What factors influence the plasticity of a facultative feeding larval predator Amblydromalus limonicus (Garman & McGregor) (Acari: Phytoseiidae)?
Liu, Wei1
; Zhang, Keshi  2
; Li, Lanjing
2
; Li, Lanjing  3
and Zhang, Zhi-Qiang
3
and Zhang, Zhi-Qiang  4
4
1State Key Laboratory of Grassland and Agro-Ecosystems, College of Ecology, Lanzhou University, Tianyan Building, No. 222 South Tianshui Road, Lanzhou 730000, Gansu Province, China.
2Manaaki Whenua – Landcare Research, 231 Morrin Road, Auckland 1072, New Zealand & School of Biological Sciences, University of Auckland, 3A Symonds Street, Auckland 1010, New Zealand.
3Manaaki Whenua – Landcare Research, 231 Morrin Road, Auckland 1072, New Zealand & School of Biological Sciences, University of Auckland, 3A Symonds Street, Auckland 1010, New Zealand.
4✉ Manaaki Whenua – Landcare Research, 231 Morrin Road, Auckland 1072, New Zealand & School of Biological Sciences, University of Auckland, 3A Symonds Street, Auckland 1010, New Zealand.
2025 - Volume: 65 Issue: 2 pages: 270-279
https://doi.org/10.24349/6poj-q5dqOriginal research
Keywords
Abstract
Introduction
Phytoseiid predators (Acari: Mesostigmata), a group of predatory mites, play a vital role in regulating populations of various phytophagous mite and insect pests (Zhang 2003). Members of this family progress through four distinct immature life stages—egg, larva, protonymph, and deutonymph—before reaching maturity or adulthood (McMurtry et al. 2015; Lopez 2023). Although the immature development period is relatively short, typically lasting less than 6 days under optimal conditions, these stages are more susceptible to mortality than adults (Zhang and Croft 1994; Zhang 2003; Vangansbeke et al. 2014; Liu et al. 2018). This vulnerability highlights the critical importance of successfully navigating these stages, particularly in unfavourable environmental conditions, for population growth (Zhang and Croft 1994).
The feeding behaviours of phytoseiid larvae, specifically their consumption of insects, mites, and pollen, vary significantly across species (Zhang and Croft 1994; Vangansbeke et al. 2014; Liu et al. 2018). These behaviours reflect diverse ecological strategies and have been extensively studied (Croft and Croft 1993; Croft et al. 1999; Chittenden and Saito 2001). Zhang and Croft (1994) classified phytoseiid larvae into three categories based on their feeding strategies:
- Non-feeding larvae: Larvae that do not feed during this stage [e.g. Neoseiulus cucumeris (Oudemans) and Phytoseiulus persimilis Athias-Henriot].
- Facultative-feeding larvae: Larvae that can develop into protonymphs with or without feeding [e.g. Amblyseius andersoni (Chant)].
- Obligatory-feeding larvae: Larvae that must feed to develop into protonymphs [e.g. Amblyseius herbicolus (Chant)].
Amblydromalus limonicus (Garman & McGregor) (Mesostigmata: Phytoseiidae), a generalist predator, feeds on mites, insects, and pollen from various species (Knapp et al. 2013; McMurtry et al. 2013; Liu and Zhang 2017). As an effective biocontrol agent, A. limonicus targets agricultural pests such as thrips and whiteflies (Knapp et al. 2013). The larvae of A. limonicus exhibit facultative feeding behaviour, providing them with flexibility during larval development (Zhang and Croft 1994; Xu and Zhang 2015; Liu et al. 2023).
Most phytoseiid species are characterised by either non-feeding or obligatory-feeding larval stages (Zhang and Croft 1994). The facultative feeding strategy represents a unique adaptation, allowing species greater flexibility in response to resource scarcity during early development. A previous study has shown that fasting during the larval stage of A. limonicus significantly extends lifespan without affecting fecundity compared to controls that did not fast (Peng and Zhang, unpublished data). Furthermore, facultative-feeding larvae of the phytoseiid predator Neoseiulus fallacis (Garman) exhibited prolonged development and increased search activity when deprived of food during the larval stage (Schausberger and Croft 1999), underscoring the role of larval feeding in the fitness of these species.
This study aims to investigate the factors influencing the larval feeding behaviour of A. limonicus by focusing on conspecific pressure, prey availability, and maternal influence. Previous research has demonstrated that increasing conspecific density negatively affects population growth in A. limonicus (Liu et al. 2018). Individuals of P. persimilis showed increased prey consumption in the presence of one conspecific compared to the those reared individually during development (Xu et al. 2023). Phytoseiid predators, which lack vision, are known to detect conspecific and interspecific individuals using chemical cues without direct physical contact (Saitoh et al. 2020; Su et al. 2021; Gu et al. 2022). Based on these findings, we hypothesised that conspecific pressure would lead to increased prey consumption in A. limonicus larvae. Furthermore, we expected that larvae with greater prey availability would exhibit higher consumption than those with limited prey.
It is well established that parental environmental conditions can influence offspring phenotypes across a range of organisms (Uller 2008; Bonduriansky and Day 2009; Bonduriansky 2021). For example, well-nourished mothers often produce larger offspring than poorly nourished ones (Donelson et al. 2009; Bonduriansky 2021), a pattern observed in phytoseiid predators such as N. cucumeris (Lee et al. 2020) and P. persimilis (Han et al. 2024). Larger eggs may confer developmental advantages (Bonduriansky 2021), leading to increased body size, but a higher prey requirement. Therefore, we hypothesised that well-nourished mothers would produce larger eggs, resulting in increased prey consumption by A. limonicus larvae. Understanding the factors influencing the feeding behaviour of the facultative feeding larvae will enhance our ability to predict and manage the population dynamics of these essential predators; and provide insight into the evolutionary route of the larval feeding strategies in phytoseiid species.
Material and methods
Mite rearing
The predator A. limonicus and its prey, the ''dried fruit mite'' Carpoglyphus lactis (L.) (Acari: Carpoglyphidae), were obtained from Bioforce Limited (Karaka, Auckland, New Zealand). Amblydromalus limonicus was provided with an ad libitum supply of mixed-stage C. lactis as food and maintained under controlled laboratory conditions at a temperature of 25 °C ± 1 °C, relative humidity of 80% ± 5%, and a light-dark cycle of 16:8 hr (light: dark). For details on rearing containers and methods, see Zhang and Zhang (2021) and Wang et al. (2024). Carpoglyphus lactis was reared on a mixture of wheat bran and yeast, both purchased from a local supermarket (Goodman Fielder Limited, New Zealand).
To obtain similarly aged cohorts of A. limonicus, approximately 50 nymphs were placed into each separate culture with ample C. lactis as their food source to allow for growth, mating, and oviposition. Nylon threads (c. 2 cm in length) were placed into the cultures overnight to collect A. limonicus eggs (< 15 hr old) for the experiments. The prey (C. lactis eggs) for the experiments were collected following the method of Liu et al. (2024a). To ensure accurate prey egg counting, the eggs were previously frozen at −18 °C for 3 days to prevent hatching. The eggs were then allowed to thaw for approximately 1 hr before being used in the experiments.
Experimental set-up
We used two types of enclosed rearing cells for rearing A. limonicus and conducting the experiments, with minor modifications to the compartments described in Liu et al. (2024b). Each cell consisted of two transparent plexiglass slides (38 mm × 25 mm × 2 mm, length × width × thickness). Plexiglass slide I featured an aperture (9 mm in diameter at the top, narrowing to 6 mm at the base), while plexiglass slide II was solid.
For experiments without conspecifics, the aperture in Plexiglass slide I was covered with a food wrap layer to allow air exchange (Figure 1A). Small holes were made in the wrap using a size 0 insect pin. A 10 mm square black plastic sheet with similar pin holes covered the base of the conical space to provide water access, while water-saturated filter paper beneath the plastic sheet ensured a continuous water supply. Plexiglass slide II sealed the cell, with two metal clips holding the assembly together.
In experiments assessing the effect of conspecifics on prey consumption, an additional compartment was added beneath the test subject (Figure 1B). A 500-mesh screen separated the two spaces, allowing airflow but preventing physical contact between the conspecifics and the larva being tested. A filter paper layer atop the plastic sheet prevented flooding of the mesh screen while still providing water to the test subject. In this set-up, the upper space housed a single larva, and the lower space housed 3 conspecific larvae to simulate conspecific pressure (Figure 1C).
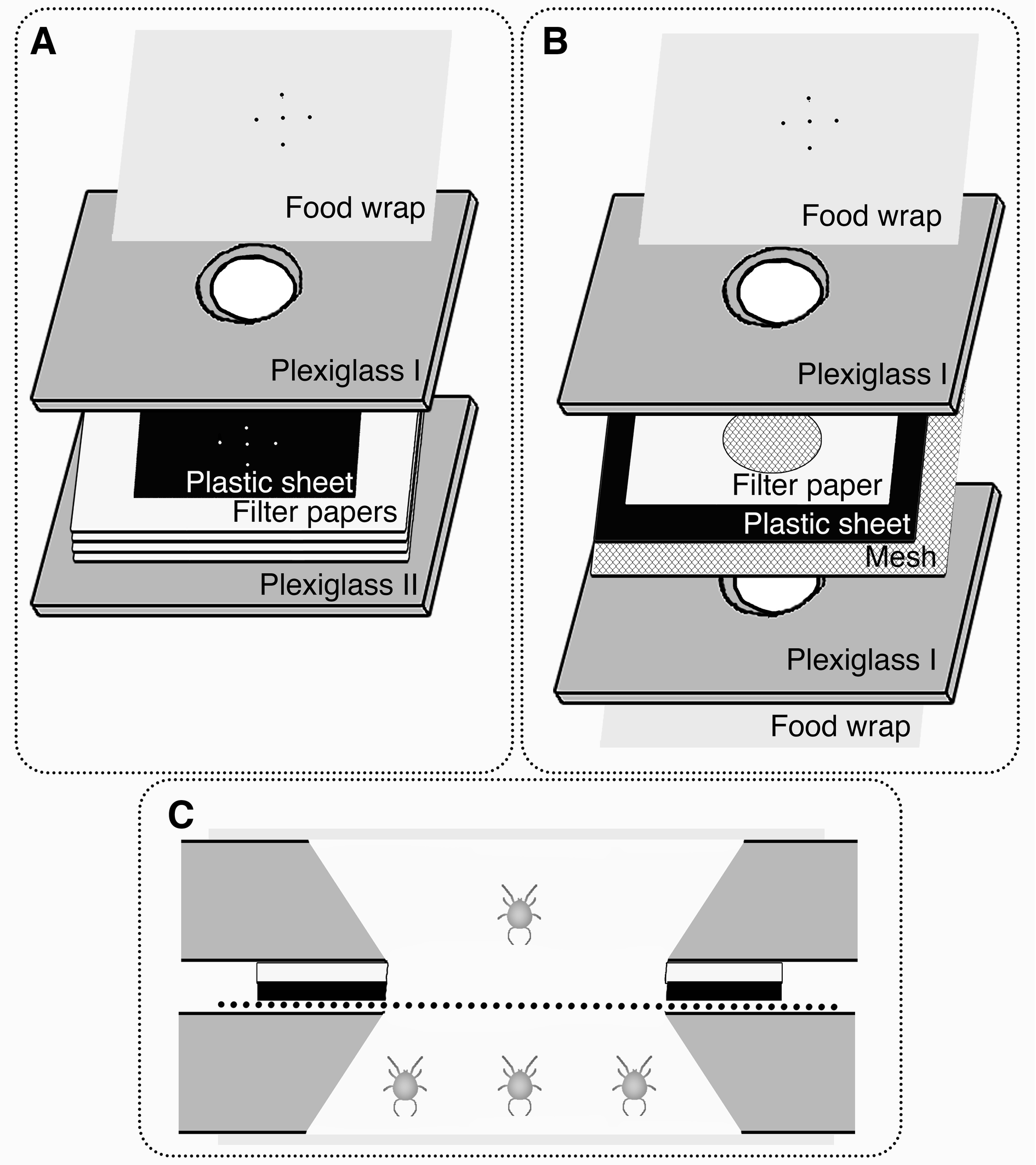


Experiment 1: Influence of conspecific presence and different prey availability on the larval feeding behaviour
To examine the influence of conspecific presence and different prey availabilities on the feeding behaviour of A. limonicus, eggs of A. limonicus (collected using the threads) were individually placed in rearing cells without food. Filtered water was provided by saturating the filter papers in the cells. Four treatments were administered to A. limonicus larvae within 15 hr of hatching: (1) 5 prey eggs without conspecifics; (2) 5 prey eggs with conspecifics; (3) 20 prey eggs without conspecifics; and (4) 20 prey eggs with conspecifics. Single-compartment cells (Figure 1A) were used for Treatments 1 and 3, while double-compartment cells (Figure 1B) were used for Treatments 2 and 4. In Treatments 2 and 4, 3 conspecific larvae (< 15 hr post-hatching) were placed in the lower compartment to simulate conspecific pressure (see Figure 1B). Feeding occurrence and prey consumption were observed every 30 min for 3 hr and once at the end of 24 hr. For both Experiments 1 and 2, prey consumption at 24 hours was excluded if the larvae had moulted into protonymphs. Therefore, only the prey consumption of individuals that remained in the larval stage at the end of the 24-hour period was considered valid data. Each treatment was replicated 30 times.
After examining prey consumption, the larvae were reared with ad libitum C. lactis until adulthood, at which point their sexes were determined using a dissecting microscope. Female A. limonicus are approximately 20% larger than males (Ma et al. 2018). A small proportion of larvae (< 10%) did not survive to adulthood, and their data were excluded from statistical analysis relating to sex.
Experiment 2: Influence of maternal diet on larval feeding behaviour
Eggs of A. limonicus collected using threads were individually placed in the single-compartment cells (Figure 1A) with ad libitum C. lactis until reaching adulthood. After moulting (< 15 hr old) into adults, one female and one male were paired for 24 hr for mating in a new cell. The females were then placed in cells with C. lactis eggs at two densities: 15 (restricted) or 40 (abundant) eggs per cell, replenished daily. Eggs laid by the females during the first 2 days were discarded to avoid influence from previous diet, and from Day 3 onward, we measured eggs using a Nikon Eclipse 90i phase-contrast microscope with NIS-Elements (version 5.10) software (Nikon, Japan) to calculate their volumes. Individual egg volume was determined using the formula:
\[V=(0.6057-0.0018 B) L B^2\]
where V represents the volume, L stands for the egg length, and B represents the maximum breadth (Narushin 2005). The eggs were then allowed to hatch individually in new cells with 5 prey eggs per cell, and larval prey consumption was observed as described in Experiment 1. Each treatment was replicated 30 times.
Statistical analyses
The statistical analysis and graph illustration were performed using R version 4.0.5 (R Core Team 2022) with ARTool (Kay et al. 2021) and ggplot2 (Wickham 2016) packages. Chi-squared tests were employed to determine the differences in offspring sex ratio. Normality of the data on prey consumption and egg volume were checked using the Shapiro–Wilk test, and means and standard errors of the means (SEMs) were calculated. Non-normal data were analysed using aligned rank transformation (ART) analyses of variance (ANOVAs) with post hoc pairwise comparisons. Linear regression was used to examine the relationship between egg volume and prey consumption. For all statistical analyses, α was set at p < 0.05.
Results
Experiment 1: Influence of conspecific presence and different prey availability on the larval feeding behaviour
Only 5% of the examined larvae (5 out of 97 valid data) did not feed during the first 3 hr and 3% for the entire (24 hr) examination period (1 out of 30 valid data). The feeding occurrence within the first 3 hr was not significantly affected by the presence of conspecifics (logistic regression: χ2 = 0.461, df = 1, p = 0.497) or by different prey availability (χ2 = 2.509, df = 1, p = 0.113). In contrast, female larvae (98%) exhibited a significantly higher feeding occurrence during the first 3 hr compared to males (89%) (χ2 = 4.616, df = 1, p = 0.032). Due to the high overall feeding occurrence (i.e. only one male did not feed during the 24 hr period), no statistical comparisons were made for the entire 24 hr period.
During the first 3 hr, larvae of A. limonicus consumed significantly more prey when given 20 prey eggs compared to those given 5 eggs (ART ANOVA: F1, 89 = 6.281, p = 0.014) (Table 1). Female larvae also consumed significantly more eggs than male larvae during the first 3 hr (F1, 89 = 13.618, p < 0.001). However, the presence of conspecifics did not influence individual prey consumption (F1, 89 = 0.996, p = 0.321), and no significant interactions were found between prey availability, conspecific presence, and sex in relation to prey consumption. The results remained qualitatively consistent when the non-significant factor (i.e. conspecific presence) was excluded from the model. Because most A. limonicus larvae moulted to protonymphs overnight, further statistical comparisons were not performed on prey consumption over the 24 hr period (Table 1).
Download as abcd Means followed by different letters are significantly different according to ART ANOVA pairwise comparisons.
Prey availability
Conspecific presence
Sex
Prey consumption
n
3 hr
n
24 hr*
5
No
Female
17
4 ± 0.4a
4
4.3 ± 0.5
Male
7
2 ± 0.6bcd
1
4
Yes
Female
12
3.7 ± 0.5ab
7
4.6 ± 0.2
Male
10
2.8 ± 0.4abc
1
5
20
No
Female
19
5.5 ± 0.4b
8
7.3 ± 0.9
Male
9
4.7 ± 1d
0
n/a
Yes
Female
14
4.6 ± 0.7bc
6
8.8 ± 0.8
Male
9
5 ± 0.7cd
2
9 ± 2
* Because of limited numbers, statistical analysis was not conducted on prey consumption after 24 hr.
n/a – No data available.
Experiment 2: Influence of maternal diet on larval feeding behaviour
Higher daily prey consumption by A. limonicus mothers resulted in the production of larger eggs (measured by individual volume) (ART ANOVA: F1, 44 = 12.424, p = 0.001) (Table 2). Overall, female eggs were significantly larger than male eggs (F1, 44 = 14.563, p < 0.001). However, when analysed by treatment, female eggs were significantly larger than male eggs when mothers were provided with 15 eggs per day, whereas no significant difference in egg size between sexes was observed when mothers were supplied with 40 eggs per day (Table 2). The interaction between maternal diet and egg sex on individual egg volume was not significant (F1, 44 = 0.475, p = 0.494). Although mothers with fewer prey produced offspring with an unbiased sex ratio, and those with more prey produced offspring with a slightly female-biased sex ratio (Table 2), the difference between these sex ratios was not statistically significant (χ2 = 1.491, df = 1, p = 0.222).
Download as abc Means followed by different letters are significantly different according to ART ANOVA pairwise comparisons.
Maternal diet (F0)
F1 sex
n
Egg volume (mm3)
Sex ratio (proportion of females)
15
Female
12
0.00140 ± 0.00003a
0.5
Male
13
0.00128 ± 0.00004b
40
Female
16
0.00145 ± 0.00002c
0.7
Male
7
0.00138 ± 0.00002ac
Approximately 10% of the examined larvae (5 males out of 48 valid data) did not feed during the first 3 hr, but all larvae consumed prey over the entire 24 hr period. The maternal diet did not influence feeding occurrence (logistic regression: χ2 = 0.704, df = 1, p = 0.402). However, female larvae (100%) had a significantly higher feeding occurrence than males (75%) (χ2 = 8.420, df = 1, p = 0.004). Since all larvae fed over the 24 hr period, no further statistical analysis was conducted.
Female larvae of A. limonicus consumed significantly more prey during the first 3 hr than males (ART ANOVA: F1, 44 = 4.363, p = 0.043) (Table 3). However, maternal diet history (F1, 44 = 0.389, p = 0.536) and the interaction between sex and maternal diet (F1, 44 = 0.030, p = 0.864) did not significantly affect prey consumption during the first 3 hr. The results were qualitatively similar when the non-significant factor (i.e. maternal diet) was removed from the model. Neither maternal diet (F1, 26 = 0.247, p = 0.623) nor sex (F1, 26 = 0.252, p = 0.620) significantly influenced prey consumption after 24 hr (Table 3). However, a significant interaction was found between maternal diet and sex on prey consumption after 24 hr (F1, 26 = 6.508, p = 0.017). Specifically, females consumed more prey than males when their mothers were provided 15 eggs daily, while males consumed more prey than females when their mothers were provided 40 eggs daily (Table 3).
Download as * Means followed by asterisks () are significantly different, using the Wilcoxon rank-sum test (p *< 0.05) within each maternal diet treatment.
Maternal diet (F0)
F1 sex
Prey consumption
n
3 hr
n
24 hr
15
Female
12
3.4 ± 0.3*
10
4.8 ± 0.1
Male
13
2.3 ± 0.5*
5
4.2 ± 0.5
40
Female
16
3.3 ± 0.3
12
4.7 ± 0.2
Male
7
2.3 ± 0.4
3
5.0 ± 0.0
The initial individual egg volumes did not influence prey consumption of A. limonicus larvae during the first 3 hr (linear regression: F1, 0.084 = 0.036, p = 0.850) or over 24 hr (F1, 0.127 = 0.347, p = 0.562). Furthermore, the relationships between egg volume and prey consumption at both time intervals were not significant (Pearson's correlation: t = −0.191, df = 56, p = 0.850 for prey consumption at 3 hr; t = 0.589, df = 23, p = 0.562 for prey consumption at 24 hr).
Discussion
This study demonstrates that sex and prey availability were the primary factors influencing prey consumption by A. limonicus larvae during the first 3 hr of feeding. Female larvae exhibited significantly higher feeding frequencies and consumed more prey than males, indicating a distinct sexual dimorphism in larval feeding behaviour. Sexual dimorphism is well documented in adult stages of phytoseiid mites, where females are generally larger and consume more prey than males (Walzer and Schausberger 2011; Han et al. 2022, 2023). However, this is the first report of such dimorphism manifesting during the larval stage in phytoseiid mites. The higher prey consumption by female larvae suggests a greater energy requirement for females even at early developmental stages, possibly due to their future reproductive roles. Further studies are needed to investigate the physiological and behavioural mechanisms behind this dimorphism and its potential implications for population dynamics and reproductive success in A. limonicus.
Our results also indicate that prey availability significantly influenced prey consumption during the first 3 hr of feeding (Experiment 1). Larvae given 20 prey eggs consumed significantly more than those given only 5 eggs. This observation supports the idea that higher prey availability and hunger stimulates greater consumption, even though A. limonicus larvae are facultative feeders (Zhang and Croft 1994). The facultative nature of larval feeding implies that larvae can develop without consuming prey, but when prey is available, they will feed. Indeed, most larvae consumed prey during the examination period. These findings emphasise the ecological importance of feeding during the larval stage. Further research could explore whether varying prey availability affects foraging strategies or metabolic adjustments in A. limonicus larvae and the fitness consequences. However, while most larvae consumed prey during the examination period, few individuals given 5 eggs fully consumed them all, especially during the first 3 hr. Whether A. limonicus larvae can assess prey abundance and adjust their consumption to conserve resources for later life stages could be investigated further.
Contrary to expectations, the presence of conspecifics did not significantly affect larval prey consumption. The lack of influence from conspecifics suggests that under the conditions provided in this study, where direct physical contact was absent, A. limonicus larvae experience no influence. The absence of a conspecific effect may indicate that interference or direct competition is necessary to alter feeding behaviour. For instance, predatory mites like P. persimilis and the seaside ladybeetle (Naemia seriata Melsheimer) have been shown to adjust their prey consumption in the presence of conspecifics when direct contact occurs (Rinehart and Long 2019; Xu et al. 2023). Thus, the lack of direct interaction among conspecifics in this study may have reduced or eliminated competitive pressures, allowing larvae to focus on prey consumption. Future studies involving direct physical contact between conspecifics larvae may provide a more complete understanding of their foraging behaviour.
In contrast to prey availability and sex, maternal diet did not influence larval prey consumption during the first 3 hr, nor did the initial egg size. This suggests that larval feeding behaviour is not strongly influenced either by maternal provisioning or by the size of the egg from which they hatched. However, we observed that maternal diet significantly affected the size of eggs produced, with females from mothers provided with more prey producing larger eggs, as expected (Lee et al. 2020; Bonduriansky 2021; Han et al. 2024). Interestingly, male eggs were more negatively affected by maternal dietary restriction, resulting in a larger size dimorphism between female and male eggs (Table 2). Further studies investigating the influence of maternal diet and offspring provisioning may enhance our understanding on the reproductive strategies of A. limonicus.
It is important to note several limitations in our study. First, the controlled laboratory conditions, while useful for reducing environmental variability, may not fully replicate the complexities of natural ecosystems. Second, we used only one prey species, C. lactis, which may limit the generalisability of our findings to other prey species. Differences in nutritional content, palatability, or defensive mechanisms across prey types may alter larval feeding behaviour. Additionally, the inability to perform statistical comparisons on prey consumption at 24 hr, because of moulting to protonymphs, limits our understanding on feeding dynamics of their whole larval stage. Future research should incorporate multiple prey species and explore the effects of maternal diet and conspecific interactions in more realistic field settings to better understand the ecological and evolutionary implications of larval feeding strategies in A. limonicus.
In conclusion, our study reveals that sex and prey availability significantly influence the feeding behaviour of A. limonicus larvae, with females consuming more prey than males. The lack of an effect from conspecific presence suggests a solitary foraging strategy during the larval stage. Although maternal diet influenced egg size, it did not significantly affect larval prey consumption. These findings contribute to our understanding of the complex factors shaping the feeding behaviour of this predatory mite and may have implications for its use in biological control programs. Further studies are needed to explore the fitness consequences of early feeding behaviour and how these behaviours may affect subsequent life stages and population dynamics under natural conditions.
Acknowledgements
We thank Helen O'Leary of Manaaki Whenua – Landcare Research for her constructive comments and suggestions that improved this manuscript. This study was supported in part by New Zealand Government core funding for Crown Research Institutes from the Ministry of Business, Innovation, and Employment's Science and Innovation Group. Wei Liu was supported by China Scholarship Council, the Natural Science Foundation of Gansu Province to grant (21JR7RA482) and Gansu Gannan Grassland Ecosystem National Observation and Research Station.
References
- Bonduriansky R. 2021. Plasticity across generations. In: Pfennig D.W. (Ed). Phenotypic plasticity & evolution: Causes, consequences, controversies. Boca Raton: CRC Press. pp. 327-348. https://doi.org/10.1201/9780429343001-17
- Bonduriansky R., Day T. 2009. Nongenetic inheritance and its evolutionary implications. Annu. Rev. Ecol. Evol. Syst., 40: 103-125. https://doi.org/10.1146/annurev.ecolsys.39.110707.173441
- Chittenden A., Saito Y. 2001. Why are there feeding and nonfeeding larvae in phytoseiid mites (Acari, Phytoseiidae)? J Ethol., 19: 55-62. https://doi.org/10.1007/s101640170018
- Croft B.A., Croft M.B. 1993. Larval survival and feeding by immature Metaseiulus occidentalis, Neoseiulus fallacis, Amblyseius andersoni and Typhlodromus pyri on life stage groups of Tetranychus urticae Koch and phytoseiid larvae. Exp. Appl. Acarol., 17: 685-693. https://doi.org/10.1007/BF00058508
- Croft B.A., Luh H.K., Schausberger P. 1999. Larval size relative to larval feeding, cannibalism of larvae, egg or adult female size and larval-adult setal patterns among 13 phytoseiid mite species. Exp. Appl. Acarol., 23: 599-610.
- Donelson J.M., Munday P.L., McCormick M.I. 2009. Parental effects on offspring life histories: when are they important? Biol. Lett., 5: 262-265. https://doi.org/10.1098/rsbl.2008.0642
- Gu X., Zhang K., Zhang Z-Q. 2022. Non-consumptive effects of intraguild predator Blattisocius dentriticus (Berlese) on the development and prey consumption of Neoseiulus cucumeris (Oudemans). Syst. Appl. Acarol., 27: 1475-1482. https://doi.org/10.11158/saa.27.7.12
- Han X., Zhang K., Xu Y., Zhang Z-Q. 2022. Prey requirement and development of a predatory mite under diet restriction: Phytoseiulus persimilis Athias-Henriot (Phytoseiidae) feeding on Tetranychus urticae Koch (Tetranychidae). Syst. Appl. Acarol., 27: 2103-2110. https://doi.org/10.11158/saa.27.10.18
- Han X., Zhang K., Xu Y., Zhang .-Q. 2023. Temporal variations in diet restrictions on prey requirement and development of a predatory mite. Int. J. Acarol., 1-6.
- Han X., Zhang K., Chen X., Zhang Z-Q. 2024. Diet restriction in parents shapes the lives of their offspring: a laboratory study using a predatory mite (Acari: Phytoseiidae). Syst. Appl. Acarol., 29: 45-59. https://doi.org/10.11158/saa.29.1.4
- Kay M., Elkin L., Higgins J., Wobbrock J. 2021. ARTool: Aligned Rank Transform for Nonparametric Factorial ANOVAs [Internet]. R package version 0.11.1. [24 Sept 2022]. Available from: https://github.com/mjskay/ARTool.
- Knapp M., van Houten Y., Hoogerbrugge H., Bolckmans K. 2013. Amblydromalus limonicus (Acari: Phytoseiidae) as a biocontrol agent: literature review and new findings. Acarologia, 53: 191-202. https://doi.org/10.1051/acarologia/20132088
- Lee M., Fan Q-H., Zhang Z-Q. 2020. Caloric restriction extends lifespan of mothers at the expense of offspring survival in a predatory mite (Neoseiulus cucumeris). Syst. Appl. Acarol., 25: 1948-1962. https://doi.org/10.11158/saa.25.11.2
- Liu J., Zhang Z-Q. 2017. Development, survival and reproduction of a New Zealand strain of Amblydromalus limonicus (Acari: Phytoseiidae) on Typha orientalis pollen, Ephestia kuehniella eggs, and an artificial diet. Int. J. Acarol., 43: 153-159. https://doi.org/10.1080/01647954.2016.1273972
- Liu J., Beggs J.R., Zhang Z-Q. 2018. Population development of the predatory mite Amblydromalus limonicus is modulated by habitat dispersion, diet and density of conspecifics. Exp. Appl. Acarol., 76: 109-121. https://doi.org/10.1007/s10493-018-0292-5
- Liu W., Zhang K., Zhang Z-Q. 2023. Larval feeding types shape the predation aggression of predatory mites in both intraspecific and interspecific encounters. Syst. Appl. Acarol., 28: 1272-1282. https://doi.org/10.11158/saa.28.7.6
- Liu Z., Zhang K., Zhang Z-Q. 2024a. Enhancing the efficiency of egg collection of the astigmatid mite Carpoglyphus lactis (Acari: Carpoglyphidae) as a diet for predatory mites. Syst. Appl. Acarol., 29: 355-358. https://doi.org/10.11158/saa.29.2.14
- Liu Z., Zhang K., Zhang Z-Q. 2024b. Unintended consequences: the adverse effects of royal jelly supplementation in the predatory mite Amblyseius herbicolus Chant (Acari: Phytoseiidae). Syst. Appl. Acarol., 29: 335-345. https://doi.org/10.11158/saa.29.2.12
- Lopez L. 2023. Meet Amblyseius swirskii (Acari: Phytoseiidae): a commonly used predatory mite in vegetable crops. J. Integr. Pest Manag., 14: 20. https://doi.org/10.1093/jipm/pmad018
- Ma M., Fan Q-H., Zhang Z-Q. 2018. Morphological ontogeny of Amblydromalus limonicus (Acari: Phytoseiidae). Syst. Appl. Acarol., 23: 1741-1765. https://doi.org/10.11158/saa.23.9.3
- McMurtry J.A., Moraes G.J.D., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 297-320. https://doi.org/10.11158/saa.18.4.1
- McMurtry J.A., Sourassou N.F., Demite P.R. 2015. The phytoseiidae (acari: Mesostigmata)
- as biological control agents. In: Carrillo D., de Moraes G.J., Peña J. (Eds). Prospects for biological control of plant feeding mites and other harmful organisms. Switzerland: Springer. p. 133-149.
- Narushin V.G. 2005. Egg geometry calculation using the measurements of length and breadth. Poult. Sci., 84: 482-484. https://doi.org/10.1093/ps/84.3.482
- R Core Team. 2022. R: A language and environment for statistical computing. Version 4.2.1. Vienna: R Foundation for Statistical Computing. [10 Feb 2022]. Available from: https://www.R-project.org/
- Rinehart S., Long J.D. 2019. Conspecifics, not pollen, reduce omnivore prey consumption. PloS One, 14: e0215264. https://doi.org/10.1371/journal.pone.0215264
- Saitoh F., Janssen A., Choh Y. 2020. The use of volatile cues in recognition of kin eggs by predatory mites. Ecol. Entomol., 45: 1220-1223. https://doi.org/10.1111/een.12872
- Schausberger P., Croft B.A. 1999. Activity, feeding, and development among larvae of specialist and generalist phytoseiid mite species (Acari: Phytoseiidae). Environ. Entomol., 28: 322-329. https://doi.org/10.1093/ee/28.2.322
- Su Y., Zhang B., Xu X. 2021. Chemosensory systems in predatory mites: from ecology to genome. Syst. Appl. Acarol., 26: 852-865. https://doi.org/10.11158/saa.26.5.3
- Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol., 23: 432-438. https://doi.org/10.1016/j.tree.2008.04.005
- Vangansbeke D., Nguyen D.T., Audenaert J., Verhoeven R., Gobin B., Tirry L., De Clercq P. 2014. Performance of the predatory mite Amblydromalus limonicus on factitious foods. BioControl, 59: 67-77. https://doi.org/10.1007/s10526-013-9548-5
- Walzer A., Schausberger P. 2011. Sex-specific developmental plasticity of generalist and specialist predatory mites (Acari: Phytoseiidae) in response to food stress. Biol. J. Linn. Soc., 102: 650-660. https://doi.org/10.1111/j.1095-8312.2010.01593.x
- Wang J., Zhang K., Li L., Zhang Z-Q. 2024. Development and reproduction of four predatory mites (Parasitiformes: Phytoseiidae) feeding on the spider mites Tetranychus evansi and T. urticae (Trombidiformes: Tetranychidae) and the dried fruit mite Carpoglyphus lactis (Sarcoptiformes: Carpoglyphidae). Syst. Appl. Acarol., 29: 269-284. https://doi.org/10.11158/saa.29.2.7
- Wickham H. 2016. Ggplot2: Elegant graphics for data analysis [Internet]. Springer-Verlag. Available from: https://ggplot2-book.org/ https://doi.org/10.1007/978-3-319-24277-4_9
- Xu Y., Zhang Z-Q. 2015. Amblydromalus limonicus: A "new association» predatory mite against an invasive psyllid (Bactericera cockerelli) in New Zealand. Syst. Appl. Acarol., 20: 375-382. https://doi.org/10.11158/saa.20.4.3
- Xu Y., Zhang K., Zhang Z-Q. 2023. The impact of conspecific presence on diet-induced developmental responses in a predatory mite. Syst. Appl. Acarol., 28: 1705-1715. https://doi.org/10.11158/saa.28.11.2
- Zhang Z-Q. 2003. Mites of greenhouses: Identification, biology and control [Internet]. CABI Publishing. Available from: http://www.cabi.org/cabebooks/ebook/20033142338
- Zhang Z.-Q., Croft B.A. 1994. A comparative life history study of immature Amblyseius fallacis, Amblyseius andersoni, Typhlodromus occidentalis and Typhlodromus pyri (Acari: Phytoseiidae) with a review of larval feeding patterns in the family. Exp. Appl. Acarol., 18: 631-657. https://doi.org/10.1007/BF00051532
- Zhang K., Zhang Z-Q. 2021. The dried fruit mite Carpoglyphus lactis (Acari: Carpoglyphidae) is a suitable alternative prey for Amblyseius herbicolus (Acari: Phytoseiidae). Syst. Appl. Acarol., 26: 2167-2176. https://doi.org/10.11158/saa.26.11.15



2024-10-09
Date accepted:
2025-03-25
Date published:
2025-04-01
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Liu, Wei; Zhang, Keshi; Li, Lanjing and Zhang, Zhi-Qiang
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



