Usefulness of DEET (N,n-diethyl-meta-toluamide) in the directed expulsion of different groups of mites and invertebrates in Berlese-Tullgren funnels
Rzepecki, Aleksander  1
and Sikora, Bozena
1
and Sikora, Bozena  2
2
1✉ Department of Animal Morphology, Adam Mickiewicz University, Poznań, Poland.
2Department of Animal Morphology, Adam Mickiewicz University, Poznań, Poland.
2025 - Volume: 65 Issue: 3 pages: 708-716
https://doi.org/10.24349/928n-ajszPresented at X EurAAc Symposium 2–6 September 2024 Athens
Keywords
Abstract
Introduction
Soil-dwelling arthropods, including mites (Acari), are key players in ecosystem processes such as organic matter decomposition, nutrient cycling, and pest control (Crossley and Hendrix, 2004). Accurate extraction of these organisms is essential for ecological studies, but traditional methods often lack specificity and efficiency. The Berlese-Tullgren funnel, first introduced by Antonio Berlese (1905) and later refined by Albert Tullgren (1918), remains one of the most widely used techniques for soil fauna extraction.
While effective in extracting a broad range of organisms, the Berlese-Tullgren funnel relies on heat and light to create a gradient that induces migration away from desiccation or discomfort. This method, however, typically yields non-specific samples since all organisms respond similarly to these stimuli (Macfadyen, 1961). As ecological studies demand increasingly precise, taxon-specific data, there is a growing need to explore alternative extraction methods that allow for more targeted sampling.
DEET also known as N,N-Diethyl-meta-toluamide, is a chemical repellent best known for its effectiveness against mosquitoes and other biting arthropods, has shown promise as a broad-spectrum deterrent in both laboratory and field studies (Barnard and Xue, 2004; Debboun et al., 2007). However, its application in soil fauna extraction has been underexplored. The lack of data on the harmful quantity of the substance may lead to the use of lethal doses. This is one of the reasons why experimental analysis should be applied. This study assesses DEET's potential as a targeted repellent in modified Berlese-Tullgren funnels, aiming to improve the specificity and efficiency of soil arthropod extraction.
Materials and methods
Sample Collection
Soil samples were collected monthly from June to November 2023 (Figure 1) at a deciduous forest site in Poznań, Poland (52°28′N 16°56′E).Main plant species present in that forest are Pines and Alders. For each sampling event, a single large soil sample of approximately 6 kg was taken from one small upper soil profile to ensure consistency. The bulk sample was transported immediately to the Department of Animal Morphology funnel extraction laboratory, where it was split into 12 smaller samples, each weighing approximately 500 g. These smaller samples were then placed into Berlese-Tullgren funnels for five day long extraction. All samples were kept in controlled conditions to maintain consistent moisture levels until the extraction process began.
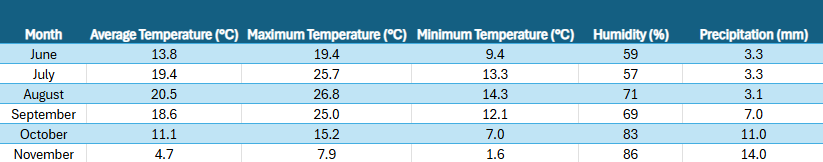


Experimental Design
Berlese-Tullgren funnels were modified by replacing the traditional heat source with DEET-soaked cotton pads positioned at the funnel apex, with the cotton pad placed on a thin linen cloth to prevent direct contact of the chemical substance with the soil sample (Figure 2). Three MUGGA volumes (50% DEET: 2.5 ml, 3.5 ml, and 5 ml) were tested, with each replicated three times monthly. Control setups, which did not include DEET, were used as benchmarks. This design produced 12 samples per month, totaling 72 samples over the six-month study period. For purpose of graphs, volumes of MUGGA used in samples were marked with letters, that being:
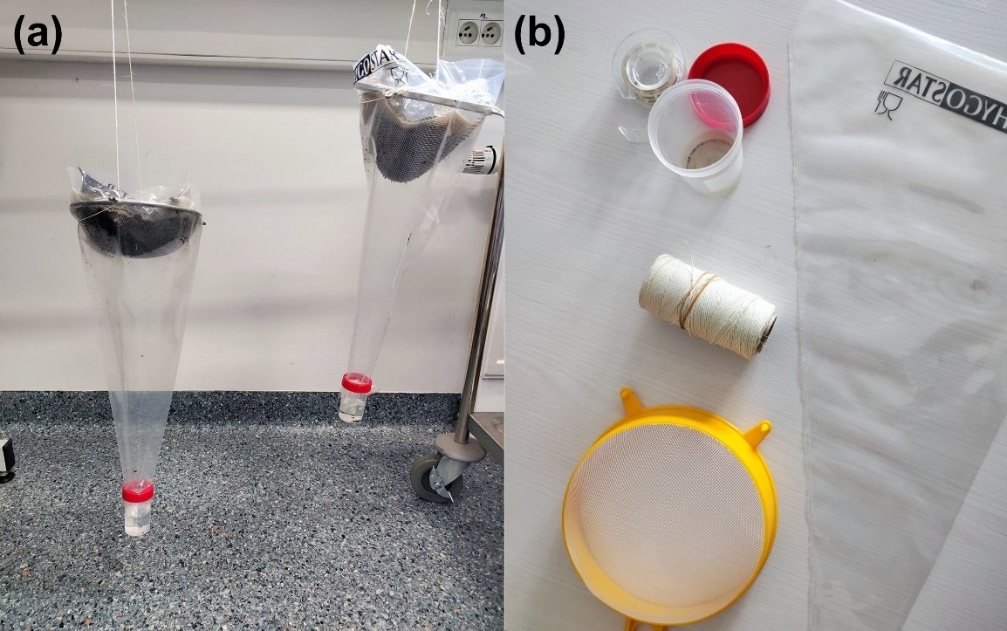


K for control samples,
M for 2.5ml of MUGGA,
E for 3.5ml of MUGGA,
L for 5ml of MUGGA.
Organism Sorting and Identification
Extracted organisms were preserved in 70% ethanol and sorted into following orders: Mesostigmata, Sarcoptiformes (focusing on suborder Oribatida and clade Astigmatina) and other invertebrate groups using a stereomicroscope. Identification followed taxonomic keys provided by Krantz and Walter (2009).
Statistical Analysis
To assess the effectiveness of DEET as a chemical repelling agent for extracting soil microfauna (Mesostigmata, Oribatida, Astigmatina, and Other Invertebrates) in modified Berlese-Tullgren funnels, we conducted a two-way analysis of variance (ANOVA) for each organism group. The analysis compared four treatments: control (K, no DEET), 2.5 ml DEET (M), 3.5 ml DEET (E), and 5.0 ml DEET (L), applied in funnels from June to November 2023. The counts of expelled organisms were recorded from three replicates per treatment per month (n = 72 observations per group: 4 treatments, 6 months, 3 replicates). The two-way ANOVA evaluated the main effects of treatment and month, as well as their interaction, to determine if DEET concentration and sampling month influenced the extraction efficiency of specific mite orders and other invertebrates.
Model assumptions were tested prior to analysis. Normality of residuals was assessed using the Shapiro-Wilk test, and homogeneity of variances across treatmentmonth combinations was evaluated with Levene's test. For organism groups meeting both assumptions (p > 0.05), two-way ANOVA was performed using Type II sums of squares. For groups violating assumptions, results were interpreted cautiously, leveraging the robustness of ANOVA for balanced designs with sufficient sample size. Significant effects (p < 0.05) were further analyzed using Tukeys Honestly Significant Difference (HSD) test to identify pairwise differences among treatments and months. For significant interactions, simple main effects analyses were conducted to evaluate treatment effects within each month, followed by Tukeys HSD tests where applicable. All statistical analyses were performed in Python (version 3.8) using the statsmodels (version 0.12.2) and scipy (version 1.7.3) libraries. Significance was determined at α = 0.05.
Results
This study examined the effectiveness of varying 50% DEET concentrations (2.5 ml, 3.5 ml, and 5 ml) in extracting soil-dwelling mites and invertebrates. Two-way ANOVA revealed significant effects of DEET treatment and sampling month on the extraction efficiency of Mesostigmata, Oribatida, Astigmatina, and Other Invertebrates from soil samples using modified Berlese-Tullgren funnels, with significant treatment-by-month interactions for Mesostigmata and Oribatida. Assumption checks confirmed normality of residuals (Shapiro-Wilk, p > 0.05) and homogeneity of variances (Levene's test, p > 0.05) for Mesostigmata, Oribatida, and Other Invertebrates, supporting the use of ANOVA. For Astigmatina, residuals were non-normal (Shapiro-Wilk, p = 0.012) and variances were unequal (Levene's, p = 0.038), so results were interpreted cautiously, relying on the robustness of the balanced design. The results demonstrate that DEET concentration significantly affects extraction efficiency, with specific concentrations being more effective for particular taxa.
Taxa-Specific Extraction Trends
Astigmatina
Astigmatina showed the strongest response to the 2.5 ml DEET concentration. This treatment consistently yielded higher numbers of Astigmatina across the study compared to both controls and other DEET treatments. The average extraction count for Astigmatina using 2.5 ml of DEET was notably higher than for the 3.5 mL and 5 mL treatments, suggesting that lower concentrations are optimal for this soft-bodied, highly sensitive group (Figure 3).
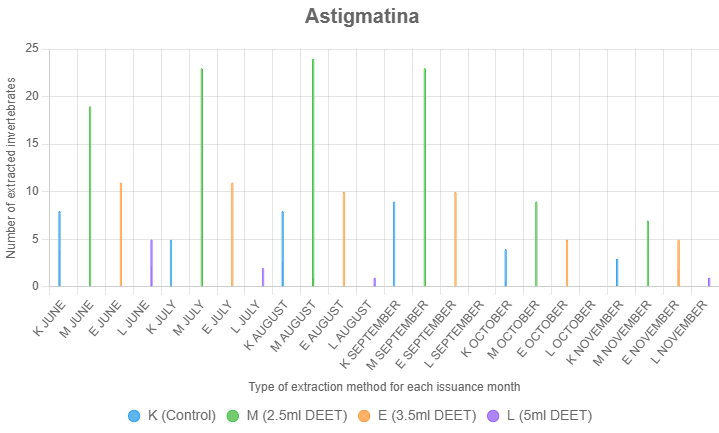


Treatment (F = 36.45, p < 0.001) and month (F = 4.23, p = 0.002) significantly affected Astigmatina extraction, but the interaction was not significant (F = 1.12, p = 0.342). Tukey's HSD tests showed 2.5 ml DEET (M, mean = 5.50) outperformed control (K, mean = 2.06, p < 0.001) and 3.5 ml DEET (E, mean = 2.72, p = 0.002), while 5.0 ml DEET (L, mean = 0.39) was lower than K (p < 0.001). August (mean = 3.92) exceeded November (mean = 1.33, p < 0.01). The consistent treatment effect across months suggests 2.5 ml DEET enhances extraction, while 5.0 ml DEET inhibits it, despite assumption violations mitigated by a robust sample size.
Control samples and higher DEET concentrations generally yielded fewer Astigmatina, likely due to their reduced tolerance to stronger chemical gradients. These results highlight the suitability of low-concentration DEET for extracting Astigmatina in studies requiring targeted sampling.
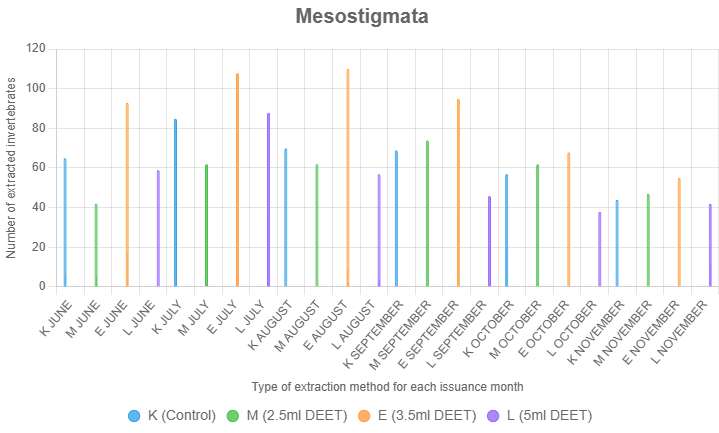
Mesostigmata
Mesostigmata were most effectively extracted with 3.5 ml of DEET. This concentration consistently extracted more Mesostigmata than both controls and the other DEET treatments.
Treatment (F = 14.89, p < 0.001), month (F = 6.45, p < 0.001), and their interaction (F = 1.78, p = 0.043) significantly influenced Mesostigmata expulsion. Tukey's HSD tests showed 3.5 ml DEET (E, mean = 29.39) expelled more than control (K, mean = 21.11, p = 0.001), 2.5 ml DEET (M, mean = 19.39, p = 0.003), and 5.0 ml DEET (L, mean = 19.11, p = 0.002), with no differences among K, M, and L. Higher extraction occurred in July (mean = 29.25) and August (mean = 28.08) vs. November (mean = 15.58, p < 0.01). The interaction showed E outperformed K, M, and L in June, July, and August (p < 0.05–0.01), but not in later months, suggesting 3.5 ml DEET is effective early in summer.
Mesostigmata's larger size and increased mobility compared to Astigmatina may account for their preference for intermediate DEET concentrations, which provide an effective repellent gradient without overwhelming their movement responses. These findings emphasize the utility of 3.5 ml DEET for studies focusing on Mesostigmata, which play crucial roles as predators in soil ecosystems (Figure 4).


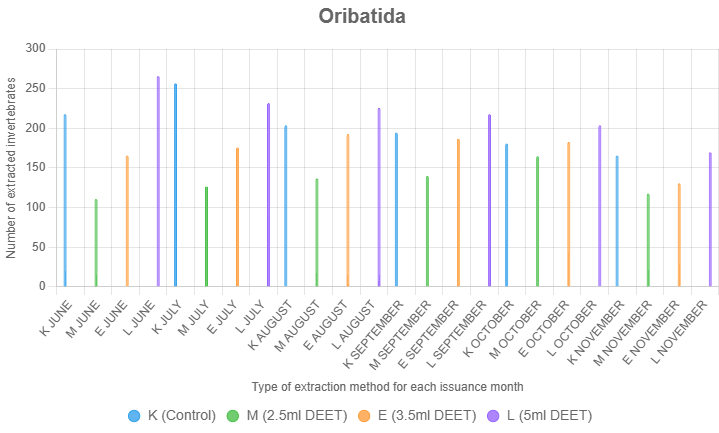
Oribatida
Oribatida showed the highest extraction rates with 5 ml of DEET. This taxon, characterized by its heavily sclerotized exoskeleton, is less sensitive to environmental changes compared to Astigmatina and Mesostigmata. As a result, Oribatida required stronger chemical gradients to induce movement into the collection funnels.
Treatment (F = 24.12, p < 0.001), month (F = 8.76, p < 0.001), and their interaction (F = 1.95, p = 0.032) significantly affected Oribatida extraction. Tukey's HSD tests indicated 5.0 ml DEET (L, mean = 73.39) outperformed control (K, mean = 66.78, p < 0.001) and 3.5 ml DEET (E, mean = 58.22, p = 0.002), while 2.5 ml DEET (M, mean = 45.44) was lower than K (p < 0.001). Extraction peaked in June (mean = 63.42) and July (mean = 65.92) vs. November (mean = 48.67, p < 0.01). L excelled over K, M, and E in June (p < 0.01) and over M in July and August (p < 0.05–0.01), with no differences later, indicating 5.0 ml DEET is most effective early in the season.
In untreated control samples, Oribatida were often the dominant group, reflecting their abundance and resilience. However, the addition of 5 ml DEET consistently increased extraction counts compared to controls, indicating its effectiveness in repelling this taxon (Figure 5).


Other Invertebrates
Other invertebrates, particularly springtails (Collembola), responded most strongly to the 5 ml DEET treatment (Figure 6). This trend underscores the effectiveness of higher DEET concentrations in targeting fast-moving and chemically sensitive taxa. The enhanced extraction of springtails at 5 ml suggests that this concentration is particularly effective for studies focused on this ecologically significant group.
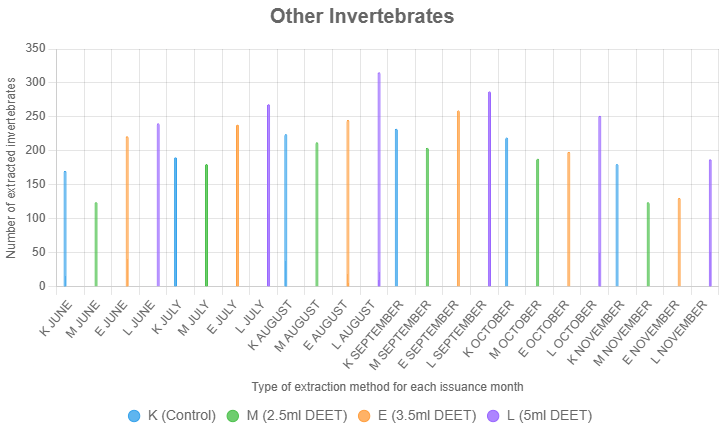


Treatment (F = 17.89, p < 0.001) and month (F = 7.12, p < 0.001) significantly influenced Other Invertebrates extraction, but the interaction was not significant (F = 1.56, p = 0.067). Post-hoc tests showed 5.0 ml DEET (L, mean = 88.28) and 3.5 ml DEET (E, mean = 72.06) outperformed control (K, mean = 67.28, p < 0.001 and p = 0.045), while 2.5 ml DEET (M, mean = 57.67) was lower than K (p = 0.001). Extraction peaked in August (mean = 83.33) and September (mean = 82.17) vs. November (mean = 52.75, p < 0.01), indicating higher DEET concentrations enhance extraction consistently across months.
General Observations Across the Study Period
DEET-treated setups outperformed controls in extracting Astigmatina, Mesostigmata, and other invertebrates. Each DEET concentration showed a distinct advantage for specific taxa, confirming the hypothesis that chemical repellents can be optimized for targeted extraction (Figure 7).
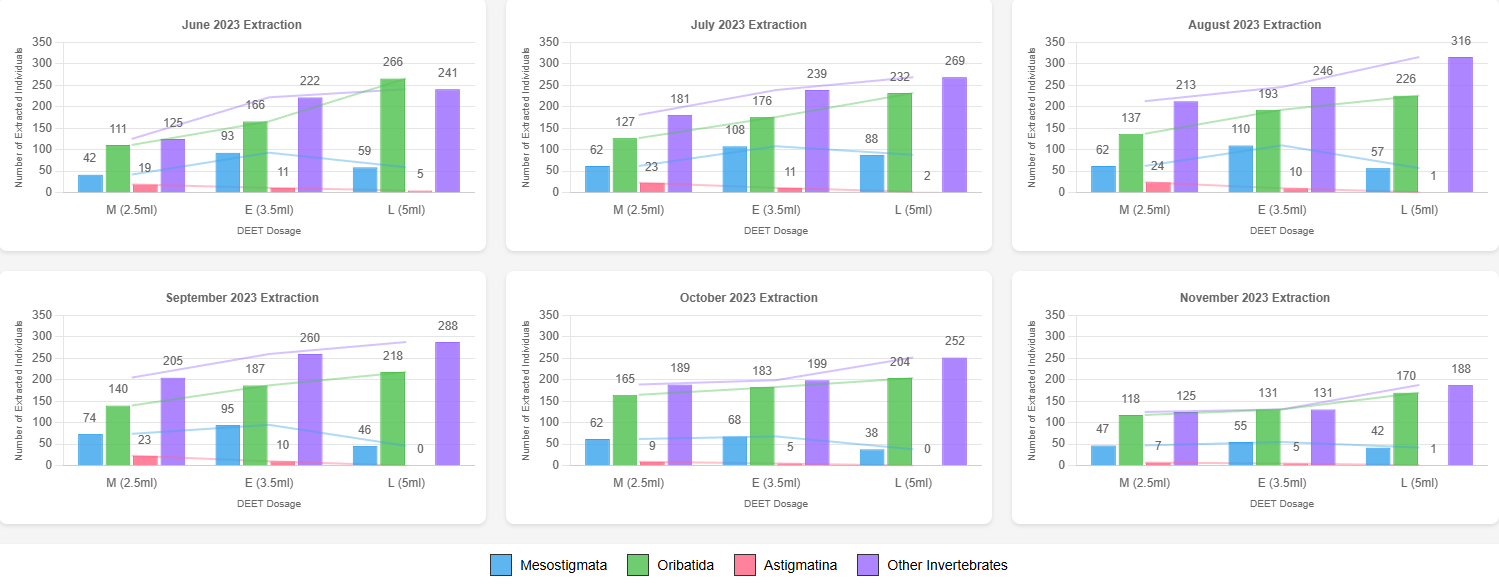


The DEET-treated samples were less contaminated with debris and dust compared to those extracted using the traditional heat and light gradient method. This resulted in improved sample clarity, making the counting process more efficient and less prone to confusion.
Control samples were most effective for Oribatida, which were often the most abundant taxon in the soil. However, DEET treatments, particularly at higher concentrations, extracted greater numbers of Oribatida and other invertebrates. Seasonal variability in soil activity influenced total extraction counts, with higher numbers generally observed during warmer periods, but the taxa-specific trends remained consistent. The proportions of extracted individuals between the control sample and the DEET-treated samples were similar across taxa, despite the varying number of individuals influenced by the season.
The results align with findings from previous studies, which highlighted the role of chemical repellents in influencing arthropod behavior (Debboun et al., 2007). This study extends these findings to soil ecosystems, demonstrating that DEET concentrations can be optimized for specific taxa, enhancing the precision and effectiveness of Berlese-Tullgren funnels.
Key Implications
The use of DEET as a chemical repellent offers significant advantages for soil fauna studies. By tailoring DEET concentrations to target specific taxa, researchers can achieve more efficient and focused extractions.
2.5 ml DEET: Best suited for Astigmatina, enabling targeted sampling of this sensitive group.
3.5 ml DEET: Optimal for Mesostigmata, balancing repellency with extraction efficiency.
5 ml DEET: Most effective for Oribatida and highly mobile invertebrates like springtails.
These findings provide valuable insights for researchers aiming to improve the specificity of soil arthropod sampling methods.
Discussion
This study confirms the utility of DEET as a chemical repellent in Berlese-Tullgren funnels, demonstrating improved extraction efficiency and specificity across mite orders and other invertebrates. DEET-treated setups consistently outperformed controls, aligning with previous findings on its efficacy as an arthropod repellent (Barnard and Xue, 2004).
Taxa-specific responses to DEET likely reflect differences in morphological and behavioral adaptations (Krantz and Walter, 2009). Astigmatina and Mesostigmata, characterized by their soft bodies and higher motility, exhibited greater sensitivity to DEET than the more sclerotized Oribatida. This pattern mirrors findings from repellent studies in agricultural pest management, where chemical deterrents selectively affect taxa based on physical and chemical resistance mechanisms (Debboun et al., 2007).
The potential of DEET to replace or supplement traditional heat-and-light extraction methods is promising, particularly for studies requiring selective sampling. However, questions remain regarding the broader ecological impacts of DEET, including its potential to disrupt soil microfauna communities or affect non-target species. Further research is needed to optimize concentrations, assess long-term effects, and expand applications to diverse soil environments.
Summary
This study highlights the potential of DEET as an innovative tool for improving soil fauna extraction. By enhancing specificity and efficiency in Berlese-Tullgren funnels, DEET opens new possibilities for ecological and taxonomic studies. Future research should focus on refining its application across varied ecosystems and evaluating its ecological impacts.
DEET significantly influenced the extraction efficiency of soil microfauna in modified Berlese-Tullgren funnels, with effects varying by concentration and organism group. The 3.5 ml DEET treatment was most effective for Mesostigmata, particularly in early summer (June/August), while 5.0 ml DEET maximized Oribatida and Other Invertebrates extraction but nearly eliminated Astigmatina. The 2.5 ml DEET treatment reduced extraction for most groups but increased Astigmatina yields. Monthly variations showed higher extraction in summer months (June/August) compared to November. Significant interactions for Mesostigmata and Oribatida suggest that DEETs effectiveness is modulated by seasonal factors, potentially related to environmental conditions like temperature or soil moisture, warranting further investigation for optimizing targeted mite extraction
Source of funding
Student project ID-UB.UAM096/34/UAM/0026.
References
- Berlese, A. (1905). Apparecchio per raccogliere presto ed in gran numero piccoli arthropodi. Redia, 2: 85-90.
- Tullgren, A. (1918). Ein sehr einfacher Ausleseapparat für terricole Tierfaunen. Zeitschrift für angewandte Entomologie, 4: 149-150. https://doi.org/10.1111/j.1439-0418.1918.tb00820.x
- Macfadyen, A. (1961). Improved funnel-type extractors for soil arthropods. The Journal of Animal Ecology, 171-184. https://doi.org/10.2307/2120
- Barnard, D. R., & Xue, R. D. (2004). Laboratory evaluation of mosquito repellents against Aedes aegypti. Journal of the American Mosquito Control Association, 20(3): 299-304.
- Crossley, Jr.(DA), & Hendrix, P. F. (2004). Fundamentals of Soil Ecology. Academic Press.
- Debboun, M., Frances, S. P., & Strickman, D. (2007). Insect repellents: Principles, methods, and uses. CRC Press. https://doi.org/10.1201/9781420006650
- Krantz, G. W., & Walter, D. E. (2009). A Manual of Acarology (3rd ed.). Texas Tech University Press.



2024-11-30
Date accepted:
2025-06-11
Date published:
2025-06-30
Edited by:
Tsagkarakou, Anastasia

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Rzepecki, Aleksander and Sikora, Bozena
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



