The effects of chlorfenapyr on the life history traits and population parameters of Neoseiulus longispinosus Evans (Mesostigmata: Phytoseiidae)
Sandeep, Veerabhadrappa  1
; Rajashekharappa, Kenchappa
1
; Rajashekharappa, Kenchappa  2
; Sharanabasappa S. Deshmukh
2
; Sharanabasappa S. Deshmukh  3
; Jayalaxmi Narayan Hegde
3
; Jayalaxmi Narayan Hegde  4
; Nagarajappa Adivappar
4
; Nagarajappa Adivappar  5
and Mallikarjuna, Hosamane Basavarajappa6
5
and Mallikarjuna, Hosamane Basavarajappa6
1✉ Department of Entomology, College of Agriculture, Shivamogga, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India.
2Department of Entomology, College of Agriculture, Shivamogga, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India.
3Department of Entomology, College of Agriculture, Shivamogga, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India.
4Department of Entomology, College of Agriculture, Shivamogga, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India.
5Department of Horticulture, College of Agriculture, Shivamogga, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India.
6Department of Agricultural Statistics. College of Agriculture, Shivamogga, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India.
2025 - Volume: 65 Issue: 3 pages: 887-899
https://doi.org/10.24349/clbm-b94oOriginal research
Keywords
Abstract
Introduction
The two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) is a cosmopolitan polyphagous mite that results in damage both directly and indirectly as a vector for many viruses to plants by reducing photosynthesis and transpiration. It has been listed as a serious pest of agricultural, horticultural, and ornamental crops worldwide (Jayasinghe and Mallik 2011; Saber et al. 2018; Sandeep et al. 2024). The control of T. urticae populations relies mostly on the use of different acaricides. However, mites rapidly develop resistance to different acaricides because of their high reproductive rate, short life cycle and arrhenotoky reproduction (McMurtry et al. 2013; Laborda et al. 2013; Mousavi et al. 2023). As resistance to acaricides in T. urticae continues to spread rapidly, the use of predatory mites for biological control is increasingly recognized as a vital strategy for managing spider mite populations.
Predatory mites are considered economically important biocontrol agents and effective alternatives to conventional chemical control in agricultural systems (Khanamani et al. 2015; Hamedi et al. 2010). Predatory mites of the Phytoseiidae family are the most popular, as they efficiently control mites in many crops around the world (Sabelis, 1982; Mollaloo et al. 2017). More than 2500 phytoseiid mite species, which affect their prey mite, are known worldwide (Demite et al. 2023). T. urticae is frequently associated with an indigenous phytoseiid predatory mite, Neoseiulus longispinosus (Evans), which serves as a promising biocontrol agent for numerous crops grown in fields as well as in protected conditions in India (Karmakar and Gupta, 2011). This predatory mite is widely distributed and adaptable to warm temperatures inside greenhouses under southern Indian conditions (Messelink et al. 2010; Lee and Lee. 2010). To conserve, enhance and exploit this predatory mite in pest management programs, it is crucial to apply selective acaricides that are effective against the pest of interest, while being less destructive to predatory mites (Ghaderi et al. 2013; Alinejad et al. 2014; Alinejad et al. 2016).
Chlorfenapyr is a broad-spectrum insecticide and acaricide known for its effectiveness against a wide range of agriculturally significant pests, including broad mites, rust mites, red spider mites, thrips, leaf miners, and whiteflies. It exhibits both contact and stomach toxicity. According to its mode of action, chlorfenapyr belongs to IRAC Group 13, which uncouplers of oxidative phosphorylation via disruption of the proton gradient (IRAC, 2025). It has been reported that exposure to a sublethal concentration of chlorfenapyr decreased the total life span and reduced the fecundity, GRR and R0 of P. persimilis and N. californicus at the LC20 and LC30 (Acari: Phytoseiidae) and prolonged the developmental times of larvae, protonymphs and deutonymphs and reduced the longevity, oviposition days and fecundity reported for Neoseiulus barkeri (Hughes) (Acari: Phytoseiidae) when it was treated with the LC10 and LC30 (Niaki et al. 2025; Cheng et al. 2025).
In the present study, we aimed to estimate the effects of chlorfenapyr on the life history traits and population parameters of N. longispinosus. Females of the predator were treated with chlorfenapyr, which was applied at the LC10, LC20 and LC30 concentrations, previously estimated for T. urticae females, and the effects were evaluated in the F1 generation of the predator, using the age-stage, two-sex life table method (Chi and Liu, 1985; Chi 1988).
Materials and methods
Rearing two-spotted spider mites (T. urticae)
Common bean plants (Phaseolus vulgaris L.) were cultivated in 15 cm diameter plastic pots inside a greenhouse at the Department of Entomology, College of Agriculture, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India. The greenhouse was maintained at 25 ± 5 °C with a 16 L:8 D photoperiod and 65 ± 5% relative humidity, and was equipped with a ventilation system to ensure proper air circulation. The plants were watered daily with tap water and supplemented with fertilizer as needed. T. urticae (green form; food source for predatory mites) were reared on bean plants under the same controlled greenhouse conditions in a separate section to prevent cross-contamination. To sustain a sufficient supply of mites for experimental use, fresh bean plants were periodically introduced into the rearing colony.
Collection and maintenance of Neoseiulus longispinosus populations
Different stages of predatory mites, N. longispinosus along with the prey mite T. urticae, were collected from infested tomato fields in Shivamogga (13.9299°N and 75.5681°E) at 2024-25. The field collected population was designated the F0 generation and sent for identification. It was identified by Dr. C. Chinnamade Gowda, an Acarologic taxonomist, who is currently working as a network coordinator of all India Network Projects on Agricultural Acarology, UAS, GKVK, Bengalore. Predatory mite rearing was conducted following the method of McMurtry and Scriven (1965). Rearing arenas consisting of black plastic discs with a 7 cm diameter were placed on moist sponges within water-filled plastic trays to maintain high humidity. To prevent escape and contamination by other arthropods, a barrier of wet cotton was placed around arena edges (Overmeer 1985). Four to five French bean (Phaseolus vulgaris) leaves heavily infested with T. urticae were placed in each arena as prey sources. An initial inoculum of N. longispinosus was released onto spider mite-infested leaves and an approximate predator-to-prey ratio of 1:25 was maintained by supplementing prey daily (Rajaee et al. 2022). The culture was maintained in a growth chamber at 25 ± 2 °C, with 65 ± 5% relative humidity and a 16:8 (L:D) h photoperiod.
Chemical tested
Chlorfenapyr (IUPAC name: 4-bromo-2-(4-chlorophenyl) pyrrole-3-carbonitrile) was used in this experiment in the form of the commercial product Intrepid (10% suspension concentrate), manufactured by BASF India Ltd.
Acute toxicity bioassay with T. urticae
To estimate the acute toxicity of chlorfenapyr to T. urticae adult females, a concentration–response bioassay was performed using the leaf dip method (Helle and Overmeer 1985). Leaf discs 3 cm in diameters were cut from Morus alba (Mulberry) plant leaves and dipped for 20 seconds in a series of chlorfenapyr concentrations (13.33, 6.66, 3.29, 1.59, 0.79, and 0.39 mg/l) and control was treated with distilled water. After 2 h of drying at room temperature, the sixty same aged female adults (24 h old) were transferred to each leaf disc. The edges of the leaf disc were surrounded by moist tissue paper to prevent mites from escaping. Mortality was recorded 72 hours post-treatment by gently probing mites with a fine brush, if individuals did not respond were considered dead. Each concentration was tested with four replications. All Petri dishes were kept inside an incubator at 25 ± 2 °C and 65 ± 5% RH with a photoperiod of 16:8 (L:D) hours.
Life table bioassay
To assess the effects of chlorfenapyr on Neoseiulus longispinosus, F1 generation was examined at previously estimated concentrations (LC10, LC20, and LC30 for T. urticae females). Prior to the introduction of predatory mites, leaf discs were dipped into each concentration, and distilled water was used as the control for 20 seconds. After 2 h of drying, the leaf discs were placed in Petri dishes containing cotton pads filled with distilled water. One hundred adult females (24 h old) of N. longispinosus were subsequently placed onto treated and control leaf discs (one mite per each leaf arena). After 24 hours of exposure, surviving females were individually transferred into individual new clean untreated leaf discs at 20 mm in diameter (89 for LC10, 78 for LC20, and 66 female mites for LC30 were survived). A male from the same treatment group (treated in separate leaf arena and only used for mating) was introduced for mating (replaced if dead) and allowed to lay eggs. After 24 hours, one egg laid by the surviving females was retained on each leaf disc (one egg for each leaf disc), while the other eggs and adult females were removed. All dishes were kept in growth chamber at 25 ± 1 °C, with 70 ± 5% RH and 16:8 h (L:D) a photoperiod. Developmental progress was recorded at 12-hour intervals. Each predatory mite was given four to six immature spider mites daily, along with an adequate supply of corn pollen provided weekly. Upon adult emergence, a male from the same treatment was paired with each female to allow mating and subsequent oviposition. The preoviposition, oviposition, and postoviposition periods, as well as fecundity and adult longevity were recorded. Eggs were removed daily after counting to ensure accurate fecundity measurements and avoid confusion with newly laid eggs. The lifespan of both females and males was monitored until natural death (Mousavi et al. 2023).
Statistical analysis
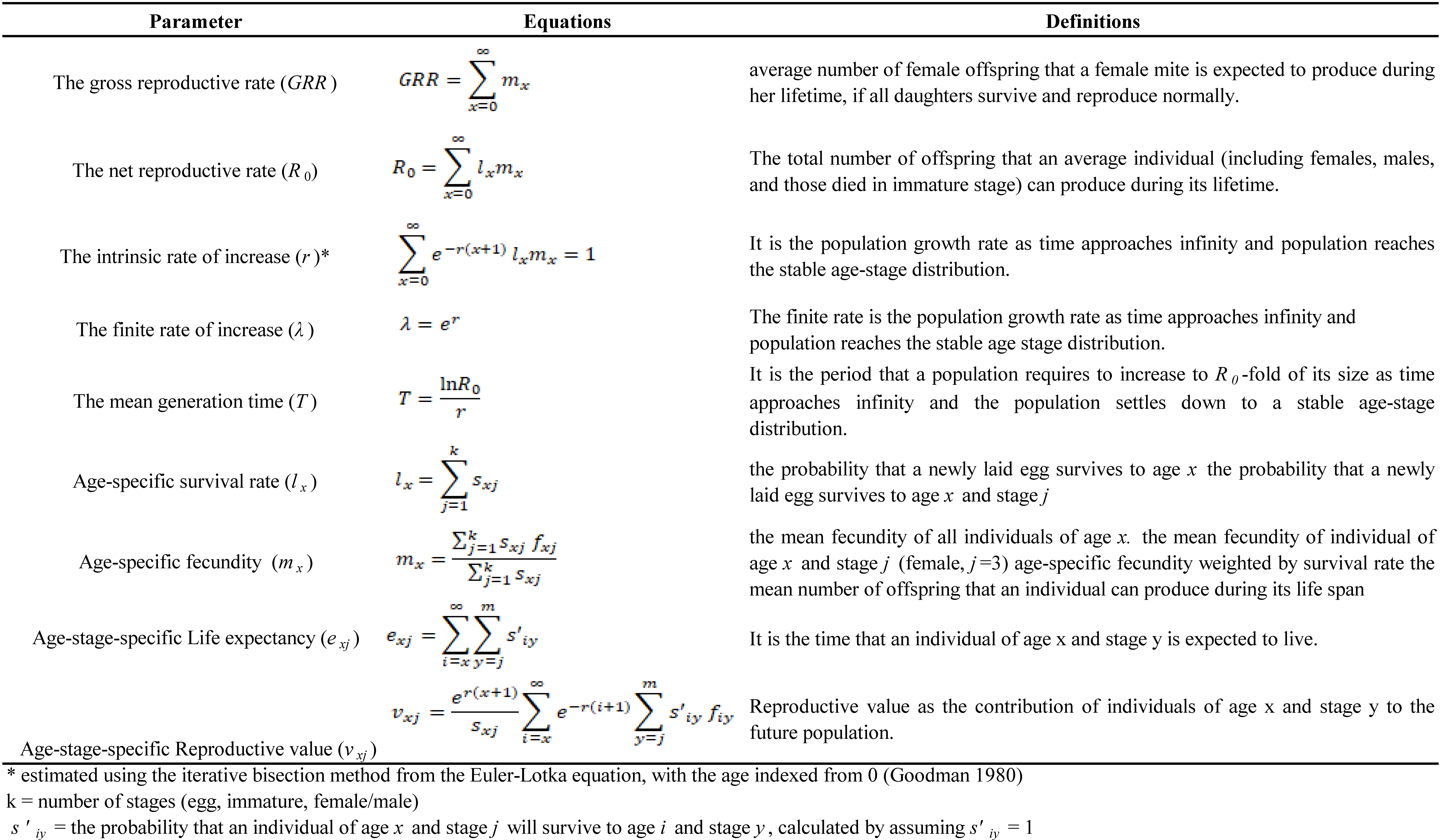


The LC values and concentration–mortality regression lines were estimated by using probit analysis using SPSS software (version 20.0) (Finney, 1952). Raw life table data of Neoseiulus longispinosus were analysed on the basis of the age-stage and two-sex life table theory using the TWOSEX-MS Chart program (Chi and Liu, 1985; Chi, 1988; Chi, 2023). The mean values and standard errors of all life history traits and population parameters were estimated by bootstrap method with 100,000 replicates. Statistical differences among the treatments were estimated the by paired bootstrap tests (Chi, 2023). The graphs for data visualization were created using Microsoft Excel 2019.
Results
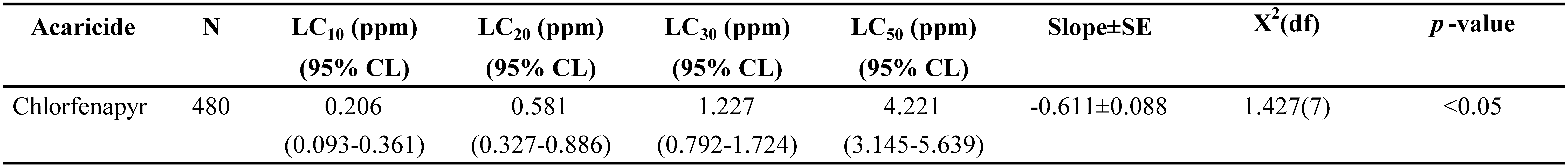
Based on probit analysis, the regression equation for the concentration–mortality relationship was determined to be Y = -0.611 + 0.974X with an LC50 value of 4.221 ppm for T. urticae. The concentrations chosen for the life table bioassay were 0.206 ppm for LC10, 0.581 ppm for LC20 treatment and 1.227 ppm for LC30 treatment, but no mortality was observed in control (Table 2). Low lethal concentrations (0.206, 0.581, and 1.227 ppm) were applied to the predatory mite, N. longispinosus to evaluate their effects on its life history traits and population parameters.


Effects on life history traits
Exposure to chlorfenapyr significantly affected the developmental duration and lifespan of both female and male Neoseiulus longispinosus (Table 3). The egg development duration was significantly longer in the LC20 and LC30 treatment groups than in the control group (p < 0.05). The longest durations were observed in the LC30 treatment, with durations of 1.85 ± 0.04 days for females and 1.75 ± 0.07 days for males, which were significantly greater than those in the control (1.34 ± 0.01 days for females and 1.26 ± 0.05 days for males). Chlorfenapyr affected the females and males in the LC30 treatment, with the longest larval period (1.59 ± 0.03 days for males and 1.50 ±0.04 days for females), followed by that in the LC20 treatment (1.42 ± 0.02 and 1.38 ± 0.04 days), which were significantly longer than those in the control (1.15 ± 0.01 and 1.14 ± 0.05 days; p < 0.05). The protonymph and deutonymph stages were also significantly prolonged in females under the LC20 and LC30 treatments compared with those under the control and LC10 treatments (p < 0.05), but in males, no significant difference was found between the LC20 and LC10 treatments (P = 0.08), and the LC30 was prolonged compared with that of the control (p < 0.05). These results indicated a dose-dependent delay in immature development. Compared with that of the control, adult longevity was significantly decreased by chlorfenapyr exposure (p < 0.05). In females, longevity decreased from 20.94 ± 0.40 to 12.71 ± 0.45 days in the control and LC30 treatments, and males followed the same trend, with longevity decreasing from 15.76 ± 0.32 to 9.38 ± 0.31 days. The total lifespans of both females and males were significantly reduced in a concentration-dependent manner (p < 0.05). The females in the control group survived the longest (26.03 ± 0.19 days), followed by those in the LC10 group (22.84 ± 0.44 days), but the lowest survival was recorded in the LC20 and LC30 groups (21.21 ± 0.19 and 19.21 ± 0.38 days, respectively). In males, the maximum duration was 21.08 ± 0.36 days in the control, and the minimum duration of 15.97 ± 0.43 days was recorded in the LC30 treatment.
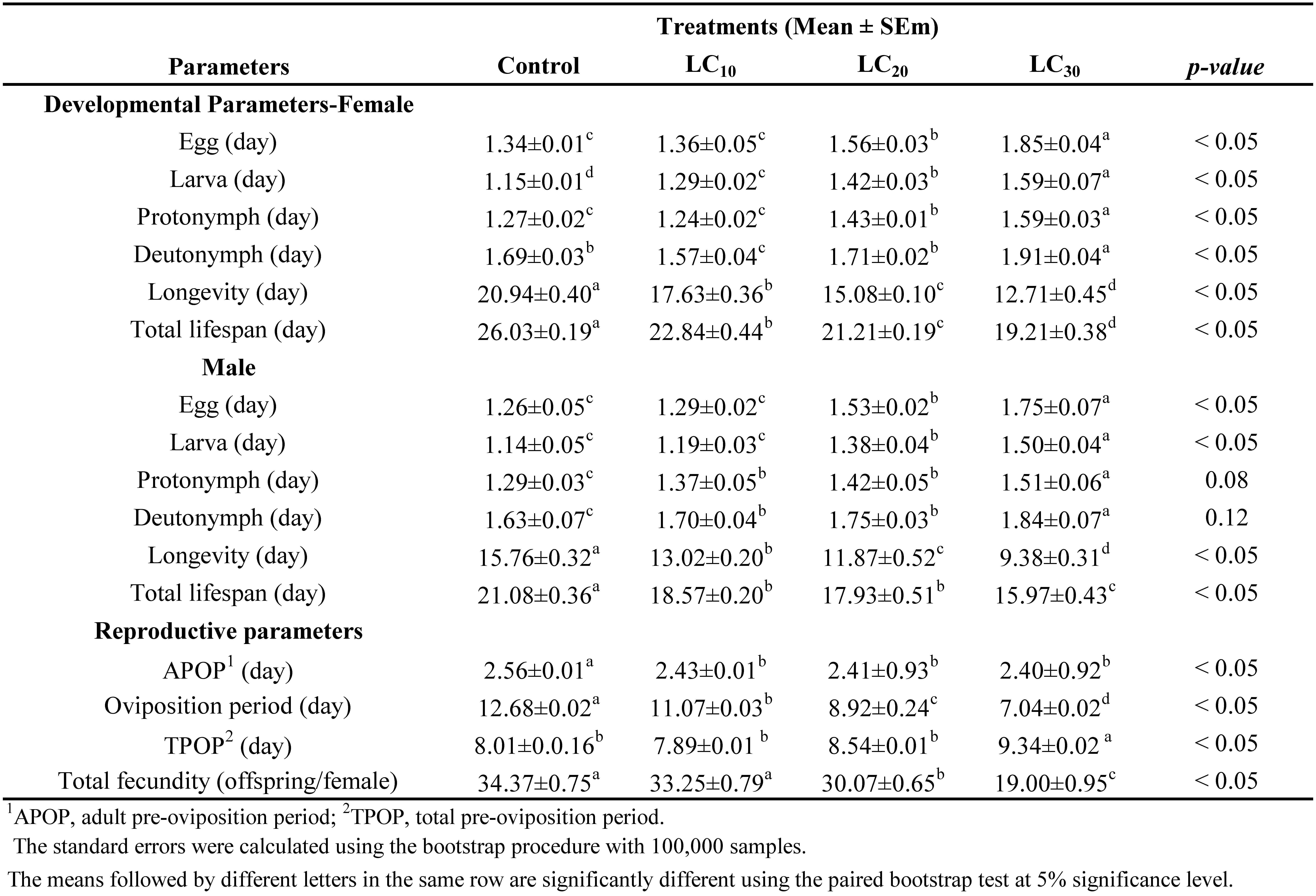


Exposure to chlorfenapyr had a significant effect on the reproductive performance of N. longispinosus, as summarized in Table 3. In the control, the mean preoviposition period was 2.56 ± 0.01 days, while there were no significant differences among the treatments. In contrast, the oviposition period decreased significantly with increasing concentrations of chlorfenapyr (p < 0.05). The control presented the longest oviposition period, 12.68 ± 0.02 days. It was significantly lower in the treatment groups, with 11.07 ± 0.03 days in the LC10 treatment group, 8.92 ± 0.24 days in the LC20 treatment group, and 7.04 ± 0.02 days in the LC30 treatment group. The total preoviposition period was significantly greater in the LC30 treatment group (9.34 ± 0.02 days) than in the control group (8.01 ± 0.16 days), indicating a delay in reproductive readiness caused by chlorfenapyr exposure (p < 0.05). The highest fecundity was observed in the control, with 34.37 ± 0.75 offspring per female. Although the LC10 treatment resulted in a nonsignificant reduction (33.25 ± 0.79 offspring/female; p > 0.05), significant reductions were recorded in the LC20 (30.07 ± 0.65 offspring/female) and LC30 treatments (19.00 ± 0.95 offspring/female; p < 0.05), indicating a strong concentration-dependent decline in reproduction.
Effects on population parameters


The gross reproductive rate (GRR) significantly decreased with increasing concentrations of chlorfenapyr (p < 0.05), as shown in Table 4. The highest GRR was recorded in the control (27.61 ± 2.23 offspring per female), which declined significantly to 24.33 ± 0.2.49 in the LC10 treatment, 22.08 ± 2.23 in the LC20 treatment, and the lowest value of 12.92 ± 1.65 offspring per female in the LC30 treatment. A similar trend was observed for the net reproductive rate (R₀). The intrinsic rate of increase (r) and the finite rate of increase (λ), indicators of population growth potential, were also significantly impacted as the concentration increased (p < 0.05). The mean generation time was greatest in the untreated group (13.03 ± 0.22 days) and the LC10 treatment group (13.01 ± 0.24 days), and there was no significant difference between the two treatments. However, a significantly shorter generation time was recorded in the LC20 (12.68 ± 0.24 days) and LC30 (12.21 ± 0.24 days; p < 0.05) groups, suggesting accelerated generational turnover under higher concentrations of chlorfenapyr.
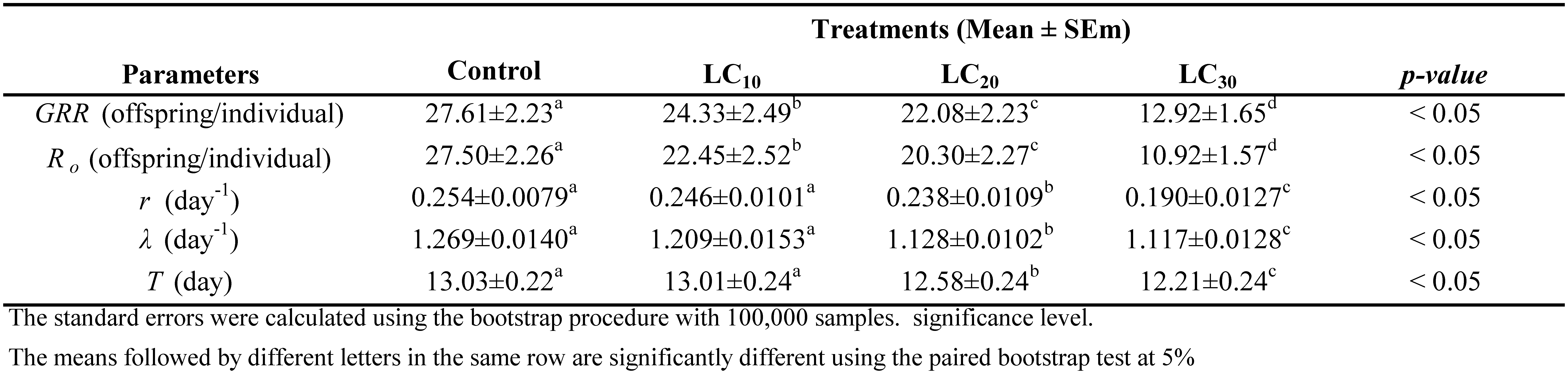


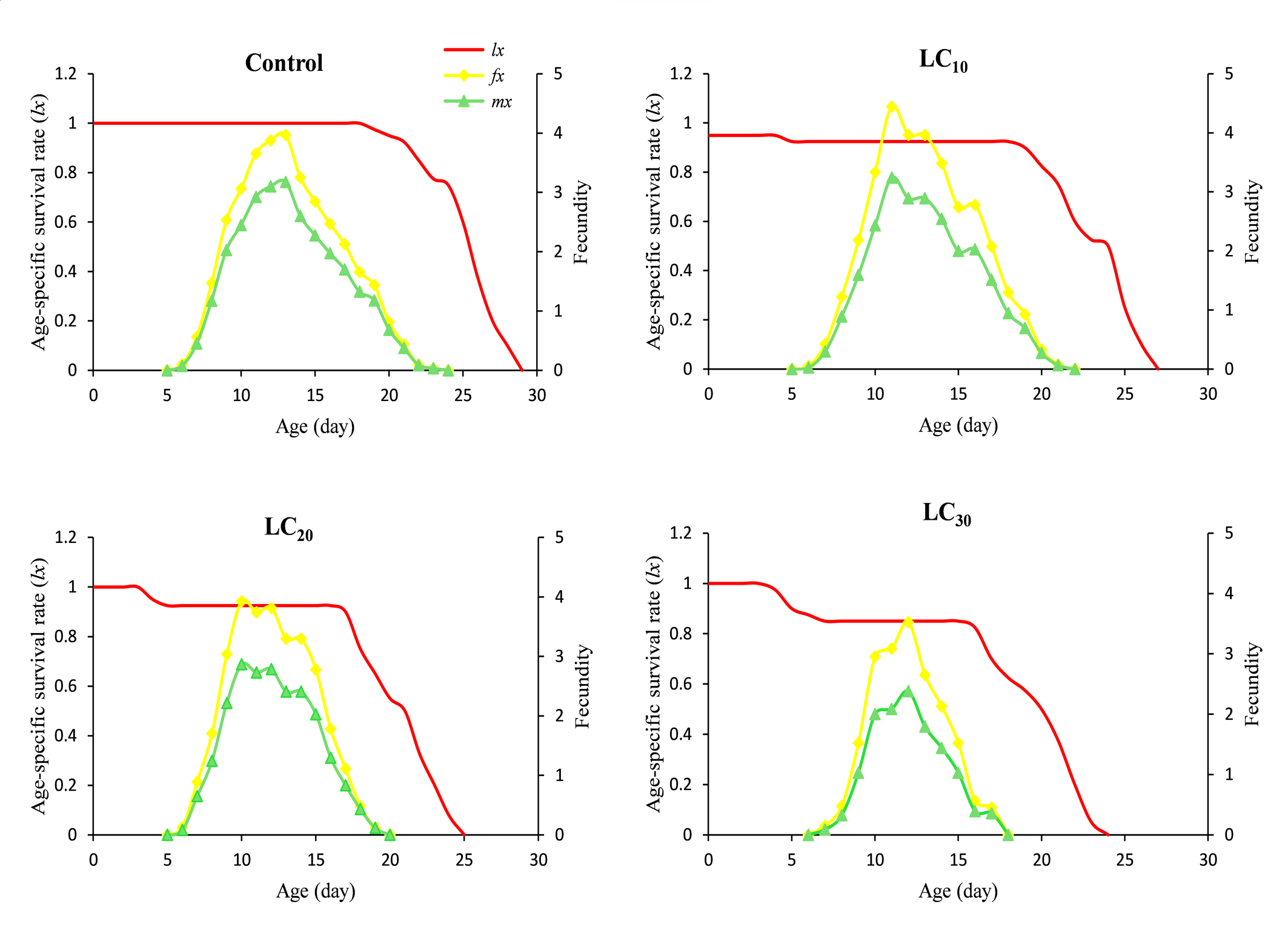


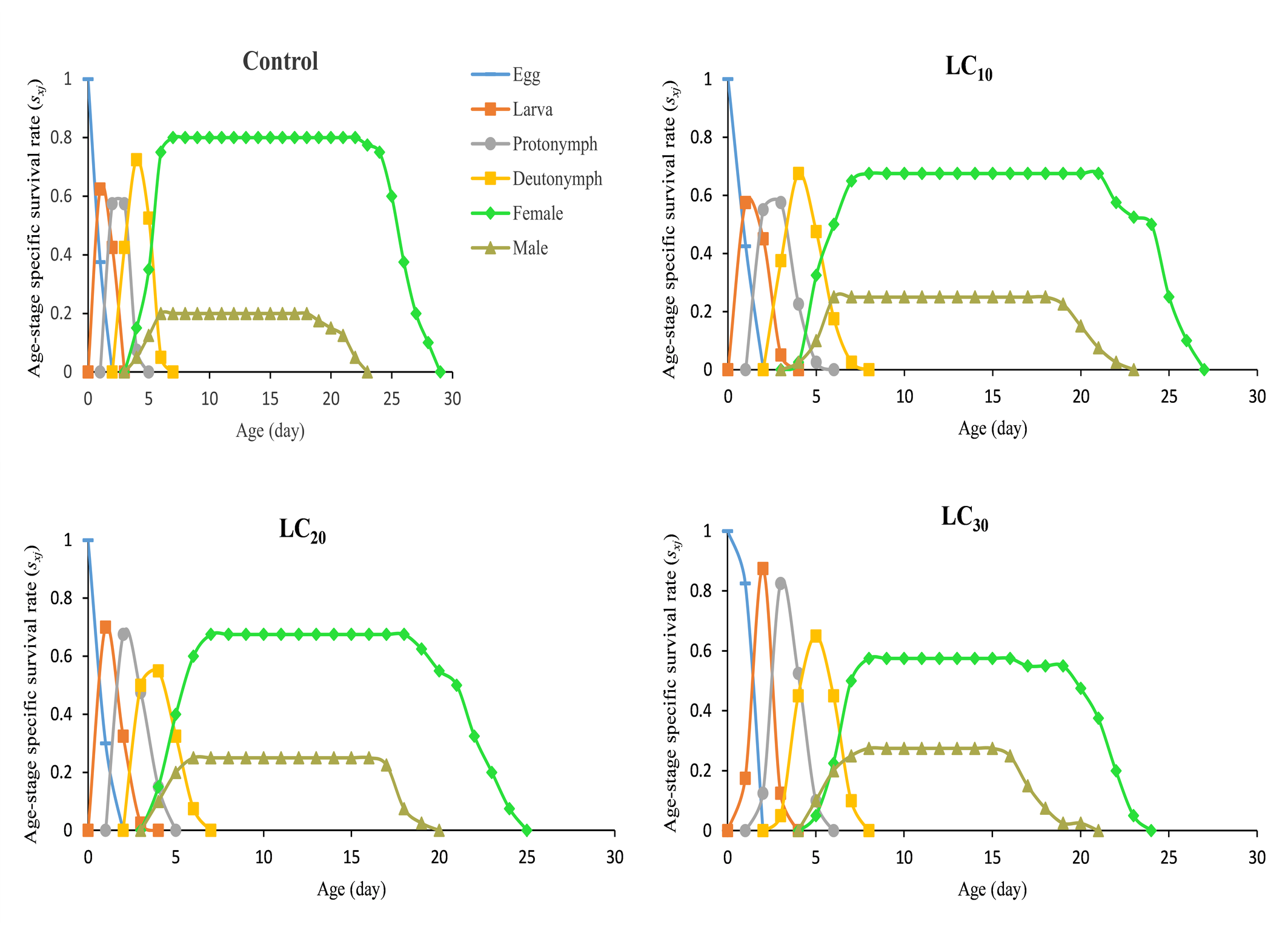
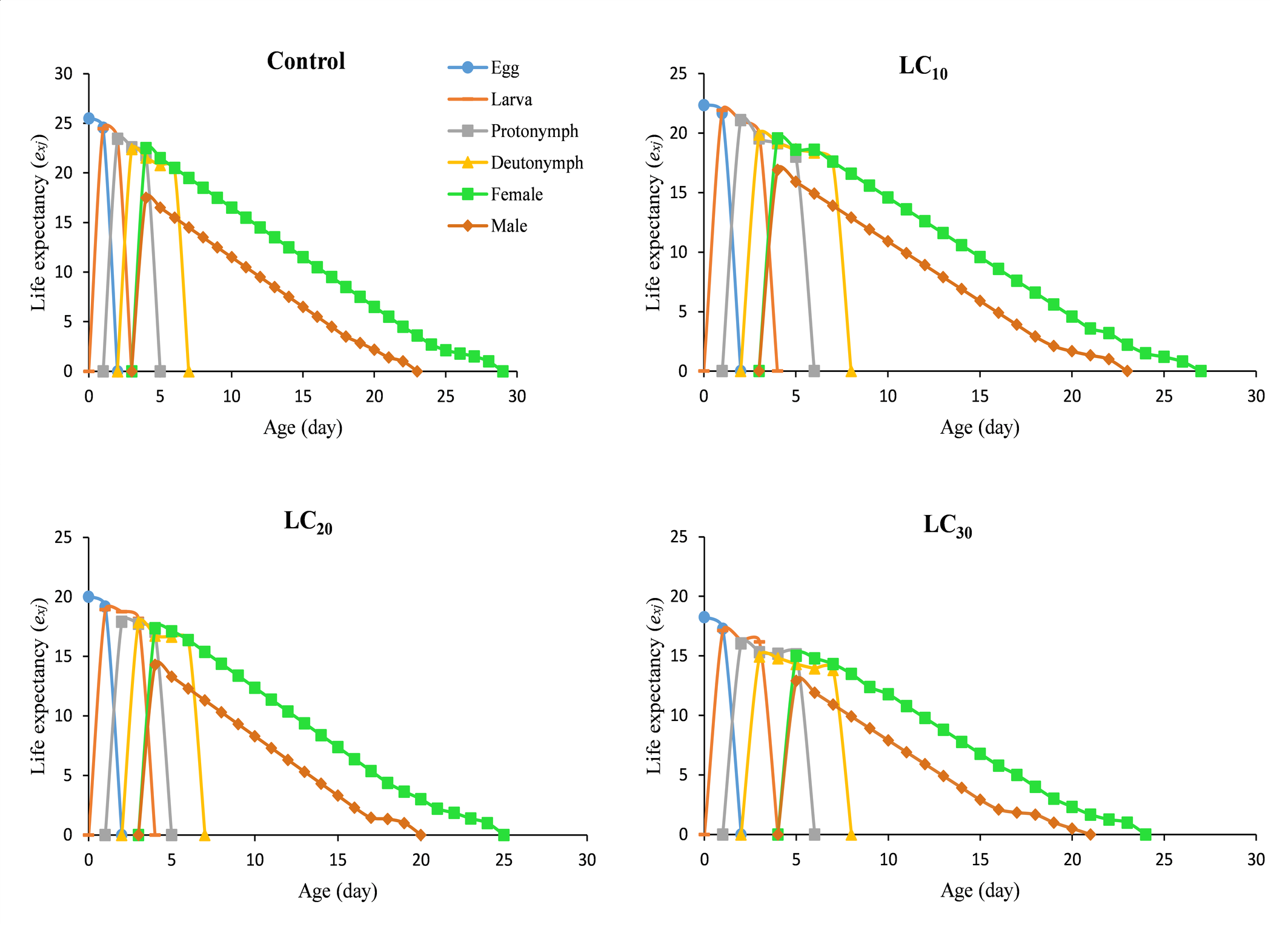
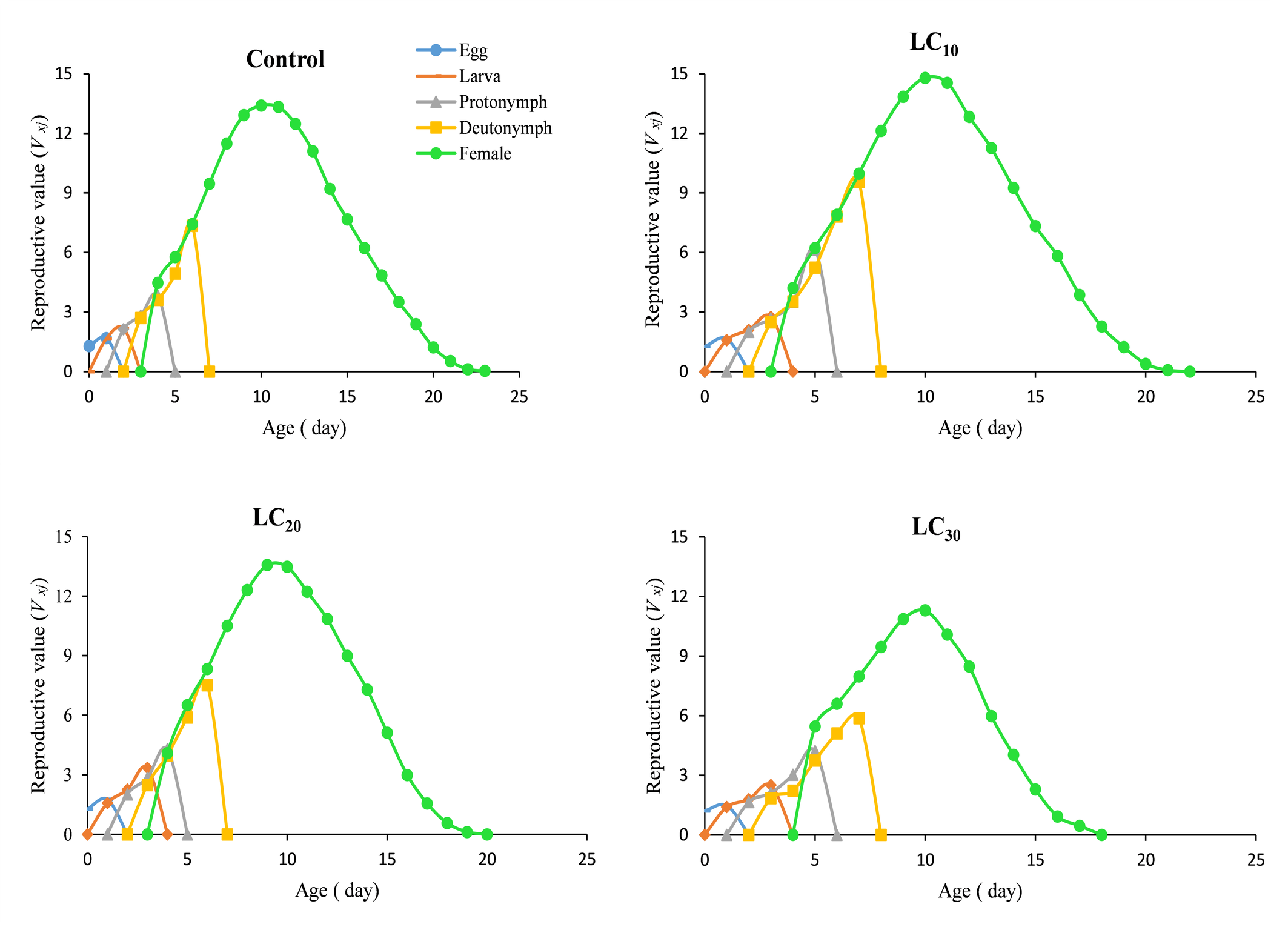
The daily and age-stage survival curves of N. longispinosus in the control and chlorfenapyr treatments are shown in Figure 1. The total lifespan of untreated individuals was 28 days, followed by 26 days for the LC10 treatment, 24 days for the LC20 treatment, and 23 days for the LC30 treatment. The maximum age-specific maternity (mₓ) values for mites treated with chlorfenapyr were approximately 3.24, 2.78, and 2.38 eggs/female/day, occurring on the 11th, 12th, and 13th days of their lifespan, respectively (Figure 2). In comparison, the control group presented a peak value of 3.17 eggs/female/day on the 13th day. The age-stage survival rate (sₓⱼ), which represents the probability that a newborn will survive to age x and stage j, is also illustrated in Figure 1. Considerable overlap was observed between the survival curves of different developmental stages (A–D). The highest female survival rate was recorded under the LC20 treatment, where 67% of eggs reached adulthood. The highest male survival rate was observed under the LC30 treatment, with 27% of eggs developing into adult males. Overall, individuals in the chlorfenapyr treatment group presented a shorter life expectancy than did those in the control group, as depicted in Figure 3. The reproductive value (vxj ) value defines the contribution of an individual at age x and stage j to the future population. The peak reproductive value of females in the first generation was observed in the control at the 13th day, followed by the 10th day in the LC10 and LC30 treatments and the 9th day in the LC20 treatment (Figure 4).




Discussion
The present study demonstrated that exposure to chlorfenapyr (applied at LC10, LC20, and LC30 for T. urticae females) significantly affected the developmental, reproductive and population parameters of the offspring of N. longispinosus, a key predatory mite in integrated pest management (IPM) in India. The prolonged development duration and reduced longevity observed at the treated concentrations reflect the physiological stress imposed by chlorfenapyr even at low concentrations (Mollaloo et al. 2017). The egg, larval, protonymph, and deutonymph stages were significantly longer in the LC20 and LC30 treatment groups than in the control group. These findings align with the results of Niaki et al. (2025), who reported prolonged durations of immature stages in Neoseiulus californicus (Acari: Phytoseiidae) under sublethal effects of chlorfenapyr at the LC20 (0.044 g/l) and LC30 (0.053 g/l) concentrations. In contrast to our findings, Sanatgar et al. (2011) reported that repeated applications of hexythiazox had no significant effect on the total developmental period of P. persimilis. This discrepancy may be attributed to differences in predatory mite species or the distinct modes of action of the acaricides used. Indeed, hexythiazox functions by disrupting chitin synthesis in mites, and the extended developmental time may be attributed to the disruption of mitochondrial oxidative phosphorylation caused by chlorfenapyr, which leads to decreased cellular energy production (Black et al. 1994). Similar developmental period prolongations were reported by He et al. (2020) in Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) when exposed to abamectin (LC20 and LC30 treatments), suggesting that a general pattern of sublethal acaricide stress disrupts normal development and growth. Interestingly, these findings contrast with some reports in which low concentrations of certain insecticides triggered hormesis-like responses and shortened development times or increased fecundity (Cutler, 2013). However, no such effect was observed in our experiments, suggesting that chlorfenapyr acts consistently as a developmental inhibitor in predatory mites.
Chlorfenapyr significantly reduced fecundity and shortened oviposition periods in a concentration-dependent manner. The application of low lethal concentrations of chlorfenapyr to newly emerged females affected the reproduction of these females and that of their progeny. Our results indicated that the decreased oviposition period of the treated females and their subsequent generation had a considerable effect on the overall reduction in their fecundity. In contrast, our results are in agreement with those reported by Kim and Paik (1996) on Amblyseius womersleyi Schicha. However, this observation contrasts with the findings of Ibrahim and Yee (2000), who reported that exposure to sublethal concentrations of abamectin suppressed the mean fecundity of N. longispinosus (Evans) by 50% at an LC50 of 0.015 ppm. This suppression could be linked to physiological damage to reproductive tissues or hormonal imbalance induced by chlorfenapyr toxicity. Preovipositional periods, particularly the total preoviposition period, are critical factors influencing the intrinsic rate of increase (Khanamani et al. 2017). According to the data analysis, the chlorfenapyr treatments did not significantly affect preoviposition periods but resulted in a shortened oviposition period and fewer oviposition days as the concentration increased. The increased total preoviposition period in the LC30 further supports the delay in reproductive maturity, possibly due to energetic trade-offs between detoxification and reproduction (Kim et al. 2004; Desneux et al. 2007). The decreased reproductive potential among the treatment groups suggested that chlorfenapyr impairs the hormones involved in vitellogenesis or ovary development.
Key life table parameters, including the gross reproductive rate (GRR), net reproductive rate (R₀), intrinsic rate of increase (r), and finite rate of increase (λ), were significantly reduced by chlorfenapyr exposure. The intrinsic rate of increase (r) is widely recognized as the most reliable indicator for assessing the overall impact of a pesticide, as it reflects both reproductive potential and survival (Hamedi et al. 2011; Moscardini et al. 2013). Furthermore, the importance of using r as an ecologically relevant bioassay parameter in toxicological studies has been highlighted by Allan and Daniels (1982). The observed declines in GRR and R₀ mirror findings in Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) exposed to pyridaben (Rahmani and Aharizad, 2019), highlighting the potential for chlorfenapyr to suppress predator population sustainability. The reduced r and λ values point to lower reproductive fitness and slower population recovery, which are key concerns in biological control scenarios due to a decreased oviposition period. In contrast to our results, beneficial mites exposed to insect growth regulators such as hexythiazox at different concentrations of 50, 12.5 and 3.125 mg/L presented lower impacts on population parameters (Musa et al. 2022), suggesting that the mode of action of chlorfenapyr is more broadly disruptive and affects energy metabolism. The shortened mean generation time (T) in the LC20 and LC30 treatments could be interpreted as a stress-induced acceleration of life history traits, the survival strategy reported in arthropods under toxic pressure (Forbes, 2000; Havasi et al. 2019). However, this acceleration is often maladaptive, as it is accompanied by reduced fecundity and survival.
Age-stage-specific survival and fecundity curves revealed significant reductions in both survival probability and reproductive outputs under sublethal treatments. The reduced maximum fecundity (mx ) at sublethal concentrations along with earlier peak days reflects the altered reproductive scheduling likely driven by oxidative stress and energy misallocation (Sparks et al. 2012). LC10 had relatively moderate effects and significantly curtailed survival and reproductive potential, which compromised the long-term viability of the predators. Interestingly, the highest female survival rate was noted in the LC20 treatment, whereas the highest number of surviving males occurred in the LC30 treatment. This could indicate sex-specific detoxification or tolerance mechanisms, as observed in other insects (Stark and Banks, 2003). The reproductive value (vxj) patterns revealed that the peak reproductive contribution occurred earlier in the treated groups, which again suggests that stress-induced early investment in reproduction at the cost of future fecundity is a well-documented phenomenon in ecotoxicology (Sibly and Calow, 1989).
Overall, the results confirmed that exposure to chlorfenapyr adversely affects the development, reproduction, and population growth of N. longispinosus. These findings have important implications for IPM programs, where the selective use of acaricides is critical for preserving natural enemies. This study highlights the need for careful evaluation of acaricide selectivity, especially for compounds such as chlorfenapyr, which has broad-spectrum and mitochondrial-disrupting activity.
Acknowledgements
The authors acknowledge the Director of Research, Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka, India for providing facilities and support.
Declarations
Conflict of interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (the study was approved).
References
- Alinejad M., Kheradmand K., Fathipour Y. 2014. Sublethal effects of fenazaquin on life table parameters of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae). Exp. Appl. Acarol., 64: 361-373. https://doi.org/10.1007/s10493-014-9830-y
- Alinejad M., Kheradmand K., Fathipour Y. 2016. Assessment of sublethal effects of spirodiclofen on biological performance of the predatory mite, Amblyseius swirskii. Syst. Appl. Acarol., 21(3): 375-384. https://doi.org/10.11158/saa.21.3.12
- Allan J. D., Daniels R. E. 1982. Life table evaluation of chronic exposure of Eurytemora affinis (Copepoda) to kepone. Mar. Biol., 66(2): 179-184. https://doi.org/10.1007/BF00397191
- Black B. C., Hollingworth R. M., Ahammadsahib K. I., Kukel C. D., Donovan S. 1994. Insecticidal action and mitochondrial uncoupling activity of AC-303,268 and related halogenated pyrroles. Pestic. Biochem. Physiol., 50(2), 115-128. https://doi.org/10.1006/pest.1994.1064
- Bozhgani N. S. S., Ghobadi H., Riahi E. 2018. Sublethal effects of chlorfenapyr on the life table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Syst. Appl. Acarol., 23(7): 1342-1351. https://doi.org/10.11158/saa.23.7.11
- Cheng S., Li H., Wei Q., Wang Z. 2025. Toxicity effects of chlorfenapyr on the biological performance of the predatory mite, Neoseiulus barkeri (Hughes)(Acari: Phytoseiidae). Crop Prot., 197: 107294. https://doi.org/10.1016/j.cropro.2025.107294
- Chi H. 1988. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol., 17: 26-34. https://doi.org/10.1093/ee/17.1.26
- Chi H., Liu H. 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. (Taipei)., 24: 225-240
- Chi H. 2023. Advances in theory, data analysis and application of the age-stage, two -sex life table for demographic control and pest management. Entomol. Gen., 43: 705-732. https://doi.org/10.1127/entomologia/2023/2048
- Cutler G. C. 2013. Insects, insecticides and hormesis: evidence and considerations for study. Dose-Response., 11(2): 154-177. https://doi.org/10.2203/dose-response.12-008.Cutler
- Demite P. R., Moraes G. J., De, Mcmurtry J. A., Denmark H. A., Castilho R. C. 2023. Phytoseiidae Database. Available from http://www.lea.esalq.usp.br/phytoseiidae.
- Desneux N., Decourtye A., Delpuech, J. M. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol., 52: 81-106. https://doi.org/10.1146/annurev.ento.52.110405.091440
- Finney D. J. 1952. Probit analysis: a statistical treatment of the sigmoid response curve.
- Forbes V. E. 2000. Is hormesis an evolutionary expectation? Funct. Ecol., 14(1): 12-24. https://doi.org/10.1046/j.1365-2435.2000.00392.x
- Ghaderi S., Minaei K., Kavousi A., Akrami M. A., Aleosfoor M., Ghadamyari M. 2013. Demographic analysis of the effect of Fenpyroximate on Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). Entomol. Gen., 34(3):225-233. https://doi.org/10.1127/entom.gen/34/2013/225
- Hamedi N., Fathipour Y., Saber M. 2010. Sublethal effects of fenpyroximate on life table parameters of the predatory mite Phytoseius plumifer. Biocontrol., 55: 271-278. https://doi.org/10.1007/s10526-009-9239-4
- Hamedi N., Fathipour Y., Saber M. 2011. Sublethal effects of abamectin on the biological performance of the predatory mite, Phytoseius plumifer (Acari: Phytoseiidae). Exp. Appl. Acarol., 53: 29-40. https://doi.org/10.1007/s10493-010-9382-8
- Havasi M., Kheradmand K., Mosallanejad H., Fathipour Y. 2019. Sublethal effects of diflovidazin on demographic parameters of the predatory mite, Neoseiulus californicus (Acari: Phytoseiidae). Int. J. Acarol., 45(4): 238-244. https://doi.org/10.1080/01647954.2019.1607550
- He Y., Zhao J., Zheng Y., Desneux N., Wu K. 2020. Sublethal effects of abamectin on Neoseiulus cucumeris (Acari: Phytoseiidae). Ecotoxic. Environ. Saf., 187: 109826.
- Ibrahim Y.B., Yee T.S. 2000. Influence of sublethal exposure to abamectin on the biological performance of Neoseiulus longispinosus (Acari: Phytoseiidae). J. Econ. Entomol., 93(4): 1085-1089. https://doi.org/10.1603/0022-0493-93.4.1085
- IRAC (Insecticide Resistance Action Committee). Susceptibility Test Methods Series, Version: 4 (July 2019). Available from http://www.irac-online.org (accessed 23 November 2024)
- IRAC 2025. Mode of Action Classification Scheme, Version 11.3, January 2025. IRAC (Insecticide Resistance Action Committee) International MoA Working Group.
- Jayasinghe G. G., Mallik B. 2011. Growth stage based economic injury levels for two spotted Spider Mite, Tetranychus urticae Koch (Acari; Tetranychidae) on tomato, Lycopersicon esculentum Mill. J. Trop. Agric., 22(1): 54-65. https://doi.org/10.4038/tar.v22i1.2670
- Karmakar K., Gupta S. K. 2011. Predatory mite fauna associated with Agri-horticultural crops and weeds from the Gangetic Plains of West Bengal, India. Zoosymposia., 6: 62-67. https://doi.org/10.11646/zoosymposia.6.1.11
- Khanamani M., Fathipour Y., Hajiqanbar H. 2015. Assessing compatibility of the predatory mite Typhlodromus bagdasarjani (Acari: phytoseiidae) and resistant eggplant cultivar in a tritrophic system. Ann. Entomol. Soc. Am., 108:501-512. 2 https://doi.org/10.1093/aesa/sav032
- Khanamani M., Fathipour Y., Talebi A. A., Mehrabadi M. 2017. Evaluation of different artificial diets for rearing the predatory mite Neoseiulus californicus (Acari: Phytoseiidae): diet-dependent life table studies. Acarologia., 57(2): 407-419. https://doi.org/10.1051/acarologia/20174165
- Kim M., Shin D., Suh E., Cho K. 2004. An assessment of the chronic toxicity of fenpyroximate and pyridaben to Tetranychus urticae using a demographic bioassay. Appl. Entomol. Zool., 39:401-409. https://doi.org/10.1303/aez.2004.401
- Kim S. S., Paik H. C. 1996. Comparative toxicity of fenpyroximate to the predatory mite, Amblyseius womersleyi Schicha and the kanzawa spider mite, Tetranychus kanzawai Kishida (Acarina: Phytoseiidae, Tetranychidae). Appl. Entomol. Zool., 31(3): 369-377. https://doi.org/10.1303/aez.31.369
- Laborda R. I., Manzano M., Gamón I., Gavidia P., Pérez-Bermúdez, Boluda R. 2013. Effects of Rosmarinus officinalis and Salvia officinalis Essential Oils on Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crop Prod., 48: 106-110. https://doi.org/10.1016/j.indcrop.2013.04.011
- Lee K. W., Lee J. H. 2010. Life table and predation capacity of Neoseiulus longispinosus (Evans) (Acari: Phytoseiidae) on Tetranychus urticae. J. Asia-Pac. Entomol., 13(3): 221-225.
- McMurtry J. A., Scriven, G. T. 1965. Insectary production of phytoseiid mites. J. Econ. Entomol., (2): 282-284. https://doi.org/10.1093/jee/58.2.282
- McMurtry J. A., Moraes G. J., Sourassou N. F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Messelink G. J., van Maanen R., van Steenpaal S. E., Janssen A. 2008. Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Bio Control., 44:372-379. https://doi.org/10.1016/j.biocontrol.2007.10.017
- Mollaloo M. G., Kheradmand K., Sadeghi B. R., Talebi A. A. 2017. Demographic analysis of sublethal effects of spiromesifen on Neoseiulus californicus (Acari: Phytoseiidae). Acarologia., 57(3): 571-580. https://doi.org/10.24349/acarologia/20174173
- Moscardini V. F., da Costa Gontijo P., Carvalho G. A., de Oliveira R. L., Maia J. B., Silva F. F. 2013. Toxicity and sublethal effects of seven insecticides to eggs of the flower bug Orius insidiosus (Say) (Hemiptera: Anthocoridae). Chemosphere., 92(5): 490-496. https://doi.org/10.1016/j.chemosphere.2013.01.111
- Mousavi A., Kheradmand K., Fathipour Y., Mosallanejad H., Havasi M. 2023. The effects of the abamectin and spirodiclofen mixture on life history and population parameters of Amblyseius swirskii. Syst. Appl. Acarol., 28(5): 971-984. https://doi.org/10.11158/saa.28.5.16
- Musa A., Međo I., Marić I., Marcic D. 2022. Transovarial toxicity matters: lethal and sublethal effects of hexythiazox on the two-spotted spider mite (Acari: Tetranychidae). Exp. Appl. Acarol., 87: 175-194. https://doi.org/10.1007/s10493-022-00733-8
- Niaki S. N., Golpayegani A. Z., Torabi E., Saboori A., Amiri-Besheli B., Havasi M. 2025. Sublethal effects of chlorfenapyr on biological parameters of Tetranychus urticae (Acari: Tetranychidae) and predatory mites Phytoseiulus persimilis and Neoseiulus californicus (Acari: Phytoseiidae). Syst. Appl. Acarol., 30(4): 754-769. https://doi.org/10.11158/saa.30.4.8
- Rahmani H., Aharizad S. 2019. Sublethal effects of pyridaben on population parameters of Amblyseius swirskii. Int. J. Acarol., 45(1): 45-50.
- Sabelis M. W. 1982. Biological control of two-spotted spider mites using phytoseiid predators. Wageningen University and Research.
- Saber M., Ahmadi Z., Mahdavinia G. 2018. Sublethal effects of spirodiclofen, abamectin and pyridaben on life-history traits and life-table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol., 75(1): 55-67. https://doi.org/10.1007/s10493-018-0226-2
- Sanatgar E., Shoushtari R. V., Zamani A. A, Nejadian E. S. 2011. Effect of frequent application of hexythiazox on predatory mite Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae). J. Entomol., 4(3): 94-101.
- Sandeep V., Chinmayi S., Bellanki A. 2024. Two-spotted spider mite: Biology, damage symptoms and integrated pest management in crop ecosystem. Vigyan Varta., 5: 163-165.
- Sibly R. M., Calow P. 1989. A life-cycle theory of responses to stress. Biol. J. Linn. Soc., 37(1-2): 101-116. https://doi.org/10.1111/j.1095-8312.1989.tb02007.x
- Sparks T. C., Crouse G. D., Durst G. 2012. Natural products as insecticides: The biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pestic. Biochem. Physiol., 102(3): 168-174.
- Stark J. D., Banks J. E. 2003. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol., 48: 505-519. https://doi.org/10.1146/annurev.ento.48.091801.112621
- Taghizadeh R., Chi H. 2022. Demography of Tetranychus urticae (Acari: Tetranychidae) under different nitrogen regimes with estimations of confidence intervals. Crop Prot., 155: 105920. https://doi.org/10.1016/j.cropro.2022.105920
- Tuan S. J., Lin Y. H., Yang C. M., Atlihan R., Saska P., Chi H. 2016. Survival and reproductive strategies in two spotted spider mites: demographic analysis of arrhenotokous parthenogenesis of Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol., 109: 502-509. https://doi.org/10.1093/jee/tov386



2025-07-15
Date accepted:
2025-08-27
Date published:
2025-09-03
Edited by:
Marčić, Dejan

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Sandeep, Veerabhadrappa; Rajashekharappa, Kenchappa; Sharanabasappa S. Deshmukh; Jayalaxmi Narayan Hegde; Nagarajappa Adivappar and Mallikarjuna, Hosamane Basavarajappa
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



