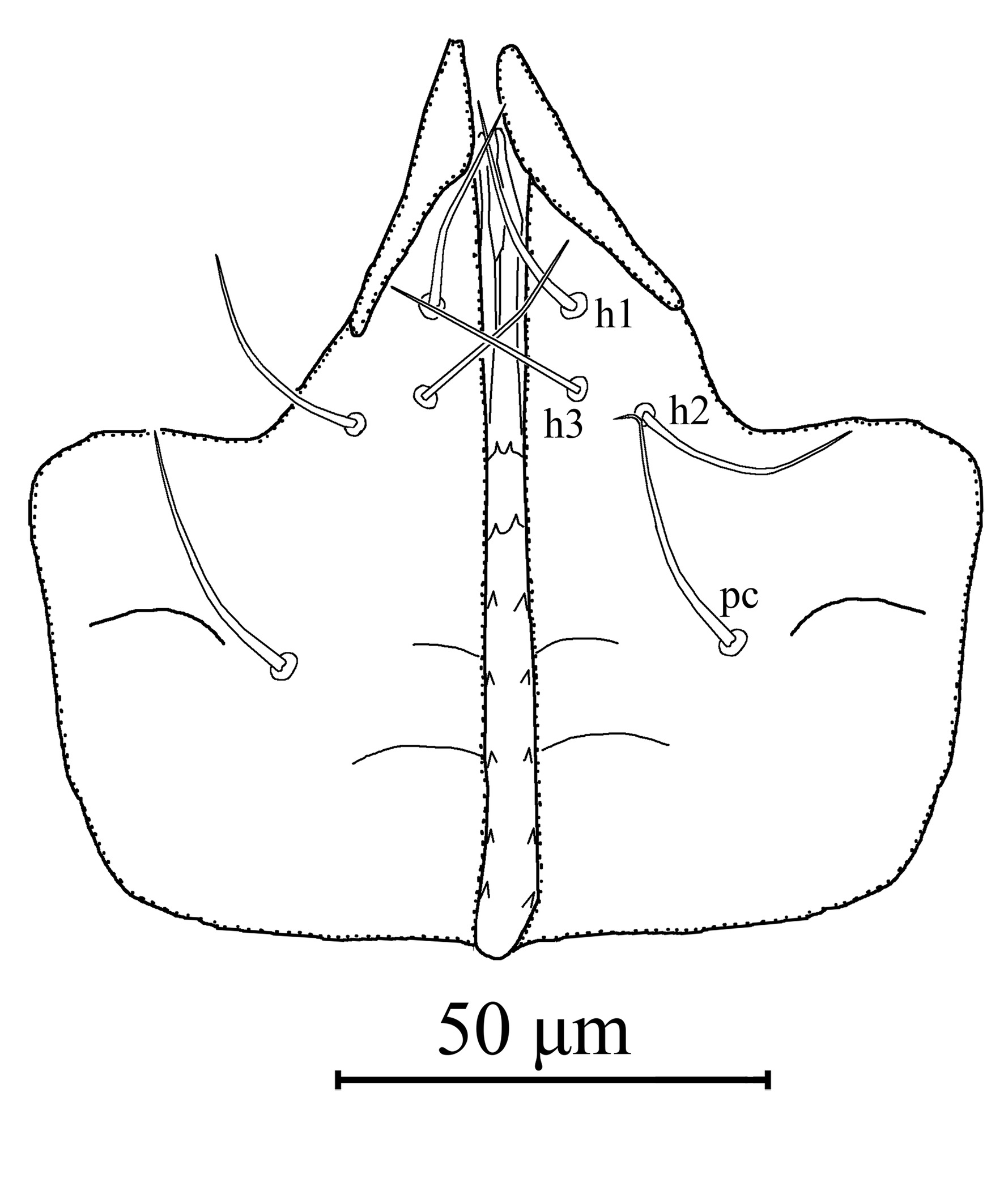


Gnathosoma – (Figures 1C, 2). Subcapitulum sclerotized, with three pairs of hypostomal setae h1, h2, and h3 each 26 in length, and one pair of palp coxal setae pc 30 in length; internal malae paired, not extending tip of corniculi; deutosternal groove with seven transverse rows of denticles, each with two lateral denticles. Second segment of chelicera 90 in length. Antiaxial poroid and external arthrodial brush visible. Fixed digit 31 long, with 10 teeth, and pilus dentilis; movable digit 34 long, with three teeth.
Spermatheca – (Figure 1D). Calyx, bell-shaped, widening apically, 14 long, 9 wide at mid-point; atrium nodular incorporated with calyx without neck; major duct visible, minor duct not visible.
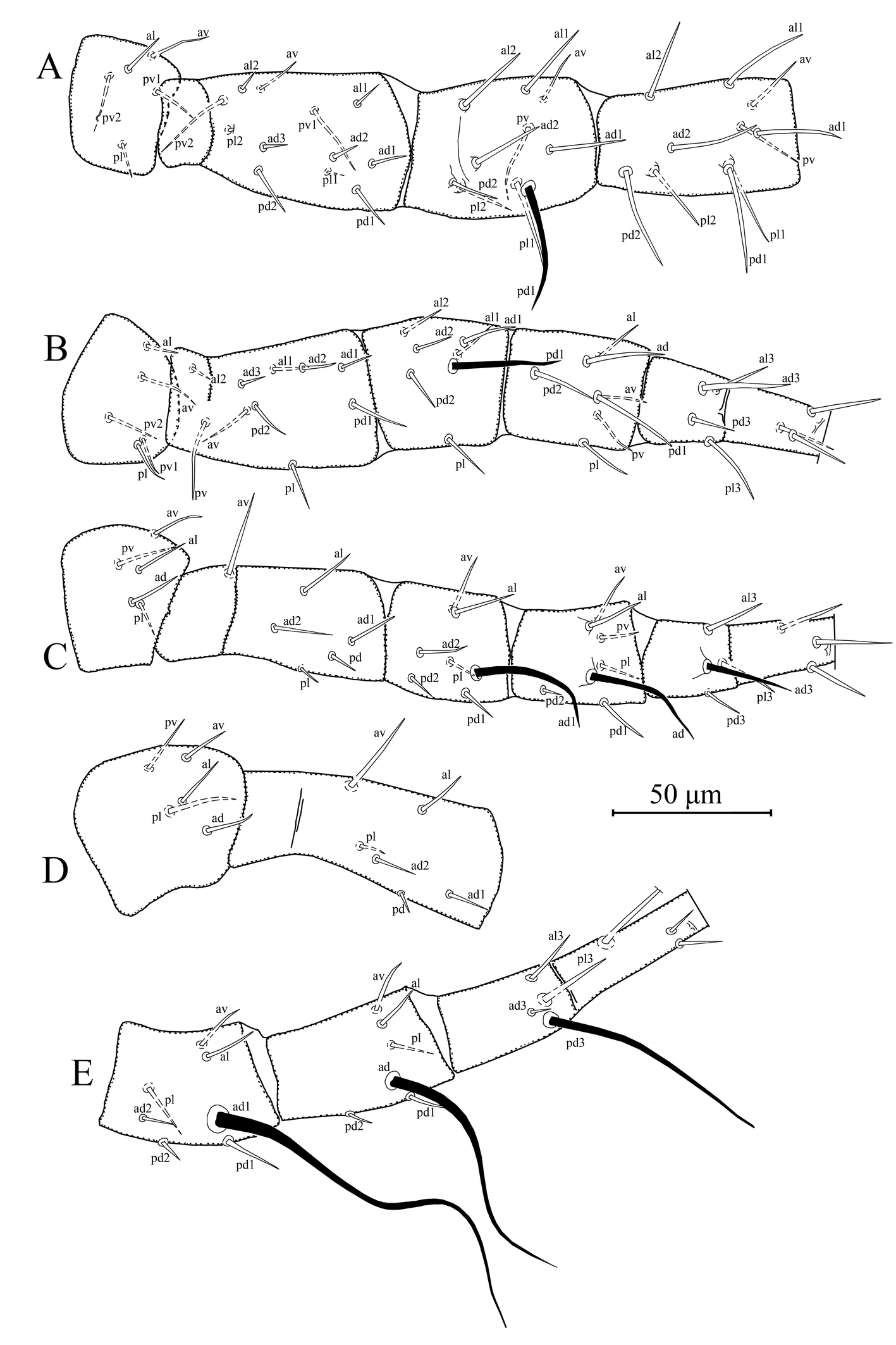


Legs – (Figures 3A–E). Length of legs I–IV 405, 334, 340, and 427, respectively. Chaetotaxy of legs as follows; leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/0 1, tibia 1 1/1 2/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 1/1 0/1 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 1/1 0/1 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/0 1. Chaetotaxy of tarsi II–IV typical for Phytoseiidae and bears 18 setae 3 3/2 3/2 3 + mv and md. Macrosetae present in all legs. Tarsus I with two erect setae, both sharp pointed and 46–49 in length. Measurements of macrosetae as follows: SgeI (pd1) 41, SgeII (pd1) 35, SgeIII (ad1) 42, StiIII (ad) 42, StIII (ad3) 28, SgeIV (ad1) 120, StiIV (ad) 85, and StIV (pd3) 73.
Remarks
Amblyseius cucurbitae was originally described by Rather (1985) based on specimens collected from various host plants in Srinagar, Jammu and Kashmir, India. The original description is limited, relying on simplified illustrations and providing only dorsal setal measurements. It lacks several critical diagnostic characters that are essential for distinguishing phytoseiid species, such as number of dorsal solenostomes, complete leg chaetotaxy, and measurements of macrosetae on the first three legs. Although Denmark and Muma (1989) and Gupta (2003) later redescribed the species, they repeated the same setal measurements, which were most likely taken directly from the original description. To address these limitations, we redescribed a female paratype of A. cucurbitae and provide a more comprehensive and reliable morphological diagnosis for future studies.
We examined the original descriptions of several species, all of them described from India. These species include, A. amorphalae, A. bhadrakshae, A. coffeae, A. indirae, A. kundurukkae, A. mohanasundarami, and A. velayudhani. Except for A. indirae, which was described from a somewhat more distant location (approximately 300–350 km away), all other species were described based on specimens collected within a restricted area of about 50–80 km in Kerala. In fact, A. kundurukkae and A. bhadrakshae were even collected from the same location (the Ayurvedic Herbal Garden of Kottakal Arya Vaidya Sala in Malappuram District), but from different host plants. Considering the close proximity of these collection sites, it seems unlikely that these specimens represent truly distinct species. Nevertheless, the authors described them as separate taxa without providing any differential diagnoses or comparisons, either among themselves or with the valid species proposed in this study, A. cucurbitae.
Despite repeated efforts to contact the authors and their collaborators, we were unable to obtain any verifiable access to the type specimens of A. amorphalae, A. bhadrakshae, A. coffeae, A. kundurukkae, A. mohanasundarami, and A. velayudhani. Dr. Anithalatha-Sadanandan, who authored all of these taxa, stated that the materials were deposited at the Zoological Survey of India, Calicut, but could not provide any documentation or accession information, referring us instead to colleagues who did not respond to our queries. According to Dr. Ramani, her former Ph.D. advisor, the type specimens were initially kept in their university lab but were later taken by her students. Following her retirement, the acarology laboratory was reassigned to new faculty members with entirely different specializations, and as a result, all taxonomic material, literature, and equipment were lost. No new acarology personnel were appointed, and currently, the department lacks successors in the field. We also attempted to contact directly the Zoological Survey of India in Calicut over a period of two years regarding these types, but received no response. Therefore, we must rely on the original descriptions and make our conclusions based on the morphological characteristics and other information provided therein.
In nearly all cases, morphological differences were neither consistent nor reliable; most were vague, involving slight variations in setal lengths or, in a few instances, questionable observations of movable digit dentition, likely due to limitations of the optic equipment used at the time. For instance, the observed differences between A. mohanasundarami, A. kundurukkae, and A. cucurbitae, specifically in the number of teeth on the movable digit of the chelicera (one, none, and three, respectively) and in certain dorsal setal and macrosetal lengths (e.g., j3, and SgeIV) show less than 20% variation and are considered to fall within the range of intraspecific variation observed among different populations of the same species (Tixier 2012). Therefore, in the absence of accessible type materials and given the vague, overlapping, and inconsistent morphological characters provided in the original descriptions, we consider these species to be junior synonyms of A. cucurbitae. Although these synonymies are strongly supported by the evidence presented above, they should be reconsidered if the type material becomes accessible in the future.
Similarly, based on our examination of the original descriptions, the only difference between A. cucurbitae and A. indirae is the number of teeth on the movable digit of the chelicera: three in the former and none in the latter. Considering the overall morphological similarity and the fact that members of the largoensis species group typically possess three or four teeth on the movable digit (Zannou et al. 2007; Liao et al. 2020; Döker et al. 2022a, b), we consider the toothless state in A. indirae to be a potential error in the original description from earlier optics. Furthermore, as A. cucurbitae was published one month earlier (August 1985) than A. indirae (September 1985), it has priority. Therefore, we consider A. indirae to be a junior synonym of A. cucurbitae.
Amblyseius paraaerialis Muma
Amblyseius paraaerialis Muma, 1967: 270.
Amblyseius (Amblyseius) hainanensis Wu, in Wu and Qian 1983: 264. New synonym.
Amblyseius brachycalyx Karmakar, Bowmik and Sherpa, 2017: 44. New synonym.
Material examined
Holotype female, Amblyseius paraaerialis, on Citrus sp. (Rutaceae), 12 December 1962, Palghat, Kerala, India, coll. V. P. Rao. A paratype female, Amblyseius hainanensis, on Citrus sp. (Rutaceae), 3 October 1980, Xinglong, Hainan, China, coll. X. Qian.
Diagnosis
Idiosomal setal pattern 10A: 9B/14 JV-3: ZV (r3 and R1 off shield). Dorsal shield smooth with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9). Dorsal setae smooth, except Z5 with a few barbs and J5 with one barb. Dorsal setae Z4, Z5, and s4 greatly elongated; j1, and j3 moderately elongated; remaining setae short/minute. Peritremes long, extending bases of setae j1. Solenostome gd3 and gd10 present on peritrematal shield. Sternal shield smooth with three pairs of setae (st1–3). Genital shield smooth as wide as ventrianal shield. Ventrianal shield, pentagonal, smooth, with three pairs of pre-anal setae (JV1, JV2, and ZV2); one pair of large crescentic solenostomes. Spermatheca with calyx cup-shaped with very small atrium attached to base of calyx, neck absent, with broad major duct. Fixed digit of chelicera with nine teeth and movable digit with three teeth. Trochanter I with five (1 0/1 0/2 1) and genu II with seven setae (2 2/0 2/0 1). Macrosetae present on all legs. Tarsus I with two erect setae, both sharp pointed and 26–28 in length. SgeI (pd1), SgeII (pd1), SgeIII (ad1), StiIII (ad), SgeIV (ad1), StiIV (ad), and StIV (ad3) present.
Re-description
Female (n = 1) (Figures 4–6)
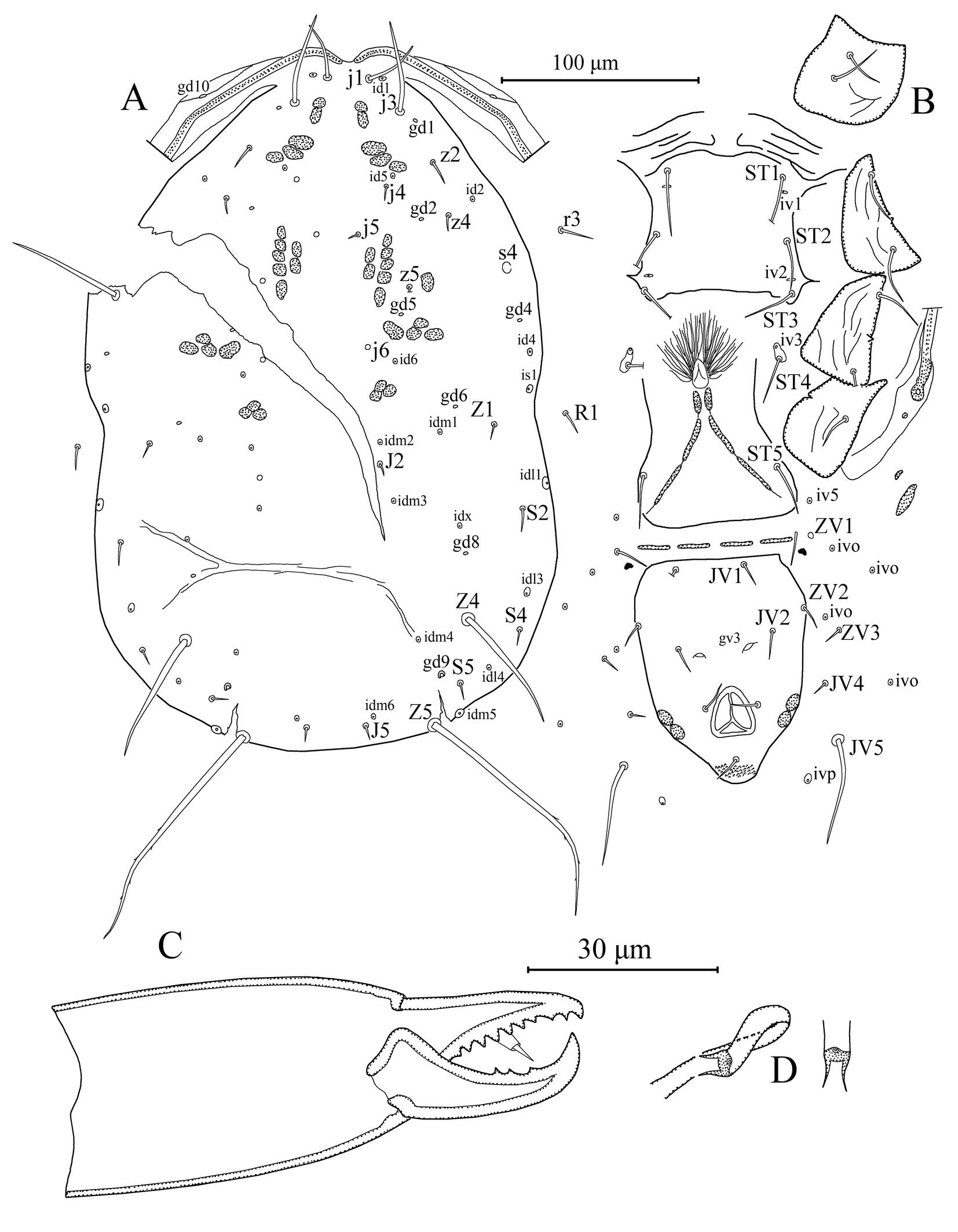


Dorsal idiosoma – (Figure 4A). Dorsal setal pattern 10A:9B (r3 and R1 off shield). Dorsal shield sclerotized, smooth; with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, and gd9), gd9 conspicuously larger than others; 16 pairs of visible poroids (id1–id6, idm1–6, is1, idl1, idl3, and idl4). Muscle marks (sigilla) visible on podosoma. Length of dorsal shield 329, width at level of s4 202, width at level of S2 228. All dorsal setae smooth except Z5 with a few barbs and J5 with one barb. Measurements of dorsal setae as follows: j1 28, j3 46, j4 7, j5 7, j6 broken, J2 7, J5 7, z2 13, z4 9, z5 broken, Z1 9, Z4 67, Z5 129, s4 60, S2 14, S4 9, S5 9, r3 18, and R1 12.
Peritrematal shield – Peritrematal shield fused with dorsal shield at level of seta j1. Peritremes begin at the stigmatic opening with three to four lines of microvilli, continuing with two lines anteriorly; extending bases of setae j1. Solenostomes gd3 and gd10, and poroid id3 visible. Solenostome gdp large inserted posterior to stigmatic opening.
Ventral idiosoma – (Figure 4B). Ventral setal pattern 14: JV–3: ZV. Sternal shield sclerotized, smooth; with three pairs of setae (st1–3) and two pairs of elliptical poroids (iv1 and iv2); distance between st1–st3 60, distance between setae st2 66; metasternal setae st4 and poroid iv3 on metasternal platelets. Genital shield smooth, as wide as ventrianal shield, with one pair of setae st5 and three pairs of sigilla (gs1–3); 120 in length, 74 width at level of st5. Two pairs of genital sigilla (gs 4–5) inserted on soft cuticle between genital and ventrianal shields and a pair of genital sigilla (sgpa) inserted posterior to setae ZV1. Ventrianal shield pentagonal, smooth; with three pairs of pre-anal setae (JV1, JV2, and ZV2), one pair of para-anal setae PA, unpaired post-anal seta PST, and a pair of large crescentic pre-anal solenostomes gv3, located posteromesad to setae JV2, distance between solenostomes 25. Length of ventrianal shield 113, width at level of ZV2 87, width at level of paraanal setae 74. Four pairs of caudoventral setae (ZV1, ZV3, JV4, and JV5) and five pairs of poroids (four pairs of ivo, and ivp) on soft cuticle surrounding ventrianal shield. Setae JV5 smooth, 53 in length.



Gnathosoma – (Figures 4C, 5). Subcapitulum sclerotized, with three pairs of hypostomal setae h1, h2, and h3 each 26 in length, and with one pair of palp coxal setae pc 30 in length; internal malae paired, not extending tip of corniculi; deutosternal groove with seven transverse rows of denticles, each with two lateral denticles, except two basal rows with two vertical teeth on each side. Second segment of chelicera 82 in length. Antiaxial poroid and external arthrodial brush not visible. Fixed digit 29 long, with 9 teeth, and pilus dentilis; movable digit 30 long, with three teeth.
Spermatheca – (Figure 4D). Calyx, cup-shaped, widening apically, 6 long, 4 wide at widest point; atrium very small nodular incorporated with calyx without neck; major duct well-developed and broad, minor duct not visible.
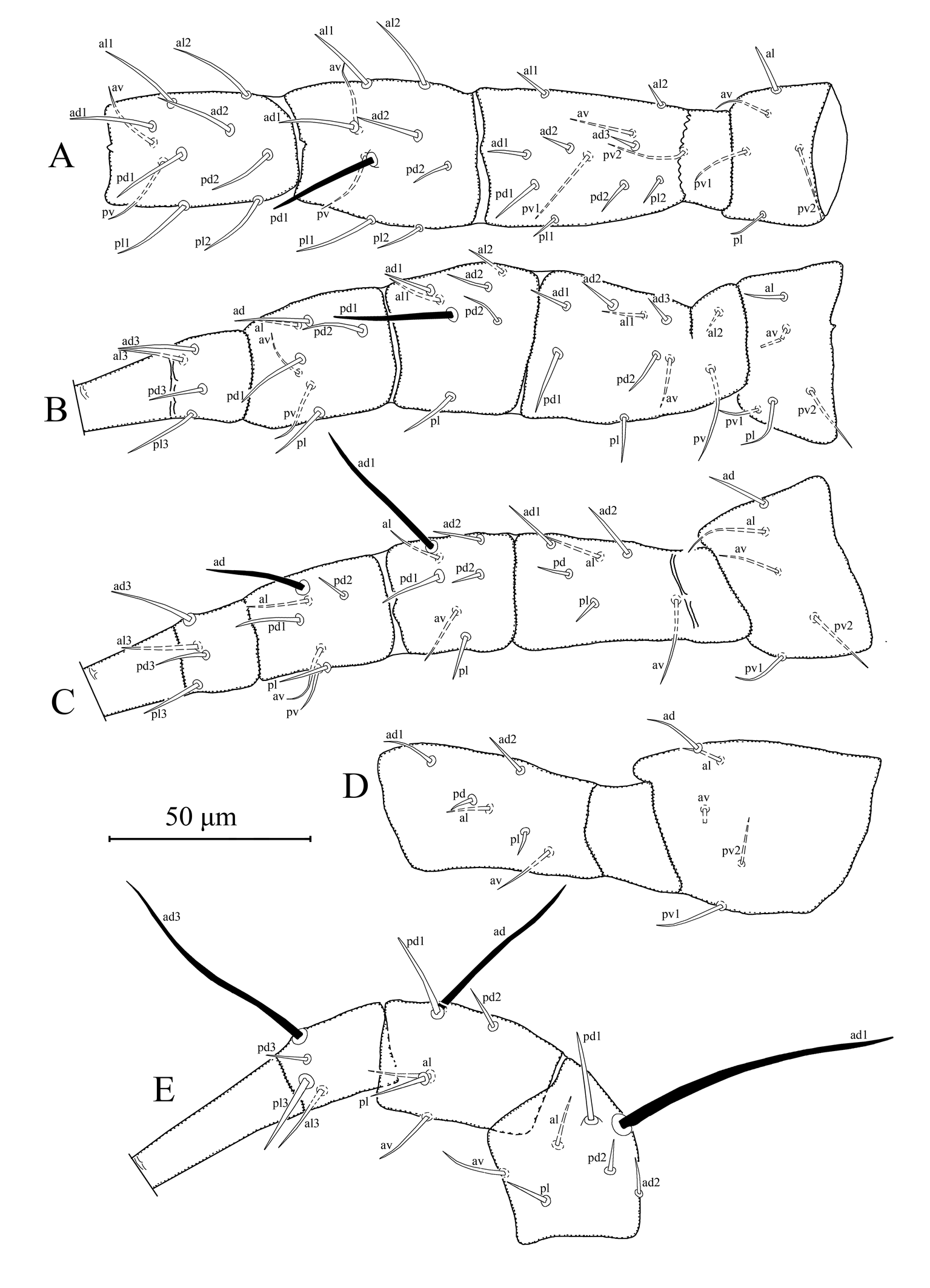


Legs – (Figures 6A–E). Length of legs I–IV 310, 264, 255, and 352, respectively. Chaetotaxy of legs as follows; leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/0 1, tibia 1 1/1 2/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 1/1 0/2 0, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 1/1 0/2 0, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/0 1. Chaetotaxy of tarsi II–IV typical for Phytoseiidae and bears 18 setae 3 3/2 3/2 3 + mv and md. Macrosetae present in all legs. Tarsus I with two erect setae. Measurements of macrosetae as follows: SgeI (pd1) 29, SgeII (pd1) 30, SgeIII (ad1) 34, StiIII (ad) 25, StIII (ad3) 28, SgeIV (ad1) 72, StiIV (ad) 45, and StIV (ad3) 59.
Remarks
Amblyseius paraaerialis was originally described by Muma (1967) based on specimens collected from Citrus sp. in two different locations in India. The original description is limited, relying on simplified illustrations and lacking several critical diagnostic characters that are essential for distinguishing phytoseiid species today, such as measurements of dorsal setae and macrosetae, number of dorsal solenostomes, and complete leg chaetotaxy. Although Denmark and Muma (1989) later provided a redescription with more details, their redescription still lacks several important morphological features.
Due to its morphological similarity with two other species, A. brachycalyx (described from India) and A. hainanensis (described from China), we examined a paratype specimen of A. hainanensis and confirmed that it is a junior synonym of A. paraaerialis. Unfortunately, we were unable to access the type material of A. brachycalyx. The only observed difference between A. paraaerialis and A. brachycalyx is the length of seta Z5 (129 vs. 150–155). However, this variation is likely due to intraspecific variation between geographically distinct populations and may be correlated with overall body size, as similarly reported for A. paraaerialis specimens from Thailand by Ehara & Bhandhufalck (1977) and Oliveira et al. (2012).
The holotype specimen of A. hainanensis could not be located in the respective collection during our study. Nevertheless, it should be noted that the setal measurements provided in the original description of A. hainanensis, specifically for j1, j3, Z4, Z5, s4, JV5, SgeIV, StiIV, and StIV, differ from those observed in the paratype specimen examined in this study. The measurements obtained from the paratype are as follows: 27, 43, 73, 148, 58, 54, 74, 47, and 59, respectively. These measurements are consistent with those observed in A. paraaerialis. The discrepancy may reflect variation among type specimens or differences in measurement methods.
Acknowledgements
We are grateful to Drs. Serge Kreiter (France), Peterson Demite (Brazil), and Emilie Demard (USA) for sharing soft copies of some papers. This study was supported by the Cukurova University Scientific Projects Foundation Units, project number FAY-2022-14495. We are grateful to three anonymous reviewers for their constructive comments on an early version of the manuscript.
References
- Anithalatha M. 2005. Two new species of predatory mites (Acarina: Phytoseiidae) from Kerala (India). Uttar Pradesh J. Zool, 25: 81-84.
- Anithalatha M., Ramani N. 2004. Two new species of predatory mites (Acarina: Phytoseiidae) from Kerala, India. J. Adv. Zool., 25: 58-60.
- Athias-Henriot C. 1957. Phytoseiidae et Aceosejidae (Acarina, Gamasina) d'Algérie. I. Genres Blattisocius Keegan, Iphiseius Berlese, Amblyseius Berlese, Phytoseius Ribaga, Phytoseiulus Evans. Bulletin de la Societé d'Histoire Naturelle de l′Afrique du Nord, 48: 319-352.
- Athias-Henriot C. 1962. Amblyseius swirskii, un nouveau phytoseiide voisin d'A. andersoni (Acariens anactinotriches). Annales de l′Ecole Nationale d'Agriculture d'Alger, 3: 1-7.
- Athias-Henriot C. 1971. La divergence néotaxique des Gamasides (Arachnides). Bull. Scientif. Bourgogne, 28: 93-106.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides, protoadéniques, Phytoseiidae). Acarologia, 17: 20-29.
- Chant D.A. (1957) Descriptions of some phytoseiid mites (Acarina, Phytoseiidae). Part I. Nine new species from British Columbia with keys to the species of British Columbia. Part II. Redescriptions of eight species described by Berlese. Can. Entomol., 89: 289-308. https://doi.org/10.4039/Ent89289-7
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). West Bloomfield, Indira Publishing House, 219 pp.
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Int. J. Acarol., 17: 187-199. https://doi.org/10.1080/01647959108683906
- Demite P.R., McMurtry J.A., Moraes G.J. de 2014. Phytoseiidae Database: a website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa, 3795: 571-577. https://doi.org/10.11646/zootaxa.3795.5.6
- Denmark H.A., Muma M.H. 1989. A revision of the genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). Occasional Papers of the Florida State Collection of Arthropods, 4, 149 pp.
- Döker I., Khaustov V.A., Joharchi O. 2022a. First report of plant inhabiting predatory mites (Acari: Phytoseiidae) in Maldives. Acarologia, 62: 865-878. https://doi.org/10.24349/ocuh-rrin
- Döker I., Vangansbeke D., Merck J. 2022b. New records of phytoseiid mites (Acari: Mesostigmata: Phytoseiidae) in Belgium with an identification key to Belgian species. Acarologia, 62: 1070-1083. https://doi.org/10.24349/yshq-dgl8
- Ehara S., Bhandhufalck A. 1977. Phytoseiid mites of Thailand (Acarina: Mesostigmata). Journal of the Faculty of Education, Tottori University, Natural Science, 27: 43-82.
- Evans G.O. 1963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Br. Mus. Nat. Hist., Zool., 10: 275-303. https://doi.org/10.5962/bhl.part.20528
- Evans G.O., Fain A. 1995. A new phoretic deuteronymph of Halodarcia Karg (Acari: Mesostigmata: Halolaelapidae) associated with carabid beetles in Belgium with a review of the genus. Bull. Inst. R. Sci. Nat. Belg. Entomol., 65: 63-71.
- Ferragut F., Navia D. 2015. Phytoseiid mites (Acari: Phytoseiidae) from Patagonia and Tierra Del Fuego. Zootaxa, 3990: 525-550. https://doi.org/10.11646/zootaxa.3990.4.3
- Gupta S.K. 1985. Three new Phytoseiidae from India (Acari: Mesostigmata). Entomon, 10: 209-214.
- Gupta S.K. 2003. A monograph on plant inhabiting predatory mites of India. Pat II: Order: Mesostigmata. Memoirs of the Zoological Survey of India, 20: 1-185.
- Karmakar K., Bhowmik S., Sherpa C. 2017. Description of five new species and re-description of two species of Amblyseius (Acari: Phytoseiidae) from West Bengal, India. Zootaxa, 4311: 39-61. https://doi.org/10.11646/zootaxa.4311.1.3
- Khaustov V.A., Döker I., Joharchi O., Marchenko I.I. 2023. Three new species of Amblyseius Berlese (Mesostigmata: Phytoseiidae) from Altai Republic, Russia. Syst. Appl. Acarol., 28: 1914-1927. https://doi.org/10.11158/saa.28.12.6
- Kreiter S., Payet R.-M., Abo-Shnaf R., Douin, M. 2021. New Phytoseiidae (Acari: Mesostigmata) of Mascareignes and Comoros Archipelagos (Indian Ocean): one new record, three new species groups and description of six new species and of six unknown males. Acarologia, 61: 845-889. https://doi.org/10.24349/Krky-e23s
- Liao L.-R., Ho C.-C., Lee H.-C., Ko C.-C. 2020. Phytoseiidae of Taiwan (Acari: Mesostigmata). Taipei, National Taiwan University Press, 538 pp.
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Ent. Soc. Can., 47: 1-64. https://doi.org/10.4039/entm9747fv
- McMurtry J.A., Moraes G.J. de., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Muma M.H. 1967. New Phytoseiidae (Acarina: Mesostigmata) from southern Asia. Fla. Entomol., 50: 267-280. https://doi.org/10.2307/3493156
- Oliveira D.C., Charanasri V., Kongchuensin M., Konvipasruang P., Chandrapatya A., Moraes G.J. de 2012. Phytoseiidae of Thailand (Acari: Mesostigmata), with a key for their identification. Zootaxa, 3453: 1-24. https://doi.org/10.11646/zootaxa.3453.1.1
- Rather A.Q. 1985. On some phytoseiid mites from India. Riv. Parassitol., 2: 291-295.
- Rowell H.L., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. Entomol., 110: 859-876. https://doi.org/10.4039/Ent110859-8
- Sadanandan M.A. 2006. A new species of predatory mite, Amblyseius (Acarina: Phytoseiidae) from stored coffee berries. J. Bio-Sci., 14: 39-41. https://doi.org/10.3329/jbs.v14i0.440
- Sadanandan M.A., Ramani N. 2006. Two new species of predatory mites Acarina: Phytoseiidae from Kerala, Índia. Zoos'Print J., 21, 2267-2269. https://doi.org/10.11609/JoTT.ZPJ.1221.2267-9
- Santhosh P.P., Sadanandan M.A. 2015. A new species of Amblyseius Berlese (Acari: Phytoseiidae) from Kerala, India. Entomon, 41: 67-70. https://doi.org/10.33307/entomon.v41i1.124
- Tixier M.-S. 2012. Statistical approaches to assess intraspecific variations of morphological continuous characters: the case study of the family Phytoseiidae (Acari: Mesostigmata). Cladistics, 28: 489-502. https://doi.org/10.1111/j.1096-0031.2012.00394.x
- Tsolakis H., Ragusa S. 2016. On the identity of Neoseiulus fallacis (Garman, 1948) (Parasitiformes, Phytoseiidae): redescription of the species and description of the new species Neoseiulus garmani. Int. J. Acarol., 42: 394-404. https://doi.org/10.1080/01647954.2016.1205134
- Wu W.N., Qian X. 1983. Two new species of the genus Amblyseius Berlese (Acari.: Phytoseiidae). Entomotaxonomia, 5: 263-266 [in Chinese].
- Zannou I.D., Moraes G.J. de, Ueckermann E.A., Oliveira A.R., Yaninek J.S., Hanna R. 2007. Phytoseiid mites of the subtribe Amblyseiina (Acari: Phytoseiidae: Amblyseiini) from sub-Saharan Africa. Zootaxa, 1550: 1-47. https://doi.org/10.11646/zootaxa.1550.1.1



2025-06-11
Date accepted:
2025-08-29
Date published:
2025-09-16
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Döker, Ismail; Atchia, Isabelle; Gasparro-Brewer, Jude; Fang, Xiao-Duan and Bolton, Samuel J.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



