Pronematinae (Trombidiformes: Iolinidae) from Brazil, with original and complementary descriptions of taxa as well as with a reappraisal of the subfamily and of Parapronematus
De Vis, Raf M. J.  1
; Araujo Lacerda, José Dantas
1
; Araujo Lacerda, José Dantas  2
; de Moraes, Gilberto J.
2
; de Moraes, Gilberto J.  3
and Ueckermann, Edward A.
3
and Ueckermann, Edward A.  4
4
1Research Station for Vegetable Production (PSKW), 2860 Sint-Katelijne-Waver, Belgium.
2Departamento de Entomologia e Acarologia, Escola Superior de Agricultura "Luiz de Queiroz", Universidade de São Paulo (ESALQ-USP), 13418-900 Piracicaba, São Paulo, Brazil.
3Departamento de Entomologia e Acarologia, Escola Superior de Agricultura "Luiz de Queiroz", Universidade de São Paulo (ESALQ-USP), 13418-900 Piracicaba, São Paulo, Brazil.
4✉ Unit for Environmental Sciences and Management, Potchefstroom Campus, North-West University, Private Bag X6001, Potchefstroom, 2520, South Africa.
2025 - Volume: 65 Issue: 2 pages: 331-372
https://doi.org/10.24349/7w23-xsl9ZooBank LSID: E317A260-87BA-4FD9-A6D3-C57DABBD396A
Original research
Keywords
Abstract
In memory of the senior author who passed away in December 2024 before the completion of this article.
Introduction
An effort has been dedicated in Brazil to the knowledge of the mite species found on plants and in the soil. One of the main objectives is to determine the species potentially useful as biological control agents. Studies have focused mainly on taxa belonging to Phytoseiidae, given their recognized importance as biological control agents (Carrillo et al. 2015; Lofego et al. 2024).
Little effort has been dedicated to the study of mites of the family Iolinidae Pritchard, 1956 in Brazil (Da Silva et al. 2017). Although apparently omnivorous, some species of this family have been reported as predators (De Vis et al. 2006a; Hernandes et al. 2015; Marcossi et al. 2024). The present work refers to species of Pronematinae André, 1980, originally described in Tydeidae Kramer, 1877 and transferred to Iolinidae by André and Fain (2000). Ueckermann et al. (2024) and De Vis et al. (2024a, b) provided new information about Pronematinae, but no recent publication provides a key for the separation of the nearly 20 genera presently placed in this subfamily. Before that is done, it is necessary to confirm the validity of the synonymy between Pausia Kuznetsov & Livshits, 1972 and Naudea Meyer & Rodrigues, 1966, proposed by Baker and Delfinado (1976). André (1980) did not accept this synonymy.
Parapronematus Baker, 1965, the type genus of Pronematinae, was originally described in Tydeidae and transferred to Iolinidae by André and Fain (2000). A recent unpublished evaluation showed that a species of this genus reported by De Vis et al. (2006b) as Parapronematus sp. in southern Brazil and deposited at Escola Superior de Agricultura ''Luiz de Queiroz'', Universidade de São Paulo (ESALQ-USP) was recently collected in northern Brazil and it has still not been reported in the literature. Baker (1965) created Parapronematus based on a single species, P. acaciae Baker, 1965. Six additional species have been described, not always completely complying with all the reported generic characteristics: P. geminus Meyer and Rodrigues, 1966, P. citri Salviejo, 1969, P. formosanus Tseng, 1985, P. ferox Gupta, 1991, P. camelia Gupta, 1992 and P. murshidabadensis Gupta, 1992 (https://species.wikimedia.org/wiki/Pronematinae ![]() , accessed on 11/10/2024, André 2021). The genus has distinctive features facilitating its separation from other genera of the same subfamily: setae la absent, three pairs of ag setae, dorsal setae of femora III and IV forked and the following leg chaetotaxy (from tarsus to trochanter, solenidia in brackets): leg I (8 (ω) – 2 (φ + κ) – 2 – 3 – 1), leg II (6 (ω) – 2 – 2 – 3 – 0), leg III (5 – 2 – 2 – 2 – 1) and leg IV (5 – 2 – 1 – 1 – 0).
, accessed on 11/10/2024, André 2021). The genus has distinctive features facilitating its separation from other genera of the same subfamily: setae la absent, three pairs of ag setae, dorsal setae of femora III and IV forked and the following leg chaetotaxy (from tarsus to trochanter, solenidia in brackets): leg I (8 (ω) – 2 (φ + κ) – 2 – 3 – 1), leg II (6 (ω) – 2 – 2 – 3 – 0), leg III (5 – 2 – 2 – 2 – 1) and leg IV (5 – 2 – 1 – 1 – 0).
The objectives of this work were: a) to present new records of Pronematinae species from Brazil, describing some new taxa and complementarily describing others; b) to conduct a reappraisal of Pronematinae and Parapronematus, presenting keys for the separation of the respective genera and species; c) to suggest new generic characteristics for Pronematinae.
Material and methods
Samples of leaves, inflorescences and fruits of plants of economic interest as well as of surrounding vegetation were collected by J.D.A. Lacerda in July 2023 in rural communities and urban areas of Parauapebas (about 150–165 m altitude), southeastern municipality of Pará State, in the Amazon region of Brazil. These were transported to the Agricultural and Forestry Entomology Laboratory of Universidade Federal Rural do Amazonas, where mites were collected and preserved in 70% ethanol until mounted in Hoyer's medium (Krantz and Walter 2009) at ESALQ-USP. Those belonging to Iolinidae were sent to PSKW where taxonomic studies were conducted. Additionally, slides with Parapronematus specimens reported by De Vis et al. (2006a, b) and slides of other Brazilian localities deposited at the Mite Reference Collection of ESALQ-USP were also included in the study.
Morphological examination and principal component analysis
Specimens were measured using a Leica DM750 microscope with a Leica Flexacam c3 camera and Leica LAS-X software. Line drawings were made with a Leica DM750 equipped with a phototube and Wacom Cintiq 16 pen display and drawn using Adobe Illustrator 2024.
Nomenclature of the body regions, setae and organotaxy is that of Kaźmierski (1989). Leg setae nomenclature is that of André (1981b) and Khaustov et al. (2020). The tables of Kaźmierski (1989) were used for the interpretation of the setal nomenclature of different authors. In the text, measurements are given in micrometres, following the name of each structure. A principal component analysis (PCA) based on setal lengths (Ueckermann and De Vis et al. 2024) of specimens of Parapronematus connarus n. sp. was done with PAST (version 4.03).
Key to developmental stages and sexes of Pronematinae
As in any other mite group, the characterization of the specimens under analysis initially requires the identification of their developmental stage. In this study, that was done basically with the help of the key provided by André (1980), with some additional information considered relevant. That is important because most descriptions in the literature refer to adult females. Larvae are recognized by having only three pairs of legs, as in most mite groups. Other stages as well as adult sexes were separated according to the following characteristics:
A. With a dehiscence line (breakage line for ecdysis) anteriad the prodorsal setae, curved backwards towards la (or ro setae when la are absent; procurved)
...... nymph
• Leg IV with tarsus bearing five setae or more and other segments nude; reduced number of ventral idiosomatic setae (lacking mtβ, 3c, 3d and all ag setae)
...... protonymph
• Leg IV with all segments, except trochanter, bearing setae; venter of idiosoma lacking only mtβ, 3d, ag3 and either ag4 (in species with four pairs of ag setae) or ag2 (in species with three pairs of ag setae)
...... deutonymph
• Leg IV with all segments, often including trochanter, bearing setae; no idiosomatic or leg setae absent (as in adult)
...... tritonymph
B. Without dehiscence line
...... adult
• Genital opening shaped as an inverted T (exceptionally completely opened, then as a large circle), with 2–4 pairs of ag setae
...... female
• Genital opening not shaped as an inverted T; with two rounded dorsal structures laterad e1; with stout solenidion extending beyond the tip on tarsus I (in Pronematus, Neopronematus); with a dorso-distal adaxial spur on femur IV; aedeagus T-shaped (usually indistinguishable), with the same number of ag setae as in female or less (only one in Homeopronematus anconai), at least in Parapronematus connarus n. sp., with two pairs of eugenital setae; smaller than female
...... male
Estimation of length of structures of previously described species in the review section
As we could not have access to the type specimens of P. acaciae, P. citri and P. ferox, the length of different structures of these species was estimated based on convertion factors calculated from the given values of the length and width of the idiosoma of those species and the length of those structures in the drawings. The calculated values are given in bold italics in Table 1. Additionally, for P. acaciae, the estimated length of the tarsus of Figure 84 of the original description was used as basis for the estimation of the length of the setae of tarsus I, depicted in Figure 85.
Results
New collection records of Pronematinae from Brazil
Iolinidae Pritchard, 1956
Pronematinae André, 1980
Pronematus Canestrini, 1886
Pronematus brasiliensis Ueckermann & De Vis, 2024
Specimens examined — all collected in Parauapebas; 5°59′11.68″ S, 49°52′34.73″ W, on Vitis labrusca L. (MZLQ 4415: 1♂); 6° 4′35.49″ S, 49°52′51.54″ W, on Senna occidentalis (L.) Link (MZLQ 4416: one ♂); 6° 4′13.53″ S, 49°54′1.40″ W, on Adenanthera pavonina L. (MZLQ 4417: one protonymph).
Remarks — The following arthropods were found in association with P. brasiliensis: on leaves of A. pavonina, unidentified Euseius Wainstein species (Phytoseiidae) and insects of the order Psocoptera; on V. labrusca, the pronematids Dasilcoferla nadirae (Da Silva et al.(2017) and Peridasilcoferla paraensis n. gen. n. sp., and unidentified Amblyseius Berlese and Iphiseiodes De Leon species (Phytoseiidae); on S. occidentalis, Eutetranychus banksi (McGregor) (Tetranychidae).
Peridasilcoferla De Vis & Ueckermann n. gen.
ZOOBANK: A2AB2608-895A-4D9D-9FBB-6BF0B4AAE4F8 ![]()
Diagnosis
Adult females with four pairs of setae on aspidosoma, 11 pairs on the opisthosoma, one on trochanters I to III, six on tarsus II and five on each of tarsi III and IV. The leg chaetotaxy is the same as in Neopronematus Panou, Emmanouel & Kaźmierski, 2000 and Dasilcoferla De Vis & Ueckermann, 2024a, the former differing in having nine and the latter ten pairs of opisthosomal setae.
Description
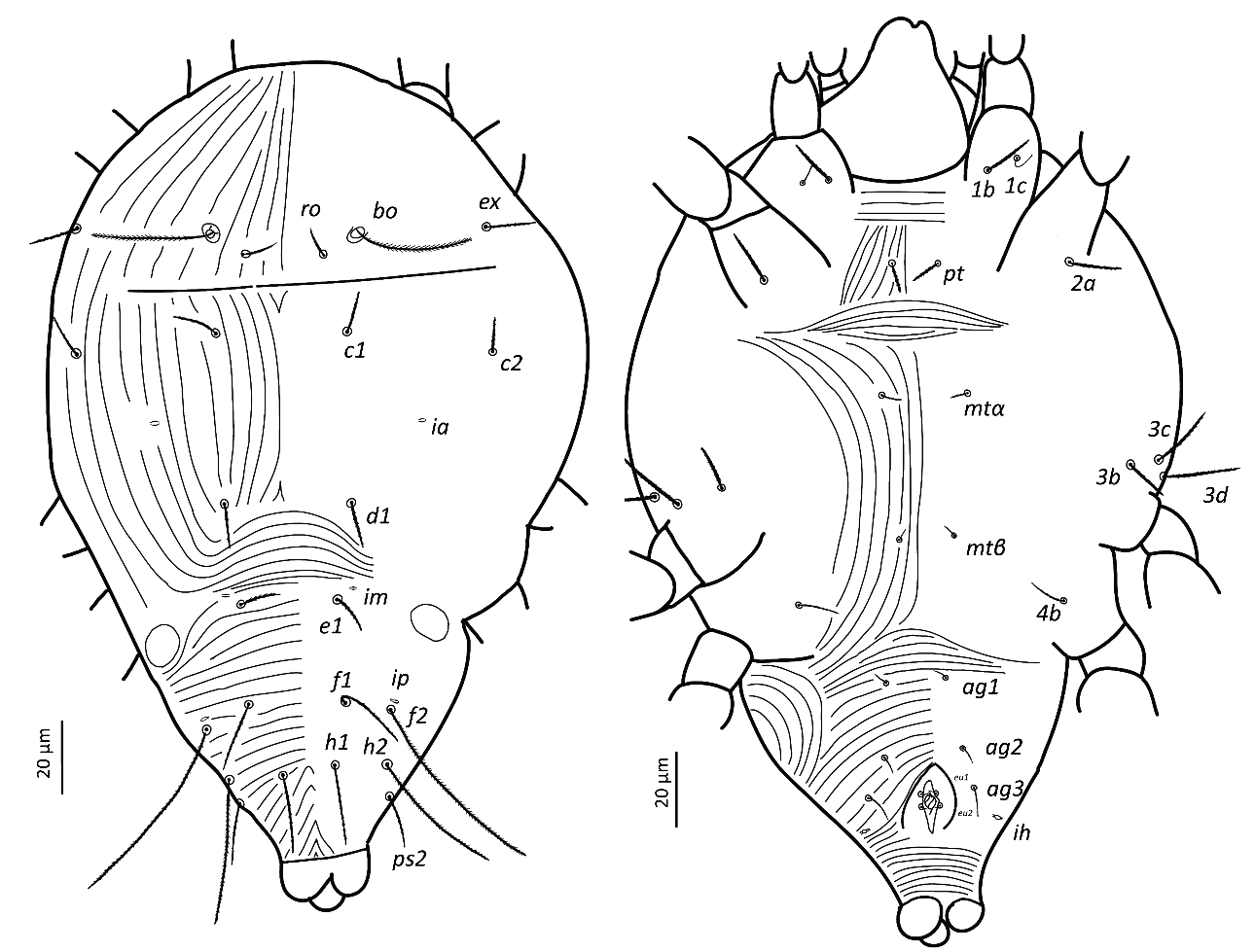
Adult female. Aspidosoma procurved with four pairs of setae (bo, ro, la and ex); opisthosoma with 11 pairs of setae (c1, c2, d1, e1, f1, f2, h1, h2, ps1, ps2 and ps3) and four pairs of lyrifissures (ia, im, ip and ih). Eye spots absent. Four pairs of aggenital setae. One pair of genital papillae. Leg I without apotele; each of other legs with ciliate empodium and two claws, without empodial hooks. Seta homologous to seta s (see Ueckermann and De Vis et al, 2024) on tarsus I absent. Femur IV undivided. Leg chaetotaxy (tarsus to trochanter): leg I (8 + ω – 3 + φ + κ – 3 – 3 – 1); leg II (6 + ω – 2 – 3 – 3 – 1); leg III (5 – 2 – 2 – 2 – 1); leg IV (5 – 2 – 1 – 2 – 0). Epimeral formula: 3-1-4-2.
Etymology
The ancient Greek prefix ''peri'' means ''near''; hence, the Peridasilcoferla refers to the similarity between the new genus and Dasilcoferla.
Remark
The new genus differs from Dasilcoferla (also described from Brazil) by having setae ps1 (absent in Dasilcoferla). Some other characteristics distinguish the new genus from Dasilcoferla as well as from Neopronematus, but these are not included in the description of the genus (adopting the model of generic characterization proposed by André, 1980). In Dasilcoferla, the eupathidia of tarsus I with fine instead of course barbs in Peridasilcoferla n. gen. and Neopronematus spp. In all Neopronematus, except N. aegeae Panou, Emmanouel & Kaźmierski, 2000 (Kuznetsov 1972; Panou et al. 2000; Sadeghi et al. 2012; Darbemamieh et al. 2015), and Dasilcoferla (De Vis et al. 2024a), the eupathidia are thoroughly barbed whereas in Peridasilcoferla n. gen. the distal third is smooth. Conversely, the body and leg setae have heavier barbs in Dasilcoferla and in Neopronematus (except N. aegeae), compared to those in Peridasilcoferla n. gen. The placement of the ag setae in the new genus is similar to that in Dasilcoferla (well separate from each other), while in Neopronematus species ag3 setae are inserted close to each other, at the anterior end of the genital opening and near ag2. Additionally, ag3 is simple in the former two genera but forked in Neopronematus, except in N. aegeae. With the description of other Neopronematus species, N. aegeae seems to differ in many respects from those species, suggesting that their generic classification should be re-evaluated.
Type species
Peridasilcoferla paraensis n. gen. n. sp.
Peridasilcoferla paraensis De Vis & Ueckermann n. gen. n. sp.
ZOOBANK: A004CFA0-A311-4917-B8E5-DC9E3137F14A ![]()
(Figures 1–4)
Adult female (n = 3)
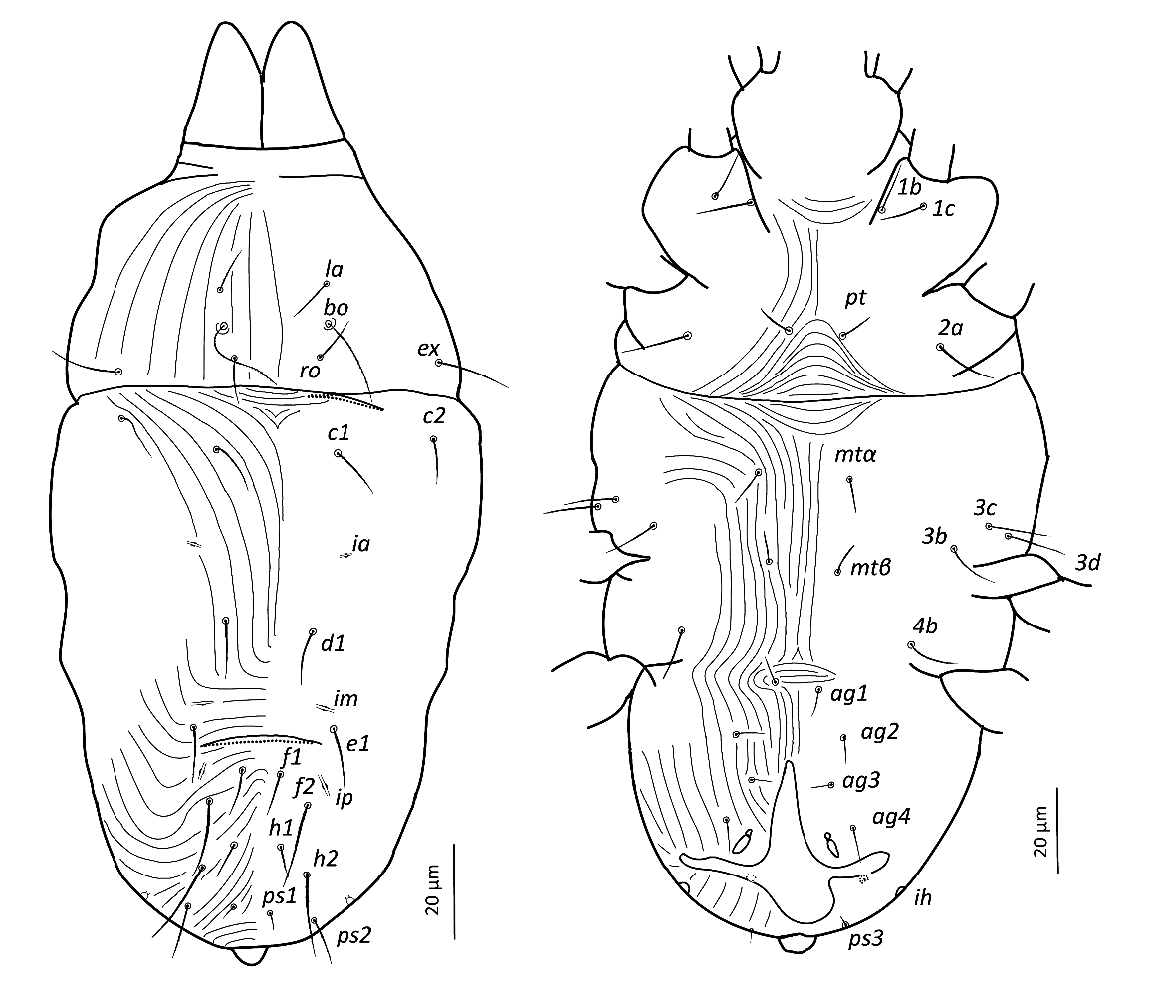
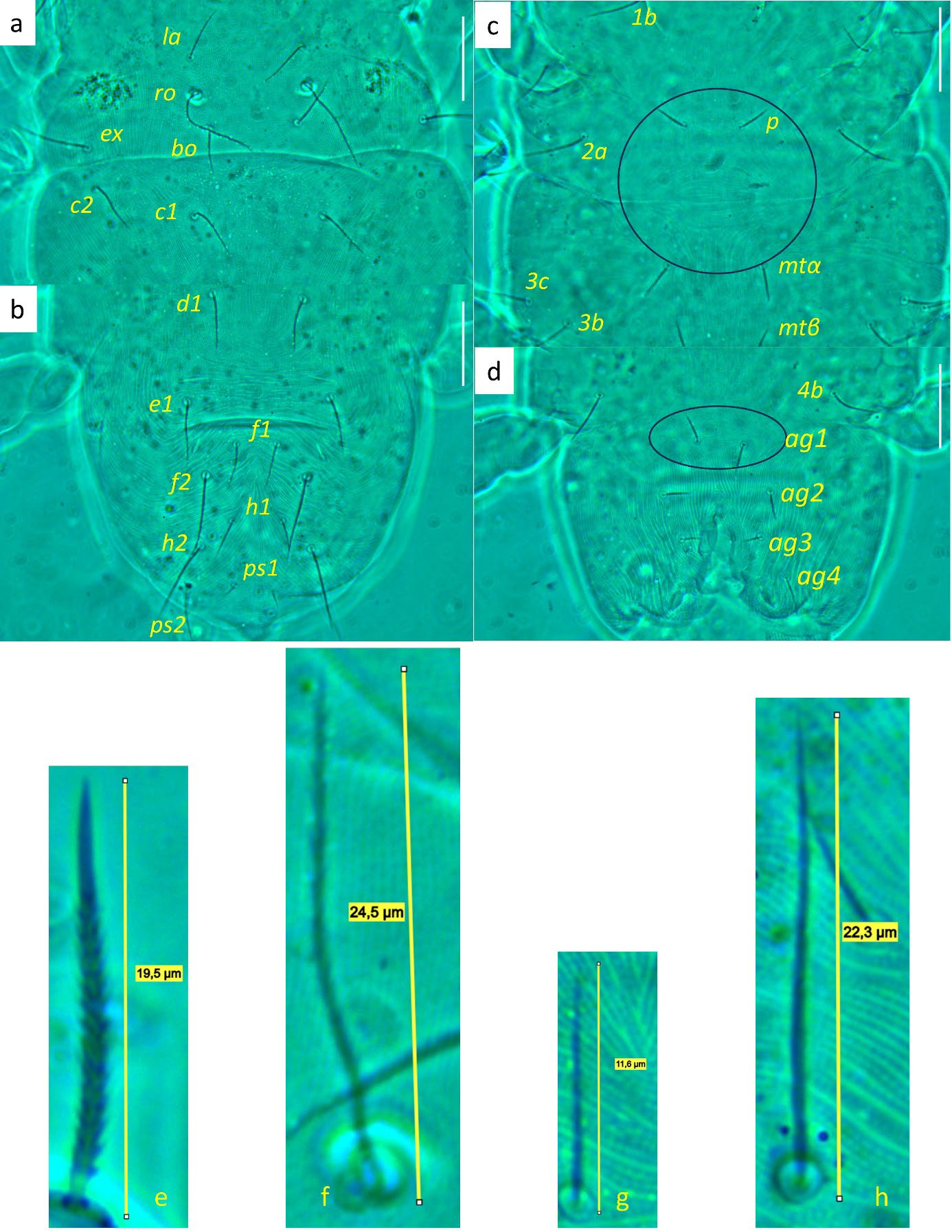
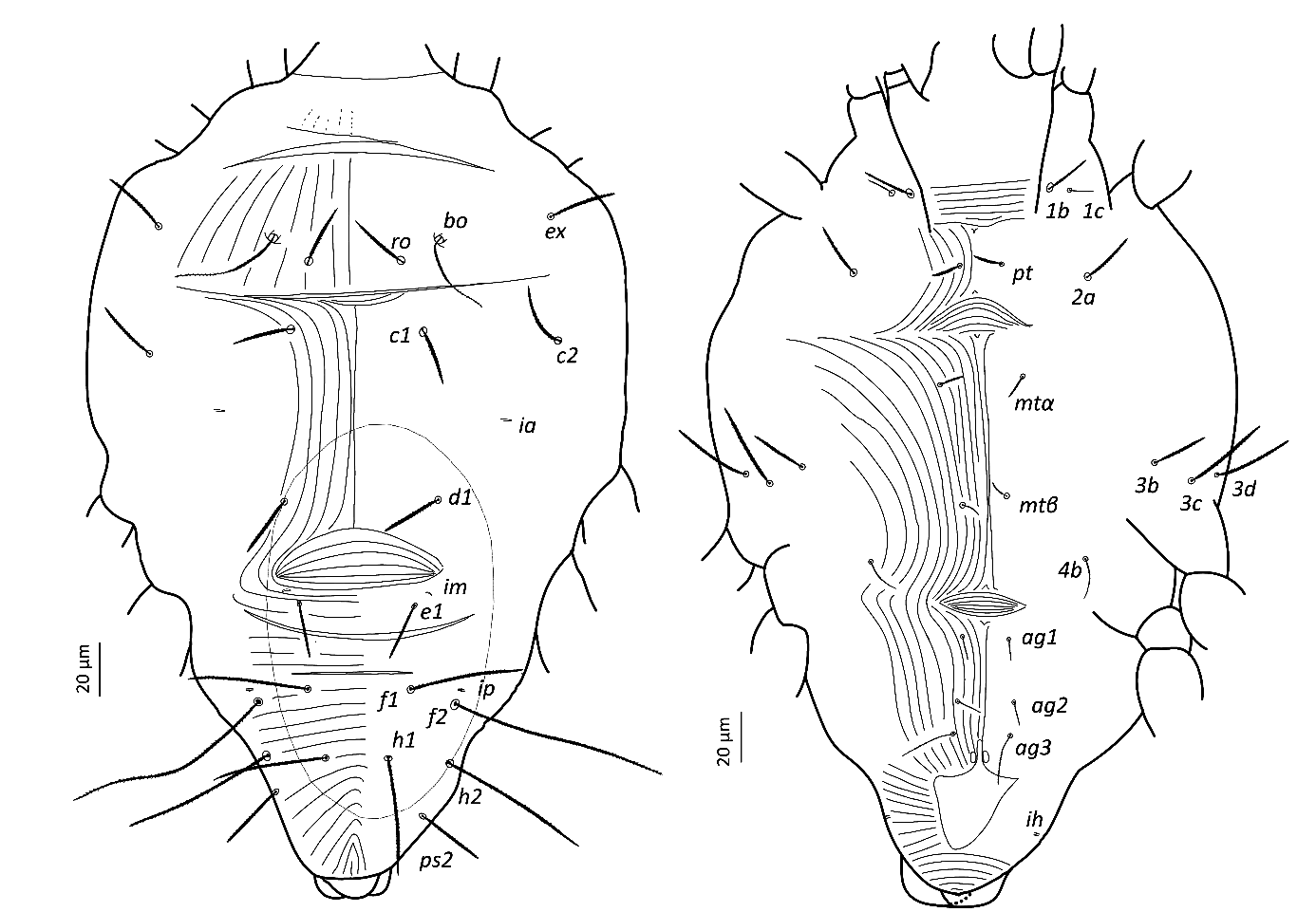
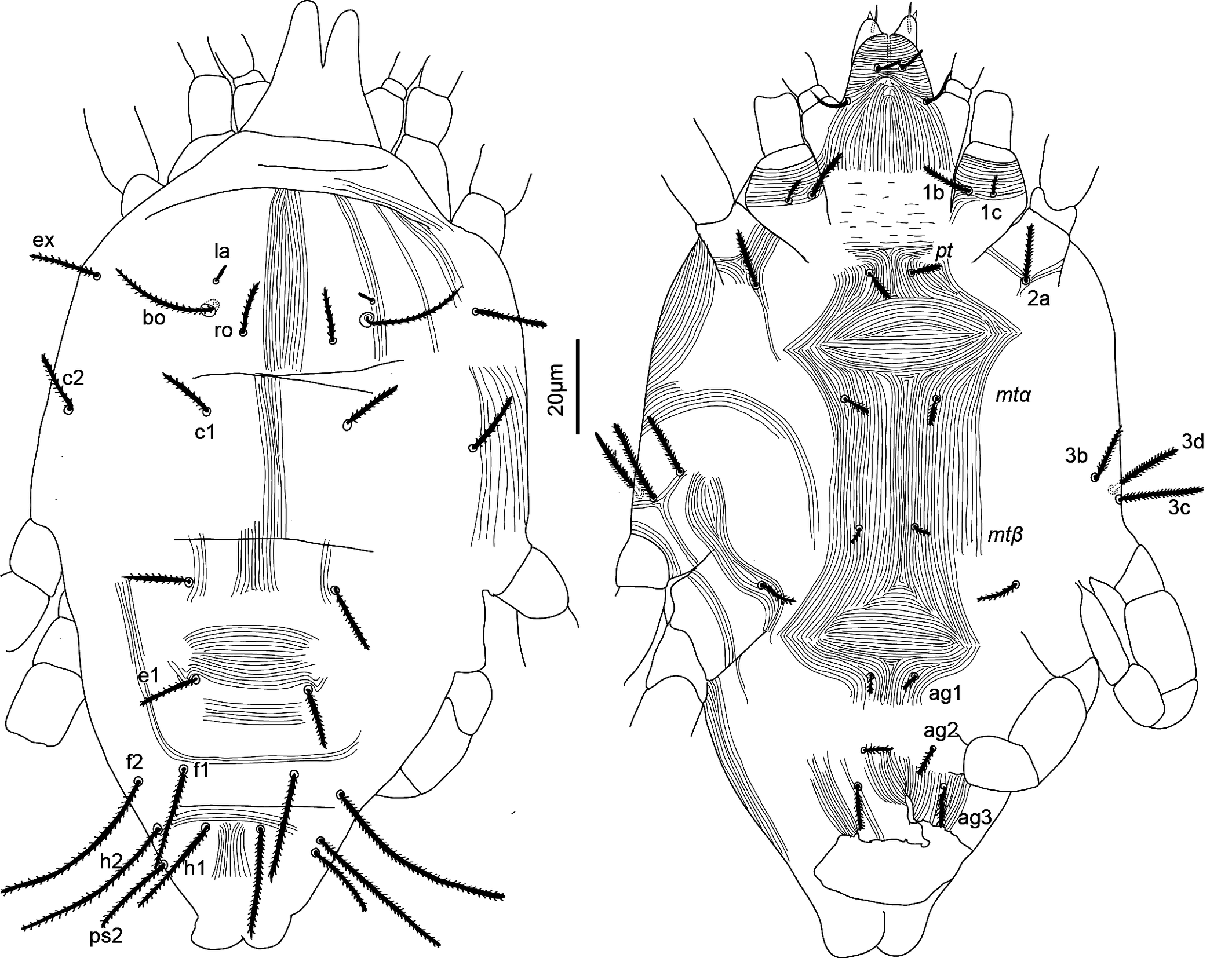
Idiosoma — (Figures 1, 4). Ovaloid, tapering posteriorly. Distance bo-h1 134 (127–140), width at level of c1 118 (106–131). Completely striate, and striae with tubercles.
Dorsum — (Figures 1, 4a-b). Aspidosoma with striation diverging towards the das (sejugal) furrow (Kaźmierski 1989); dorsal part of opisthosoma with longitudinal striation between c1 and d1; diverging towards das, to form a short but wide spindle-shaped striation pattern between ro and c1; also diverging posteriorly to form a spindle-shaped pattern between d1 and e1; striation transverse between f1 and h1 and inversed V-shaped posteriorly, up to ps1, and about transverse between ps1 and the posterior margin.
Most idiosomal setae aciculate (Figures 4a, b, g, h), apparently serrate, their surface at least with a rough aspect (overlapping with striae makes it unclear); trichobothrial setae (bo) on aspidosoma longer than ex, which in turn is longer than subequal la and ro; la somewhat thinner than others of subequal thickness (Figures 1, 4a); bo finely barbed, except for a smooth proximal section (Figure 4f). Dorsal setae f2 (Figure 4h) and h1 longest followed by c1, e1 and ps2; other setae shorter, with ps1 very short. Setae f1 (Figure 4g), h1 and ps1 almost in a straight row and decreasing in length and much shorter than respectively f2, h2 and ps2, also in a straight row and decreasing in length. Setae c1 and d1 not reaching bases of respectively d1 and e1 but e1 about as long as distance between its base and the base of f1. Setal lengths and distances between setae: bo 27 (24–28), bo-bo 26 (26–27), ro 12 (12–13), ro-ro 21 (20–21), la 15 (14–16), la-la 27 (27–28), ex 19 (19–20), c1 15 (14–15, c2 15 (15–16), c1-c1 33 (31–35), d1 15 (15–16), d1-d1 25 (22–27), e1 16 (15–17), e1-e1 34 (33–35), f1 12 (11–13), f1-f1 10 (9–10), f2 24 (22–26), h1 10 (9–11), h2 22 (21–23), ps1 4 (3–4), ps2 14 (13–14).
Lyrifissure ia located between setae c1 and d1, slightly laterad hypothetical line connecting the bases of these setae; lyrifissure im immediately anteriad e1, in hypothetical line between d1 and e1; lyrifissure ip immediately anteriad f2, slightly laterad hypothetical line between e1 and f2.
Venter — (Figures 1, 4 c-d). Striation transverse immediately behind gnathosoma, longitudinal between coxae I and II and between coxae III and IV; striation spindle-shaped between setae pt and mtα and anteriad ag1, the former much larger than the latter; striae converging from ag1 to level of genital opening.
With four pairs of ag setae; distances between ag1-ag1 11 (11–12), ag2-ag2 21 (17–25), ag3-ag3 18 (17–19) and ag4-ag4 30 (30–31). Setae ag1 positioned within the spindle-shaped striation pattern. One pair of genital papillae. Seta ps3 very short, anterolaterad anal opening, without small excrescence in between them (which is present in other genera). Lyrifissure ih laterad genital opening.
Setal lengths and distances: 1b 13 (11–14), 1c 12 (11–14), pt 8 (8–9), pt-pt 14 (13–14), 2a 17 (16–17), mtα 9 (9–10), mtα-mtα 26 (22–31), 3b 14 (13–14), 3c 14 (14–16), 3d 15 (14–16), mtβ 8 ± (8–9), mtβ-mtβ 19 (16–24), 4b 14 (13–15), ag1 7 (7–8), ag2 7 (7–8), ag3 5 (4–5), ag4 10 (9–11), ps3 4 (4–5).


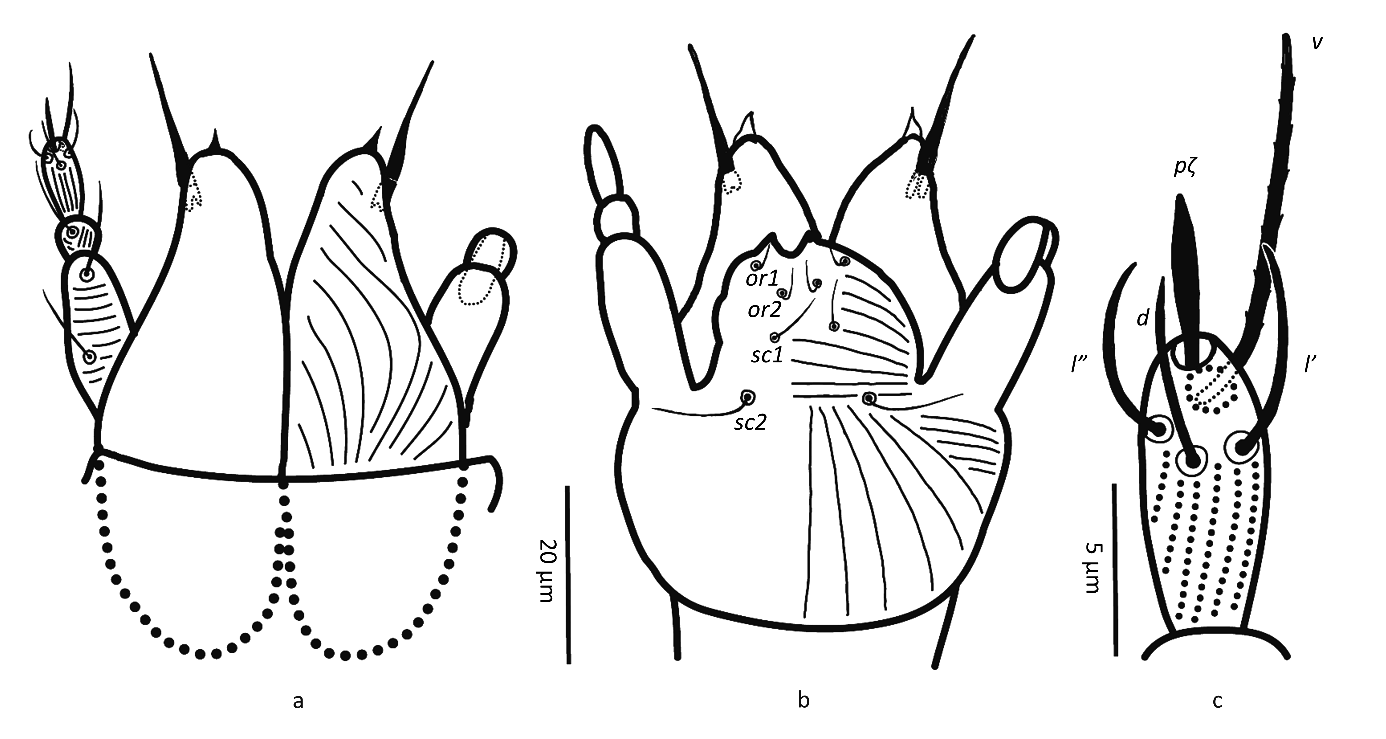
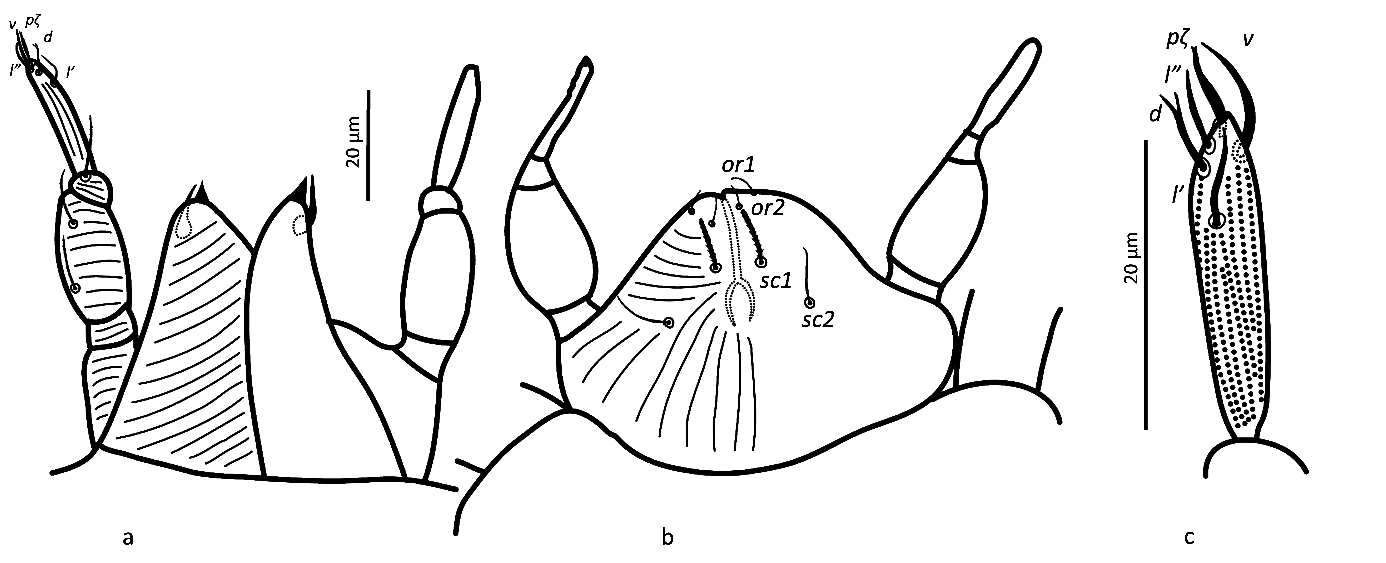
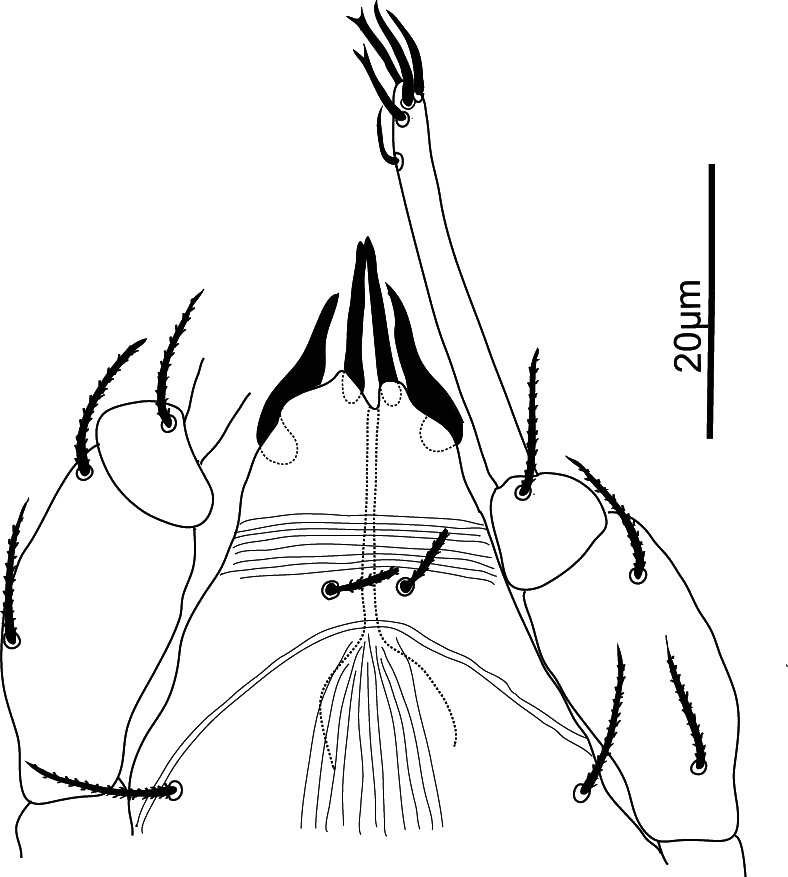
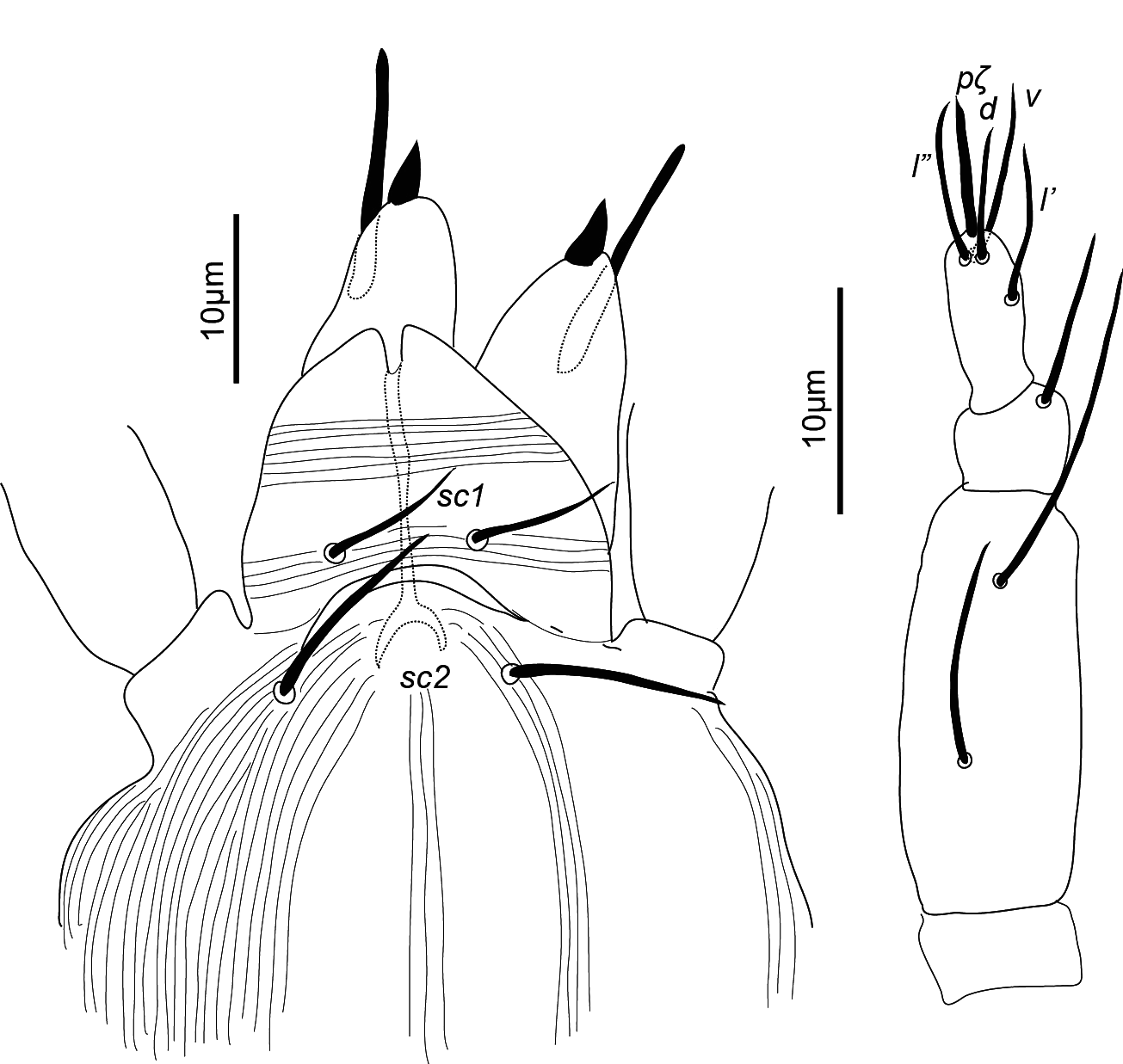
Gnathosoma — (Figure 2). Chelicera (abaxial view) with parallel diagonal striae converging posteriorly; stylet 17 (16–18). Subcapitulum with striae in section anteriad sc2 almost transverse; posteriad sc2 diverging posteriorly, except behind palp, where they are transverse; with two pairs of oral setae, or1 5 and or2 5 and two pairs of scapular setae, sc1 8, sc2 11. Palp tarsus 7 (7–8) long, with lobed longitudinal striae, bearing distally a lanceolate and smooth eupathidium pζ 4 (4–5); setae l′ and l″ curved towards eupathidium, seta v stout, longer than other setae and finely serrated (barely distinguishable); setae l′, l″ and d smooth, l′ a bit shorter than the other two setae, solenidion and ba not visible; l′, l″ and d inserted at about same level, slightly more proximal than v; chaetotaxy from distal to proximal segment 5-1-2. Femorogenu 21 long and 8 in diameter.


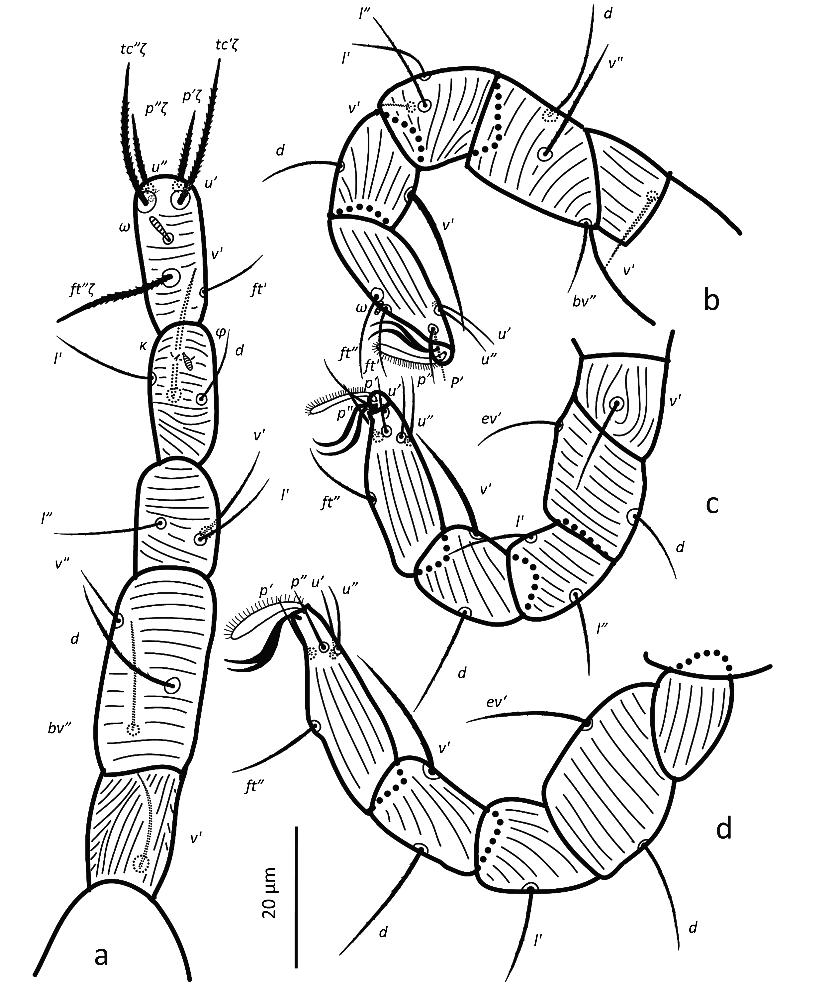
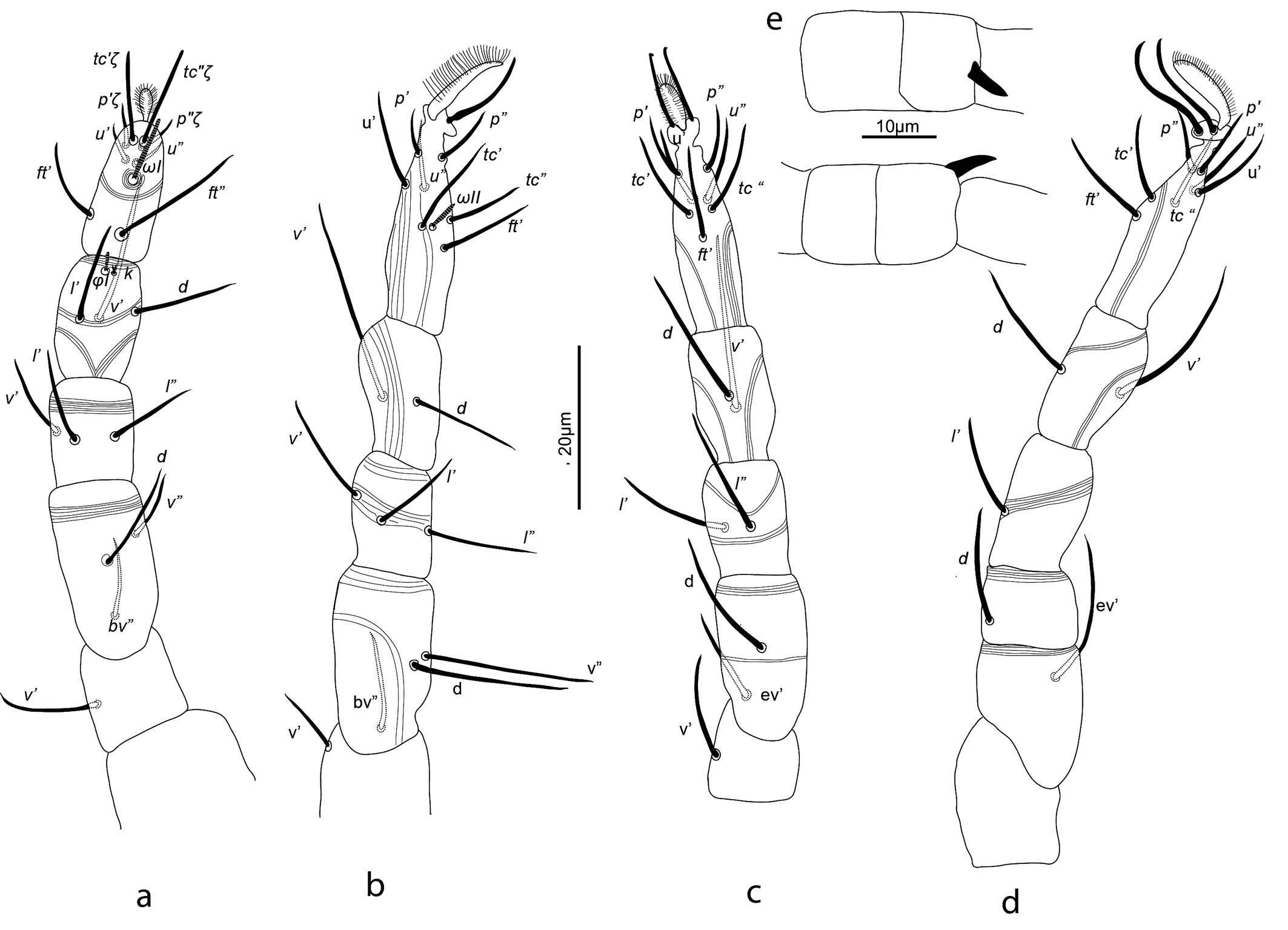
Legs — (Figure 3). Leg lengths (from base of trochanter to base of claws): I 96 (95–98), II 81 (79–85), III 78 (77–80), IV 88 (83–93), tarsus I length 20 (20–21), tibia I length 19 (18–20). Leg I without pretarsus; each of other legs with ciliate empodium and two claws, without empodial hooks. All leg setae aciculate, finely serrate. Ventral setae of all tibiae stouter than other leg setae, serration hardly visible. Leg I with solenidion ωI not reaching tip of tarsus, eupathidia ft″ζ, tc′ζ, tc″ζ (Figure 4e), p′ζ and p″ζ barbed, except distal third; subequal prorals about 0.6 as long as subequal tectals, and a pair of small forked unguinal setae proximal to prorals on ventral side, seta homologous to s absent (see Ueckermann and De Vis et al. 2024). Tibia I with bifurcated famulus κ, about half as long as solenidion φI. Femur IV undivided. Setal lengths: leg I: ωI 4 (4–5), ft′ζ 11 (11–12), ft″ζ 18 (18–19), tc′ζ 19 (19–20), tc″ζ 20 (19–22), p′ζ 12 (11-12), p″ζ 11 (10–11), u′ 3 (2–3), u″ 2 (2–3), φI 3 (2–3), κ″ 1 (1–2); leg II :ωII 2 (1–2), tibia v′ 23 (22–23), femur v″ 18 (16–19); leg III: tibia v′ 21 (20–22), tibia d 17 (16–18); leg IV: tibia v′ 21 (19–22), tibia d 21 (20–22), femur ev′ 18 (17–18), femur d 17 (16–18).




Protonymph (n = 1)
Idiosomal chaetotaxy as in female but mtβ and all aggenital setae absent, two setae on coxa III (3d absent) and 4b absent, hence epimeral formula 3-1-3-0. Leg chaetotaxy like female but with no setae on trochanters I and II (instead of one in each), and segments of leg IV nude except for tarsus IV (as in adult female). Hence, protonymph with the following chaetotaxy: I (8+ω-3+k+φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-1), IV (5-0-0-0-0).
Male and other immature stages
Not found.
Differential diagnosis
This is the only species in this genus, but differences with related species of other genera are subsequently mentioned. The placement of the ag setae is similar to that of the closely related D. nadirae, but it differs by having 11 pairs of opisthosomal setae, seta l″ on palp not sigmoid, setae p subequal to each other, tc also subequal to each other, setae tc about twice as long as setae p; eupathidia not completely barbed, spindle-form striae near ag1 small, only a bit broader than distance ag1-ag1. It can be distinguished from the closely related Neopronematus species by the presence of 11 pairs of opisthosomals, non-forked ag3 and the position of ag setae.
Type locality and habitat
Collected by J. D. A. Lacerda on July 13, 2023, on leaves of an unidentified Bauhinia L. (Fabaceae) species at Fazenda Vitória (5°58′6.79″ S, 49°47′39.71″ W, and 166 m altitude), Palmares II, Parauapebas, Pará State, Brazil.
Type specimens and depository
Holotype female (MZLQ 4382), two paratype females (MZLQ 4383 and MZLQ 4384) and one paratype protonymph (MZLQ 4385) deposited at the Mite Reference Collection of Departamento de Entomologia e Acarologia, Escola Superior de Agricultura ''Luiz de Queiroz'' (ESALQ-USP), Universidade de São Paulo (USP), Piracicaba, São Paulo state, Brazil, ESALQ (Zhang 2018).
Etymology
The species name refers to Pará, the Brazilian state where the type specimens were collected.
Parapronematus Baker, 1965
Parapronematus connarus De Vis & Ueckermann n. sp.
ZOOBANK: 8DEAC753-7D23-4907-B906-A6D7D596FCC6 ![]()
(Figures 5–11)
Measurements — Measurements of different structures of each life stage shown in Table 1.
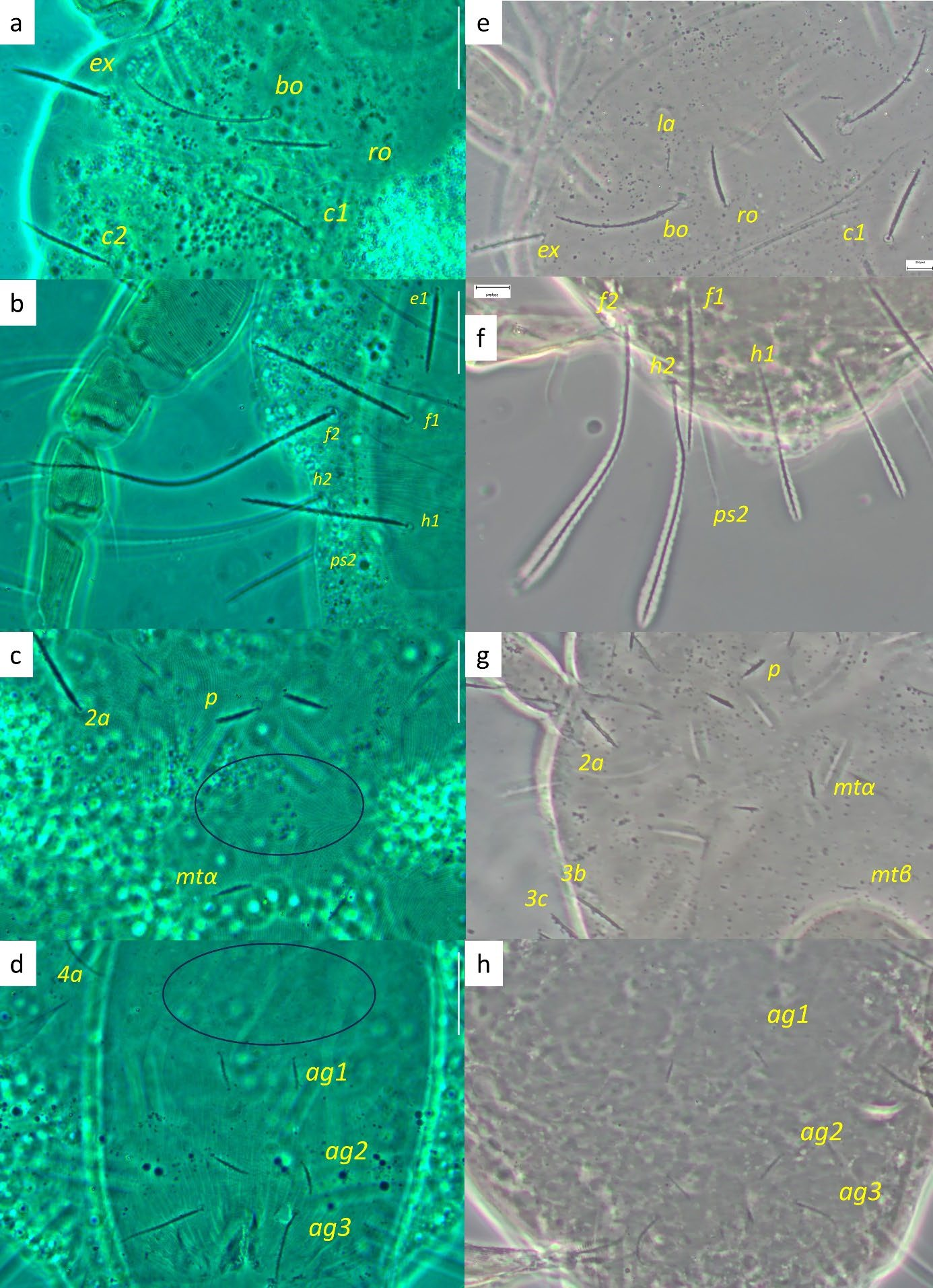
Adult female (n = 20)


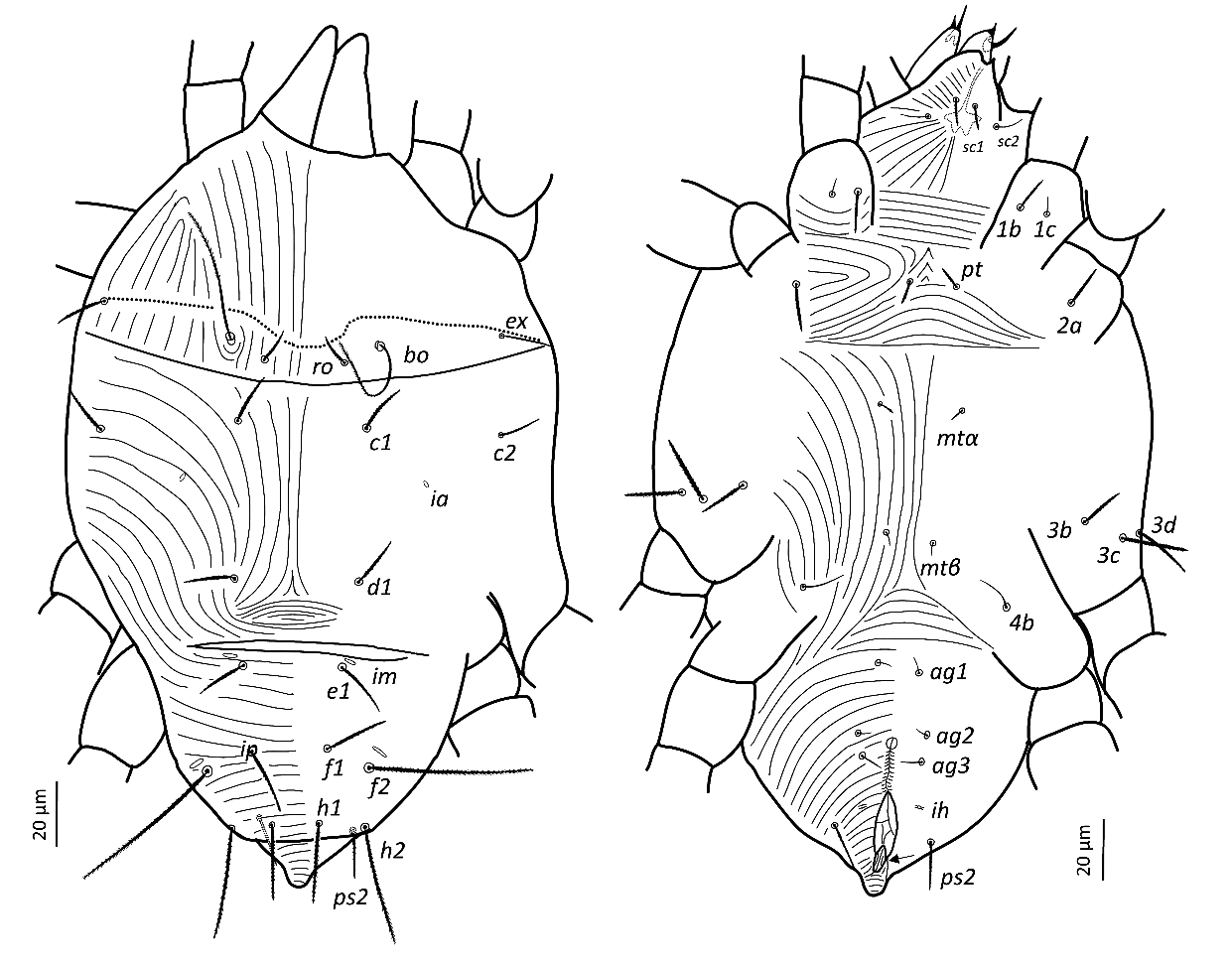
Idiosoma — (Figures 5, 8). Broad, pyriform, tapering posteriorly. Completely covered with fine tuberculate striae, less discernible than in other pronematid genera. Aspidosoma with three pairs of setae (bo, ro and ex; la absent) and opisthosoma with nine pairs of setae (c1, c2, d1, e1, f1, f2, h1, h2 and ps2; ps1 and ps3 absent); lyrifissures ia, im, ip and ih present, but mostly not discernible in adult female, most distinguishable in adult males and immatures.
Dorsum — (Figures 5, 8a-b). Aspidosoma with diverging striation towards das furrow (Kaźmierski 1989); dorsal part of opisthosoma with longitudinal striation between c1 and d1; diverging towards das to form a short but wide spindle-shaped striation pattern between ro and c1; also diverging posteriorly to form a spindle-shaped pattern between d1 and e1; striation transverse between f1 and h1 and inversed V-shaped posteriorly.
Idiosomal setae aciculate and distinctly serrate (Figures 5, 8a-b, 9c-d); trichobothrial setae bo (Figures 5, 9b) on aspidosoma longer than ex, which is in turn longer than ro; ex and ro stouter than bo (Figures 1, 4b); bo finely barbed, barbs longer in distal half (Figure 9b). Dorsal setae f2 (Figure 9d) and h2 longest, followed by f1 (Figure 9c) and h1, and then c2 and ps2; c1 shortest. Setae c1, d1 and e1 not reaching bases of respectively d1, e1 and f1, but f1 passing base of h1.
Lyrifissures not always distinguishable, ia located between setae c2 and d1; lyrifissure im immediately anteriad e1; lyrifissure ip immediately anteriad f2.
Venter — (Figures 5, 8c-d). Striae transverse between coxae I; concave outwards in the region of pt setae; about longitudinal medially between levels of mtα and 4b; spindle-shaped between setae pt and mtα and anteriad ag1; converging posteriorly to level of genital opening, around which it is radiating and behind of which it is about transverse.
With three ag setae. One pair of genital papillae, only occasionally distinct. Lyrifissure ih between genital opening and seta ps2.
Gnathosoma — (Figure 6). Chelicera with parallel diagonal striae in dorsal view. Striae in section anteriad sc2 of subcapitulum almost transverse, posteriad sc2 almost longitudinal medially, diverging posteriorly elsewhere; with two pairs of oral setae (or1 4 and or2 4) and two subcapitular setae (sc1 10, stout and barbed and sc2 13, thin and smooth). Palp tarsus long and narrow, with lobed longitudinal striae, bearing distally a slightly forked eupathidium pζ; setae l′ and l″ curved towards eupathidium; seta d distally forked; seta v stout and barely longer than eupathidium; all setae smooth, solenidion ω and ba not distinguishable; l′ inserted more proximal than d, which is more proximal than l″, which is about at the same level as v; chaetotaxy (5-1-2). Femorogenu 21 long and 12 in larger diameter.


Legs — (Figure 7). All leg setae aciculate, finely serrate with dorsal setae, in general, stouter and longer than ventral setae of the same leg segment (although less clearly on leg III). Ventral setae on tibiae gradually varying from stout and long on tibia I to fine and short on tibia IV. Leg I with solenidion ω not reaching tip of tarsus, eupathidia ft″ζ, tc′ζ, tc″ζ, p′ζ and p″ζ barbed for their entire length, except for smooth tip 2 µm, tectals subequal, prorals shorter and unequal, with p″ζ the shortest, and a pair of small forked unguinal setae proximal to prorals on ventral side, seta homologous to s absent (see Ueckermann and De Vis et al. 2024). Tibia I with bifurcated famulus κ but this not always visible, and tip can also appear as a knot (bifurcation not visible), about half as long as solenidion φ. Tarsus III with six setae, but two specimens with only five (specimens 113 and 112 in Table 1; probably shortest, most distal ft′ absent and no setal base visible); these two specimens lack the other leg III, so double checking is not possible. Femur IV undivided. Dorsal setae d on both femora III and IV forked.
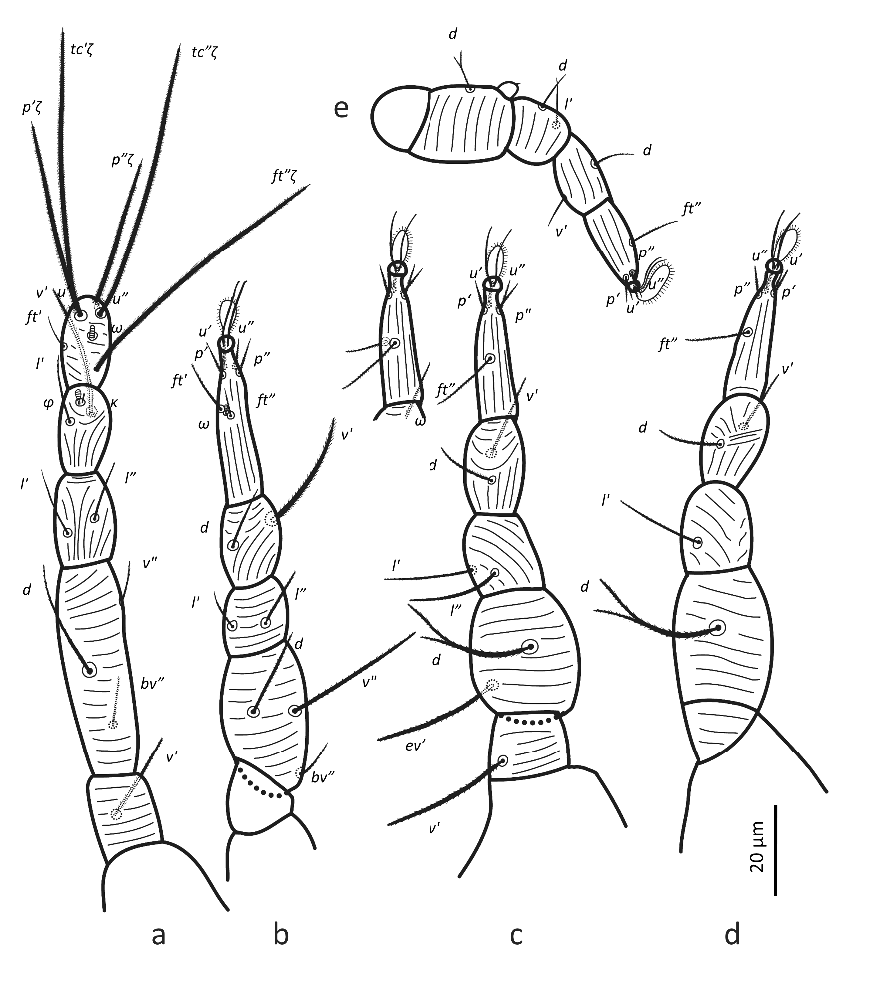




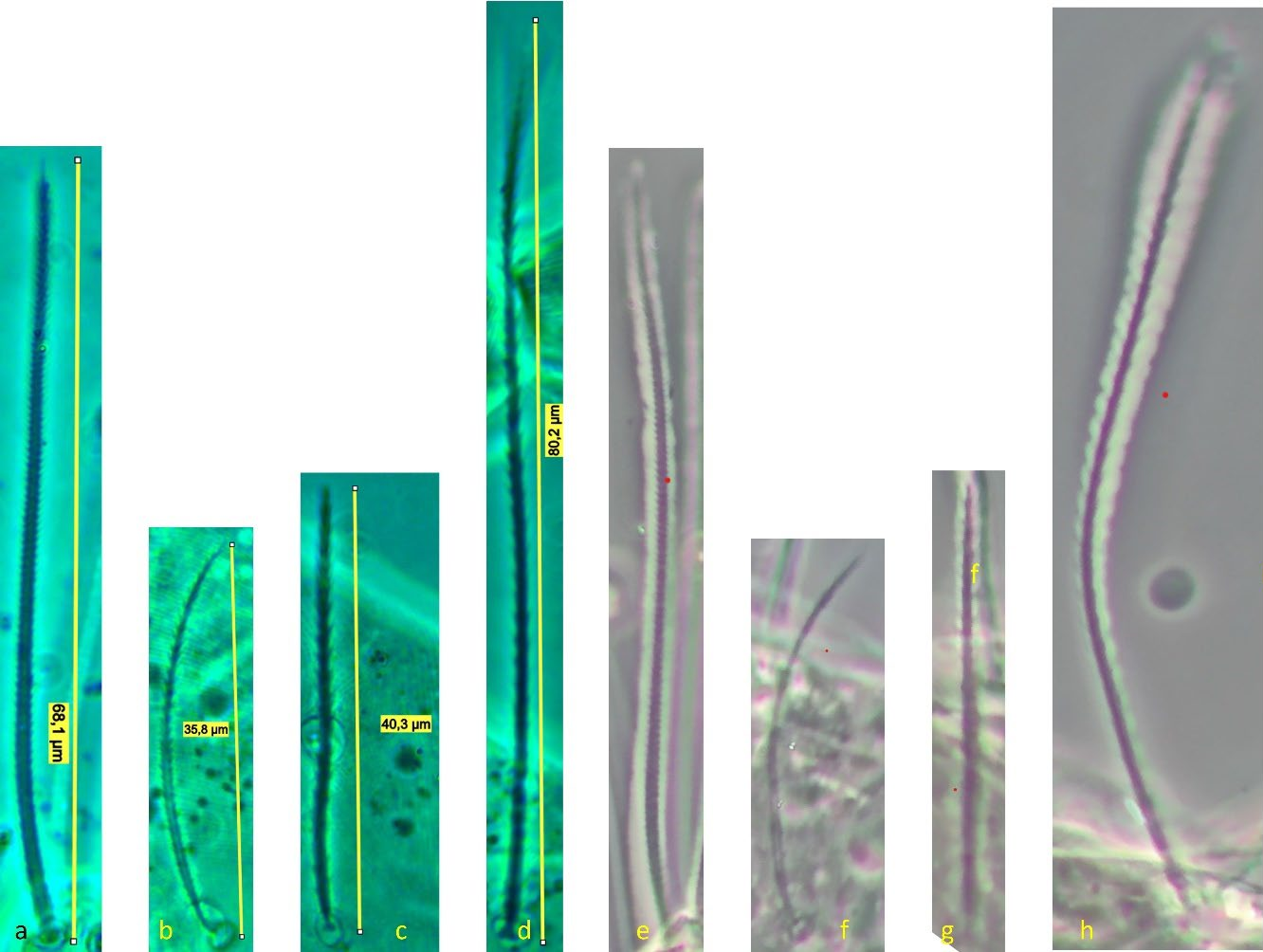


Male (n = 7) (Figures 7, 10)
Idiosoma — Most characters as in female, except: dorsal setae shorter, serrate; with one pair of rounded structures of 11 µm in diameter posterolaterad e1, genital opening with two pairs of eugenital setae. Femora III and IV each with forked seta relatively shorter than in female. Femur IV with a small and pointed dorso-distal adaxial spur inserted on a thumb-like process (Figure 7).


Tritonymph (n = 5) (Figure 11)
Dehiscence line anteriad bo, ro and ex, projecting toward ro; idiosomal and leg chaetotaxy as in adults. Lyrifissures clearly visible. Epimeral formula as in female. With longitudinal invagination connecting genital and anal openings. With distal excrescence, inserted anteriad anal opening and bending over it. Seta ft′ on tarsus III present. Dorsal setae on femur III not forked or with one very short branch (1 µm); femur IV with dorsal seta not forked.
Deutonymph (n = 1)
Dehiscence line as in tritonymph. Idiosomal chaetotaxy as in female but with only one ag seta instead of three. Epimeral formula as in female. With longitudinal invagination connecting genital and anal openings. With distal excrescence as in tritonymph. Leg chaetotaxy as in female, but with trochanter I nude instead of with one seta; with one dorsal seta on tarsus III instead of two (ft′ lacking); and setae on femora III and IV not forked. Hence, leg chaetotaxy as follows: leg I (8 + ω – 2 + φ + κ – 2 – 3 – 0); leg II (6 + ω – 2 – 2 – 3 – 0); leg III (5 – 2 – 2 – 2 – 1); leg IV (5 – 2 – 1 – 1 – 0).
Protonymph (n = 2)
Dehiscence line as in tritonymph. Idiosomal chaetotaxy as in female, but without ag setae, with only two setae on coxa III and without setae mtβ and 4b, hence epimeral formula 3-1-3-0. With longitudinal invagination connecting genital and anal opening. With distal excrescence as in tritonymph. Leg chaetotaxy as in female but trochanter I nude instead of with one seta, with only five setae on tarsus III instead of six, and leg IV segments nude except tarsus, as in female. Hence, protonymph with the following chaetotaxy: leg I (8 + ω – 2 + φ + κ – 2 – 3 – 0), leg II (6 + ω – 2 – 2 – 3 – 0); leg III (5– 2 – 2 – 2 – 1); leg IV (5– 0 – 0 – 0 – 0).


Larva (n = 2)
Idiosomal chaetotaxy as female but all ag setae absent and coxa III with only one seta (3c and 3d absent); epimeral formula 3-1-2. Leg chaetotaxy as in female, but with no unguinal setae (u), trochanter III nude instead of with one seta. Thus, chaetotaxy: leg I (6 + ω – 2 + φ + κ – 2 – 3 – 0), leg II (6 + ω – 2 – 2 – 3 – 0), leg III (5– 2 – 2 – 2 – 0). Tarsus I with small pad like empodium, without claws (om), setae p and ft not eupathidial, setae tcζ eupathidial.
Differential diagnosis
This species differs from P. geminus by having three setae on aspidosoma instead of four; from P. formosanus by having three setae on each of femora I and II instead of two; from P. citri by having dorsal setae ro, ex, c1, c2, d and e2 longer than 13 µm; from P. acaciae by having setae f1 30 ± 0,9 (25–41) 19, h1 33 ± 0,7 (28–43), h2 56 ± 0,6 (51–63) shorter and p″ζ 42 ± 0,6 (38–47), instead of f1≈49 , h1≈49, h2≈68 and p″ζ≈34; and from P. camelia, P. ferox and P. murshidabadensis by having tc″/tarsus I > 3 instead of < 2 (see Table 3).
Remarks
Parapronematus connarus De Vis & Ueckermann n. sp. shows great variability in relation to the number of setae on tarsus III and some setal lengths. Two specimens (112 and 113) have five setae (ft′ absent) on tarsus III instead of six. Together with specimen 121, these have longer setae ro, c2, f1, f2, h1, 3d, ag2 and ag3 than the other specimens of the same new species (Table 1), with specimen 122 (MZLQ 4386) showing other aberrations. Still, other characteristics of these specimens were the same as in other specimens. The bold setae of specimens 112 (MZLQ 4387), 113 (MZLQ 4388) and 121 (MZLQ 4404) do not differ from the holotype. Therefore, we do not consider these three specimens to belong to a different species. Baker (1965) and André (1980) were not clear on that, mentioning the presence of five or six setae on tarsus III in species of this genus. Additionally, although the differences in setal lengths might support the thesis that they belong to a different species, the two specimens with five setae on tarsus III were found together with three specimens with six setae on a single host plant. Seta ft′ appears during ontogeny in the tritonymph.
The seta on trochanter I and the second dorsal seta on tarsus III also appear in the tritonymph of Homeopronematus, Quasihomeopronematus (André 1980; De Vis and Ueckermann 2024b), Pseudopronematulus (De Vis and Ueckermann 2024a) and Proctotydaeus (André 1980). This seems to be a general pattern.
Type specimens and depository
Parauapebas, Pará, J.D. Lacerda, on Connarus sp., July 2023, holotype female (MZLQ 4386); on Clusia rosea, July 2023, 5 paratype females (MZLQ 4387–4391); on Eucalyptus spp., July 2023, paratype female (MZLQ 4392), 2 paratype deutonymphs (MZLQ 4393 and MZLQ 4394); on Andropogon gayanus, July 2023, paratype female (MZLQ 4395); on Arrabidaea chica, July 2023, paratype female (MZLQ 4396); on Musa paradisiaca, July 2023, paratype male (MZLQ 4397); on Sida rhombifolia, July 2023, paratype tritonymph (MZLQ 4398); on Stachytarpheta sp., July 2023, paratype female (MZLQ 4399); on Connarus sp., July 2023, paratype male (MZLQ 4400); on Syzygium malaccense, July 2023, 2 paratype males (MZLQ 4401 and MZLQ 4402). Piracicaba, São Paulo State, R.M.J De Vis, on Hevea brasiliensis, July, 2002, paratype male (MZLQ 4403); on H. brasiliensis, April- May, 2003, 4 paratype females (MZLQ 4404, MZLQ 4405, MZLQ 4406, MZLQ 4407, MZLQ 4408), 2 paratype larvae (MZLQ 4409 and MZLQ 4410), 2 paratype protonymphs (MZLQ 4409), 3 paratype tritonymphs (MZLQ 4404, MZLQ 4411 and MZLQ 4412). Pontes e Lacerda, Mato Grosso State, N.J. Ferla, on H. brasiliensis, date unknown, paratype female (MZLQ 4413); H. brasiliensis, September-October 1999, 2 paratype females (MZLQ 5658 and MZLQ 5659), paratype male (MZLQ 5659). Itambé, Pernambuco, M.G.C. Gondim Jr., on Acrocomia intumescens, July 1998, paratype female (MZLQ 4414), paratype tritonymph (MZLQ 4473). All types deposited at the Mite Reference Collection, ESALQ-USP, Piracicaba, São Paulo State, Brazil.
Etymology
The species is named after Connarus, the genus of the host plant of the holotype.
PCA analysis
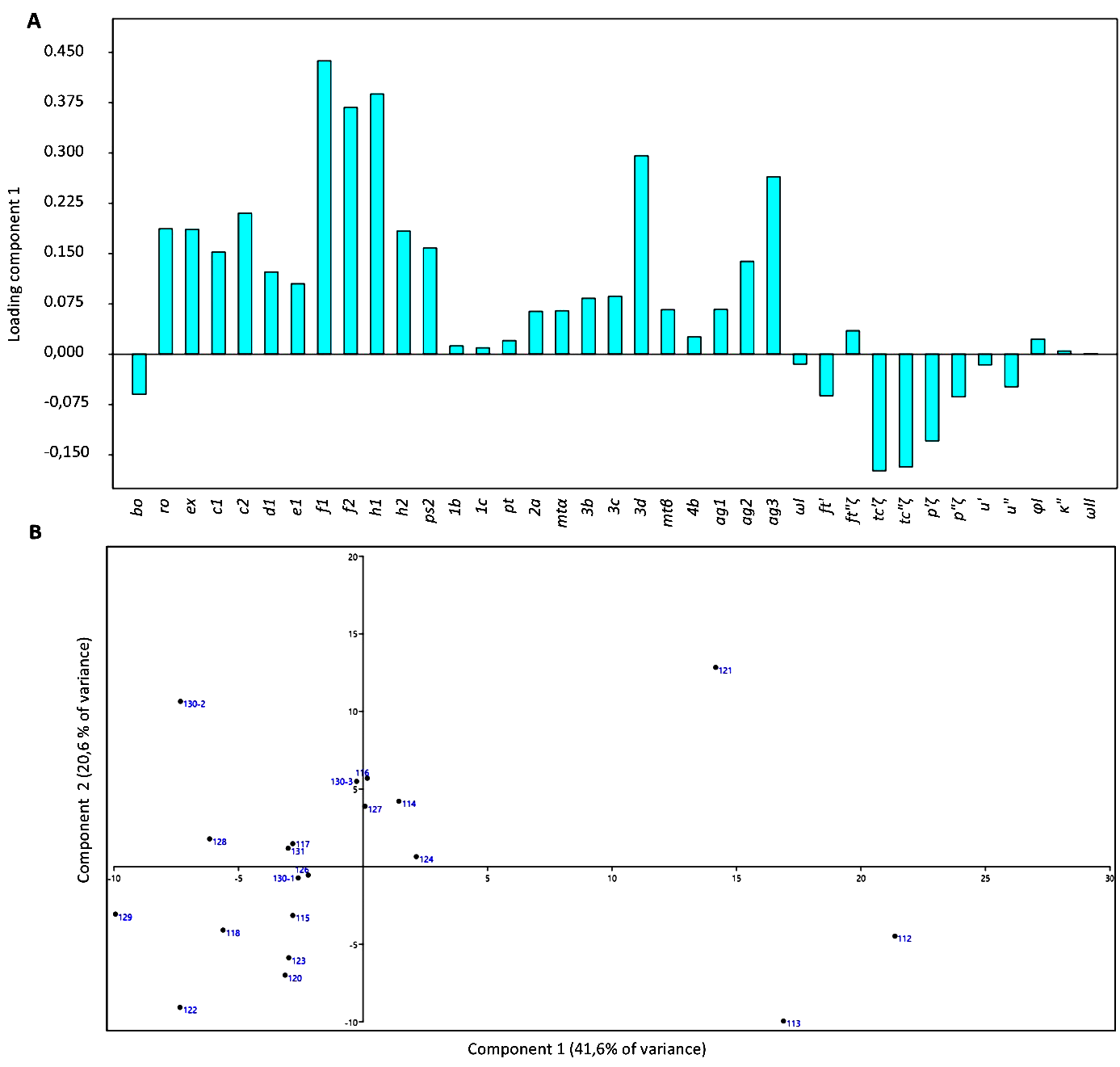
The characteristics that define the major differences between the specimens are setae f1, f2, h1, 3d, ag3, tc′ζ, tc″ζ and p′ζ (Figure 12A, Table 1). Specimens 112, 113 and 121 stand out from the other specimens (Figure 12B).


Download as
Female
Male
Tritonymph
Deutonymph
Protonymph
Larva
All
Spc. 112
Spc. 113
Spc. 121
Spc. 122
bo-h1
190 (170–231)
191
198
182
188
134 (126–146)
156 (146–172)
112
108 (97–120)
89 (88–89)
width (at level c1)
184 (155–212)
198
194
168
196
124 (109–138)
151 (147–158)
133
110 (103–116)
102 (101–103)
bo
44 (39–50)
41
39
50
40
33 (32–35)
42 (40–44)
39
36 (35–37)
33 (33–34)
bo-bo
52 (46–62)
58
62
51
56
37 (33–45)
46 (40–50)
44
37 (37–38)
29 (29–30)
ro
18 (16–22)
22
22
20
16
10 (8–12)
11 (9–13)
117
9 (8–9)
9 (8–10)
ro-ro
27 (23–36)
34
36
28
28
19 (16–21)
24 (21–26)
23
21 (19–22)
16 (15–16)
ex
23 (20–27)
27
25
26
23
13 (10–15)
15 (13–17)
14
11 (10–11)
12 (11–12)
c1
21 (18–29)
21
21
29
19
12 (9–15)
15 (13–17)
17
13 (13–14)
14 (13–14)
c2
22 (18–26)
26
24
26
18
9 (8–11)
14 (13–16)
13
11 (11–12)
14 (13–16)
c1-c1
52 (39–66)
48
49
48
54
33 (31–35)
41 (36–46)
35
34 (34–35)
35 (34–37)
d1
21 (19–26)
23
22
26
20
13 (11–16)
15 (13–17)
15
12 (12–13)
12 (11–13)
d1-d1
51 (34–60)
55
59
53
60
32 (31–33)
40 (38–43)
34
31 (31–32)
32 (31–32)
e1
21 (19–26)
22
20
26
19
13 (10–15)
15 (13–19)
15
11 (10–11)
10 (10–11)
e1-e1
42 (36–63)
43
49
42
43
25 (22–27)
30 (28–34)
26
22 (21–22)
24 (24–25)
f1
30 (25–41)
39
41
35
30
19 (17–21)
19 (16–22)
17
13 (13–14)
11 (11–12)
f1-f1
36 (29–45)
44
45
37
34
24 (24–26)
26 (23–33)
20
17 (16–17)
12 (12–13)
f2
73 (67–81)
81
77
79
70
47 (45–52)
52 (49–55)
45
34 (33–344)
26 (24–28)
h1
33 (28–43)
43
39
38
32
22 (21–24)
22 (19–26)
19
14 (14–15)
11 (10–11)
h2
56 (51–63)
58
56
63
51
34 (32–37)
36 (35–37)
19
21 (20–22)
15 (14–15)
ps2
24 (21–29)
29
28
25
21
16 (14–18)
17 (15–21)
19
9 (8–9)
4 (3–4)
1b
18 (17–20)
19
17
19
18
13 (12–15)
13 (12–13)
12
9 (9–10)
9 (9–10)
1c
8 (6–10)
9
7
8
6
6 (5–7)
6 (5–9)
5
4 (4–5)
5 (4–5)
pt
11 (10–13)
11
11
13
11
9 (8–10)
8 (7– 9)
6
6 (5–6)
5 (5–6)
pt-pt
16 (11–21)
16
21
15
17
13 (11–16)
16 (15–17)
14
14 (14–15)
15 (14–15)
2a
21 (17–23)
22
21
23
21
15 (12–16)
14 (12–16)
12
11 (10–12)
10 (10–11)
mtα
9 (6–11)
9
11
10
9
7 (6–8)
6 (5–8)
5
4 (4–5)
4 (4–5)
mtα-mtα
31 (22–42)
33
37
30
22
22 (18–25)
26 (219–31)
25
23 (22–24)
24 (23–25)
3b
20 (17–22)
22
19
20
19
13 (11–14)
13 (11–15)
12
9 (9–10)
10 (9–10)
3c
32 (28–36)
33
30
35
30
18 (15–20)
22 (20–24)
22
15 (15–16)
Absent
3d
26 (21–32)
32
31
32
25
16 (14–17)
18 (13–21)
14
Absent
Absent
mtβ
7 (5–9)
8
9
7
6
5 (4–7)
5 (4–5)
4
Absent
Absent
mtβ-mtβ
21 (17–26)
20
-
19
22
15 (13–18)
16 (15–20)
19
Absent
Absent
4b
15 (13–18)
17
-
17
13
11 (9–14)
10 (8–12)
8
Absent
Absent
ag1
7 (6–9)
8
9
7
6
5 (5–,6)
4 (4–6)
4
Absent
Absent
ag1-ag1
17 (13–24)
18
24
15
15
9 (9–10)
11 (8–14)
11
Absent
Absent
ag2
8 (7–12)
12
11
8
7
5 (4–6)
5 (3–7)
absent
Absent
Absent
ag2-ag2
29 (14–47)
22
24
29
30
20 (18–22)
20 (18–22)
absent
Absent
Absent
ag3
15 (12–22)
22
21
14
20
8 (8–10)
7 (2–10)
absent
Absent
Absent
ag3-ag3
37 (22–65)
23
22
34
45
28 (24–32)
21 (18–24)
absent
Absent
Absent
eu1
Absent
absent
absent
absent
absent
3 (3–4)
Absent
absent
Absent
Absent
eu2
Absent
absent
absent
absent
absent
4 (3–5)
Absent
absent
Absent
Absent
palptarsus
23 (20–26)
24
24
26
21
16 (14–18)
20 (19–22)
13
13 (12–13)
12 (12–13)
palp p ’ζ
6 (4–8)
8
7
6
7
4 (4–5)
5 (5–6)
3
3 (3–4)
3 (3–4)
stylet
13 (11–15)
11
14
14
14
10 (10–11)
12 (10–12)
10
10 (10–11)
9 (8–9)
leg I
142 (129–154)
140
139
147
141
110 (100–123)
118 (106–132)
84
68 (67–70)
67 (66–68)
leg II
121 (112–134)
116
114
128
122
99 (94–112)
108 (101–118)
83
66 (65–66)
64 (64–65)
leg III
119 (109–147)
125
113
147
124
89 (82–101)
109 (100–125)
77
70 (68–71)
69 (67–70)
leg IV
123 (111–135)
127
111
131
127
95 (86–107)
108 (91–129)
76
65 (63–67)
Absent
tarsus I length
24 (23–26)
24
23
24
23
21 (18–24)
22 (20–24)
18
16 (15–16)
17(16–17)
tibia I length
22 (20–25)
22
20
24
22
18 (17–20)
19 (18–21)
14
12 (12–13)
12 (11–12)
ωI
5 (4–6)
4
4
6
4
4 (3–5)
3 (3–4)
2
2 (1–2)
2 (2–3)
ft’ζ
12 (9–16)
9
9
16
-
9 (7–11)
10 (8–11)
8
6 (6–7)
6 (6–7)
ft"ζ
69 (63–73)
69
69
-
63
539 (49–61)
44 (38–52)
29
11 (10–11)
9 (9–10)
tc’ζ
72 (67–79)
67,5
67
-
-
56 (47–63)
51 (47–59)
41
31 (31–32)
22 (21–23)
tc"ζ
70 (65–76)
66,8
65
-
-
55 (51–60)
49 (44–55)
39
32 (32–33)
30 (29–31)
p’ζ
51 (44–58)
46,7
47
-
48
39 (36–45)
34 (31–40)
26
19 (19–20)
7 (6–7)
p"ζ
42 (38–47)
39,1
40
-
38
31 (26–345)
25 (23–32)
20
16 (16–17)
6 (6–7)
u’
4 (2–5)
3
3
4
2
3 (2–5)
3 (2–3)
4
3 (2–3)
Absent
u"
4 (2–5)
3
3
3
2
3 (2–4)
2 (2–3)
3
3 (2–3)
Absent
φI
3 (2–4)
3
3
3
2
2 (2–3)
3 (2–4)
-
2 (1–2)
κ"
2 (2–3)
3
2
3
-
2 (1–2)
2 (1–2)
2
-
2 (2–3)
ωII
3 (2–4)
3
2
3
2
2 (2–3)
1 (1–2)
13
2 (1–2)
1 (1–2)
tibia II v’
32 (30–34)
31
30
34
31
24 (22–25)
24 (22–27)
22
18 (17–18)
16 (15–17)
tibia II d
19 (17–22)
18
19
22
18
12 (11–13)
13 (12–15)
10
8 (7–8)
7 (6–7)
femur II v"
36 (31–40)
37
39
40
33
25 (23–28)
26 (24–27)
23
17 (16–18)
17 (16–17)
femur II d
25 (23–29)
24
25
27
23
14 (12–17)
20 (18–23)
15
13 (12–13)
13 (12–13)
tarsus III ft’
10 (8–11)
-
-
10
9
8 (7–8)
7 (5–9)
absent
Absent
Absent
tarsus III ft"
19 (17–21)
19
17
20
17
14 (14–15)
14 (13–15)
11
9 (9–10)
10 (9–10)
tibia III v’
20 (18–23)
23
20
22
19
15 (13–16)
15 (15–16)
10
9 (8–9)
8 (8–9)
tibia III d
16 (13–17)
16
16
17
13
11 (9–13)
12 (11–13)
9
8 (8–9)
8 (8–9)
femur III ev’
31 (28–34)
34
32
32
28
21 (21–23)
22 (21–24)
18
15 (15–16)
13 (12–13)
femur III d
36 (32–40)
36
35
40
34
17 (13–20)
30 (27–32)
27
22 (22–23)
20 (20–21)
long fork
16 (10–21)
16
14
21
16
7 (3–10)
11 (8–15)
absent
Absent
Absent
short fork
12 (8–15)
9
11
15
10
5 (4–8)
2 (1–3)
absent
Absent
Absent
tibia IV v’
16 (14–22)
16
22
17
12 (9–13)
12 (11–14)
9
Absent
Absent
tibia IV d
17 (16–20)
18
19
17
16
12 (11–14)
13 (11–15)
10
Absent
Absent
femur IV d
39 (34–44)
38
34
44
36
15 (13–18)
33 (26–36)
24
Absent
Absent
long fork
19 (14–25)
17
15
25
15
8 (6–9)
7 (10–15)
absent
Absent
Absent
short fork
14 (10–18)
13
12
18
13
5 (4–6)
3 (6–9)
absent
Absent
Absent
Reappraisal of Parapronematus
Most of the descriptions of species of this group are rather incomplete. Several of them do not have measurements and none indicates the variability of measurements. Most do not provide scale bars in illustrations. Characters of doubtful value have been used to distinguish species while characters of recognizable value are not always considered. Although complementary descriptions should be done, in this work we could only redescribe P. geminus, as in most cases the museums in which they are deposited do not allow their shipment.
None of the descriptions mentions lyrifissures. However, in our specimens, lyrifissures could be seen in most males and all immatures, despite not always visible in adult females.
Similarly, striae are very fine and indistinct in part of the body of several species. We suppose that they are present but not always visible. In our specimens, digital augmentation shows striae in specimens where striae are not distinguishable under phase contrast microscopic examinations at 1000 X. It seems that this characteristic is influenced by mounting conditions. We therefore do not consider this a reliable characteristic for distinguishing species based on publications of distinct authors (as used for the characterization of P. citri, e.g.).
As the species are not sclerotized, distances between setae are influenced by the state of the mite when mounted, mounting conditions, as the amount of medium used and the ′pressure′ on the specimens, the presence of folds, etc. These aspects will lead to inaccurate measurements of distance between setae. Thus, we also consider this characteristic inadequate to separate species.
Likewise, length of the dorsal setae on femora III and IV in relation to the width of the segment seems a characteristic of questionable value. We believe that the width or height of femora are also influenced by the mounting conditions. So, any parameter comparing the length of the dorsal setae with the width of the segment may be inaccurate and should preferably not be used to separate species. This type of problem seems of lesser importance in relating the length of eupathidia and length of tarsus I. Hence, in this publication the ratio between the lengths of tc″ζ and of tarsus I is taken into account in the separation of species.
The length of individual setae, even on the same species, can differ significantly, so relative length can also vary, for practical reasons. The tips of the setae can break off and that may be overlooked. We therefore recommend measuring setae of Pronematinae at 1000 X for higher confidence on measurements.
André (1979: 204) considered the description of a new genus based on a single specimen to be unadvisable, except when the specimen exhibits very special characteristics. We propose to extend this suggestion to the description of new species. For Phytoseiidae, Tixier (2012) concluded that measurement of at least ten specimens is necessary to express variability reliably. We, therefore, suggest describing new taxa only when several specimens are available, unless the specimen exhibits special characteristics assumed to be of lower variability.
The position of the solenidion on tarsus I is used as a distinctive character in the Parapronematus key of Gupta (2002). However, this is not always clearly presented in illustrations of different species.
The number of setae on tarsi III and IV varies between species, so it could be considered a reliable feature to separate species. However, in P. connarus n. sp., we found two specimens with five setae on tarsi III and IV instead of six in the majority of the specimens. This characteristic is not always clearly stated in the descriptions, and we see discrepancies between the text of descriptions and the respective illustrations (e.g. P. acaciae Baker, 1965) and in all descriptions but that of P. formosanus, leg figures are unclear or incomplete.
It is concluded that a revision of the whole genus is necessary, starting with a redescription of its type species and the creation of eventual new genera according to the generic concept of André (1980). Types of P. acaciae can only be studied in the museum where they are deposited. Thus, in this publication we selected characteristics that allow the separation of the species, although many uncertainties remain concerning other characteristics. A provisional re-characterization of Parapronematus is subsequently provided, based on what is available in the literature, the examination of the type specimens of the new species here described (P. connarus n. sp.) and the complementary description of P. geminus, provided in this publication. Additionally, a discussion about the other species placed in the genus is presented, concerning their main characteristics and the limitation of the information available in the literature.
Diagnosis of Parapronematus
Adult females with three pairs of setae on the aspidosoma (la lacking, except in one species), nine pairs on the dorsum of the opisthosoma (ps1 and ps3 lacking), three pairs of ag, three setae on tibia I and two on genu I instead of respectively four and three in all other pronematid genera. Two setae on genu II, trochanter II nude, and one seta on femur IV. Only two other genera have three ag setae, but they have different leg I setation. Aberrations of some species concerning the type species: one species with four setae (la present) on aspidosoma, three of the seven species with five setae on tarsus III instead of six (the species described below has five or six setae on tarsus III), one species with two setae on each of femora I and II instead of three.
Complementary description
Adult female. Aspidosoma procurved, with 3 –4 pairs of setae (bo, ro and ex; la absent in all but one species); opisthosoma with nine pairs of setae (c1, c2, d1, e1, f1, f2, h1, h2 and ps2; ps1 and ps3 absent) and four pairs of lyrifissures (ia, im, ip and ih). Eye spots absent. Three pairs of ag setae. One pair of genital papillae. Leg I without pretarsus; each of other legs with ciliate empodium and two claws, without empodial hooks. Seta homologous to s on tarsus I absent. Femur IV undivided. Leg chaetotaxy (tarsus to trochanter; aberrations from type species in bold): leg I (8 + ω – 2 + φ + κ – 2 – 2 or 3 – 1); leg II (6 + ω – 2 – 2 – 2 or 3 – 0); leg III (5 or 6 – 2 – 2 – 2 – 1); leg IV (5 – 2 – 1 – 1 – 0). Epimeral formula 3-1-4-2.
Remarks
According to the concept of André (1980), new genera should be defined for: i) species having setae la; ii) species having two setae on each of femora I and II; and iii) species having five setae on tarsus III. However, to describe a new genus, a full description of the type species is needed. A redescription of P. geminus (seta la present) is presented underneath based on two paratypes; but more specimens are needed to determine variability. Type specimens of other species could not be studied, and the respective descriptions are sometimes ambiguous, with contradictions between the text and the illustrations, including the type species of Parapronematus. So, we prefer not to create new genera, given the impossibility of making a complete description of the type species, adapting the generic descriptions of Baker (1965) and André (1980). The latter author mentioned 5–6 setae on tarsus IV in his diagnosis of the genus. None of the descriptions of Parapronematus species mentions tarsus IV with six setae. Hence, in the complementary description of this genus in this publication, the variation of six setae on tarsus IV is not mentioned, despite being detected in some of the specimens of the new species described in this work, as this could be just a case of aberration.
We suppose that the following setae are absent in Parapronematus (not mentioned in original descriptions): tibia I, d; genu I, v′; tarsus II tc″; genu II, v′; trochanter II, v′; tarsus III, ft ′(shortest seta); tarsus IV, ft′ (shortest seta); and femur IV, ev′.
Type species
Parapronematus acaciae Baker 1965.
Parapronematus acaciae Baker, 1965
Parapronematus acaciae Baker, 1965: 116.
Parapronematus acacia, Gupta 2002: 121.
The original description of this species is extremely basic, with no measurements or details in the illustration. In the complementary description of Gupta (2002), he used part of the original description and all drawings of the original description, with no new measurements.
The type specimens could not be studied because they could not be loaned; hence, in this publication, the length of setae of the dorsal part of opisthosoma and of tarsus I were estimated (Table 2) based on the illustrations provided in the original description.
We found the following inconsistencies between the description and the illustrations: tarsus III is depicted with five setae, instead of six in the description of the genus. Also, according to the original description, ''The forked seta of femur IV is at least twice as long as the width of the segment'', but in the illustration, the length of the seta and the width of the segment are similar. For the moment, we retain the genus description with six setae on tarsus III and the estimated setal lengths as distinguishing characteristics and we consider the relative length of the dorsal setae of femora III and IV to the width of the segment as an unreliable characteristic. However, we are convinced that the species needs a redescription, especially for being the type of the genus.
As distinguishing characters, we retain: f1 49, h1 49 and h2 68, which are clearly longer than in P. camelia, P. formosanus, P. murshidabadensis and P. connarus n. sp. Eupathidia long, tc″ζ /tarsus I≈3.0 but seta p″ζ 34 is shorter than in P. connarus n. sp. (37.5–47.3 µm), tarsus III with six and tarsus IV with five setae, according to the text of the original description of the genus.
Parapronematus camelia Gupta, 1992
Parapronematus camelia Gupta, 1992: 126.
Parapronematus cameliae, Gupta 2002: 121.
The description of this species is basic and lacks many measurements and detailed figures. We see inconsistencies between the textual description and the illustrations: Figure 58 shows a decrease in length of setae f2, h2, f1 and h1; seta ps2 is not depicted, while in the description the lengths of these setae are D4 (f1) = 31, L2 (f2) 56, D5 (h1) 36 and L3 (h2) 23. It is unclear which setal nomenclature is followed. On basis of the figure, we would switch the length of setae mentioned in the text (h) to h1 23 and h2 36. Figure 59 seems to be leg I instead of palp and the limit between tarsus and tibia as well as between femur and trochanter are not depicted, nor are the solenidia. In the text, ''ωI thick and φI very small'', but neither of them is illustrated. We suppose that φI has been confounded with κ. So, we cannot use the form of φI as a distinguishing parameter.
As distinguishing characters, we retain: f2 short (56 µm); seta c2 stout and lanceolate, other setae thin and pointed; eupathidia intermediate, tc″/tarsus I≈2.2; tarsus III with six and tarsus IV with five setae, according to the illustration of the original description.
In the original description, the species was named Pronematus camelia (the designation camelia is a name in apposition). In his monograph, Gupta (2002) changed the name to P. cameliae. We believe that that change is not justified.
Parapronematus citri Salviejo, 1969
Parapronematus citri Salviejo, 1969: 272.
The description of this species is also basic and lacks measurements, although the illustrations are rather detailed, showing some important characteristics, as follows: setae ro, ex, c1, c2, d1 and e1 very short (less than half as long as f1), thick and lanceolate; f1 about 2/3 as long as h1; f2 slightly over twice as long as f1; h2 about 1.2 times as long as h1 (Table 1). The number of setae on tarsus IV is mentioned in the text to be ''as for the genus'' (five setae), whereas the illustration seems to show six setae (not totally clear). We suppose it to be five.
We consider to be distinctive characteristics of this species: setae ro, ex, c1, c1, d1 and e1 11–13, shorter than all other setae; eupathidia long (tc″/tarsus I≈3.0); tarsi III and IV respectively with six and five setae.
Parapronematus ferox Gupta, 1991
Parapronematus ferox Gupta, 1991: 231.
Parapronematus ferox, Gupta, 2002: 123.
The description of this species is very basic and lacks most measurements and detailed illustrations. The long f1 in relation to f2 in Figure 58 of the original description is noteworthy. Double checking it would be convenient; if correct, this characteristic would be sufficient to distinguish this from all other species. The lengths of the dorsal setae of femora III and IV were not provided, but they seem to be longer than in other species. There is a contradiction between the descriptive text and the illustration concerning the number of setae on tarsus III; in the text it is mentioned to be the same as reported for the genus, while the illustration shows both tarsi III and IV with five setae.
Distinguishing characteristics: f1 almost twice as long as f2 (the opposite in all other species); setae ro, ex, c1, c2, d1 stout and lanceolate; other dorsal setae on opisthosoma, thin and pointed; eupathidia short (tc″/tarsus I≈1.5); tarsi III and IV each with five setae (based on the illustration of the original description).
Parapronematus formosanus Tseng, 1985
Parapronematus formosanus Tseng, 1985: 85.
The description of this species is detailed, missing few measurements, although information on variability was not provided. The illustrations are detailed, though without scale bars. Striae are shown on dorsum and venter of the idiosoma. The ventral setae are illustrated in detail, varying from the very broad and leaflike pt to the lanceolate mtβ.
Calls attention the statement ''legs strongly sclerotized, brown in colour''. As far as we know no other sclerotised species have been reported in Iolinidae. Two inconsistencies were detected between the descriptive text and the illustrations: in the differential diagnosis with P. acaciae, femora III and IV are mentioned to have two pairs of setae each. We suppose that this should refer to femora I and II, as described in the table with the number of setae on the legs and the depicted number of setae in the illustrations. Secondly, the text mentions the presence of striae in a diamond pattern anteriad ag1, which seems to be correct, while the pattern is illustrated in Figure 130 anteriad mtβ.
As distinguishing characteristics, we retain: setae ro, ex, c1, c2, d1 and e1 stout and lanceolate, other dorsal setae on opisthosoma long, thin and pointed; eupathidia of intermediate length (tc″/tarsus I≈2.2); two setae on each of femora I and II (instead of the usual three); tarsi III and IV each with five setae; setae pt, mtα and mtβ barbed, from leaflike to lanceolate.
Parapronematus geminus Meyer & Rodrigues, 1966
Parapronematus geminus Meyer & Rodrigues, 1966: 21.
(Figures 8g-h, 13–15)
Adult female (n = 2)
Measurements — Measurements of different structures are shown in Table 2.
Idiosoma — (Figure 13). Ovaloid, tapering posteriorly. Completely covered with fine striae with tiny tubercles, not clearly visible. Aspidosoma with four pair of setae (bo, ro, la and ex), of which la very short, and opisthosoma with nine pairs of setae (c1, c2, d1, e1, f1, f2, h1, h2 and ps2; ps1 and ps3 absent).
Dorsum — (Figures 8e-f, 13). Striae barely visible, in parts of the dorsum. Aspidosoma with striae longitudinal medially, progressively more divergent towards das furrow laterally. Opisthosoma with median longitudinal striation, divergent towards das furrow, but only patches of striae visible, divergent between levels of d1 and e1, transverse between setae e1 and h1 and then divergent towards posterior margin.
Idiosomal setae acute and clearly serrate (Figures 8e-f, 9g-h, 13); trichobothrial seta (bo) longer than ex, which is in turn longer than ro; bo barbed (Figures 8e, 9b, 13); seta la very short and smooth. Setae f2 (Figures 9h, 13) and h2 longest followed by f1 (Figures 9g, 13) and h2, and then ps2, d1 and c1, with c2 shortest. Setae c1, d1 and e1 not reaching bases of d1, e1 and f1, but f1 longer than distance between its base and the base of h1. Seta ps1 absent.
Venter — (Figures 8g-h, 13). Area immediately posteriad gnathosoma with transverse striation; striation longitudinal between setae pt and between setae mtα and mtβ; two areas of spindle-shaped striation pattern between pt and mtα and immediately anteriad ag1.
With three pairs of ag setae anterolaterad anal opening; genital papillae not distinguishable. Seta ps3 absent. Lyrifissure ih not distinguishable.


Gnathosoma — (Figure 14).
With two pairs of scapular setae, sc1 8 (8) and sc2 10 (12), both serrate and slender; setae or1 and or2 not distinguishable; striae in section anteriad sc1 almost transverse; in section between sc1 and sc2 converging anteromedially. Palptarsus elongate, with a distal forked eupathidium pζ; setae l′ and l″ curved towards eupathidium; seta d forked at tip; l′ the most proximal, followed by seta d; setae l″ and v at about same levels; all setae smooth and of similar thickness; solenidia ω and ba not distinguishable, chaetotaxy (5-1-2). Femorogenu length 20 (23), width 11 (11).


Legs — (Figure 15). Chaetotaxy: leg I (8 +ω–2+φ+k – 2 – 3 – 1); leg II (6+ ω – 2 – 2 – 3 – 0); leg III (5 – 2 – 2 – 2 – 1); leg IV (5 – 2 – 1 – 1 – 0). Epimeral formula: 3 – 1–4 – 2. All non-solenidion leg setae acute, serrate and stout. Leg I with solenidion ω not reaching tip of tarsus, eupathidia ft″ζ, tc′ζ, tc″ζ (Figure 9a), p′ζ and p″ζ barbed for the entire length, tectals subequal, shorter prorals not as long, with p″ζ the shortest; with a pair of small forked unguinal setae proximal to prorals on ventral side; seta homologous to s between prorals absent (see Ueckermann and De Vis et al. 2024). Tibia I with bifurcated famulus κ but this is not always visible, about same length as solenidion φ. Seta d on femur III forked. Femur IV undivided and with seta d also forked.
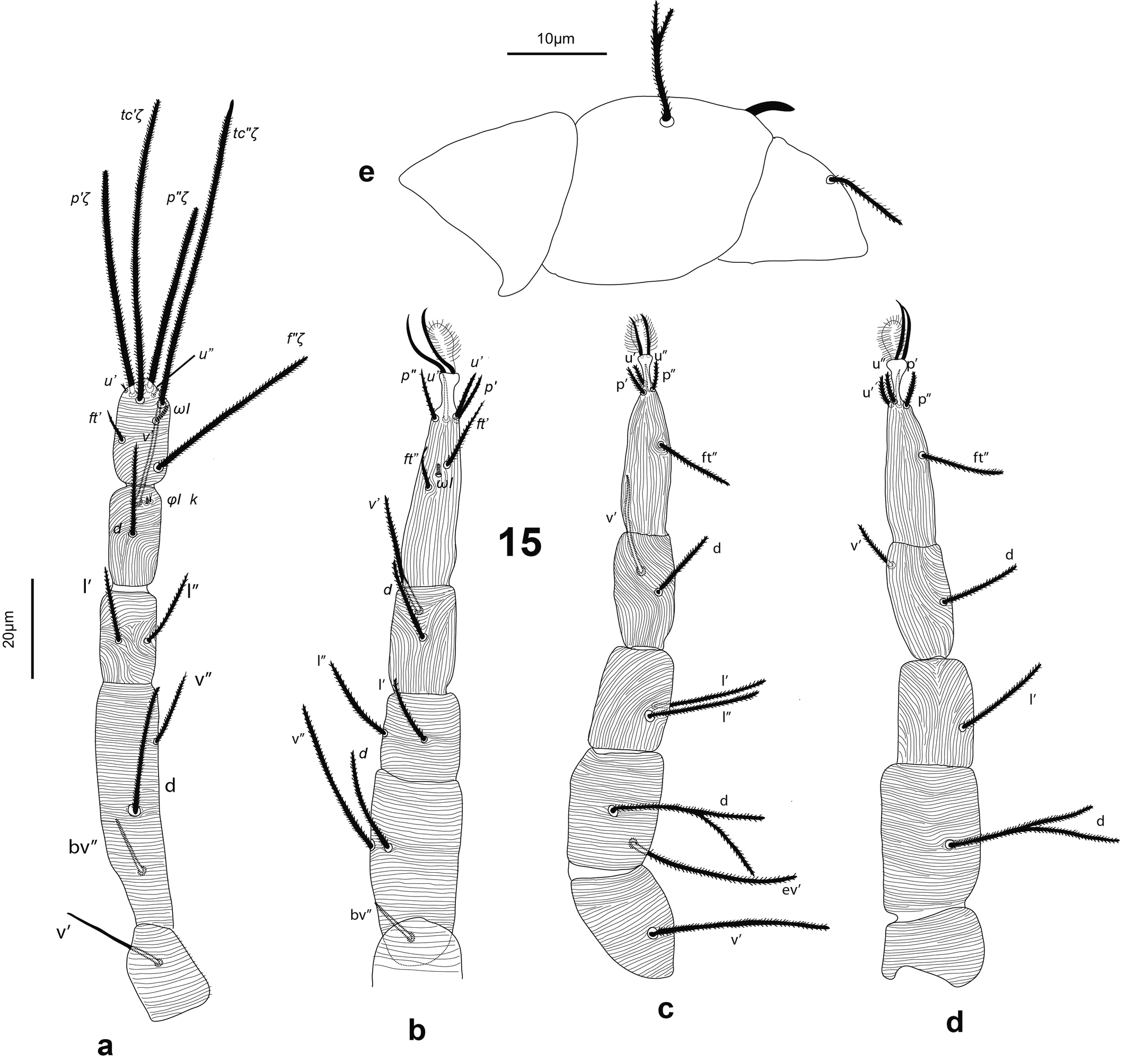


Adult male (n = 2 paratypes, in poor condition)
Idiosoma — Most characters same as in female. Dorsal setae shorter. Distance bo-h1 140, width at level c1 134, bo 30; bo-bo 43; ro 12; ro-ro 21; la 4; la-la 38; ex 16; c1 16; c1-c1 36; c2 10; d1 14; d1-d1 31; e1 12; e1-e1 30; f1 21; f1-f1 24; f2 40; h1 23; h2 37; ps2 17, 1b 12; 1c 5; pt 9; pt-pt not possible to measure; 2a 16; mtα 6; mtα-mtα 24, remaining setae cannot be measured.
Legs — Femora III and IV with forked setae. Femur IV with a dorso-distal, adaxial pointed spur (Figure 15e). Other measurements: leg I 102; tarsus I 19; tibia I 16; tarsus I setae: ft′ 8; ft″ζ 42; tc′ζ 53; tc″ζ 48 p′ζ 37; p″ζ 31.
Differential diagnosis
This species closely resembles P. connarus n. sp., by having similar setal lengths and shapes. It can be distinguished from P. connarus n. sp. and other species of the same genus by having seta la and five setae on each of tarsi III and IV.
Type specimens and depository
Holotype female, from Gossypium sp., R. Quetxoaio, Mozambique, April 16, 1964, M.C. Rodrigues; paratype female, from Mangifera indica, Morrumbala, Mozambique, March 25, 1964, M.K. P. Meyer; paratype male, from Gossypium sp., Kaidundjua, Mozambique, April 17, 1964, M.C. Rodrigues; paratype male, from shrub, Raraga, Mozambique, March 18, 1964, M.K.P. Meyer.
Holotype female (accession number Acy 65/30) and paratype female (accession number Acy 65/137), paratype male (accession number Acy 65/37), paratype male (accession number Acy 65/142) at Mite Collection, ARC-Plant Health Protection, Pretoria, South Africa, NCA (Zhang 2018).
Parapronematus murshidabadensis Gupta, 1992
Parapronematus murshidabadensis Gupta, 1992: 126.
Parapronematus murshidabadensis, Gupta, 2002: 123.
The description of this species is basic, missing many measurements and detailed illustrations. In contrast with the description of P. camelia, in the description of P. murshidabadensis the relative lengths of the setae in the description coincide with what is shown in the illustration: D4 (f1) 44, L2 (f2)78, D5 (h1) = 40, L3 (h2) 56. The used nomenclature does not coincide with that of Baker (1965). Solenidion ωI stout, φI extremely small and blunt. We suppose that in this case φ1 is confused with κ. As an inconsistency within the text of the original description, it is stated in the key to species that the solenidion on tarsus I is placed in the middle of the segment, whereas in Figure 65 of Gupta (1992) it appears in the distal half.
Distinguishing characteristics: seta f1 nearly twice as long as e1 (44 and 21). Setae f1, f2, h1 and h2 (44, 78, 40, 56) longer than in other species and ft″ζ relative short, less than half length of p″ζ (figure 65 of Gupta, 1992). Setae ro, ex, c1, d1 and e1 thick and lanceolate; other dorsal setae of opisthosoma long, thin and pointed (c2 unclear); eupathidia intermediate; tc″/tarsus I≈1.7, but tarsus I seems long; tarsi III and IV each with five setae.
Download as * Holotype, paratype between brackets
Characteristic
P. acaciae
P. camelia
P. citri
P. ferox
P. formosanus
P. geminus _*
P. murshidabadensis
P. connarus n. sp.
Baker (1965)
Gupta (1992, 2002)
Salviejo (1969)
Gupta (1991, 2002)
Tseng (1985)
This paper
Gupta (1992, 2002)
This paper
body length
280
224
265
266
326
278
bo-h1
192 (180)
190 (170–231)
width at level c1
97
145
152
156
179
172 (165)
168
184 (155–212)
bo
41
31
30
49
35
40 (39)
47
44 (39–50)
bo-bo
51
48
40
56 (52)
52 (46–62)
ro
20
25
12
24
18
19 (19)
22
18 (16–22)
ro-ro
33
22
21
33(31)
27 (23–36)
la
Absent
Absent
Absent
Absent
Absent
7 (5)
Absent
Absent
la-la
Absent
Absent
Absent
Absent
Absent
55 (55)
Absent
Absent
ex
27
22
13
20
24
23 (26)
24
23 (20–27)
c1
23
22
13
16
19
23 (23)
22
21 (18–29)
c2
23
22
11
63 (48)
24
22 (19–26)
c1-c1
56
47
47
22 (21)
52 (39–66)
d1
23
13
19
19
25 (25)
21
21 (19–26)
d1-d1
51
38
53
62(51)
51 (34–60)
e1
26
22
13
23
16
23 (22)
21
21 (19–26)
e1-e1
44
32
62
42 (39)
42 (36–63)
f1
49
31
27
82
40
34 (38)
44
30 (25–41)
f1-f1
44
29
36
41 (38)
36 (29–45)
f2
77
59
45
70
69 (68)
78
73 (67–81)
h1
49
23
35
31
26
35 (39)
40
33 (28–43)
h2
68
42
54
54
57 (58)
56
56 (51–63)
ps2
26
24
24
26
27 (29)
25 (21–29)
1b
18 (19)
18 (17–20)
1c
6 (7)
8 (6–10)
Pt
11
11 (12)
11 (10–13)
pt-pt
16
16 (14)
16 (11–21)
2a
19 (21)
21 (17–23)
mtα
13
9 (10)
9 (6–11)
mtα-mtα
34
34 (32)
31 (22–42)
3b
20 (23)
20 (17–22)
3c
29 (29)
32 (28–36)
3d
26 (24)
26 (21–32)
mtβ
7
7 (7)
7 (5–9)
mtβ-mtβ
22
27 (19)
21 (17–26)
4b
13 (15)
15 (13–18)
ag1
7
6 (7)
7 (6–9)
ag1-ag1
19 (16)
17 (13–24)
ag2
7
8 (8)
8 (7–12)
ag2-ag2
26 (24)
29 (14–47)
ag3
14 (16)
15 (12–22)
ag3-ag3
18
32 (31)
37 (22–65)
palptarsus
29
29 (28)
23 (20–26)
palp p ’ζ
4 (5)
6 (4–8)
Stylet
10 (11)
13 (11–15)
leg I
120 (141)
142 (129–154)
leg III
118 (120)
121 (112–134)
leg III
121 (126)
119 (109–147)
leg IV
127 (131)
123(111–135)
tarsus I length
23
18
29
23 (24)
24 (23–26)
tibia I length
22
15
26
19 (21)
22 (20–25)
ωI
5
Stout
7 (6)
5 (4–6)
ft’ζ
12
20
9 (12)
12 (9–16)
ft"ζ
69
54
73 (72)
69 (63–73)
tc’ζ
71
67
75 (absent)
72 (67–79)
tc"ζ
70
59
71(absent)
70 (65–76)
p’ζ
43
47
49 (51)
51 (44–58)
p"ζ
34
31
40 (39)
42 (38–47)
u’
0 (3)
4 (2–5)
u"
2 (3)
4 (2–5)
φI
2 (2)
3 (2– 4)
κ"
2 (2)
2 (2–3)
ωII
3 (3)
3 (2–4)
tibia II v’
24 (31)
32 (30–34)
tibia II d
16 (18)
19 (17–22)
femur II v"
39 (37)
36 (31–40)
femur II d
25 (24)
25 (23–29)
tarsus III ft’
Present
Present
Present
Absent
Absent
Absent
Absent
10 (8–11)
tarsus III ft"
17 (18)
19 (17–21)
tibia III v’
18 (18)
20 (18–23)
tibia III d
12 (15)
16 (13–17)
femur III ev’
29 (31)
31 (28–34)
femur III d
33 (26)
36 (32–40)
long part
17–20
16 (10–21)
short part
13–15
12 (8–15)
tibia IV v’
13 (15)
16 (14–22)
tibia IV d
13 (17)
17 (16–20)
femur IV d
32 (37)
39 (34–44)
long part
17–20
19 (14–25)
short part
13–14
14 (10–18)
Key to the Parapronematus species
Based on the previous considerations, a key is subsequently provided to separate the species of this genus. This should be considered provisional, until a thorough re-examination of type specimens or newly collected specimens is done.
1. Aspidosomal seta la present (despite very short, ≈5–7 µm)
...... P. geminus
— Aspidosomal seta la absent
...... 2
2 (1). Two setae on each of femora I and II; setae ro, ex, c1, c2, d1 and e1 (narrowly) lanceolate and serrate; setae pt mtα and mtβ barbed, from leaflike to lanceolate
...... P. formosanus
— Three setae on each of femora I and II; dorsal and ventral setae thinner
...... 3
3 (2). Dorsal setae ro, ex, c1, c2, d1 and e2 short (11–13 µm)
...... P. citri
— Dorsal setae ro, ex, c1, c2, d1 and e2 longer than 13 µm
...... 4
4 (3). Ratio tc″/tarsus I at least 2.9
...... 5
— Ratio tc″/tarsus I at most 2.2
...... 6
5 (4). Setae f1 30 ± 0,9 (25–41) 19, h1 33 ± 0,7 (28–43), h2 56 ± 0,6 (51–63), and p″ζ 42 ± 0,6 (38–47)
...... P. connarus De Vis & Ueckermann n. sp.
— Setae f1≈49, h1≈49, h2≈68 longer and p″ζ≈34 shorter
...... P. acaciae
6 (4). Seta bo≈31, f1≈31 and h1≈23; six setae on tarsus III
...... P. camelia
— Seta bo, f1 and h1 at least 40% longer than above; five setae on tarsus III
...... 7
7 (6). Seta f1 twice as long as f2; seta h1≈31
...... P. ferox
— Seta f1 slightly shorter than f2; seta h1≈40
...... P. murshidabadensis
In addition to the key provided, Table 3 brings an overview of selected characteristics to separate the Parapronematus species.
Download as * The italic bold values are estimated from the respective illustrations (see Material & Methods)
Characteristic
Parapronematus species
acaciae
camelia
citri
ferox
formosanus
geminus
murshidabadensis
connarus n. sp.
Presence/absence setae
la
Absent
Absent
Absent
Absent
Absent
Present
Absent
Absent
# setae on femora I and II
3
3
3
3
2
3
3
3
# setae on tarsus III
6
6
6
5
5
5
5
6 (5 in few specimens)
Shape of setae
ro, ex, c1, c2, d1, e1
Stout, serrate lanceolate
Stout, lanceolate
Stout, lanceolate
Stout, barbed
Narrow, lanceolate, barbed
Narrow, setose
Stout, lanceolate
Aciculate, broader at base, barbed
pt mtα mtβ
Barbed, stout to very thin
Barbed, leaflike to lanceolate
Barbed, stout to very thin
Barbed, stout to very thin
Length of setae
ro, ex, c1, c2, d1, e1
23–27
22–25
Short (11–13)
≈16–24
16–24
19–26
21–24
16–25
bo
≈41*
31
≈30
≈49
35
39–40
47
44 (38–50)
e1
≈26
22
≈13
≈23
16
22–23
21
21 (19–26)
f1
≈49
31
≈27
≈82
40
34–38
44
30 (25–41)
f2
≈77
≈59
≈45
70
68–69
78
73 (67–81)
h1
≈49
23
≈35
≈31
26
35–39
40
33 (28–43)
h2
≈68
≈42
≈54
54
54
56
56 (51–63)
p”ζ
≈34
≈31
39–40
42 (38–47)
tc"/tarsus I
≈3.0
≈2.2
≈3.0
≈1.5
≈2.2
≈3.0
≈1.7
≈2.9
Reappraisal of Pronematinae
As presently conceived, Pronematinae is composed of 19 genera, depending upon a definition of the validity of the synonymy of Pausia Kuznetsov & Livshitz, 1972 under Naudea Meyer & Rodrigues, 1966. The latter two genera were synonymized by Baker and Delfinado (1976), who considered them to differ only in the number of opisthosomal setae (ten pairs in Pausia and nine in Naudea). André (1980) considered both valid, without referring to Baker and Delfinado (1976) and providing a brief characterization of each.
Thus, the structure of this section starts with a characterization of the sole species included in Naudea, followed by a comparative characterization of the genera, subgenera (of Proctotydaeus Berlese, 1911) and species of this subfamily, ending with the presentation of a key to separate the genera and subgenera.
Our examination of the holotype female, one paratype female and the paratype male of the single known species of Naudea led to the following characterization.
Naudea richinda Meyer & Rodrigues, 1966
Naudea richinda Meyer & Rodrigues, 1966: 23.
Pausia richinda, Baker & Delfinado, 1976: 35.
Adult female
(Figures 18, 19)
Measurements — (Holotype followed by paratype, the latter in brackets).
Idiosoma — (Figure 16). Elongate-oval. Distance bo-h1 137 (140), width at level c1 113 (112). Completely covered with fine striae with tiny tubercles, not clearly visible. Aspidosoma with four pairs of setae (bo, ro, la and ex) and opisthosoma with ten pairs of setae (c1, c2, d1, e1, f1, f2, h1, h2, ps2 and ps3); ps1 absent.
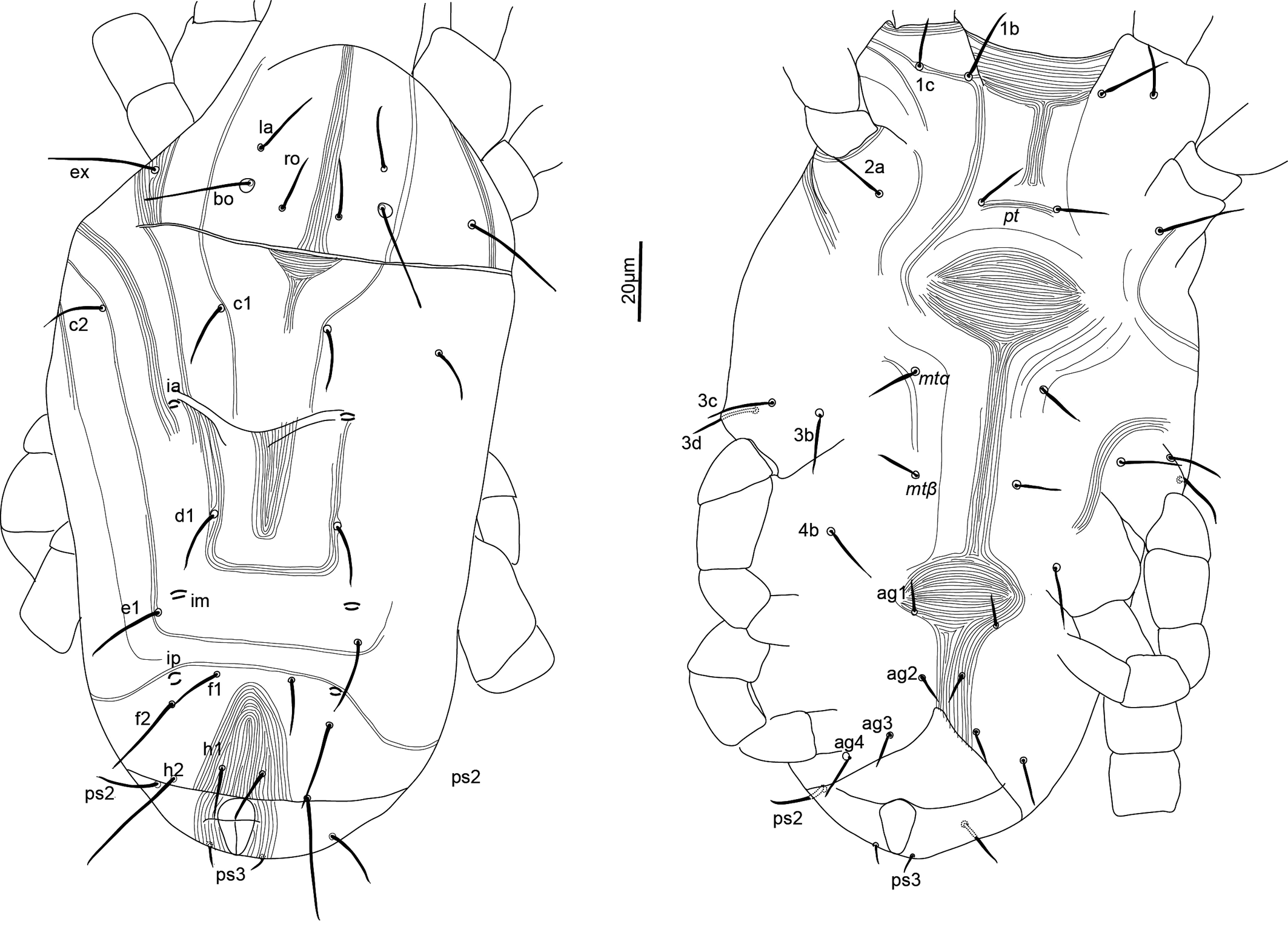


Dorsum — Aspidosoma with striae longitudinal centrally, diverging toward das furrow laterally; dorsal part of opisthosoma with median longitudinal striation, diverging toward das furrow, about transverse from the level between d1 and im to the level of f1 and then diverging toward posterior margin.
Eye spots absent. Idiosomal setae aciculate and smooth; seta bo about as long as ex, which in turn is longer than ro and la, the latter two subequal. Setae f2 and h2 subequal to bo and ex, longer than other dorsal idiosomal setae; ro, f2 and h2 the shortest. Setae c1, d1, e1 and f1 not reaching bases of respectively d1, e1, f1 and h1.
Setal lengths and distances between setae: bo 27 (27), bo-bo 33 (33), ro 15 (15), ro-ro 18 (14), la 17 (18), la-la 30 (31), ex 25 (27), c1 21 (17), c1-c1 25 (27), c2 17 (16), d1 17 (17), d1-d1 25 (26), e1 19 (18), e1-e1 45 (44), f1 14 (13), f1-f1 15 (15), f2 25 (21), h1 14 (14), h2 25 (29), ps2 16 (15) and ps3 6 (7). Lyrifissures ia located between setae c1 and d1, im immediately anteriad e1 and ip immediately anteriad f2.
Venter — Striation mostly longitudinal, except for transverse region immediately behind gnathosoma, between pt and mtα and immediately anteriad ag1; in the latter two regions, striation forming a spindle-shaped pattern, respectively wider than the distance between setae mtα and wider than the distance between setae ag1.
With four pairs of ag setae anterolaterad anogenital area; genital papillae and lyrifissure ih not visible.
Setal lengths and distances between setae: 1b 19 (16), 1c 13 (11), pt 13 (13), 2a 17 (20), mtα 11 (13), 3b 16 (15), 3c 15 (16), 3d 17 (16), mtβ 11 (11), 4b 16 (14), ag1 9 (10), ag2 8 (8), ag3 9 (0), ag4 12 (0), pt–pt 19 (18), mtα–mtα 37 (31), mtβ–mtβ 29 (24), ag1-ag1 25 (22), ag2-ag2 12 (12). Anogenital area in both holotype and paratype ruptured, hence distances ag3-ag3 and ag4-ag4 are not possible to measure.
Gnathosoma — (Figure 17). Chelicera, with short stylets 14 (17). Subcapitulum with striae almost transverse in section anteriad sc2 and diverging posteriorly; with two pairs of smooth scapular setae, sc1 11 (11) and sc2 13 (13); orals or1 and or2 not distinguishable. Palptarsus elongate, with a distal eupathidium pζ 6 (8), setae l′, l″, v and d long and smooth, l′ inserted more proximal than d and l″ (both at about same level); solenidion ω and ba not distinguishable, chaetotaxy (5-1-2). Femorogenu length 18 (17), width 8 (9).


Legs — (Figure 18). Chaetotaxy: leg I (8 +ω–3+φ+k – 3 – 3 – 1); leg II (7+ ω – 2 – 3 – 3 – 1); leg III (7 – 2 – 2 – 2 – 1); leg IV (7 – 2 – 1 – 1 + 1– 0). Epimeral formula: 3 – 1 – 4 – 2. All leg setae aciculate and smooth, some ventral setae stouter. Tarsus I without claws, but with empodium; ft″ζ, tc′ζ and tc″ζ the longest setae. Tibia I with bifurcated famulus κ, 2(2), which is not always visible, about half length of solenidion φI, 2 (3); and seta v′ very long and stout. Leg II with ωI 5(5), tibia with ventral seta v′ very long 25(24) and stout, seta d 16 (16); femur seta v″ 23(21) and d 20(18). Leg III with setae v′ and d of tibia 25(24) and 19(19), respectively, and ev′ and d of femur 17(17) and 16(17), respectively. Leg IV with femur divided, with setae ev′ 22(18) and d 18(16), tibia with setae v′ and d 23(25) and 19(19), respectively. Legs II to IV each with an empodium and two claws distally.


Adult male
(Figure 19)
Idiosoma: most characters same as in female with the following exceptions: dorsal setae subequal and some even longer than those of female; dorsal part of opisthosoma medially covered with broken striae. Femur IV with a dorso-distal adaxial spur (Figure 18e). Measurements: distance bo-h1 117, width at level c1 88, bo 26, bo-bo 25, ro 14, ro-ro 13, la 16, la-la 25, ex 28, c1 23, c1-c1 25, c2 20, d1 24, d1-d1 14, e1 23, e1-e1 29, f1 15, f1-f1 10, f2 24, h1 16, h2 26, ps2 17, ps3 5, 1b 17, 1c 12, pt 12, pt-pt 16, 2a 17, mtα 12, mtα-mtα 29, 3b 16, 3c 18, 3d 18, mtβ 10, mtβ-mtβ 23, 4b 13, ag1 8, ag1-ag1 18, ag2 8, ag2-ag2 13, ag3 7, ag3-ag3 11, ag4 11, ag4-ag4 20, palptarsus 7, pζ 5, stylet 13, sc1 11, sc2 12, femorogenu length 16, femorogenu width 7, leg I 104, leg II 78, leg III 82, leg IV 93, tarsus I 28, tibia I 20, leg I ωI 17, leg I φI 3, leg I k 2, leg II ωII 4, leg II tibia v′ 21 and d 15, leg IV femur v″ 24 and d 18, leg III tibia v′ 23 and d 20, leg III femur ev″ 16 and d 17, leg IV tibia v′ 20 and d 22 femur ev″ 18 and d 13.
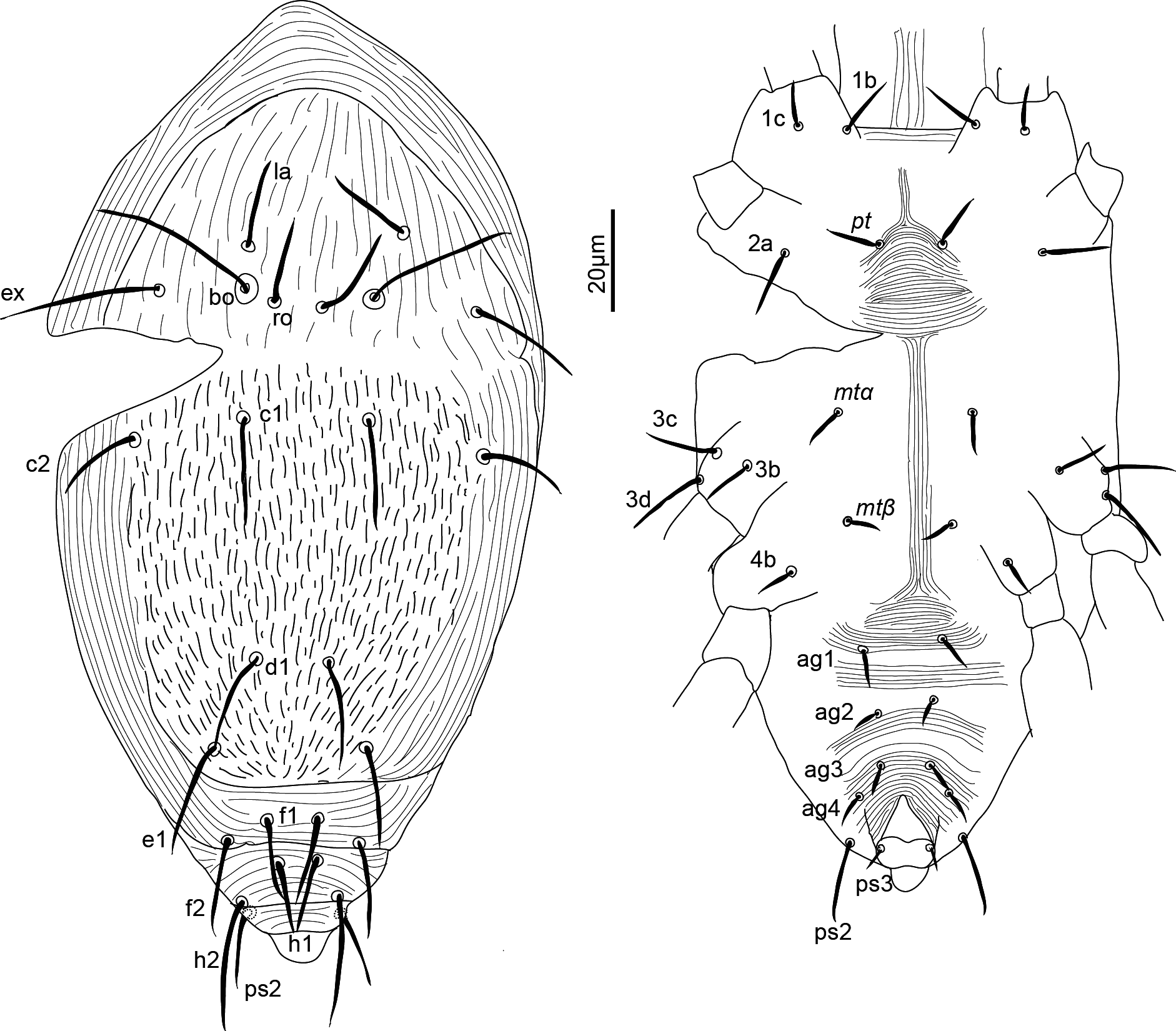


Type specimens and depository
Holotype female, paratype female and paratype male from Gossypium herbaceum, Tshipise, Limpopo Province, South Africa, 24 March 1965, M.K.P. Smith-Meyer and M. Rodrigues.
Holotype female, paratype female and paratype male (all under the same accession number Acy 65/123), at Mite Collection, ARC-Plant Health Protection, Pretoria South Africa, NCA (Zhang 2018).
Remarks
The characteristics of the type specimens examined led to the conclusion that the types of the only species of this genus differ from the four described Pausia species by the number of setae on tarsus II (seven, instead of six), tarsus III (seven, instead of six), the number of setae on tarsus IV (seven, instead of 5–6) and the number of opisthosomal setae (ten instead of 10–11). Moreover, Pausia shows several variations in number of setae on different leg segments and on the opisthosoma among species. Pausia litchiae Silva, Da-Costa & Ferla is the only species with 10 pairs of setae on the opisthosoma, five setae on tarsus III (instead of six) and two setae on genu IV (instead of one). Pausia magdalenae Baker & Definado has only two setae on tibia I (l′ ? absent) and femur II (bv″ absent) instead of three in the type species (Baker and Delfinado 1976, Da Silva et al. 2017). We recommend studying and/or redescribing, if necessary, P. litchiae and P. magdalenae, to confirm their aberrant leg chaetotaxy and eventually to transfer them to new genera. Hence, in this publication Naudea and Pausia are considered different genera. As a result, Pronematinae presently congregates 19 genera, one of which is composed of four subgenera (Kazmierski 1998a), based on Baker (1968b, 1969), Kaźmierski (1998a) and André (2004).
Characterization of genera of Pronematinae
Table 4 shows the diagnostic characteristics of the taxa presently included in Pronematinae, mostly based on an evaluation of the literature, but also including our examination of the specimens collected in this study as well as of type specimens of two previously described species that are redescribed in this publication.
Download as * Do not vary among genera: tibiae II, III and IV, each with two setae, femur I and III with respectively three (except **) and two setae and trochanters III and IV respectively with one seta and nude, totalling 12 setae on these segments.
Genus/ species
Sp.
Asp.
Opisthosoma
ag
Leg I *
Leg II
Leg III
Leg IV
Total
#
#
#
#
apotele
ta
Ti
ge
tr
ta
ge
fe
tr
ta
ge
ta
ge
fe
fe div.
#
Naudea richinda
1
4
10–ps1
4
1
8+ω
3+κ+φ
3
1
7+ω
3
3
1
7
2
7
1
2
1
60
Neonaudea gossypii
1
4
11
4
1
8+ω
3+κ+φ
3
1
7+ω
3
3
1
5
2
5
1
2
1
56
Pausia ***
4
4
10 or 11
4
1
8+ω
2or3+κ+φ
3
1
6+ω
3
2or3
1
5 or 6
2
5
1or2
2
1
56
taurica, colonus
2
11
3+κ+φ
3
6
1
56
magdalenae
1
11
2+κ+φ
2
6
1
54
litchiae
1
10–ps3
3+κ+φ
3
5
2
56
Pronecupulatus anahuacensis
1
4
11
?
1
8+ω
3+κ+φ
3
1
6+ω
1
3
5
1
5
1
2
0
52
Duoparus hyeresensis
1
4
11
2
0
9+ω
3+κ+φ
3
1
7+ω
3
3
1
6
2
6
1
2
1
59
Metapronematus leucohippeus
1
4
10–ps1
3
0
8+ω
3+κ+φ
3
1
6+ω
3
3
1
6
2
5
1
1
0
55
Parapronematus
8
3 or 4
9–ps1–ps3
3
0
8+ω
2+κ+φ
2
1
6+ω
2
2 or3
0
5 or 6
2
6
1
1
0
49-52
acaciae, camelia, citri
3
3
3
6
52
connarus n. sp.
1
3
3
6 (5)
52(51)
geminus
1
4
3
5
52
ferox, murshidabadensis
2
3
3
5
51
formosanus **
1
3
2**
5
49
Apopronematus bakeri
1
4
11
3
0
8+ω
3+κ+φ
2
1
6+ω
2
3
1
5
1
5
1
1
1 ?
51
Proctotydaeus
22
4
10 or 11
4
0
8+ω
3+κ+φ
3
1
7+ω
3
3
1
7
2
7
1
2
1/0
60
P. (Proctotydaeus)
5
11
0
60
P. (Oriola)
6
11
0
60
P. (Neotydeolus)
4
10–ps2
0
60
P. (Proctotydulus)
7
11
1
60
Pronematulus
3
4
11
4
0
8+ω
3+κ+φ
3
1
7+ω
2
3
1
6
2
6
1
2
1 ?
57
Pseudopronematulus
5
4
11
4
0
9+ω
3+κ+φ
3
1
7+ω
3
3
1
6
2
6
1
2
1
59
Ueckermanniella cataracta
1
4
11
4
0
8+ω
3+κ+φ
3
1
6+ω
3
3
1
6
2
5
1
1
1
55
Homeopronematus anconai
1
4
11
1 or 4
0
9+ω
3+κ+φ
3
1
6+ω
3
3
1
6
2
6
1
2
0
58
Quasihomeopronematus nordestinus
1
4
10–ps1
4
0
9+ω
3+κ+φ
3
1
6+ω
3
3
1
6
2
6
1
2
0
58
Pronematus
8
4
10–ps2
4
0
9+ω
3+κ+φ
3
0
6+ω
3
3
0
5
2
5
1
2
0
54
Lourus
2
4
11
4
0
8+ω
3+κ+φ
3
1
6+ω
3
2
1
5
2
5
1
2
0
54
Neopronematus
8
4
9–ps1, ps2
4
0
8+ω
3+κ+φ
3
1
6+ω
3
3
1
5
2
5
1
2
0
55
Dasilcoferla nadirae
1
4
10–ps1
4
0
8+ω
3+κ+φ
3
1
6+ω
3
3
1
5
2
5
1
2
0
55
Peridasilcoferla paraensis n. gen. n. sp.
1
4
11
4
0
8+ω
3+κ+φ
3
1
6+ω
3
3
1
5
2
5
1
2
0
55
Total 20 genera
71 species
** Parapronematus formosanus has two setae on each femur I and II instead of three.
*** We include the species of Pausia in this table because the chaetotaxy of the species belonging to that genus differs from each other.
Key to the genera and subgenera of Pronematinae
The Pronematinae contains several monotypic genera; for those, the name of the corresponding species is shown immediately after reaching the name of the genus. For genera consisting of more than one species, the number of species is presented in Table 4. The distribution of the genera is based on type localities of the type species except for Pronematus and Homeopronematus, for which we refer to Ueckermann and De Vis et al. (2024) and De Vis et al. (2024b).
1. Apotele I with vestigial claws or with no claws (empodium usually present); femur IV divided
...... 2
— Apotele I absent
...... 5
2 (1). Tarsi II–IV each with seven setae; South Africa
...... Naudea Meyer & Rodrigues, 1966
— Tarsus II with seven or less setae; tarsi III and IV each with at most six setae
...... 3
3 (2). Tarsus II with seven setae; Egypt
...... Neonaudea El Bagoury & Abou Awad, 1966
— Tarsus II with 5–6 setae
...... 4
4 (3). Genu II with three setae; genu III with two and tarsus III with 5–6 setae, Brazil, Crimea, Greece, South Africa
...... Pausia Kuznetsov & Livshitz, 1972
— Genua II and III each with only one seta; tarsus III with five setae; Mexico
...... Pronecupulatus Baker, 1944
5 (1). One pair of aggenital setae (known only from males)
...... 6
— More than one pair of aggenital setae (known from males and females)
...... 7
6 (5). Ten pairs of opisthosomal setae (ps1 absent); Brazil
...... Quasihomeopronematus De Vis & Ueckermann, 2024b (Q. nordestinus male)
— Eleven pairs of opisthosomal setae; worldwide except Africa and Oceania
...... Homeopronematus André, 1980 (Homeopronematus anconai (Baker, 1943) male)
7 (5). Two pairs of aggenital setae, France
...... Duoparus De Vis & Ueckermann, 2024a
— More than two pairs of aggenital setae
...... 8
8 (7). Three pairs of aggenital setae
...... 9
— Four pairs of aggenital setae
...... 11
9 (8). Genua I and II each with three setae; USA
...... Metapronematus André, 1980
— Genua I and II each with two setae
...... 10
10 (9). Seta la reduced or absent; tibia II with two setae + φ + κ; genu III with two setae; Brazil, Democratic Republic of Congo, India, Mozambique, Philippines, Taiwan
...... . Parapronematus Baker, 1965
— Seta la normal; tibia II with three setae + φ + κ; genu III with one seta; USA
...... Apopronematus André, 1980 (A. bakeri André, 1980)
11 (8). Tarsi III and IV each with seven setae; in general insect associates
...... Proctotydaeus Berlese, 1911 12
— Tarsi III and IV each with at most six setae, in general not associated with insect
...... 15
12 (11). Seta bo club-shaped; ten pairs of opisthosomal setae (ps2 absent); femur IV not divided; Brazil
...... Proctotydaeus (Neotydeolus) Flechtmann & Camargo, 1979
— Seta bo whip-shaped; 11 pairs of opisthosomal setae (ps2 present), femur IV divided or not
...... 13
13 (12). Paraproctal suckers distinctly developed (especially in females); anus terminally surrounded by sucker-like papillae; tectal setae (tc) not longer than tarsus; unguinal setae (u) on tarsus I normal; femur IV not divided; Galapagos Islands, Indonesia
...... Proctotydaeus (Proctotydaeus) Berlese, 1911
— Paraproctal suckers weakly developed; setae (tc) longer than tarsus; unguinal setae (u) on tarsus I shorter than other setae; femur IV divided or not
...... 14
14 (13). Femur IV not divided; males with some bifurcate setae; Brazil, Canada, Poland, USA
...... Proctotydaeus (Oriola) (Baker, 1968b)
— Femur IV divided; males without bifurcate setae; Crimea, Iran, Mozambique, South Africa, Ukraine, US
...... Proctotydaeus (Proctotydulus) Kaźmierski, 1998a
15 (11). Tarsus II with seven setae
...... 16
— Tarsus II with six setae
...... 17
16 (15). Genu II with two setae; Crimea, Taiwan, USA, Uzbekistan
...... Pronematulus Baker, 1965
— Genu II with three setae; Belgium, Brazil, China, Mozambique, South Africa
...... Pseudopronematulus Fan & Li, 1992
17 (15). Tarsus III and femur IV respectively with six and one seta; South Africa
...... Ueckermanniella Beron, 2012
— Tarsus III and femur IV respectively with five and two setae
...... 18
18 (17). Trochanters I and II each with one seta
...... 19
— Trochanters I and II nude; worldwide
...... . Pronematus Canestrini, 1886
19 (18). Femur II with two setae; opisthosoma with 11 pairs of setae; Crimea, Mozambique
...... Lourus Ueckermann and Grout 2007
— Femur II with three setae; opisthosoma with 9–11 pairs of setae
...... 20
20 (19). Nine pairs of opisthosomal setae (ps1 and ps2 absent); Greece, Hungary, Crimea, Iran
...... Neopronematus (Panou, Emmanouel & Kaźmierski, 2000)
— More than nine pairs of opisthosomal setae
...... 21
20 (21). Ten pairs of opisthosomal setae (ps1 absent); Brazil
...... Dasilcoferla De Vis & Ueckermann, 2024a
— Eleven pairs of opisthosomal setae; Brazil
...... Peridasilcoferla De Vis & Ueckermann n. gen. (This paper)
For the following genera, keys to the species have been developed (reference in brackets): Neopronematus (Darbemamieh et al. 2015), Parapronematus (this work), Proctotydaeus (Kaźmierski 1998a); Pronematus (Ueckermann et al. 2024) and Pseudopronematulus (De Vis et al. 2024a).
Discussion
Presence/ absence of one or more setae is a key feature in the taxonomy of Pronematinae. Thus, an important aspect is to decide which dorsal seta/setae is/are absent: ps1, ps2 and/or h2? In his diagnoses of the genera Naudea, Pronematus, Metapronematus and Parapronematus, André (1980) found it difficult to believe the missing seta to be ps1 in one species and ps2 in another. Without specifying the species and the genus he referred to, Kaźmierski (1998a: 44) expressed the same idea. However, in our understanding, the loss of setae on the body or legs does not merely follow a straight line and might occur at different times in evolution on different continents and ecosystems. More recently, Panou et al. (2000) described the genus Neopronematus, with two missing opisthosomal setae, stating that these were h1 and ps1, while the most logic would be ps1 and ps2, with h2 (which is long) present. Authors of other new Neopronematus species (Sadeghi et al. 2012; Ripka et al. 2013; Darbemamieh et al. 2015; Ahmad-Hosseini et al. 2017) agreed with Panou et al. (2000). However, we do not agree with this point of view and think that both ps1 and ps2 setae are lost in Neopronematus following thereby André (1981a), who stated that the distal setae likely to be lost are all ps and that the only seta that tends to move laterally is e1. So, in our opinion the missing setae in the different genera might be: ps1 (Naudea, Metapronematus, Quasihomeopronematus, Dasilcoferla); ps2 (Proctotydaeus (Neotydeolus), Pronematus); both ps1 and ps2 (and not h2 and ps2) (Neopronematus), ps3 [in Pausia litchiae Da Silva et al. (2017), but this should be confirmed] or ps1 and ps3 (Parapronematus), according to Table 4. Unstable setae may either become very small or disappear (Coineau 1974; André 1981a). Examples can be found in seta la. In several genera, this seta is thinner than other setae of the aspidosoma (De Vis et al. 2024a). In P. geminus, it is reduced, tiny, while absent in all other Parapronematus species. A second example refers to ps1, very small in Peridasilcoferla n. gen. and Homeopronematus, and absent in the closely related Dasilcoferla (De Vis et al. 2024a) and Quasihomeopronematus (De Vis et al. 2024b).
It would not seem plausible that instead, ps2 was lost, ps1 moved to the usual position of ps2 and in parallel became as large as ps2. Dasilcoferla has the same chaetotaxy as Neopronematus, but with one less dorsal seta, which would be logically the loss of ps2, although Neopronematus is not comparable in the same way; it has many differences and stands probably far away from Dasilcoferla or Peridasilcoferla n. gen.
It has been considered that the loss of opisthosomal ag or leg setae justifies the description of a new genus of Pronematinae (André 1980; Kaźmierski 1998b; Khaustov 2022). The adoption of this concept could explain the existence of several monotypic genera. Alternatively, the existence of many monotypic genera in Pronematinae could be due to the insufficient effort dedicated to the study of these mites, in comparison with other groups, e.g. the Phytoseiidae, which are used broadly in biocontrol programs (Van Lenteren et al. 2018). The interest in using Pronematinae in biocontrol has recently increased (Vervaet et al. 2022; Pijnakker et al. 2022a; Pijnakker et al. 2022b; Marcossi et al. 2024). So, the number of monotypic genera might diminish as more species are discovered.
Related to that, studies are necessary to define the ecological and evolutionary importance of the loss of body or leg setae, to strengthen this generic concept. In the meantime, we consider it important to maintain the actual concept to avoid confusion, although not all authors followed it in the past (see also De Vis et al. 2024a). This concept may also lead to the believe that different species within a genus can be defined based on differential lengths of setae. This is a characteristic not well defined in early descriptions, but it could support the need for redescriptions of the early described species, to ensure their validity. Baker was the first to introduce leg chaetotaxy in the description of genera (Baker 1965). However, the lack of leg chaetotaxy in his species descriptions (Baker 1968a) led to the presently high number of species inquirendae (Kaźmierski 1998b; Ueckermann et al. 2024).
Naudea has the same leg chaetotaxy as Proctotydaeus, but the latter lacks empodium on leg I (Table 4). However, two Pausia species have aberrant chaetotaxy; P. magdalenae has only two setae on tibia I (l′ ? absent) and femur II (bv″ absent) (!) instead of three in the type species (Baker and Delfinado 1976). Proctotydaeus litchiae has five setae on tarsus III (ft′ absent) instead of six, two setae on genu IV instead of one and it lacks ps3 (ten opisthosomals) when compared to the type species of the genus (Da Silva et al. 2017). These findings need confirmation, and new genera should be described within the current generic concept if these characteristics are confirmed. If the presence of two setae on genu IV is confirmed, that would be the first species within the Pronematinae to show that characteristic (André 1980).
We think that other aspects of the morphology could be introduced in the genus descriptions and might even have priority over the presence or absence of setae. The difficulty in this is that early described genera and species have been poorly defined and redescriptions of those are therefore needed before these characters can be used. An additional problem is that the types of at least some species are in inadequate condition to be further studied. We have recently described new species in the following genera of Pronematinae: Pronematus (Ueckermann et al. 2024), Duoparus, Pseudopronematulus and Dasilcoferla (De Vis et al. 2024a), Homeopronematus and Quasihomeopronematus (De Vis et al. 2024b), Peridasilcoferla n. gen. and Parapronematus (this paper). While doing so, we were convinced that the following characteristics seemed of generic value:
a) in the idiosoma:• Presence or absence of tubercles on the striae: this seems to be fixed within at least two genera: absent in Pseudopronematulus (five species examined) and present in Pronematus (eight species examined).
• Presence/absence of spindle-shaped pattern of striation anteriad e1: present in Parapronematus, absent in all other genera (compare Figures 1 and 5 of this publication).
• Extent of the spindled pattern of striae anteriad mtα: in Peridasilcoferla n. gen. it extends anteriorly to level of pt; in others (Pronematus, Homeopronematus; Quasihomeopronematus, Dasilcoferla, Parapronematus) it almost reach level of pt; in still others (Duoparus, Pseudopronematulus) it is much smaller. Unfortunately, this characteristic is not pointed out in the description of many species.
• Extent of the spindled pattern of striae anteriad ag1: Smaller than distance between setae ag1 (Duoparus), a bit wider than the distance between those setae (Pseudopronematulus, Peridasilcoferla n. gen, Parapronematus) or much broader, about twice the distance between those setae (Pronematus, Homeopronematus). Also in this case, the characteristic is not properly detailed in the description of many species.
• Presence and form of la: Somewhat thinner than bo and ex (most genera), thin and small (Parapronematus geminus) or absent (all other Parapronematus spp.). In the generic concept of André (1980), a new genus should be described for species with la absent.
• Forked ag3: present in Homeopronematus, Quasihomeopronematus and Neopronematus (except N. aegeae, Panou et al. 2000; in fact, the generic placement of N. aegeae should be reevaluated based on that characteristic).
• Positioning of ag setae: Pseudopronematulus-type, positioned in two eccentric lines (Pseudopronematulus, Duoparus); or Pronematus-type with distances ag2-ag2 and ag3-ag3 short and near base of the longitudinal part of the genital opening (Neopronematus (Panou et al. 2000), Pronematus, Homeopronematus, Quasihomeopronematus); or Dasilcoferla-type with ag1 and ag3 close to each other while ag2 and ag4 widely separated (Dasilcoferla and Peridasilcoferla n. gen.).
• The (relative) lengths of setae 3b, 3c and 3d: 3c longer than the other two subequal setae (Pseudopronematulus), 3d longer than the other two subequal setae (Homeopronematus, Quasihomeopronematus); subequal 3c and 3d and these longer than 3b (Pronematus, Dasilcoferla); 3d longer than 3c which is longer than 3b (Duoparus, Peridasilcoferla n. gen.). As differences may not be much, measurement of several specimens seems necessary.
b) in the gnathosoma:• Status of seta v on palptarsus: smooth (Pronematus, Homeopronematus, Quasihomeopronematus, Pseudopronematulus, Dasilcoferla), finely barbed (Peridasilcoferla n. gen.) or heavily barbed (Duoparus). This characteristic is very clear in Duoparus but not in other genera under phase microscopy.
c) in the legs:• Presence of seta homologous to s (Ueckermann et al. 2024), present in Pronematus, Homepronematus and Quasihomeopronematus (De Vis et al. 2024b); vestigial or absent in Pseudopronematulus and Duoparus (De Vis et al. 2024a); absent in Dasilcoferla, Peridasilcoferla n. gen., and Parapronematus (De Vis et al. 2024a and this paper); unknown for other genera.
• Length of ωI. We prefer to use the absolute length as the width of leg is dependent on mounting conditions. In most species, the relative length of the eupathidia: (tc) subequal (most species) or not (Dasilcoferla); (p) subequal (Duoparus, Pseudopronematulus), p″ζ shorter (Pronematus, Homeopronematus Quasihomeopronematus, Peridasilcoferla n. gen., Parapronematus) or longer than p′ζ. (Dasilcoferla, Neopronematus).
• The status of the eupathidia on tarsus I: smooth (Duoparus, some Pseudopronematulus), partially barbed (Homeopronematus, Quasihomeopronematus, Peridasilcoferla n. gen., Neopronematus, some Pseudopronematulus,) or completely barbed (Dasilcoferla, Pronematus (but not tip), Parapronematus, some Pseudopronematulus); the type of barbs on the eupathidia also differ.
• Presence of forked dorsal setae: present on femur III only (at least some Pseudopronematulus; to be confirmed in others); present on femora III and IV (Parapronematus); or absence (all other genera).
• Pending upon confirmation of studies to be conducted, other characteristics seemingly important to consider in generic characterization include: the ornamentation of eupathidia of tarsus I, as well as the distance along the main axis of the two most distal setae of tarsus IV. Still, given the small dimensions of those setae, their usefulness as diagnostic features remains to be determined.
Summarizing our vision of the important characteristics in the definition of taxa of Pronematinae, we retain the listed characteristics as of generic importance and recommend them to be part of genus descriptions and not of species description (although further study of described species should confirm this). For body characteristics: the presence or absence of tubercles on the striae, the presence/absence of spindle form striae anterior to setae e1 on the dorsum; the form of spindle form striae anterior to setae mtα; the form of spindle form striae anterior to setae ag1 on the venter; the presence and form of seta la; the form of ag3 (forked or not). For gnathosoma characteristics: the status of seta v on palptarsus. For leg characteristics: the relative length of the eupathidia.
Acknowledgements
The seed of this work was planted during the post-doctoral fellowship nr 01/13725-6 at ESALQ provided by FAPESP (State of São Paulo Research Foundation) to the first author in 2002. The Biodiversity Virtual Institute Program (BIOTA) of FAPESP (http://www.biotasp.org.br ![]() ); it was in part supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant No. 88887.827149/2023-00, awarded to J. Lacerda; Universidade Federal Rural da Amazônia Campus Parauapebas provided logistic support; and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) issued collection authorization (nr.89189-2) and provided logistical assistance in expeditions.
); it was in part supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant No. 88887.827149/2023-00, awarded to J. Lacerda; Universidade Federal Rural da Amazônia Campus Parauapebas provided logistic support; and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) issued collection authorization (nr.89189-2) and provided logistical assistance in expeditions.
Arne Jansen and Irene Cardoso were so kind to transport slides of Brazilian mites to the first author and Christine Muggler was our postal hub in Rio de Janeiro.
Distribution of work
Raf M.J. De Vis collected samples in 2002–2003, made the description of P. connarus n. sp., developed keys and wrote the text. José P. Lacerda collected samples and mounted the mites in Brazil; Gilberto de Moraes supervised the work of José P. Lacerda and this work as senior acarologist. Edward A. Ueckermann redescribed P. geminus and N. richinda and supervised the work as senior acarologist and Pronematinae specialist. We want to acknowledge explicitly Henri André for his initiative to build the Tydeoidea database in the wikispecies platform (André 2021). It was an invaluable tool during the preparation of this manuscript and all previous ones.
References
- Ahmad-Hosseini M., Khanjani M., Karamian R. 2017. A new species of Neopronematus and a redescription of Pronematus rykei Meyer & Rodrigues (Acari: Iolinidae) from Iran. Zootaxa, 4337(4): 493-508. https://doi.org/10.11646/zootaxa.4337.4.2
- André H.M. 1979. A generic revision of the family Tydeidae (Acari\,: Actinedida) I. Introduction, paradigms and general classification. Ann. Soc. R. Zool. Belg., 3-4(1978): 189-208.
- André H.M. 1980. A generic revision of the family Tydeidae (Acari: Actinedida) IV. Generic descriptions, keys and conclusions. Bull. Ann. Soc. R. Belge Ent., 116(7-9): 103-168.
- André H.M. 1981a. A generic revision of the family Tydeidae (Acari, Actinedida) II. Organotaxy of the idiosoma and gnathosoma. Acarologia, 22(1): 31-46.
- André H.M. 1981b. A generic revision of the family Tydeidae (Acari, Actinedida) III. Organotaxy of the legs. Acarologia, 22(2): 165-178.
- André H.M. 2004. Revalidation of Oriola and replacement name for Meyerella (Acari: Tydeoidea). Int. J. Acarol., 30(3): 279-280. https://doi.org/10.1080/01647950408684395
- André H.M. 2021. The Tydeoidea (Ereynetidae, Iolinidae, Triophtydeidae and Tydeidae)-An online database in the Wikispecies platform. Acarologia, 61(4): 1023-1035. https://doi.org/10.24349/6yc5-1lxw
- André H.M., Fain A. 2000. Phylogeny, ontogeny and adaptive radiation in the superfamily Tydeoidea (Acari: Actinedida), with a reappraisal of morphological characters. Zool. J. Linn., 130(3): 405-448. https://doi.org/10.1111/j.1096-3642.2000.tb01636.x
- Baker E.W. 1943. Nuevos tydeidos mexicanos (Acarina). Rev. Soc. Mex. Hist. Natur., 4(3-4): 181-189.
- Baker E.W. 1944. Pronecupulatus, a new genus of Tydeidae (Acarina) from Mexico. J. Kans. Entomol. Soc., (17): 72-73.
- Baker E.W. 1965. A review of the genera of the family Tydeidae (Acarina). Adv. Acarol., 2: 95-133.
- Baker E.W. 1968a. The genus Pronematus Canestrini. Ann. Entomol. Soc. Am., 61(5): 1091-1097. https://doi.org/10.1093/aesa/61.5.1091
- Baker E.W. 1968b. Two new genera of Tydeidae (Acarina). Ann. Entomol. Soc. Am., 61(4): 968-970. https://doi.org/10.1093/aesa/61.4.968
- Baker E.W. 1969. Oriolella, a new name for Oriola (Acarina: Tydeidae). Entomol. Soc. Wash. Proc., 71(2): 204.
- Baker E.W., Delfinado M.D. 1976. Notes on the genus Naudea Meyer and Rodrigues, with description of a new species (Acarina: Tydeidae). Int. J. Acarol., 2(1): 35-38. https://doi.org/10.1080/01647957608683754
- Berlese A. 1911. Acarorum species novae quindecim. Gen. Proctotydaeus n. gen. Redia, (7): 430-431.
- Berlese A. 1914. Acari nuovi. Manipulus IX. Redia, (10): 113-150.
- Beron P. 2012. Ueckermanniella nom. nov. pro Kakamasia Ueckermann et Grout, 2007 (Acari, Iolinidae) nec Kakamasia Lawrence, 1944 (Acari, Erythraeidae). Hist. Nat. Bulg., (20): 67-68.
- Canestrini G. 1886. Prospetto dell′ Acarofauna italiana (Continuazione). Famiglia degli Eupodini. Atti del R. Istituto Veneto di Scienze, Lettere ed Arti (ser. 6), 4(5): 693-734.
- Carrillo D., De Moraes G.J., Peña J.E. eds. 2015 . Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms. Cham: Springer International Publishing. pp. https://doi.org/10.1007/978-3-319-15042-0
- Coineau Y. 1974. Éléments pour une monographie morphologique, écologique et biologique des Caeculidae (Acariens). Mem. Mus. Natl. Hist.Nat., A(81): 330.
- Da Silva G.L., Da-Costa T., Ferraz C.S., Pallini A., Ferla N.J. 2017. First description of iolinid mites (Acari: Tydeoidea) from Brazil. Syst. Appl. Acarol., 22(5): 694–701. https://doi.org/10.11158/saa.22.5.8
- Darbemamieh M., Hajiqanbar H., Khanjani M., Kaźmierski A. 2015. New species and records of Neopronematus (Acari: Iolinidae) from Iran with a key to world species. Zootaxa, 3990(2): 235–246. https://doi.org/10.11646/zootaxa.3990.2.4
- De Leon D. 1966. Phytoseiidae of British Guyana with keys to species. Studies on the Fauna of Suriname and other Guyanas, 8(1): 81-102.
- De Vis R.M.J., de Moraes G.J., Bellini M.R. 2006a. Initial screening of little known predatory mites in Brazil as potential pest control agents. Exp. Appl. Acarol., 39(2): 115-125. https://doi.org/10.1007/s10493-006-9004-7
- De Vis R.M.J., Moraes G.J. de, Bellini M.R. 2006b. Mites (Acari) of rubber trees (Hevea brasiliensis Muell. Arg., Euphorbiaceae) in Piracicaba, state of São Paulo, Brazil. Neotrop. Entomol., 35(1): 112-120. https://doi.org/10.1590/S1519-566X2006000100015
- De Vis R.M.J., Vervaet L., Van Leeuwen T., Braga A.D.F., Castilho R.D.C., Ueckermann E.A. 2024a. Description of two new genera, three new species, and redescription of two species of Pronematinae (Acari: Iolinidae). Acarologia, 64(4): 1063-1099. https://doi.org/10.24349/z0vq-ji83
- De Vis R.M.J., Vervaet L., Reybroeck E., Vanlommel W., Van Havermaet R., Foque, D., Marcossi, I., Pallini, A., Binda de Assis, C. H., Van Leeuwen, T., Ueckermann E.A. 2024b. Rescription of Homeopronematus anconai (Baker), and description of Quasihomeopronematus nordestinus n. gen. n. sp. (Acari: Iolinidae). Acarologia, 64(4): 1283-1311. https://doi.org/10.24349/cu4t-4h6v
- El-Bagoury M.E., Abou-Awad B.A. 1966. Neonaudea gen. n. of the family Tydeidae from Egypt (Acari, Tydeoidea). Acarologia, 27(2): 121-123.
- Fan Q.-H., Li L.-S. 1992. A new genus and three new species of Tydeidae (Acari: Actinedida) from China. J. Fujian. Agric. Coll., 21: 396-400.
- Flechtmann C.H.W., Camargo C. de 1979. Acari associated with stingless bees (Meliponidae, Hymenoptera) from Brazil. In: Proc. 4th Int. Congr. Acarol., Budapest: Akademiai Kiado. pp. 315-319.
- Gupta S.K. 1991. Studies on predatory prostigmatid mites of North East India with descriptions of new dpecies and dew records from India. Records of the Zoological Survey of India, 88(2): 207-239. https://doi.org/10.26515/rzsi/v88/i2/1991/161345
- Gupta S.K. 1992. Arachnida: Plant mites (Acari). In: Fauna of West Bengal, Part 3. State fauna Series 3, Calcutta: Zoological Survey of India. pp. 61-211.
- Gupta S.K. 2002. A monograph on plant inhabiting predatory mites of India. Part 1: Orders: Prostigmata, Astigmata and Cryptostigmata. A monograph on plant inhabiting predatory mites of India. Part 1: Orders: Prostigmata, Astigmata and Cryptostigmata., 19(2): 1-183.
- Hernandes F.A., De Castro T.M.M.G., Venancio R. 2015. Prostigmata (Acari: Trombidiformes) as Biological Control Agents. In: Carrillo D., De Moraes G. J., Peña J. E. (Eds) Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms, Cham: Springer International Publishing. pp. 151-184. https://doi.org/10.1007/978-3-319-15042-0_6
- Kaźmierski A. 1989. Morphological studies on Tydeidae (Actinedida, Acari). I: Remarks about the segmentation, chaetotaxy and poroidotaxy of idiosoma. Acta zool. Cracov, 32(4): 69-83.
- Kaźmierski A. 1998a. A review of the genus Proctotydaeus Berlèse (Actinedida: Tydeidae: Pronematinae). Acarologia, 39(1): 33-47.
- Kaźmierski A. 1998b. Tydeinae of the world: generic relationships, new and redescribed taxa and keys to all species. A revision of the subfamilies Pretydeinae and Tydeinae [Acari: Actinedida: Tydeidae]-part IV. Acta Zoologica Cracoviensia, 41(2): 283-455.
- Khaustov A.A. 2022. A new genus and species of Tydeidae (Acari: Prostigmata) from Western Siberia, Russia. https://doi.org/10.24349/1dwx-x5h2
- Khaustov A.A., Hugo-Coetzee E., Ermilov S.G. 2020. A new species of Lorryia (Acari: Tydeidae) from a termite nest in South Africa. Acarina, 28(1): 47-53. https://doi.org/10.21684/0132-8077-2020-28-1-47-53
- Kramer P. 1877. Tydeidae. Arch. Naturgesch., (43): 232-246.
- Krantz G.W., Walter D.E. eds. 2009. A manual of acarology. 3rd ed. Lubbock, Texas: Texas Tech
- University Press. pp. 807.
- Kuznetsov N.N. 1972. Mites of the genus Pronematus Canestrini (Acarina, Tydeidae) from Crimea. Nauch Dokl Vyssh Shk, Biol Nauk, (5): 11-16.
- Kuznetsov N.N., Livshits I.Z. 1972. A new genus and species of Tydeidae (Acariformes) from Crimea (In Russian). Zool. Zhurnal, 51(11): 1738-1740.
- Lofego A.C., Barbosa M.F.D.C., Demite P.R., Moraes G.J.D. 2024. Phytoseiidae (Acari: Mesostigmata) of the subfamily Amblyseiinae from Brazil. Zootaxa, 5439(1): 1-306. https://doi.org/10.11646/zootaxa.5439.1.1
- Marcossi Í., Francesco L.S., Fonseca M.M., Pallini A., Groot T., De Vis R., Janssen A. 2024. Predatory mites as potential biological control agents for tomato russet mite and powdery mildew on tomato. J. Pest. Sci., 251-263. https://doi.org/10.1007/s10340-024-01802-0
- McGregor E.A. 1914. Four new tetranychids. Ann. Entomol. Soc. America, 7(4): 354-360. https://doi.org/10.1093/aesa/7.4.354
- Meyer M.K.P., Rodrigues M.C. 1966. Acari associated with cotton in Southern Africa (with reference to other plants). Garcia de Orta, Ser. Bot., 13(2): 195-226.
- Panou H.N., Emmanouel N.G., Kaźmierski A. 2000. Neopronematus, a new genus of the subfamily Pronematinae (Acari: Prostigmata: Tydeidae) and a new species from Greece. Acarologia, 41(3): 321-325.
- Pijnakker J., Hürriyet A., Petit C., Vangansbeke D., Duarte M.V.A., Arijs Y., Moerkens R., Sutter L., Maret D., Wäckers F. 2022a. Evaluation of phytoseiid and iolinid mites for biological control of the tomato russet mite Aculops lycopersici (Acari: Eriophyidae). Insects, 13(12): 1146. https://doi.org/10.3390/insects13121146
- Pijnakker J., Moerkens R., Vangansbeke D., Duarte M., Bellinkx S., Benavente A., Merckx J., Stevens I., Wäckers F. 2022b. Dual protection: a tydeoid mite effectively controls both a problem pest and a key pathogen in tomato. Pest Manag. Sci., 78(1): 355-361. https://doi.org/10.1002/ps.6647
- Pritchard A.E. 1956. A New superfamily of trombidiform mites with the description of a new family, Genus, and Species (Acarina: Iolinoidea: Iolinidae: Iolina nana). An. Esc. Nac. Cienc. Biol., 49(3): 204-206. https://doi.org/10.1093/aesa/49.3.204
- Ripka G., Laniecka I., Kazmierski A. 2013. On the arboreal acarofauna of Hungary: Some new and rare species of prostigmatic mites (Acari: Prostigmata: Tydeidae, Iolinidae and Stigmaeidae). Zootaxa, 3702(1): 1–50. https://doi.org/10.11646/zootaxa.3702.1.1
- Sadeghi H., Łaniecka I., Kaźmierski A. 2012. Tydeoid mites (Acari: Triophtydeidae, Iolinidae, Tydeidae) of Razavi Khorasan Province, Iran, with description of three new species. Ann. Zool., 62(1): 99-114. https://doi.org/10.3161/000345412X633685
- Salviejo P.B. 1969. Some Philippine tydeid mites (Tydeidae: Acarina). Philippine Entomologist, 1(4): 261-276. https://doi.org/10.59852/TPE-A27V1I4
- Tixier M. 2012. Statistical approaches to assess intraspecific variations of morphological continuous characters: the case study of the family Phytoseiidae (Acari: Mesostigmata). Cladistics, 28(5): 489-502. https://doi.org/10.1111/j.1096-0031.2012.00394.x
- Treat A.E. 1970. Two tydeid mites from the ears of noctuid moths. American Museum Novitates, 2426: 1-14.
- Tseng Y.-H. 1985. A Taxonomic Study of the Tydeidae from Taiwan (Acarina: Trombidiformes) (Part II). J. Taiwan Mus., 38(2): 65-93.
- Ueckermann E.A., De Vis R.M.J., Reybroeck E., Vervaet L., Vanlommel W., Dewitte J., Foqué D., Gautam S., Ouyangh Y., Vangansbeke D., De Clercq P., Van Leeuwen T. 2024. Redescription of Pronematus ubiquitus (McGregor, 1932) (Acari, Iolinidae), description of two new species and redescription of two additional species with a review of and key to all Pronematus species. Acarologia, 64(1): 277-311. https://doi.org/10.24349/tyki-9xlp
- Ueckermann E.A., Grout T.G. 2007. Tydeoid mites (Acari: Tydeidae, Edbakerellidae, Iolinidae) occurring on Citrus in southern Africa. J. Nat. Hist., 41(37-40): 2351-2378. https://doi.org/10.1080/00222930701589921
- Van Lenteren J.C., Bolckmans K., Köhl J., Ravensberg W.J., Urbaneja A. 2018. Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl, 63(1): 39-59. https://doi.org/10.1007/s10526-017-9801-4
- Vervaet L., Parapurath G., De Vis R., Van Leeuwen T., De Clercq P. 2022. Potential of two omnivorous iolinid mites as predators of the tomato russet mite, Aculops lycopersici. J. Pest. Sci., 95(4): 1671-1680. https://doi.org/10.1007/s10340-022-01544-x
- Wainstein B.A. 1962. Révision du genre Typhlodromus Scheuten, 1857 et systématique de la famille des Phytoseiidae (Berlese, 1916)(Acarina: Parasitiformes). Acarologia, 4: 5-30.
- Zhang Z.-Q. 2018. Repositories for mite and tick specimens: acronyms and their nomenclature. Syst. Appl. Acarol., 23(12): 2432-2446. https://doi.org/10.11158/saa.23.12.12



2024-11-03
Date accepted:
2025-03-31
Date published:
2025-04-14
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 De Vis, Raf M. J.; Araujo Lacerda, José Dantas; de Moraes, Gilberto J. and Ueckermann, Edward A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



