Functional response of two native species Typhlodromus khosrovensis and Euseius amissibilis (Acari: Phytoseiidae) feeding on eggs of Tetranychus urticae (Acari: Tetranychidae)
Afkhami, Maryam  1
; Farazmand, Azadeh
1
; Farazmand, Azadeh  2
; Rahmani, Shima
2
; Rahmani, Shima  3
and Golpayegani, Azadeh Zahedi
3
and Golpayegani, Azadeh Zahedi  4
4
1Department of Plant Protection, Science and Research Branch, Islamic Azad University, Tehran, Iran.
2✉ Department of Agricultural Zoology, Iranian Research Institute of Plant Protection, Agricultural Research, Education and Extension Organization (AREEO). Tehran, Iran.
3Department of Plant Protection, Science and Research Branch, Islamic Azad University, Tehran, Iran.
4Department of Plant Protection, Science and Research Branch, Islamic Azad University, Tehran, Iran & Department of Plant Protection, Faculty of Agriculture, University of Tehran, Karaj, Iran.
2025 - Volume: 65 Issue: 2 pages: 497-504
https://doi.org/10.24349/7hph-sayxOriginal research
Keywords
Abstract
Introduction
The two-spotted spider mite Tetranychus urticae Koch (Acari: Tetranychidae) is one of the most serious agricultural pests found in both field and greenhouse crops in various agroecosystems worldwide. This mite damages over 1,100 plant species from 140 families, with more than 150 of these plant species, having significant economic value (Takafuji 1994; Bolland et al. 1998; Migeon et al. 2011). Indeed, T. urticae poses a significant threat to various crops, including vegetables, large trees, horticultural plants, and deciduous fruit trees (Oliveira et al. 2007; Migeon et al. 2010).
Considering the evolution of pesticide resistance, the use of biological control agents is a necessary method against T. urticae all over the world (Gerson and Weintraub, 2007; Gerson, 2014; Amoah et al. 2016; Gigon et al. 2016). Predatory mites in the family Phytoseiidae are highly effective biological control agents. They play a critical role in controlling small-bodied pests such as spider mites, thrips, whiteflies, and nematodes in crops (Williams et al. 2004; Rahmani et al. 2009). Extensive research has been conducted on the distribution, ecology, biology, and behavior of this family in Iran (Hajiqanbar and Farazmand, 2021). Most studies have focused on the behavioral aspects and life table parameters of the phytoseiid species (Seiedy et al. 2012; Solymani et al. 2016; Fathipour et al. 2017; 2018; 2020; Dalir et al. 2021). However, there is limited information available about the biology and behavior of native phytoseiids (Ganjisaffar et al. 2011). Until now, the distribution of 76 native species in Iran has been recorded (Hajizadeh and Faraji 2016; Demite et al. 2024), but only four species, namely Typhlodromus (Anthoseius) bagdasarjani Wainstein and Arutunjan, Phytoseius plumifer (Canestrini & Fanzago), Amblyseius herbicolus Chant and Neoseiulus barkeri Hughes were considered in the biological and behavioral studies (Notghi Moghadam et al. 2010; Ganjisaffar et al. 2011; Jafari et al. 2012; Farazmand et al. 2012; Moghadasi et al. 2014; Riahi et al. 2016).
Functional response is an ecological parameter used to evaluate predatory mites' efficiency. It is often applied to estimate the effectiveness of natural enemies before releasing them (Xiao & Fadamiro, 2010). This parameter illustrates important information about the voracity of a biocontrol agent. It can be affected by abiotic factors such as temperature, experimental units, and applying pesticides, as well as biotic factors including prey species, prey stage, host plant, natural enemies' generation, and age (De Clercq et al. 2000; Mohaghegh et al. 2001; Skirvin and Fenlon 2001; Mahdian et al. 2006; Li et al. 2007). Holling (1959) was the first one who propose a functional response and classified it into three types. Most phytoseiids have shown functional response type II (Castagnoli and Simoni, 1999; Cedola et al. 2001; Reis et al. 2003; Ahn et al. 2010) in which the number of consumed prey approaches the asymptote hyperbolically as prey density increases. In type III, the number of consumed prey approaches the asymptote as a sigmoid function and the proportion of consumed prey exhibits a positive density dependence over some regions of prey density. Although functional response type III has also been recorded in different ages of some phytoseiid species (Fathipour et al. 2017; 2018).
Numerous studies in Iran have dealt with this aspect of phytoseiid mites which are important commercially (Fathipour et al. 2017; 2018; 2020). On the other hand, studies held on native phytoseiids are rare (Kouhjani Gorji et al. 2009; Farazmand et al. 2012; Jafari et al. 2012).
During a study on phytoseiids of Tehran city, three species were identified namely Typhlodromus (Anthoseius) khosrovensis Arutunjan, Euseius amissibilis Meshkov, and T. bagdasarjani (Afkhami et al. 2024). The functional response of T. bagdasarjani, as well as biology, behavioral characteristics, and its interaction with other predators have already been studied (Ganjisaffar et al. 2011; Farazmand et al. 2012, 2015; Moghadasi et al. 2014; Jafarian et al. 2022). van Houten et al. (2016) reported that E. amissibilis has the potential to control whiteflies and thrips. However, there are no studies about the biology and behavioral/ecological features of E. amissibilis and T. khosrovensis while feeding on T. urticae.
In this study, two phytoseiid mites T. khosrovensis and E. amissibilis were collected from mulberry trees in Tehran city, Iran to evaluate their functional response when preying on T. urticae eggs. Therefore, the potential of both phytoseiid species as a native biological control agent could be explored.
Materials and methods
Stock prey colony
The colony of Tetranychus urticae used in this study was obtained from the Department of Agricultural Research Zoology, Iranian Research Institute of Plant Protection, Tehran, Iran. The mites were reared on lima bean (Phaseolus vulgaris) at 25 ± 1 °C, 60–70% RH, and a photoperiod of 16:8 h (L: D).
Collecting and rearing predators
Typhlodromus khosrovensis, and E. amissibilis were collected from mulberry tree leaves in Chitgar Forest Park and Shad Park, respectively in Tehran, Iran, in 2023. For rearing both predator species, they were transferred into an arena consisting of a plastic sheet putting on water-saturated foam in a Plexiglas box (26 × 18 × 10 cm), which was half filled with water. The edges of the plastic sheet were covered with moist tissue paper to prevent predators from escaping (Walzer and Schausberger 1999). Bean leaves fully infested with T. urticae were put on the arena three times a week. The phytoseiid colony was kept in a growth chamber in constant condition (25 ± 1 °C, 60± 70% RH, and a photoperiod of 16:8 h L: D). The age of the adult females used for T. khosrovensis and E. amissibilis was 24 hours for the functional response experiments.
Functional response
The experimental unit was a 3.5 cm diameter mulberry leaf disc placed upside down on a thin layer of cotton in a petri dish with a 6 cm diameter, and 1 cm height. Six densities (2, 4, 8, 16, 32, and 64) of T. urticae eggs were used in functional response experiments. 44 adult females of T. urticae from the stock culture were transferred onto the leaf discs and allowed to oviposit for 24 hours, then they were removed. The eggs deposited were counted, and only the specified number was kept in the experimental unit, and the remaining eggs were removed. After that, the adult female predatory mites were starved for 6 hours and transferred to the leaf discs to feed on the prey for 24 hours. To prevent the escape of predatory mites, the petri dishes' openings were sealed with parafilm, and to provide ventilation, about 10 tiny holes were made in parafilm. Each experiment was replicated 10 times at temperature of 25 ± 1 °C, 60–70% RH, and a photoperiod of 16:8 h (L: D). After 24 h, the number of intact eggs remaining was counted. The data on functional response were analyzed in two steps (Juliano, 2001). First, the type of functional response was determined by logistic regression of the proportion of prey consumed (Na / N0 ) as a function of prey density (N0 ):
\[\frac{N_e}{N_o}=\frac{\exp \left(P_0+P_1 N_0+P_2 N_0^2+P_3 N_0^3+P_4 N_0^4\right)}{1+\exp \left(P_0+P_1 N_0+P_2 N_0^2+P_3 N_0^3+P_4 N_0^4\right)} (1)\]
Where P0 , P1 , P2, and P3 are the intercept, linear, quadratic, and cubic coefficients, respectively, which were estimated using the method of maximum likelihood. The sign of the P1 from equation (1) can be used to distinguish the type of the functional response curve. If P1 < 0, it describes a type II functional response. If P1 > 0 and P2 < 0, it shows a type III functional response (Juliano, 2001). After determining the type of functional response, the next step is to estimate the handling time and attack rate coefficients. In this study, we used an explicit deterministic model for type II functional response (Royama, 1971; Rogers, 1972):
\[N a=N t[1-\exp (a(T h N a-T))] (2)\]
Where Na is the number of prey killed, Nt is the initial number of prey, T is the total time available for the predator, a is the attack rate, and Th is the handling time. For a type III response, the attack rate is assumed to increase with host density according to the equation a = (d + bN0)/ (1 + cN0) (Hassell et al. 1977). In cases where both d and c are not significantly different from 0, this leads to a = bN0 which can be inserted into equation (2). Then, for each host density, the attack coefficient (a) can be determined as a = bN.
Results
Download as
Prey stage
Parameters
Euseius amissibilis
Typhlodromus khosrovensis
Estimate (±SE)
X2
P
Estimate (± SE)
c2
P
Egg
P0
-0.2223 ± 0.3953
0.32
0.5738
-0.6342 ± 0.3884
2.67
0.1025
P1
–0.1222 ± 0.0621
3.87
0.0491
0. 0204 ± 0.0594
0.12
0.0305
P2
0.00482 ± 0.00236
4.16
0.0414
-0.00158 ± 0.00225
0.49
0.4837
P3
–0.00005 ± 0.00002
3.93
0.0474
0.000020 ±^ ^0.000022
0.84
0.36
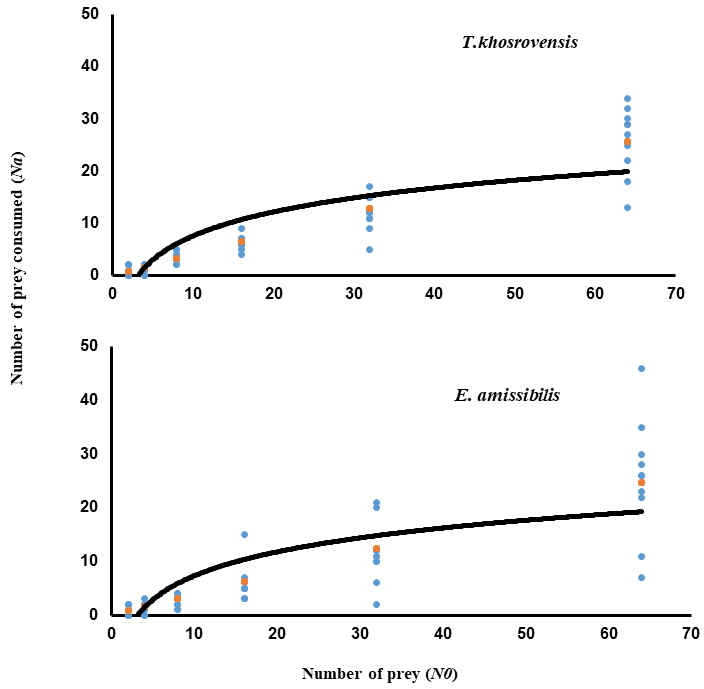


The functional response of both phytoseiid species showed a type II on eggs of T. urticae (Figure 1). The linear coefficient of Equation (1) in both cases was negative and significantly different from 0 (P < 0.01), indicating that the consumption rate of T. urticae eggs declined as prey density increased (Table 1). The highest attack rate, and the lowest handling time were recorded for E. amissibilis on the eggs of T. urticae (Table 2). Euseius amissibilis consumed higher numbers of eggs compared to T. khosrovensis, particularly at higher prey densities (Table 2). The maximum attack rate (T / Th ) on eggs of T. urticae was estimated to be 9.98 for E. amissibilis, and 8.57 for T. khosrovensis, respectively (Table 2).
Download as The values in parentheses represent 95% confidence intervals. a; attack rate (h-1); Th, handling time (h); T/Th, maximum attack rate.
Predator species
Prey stage
Functional response parameters
a±SE
Th±SE
T / Th
R2
E. amissibilis
Egg
0.0204 ± 0.0014
2.3018 ± 0.0943
9.98
0.84
(0.0174 – 0.0234)
(2.2156 – 2.5919)
T. khosrovensis
0.0213 ± 0.0009
2.8013 ± 0.0791
8.57
0.88
(0.0195 – 0.0231)
(2.2134 – 2.9592)
Discussion
In this study, the functional response of both phytoseiids, T. khosrovensis, and E. amissibilis feeding eggs of T. urticae showed a type II, where the predation rate decreases as prey density increases. This type of functional response has been reported in phytoseiid mites in previous studies (Ahn et al. 2010; Castagnoli et al. 1999; Farazmand et al. 2012; Jafari et al. 2011) and other species of the genus Typhlodromus and Euseius showed the same type of functional response on eggs and immature stages of T. urticae (Badii et al., 2004; Farazmand et al., 2012).
According to the lifestyle classification of Phytoseiidae family, T. khosrovensis, and E. amissibilis are categorized in type III and IV, respectively (McMurtry et al. 2013). The type IV predators are generally pollen feeders. They have their highest reproductive capacity when feeding on pollen, and populations in the field often increase significantly when the crop or the surrounding vegetation is flowering. Also, type III phytoseiids feed on pollen, though they prefer, or show better performance on prey such as pest species in Acaridae, Eriophyidae, Tetranychidae, Tenuipalpidae, Tydeidae, as well as thrips, whiteflies, and mealybugs (McMurtry et al. 2013). Euseius amissibilis as a phytoseiidae type IV prefer to feed on pollen grains (Badii et al. 2004). However, some reports are showing the pollen-feeding generalist predators in the genus Euseius can also prey on some small arthropods (de Moraes and Lima, 1983; Grout et al. 1992; Stathakis et al. 2021). Several studies have indicated that species type IV are effective biocontrol agents of spider mites on some crops (McMurtry et al. 2013).
In this study, only eggs of the two-spotted spider mites were used for the evaluations of the functional response. According to Badii et al. (2004), T. urticae consumption as a prey was inversely related to its size; thus, eggs were the most preferred stage that the predators could consume. On the other hand, ingestion of the deutonymphs or adults was very rare.
The maximum handling time, which shows the time required for capturing and feeding on prey was estimated as 2.30 (h), and 2.80 (h) for T. khosrovensis and E. amissibilis, respectively, for 24 h. Based on the attack rate, and handling time, both phytoseiids in this study performed lower ability compared to the other native phytoseiid mite T. bagdasarjani when preyed on eggs of T. urticae (a = 0.089 h-1 and Th=1.80), although T. bagdasarjani was still a weaker predator in comparison to Neoseiulus californicus (a=0.09 h-1 and Th=1.61) (Farazmand et al. 2012). In addition, the ability of T. khosrovensis and E. amissibilis to catch the spider mites' eggs was estimated lower in comparison to P. plumifer (Kouhjani Gorji et al. 2009), and E. hibisci in the study conducted by Badii et al (2004).
Most functional response studies have been conducted on the specialist phytoseiid, Phytoseiulus persimilis, as well as selective and generalist phytoseiids, N. californicus and Amblyseius swirskii Athias-Henriot (Fathipour et al. 2017, 2018, Ahn et al. 2010), which are recommended for biological control of two-spotted spider mites, thrips, and whiteflies (Xiao and Fdamiro, 2010, Castagnoli & Simoni 1999). On the other hand, phytoseiid mites within the type III and IV lifestyles which are generally pollen feeders, have a moderate and partial impact on spider mite eggs and immatures. However, these potential predators can be considered as an auxiliary biocontrol agent. In augmentative release programs, they could play a supporting role in achieving biological control of T. urticae when used in combination with specialized imported predatory mites, which appear to be more effective.
In conclusion, neither E. amissibilis nor T. khosrovensis are specialist predators of T. urticae, but they have the potential to improve the control of this mite on mulberry trees existing in urban green spaces. However, extra studies are necessary to obtain the knowledge about behavior of these predators in their natural environment. Their ecological attributes are somewhat different and they seem to act quite independently.
In this study, only the eggs of T. urticae were provided to the phytoseiids, but in the field, the predators must overcome the dense web of the two-spotted spider mites to catch the prey. Spider mites, particularly species of the genus Tetranychus create complicated webs to hinder the movement of the phytoseiids categorized as ecological types III, and IV. Also, more studies about the other ecological aspects of predatory mites including prey preference, switching behavior, and intra-specific interaction, help us to obtain much more information to use them as an auxiliary and potential biocontrol agent to prevent applying extra pesticides on urban pests.
References
- Afkhami M., Farazmand A., Faraji F. 2024. Morphological variation and abnormalities in two species of Typhlodromus Scheuten (Acari: Phytoseiidae). Syst. Appl. Acarol., 29(5): 570-578. https://doi.org/10.11158/saa.29.5.3
- Ahn J.J., Kim K.W., Lee J.H. 2010. Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on strawberry leaves. J. Appl. Entomol., 134: 98-104. https://doi.org/10.1111/j.1439-0418.2009.01440.x
- Amoah B., Anderson J., Erram D., Gomez J., Harris A., Kivett J. 2016. Plant spatial distribution and predator-prey ratio affect the biological control of the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae) by the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). Biocontrol Sci. Technol., 26: 548-561. https://doi.org/10.1080/09583157.2015.1133807
- Badii M.H., Hernandez-ortiz E., Flores A.E., Landeros J. 2004. Prey stage preference and functional response of Euseius hibisci to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp. Appl. Acarol., 34(3-4): 263-273. https://doi.org/10.1007/s10493-004-1180-8
- Bolland H.R., Gutierrez J., Flechtmann C.H.W. 1998. World catalogue of the spider mite family (Acari: Tetranychiade). Leiden, Brill Academic publishers., 392 pp.
- Castagnoli M., Simoni S. 1999. Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 23(3): 217-234.
- Cedola C.V., Sanchez N.E., Liljesthrom G.G. 2001. Effect of tomato leaf hairiness on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 25(10-11): 819-831.
- Dalir S., Hajiqanbar H., Fathipour Y., Khanamani M. 2021. Age-dependent functional and numerical responses of Neoseiulus cucumeris (Acari: Phytoseiidae) on two-spotted spider mite (Acari: Tetranychidae). J. Econ. Entomol., 114(1): 50-61. https://doi.org/10.1093/jee/toaa266
- De Clercq P., Mohaghegh J., Tirry L. 2000. Effect of host plant on the functional response of the predator Podisus nigrispinus (Heteroptera: Pentatomidae). Biol. Control., 18: 65-70. https://doi.org/10.1006/bcon.1999.0808
- Farazmand A., Fathipour Y., Kamali K. 2012. Functional response and mutual interference of Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Int. J. Acarol., 38: 369-376. https://doi.org/10.1080/01647954.2012.655310
- Farazmand A., Fathipour Y. Kamali K. 2015. Intraguild predation among Scolothrips longicornis (Thsanoptera: Thripidae), Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) under laboratory conditions. Insect science. 263-272. https://doi.org/10.1111/1744-7917.12047
- Fathipour Y., Karimi M., Farazmand A., Ali A.T. 2018. Age-specific functional response and predation capacity of Phytoseiulus persimilis (Phytoseiidae) on the two-spotted spider mite. Acarologia, 58(1): 31-40. https://doi.org/10.24349/acarologia/20184225
- Fathipour Y., Karimi M., Farazmand A., Talebi A.A. 2017. Age-specific functional response and predation rate of Amblyseius swirskii (Phytoseiidae) on two-spotted spider mite. Syst. Appl. Acarol., 22(2): 159-169. https://doi.org/10.11158/saa.22.2.1
- Fathipour Y., Maleknia B., Bagheri A., Soufbaf M., Reddy G.V. 2020. Functional and numerical responses, mutual interference, and resource switching of Amblyseius swirskii on two-spotted spider mite. Biol. Control., 146: 104266. https://doi.org/10.1016/j.biocontrol.2020.104266
- Ganjisaffar F., Fathipour Y., Kamali K. 2011. Temperature-dependent development and life table parameters of Typhlodromus bagdasarjani (Phytoseiidae) fed on two-spotted spider mite. Exp. Appl. Acarol., 55: 256-272. https://doi.org/10.1007/s10493-011-9467-z
- Gerson U. 2014. Pest control by mites (Acari): present and future. Acarologia., 54(4): 371-394. https://doi.org/10.1051/acarologia/20142144
- Gerson U., Weintraub P.G. 2007. Mites for the control of pests in protected cultivation. Pest Manag. Sci., 63(7): 658-676. https://doi.org/10.1002/ps.1380
- Grout T.G., Richards G.I. 1992. Euseius addoensis addoensis, an effective predator of citrus thrips,Scirtothrips aurantii, in the eastern Cape Province of South Africa. Exp. Appl. Acarol., 15: 1-13. https://doi.org/10.1007/BF01193963
- Hassell M.P., Lawton J.H., Beddington J.R. 1977. Sigmoid functional response by invertebrate predators and parasitoids. J. Anim. Ecol., 46: 249-262. https://doi.org/10.2307/3959
- Holling C.S. 1959. The components of predation as revealed by a study of small mammal predation of the European pine sawfly. Can. Entomol., 91: 293-320. https://doi.org/10.4039/Ent91293-5
- Jafari Sh., Fathipour Y., Faraji F. 2012. The influence of temperature on the functional response and prey consumption of Neoseiulus barkeri (Phytoseiidae) on two-spotted spider mite. J. Entomol. Soc. Iran., 31(2): 39-52.
- Jafarian F., Jafari Sh., Fathipour Y. 2022. Functional response of the predatory mite, Typhlodromus bagdasarjani (Acari: Phytoseiidae) to protonymphs of Eotetranychus frosti (Acari: Tetranychidae) on four apple cultivars. Acarologia., 62 (2):454-464. https://doi.org/10.24349/7ejy-uk7s
- Juliano S.A. 2001. Nonlinear curve fitting: predation and functional response curves. In: Scheiner, S.M. and J. Gurevitch (eds.), Design and Analysis of Ecological Experiments. New York, Oxford University Press., 178-196 pp https://doi.org/10.1093/oso/9780195131871.003.0010
- Kouhjani Gorji M., Fathipour Y., Kamali K. 2009. The effect of temperature on the functional response and prey consumption of Phytoseius plumifer (Acari: Phytoseiidae) on the two-spotted spider mite. Acarina., 17(2): 231-237.
- Li D.X., Tian J., Shen Z.R. 2007. Functional response of the predator Scolothrips takahashii to hawthorn spider mite, Tetranychus viennensis: effect of age and temperature. Biol. Control., 52: 41-61. https://doi.org/10.1007/s10526-006-9015-7
- Mahdian K., Vantornhout I., Tirry L., De Clercq P. 2006. Effects of temperature on predation by the stinkbugs Picromerus bidens and Podisus maculiventris (Heteroptera: Pentatomidae) on noctuid caterpillars. Bull. Entomol. Res., 96: 489-496. https://doi.org/10.1079/BER2006450
- Maleknia B., Fathipour Y., Soufbaf M. 2016. How greenhouse cucumber cultivars affect population growth and two-sex life table parameters of Tetranychus urticae (Acari: Tetranychidae). Int. J. Acarol., 42: 70-78. https://doi.org/10.1080/01647954.2015.1118157
- McMurtry J. A., De Moraes G. J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae). Sys. Appl. Acarol., 18, 297-320. https://doi.org/10.11158/saa.18.4.1
- Migeon A., Nouguier E., Dorkeld F. 2010. Spider mites web: a comprehensive database for the Tetranychidae. Trends. Acarol., 557-560. https://doi.org/10.1007/978-90-481-9837-5_96
- Moghadasi M., Saboori A., Allahyari H., Zahedi Golpayegani A. 2014. Prey stages preference of different stages of Typhlodromus bagdasarjani (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on rose. Persian. J. Acarol., 2(3): 531-538.
- Mohaghegh J., De Clercq P., Tirry L. 2001. Functional response of the predators Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae) to the beet armyworm, Spodoptera exigua (Hubner) (Lep., Noctuidae): Effect of temperature. J. Appl. Entomol., 125: 131-134. https://doi.org/10.1046/j.1439-0418.2001.00519.x
- Moraes G.J., Lima H.C. 1983. Biology of Euseius concordis (Chant) (Acarina: Phytoseiidae). Acarologia., 24(3): 251-255.
- Notghi Moghadam B.A., Hajizadeh J., Jalali Sendi J. Rafati Fard M. 2010. Influence of three diets on development and oviposition of Amblyseius herbicolus under laboratory conditions. J. Entomol. Soc. Iran., 30(1): 51-6.
- Oliveira H., Janssen A., Pallini A., Venzon M., Fadini M., Duarte V. 2007. A phytoseiid predator from the tropics a potential biological control agent for the spider mite Tetranychus urticae Koch (Acari: Tetranychidae). Biol. Control., 42(2):105-109. https://doi.org/10.1016/j.biocontrol.2007.04.011
- Rahmani H., Fathipour Y., Kamali K. 2009. Life history and population growth parameters of Neoseiulus californicus (Acari: Phytoseiidae) fed on Thrips tabaci (Thysanoptera: Thripidae) in laboratory condition. Syst. Appl. Acarol., 14(2): 91-100. https://doi.org/10.11158/saa.14.2.2
- Reis P.R., Sousa E.O., Teodoro A.V., Neto M.P. 2003. Effect of prey densities on the functional and numerical response of two species of predaceous mites (Acari: Phytoseiidae). Neotrop. Entomol., 32(3): 461-467. https://doi.org/10.1590/S1519-566X2003000300013
- Riahi E., Fathipour Y., Talebi A.A. Mehrabadi M. 2016. Pollen quality and predator viability: life table of Typhlodromus bagdasarjani on seven different plant pollens and two-spotted spider mite. Syst. Appl. Acarol., 21: 1399-1412. https://doi.org/10.11158/saa.21.10.10
- Rogers D. 1972. Random search and insect population models. J. Anim. Ecol., 41(2): 369-383. https://doi.org/10.2307/3474
- Royama T. 1971. A comparative study of models for predation and parasitism. Res. Popul. Ecol., 1: 1-90. https://doi.org/10.1007/BF02511547
- Seiedy M., Saboori A., Allahyari H., Talaei-Hassanloui R., Tork M. 2012. Functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) on untreated and Beauveria bassiana- treated adults of Tetranychus urticae (Acari: Tetranychidae). J. Insect Behav., 25: 543-553. https://doi.org/10.1007/s10905-012-9322-z
- Skirvin D.J., Fenlon J.S. 2001. Plant species modifies the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae): Implications for biological control. Bull. Entomol. Res., 91: 61-67. https://doi.org/10.1079/BER200063
- Soleymani S., Hakimitabar M., Seiedy M. 2016. Prey preference of predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) and Bemisia tabaci (Hemiptera: Aleyrodidae). Biocontrol Sci. Technol., 26: 562-569. https://doi.org/10.1080/09583157.2015.1133808
- Stathakis T.I., Kapaxidi E.V., Papadoulis G.T., Papanikolaou N.E. 2021. Predation by Euseius scutalis (Acari: Phytoseiidae) on Tetranychus urticae and Eutetranychus orientalis (Acari: Tetranychidae): effect of prey density and developmental stage. Syst. Appl. Acarol., 26: 1940-1951. https://doi.org/10.11158/saa.26.10.8
- Walzer A., Schausberger P. 1999. Predation preferences and discrimination between con-and heterospecific prey by the phytoseiid mites Phytoseiulus persimilis and Neoseiulus californicus. Biol. Control., 43(4): 469-478.
- Williams M.E.D.C., Kravar-Garde L., Fenlon J.S., Sunderland K.D. 2004. Phytoseiid mites in protected crops: the effect of humidity and food availability on egg hatch and adult life span of Iphiseius degenerans, Neoseiulus cucumeris, N. californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol., 32 (1-2):1-3. https://doi.org/10.1023/B:APPA.0000018170.46836.11



2024-09-03
Date accepted:
2025-03-10
Date published:
2025-05-07
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Afkhami, Maryam; Farazmand, Azadeh; Rahmani, Shima and Golpayegani, Azadeh Zahedi
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



