Phytoseiidae mite (Parasitiformes: Phytoseiidae) assemblages from different Cerrado vegetation types
Rossetti, Octavio César  1
; Barroso, Geovanny
1
; Barroso, Geovanny  2
; Demite, Peterson Rodrigo
2
; Demite, Peterson Rodrigo  3
; de Lima, Edgar Luiz
3
; de Lima, Edgar Luiz  4
and Daud, Rodrigo Damasco
4
and Daud, Rodrigo Damasco  5
5
1Laboratório de Taxonomia, Ecologia e Interações de Aracnídeos (TEIA), Departamento de Ecologia, Instituto de Ciências Biológicas - ICB V - UFG, Campus Samambaia, Avenida Esperança s/n, 74690-900, Goiânia, Goiás, Brazil.
2Departamento de Biologia Geral e Aplicada, Universidade Estadual Paulista (UNESP), Rio Claro, São Paulo, Brazil.
3Instituto Federal de Educação, Ciência e Tecnologia de Roraima, Campus Bonfim, Bonfim, Roraima, Brazil.
4Departamento de Ecologia, Instituto de Ciências Biológicas – ICB V, UFG, Goiânia, Goiás, Brazil.
5✉ Laboratório de Taxonomia, Ecologia e Interações de Aracnídeos (TEIA), Departamento de Ecologia, Instituto de Ciências Biológicas - ICB V - UFG, Campus Samambaia, Avenida Esperança s/n, 74690-900, Goiânia, Goiás, Brazil.
2025 - Volume: 65 Issue: 2 pages: 505-518
https://doi.org/10.24349/3rdh-xfojOriginal research
Keywords
Abstract
Introduction
The Brazilian Cerrado represents the second largest biome in Latin America, encompassing an approximate area of 2 million of km² (Ribeiro and Walter 2008). Due to its considerable species richness and notable levels of endemism, the Cerrado biome is recognized as one of the world's biodiversity hotspots (Myers et al. 2000; Myers 2003; Brooks et al. 2006; Loyola et al. 2009; Sano et al. 2019). Cerrado is characterized by diverse mosaic of vegetation types, including savannas, grasslands, and forests, which harbour a rich array of flora and fauna (Mendonça et al. 1998). Beyond its biodiversity significance, the Cerrado provides crucial ecosystem services to society, including agricultural benefits such as crop pollination and natural biological control (Teixeira et al. 2017).
Phytoseiid mites (Parasitiformes: Mesostigmata: Phytoseiidae) are predatory commonly used as biological control agents targeting phytophagous mites, whiteflies, and thrips (McMurtry and Croft 1997; McMurtry et al. 2013, 2015). These mites show distinct feeding behaviour (McMurtry et al. 2013), while the majority is generalist targeting a wide range of pest species, which contributes to their utility in pest management strategies. Furthermore, these mites inhabit a variety of habitats, including agricultural fields, orchards, and natural vegetation (McMurtry et al. 2013, 2015). Up to now 2,985 phytoseiid species have been taxonomically described, with 260 of these species recorded within Brazil (Demite et al. 2025; Lofego et al. 2024). Despite the vast extent and complexity of the Cerrado biome, numerous mite species inventories have been conducted on the fauna of this important family of predatory mites, but most of them have been conducted on vegetation remnants close to agriculture, with little attention to species occurrence according to Cerrado vegetation formations (Lofego et al. 2004; Demite et al. 2009, 2016, 2017, 2021; Rezende and Lofego 2011; Rezende et al. 2014; Abreu et al. 2017; De Araújo and Daud 2017; Teixeira et al. 2017; Conceição et al. 2021; Duarte et al. 2021; Moraes et al. 2022).
At the same time as agricultural intensification occurred, there is an increase in nickel exploration at the Cerrado biome of Goiás State (Neri et al. 2011). In regions designated for mineral exploration, the land has become degraded and generally loses its structure and function (Primack and Massardo 2001). However, despite the intense impact generated by nickel mining activities, a high diversity of predatory mites is expected to occur in the remaining vegetation. The remnants of Cerrado vegetation can serve as important indicators of ecosystem sustainability by harbouring mite species that contribute to ecological balance and enhance ecosystem resilience (Medeiros 2019; Abreu et al. 2017; Teixeira et al. 2017).
To enhance our knowledge of phytoseiid diversity within the native vegetation of the Cerrado, this study investigated the phytoseiid mite assemblages in three Cerrado vegetation formations adjacent to nickel mining areas. Additionally, we assess potential disparities in species richness and composition among these formations to pinpoint which habitats harbour the highest diversity of Phytoseiidae, thereby offering ecosystem services. In addition, we analysed the relationship between the estimated richness of Phytoseiidae and the distance from natural vegetation remnants to mining sites, aiming to assess the possible effect of this activity on the community structure of Phytoseiidae mites. We expect to find greater diversity of mites in environments with greater heterogeneity and structural complexity, since they present greater availability of ecological niches and resources, allowing to support greater abundance and diversity of fauna (Schuelze and Mooney 1997; Altieri 2002). Moreover, we expect to find higher estimated richness of Phytoseiidae in the most distant natural vegetation remnants from nickel mining sites, as these natural areas are less affected by mining activities.
Material and methods
Sampling areas
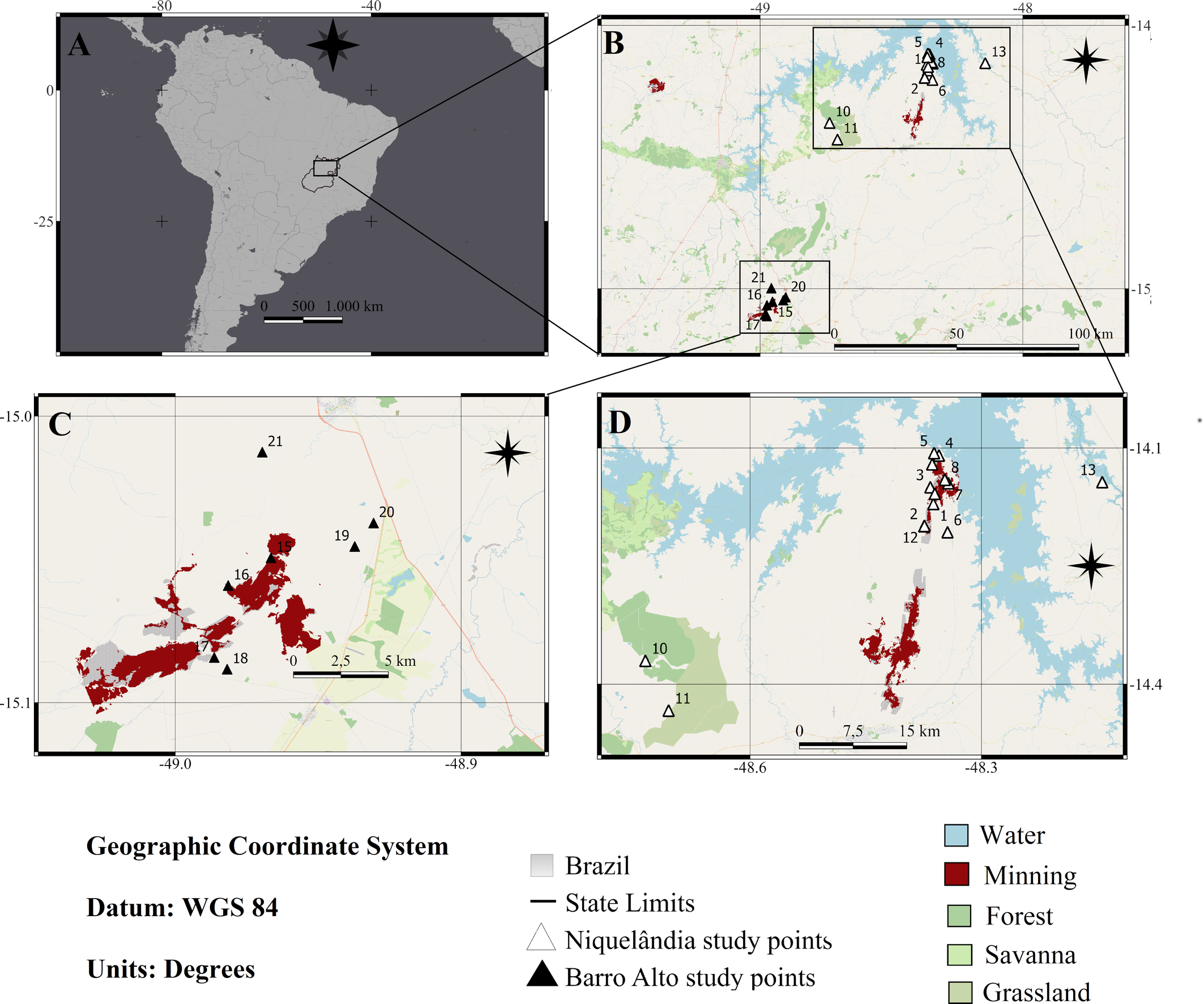
A total of 81 plots were established for analysis at 21 sampling sites within the natural vegetation remnants of the Cerrado. These sampling sites were situated in the municipalities of Niquelândia and Barro Alto, Goiás State, Brazil (Figure 1 and Table 1). Most of the sampling sites (13) were located within or immediately adjacent to the mining area at distances of less than 600 meters. Five sampling sites were located within a range of 1 to 5 km from the mining area, while three were located between 10 and 30 km away.
The natural vegetation remnants sampled were categorized into three primary plant formations based on the classification by Ribeiro and Walter (2008): forest (F), savannah (S), and grassland (G). According to Ribeiro and Walter (2008), forest formations represent regions primarily covered by trees, capable of creating either a continuous or fragmented canopy. The savannah formation denotes a landscape where trees and shrubs are spaced apart and spread across a ground mostly covered by grasses (Coutinho 2006; Ribeiro and Walter 2008; Batalha 2011). In contrast, grassland formations encompass areas where grasses dominate alongside sparse shrubs, without a significant presence of trees (Ribeiro and Walter 2008). In this way, 11 natural vegetation remnants sampled were identified as belonging to the savannah type, while eight of these remnants were classified as forest and two as grassland.
Sampling and identification
At each of the 21 sampling sites, we conducted two sampling lines, spaced 150 meters apart. For each sampling line, we established two 5 x 5 m quadrat samples (sampling plots): one located 10 meters from the edge of the vegetation remnant and the other 50 meters from the edge. This approach resulted in four sample plots per sampling site, for a total of 84 sample plots. Due to inaccessibility, three samples could not be collected, resulting in a final total of 81 sample plots available for mite collection. Two surveys were conducted, one in March 2015 and one in January 2016, both during the rainy season. In each survey, 20 leaves were collected from around the mid-canopy of all trees present in each sample plot, regardless of the surrounding vegetation type. Although some plots were located in areas classified as grassland, a vegetation formation within the Cerrado domain, this vegetation type is characterized by scattered and widely spaced trees. The focus of our sampling was solely on the trees. The 20 sampled leaves were placed in individually labelled paper bags, with one bag for each plant specimen. The bags were then stored in refrigerated Styrofoam boxes. It was not possible to identify the species of all plants sampled in the sampling plots because the field collection team did not have a botanist available to assist with this task. The lack of specialized botanical expertise during sampling limited the taxonomic resolution of the collected specimens, which affected the completeness of the floristic inventory.
The extraction of mites was conducted by washing the sampled leaves with 30% alcohol, following the protocol of Araújo et al. (2022). The samples were examined under a stereomicroscope and all mites found were mounted on slides with Hoyer's medium (Moraes and Flechtmann 2008). Specimen identification and counting were performed under a phase-contrast optical microscope using specialized dichotomous keys for genera and subgenera (Chant and McMurtry 2007) and works on descriptions, redescriptions, and revisions of Phytoseiidae to identify taxa at the species level (e.g., Denmark 1966; Demite et al. 2016, 2021; Barbosa and Demite 2023; Silva et al. 2024; Lofego et al. 2024; Ferragut and Navia 2024). The identified specimens were deposited in the Acari Collection at the Universidade Estadual Paulista (UNESP), São José do Rio Preto, São Paulo, Brazil.
Data analysis
Sampling effort varied between vegetation types due to differences in the number of trees in each sampling plot, which could bias direct species richness comparisons. Therefore, we used the first-order Jackknife method (Jackknife 1) to mitigate this effect and obtain a more reliable estimate of expected richness. This method minimizes the effects of differences in sampling effort and provides a more accurate approximation of true species richness in each plot. Since our primary focus is on species composition rather than abundance, Jackknife 1 is the most appropriate choice, as it corrects for the underestimation of richness due to insufficient sampling (Heltshe and Forrester 1983). The species richness of Phytoseiidae was estimated for each Cerrado vegetation type using the first-order Jackknife method (Jackknife 1) (Heltshe and Forrester 1983), performing 999 permutations with the ''vegan'' package (Oksanen et al. 2013) in RStudio 4.3.1 (R Core Team 2025). The estimated richness for each vegetal type (forest, grassland or savannah) was represented in a graph built with ''ggplot'' package (Wickham 2006) in RStudio 4.3.1 (R Core Team 2025). The comparison of estimated mite richness among Cerrado formations was calculated using cumulative Jackknife values and was subsequently visually assessed by checking the overlap of error bars (confidence intervals) with the mean, as proposed by Cumming et al. (2007). This approach allows us to compare Jackknife values while accounting for the progressive variability of Jackknife 1 values across samples, providing a more accurate understanding of how estimated species richness values vary among vegetation types. To complement this approach, we performed a Kruskal-Wallis statistical test using the cumulative Jackknife 1 richness to assess significant differences in estimated species richness values among vegetation types, as the assumptions of normality in the data distribution and homogeneity of variances were not met. Subsequently, if the Kruskal-Wallis test shows a statistically significant difference in the estimated predatory mite richness among the three vegetation types, we use Dunn's test post-hoc to determine which vegetation types are significantly different. We used the ''stats'' package in Rstudio for the Kruskal-Wallis and Dunn analyses (R Core Team 2025). Additionally, we used the estimated richness (Jackknife 1) separately for each sample plot to determine possible differences among the sampling plots located at the edge (10 m) and interior (50 m) in the natural vegetation remnants. Since the assumptions of normal data distribution and homogeneity of variances were not satisfactorily met, we opted for the non-parametric statistical Mann-Whitney U test to assess differences in estimated richness between the sampling plots established at the edge and interior in the natural vegetation remnants. We also verified the effect of distance of each sample plot from the nickel mining areas by Spearman correlation using Jackknife 1 estimated richness values for each sample plot as response variable. Additionally, we generated a graph illustrating the relationship between distance to mining sites and estimated richness (Jackknife 1) of predatory mites. For these analyses, we used the ''stats'' package to perform the Mann-Whitney U test and Spearman correlation, and the ''ggplot2'' package for data visualization and graphical representation (Wickham 2016; R Core Team 2025). All statistical analyses were conducted in Rstudio (R Core Team 2025). For all statistical analyses, we considered α = 0.05 as the significance level.
Moreover, we applied a non-metric multidimensional scaling (NMDS) test using a Jaccard matrix to evaluate the similarity of mite communities among the three different vegetation formations. The statistical significance of the groups generated by NMDS was evaluated through Analysis of Similarity (ANOSIM) (Bootstrap = 9,999 permutations; Clarke 1993). The ANOSIM and NMDS tests were performed using the Past 3.23 software (Hammer et al. 2001).


Download as
Remnants
Municipality
Formation
Latitude(S)
Longitude(W)
1
Niquelândia
Savannah
14º10′15.8″
48º21′44.5″
2
Niquelândia
Grassland
14º11′57.5″
48º22′27.7″
3
Niquelândia
Savannah
14º08′59.5″
48º21′58.7″
4
Niquelândia
Forest
14º06′34.4″
48º21′18.4″
5
Niquelândia
Savannah
14º06′23.3″
48º21′41.1″
6
Niquelândia
Forest
14º11′85.3″
48º20′38.4″
7
Niquelândia
Forest
14º08′40.7″
48º20′34.6″
8
Niquelândia
Savannah
14º08′25.1″
48º20′51.1″
9
Niquelândia
Savannah
14º07′14.4″
48º21′50.1″
10
Niquelândia
Forest
14º22′13.2″
48º44′09.1″
11
Niquelândia
Grassland
14º26′01.0″
48º42′20.6″
12
Niquelândia
Savannah
14°11′56″
48°22′26″
13
Niquelândia
Forest
14°08′36″
48°08′36″
14
Niquelândia
Savannah
14°09′29″
48°21′38″
15
BarroAlto
Savannah
15º02′57.1″
48º57′11.2″
16
BarroAlto
Savannah
15º03′45.9″
48º58′26.7″
17
BarroAlto
Forest
15º05′52.5″
48º58′51.3″
18
BarroAlto
Forest
15º06′13.1″
48º58′28.5″
19
BarroAlto
Forest
15º02′37.2″
48º54′43.6″
20
BarroAlto
Savannah
15º01′56.0″
48º54′10.4″
21
BarroAlto
Savannah
14º59′51.3″
48º57′26.6″
Results
A total of 279 Phytoseiidae mites, distributed in 19 species, were collected across all the samples carried out (Table 2). Of these, 130 mites of 15 species were collected in 40 forest plots, 137 mites of 15 species in 38 savannah plots, and only 12 mites of four species in three grassland plots. The most abundant species in all samples were Euseius sibelius (De Leon), E. citrifolius Denmark and Muma, and Transeius bellottii (Moraes and Mesa), with these three species dominating sampling sites 17 (Forest), 5 (Savannah), and 5 (Savannah), respectively (Table 3). The species with the lowest recorded frequency were Arrenoseius lofegoi Barbosa and Demite, Galendromimus (G.) kynolithus Silva, Gondim Jr. and Demite, Neoseiulus goiano Demite, Cavalcante and Lofego, Proprioseiopsis dominigos (El-Banhawy), and Typhlodromus (Anthoseius) transvaalensis (Nesbitt), with only one individual collected for each (Table 2).
Amblydromalus insolitus Nuvoloni and Lofego, Galendromus (G.) annectens (De Leon), Phytoseius intermedius Evans and MacFarlane, and P. dominigos were only recorded in the municipality of Niquelândia. In contrast, the species Amblyseius compositus Denmark and Muma, A. lofegoi, G. (G.) kynolithus, Neoseiulus californicus (McGregor), N. goiano, N. tunus, and T. (A) transvaalensis were only sampled in remnants within the municipality of Barro Alto (Table 3).
Regarding the vegetation formations, P. nahuatlensis De Leon and E. citrifolius were the most abundant species in the grassland formations, while E. sibelius, E. citrifolius, and T. bellottii were the most abundant in the forest and savanna vegetations (Table 2). Euseius citrifolius, E. sibelius, P. nahuatlensis, and T. bellottii were recorded in all sampled vegetation types (Table 2). In contrast, Amblyseius chiapensis De Leon, A. compositus, N. californicus, and N. goiano were found only in forest formations, while A. lofegoi, G. (G.) kynolithus, P. dominigos, and T. (A.) transvaalensis were exclusive to savannah. Eleven species were common to both forest and savannah formations. Grasslands did not show any exclusive species, indicating that the species in this vegetation formation represent a subset of the Phytoseiidae assemblages from forest and savannah formations (Table 2).
Download as
Species
Vegetation type
Total
G
F
S
Amblydromalus insolitus Nuvoloni and Lofego
0
1
1
2
Amblyseius chiapensis De Leon
0
2
0
2
Amblyseius compositus Denmark and Muma
0
2
0
2
Arrenoseius lofegoi Barbosa and Demite
0
0
1
1
Euseius citrifolius Denmark and Muma
2
24
21
47
Euseius sibelius (De Leon)
1
37
29
67
Euseius uai Demite and Lofego
0
2
3
5
Galendromimus (G.) kynolithus Silva, Gondim Jr. and Demite
0
0
1
1
Galendromus (G.) annectens (De Leon)
0
1
1
2
Neoseiulus californicus (McGregor)
0
4
0
4
Neoseiulus goiano Demite, Cavalcante and Lofego
0
1
0
1
Neoseiulus tunus (De Leon)
0
8
5
13
Phytoseius guianensis De Leon
0
1
1
2
Phytoseius intermedius Evans and MacFarlane
0
1
1
2
Phytoseius kaapre Demite, Lofego and Feres
0
3
1
4
Phytoseius nahuatlensis De Leon
3
2
8
13
Proprioseiopsis dominigos (El-Banhawy)
0
0
1
1
Transeius bellottii (Moraes and Mesa)
1
12
16
29
Typhlodromus (Anthoseius) transvaalensis (Nesbitt)
0
0
1
1
Unidentified immatures
5
29
46
80
Richness
4
15
15
19
Abundance
12
130
137
279
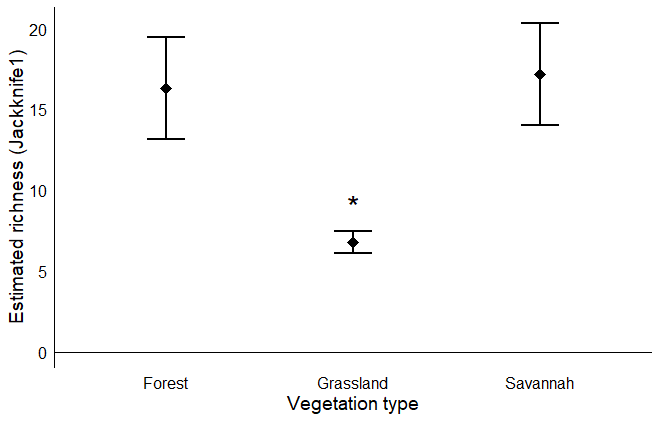
The estimated species richness of Phytoseiidae was higher in savannah and forest formations compared to grassland formations (Kruskal-Wallis test, χ² = 13.13, df = 2, P = 0.001) when analysed using the cumulative approach of estimated richness (Figure 2). The results of Dunn's test confirm the pattern observed in Figure 2, indicating that grassland had a significantly lower estimated richness compared to forest (Dunn's test, Z = 3.57, P = 0.003) and savannah (Dunn's test, Z = -3.56, P = 0.0003). However, the estimated richness (Jackknife 1) of Phytoseiidae mites did not differ between forest and savannah (Dunn's test, Z = 0.61, P = 0.56).
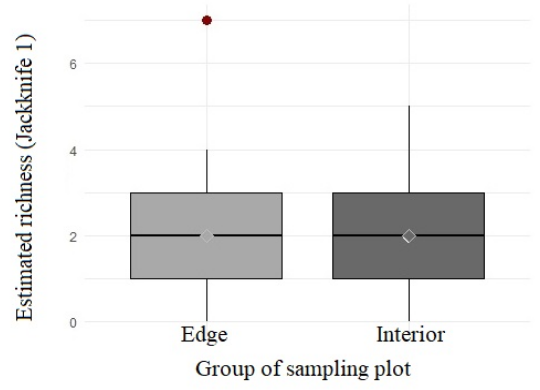
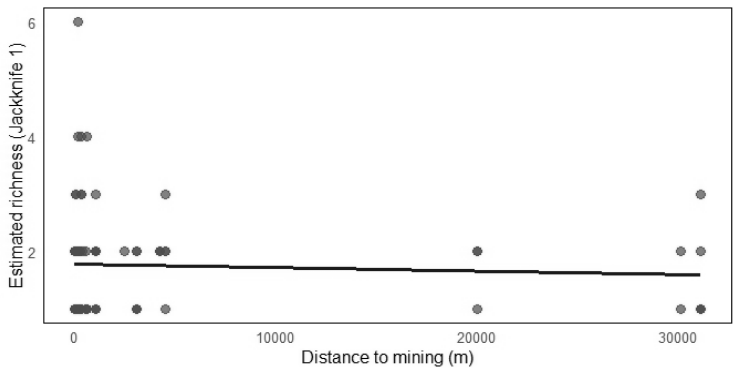
On the other hand, no differences were found between the plots established in the edge (10 m) and interior (50 m) of natural vegetation remnants (Mann-Whitney U test, W = 593.50, P = 0.99) (Figure 3). Furthermore, the distance to the nickel mining sites did not affect the estimated richness of Phytoseiidae mites in Cerrado vegetation remnants (Spearman's correlation, ρ = -0.008, n = 81, P = 0.59) (Figure 4).
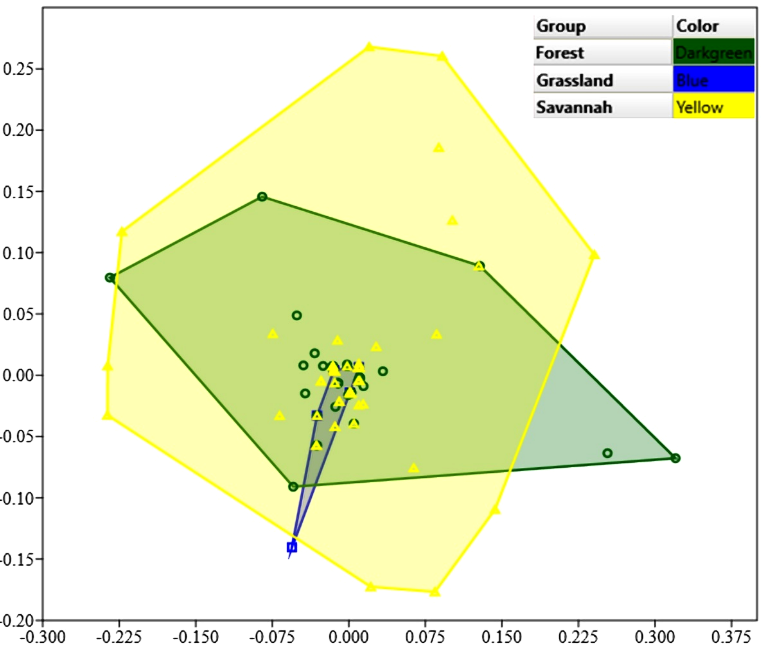
The NMDS analysis revealed overlap in mite species composition among the three vegetation formations, indicating a high level of similarity in Phytoseiidae species composition across the three Cerrado vegetation types (Figure 5). This result is consistent with the ANOSIM test, which also showed no significant differences in Phytoseiidae species composition among the three Cerrado vegetation formations (ANOSIM, r = 0.007, P = 0.65).
Download as * Coordinates of the sample points in Table I.
** Previously reported for the Cerrado in the Goiás State, Brazil (Rezende and Lofego 2011; Rezende et al. 2012; Demite et al. 2017; Teixeira et al. 2017; Barroso et al. 2019; Duarte et al. 2021; Moraes et al. 2022; Demite et al. 2025).
Species
Sampling sites
Niquelândia
Barro Alto
Total
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
Amblydromalus insolitus Nuvoloni and Lofego
1
1
2
Amblyseius chiapensis De Leon**
1
1
2
Amblyseius compositus Denmark and Muma**
2
2
Arrenoseius lofegoi Barbosa and Demite
1
1
Euseius citrifolius Denmark and Muma**
3
1
8
3
1
1
2
3
1
1
5
5
6
5
2
47
Euseius sibelius (De Leon)**
3
1
6
2
9
1
1
2
2
4
8
11
7
1
9
67
Euseius uai Demite and Lofego**
1
1
1
2
5
Galendromimus (G.) kynolithus Silva, Gondim Jr. and Demite
1
1
Galendromus (G.) annectens (De Leon)**
1
1
2
Neoseiulus californicus (McGregor)
2
1
1
4
Neoseiulus goiano Demite, Cavalcante and Lofego**
1
1
Neoseiulus tunus (De Leon)**
3
3
5
2
13
Phytoseius guianensis De Leon**
1
1
2
Phytoseius intermedius Evans and MacFarlane**
1
1
2
Phytoseius kaapre Demite, Lofego and Feres
1
2
1
4
Phytoseius nahuatlensis De Leon**
1
1
3
1
1
4
2
13
Proprioseiopsis dominigos (El-** Banhawy)
1
1
Transeius bellottii (Moraes and Mesa)**
2
1
5
1
6
1
4
1
2
1
1
1
2
1
29
Typhlodromus (Anthoseius) transvaalensis (Nesbitt)
1
1
Unidentified immatures
4
2
2
4
6
6
1
1
3
3
1
2
8
6
12
3
2
3
11
80
Richness
4
2
3
6
4
2
4
2
2
6
2
4
3
7
6
8
6
3
3
4
19
Abundance
13
4
9
15
23
2
23
2
3
10
8
3
8
5
20
23
39
24
9
10
26
279








Discussion
Despite the proximity to nickel mining sites, the Cerrado remnants evaluated in this study supported a considerable diversity of Phytoseiidae mite species, including those with potential agricultural importance, such as E. sibelius, E. citrifolius, N. tunus, and T. bellottii (McMurtry et al. 2015). Our results were comparable to the findings of Demite et al. (2021), who reported 21 Phytoseiidae species in remnants of natural vegetation and cultivated forests. These results suggest that the diversity of Phytoseiidae mites in mining affected areas or adjacent regions is similar to that observed in native vegetation remnants and cultivated forests, highlighting the resilience of these predatory mite species under different environmental conditions.
Although several scientific studies have investigated the diversity of Phytoseiidae mites in the natural vegetation of the Cerrado biome (Lofego et al. 2009; Rezende and Lofego 2011; Rezende et al. 2014; Abreu et al. 2017; Demite et al. 2017; Teixeira et al. 2017; Silva et al. 2020; Duarte et al. 2021; Moraes et al. 2022), our study provides a comprehensive assessment of Phytoseiidae diversity, identifying all taxa to the species level. Furthermore, this study introduces a novel ecological analysis by examining how proximity to mining areas may influence the diversity and composition of Phytoseiidae mite communities, considering the Cerrado vegetation formations in which the sampling sites are located.
Amblydromalus insolitus, A. lofegoi, G. (G.) kynolithus, P. kaapre, T. (A.) transvaalensis and N. californicus are reported here for the first time in the state of Goiás. Moreover, A. lofegoi and G. (G.) kynolithus were described from individuals sampled in Mata Atlântica (Barbosa and Demite 2023) and Caatinga (Silva et al. 2024) biomes, in Bahia and Pernambuco states, respectively. Here we reported A. lofegoi and G. (G.) kynolithus for the first time for Cerrado remnants from Goiás State.
In this study, E. sibelius, E. citrifolius, P. nahuatlensis and T. bellottii were identified in the three vegetation types (grassland, forest and savannah). These species were also most abundant in forest and savannah formations, suggesting remarkable resilience in their ability to persist in different vegetation formations within the same biome, including grasslands, a more homogeneous environment composed of grasses with more widely spaced trees. This finding is particularly relevant given that the Cerrado biome is experiencing a trend toward the reduction of natural vegetation fragments (Primack and Massardo 2001; Neri et al. 2011), which may provide further insight into which Phytoseiidae mite species are more resilient to declines in abundance even under the effects of habitat loss.
On the other hand, A. chiapensis, A. compositus, N. californicus, and N. goiano were found exclusively in sampling plots within forest formations; A. chiapensis and A. compositus were recorded in two different sampling plots, with one individual per sample, while N. californicus was found in three different samples, totalising four individuals, and a single individual of N. goiano was recorded in one sampling plot. Arrenoseius lofegoi, G. (G.) kynolithus, P. dominigos and T. (A.) transvaalensis were found exclusively in the savannah formation, with a single individual sampled for each species in different sampling plots. The low abundance and limited occurrence of these species raises uncertainties as to whether factors such as vegetation formation type, proximity to mining areas, or the location of sampling plots within the natural vegetation remnant play a determining role in the occurrence of these Phytoseiidae species. In addition, other factors that may operate at different levels or smaller scales, such as intrinsic characteristics of host plants, may also influence community abundance and species composition.
There remains a knowledge gap regarding recently described Phytoseiidae species, such as A. lofegoi and G. (G.) kynolithus, mainly due to the limited time available to conduct studies that could elucidate their feeding preferences and potential as natural enemies of agricultural pests. Among other Phytoseiidae species, N. californicus is widely recognized as an effective natural enemy of several pests, especially tetranychid mites such as Tetranychus urticae Koch, Panonychus ulmi (Koch), and Panonychus citri (McGregor) (Monteiro 2002). Amblyseius chiapensis has been proposed as a potential natural enemy of T. urticae (Amaral et al. 2020), and information on its oviposition rate is available in Amaral et al. (2018). Amblyseius compositus has been studied and confirmed as a potential predator of Brevipalpus species (Reis et al. 2007). In addition, Amblyseius species have been classified as subtype III-b of predatory mites, which includes generalist predators with a preference for plants with glabrous leaves (McMurtry et al. 2013).
There is insufficient information on N. goiano and P. dominigos to determine their life history parameters or to assess their potential as natural enemies of agricultural pests. However, Neoseiulus species are classified as type II, referring to selective predators of tetranychid mites while Proprioseiopsis exhibit vertical movement among their plant hosts and are classified as subtype III-e, generalist predators in leaf litter and debris environments (McMurtry et al. 2013). In addition, T. (A.) transvaalensis has been studied as a potential biological control agent for pests affecting physic nut (Jatropha curcas L.), showing high potential as a generalist predator in the regulation of Polyphagotarsonemus latus (Smiley) populations (Cañarte et al. 2017).
The estimated species richness for the Cerrado remnants indicated that savannah areas hosted a comparable mite species richness to forest formations, demonstrating that different Cerrado vegetation types exhibit considerable mite diversity. Moraes et al. (2022) also found greater plant mite species richness in savannah than grassland in the Parque Nacional das Emas, an important Cerrado conservation unit in Brazil. Therefore, these areas should be considered in public policies for biodiversity conservation (Demite et al. 2017). The diversity of mite fauna in Cerrado formations may be related to environmental heterogeneity. Cerrado biome is characterized by a mosaic of different types of environments, ranging from grasslands to forests (Ribeiro and Walter 2008), represented by its distinct vegetation formations (e.g., forest, rocky fields, savannah, riparian forests), creating a heterogeneous gradient within a given area (Silva et al. 2002). Greater environmental heterogeneity promotes a high functional diversity of species, as it increases the likelihood of the ecosystem supporting more species that can respond favourably to various environmental disturbances due to the abundance of resources and diverse environmental conditions provided by a more complex ecosystem (e.g., food, refuge, mating sites) (Schuelze and Mooney 1997; Levin 1999; McCann 2000; Montoya et al. 2001; Altieri 2002). Phytoseiidae mites are often found on the undersides of leaves where they can avoid excessive exposure to desiccation or predators while benefiting from a stable microenvironment. In addition, they can adapt to environmental fluctuations by using plant structures (e.g., domatia) as refugia that provide shelter from extreme weather conditions or disturbances. Consequently, the presence of these favourable conditions directly influences their reproductive success and overall population dynamics (McMurtry et al. 2013). Both forest and savannah are more structurally and functionally complex habitats than grassland (Ribeiro and Walter 2008).
Each vegetation formation is shaped by a particular climatic regime, which influences the presence of plant species with specialized structures adapted to that regime, such as spines, domatia, pubescent or glabrous leaves (Ribeiro and Walter 2008). These structures, in turn, may influence the composition of Phytoseiidae mites, as certain species prefer to inhabit plants that provide such characteristics (McMurtry et al. 2013). However, as shown by the NMDS and ANOSIM analyses, the vegetation types of the Cerrado harbour a similar species composition of the Phytoseiidae fauna, suggesting that factors operating at smaller scales may play a critical role in structuring these communities. For example, some Phytoseiidae species (Type III species according to McMurtry et al. 2013) exhibit preferences for certain characteristics of their host plants that facilitate the development and reproduction of these mite species (McMurtry et al. 2013). Therefore, further studies are needed to elucidate the effect of possible influences of plant traits from different vegetation types on mite fauna composition.
Apart from the similarity in the species composition among vegetation types, the estimated richness of Phytoseiidae did not differ between the sampling plots located in the interior (50 m) and those at the edge of the natural vegetation remnant (10 m). Moreover, the distance from mining sites did not affect the estimated richness of mites. Mining activities result in loss of native vegetation, habitat fragmentation, and soil contamination (Neri et al. 2011). All the vegetation remnants studied are highly fragmented and probably they suffer the same effects of mining activities, which can promote homogenization in the Phytoseiidae fauna on plants from these vegetation remnants. This means that fewer species are exclusive to a particular vegetation type, and a greater number of mite species are shared among vegetation types. These findings raise new questions about how Phytoseiidae mite communities respond to habitat fragmentation and loss in threatened natural vegetation remnants. In order to achieve a comprehensive understanding of the impact of human activity on mite structure assemblages, it is imperative to conduct comparative studies between preserved and highly fragmented vegetation remnants.
Conclusion
This study reported some mites previously found in the Cerrado vegetation and noted new occurrences of phytoseiid mites in the bioma and Goiás, Brazil. This is the second study to compare mite assemblages among different vegetation formations within the Cerrado biome (Moraes et al. 2022). In this study, we also explore how environmental impact factors, such as mining activities, can modify the composition of Phytoseiidae mite communities in the Cerrado. We also aim to conduct an exploratory ecological analysis to ascertain how the distance of natural vegetation remnants from mining areas affects these arthropods. Cerrado vegetation remnants with greater structural complexity can support a higher species richness of Phytoseiidae compared to more homogeneous vegetation formations, such as grassland formations. Additionally, Cerrado remnants harboured potential species for biocontrol programs, such as E. citrifolius, E. sibelius, N. californicus, N. tunus, and T. bellottii, even in close proximity to nickel mining areas. This highlights the importance of conserving the biome, as these natural areas can serve as crucial reservoirs of Phytoseiidae biodiversity with potential for natural pest regulation in agriculture.
Acknowledgements
This work was funded by the Anglo-American Brazil – FUNAPE agreement, CNPq (Universal Call, process 456538/2014-3), and FAPEG - Fundação de Amparo à Pesquisa do Estado de Goiás (CH 07/2014 – Universal Call, process 201410267001741).
References
- Abreu K.M., Araújo F.G., Lima E.L., Daud R.D. 2017. Mites (Arachnida, Acari) on Astronium fraxinifolium Schott (Anacardiaceae) from the Cerrado remnants associated with nickel mining areas. Acarologia, 57(2): 223-232. https://doi.org/10.1051/acarologia/20164151
- Altieri M.A. 2002. Agroecología: principios y estrategias para diseñar sistemas agrarios sustentables. In: Sarandon, S.J. (Ed.), Agroecología: el camino hacia una agricultura sustentable, Buenos Aires–La Plata, p. 49-56.
- Amaral F.S., Cavalcante A.C., Lofego A. C. 2020. Amblyseius chiapensis (Acari: Phytoseiidae) as natural enemy of Tetranychus urticae (Acari: Tetranychidae). Syst. Appl. Acarol., 25(2): 173-177. https://doi.org/10.11158/saa.25.2.1
- Amaral F.S., Lofego A.C., Cavalcante A.C. 2018. Oviposition rates of Amblyseius aerialis (Muma) and Amblyseius chiapensis DeLeon (Acari: Phytoseiidae) under seven foods different patterns for the same genus. Syst. Appl. Acarol., 23(5), 795-798. https://doi.org/10.11158/saa.23.5.1
- Araújo F.G., Lima E.L., Costa E., Daud R.D. 2022. Influence of natural vegetation conservation on the distribution of mites in rubber tree crops. Syst. Appl. Acarol., 27(8): 1629-1647. https://doi.org/10.11158/saa.27.8.13
- Barbosa M.F. de C., Demite P.R. 2023. A new species of Arrenoseius Wainstein (Mesostigmata: Phytoseiidae) from Brazil, with a world key to the genus. Int. J. Acarol., 49(1): 49-53. https://doi.org/10.1080/01647954.2023.2178505
- Barroso G., da Rocha C.M., Moreira G.F., Hata F.T., Roggia S., Ventura M.U., Pasini A., Silva J.E.P., Holtz A.M., Moraes G.J. de. 2019. What is the southern limit of the distribution of red palm mite, Raoiella indica (Acari: Tenuipalpidae), in agricultural lands in Brazil? Fla. Entomol., 102(3): 581-585. https://doi.org/10.1653/024.102.0334
- Batalha M.A. 2011. O cerrado não é um bioma. Biota. Neotrop., 11: 21-24. https://doi.org/10.1590/S1676-06032011000100001
- Brooks T.M., Mittermeier R.A., da Fonseca G.A.B., Gerlach J., Hoffmann M., Lamoreux J.F., Mittermeier C.G., Pilgrim J.D., Rodrigues A.S.L. 2006. Global biodiversity conservation priorities. Science, 313: 58-61. https://doi.org/10.1126/science.1127609
- Cañarte E., Sarmento R.A., Venzon M., Pedro-Neto M., Junior D.F.F., Santos F.A., Pallini A. 2017. Suitability and nutritional requirements of the predatory mite Typhlodromus transvaalensis, a potential biological control agent of physic nut pest mites. Biol. Control, 115: 165-172. https://doi.org/10.1016/j.biocontrol.2017.10.008
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House. pp. 219.
- Clarke K.R. 1993. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol., 18(1): 117-143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
- Conceição E.M., Demite P.R., Rezende J.M., Carniello M.A., Lofego A.C. 2021. Phytoseiidae (Acari: Parasitiformes: Mesostigmata) inhabiting native plants from three biomes in Mato Grosso State, Brazil, with description of a new species. Syst. Appl. Acarol., 26(12): 2268-2286. https://doi.org/10.11158/saa.26.12.6
- Coutinho L.M. 2006. O conceito de bioma. Acta Bot. Brasílica., 20: 13-23. https://doi.org/10.1590/S0102-33062006000100002
- Cumming G., Fidley F., Vaux D.L. 2007. Error bars in experimental biology. J. Cell Biol., 177(1): 7-11. https://doi.org/10.1083/jcb.200611141
- De Araújo W.S., Daud R.D. 2017. Insights on plant mite occurrence in natural vegetation remnants from Brazil. Syst. Appl. Acarol., 22(2): 302-322. https://doi.org/10.11158/saa.22.2.12
- Demite P.R., Cavalcante A.C., Dias M.A., Lofego A.C. 2016. A new species and redescription of two species of Euseius Wainstein (Acari: Phytoseiidae) from Cerrado biome areas in Brazil. Int. J. Acarol., 42(7): 334-340. https://doi.org/10.1080/01647954.2016.1197311
- Demite P.R., Dias M.A., Cavalcante A.C.C., Ramos M.V.V., Lofego A.C. 2017. Phytoseiid mites (Acari: Mesostigmata: Phytoseiidae) associated with Cerrado biome plants in Brazil, with description of a new species. Syst. Appl. Acarol., 22(12): 2141-2177. https://doi.org/10.11158/saa.22.12.9
- Demite P.R., Feres R.J., Lofego A.C., Oliveira A.R. 2009. Plant inhabiting mites (Acari) from the Cerrado biome of Mato Grosso State, Brazil. Zootaxa, 2061(1): 45-60. https://doi.org/10.11646/zootaxa.2061.1.3
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2025. Phytoseiidae Database. Available from: http://www.lea.esalq.usp.br/phytoseiidae (accessed on 10/04/2025).
- Demite P.R., Rezende J.M., Dahmer P.C., Cavalcante A.C.C., Lofego A.C. 2021. A new species of Amblydromalus Chant and McMurtry (Acari: Phytoseiidae), with notes on occurrence of genus in South America. Acarologia, 61(3): 527-537. https://doi.org/10.24349/acarologia/20214445
- Demite P.R., Rezende J.M., Lofego A.C., Amaral F.S., Barreto M.R., Moraes G.J. de 2021. Phytoseiid mites (Acari: Mesostigmata: Phytoseiidae) from Mato Grosso State, Central-Western Brazil. An. Acad. Bras. Ciênc., 93: e20200729. https://doi.org/10.1590/0001-3765202120200729
- Denmark H.A. 1966. Revision of the genus Phytoseius Ribaga, 1904 (Acarina: Phytoseiidae). Fla. Dep. Agric. Bul., 6: 1-105.
- Duarte M.E., Demite P.R., De Mendonça R.S., Michereff-Filho M., De Mesquita M.L.S.C., Peixoto J.R., Navia D. 2021. Phytoseiidae mites associated with native and cultivated solanaceous in Central-West Brazil. Syst. Appl. Acarol., 26: 2358-2384. https://doi.org/10.11158/saa.26.12.13
- Ferragut F., Navia D. 2024. Phytoseius Ribaga (Mesostigmata: Phytoseiidae) in the Brazilian Espinhaço Range: seven new species, renaming of the plumifer species group, and a critical review of its taxonomic characters. Zootaxa, 5493(3): 201-245. https://doi.org/10.11646/zootaxa.5493.3.1
- Hammer Ø., Harper A.T., Ryan P.D. 2001. Past: Paleontological statistics software package for education and data analysis. Paleont. Electr., 4(1). http://palaeoelectronica.org/2001_1/past/issue1_01.htm
- Heltshe J.F., Forrester N.E. 1983. Estimating species richness using the jackknife procedure. Biometrics, 1-11. https://doi.org/10.2307/2530802
- Levin S. 1999. Fragile Dominion. Perseus Books, Reading, Massachusetts. pp. 254.
- Lofego A.C., Barbosa M.F. de C., Demite P.R., Moraes G.J. de. 2024. Phytoseiidae (Acari: Mesostigmata) of the subfamily Amblyseiinae from Brazil. Zootaxa, 5439(1): 1-306. https://doi.org/10.11646/zootaxa.5439.1.1
- Lofego A.C., Demite P.R., Kishimoto R.G., Moraes G.J. de. 2009. Phytoseiid mites on grasses in Brazil (Acari: Phytoseiidae). Zootaxa, 2240(1): 41-59. https://doi.org/10.11646/zootaxa.2240.1.3
- Lofego A.C., Moraes G.J. de, Castro L.A. S. 2004. Phytoseiid mites (Acari: Phytoseiidae) on Myrtaceae in the State of São Paulo, Brazil. Zootaxa, 516(1): 1-18. https://doi.org/10.11646/zootaxa.516.1.1
- Loyola R.D., Oliveira-Santos L.G.R., Almeida-Neto M., Nogueira D.M., Kubota U., Diniz-Filho J.A.F., Lewinsohn T.M. 2009. Integrating economic costs and biological traits into global conservation priorities for carnivores. PLoS One, 4(7): e6824. https://doi.org/10.1371/journal.pone.0006807
- McCann K.S. 2000. The diversity-stability debate. Nature, 405: 228-233. https://doi.org/10.1038/35012234
- McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol., 42: 291-321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., Moraes G.J. de, Famah-Sourassou N. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- McMurtry J.A., Sourassou N.F., Demite P.R. 2015. The Phytoseiidae (Acari: Mesostigmata) as biological control agents. In: Carrillo D., Moraes G.J de, Peña J. (Eds.), Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms. Springer, Cham. p. 133-149. https://doi.org/10.1007/978-3-319-15042-0_5
- Medeiros, N.F. 2019. Restoration practices and studies in the Brazilian Cerrado. [Master's dissertation, Universidade Federal de Minas Gerais]. http://hdl.handle.net/1843/33895
- Mendonça R.C., Felfili J.M., Walter B.M.T., da Júnior M.C.S., Rezende A.V., de Filgueiras T.S., Fagg C.W. 2008. Flora vascular do bioma Cerrado. In Sano S.M., Almeida S.P., Ribeiro R.F. (Eds.), Cerrado: ecologia e flora. Embrapa Informação Tecnológica, Brasília, DF. pp. 1028-1059.
- Monteiro L.B. 2002. Manejo integrado de pragas em macieira no Rio Grande do Sul II: uso de Neoseiulus californicus para o controle de Panonychus ulmi. Rev. Bras. Frutic., 24: 395-405. https://doi.org/10.1590/S0100-29452002000200024
- Montoya J.M., Solé R.V., Rodríguez M.A. 2001. La arquitectura de la naturaleza: complejidad y fragilidad en redes ecológicas. Ecosistemas, 10(2): 1-14.
- Moraes G.J. de, Flechtmann C.H.W. 2008. Manual de Acarologia: Acarologia básica e ácaros de plantas cultivadas no Brasil. Holos Editora, Ribeirão Preto. pp. 308.
- Moraes V.D.S., Nunes S.N., Demite P.R., Daud R.D. 2022. Vegetation structure defines mite assemblage on plants: a case study in Cerrado biome. Entomol. Commun., 4: e2022. https://doi.org/10.37486/2675-1305.ec04029
- Myers, N. 2003. Biodiversity hotspots revisited. Bioscience, 53: 916-917. https://doi.org/10.1641/0006-3568(2003)053%5B0916:BHR%5D2.0.CO;2
- Myers N., Mittermeier R.A., Mittermeier C.G., da Fonseca G.A.B., Kent, J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. https://doi.org/10.1038/35002501
- Neri A.V., Soares M.P., Meira Neto J.A.A., Dias L.E. 2011. Espécies de Cerrado com potencial para recuperação de áreas degradadas por mineração de ouro, Paracatu-MG. Rev. Árvore, 35: 907-918. https://doi.org/10.1590/S0100-67622011000500016
- Oksanen, J. 2013. Package ′vegan′. Community ecology package, version 2.9. https://cran.r-project.org/web/packages/vegan/index.html
- Primack R., Massardo F. 2001. Restauración ecológica. In: Primack R., Rozzi R., Feinsinger P., Dirzo R., Massardo F. (Eds.), Fundamentos de conservación biológica: perspectivas latinoamericanas. Fondo de Cultura Económica, México DF. pp. 559-582.
- R Core Team. 2025. R: A language and environment for statistical computing. Version 4.2.1. Vienna: R Foundation for Statistical Computing. [1 Apr 2025]. Available from: https://www.R-project.org/
- Reis P.R., Teodoro A.V., Pedro Neto M. 2007. História de vida de Amblyseius compositus Denmark e Muma predando Brevipalpus phoenicis (Geijskes) (Acari: Phytoseiidae, Tenuipalpidae). Coffee Sci., 2(2): 150-158.
- Rezende J.M., Lofego A.C. 2011. Phytoseiidae (Acari: Mesostigmata) on plants of the central region of the Brazilian Cerrado. Acarologia, 51(4): 449-463. https://doi.org/10.1051/acarologia/20112027
- Rezende J. M., Lofego A. C., Navia D., Roggia S. 2012. Mites (Acari: Mesostigmata, Sarcoptiformes and Trombidiformes) associated to soybean in Brazil, including new records from the Cerrado areas. Fla. Entomol., 95(3): 683-693. https://doi.org/10.1653/024.095.0319
- Rezende J.M., Lofego A.C., Nuvoloni F.M., Navia D. 2014. Mites from Cerrado fragments and adjacent soybean crops: does the native vegetation help or harm the plantation? Exp. Appl. Acarol., 64(4): 501-518. https://doi.org/10.1007/s10493-014-9844-5
- Ribeiro J.F.,Walter B.M.T. 2008. As principais fitofisionomias do bioma Cerrado. In: Sano S.M., Almeida S.P. (Eds.) J. F. Ribeiro (Eds.), Cerrado: ecologia e flora (pp. 151-212).
- Sano E.E., Rodrigues A.A., Martins E.S., Bettiol G.M., Bustamante M.M., Bezerra A.S., Bolfe E. L. 2019. Cerrado ecoregions: A spatial framework to assess and prioritize Brazilian savanna environmental diversity for conservation. J. Environ. Manage., 232: 818-828. https://doi.org/10.1016/j.jenvman.2018.11.108
- Schulze E.D., and Mooney H.A. 1994. Ecosystem function of biodiversity: a summary. In Schulze E.D., Mooney H.A (Eds.), Biodiversity and ecosystem function. Springer-Verlag, Berlín. pp. 497-510
- Silva J.F., Pereira J.M., Rocha C.B.S., Peres A.J.A., de Lima E.L., Daud R.D. 2020. Composition and abundance of mites (Arachnida: Acariformes: Parasitiformes) on Hancornia speciosa Gomes varieties. Int. J. Acarol., 46(6): 394-400. https://doi.org/10.1080/01647954.2020.1805511
- Silva L.O., Costa D.A., Santo Filho K. do E., Ferreira H.D., Brandão D. 2002. Levantamento florístico e fitossociológico em duas áreas de cerrado sensu stricto no Parque Estadual da Serra de Caldas Novas, Goiás. Acta Bot. Brasílica, 16: 43-53. https://doi.org/10.1590/S0102-33062002000100006
- Silva L.R.A., Gondim Jr M.G.C., Demite P.R. 2024. A new species of Galendromimus Muma (Acari: Phytoseiidae) from the Caatinga biome, Brazil. Acarologia, 64(1): 32-39. https://doi.org/10.24349/cny5-hj6f
- Teixeira J.V., Ribeiro R.N., Daud R.D. 2017. Mites on Curatella americana L. (Dilleniaceae) from Cerrado vegetation remnants in mining site vicinities. Int. J. Acarol., 43(4): 302-307. https://doi.org/10.1080/01647954.2017.1284899
- Wickham H. 2006. An introduction to ggplot: An implementation of the grammar of graphics in R. Statistics, 1-8.
- Wickham H. 2016. ggplot2: Elegant Graphics for Data Analysis, Version 4.3.1. Springer, 978-981.



2024-09-25
Date accepted:
2025-04-17
Date published:
2025-05-12
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Rossetti, Octavio César; Barroso, Geovanny; Demite, Peterson Rodrigo; de Lima, Edgar Luiz and Daud, Rodrigo Damasco
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



