A new species of Neoseiulella Muma (Parasitiformes: Phytoseiidae) from Northwestern India
Robin  1
; Döker, Ismail
1
; Döker, Ismail  2
; Kaur, Paramjit
2
; Kaur, Paramjit  3
; Brar Bhullar, Manmeet
3
; Brar Bhullar, Manmeet  4
and Chinnamade Gowda, Channegowda
4
and Chinnamade Gowda, Channegowda  5
5
1Punjab Agricultural University, Department of Entomology, 141004 Ludhiana, Punjab, India.
2✉ Cukurova University, Agricultural Faculty, Department of Plant Protection, Acarology Lab, Adana, Türkiye.
3✉ Punjab Agricultural University, Department of Entomology, 141004 Ludhiana, Punjab, India.
4Punjab Agricultural University, Department of Entomology, 141004 Ludhiana, Punjab, India.
5University of Agricultural Sciences, Department of Agricultural Entomology, Bangalore, India.
2025 - Volume: 65 Issue: 3 pages: 762-770
https://doi.org/10.24349/wpop-gesjZooBank LSID: A1FB4A1E-8994-4B5B-B015-928FF39E5E35
Original research
Keywords
Abstract
Introduction
Phytoseiid mites (Parasitiformes: Phytoseiidae) are a diverse and important group of predatory mites, playing a crucial role in controlling pest mite populations across various ecosystems, including both agricultural and natural environments (McMurtry et al. 2013). Despite the significance of phytoseiid mites, the biology and feeding habits of almost all species within the genus Neoseiulella Muma, 1961, remain poorly understood. Among them, N. tiliarum (Oudemans, 1930), the oldest and most widely distributed species in the genus, is known to inhabit pubescent domatia (Kabicek 2008; Kolodochka 2009).
A total of 49 nominal species of Neoseiulella have been described globally, with 42 of them currently considered valid (Ferragut and Peña-Estévez 2003; Kanouh et al. 2012; Demite et al. 2025). Many of these species are widely distributed across diverse geographical regions, inhabiting various habitats such as forests, wetlands, and other natural environments (Stathakis et al. 2016; Döker 2018).
The current study aims to describe a new species, Neoseiulella pentaporus Döker, Robin, and Kaur sp. nov., discovered in the northwestern region of India, specifically in the state of Himachal Pradesh.
Material and methods
Plant leaves were carefully examined using a 10x hand magnifier to identify those colonized by phytoseiid mites. Adult phytoseiid mites were collected from apple trees by beating the branches over a plastic plate with a dark background using a wooden stick. In the field, mites were dislodged and collected on a plate. Dislodged mites were immediately picked up using a fine camel hair brush (size 000) and transferred into 2 mL screw-cap glass vials which were filled with 70% ethyl alcohol. For further preservation, the mites were then stored in 60% lactic acid. Specimens preserved in cavity slides with 60% lactic acid were heated on a hotplate set to 50 °C for 24 hours. The mites were then mounted on microscope slides under a stereozoom microscope (Olympus, Magnus) using Hoyer's medium. The slides were dried at 50-55 °C in a hot air oven for 48 hours and sealed with nail polish.
All essential morphological characters, commonly used in phytoseiid mite descriptions, were measured with an Olympus® CX-41 microscope. Illustrations were prepared using a Camera Lucida, and final image adjustments were made in Adobe Photoshop® (version CS6). The classification system proposed by Chant and McMurtry (2007) was followed. The setal nomenclature for the dorsal idiosoma follows Lindquist and Evans (1965), as modified for phytoseiid mites by Rowell et al. (1978), while the ventral idiosomal setal nomenclature follows Chant and Yoshida-Shaul (1991). The adenotaxy and poroidotaxy of the idiosoma were interpreted according to Athias-Henriot (1971, 1975), and the chaetotaxy of the legs was based on the system described by Evans (1963), and Evans and Till (1979).
Measurements are given in micrometers (µm) and are presented as the mean with the range in parentheses. The length of the dorsal shield was measured along the midline, from the anterior to the posterior margin. The ventrianal shield was measured along the midline, including the cribrum. The length of the legs was measured from the basal margin of the coxa to the apex of the tarsus, excluding the ambulacrum.
Results
Neoseiulella pentaporus Döker, Robin and Kaur sp. nov.
ZOOBANK: 69FCD422-D837-41B6-B85A-48C8113FFC20 ![]()
Type material and depository
Eight females collected on apple tree, Malus domestica L. (Rosaceae), Kukumseri, Udaipur, Lahaul and Spiti, Himachal Pradesh, India, 32°42′03.48″N, 76°41′19.83″E, 30 September 2024, 2753 m above sea level, collectors Paramjit Kaur and Robin.
The holotype female and two paratype females were deposited in PC Unit Repository, UAS, GKVK, Bangalore, India; two paratype females in Acarology Laboratory, Punjab Agricultural University, Ludhiana, India; and three paratype females in Acarology Laboratory, Department of Plant Protection, Cukurova University, Adana, Türkiye.
Diagnosis
Idiosomal setal pattern 12A: 9B/15 JV:ZV (r3 and R1 off shield). Dorsal shield strongly reticulated with five pairs of solenostomes (gd2, gd4, gd6, gd8 and gd9). Dorsal setae smooth, except Z4 and Z5 serrated and Z5 knobbed apically, most setae arising on small tubercles. Peritremes long, extending between setae j1. Solenostomes gd3 present on peritrematal shield. All ventral shields smooth; sternal shield with three pairs of setae (ST1–ST3). Ventral seta ST4 on soft integument. Ventrianal shield pentagonal with four pairs of pre-anal setae (JV1, JV2, JV3, and ZV2); one pair of small crescentic solenostomes. Spermatheca with calyx bell-shaped with large nodular atrium attached to calyx. Fixed digit of chelicera with six-seven teeth and movable digit with two teeth. Trochanter I with six (1 0/1 1/2 1) and genu II with seven setae (2 2/0 2/0 1). Seta al on trochanter I subulate. Leg IV with two macrosetae on genu and basitarsus each knobbed apically; other legs without macroseta.
Description
Female (n = 8)
(Figures 1–4)
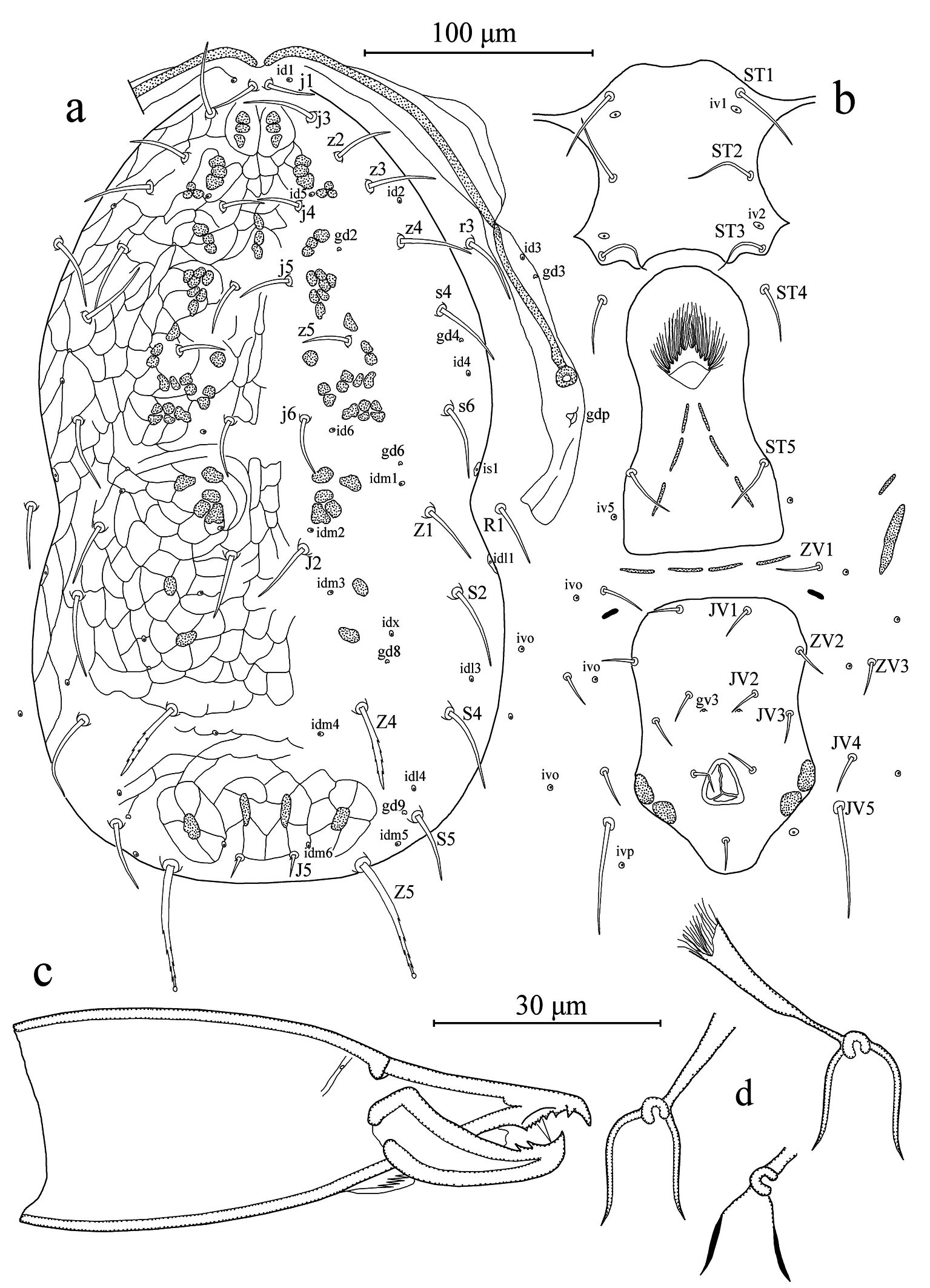


Dorsal idiosoma (Figures 1a, 2a-b). Dorsal setal pattern 12A:9B (r3 and R1 off shield). Dorsal shield strongly reticulated; with five pairs of solenostomes (gd2, gd4, gd6, gd8, and gd9) and 16 pairs of poroids (id1, id2, id4, id5, id6, idm1, idm2, idm3, idm4, idm5, idm6, idx, is1, idl1, idl3, and idl4). Muscle-marks (sigilla) visible mostly on podosoma, length of dorsal shield 343 (335–348), width at level of s4 195 (188–200), width at level of S2 209 (200–213). Dorsal setae smooth except Z4 and Z5 serrated and J5 with one barb; seta Z5 knobbed apically. Measurements of dorsal setae as follows: j1 20 (18–22), j3 28 (26–30), j4 20 (19–20), j5 20 (18–22), j6 23 (22–24), J2 28 (27–29), J5 11 (10–12), z2 24 (23–25), z3 28 (25–29), z4 29 (28–29), z5 21 (20–22), Z1 26 (25–27), Z4 36 (35–37), Z5 52 (51–53), s4 31(30–32), s6 32 (32–33), S2 35 (34–37), S4 37 (35–38), S5 27 (25–28), r3 29 (28–30), and R1 26 (25–27). Peritremes extending between setae j1; solenostome gd3 and poroid id3 visible on peritrematal shield; solenostome gdp large, posterior to the stigmatic opening.
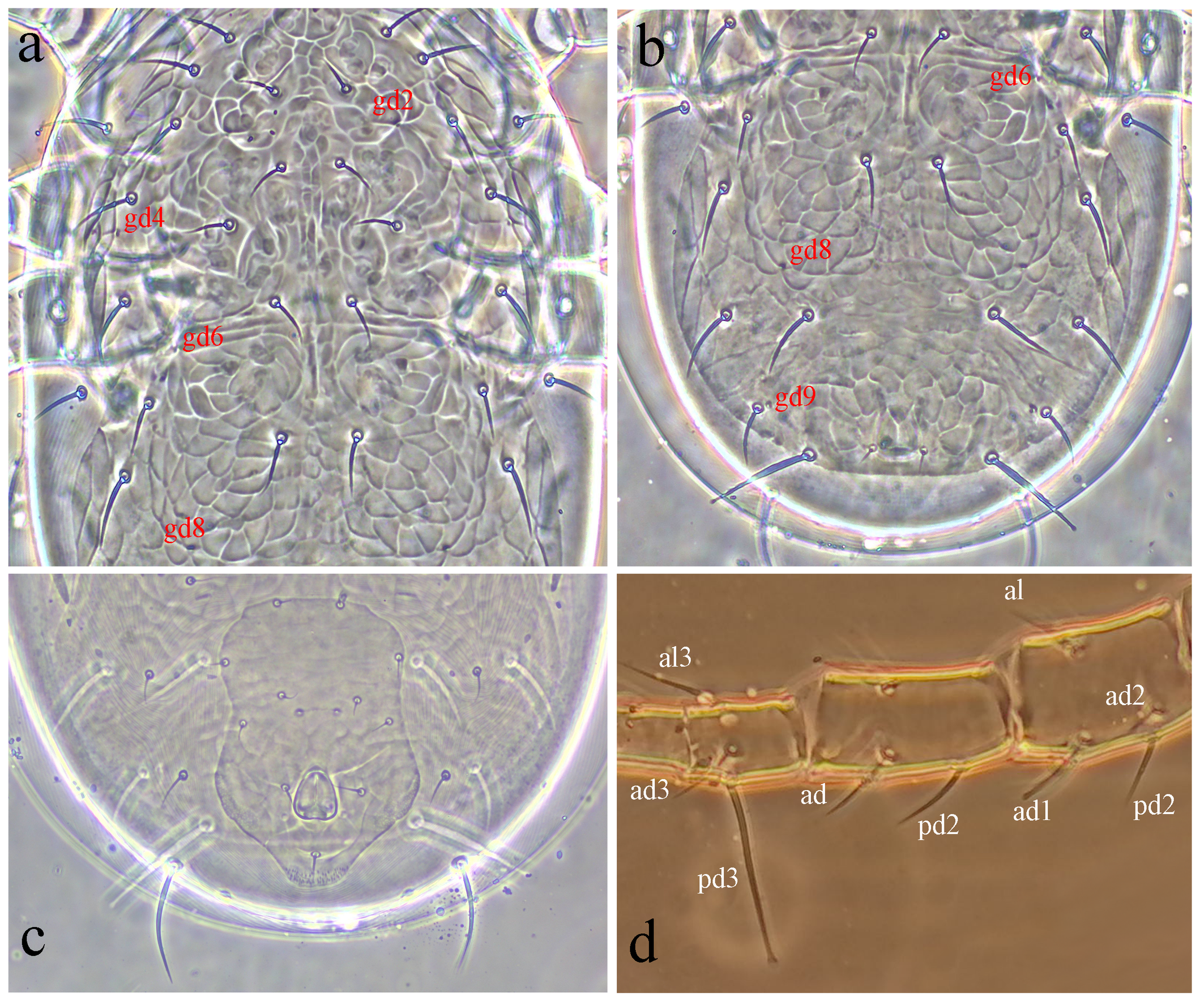


Ventral idiosoma (Figures 1b, 2c). Ventral setal pattern 15:JV:ZV. Sternal shield poorly sclerotized, smooth; with three pairs of setae (ST1–ST3) and two pair of poroids (iv1 and iv2); distance between ST1–ST3 67 (65–68), distance between setae ST2 60 (59–62); metasternal setae ST4 on soft integument, and poroids iv3 indiscernible. Genital shield smooth, with one pair of setae ST5; width at level of ST5 60 (59–61); one pair of para-genital poroids iv5 on soft cuticle. Ventrianal shield pentagonal, smooth, with a slight waist; with four pairs of pre-anal setae (JV1, JV2, JV3, and ZV2); one pair of para-anal setae PA, unpaired post-anal seta PST, and a pair of small crescentic pre-anal solenostomes gv3 posteromesad JV2, distance between solenostomes 16 (15–16). Length of ventrianal shield 114 (110–118), width at level of ZV2 78 (76–80), width at level of muscle marks (widest point) 82 (79–85), width at level of waist (narrowest point) 68 (64–70). Four pairs of caudoventral setae (ZV1, ZV3, JV4, and JV5) and five pairs of poroids (four pairs of ivo, and ivp) on soft cuticle surrounding ventrianal shield. Setae JV5 smooth, 47 (44–49) in length.
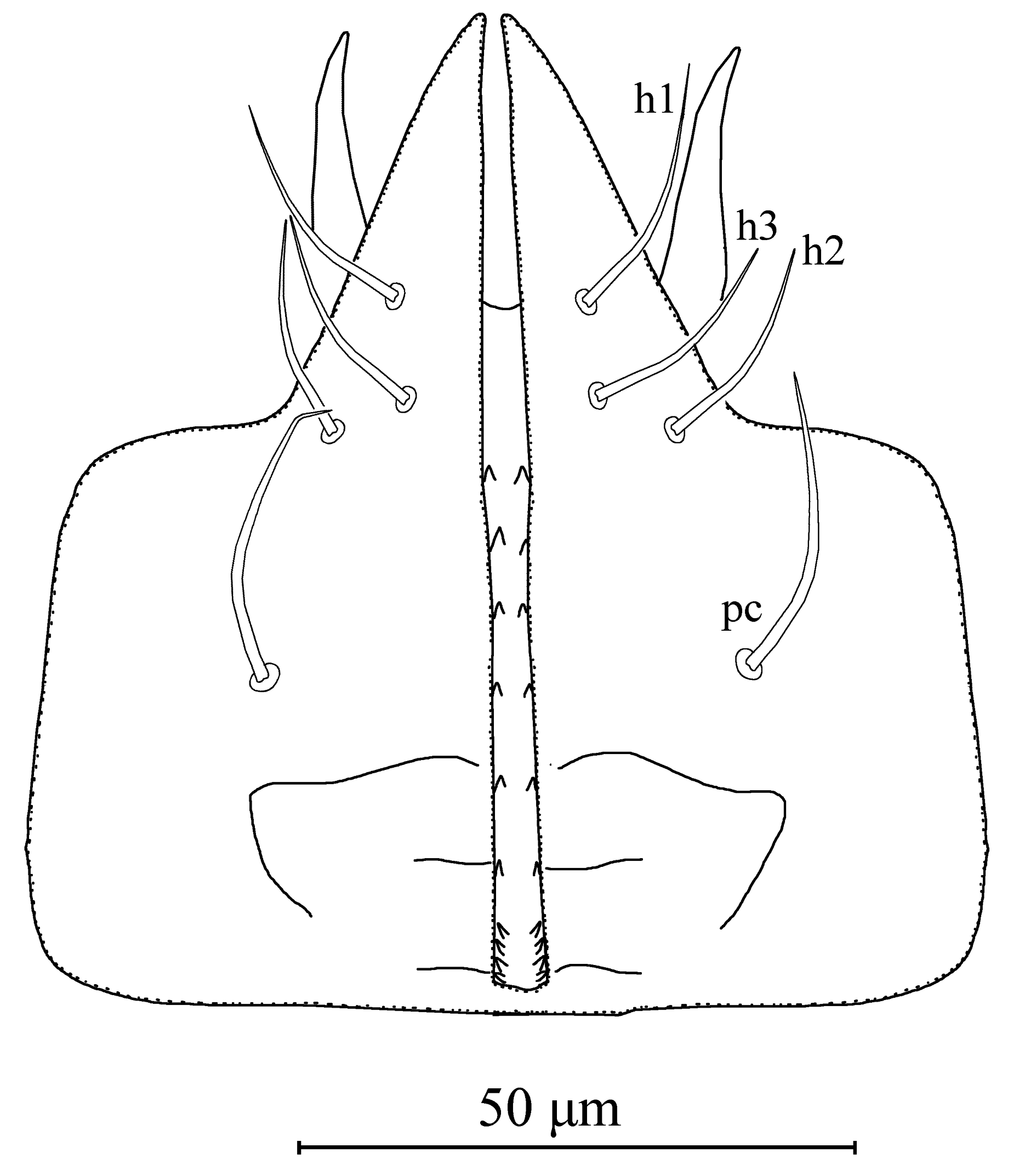


Gnathosoma (Figures 1c, 3). The hypostomal groove narrow, with seven transverse rows of denticles; basal row consists of four to five denticles, vertically aligned; other six rows each contain two denticles. Subcapitular setae h1 23 (22–24), h2 21 (19–22) and h3 21 (19–22), slightly shorter than palp coxal setae (pc) 26 (25–27). Fixed digit 28 (27–29) long, with six-seven teeth, and pilus dentilis; movable digit 27 (26–29) long, with two teeth.
Spermatheca (Figure 1d). Calyx bell-shaped, flaring distally, 12 (10–14) long; atrium large nodular, attached to calyx; major duct broad and minor duct not visible.
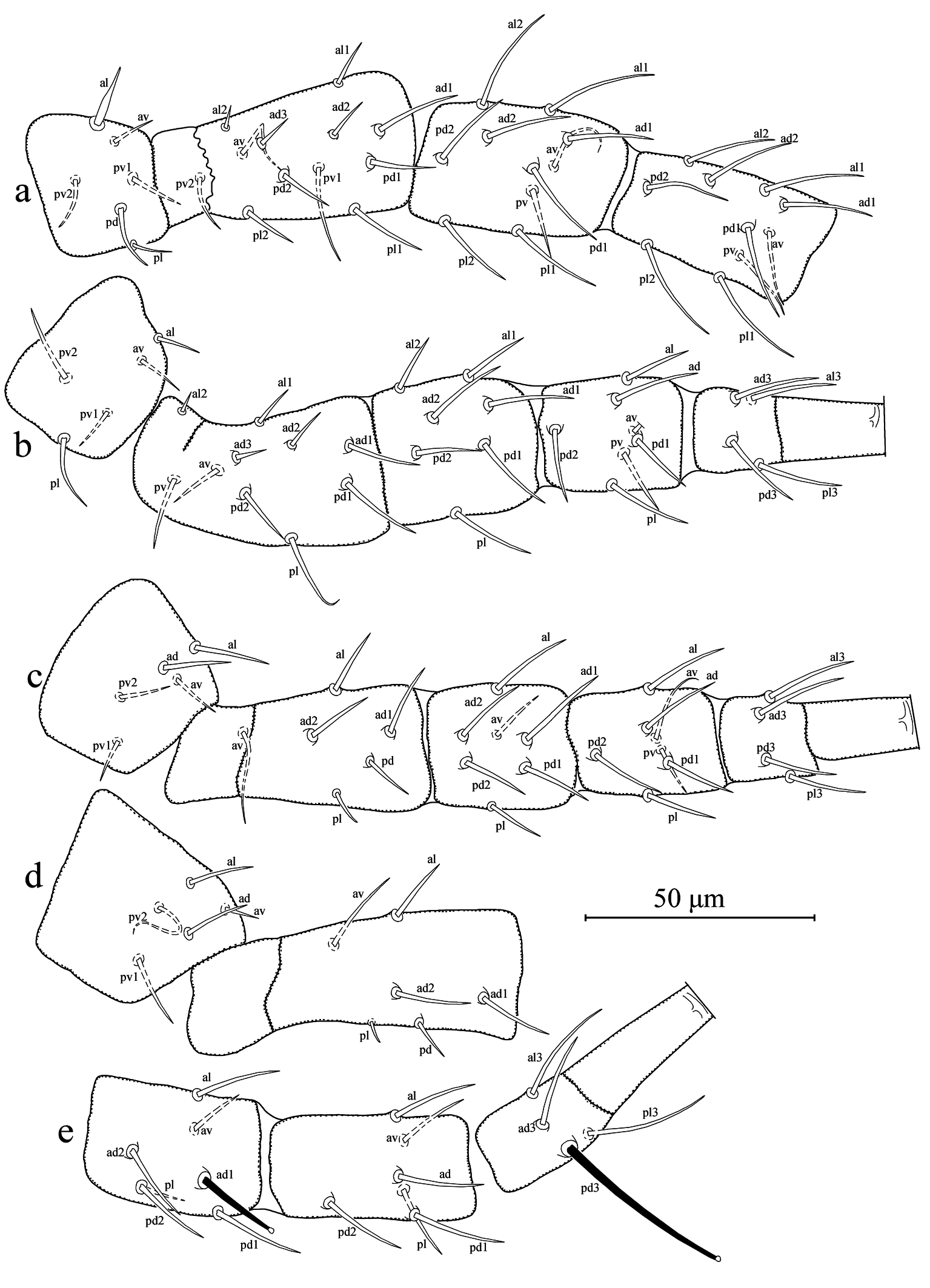


Legs (Figures 4a–e). Length of legs I-IV 301 (295–310), 258 (250–265), 261 (250–270), and 333 (325–340), respectively. Chaetotaxy of legs as follows; leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 1/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/0 1, tibia 1 1/1 2/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 1/1 0/2 0, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 1/1 0/2 0, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/0 1. Most of dorsal setae arising on tubercles. Seta al on trochanter I subulate; two macroseta present on genu and basitarsus of leg IV, both knobbed apically; SgeIV 21 (20–22) and StIV (pd3) 42 (39–45) in length. Distance between pd3 and dorsal slit organ (lyrifissure) 43 (41–43).
Etymology
The name of the species is derived from the Greek word ''πέντα- (penta-)'' + porus (pore) due to the presence of five pairs of solenostomes on the dorsal shield. Noun in apposition.
Differential Diagnosis
Neoseiulella pentaporus Döker, Robin, and Kaur sp. nov. belongs to the tiliarum species group of Chant (1959) due to its dorsal setae of approximately uniform length and the presence of ventral seta JV3. Among the 30 nominal species in this group, the new species shows close affinity to N. vollsella (Chaudhri, Akbar and Rassol, 1974), N. prunus (Denmark and Rather, 1984), N. celtis Denmark and Rather, 1996, and N. transitans (Gupta, 1981) in terms of the nature of dorsal and ventral aspects, including the ventrianal shield and chelicera dentition (Chant and Yoshida-Shaul 1989; Kanouh et al. 2012; Stathakis et al. 2016; Döker 2018). The former was described from Pakistan, while the latter three were described from Jammu & Kashmir, India. However, N. prunus and N. transitans are both considered junior synonyms of N. vollsella (Chant and Yoshida-Shaul 1989; Kanouh et al. 2012).
The new species is distinctly different from all of these species by possessing five pairs of solenostomes (gd4 and gd6 present) on the dorsal shield and only two knobbed macrosetae on leg IV. In contrast, N. vollsella have only three pairs of solenostomes and three knobbed macrosetae on leg IV (Chant and Yoshida-Shaul 1989; Kanouh et al. 2012). Some of dorsal setae, ventral seta JV5, and macroseta on basitarsus IV of the new species are generally longer compared to the type material descriptions of other species. These setae are between 25–33% longer, with the new species falling outside the range previously considered as intraspecific variation but now falling within interspecific variation between the two species (Tixier 2012). In addition, N. celtis has only one pair of solenostomes (gd9) on the dorsal shield and a single knobbed macroseta on leg IV. The dorsal seta Z4 in N. celtis (20) is conspicuously shorter than that of the new species (35–37).
The significance of a single pair of solenostomes in distinguishing closely related species within the genus Kampimodromus Nesbitt and Neoseiulus Hughes, has been previously confirmed by molecular analyses in several studies (Döker et al. 2018; Khaustov et al. 2022). Moreover, variation in the number of knobbed macrosetae is linked to species complexes in the subgenus Anthoseius De Leon, another taxonomic group in the family Phytoseiidae, but still within the subfamily Typhlodrominae (Ueckermann et al. 2008, 2021; Döker et al. 2024a).
Remarks
Interestingly, our examination revealed that the anterolateral seta on the first trochanter of the new species is consistently modified into a subulate form in all type specimens, characterized by a robust base that gradually tapers to a fine point, as previously reported for several species belongs to the genus Euseius Wainstein (Döker et al. 2024b; 2025a, b; Tsolakis and Ranja-William 2025). While the function of these modified setae remains unclear, it seems that this modification is common in various groups within the family Phytoseiidae. Further investigations should confirm whether this feature is present in other Neoseiulella species as well as other groups within the family Phytoseiidae.
Acknowledgements
We are grateful to Prof. Serge Kreiter (France) for helpful discussions on the species status described in this paper. We thank the Science and Engineering Research Board, DST, Government of India (CRG/2021/006434), for funding and manpower support for surveillance in the Trans Gangetic Plain and North Western Himalaya. We also acknowledge the All India Network Project on Agricultural Acarology (ICAR-34), Department of Entomology, Punjab Agricultural University, Ludhiana, and the Network Unit at the University of Agricultural Sciences, GKVK, Bangalore, for providing necessary facilities. Additional support was provided under the DST-FIST project (SR/FST/LSI/636/2015(c)). This study was also supported by the Cukurova University Scientific Projects Foundation (FAY-2022-14495).
References
- Athias-Henriot C. 1971. Nouvelles notes sur les Amblyseiini (Gamasides podospermiques, Phytoseiidae) I. La dépilation des génuaux et tibias des pattes. Acarologia, 13: 4-15.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides protoadéniques, Phytoseiidae). Acarologia, 17: 20-29.
- Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. The Canadian Entomologist, 61: 1-166. https://doi.org/10.4039/entm9112fv
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). West Bloomfield, Indira Publishing House, 219 pp.
- Chant D.A., Yoshida-Shaul E. 1989. A world review of the tiliarum species group in the genus Typhlodromus Scheuten (Acari: Phytoseiidae). Canadian Journal of Zoology, 67: 1006-1046. https://doi.org/10.1139/z89-144
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). International Journal of Acarology, 17: 187-199. https://doi.org/10.1080/01647959108683906
- Chaudhri W.M., Akbar S., Rasool A. 1974. Taxonomic studies of the mites belonging to the families Tenuipalpidae, Tetranychidae, Tuckerellidae, Caligonellidae, Stigmaeidae and Phytoseiidae. Lyallpur, Pakistan, University of Agriculture Technical Bulletin, 250 pp.
- Demite P.R., de Moraes G.J., McMurtry J.A., Denmark H.A., Castilho R.C. 2025. Phytoseiidae database. [accessed 2025 March 22]. https://www.lea.esalq.usp.br/phytoseiidae/
- Denmark H.A., Rather A.Q. 1984. Revision of the genus Typhloctonus Muma, 1961 (Acarina: Mesostigmata). International Journal of Acarology, 10: 163-177. https://doi.org/10.1080/01647958408683371
- Denmark H.A., Rather A.Q. 1996. Revision of the genus Neoseiulella Muma (Acari: Phytoseiidae). International Journal of Acarology, 22: 43-77. https://doi.org/10.1080/01647959608684080
- Döker I. 2018. Re-description of two new records and description of Neoseiulella kazaki sp. nov. (Acari: Phytoseiidae) from Turkey. Systematic and Applied Acarology, 23: 113-122. https://doi.org/10.11158/saa.23.1.9
- Döker I., Atchia I., Jose A., Bolton S. 2024a. Complementary description of two Anthoseius De Leon (Acari: Phytoseiidae) species based on their holotypes with new synonyms. Acarologia, 64: 192-201. https://doi.org/10.24349/gt2k-nkkv
- Döker I., Karut K., Karaca M.M., Cargnus E., Kazak C. 2018. Internal Transcribed Spacer (ITS) sequences of some Kampimodromus (Acari: Phytoseiidae) species: Is Kampimodromus ragusai a valid species or a synonym of Kampimodromus aberrans? Systematic and Applied Acarology, 23: 2237-2243. https://doi.org/10.11158/saa.23.11.15
- Döker I., Çelik S.O., Karaca, M.M. 2025a Review of Euseius Wainstein (Parasitiformes: Phytoseiidae) in Türkiye: An integrative taxonomic approach using morphological and molecular data with a new combination Euseius degenerans (Berlese). Systematic and Applied Acarology, 30: 560-591. https://doi.org/10.11158/saa.30.3.6
- Döker I., Khaustov V.A., Joharchi O., Khaustov A., Kazakov D.V., Meshkov Y.I. 2024b. Integrative taxonomy demonstrates synonymy between Euseius amissibilis Meshkov and Euseius gallicus Kreiter & Tixier (Acari: Phytoseiidae). Systematic and Applied Acarology, 29: 60-77. https://doi.org/10.11158/saa.29.1.5
- Döker,I., Zidan I.M., Ueckermann E.A., Momen F.M. 2025b. A supplementary description of Euseius talinga (Pritchard & Baker) (Acari: Phytoseiidae) from Egypt. International Journal of Acarology, 51: 122-127. https://doi.org/10.1080/01647954.2025.2449939
- Evans G.O. 1963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bulletin of the British Museum (Natural History) Zoology, 10: 275-303. https://doi.org/10.5962/bhl.part.20528
- Evans G.O., Till W.M. 1979. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes): An introduction to their external morphology and classification. The Transactions of the Zoological Society of London, 35: 139-262.
- Ferragut F., Peña-Estévez M.A. 2003. Phytoseiid mites of the Canary Islands (Acari: Phytoseiidae): 1. Gran Canaria Island. International Journal of Acarology, 29: 149-170. https://doi.org/10.1080/01647950308683654
- Gupta S.K. 1981. Phytoseiidae (Acari: Mesostigmata) from Jammu and Kashmir, India, with descriptions of five new species. Indian Journal of Acarology, 5: 37-49.
- Kabicek J. 2008. Cohabitation and intra-leaf distribution of phytoseiid mites (Acari: Phytoseiidae) on leaves of Corylus avellana. Plant Protection Science, 44: 32-36. https://doi.org/10.17221/3/2008-PPS
- Kanouh M., Kreiter S., Douin M., Tixier M.-S. 2012. Revision of the genus Neoseiulella Muma (Acari: Phytoseiidae): re-description of species, synonymy assessment, biogeography, plant supports and key to adult females. Acarologia, 52: 259-348.
- Khaustov V.A., Döker I., Joharchi O., Kazakov D.V., Khaustov A.A., Moradi M., Fang X.-D., Klimov P. 2022. A new, broadly distributed species of predacious mites, Neoseiulus neoagrestis sp. nov., (Acari: Phytoseiidae) discovered through GenBank data mining and extensive morphological analyses. Systematic and Applied Acarology, 27: 2038-2061. https://doi.org/10.11158/saa.27.10.14
- Kolodochka L.A. 2009. A review of predaceous mites of the genus Typhloctonus Muma (Parasitiformes, Phytoseiidae) in Ukraine with description of unknown male of T. tuberculatus. Vestnik Zoologii, 43: e1-e12.
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina Acarina: Mesostigmata. The Memoirs of the Entomological Society of Canada, 47: 1-64. https://doi.org/10.4039/entm9747fv
- McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Systematic and Applied Acarology, 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Muma, M.H. 1961. Subfamilies, genera, and species of Phytoseiidae (Acarina: Mesostigmata). Bulletin of the Florida State Museum, 5: 267-302. https://doi.org/10.58782/flmnh.tqpo4380
- Oudemans, A.C. 1930. Acarologische Aanteekeningen. CI. Entomologische Berichten, 8: 48-53.
- Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). The Canadian Entomologist, 110: 859-876. https://doi.org/10.4039/Ent110859-8
- Stathakis T., Kapaxidi E.V., Papadoulis G.Th. 2016. A new species and three new records of Phytoseiidae (Acari: Mesostigmata) found on coastal and wetland vegetations in Greece. Systematic and Applied Acarology, 21: 567-582. https://doi.org/10.11158/saa.21.5.2
- Tixier M.-S. 2012. Statistical approaches to assess intraspecific variations of morphological continuous characters: the case study of the family Phytoseiidae (Acari: Mesostigmata). Cladistics, 28: 489-502. https://doi.org/10.1111/j.1096-0031.2012.00394.x
- Tsolakis H., Ranja-William F.A. 2025. New records of Phytoseiid phytoseiid mites from Madagascar with description of two new species. Acarologia: 65: 448-460. https://doi.org/10.24349/t5zy-f3cc
- Ueckermann E.A., Situngu S., Barker N.P. 2021. Checklist of Phytoseiidae (Acari: Mesostigmata) species from plants bearing leaf domatia, from the Eastern Cape Province, South Africa, with the description of a new species. Journal of Natura History, 55: 683-697. https://doi.org/10.1080/00222933.2021.1919777
- Ueckermann E.A., Zannou I.D., Moraes G.J. de, Oliveira A.R. de, Hanna R., Yaninek J.S. 2008. Phytoseiid mites of the tribe Typhlodromini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1901: 1-122. https://doi.org/10.11646/zootaxa.1901.1.1



2025-05-12
Date accepted:
2025-07-02
Date published:
2025-08-05
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Robin; Döker, Ismail; Kaur, Paramjit; Brar Bhullar, Manmeet and Chinnamade Gowda, Channegowda
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



