Two new species of gall mites (Acariformes, Eriophyoidea) from ferns (Polypodiopsida) from South Africa and Vietnam
Chetverikov, Philipp E.  1
; Craemer, Charnie
1
; Craemer, Charnie  2
; Gankevich, Vladimir D.
2
; Gankevich, Vladimir D.  3
; Kudryavtzev, Andrey T.4
; Desnitskiy, Alexey G.
3
; Kudryavtzev, Andrey T.4
; Desnitskiy, Alexey G.  5
; Le, Thi Tuyet Nhung
5
; Le, Thi Tuyet Nhung  6
; Nguyen, Duc Viet
6
; Nguyen, Duc Viet  7
and Trinh Xuan, Hoat
7
and Trinh Xuan, Hoat  8
8
1✉ Saint Petersburg State University, Universitetskaya Nab. 7/9, St. Petersburg, 199034, Russia & Zoological Institute of Russian Academy of Sciences, Universitetskaya Nab. 1, Saint Petersburg, 199034, Russia.
2Landcare Research, 231 Morrin Road, Auckland, 1072, New Zealand.
3Zoological Institute of Russian Academy of Sciences, Universitetskaya Nab. 1, St. Petersburg, 199034, Russia.
4Zoological Institute of Russian Academy of Sciences, Universitetskaya Nab. 1, St. Petersburg, 199034, Russia & St. Petersburg State Agrarian University, Peterburgskoe shosse 2, Pushkin, St. Petersburg, 196601, Russia.
5Saint Petersburg State University, Universitetskaya Nab. 7/9, St. Petersburg, 199034, Russia.
6Plant Protection Research Institute, 10 Pho Vien, Duc Thang ward, Bac Tu Liem district, Hanoi City, Vietnam.
7Plant Protection Research Institute, 10 Pho Vien, Duc Thang ward, Bac Tu Liem district, Hanoi City, Vietnam.
8Plant Protection Research Institute, 10 Pho Vien, Duc Thang ward, Bac Tu Liem district, Hanoi City, Vietnam.
2025 - Volume: 65 Issue: 3 pages: 868-886
https://doi.org/10.24349/9qu7-i1j8ZooBank LSID: D48F0248-D9DF-47BB-83BE-D2A368788288
Original research
Keywords
Abstract
Introduction
Eriophyoid mites (Eriophyoidea Nalepa, 1898) are an ancient lineage of highly host-specific phytoparasites closely related to soil mites of the family Nematalycidae Strenzke, 1954 (Bolton et al. 2017; Klimov et al. 2018, 2022). In extant ecosystems, they exhibit peak species richness on angiosperms, with significantly fewer representatives on conifers and ferns (Gerson 1996; Amrine et al. 2003). Evolutionary evidence indicates that eriophyoid mites originated as symbionts of ancient land plants, with their earliest associations likely occurring on now-extinct lineages such as seed ferns, progymnosperms, and proangiosperms (Sukhareva 1994; Sidorchuk et al. 2014; Klimov et al. 2025). The most morphologically archaic extant eriophyoids of the family Pentasetacidae Schliesske, 1985, which retain a complete set of plesiomorphic traits, are associated with ancient coniferous hosts in the genera Araucaria and Cupressus (Chetverikov et al. 2019). While theoretically, similar relict Eriophyoidea taxa could have persisted on ferns (Polypodiopsida), no pentasetacid-like eriophyoids have been documented on these hosts (Gerson 1996; Lotfollahi and de Lillo 2017). Recent molecular phylogenetic analyses – albeit limited in taxon sampling – suggest that fern-associated eriophyoid lineages evolved through secondary host shifts from non-fern hosts (Li et al. 2014; Chetverikov et al. 2023; Zhang et al. 2024).
At present, ~40 eriophyoid mites species from 20 genera (Aceria, Acerimina, Aculus, Calacarus, Cosella, Cymeda, Dicolopodacus, Diphytoptus, Eriocaenus, Eriophyes, Esalquia, Floracarus, Jutarus, Leipothrix, Litaculus, Metaculus, Nothopoda, Phyllocoptes, Rhinophytoptus, and Stenacis) are known from ferns (Petanović et al. 2015; Lotfollahi and de Lillo 2017; Chetverikov et al. 2023). The greatest generic diversity of fern-associated eriophyoid mites occurs on fern orders Polypodiales and Cyatheales, followed by Equisetales, Gleicheniales, and Schizaeales, whereas none have been described from Marattiales (Petanović et al. 2015). Most eriophyoid mite species inhabiting ferns belong to the subfamilies Phyllocoptinae (~45%), Eriophyinae (~34%), and Nothopodinae (~18%) of the family Eriophyidae. Only one species (Rhinophytoptus plagiogyrus Huang, 2001 from Plagiogyria formosana Christ) reperesents the family Diptilomiopidae. Approximately half of the known fern-associated eriophyid mite species are vagrants, causing no visible damage to their hosts. The other species induce various frond distortions (including marginal rolling, discoloration, thickening, and curling) or, more rarely, form erinea, galls, or witches' brooms (Gerson 1996; Lotfollahi and de Lillo 2017).
From 2017 to 2024, a series of expeditions were conducted to study the eriophyoid fauna of South Africa and Vietnam. These field surveys were organized by the ARC Plant Protection Research Institute (Roodeplaat, Gauteng, South Africa) and Plant Protection Research Institute (Hanoi, Vietnam) in collaboration with Saint-Petersburg State University (Russia). During these surveys, two new species of eriophyoid mites of the family Eriophyidae were discovered on ferns of the genera Ptisana (Marattiales) and Nephrolepis (Polypodiales). In this paper, we present descriptions of these species, supported by partial sequences of the mitochondrial Cox1 and nuclear 28S rRNA genes, and photographs of the damage symptoms caused to the infested host plants. Additionally, we document the stages of gallogenesis on Nephrolepis through a series of sequential images.
Material and methods
Collection and morphological measurements
Eriophyoid mites were collected from ferns in South Africa (2017) and Vietnam (2023–2024) using a fine minute pin under a stereomicroscope. They were kept in Eppendorf tubes filled with 96% ethanol in a freezer at −20\, °C prior to transport to the Zoological Institute of the Russian Academy of Sciences (ZIN RAS), where the mites were mounted in modified Berlese medium with iodine (Amrine and Manson 1996) and cleared on a heating block at 90 °C for 3 hours. The slide-mounted specimens were examined with differential interference contrast light microscopy (DIC LM) and phase contrast light microscopy (PC LM) in the Laboratory of Parasitic Arthropods of the ZIN RAS. In the species description, measurements of the holotype (female) are presented along with ranges based on measurements of paratype females; in the descriptions of males, only ranges are given. All measurements are given in micrometers (μm) as lengths unless stated otherwise. The classification and terminology of eriophyoid genital anatomy and external morphology follow that of Amrine et al. (2003); Chetverikov (2014), and Lindquist (1996), respectively. Drawings of the mites were produced with the aid of a video projector as described by Chetverikov (2016). Host plant taxonomy follows Senterre et al. (2014) and Hovenkamp and Miyamoto (2005).
DNA extraction, PCR and sequencing
For DNA extraction, 1‒3 mite specimens of each species were crushed with a fine pin in a 3 μL drop of distilled water on a cavity well microscope slide. The drop was then pipetted into a thin-walled PCR tube containing 20 μL of a 5% solution of Chelex® 100 Resin (Bio-Rad Laboratories, Inc., Hercules, California, USA) followed by two heating steps (5 min at 95 °C). The supernatant above the settled Chelex® granules was used as the DNA template for PCR to amplify a fragment of subunit I of the Cox1 gene and the D1D2 fragment of the 28S rDNA gene. Thermal cycling profiles and primers (for both PCR and sequencing) followed those specified by Chetverikov and Bertone (2022). After amplification, 4.5 μL of each reaction product was mixed with 0.5 μL of SYBR Green I (Lumiprobe, Hannover, Germany) and analyzed by electrophoresis in a 1% agarose gel to assess product size and concentration. Sequencing was performed using BigDye Terminator v.3.1 chemistry in a 3500xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Results
Family Eriophyidae Nalepa, 1898
Subfamily Eriophyinae Balepa, 1898
Tribe Aceriini Amrine & Stasny, 1994
Genus Cymoptus Keifer, 1946
Remarks — This genus is very close to the large genus Aceria Keifer, 1944. Unlike Aceria, Cymoptus lacks tibial seta l′ I. Keifer (1946) and Amrine et al. (2003) reported that dorsal opisthosomal annuli in Cymoptus are smooth and sinuate, whereas ventral annuli are reinforced with spine-like microtubercles. These traits (undulation of annuli and spine-like shape of microtubercles) are variable at the species level, e.g. Cymoptus bengalensis Ghosh, Mondal and Chakrabarti, 1984 lacks annular undulation and spine-like microtubercles. Additionally, in various eriophyoid taxa deutogyne and protogyne forms of the same species differ in the shape of opisthosomal annuli and microtubercles (Manson and Oldfield 1996; Sukhareva and Chetverikov 2013). We propose relaxing the generic concept of Cymoptus by excluding characteristics of the opisthosomal annuli from its diagnosis, and treating Cymoptus as a taxon with all morphological traits typical of the genus Aceria, except for the absence of tibial seta l′ I. Although future molecular phylogenetic studies may reveal non-monophyly of the ''relaxed» Cymoptus, we consider this interpretation convenient for the systematics of Eriophyoidea.
Type species — Cymoptus spiniventris Keifer, 1946
Species included — Five (Table 1).
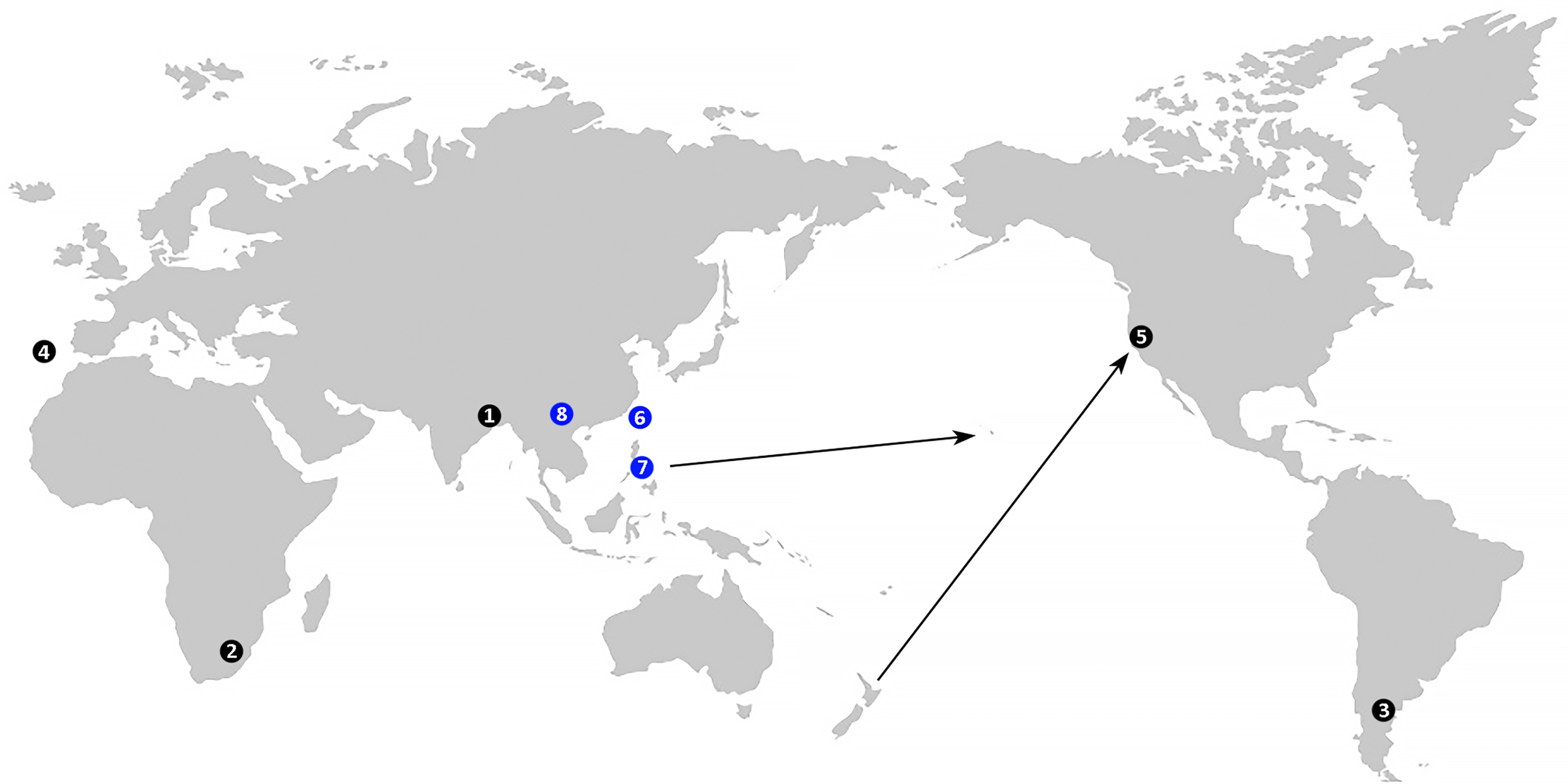
Hosts — Four Cymoptus spp. are known from eudicot angiosperms (Nothofagaceae, Euphorbiaceae, Lauraceae), and one species (C. ptisanis n. sp.) from ferns (Marattiaceae).
Relation to hosts — Except for one presumably vagrant species (C. bengalensis), the other four Cymoptus spp. are associated with one of two types of plant growth abnormalities: erineum (C. spiniventris, C. ptisanis n. sp., C. vieirai) and withches' broom (C. waltheri).
Deuterogyny — Based on material from New Zealand, Manson (1984) reported deuterogyny in C. waltheri (Keifer, 1939). No data on deuterogyny in other Cymoptus spp.
Distribution — (Figure 1). Current data on the global distribution of Cymoptus spp. remain limited. Records include findings on native hosts in type localities in Argentina, India, Portugal, and South Africa, as well as one record from the USA, where C. waltheri was detected on Nothofagus menziesii introduced from New Zealand (Keifer 1939). Additionally, C. waltheri has been reported in New Zealand on native Nothofagus spp. (Manson 1984).


Download as
Mite species
Host plant
Relation to host
Type locality
Reference
C. bengalensis Ghosh, Mondal and Chakrabarti, 1984
Mallotus repandus (Rottler et Willd.) Muell. (Euphorbiaceae)
vagant on lower leaf surface
West Bengal, India
Ghosh et al. (1984)
C. ptisanis n. sp.
Ptisana salicifolia (Schrad.) Senterre et Rouhan (Marratiaceae)
in erineum around sporangia on lower surface of fronds
South Africa
this paper
C. spiniventris Keifer 1946
Nothofagus dombeyi (Mirb.) Oerst. (Nothofagaceae)
erineum on lower leaf surface
Argentina
Keifer (1946)
C. vieirai Carmona, 1988
Apollonias barbujana (Cav.) Bornm. (Lauraceae)
Inhabiting patches and felty erineum with Eriophyes barbujanae Carmona, 1988 on the lower leaf surface
Madeira Island, Portugal
Carmona (1988)
C. waltheri (Keifer, 1939)
Nothofagus menziesii Orst. (Nothofagaceae)
terminal stunting and branching, resulting in a heavy cluster of large, aborted buds (withches’ broom)
California, USA
Keifer (1939)
Cymoptus ptisanis n. sp.
ZOOBANK: 32074337-C04A-45F0-B20C-5D90E2498CCE ![]()
Figures 2 – 5
Female (n = 11)
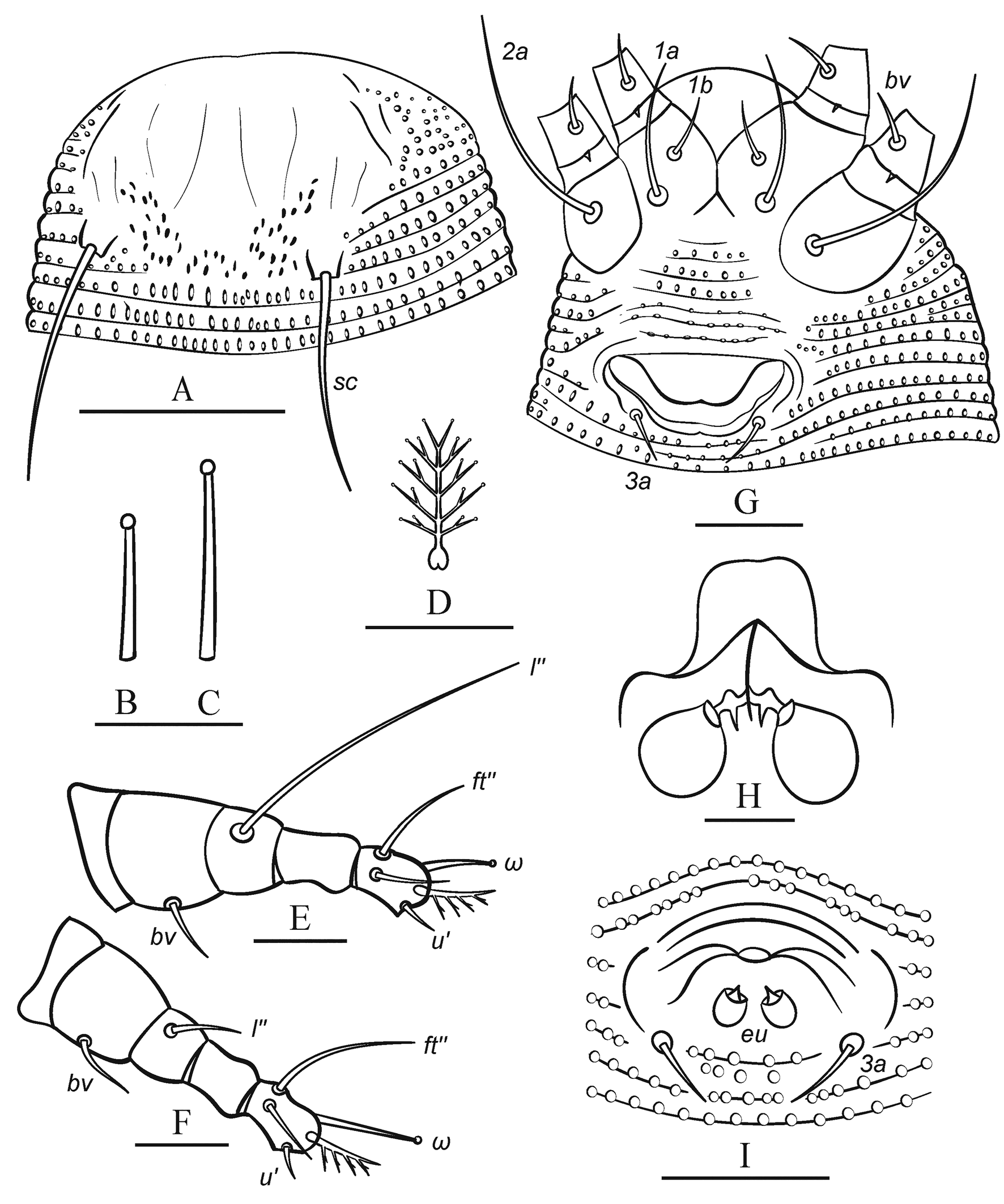


Idiosoma elongate, vermiform, whitish, 143 (130─164), 49 (38─55) wide at level of seta c2. Prodorsal shield semicircular, 19 (17─22), 30 (27─33) wide, without frontal lobe. Prodorsal shield ornamentation weak consisting of faint longitudinal lines (representing putative traces of admedians and submedians) and a group of about 30–50 distinct microtubercules in the posteromedial part of prodorsal shield between tubercles of sc. Scapular setae sc 23 (19─26), 22 (21─23) apart, directed divergently posteriad. Gnathosoma directed anteriad and slightly downward, 18 (16─21), ep 2 (1─3), d 8 (6─10), ν 2 (2─3); chelicera 15 (13─19).
Leg I 28 (25─31), femur 7 (6─8), bv 4 (3─6), genu 3 (3─5), l″ 19 (17─22), tibia 5 (4─6), l′ absent; tarsus 5 (4─6), u′ 4 (3─6), ft′ 4 (3─6), ft″ 10 (8─14), ω 6 (5─7) with small subspherical knob; empodium 5‒rayed, 4 (4─6). Leg II 27 (24─28), femur 6 (5─7), bv 3 (2─5), genu 3 (3─5), l″ 7 (5─9), tibia 5 (4─5); tarsus 4 (4─6), u′ 2 (2─3), ft′ 4 (3─7), ft″ 11 (7─16), ω 6 (5─7) with small subspherical knob; empodium 5‒rayed, 4 (4─5).
Infracapitular plate subtriangular, rounded anteriorly, smooth, 10 (8─13), 10 (9─13) wide; the prosternal apodeme is indistinct in most specimens but appears forked posteriorly when clearly visible.
Coxal plates smooth or with several faint curved ridges. Setae 1b 4 (3─6), 8 (7─11) apart; 1a 15 (10─17), 10 (9─12) apart; 2a 20 (17─25), 21 (20─25) apart; 6 (5─7) incomplete coxigenital annuli bearing tiny round microtubercles before epigynium.


External genitalia. Genital coverflap, smooth, narrow, 11 (9─13), 20 (19─23) wide; setae 3a 3 (3─5), 12 (10─13) apart; pregenital plate (sensu Flechtmann et al. 2015) absent.
Internal genitalia (n=4). Spermathecae globose or tear-drop shaped, 5–8 in diameter; spermathecal tubes short, about 2─3, 2─3 wide, directed laterad; rudimentary spermathecal process (sensu Duarte et al. 2016), associated with spermathecal tube, present and about 1─2; longitudinal bridge 7─9, anterior (transverse) genital apodeme trapezoidal; oblique apodeme distinct.
Opisthosoma with 69 (63─75) dorsal and 70 (67─77) ventral annuli. Dorsal and ventral annuli with distinct oval microtubercles. Dorsally they are present on anterior 60 annuli, and absent on rear 9─10 annuli. Setal lengths: c2 29 (20─36), d 32 (26─40), e 5 (4─8), f 11 (11─14); h1 2 (1─2); h2 43 (38─50); 15 (11─17) annuli from rear shield margin to c2; 12 (9─12) annuli between c2–d; 12 (9─14) annuli between d and e; 24 (22─27) annuli between e and f; and 8 (8─11) annuli between f and h2.
Male (n = 3)
Idiosoma fusiform, whitish, 119─126, 42─46 wide. Prodorsal shield 17─21, 27─31 wide. Prodorsal shield ornamentation similar to that in female. Scapular setae sc 19─20, 19─22 apart. Gnathosoma 16─18, ep 1─2, d 6─9, ν 1─2; chelicera 11─16.
Leg I 25─30, femur 6─7, bv 3─4, genu 3─4, l″ 14─20, tibia 4─5, l′ absent; tarsus 4─5, u′ 2─3, ft′ 3─4, ft″ 10─12, ω 6─7 with small subspherical knob; empodium 5‒rayed, 4─5. Leg II 26─28, femur 5─7, bv 2─4, genu 3─4, l″ 5─8, tibia 4─5; tarsus 3─4, u′ 2─3, ft′ 3─4, ft″ 11─13, ω 6─8 with small subspherical knob; empodium 5‒rayed, 4─5.
Coxal plates smooth, 1b 2─3, 6─9 apart; 1a 5─9, 9─11 apart; 2a 15─18, 18─20 apart; 6─7 incomplete coxigenital annuli before epiandrium.
External genitalia. Genital area about 10─12, 14─16 wide; setae 3a 3─4, 9─12 apart; eu about 0.5─1.
Opisthosoma with 61─68 dorsal and 61─64 ventral annuli microtuberculated similar to those in female. Setal lengths: c2 23─28, d 25─33, e 4─6, f 9─11, h1 2─3, h2 39─46; 9─14 annuli from rear shield margin to c2; 7─10 annuli between c2–d; 10─12 annuli between d and e; 20─24 annuli between e and f; 7─8 annuli between f and h2.
Gen Bank data
PV698369 (Cox1, 1151 bp), PV711119 (28S, 1009 bp).
Host plant
Ptisana salicifolia (Schrad.) Senterre and Rouhan (Polypodiopsida: Marattiales: Marratiaceae).
Relation to the host
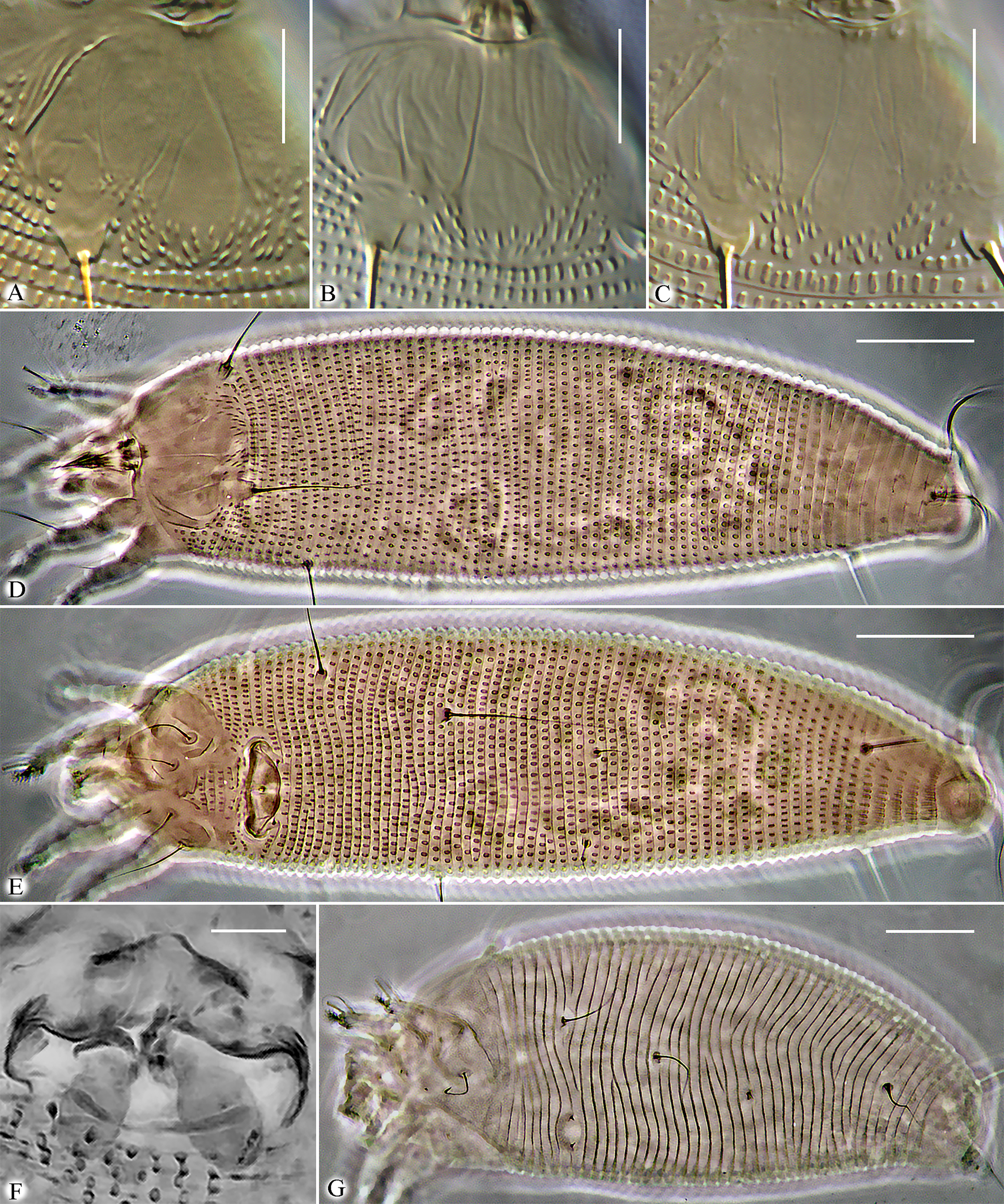
Mites live in brownish erinea, which they cause around synangia on lower surface of frond pinnae (Figure 4).



Remarks — Erinea were found exclusively around synangia of P. salicifolia, indicating that mites (a) can locate and distinguish these structures and (b) selectively target epidermal cells near synangia while ignoring other cells. Intriguingly, a similar ability to induce morphologically distinct galls in specific leaf zones is known in eriophyoid mites associated with angiosperms, for example, Eriophyes spp. galls on Tilia spp. (Soika and Kozak 2013). Our long-term observations suggest that eriophyoid mites very often move along veins and possibly recognize them when crossing. The mites' capacity to detect veins (via sensory structures on their distal leg segments and gnathosoma) and follow them may be key to locating precise regions on fern fronds or angiosperm leaves for inducing galls. Future experimental work should explore how eriophyoids identify suitable sites for gall induction. In P. salicina, colonization by C. ptisanis n. sp. produces a distinctive dark, ring-like epidermal area surrounding synangia (Figure 4D, arrows). A similar ring-like zone appears during early-stage chambered gall formation in certain angiosperms (e.g., Betula or Alnus; see fig. 1a, c in Desnitskiy et al. 2024). Whether these rings are structurally and genetically homologous remains unclear. Comparative histological and genetic studies of eriophyoid galls on ferns and angiosperms could resolve this question.
Type locality
SOUTH AFRICA: Mpumalanga Prov., near Sabie, Bridal Veil Falls, 25°04′55.2″S 30°43′29.2″E (Figure 5).



Type material
Holotype female in slide #M110-1, 18 paratypes on slide series #M110, collected on 14 November 2017 at the type locality, coll. P. Chetverikov, C. Craemer, S. Neser. Type material is deposited in Acarological collection of ZIN RAS.
Etymology
The species epithet ptisanis is an adjective, gender masculine, derived from the generic name of the host plant, Ptisana.
Differential diagnosis
Among other Cymoptus spp., the new species is closest to C. waltheri (Keifer, 1939). The main differences concern ornamentation of prodorsal shield and number of empodial rays. In C. walteri a short median line is present in posterior half of the shield, the admedian lines are long and complete, the internal submedian lines converge anteriorly, the cuticle between tubercles of sc is smooth, and the empodium is 3-rayed (Keifer 1939, Plate LXXXIV). In C. ptisanis n. sp. median line is absent; submedian lines faint, non-converging; the cuticle between tubercles of sc with 30–50 distinct microtubercles; and the empodium is 5-rayed.
Subfamily Nothopodinae Keifer, 1956
Tribe Nothopodini Keifer, 1956
Genus Nothopoda Keifer, 1951
Remarks on the generic diagnosis of Nothopoda — This genus is poorly distinguished from another nothopodine genus, Floracarus Keifer, 1953. They differ only in the presence (in Floracarus) and absence in (Nothopoda) of a relatively small subtriangular frontal lobe of the prodorsal shield (Keifer 1953, p. 69; Amrine et al. 2003). Presence or absence of this structure is a weak discriminating character, also varying among members of different eriophyid genera (e.g. in Aceria and Eriophyes). Recent phylogenies based on limited set of nothopodine taxa, did not support monophyly of Nothopoda and inferred Floracarus a basal taxon in a clade comprising three nothopodine genera – Nothopoda, Cosella, and Floracarus (Chetverikov et al. 2023). In this study, we follow the traditional concept of Nothopoda as a Floracarus-like taxon lacking a frontal lobe. However, future morphological revisions and molecular phylogenetic studies may confirm synonymy of Nothopoda and Floracarus.
Nothopoda vietnamensis n. sp.
ZOOBANK: B7C38800-01F0-4069-9D12-7785DBFE6615 ![]()
Figures 6 – 7
Female (n = 19)
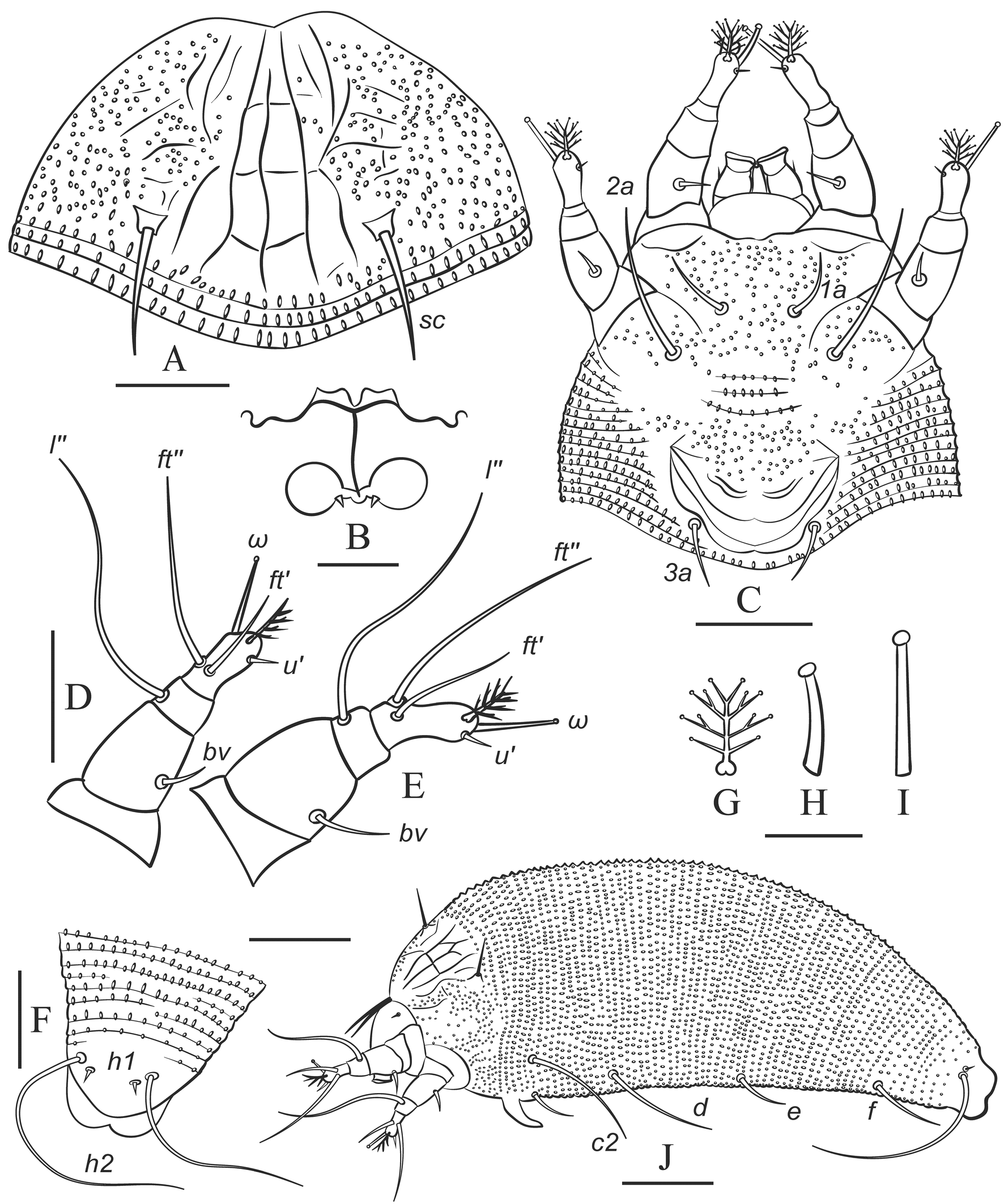


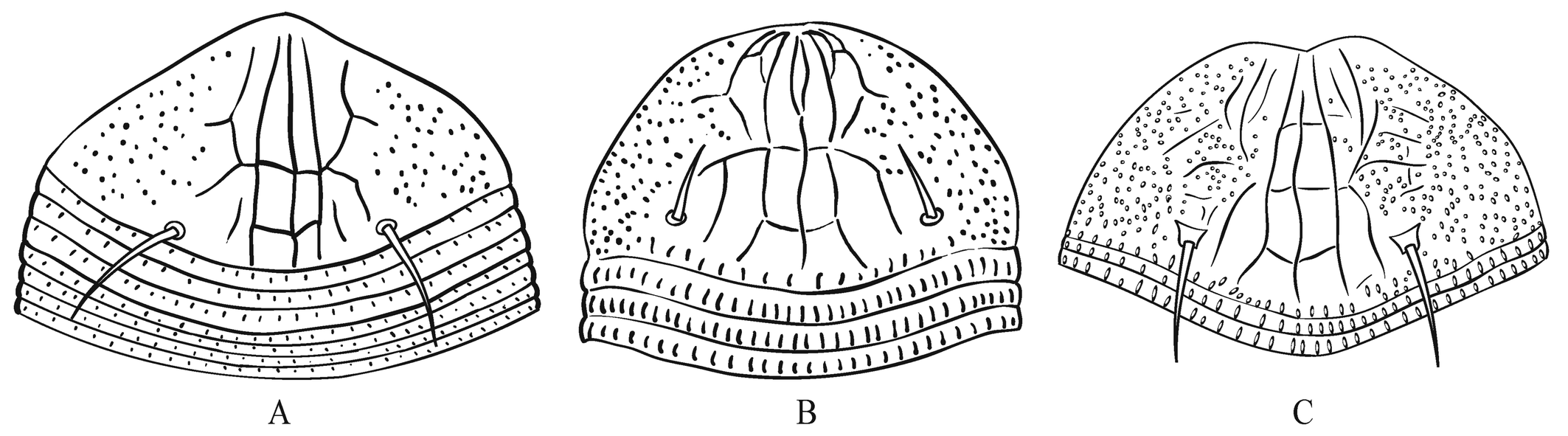
Idiosoma elongate, vermiform, white to slightly yellowish, 195 (170─241), 55 (50─62) wide at the level of seta c2. Prodorsal shield subtriangular, rounded and slightly notched anteriorly, 28 (24─30), 36 (30─40) wide, without frontal lobe. Prodorsal shield with variable linear ornamentation in medial part. Lateral parts of prodorsal shield with numerous microtubercles. Most commonly, the linear pattern is subsymmetrical and includes median line, two admedians, submedian I and a series of longitudinal and transversal lines. Median line entire, slightly sinuous, incomplete (absent in anterior 1/5 part of prodorsal shield). Admedians entire, complete, converging anteriorly. Usually three short transverse lines crossing median line are present between admedians. Submedian line I broken, consisting of two approximately equal fragments. Three to six short transverse lines present near anterior fragment of submedian I. Some females possess aberrant ornamentation of prodorsal shield with median line broken and additional transverse lines present and forming cells along anterior margin of prodorsal shield and between long lines (Figure 7). Scapular setae sc 9 (7─12), 21 (17─24) apart, directed dorsally and slightly posteriad. Gnathosoma directed anteriad and obliquely downward, 19 (18─23), ep 2 (1─3), d 2 (2─3), simple, ν 2 (1.5─2); chelicerae 13 (11─14).
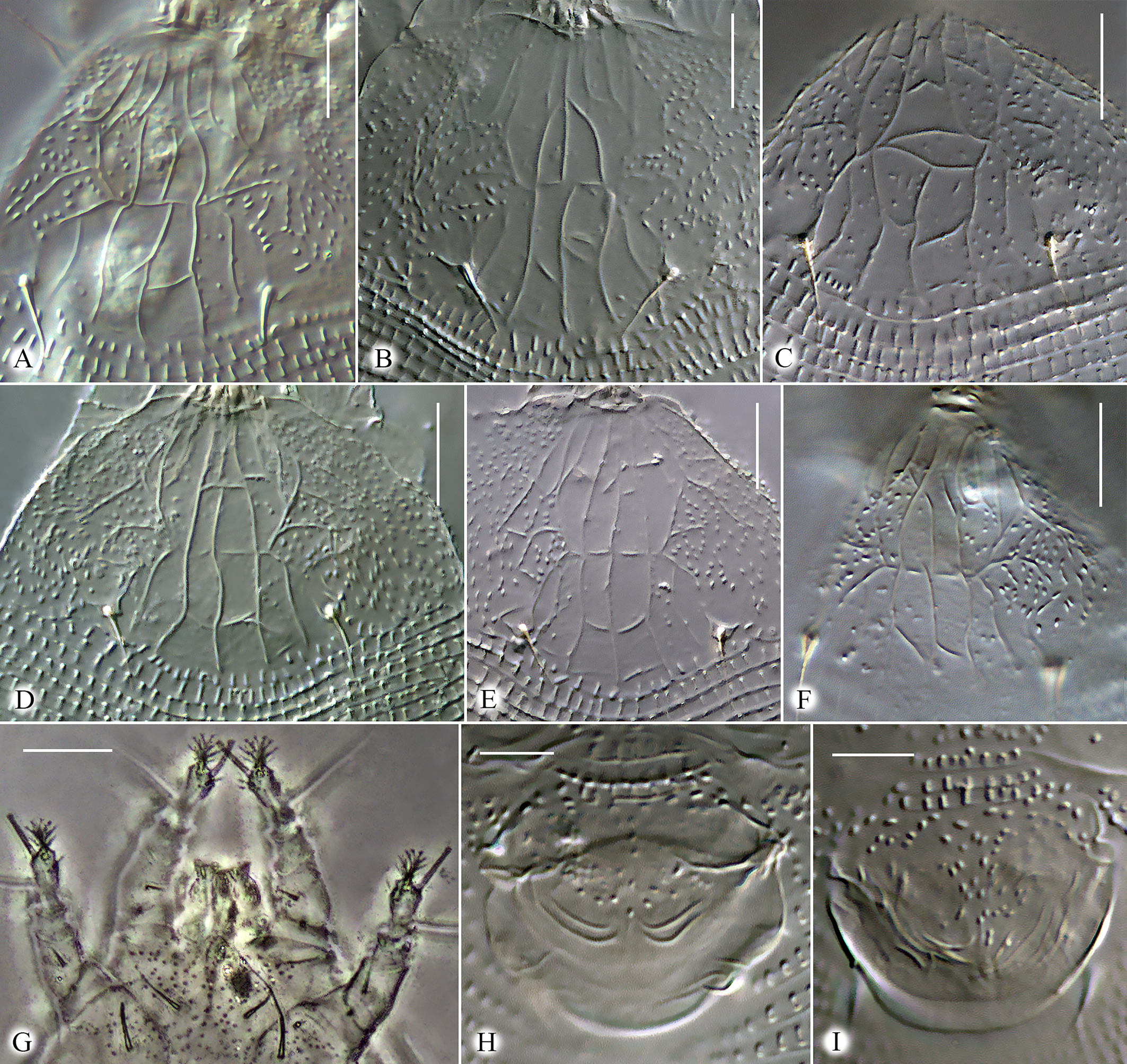


Leg I 35 (30─39), femur 9 (9─10), bv 5 (4─8), genu 4 (3─4), l″ 23 (20─30), tibia absent (or fused with tarsus), l′ absent; tarsus 7 (7─8), u′ 2 (1─2), ft′ 8 (4─10), ft″ 19 (15─24), ω 7 (5─8) with small subspherical knob, displaced on medially; empodium 4‒rayed, 5 (4─6) long. Leg II 33 (29─36), femur 9 (8─9), bv 4 (3─5), genu 3 (3─4), l″ 20 (17─26), tibia absent (or fused with tarsus); tarsus 7 (7─8), u′ 2 (1─2), ft′ 4 (3─6), ft″ 19 (15─23), ω 7 (5─8) with small subspherical knob, commonly located in dorsal position above empodium; empodium 4‒rayed, 5 (4─6) long.
Infracapitular plate suboval, rounded anteriorly, smooth or with indistinct small microtubercles, 7 (6─9), 11 (10─13) wide; prosternal apodeme absent.
Coxal plates with numerous small microtubercles. Setae 1b absent; 1a 9 (7─12), 9 (8─10) apart; 2a 20 (17─28), 21 (18─22) apart; 4 (3─7) incomplete coxigenital annuli bearing tiny, round microtubercles anterior to the epigynium.
External genitalia. Genital coverflap with a group of microtubercles in medio-proximal region and in most females with two paired symmetrically located transverse ridges near distal margin of coverflap (Figure 7 H), which in some individuals may be replaced by several thinner ridges (Figure 7 I), 11 (9─13), 20 (19─23) wide; setae 3a 5 (4─8), 16 (14─17) apart; basal part of epigynium and pregenital area with numerous tiny microtubercles.
Internal genitalia (n=5). Spermathecae globose, 6–8 in diameter; spermathecal tubes short, about 2─3, 1─3 wide, directed laterad; rudimentary spermathecal process (sensu Duarte et al. 2016), associated with spermathecal tube, present and about 0.5–1 long; longitudinal bridge 11─14, anterior (transverse) genital apodeme consists of two fragments separated with a notch, putatively situated within the transverse plane (orthogonal to the longitudinal body axis, as in cecidophyines); oblique apodeme distinct, sinuous, almost orthogonal to longitudinal bridge.
Opisthosoma with 73 (65─76) dorsal and 74 (68─79) ventral annuli. Dorsal and ventral annuli with distinct oval microtubercles. Setal lengths: c2 25 (20─30), d 43 (33─49), e 5 (4─7), f 16 (14─20); h1 2 (2─3); h2 48 (38─59); 8 (5─9) annuli from rear shield margin to c2; 14 (10─16) annuli between c2–d; 18 (15─19) annuli between d and e; 23 (20─25) annuli between e and f; and 9 (7─10) annuli between f and h2.
Gen Bank data
PV698368 (Cox1, 1161 bp), PV711118 (28S, 1005 bp).
Host plant
Nephrolepis cordifolia (L.) C.Presl (Polypodiales: Nephrolepidaceae).
Relation to the host
Mites live in galls on frond pinnae (Figures 8, 9).



Remarks — The gall formation process in N. cordifolia resembles the development of marginal leaf-rolling galls induced by eriophyoid mites on leaves of various dicotyledonous plants. These pathological morphogenetic processes in ferns and angiosperms may share elements of genetic developmental programs common across different phylogenetic lineages of land plants, which are exploited by gall-inducing mites. Remarkably, recent studies on regulatory gene expression in Fragaria viridis (Rosaceae) leaf galls caused by the eriophyoid mite Fragariocoptes setiger (Nalepa, 1894) (Eriophyoidea: Phytoptidae) revealed localized alterations in leaf epidermis dorsoventral polarity (Paponova et al. 2021). Future research should examine whether similar polarity changes occur in eriophyoid mite galls on other hosts, including ferns.



Type locality
VIETNAM: Tam Dao, near Silver waterfall (Figure 10), 21°27′13.2″N 105°38′38.1″E



Type material
Holotype — female in slide #V21-1, 25 paratypes on slide series #V21 and #V23, collected 22 February 2023 in type locality, coll. P. Chetverikov and Viet D. Nguyen. Type material deposited in Acarological collection of ZIN RAS.
Additional material — Numerous adults and immatures in slide series #V61 and #V62 collected 8 March 2024 in VIETNAM: Moc Chau, 20°50′56.7″N 104°39′55.4″E, coll. P. Chetverikov and Viet D. Nguyen.
Etymology
The species epithet vietnamensis, gender feminine, is an adjective derived from ''Vietnam'', indicating the country where the type locality of the new species is situated.
Differential diagnosis
The new species, Nothopoda vietnamensis n. sp., is morphologically very close to Floracarus footei Keifer, 1953 (assigned to Nothopoda by Amrine and Stasny (1994)) and F. biserratae Huang, 2001. These three species have been recorded from Southeast Asia (Figure 1) parasitizing ferns of the genus Nephrolepis (Polypodiales: Nephrolepidaceae) and causing presumably similarly shaped galls on pinnae (Table 2). Considering high morphological variability observed in N. vietnamensis n. sp. and possible synonymy of Nothopoda and Floracarus (see above), the three species discussed may either belong to one polymorphic widely distributed olygophagous species or represent a complex of geographically isolated species, discrimination of which is possible only based on DNA comparison. Because molecular data on F. footei and F. biserratae is unavailable, following morphological concept of species, here we treat the Vietnamese mites as a new taxon and differentiate it from F. footei and F. biserratae based on ornamentation of prodorsal shield, width of first three dorsal opisthosomal annuli and presence/absence of coxigenital annuli (Table 2, Figure 11).
Download as
Character
Nothopoda vietnamensis n. sp.
Floracarus footei Keifer, 1953
Floracarus biserratae Huang, 2001
Bifurcation of apical part of median line of prodorsal shield forming a small elliptical closed cell
Absent
Present
Absent
(Figure 11 C)
(Figure 11 B)
(Figure 11 A)
Number of transverse lines crossing median line
3 or more
2
2
Posterior part of submedian line I
connected anteriorly with neighbor lines
connected anteriorly with neighbor lines
separated anteriorly from neighbor lines
Coxigenital annuli between coxae II and external genitalia
5–6
3–4
absent
Width of the first three dorsal annuli relative to other dorsal annuli
same
same
the first three dorsal annuli are three times wider than other annuli (Figure 11 A)
Number of dorsal opisthosomal annuli
69–79
69
74
Type locality
Northern Vietnam
“the Philippines, intercepted in quarantine at Honolulu, Hawaii”
Taiwan
Host plant
Nephrolepis cordifolia (L.) K. Presl
Nephrolepis sp.
Nephrolepis biserrata (Sw.) Schott
Relation to host
in galls on frond pinnae (Figures 8, 9)
“the mites make terminal galls on the frond branches”
“this species causes scrolling at the edge of the leaf”
Reference
this paper
Keifer 1953
Huang 2001


Acknowledgments
We are grateful to Dr. Enrico de Lillo (Italy) for providing copies of rare taxonomic publications on eriophyoid mites and to Dr. Bruno Senterre (Biodiversity and Conservation Centre, University of Seychelles) for his help with plant identification. The light microscopy study was funded by the Zoological Institute of Russian Academy of Sciences (ZIN RAS project # 125013001089-0). PCR and sequencing were conducted with the equipment of the ''Development of Molecular and Cellular Technologies″, ''The Bio-Bank″, and ''Microscopy and Microanalysis» Resource Centers and supported by Saint Petersburg State University (Russia).
References
- Amrine J.W. Jr., Manson D.C.M. 1996. Chapter 1.6.3 Preparation, mounting and descriptive study of eriophyoid mites. In: Lindquist E.E., Sabelis M.W., Bruin J. (Eds). Eriophyoid Mites: Their Biology, Natural Enemies and Control. World Crop Pests, vol. 6. Amsterdam: Elsevier Science Publishing: 383-396. https://doi.org/10.1016/S1572-4379(96)80023-6
- Amrine J.W. & Stasny T.A. 1994. Catalog of the Eriophyoidea (Acarina: Prostigmata) of the world. West Bloomfield: Indira Publishing House. 798 pp.
- Amrine J.W. Jr., Stasny T.A.H., Flechtmann C.H.W. 2003. Revised keys to the world genera of the Eriophyoidea (Acari: Prostigmata). Michigan: Indira Publishing House. 244 pp.
- Bolton S.J., Chetverikov P.E., Klompen H. 2017. Morphological support for a clade comprising two vermiform mite lineages: Eriophyoidea (Acariformes) and Nematalycidae (Acariformes). Syst. Appl. Acarol., 22(8): 1096-1131. https://doi.org/10.11158/saa.22.8.2
- Carmona M.M. 1988. Cymoptus vieirai n. sp. (Acarina: Eriophyidae). Bol. Mus. Mun. Funchal, 40(205): 239-242.
- Chetverikov P.E. 2014. Comparative confocal microscopy of internal genitalia of phytoptine mites (Eriophyoidea Phytoptidae): new generic diagnoses reflecting host-plant associations. Exp. Appl. Acarol., 62(2): 129-160 https://doi.org/10.1007/s10493-013-9734-2
- Chetverikov P.E. 2016. Video projector: a digital replacement for camera lucida for drawing mites and other microscopic objects. Syst. Appl. Acarol., 21(9): 1278-1280. https://doi.org/10.11158/saa.21.9.10
- Chetverikov P.E., Craemer C., Cvrković T., Efimov P.G., Klimov P.B., Petanović R.U., Sukhareva S.I. 2019. First pentasetacid mite from Australasian Araucariaceae: morphological description and molecular phylogenetic position of Pentasetacus novozelandicus n. sp. (Eriophyoidea, Pentasetacidae) and remarks on anal lobes in eriophyoid mites. Syst. Appl. Acarol., 24(7): 1284-1309. https://doi.org/10.11158/saa.24.7.12
- Chetverikov P.E., Bertone M. 2022. First rhyncaphytoptine mite (Eriophyoidea, Diptilomiopidae) parasitizing American hazelnut (Corylus americana): molecular identification, confocal microscopy, and phylogenetic position. Exp. Appl. Acarol., 88: 75-95. https://doi.org/10.1007/s10493-022-00740-9
- Chetverikov P.E., Craemer C., Gankevich V.D., Zhuk A.S. 2023. Integrative taxonomy of the gall mite Nothopoda todeica n. sp. (Eriophyidae) from the disjunct Afro-Australasian fern Todea barbara: morphology, phylogeny, and mitogenomics. Insects, 14(6): 507. https://doi.org/10.3390/insects14060507
- Desnitskiy A.G., Chetverikov P.E., Ozman-Sullivan S.K. 2024. Advances in the study of mite gallogenesis and its comparison with the development of insect-induced galls. Acarina, 32(1): 43-57. https://doi.org/10.21684/0132-8077-2024-32-1-43-57
- Duarte M.E., Chetverikov P.E., Silva E.S., Navia D. 2016. Three new species of eriophyoid mites (Acariformes, Eriophyoidea) from Lippia alba (Verbenaceae) from Brazil, and remarks on the thorn-like spermathecal process. Syst. Appl. Acarol., 21(9): 1225-1249. https://doi.org/10.11158/saa.21.9.7
- Gerson U. 1996. Secondary associations: Eriophyoid mites on ferns. In: Lindquist E.E., Sabelis M.W., Bruin J. (Eds). Eriophyoid mites: their biology, natural enemies and control. World Crop Pests 6. Elsevier, Amsterdam, pp. 227-230. https://doi.org/10.1016/S1572-4379(96)80013-3
- Ghosh B., Mondal S., Chakrabarti S. 1984. Four new species of eriophyid mites (Acarina: Eriophyoidea) from West Bengal, India. Entomon, 9(3): 231-237.
- Hovenkamp P.H., Miyamoto F. 2005. A conspectus of the native and naturalized species of Nephrolepis (Nephrolepidaceae) in the world. Blumea, 50(2): 279-322. https://doi.org/10.3767/000651905X623003
- Huang K.W. 2001. The eriophyid mites of Taiwan: description of twenty-three species from Lanyu. Bull. Natl. Mus. Nat. Sci., 13: 37-64.
- Keifer H.H. 1939. Eriophyid studies VI. Bull. Calif. Dep. Agric., 28: 416-426.
- Keifer H.H. 1946. Eriophyid studies XVI. Bull. Calif. Dep. Agric., 35: 39-48. https://doi.org/10.1093/jee/39.5.563
- Keifer H.H. 1953. Eriophyid studies XXI. Bull. Calif. Dep. Agric., 42: 65-79.
- Klimov P.B., O'Connor B.M., Chetverikov P.E., Bolton S.J., Pepato A.R., Mortazavi A.L., Tolstikov A.V., Bauchan G.R., Ochoa R. 2018. Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch. Mol. Phylogenet. Evol., 119: 105-117. https://doi.org/10.1016/j.ympev.2017.10.017
- Klimov P.B., Chetverikov P.E., Dodueva I.E., Vishnyakov A.E., Bolton S.J., Paponova S.S., Lutova L.A., Tolstikov A.V. 2022. Symbiotic bacteria of the gall-inducing mite Fragariocoptes setiger (Eriophyoidea) and phylogenomic resolution of the eriophyoid position among Acari. Sci. Rep., 12: 3811. https://doi.org/10.1038/s41598-022-10467-7
- Klimov P.B., Kolesnikov V.B., Vorontsov D.D., Ball A.D., Bolton S.J., Mellish C., Edgecombe G.D., Pepato A.R., Chetverikov P.E., He Q., Perotti M.A., Braig H.R. 2025. The evolutionary history and timeline of mites in ancient soils. Sci. Rep., 15(1): 13555. https://doi.org/10.1038/s41598-025-96115-2
- Li H.-S., Xue X.-F., Hong X.-Y. 2014. Homoplastic evolution and host association of Eriophyoidea (Acari, Prostigmata) conflict with the morphological-based taxonomic system. Mol. Phylogenet. Evol., 78: 185-198. https://doi.org/10.1016/j.ympev.2014.05.014
- Lindquist E.E. 1996. Chapter 1.1 External anatomy and systematics 1.1.1. External anatomy and notation of structures. In: Lindquist E.E., Sabelis M.W., Bruin J. (Eds). Eriophyoid mites: their biology, natural enemies and control. World Crop Pests 6. Elsevier, Amsterdam, 331 pp. https://doi.org/10.1016/S1572-4379(96)80003-0
- Lotfollahi P., de Lillo E. 2017. Eriophyoid mites from ferns: description of a new Leipothrix Keifer species (Eriophyidae: Phyllocoptinae) from the Arasbaran forests (Iran) and a key to the world species. Acarologia, 57(4): 731-745. https://doi.org/10.24349/acarologia/20174179
- Manson D.C.M. 1984. Eriophyinae (Arachnida: Acari: Eriophyoidea). Fauna N. Z., 5: 1-123.
- Manson D.C.M., Oldfield G.N. 1996. Life forms, deuterogyny, diapause and seasonal development. In: Lindquist E.E., Sabelis M.W., Bruin J. (Eds). Eriophyoid mites: their biology, natural enemies and control. World Crop Pests 6. Elsevier, Amsterdam: 173-183. https://doi.org/10.1016/S1572-4379(96)80009-1
- Paponova S.S., Chetverikov P.E., Pautov A.A., Yakovleva O.V., Zukoff S.N., Vishnyakov A.E., Sukhareva S.I., Krylova E.G., Dodueva I.E., Lutova, L.A. 2018. Gall mite Fragariocoptes setiger (Eriophyoidea) changes leaf developmental program and regulates gene expression in the leaf tissues of Fragaria viridis (Rosaceae). Annals of Applied Biology, 172(1): 33-46. https://doi.org/10.1111/aab.12399
- Petanović R.U., Amrine J.W. Jr., Chetverikov P.E., Cvrković T.K. 2015. Eriocaenus (Acari: Trombidiformes: Eriophyoidea), a new genus from Equisetum spp. https://doi.org/10.11646/zootaxa.4013.1.3
- Senterre B., Rouhan G., Fabre I., Morel C., Christenhusz M.J.M. 2014. Revision of the fern family Marattiaceae in the Seychelles with two new species and a discussion of the African Ptisana fraxinea complex. Phytotaxa, 158(1): 57-75. https://doi.org/10.11646/phytotaxa.158.1.4
- Sidorchuk E.A., Schmidt A.R., Ragazzi E., Roghi G., Lindquist E.E. 2014. Plant-feeding mite diversity in Triassic amber (Acari: Tetrapodili). J. Syst. Palaeontol., 13: 129-151. https://doi.org/10.1080/14772019.2013.867373
- Soika G., Kozak M. 2013. Eriophyes species (Acari: Eriophyoidea) inhabiting lime trees (Tilia spp.: Tiliaceae) - supplementary description and morphological variability related to host plants and female forms. Zootaxa, 3646(4): 349-385. https://doi.org/10.11646/zootaxa.3646.4.3
- Sukhareva S.I. 1994. Family Phytoptidae Murray 1877 (Acari: Tetrapodili), its composition, structure and suggested ways of evolution. Acarina, 2(1): 47-72.
- Sukhareva S.I., Chetverikov P.E. 2013. Morphological transformations in the course of transition from protogyne to deutogyne form of female in eriophyoid mites (Acari: Eriophyoidea). Vestn. S.-Peterb. Univ., 3: 3-15. [in Russian].
- Zhang Q., Lu Y.-W., Liu X.-Y., Li Y., Gao W.-N., Sun J.-T., Hong X.-Y., Shao R., Xue X.-F. 2024. Phylogenomics resolves the higher-level phylogeny of herbivorous eriophyoid mites (Acariformes: Eriophyoidea). BMC Biol., 22: 70. https://doi.org/10.1186/s12915-024-01870-9



2025-06-07
Date accepted:
2025-08-27
Date published:
2025-09-02
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Chetverikov, Philipp E.; Craemer, Charnie; Gankevich, Vladimir D.; Kudryavtzev, Andrey T.; Desnitskiy, Alexey G.; Le, Thi Tuyet Nhung; Nguyen, Duc Viet and Trinh Xuan, Hoat
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



