Progress in understanding the world mesostigmatic mites, with emphasis on the family Phytoseiidae (Acari: Mesostigmata)
de Moraes, Gilberto J.  1
; Castilho, Raphael de Campos
1
; Castilho, Raphael de Campos  2
; Flechtmann, Carlos H.W.
2
; Flechtmann, Carlos H.W.  3
; Demite, Peterson R.
3
; Demite, Peterson R.  4
and Halliday, Bruce
4
and Halliday, Bruce  5
5
1✉ Depto. Entomologia e Acarologia, Escola Superior de Agricultura "Luiz de Queiroz" (ESALQ), Universidade de São Paulo (USP), 13418-900 Piracicaba, São Paulo, Brazil.
2Depto. Entomologia e Acarologia, Escola Superior de Agricultura "Luiz de Queiroz" (ESALQ), Universidade de São Paulo (USP), 13418-900 Piracicaba, São Paulo, Brazil.
3Depto. Entomologia e Acarologia, Escola Superior de Agricultura "Luiz de Queiroz" (ESALQ), Universidade de São Paulo (USP), 13418-900 Piracicaba, São Paulo, Brazil.
4Laboratório de Acarologia, Departamento de Ciências Biológicas, Universidade Estadual Paulista (UNESP), 15054-000, São José do Rio Preto, São Paulo, Brazil & Campus Bonfim, Instituto Federal de Roraima, 69380-000, Bonfim, Roraima, Brazil.
5Australian National Insect Collection, CSIRO, Canberra, Australia.
2025 - Volume: 65 Issue: 3 pages: 647-676
https://doi.org/10.24349/q3gy-1vygPresented at X EurAAc Symposium 2–6 September 2024 Athens
Keywords
Abstract
Introduction
This paper is an adaptation of a talk presented by the first author in the 10th EURAAC (European Association of Acarologists) Symposium, held at Athens, Greece in September 2024. Acarology is a relatively young science that has evolved quickly in recent years because of the growing importance of mites in agriculture as pests and biological control agents, and because of their role as vectors of pathogens of human beings, domesticated animals, and plants (Bregetova 1966; Krantz and Walter 2009; Walter and Proctor 2013; Vacante 2015).
The objective of this publication is to review the history of research on the mites of the order Mesostigmata. The historical review provides the context for a discussion of the present state and future development of studies of the Mesostigmata, with emphasis on the family Phytoseiidae. This family contains most of the species of predatory mites that have been used for the biological control of harmful arthropods in agriculture worldwide.
Factors affecting the early development of Acarology
Prasad (1982) provided a comprehensive account on the history of Acarology, providing important information from 25 countries. That historical review identified several factors that have had significant impact on the development of Acarology worldwide, as summarised in Table 1.
Download as
1750’s
Compound microscopes
1900’s
Berlese-Tullgren funnel
1940’s
Synthetic pesticides widespread use
1950’s
Research in mite-borne disease
1950’s
Training of new acarologists, especially from mid 1900’s
1960’s
Establishment of specialised acarology journals
1960’s
Commercial interest in biological control
Perhaps because they are visible to the naked eye, and because they directly affect human beings and domesticated animals, ticks were the first mites to be referred to in art and literature. Objects that appear to be ticks can be seen in a sculpture from an Egyptian tomb dated about 1,500 years BCE (Arthur 1965). Although not named, references to animals believed to be ticks as the cause of diseases were also found in an ancient Egyptian papyrus written over 1,000 years BCE. Homer also referred to ticks in his classic books Odyssey and Iliad in about 800 BCE. About 500 years later, Aristoteles was not only able to see ticks on different animals, which he called kroton and kynoraistes, but also smaller mites, which he called akari, scolekes and phtheires (Emmanuel 1982). Ticks were also shown in sculptures in a Bacchus temple in Lebanon, about 150 CE (Gorirossi-Bourdeau 1995).
Hildegard von Bingen was probably the first person in Germany to publish information about mites, when she referred to a mite that parasitises both human beings and domesticated animals, and which is much smaller than ticks (Alberti 2005). She lived from 1098 to 1179, and wrote a note about Sarcoptes scabiei (DeGeer) (the itch mite; Sarcoptidae) in the book Liber Subtilitatum Diversarum Naturarum Creaturarum.
Because of the small size of most mites, more significant reports about them were only possible from the 1700's, when major improvements of compound microscopes took place in Europe (Joblot 1754). The small size of most mites and the need for microscopes to study them was stressed early in the history of Acarology (Geoffroy 1762; DeGeer 1778). Even at this early stage, improved instruments allowed the observation of mite morphology and behaviour in amazing levels of detail. This was the first main characteristic highlighted in the opening speech of the Third International Congress of Acarology (Rosicky 1971) as important in driving the development of our knowledge about mites. Occurrence of major pandemics also seems to have affected the development of Acarology, by drawing attention to vector-borne diseases.
The early development of Acarology was hampered by the fact that most mites are found in the soil, which made collecting them difficult for early workers. The successful collecting of large numbers of soil mites was greatly facilitated by the extraction methods developed by the Italian Antonio Berlese and the Swedish Hugo Albert Tullgren. The collecting devices they developed, or further adaptations of them, are still widely used today for the same purpose. The development of effective slide mounting media also had a tremendous impact on the study of microarthropods, as described by Micherdziński (1966).
The use of chemical pesticides for pest control was widely adopted worldwide soon after World War II, especially following the development of DDT and BHC. That development seemed at first to be the solution to pest problems in wide-scale food production, but these products and others soon became the cause of recurrent problems, in part by the elimination of natural enemies, and in part by the use of plant varieties that were more readily attacked by pests (Chant 1959a). The increased problem with arthropod pests, including plant-feeding mites, led to increasing interest in studying those harmful organisms and their natural enemies, in an attempt to improve the efficacy of control practices. Reference to the beneficial value of predatory mites was made as early as Ewing (1913), and the commercial production of predatory mites for the biological control of pests developed significantly in the 1960–1970's.
The increasing interest in those studies resulted in the need for the establishment of training programs and the concurrent development of appropriate educational support. An international training program in Acarology was put in place in the USA, initiated at Duke University (North Carolina) in 1951, moved to the University of Maryland in 1954, and then to the Ohio State University in 1961, where it was taught annually until 2018. During that period, over 1,700 professionals or students were trained in at least one of its three modules (Introduction to Acarology, Agricultural Acarology, and Medical and Veterinary Acarology). More recently, that training program moved to its current location at the University of Arkansas. Another program of similar nature was offered at the University of Nottingham, England, from1962 to 1987. The Société des Acarologues de Langue Française conducted an acarology training program in the French language at several European universities during the 1980's. Other training programs were or have been held in different countries on a smaller scale.
Those training programs, and the science of Acarology in general, made use of a growing number of textbooks and other important publications, including Murray (1877), Banks (1905, 1915), Vitzthum (1931, 1943), Baker and Wharton (1952), Baker et al. (1956, 1958), Strandtmann and Wharton (1958), Krantz (1970, 1978), Jeppson et al. (1975), Evans (1992), Coineau et al. (1997), Camicas et al. (1998), Fernandez and Coineau (2007), Pérez-Eid (2007), Krantz and Walter (2009), Hoy (2011), Walter and Proctor (2013) and Vacante (2015). Another important development was the launching of journals specialised in Acarology, such as Acarologie – Schriftenreihe für Vergleichende Milbenkunde, published privately by Werner Hirschmann between 1957 and 1993, as well as Acarologia (starting in 1959), International Journal of Acarology (1975), Experimental and Applied Acarology (1985), Acarina (1993), Systematic and Applied Acarology (1996) and others. Another extremely helpful trend in the promotion of Acarology was the initiation of the International Congress of Acarology. This worldwide gathering of acarologists was first held at Colorado State University, Fort Collins, USA, in 1963, and then approximately every four years, with the most recent meeting in New Zealand in 2022.
The initial development of mite taxonomy and biology
Animal systematics as presently understood began in 1758, when the 10th edition of Systema Naturae was published by Carolus Linnaeus (Linnaeus 1758). That publication marked the introduction of binominal nomenclature, in which the scientific name of a species consists of a statement of the genus name followed by its specific name. In that particular publication, each name was followed by a brief description of the species.
Linnaeus listed 31 species of mites (and two species of pseudoscorpions) in a genus he then designated as Acarus, in the order Aptera of the class Insecta. That number seems to have been much lower than reported in the literature at the time, which was about 90 according to Micherdziński (1966). Linnaeus' list included the following numbers of mite species, here presented in their current higher mite taxa: Mesostigmata, 3; Oribatida: Astigmatina (Sarcoptiformes), 5; other Oribatida, 4; Ixodida, 6; and Prostigmata (Trombidiformes), 13. It is noteworthy that Linnaeus' list included most of the different major mite groups recognised today, missing only Opilioacarida and Holothyrida, which were established much later. The listed Mesostigmata are presently placed in three different genera, as Asca aphidioides (Linnaeus) (Ascidae), Parasitus coleoptratorum (Linnaeus) and Pergamasus crassipes (Linnaeus) (both Parasitidae). Each of these species was designated as type species of its respective genus. In a footnote on page 617, Linnaeus (1758) noted the small size of mites, their ubiquity and abundance, and their importance as pests. Apparently for the first time, he referred to them as Acari, the plural of Acarus.
Linnaeus (1758) also listed the species Pediculus vespertilionis in Insecta – Aptera. It was later considered to belong to the mite family Spinturnicidae, but it was the subject of great taxonomic and nomenclatural confusion (Collins 1930) and was eventually invalidated by the International Commission on Zoological Nomenclature (Opinion 128, 1936).
Two other mite genera were described soon after Linnaeus (1758). Hydrachna Müller, 1769 (apud Müller 1776), with 48 species of aquatic mites, and Trombidium Fabricius, 1775, with three species of distinctly red terrestrial mites. Both generic names are still associated with the same groups of species.
Latreille (1795) used the French term ''Tiques'' for a series of species in the genera Argas, Atomus, Ixodes, Pycnogonum, Bdella, Hydrachna, Trombidium, Acarus, Parasitus, Siro and Chelifer. While most of these genera are still recognised as mites today, they also include a sea-spider, a pseudoscorpion, and a harvestman. Latreille (1796) then used the name ''Acephales'' for the same group, with the addition of the genera Nycteribia, Carios, Leptus, Cheyletus, Smaris, Limnochares, Eylais, Carpais, Tyroglyphus, Tarantula, Aranea, Galeodes, Scorpio and Phalangium. As before, several of these genera are now placed in other groups of Arachnida or Insecta. Latreille (1802) arranged these genera in a series of groups called Acaridies (= Acaridiae), Hydracnelles (= Hydrachnellae), and Tiques (= Riciniae), which excluded the other groups of Arachnida. Latreille (1806) continued to place the mites and other arachnids in the Insecta Aptera, in a group he called Acera (= Acères), with a greatly increased number of genera.
Oudemans (1896a, b) presented some notes on the history of previous work on mites, especially the work of Linnaeus, Fabricius, Latreille, and Müller, but we cannot confirm all his interpretations.
The early (1795) description of the two speciose tick genera (Argas and Ixodes) is consistent with the importance of ticks as mammal parasites, and their relatively large size. The early description of the mesostigmatic genus Parasitus also suggests the common occurrence of this group in Europe, and its early recognition led to the importance of this name in mite nomenclature, and its adoption as the root of names of major mite taxa (Parasitiae; Parasitidae Oudemans, 1901; Parasitinae; Parasitoidea, Parasitina Johnston, 1982 and Parasitiformes Krantz, 1978). The name Parasitus was first used by Latreille (1795), but Latreille (1796) replaced Parasitus with Carpais without any explanation. Latreille (1802) then further replaced Carpais with Gamasus, referring to Carpais as a ''trop dure'' (too harsh). According to the author, Gamasus was a suitable Greek word to express the agility of these mites, but his indecision led to later ambiguity in the use of the alternative names Parasitidae Oudemans, 1901 and Gamasidae Leach, 1815 for the same family of mites. Despite the earlier availability of Gamasidae in the literature, most authors use the name Parasitidae Oudemans, probably because Gamasus is a junior synonym of Parasitus. These nomenclatural changes were reviewed by Oudemans (1896b) and Hrúzová and Fenďa (2018).
Cuvier (1798) listed five Acarus species (sensu Linnaeus) referred to as mites, and an unspecified number of species of Hydrachna that he cited as synonymous with Trombidium. He associated the latter with harvestmen, and provided brief details on the morphology and ecology of all of these organisms. Lamarck (1801) was the first to distinguish the arachnids as a separate class, placing them among his ''Arachnides palpistes'', which included the mite genera Acarus, Bdella, Eylais, Hydrachna and Trombidium, as well as the non-mite groups mentioned by Latreille (1796), in addition to amblypygids and harvestmen. However, Latreille (1806, 1810) still referred three mite genera he had described in 1796 to the Insecta - Aptera. Leach (1815) also grouped the mites known at the time with several members of present-day Arachnida in what he called ''class Arachnides″. Conversely, Sundevall (1835) cited the genera described by Latreille (1796) (Astoma [sic], Caris [sic] and Leptus) as well as Achlysia and Ocypeta, in a''family" of the ''order'' Acari. Haller (1881) and Canestrini (1891) placed the mites in a new ''class'' (Acaroidea), at the same level as crustaceans, arachnoids, myriapods and insects.
In terms of mesostigmatic biology, important discoveries were reported by different authors as early as the beginning of the 19th century. Amazing details about the behaviour of these mites, the importance of their first pair of legs as a sensor of environmental stimuli and the relation of these and other structures with those of insects and crustaceans were published almost 200 years ago (Robineau-Desvoidy 1830). And that is despite the major limitation of the time in terms of quality of available microscopes. Dugès (1834b) observed the quick death of most ''Gamasus'' species when taken away from their natural habitat, unless kept in a jar with a water source. The requirement of ''some moisture in the atmosphere'' for the survival of ''Gamasinae'' was later stressed by Michael (1892). This discovery is of major importance in the practical use of some of mesostigmatic species for pest control.
Dugès (1834b) also detailed the process of attachment and detachment of uropodines (Mesostigmata) to their coleopterous carriers. In addition, he reported the parasitism of vertebrates by Dermanyssus species (Mesostigmata: Dermanyssidae), as well as predation by ''Gamasus'' on other mites, including tetranychids, with details of the relationship between the morphology of these mites and their feeding behaviour (Dugès 1834a, b). Dugès (1834b) also referred to the considerable morphological differences between the chelicerae of males and females of Dermanyssus, which led him to believe that different sexes of the same species belonged to different species. A detailed description of the morphology, biology and behaviour of Mesostigmata was provided in the late 19th century by Mégnin (1876).
The considerable alteration in morphology from the first to the subsequent developmental stages led Trouessart (1892) to realise that mites in general undergo metamorphosis during their developmental process, including a six-legged larval stage. The presence of only six legs in the first post-embryonic stage had already been reported by Dugès (1834a) and Mégnin (1876), both of whom referred to it as a larva. Canestrini (1891) provided details of the different mite developmental stages, based on his own observations as well as those of other renowned acarologists, and used them as the basis of his proposed classification system (Ragusa 2002).
The absence of basic data on mite biology and taxonomy led to some incorrect conclusions in the early literature. An example is the confusion concerning the deutonymphal stage of some astigmatic mites. Dugès (1834b) described the genus Hypopus for a minute mite he found attached to a beetle (Hister sp.), and included several other species in the genus. Dujardin (1849) mistook Hypopus for the juvenile stage of a gamasid species, and Nicolet (1855) interpreted it as a parasitic stage of a gamasid that attacked oribatids. Mégnin (1876) later attributed that mistake to the high degree of sclerotisation of the idiosoma of those deutonymphs. The convoluted history of the name Hypopus was reviewed by Michael (1884).
Mégnin (1876) also discussed synonymies presented by some early authors, who applied different names to different stages and sexes of the same mesotigmatid species. He also corrected a common assumption of the time, presented by Dugès (1834b), that gamasids were mostly parasites of invertebrates and vertebrates. Michael (1892) supported Mégnin's concept, indicating that those mites were mostly predators, and corrected Dugès' conclusions about the morphology and behaviour of the structures involved in the mating process.
The number of described mite taxa increased considerably between 1758 and 2011 (Table 2). In that period, the number of species increased from nearly 30 to about 55,000, across all mite orders (Beaulieu et al. 2011). This enormous increase in the number of species was followed by efforts to determine the relationships among them, and their possible evolutionary relationships.
Download as
Linnaeus (1758)
Dugès (1834a)
Koch (1842)
Canestrini (1891)
Vitzthum (1931)
Baker et al. (1958)
Wharton (1964)
Zhang et al. (2011)
Families
–
7
13
33
39
189
–
526
Genera
1
24
75
199
259
–
–
5,611
Species
29
>60
832
–
5,376
–
17,5
54,617
Mite classification systems
About 20 years after the publication of the 10th edition of Systema Naturae, DeGeer (1778) highlighted the difficulty of grouping mites according to their morphological features. He mentioned that despite the observed differences in dimensions of the legs between different mite species, it remained difficult to establish the limits of these variations, and proposed instead grouping mites according to their preferred microhabitats. Latreille (1795) referred to that conclusion, and separated the mites into distinct genera without referring to the relationships among them. Micherdziński (1966) pointed out that the small number of acarologists were overwhelmed by the abundance of mites available for study, which lead to a lack of solid, lasting foundations for the development of mite systematics. In the 19th Century the overwhelming numbers of new forms discovered was so great that new species and genera were given very brief descriptions, often without illustrations, and little time was devoted to more careful revisionary taxonomic study.
The first proposals for the classification of mites in the beginning of the 1800's, were based on their morphological similarities, such as Hermann (1804). At that time, the taxonomic value of different structures was not yet understood. Initial classifications were based on characteristics of the gnathosoma (palp, chelicera, epistome) and idiosoma (shape, degree of sclerotisation, degree of fusion of dorsal plates), as well as the number of legs. Other characteristics taken into account included the respiratory system, followed by the extent and degree of fusion of the ventral idiosomatic plates. More recently, information from the chaetotaxy of the idiosoma and appendices, the basic types of setae, the presence and constitution of the pretarsi, and the spermathecae, was added to the analysis.
Berlese (1896) published a detailed account of the variation in the digestive system in mites in general, referring to previous works on that subject published by other authors. However, Micherdziński (1966) pointed out that these structures were not useful in identifying major mite groups. Oudemans (1906) developed a classification based on the characteristics of the respiratory system, but most of his taxa and the names he applied to them did not survive revisions by later authors.
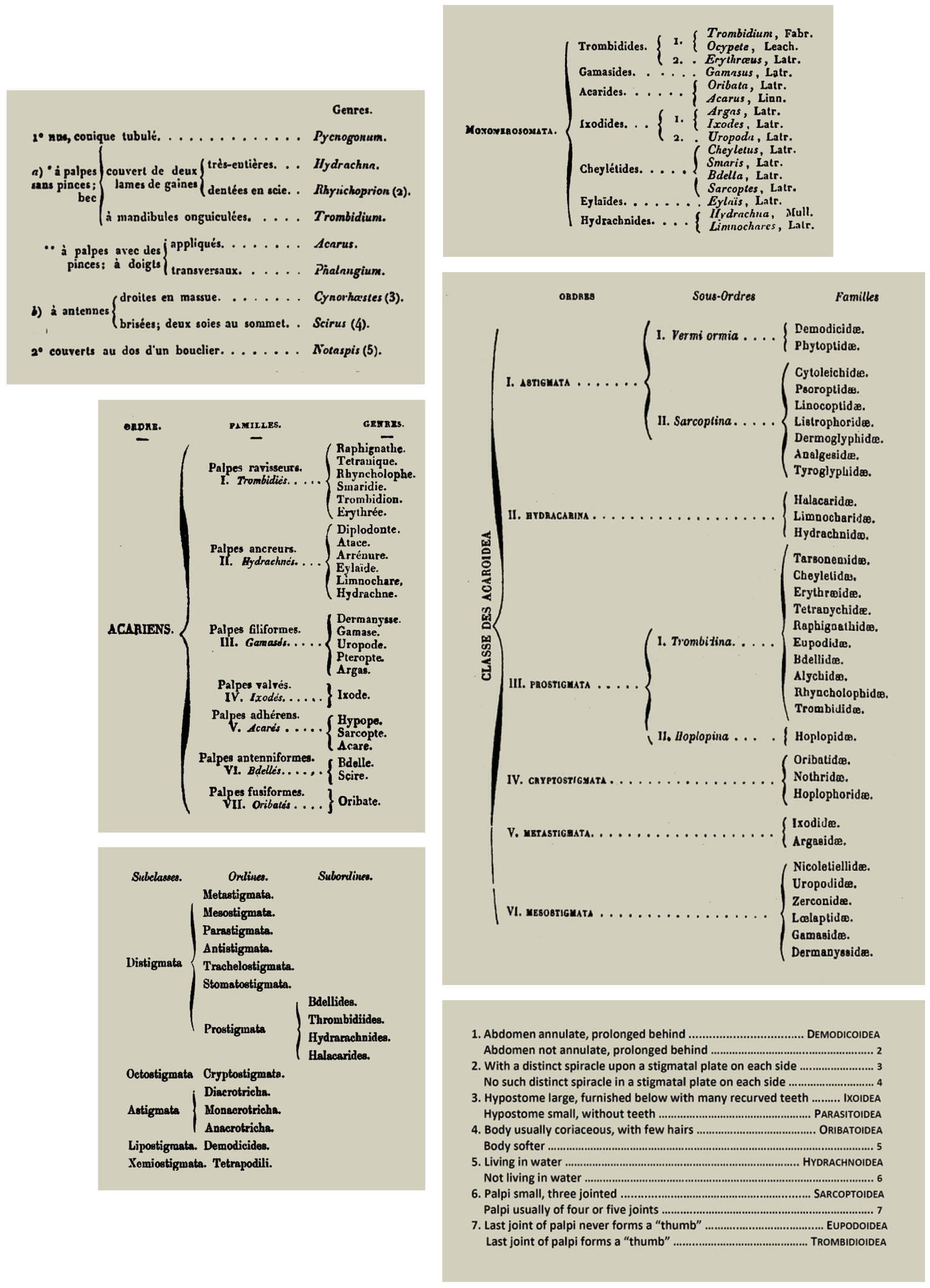


Many different classification systems for the suprageneric groups of mites have been proposed (Table 3). Differences between these systems reflect differing opinions as to the taxonomic importance of different sets of structures. The discovery of new taxa and new character states of the selected structures led to many changes in the concepts of previously recognised taxa. The resulting classifications are only of historic interest, but they represent important steps in the development of the modern system (Figure 1).
Download as
Hermann (1804)
Trouessart (1892)
Latreille (1806, 1810)
Berlese (1899, 1913)
Leach (1815)
Oudemans (1896–1936)
von Heyden (1826)
Banks (1915)
Dugès (1834a)
Vitzthum (1931, 1942)
Sundevall (1835)
Hirschmann (1938–1946)
Koch (1842)
Johnston (1965)
Gervais (1844)
Krantz (1970, 1978)
Nicolet (1855)
Evans (1992)
Gervais and van Beneden (1859)
Krantz and Walter (2009)
Kramer (1877) + Canestrini (1891)
Many authors attempted revisions of these early systems of mite classification, including Gervais (1844), Trouessart (1891), Oudemans (1896a), the monumental contribution by Vitzthum (1943), and importantly the textbook by Baker and Wharton (1952). The influential work published by Johnston (1965) represents an important transition between the initial and later proposals, especially in the increased subdivision of the Acariformes (Table 4). Significantly, Donald Johnston was the scientific coordinator of the Acarology Summer Program at the Ohio State University, and the views presented there proved to be very influential in the development of modern acarology.
Download as
Order Opilioacariformes
Order Acariformes
Suborder Opilioacarina (or Notostigmata)
Suborder Tetrapodili
S. Eleutherengona
Order Parasitiformes
S. Tarsonemini
Suborder Holothyrida
S. Labidostommei
S. Mesostigmata
S. Parasitengona
S. Ixodides
S. Endeostigmata
S. Palaeacari
S. Oribatei
S. Acaridei
Molecular data is being taken into account to an increasing degree as a source of information in systematics, including in Acarology. This new tool is playing a growing role in the analysis of the classification of the taxa described at all taxonomic levels, and has largely confirmed early decisions based on characteristics of the respiratory system, such as those of Kramer (1877) and Canestrini (1891).
Basic structure of the Mesostigmata
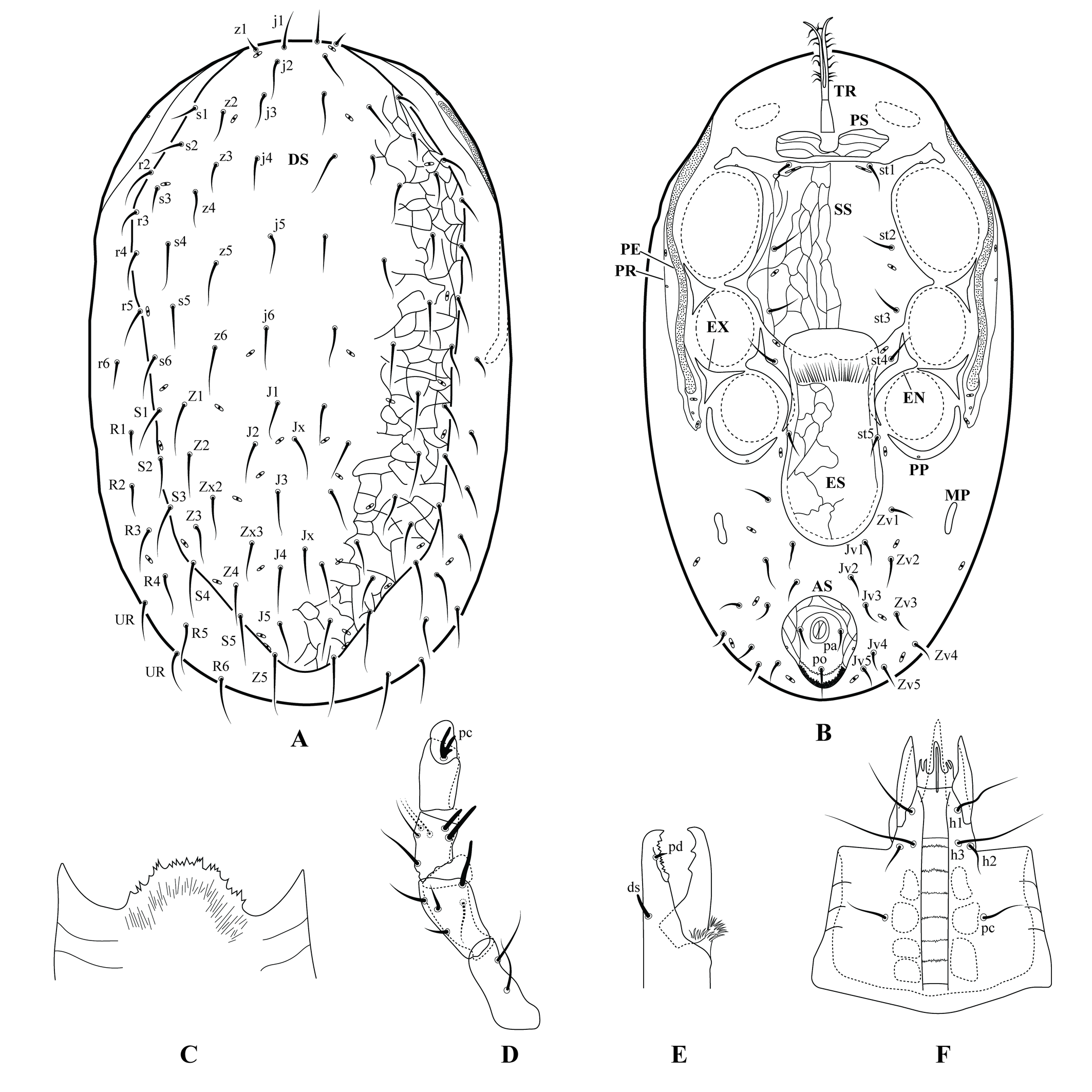
Berlese (1892) presented an early detailed characterization of Mesostigmata, using the basic structures of a typical species. These structures are shown in Figure 2 of this publication. This group is commonly used as a model for training in the skills required for dissection, illustration and recognition of mite structures, because they clearly display the structures used in the identification of most mite species. Early acarologists such as Mégnin (1876) and Michael (1892) considered them the most highly organised of all mites.


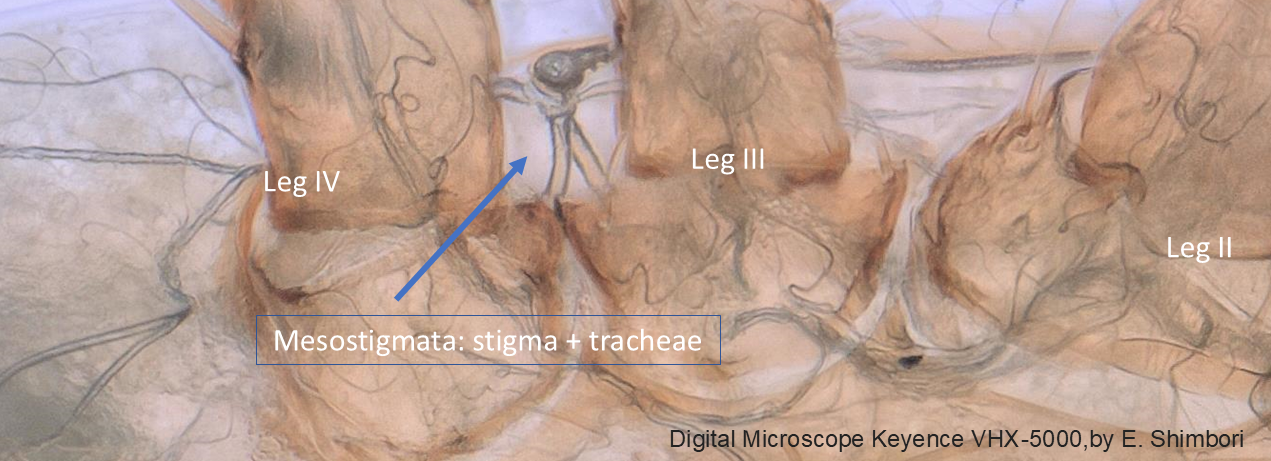
The most important diagnostic character state for the Mesostigmata is the presence of a pair of respiratory openings (the stigmata), generally between the insertions of legs III and IV (Figure 3), or less commonly between legs II and III. Internally, the openings lead to a complex system of ducts, called tracheae, which allow the entry of air to all parts of the organism. Externally, each opening is usually connected to a latero-longitudinal furrow called peritreme. Details of these structures were first reported long ago by authors including Mégnin (1876) and Kramer (1877). Mégnin (1876) believed the peritreme had a protective role in preventing the entry of dust into the tracheae, but that it may also be an air storage structure.


The gnathosoma of these mites is composed of a pair of separate chelicerae and a pair of palps. Each mesostigmatid chelicera is composed of three segments, the basal most of which is distinctly shorter than the median, and the distal segment is most often a dorsal elongate extension called the fixed digit. There is also a distal movable digit that is hinged at the base of the fixed digit. The two digits combine together to form a scissors- or pincer-like structure. The fixed digit most often bears a subdistal, ventral and antiaxial seta-like structure called the pilus dentilis or, less often, a lobe-shaped structure. The fixed digit is usually delimited dorso-basally by a transverse lyrifissure (sensory structure), next to which is the so-called dorsal seta. Another lyrifissure is often present antiaxially on the same digit, oriented diagonally to the ventral face of the chelicera, and lying next to the arthrodial membrane that connects the movable and fixed digits. The fixed digit is greatly reduced in some taxa. In adult males, the movable digit may have an elongate structure called spermatodactyl, used for transferring sperm to the females. Both digits can have the opposable margins smooth or variously dentate (Bowman 2021). Other structures can occasionally be found on the chelicerae, such as arthrodial brushes or excrescences.
The palps usually have five free segments (basal to distal - trochanter, femur, genu, tibia and tarsus), while the right and left coxae are fused to form a broad tube often referred to as the ''capitulum'', through which the chelicerae move back and forth during feeding. The most distal palp segment (tarsus) usually bears a basal claw that can be entire or (more often) divided into 2–4 distal branches.
The dorsal part of capitulum (epistome) is a thin, lightly sclerotised structure that extends to a variable distance beyond the basalmost articulation of the palp. This structure is often lightly sclerotised and a good quality microscope is required for its study, except for example in the Parasitidae in which is it more strongly sclerotised (Haller 1881). The epistome can be distally truncate, rounded, acuminate, or provided with extensions, and its margin may be smooth, serrate or fimbriate. The ventral surface of the capitulum has a median longitudinal groove known as the deutosternum, which bears a variable number of transverse, usually denticulate lines (Bowman 2023), flanked by three pairs of hypostomal and one pair of subcapitular setae. The anterior edge of the ventral surface of the capitulum bears a pair of usually conical spine-like corniculi of different lengths. The corniculi may be membranous, truncate or bifurcate. Between the corniculi there are one or more pairs of unsclerotised, tapering or subdivided, fimbriate or smooth structures called internal malae. Other single (labrum, supralabral process) or paired (salivary styli) structures may also be distinguished as parts of the gnathosoma.
The dorsum of the idiosoma is usually covered by a large dorsal shield, which can be divided into a variable number of plates, the anteriormost of which is usually the largest. The entire or divided shield is provided with few to many setae, a few pairs of pores (solenostomes) and lyrifissures (poroids). Ventrally (except in some endoparasitic species), there is almost always an elongate narrow structure arising from the membrane just posterior to the gnathosoma known as the tritosternum. The tritosternum is usually distally divided and fimbriate, and movable by muscular activity. It has been shown to play an important role in directing fluid food toward the mouth, in conjunction with the deutosternum (Wernz and Krantz 1976). Posterior to the tritosternum there is often one or more pairs of slender and transversely oriented platelets (presternal platelets), which in turn are followed by the much larger sternal shield, which usually bears three pairs of setae and 2–3 pairs of lyrifissures. The sternal shield is usually followed posteriorly by a pair of platelets (metasternal platelets), each bearing one seta and, often, one lyrifissure. The oopore lies behind the sternal shield, and is covered by one or more platelets which together constitute an epigynal shield. The epigynal shield may be fused with a more posterior ventral shield to form a genitoventral shield. The separate or fused epigynal and ventral shields may have none to several pairs of setae and a pair of lyrifissures, or the lyrifissures may be in the surrounding unsclerotised cuticle. At the posterior end of the ventral idiosoma there is almost invariably a shield surrounding the anal opening (anal shield), which is separate or may be fused with the ventral shield to form a ventrianal shield. In some groups, the anal shield is also fused with the neighbouring plates or with the dorsal shield. The ventral shield bears all or part of the opisthogastric setae, while the anal shield bears three circum-anal setae (a pair of para-anal setae and one post-anal seta). One or two platelets are found in the soft integument behind each leg IV, known as the metapodal platelets. External to the coxae of each side there is an elongate structure, entire or variously fragmented, known as the exopodal plate. A similar structure, known as the endopodal plate, entire or fragmented, is located medial to the coxae. The anterior section of the endopodal plate is usually fused to the sternal shield. A variously developed platelet (parapodal platelet; varying from crescent moon-shaped to subtriangular) is often found around the posterior margin of each coxa IV.
Another (usually) slender plate supports the peritreme, and is commonly fused to the anterolateral region of the dorsal shield (peritrematal plate). It may also be fused to the posterior region of the exopodal plate or to the parapodal platelet.
Slightly darker and somewhat punctate or irregular patches may be visible on the inner surface of the capitulum, and the dorsal and ventral shields. These are the sites of muscle attachments, known as sigilla.
Each leg has six free segments (coxa, trochanter, femur, genu, tibia, tarsus), and each usually ends in a pretarsus. Vacante and Flechtmann (2025) argued that this structure should more appropriately be called the post-tarsus, given that leg structures are usually named from base to apex; the term post-tarsus is occasionally used for insects and some other arachnids. The pretarsus consists of a pair of curved claws and a median membranous structure called the pulvillus, which is composed of three distally tapering or rounded lobes. Transversely-oriented lyrifissures are usually found around the base of the tarsus, separating the short basitarsus and the longer telotarsus, as well as around the base of the femur where they separate the basifemur and the telofemur. The distal section of the tarsus of leg I is occasionally separated from the remainder of the segment, constituting an acrotarsus.
In contrast with many other mites, the Mesostigmata do not have eyes (Mégnin 1876; Michael 1892), trichobothria or solenidia, but they may have modified setae on the tarsi of the palps and leg I, which appear to have a chemosensory function.
Morphological diversity of Mesostigmata
Despite the relative morphological homogeneity of most mites of this order, a considerable level of variation can still be found (Figure 4). The differences mostly concern variation in size, from 300 to more than 2,000 µm in length; in the shape of the idiosoma, from ovate to worm-like; the shape and length of the chelicera, with both digits well developed or lacking a fixed digit; the degree of development of the peritreme; and characteristics of the reproductive structures. Other important morphological differences between species can be found in the placement, length, and morphology of setae on the idiosoma and legs, which provide a rich source of taxonomic information.



How are the Mesostigmata presently classified in relation to other mites?
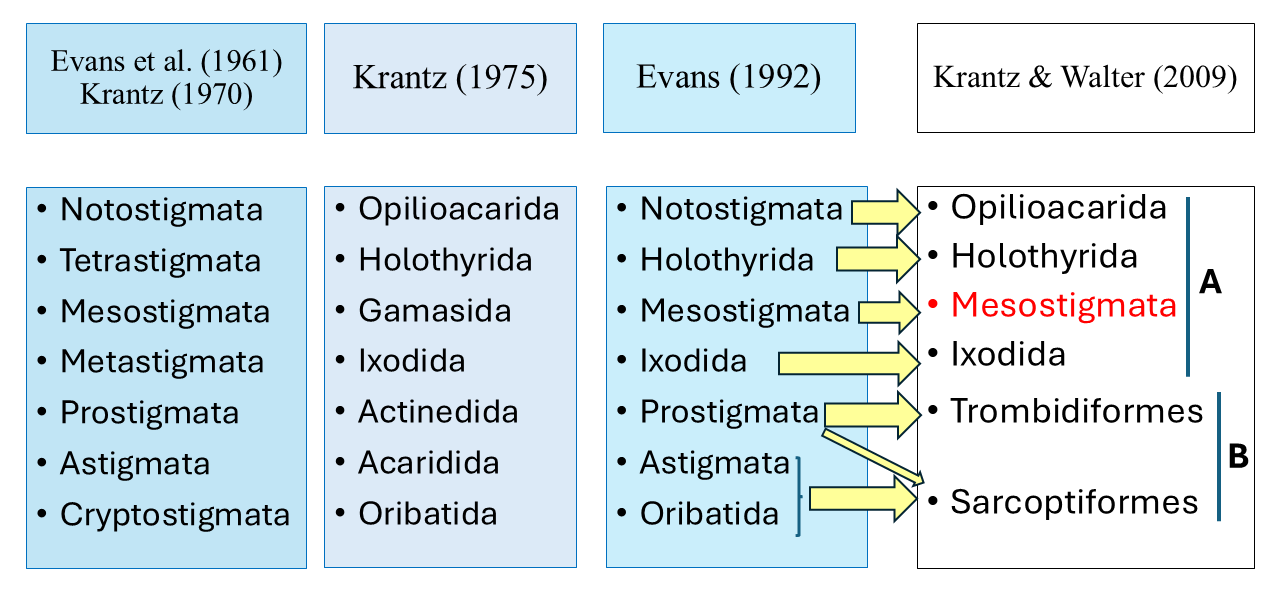
While the class Insecta of the subphylum Hexapoda is presently considered to comprise 28 orders (Vanin et al. 2024), the subclass Acari of the class Arachnida is presently considered to consist of only six orders distributed in two superorders (Krantz and Walter 2009), namely Parasitiformes (orders Opiliocacarida, Holothyrida, Mesostigmata and Ixodida) and Acariformes (orders Trombidiformes and Sarcoptiformes). It is not clear whether the mites as a whole constitute a single monophyletic taxon, and the relationships between the mites and other groups of Chelicerata remain unresolved (for example Dabert et al. 2010; Lozano-Fernandez et al. 2019).
A comparison of the classification system in Krantz and Walter (2009) with that in (Krantz, 1978) shows that the main differences refer to the names adopted for each group, and the fusion of the former order Astigmata (= Acaridida) with the former order Oribatida, to form the order Sarcoptiformes (Figure 5). Another important change was the transfer of most families of Endeostigmata from the Prostigmata to the Sarcoptiformes, with only two of the 12 families remaining in the Trombidiformes. The Trombidiformes now consist of what was then referred to as Prostigmata, except for the large part of the Endeostigmata. Mégnin (1876) had already mentioned the closeness between ''Oribatida'' and ''Sarcoptides''. A more recent suggestion for change (Bolton et al. 2023) refers to the reclassification of the Eriophyoidea, moving it from Trombidiformes into Sarcoptiformes.


Main contributors to the taxonomy of Mesostigmata
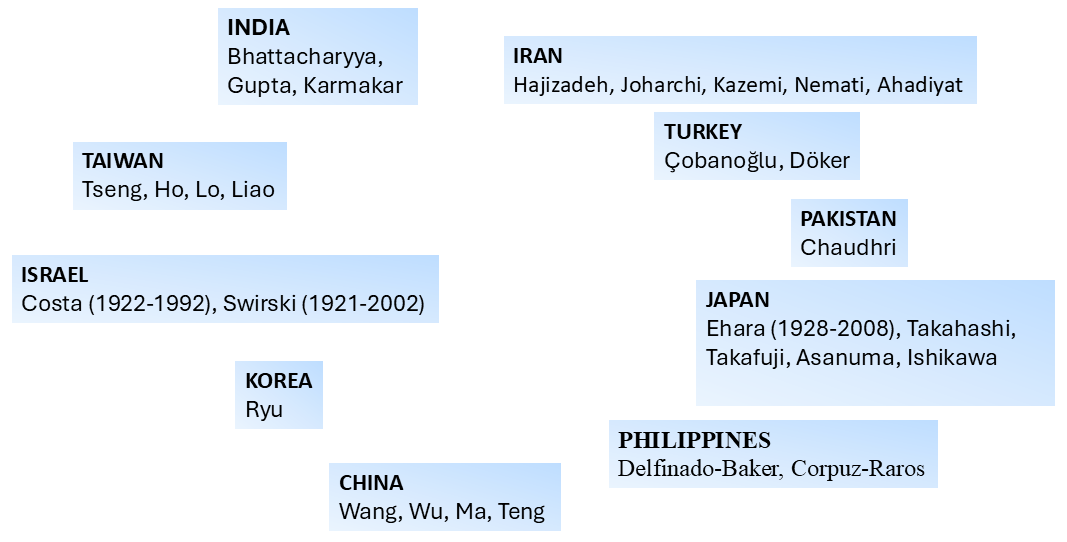
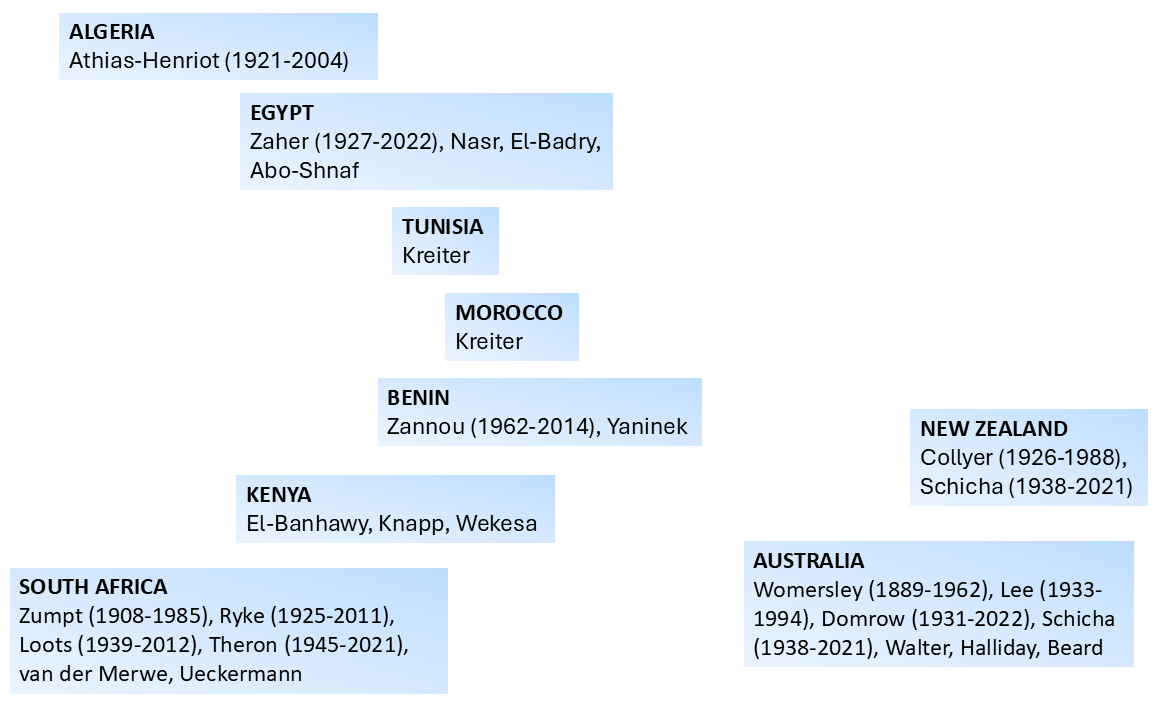
A large number of acarologists has contributed to the development of the understanding of mite morphology and the subsequent determination of the relationship between the Mesostigmata and other mite groups. In this work we provide a pictorial representation of those that most significantly participated in that process in different parts of the globe, including the deceased as well as representative leading active acarologists (Figures 6–9). Many of the acarologists currently working in Mesostigmata can be found in the databases cited in Table 8.
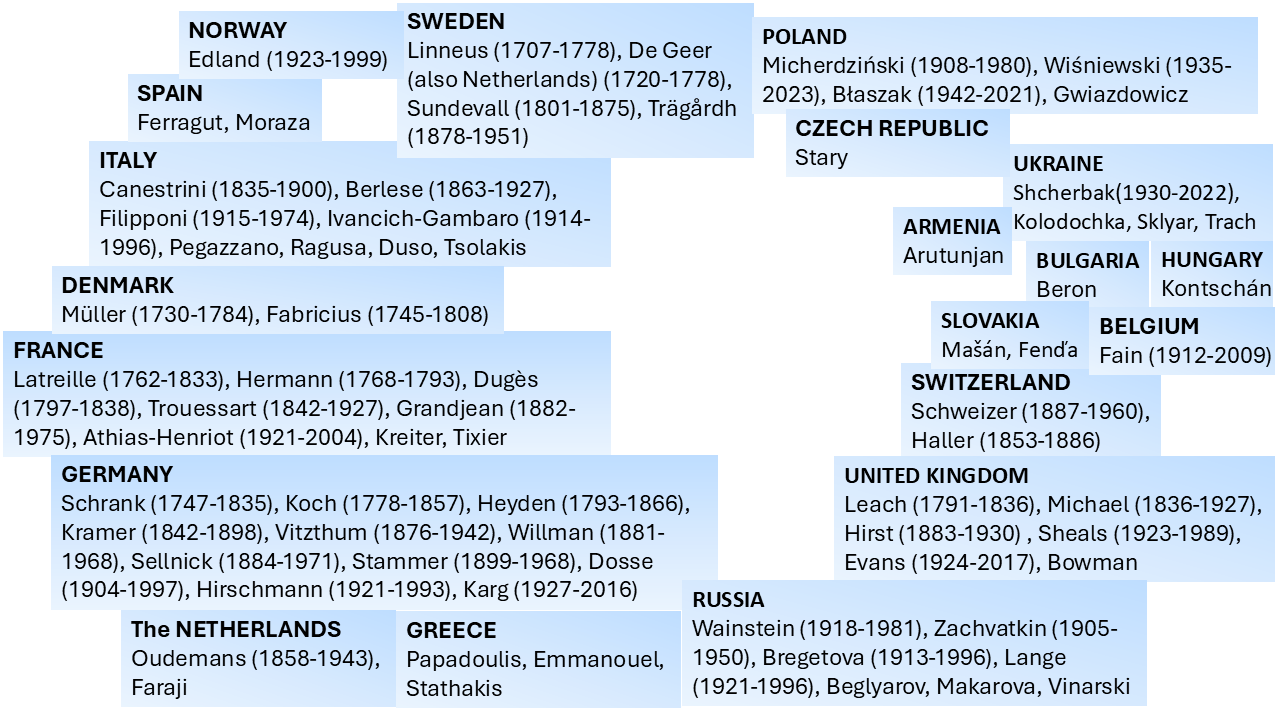


A quick inspection of Figures 6–9 suggests that the early work on systematics of Mesostigmata was dominated by European acarologists. The most influential early contributions came from naturalists in Scandinavian countries, followed by French, then German, Italian, Dutch and British acarologists. Contributions of French, German and Italian acarologists were summarised respectively by Bertrand et al. (2008), Alberti (2005) and Ragusa (2002). North Americans only became significantly involved in this process in the beginning of the 20th century, followed by the Russian school and the more recent growth in contributions from South America, Australia and Africa.



According to Oudemans (1896b), Leach (1815) was the first to propose the establishment of a group of ''Gamasus-like mites'' (the present-day Mesostigmata), which he called ''Gammasides'', consisting of a single genus, ''Gammasus''. Lists of related names and the corresponding references were presented by Berlese (1892) and Oudemans (1896b). A few years after Leach's publication, Gamases was used by Dugès (1834a) to refer to a group that he termed ''family'', mostly composed of genera currently placed in the Mesostigmata (Dermanyssus, Gamasus, Pteroptus and Uropoda) as well as Argas, which is now placed in the order Ixodida. Gervais and van Beneden (1859) presented what they considered to be the nine-member mite groups. Referring to those same groups, Mégnin (1876) called for the need to combine two of them, Gamasides and Ixodides, based on the constitution of the chelicera, presence of peritreme and location of the respiratory openings. A group composed of the present-day Mesostigmata and Ixodida was established as the order Mesostigmata by Berlese (1899) and as the suborder Peritremata by Ewing (1913), who also mentioned the closeness of Argas and ''Ixodes''. Mégnin (1876) also provided detailed characterisations of the genera then included in Gamasides (Gamasus, Pteropus and Uropoda). Based on the characteristics of the gnathosoma and the first pair of legs, he considered the Uropodina to represent a link, or an evolutive stage, between insects and Arachnida, but that possibility was not fully discussed. Kramer (1881) referred to Gamasidae, a group composed of essentially the same suprageneric taxa mentioned by Dugès (1834a), but not including Argas. Kramer also specified the genera included in the groups previously proposed by Dugès (1834a) and by himself: Pteroptina (with Pteroptus Dufour, a junior synonym of Spinturnix von Heyden); Uropodina – Trachynotus and Uropodus; and Gamasina – Dermanyssus, Gamasus and Sejus. Canestrini (1891) was the first to use the name Mesostigmata to designate this order, placing in it the mites with stigmata between the second and third or between the third and fourth coxae.


At the end of the 1800's and the beginning of the 1900's, there were disagreements among acarologists in relation to the existing number of mesostigmatic families. Based on Beaulieu et al. (2011), the first mesostigmatic families (in the present sense of this taxonomic rank) reported in the literature seem to have been Dermanyssidae Kolenati, 1859; Uropodidae Kramer, 1881; Epicriidae Berlese, 1885; Sejidae Berlese, 1885; Iphiopsidae Kramer, 1886; Laelapidae Canestrini, 1891; and Zerconidae Canestrini, 1891.


Canestrini (1891) considered the existence of six families (Demanyssidae; Gamasidae; Laelaptidae; Nicoletiellidae Canestrini, 1891; Uropodidae; and Zerconidae). Nicoletiellidae was considered a junior synonym of Labidostommatidae Oudemans, 1904 (currently Trombidiformes) by Bertrand (1990). Berlese (1899) included two groups in the order Mesostigmata, Gamasida and Ixodida. In turn, Gamasida was considered to include eight families (Antennophoridae, Celaenopsidae, Dermanyssidae, Gamasidae, Laelapidae, Pteroptidae, Uropodidae and Zerconidae) and Ixodida to include two (Argasidae and Ixodidae).
Oudemans (1903) considered the Mesostigmata to consist of three families, namely Parasitidae (then indicated to be a senior synonym of Gamasidae), Spelaeorhynchidae and Ixodidae. He considered Parasitidae to contain nine subfamilies, all of which had been considered at the family level by previous authors, and these groups were all once again treated at the family level by Oudemans (1906).
Years later, Vitzthum (1943) considered Laelapidae to consist of 13 subfamilies, most of which had also been considered by other authors at the family level (Beaulieu et al. 2011). Trägårdh (1938, 1946) proposed a classification of the Mesostigmata that comprised 13 new families, including new information from the extent and fusion of the plates covering the genital opening. Camin and Gorirossi (1955) extended that idea and established two more families in the suborders Monogynaspida and Trigynaspida, as well as the infraorders Antennophorina and Cercomegistina. More recently, Lindquist et al. (2009) and Beaulieu et al. (2011) moved part of the Monogynaspida out to constitute the suborder Sejida Kramer, 1885 (originally spelled as Sejina), as shown in Table 5.
Download as
Suborder Sejida
Suborder Monogynaspida
Sejoidea
Infraorder Uropodina
Heterozerconoidea
Microgynioidea
Thinozerconoidea
Suborder Trigynaspida
Diarthrophalloidea
Infraorder Cercomegistina
Uropodoidea
Cercomegistoidea
Infraorder Gamasina
Infraorder Antennophorina
Epicrioidea
Aenictequoidea
Heatherelloidea
Antennophoroidea
Zerconoidea
Celaenopsoidea
Arctacaroidea
Fedrizzioidea
Parasitoidea
Megisthanoidea
Veigaioidea
Paramegistoidea
Eviphidoidea
Parantennuloidea
Rhodacaroidea
Dermanyssoidea
Ascoidea
Phytoseioidea
Hence, based mostly on the information provided by Beaulieu et al. (2011), it can be concluded that the major recent changes in the classification of the Mesostigmata included (a) an increase in the number of superfamilies from one (Parasitoidea) in Banks (1915) to seven in Krantz (1978), to ten in Lindquist et al. (2009); (b) the addition of numerous new families since the publication of Krantz (1970) – 17 families in Uropodina, 11 in Trigynaspida, one in Rhodacaroidea, one in Zerconoidea, one in Dermanyssoidea and one in Heatherelloidea; (c) the addition of two new families of Ascoidea by Lindquist and Moraza (2014, 2023).
Among key contributors for the understanding of the morphology and or taxonomy of the Mesostigmata, Micherdziński (1966) highlighted the works of H. Vitzthum, A. Berlese, A.C. Oudemans, I. Trägårdh and K. Viets. One should also include A.D. Michael, mostly active in the late 1800's and early 1900's, concerning details of the internal morphology (e.g., Michael 1892), as well as N.G. Bregetova, G.O. Evans and E.E. Lindquist. Among the many major contributions of the latter two authors, one should list their contribution to the adoption of chaetotaxy and setal nomenclature, extensively used today for mites of this order (Evans 1963; Lindquist and Evans 1965). Add to that the publications about the Laelapidae mites from the United Kingdom by Evans and Till (1965, 1966, 1979), which established a model for similar publications about mesostigmatic mites in general across the world.
Major contributions were also made by C. Athias-Henriot, including the determination of the variability of the morphology of the spermathecal structures of Mesostigmata, and the possibility of using this information for taxonomic purposes (Athias-Henriot 1968). A substantial period of time was required from the early report of mesostigmatic spermathecae in the beginning of the 19th century (referred to as ''copulatory structure'', by Oudemans 1905) and the detailed study by Athias-Henriot. The contribution of the latter author was also important in the determination of the arrangement of pores and lyrifissures of Mesostigmata (Athias-Henriot 1975). Many other prominent acarologists are still making important contributions to the Mesostigmata, but space limitations prevent our discussing them all in detail.
Increasing numbers of named Mesostigmata
Download as
Linnaeus (1758)
Leach (1815)
Dugès (1834a)
Koch (1842)
Canestrini (1891)
Vitzthum (1931)
Baker et al. (1958)
Beaulieu et al. (2011)
Families
–
7
1
1
6
39
62
109
Genera
1
16
4
10
34
259
–
880
Species
3
-
16
127
–
–
–
11,424
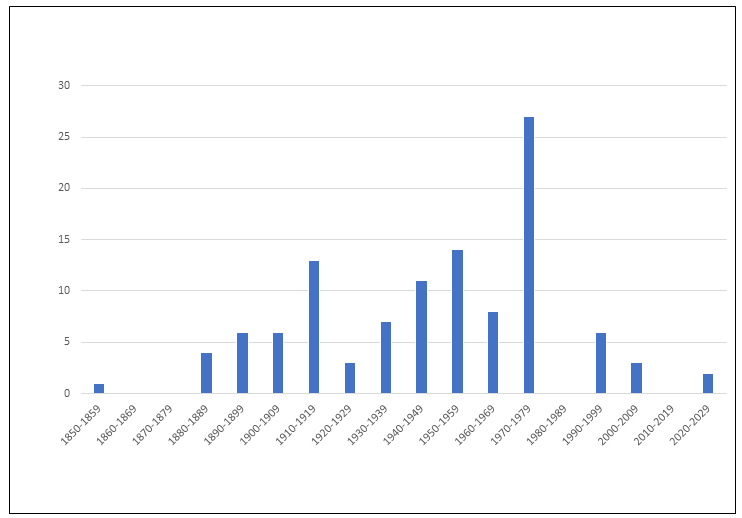
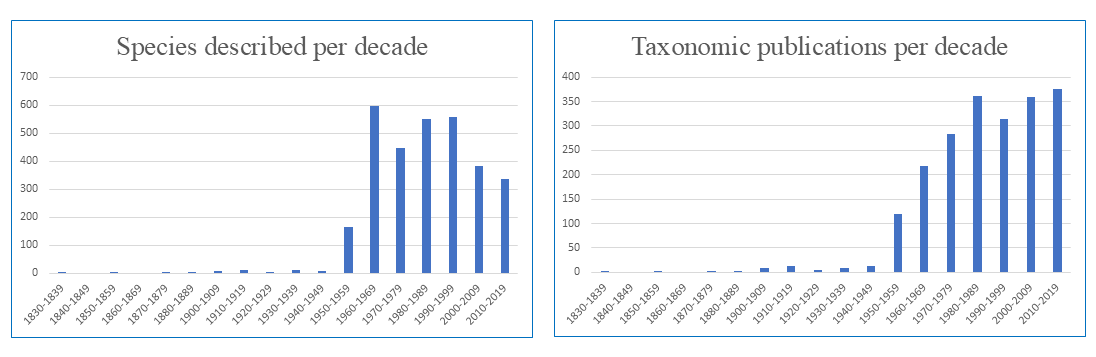
In parallel with the growing numbers of descriptions of mites in general, the number of descriptions of Mesostigmata has increased considerably, jumping from three species in a single genus in 1758 to approximately 12,000 species in almost 900 genera and 109 families in 2011 (Table 6). However, the rate of description of new families per decade has begun to decrease (Figure 10), probably because poorly studied areas have still not received adequate attention, including many countries in Africa, Asia and the Americas. The seven most speciose families of Mesostigmata now contain about 50% of all species of this order (Table 7, based on Beaulieu et al. 2011). Phytoseiidae is by far the most speciose, with about 2,700 valid species, followed by Laelapidae, with an estimated 1,700 species.
Download as
Family
Number
Source
Phytoseiidae
2,700
Demite et al. (2024)
Laelapidae
1,700
This publication
Rhinonyssidae
510
Beron (2020)
Zerconidae
500
Mohammad-Doustaresharaf et al. (2023)
Macrochelidae
470
This publication
Ologamasidae
472
This publication
Parasitidae
426
Beaulieu et al. (2011)


Development of consolidated databases
Advances in internet technology in the last few decades have made it possible to compile databases and catalogues of taxonomic information of several mesostigmatic families (Table 8). This is considered a positive trend for at least two reasons. First, to demystify what is effectively known about the taxonomy of those groups, given the widespread distribution of those publications. Second, to facilitate access to the available information by new researchers of each group.
Download as
Ameroseiidae (Mašán, 2017)
Ascidae (www.lea.esalq.usp.br/acari/ascidae/)
Blattisociidae (www.lea.esalq.usp.br/acari/blattisociidae/)
Dasyponyssidae, Dermanyssidae, Entonyssidae, Halarachnidae, Hystrichonyssidae, Macronyssidae, Manitherionyssidae, Raillietiidae, Spelaeorhynchidae (Beron, 2014)
Laelapidae (Moraes et al., 2022)
Melicharidae (www.lea.esalq.usp.br/acari/melicharidae/)
Ologamasidae (Castilho et al., 2016)
Parholaspididae (Quintero-Gutiérrez and Halliday, 2021)
Pachylaelapidae (Mašán and Halliday, 2014)
Phytoseiidae (www.lea.esalq.usp.br/phytoseiidae/)
Podocinidae (Barros et al., 2020)
Rhodacaridae (Castilho et al., 2012)
Rotundabaloghiidae (Kontschán, 2016)
Rhinonyssidae (Beron, 2020)
Spinturnicidae (Beron, 2020)
Trachyuropodidae (Kontschán and Ermilov, 2023)
Uropodina (Wísniewski and Hirschmann, 1993; Kontschán, 2024)
Special attention will be given to Phytoseiidae in the subsequent section of this publication. As to other families (Laelapidae, Ascidae, Blattisociidae and Melicharidae), an analysis of the available data suggests a peak of the number of species described, between 1960 to 1969. The cause for this trend was not determined, but could be related to the establishment of two scientific journals totally dedicated to the publication of acarological themes at that time (Acarologia and International Journal of Acarology), and to the establishment of the International Congresses of Acarology.
Practical importance of Mesostigmata
As presently interpreted, Mesostigmata is the group of Parasitiformes containing by far the largest number of species (over ten times more than the second most speciose order, Ixodida). But what is known about the practical importance of mesostigmatic species? Bregetova (1966) presented a discussion of the economic importance of the Mesostigmata in agriculture and human and veterinary medicine, and that review should now be revised to incorporate more modern trends.
Mesostigmata are not known to cause major damage to wild or cultivated plants, despite the fact that some have been reported to obtain some nourishment from plants, as first assumed by Gilliatt (1935) and later proved by other authors (Porres et al. 1975; Adar et al. 2012) for mites of the family Phytoseiidae. Some species of Uropodina (especially Uropoda abnoxia Reuter) have been reported to attack the base of some horticultural crops when at high population levels in protected cultivations, as summarised by Bregetova (1966). It is well known that pollen is an important source of food for some phytoseiids (McMurtry et al. 2013), while some species of Ameroseiidae, Melicharidae and Phytoseiidae are known to develop and reproduce when feeding on nectar, as discussed in Moraes et al. (2015).
Several species of Mesostigmata are known to feed on fungi (as extensively discussed in different chapters of Carrillo et al. 2015), and they may be numerous in cultivated fungi or in laboratory fungal cultures, but none is considered a pest of cultivated mushrooms (Flechtmann 1976; Moraes et al. 2015). Some Uropodina have been blamed for the overexploitation of food added in commercial production of earthworms (Bregetova 1966).
An early account on the importance of the Mesostigmata as parasites of vertebrates and invertebrates was published by Gervais and van Beneden (1859). Many families of Mesostigmata contain species reported as parasites of those organisms (Bregetova 1966; Lindquist et al. 2009) (Figure 11). Some species have been reported to bite humans and cause harmful skin reactions. A detailed account of the morphology, biology, behaviour, type of effect on the parasitised vertebrates (especially of medical importance) and control of these mites was presented by Alexander (1984). These mites are especially important in poultry houses, where they cause serious health problems to chickens, and attack human workers. They have been extensively studied as possible parasites or vectors of organisms pathogenic to humans and wild and domesticated animals (Tiraboschi 1904; Strandtmann and Wharton 1958; Bregetova 1966). The most important species include Dermanyssus gallinae (DeGeer) (Dermanyssidae) and Ornithonyssus sylviarum (Canestrini & Fanzago) (Macronyssidae), both parasites of chickens. Others are important endoparasites in the nares of birds (Rhinonyssidae) or the ears of domesticated ruminants (Halarachnidae, Raillietinae). Also important are Varroa species (Varroidae), which play an important role as parasites of honeybees worldwide (Klimov et al. 2016). Strandtmann and Wharton (1958), Bregetova (1966), Lindquist et al. (2009) and Radovsky (2010) also provided valuable reviews of parasitic Mesostigmata. Work on the association between Mesostigmata and pathogens of different groups was reviewed in detail by Bregetova (1966). A recent revision of basic aspects of the mesostigmatic families of vertebrate parasites was recently published (in Portuguese) by Barros-Battesti et al. (2024), dealing mostly with the species found in Brazil.
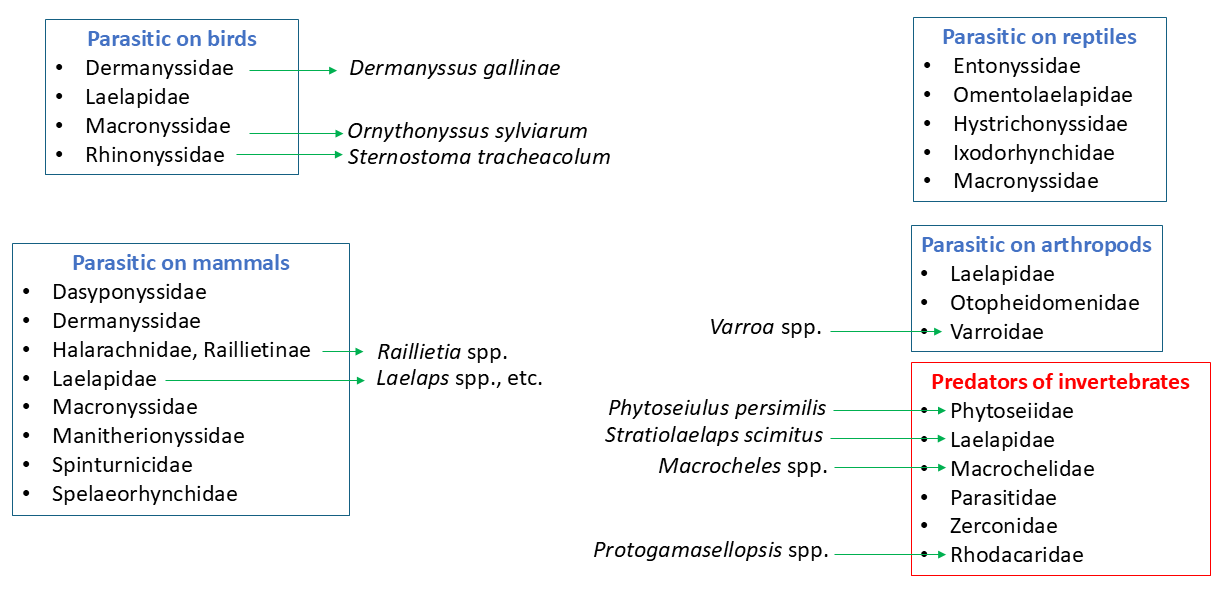


Some species of Uropodina, especially Fuscuropoda agitans (Banks), prey on earthworms (Stone and Ogles 1953). Additionally, several species of different mesostigmatic families (mainly Phytoseiidae, Laelapidae and Macrochelidae) have been reported to play a role as natural enemies of harmful arthropods (see different chapters in Carrillo et al. 2015). Some of these have been mass-produced under controlled conditions for commercial use as biological control agents, especially some species in the family Phytoseiidae.
Phytoseiidae
This group was first reported by Berlese (1916) as the tribe Phytoseiini of the family Laelapidae, and was raised to the family level by Baker and Wharton (1952). A detailed account of the initial studies of the morphology, taxonomy, biology and possible importance of the Phytoseiidae for pest control was published by Chant (1959a) in the first issue of the first volume of Acarologia. Phytoseiidae is composed of species considered beneficial for their predatory behaviour on harmful mites and insects (McMurtry et al. 2013, 2015). Hence, in her evaluation of the economic importance of Mesostigmata in agriculture, Bregetova (1966) mentioned the expectation then held for the possible use of phytoseiids as biological control agents. A few years later, a revision of the importance of phytoseiid and other predatory arthropods in the biological control of harmful organisms was published by Huffaker et al. (1970).
In contrast to most other mesostigmatic families, phytoseiids are mainly plant inhabitants, and only a minority can be found in the soil. The inability of mites of most other mesostigmatic families to remain on plants is probably related to their requirement for high humidity levels. This seems consistent with the presence of some members of other families on (at least) low growing plants at night (Esteca et al. 2021), when air saturation deficit reaches the lowest levels. This trend favours the phytoseiids, by releasing them from competition with other mites of the same guild. Some other arboreal groups such as the Stigmaeidae (Trombidiformes) have been shown to interfere with and compete with phytoseiids (Fan and Flechtmann 2015).
Presently, phytoseiids constitute the most important group of predatory mites used commercially for the biological control of mite and small insect pests, in all three types of biological control strategies: classic, conservation and augmentation. Over 35 species of this family have been commercially mass produced and sold to growers for pest control around the world (van Lenteren 2012; Knapp et al. 2018; van Lenteren et al. 2018), especially of spider mites (Tetranychidae), whiteflies (Hemiptera) and thrips (Thysanoptera). These mites are naturally found on both cultivated plants and natural vegetation. They move quickly when disturbed or in search of prey, but during the day they usually hide most on the underside of the leaves, along the main veins. Others protect themselves by hiding in the growing tips of plants or in domatia (McMurtry et al. 2015), or within natural refuges in the structure of the plant (Beard and Walter 2001). They are rather uniform morphologically, but some unusual forms have been recently reported from different habitats, such as the Neotropical forests (Figure 12), bearing setae that are uncommon in this family, or unpaired setae, among other differences.
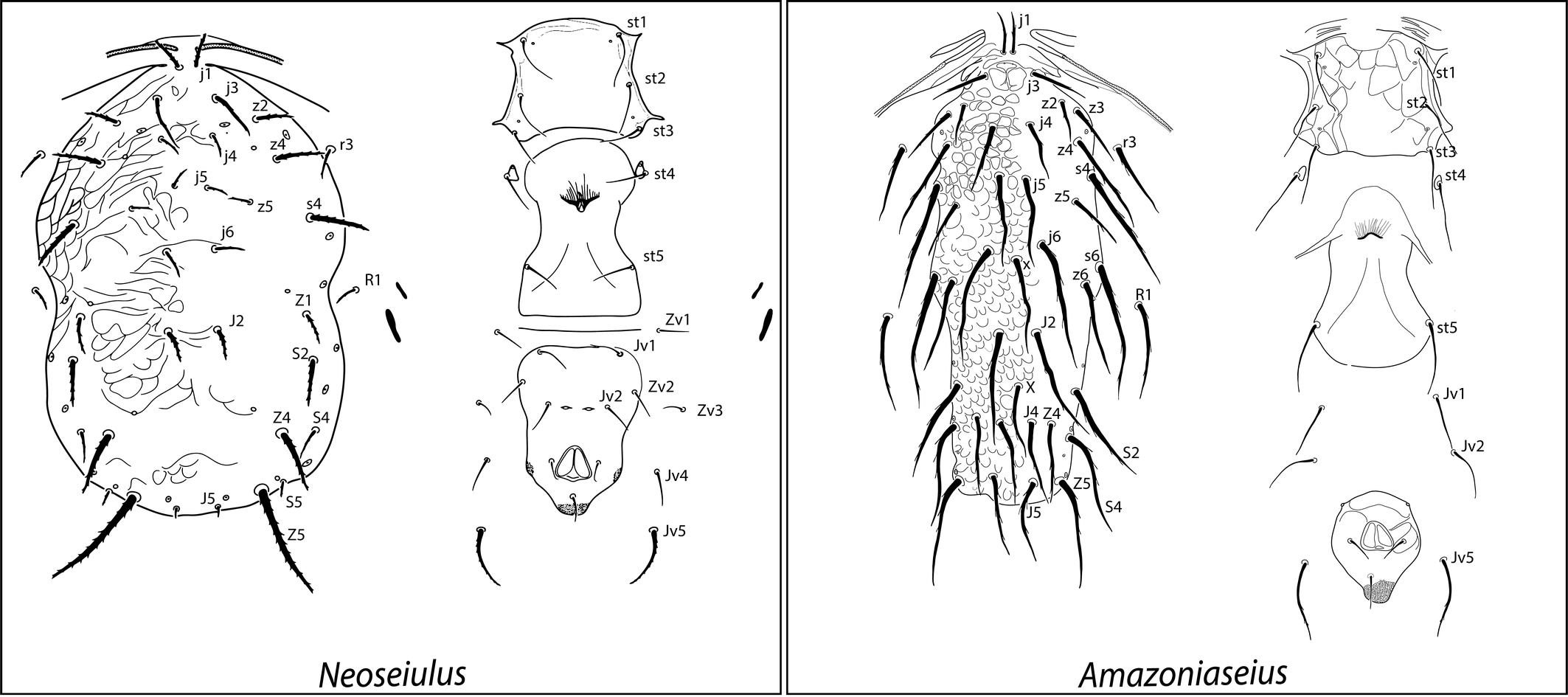


Table 9 shows records of the numbers of described phytoseiid species from different sources. Despite a reduction in the rate of new species discovery, the absolute number of species described is growing continuously. An evaluation of the number of phytoseiid species described in each decade (Figure 13) shows a pattern similar to that reported for mesostigmatic mites in general. A peak in the number of species described in 1960–1969 was followed by a slight reduction in the number of new species described in recent decades. Yet, the data suggest a continuously growing trend for the number of publications about taxonomy since 1950. Hence, efforts to understand these mites have increased, potentially contributing to improvements in their conservation and use.


What are the factors contributing to that growth? Certainly, the first two revisions of the phytoseiid mites (Nesbitt 1951; Chant 1959b) were fundamental for the establishment of the basis on which the extensive successive works could be conducted. Only 26 species of the group presently known as Phytoseiidae (then as a subfamily of Laelapidae) were mentioned by Nesbitt (1951), most of which had been described previously by other authors. Just a few years later, Chant (1959b) reported 148 species, including 38 new species, as well as a few species that were overlooked by Nesbitt (1951) and many species that had been published since 1951.
The subsequent series of revisions by Chant and Yoshida-Shaul during the 1980's and 1990's, followed by the publication of the two editions of the Phytoseiidae Catalogue (Moraes et al. 1986, 2004) were important in facilitating the work of new taxonomists interested in studying these mites. The comprehensive revision of the family by Chant and McMurtry from the 1990's to the 2000's, a series of revisionary works by different authors around the globe, and the creation of the Phytoseiidae Database in 2014 (Demite et al. 2014), further contributed to the development of study of mites of this family. References to the papers by Chant and co-workers can be found in Demite et al. (2024).
The interest by the private sector in the commercial development of biological control was also important in the evolution of our knowledge of these mites. This interest has prompted private companies to collaborate with research institutions, including the establishment of research units dedicated to the experimental evaluation of prospective species. The commercial use of predatory mites depends on the successful development of mass-rearing methods, and the difficulties involved in that process mean that only a few species have been used in this way on a large scale.
Download as
Species
Genera
References
26
4
Nesbitt (1951)
148
9
Chant (1959b)
1,500
79
Moraes et al. (1986)
2,200
67
Moraes et al. (2004)
2,416
91
Demite et al. (2014)
2,985
102
Demite et al. (2024)
Despite the great diversity of the Phytoseiidae, about 83% of all known species belong to one of the ten most speciose genera (Table 10). However, the search for new biological control agents should not be limited to those genera. At least two of the most commonly used phytoseiids internationally belong to the genus Phytoseiulus, which has a relatively restricted natural distribution in southern South America, and low diversity, with only four species.
Download as
Genus
Main habitat
Number of species
Typhlodromus
Temperate
464
Amblyseius
High humidity
389
Neoseiulus
World
382
Phytoseius
Temperate
232
Euseius
Tropical
218
Proprioseiopsis
World, soil
149
Typhlodromips
Subtropical
102
Transeius
World
64
Scapulaseius
Asia, Oceania
63
Kuzinellus
World (uncommon in tropics)
53
Suggestions to increase the understanding and practical use of Mesostigmata
Priority studies aimed at better understanding the Mesostigmata and expanding their practical use are certainly tied to the particular geographic location under consideration. In some regions, virtually nothing is known about the local fauna, whereas in others, at least some knowledge about the species composition and perspective for their use is available. Taking that into consideration, we present a list of subjects considered relevant at this stage to promote the practical use of those mites (Table 11).
Download as
· Synthesis of information: taxonomic databases, photographic databases, revisionary works
· Provision of quality descriptions
· Complementing molecular characterisation (DNA barcoding)
· Survey in underexplored areas
· Training of new mite taxonomists
· Main challenges for practical use:
• Develop viable use of predatory mites in large cultivation areas
• Conservation of predatory mites by supplying alternative food
• Selection of predator strains with higher efficacy in the control of pest organisms
In terms of research, we emphasise the need to continue the efforts to synthesise the data that has already been published, including databases of taxonomy and biology. The present easy access to searching tools in the internet has tremendously facilitated this type of work. Some work has been done in this area but much more can be achieved, as some mesostigmatic groups or some geographic regions have still not received the attention they deserve. In terms of taxonomy, not only written information should be synthesised, but effort should also be dedicated to the establishment of image libraries, especially of type specimens, making the images available electronically, eliminating the need for the eventual shipment of type specimens by regular mail for taxonomic evaluation. That could prevent not only their eventual damage or loss during transport, but also a more thorough and permanent registration of their morphological features. One such image library is available as VIRMISCO — The Virtual Microscope Slide Collection, at the Senckenberg Museum of Natural History, Görlitz (Decker et al. 2018).
For the same reason, and of similar importance, is the preparation of more detailed original descriptions or redescriptions of taxa, based on a reasonable number of type specimens and measurements of taxonomically relevant structures of all types, to allow a study of their morphological variability (Tixier 2012). Detailed illustrations, whenever possible including good quality photographs, are also desirable.
It has become progressively easier to include molecular data in descriptions of new taxa. According to availability of appropriate specimens, this should also be considered, using one of the adequate markers for the more plesiomorphic characters, for the study of phylogenetic relationships, or more recently derived, to distinguish species. This type of information has become progressively more common in the description of new species, but a critical analysis of what has been achieved in this area is not yet available.
In seeking adequate representation of the members of the group, in attempting to produce a meaningful classification system, efforts could be dedicated to the conduct of surveys in inadequately explored regions around the globe. For example, little is known about the soil mesostigmatic mites from most of South America and Africa. Endemic species collected in those areas may significantly affect the concepts of their taxonomic groupings. Many examples of considerable changes can be found in the literature. As an example, Berlese (1906) referred to the need to change his own previous concept (Berlese 1892) of the family Gamasidae (Parasitidae), which considered it necessary and sufficient for males to have spurs on leg II, because of the exceptions he became aware of inside and outside of the group. A more recent example concerns the modification of the concept of the family Ameroseiidae, given the finding of a few new species in a new genus of this family (Endopodoseius Abo-Shnaf & Moraes), which was distinguished from the other genera of the same family by the presence of seta J5 and divided dorsal shield, among other important features that were until recently taken as distinguishing characteristics of the whole family (Abo-Shnaf et al. 2023).
In terms of practical applications, biological control of pest organisms with predatory mites has been used mostly in protected crops, or in small open fields of crops of high economic return (e.g., strawberry, some fruit trees). However, the bulk of food around the globe is produced in open fields, where crops are subject to attack by several mite species that are not currently controlled biologically, most probably because of cost and difficulty in handling the natural enemies under those conditions. Efforts could be dedicated to change this pattern. The use of biological control with predatory mites in extensively grown crops such as soybean and cotton could lead to important reductions of the use of chemicals in agriculture. Hence, the development of techniques to change that status quo is highly desirable.
Present regulations concerning biological control and conservation efforts of local fauna and flora have made the importation of exotic biological control agents more difficult. Hence, better use of local species becomes more desirable. For that purpose, for each promising biological control agent, the selection of populations with higher efficacy in the control of a particular pest could be sought, making use of selection practices developed by plant and animal breeders. The artificial selection of genotypes of promising species within the range of its natural genetic variability has been proposed for quite a long time for different groups of natural enemies. A recent revision on this subject was published by Lommen et al. (2017). For predatory mites, this type of study has been conducted mostly seeking for enhanced resistance to some types of pesticides (Hoy 1986) or, at some degree, for reduced capacity of the predator to diapause (Veerman 1992; van Houten et al. 1995). However, several other traits could be studied in attempts to improve the performance of predatory mites or their more economic commercial production, such as higher predation rates (Lommen et al. 2017). For example, Beard and Walter (2001) showed that species of Neoseiulus can be highly host plant specific in their natural habitat, and are not the free-ranging generalist predators they were thought to be. This finding has far-reaching implications for the efficient use of phytoseiids as control agents. Aspects of the host plant influence the behaviour and distribution of phytoseiids independently of prey availability and distribution (Bakker and Klein 1992; Beard and Walter 2001), so phytoseiids with specific needs in terms of host plants may not be amenable for use as generalist predators in biological control. The phytoseiid most appropriate to a particular host plant should be sought, and emphasis in phytoseiid studies should be redirected from diet alone and placed on the range of specific requirements of that particular species.
This type of study, which depends heavily on genetic tools, can be adapted for the inclusion of mites as the subject to be studied. That would require the precise determination of attributes to be considered for selection as well as of the possible effects that such endeavour could have on the natural populations of the same and other organisms, envisioning the better performance of the predator with no environmental disturbances. It is also important is to study the development of conservation practices fostering the efficacy of locally occurring predatory mites, especially in extensively cultivated crops, when the release of commercial predators is less suitable (Azevedo et al. 2020).
Acknowledgements
We are grateful for the partial support provided by Institut National de Recherche pour l'Agriculture, l'Alimentation et l'Environment (INRAE) and Agropolis Fondation (both France) during the time spent in the preparation of this manuscript; to Denise Navia, Thais Juliane do Prado (both INRAE), Eduardo Shimbori (CIRAD, France) and Lucas Lorena Godoy (ESALQ-USP, Brazil) for their logistical support; to Sam Bolton, for sharing information of some of his on-going work; to Fernando Angelo Piotto, for his enlightening discussion about the genetic aspects of artificial selection techniques; to Dariusz Gwiazdowicz (FFWT-PULS, Poland), Eddie Ueckermann, Markus Knapp (Koppert, The Netherlands) and Salvatore Ragusa (S.EN.FI.MI.ZO-UP, Italy) for providing some of the cited literature; and to Serge Kreiter, for calling our attention to the availability of the unpublished work presented by him in the 6th EURAAC, in 2008 in Montpellier, France. We greatly appreciate the contribution provided by Frédéric Beaulieu and anonymous reviewers of the previous version of this publication, for the numerous important suggestions and major contributions that they provided.
References
- Abo-Shnaf R., Castilho R.C., Marticorena J.L.M., Moraes G.J. de. 2023. A new genus and three new species of mites, with a revised concept of the family Ameroseiidae (Acari: Mesostigmata: Ascoidea). Zootaxa, 5231: 249-272. https://doi.org/10.11646/zootaxa.5231.3.2
- Adar E., Inbar M., Gal S., Doron N., Zhang Z.-Q., Palevsky E. 2012. Plant-feeding and non plant-feeding phytoseiids: differences in behaviour and cheliceral morphology. Exp. Appl. Acarol., 58: 347-358. https://doi.org/10.1007/s10493-012-9589-y
- Alberti G. 2005. Tribute to the past - Notes on the history of acarology in Germany. Phytophaga, 14: 13-56.
- Alexander J.O. 1984. Infestation with Gamasid Mites. In: Alexander J.O. (Ed). Arthropods and Human Skin. London: Springer, p. 303-315. https://doi.org/10.1007/978-1-4471-1356-0_17
- Arthur D.R. 1965. Ticks in Egypt in 1500 B.C.? Nature, 206: 1060-1061. https://doi.org/10.1038/2061060a0
- Athias-Henriot C. 1968. L'appareil d'insémination laelapoïde (Acariens anactinotriches: Laelapoidea, ♀♀). Premières observations. Possibilité d'emploi à des fins taxonomiques. Bull. Scient. Bourgogne, 25: 229-274.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. 2. Le relevé organotaxique de la face dorsale adulte (gamasides, protoadéniques, Phytoseiidae). Acarologia, 17: 20-29.
- Azevedo L.H., Moreira M.F.P., Pereira G.G., Borges V., Moraes G.J. de, Inomoto M.M., Vicente M.H., Siqueira Pinto M., Peres L.E.P., Rueda-Ramírez D., Carta L., Meyer S.L.F., Mowery J., Bauchan G., Ochoa R., Palevsky E. 2020. Combined releases of soil predatory mites and provisioning of free-living nematodes for the biological control of root-knot nematodes on `Micro Tom tomato'. Biol. Control, 146: 10 pp. https://doi.org/10.1016/j.biocontrol.2020.104280
- Baker E.W., Wharton G.W. 1952. An introduction to Acarology. New York: The Macmillan Company. pp. 465.
- Baker E.W., Evans T.M., Gould D.J., Hull W.B., Keegan, H.L. 1956. A Manual of Parasitic Mites of Medical or Economic Importance. New York: A Technical Publication of the National Pest Control Association. pp. 170.
- Baker E.W., Camim J.H., Cunliffe F., Wooley T.A., Yunker C.E. 1958. Guide to the Families of Mites. College Park: The Institute of Acarology, University of Maryland. pp. 242+ix pp.
- Bakker, F.M., Klein, M.E. 1992. Transtrophic interactions in cassava. Exp. Appl. Acarol., 14: 293-311. https://doi.org/10.1007/BF01200569
- Banks N. 1905. A treatise on the Acarina or mites. Proc. Unit. Stat. Nat. Mus., 28, 114 pp. https://doi.org/10.5479/si.00963801.28-1382.1
- Banks N. 1915. The Acarina or mites. United States Department of Agriculture Report, 108. pp. 153.
- Barros-Battesti D.M., Zacarias Machado R., André M.R. 2024. Ectoparasitofauna Brasileira de Importância Veterinária. Volume 2. Acarofauna de Importância Veterinária: Parasitiformes - Mesostigmata. Colégio Brasileiro de Parasitologia Veterinária, Jaboticabal, 262 pp.
- Barros A.R.A., Castilho R.C., Moraes G.J. de. 2020. Catalogue of the mite family Podocinidae Berlese (Acari: Mesostigmata). Zootaxa, 4802(1): 141-156. https://doi.org/10.11646/zootaxa.4802.1.9
- Beard J.J., Walter G.H. 2001. Host plant specificity in several species of generalist mite predators. Ecol. Entomol., 26: 562-570. https://doi.org/10.1046/j.1365-2311.2001.00367.x
- Beaulieu F. 2009. Review of the mite genus Gaeolaelaps Evans & Till (Acari: Laelapidae), and description of a new species from North America, G. gillespiei n. sp. Zootaxa, 2158: 33-49. https://doi.org/10.11646/zootaxa.2158.1.3
- Beaulieu F., Dowling A.P.G., Klompen H., Moraes G.J. de, Walter D.E. 2011. Superorder Parasitiformes Reuter, 1909. In: Zhang Z.-Q. (Ed). Animal Biodiversity: An outline of Higher-Level Classification and Survey of Taxonomic Richness. Zootaxa, 3148: 123-128. https://doi.org/10.11646/zootaxa.3148.1.23
- Berlese A. 1885. Acarorum sistematis. Specimen. Bull. Soc. Ent. Ital., 17: 121-144.
- Berlese A. 1892. Acari, Myriopoda et Scorpiones Hucusque in Italia Reperta. Ordo Mesostigmata (Gamasidae). Padua: Tipografia del Seminario, 143 pp. [reprint by Junk, The Hague, 1979].
- Berlese A. 1896. Ricerche sugli organi e sulła funzione della digestione negli Acari. Riv. Patol. Veget., 5: 129-195 + plates 8, 9.
- Berlese A. 1899. Gli Acari agrari. III. - Ordini, famiglie e generi degli Acari. Riv. Patol. Veget., 7: 312-344.
- Berlese A. 1906. Monografia del genere Gamasus Latr. Redia, 3: 66-304 + plates 2-19.
- Berlese A. 1913. Acarotheca Italica. Fasciculus 1, 2. Firenze: Tipografia di M. Ricci. pp. 221.
- Berlese A. 1916. Centuria prima di Acari nuovi. Redia, 12: 19-66.
- Beron P. 2014. Acarorum Catalogus. III, Superorder Parasitiformes, order Opilioacarida (Opilioacaridae), order Holothyrida (Holothyridae, Allothyridae, Neothyridae), order Mesostigmata, Gamasina, Dermanyssoidea (Dermanyssidae, Macronyssidae, Halarachnidae, Raillietiidae, Entonyssidae, Manitherionyssidae, Hystrichonyssidae, Dasyponyssidae), Spelaeorhynchoidea (Spelaeorhynchidae). Pensoft. National Museum of Natural History, Sofia. Bulgarian Academy of Sciences, 285 pp.
- Beron P. 2020. Acarorum Catalogus. VI. Order Mesostigmata. Gamasina: Dermanyssoidea (Rhinonyssidae, Spinturnicidae). Pensoft. National Museum of Natural History, Sofia. Bulgarian Academy of Sciences, 265 pp. https://doi.org/10.3897/ab.e54206
- Bertrand M. 1990. La famille des Labidostomidae Oudemans, 1904 (Acari: Actinedida). Révision des genres et sous-genres et catalogue des espèces décrites. Acarologia, 31: 31-38.
- Bertrand M., Kreiter S., McKoy K., Migeon A., Navajas M., Tixier M.-S., Vial L. 2008. A quick overview of the story of French acarologists [Internet]. Available from: https://studylib.net/download/8071941. Accessed 21 October 2024.
- Bolton S.J., Chetverikov P.E., Ochoa R., Klimov P.B. 2023. Where Eriophyoidea (Acariformes) belong in the tree of life. Insects, 14: 527. https://doi.org/10.3390/insects14060527
- Bowman C.E. 2021. Feeding design in free‑living mesostigmatic chelicerae (Acari: Anactinotrichida). Exp. Appl. Acarol., 84: 1-119. https://doi.org/10.1007/s10493-021-00612-8
- Bowman C.E. 2023. Looking for future biological control agents: the comparative function of the deutosternal groove in mesostigmatic mites. Exp. Appl. Acarol., 91: 139-235. https://doi.org/10.1007/s10493-023-00832-0
- Bregetova N.G. 1966. Economic importance of Mesostigmata in medicine, veterinary and agriculture. In: Kochman J., Boczek J., Rajski A. (Eds). Zagadnienia Akarologii, Zeszyty Problemowe Postepow Nauk Rolniczych. Warsaw: Polska Akademia Nauk, Wydzial Nauk Rolniczych i Lesnyc, p. 51-76.
- Camicas J.L., Hervy J.P., Adam F., Morel P.C. 1998. Les tiques du monde (Acarida: Ixodida). Nomenclature, Stades décrits, Hôtes, Répartition (espèces decrites avant le 01/1/96). Paris: Éditions de l'Orstom. pp. 233.
- Camin J.H., Gorirossi F.E. 1955. A revision of the Suborder Mesostigmata (Acarina), based on New Interpretations of Comparative Morphological Data. Lincoln Park: The Chicago Academy of Sciences Special Publications. pp. 70.
- Canestrini G. 1891. Abrozzo del sistema acarologico. Atti del Reale Istituto Veneto di Scienze, Lettere ed Arti, 38 (serie 7, tomo 2), pp. 699-725. https://doi.org/10.1007/BF03017253
- Carrillo D., Pena J.E., Moraes G.J. de. 2015. Prospects for biological control of plant feeding mites and other harmful organisms. Cham: Springer. pp. 328. https://doi.org/10.1007/978-3-319-15042-0
- Castilho R.C., Moraes G.J. de, Halliday B. 2012. Catalogue of the mite family Rhodacaridae Oudemans, with notes on the classification of the Rhodacaroidea (Acari: Mesostigmata). Zootaxa, 3471: 1-69. https://doi.org/10.11646/zootaxa.3471.1.1
- Castilho R.C, Silva E.S., Moraes G.J., Halliday B. 2016. Catalogue of the family Ologamasidae Ryke (Acari: Mesostigmata). Zootaxa, 4197(1): 1-147. https://doi.org/10.11646/zootaxa.4197.1.1
- Cavalcante A.C.C., Demite P.R., Amaral F.S.R., Lofego A.C., Moraes G.J. de. 2017. Complementary description of Neoseiulus tunus (De Leon) (Acari: Mesostigmata: Phytoseiidae) and observation on its reproductive strategy. Acarologia, 57: 591-599. https://doi.org/10.24349/acarologia/20174178
- Chant D.A. 1959a. Observations sur la famille des Phytoseiidae. Acarologia, 1: 11-22+plate.
- Chant D.A. 1959b. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Canad. Entom., Supplement 12, 166 pp.
- Coineau Y., Cléva R., Chatenet G. du. 1997. Ces Animaux Minuscules qui Nous Entourent. Paris: Delachaux et Niestlé, 80 pp.
- Collins B.J. 1930. The Confused Nomenclature of Nycteribia vespertillonis and Spinturnix vespertilionis. M.Sc. Dissertation, Faculty of the American University, Washington, D.C., 72 pp. + 4 plates.
- Cuvier, G. 1798. Tableau élémentaire de l'histoire naturelle des animaux. Paris: Baudouin, vi + 710 pp. + 14 plates. https://doi.org/10.5962/bhl.title.11203
- Dabert M., Witalinski W., Kazmierski A., Olszanowski Z., Dabert J. 2010 Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Molecular Phylog. Evol., 56: 222-241. https://doi.org/10.1016/j.ympev.2009.12.020
- Decker P., Christian A, Xylander W.E.R. 2018. VIRMISCO - The Virtual Microscope Slide Collection. ZooKeys 741: 271-282. https://doi.org/10.3897/zookeys.741.22284
- DeGeer C. 1778. Memoires pour servir a l'histoires des insects. Volume 7. Stockholm: L'Imprimerie de Pierre Hesselberg. pp. 950+49 plates.
- Demite P.R., Cruz W.P. da, McMurtry J.A., Moraes G.J. de. 2017. Amazoniaseius imparisetosus n.sp., n.g.: an unusual new phytoseiid mite (Acari: Phytoseiidae) from the Amazon forest. Zootaxa, 4236(2): 302-310. https://doi.org/10.11646/zootaxa.4236.2.5
- Demite P.R., McMurtry J.A., Moraes G.J. de. 2014. Phytoseiidae Database: a website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa, 3795: 571-577. https://doi.org/10.11646/zootaxa.3795.5.6
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2024. Phytoseiidae Database. Available from: https://www.lea.esalq.usp.br/phytoseiidae. Accessed 28 October 2024.
- Dugès A. 1834a. Recherches sur l'ordre des acariens en général et la famille des trombidiés en particulier. Article Premier. Considérations générales sur les acariens comparés aux autres arachnides, aux insectes et aux crustacés. Premier Mémoire. Annales des Sciences Naturelles. Partie Zoologie. Crochard: Libraire-Éditeur. p. 5-46 +plate.
- Dugès A. 1834b. Recherches sur l'ordre des acariens. Troisième Mémorie. Annales des Sciences Naturelles. Seconde série. Tome Second - Zoologie. Crochard: Libraire-Éditeur. p. 18-63 +plates 7, 8.
- Dujardin F. 1849. Mémoire sur des acariens sans bouche. Dont on a fait le genre Hypopus et qui sont le premier age des Gamases. Annales des Sciences Naturelles. 3ème sér., 12: 243-265 + plate 11.
- Elzinga, R.J. 1993. Larvamimidae, a new family of mites (Acari: Dermanyssoidea), associated with army ants. Acarologia, 34: 95-103.
- Emmanuel N.G. 1982. Greece. In: Prasad V. (Ed). History of Acarology. West Bloomfield: Indira Publishing House, p.195-210.
- Esteca F.C.N., Trandem N., Klingen I., Santos J.C., Delalibera Jr. I., Moraes, G.J. de. 2021. Cereal straw mulching in strawberry - A facilitator of plant visits by edaphic predatory mites at night? Diversity, 12, 242: 145-160. https://doi.org/10.3390/d12060242
- Evans G.O. l963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Brit. Mus. (Nat. Hist.) Zool., 10: 277-303. https://doi.org/10.5962/bhl.part.20528
- Evans G.O. 1992. Principles of Acarology.CAB International, Wallingford, 563 pp. https://doi.org/10.1079/9780851988221.0000
- Evans G.O., Till W.M. 1965. Studies on the British Dermanyssidae (Acari: Mesostigmata). I. External morphology. Bull. Brit. Mus. (Nat. Hist.) Zool., 13: 249-294. https://doi.org/10.1080/00222936508651622
- Evans G.O., Till W.M. 1966. Studies on the British Dermanyssidae (Acari: Mesostigmata). II. Classification. Bull. Brit. Mus. (Nat. Hist.) Zool., 14: 109-370.
- Evans G.O., Till W.M. 1979. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes). An introduction to their external morphology and classification. Trans. Zool. Soc. Lond., 35: 139-270. https://doi.org/10.1111/j.1096-3642.1979.tb00059.x
- Ewing H.E. 1913. New Acarina. Part I. General considerations and descriptions of new species from Minnesota, Wisconsin and Michigan. Bull. American Mus. Nat. Hist. 32: 93-121.
- Fabricius I.C. 1775. Systema Entomologiae Insectorum. Havniae: Historiae Naturalis Fautoribus. pp. 832.
- Fan Q.H., Flechtmann C.H.W. 2015. Stigmaeidae. In: Carrillo D., Pena J.E., Moraes G.J. de (Eds). Prospects for Biological Control of Plant Feeding Mites and other Harmful Organisms. Cham: Springer, p. 185-206. https://doi.org/10.1007/978-3-319-15042-0_7
- Fernandez N., Coineau Y. 2007. Maladies, Parasites et autres Ennemies de l'Abeille Mellifère. Paris: Editions Atlantica. pp. 504.
- Flechtmann C.H.W. 1976. Sobre dois ácaros (Arthropoda: Acari) frequentes em criações da broca da cana-de-açúcar (Insecta: Lepidoptera). Ecossistema, 1: 62-63.
- Geoffroy M. 1762. Histoire abregée des insectes qui se trouvent aux environms de Paris dans laquelle ces animaux son rangés suivant um order metododique. Paris: Durand. pp. 690+12 plates. https://doi.org/10.5962/bhl.title.154842
- Gervais P. 1844. Histoire naturelle des insectes. Aptères. Acères Phrynéides, Scorpionides, Solpugides, Phalangides et Acarides; Dicères Épizoïques, Aphaniptères et Thysanoures. Tome troisième. Paris: Libririe Encyclopédique re Roret. pp. 476.
- Gervais P., van Beneden P.-J. 1859. Zoologie Médicale. Exposé Méthologique du Règne Animal Basé sur l'Anatomie, l'Embryologénie et la Paléontologie comprenant la Description des Espèces Employées en Médecine de celles qui sont Venimeuses et de celles qui sont parasites de l'homme et des animaux. Vol. 1. J.-B. Baillière et Fils. Paris, 528 pp. https://doi.org/10.5962/bhl.title.130910
- Gilliatt F.C. 1935. Some predators of the European red mite, Paratetranychus pilosus C. & F., in Nova Scotia. Canad. J. Res., 39d: 19-38. https://doi.org/10.1139/cjr35d-002
- Gorirossi-Bourdeau F. 1995. A documentation in stone of Acarina at the Roman temple of Bacchus in Baalbek, Lebanon about 150 AD. Bull. Ann. Soc. Roy. Belge d'Entom., 131: 3-15.
- Haller G. 1881. Die Mundtheile und systematische Stellung der Milben. Zool. Anzeiger, 88: 380-386.
- Hermann J.F. 1804. Mémoire Apterológique. Strasbourg: De L'imprimerie de F.G. Levrault. pp. 144+9 plates. https://doi.org/10.5962/bhl.title.137910
- Hoy M. 1986. Use of genetic improvement in biological control. Agric., Ecos. Env., 15: 109-119. https://doi.org/10.1016/0167-8809(86)90084-8
- Hoy M. 2011. Agricultural Acarology. Introduction to Integrated Mite Management. Boca Raton: CRS Press. pp. 430. https://doi.org/10.1201/b10909
- Hrúzová, K., Fenďa, P. 2018. The family Parasitidae (Acari: Mesostigmata) - history, current problems and challenges. Acarologia, 58 (Suppl.): 25-42. https://doi.org/10.24349/acarologia/20184280
- Huffaker C.B., van de Vrie M., McMurtry J.A. 1970. Ecology of tetranychid mites and their natural enemies: a review. II. Tetranychid populations and their possible control by predators: an evaluation. Hilgardia, 40: 391-458. https://doi.org/10.3733/hilg.v40n11p391
- Jeppson L.R., Keifer H.H., Baker E.W. 1975. Mites injurious to economic plants. Berkeley: University of California Press. pp. 614+74 plates. https://doi.org/10.1525/9780520335431
- Joblot L. 1754. Observations d'Histoire Naturelle, faites avec le Microscope. Second Edition, 2 volumes. Paris: Briasson Libraire. pp. 399. https://doi.org/10.5962/bhl.title.46942
- Johnston D.E. 1965. Comparative studies on the mouth-parts of the mites of the suborder Acaridei (Acari) [PhD Dissertation]. Columbus: Ohio State University. pp. 192.
- Johnston D.E. 1982. Acari, Opilioacariformes, Parasitiformes. In: Parker, 2: 111-116.
- Kazemi S., Rajaei A., Beaulieu F. 2014. Two new species of Gaeolaelaps (Acari: Mesostigmata: Laelapidae) from Iran, with a revised generic concept and notes on significant morphological characters in the genus. Zootaxa, 3861 (6): 501-530. https://doi.org/10.11646/zootaxa.3861.6.1
- Klimov P.B., OConnor B., Ochoa R., Bauchan G.R., Redford A.J., Scher J. 2016. Bee Mite ID: Bee-Associated Mite Genera of the World. USDA APHIS Identification Technology Program (ITP), Fort Collins, CO. https://idtools.org/id/mites/beemites [date of access on April 7, 2025].
- Knapp M., van Houten Y., van Baal E., Groot T. 2018. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia, 58 (Suppl), 72-82. https://doi.org/10.24349/acarologia/20184275
- Koch C.L. 1842. Übersicht des Arachnidensystems. C.H. Nürnberg: Zeh'schen Buchhandlung. pp. 130+13 plates.
- Kolenati F.A. 1859 Beiträge zur Kenntniss der Arachniden. Sitzungsb. d. k. Akad. d. W. math. Naturw., 35(7-12): 155-190 + 8 plates.
- Kontschán J. 2024. Catalogue of the Uropodina species (Acari: Mesostigmata) described between 1993 and 2023. Acta Phyt. Entom. Hungarica, 59(1): 29-62. https://doi.org/10.1556/038.2024.00216
- Kontschán J., Ermilov S.G. 2023. Catalogue of trachyuropodid mites (Acari:Mesostigmata: Uropodina: Trachyuropodidae) of the world, with the description of Trachyibana kozari n. sp. from Singapore. Acta Phyt. Entom. Hungarica 58(1): 18-50. https://doi.org/10.1556/038.2023.00183
- Kramer P. 1877. Grundzüge zur Systematic der Milben. Archiv für Naturgeschichte, 43: 215-247.
- Kramer, P. 1881. Ueber die Prinzipien der Classification bei den Gamasiden. Zeitschrift für die Gesamte Naturwissenschaften Halle, 54: 638-642.
- Kramer P. 1885. Uber Halarachne Halichoeri Allm. Zeitschrift für die Gesammten Naturwissenschaften, 58: 46-74.
- Kramer P. 1886. Ueber Milben. I Zur Kenntnis einiger Gamasiden. Archiv für Naturgeschichte, 52: 241-268 + 3 plates. https://doi.org/10.5962/bhl.part.28438
- Krantz G.W. 1970. A Manual of Acarology. Corvallis: Oregon State University Bookstores. pp. 335.
- Krantz G.W. 1978. A Manual of Acarology, 2nd. edit. Corvallis: Oregon State University Bookstores. pp. 578.
- Krantz G.W., Walter D.E. 2009. A Manual of Acarology, 3rd. edit. Lubbock: Texas Tech University Press. pp. 807.
- Lamarck J.B. 1801. Systême des animaux sans vertèbres. Paris. pp. 452.
- Latreille P.A. 1795. Observations sur la variété des organes de la bouche des tiques, et distribution méthodique des insectes de cette famille d'après les caractères établis sur la conformation de ces organes. Rev. Encyclop., 4: 15-20.
- Latreille P.A. 1796. Précis des caractères génériques des insectes, disposés dans um ordre naturel. Paris: Prévôt. pp. 208. https://doi.org/10.5962/bhl.title.58411
- Latreille P.A. 1802. Histoire Naturelle, Générale et Particulière des Crustacés et des Insectes, vol. 3. Paris: L'Imprimerie de F. Dufart, xii + 467 pp. https://doi.org/10.5962/bhl.title.15764
- Latreille P.A. 1806. Genera crustaceorum et insectorum. Tomus primus. Parasiis et Argentorati, apud Amand Koenig, Bibliopolam, 296 pp.
- Latreille P.A. 1810. Considérations générales sur l'ordre naturel des animaux composant les classes des Crustacés, des Arachnides et des Insectes avec un tableau méthodique de leurs genres, disposés en familles. Paris: F. Schoell. pp. 444. https://doi.org/10.5962/bhl.title.39620
- Leach W.E. 1815. A tabular view of the external characters of four classes of animals, which Linné arranged under Insecta; with the distribution of the genera composing three of these classes into orders, &c. and descriptions of several new genera and species. Trans. Linn. Soc. Lond., 11: 306-400. https://doi.org/10.1111/j.1096-3642.1813.tb00065.x
- Lindquist, E.E. (1994) Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for same ventrolateral body setae. Acarologia, 35, 323-326.
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entom. Soc. Can., 47, 64 pp. https://doi.org/10.4039/entm9747fv
- Lindquist E.E., Moraza M.L. 2014. Mites coexistent with neotropical hispine beetles in unfurled leaves of Heliconia: a new genus and family of the Ascoidea (Acari: Mesostigmata: Gamasina). J. Nat. Hist., 48: 1611-1651. https://doi.org/10.1080/00222933.2013.877995
- Lindquist E.E., Moraza M.L. 2023. A newly recognized family of the Ascoidea (Acari: Mesostigmata: Gamasina), based on a revised concept of the subgenus Lasioseius (Endopodalius) Christian and Karg, 2006. Acarologia, 63: 998-1016. https://doi.org/10.24349/4izt-pj1n
- Lindquist E.E., Krantz G.W., Walter D.E. 2009. Order Mesostigmata. In: Krantz G.W., Walter D.E. (Eds). A manual of Acarology, 3rd. edit. Lubbock: Texas Tech University Press, p. 124-232.
- Linnaeus C. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymys, locis. Vol. 1, tenth edit., reformata. Holmiae, Impensis Direct. Laurentii Salvii, 824 pp. https://doi.org/10.5962/bhl.title.542
- Lommen S.T.E., Jong P.WS. de, Pannebakker B.A. 2017. It is time to bridge the gap between exploring and exploiting: prospects for utilizing intraspecific genetic variation to optimize arthropods for augmentative pest control - a review. Entomol. Exp. Appl., 162: 108-123. https://doi.org/10.1111/eea.12510
- Lozano-Fernandez J., Tanner A.R., Giacomelli M., Carton R., Vinther J., Edgecombe G.D., Pisani D. 2019. Increasing species sampling in chelicerate genomic-scale datasets provides support for monophyly of Acari and Arachnida. Nature Communications, 2019: 10:2295. https://doi.org/10.1038/s41467-019-10244-7
- Mašán P. 2017. A revision of the family Ameroseiidae (Acari, Mesostigmata), with some data on Slovak fauna. ZooKeys, 704: 1-228. https://doi.org/10.3897/zookeys.704.13304
- Mašán P., Halliday B. 2014. Review of the mite family Pachylaelapidae (Acari: Mesostigmata). Zootaxa, 3776 (1): 1-66. https://doi.org/10.11646/zootaxa.3776.1.1
- McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- McMurtry J.A., Famah Sourassou N., Demite P.R. 2015. The Phytoseiidae (Acari: Mesostigmata) as biological control agents. In: Carrillo D., Peña J.E., Moraes G.J. de (Eds). Prospects for Biological Control of Plant Feeding Mites and other Harmful Organisms. Cham: Springer, p. 133-149. https://doi.org/10.1007/978-3-319-15042-0_5
- Mégnin P. 1876. Mémoir sur l'organisation et la distribution zoologique des acariens de la famille des Gamasidés. Journal de l'Anatomie et de la Physiologie Normales et Pathologics de l'Homme et des Animaux, 12: 288-336+plates 7, 8.
- Michael A.D 1884. The hypopus question, or the life-history of certain Acarina. Zoological J. Linnean Soc., 17: 371-394. https://doi.org/10.1111/j.1096-3642.1884.tb02247.x
- Michael A.D. 1892. On the variations in the internal anatomy of the Gamasinae, especially in that of the genital organs and their mode of coition. Trans. Linn. Soc. Lond. (ser. 2), 5: 281-324. https://doi.org/10.1111/j.1096-3642.1892.tb00175.x
- Micherdziński W. 1966. Historia Akarologii. Zeszyty Problemowe. Postepow Nauk Rolniczych, 65: 7-21.
- Mohammad-Doustaresharaf M., Karaca M., Bagheri M., Urhan R. 2023. A taxonomic study on the zerconid mites (Acari: Zerconidae) in northwestern Iran: descriptions of three new species with three new records. Syst. Appl. Acarol., 28: 429-460. https://doi.org/10.11158/saa.28.3.3
- Moraes G.J. de, McMurtry J.A., Denmark H.A. 1986. A Catalog of the Mite Family Phytoseiidae. References to Taxonomy, Synonymy, Distribution and Habitat. Brasilia: Embrapa. pp. 353.
- Moraes G.J. de, McMurtry J.A., Denmark H.A., Campos C.B. 2004. A Revised Catalog of the Mite Family Phytoseiidae. Zootaxa, 434, 1-494. https://doi.org/10.11646/zootaxa.434.1.1
- Moraes G.J. de, Venancio R., Santos V.L.V. dos, Paschoal A.D. 2015. Potential of Ascidae, Blattisociidae and Melicharidae (Acari: Mesostigmata) as Biological Control Agents of Pest Organisms. In: Carrillo D., Moraes G.J. de, Peña J.E. (Eds). Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms, Progress in Biological Control Series, 19, pp. 33-75. https://doi.org/10.1007/978-3-319-15042-0_2
- Moraes G.J., Moreira G.F., Freire R.A.P., Beaulieu F., Klompen H., Halliday B. 2022. Catalogue of the Free-Living and Arthropod-Associated Laelapidae Canestrini (Acari: Mesostigmata), with Revised Generic Concepts and a Key to Genera. Zootaxa, 5184(1): 1-509. https://doi.org/10.11646/zootaxa.5184.1.1
- Müller O.F. 1776. Prodromus, seu animalium daniae et norvegiae indigenarum. characteres, nomina, et synonymia imprimis popularium. Hauniae: Typis Halageriis. pp. 282. https://doi.org/10.5962/bhl.title.13268
- Murray A. 1877. Economic Entomology. Aptera. London: Chapman & Hall. pp. 433. https://doi.org/10.5962/bhl.title.51877
- Nesbitt H.H.J. 1951. A taxonomic study of the Phytoseiinae (family Laelaptidae) predaceous upon Tetranychidae of economic importance. 12. The Netherlands: Zoologische Verhandelingen. pp. 64+32 plates.
- Nicolet M.H. 1855. Histoire naturelle des acariens qui se trouvent aux environs de Paris. Archiv. Mus. d'Hist. Natur., 7(4): 381-482 + 10 plates. https://doi.org/10.5962/bhl.title.66066
- Oudemans A.C. 1896a. List of Dutch Acari Latr. First part: Oribatei Dug. with synonymical notes and other remarks. Tijdschr. Entomol., 39: 53-65.
- Oudemans A.C. 1896b. List of Dutch Acari. Second part: Gamasides, with notes on synonymies and other remarks. Tijdschr. Entomol., 39: 131-141.
- Oudemans A.C. 1901. Notes on Acari. Third series. Tjdschr. Ned. Dierkd. Ver., 2de Serie, 7: 50-88.
- Oudemans A.C. 1903. Verslag van de 57ste Zomervergadering der Nederlandsche Entomologische Vereeniging, te Zutphen, op 7 Juni 1902. Tijdschr. Entomol., 45: 50-64.
- Oudemans, A.C. 1904. Notes on Acari. Eleventh series. Tijdschr. Entomol., 45: 93-134 + plates 11-13.
- Oudemans A.C. 1905. Verslag van de zestigste zomervergadering der Nederlandsche Entomologische Vereeniging, gehouldem te driebergen op zaterdag, 20 Mei 1905, des morgens ten 11 ure. Tijdschr. Entomol., 48: 77-81.
- Oudemans A.C. 1906. Nieuwe classificatie der Acari. Entomologische Berichten, 2 (27): 43-48.
- Pérez-Eid C. 2007. Les tiques: identification, biologie, importance médicale et vétérinaire. Paris: Lavoisier - Technique et Documentation. pp. 328.
- Porres M.A., McMurtry J.A., March R.B. 1975. Investigations of leaf sap feeding by three species of phytoseiid mites by labelling with radioactive phosphoric acid (H332PO4). Ann. Entomol. Soc. Amer., 68: 871-872. https://doi.org/10.1093/aesa/68.5.871
- Prasad V. 1982. History of Acarology. Oak Park: Indira Publishing House. pp. 472.
- Quintero-Gutiérrez E.J., Halliday B. 2021. Review of the mite family Parholaspididae Evans, 1956 (Acari: Mesostigmata). Zootaxa, 5005(4): 401-459. https://doi.org/10.11646/zootaxa.5005.4.1
- Radovsky F.J. 2010. Revision of genera of the parasitic mite family Macronyssidae (Mesostigmata: Dermanyssoidea) of the world. West Bloomfield: Indira Publishing House. pp. 160.
- Ragusa S. 2002. As Time goes by: a Profile of Italian Acarology. In: Bernini F., Nannelli R., Nuzzaci G., de Lillo E. (Eds). Acari Phylogeny and Evolution. Adaptations in Mites and Ticks. Dordrecht: Springer, p.1-20. https://doi.org/10.1007/978-94-017-0611-7_1
- Robineau-Desvoidy, J.B. (1830) Sur un nouveau genre de parasites de la classe des acaridiens. Annales des Sciences d'Observation, 3, 122-127 + Plate VI.
- Rosicky B. 1971. Acarology and its practical importance. In: Proceedings of the 3rd. International Congress of Acarology, Prague, p. 21-32. https://doi.org/10.1007/978-94-010-2709-0_3
- Stone P.C., Ogles G.D. 1953. Uropoda agitans, a mite pest in commercial fishworm beds. J. Econ. Entom., 46: 711. https://doi.org/10.1093/jee/46.4.711
- Strandtmann R.W., Wharton G.W. 1958. Manual of Mesostigmatic Mites. College Park: The Institute of Acarology, University of Maryland. pp. 330+69 figures.
- Sundevall C.J. 1835. Conspectus arachnidum. Basel: Naturforschenden Gesellschaft in Basel, Londini Gothorum, Universitatis Typographus. pp. 39.
- Tiraboschi C. 1904. Les rats, les souris et leurs parasites cutanés dans leurs rapports avec la propagation de la peste bubonique. Archives de Parasitologie, 7: 161-349.
- Tixier M.-S. 2012. Statistical approaches to assess intraspecific variations of morphological continuous characters: the case study of the family Phytoseiidae (Acari: Mesostigmata). Cladistics, 28: 489-502. https://doi.org/10.1111/j.1096-0031.2012.00394.x
- Trägårdh I. 1938. Further contributions towards the comparative morphology and classification of the Mesostigmata. Entomol. Tidskr., 59: 123-158.
- Trägårdh I. 1946. Outlines of a new classification of the Mesostigmata (Acarina) based on comparative morphological data. Lunds Universitets Årsskrift, 57: 3-37.
- Trouessart E. 1891. Considérations générales sur la classification des acariens suivies d'un essai de classification nouvelle. Revue des Sciences Naturelles de l'Ouest, 1: 289-308.
- Trouessart E. 1892. Considérations générales sur la classification des acariens suivies d'un essai de classification nouvelle (continuation). Rev. Scien. Natur. l'Ouest, 2: 20-54.
- Vacante V. 2015. The handbook of mites of economic plants. Identification, bio-ecology and control. Oxfordshire: CAB International pp. 872. https://doi.org/10.1079/9781845939946.0001
- Vacante C., Flechtmann C.H.W. 2024. The Handbook of Mites of Stored Products. Oxfordshire: CABI, pp. 671. https://doi.org/10.1079/9781786390912.0000
- van Houten Y.M., van Rijn P.C.J., Tanigoshi L.K., van Stratum P., Bruin J. 1995. Preselection of predatory mites to improve year-round biological control of western flower thrips in greenhouse crops. Ent. Exp. Appl., 74: 225-234. https://doi.org/10.1111/j.1570-7458.1995.tb01895.x
- van Lenteren J.C. 2012. The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl, 57: 1-20. https://doi.org/10.1007/s10526-011-9395-1
- van Lenteren J.C., Bolckmans K., Köhl J., Ravensberg W.J., Urbaneja A. 2018. Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl, 63: 39-59. https://doi.org/10.1007/s10526-017-9801-4
- Vanin S.A., Nihei S.S., Souza-Dias, P.G.B. 2024. Cap. 3, Filogenia e classificação. In: Rafael J.A., Melo G.A.R., Carvalho C.J.B., Casari S., Constantino, R. (Eds). Insetos do Brasil: Diversidade e Taxonomia. 2ª ed. Manaus: Instituto Nacional de Pesquisas da Amazônia, p. 57-87. https://doi.org/10.61818/56330464c03
- Veerman A. 1992. Diapause in phytoseiid mites: a review. Exp. Appl. Acarol., 14: 1-60. https://doi.org/10.1007/BF01205351
- Vitzthum H. 1931. Ordnung der Arachnida: Acari. Handbuch der Zoologie, 3(9), Krumbach, T. (Ed). Berlin: Walter de Gruyter, pp. 160.
- Vitzthum H. 1943. Acarina. Klassen und Ordnung des Tierreichs. Bronns H.G. (Ed). Band 5: Arthropoda; Abteilung IV: Arachnoidea. Akademische Verlagsgesellschaft Becker & Erler Kom. Leipzig: G.E.S., pp. 1011.
- von Heyden C.H.G. 1826. Versuch einer systematischen Eintheilung der Acariden. Isis, 18: 608-613.
- Walter D.E. 2009. Suborder Endeostigmata. In: Krantz G.W., Walter D.E. (Eds). A Manual of Acarology, 3rd. edit. Lubbock: Texas Tech University Press. p. 421-429.
- Walter D.E., Proctor H.C. 2013. Mites: Ecology, evolution & behaviour. Life at a Microscale. Sec. edition. Dordrecht: Springer. pp. 494. https://doi.org/10.1007/978-94-007-7164-2
- Wernz J.G., Krantz G.W. 1976. Studies on the function of the tritosternum in selected Gamasida (Acari). Canad. J. Zool., 54: 202-212. https://doi.org/10.1139/z76-022
- Wharton G.W. 1964. First International Congress of Acarology. Keynote address. Acarologia, Special Issue: 37-43.
- Wiśniewski J., Hirschmann W. 1993. Katalog der Ganggattungen, Untergattungen, Gruppen und Arten der Uropodiden der Erde (Taxonomie, Literatur, Grösse, Verbreitung, Vorkommen). Gangsystematic der Parasitiformes 548. Acarologie, 40: 1-220.
- Zhang Z.-Q., Hooper J.N.A., van Soest R. et al. 2011. (Eds). Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 7-237. https://doi.org/10.11646/zootaxa.3148.1.2



2025-01-10
Date accepted:
2025-05-06
Date published:
2025-06-30
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 de Moraes, Gilberto J.; Castilho, Raphael de Campos; Flechtmann, Carlos H.W.; Demite, Peterson R. and Halliday, Bruce
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



