Bottle-Acaricide-Impregnation-Test (BAIT): an alternative novel bioassay for diagnosis of acaricide tick-tolerance and/or resistance
Ndawula, Charles Junior  1
; Sempijja, Fred2
; Emudong, Patrick3
; Muwereza, Nelson
1
; Sempijja, Fred2
; Emudong, Patrick3
; Muwereza, Nelson  4
; Abila, Patrick P'Odyek
4
; Abila, Patrick P'Odyek  5
and Kalyetsi, Rogers
5
and Kalyetsi, Rogers  6
6
1✉ National Agricultural Research Organization, P.O Box 295, Entebbe, Uganda & National Livestock Resources Research Institute, P.O Box 5704, Wakiso, Uganda.
2Uganda institute of Allied health and management Sciences-Mulago P. O Box 34025, Kampala, Uganda & Mbarara University of Science and Technology P.O Box 1410, Mbarara, Uganda.
3National Agricultural Research Organization, P.O Box 295, Entebbe, Uganda & National Livestock Resources Research Institute, P.O Box 5704, Wakiso, Uganda.
4National Agricultural Research Organization, P.O Box 295, Entebbe, Uganda & National Livestock Resources Research Institute, P.O Box 5704, Wakiso, Uganda.
5National Agricultural Research Organization, P.O Box 295, Entebbe, Uganda & National Livestock Resources Research Institute, P.O Box 5704, Wakiso, Uganda.
6Mbarara University of Science and Technology P.O Box 1410, Mbarara, Uganda.
2025 - Volume: 65 Issue: 2 pages: 575-583
https://doi.org/10.24349/jglt-6lu6Original research
Keywords
Abstract
Introduction
Ticks are blood-feeding ectoparasites capable of transmitting a broad range of pathogens, including bacteria, protozoa, viruses, nematodes, and fungi to humans and animals (de la Fuente et al. 2008). Tick-borne-disease (TBD) sustainable-control is attainable mainly through chemoprophylaxis and vaccination. However, due to the complexity of tick-borne pathogens, TBD-specific vaccines are elusive, yet chemoprophylactic drugs are expensive. Consequently, reduction of the animal-tick burden using acaricides remains the first line for control of TBDs (Rodriguez-Vivas et al. 2018). Although there are several ways of applying acaricides, such as spraying, dipping, and pour-on (Moraes et al. 2023), all methods aim to impregnate the acaricide onto the animal skin, which serves as a tick-decoy. Similarly, Davidson and colleagues (2024) have explored this approach in developing acaricide impregnated military uniforms toward control of human tick bite exposure. However, the prolonged and improper use of acaricides has led to the emergence of acaricide-resistant tick populations (Rodriguez-Vivas et al. 2018), thereby downplaying the significance of acaricides in TBD-control. Therefore, regular surveillance of the ticks' acaricide susceptibility is critical in determining the acaricide withdraw period and the effective acaricides. Until now, researchers have illustrated several assays for monitoring the acaricide resistant tick populations. These include the Larval Immersion Test (LIT) (Shaw 1966), Larval Tarsal Test (LTT) (Lovis et al. 2011), Syringe immersion test (SIT) (Shindhu et al. 2012), Nymph Immersion test (NIT) (Baker and Shaw 1965), and RaTexT® (Jongenjan et al. 2024). However, the Food and Agricultural Organization (FAO 2004) recommends only two methods: Larval Packet test (LPT) (Stone and Haydock 1962), and the Adult Immersion test (AIT) (Drummond et al. 1973). However, these methods have several drawbacks. For instance, (1) both LPT and AIT undermine the fundamental biological differences between one-host and multiple-host ticks. In particular, whereas the entire life cycle of the one-host ticks, such as R. microplus, prevails on the cattle for 30 days (Senbill et al. 2018), at each stage of the life cycle, multiple-host ticks, such as R. appendiculatus, stay on the animal for fewer days, feed to engorgement and detach to the ground (Troughton and Levin 2007). The implication is that, in contrast to multiple-host ticks, one-host ticks are consistently exposed to acaricides. Therefore, the accuracy of the acaricide bioassay could vary between the tick life cycle stages of the three-host ticks versus one-host tick. Additionally, differences in response to acaricides have also been observed between engorged (blood-fed) and unfed ticks (Delmonte et al. 2017). (2) assembly of the LPT assay requires specific additional reagents and materials, such as olive oil, bull-dog clips, Whatman filter paper (grade 1), and Trichloroethylene (FAO 2004), making the assay costly. These drawbacks highlight the need for novel methods which are simpler, cheaper, and universal to all tick life cycle stages. This research addresses FAO's (2004) long-standing call for the development of novel diagnostic tools to detect acaricide resistance in ticks that are affordable in resource-limited areas. Therefore, we propose an alternative novel assay, the Bottle-Acaricide-Impregnation-Test (BAIT). The proposed assay mimics the CDC bottle assay (Brogdon and McAlister 1998), but with modifications. Unlike the CDC bottle assay, the BAIT assay exploits the presence of the surface-active agent (surfactant) in the acaricide formulation. Similar to plant pesticides (Parr and Norman 1965), the surfactant promotes the reduction of the acaricide surface tension, leading to increased surface adsorption of the acaricide. Our hypothesis was that acaricides can independently adsorb onto other surfaces, such as plastic bottles, therefore, can be used for examining acaricide tolerance and/or resistance of ticks. This study addressed four fundamental research questions: (1) Is the BAIT assay as efficient as the currently FAO (2004) recommended methods (LPT and AIT)? (2) Is the BAIT-detection efficacy influenced by the class of acaricides? (3) Is the efficacy of the BAIT assay dependent on acaricide concentration? (4) Is the BAIT assay applicable to all stages of the tick's life cycle (larvae, nymphs, and adults)? Therefore, the goal of this study was to investigate the efficacy of the BAIT-assay in diagnosing acaricide resistant and/or tolerant tick-populations. The development of the novel alternative assay involves the impregnation of plastic sample bottles with selected types of acaricides from different classes diluted at single (1x) and double (2x) working concentrations. The acaricide-impregnated bottles were used to examine acaricide resistance among laboratory-bred Rhipicephalus appendiculatus ticks. The BAIT method was examined in comparison with the FAO (2004) recommended methods: LPT, NIT, and AIT. This novel assay could be applied globally for (1) laboratory and field conditions (2) diagnosis of acaricide-resistant or tolerant tick populations (3) assessing the efficacy of new acaricide formulations. Ultimately, diagnosis of acaricide resistant and/or tolerant tick populations guides farmers and decision makers; on the acaricide withdraw period and on the choice of the appropriate effective alternative acaricide(s).
Materials and Methods
Ethical review
The study protocol was reviewed by the Research Ethical Committee and the Faculty Research Committee of the Faculty of Medicine, Mbarara University of Science and Technology (Reference: MUST 2023-1166).
Materials and Methods
Ticks
The R. appendiculatus ticks used in this study were collected from Nakasongola district which is located in the cattle corridor of Uganda. Farmers in Nakasongola use different acaricides for tick control namely Amitraz, DuoDip (data unpublished). The ticks used in this study were maintained in the colony (Bailey 1960) at NaLIRRI-NARO for up to five generations without exposure to acaricides.
Chemicals
Three classes of acaricides: DuoDip 55% E.C. (organophosphate), Norotraz 12.5% E.C. (amidine) and SyperTix 10% E.C. (synthetic pyrethroid) were used for all assays. These represent the classes of acaricides used by the farmers in the study area. The acaricides were obtained from a farm supply shop and were certified by the National Drug Authority of Uganda. All the acaricides were independently diluted to single (1x) and double (2x) working concentration. At 1x concentration, the acaricide was diluted as recommended by the manufacturer whereas at 2x the acaricide recommended concentration was doubled.
Consumables
Magnifying lens, tally counter, white tray, white paper (A4 size), masking tape, fine art soft brushes, petri dishes (5 x 5 cm), forceps, olive-oil, trichloroethylene, bull dog clips, Whatman filter (grade 1, 25mm) paper, plastic sample bottles (100 ml) and cut pieces of nylon cloth.
The Bioassays
The Larval and Nymph packet Test
The LPT and NIT assay was conducted as described by Stone and Haydock (1962) and by Baker and Shaw, (1965) using unfed larvae and nymphs respectively, but with slight modifications. To prepare the LPT, the acaricides were independently diluted with two parts of Trichloroethylene and one part of Olive oil to single (1x) and double (2x) working concentration. Twenty milliliters of the mixture were dispensed into a petri dish and a filter paper of was immersed for 1 min to allow impregnation. The wet filter paper was left to stand for one hour in a running fume hood to allow air drying. The dried filter paper was folded in the middle and sealed at the sides using bulldog clips after which the unfed larvae were added. To prepare the NIT, the acaricides were diluted in water to single (1x) and double (2x) working concentration. Thereafter, the unfed nymphs were immersed into 5 ml of the diluted acaricide for one minute, removed, dried on a paper towel and finally transferred to the packet made-up of non- impregnated filter papers. For each assay, approximately, 100 active unfed larvae or nymphs were counted and gently dispensed into the packets and sealed using a third clip. The tick-packets was placed in an incubator at 27 °C, 85-90% RH and the acaricide-induced mortality was assessed after 24 h based on which the efficacy of was determined as described in the formular below.
Adult Tick Immersion test (AIT)
The AIT assays were conducted following the FAO, (2004) guidelines. The acaricides were independently diluted with water to 1x and 2x working concentration. The adult female unfed ticks were immersed into the test-acaricide formulation in a petri dish and let to immerse for 30 minutes with periodical agitation after every 10 minutes. Then the immersed ticks were transferred onto an acaricide-free dry paper towel. The dry ticks were placed into acaricide-free sample bottles, incubated at 27 °C, 85-90% RH and the acaricide effect assessed after 24 h. All assays were performed in triplicate for each test acaricide. Additionally, following the procedure described by Drummond and colleagues (1973) AIT assay was performed to assess effect of the acaricides on 10 engorged adult female ticks.
The BAIT assay for Larvae, Nymph and Adults
All acaricides were independently diluted in water to 1x and 2x working concentration Five milliliters of the diluted acaricide were dispensed into the new 50 ml polypropylene sample bottles (regularly used in hospitals for urine sample collection), covered with a nylon cloth (0.8- 1 mm mesh size), tilted at 45° while swirling for 5 min, after which the bottle was let to stand for 5 min. This was repeated three times to ensure total drenching and impregnation of the sample-bottle interior surface. Thereafter, the test-acaricide was discarded, the impregnated sample-bottles were thoroughly drained and let to air-dry for 1 hour at room temperature. At least 100 unfed larvae, nymphs or adults were counted and transferred to the acaricide impregnated bottles. All assays were performed in triplicates. Additionally, the efficacy of the BAIT assay against engorged female ticks was assessed following the same procedure but with a slight modification. Particularly in this case, because the engorged ticks are bigger than the unfed ticks, a bigger surface area is required to ensure tick-total contact with the surface of the product impregnated container. Therefore, petri dishes were used to ensure uniform acaricide-tick exposure and separation of the ticks to track their individual ability to lay eggs. After exposure to the acaricide impregnated petri dish, the ticks were assessed as described by (Drummond et al. 1973; FAO 2004).
Data collection and analysis
Assay efficacy assessment
Tick mortality
The assay-efficacy against the unfed ticks (larvae, nymph and adults) was assessed 24 h after test-acaricide exposure based on the acaricide-induced tick mortality. The acaricide-induced mortality was depicted based on the number of the immobilized ticks.
Tick resistance
The assay-efficacy against engorged adult female ticks was assessed 7 days post exposure. The percentage resistance was determined based on tick-egg laying ability of the acaricide-treated ticks versus the untreated ticks (FAO, 2004). The percentage resistance is calculated as indicated below:
Resistance (%) = (Nt/Nw) x 100
Nt is number of treated ticks laying eggs
Nw is number of untreated ticks laying eggs
Statistical Data analyses
Data were subjected to ANOVA at 99.05% (p=0.05) confidence level using R program (R-4.32 version). Where the mean square value was significant, data were further subjected to Post hoc test using Tukey's procedure Shapiro-Wilkson test (P=0.05).
Results
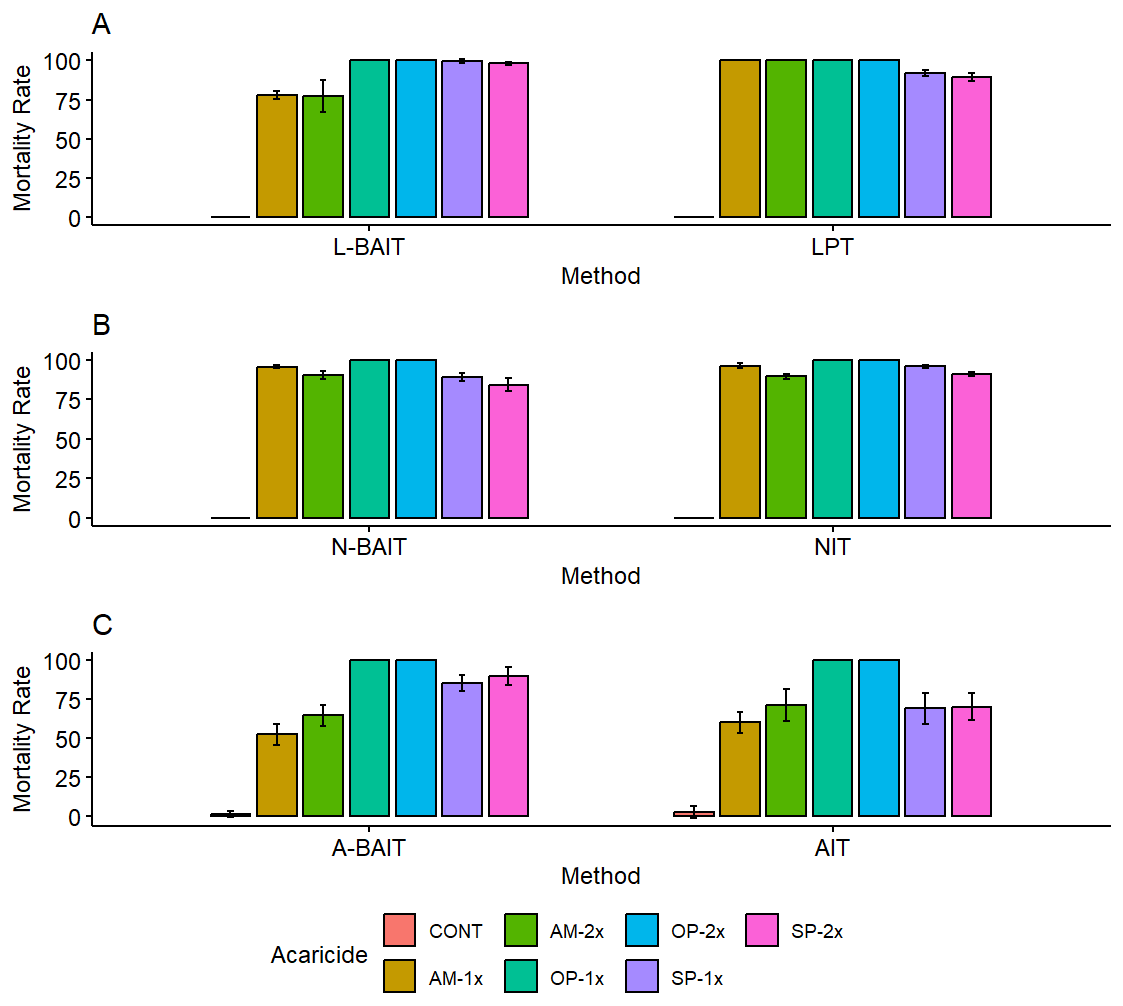
Based on the unfed-tick mortality rate, the efficacy of the BAIT assay was determined in comparison to the three commonly used assays (LPT, NIT and AIT) using three test acaricides diluted to single (1X) and at double (2x) working concentration. The BAIT assay was as efficient as the LPT, NIT and AIT (figure 1 A–C). With all the test acaricide, no difference in mortality was noted at single (1x) and double working concentration (p=0.381). A difference in mortality was noted among the three classes of acaricides and at the stage of tick development (larvae, nymph and adult). At the larval-stage, higher mortality was shown with the organophosphate (DuoDip) and Synthetic pyrethroid (SyperTix) acaricide than with the amidine (Nortotraz). A similar pattern was noted at the nymphal and the adult-stage. At all the tick-development stages, Amidines induced the lowest tick-mortality rate. Another research question was; is the BAIT assay also efficient in detecting resistance among engorged/fed ticks? To answer the question, the BAIT assay was compared with the AIT. Similarly, 3 test acaricides were used but at single (1x) concentration. The efficacy was determined based on the egg-laying ability post acaricide exposure.


Our data (Table 1) revealed difference between the efficacy of the BAIT and the AIT assay with different test acaricides. Both assays were equally efficacious with synthetic pyrethroids (SP) whereas with amidines (AM) and organophospates (OP), AIT was more efficacious than BAIT assay.
Download as
Method
Treatment
Conc
No. Treated-ticks
No. laying-Ticks
% Resistance
AIT
Water
0
10
10
100
AIT
OP
1x
10
0
0
AIT
AM
1x
10
0
0
AIT
SP
1x
10
10
100
A-BAIT
Water
0
10
10
100
A-BAIT
OP
1x
10
2
20
A-BAIT
AM
1x
10
10
100
A-BAIT
SP
1x
10
10
100
Discussion
Ticks are ranked second to mosquitoes in pathogen-transmission, because they are capable of transmitting pathogens ranging from viruses, bacteria, protozoa, to humans and animals (de la Fuente et al. 2008). One of the key factors that foster tick-pathogen transmission is the high egg-laying capacity and the ability to thrive under harsh field conditions. For instance, Ixodid ticks such as Amblyomma variegatum, R. appendiculatus lay at least 2000 eggs with over 90% egg viability (Leal et al. 2020). Therefore, tick-population reduction using acaricides remains fundamental in tick-borne disease control. However, after decades of acaricide application, populations of acaricide-resistant ticks have emerged globally (Rodriguez-Vivas et al. 2018).
Since the 1960s, detection of the acaricide-resistant tick population has relied on two FAO recommended methods: LPT and AIT (FAO 2004). Although the two methods are widely used, they have limitations. For instance, each method is specific to a particular tick stage of development, and both are limited to laboratory conditions. This study illustrates a novel alternative method for detecting acaricide resistant/ tolerant tick population; the Bottle Acaricide Impregnation Test (BAIT). The BAIT assay is similar to the CDC bottle assay that was developed by Brogdon and McAlister (1998). However, several modifications were made in the BAIT assay. For instance, whereas the CDC assay is performed using glass bottles, we opt to use plastic bottles which are cheaper and readily available. Additionally, unlike with CDC assay, the BAIT assay exploits the properties of acaricide formulation without addition of surface binding synergists. Therefore, while preparing the BAIT assay only was water added to the acaricide as per the manufacturer's instructions.
We also examined whether doubling the acaricide working-concentration could impact the BAIT method proficiency. Note that this mimics one of the common practices applied by farmers to enhance the efficacy of acaricides (Mugabi et al. 2010). On comparing the BAIT assay, with the LPT, NIT and AIT, we established that there was no significant difference in efficiency (figure 1 A–C) at both acaricide working concentrations (1x and 2x). The proficiency of the BAIT method could be attributed to presence of surfactant within the acaricide formulations which promotes lowering of the acaricide surface tension and formation of the surface film leading to: (1) increase in acaricide surface-dispersion (2) reduction of the acaricide wash-down. The role of surfactants is extensively discussed, for instance, in formulation of plant pesticides (Parr and Norman, 1965). Considering that acaricides constitute surfactant(s), addition of trichloroethylene and olive oil while preparing the LPT assays does not impact on the proficiency of the test, hence the additional chemicals are redundant. Instead, adding other chemicals increases the cost of the methods, and yet for instance, trichloroethylene is carcinogenic to the end user (Chiu et al. 2013). Despite their proficiency, the currently used methods (LPT, NIT and AIT) are restricted to a specific stage of tick development whereas the BAIT method is universal to all the stages. Additionally, the LPT, AIT and NIT require use ticks with a different feeding status. However, while developing the LPT versus AIT and NIT, the researchers neglected the physiological differences among the fed or unfed ticks and at different stages of development. For instance, differences in gene transcript expression have been revealed between fed and unfed ticks (Charrier et al. 2018; Ring et al. 2022). The acaricides could also influence the gene transcripts among fed and unfed ticks. We noted an inconsistence in response among the fed (engorged) and unfed adult ticks using the AIT and A-BAIT method (Table 1) (figure 1C). Similarly, Delmonte and colleagues (2017) illustrated differences in response to acaricides among fed and unfed nymphs. These findings emphasize the potential role of physiological differences in acaricide response; an aspect neglected by the currently recommended methods (FAO, 2004): LPT (Stone & Haydock, 1962) and AIT (Drummond et al. 1973). Our findings suggest concern over the accuracy of the current AIT method. We suggest that the BAIT method is conducted using unfed ticks (larvae, nymphs, adults). However, if the users intend to use engorged ticks, calculations should take the acaricide response against the emerging unfed larvae into account. The reason is that, as often shown in other drug-resistant biological organisms, the gene(s) coding for acaricide tick resistance is hereditable (Rodriguez-Vivas et al. 2018).
In addition to illustrating an alternative novel method for examining acaricide resistant/tolerant population, this study highlights the impact of concentration on acaricide potency. Doubling the acaricide concentration is a common practice used by farmers in desperate search for ways to kill acaricide resistant/ tolerant ticks (Mugabi et al. 2010). However, our findings show that doubling the acaricide concentration does not lead to increased acaricide potency, hence not relevant in controlling the resistant tick population.
Finally, whereas the study aim was to compare the efficacy of the BAIT assay versus the current assays (AIT, LPT, NIT), our data reveal difference in acaricide potency . This trend could be attributed to a difference in the active chemical ingredient. For instance, unlike the other test-acaricides (Norotraz and Sypertix), DuoDip consists of two active chemical ingredients: Chlorpyrifos and Cypermethrin (as per the manufacturers' description) explaining why it is more lethal than other acaricides.
Conclusion
This study demonstrates an alternative novel bioassay for detection of acaricide tick resistance and/or tolerance. In addition to showing an no significant difference in proficiency with the FAO recommended methods (LPT and AIT), the BAIT method has several merits over the other assays. (1) The novel method is less costly especially because apart from the plastic sample bottles no other chemicals and consumables are required. Moreover, because of the acaricide-surface adsorption effect, once impregnated, the novel assay bottles could be re-used without re-addition of the test acaricide (data unpublished). (2) It is applicable to all stages of the tick life cycle and potentially to all tick species. (3) It is applicable both under field and laboratory conditions. (4) It does not require highly trained personnel. Ultimately, because of the aforementioned properties, the BAIT method simplifies diagnosis of the acaricide tick resistance/ tolerance, and it is affordable to the resource-limited areas, hence suited for global use.
Role of the funding source
This article has been produced with the financial assistance of the European Union (Grant no. DCI-PANAF/2020/420–028), through the African Research Initiative for Scientific Excellence (ARISE), pilot program. ARISE is implemented by the African Academy of Sciences with support from the European Commission and the African Union Commission. The contents of this document are the sole responsibility of the author(s) and can under no circumstances be regarded as reflecting the position of the European Union, the African Academy of Sciences, and the African Union Commission.
Acknowledgments
We thank (1) Dr. Dania Richter of the Technische Universität Braunschweig, Institute of Geoecology, Landscape Ecology & Environmental Systems Analysis for the support offered during preparation of the manuscript. (2) Mr. Omwene E, Mr. Mayeku M and Mr. Omuno E for the support offered in maintaining the tick colony and assessing the efficacy of the novel assay. (3) The National Livestock Resources Research Institute which is under the National Agricultural Research Organization for hosting this research study.
References
- Bailey K.P. 1960. Notes on the rearing of Rhipicephalus appendiculatus and their infection with Theileria parva for experimental transmission. Bull. Epizoot. Dis. Afr. 8, 33-43.
- Baker J.F.A, Shaw R.D. 1965. Toxaphene and lindane resistance in Rhipicephalus appendiculatus, the brown ear tick of equatorial and southern Africa. J S Afr Vet Med Ass. 36:321-330.
- Brogdon W.G, McAllister J.C. 1998. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc. 14:159-164.
- Charrier N.P., Couton M., Voordouw M.J., Rais O., Durand-Hermouet A., Hervet C., Plantard O., Rispe C. 2018. Whole body transcriptomes and new insights into the biology of the tick Ixodes ricinus. Parasit Vectors. 11: 364. https://doi.org/10.1186/s13071-018-2932-3
- Chiu W.A., Jinot J., Scott C.S., Makris S.L., Cooper G.S., Dzubow R.C., Bale A.S., Evans M.V., Guyton K.Z., Keshava N., Lipscomb J.C., Barone S., Jr Fox J.F., Gwinn M.R., Schaum J., Caldwell J. C. 2013. Human health effects of trichloroethylene: key findings and scientific issues. Environ Health Perspect. 121: 303-311. https://doi.org/10.1289/ehp.1205879
- Davidson S.A, Nun D.J, Chellaraj A.H., Johnson J.Y, Burgess AM, Dehemer S, Milner EE. 2024. Reduced effectiveness of permethrin-treated military uniforms after prolonged wear measured by contact irritancy and toxicity bioassays with Ixodes scapularis (Acari: Ixodidae) nymphs. J Med Entomol. 61:1181-1189. https://doi.org/10.1093/jme/tjae080
- de la Fuente J., Estrada-Pena A., Venzal J.M., Kocan K.M., Sonenshine D.E. 2008. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13: 6938-6946. https://doi.org/10.2741/3200
- Delmonte C., Cruz P.B., Zeringóta V., de Mello, V., Ferreira, F., Amaral, M.D.P.H., Daemon, E. 2017. Evaluation of the acaricidal activity of thymol incorporated in two formulations for topical use against immature stages of Rhipicephalus sanguineus sensu lato (Latreille, 1806) (Acari: Ixodidae). Parasitol Res. 116: 2957-2964. https://doi.org/10.1007/s00436-017-5604-x
- Drummond R.E.A., Ernst S.E., Trevino J.L., Gladney W.J., Graham O.H. 1973. Boophilus annulatus and B. Microplus: Laboratory Tests of Insecticides. J. Econ. Entomol. 66: 130-133. https://doi.org/10.1093/jee/66.1.130
- Food and Agriculture Organization (FAO) of the United Nations. 2004. Guidelines Resistance Management Integrated Parasite Control Ruminants, Module 1:56
- Jongejan F., Berger L., Reck J., Ferreira P.T., de Jesus M.S., Scott F.B., de Avelar B.R., Guimarães BG, Correia TR., Muhanguzi D., Vudriko P., Byaruhanga J., Tumwebaze M., Nagagi Y., Temba V., Biguezoton AS., Farougou S., Adehan S., Jumba H., Homminga L., Hulsebos I., Petersen A., Klafke G. 2024.
- RaTexT®: a novel rapid tick exposure test for detecting acaricide resistance in Rhipicephalus microplus ticks in Brazil. Parasit Vectors. 2024 Aug 28;17(1):365. https://doi.org/10.1186/s13071-024-06448-6
- Leal B., Zamora, E., Fuentes A., Thomas D.B., Dearth R.K. 2020. Questing by Tick Larvae (Acari: Ixodidae): A Review of the Influences That Affect Off-Host Survival. Ann. Entomol. Soc. Am. 113: 425-438. https://doi.org/10.1093/aesa/saaa013
- Lovis L., Reggi J., Berggoetz M., Betschart B., Sager H. 2013. Determination of acaricide resistance in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) field populations of Argentina, South Africa, and Australia with the Larval Tarsal Test. J. Med. Entomol. 50: 326-335.
- Moraes N., Nicaretta J.E., Rodrigues D.C., Gonzaga B.C.F., Barrozo M.M., Vale F.L., Pereira E Sousa L.J., Coutinho A.L., Gomes G.W., Teixeira W.F.P., Lopes W.D.Z., Monteiro C. 2023. Comparison of the efficacy of different methods to apply acaricides for control of Rhipicephalus (Boophilus) microplus. Ticks Tick Borne Dis. 14: 102190. https://doi.org/10.1016/j.ttbdis.2023.102190
- Mugabi K.N., Mugisha, A., Ocaido, M. 2010. Socio-economic factors influencing the use of acaricides on livestock: a case study of the pastoralist communities of Nakasongola District, Central Uganda. Trop Anim Health Prod. 42: 131-136. https://doi.org/10.1007/s11250-009-9396-6
- Parr J.F., Norman A.G. 1965. Considerations in the Use of Surfactants in Plant Systems: A Review. Bot. Gaz. 126: 86-96. https://doi.org/10.1086/336300
- Ring K., Couper L.I., Sapiro A.L., Yarza F., Yang X.F., Clay K., Mateusiak C., Chou S., Swei A. 2022. Host blood meal identity modifies vector gene expression and competency. Mol. Ecol. 31: 2698-2711. https://doi.org/10.1111/mec.16413
- Rodriguez-Vivas R.I., Jonsson N.N., Bhushan C. 2018. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol. Res. 117: 3-29. https://doi.org/10.1007/s00436-017-5677-6
- Senbill H., Hazarika L.K., Baruah A., Borah D.K., Bhattacharyya B., Rahman S. 2018. Life cycle of the southern cattle tick, Rhipicephalus (Boophilus) microplus Canestrini 1888 (Acari: Ixodidae) under laboratory conditions. Syst. Appl. Acarol. 23: 1169 - 1179. https://doi.org/10.11158/saa.23.6.12
- Shaw R.D. 1966. Culture of an organophosphorus-resistant strain of Boophilus microplus (Can.) and an assessment of its resistance spectrum. Bul. Entomol. Res. 56: 389-405. https://doi.org/10.1017/S0007485300056480
- Sindhu Z.U., Jonsson NN., Iqbal Z. 2012. Syringe test (modified larval immersion test): a new bioassay for testing acaricidal activity of plant extracts against Rhipicephalus microplus. Vet. Parasitol, 188: 362-367. https://doi.org/10.1016/j.vetpar.2012.03.021
- Stone B.F., Haydock K.P. 1962. A method for measuring the acaricide-susceptibility of the cattle tick Boophilus microplus (Can.). Bul. Entomol. Res. 53: 563-578. https://doi.org/10.1017/S000748530004832X
- Troughton D.R., Levin M.L. 2007. Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J. Med. Entomol. 44: 732-740. https://doi.org/10.1093/jmedent/44.5.732



2025-02-14
Date accepted:
2025-05-27
Date published:
2025-06-11
Edited by:
Marčić, Dejan

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Ndawula, Charles Junior; Sempijja, Fred; Emudong, Patrick; Muwereza, Nelson; Abila, Patrick P'Odyek and Kalyetsi, Rogers
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



