Redescription of the feather mite Ingrassia aequinoctialis (Trouessart, 1899) (Acariformes: Xolalgidae) with notes on systematics and host associations of the Ingrassia genus-group
Mironov, Sergey V.  1
; Stormer, Hannah
1
; Stormer, Hannah  2
and Proctor, Heather C.
2
and Proctor, Heather C.  3
3
1✉ Zoological Institute, Russian Academy of Sciences, Universitetskaya embankment 1, 199034, Saint Petersburg, Russia.
2Department of Biological Sciences, University of Alberta, Edmonton, Alberta, T6G 2E9, Canada.
3Department of Biological Sciences, University of Alberta, Edmonton, Alberta, T6G 2E9, Canada.
2025 - Volume: 65 Issue: 2 pages: 482-496
https://doi.org/10.24349/f5zx-leveZooBank LSID: 0936B9ED-6025-4C6D-AFB6-555A4511F154
Original research
Keywords
Abstract
Introduction
The feather mite genus Ingrassia Oudemans, 1905, with 30 currently known species, is the most species-rich genus in the subfamily Ingrassiinae (Analgoidea: Xolalgidae) (Oudemans 1905; Černý 1967; Gaud 1972; Gaud and Atyeo 1981a; Chirov and Mironov 1990; Dabert and Ehrnsberger 1991; Vasyukova and Mironov 1991; Dabert 2000; Mironov and Palma 2006; Mironov and Proctor 2008; Stefan et al. 2013; Han et al. 2021). As for all representatives of the family Xolalgidae, these mostly small feather mites (with body size of adults about 300 μm) mainly inhabit the downy and covert feathers in the plumage of their avian hosts. Species of this genus have been recorded so far from hosts of five orders of aquatic birds: Charadriiformes, Phaethontiformes, Podicipediformes, Procellariiformes and Sphenisciformes. The majority of known Ingrassia species (20 species) are associated with charadriiforms, seven species live on procellariiforms, and one species is known from each of the three remaining host orders (Table 1).
In their generic revision of the family Xolalgidae, Gaud and Atyeo (1981a, 1981b) provided a modern taxonomic diagnosis of the genus Ingrassia and a review of its species content. Investigations on the systematics and diversity of Ingrassia in the past fifty years have been mainly focused on species associated with hosts from the order Charadriiformes (Gaud 1972; Vasyukova and Mironov 1986, 1991; Chirov and Mironov 1990; Dabert and Ehrnsberger 1991; Dabert 2000; Mironov and Palma 2006; Han et al. 2021). To date, identification keys to charadriiform-associated Ingrassia species are available for those in Africa (Gaud 1972) and in northern Eurasia (Chirov and Mironov 1990; Vasyukova and Mironov 1991), while all descriptions of the species associated with birds from other orders are scattered in various taxonomic papers (Černý 1967; Mironov and Proctor 2008; Stefan et al. 2013).
In the present paper, we redescribe the species Ingrassia aequinoctialis (Trouessart, 1899), which has been recorded from three species of tropicbirds (Phaethontidae): the Red-billed Tropicbird Phaethon aethereus Linnaeus, 1758 (type host), White-tailed Tropicbird P. lepturus Daudin, 1802 (=P. candidus Brisson, 1760), and Red-tailed Tropicbird P. rubricauda Boddaert, 1783 (=P. phoenicurus Gmelin, 1789). Although I. aequinoctialis is readily recognized because of its extremely large size compared to other Ingrassia species, it has never been illustrated or carefully redescribed since its brief initial text-only description by Trouessart (1899: 20). Apparently for these reasons, this mite species for a long time remained in the genus Megninia Berlese, 1881 (Analgidae) (Radford 1953, 1958), and was placed in Ingrassia only in the revision of the family Xolalgidae (Gaud and Atyeo 1981a). In addition to this redescription, we provide an updated world checklist of Ingrassia species together with those of three closely related genera, and discuss relationships and host associations of the Ingrassia genus-group.
Material and methods
Feather samples from Red-tailed Tropicbirds, Phaethon rubricauda, were collected under permit from the US Fish and Wildlife Service in June 2002 at Johnston Island, a part of Johnston Atoll National Wildlife Refuge, located in the central Pacific Ocean, a part of the United States. Capturing, handling and feather sampling was done by Julian P. Donahue and Dr. E. A. Schreiber, both from the Los Angeles County Museum of Natural History. Collected material was preserved in vials with 70% ethanol. These samples were subsequently shipped to the Proctor Lab at the University of Alberta. Mite specimens were cleared for 24 hours in lactic acid, and then mounted by HP and HS on microscope slides in commercially prepared PVA medium (BioQuip Products, Rancho Dominguez, California). Slides were cured for four days on a slide warmer set at ~40°C prior to being examined using DIC light microscopy on a Leica DMLB compound microscope. Slide-mounted specimens of Ingrassia aequinoctialis were sent to SM for examination and illustration.
The redescription and measuring techniques follow the standard formats used for ingrassiine feather mites (Mironov and Palma 2006; Mironov and Proctor 2008; Stefan et al. 2013; Mironov et al. 2017; Han et al. 2021). Morphological terms follow Gaud and Atyeo (1981a, 1996); the idiosomal and leg chaetotaxies also follow these authors with corrections for coxal setae by Norton (1998). Primary drawings and measurements were made using Leica light microscopes (DM2500 and DM 5000B, Leica Microsystems, Inc.) with DIC illumination and camera lucida; and final digital drawings were prepared using a Wacom Cintiq 22 graphics tablet (Wacom Co., Ltd). All the measurements are in micrometres (μm). Systematics and scientific names of birds follow the IOC World Bird List, v 14.2 (Gill et al. 2024). The examined material is deposited at the Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia.
Systematics
Family Xolagidae Dubinin, 1953
Subfamily Ingrassiinae Gaud & Atyeo, 1981
Genus Ingrassia Oudemans, 1905
Type species: Megninia veligera Oudemans, 1904 by original designation.
Ingrassia aequinoctialis (Trouessart, 1899)
(Figures 1–6)
Megninia aequinoctialis Trouessart 1899: 20; Radford 1953: 206, 1958: 112.
Ingrassia aequinoctialis: Gaud and Atyeo, 1981a: 71.
Material examined
3 males and 3 females (ZISP 23748–23753) from Phaethon rubricauda Boddaert, 1783 (Phaethontiformes: Phaethontidae), Johnston Island, Johnston Atoll National Wildlife Refuge (16°44′13″N, 169°31′26″W), U.S.A.; June 2002; coll. J. P. Donahue and E. A. Schreiber (see Material & Methods for more details).
Description
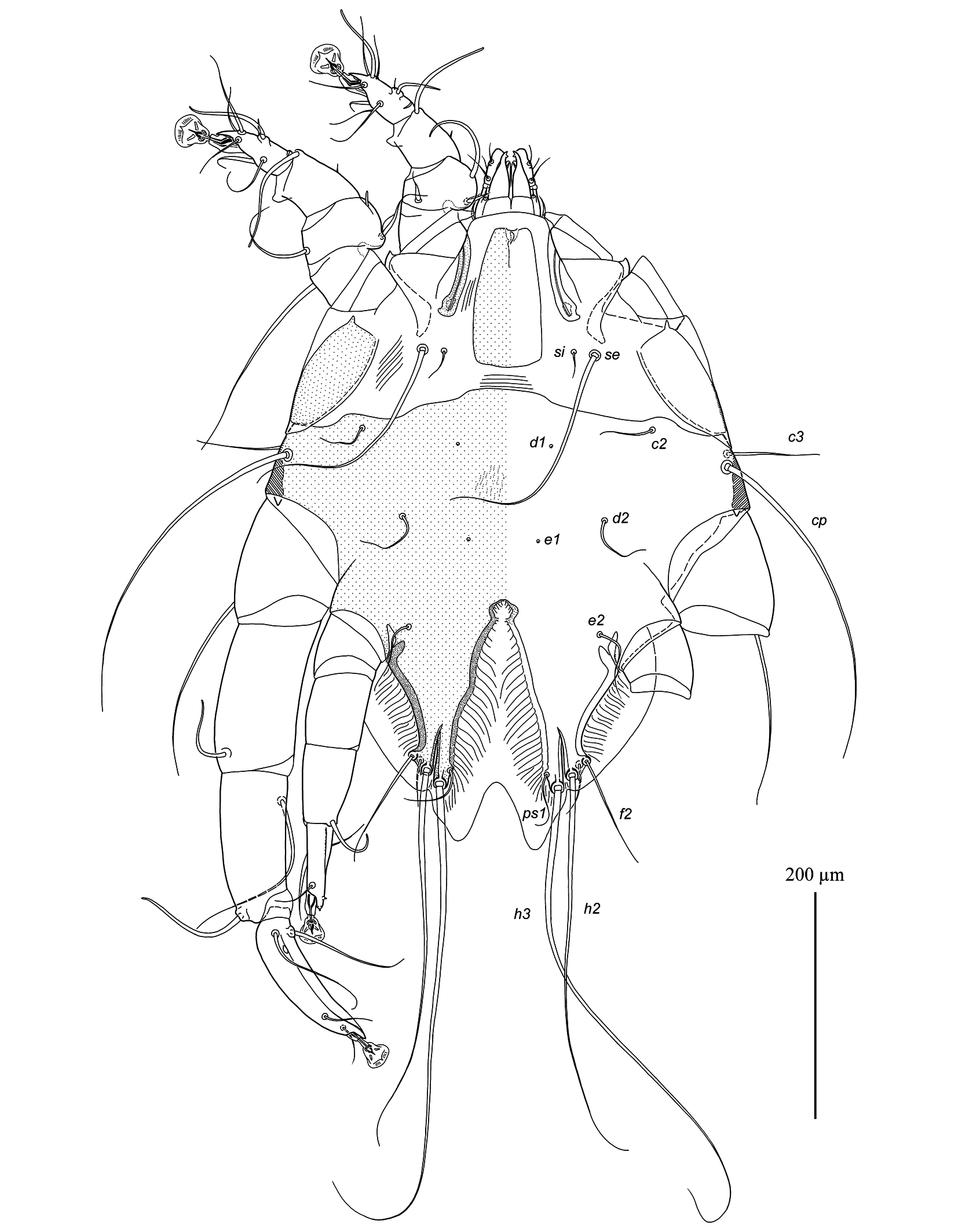




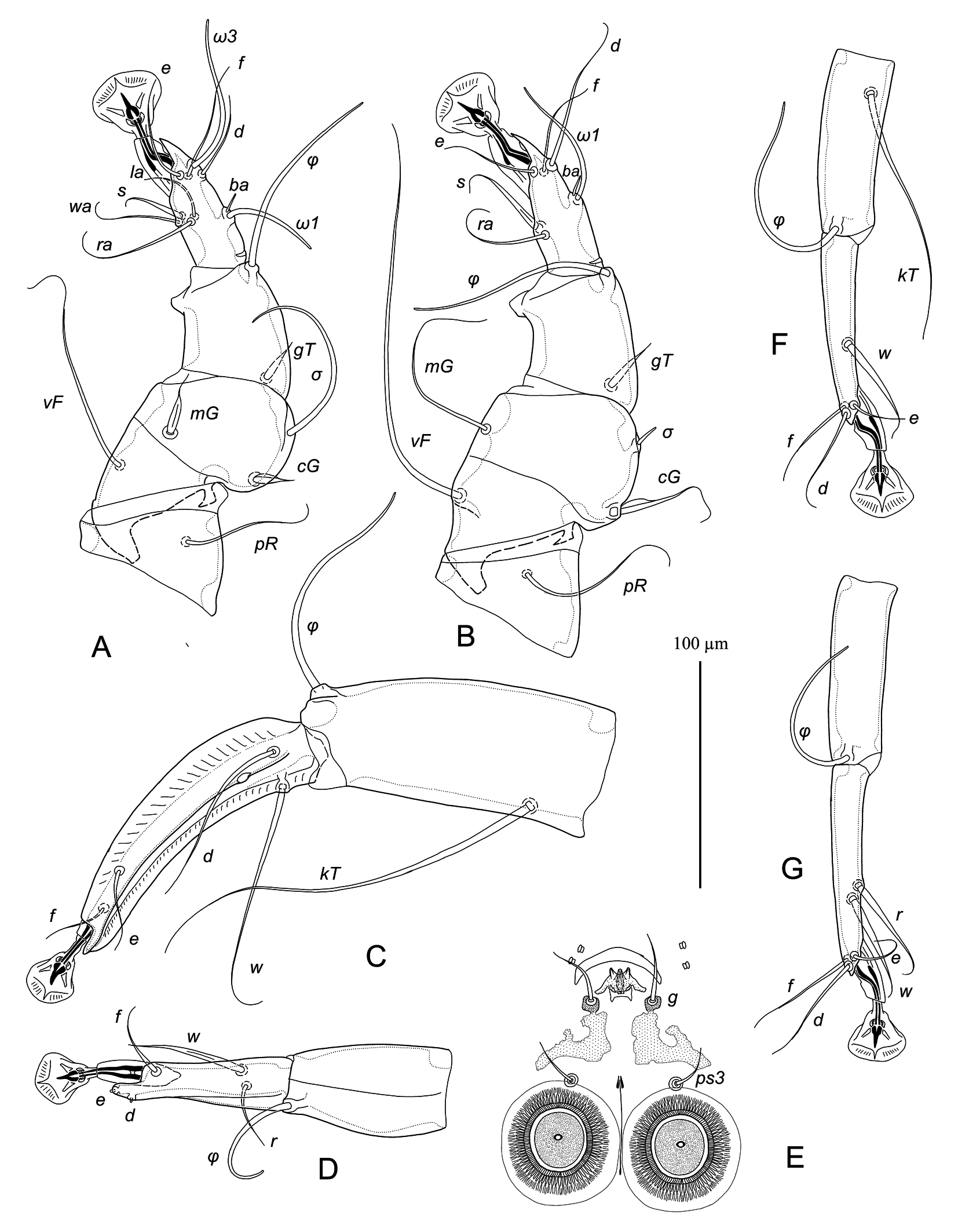
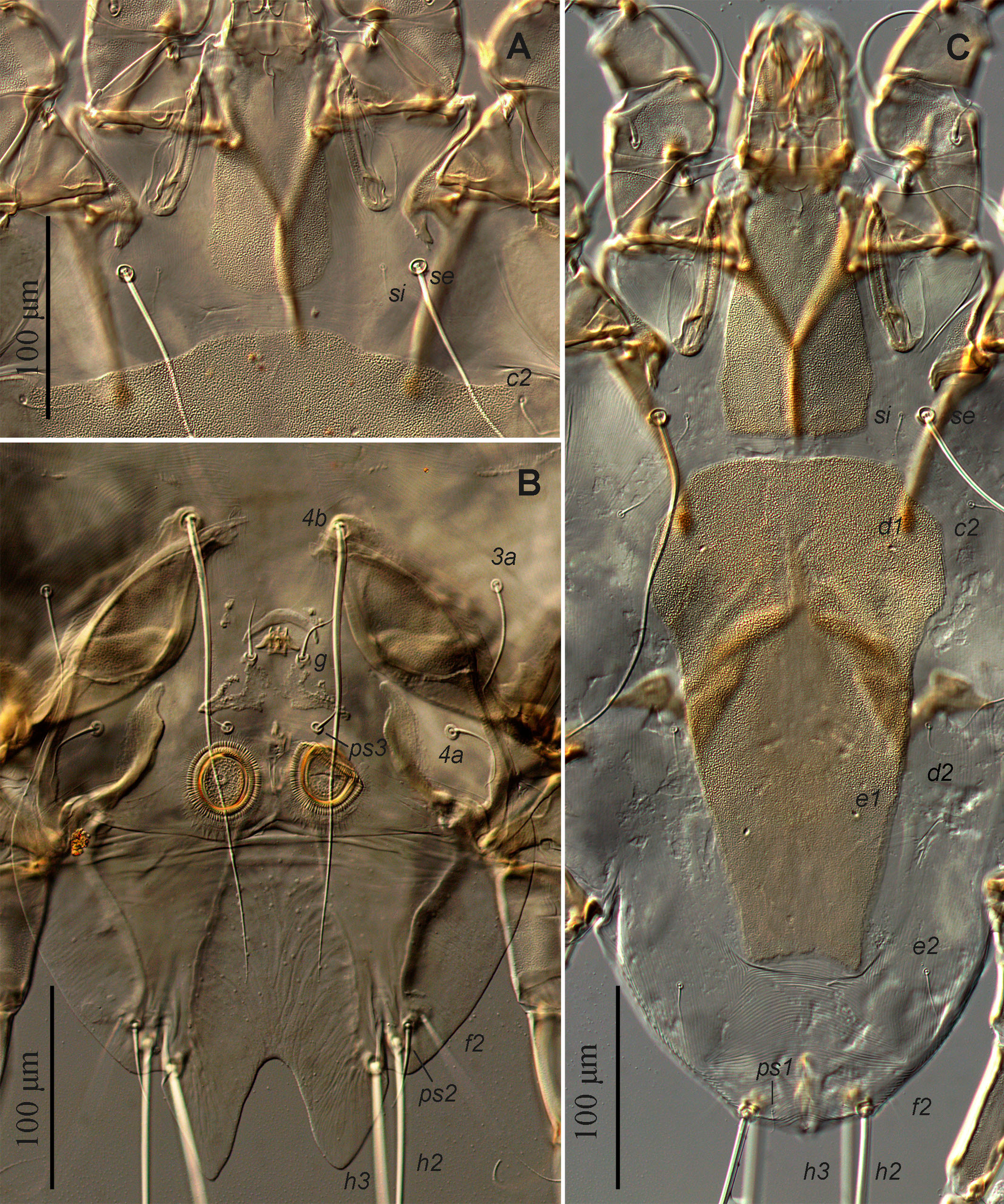
Male — (range for 3 specimens) (Figs. 1, 2, 5A-E). Idiosoma: length from anterior end to bases of setae h3 475–500, greatest width 405–415, length of hysterosoma 300–330. Prodorsal shield: nearly rectangular longitudinal plate slightly attenuate anteriorly, posterior margin almost straight or slightly convex, length 115–120, width at posterior margin 60–65, anterior end with longitudinal crest about 10 long, posterior end extending slightly beyond level of scapular setae se. Scapular setae se, si situated on striated tegument at the same transverse level; base of setae se separated by 135–150. Scapular shields: wide, inner margins of shield convex with narrow suprategumental fold, posterior end with angular suprategumental extension. Distance between prodorsal and hysteronotal shield 10–20. Hysteronotal shield: anterior margin convex and slightly sinuous, length of shield from anterior end to bases of setae h3 330–350, surface uniformly punctured, traces of fusion with humeral shields indistinct. Setae c2, d2, e2 and c3 short filiform, shorter than trochanter III. Setae d1 and setae e1 situated posterior to levels of setae c2 and d2, respectively. Supranal concavity circular, open posteriorly into terminal cleft. Terminal cleft long and roughly triangular, extending to level of trochanters III, length of the cleft from anterior end of concavity to bases of setae h3 155–160, greatest width at level of seta ps1 85–90. Terminal extensions on interlobar membrane wide triangular, with rounded apices, length of extensions from base of setae h3 to apices 50–55; incision between extensions wide triangular with rounded anterior extending to level of setae h3, length of incision 53–55. Setae h3 situated posterior to level of setae h2. Setae ps1 situated approximately at level of setae h2, not extending to apices of terminal extensions of interlobar membrane. Setae f2 extending slightly beyond terminal extensions. Distances between dorsal setae: c2:d2 70–80, d2:e2 85–98, e2:h3 125–140, c2:c2 245–250, h2:h2 120–140, h3:h3 100–105, ps1:ps1 85–88. Lengths of dorsal setae: c2 45–50, d2 and e2, 55–60, c3 85–100, f2 105–135, ps1 50–60.
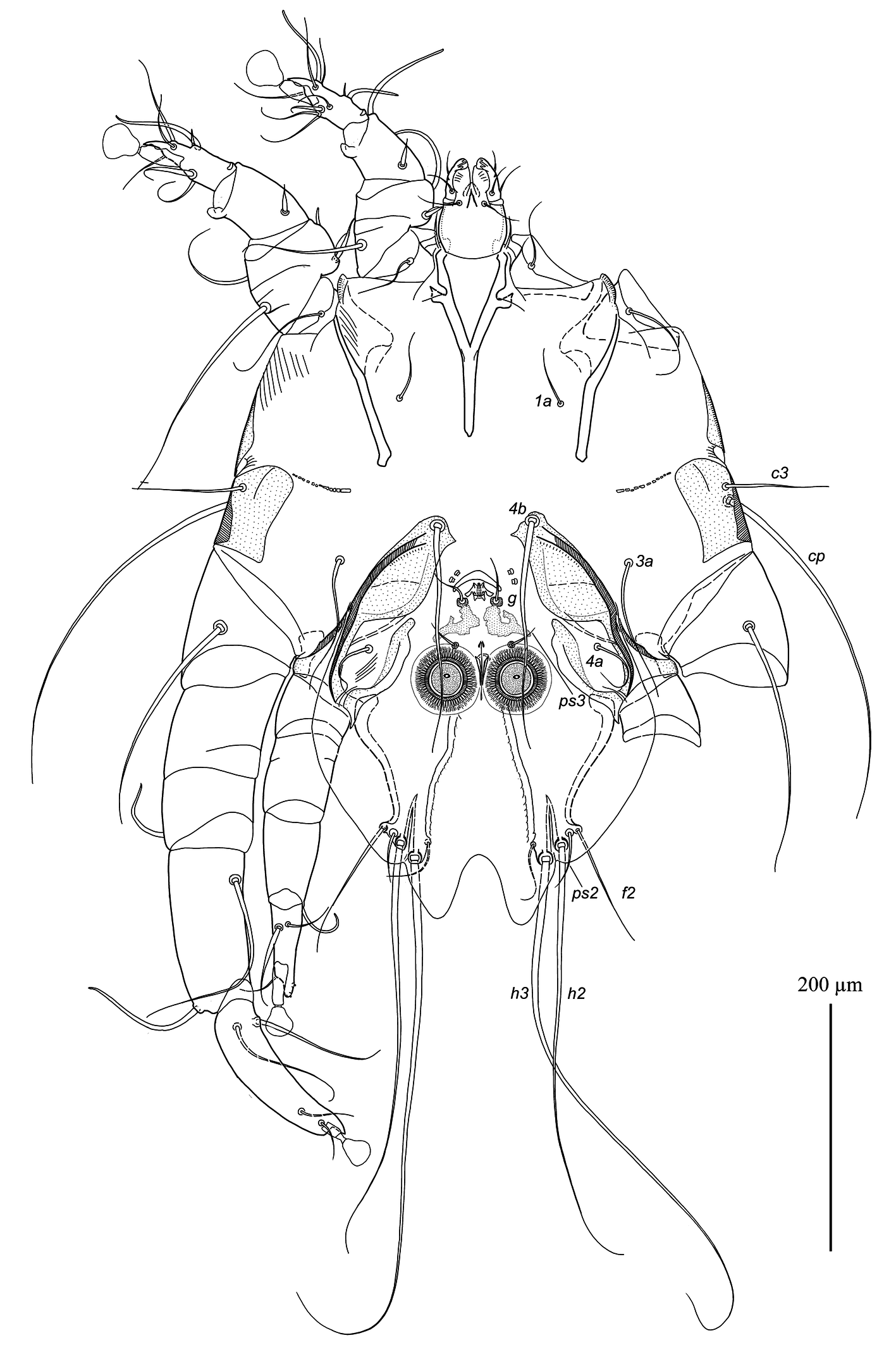
Epimerites I fused in a Y, with stem (sternum) approximately half as long as total length of epimerites I (Fig. 2). Epimerites III rudimentary. Inner ends of epimerites IIIa free (connected by narrow sclerotized bridge in one specimen). Epimerites IVa long, almost completely enclosing coxal fields IV. Central part of coxal fields IV bearing setae 4a not sclerotized. Setae 1a short filiform, extending to level of tips of epimerites II. Setae 3a extending to level of trochanters IV. Setae 4b situated on inner ends of epimerites IIIa, represented by macrosetae and extending approximately to midlength of opisthosomal lobes. Setae ps3 and 4a approximately at same transverse level. Epiandrum (pregenital apodeme) bow-shaped, 15–20 long, 36–38 wide. Genital apparatus small, 11–13 × 22–23; aedeagus minute, barely extending from genital arch. Genital shields roughly L-shaped, with strongly irregular margins (Fig. 5E). Genital setae g on anterior ends of genital shields. Adanal shields absent, setae ps3 on soft tegument anterior to adanal suckers. Adanal suckers circular, without denticles on anterior margin, 30–33 in diameter; surrounding membrane with extensive radial striation. Distances between ventral setae: 4b:3a 32–38, 4b:g 55–73, g:ps3 30–35, ps3:h3 165–170, 4b:4b 55–75, g:g 24–28, ps3:ps3 45–50. Lengths of ventral setae: 1a 27–33, 3a 65–80, 4a 42–48, 4b 220–230, g 25–28, ps2 38–45.
Tarsi I, II, each with a long apicodorsal claw-like projection, approximately 2.5 times longer than wide at base (Fig. 5A, B). Setae s of tarsi I, II spiculiform with short filiform apex. Tibia I, II, each with spine-like ventral processes, rounded apically; setae gT of tibiae I, II spiniform with short filiform apex. Seta mG of genu I spiniform with filiform apex, seta mG of genu II long filiform. Setae cG I, II with narrow membranous extension in basal part. Tibia III without angular extension at base of solenidion φ (Fig. 5C). Tarsus III 130–135 long, slightly curved, with crest-like inner and outer margins, and with short finger-like apical projection. Tarsus IV 75–78 long, with short finger-like apical extension bearing setae d, e; seta w spiculiform with short filiform apex, extending to midlength of ambulacral disc; modified setae d, e button-like, with minute central nipple (Fig. 5D). Legs IV excluding pretarsus 250–255 long, with tarsus and distal half of tibia extending beyond lobar apices (level of setae h3). Lengths of solenidia: ω1I 38–40, ω1II 58–63, σI 82–85, σII 10–12, σIII 62–65.
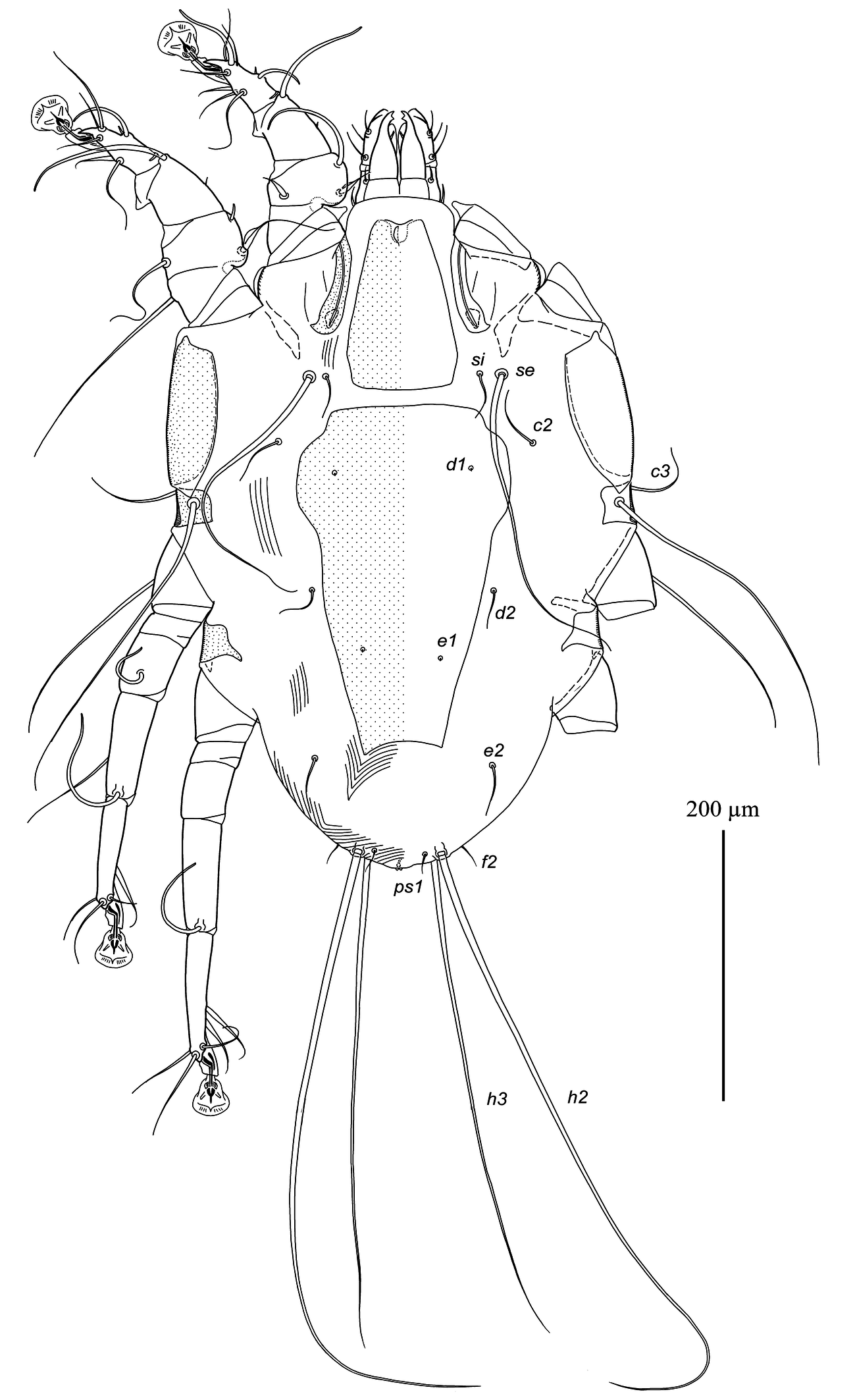




Female — (range for 3 specimens) (Figs. 3, 4, 5F, G). Idiosoma, length × width, 480–485 × 325–335; length of hysterosoma 275–280. Prodorsal shield: longitudinal plate shaped as very tall trapezoid; anterior end with short longitudinal crest about 5 long; posterior margin straight, posterior end slightly extending beyond level of scapular setae, length 115–125, width at posterior margin 78–83. Scapular setae se, si situated on striated tegument at the same transverse level, bases of setae se separated by 135–140. Scapular shields as in the male. Hysteronotal shield shaped as large longitudinal plate occupying median area of hysterosoma and stretching from midlevel of scapular shields to level slightly beyond trochanters IV, anterior margin straight, lateral margins strongly attenuate to posterior end, posterior margin concave, greatest length 240–250, greatest width of anterior part 145–150, width at posterior margin 60–68. Distance between prodorsal and hysteronotal shield 10–22. Setae c2, d2, e2 filiform, shorter than trochanters III, situated on striated tegument. Setae c3 slightly longer than trochanters III. Setae d1, e1 situated on hysteronotal shield, posterior to levels of setae c2 and d2, respectively. Setae e2 approximately at level of posterior margin of hysteronotal shield. Distances between dorsal setae: c2:d2 100–110, d2:e2 125–130, e2:h3 65–70, h2:h2 60–65, h3:h3 45–58. Lengths of dorsal setae: c2, d2 25–30, setae e2 35–45, c3 55–63. Macrosetae h3 about 3/4 the length of macrosetae h2.
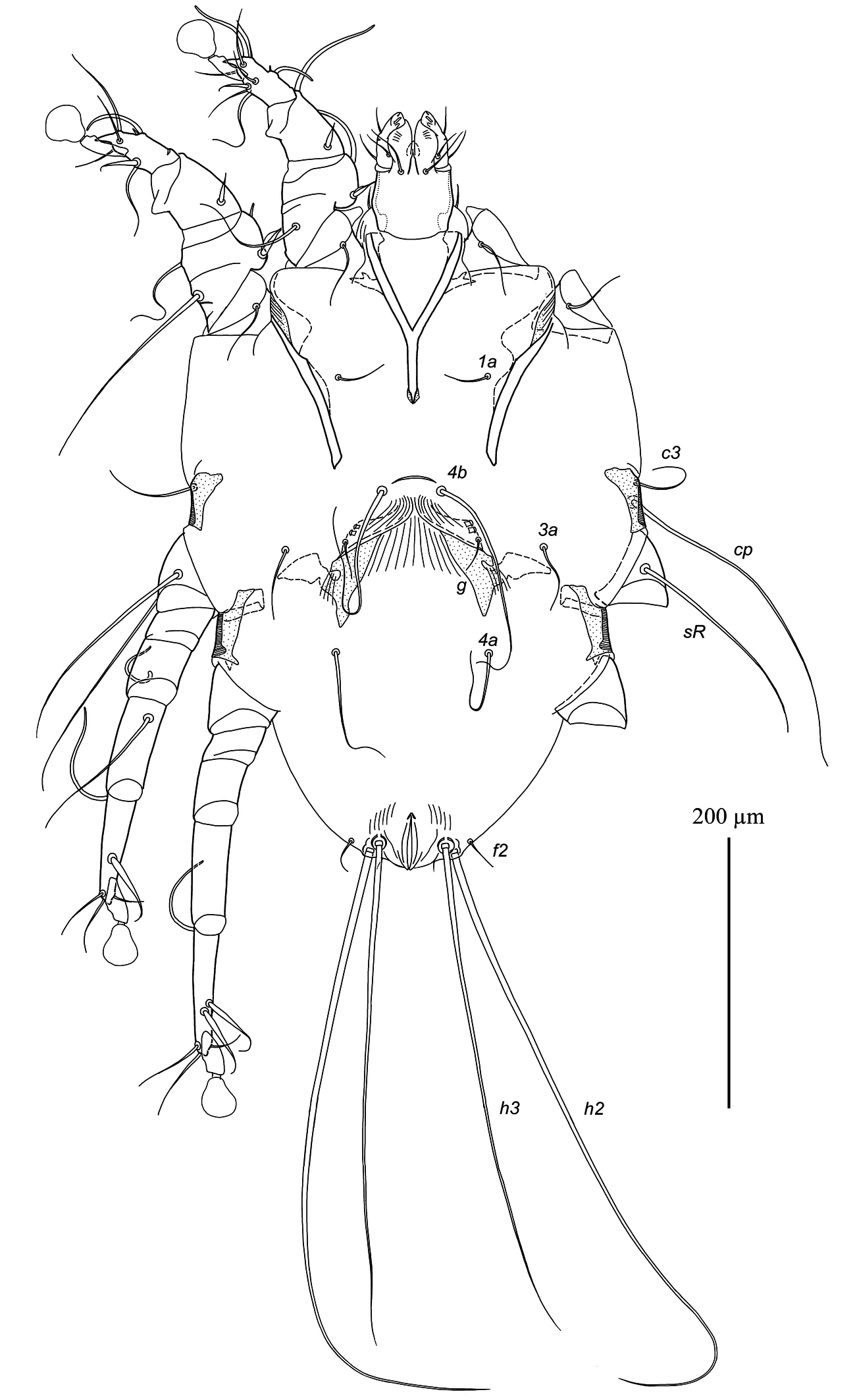
Epimerites I fused into a Y, with stem about 1/3 the total length of epimerites I (Fig. 4). Epigynum shaped as almost straight narrow transverse sclerite between bases of setae 4b, 25–30 wide. Apodemes of oviporus with posterior ends extending beyond trochanters III and with large lateral wing-like projection. Setae g situated at level of setae 3a or slightly anteriorly. Setae 4b long, extending to level of trochanters IV; setae 4a half as long as distance between their bases and posterior end of opisthosoma. Copulatory opening subterminal, covered by posterior ends of flaps of anal opening; spermatheca indistinct. Distances between ventral setae: 4b:3a 38–45, 4b:g 35–38, g:4a 83–88, 4a:4a 110–130. Lengths of ventral setae: 1a 35–40, 3a 45–52, 4a 80–85, 4b 160–180, g 8–10.
Legs I, II as in the male. Legs IV with tarsus and distal half of tibia extending beyond posterior margin of opisthosoma (Fig .3). Tarsi III, IV with small and rounded apicodorsal spine, 80–85 and 88–95 long, respectively (Fig. 5F, G). Trochanteral setae sRIII long, approximately equal to summed length of corresponding femur, genu and tibia. Seta w of tarsus III and setae r, w of tarsus IV thick spiculiform, with short filiform apices. Lengths of solenidia: ω1I 28–35, ω1II 50–53, σI 70–78, σII 10–11, σIII 40–48.




Remarks
Ingrassia aequinoctialis is an unmistakable species of the genus Ingrassia. This is an uncommonly large species with body length 480–500 µm, while in all other species of the genus, the length of idiosoma ranges from 250–350 µm. Additionally, Ingrassia aequinoctialis differs from other known species of the genus in the following features in both sexes: the prodorsal shield is a longitudinal plate shaped as a rectangle (male) or tall-trapezoid (female), and tarsi I, II have a long claw-like apicodorsal extension, about ¼ the total length of corresponding tarsi. In other known species of the genus, the prodorsal shield is shaped as a narrow median band with pointed posterior end or narrow longitudinal plate with an angular or rounded posterior margin; and the apicodorsal extension of tarsi I, II is shaped as a short acute or rounded spine. It is interesting to note that the strongly widened idiosoma and the convex anterior margin of the hysteronotal shield in the males of I. aequinoctialis and the hysteronotal shield enlarged anteriorly in the females of this species resemble those in mites of the genus Vingrassia Vasyukova & Mironov, 1991. However, in males of Vingrassia species, the anterior margin of the hysteronotal shield is bluntly angular (vs. rounded in I. aquinoctialis), and in females, the hysteronotal shield is roughly T-shaped (vs. lateral margins of anterior part convex).
It is interesting to note that all three species of tropicbird harbor another feather mite of extremely large size: Laminalloptes phaetontis (Fabricius, 1775) (Analgoidea: Alloptidae). The length of the idiosoma in the males and females of L. phaetontis can reach 1100 and 900 µm, respectively, making this species one of the largest among feather mites (Atyeo and Peterson 1967; Mironov and Stefan 2016). The phenomenon of the origin of very large feather mites from different families on tropicbirds remains enigmatic.
Discussion
In the discussion below, we consider taxonomic characteristics of the genera comprising the Ingrassia genus-group, their host associations and possible relationships. Authorities for host species mentioned in the Discussion are shown in Table 1. The genus Ingrassia was originally established by Oudemans (1905) for a single species, Megninia veligera Oudemans, 1904 from Tringa flavipes (Charadriiformes). Vitzthum (1921, 1922) described two new species, I. oceanica Vitzthum, 1921 from Hydrobates pelagicus (Procellariiformes) and I. tringae Vitzthum, 1922 from Calidris minuta (Charadriiformes), and transferred to this genus one more species, Analges velatus Mégnin, 1877 from Anas platyrhynchos (Anseriformes). Soon after, Hull (1934) established the genus Leptosphyra Hull, 1934 based on a single species Analges centropodus Mégnin, 1877 from Vanellus vanellus (Charadriiformes). Both Ingrassia and Leptosphyra were listed in the world checklists of feather mites summarized by Radford (1953, 1958).
Download as Remarks: *—type species of mite genera, (?)—questionable host association.
Mite genus and species
Type host
Host family
Host order
References
Ingrassia Oudemans, 1905
Oudemans 1905; Gaud 1972; Gaud and Atyeo 1981a
I. aequinoctialis (Trouessart, 1899)
Phaethon aethereus Linnaeus
Phaethontidae
Phaethontiformes
Trouessart 1899; Gaud and Atyeo 1981a
I. americanaDabert & Ehrnsberger, 1991
Tringa melanoleuca (Gmelin, JF)
Scolopacidae
Charadriiformes
Dabert and Ehrnsberger 1991
I. antarctica (Gaud, 1952)
Pelecanoides georgicus Murphy & Harper
Procellariidae
Procellariiformes
Gaud 1952
I. aphrizae Dabert & Ehrnsberger, 1991
Aphriza virgata (Gmelin, JF)
Scolopacidae
Charadriiformes
Dabert and Ehrnsberger 1991
I. arenaria (Gaud, 1972)
Arenaria interpres (Linnaeus)
Scolopacidae
Charadriiformes
Gaud 1972
I. calonectris* Stefan, Gomez-Diaz & Mironov, 2013
Calonectris borealis Cory
Procellariidae
Procellariiformes
Stefan et al. 2013
I. centrotibia Gaud, 1972
Himantopus himantopus (Linnaeus)
Recurvirostridae
Charadriiformes
Gaud 1972
I. chionis* Han, Mironov, Kim & Min, 2021
Chionis albus (Gmelin, JF)
Chionididae
Charadriiformes
Han et al. 2021
I. colymbi Gaud, 1974
Tachybaptus ruficollis (Pallas)
Podicipedidae
Podicipediformes
Gaud 1974
I. dubinini Černý, 1967
Ardenna tenuirostris (Temminck)
Procellariidae
Procellariiformes
Dubinin 1949; Černý 1967
I. eudyptula Mironov & Proctor, 2008
Eudyptula minor (Forster)
Spheniscidae
Sphenisciformes
Mironov and Proctor 2008
I. falcifera (Gaud, 1953)
Hydrobates castro (Harcourt)
Hydrobatidae
Procellariiformes
Gaud 1953
I. fissitarsa (Gaud, 1958)
Gallinago gallinago (Linnaeus)
Scolopacidae
Charadriiformes
Gaud 1958
I. forcipata (Haller, 1882)
Actitis macularius (Linnaeus)
Scolopacidae
Charadriiformes
Haller 1882
I. limnodromi Vasyukova & Mironov, 1986
Limnodromus scolopaceus (Say)
Scolopacidae
Charadriiformes
Vasyukova and Mironov 1986
I. limosae Gaud, 1972
Limosa limosa (Linneaeus)
Scolopacidae
Charadriiformes
Gaud 1972
I. micronota Stefan, Gomez-Diaz & Mironov, 2013
Bulweria bulwerii (Jardine & Selby)
Procellariidae
Procellariiformes
Stefan et al. 2013
I. oceanica Vitzthum, 1921
Hydrobates pelagicus (Linnaeus)
Hydrobatidae
Procellariiformes
Vitzthum 1921
I. oceanodromae Černý, 1967
Hydrobates leucorhous (Vieillot)
Hydrobatidae
Procellariiformes
Černý 1967
I. phalaropi Gaud, 1972
Phalaropus fulicarius (Linneaus)
Scolopacidae
Charadriiformes
Gaud 1972
I. philomachi Gaud, 1972
Calidris pugnax (Linnaeus)
Scolopacidae
Charadriiformes
Gaud 1972
I. platyspina Mironov & Palma, 2006
Prosobonia parvirostris (Peale)
Scolopacidae
Charadriiformes
Mironov and Palma 2006
I. semifissa Gaud, 1972
Glareola pratincola (Linnaeus)
Glareolidae
Charadriiformes
Gaud 1972
I. slonskiana Dabert, 2000
Tringa ochropus Linnaeus
Scolopacidae
Charadriiformes
Dabert 2000
I. spinata (Gaud & Mouchet, 1959)
Glareola nuchalis Gray
Glareolidae
Charadriiformes
Gaud and Mouchet, 1959
I. strictior (Berlese, 1898)
Calidris pugnax (Linnaeus) (?)
Scolopacidae
Charadriiformes
Berlese 1898; Hull 1934; Turk 1953; Gaud and Atyeo 1981a
I. tridactyla (Gaud & Mouchet, 1959)
Actophilornis africanus (Gmelin, JF)
Jacanidae
Charadriiformes
Gaud and Mouchet 1959
I. tringae Vitzthum, 1922
Calidris minuta (Leisler)
Scolopacidae
Charadriiformes
Vitzthum 1922; Gaud 1972; Gaud and Atyeo 1981a
(=I. minuta Gaud, 1972)
I. veligera (Oudemans, 1904)*
Tringa flavipes (Gmelin, JF)
Scolopacidae
Charadriiformes
Oudemans 1904, 1905
I. xiphidiopteri Gaud, 1972
Vanellus albiceps Gould
Charadriidae
Charadriiformes
Gaud 1972
Leptosphyra Hull, 1934
Hull 1934; Gaud 1974; Gaud and Atyeo 1981a
L. acuticubita (Gaud, 1953)
Pluvianus aegyptius (Linnaeus)
Pluvianidae
Charadriiformes
Gaud 1953
L. centropoda (Mégnin, 1877)*
Vanellus vanellus (Linnaeus)
Charadriidae
Charadriiformes
Robin and Mégnin 1877; Gaud 1972
L. labeotibia Gaud, 1968
Burhinus capensis (Linnaeus)
Burhinidae
Charadriiformes
Gaud 1968
L. semidentata Gaud, 1972
Anarhynchus alexandrinus (Linnaeus)
Charadriidae
Charadriiformes
Gaud 1972
Tectingrassia Gaud, 1972
Gaud 1972, 1974; Gaud and Atyeo 1981a
T. avosetta Gaud, 1974
Recurvirostra avosetta Linnaeus
Recurvirostridae
Charadriiformes
Gaud 1974
T. calidris Vasyukova & Mironov, 1986
Calidris alpina (Linnaeus)
Scolopacidae
Charadriiformes
Vasyukova and Mironov 1986
T. gracilipes (Trouessart & Neumann, 1888)
Tringa totanus (Linnaeus)
Scolopacidae
Charadriiformes
Trouessart and Neumann 1888; Gaud 1958; Gaud and Atyeo 1981a
(=T. foliata Gaud, 1958)
T. holoplax Chirov & Mironov, 1990
Calidris ferruginea (Pontoppidan)
Scolopacidae
Charadriiformes
Chirov and Mironov 1990
T. lativelata Gaud, 1972
Calidris pugnax (Linnaeus)
Scolopacidae
Charadriiformes
Gaud 1972
T. pilosa (Gaud, 1958)*
Tringa glareola Linnaeus
Scolopacidae
Charadriiformes
Gaud 1958, 1972
T. truncatifolia Gaud, 1972
Arenaria interpres (Linnaeus)
Scolopacidae
Charadriiformes
Gaud 1972
Vingrassia Vasyukova and Mironov, 1991
Vasyukova and Mironov 1991
V. cygni Mironov & Galloway, 2002
Cygnus columbianus (Ord)
Anatidae
Anseriformes
Mironov and Galloway 2002
V. latior (Trouessart, 1899) comb. n.
Anas bahamensis Linnaeus
Anatidae
Anseriformes
Trouessart 1899
V. velata (Mégnin, 1877)*
Anas platyrhynchos Linnaeus
Anatidae
Anseriformes
Robin and Mégnin 1877
For unclear reasons, Gaud ignored the genus Ingrassia in his early works on feather mites from Africa and described all ingrassine species from charadriiforms in the content of the genus Leptosphyra (Gaud 1952, 1953, 1958; Gaud and Mouchet 1959). Later on, in a taxonomic review of feather mites associated with charadriiforms in Africa, Gaud (1972) recognized the priority of the genus Ingrassia and significantly reformed its species content. Along with descriptions of 11 new species, this author enlarged the content of Ingrassia by including Leptosphyra as a subgenus and establishing the new subgenus Tectingrassia Gaud, 1972.
In their generic review of the family Xolalgidae, Gaud and Atyeo (1981a, 1981b) provided uniform diagnoses for all genera of the family and revised their species contents. In the part dealing with the subfamily Ingrassiinae, these authors (Gaud and Atyeo, 1981a) elevated the subgenera Ingrassia, Leptosphyra and Tectingrassia to full generic rank, but stressed their very close affinity to each other. Within the subfamily Ingrassiinae, these three genera can be referred to as members of the Ingrassia genus-group. Along with restriction to particular aquatic bird orders, these genera share the following combination of morphological features. In both sexes, idiosomal setae c1 are absent, tarsus I has 4 ventral setae (la, ra, wa, and s), tarsus II has two ventral setae (ra and s), and tibiae I, II have ventral spine-like projections; in males, the opisthosoma has a pair of long lobes separated by the deep terminal cleft and surrounded by lateral and interlobar membranes, the distal ends of interlobar membrane spreading onto the lobar apices form angular or semi-ovate terminal projections, legs III are hypertrophied, and the inner tips of epimerites IIIa bear the bases of setae 4b; in females, idiosomal setae ps2 and ps3 are absent.
The genera Ingrassia, Leptosphyra and Tectingrassia differ from each other by the following characters. In representatives of the genus Tectingrassia, the prodorsal shield in both sexes is shaped as a trapezoid, the posterior angles of which incorporate the bases of scapular setae se and si, while in two other genera the prodorsal shield is a narrow longitudinal band or plate with angular or rounded posterior margin and scapular setae are situated on soft tegument or on small teardrop-like sclerites. Additionally, in females of Tectingrassia, the hysteronotal shield occupies the entire median area of the hysterosoma and its anterolateral angles are close to or fused with the humeral shields. In females of Ingrassia and Leptosphyra, the hysteronotal shield is variable in size, from a very small plate (as in Ingrassia micronota Stefan, Gomez-Diaz & Mironov, 2013) to a relatively large longitudinal rectangle (as in many Ingrassia species from charadriiforms), but its anterolateral parts are never close to the humeral shields (see Gaud 1972; Gaud and Atyeo 1981a; Chirov and Mironov 1990; Vasyukova and Mironov 1991).
Mites of the genus Leptosphyra differ from those of two other genera in having a retrograde hook-like lateral projection on femora II, while in the two other genera, this projection is absent or barely developed. Representatives of the genus Ingrassia are distinguished by the absence of the aforementioned character states characterizing Leptosphyra and Tectingrassia, which are obviously apomorphic. Compared to the two latter genera, members of Ingrassia are more diverse in morphological features, in particular, in the shape of the prodorsal shield in both sexes, which varies from a narrow median band (in species from Procellariiformes) to a longitudinal plate with lateral margins parallel or divergent posteriorly (in species from Charadriiformes), and in the shape and size of the hysteronotal shield in females (see Gaud, 1972; Chirov and Mironov 1990; Vasyukova and Mironov 1986, 1991; Mironov and Proctor 2008; Stefan et al. 2013; Han et al. 2021)
Vasyukova and Mironov (1991) established Vingrassia, one more genus of this genus-group, which initially included only Ingrassia velata (Mégnin, 1877), which they transferred from Ingrassia. The genus Vingrassia, representatives of which are associated with anseriform hosts, differs from other genera of the Ingrassia genus-group in having epimerites I fused as a V and the idiosoma noticeably widened in both sexes, the hysteronotal shield with blunt-angular extension on the anterior margin in males, and T-shaped hysteronotal shield in females. In contrast, the other three genera of the group have epimerites I fused in the form of a Y with a very long stem, the anterior margin of the male hysteronotal shield is straight, wavy or concave, and the hysteronotal shield in females is of another form and in most species is not enlarged anteriorly. The second described species of Vingrassia, V. cygni Mironov & Galloway, 2002, has epimerites I fused as a Y with a very short stem (Mironov and Galloway 2002). In their review of supraspecific feather mite taxa of the world, Gaud and Atyeo (1996) did not recognize Vingrassia as a genus and considered it as a synonym of Ingrassia. Unlike these authors, we recognize Vingrassia as a morphologically distinct genus and herein include one more species with a new name Vingrassia latior (Trouessart, 1899) comb. n. (Table 1). This species, originally described from one male and named Megninia centropodos latior Trouessart, 1899, was collected from Anas bahamensis (Anseriformes) in Central America. Although it was described extremely briefly, the original description indicates that it is a wide-bodied mite, with the opisthosomal membrane forming two widely rounded lobes (Trouessart 1899: 57). These features, which are also stressed in the subspecific name ''latior″, along with association with an anseriform host, give evidence that this mite is close to other species from anseriforms, like Vingrassia velata and V. cygni, rather than to narrow-bodied Ingrassia species living on charadriiforms. Gaud and Atyeo (1981a) conventionally referred this species to the genus Ingrassia.
Phylogenetic analysis of the family Xolalgidae based on morphological characters (Mironov 2005) supported the monophyly of the cluster corresponding to the Ingrassia genus-group and clades corresponding to the genera Leptosphyra, Tectingrassia and Vingrassia. However, the genus Ingrassia in this analysis appeared to be paraphyletic, because the clades of three latter genera branched out of the crown of the Ingrassia group cluster.
Considering host associations of Ingrassia and related genera, we follow the current taxonomic concept (Gaud and Atyeo 1981a; Mironov and Galloway 2002) and recognize four genera in the Ingrassia genus-group (Table 1). This updated world checklist provides data on type hosts of mites and corresponding references containing adequate descriptions and taxonomic notes. It is clear that three genera of the group are restricted to particular orders: Leptosphyra and Tectingrassia are restricted to Charadriiformes, and Vingrassia species to Anseriformes. At the same time, mites of the genus Ingrassia are distributed on members of the Charadriiformes, Phaethontiformes, Podicipediformes, Procellariiformes and Sphenisciformes. Within Neoaves, these five orders belong to the major clade Aequornithes uniting aquatic birds except Anseriformes (e.g. Prum et al. 2015). This host distribution suggests that the genus Ingrassia probably originated on the ancestors of Aequornithes and further evolved on some lineage of this major clade, while Leptosphyra and Tectingrassia were formed and evolved on representatives of Charadriiformes. The genus Vingrassia is restricted to Anseriformes, which belongs to the archaic avian clade Galloanserae. The origin of this genus on these hosts is enigmatic, because any representatives of the family Xolalgidae are absent on galliforms, a sister lineage of anseriforms in Galloanserae. It is highly likely that primary appearance of Vingrassia on the ancestor of Anseriformes is the result of a horizontal transfer event from some representatives of Aequornithes.
Taking into account the relatively weak morphological features differentiating members of the Ingrassia genus-group, their host distributions, and the results of morphologically based phylogenetic analysis of Xolalgidae, it is possible to hypothesize that the genera Leptosphyra, Tectingrassia and Vingrassia could represent derived lineages of Ingrassia that originated within corresponding bird orders. If this turns out to be the case, perhaps it would be expedient to decrease the rank of Leptosphyra, Tectingrassia and Vingrassia and include them as subgenera in Ingrassia. The taxonomic structure of the Ingrassia genus-group certainly needs careful revision, and for this purpose, molecular based phylogenetic analysis would be quite desirable.
Acknowledgements
The authors thank Julian P. Donahue and Dr. Betty Elizabeth Anne Schreiber from the Los Angeles County Museum of Natural History Museum of Los Angeles County, who provided us with mites from their collecting activities. We are also grateful to Dmitri Logunov (The Manchester Museum, U.K.) for assisting with delivery of slide-mounted mites to SM. The study was supported in part by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to HP. The taxonomic part of the study was supported by the Ministry of Science and Higher Education of the Russian Federation (project No. 125013001089-0) for SM.
References
- Atyeo W.T., Peterson P.C. 1967. The feather mite genus Laminalloptes (Proctophyllodidae: Alloptinae). Journal of the Kansas Entomological Society, 40 (4): 447-458.
- Berlese A. 1898. Megninia centropodos var. strictior. Acari, Myriopoda et Scorpiones hucusque in Italia reperta, Padova, Fascocolo 87, No 6.
- Černý V. 1967. Trois espèces nouvelles des Acariens plumicoles (Analgoidea) parasites des Procellariiformes. Folia Parasitologica, 14: 87-91.
- Chirov P.A., Mironov S.V. 1990. Feather mites of the subfamily Ingrassiinae of limicolines and ducks. Izvestiya Akademii Nauk Kirgizkoi SSR, Khimiko-Teknologicheskie i Biologicheskie Nauki, 3: 74-83. [In Russian]
- Dabert J. 2000. Feather mites (Acari: Astigmata) of water birds of the Słońsk nature reserve with the description of a new species. Biological Bulletin of Poznań, 37: 303-316.
- Dabert J., Ehrnsberger R. 1991. Zwei neue Federmilben-Arten aus der Gattung Ingrassia Oudemans, 1905 (Analgoidea; Xolalgidae; Ingrassiinae). Osnabrücker naturwissenshaftliche Mitteilungen, 17: 127-142.
- Dubinin V.B. 1949. Feather mite fauna of birds of the order Procellariiformes and its features. Parazitologicheskii Sbornik, 11: 201-228. [In Russian]
- Gaud J. 1952. Acariens plumicoles (Analgesidae) de quelques oiseaux des Iles Kerguelen (Récolte P. Paulian). Mémoires de l′Institut scientifique de Madagascar, Séries A, 7: 161-166.
- Gaud J. 1953. Sarcoptides plumicoles des oiseaux d'Afrique occidentale et centrale. Annales de Parasitologie Humaine et Comparée, 28: 193-226. https://doi.org/10.1051/parasite/1953283193
- Gaud J. 1958. Acariens plumicoles (Analgesoidea) parasites des oiseaux du Maroc. II. Analgidae. Bulletin de la Société de Sciences Naturelles et Physiques du Maroc, 38: 27-49.
- Gaud J. 1968. Acariens sarcoptiformes plumicoles (Analgoidea) parasites sur les oiseaux Ralliformes et Gruiformes d'Afrique. Musée Royale de l′Afrique Centrale, Annales, Série in-8°, Sciences Zoologiques, 164: 1-101.
- Gaud J. 1972. Acariens Sarcoptiformes plumicoles (Analgoidea) parasites sur les oiseaux Charadriiformes d'Afrique. Musée Royale de l′Afrique Centrale, Annales, Série in-8°, Sciences zoologiques. 193: 1-116.
- Gaud J. (1973) 1974. Quelques espèces nouvelles de Sarcoptiformes plumicoles (Analgidae et Dermoglyphidae) parasites d'oiseaux d'Europe. Acarologia, 15: 727-758.
- Gaud J., Atyeo W.T. 1981a. La famille Xolalgidae Dubinin, nouveau statut (Sarcoptiformes plumicoles, Analgoidea). I. Sous-famille Ingrassiinae, n. sub. fam. Acarologia, 22: 63-79.
- Gaud J., Atyeo W.T. 1981b. La famille Xolalgidae, Dubinin (Acariens plumicoles, Analgoidea). II. Sous-familles Xolalginae et Zumptiinae, n. sub. fam. Acarologia, 22: 313-324.
- Gaud J., Atyeo W.T. 1996. Feather mites of the World (Acarina, Astigmata): the supraspecific taxa. Musée Royal de l′Afrique Centrale, Annales, Sciences Zoologiques, 277: 1-193 (Pt. 1, text), 1-436 (Pt. 2, illustrations).
- Gaud J., Mouchet J. 1959. Acariens plumicoles (Analgesoidea) parasites des oiseaux du Cameroun. II. Analgesidae. Annales de Parasitologie Humaine et Comparée, 34: 149-208. https://doi.org/10.1051/parasite/1959341149
- Gill F., Donsker D., Rasmussen P. (Eds.). 2024. IOC World Bird List (v 14.2). Available from http://www.worldbirdnames.org/ (accessed 10 January 2025). https://doi.org/10.14344/IOC.ML.14.2
- Haller G. 1882. Zur Kenntniss der Dermaleichiden. Archiv für Naturgeschichte, 48: 47-79.
- Han Y.-D., Mironov S.V., Kim J.-H., Min G.-S. 2021. Feather mites (Acariformes: Astigmata) from marine birds of the Barton Peninsula (King George Island, Antarctica), with descriptions of two new species. ZooKeys, 1061: 109-130. https://doi.org/10.3897/zookeys.1061.71212
- Hull J.E. 1934. Concerning British Analgidae (Feather-mites). Transactions of the Northern Naturalists' Union, 1: 200-206.
- Mironov S.V. (2004) 2005. Phylogeny of the feather mite family Xolalgidae (Astigmata: Analgoidea) and revolutionary trends with non-passerine birds. Phytophaga, 14: 433-449.
- Mironov S.V., Galloway T.D. 2002. Four new species of feather mites (Acari: Analgoidea). The Canadian Entomologist, 134: 605-618. https://doi.org/10.4039/Ent134605-5
- Mironov S.V., Palma R.L. 2006. Two new feather mite species (Acari: Analgoidea) from the Tuamotu sandpiper Aechmorhynchus parvirostris (Charadriiformes: Scolopacidae). Tuhinga - Records of the Museum of New Zealand Te Papa Tongarewa, 17: 49-59.
- Mironov S.V., Proctor H.C. 2008. The probable association of feather mites of the genus Ingrassia (Analgoidea: Xolalgidae) with the blue penguin Eudyptula minor (Aves: Sphenisciformes) in Australia. Journal of Parasitology, 94: 1243-1248. https://doi.org/10.1645/GE-1579.1
- Mironov S.V., Ehrnsberger R., Dabert J. 2017. Feather mites of the genera Dubininia and Cacatualges (Acari: Xolalgidae) associated with parrots (Aves: Psittaciformes) of the Old World. Zootaxa, 4272 (4): 451-490. https://doi.org/10.11646/zootaxa.4272.4.1
- Mironov S.V., Stefan L.M. 2016. On identification of species in the feather mite genus Laminalloptes Dubinin, 1955 (Acari: Alloptidae). Acarina, 24 (1): 77-85. https://doi.org/10.21684/0132-8077.2016.24.1.77.85
- Norton R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Experimental and Applied Acarology, 22: 559-594. https://doi.org/10.1023/A:1006135509248
- Oudemans A.C. 1904. Acarologische Aanteekeningen XIV. Entomologische Berichten, 1: 190-195. https://doi.org/10.5962/bhl.part.1123
- Oudemans A.C. 1905. Acarologische Aanteekeningen XVII. Entomologische Berichten, 1: 222-226. https://doi.org/10.5962/bhl.part.1126
- Prum R.O., Berv J.S., Dornburg A., Field D.J., Townsend J.P., Lemmon E.M., Lemmon A.R. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature, 526: 569-573. https://doi.org/10.1038/nature15697
- Radford C.D. 1953. The mites (Acarina: Analgesidae) living on or in the feathers of birds. Parasitology, 42: 199-230. https://doi.org/10.1017/S0031182000084468
- Radford C.D. 1958. The host-parasite relationships of the feather mites (Acarina: Analgesoidea). Revista Brasileira de Entomologia, 8: 107-170.
- Robin C., Mégnin P. 1877. Mémoire sur les Sarcoptides plumicoles. Journal de l′Anatomie et de la Physiologie Normales et Pathologiques de l′Homme et des Animaux, Paris, 13: 209-248, 391-429, 498-520, 629-656 + pls. XII, XIII, XXII-XXIX, XXXVI-XXXVIII.
- Stefan L.M., Gomez-Diaz E., Mironov S.V. 2013. Three new species of the feather mite subfamily Ingrassiinae (Acariformes: Xolalgidae) from shearwaters and petrels (Procellariiformes: Procellariidae). Zootaxa, 3682 (1): 105-120. https://doi.org/10.11646/zootaxa.3682.1.4
- Trouessart E.L. (1898) 1899. Diagnoses préliminaires d'espèces nouvelles d'Acariens plumicoles. Additions et corrections à la sous-famille des Analgésinés. Bulletin de la Société d'Études scientifiques d'Angers, 28: 1-62.
- Trouessart E.L., Neumann G. 1888. Diagnoses d'espèces nouvelles de Sarcoptides plumicoles (Analgesinae). Bulletin scientifique de la France et de la Belgique, 19: 325-380 + pls. XXII-XXVII.
- Turk F.A. 1953. A synonymic catalogue of British Acari; Part II. Annals and Magazine of Natural History, 6: 81-89. https://doi.org/10.1080/00222935308654402
- Vasyukova T.T., Mironov S.V. 1986. New species of feather mites of Siberia. Publisher: Nauka, Siberian Dept., Novosibirsk, 72 pp. [In Russian]
- Vasyukova T.T., Mironov S.V. 1991. Feather mites of Anseriformes and Charadriiformes of Yakutia, Systematics. Publisher: Nauka, Siberian Dept., Novosibirsk, 201 pp. [In Russian]
- Vitzthum H. 1921. Acarologische Beobachtungen. (5. Reihe). Archiv für Naturgeschichte, 87A (4): 1-77.
- Vitzthum H. 1922. Acarologische Beobachtungen. Zoologische Jahrbücher, Abteilung für Systematik Ökologie und Geographie der Tiere, 44 (6): 517-564.



2025-02-04
Date accepted:
2025-04-25
Date published:
2025-05-07
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Mironov, Sergey V.; Stormer, Hannah and Proctor, Heather C.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



