Insectolaelaps zvoleniensis (Wiśniewski & Hirschmann), a common but little known digamasellid mite (Acari: Mesostigmata) of subcortical microhabitats in Slovakia
Mašán, Peter  1
1
1✉ Institute of Zoology, Slovak Academy of Sciences, Dúbravská cesta 9, 845 06 Bratislava, Slovakia.
2025 - Volume: 65 Issue: 2 pages: 461-481
https://doi.org/10.24349/uv0y-d60oZooBank LSID: 5ACD2ED5-1ACD-4357-BFFD-1BB19180199A
Original research
Keywords
Abstract
Introduction
The genus Insectolaelaps is a rather small group of digamasellid mites distributed in the Holarctic, currently comprising 15 known species from Europe (10 spp.), Asia (3 spp.) and North America (2 spp.), mainly found in saproxylic habitats, especially in subcortical spaces associated with galleries of bark- and wood-boring beetles (Castilho 2012). The phoretic activity of their deutonymphs is common in many xylophagous beetles, especially in the scolytine curculionids (Hirschmann and Wiśniewski 1982).
Insectolaelaps was originally described by Shcherbak (1980) as a genus of Rhodacaridae Oudemans and later treated as a subgenus of Dendrolaelaps Halbert by Hirschmann and Wiśniewski (1982). Karg (1993) and Castilho (2012) considered Insectolaelaps as a separate genus of Dendrolaelapinae Hirschmann and Digamasellidae Evans, respectively (both as equivalent taxa), with Ipidodendrolaelaps Hirschmann & Wiśniewski as a conspecific taxon, whose species were also included in the genus by Shcherbak (1980), as species of the quadrisetus group sensu Lindquist, 1975. In my opinion, the separate position of Ipidodendrolaelaps is justified and should be considered as a valid taxon in future taxonomic revisions.
The aim of this study is to describe both the adults and the deutonymphal stage of Insectolaelaps zvoleniensis, to compare them with other similar species and to provide some data on the habitat preference of the species. This topic provides insights into the relationships between mites, beetles, decomposing wood and their shared environment. This work is part of a project that aims to increase our knowledge about the diversity of saproxylic mites in Slovakia.
Material and methods
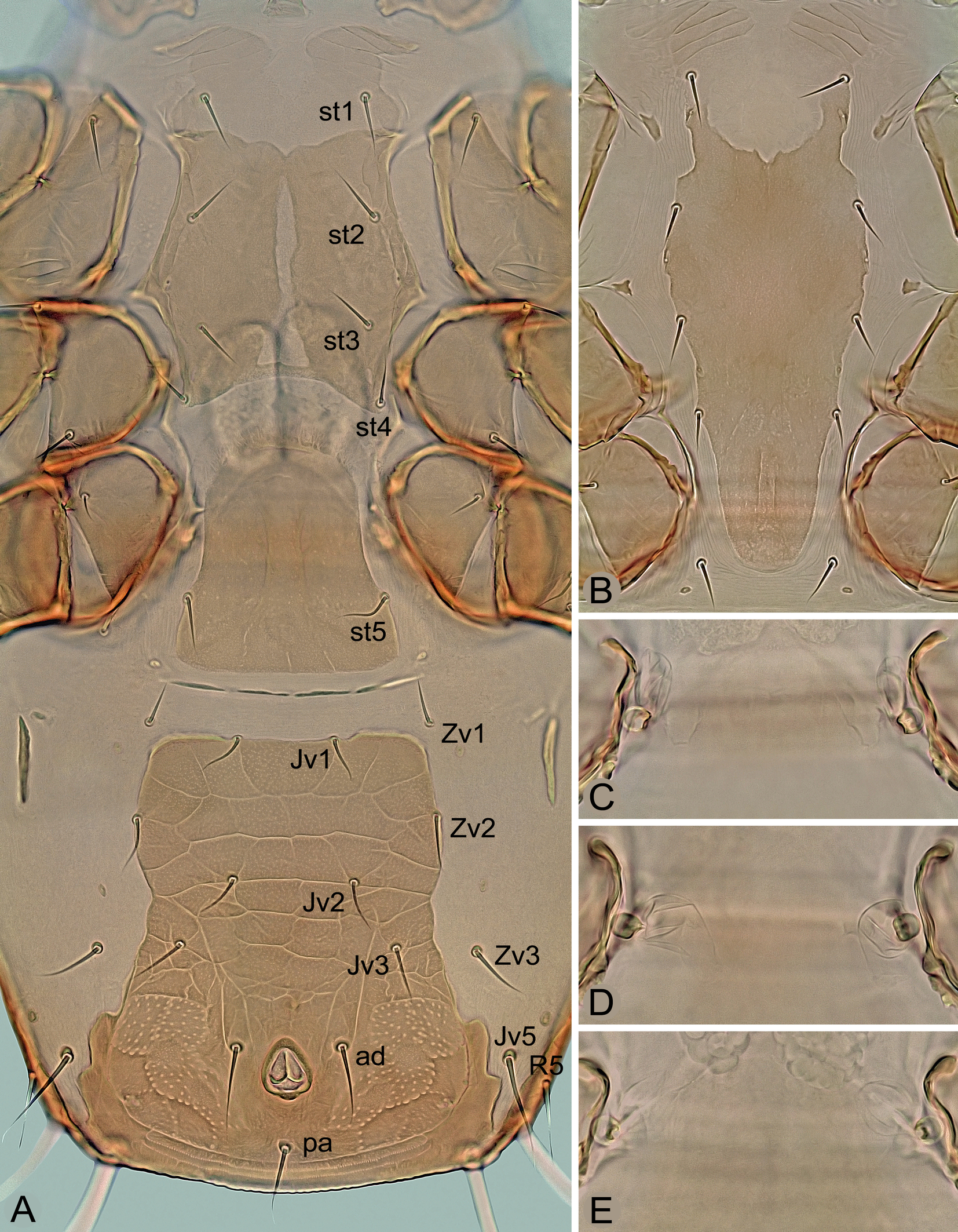
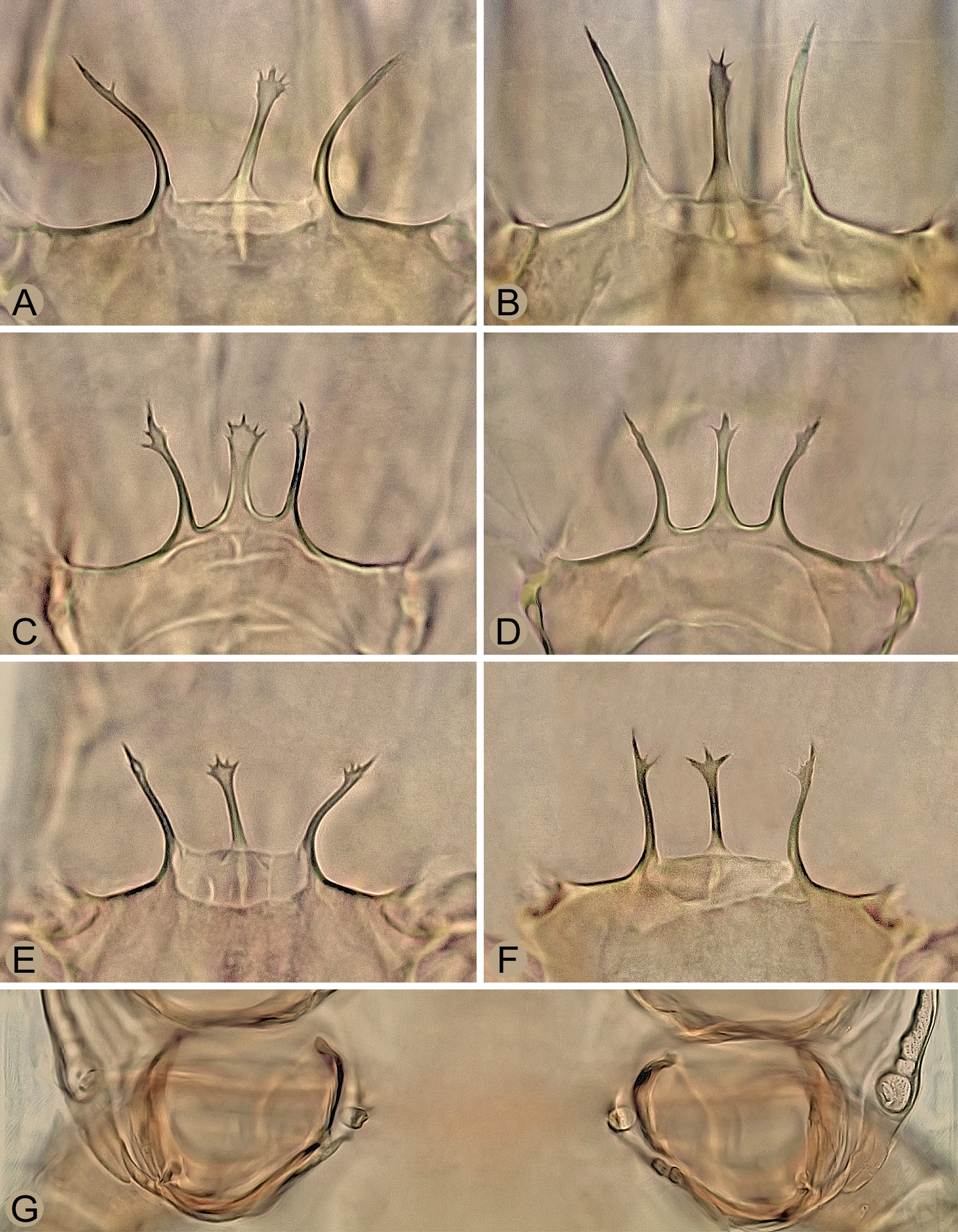
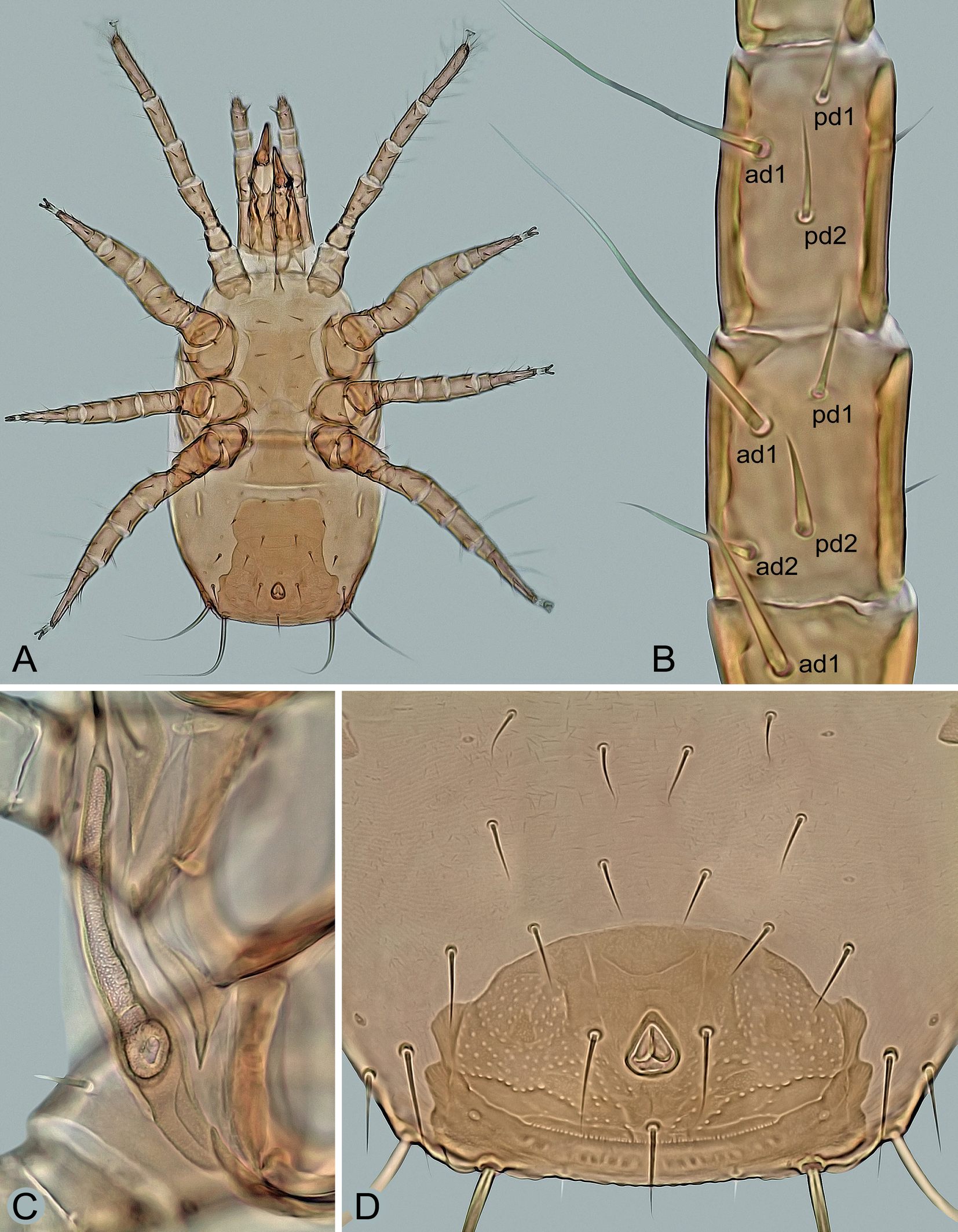
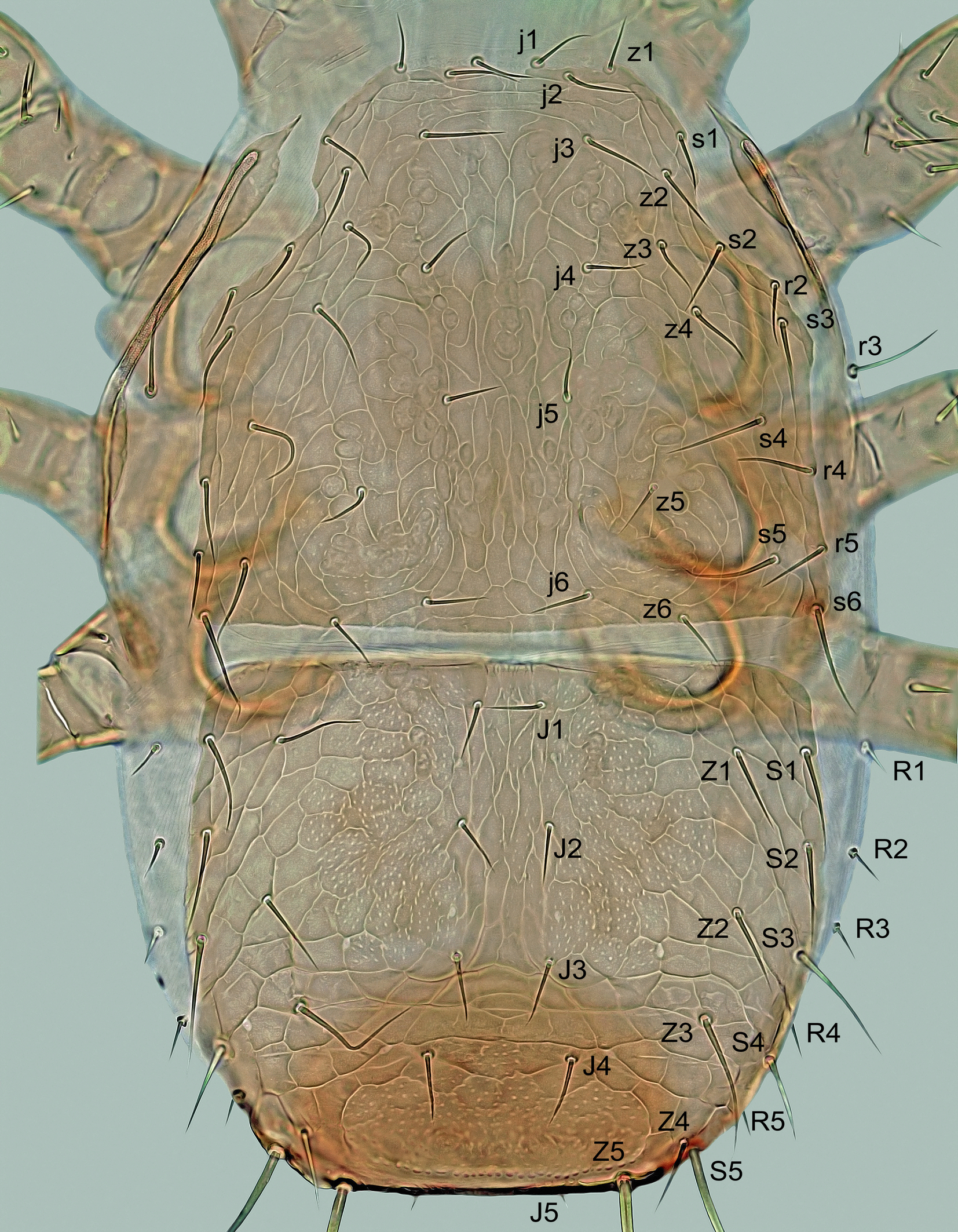
Mites were collected individually directly from both sides of the subcortical microhabitats with a moistened toothpick, preserved in ethyl alcohol, and then mounted on slides with Swan's chloral hydrate medium. The specimens were measured and photographed using a Leica DM 1000 light microscope and a Leica EC3 digital camera. The length of the shields and legs was measured along the midline, the width at the widest point, unless otherwise stated in the description. The dorsal setae were measured from the base to the tip, the legs I–IV with the coxa, but without the pretarsal ambulacrum. The number of teeth on the chelicerae is given without their apical hooks. The measurements are given as ranges (minimum to maximum) in micrometers, and the photomicrographs on the plates are not scaled. The notation symbols for the idiosomal setae follow Lindquist and Evans (1965), slightly modified by Lindquist (1994), and the notation symbols for the leg setae follow Evans (1963). The terminology for the other anatomical structures follows Evans and Till (1979). The chaetotactic symbols used here are shown in Figures 3A, 4A, 7B and 8.
Results and discussion
Insectolaelaps zvoleniensis (Wiśniewski & Hirschmann)
Dendrolaelaps (Insectolaelaps) zvoleniensis Wiśniewski and Hirschmann, in Hirschmann and Wiśniewski, 1984: 88.
Multidendrolaelaps putte Huhta and Karg, 2010: 341. New synonymy.
Insectolaelaps zvoleniensis.— Castilho, 2012: 206.
Multidendrolaelaps putte.— Castilho, 2012: 216; Qayyoum et al., 2016: 326; Erman et al., 2024: 82.
Dendrolaelaps (Multidendrolaelaps) putte.— Huhta, 2016: 138.
Diagnosis
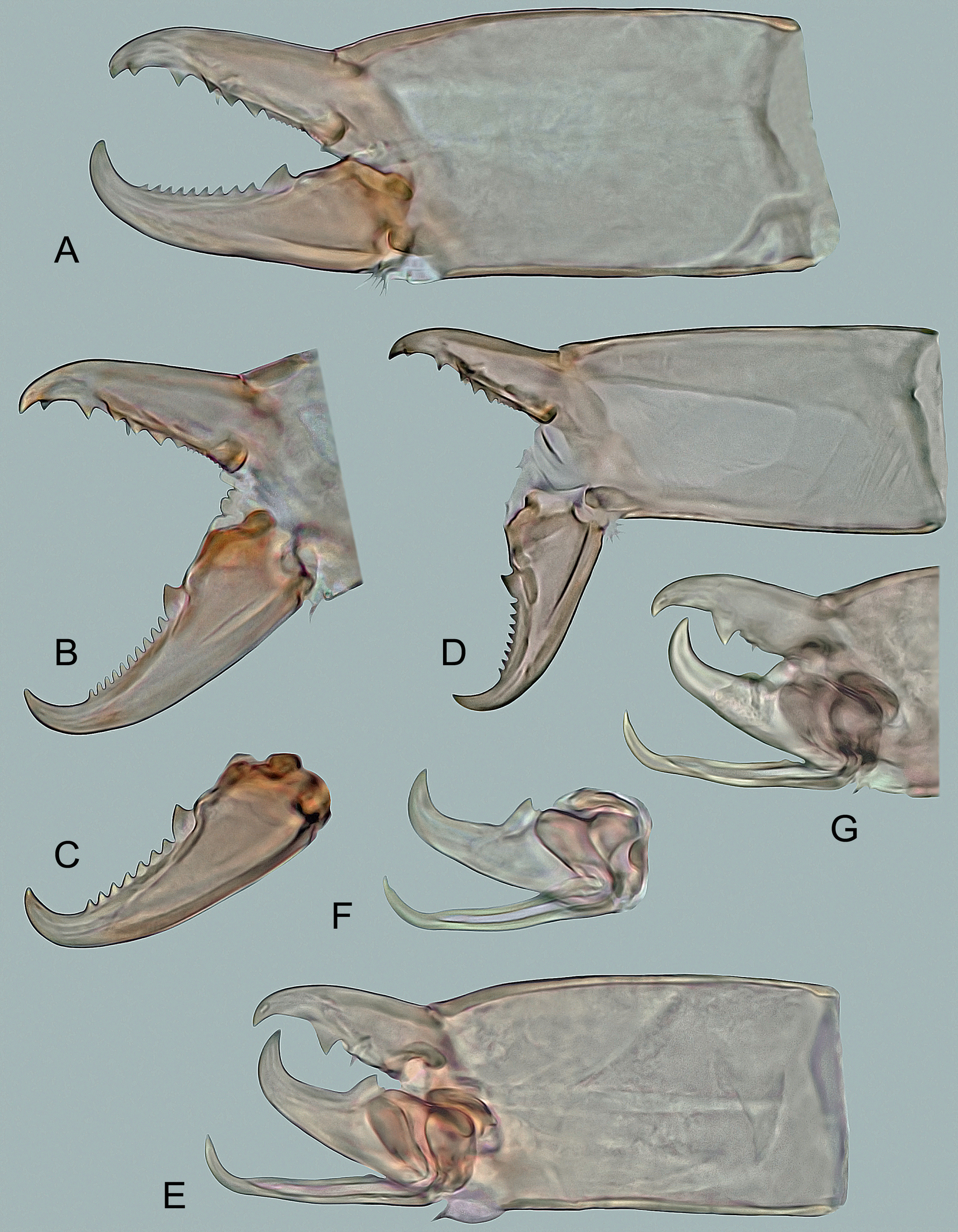
Idiosoma suboval, in females moderately widened to two thirds of the length. Reticulation of dorsal shields complete in deutonymph or only marginally developed in adults. Strong punctation of shields well developed, especially on anteromedial and posteriormost areas of opisthonotum and on surface lateral to anus. Length of dorsal setae j1–j3, Z1–Z3, S1–S3 and R3–R5 gradually increasing posteriorly in each of these rows, including J1–J4 in female; seta r3 on soft integument in deutonymph. In female, the sternal shield is provided with a longitudinal medial cleft of only weakly sclerotized integument and setae st3, which are widely separated and of similar thickness to the other sternal setae; ventrianal shield with four pairs of opisthogastric setae (Jv1‒Jv3, Zv2), laterally moderately narrowed in medial part, conspicuously widened in posterior part and with a wavy border in posterolateral corners (such a border is also formed in anal shield of deutonymph); spherical structures of the sperm induction system near coxae IV, connected to a membranous small sac, without a pair of tubular appendages. Peritremes of adults short and with anterior end reaching approximately to middle of coxae II, peritremes of deutonymph normal and reaching to setae s1. Exopodal platelets II/III and III/IV of adults separate and free of peritrematal shields. In male, triangular platelets with setae st5 free of sternitogenital shield. Movable cheliceral digit with eight to eleven teeth in females and six to nine teeth in deutonymphs; male spermatodactyl thin, narrowed and hook-shaped in apical part and about as long as movable digit. Seta pv1 absent on genu III; dorsal setae ad1 on genua III‒IV and tibia IV and ad2 on tarsi II‒IV conspicuously long, distally attenuate and erect. Male legs II spurred, legs IV thickened like legs II, but without spurs or spines.
Description
Female — (Figures 1, 2, 3A, 3C‒E, 4A, 4D, 5A‒C, 6A, 6B, 6G and 7A‒C).



Dorsal idiosoma – (Figure 1). Idiosoma 500–600 μm long (one specimen 450 μm), 270–370 μm wide, dorso-ventrally flattened, elongate, suboval, widest in posterior part at about level of R2, largely covered by two dorsal shields. Podonotal shield 250–290 μm long, 255‒310 μm wide, semicircular, convex anteriorly, straight or slightly convex laterally and posteriorly, with four small and rounded medial scleronodules between setae j5 and j6, irregular reticulation on marginal surface and mostly smooth on medial surface, scattered punctation and 22 pairs of setae (j1–j6, z1–z6, s1–s6, r2‒r5), of which r3 usually located on peritrematal part of the shield. Opisthonotal shield 250–320 μm long, 265‒340 μm wide, subhexagonal, anteriorly and posteriorly almost straight to slightly convex, laterally well rounded (widest at level between setae R2 and R3), with posterolateral margins slightly expanded ventrally and projected into distinct angles bearing elongate setae Z5 and S5; surface punctate-reticulate: reticulate in anterolateral and posterior regions and distinct punctation in anteromedial region between setae J1, Z1, Z2 and J3 and on longitudinally narrow posterior region between setae Z4; 20 pairs of opisthonotal setae, of which only R1 is located on the soft integument outside the shield. Dorsal shields with very fine punctation on entire surface. Dorsal setae needle-like, relatively short or moderately long, with exception of two pairs of elongate, similarly sized and whip-like setae (Z5 and S5) at posterolateral corners of opisthonotal shield. Length of dorsal setae j1–j3, J1–J4, Z1–Z3, S1–S3 and R2–R5 gradually increasing posteriorly in each of these rows. Lengths of selected dorsal setae: j1 28–35 μm, j2 32–39 μm, j3 34–42 μm, j4 27–33 μm, j5 26–32 μm, j6 28–35 μm, z1 24–31 μm, z5 28–33 μm, s5 40–46 μm, s6 43–49 μm, r2 27–32 μm, r3 43–53 μm, r5 29–34 μm, J1 24–30 μm, J2 26–33 μm, J3 32–40 μm, J4 36–46 μm, J5 17–23 μm, Z1 36–42 μm, Z2 42–49 μm, Z3 64–73 μm, Z4 31–38 μm, Z5 150–185 μm, S1 38–48 μm, S2 43–51 μm, S3 53–61 μm, S4 48–55 μm, S5 145–180 μm, R1 and R2 17–24 μm, R3 19–25 μm, R4 26–32 μm, R5 33–41 μm.
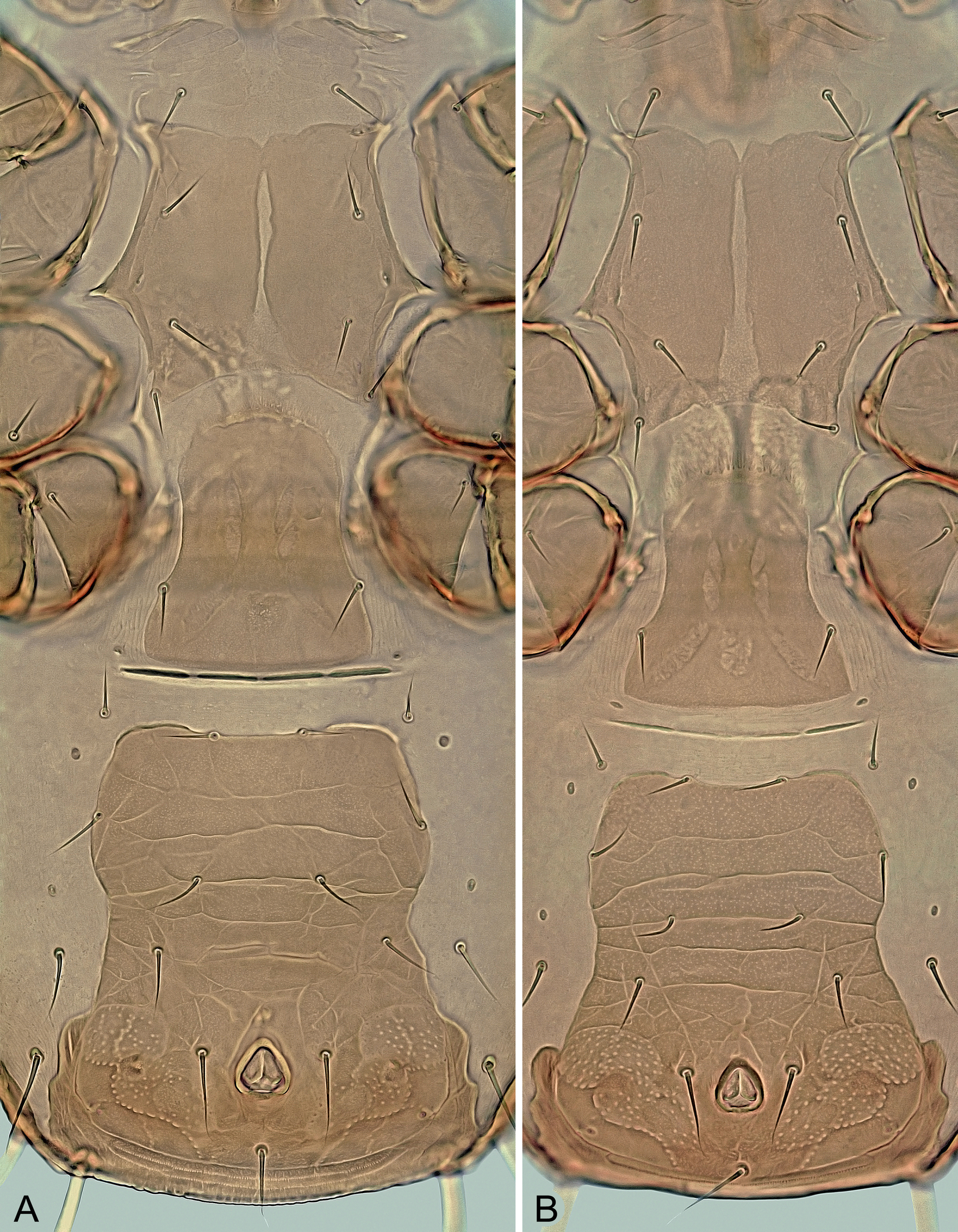


Ventral idiosoma – (Figures 2 and 3A). Tritosternum normal for the genus. Presternal plates weakly sclerotized, transversely striate and separately connected to anterolateral margins of sternal shield. Sternal shield 92–112 μm long in its better sclerotized and defined part, 77–103 μm wide at the narrowest part between coxae II, with well-developed anterolateral and lateral corners, concave posterior posterior margin, three pairs of slit-like lyrifissures (iv1–iv3) and four pairs of sternal setae (st1–st4), of which st1 located on weakly sclerotized anterior part of shield; bases of st3 usually not conspicuously adjacent compared to other sternal setae, especially st1; medial surface also unevenly sclerotized, with a narrow longitudinal stripe of weak sclerotization giving the impression of a longitudinal division or cleavage of the shield into two halves, this sternal cleft being variably pronounced and sometimes absent, often in females shortly after ecdysis; shield finely punctate like other ventrally located scutal structures, with some lines of reticulation only in areas near lateral margins; st1–st4 similar in length and thickness, with following distances between them: st3↔st3 (57–78 μm) ≈ st1↔st1 (60–75 μm) < st2↔st2 (77–89 μm) < st4↔st4 (84–93 μm). Epigynal shield elongate, 115–135 μm long, 85–108 μm at widest part behind genital setae (st5) and 55–75 μm at narrowest part in anterior half between coxae IV, with convex hyaline anterior margin extending to posterior margin of sternal shield, concave lateral margins, straight to slightly convex posterior margin, smooth or sparsely punctate surface and a pair of setae on posterolateral margins; genital lyrifissures (iv5) on soft integument near posterolateral corners of shield. Area between epigynal and ventrianal shields with four transverse slit-like sclerites. Ventrianal shield longer than wide or as long as wide, 160–215 μm long, 110–150 μm wide in widest anterior part at level of Zv2 and 160–190 μm wide in widest posterior part at level of anus, straight or convex anteriorly between setae Jv1, laterally narrowed medially, conspicuously widened in posterior part, with four pairs of opisthogastric setae (Jv1‒Jv3, Zv2; of which Jv1 and Zv2 on shield margin) and three circumanal setae; almost entire surface reticulate or with transverse lines, except for punctate area in posterior part around anus; circumanal setae similar in length (ad 36–49 μm, pa 34–48 μm); posterolateral margins distinctly wavy and posterior margin connected to opisthonotal shield; cribrum anteriorly delimited by a curved line, with two transverse and slightly curved rows of denticles. Endopodal platelets III/IV free, small and narrow, subtriangular. Exopodal platelets II/III and III/IV separated, subtriangular, free of peritrematal shields. Peritrematal shields narrow, free along almost entire length except for tip of poststigmatic parts connected to narrow parapodal plates and anterior part fused to podonotal shield between setae r2 and r3; peritremes short (Figure 7C), 70–90 μm long, with anterior tip not reaching anterior margin of coxae III. Two pairs of metapodal platelets present (Figure 3A); larger platelets narrow and long, 30‒45 μm long, rod-shaped; smaller platelets tiny, slit-shaped or irregular. Soft opisthogastric integument with three pairs of setae (Jv5, Zv1, Zv3). All sternal and opisthogastric setae similar to those on dorsum, usually with attenuate terminal parts, with following lengths: st1 24–31 μm, st2 and st3 21–31 μm, st4 20–27 μm, st5 20–31 μm, Jv1 20–28 μm, Jv2 21–31 μm, Jv3 24–33 μm, Jv5 45–56 μm, Zv1 20–26 μm, Zv2 21–33 μm, and Zv3 24–36 μm.


Sperm induction system – (Figures 3C–E and 6G). Normal for the genus, with a pair of well-sclerotized spherical structures (8–9 μm in size), each connected to inner margin of coxa IV and a hyaline sac-like membrane.
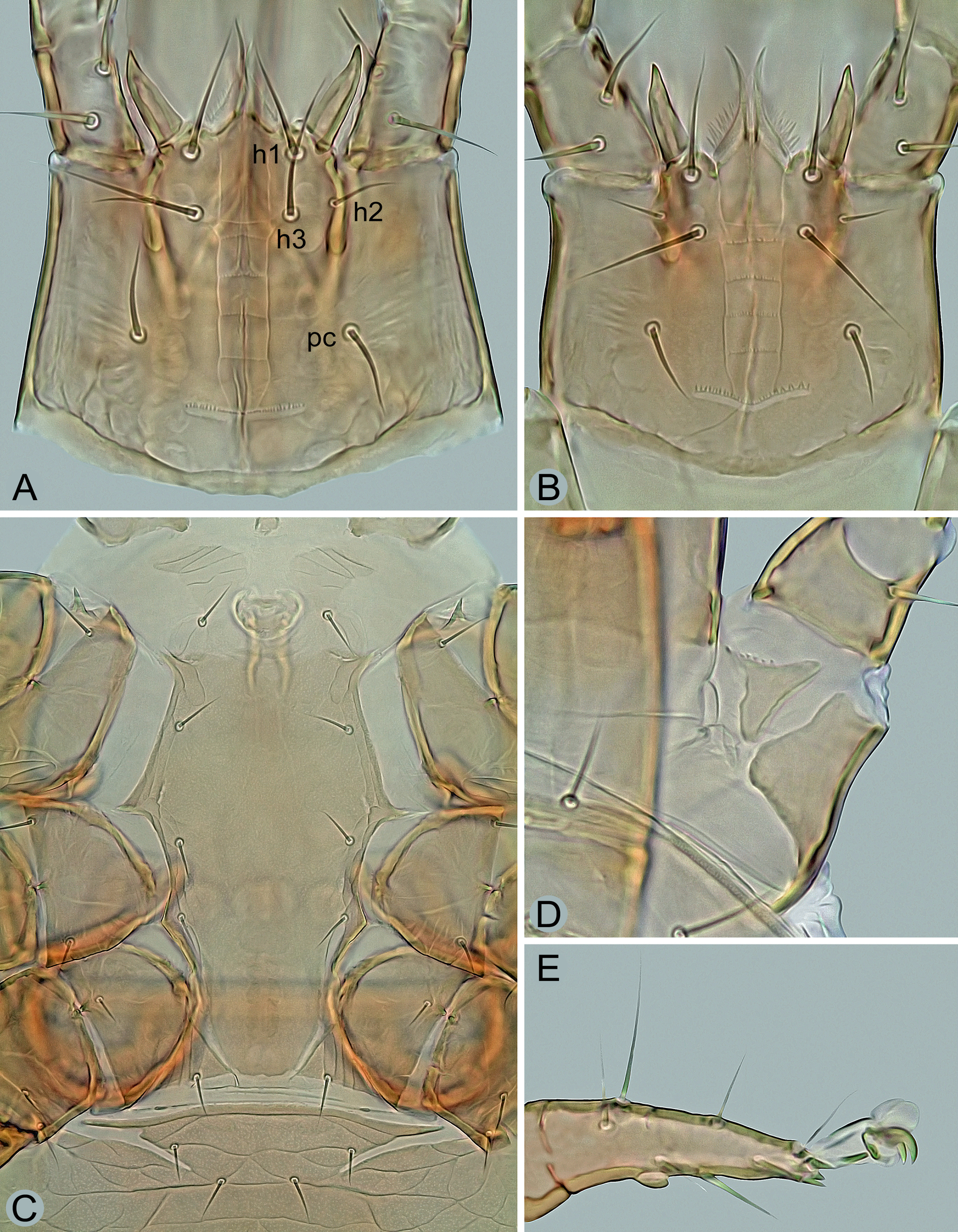


Gnathosoma – (Figures 4A, 5A–C, 6A and 6B). Deutosternal furrow normal for genus, with five transverse rows of many denticles connected laterally by longitudinal lines (Figure 4A); posteriormost row widest and extending beyond longitudinal lines; corniculi well sclerotized and spaced, horn-like, divergent to each other; internal malae apically pointed, with densely fimbriated outer margins and extending slightly beyond corniculi. Hypostomal setae smooth and needle-like, usually with attenuated apical part in h1 and h3; setae h2 shortest and h3 longest (h1 34–41 μm, h2 17–21 μm, h3 38–45 μm, pc 23–28 μm). Middle article of chelicerae relatively short and broad, with similar width along entire length, 135–150 μm long (Figure 5A); digits relatively robust, movable digit 60–70 μm long, with well-developed terminal hook and usually eight to eleven teeth, including the largest and most proximal tooth (Figures 5A–C); fixed digit with bidentate terminal hook, one distal tooth and a laterally located row of three larger medial teeth, followed by usually 7–11 tiny proximal teeth; pilus dentilis short and thin, inconspicuous (Figures 5A and 5B); dorsal seta short, hyaline and barely visible; arthrodial membrane well developed, usually with a row of several spines near ventral base of movable digit. Anterior margin of epistome triramous; lateral processes thin and pointed, with fine apical serration; medial process slightly shorter than lateral processes, with apical part moderately widened and shortly spiny (Figures 6A and 6B).


Legs – (Figures 4D, 7A and 7B). All legs shorter than idiosoma, with well-developed pretarsus and ambulacral apparatus including pulvillus and two claws; pulvillus and claws of legs I smaller than those of other legs; legs I longer than legs IV (Figure 7A); dorsal surface of coxae I with two longitudinal clefts forming a subtriangular platelet surrounded by a soft cuticle (Figure 4D), the platelet with a row of several denticles on anterior margin; anterior margin of coxae II with a sharp spine as in Figure 4C; legs I 370–460 μm, legs II 285–370 μm, legs III 275–355 μm, legs IV 350–440 μm long. Chaetotaxy of the legs: leg I – coxa (2), trochanter 1-1/3-1 (6), femur 2-3/1, 2/3-2 (13), genu 2-3/2, 2/1-2 (12), tibia 2-3/2, 2/1-2 (12); leg II – coxa (2), trochanter 1-0/3-1 (5), femur 2-3/1, 2/2-1 (11), genu 2-3/1, 2/1-2 (11), tibia 2-2/1, 2/1-2 (10); leg III – coxa (2), trochanter 1-1/3-0 (5), femur 1-2/1, 1/0-1 (6), genu 2-2/1, 2/0-1 (8), tibia 2-1/1, 2/1-1 (8); leg IV – coxa (1), trochanter 1-1/3-0 (5), femur 1-2/1, 1/0-1 (6), genu 1-2/1, 2/0-1 (7), tibia 1-1/1, 2/1-1 (7); tarsi II–IV with 18 setae; genu III with eight setae (pv1 absent). Leg setae smooth and thin, mostly needle-like, except for slightly thickened dorsal setae ad1 and ad2 on femora IV, conspicuously shortened and thickened apicoventral setae (av1, pv1) on tarsi II‒IV and elongated, distally attenuated and more or less erect whip-like dorsal setae ad1 on genua III and IV, ad1 on tibia IV (Figure 7B), and ad2 on tarsi II‒IV; ad1 on genu IV 75–87 μm long and ad1 on tibia IV 82–94 μm long.
Male — (Figures 4B, 4C, 4E, 5E–G, 6C and 6D).




Idiosoma – 435–530 μm long, 250–320 μm wide. Podonotal shield 225–270 μm long and 250–315 μm wide, laterally fused with peritrematal shield, located at anterolateral margins of venter; opisthonotal shield 220–265 μm long and 245–315 μm wide, extensively united laterally and posteriorly with ventral shield. All dorsal setae on podonotal and opisthonotal shields, including marginal setae R1–R5. Sternitogenital shield as in Figure 4C, 205–230 μm long, with well-developed endopodal corners between coxae II‒III and III‒IV, completely micropunctate, with reticulate pattern only near lateral margins, and four pairs of similar sternal setae (st1, st2 and st5 21–26 μm, st3 and st4 18–23 μm); genital setae (st5) on free triangular plates next to posteriormost margins of sternitogenital shield, each plate with several longitudinal lines on surface. Anterior margin of ventral shield widely convex and deeply notched behind coxae IV, each notch narrow, long, directed diagonally and ending between bases of setae Jv1 and Zv1; shield with same number of opisthogastric setae as in female. Poststigmatic part of peritrematal shields free. Ventral gnathosoma with similar features as in female (Figure 4B), but with slightly wider deutosternal furrow; length of rostral setae as follows: h1 31–37 μm, h2 14–18 μm, h3 35–39 μm, pc 20–24 μm. Middle article of chelicerae similar in proportions to that of female, 110–120 μm long; movable digit 46–54 μm long, regularly curved distally, with well-developed terminal hook, large submedial tooth having denticle at proximal base, and spermatodactyl (Figures 5E–G); spermatodactyl about as long as fixed digit, narrow and 49–54 μm long, directed anteriorly, slightly narrowed towards terminal part, curved at the end and with pointed tip; sperm canal relatively wide, situated approximately along median axis of spermatodactyl and apparently open before subterminal curvature of spermatodactyl; fixed digit with short terminal hook, subdistal tooth and small pilus dentilis. Epistome as in Figures 6C and 6D, similar to that of female. Legs II spurred: femur, genu, tibia and tarsus with anteroventral seta modified into a robust spur (femur) or a small hemispherical tubercle (genu, tibia, tarsus); all three hemispherical tubercles of equal size; ventral telotarsus II without conspicuous projection in subterminal part (in contrast to most other congeners; Figure 4E). No segment of legs IV spurred; sexual dimorphism of dorsal setae of legs IV not pronounced, although legs IV comparatively thicker than in female; legs I 365–425 μm, legs II 280–345 μm, legs III 270–335 μm, legs IV 340–420 μm long. Other characteristics as in female.
Deutonymph — (Figures 3B, 5D, 6E, 6F, 7D and 8–10).


Dorsal idiosoma – (Figure 8). Idiosoma 390–470 μm long, 205–305 μm wide, elongate, suboval, covered by two dorsal shields and with the same number of setae as in adults. Podonotal shield 210–250 μm long, 200‒250 μm wide, subpentagonal, convex anteriorly, concave anterolaterally between setae s1 and s3, more or less straight posterolaterally and posteriorly, without distinct medial scleronodules between setae j5 and j6 and with 21 pairs of setae (r3 located on soft integument outside shield). Opisthonotal shield 175–225 μm long, 190‒255 μm wide, subquadrate, with straight to moderately convex anterior and lateral margins, truncated posterior margin between setae Z5 and similar setation as in female, except for presence of four pairs of marginal setae (R2‒R5 on soft integument adjacent to lateral margins of shield). Surface of both dorsal shields completely reticulate, some reticulate cells strongly punctate, especially in areas around setae z5 of podonotum and in large anteromedial and small posteromedial parts of opisthonotum (this punctation sometimes strongly reduced). Dorsal setae as in female. Soft integument densely striated. Lengths of selected dorsal setae as follows: j1 23–27 μm, j2 28–32 μm, j3 32–36 μm, j4, j6 and z5 24–28 μm, j5 and z1 22–26 μm, s5 37–42 μm, s6 44–49 μm, r3 35–41 μm, r5 27–32 μm, J1 and J2 22–27 μm, J3 25–30 μm, J4 27–32 μm, J5 8–12 μm, Z1 and Z2 33–39 μm, Z3 60–66 μm, Z4 29–36 μm, Z5 150–170 μm, S1 37–44 μm, S2 40–47 μm, S3 52–60 μm, S4 34–42 μm, S5 145–160 μm, R1–R4 14–19 μm, R5 26–32 μm.



Ventral idiosoma – (Figures 3B and 7D). Presternal plates weakly sclerotized, diagonally or transversely striated and completely connected to anterior margin of sternal shield (Figure 3B). Sternal shield 185–220 μm long (measured from level of st1), 72–86 μm wide (at widest part of iv2), wedge-shaped, weakly sclerotized in anteriormost part with st1, usually smooth over entire surface (sometimes with sparse dots), with anterolateral margins between iv2 and st2 deeply concave, and four pairs of setae on lateral margins; sternal setae st5 on soft integument adjacent to posterior margin of shield (Figure 3B). Seven pairs of opisthogastric setae (Jv1‒Jv3, Jv5, Zv1‒Zv3), all on soft integument (Figure 7D). Anal shield distinctly wider than long, 75–95 μm long, 135–160 μm wide, as in Figure 7D, rounded anteriorly, undulating laterally, straight to slightly convex posteriorly, densely and distinctly punctate except for anteromedial surface and area posterior to postanal seta; cribrum with a slightly curved row of denticles posterior to postanal seta. A pair of irregularly suboval or subcircular metapodal platelets, sometimes accompanied by a pair of slit-shaped sclerites near their inner margin. Peritreme long, with only narrow fragments of peritrematal shield in anterior and submedial parts; anterior tip free of podonotal shield and reaching close to setae s1. Subtriangular exopodal platelets II/III and III/IV connected by thin filamentous parts of their ends. Lengths of ventrally located setae as follows: st1–st3 18–24 μm, st4 and st5 15–22 μm, ad 32–40 μm, pa 31–37 μm, Jv1–Jv3 and Zv2 20–25 μm, Jv5 42–49 μm, Zv1 17–21 μm, and Zv3 24–30 μm.


Gnathosoma – (Figures 5D, 6E and 6F). Ventral gnathosoma similar to that of female. Middle article of chelicerae 104–115 μm long; movable digit 40–50 μm, usually with eight or nine teeth, but often also with seven and rarely six teeth; fixed digit with similar dentition as in female, except for number of only five or six minute proximal teeth in lateral row (Figure 5D). Anterior margin of epistome as in Figures 6E and 6F, similar to that of female.
Legs – All legs shorter than idiosoma: legs I 315–375 μm, legs II 260–300 μm, legs III 255–290 μm and legs IV 300–355 μm long. Setae, chaetotaxy and ambulacral apparatus as in adults, but apicoventral setae (av1 and pv1) of tarsi II‒IV thin, needle-like and with subapical arrangement.
Material examined
Slovakia: 19 deutonymphs ‒ Borská Nížina Lowland, Lozorno Village, Dlhá Mláka Forest, pine forest with birch admixture, on Cucujus cinnaberinus (Scopoli) (Coleoptera: Cucujidae), elevation 180 m, 29 March 2020; 24 deutonymphs ‒ Malé Leváre Village, hardwood floodplain forest, on C. cinnaberinus, elevation 150 m, 30 Oct. 1990; 33 females, 41 males, 125 deutonymphs ‒ Vysoká pri Morave Village, Horný Les Forest, hardwood floodplain forest, under bark of Fraxinus sp., Salix sp., Populus sp., elevation 145 m, 24 Oct. 2019; two females, one male ‒ Bukovské Vrchy Hills, Nová Sedlica Village, Medová Baba Pond, under the bark of alder (Alnus incana), elevation 635 m, 28 May 2020; one female ‒ Burda Hills, Chľaba Village, Kováčov Settlement, edge of deciduous forest (Carpineto-Quercetum) and meadow, underground nest of Lasius umbratus (Nylander) colonized by Lasius fuliginosus (Latreille) (Hymenoptera, Formicidae) in rotting tree stump (Quercus sp.), elevation 190 m, 24 Oct. 2018; eight females, three males ‒ Little Carpathians Mountains, Bratislava Capital, Horský Park, deciduous forest, under the bark of oak (Quercus sp.), elevation 220 m, 5 June 2019; 5 deutonymphs ‒ with the same data as in previous record, on C. cinnaberinus, 7 May 2019; 3 deutonymphs ‒ Bratislava Capital, zoological garden, deciduous forest, on C. cinnaberinus, under the bark of oak (Quercus sp.), elevation 200 m, 29 March 2009; 118 deutonymphs ‒ Podunajská Rovina Flatland, Bodíky Village, softwood floodplain forest, on C. cinnaberinus, under poplar bark, elevation 120 m, 25 Sept. 2019; eight females, seven males ‒ Považský Inovec Mountains, Lúka Village, Srnia Dolina Valley, deciduous forest, under the bark of sweet chestnut (Castanea sativa), elevation 230 m, 12 Oct. 2019; 17 deutonymphs ‒ Srnia Dolina Valley, edge of deciduous forest, on C. cinnaberinus, elevation 250 m, 12 Apr. 1992; one female ‒ Revúcka vrchovina Highlands, Sirk Village, under bark of common walnut (Juglans regia), elevation 340 m, 8. Aug. 1997; 9 deutonymphs ‒ Trnavská Pahorkatina Wold, Horná Streda Village, inundation area of Váh River, poplar forest, on C. cinnaberinus, elevation 165 m, 7. Oct. 1990; 8 deutonymphs ‒ with the same data as in previous record, 4 March 1995.
The above collection data for Insectolaelaps zvoleniensis represent only a small proportion of the individuals examined and stored in a collection repository. Although the species was originally reported from Slovakia only from Zvolen (Kováčová Forestry), it is widespread throughout the country and has also been found at other collection sites, namely Borský Svätý Jur Village, Bratislava Capital (Kamzík Forest, Lieskovec Forest, Rusovce Settlement, Železná Studnička Forest), Brunovce Village, Drienčany Village, Hnúšťa Town, Hrabušice Village, Hrádok Village (Hrádocká Valley), Hubina Village, Malá Tŕňa Village, Pruské Village, Skalka nad Váhom Village, Staré Hory Village, Stupava Town, Svätý Jur Town (Šúr Forest), Šaštín-Stráže Town (Gazárka Forest), Špania Dolina Village, Tomky Village, Topoľa Village, Turček Village, and others. All these specimens were collected by the first author and are deposited at the Institute of Zoology of the Slovak Academy of Sciences, Bratislava, Slovakia.
Taxonomic notes
During his short visit to Slovakia in 1983, Wiśniewski and his team collected several individuals of a previously unknown digamasellid species. One year later, the species was described by Wiśniewski and Hirschmann (in Hirschmann and Wiśniewski 1984: 88) in Dendrolaelaps (Insectolaelaps) as ''zvoleniensis'', named after the town of Zvolen, which is located near the type locality (Kováčová Forestry). It is based on seven deutonymphs found under the bark of Pinus sp. and Quercus sp., including five phoretic deutonymphs attached to the host beetle Cucujus cinnaberinus and one male found in an anthill of Formica polyctena Förster. Both described stages are more difficult to identify without experience with the largely uniform digamasellids than the adult female, which is characterized by a greater number of distinct diagnostic features. The female stage of I. zvoleniensis has never been described, and since the first publication this species has not been mentioned by subsequent authors. These circumstances may have contributed to the later misidentification of I. zvoleniensis and the description of an identical species from Finland, Multidendrolaelaps putte, whose female can be easily recognized among the digamasellids by the longitudinal cleft on the sternal shield (Huhta and Karg 2010).
In the Wiśniewski collection in Poznan there are two slides, each bearing a deutonymph and the following label: Dendrolaelaps zvoleniensis, Paratyp, CSSR ‒ Zvolen, IX. 1983, leg. J. Wiśniewski. These two deutonymphs that I was able to examine, as well as the type material of the species Multidendrolaelaps putte from Finland, including adults and a deutonymph, are identical to the individuals from Slovakia. I have not found any differences that would support the validity of the two species. Therefore, I propose the recognition of M. putte as a junior synonym of D. zvoleniensis.
In the above-mentioned collection in Poznan there is also a female of Insectolaelaps zvoleniensis, incorrectly identified as Insectolaelaps armatus (Hirschmann, 1960), on a slide with the number JW-1161, i.e. the collection code also given in the original description of I. zvoleniensis, and on the slide with the only described male of the species (holotype) deposited in the Hirschmann collection in Munich. This male in Munich (labelled A 20041043) was also provisionally thought to be Insectolaelaps armatus, as its original label reads: ''Dendrolaelaps armatus oder zvoleniensis''. It should be noted that the slide with the male is not designated as a type or holotype, but undoubtedly belongs to the original series. The female in the Poznan repository was probably overlooked, otherwise it cannot be explained why it was not used in the description. Perhaps the female was intentionally not used for the description because the split sternal shield seems unusual and rather an anomaly, unless more individuals are available for comparison.
Huhta and Karg (2010) provided the following list of general diagnostic characters for the classification of Multidendrolaelaps putte into the genus: palptarsal apotele with two tines, two pairs of mediodorsal scleronoduli, solenostomes in coxae III, and more than five teeth on the movable digit of the female chelicerae. The comparison with other ''congeneric species'', especially Multidendrolaelaps bispinosus (Karg), is based on the inconspicuous features such as the distally split median process of the triramous epistome and the thick and strongly elongated setae Z5 and S5.
Multidendrolaelaps putte was originally reported from ''decaying trunk of aspen'' in Finland (Huhta and Karg 2010). I examined ten females, including the holotype, one male and one deutonymph from the original type series. These individuals matched morphologically perfectly with the specimens found in Slovakia, as well as with the type deutonymphs deposited in Poznan. It is interesting to note that these two identical species proposed here for synonymization were placed in two different (sub)genera by their original describers, namely ''zvoleniensis'' in Insectolaelaps and ''putte'' in Multidendrolaelaps. This difference in placement was mainly a consequence of Huhta and Karg (2010) incorrectly considering the solenostomes (given as gonopores) as part of coxae III instead of IV. The classification system of the individual taxa of the family Digamasellidae is primarily derived from the type and position of the sperm induction pores (= solenostomes) in females. In Insectolaelaps, a pair of spherical structures representing the sperm induction pores is developed near the inner side of coxae IV (Figures 3B‒E and 6G).
In their original description, Hirschmann and Wiśniewski (1984) placed Dendrolaelaps zvoleniensis in the subgenus Insectolaelaps, but without providing arguments for this classification, as they could not use the morphological structures of the spermathecal structures of the female, which was not represented in their type material. In the differential diagnosis, they compared some morphological features of the new species with those of Insectolaelaps armatus (Hirschmann, 1960), as the chaetotaxy and the shape of the idiosoma are similar.
The females of Insectolaelaps zvoleniensis can be easily and reliably distinguished from those of other related species, especially due to the uneven sclerotization of medial part of the sternal shield and the unusual wavy border of widened posterolateral angles of the ventrianal shield (this specific border is also developed in the deutonymph). Adults and deutonymphs are distinguished by the relative length of the opisthonotal setae, which gradually lengthen posteriorly in some rows, by the presence of several elongate, erect and distally attenuated setae on the dorsal side of legs III and IV, and by a distinct punctate pattern on the anteromedial surface of the opisthonotal shield. The male is characterised by the absence of spurs on legs IV and a peculiar appendage in the ventral subterminal part of telotarsus II.
A completely or partially longitudinally divided sternal shield is also rarely found in other mesostigmatic mites. For example, it is one of the diagnostic features of the iphiopsidine laelapids of the genera Scissuralaelaps Womersley and Iphiolaelaps Womersley, which are known as symbionts of myriapods and cockroaches in Southeast Asia and Australia (Moraes et al. 2022). The genus Schizosthetus Athias-Henriot (Parasitidae), whose members live in subcortical microhabitats in the Holarctic and maintain phoretic relationships with bark- and wood-boring beetles, is also characterized by a similarly shaped sternal shield (Athias-Henriot 1982). Finally, some blattisociid species of the genus Lasioseius Berlese, which live under the bark of trees and in decaying wood in Europe, have a sternal shield with a significantly reduced sclerotization in its medial and posterior parts (Mašán and Halliday 2023).
Ecological notes
Among saproxylic mesostigmatic mites inhabiting subcortical microhabitats, Insectolaelaps zvoleniensis is the most common and abundant species in Slovakia, as it is able to colonize the widest range of host trees, including coniferous species, and at the same time colonize trees at different stages of their decomposition. I have collected it under the bark of the following host tree species, namely Acer pseudoplatanus, Acer spp., Aesculum hippocastanum, Alnus glutinosa, Alnus incana, Carpinus betulus, Castanea sativa, Cerasus avium, Fagus sylvatica, Fraxinus spp., Juglans regia, Malus domestica, Picea abies, Populus spp., Prunus domestica, Quercus spp., Robinia pseudoacacia, Salix spp., Tilia spp. and Ulmus spp. It clearly prefers deciduous trees, especially those with lighter and more porous wood such as poplars and willows. Occasionally, however, it is also found under the bark of conifers, especially spruce, when these are at an advanced stage of decomposition.
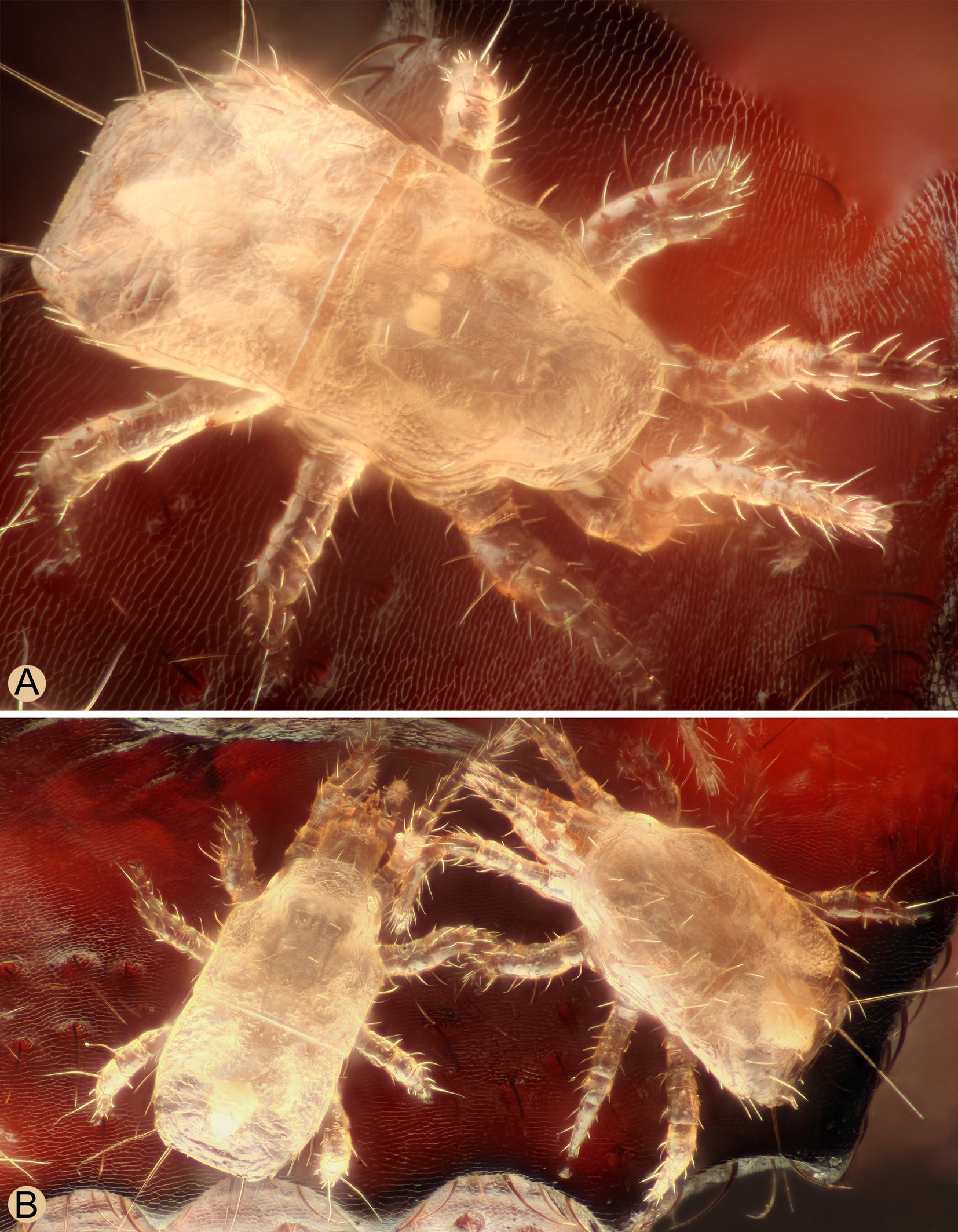
The effective dispersal of Insectolaelaps zvoleniensis, which colonizes the specific subcortical microhabitats, is ensured by the phoresy of deutonymphs on the flat bark beetles of the family Cucujidae, Cucujus cinnaberinus, a species with xylophagous larvae belonging to the larger ecological group of saproxylic insects (Speight 1989). This host beetle appears to be endemic to Europe and is distributed from Spain to Ukraine and Sweden, although its populations are more widespread only in Eastern Europe, from Austria and Bavaria eastwards (Horák and Chobot 2009). In Slovakia and Central Europe there is another representative of the genus Cucujus, C. haematodes Erichson, which is widespread in the Palaearctic region. As some photos on the internet show, this species is also a phoretic host of digamasellid mites in Europe and Asia (Russia ‒ Karachay-Cherkessia in the North Caucasus, Krasnoyarsk region in Siberia, Nizhegorodskaya Oblast), as well as other related species C. clavipes (Fabricius) and C. puniceus Mannerheim in North America or C. coccinatus Lewis in East Asia (Japan).
It can be assumed that the distribution area of Insectolaelaps zvoleniensis could be very similar to that of its phoretic host Cucujus cinnaberinus. This beetle is an attractive species for photographers due to its size, bright coloration and extremely flat body. Therefore, numerous photos of this species can be found on the internet, including photos with phoretic digamasellid mites (probably I. zvoleniensis) taken in different parts of Europe, e.g. in the Austria (Graz Region, Vienna Region), Belarus (Belovezhskaya Pushcha National Park), Czech Republic (Prague Region), Germany (Berlin Region), Hungary (Heves County), Lithuania (Kaunas Region), Poland (Podlaskie Voivodeship), Russia (Moskovskaya Oblast, Nizhegorodskaya Oblast, Republic of Karelia) and Ukraine (Donetsk Oblast, Kharkiv Oblast, Kyiv Oblast). The deutonymphs on the host beetle from Hungary were photographed in such high resolution and quality that they can be identified with certainty as I. zvoleniensis (Figures 9 and 10).
In connection with the known distribution area of the beetles of the genus Cucujus, it is remarkable that their occurrence in Turkey has not yet been recorded (Horák and Chobot 2009). Specifically from Turkey, there is an atypical record of Insectolaelaps zvoleniensis (under the synonymous name Multidendrolaelaps putte) recently reported from ''domestic poultry manure'' (Qayyoum et al. 2016). This published report should therefore be considered untrustworthy, even if it is supplemented by drawings that allow identification of the species. However, these drawings are very similar to the original illustrations by Huhta and Karga (2010), which also reduces the reliability of the published finding.
I examined eight adult beetles of Cucujus cinnaberinus with the deutonymphs of Insectolaelaps zvoleniensis. The number of deutonymphs varied between three and 118 individuals, which were mostly attached to the ventral side of the body, mainly on the thorax, and to the inner side of the elytra. It should be mentioned that I. zvolenensis was occasionally found under bark, where no larval galleries of cucujids or other xylophagous beetles were detected. Interestingly, no phoretically active deutonymphs of I. zvoleniensis were found on other xylobiont beetles with similar bionomy, namely the pyrochroid beetles Pyrochroa coccinea (Linnaeus) or Schizotus pectinicornis (Linnaeus), both of which have a softer and less chitinized integument and elytra and whose morphologically very similar xylophagous larvae often co-occur with those of C. cinnaberinus and could possibly serve as a potential secondary host. I was also unable to detect phoresy of I. zvoleniensis in other saproxylic beetle or arthropod species.
Many digamasellid species inhabit narrow spaces in subcortical microhabitats and have therefore developed a pronounced dorsoventral flattening of the body, as is also strikingly pronounced in the host beetle Cucujus cinnaberinus. Another important morphological adaptation that enables them to live in a spatially restricted environment and facilitate locomotion appears to be the peculiarity of coxae I, the dorsal side of which is split longitudinally and diagonally, with the edges well separated by a band of soft interstitial integument. This morphological adaptation is particularly pronounced in I. zvoleniensis, where the dorsal side of coxa I has two longitudinal clefts forming a separate, subtriangular plate (Figure 4D).
Acknowledgements
I would like to express my sincere gratitude to Dariusz Gwiazdowicz (Poznan University of Life Sciences, Faculty of Forestry and Wood Technology, Poland) for providing the type material of the species described by Jerzy Wiśniewski and for his support and provision of laboratory space, equipment and assistance during my stay in Poland. I thank the following zoologists for providing the type specimens used in this work: Varpu Vahtera (Zoological Museum, University of Turku, Finland), Stefan Friedrich (Bavarian State Collection of Zoology, Munich, Germany) and Pedro Cardoso (Finnish Museum of Natural History, Zoological Museum, Helsinki, Finland). Many thanks also to the photographers for permission to use their photos in this publication, especially to Nikola Rahmé, a well-known nature photographer from Hungary, for his truly impressive macro shots of phoretically active mites on a beetle host.
This work was fully supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Academy of Sciences [VEGA Grant No. 2/0007/22: Mesostigmatic mites associated with subcortical habitats and wood-destroying insects in Slovakia ‒ taxonomy, ecology and chorology of the species of Digamasellidae (Acari: Parasitiformes)].
References
- Athias-Henriot C. 1982. Schizosthetus n. g. (type Eugamasus lyriformis McGr. & Farr., 1969) avec deux espèces nouvelles (Parasitiformes, Parasitidae). Acarologia, 23: 207-214.
- Castilho R.C. 2012. Taxonomy of Rhodacaroidea mites (Acari: Mesostigmata) [Phd Thesis]. Piracicaba: Universidade de São Paolo. pp. 579.
- Erman O., Doğan S., Ayyıldız N., Özkan M. 2024. Checklist of the mites (Acari) of Türkiye. Third supplement. Acarol. Stud., 6: 81-111. https://doi.org/10.47121/acarolstud.1500691
- Evans G.O. 1963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Br. Mus. Nat. Hist., Zool., 10: 275-303. https://doi.org/10.5962/bhl.part.20528
- Evans G.O., Till W.M. 1979. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari - Parasiti¬formes). An introduction to their external morphology and classification. Trans. Zool. Soc. London, 35: 145-270. https://doi.org/10.1111/j.1096-3642.1979.tb00059.x
- Hirschmann W. 1960. Gangsystematik der Parasitiformes. Teil 3. Die Gattung Dendrolaelaps Halbert 1915. Acarologie, Schriftenreihe für Vergleichende Milbenkunde, 3: 1-27.
- Hirschmann W., Wiśniewski J. 1982. Weltweite revision der Gattungen Dendrolaelaps Halbert 1915 und Longoseius Chant 1961 (Parasitiformes). Acarologie, Schriftenreihe für Vergleichende Milbenkunde, 29(1): 1-190, 29(2): 1-48 + pls I-XIV + pls 1-94.
- Hirschmann W., Wiśniewski J. 1984. Gangsystematik der Parasitiformes. Teil 458. Teilgang, Stadien von 9 neuen Dendrolaelaps-Arten aus den Ländern Tschechoslowakei, Polen, Vietnam, Südafrika, Brasilien und Ekuador (Trichopygidiina). Acarologie, Schriftenreihe für Vergleichende Milbenkunde, 31: 87-98.
- Horák J., Chobot K. 2009. Worldwide distribution of saproxylic beetles of the genus Cucujus Fabricius,1775 (Coleoptera: Cucujidae). In: Buse J., Alexander K.N.A., Ranius T., Assmann, T. (Eds). Saproxylic beetles - their role and diversity in European woodland and tree habitats. Sofia - Moscow: Pensoft Publishers. p. 189-206.
- Huhta V. 2016. Catalogue of the Mesostigmata mites in Finland. Memoranda Soc. Fauna Fl. Fenn., 92: 129-148.
- Huhta V., Karg W. 2010. Ten new species in genera Hypoaspis (s. lat.) Canestrini, 1884, Dendrolaelaps (s. lat.) Halbert, 1915, and Ameroseius Berlese, 1903 (Acari: Gamasina) from Finland. Soil Org., 82: 325-349.
- Karg W. 1993. Acari (Acarina), Milben Parasitiformes (Anactinochaeta) Cohors Gamasina Leach, Raubmilben. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und ihrer Lebensweise. 59. Teil. 2., überarbeitete Auflage. Jena, Stuttgart, New York: Gustav Fischer Verlag. pp. 523.
- Lindquist E.E. 1975. Digamasellus Berlese, 1905, and Dendrolaelaps Halbert, 1915, with description of new taxa of Digamasellidae (Acarina: Mesostigmata). Can. Entomol., 107: 1-43. https://doi.org/10.4039/Ent1071-1
- Lindquist E.E. 1994. Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for some ventrolateral body setae. Acarologia, 35: 323-326.
- Lindquist E.E., Evans G.O. 1965. Taxonomic concept in the Ascidae, with a modified setae nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Can., 47: 1-64. https://doi.org/10.4039/entm9747fv
- Mašán P., Halliday B. 2023. Two new species of Lasioseius (Acari: Mesostigmata: Blattisociidae) with reduced sclerotisation of the sternal shield. Int. J. Acarol., 49: 24-33. https://doi.org/10.1080/01647954.2023.2177343
- Moraes G.J.de, Moreira G.F., Freire R.A.P., Beaulieu F., Klompen H., Halliday B. 2022. Catalogue of the free-living and arthropod-associated Laelapidae Canestrini (Acari: Mesostigmata), with revised generic concepts and a key to genera. Zootaxa, 5184(1): 1-509. https://doi.org/10.11646/zootaxa.5184.1.1
- Qayyoum M.A., Ozman-Sullivan S.K., Khan B.S. 2016. Description of new records of the family Digamasellidae (Acari: Mesostigmata) from Kızılırmak Delta, Samsun Province, Turkey. Turk. J. Zool., 40: 324-327. https://doi.org/10.3906/zoo-1502-28
- Shcherbak G.I. 1980. The Palearctic Mites of the Family Rhodacaridae. Kiev: Naukova Dumka. pp. 216. [in Russian]
- Speight M.C.D. 1989. Saproxylic invertebrates and their conservation. Nature and environment series 42. Strasbourg: Council of Europe. p. 1-81.



2025-03-24
Date accepted:
2025-04-23
Date published:
2025-05-06
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Mašán, Peter
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



