New data on Leptotrombidium miniopteri (Acariformes: Trombiculidae) with a description of the adult stage
Stekolnikov, Alexandr A.  1
; Karağaç, Rümeysa
1
; Karağaç, Rümeysa  2
; Sevsay, Sevgi
2
; Sevsay, Sevgi  3
and Elverici, Mert
3
and Elverici, Mert  4
4
1✉ Laboratory of Parasitic Arthropods, Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia.
2Department of Biology, Institute of Science, Erzincan Binali Yıldırım University, Erzincan, Türkiye.
3Department of Biology, Institute of Science, Erzincan Binali Yıldırım University, Erzincan, Türkiye.
4Department of Biology, Faculty of Sciences and Arts, Erzincan Binali Yıldırım University, Erzincan, Türkiye.
2025 - Volume: 65 Issue: 2 pages: 380-395
https://doi.org/10.24349/ms7d-ocawZooBank LSID: 57635A4A-FB04-4E5D-8EB9-AC8AA2360C81
Original research
Keywords
Abstract
Introduction
Family Trombiculidae comprises more than 3000 species known almost exclusively from their parasitic larval stage, chigger (Nielsen et al. 2021). A fully engorged trombiculid larva detached from its host can be easily reared to the deutonymphal stage in the laboratory; the exuvium of such larva kept after the metamorphosis and the deutonymph constitutes a basis for the description of two stages of the same life cycle. Tens of trombiculid species were supplied with the descriptions of their nymphs by this method (Vercammen-Grandjean 1958, 1965b; Crossley 1960; Mąkol et al. 2010). At the same time, rearing nymphs into adults is much more difficult and takes a lot of time; such attempts involved a much lesser number of species (Womersley 1952; Southcott & Frances 1991; Moniuszko et al. 2017). An especially rare case is obtaining eggs and then larvae from adults collected in nature because postlarval trombiculids are usually cryptozoic. The exclusion is cave-dwelling Trombiculidae with their larvae parasitizing bats. Thus, de la Cruz & Socarras (1993) obtained larvae from the adults of Ischnothrombium diploctenum Feider, 1983 and Tectumpilosum negreai Feider, 1983; these two genera and species were described from adult instars collected on guano in Cuban caves (Feider 1983a, b). Examination of larvae allowed synonymization of Ischnothrombium Feider, 1983 with the bat-parasitizing chigger genus Whartonia Ewing, 1944 (Daniel & Stekolnikov 2002).
Our finding of adult trombiculids in a cave located in the Western Taurus Karst Region in Anatolia and obtaining offspring from one female allowed us to prepare a description of the adult stage for one more species with an identification confirmed by its larval stage. This species, Leptotrombidium miniopteri Feider, 1966, was previously known only from its type locality in Romania; it was described from larvae parasitizing the common bent-wing bat Miniopterus schreibersii (Kuhl, 1817) (Feider 1966). The species was re-described by Vercammen-Grandjean & Langston (1976), who examined the larval holotype. Besides describing the adult, we give a re-description of L. miniopteri larvae to provide additional data on their morphology and variation.
Material and methods
Our collection locality, Hacı Akif Cave (37°37.527′N, 031°29.001′E, 1210 m a.s.l.), is located on an island of the same name on Lake Beyşehir in the Konya Province of Türkiye. It has been a popular tourist destination considered within the scope of alternative tourism, preserved in its natural state. It is also fascinating with its diverse community of enigmatic invertebrate species, many of which display extreme levels of troglomorphism. It was subject to extensive taxonomic research by multiple authors, comprising a fauna of 11 species published in the literature (Kunt et al. 2010). Currently, the cave is protected in various scales: Lake Beyşehir and its environs is a national park, and the cave has been recognized as a natural asset since 10 October 2023 under the highest rank recognized by the management authority (MoEUCC, 2023).
Hacı Akif is a moderately long fossil cave with a planimetric length of 270 m and a small entrance, almost entirely composed of the dark zone. It is horizontal, with the deepest point –8 m below the entrance level, and rich in speleothem formations. The cave is consistently used by a crowded bat colony: the earliest known record of guano there dates back to 55 years ago (Bakalowicz 1970). Mert Elverici visited the cave on 16 Jul. 2011, 7 Oct. 2022, and 18 Nov. 2022, observing the colony in various stages (pub rearing, hibernation). The known host of L. miniopteri, the common bent-wing bat, is the dominant bat species roosting in the cave (Tarkan Yorulmaz, personal communication).
Adult mites and larvae were collected on 7 Oct. 2022 by Mert Elverici. Precision tweezers or aspirator were used to collect the mites from fresh bat guano or the water surface of a small pond on the cave floor in a gallery crowded with bats. A single adult mite was found and collected on 18 Nov. 2022 under a stone in a separate gallery free of guano. A total of 12 adults and nine larvae were collected. One adult mite among those collected 7 Oct. 2022, which turned out to be an ovigerous female, was kept alive and transported to the laboratory. Other material was preserved in 70% ethanol. The live female was placed in a glass vial (25x30 mm) filled with charcoaled Plaster-of-Paris and closed with a tight, semi-transparent lid. The rearing experiment was carried out at room temperature with a natural light/dark cycle. This female laid five eggs on 12 Oct. 2022, followed by additional 25 eggs on 17 Oct. and seven more on 21 Oct. All eggs were laid one by one and were white in color. The first prelarva was observed on 8 Nov. 2022, and from the total of 37 eggs, 17 larvae were obtained successfully. The total time between oviposition and emergence of larvae was 37 days. One postlarval mite was used for a COI sequence analysis, but this attempt was not successful. Specimens for the light microscope examination were mounted on glass slides in Hoyer's medium (Walter & Krantz 2009); postlarval mites were cleared in KOH before the mounting (Mąkol & Sevsay, 2011).
Twelve slides were examined and then deposited in the Laboratory of Parasitic Arthropods, Zoological Institute of the Russian Academy of Sciences (ZIN, St Petersburg, Russia). Their collection numbers are ZIN 18585 – 18588 (larvae obtained from the gravid female in the laboratory), ZIN 18589 (mother of these larvae), ZIN 18590 – 18593 (other adult females), and ZIN 18594 – 18596 (larvae collected in nature). The slides were examined under a Leica DM 2500 compound microscope (Leica Microsystems GmbH, Wetzlar, Germany) using differential interference contrast (DIC). Microphotographs were taken using a Leica DMC 4500 digital camera. Morphological drawings were prepared using a drawing tube. Measurements of adult mites were made on the microphotographs using the means of Adobe Photoshop CC 2014 software (Adobe Inc., San Jose, CA, US), with a prior calibration by a stage micrometer. Measurements and drawings of larvae (except drawings of legs) were prepared under a MBI-3 microscope (LOMO plc, St Petersburg, Russia) with phase contrast optics, a drawing tube, and an ocular micrometer. Count of setae on palps and legs of adult specimens was made by a preparation of schematic drawings of their segments (not published). In total, seven larvae and three adult females were examined entirely. Measurements and counts of setae were not done for two females because of the insufficient quality of the slides.
Four additional slides with larvae obtained from the egg-laying mother, 10 with larvae collected in nature, and six with postlarvae are deposited in the Acarology Laboratory of Erzincan Binali Yıldırım University (EBYU, Erzincan, Türkiye).
To compare L. miniopteri with closely related larval species of Trombiculidae, we used the following previously obtained metric and meristic data stored in electronic tables: (1) data for 45 specimens of L. montanum Stekolnikov, 2004 from the Caucasus later synonymized with L. paradux Vercammen-Grandjean & Langston, 1976 (Stekolnikov 2004); (2) data for three specimens of L. paradux from Ethiopia (Antonovskaia et al. 2024); (3) literature data for the holotype of L. paradux (Vercammen-Grandjean & Langston 1976); (4) data for 236 specimens of L. alanicum Stekolnikov, 2004 from the Caucasus and Transcaucasia; (5) data for 65 specimens of L. europaeum (Daniel & Brelih, 1959) from the Dagestan and Northeast Türkiye (Stekolnikov 2004). Each specimen was characterized by 22 metric and meristic traits. Means and ranges for the samples 1, 4, and 5 were published previously (Stekolnikov 2004, 2013). The small size of the L. miniopteri larval sample (7 specimens) did not allow the application of the discriminant analysis. Therefore, the comparison was carried out by the creation of two-dimensional scatterplots for each pair of traits. When a scatterplot gave the complete separation of two species, a rate of the two traits was constructed that allowed species discrimination. Finally, only the indices with non-overlapping intervals were kept.
We use the standard terminology, abbreviations, and diagnostic formulas for larval trombiculids summarized by Goff et al. (1982). In the description of the adult stage, we follow mainly Moniuszko et al. (2017) who, in turn, referred to Crossley (1960) and Wohltmann et al. (2007).
Results
Leptotrombidium miniopteri Feider, 1966
(Figures 1 – 6)
Diagnosis (larvae)
SIF = 7B-B-3-2111.0000; fPp = N/N/BNN; fCx = 1.1.1; fSt = 2.2; PL > AM > AL; fD = 2H-(10 – 15)-(8 – 10)-8 + (10 – 25); DS = 44 – 59; V = 33 – 35; NDV = 78 – 92; Ip = 819 – 963; color in life pale yellow, with orange eyes; scutum with broadly rounded posterior corners, posterior scutal margin nearly straight in center; sensilla (trichobothria) with ca. 8 branches in distal half; sensillary bases anterior to level of PLs by 1 – 5 µm. Standard measurements are given in Table 1.
Download as
Our material (n = 7)
Type series*
L. avonense *
Range
Mean
Holotype
Mean of 8
Range
Mean
Holotype
AW
70-86
76
73
67
72-76
74
72
PW
82-96
86
82
85
80-86
83
80
SB
25-33
31
31
31
31-35
33
32
ASB
27-32
30
27
26
27-30
28
29
PSB
14-20
17
15
14
16-18
17
17
SD
44-50
47
42
40
44-47
45
46
P-PL
12-17
14
-
-
-
-
-
AP
27-31
29
26
23
26-28
27
28
AM
47-58
55
50
38
47-54
51
51
AL
40-49
44
43
30
38-42
40
42
PL
67-74
70
60
57
54-64
59
59
S
63-63
63
72
58
70-78
74
78
H
63-72
67
60
50
57-63
59
58
Dmin
38-49
44
38
35
36-39
38
36
Dmax
61-72
66
56
45
53-61
55
54
Vmin
34-40
37
31
23
27-32
30
27
Vmax
50-60
54
46
29
44-47
45
45
pa
266-328
303
299
275
292-300
295
296
pm
252-295
275
257
247
263-272
267
266
pp
295-340
314
298
305
294-308
300
294
Ip
819-963
893
854
827
852-878
862
856
TaIIIL
83-92
86
-
-
-
-
-
TaIIIW
18-22
19
-
-
-
-
-
S1
20-27
23
28
-
22
-
-
S2
16-23
19
-
-
-
-
-
DS
44-59
50
44
36
40-44
-
-
V
33-35
34
32
34
35-40
-
-
NDV
78-92
84
76
70
79-87
-
-
Diagnosis (adult females)
Trombiculidae with deep yellow color when alive. Idiosoma constricted at level of posterior leg coxae, densely covered with barbed setae. Dorsal setae inserted on short papillae and have spoon-like dilation at their end. Eyes absent. Palp tarsus with 14 barbed setae, one thin solenidion (ω) laterally, and five eupathidia (ζ) distally. Palp tibia with odontus (claw), four terminally rounded, spatulate paradonts medially (three distal and one proximal), two or three nude setae in distal part of segment (at level of paradonts), and 7 – 10 barbed setae in proximal part; total number of setae 10 – 12 (excluding paradonts). Tectum nearly round, with few minute tectal teeth. Sensilla flagelliform, with short spike-like setulae basally and multiple longer branches in distal part. Sensillary area concave anteriorly, posterior apodeme triangular, with anchor-like crest at end. Standard measurements are given in Table 2.
Download as
ZIN 18589
ZIN 18590
ZIN 18591
Ta I (L)
271
293
318
Ta I (W)
114
116
129
Ti I
169
200
200
Ge I
147
168
157
tFe I
134
150
161
bFe I
149
171
182
Tr I
109
104
104
Cx I
200
214
236
Ta II
183
211
225
Ti II
102
118
118
Ge II
95
104
111
tFe II
90
104
107
bFe II
120
132
139
Tr II
92
96
100
Cx II
136
132
143
Ta III
179
214
229
Ti III
113
118
118
Ge III
89
96
104
tFe III
88
100
104
bFe III
116
136
143
Tr III
63
111
111
Cx III
170
171
175
Ta IV
214
257
268
Ti IV
128
150
154
Ge IV
108
125
136
tFe IV
110
129
136
bFe IV
141
161
171
Tr IV
124
139
150
Cx IV
163
186
189
SB
56
60
59
PSL
54
70
72
ASL
209
230
254
CTL
284
-
357
S
149
-
184
T
48
-
-
Odo
40
43
43
PTa (L)
65
74
76
PTa (W)
27
27
27
PTi
79
88
90
PGe
92
108
110
PFe (L)
133
155
153
PFe (W)
72
84
86
PTr
54
63
61
Cs
99
112
113
Cb (L)
217
225
229
Cb (W)
62
68
68
Dmin
31
43
-
Dmax
67
83
-
Description of larva



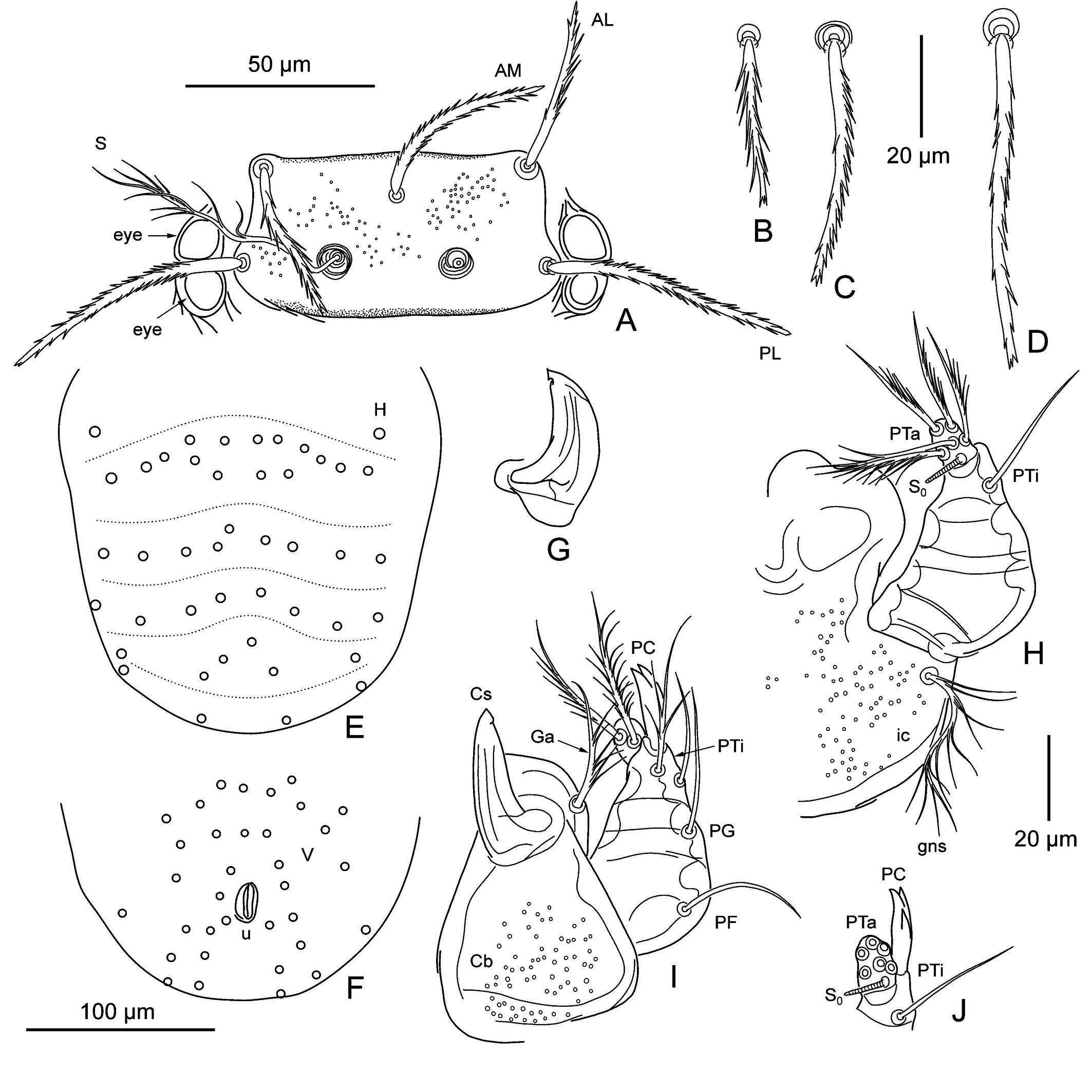


Idiosoma — (Figures 1B, C, 2B – F). Color in life pale yellow; eyes 2 + 2, color in life orange; 44 – 59 moderately barbed dorsal idiosomal setae, including two clearly separated humeral setae; 1st posthumeral row (C excluding humeral setae) with 10 – 15 setae, 2 – 4 of its setae inserted posterior to main row, in its central part; 2nd row (D) with 8 – 10 setae; 3rd (E) row with 8 setae; remaining caudal setae 10 – 25 in number; two sternal setae between coxae I and two between coxae III; 33 – 35 ventral setae; NDV = 78 – 92.
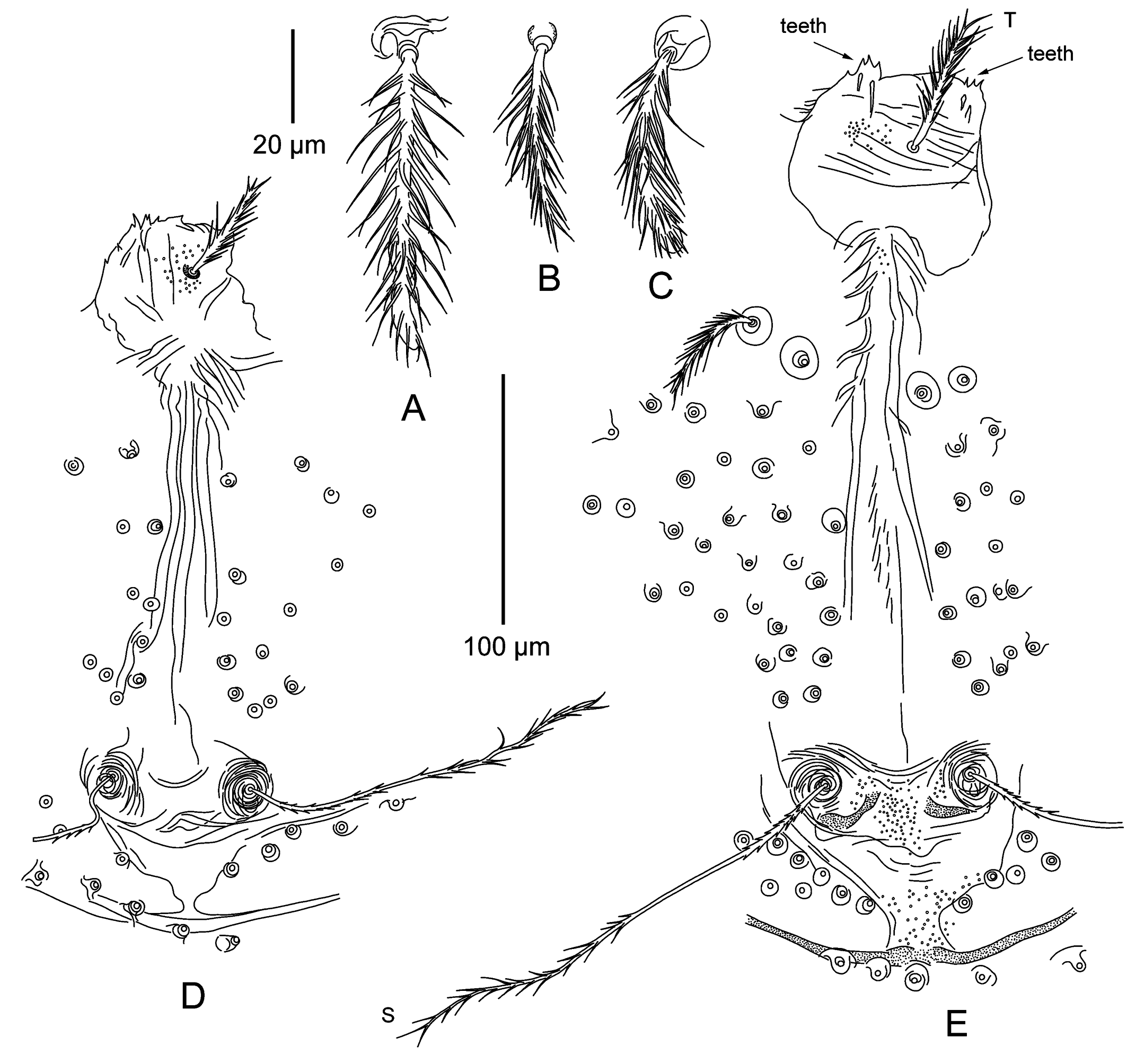
Gnathosoma — (Figure 2G – J). Cheliceral blade with tricuspid cap; cheliceral base moderately punctate; gnathobase (infracapitulum) moderately punctate, bears one pair of branched gnathocoxal (tritorostral) setae; galeal (deutorostral) seta branched; palp claw (odontus) with three prongs, two subequal terminal and one in middle part of claw; palp femoral and genual setae nude, dorsal palp tibial seta branched, lateral and ventral setae nude; palp tarsus with seven branched setae and tarsala (ω).
Scutum — (Figures 1B, 2A). Rectangular, with small and rather sparse puncta visible mainly in its anterior part, with broadly rounded posterior corners, posterior scutal margin nearly straight in center; all scutal setae barbed similarly to dorsal idiosomal setae; AM placed posterior to level of ALs; PL > AM > AL; sensillary bases anterior to level of PLs by 1 – 5 µm; sensilla (trichobothria) with ca. 8 branches in distal part.
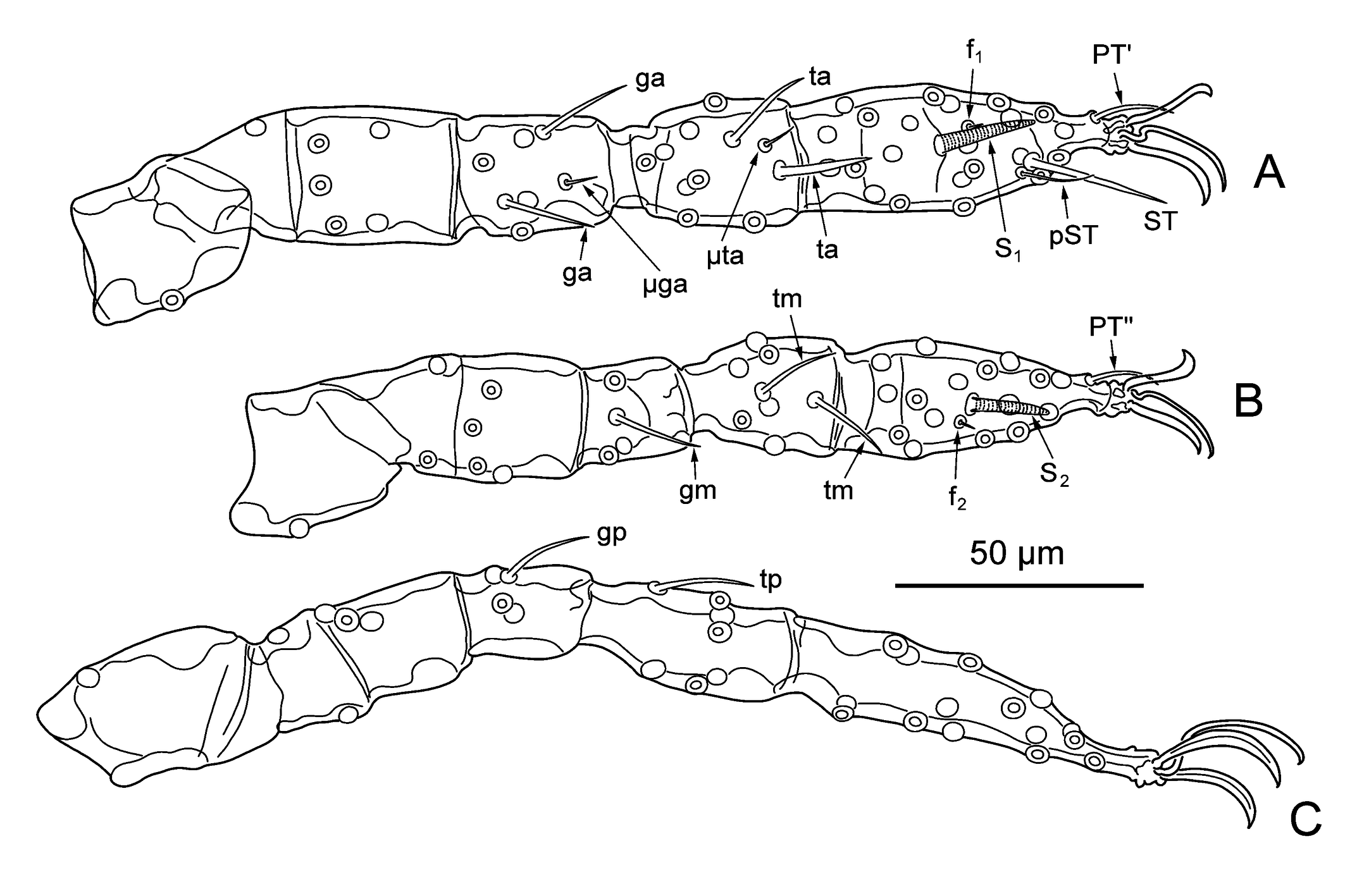


Legs — (Figure 3). Seven-segmented (with divided femur), with one pair of claws and claw-like empodium. Specialized setae: Leg I: 2 genualae (σ), microgenuala (κ), 2 tibialae (φ), microtibiala (κ), tarsala (ω), famulus (ε) distal to tarsala, subterminala (ζ), parasubterminala (z), pretarsala (ζ). Leg II: genuala (σ), 2 tibialae (φ) in tandem, tarsala (ω), famulus (ε) proximal to tarsala, pretarsala (ζ). Leg III: genuala (σ), tibiala (φ). Unspecialized setae (legs I, II, III): coxae 1, 1, 1; trochanters 1, 1, 1; basifemora 1, 2, 2; telofemora 5, 4, 3; genua 4, 3, 3; tibiae 8, 6, 6; tarsi 22, 16, 15 (all branched).
Remarks
Three larvae collected in nature have the palp femoral seta forked on one side. They also differed from the four examined larvae produced by the female ZIN 18589 by longer leg tarsalae (ω) (S1 24 – 27 vs. 20 – 21 and S2 21 – 23 vs. 16 – 18) and lesser number of setae in 1st posthumeral row (10 – 13 vs. 14 – 15).
According to the description of L. avonense Vercammen-Grandjean & Langston, 1976, this species differs from L. miniopteri by more strongly barbed body setae, shorter leg tarsala I (S1 22 vs. 28), more prominent posterior scutal margin (characterized by the measurement PSB), and a little larger but more sparse puncta of the scutum (Vercammen-Grandjean & Langston 1976). However, intervals for S1 and PSB in our material overlap those for the type series of both species (Table 1). Therefore, L. avonense can probably be synonymized with L. miniopteri. Re-examination of type series is necessary to confirm the identity of these species. Their comparison with a very similar American bat chigger L. myotis (Ewing, 1929) and other species of the species group myotis (Stekolnikov 2013) would also be desirable.
Leptotrombidium miniopteri can be separated from three closely related species, L. paradux, L. europaeum, and L. alanicum, first of all, by a longer leg III tarsus in relation to the whole leg (ratio pp/TaIIIL) (Fig. 7, Table 4). The absolute length of leg III tarsus (TaIIIL) is also higher in L. miniopteri than in L. alanicum (Table 4). The next difference is longer PLs in relation to the distance between them (ratio PW/PL). In addition, L. miniopteri differs from L. paradux by a lesser number of ventral idiosomal setae (V) and a lesser distance between sensillary bases (SB); from L. europaeum, by a lesser SB in relation to the lengths of setae (ratios AL/SB, PL/SB, and H/SB) and to the length of leg III tarsus (TaIIIL/SB). Some additional ratios allowing discrimination of L. miniopteri are given in Table 4.
Description of adult female







Idiosoma — (Figures 4A – C, 6A – C). Color in life deep yellow. Constricted at level of posterior leg coxae, densely covered with setae. All setae densely covered with long barbs, caudal setae longest. Dorsal setae inserted on short papillae and have spoon-like dilation at their end. Eyes absent. Genital area with three pairs of genital suckers (acetabula) and surrounded by paired valves covered with barbed setae. Anal orifice surrounded by paired valves covered with barbed setae.
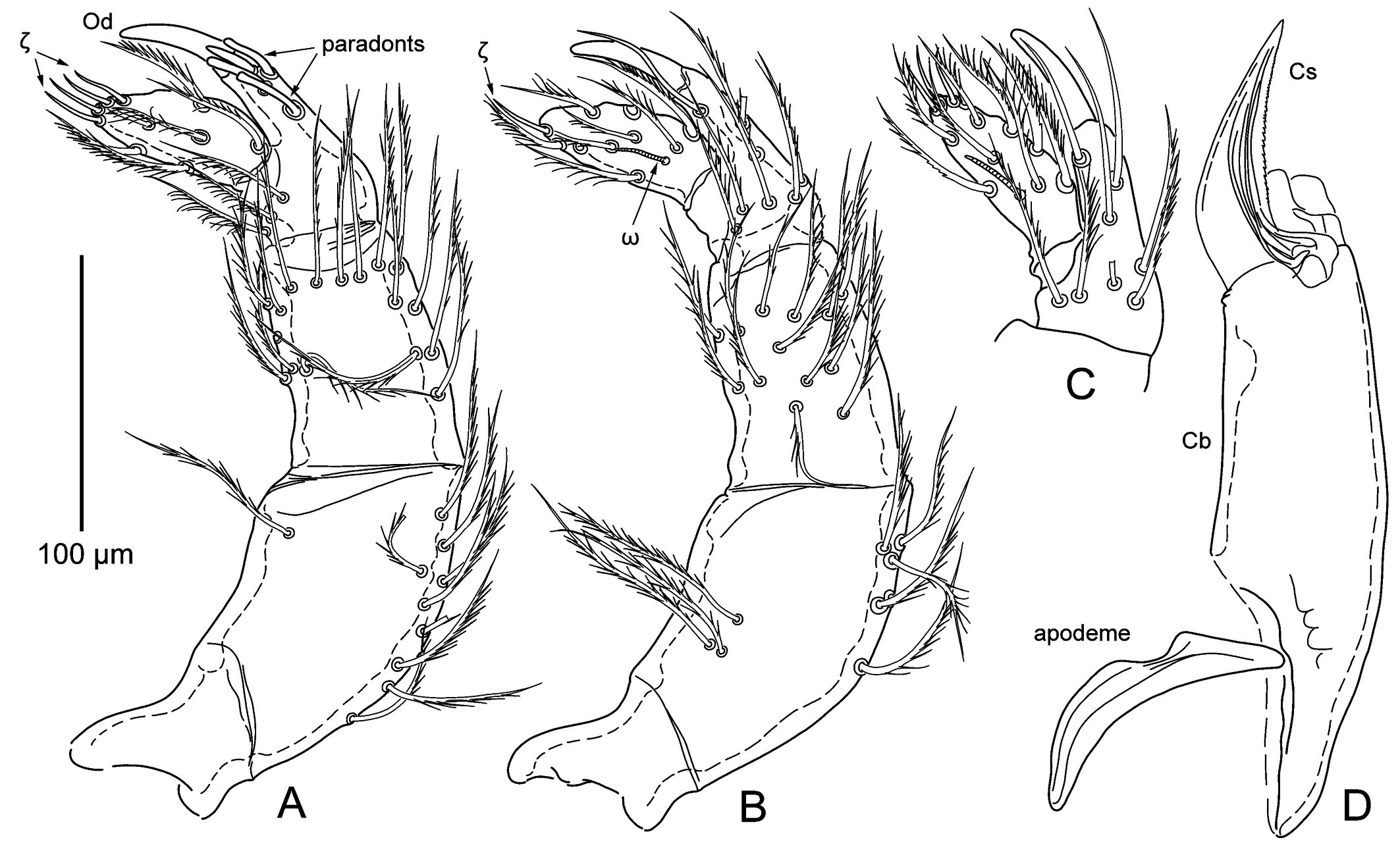
Palps — (Figure 5A – C). Palp tarsus with 14 barbed setae, one thin solenidion (ω) laterally and five eupathidia (ζ) distally. Palp tibia with odontus (claw), four paradonts medially (distal lanceolate with a blunt end, two spatulate on sides of it, and one spatulate proximally, at distance from other three), two or three nude setae in distal part of segment (at level of paradonts) and 7 – 10 barbed setae in proximal part; total number of setae 10 – 12 (excluding paradonts). Palp genu with 30 – 50 barbed setae, palp femur with 19 – 29 setae (Table 3). Barbs of setae long and thin.
Chelicerae — (Figure 5D). Cheliceral base long, rather slender; area of articulation slightly shorter than half of cheliceral base (excluding pseudochela); cheliceral blade with distinct bend ventrally, evenly tapering to end, with minute serration on dorsal edge; cheliceral apodeme evenly curved, with anterior concavity in center.
Crista metopica — (Figures 4D, 6 D – E). Tectum nearly round, with few minute tectal teeth. Tectal seta covered with long thin barbs. Sensilla flagelliform, with short spike-like setulae basally and multiple longer branches in distal part. Sensillary area concave anteriorly, posterior apodeme triangular, with anchor-like crest at end. Multiple body setae surround crista and sensillary area.
Legs — (Figure 4E). Seven-segmented (with divided femur), all segments covered with multiple barbed setae, tarsi with pair of claws. Specialized setae: Leg I: tFe 15 – 22 ζ on dorsal side; Ge 4 – 6 σ and 63 – 89 ζ on dorsal side and upper halves of lateral sides; Ti 15 – 27 φ and 61 – 71 ζ over entire surface and 1 κ at distal end; Ta at least 73 ω and at least 83 ζ (total number of ω + ζ = 248) over entire surface and 1 ε in distal part of segment. Leg II: tFe 1 – 3 ζ on dorsal side; Ge 12 – 17 ζ on dorsal side; Ti 1 – 2 φ and 9 – 10 ζ on dorsal side; Ta 5 – 9 ω, 1 ζ (near claws laterally), and 1 ε in middle part of segment. Leg III: tFe 1 – 2 ζ on dorsal side; Ge 8 – 13 ζ on dorsal side; Ti 9 – 13 ζ on dorsal side; Ta 1 – 2 ω and 1 ζ on dorsal side. Leg IV: tFe 1 – 2 ζ at distal end dorsally; Ge 10 – 11 ζ on dorsal side; Ti 12 – 20 ζ on dorsal side; Ta 1 ω and 6 – 8 ζ on dorsal side (Table 3).
Download as
Segment
ZIN 18589
ZIN 18590
ZIN 18591
N. vulgaris
PFe
19
24
29
-
PGe
30
39
50
-
PTi
10 (1 broken) + 1N
7 + 3N / 8 + 2N
10 + 2N / 8 + 2N
10 + 1N (basidont)
tFe I
15ζ
19ζ
22ζ
1ζ
Ge I
4σ, 65ζ
6σ, 63ζ
?σ, 89ζ
ca. 33σ
Ti I
15φ, 61ζ, 1κ
21φ, 67ζ, 1κ
27φ, 71ζ, 1κ
ca. 35φ
Ta I
-
-
73ω, 83ζ, 92?
ca. 50ω, ca. 22ζ
tFe II
1ζ
3ζ
3ζ
1ζ
Ge II
12ζ
13ζ
17ζ
6σ
Ti II
1φ, 9ζ
2φ, 8ζ
1φ, 10ζ
5φ, 1ζ
Ta II
5ω, ?ζ, 1ε
9ω, 1ζ, 1ε
8ω, 1ζ, 1ε
6ω
tFe III
-
2ζ
1ζ
1ζ
Ge III
-
8ζ
13ζ
4σ
Ti III
-
9ζ
13ζ
3φ, 1ζ
Ta III
-
1ω, 1ζ
2ω, 1ζ
4ω
tFe IV
-
2ζ
1ζ
1ζ
Ge IV
-
10ζ
11ζ
4σ
Ti IV
-
12ζ
20ζ
6φ, 1ζ
Ta IV
-
1ω, 6ζ
1ω, 8ζ
6ω, 1ζ
Discussion
Morphology of larvae
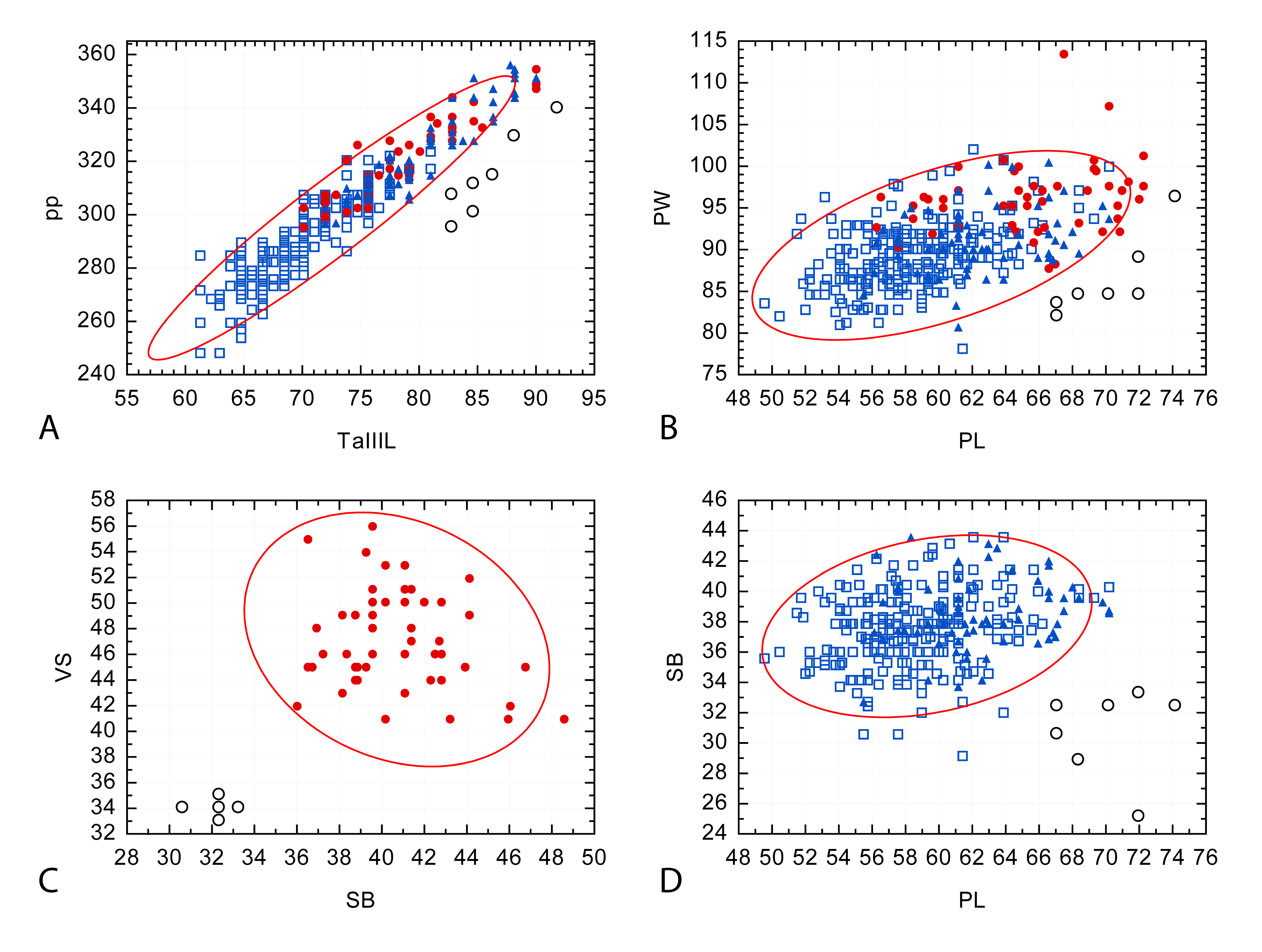


Using the key to Leptotrombidium species of the world compiled by Stekolnikov (2013), we initially identified the four larvae reared in laboratory as L. paradux. However, identification using the key published by Vercammen-Grandjean & Langston (1976) failed. Application of the discriminant functions created by Stekolnikov (2004) for the separation of L. paradux (syn.: L. montanum), L. europaeum, and L. alanicum led to identification of two specimens as L. alanicum, form 3 (described from Armenia and Azerbaijan), and two as L. europaeum, form 5 (Dagestan). In addition, the examination of three more larvae (collected in nature) showed that they have 10, 11, and 13 setae in the 1st posthumeral row vs. 12 – 21 in L. paradux. An additional search for species similar to our material and described as having 10 setae in 1st posthumeral row led us to the group of closely related species parasitizing bats and designated by Stekolnikov (2013) as the species group myotis. Among its members, L. miniopteri seems the most similar to our specimens, judging from the re-descriptions and figures of the group members published by Vercammen-Grandjean & Langston (1976). At the same time, examination of the differences between our specimens and L. paradux, L. europaeum, and L. alanicum with the use of two-dimensional scatterplots revealed a series of indices allowing reliable separation of our material from all of them (Fig. 7, Table 4).
Download as
Mean
Std.Dev.
Range
Quartile range
Mean
Std.Dev.
Range
Quartile range
L. miniopteri (n = 7)*
L. paradux (n = 49)**
SB
30.73
2.87
25-33
28.8-32.4
40.71
2.78
36-49
38.9-42.5
V
34.00
0.71
33-35
34.0-34.0
47.16
3.84
41-56
45.0-50.0
pp/TaIIIL
3.66
0.07
3.55-3.73
3.57-3.72
4.08
0.12
3.86-4.36
4.00-4.15
PW/PL
1.23
0.04
1.18-1.30
1.21-1.25
1.47
0.11
1.30-1.70
1.39-1.57
NDV/PL
1.20
0.03
1.16-1.24
1.18-1.22
1.57
0.14
1.35-1.96
1.45-1.65
PL/P-PL
4.93
0.54
4.34-5.73
4.44-5.57
3.40
0.39
2.62-4.32
3.10-3.65
NDV/TaIIIL
1.00
0.05
0.94-1.06
0.98-1.02
1.30
0.11
1.12-1.61
1.23-1.35
L. miniopteri (n = 7)*
L. europaeum (n = 65)
pp/TaIIIL
3.66
0.07
3.55-3.73
3.57-3.72
4.04
0.10
3.86-4.20
3.98-4.12
PW/PL
1.23
0.04
1.18-1.30
1.21-1.25
1.46
0.09
1.31-1.63
1.39-1.53
DS/PW
0.57
0.03
0.54-0.61
0.57-0.58
0.46
0.04
0.39-0.54
0.44-0.49
AL/SB
1.45
0.08
1.35-1.57
1.36-1.50
1.07
0.07
0.89-1.26
1.03-1.12
PL/SB
2.30
0.26
2.07-2.86
2.16-2.38
1.63
0.12
1.32-1.83
1.55-1.73
H/SB
2.21
0.29
1.94-2.79
1.97-2.38
1.66
0.12
1.27-1.86
1.59-1.75
TaIIIL/SB
2.83
0.40
2.54-3.64
2.56-3.06
2.08
0.15
1.76-2.42
1.97-2.19
L. miniopteri (n = 7)*
L. alanicum (n = 236)
TaIIIL
85.89
3.24
83-92
82.8-88.2
69.30
3.95
61-81
66.6-72.0
pp/TaIIIL
3.66
0.07
3.55-3.73
3.57-3.72
4.16
0.12
3.88-4.65
4.08-4.24
PL/V
2.06
0.12
1.97-2.25
1.97-2.12
1.40
0.16
0.98-1.79
1.29-1.52
AL/SB
1.45
0.08
1.35-1.57
1.36-1.50
1.04
0.08
0.83-1.35
0.99-1.09
TaIIIL/V
2.48
0.08
2.42-2.62
2.44-2.49
1.66
0.19
1.22-2.22
1.53-1.80
The difference between the four larvae belonging to the offspring of one female and the three larvae collected in nature cannot be estimated as a difference between species. We believe that it is a case of individual variation enhanced by the effect of family resemblance. Previously, Goksu et al. (1960) investigated morphological variation among 196 larvae, the offspring of 11 different mated pairs of laboratory-reared adults obtained, in turn, as larvae from a rodent host and identified as Leptotrombidium akamushi (Brumpt, 1910). They recorded significant variation in a series of chaetotaxic traits traditionally considered stable taxonomic characters. Noteworthy is the case of forked palpal femoral seta (Goksu et al. 1960, fig. 1, 5), which looks identical with the setae observed by us in the larvae collected in nature. The average number of dorsal body setae was shown to vary significantly more in their material from family to family than within single families. This effect can explain the high number of setae in the 1st posthumeral row (14 – 15) in our laboratory-reared larvae, which could be a rare variant in the population as a whole.
Morphology of adult females
Published diagnoses of adult trombiculids are rare, and only a small part of them include detailed descriptions. As a result, distinguishing between intraspecific variation and differences at the species or even genus level can be almost impossible. Further accumulation of descriptions is necessary to develop a taxonomy of Trombiculidae at the adult stage. In our case, only one female (ZIN 18589) can be indubitably identified as L. miniopteri, based on examination of its larval offspring. Collection of other adults at the same place does not prove their conspecificity because the cases of joint occurrence of different chigger species on one bat host (Stekolnikov et al. 2022) suggests that several trombiculid species can inhabit the same cave. Among three examined adult females, ZIN 18589 is the smallest and ZIN 18591 is the largest specimen (Table 2). The difference in size correlates with the number of unspecialized setae on palps and specialized setae on legs (Table 3). Therefore, these differences of setation can be estimated as a size-dependent intraspecific variation, until proven otherwise. Sensilla of the specimen ZIN 18591 differ from those of the specimen ZIN 18589 by the presence of a middle part lacking of branches (Figure 6).
Since we did not find a complete description of adult Leptotrombidium in the literature, we compare our results first with a description of adult Neotrombicula vulgaris (Schluger, 1955), a species from another genus published by Moniuszko et al. (2017). The setation of palp tarsus in adult L. miniopteri was found to be identical with that of adult N. vulgaris. The setation of palp tibia was different by the presence of more than one nude seta (one seta is broken in the specimen ZIN 18589, so it is not clear if it is nude or barbed). We see no shape differences between the most distal nude seta defined as basidont by these authors and one or two additional nude setae. They also designated the most proximal paradont as a ''spike-like seta″; however, this seta does not differ from other paradonts neither in their drawing of N. vulgaris, nor in our specimens of L. miniopteri.
Womersley (1952) described and illustrated adult stages of three species currently included in the genus Leptotrombidium – L. akamushi, L. deliense (Walch, 1922), and L. pallidum (Nagayo et al. 1919). He did not describe the setation of palp tibiae in these species, except for indicating the presence of three paradonts (termed ''accessory spines″). Fourth, proximal paradont, was mentioned only for L. akamushi, but it is present in the figures of all three species. The three nude distal setae, identical by their shape with proximal branched setae, can be clearly recognized in the drawings of the lateral side of the palpal tibia for all three species (Womersley 1952, Plate 90). Thus, we can assume that presence of these setae is a common trait of Leptotrombidium.
The specialized setae on leg tarsi of examined adult specimens are definitely divided into two main types: (1) thick, rod-like (not pointed at their end), with visible sculpture on their surface; (2) thin, smooth, pointed at their end. Following Moniuszko et al. (2017), we designated setae of the first type as tarsal solenidia (ω) and setae of the second type as eupathidia (ζ). We also recorded setae of both these types on tibiae I and II and on genu I. We designated setae of the first type as tibial or genual solenidia (φ or σ), respectively, and setae of the second type as eupathidia (ζ). Moniuszko et al. (2017) recorded both φ and ζ on tibiae II – IV, but only σ on genua I – IV (Table 3). We also found one microchaeta on tarsus II and one on tibia I; we designated them as ε and κ, respectively, like the famuli on tarsi I and II and the microchaeta on tibia I in larval trombiculids.
The nomenclature of specialized leg setae in larval trombiculids does not strictly follow the two main types of setae defined above. Thus, specialized setae on genua and tibiae (excluding microchaetae) are always classified as solenidia (σ and φ, respectively), although they are usually smooth, thin and pointed. Only distal tibiala I or rarely both tibialae I can be rod-like and ''striated″, i.e. having multiple transverse rows of micropores (Vercammen-Grandjean 1965a; Stekolnikov 2022). Contrary to postlarval instars, larval trombiculids never have specialized setae on telofemora.
Acknowledgements
This study is a part of the outcomes of cave exploration and registration projects conducted for conservation and management purposes, supported by the Ministry of Environment, Urbanization, and Climate Change, Directorate of Protection of Natural Assets, Republic of Türkiye. We also extend our gratitude to the professional cavers affiliated with Kaşif Consulting, Reporting, and Organization Company (Ankara, Türkiye), as well as the sportive cavers from the Turkish Caving Federation, for their invaluable assistance in organizing and implementing the fieldwork. We thank Dr. Tarkan Yorulmaz (Department of Biology, Çankırı Karatekin University, Türkiye), who accompanied Mert Elverici during the field study. We thank two anonymous reviewers for the careful review of the manuscript and useful suggestions. This research was supported by the cooperative agreement No. 125013001089-0 from the Ministry of Science and Higher Education of the Russian Federation (to Alexandr A. Stekolnikov) and by the Scientific Research Foundation of Erzincan Binali Yıldırım University Scientific Research Projects (EUBAP, Project number: FBA-2024-965, to Rümeysa Karağaç and Sevgi Sevsay).
References
- Antonovskaia A.A., Lushchekina A.A., Lavrenchenko L.A., Stekolnikov A.A. 2023 (2024). On the chigger mite (Acariformes: Trombiculidae) fauna of Ethiopia. Int. J. Acarol., 50 (1): 53-67. https://doi.org/10.1080/01647954.2023.2293878
- Bakalowicz M. 1970. Campagne spéléologique 1970 en Taurus occidental expedition française en Turquie. Grottes et gouffres, Bull. périodique du Spéléo-club de Paris. 45: 15-26.
- Crossley D.A., Jr. 1960. Comparative external morphology and taxonomy of nymphs of the Trombiculidae (Acarina). Univ. Kansas Sci. Bull., 40 (6): 135-321.
- de la Cruz J., Socarras A.A. 1993. Los generos Ischnothrombium y Tectumpilosum (Acarina: Trombiculidae) en Cuba. Poeyana, 437: 1-14.
- Daniel M., Stekolnikov A.A. 2002. New data on chigger mites of the subfamily Leeuwenhoekiinae (Acari: Trombiculidae) parazitizing bats in Cuba. Acarina, 10 (2): 149-154.
- Feider Z. 1966. Nouvelles larves de Trombiculides (Acariformes) parasites sur des petites mammifères. Zool. Anz., 176: 106-123.
- Feider Z. 1983a. Seconde contribution à la connaissance des Trombiculoidea cavernicoles de Cuba. In: Orghidan T. et al. (Eds). Résultats des expéditions biospéologiques cubano-roumaines à Cuba. Vol. 4. Bucureşti: Editura Academiei Republicii Socialiste România. pp. 115-138.
- Feider Z. 1983b. Un Leeuwenhoekiidae (Acariformes) cavernicole collecté à Cuba. In: Orghidan T. et al. (Eds). Résultats des expéditions biospéologiques cubano-roumaines à Cuba. Vol. 4. Bucureşti: Editura Academiei Republicii Socialiste România. pp. 149-153.
- Goff M.L., Loomis R.B., Welbourn W.C., Wrenn W.J. 1982. A glossary of chigger terminology (Acari: Trombiculidae). J. Med. Entomol., 19 (3): 221-238. https://doi.org/10.1093/jmedent/19.3.221
- Goksu K., Wharton P.W., Yunker C.E. 1960. Variation in populations of laboratory-reared Trombicula (Leptotrombidium) akamushi (Acarina: Trombiculidae). Acarologia, 2 (2): 199-204.
- Kunt K.B., Yağmur E.A., Özkutuk S., Durmuş H., Anlaş S. 2010. Checklist of the cave dwelling Invertebrates (Animalia) of Türkiye. Biol. Div. Conserv., 3(2): 26-41.
- Mąkol J., Cichocki J., Felska M., Kłosińska A., Łaydanowicz J., Łupicki D., Gabryś G. 2010. A new data on biology and taxonomy of Neotrombicula inopinata (Oudemans, 1909) and Leptotrombidium russicum (Oudemans, 1902) (Acari: Actinotrichida: Trombiculidae). Ann. Zool., 60 (3): 419-427. https://doi.org/10.3161/000345410X535406
- Mąkol J., Sevsay S. 2011. Notes on the genus Dolichothrombium (Acari: Prostigmata: Trombidiidae) with description of a new species. Zootaxa, 2971: 1-16. https://doi.org/10.11646/zootaxa.2971.1.1
- MoEUCC (The Ministry of Environment, Urbanization and Climate Change of the Republic of Türkiye). 2023. Konya Beyşehir, Hacı Akif Adası Mağarası′nın Tabiat Varlığı Olarak Tescili; [9 Apr 2025]. Available from: https://webdosya.csb.gov.tr/db/tabiat/duyurular/ek_ilan_haci_akif_adasi_magarasi_20231020114928.pdf
- Moniuszko H., Shatrov A.B., Mąkol J. 2017. Description of active post-larval forms of Neotrombicula vulgaris (Schluger, 1955) (Prostigmata: Trombiculidae), with notes on biology and ecology of the species. Ann. Zool., 67 (2): 243-251. https://doi.org/10.3161/00034541ANZ2017.67.2.005
- Nielsen D.H., Robbins R.G., Rueda L.M. 2021. Annotated world checklist of the Trombiculidae and Leeuwenhoekiidae (1758-2021) (Acari: Trombiculoidea), with notes on nomenclature, taxonomy, and distribution. Zootaxa, 4967: 1-243. https://doi.org/10.11646/zootaxa.4967.1.1
- Southcott R.V., Frances S.P. 1991. The life history stages of Odontacarus (Leogonius) barrinensis (Womersley) (Acari: Trombiculidae: Leeuwenhoekiinae). Int. J. Acarol., 17 (4): 275-287. https://doi.org/10.1080/01647959108683919
- Stekolnikov A.A. 2004. Variability in Leptotrombidium europaeum and two new related chigger mite species (Acari: Trombiculidae) from Caucasus. Parazitologiya, 38: 388-405 [in Russian].
- Stekolnikov A.A. 2013. Leptotrombidium (Acari: Trombiculidae) of the World. Zootaxa, 3728: 1-173. https://doi.org/10.11646/zootaxa.3728.1.1
- Stekolnikov A.A. 2022. A new genus and species of bat chiggers (Acariformes: Trombiculidae) from Kenya. Acarologia, 62 (2): 418-425. https://doi.org/10.24349/5n37-k5b9
- Stekolnikov A.A., Quetglas J., Ibáñez C., Sánchez-Navarro S. 2022. Contribution to the fauna of chiggers (Acariformes: Trombiculidae) parasitizing bats in Spain. Acarologia, 62 (4): 1201-1209. https://doi.org/10.24349/qojp-y0ow
- Vercammen-Grandjean P.H. 1958. Revision du genre Schoutedenichia Jad. et Verc. Ann. Mus. Roy. Congo Belg., sér. 8, 65: 1-103.
- Vercammen-Grandjean P.H. 1965a. On the true nature of the ''striate'' solenidion in chiggers (Trombiculidae: Acarina). Acarologia, 7 (2): 318-320.
- Vercammen-Grandjean P.H. 1965b. Revision of the genera: Eltonella Audy, 1956 and Microtrombicula Ewing, 1950, with descriptions of fifty new species and transferal of subgenus Chiroptella to genus Leptotrombidium (Acarina, Trombiculidae). Acarologia, 7 (suppl.): 34-257.
- Vercammen-Grandjean P.H., Langston R.L. 1976. The chigger mites of the World (Acarina: Trombiculidae et Leeuwenhoekiidae). Vol. III. Leptotrombidium complex. San Francisco: George Williams Hooper Foundation, University of California. pp. 1061.
- Walter D.E., Krantz G.W. 2009. Collection, rearing and preparing specimens. In: Krantz G.W., Walter D.E. (Eds.). A Manual of Acarology. 3rd edition. Lubbock: Texas Tech University Press. pp. 83-96.
- Wohltmann A., Gabryś G., Mąkol J. 2007. Terrestrial Parasitengona inhabiting transient biotopes. In: Gerecke R. (Ed.). Suswasserfauna Mitteleuropas. Vol. 7/2-1, Chelicerata, Acari I. Munchen: Elsevier Spektrum Akademischer Verlag. pp. 158-240. https://doi.org/10.1007/978-3-662-55958-1_6
- Womersley H. 1952. The scrub-typhus and scrub-itch mites (Trombiculidae, Acarina) of the Asiatic-Pacific region. Rec. South Aust. Mus., 10: 1-435.



2025-01-29
Date accepted:
2025-04-14
Date published:
2025-04-16
Edited by:
Auger, Philippe

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Stekolnikov, Alexandr A.; Karağaç, Rümeysa; Sevsay, Sevgi and Elverici, Mert
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



