An exceptionally well-preserved Eocene fossil mite, Histiogaster altilis sp. n. (Acari: Astigmata), from tree sap: Evidence of morphological and ecological niche conservatism, with a review of fossil Astigmata
Kolesnikov, Vasiliy B.  1
; Vorontsov, Dmitry D.
1
; Vorontsov, Dmitry D.  2
; Perkovsky, Evgeny E.
2
; Perkovsky, Evgeny E.  3
and Klimov, Pavel B.
3
and Klimov, Pavel B.  4
4
1Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, 152742 Borok, Yaroslavl Province, Russia & Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, 625003 Tyumen, Russia.
2Koltzov Institute of developmental biology, RAS, Russia.
3Natural History Museum of Denmark, 2100 Copenhagen, Denmark.
4✉ Department of Biological Sciences, Purdue University, Lilly Hall of Life Sciences, West Lafayette, IN 47907, USA.
2025 - Volume: 65 Issue: 1 pages: 213-241
https://doi.org/10.24349/c35e-8bmjZooBank LSID: B3A38FFE-645E-4E3C-BC3D-171AE3B5C384
Original research
Keywords
Abstract
Introduction
Mites belonging to the hyporder Astigmata (= Astigmatina) include more 6000 (Schatz et al. 2011) free-living and parasitic species living in terrestrial, freshwater and synanthropic habitats (OConnor, 2009). However, in contrast to oribatid mites, fossil records of Astigmata are scarce (Dunlop et al. 2011). Currently, there are 24 records, but only 14 can be confidently attributed to Astigmata (Table 1). Of these, eight species are known from heteromorphic deutonymphs (a specialized dispersal stage), while only five are represented by adults and other feeding stages (Table 1). Phoretic stages of astigmatid mites have a much higher likelihood of preservation in amber because their insect carriers are likely to be trapped in amber and fossilized. Once entrapped in resin, heteromorphic deutonymphs may detach from their arthropod host or be preserved while still attached to the host, providing insight into their ancient host associations (Kolesnikov et al., 2023). In contrast, adult and other feeding stages are less likely to fossilize due to their limited mobility and habitat specificity. Fossilization is most likely to occur when these mites inhabit environments in close proximity to resin-producing trees, such as subcortical habitats where resin can easily entrap them. The fossil species Glaesacarus rhombeus (Koch et Berendt, 1854) (Sidorchuk and Klimov, 2011) is an example of such a mite species, as its feeding stages are particularly abundant in Eocene amber. A modestly sized piece of Baltic amber can contain dozens of G. rhombeus specimens. These mites were reported as less common in Rovno amber (Perkovsky et al., 2007), but they are the most important mites in Rovno amber as well. While we have detailed knowledge of the external morphology of G. rhombeus, its ecology and biology remain speculative due to the lack of close extant relatives that could provide ecological insights.
Download as * The genus was originally proposed as follows (Dubunin, 1962): “Palaeotyroglyphus W. Dubinin, nom. nov. (= Gamasus Mani, 1945, 1946). Genotype—Gamasus fossilis Mani, 1945; Larva (and not protonymph as written by Mani, 1945 and George, 1952) (Fig. 1290)”. However, Gamasus fossilis Mani, 1945, clearly belongs to Mesostigmata based on the presence of peritremes, ambulacra with two claws, and the shape and setation of the legs. In contrast, George (1952) described and illustrated an astigmatid mite but identified it as a “gamasid mite”. This mite appears in Fig. 1290 of Dubinin (1962). It is therefore likely that Dubinin (1962) considered George’s mite to be identical to Mani’s mite without actually examining Mani’s description.
Identification
Developmental stage
Phoretic host
Age
Reference
Notes
Levantoglyphus sidorchukae Klimov, Vorontsov, Azar, Sidorchuk, Braig, Khaustov, Tolstikov, 2021 (Levantoglyphidae)
phoretic deutonymphs
found without a host
Early Cretaceous (Lebanese amber, 125–129 Ma)
Klimov et al 2021
Plesioglyphus lebanotermi Sendi, Klimov, Kolesnikov, Káčerová, Bonino, Azar, Robin, 2025 (Schizoglyphidae)
phoretic deutonymphs
Lebanotermes veltzae (Blattodea)
Early Cretaceous (Lebanese amber, 125–129 Ma)
Azar 2007; Sendi et al., 2025
Congovidia glesoconomorphi Kolesnikov et Klimov, 2023 (Hemisarcoptidae)
phoretic deutonymphs
Glesoconomorphus ekaterinae (Coleoptera: Mycteridae)
Late Eocene (Rovno amber, 34–37 Ma)
Telnov et al. 2021 (as “Winterschmidtiidae”); Kolesnikov et al. 2023
Glaesacarus rhombeus (Koch & Berendt, 1854) (Glaesacaridae)
all feeding stages and eggs (except phoretic deutonymphs)
free-living
Late Eocene (Baltic amber 34–37 Ma, Rovno amber, 34-37 Ma)
Koch and Berendt 1854; Sidorchuk and Klimov 2011; Perkovsky et al. 2007
Histiostoma ovalis-species group (Histiostomatidae)
phoretic deutonymph
Phloeosinus sp. (Coleoptera:Scolytidae)
Late Eocene (Baltic amber, 34–37 Ma)
Wirth & Garonna 2015
Histiogaster altilis sp.n. (Acaridae)
adult
free-living
Late Eocene (Rovno amber, 34-37 Ma)
Vorontsov et al. 2023 (as Histiogaster sp); Pepato et al. 2022 (as Histiogaster sp.)
Described in this study
Acaridae or Histiostomatidae
phoretic deutonymph
Dasumiana emicans (Araneae: Dysderidae)
Eocene (Baltic amber, 34–37 Ma)
Dunlop 2012
Acaridae
phoretic deutonymph
Collohmannia weiterschani (Acari: Oribatida: Collohmanniidae)
Eocene (Baltic amber, 34–37 Ma)
Kolesnikov et al. 2024
Astigmata, probably Acaridae
phoretic deutonymph
found without a host
Eocene (Baltic amber 34–37 Ma)
Halbwachs et al. 2021 (as unidentified palynomorph, Plate 8, Fig. 12)
Amphicalvolia hurdi Türk, 1963 (Winterschmidtiidae)
phoretic deutonymphs
found without a host
Miocene (Mexican Chiapas amber, 15–20 Ma)
Türk 1963
Belongs to either Winterschmidtia or Parawinterschmidtia (Klimov et al. 2021, OConnor 2022)
Atopomelidae (OConnor, pers. comm.)
feeding stages
Mammal hair
Early Miocene (Dominican amber, 16–18 Ma)
Poinar 1988 (as Listrophoroidea)
Astigmata
phoretic deutonymphs
ambrosia beetle (Coleoptera: Platypodinae)
Early Miocene (Dominican amber, 16–18 Ma)
Santiago-Blay et al. 2012
Astigmata (genus unrecognizable) (specimen 128-g)
unknown, probably a feeding stage
unknown
Quaternary (Mizunami copal)
Aoki 1974 (taxon names written in Japanese)
Astigmata (genus unrecognizable)
nymph
free-living
Dolomite from the base of the Saline Series at the junction of Jankush Nulla and Warchha Gorge, Warchha, Punjab, Pakistan. Early Permian (Ghazi and Mountney, 2011).
George 1952 (as “Parasitidae”)
Assigned to Palaeotyroglyphus by* Dubinin (1962) as “Gamasus fossilis Mani, 1945”, which belongs to Mesostigmata
Not Astigmata
Ectoparasitic feather mites
eggs
Dinosaur feathers
Early Cretaceous (Nova Olinda, Crato Formation, late Aptian)
Martill and Davis 1998
Not Astigmata, possible misidentification for Cheyletoidea (Bochkov, 2009).
Palaeotyroglyphus fossilis (Mani, 1945)
nymph
free-living
Worli Hill, Mumbai, India. Maastrichtian to Selandian, 66–61.7 Ma (Mantilla et al. 2022)
Dubinin, 1962 (as “larva, from the Deccan Intertrappean sample, Punjab, Neogene (?)”; type species: “Gamasus fossilis Mani, 1945”
Dubinin (1962) designated Gamasus fossilis Mani, 1945 (Mesostigmata) as the type species for the genus Palaeotyroglyphus Dubinin, 1962. However, he misidentified this species, mistaking it for “Parasitidae” described by George (1952), which actually belongs to Astigmata. According to ICZN Article 70.1.1, when designating a type species from a previously established nominal species, it is assumed that the species has been correctly identified. Therefore, despite the misidentification, Palaeotyroglyphus should be classified within Mesostigmata*
Acarus resinosus Presl, 1822
unknown
unknown
Eocene (Baltic amber, 34–37 Ma)
Presl, 1822
nomina dubia (Dunlop et al. 2011); could be a Bdellidae based on the original description: “the body is ovoid, tapering at the front, with the head projecting like a tip”.
"Sarcoptes kutchensis" Agnihotri, Singh, Subramanian et Acharya, 2023
adult
free-living
Middle Eocene (Kutch amber)
Agnihotri et al 2023
Not Astigmata, misidentification for Endeostigmata (probably Nanorchestidae)
Specimens unrecognizable as mites
free-living
Warchha 14; Salt and Marl KS from Khewra, respectively, from Salt Range, Punjab, Pakistan. Early Permian (Ghazi and Mountney, 2011).
Mani 1946 (as “Mite (leg)”; “gamasid mite (protonymph)”)
Attributed to Palaeotyroglyphus fossilis by Dubinin (1962), but both specimens are unrecognizable as mites
“Tyroglyphites miocenicus” Pampaloni, 1902
adult
free-living
Neogene (Sicily, Italy)
Pampaloni 1902
Not Astigmata, misidentification for Lohmanniidae
“Acarus indicus” Kumar, Ja Jha, Bhattacharya & Pande, 2011
nymph
free-living
Originally reported from Permian sediments in the Chamba Valley, Himachal Pradesh, India, it likely represents contamination by a modern mite
Kumar et al. 2011
Not Astigmata, misidentification for juvenile Nothridae (Norton, pers. comm.)
“Acarus siro (Linnaeus, 1758)”
nymph
free-living
Originally reported from Permian sediments in the Chamba Valley, Himachal Pradesh, India, it likely represents contamination by a modern mite
Kumar et al. 2011
Not Astigmata, misidentification for juvenile Nothridae (Norton, pers. comm.)
Our survey of Rovno amber mites yielded a single amber piece containing several exceptionally well-preserved males and females of a mite belonging to the modern genus Histiogaster Berlese, 1883, family Acaridae. This genus includes 24 modern species living in subcortical habitats, including galleries of bark beetles (Woodring, 1966), tree sap flows (Türk and Türk, 1957; Bugrov, 1997), decaying wood and polypore fungi, and other fermented materials (Zachvatkin, 1941; Woodring, 1966; Klimov and Tolstikov, 2011; Pfammatter et al., 2016; Klimov et al., 2022). Among most other acarid genera, males of Histiogaster are distinct by having characteristic four-lobed, fan-like projections on the posterior opisthosoma, which serve for attachment to females during mating and pre-copulatory guarding (Klimov et al. 2022). The only genera that have a similar structure are Paulacarellus (unrelated to Histiogaster) inhabiting sea wrack in the marine supralittoral zone (Fain, 1977; Klimov, 2000) and Horstiella (related to Histiogaster, our data) associated with bees of the genus Epicharis from the Neotropical region (Ochoa and OConnor, 2000).
In this study, we use advanced imaging (high-resolution microscopy, DIC in combination with transmitted light and CLSM with a super-resolution detector) and amber preparation techniques to examine the fine morphological details of this newly discovered fossil mite, Histiogaster altilis sp. n. We compared its morphological traits with those of modern Histiogaster species with known ecologies to infer the paleohabitat of the fossil. Additional evidence from the amber piece, such as its physical characteristics (suggestive of phloem sap presence) and syninclusions (e.g., bacterial or fungal hyphae that could indicate fermented substances), further aided our paleoecological reconstruction. Histiogaster altilis sp. n. provides valuable evidence of the long-term evolutionary stability of this genus and its specialized ecological niche since the Eocene.
Methods
Amber processing
The amber pieces were trimmed and polished following the protocols outlined by Sidorchuk and Vorontsov (2018). To optimize high-resolution immersion optics and allow observation of specimens from multiple angles, the amber was polished to leave a space of ≤100 μm between the surface and the inclusion. The processed amber pieces were then stored in water.
Imaging
For microscopic observation, processed pieces of amber were mounted on a coverslip in a saturated fructose solution, then the coverslip was inverted and studied under a microscope (see Vorontsov and Voronezhskaya 2022 for details). Images were taken in transmitted and reflected light using a Nikon E-800 compound microscope equipped with an Olympus OM-D E-M10-II digital camera. Image z-stacks (images taken consequently from multiple focal planes) were processed in Adobe Lightroom for color correction, noise reduction, and sharpness enhancement. Focus stacking, both automatic and manual, was performed in Helicon Focus. Confocal scanning was done using a Zeiss LSM-880 CLSM equipped with AiryScan detector. A 488 nm Argon laser was used for excitation, while detection range was set to 500–700 nm. To view and manipulate the confocal image stacks, we used the following software packages: Zeiss ZEN, FIJI (Schindelin et al. 2012), FastStone Image Viewer, and Helicon Focus. The original image stacks and three-dimensional reconstructions are available through Figshare (https://doi.org/10.6084/m9.figshare.28632650 ![]() ; https://doi.org/10.6084/m9.figshare.22101911
; https://doi.org/10.6084/m9.figshare.22101911 ![]() ).
).
Terminology and measurements
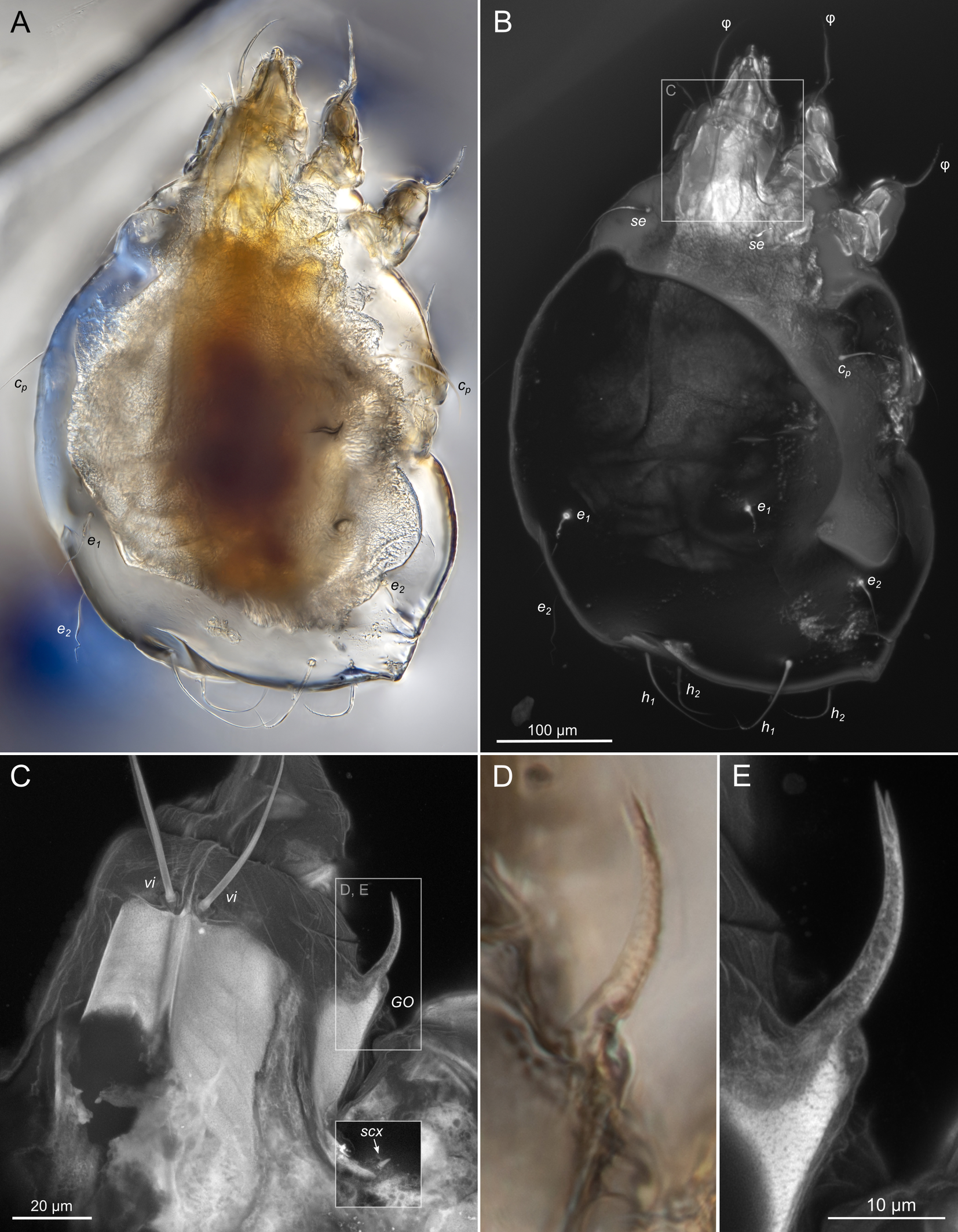
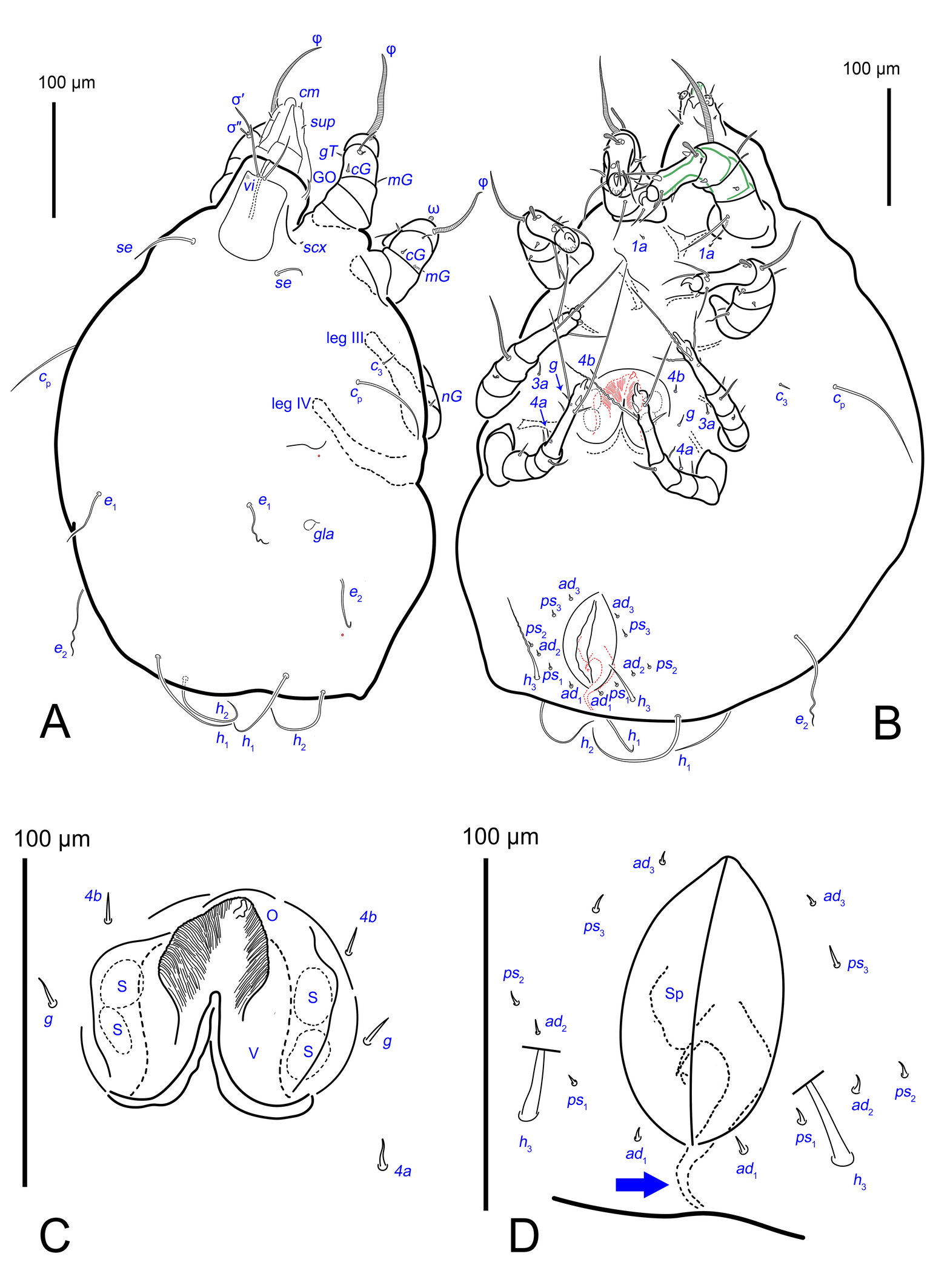
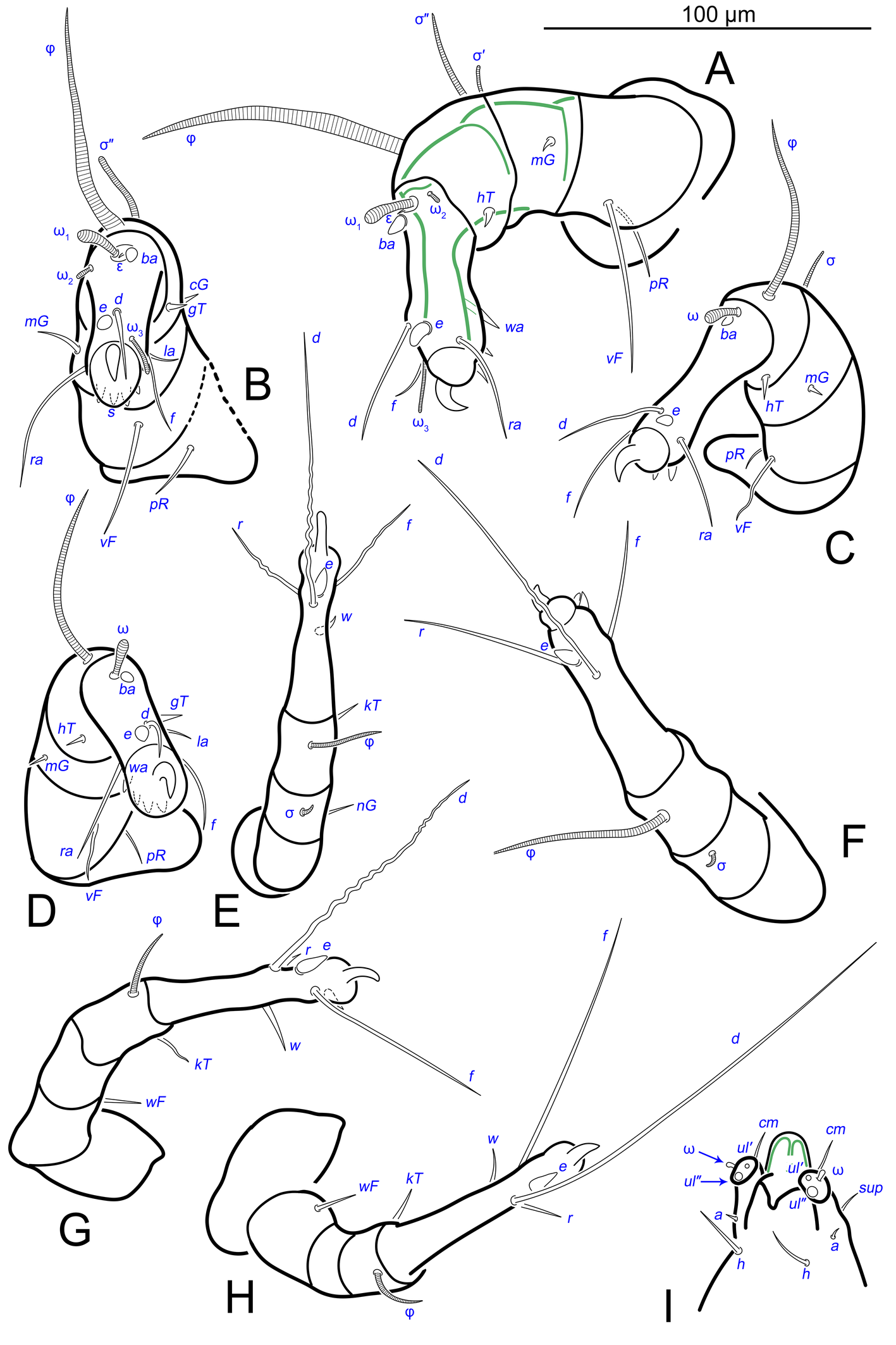
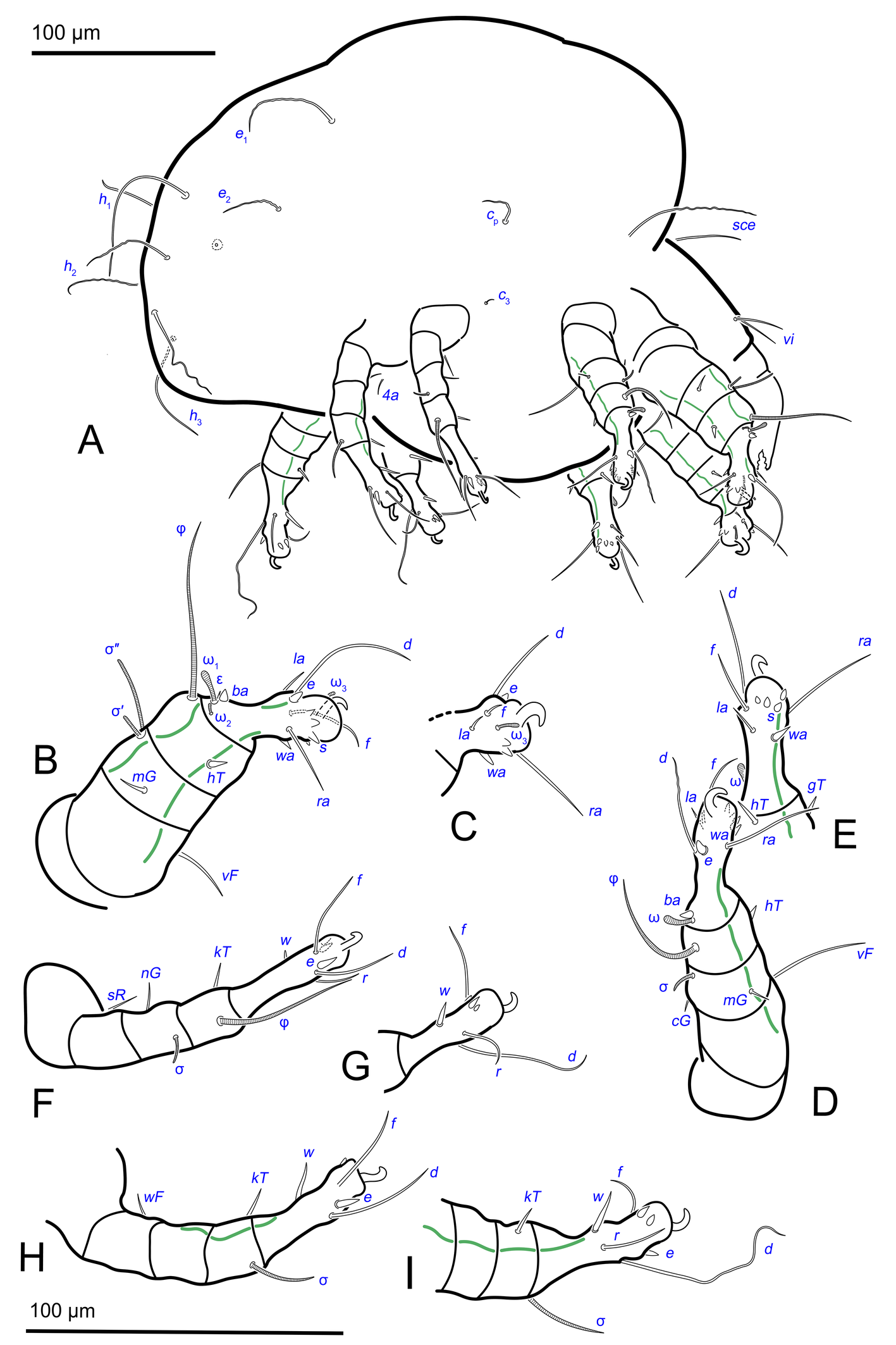
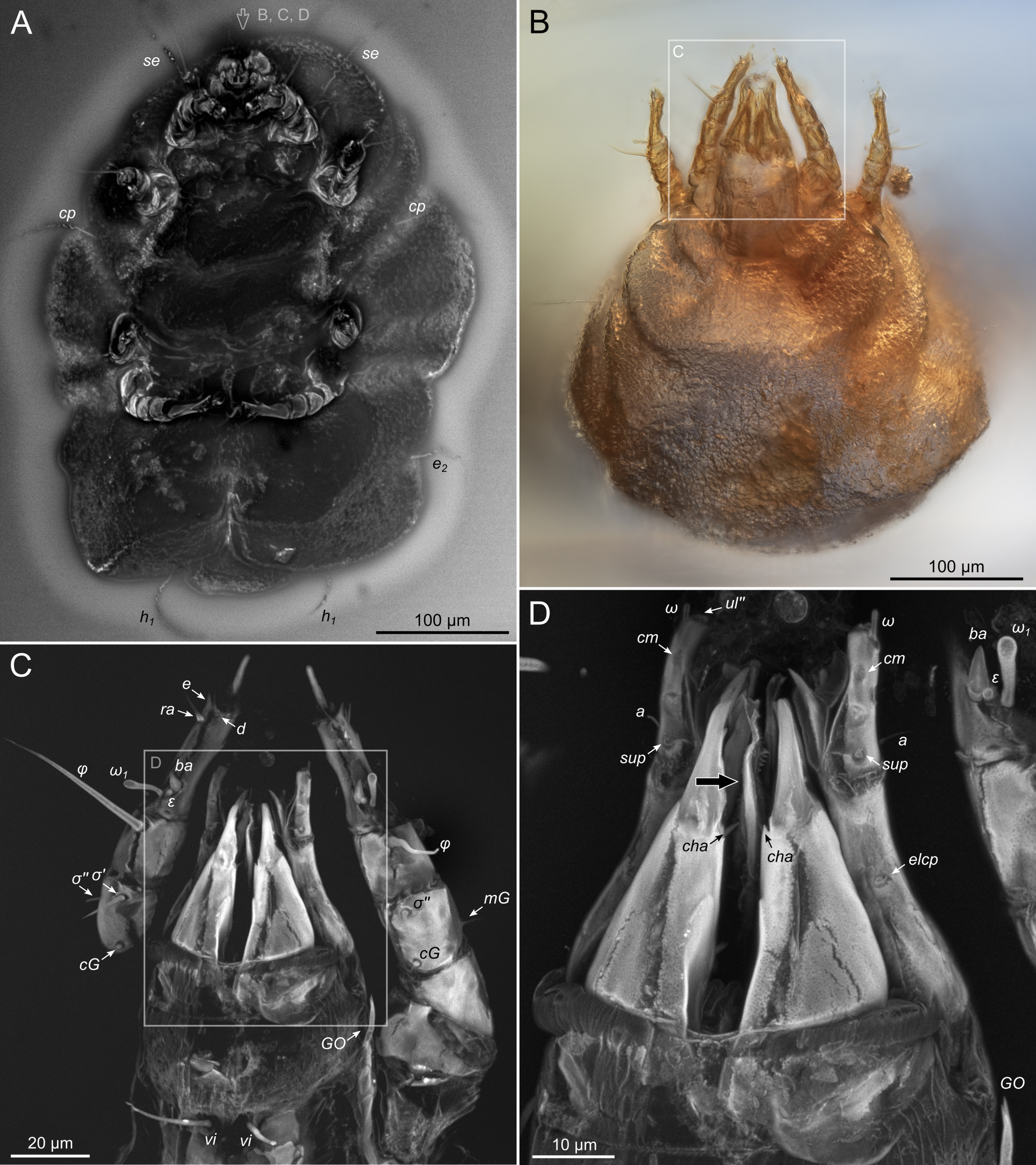
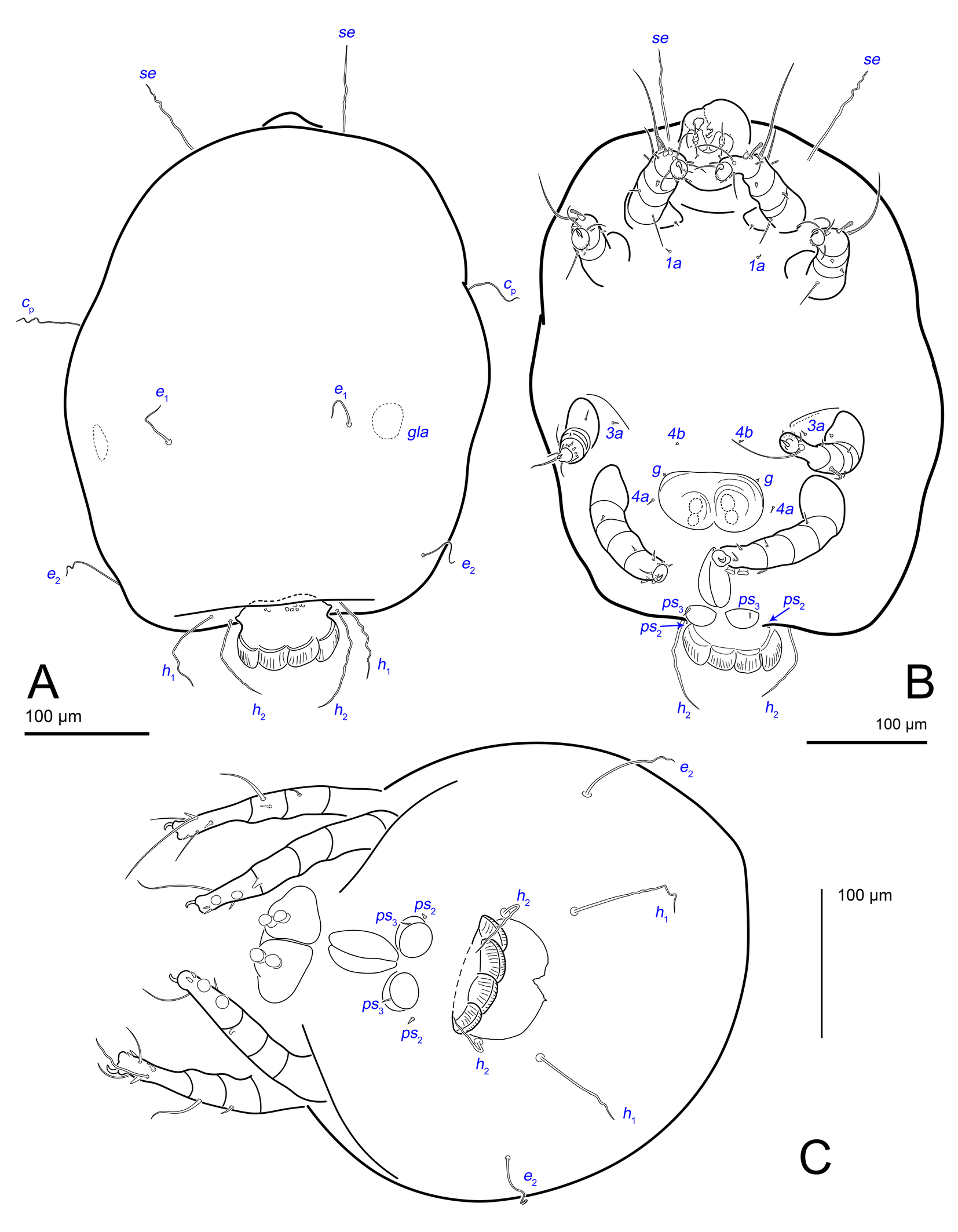
The idiosomal chaetotaxy terminology follows Griffiths et al. (1990), while the terminology for the palp and leg chaetotaxy is based on Grandjean (1939) and Griffiths (1970). The terminology of coxisternal setae follows Norton (1998). The terminology of the spermatheca follows that of Klimov and OConnor (2003). The terminology for the ovipore and related structures follows Evans (1992), as applied to oribatids. For consistency with Oribatida, the structure commonly referred to as the ′vagina′ in Astigmata is termed the ovipositor. The following abbreviations are used: A—aedeagus; GO—Grandjean's organ; gla— opisthonotal gland openings; O—plicated zone of the ovipositor; S—genital papillae; Sp—spermatheca; V—ovipositor. All measurements are provided in micrometres (μm).
Results
Systematic paleontology
Class Arachnida Cuvier, 1812
Order Sarcoptiformes Reuter, 1909
Suborder Oribatida Duges, 1834
Family Acaridae Latreille, 1802
Genus Histiogaster Berlese, 1883
Type species. Tyroglyphus carpio Kramer, 1881, by original designation.
Histiogaster altilis sp. n.
ZOOBANK: E453FB5A-EA48-4904-8CB0-D52EA6510ABF ![]()
(Figs 1–18)
Material
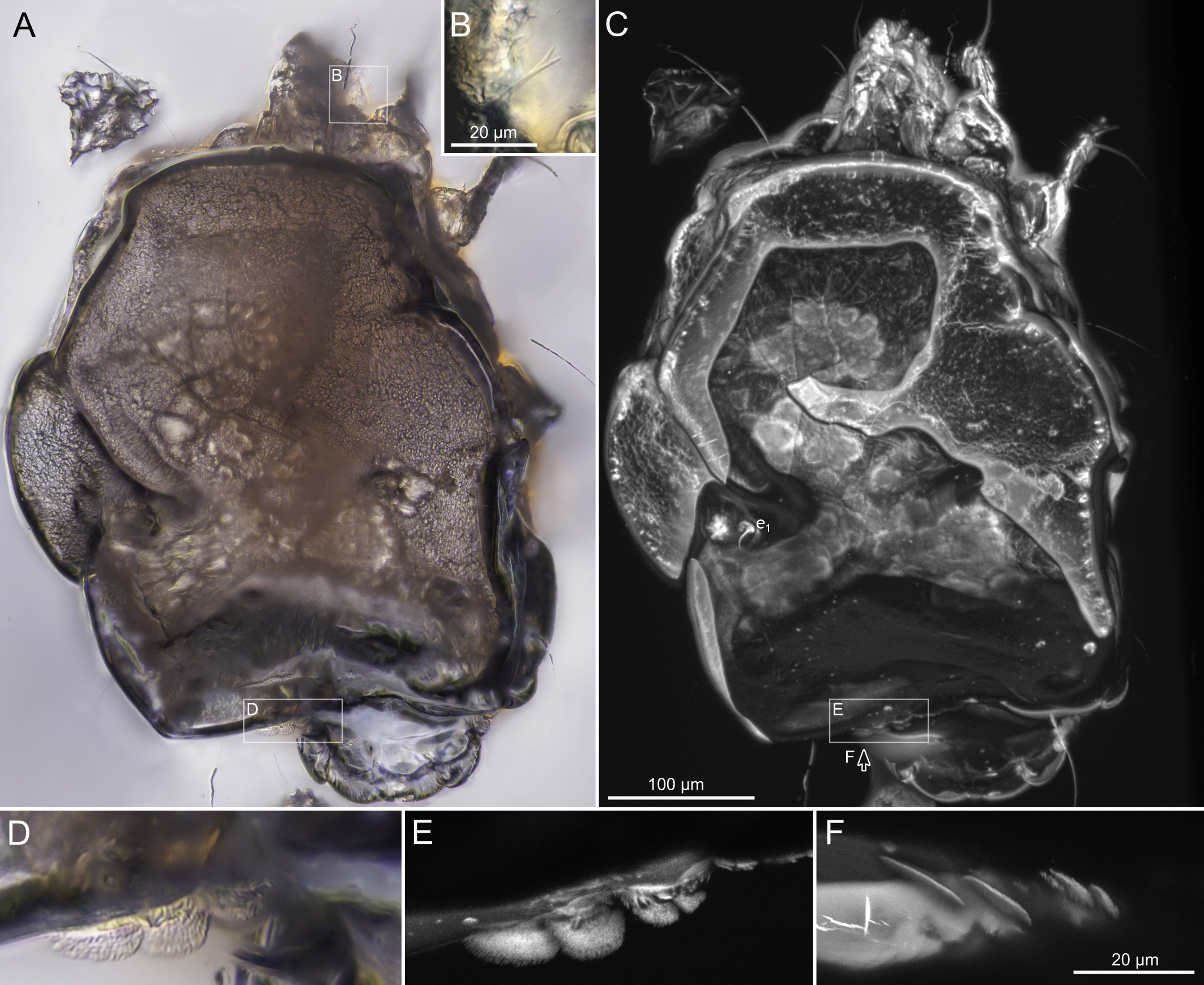
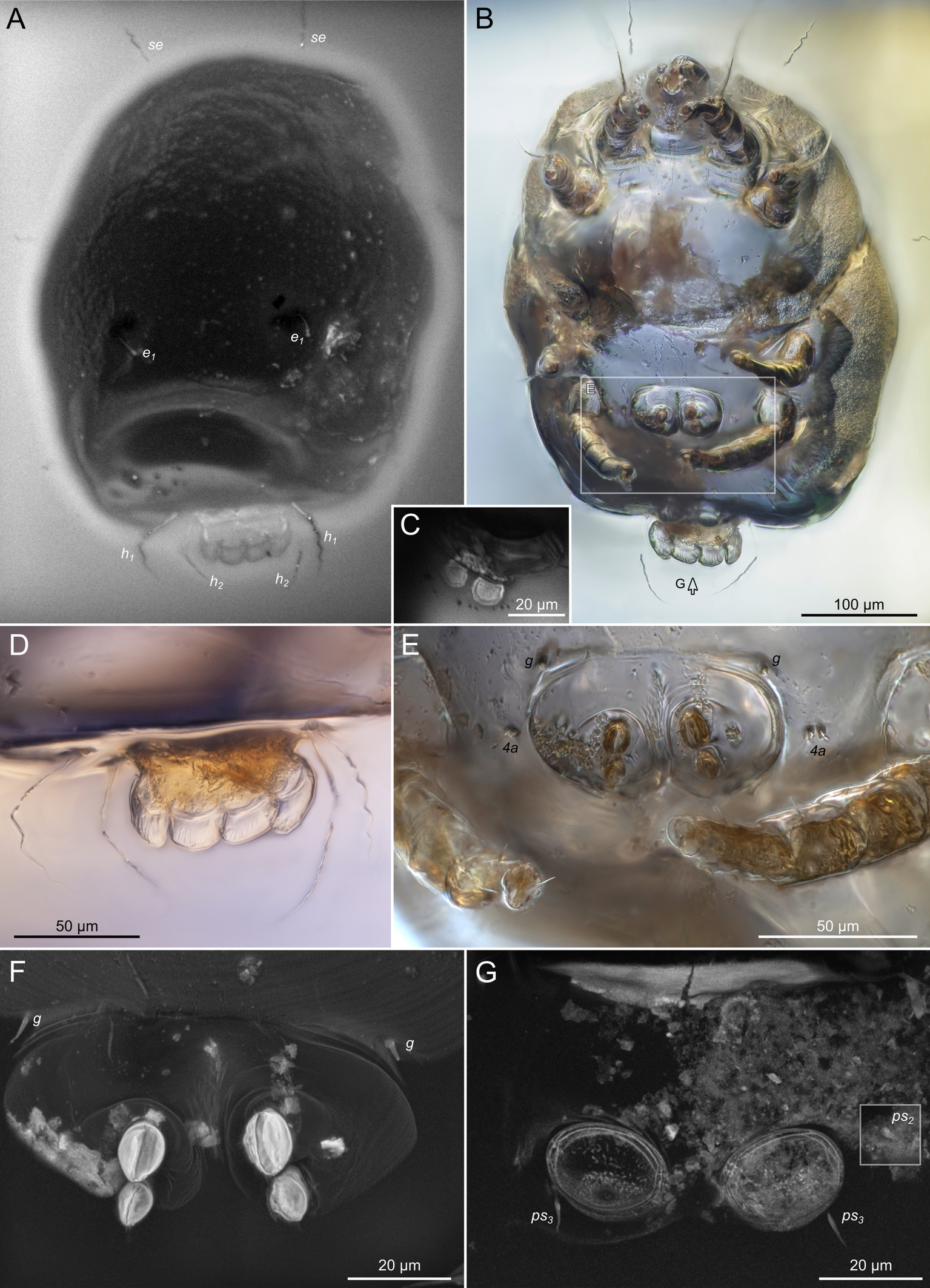
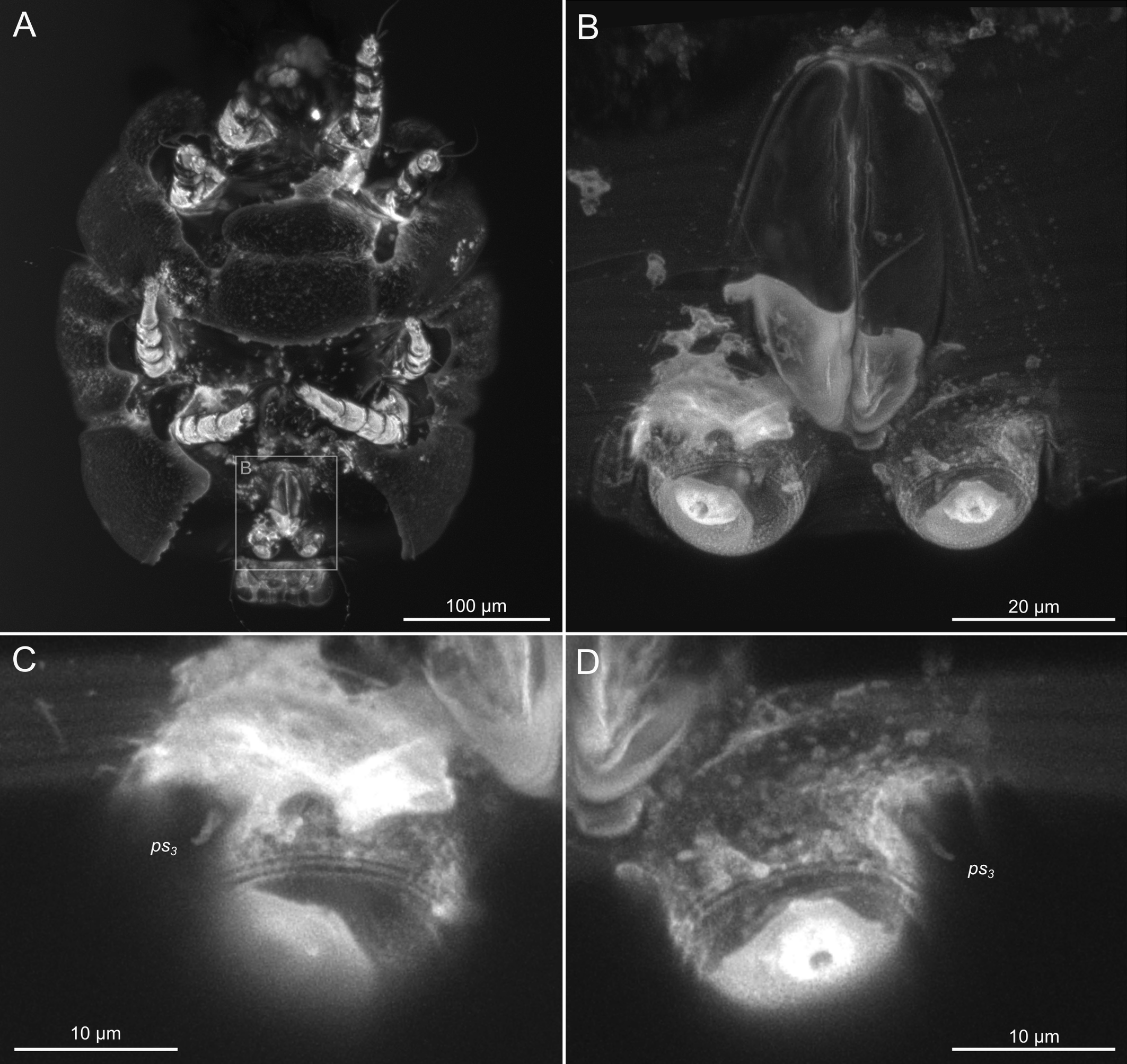
Rovno amber originated on the Volhynian Uplift (Chemyreva et al. 2024). We studied two pieces of Eocene Rovno amber mined at Pugach quarry (Klesov, Rovno Oblast) from the amber collection of the Schmalhausen Institute of Zoology, Kiev (SIZK), with the following collection numbers: SIZK K-15219 and SIZK K-15220. These two amber pieces were cut from a single original piece of amber (catalog number 3-694, weight 10.3 g, dimensions 40х34х15 mm). Amber was dark-yellow (Fig.1A–C), with many areas being nearly opaque due to numerous micro-inclusions (Fig.1D), which we interpreted as fossilized emulsion of phloem sap (Fig.1DFHI). However, at a microscopic level, the amber was transparent. One of the amber pieces (K-15219) contained filamentous fungal hyphae (Fig.1EG). A total of 11 adult mite specimens were found (8 females and 3 males). These mites were easily recognizable as a species of Histiogaster due to the presence of four fan-like projections on the posterior opisthosoma in males. Brief descriptions of these specimens are provided below.



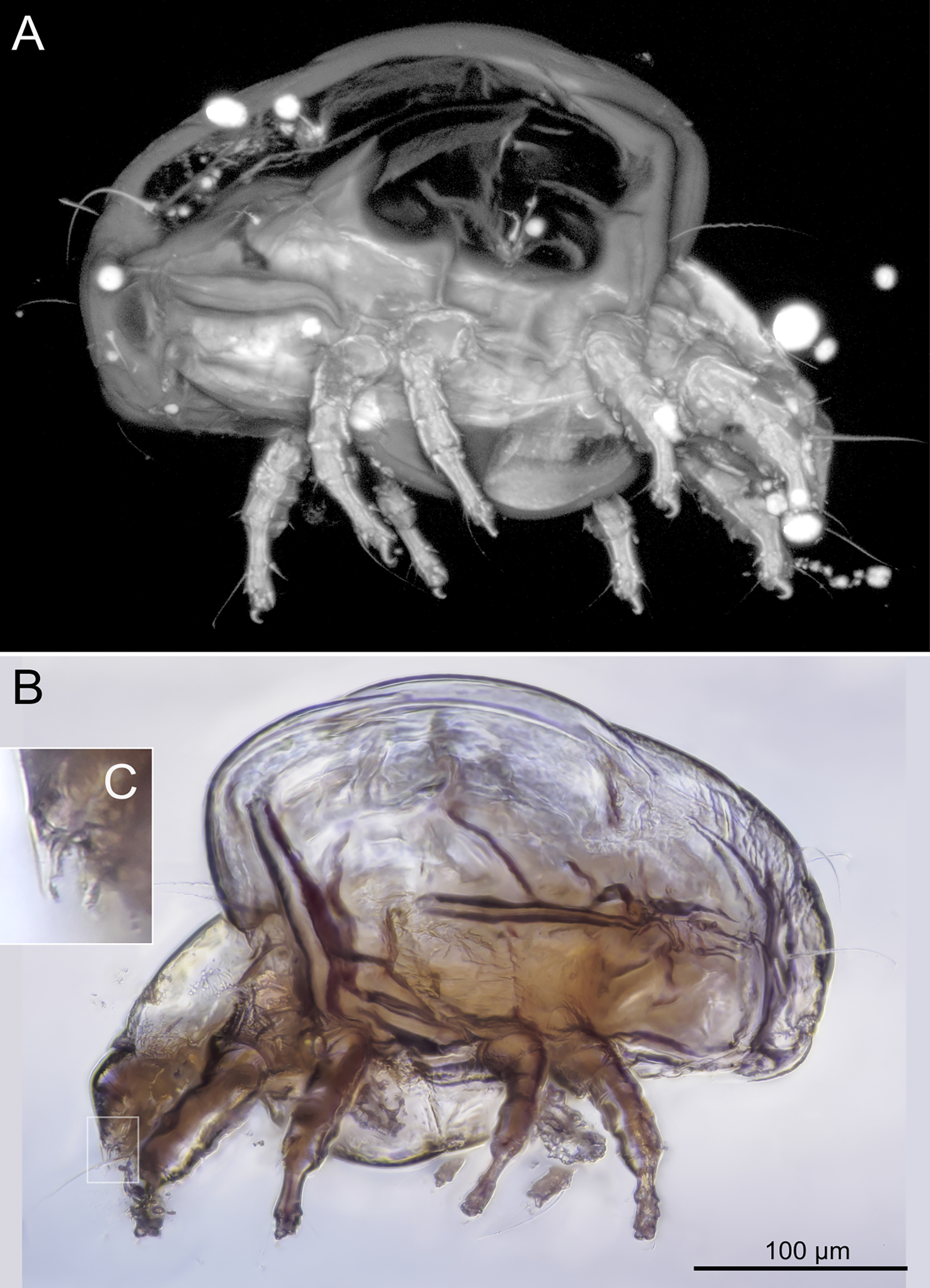
SIZK K-15220-В: holotype, a well-preserved female (Fig. 2–5). Almost all structures, including the spermatheca, are visible.


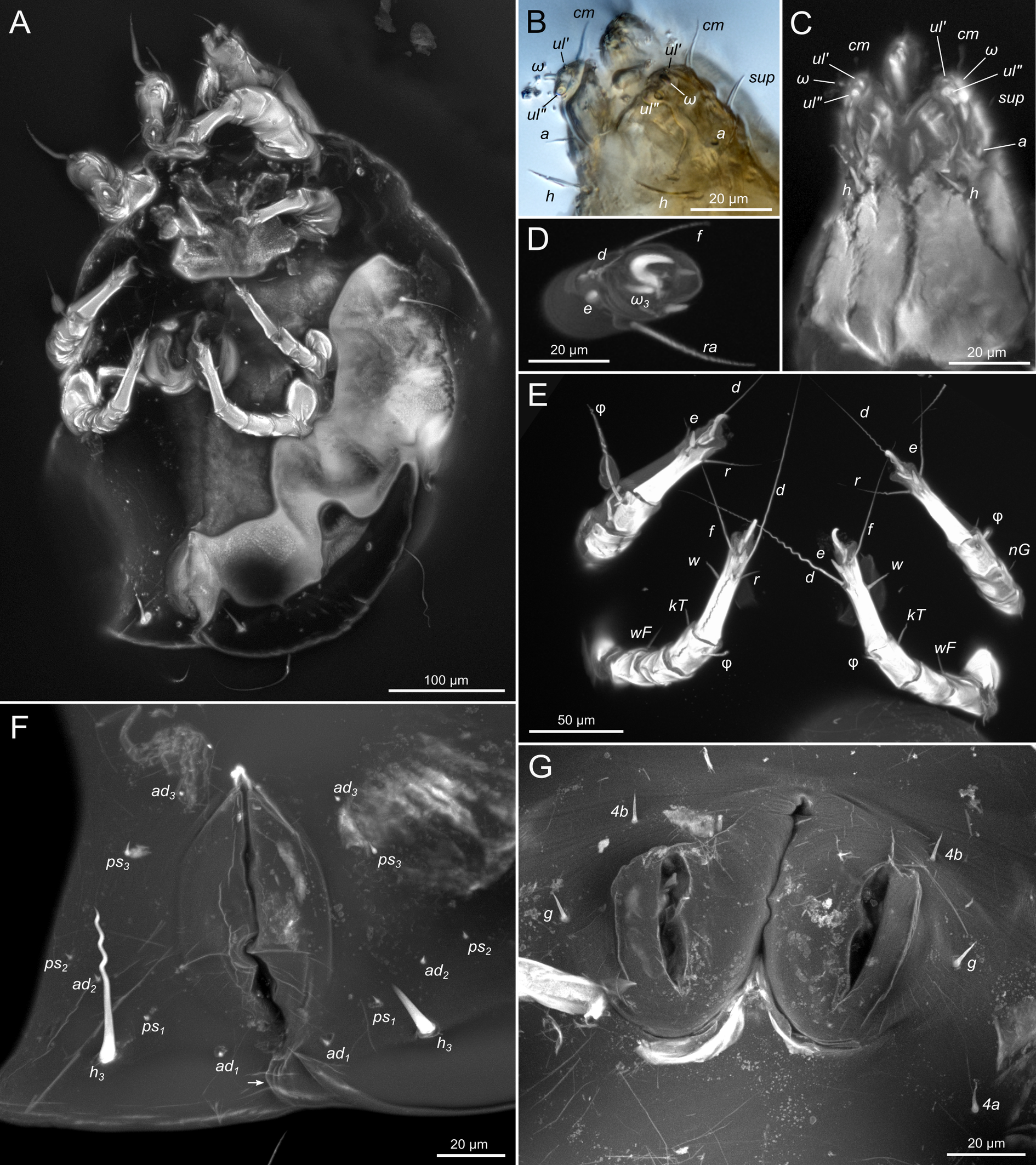






SIZK K-15219-В: paratype, a well-preserved female, compressed laterally (Fig.6–7). The legs and gnathosoma are better visible in the lateral aspects.




SIZK K-15220-A1: paratype, a moderately preserved female (Fig. 8), the gnathosoma is bent ventrally and is visible in the frontal view. Specimens A1, A2 and A3 were situated close to each other. Specimen A1 was separated for study, while specimens A2 and A3 were not separated (Fig.14A).


SIZK K-15220-A2, K-15220-A3, K-15220-С: moderately-well preserved females (Fig.9). In most specimens, legs and gnathosoma are visible, but the idiosomal cuticle is deformed and opaque, which does not allow to link setae with their alveoli or to find short setae.
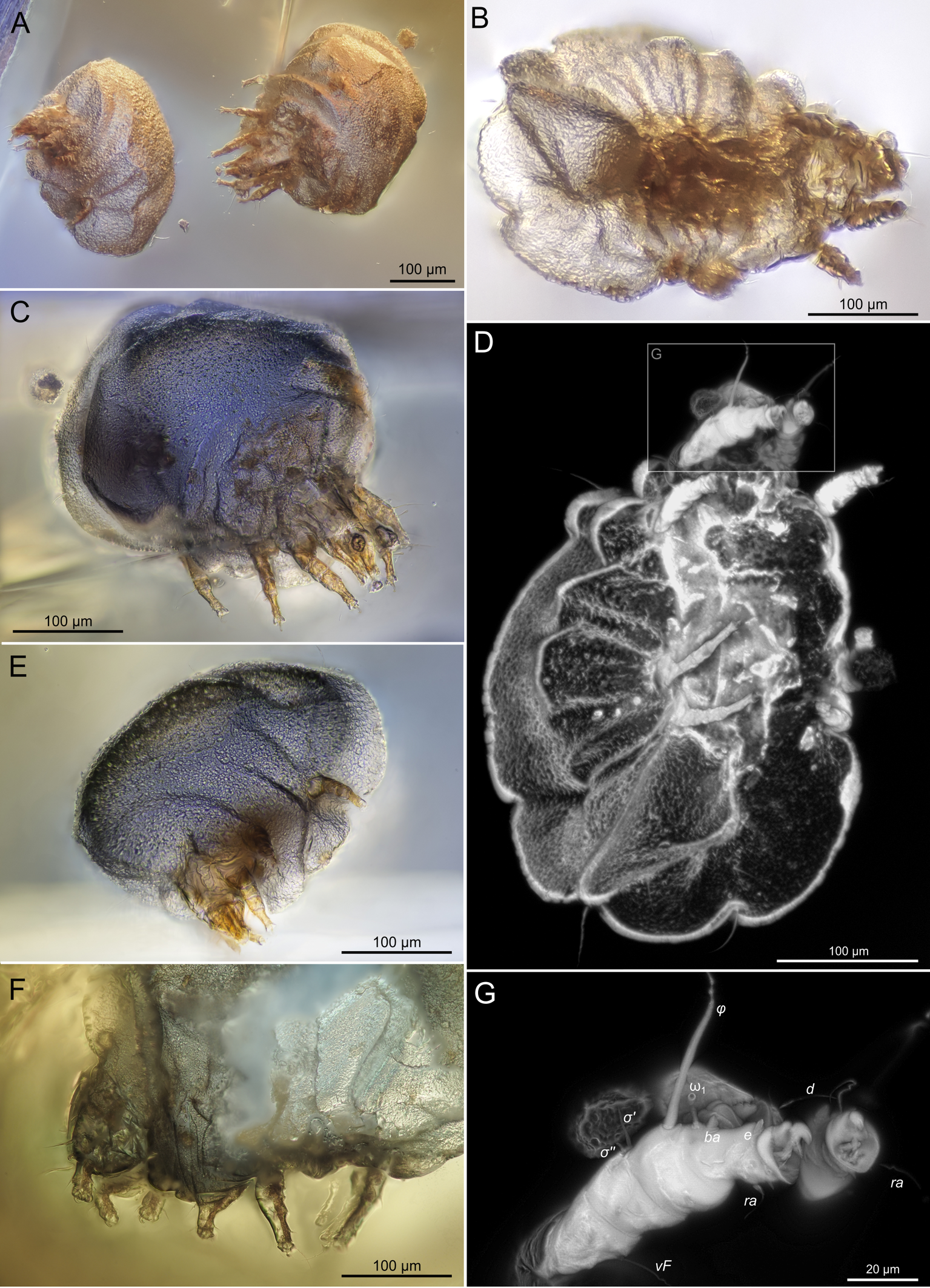


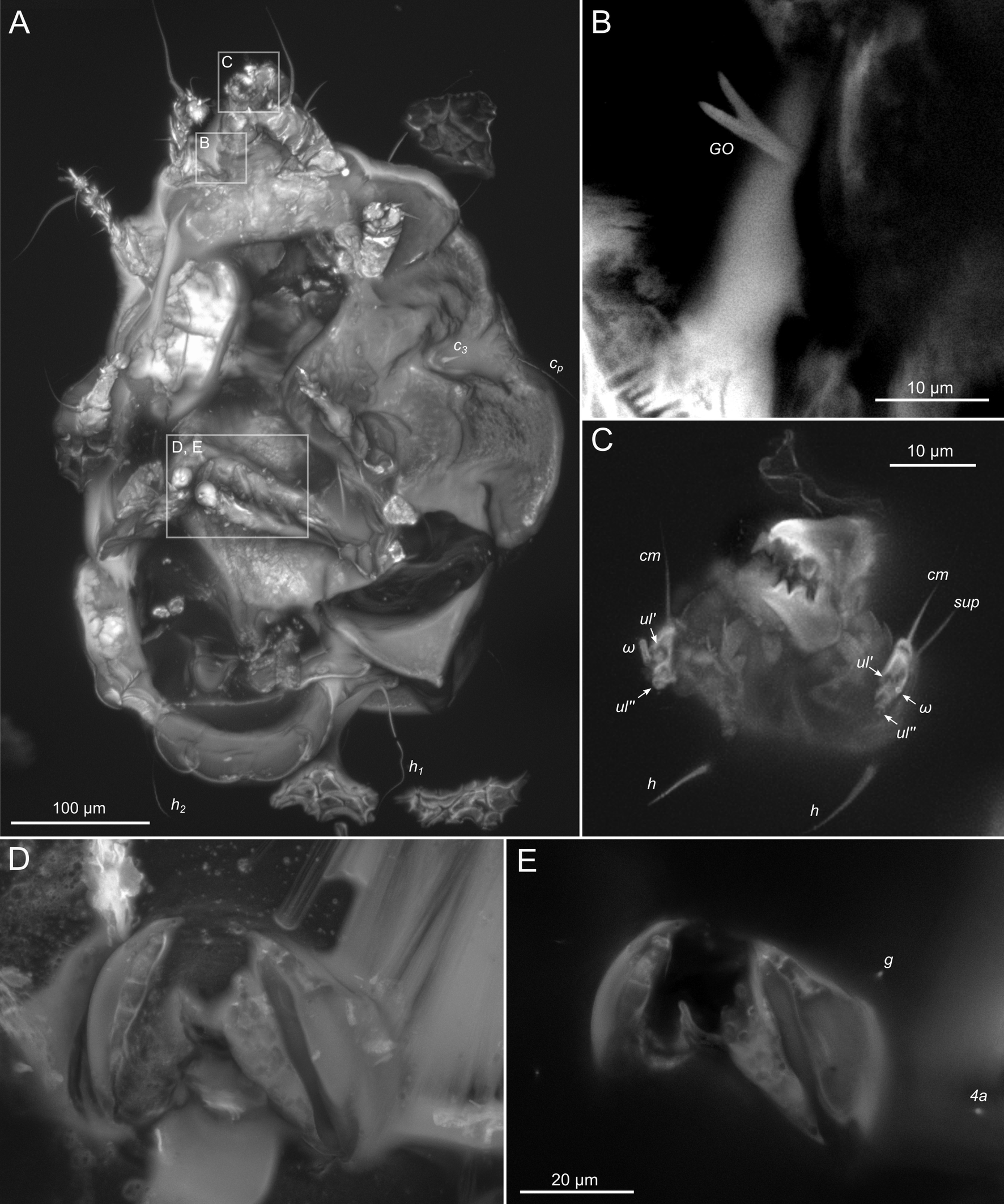
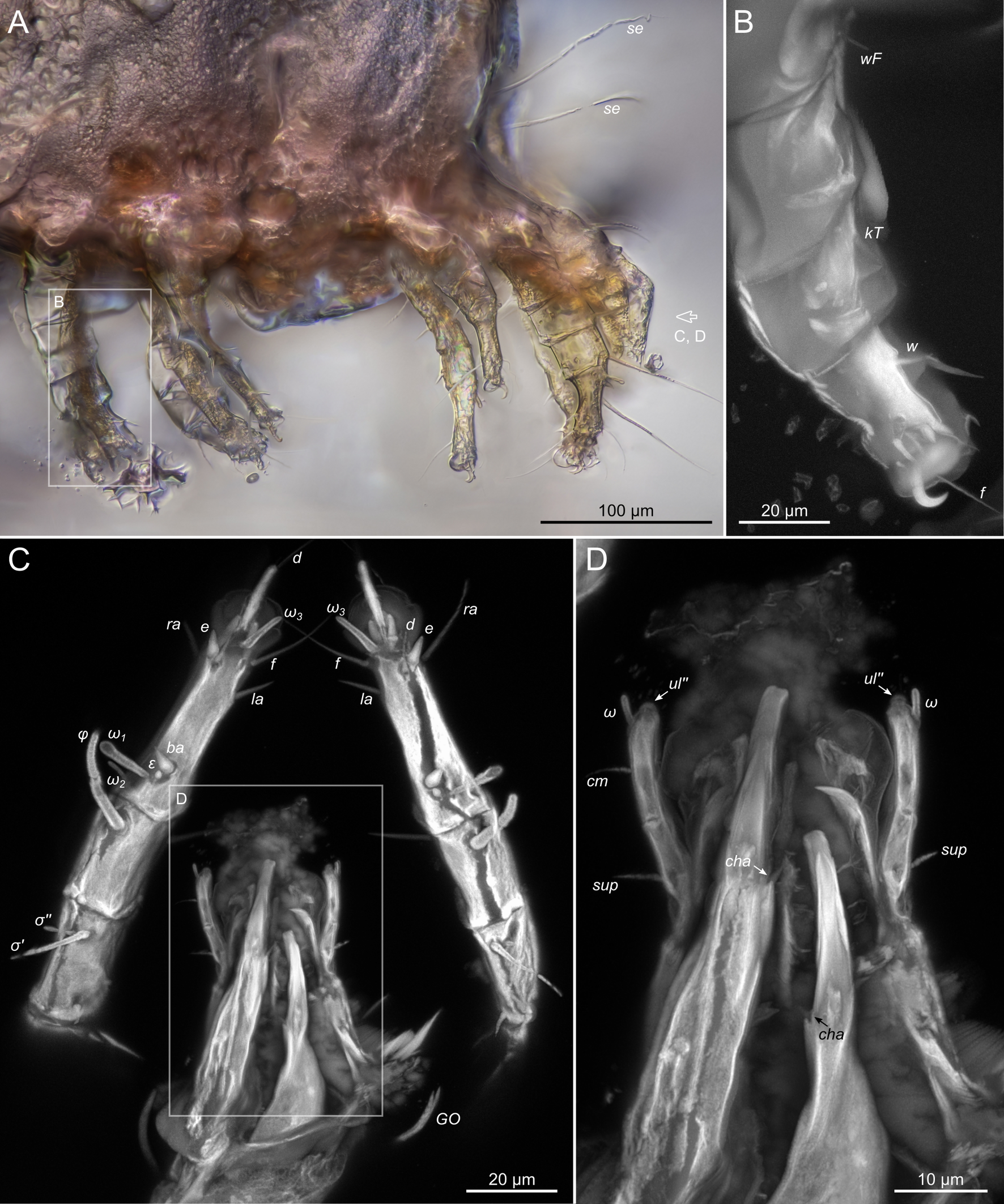
SIZK K-15219-A: a well-preserved male specimen (Fig.10–14), with visible legs, dorsal and ventral structures, gnathosoma, and genitalia. The amber piece is polished into a six-sided shape to provide access to dorsal, ventral, lateral, frontal, and caudal views. The gnathosoma is bent ventrally and visible from the frontal aspect.








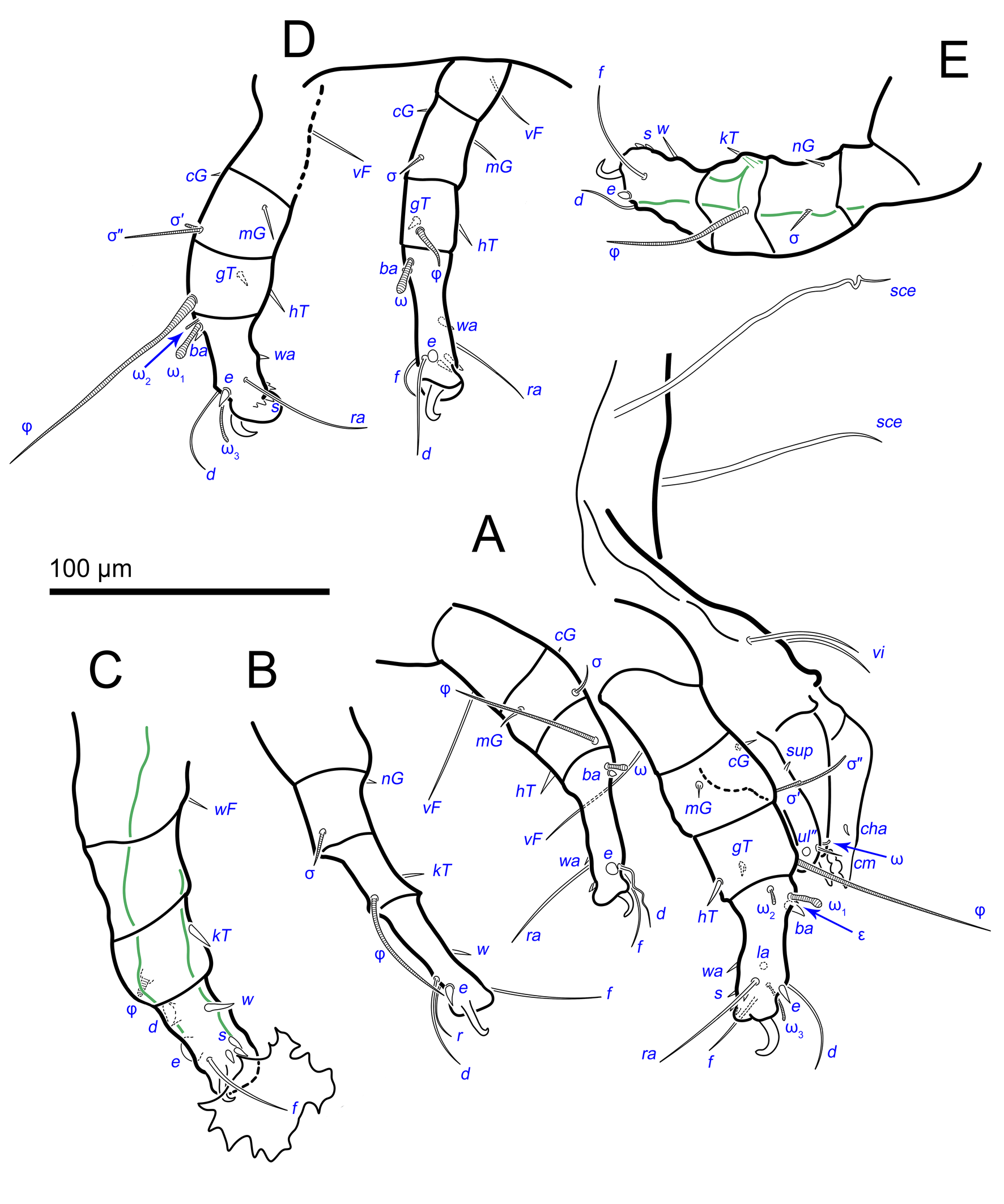


SIZK K-15219-C: a well-preserved male (Fig.15, 17–18) from a partially degraded layer of amber.








SIZK K-15219-D: a partially preserved female.
SIZK K-15219-E: a moderately-well preserved male (Fig.16), anal suckers are visible in the ventral aspect.
SIZK K-15220-D: a partially preserved female (Fig.9 F).
Type deposition
The holotype (SIZK K-15220-В), 2 paratypes (SIZK K-15219-B, K-15220-A1), non-type specimens of H. altilis (SIZK K-15219-A, K-15219-C, K-15219-D, K-15219-E, K-15220-A2, K-15220-A3, K-15220-С, K-15220-D) and syninclusions are deposited in the amber collection of the Schmalhausen Institute of Zoology NAS of Ukraine, Kiev (SIZK).
Etymology
The specific name (altilis Lat. adj., fattened) refers to the large, corpulent body of the fossil mite.
Diagnosis
Body length 280–520, width 280–360. Grandjean's organ arched, with forked tip. Supracoxal setae scx short, spiniform, twice as long as its width at base. Female with 3 pseudanal ps1–3 and 3 adanal setae ad1–3; setae ad1, ps1, ad2 and ps2 situated in one diagonal row. Canal of spermatheca relatively wide (wider than bases of ps and ad), curved and not looped, shorter than anal slit. Internal canal of spermatheca not visible. Plicated zone of ovipositor well developed, its length and width at least 1/3 length and width of the non-plicated zone of the ovipositor. Coxal apodemes free, distance between posterior coxal apodemes II and apodemes III shorter than length of coxal apodemes III. In male, pseudanal setae ps3 filiform (weakly spiniform), situated at anterior margin of anal suckers; setae ps2 short, spiniform, wider than ps3. In male, dorsal part of opisthosomal shield shorter than wide; pattern of dorsal opisthosomal shield absent. In male, opisthosomal fan-like lobes overlap at bases. Male genital apparatus situated between coxal fields IV. Lateral edge of genital capsule forms a very broad arc, not bent medially. Aedeagus long (more 2 times longer than anal setae ps3), not protruding beyond anterior margin of genital capsule.
Description
Female
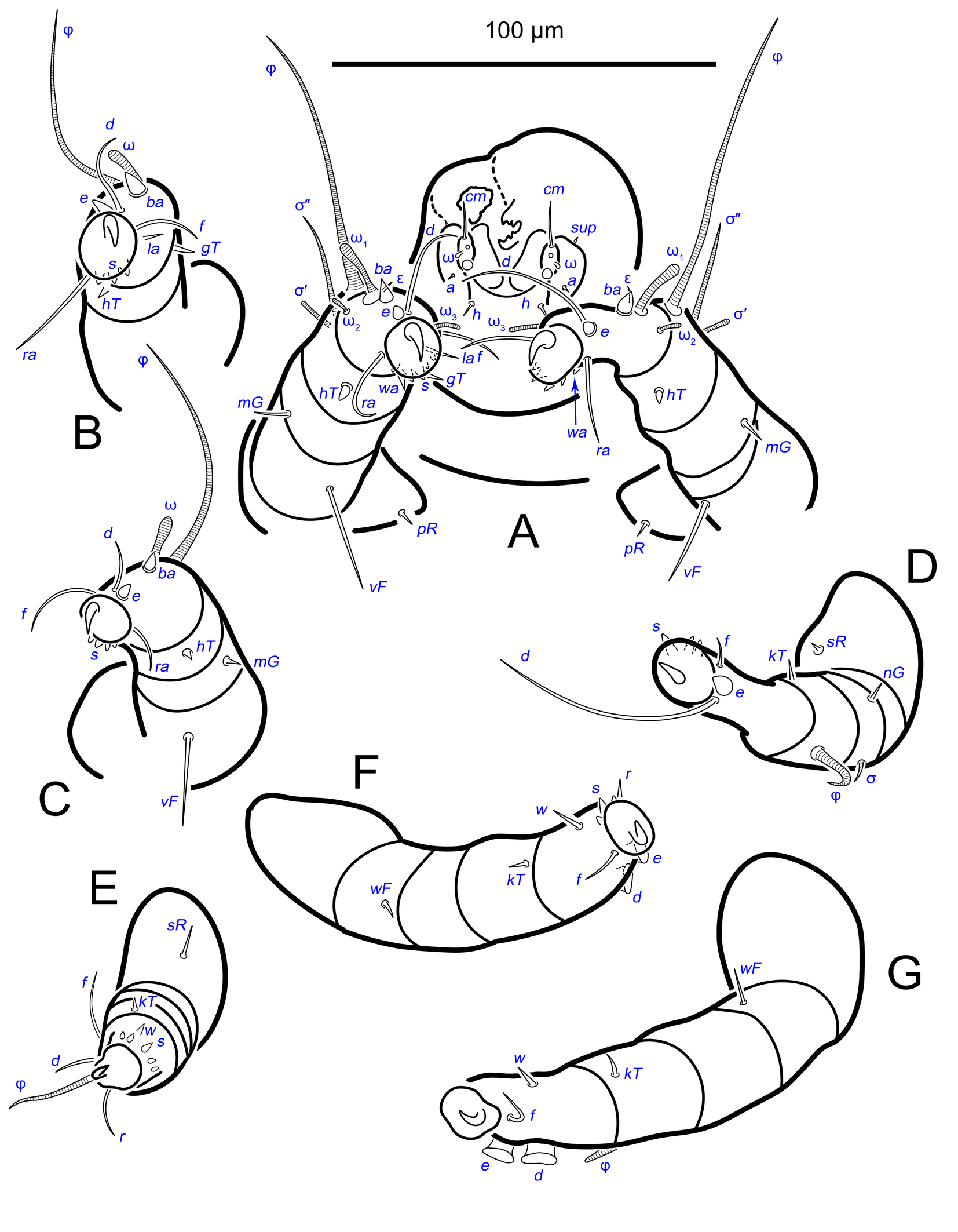
Gnathosoma. Subcapitulum elongated, seta h present (Figs 3B,C, 5I). Chelicerae elongated; movable finger with 3 teeth observed, fixed finger with 3 teeth observed (Fig. 6C, 7A, 9G); seta cha short, needle-like (Fig. 8D). Palps (Figs 8D, 3B, C, 5I) with setae a, cm and sup, elcp and solenidia ul″, ul′, ω. Labrum serrate distally (Fig.8D, arrow); pectinate processes at the labrum base not observed.
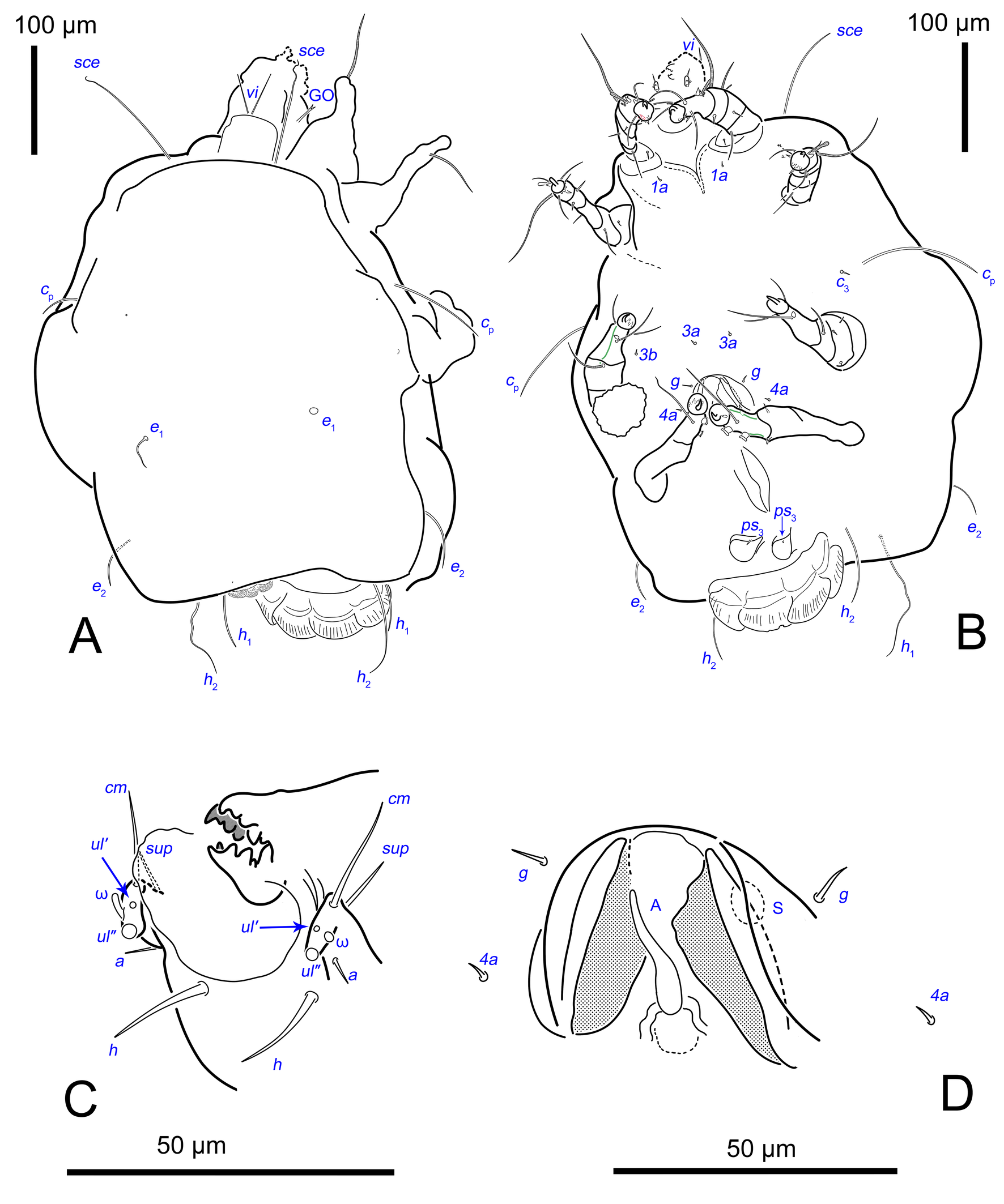
Idiosoma. Prodorsal shield (Figs 2C, 4A) long, evenly punctate, lateral margins almost straight. Setae vi filiform, distance between vi-vi 4–5 (n=4), setae ve absent. Setae se long, distance between se-se 78–80. Setae si absent. Supracoxal setae scx thick, twice as long as width at its base (Fig. 2C). Grandjean's organ (GO) arched, bifurcated (Figs 2D, E). Setae cp long, filiform, setae c3 short, filiform. Setae e1, e2, h1, h2 and h3 filiform, subequal. Setae e1 not reaching posterior end of body. Setae c1, c2, d1, and d2 absent. Opisthonotal gland openings (gla) slightly posterior to setae e1 (Fig. 4A). Coxal setae 1a, 3a, 3b, 4a and genital setae g subequal (Figs 3G, 4B, C). Setae 4a posterior to setae g. Sternum simple (Fig. 4B). Distance between anterior apodemes II–III shorter than length of apodemes III. Anterior apodemes III and IV, free, not fused medially (coxal fields III open) (Figs 3A, 4B). Anal opening (Figs 3F, 4D) with 3 pairs of short pseudanal setae (ps1, ps2, ps3) and three pairs of adanal setae (ad1, ad2, ad3). Setae ad1, ps1, ad2 and ps2 situated in a diagonal row. Setae ps3 and ad3 situated near anterior end of anal opening; ps3 closer to the middle of the anal opening, ad3 closer to anterior end of anal opening. Distance between ps1-ps1 and ad1-ad1 less than distance between h3-h3; distances ad2-ad2 and h3-h3 subequal; distance ps2-ps2 longer than distance h3-h3. Inseminatory canal of spermatheca (Figs 3F, 4D) relatively wide, 2–3 times wider than bases of setae ps and ad; curved and not looped; length shorter than anal slit, directly enters to spermatheca. Internal canal of spermatheca absent. Efferent ducts and their transverse base not observed. Spermatheca (Sp) deformed, poorly visible. Ovipore (V) situated between epimers III and IV, wide (Figs 3A, 4B). Plicated zone (O) of the ovipositor (Figs 3G, 4C) well developed, its length and width at least 1/3 length and width of the non-plicated zone of the ovipositor. Genital papillae (S) larger than diameter of base of genital setae (Fig. 4C).
Legs. Legs shorter than body, all segments free; empodial claws short, no longer that ambulacra (Figs 3A,D,E, 4A, B, 5A–H, 6, 7, 9). Leg setation: trochanters 1-1-1-0, femora 1-1-0-1, genua 2(2)-2(1)-1(1)-0, tibia 2(1)-2(1)-1(1)-1(1), tarsus 12(3+ε)-12(1)-10-10. Trochanters I and II with filiform setae (pR I-II, sR III). Femoral setae vF I–II and wF IV filiform. Genual setae cG I–II, mG I–II and nG III weakly spiniform. Tibial setae gT I–II, hT I–II and kT III–IV weakly spiniform. Tarsus I–II without foliate setae; setae ra, d, f, la filiform, long, e and ba spiniform, short; wa weakly spiniform, short; p, u, s, q, v very short, spiniform. Distance between wa-s much larger than diameter of bases of s. Tarsus III with filiform setae d, r, f; weakly spiniform w; spiniform e; and very short, spiniform p, u, s, q, v. Tarsus IV with filiform setae d, f; weakly spiniform w and r; strongly spiniform e; very short, spiniform p, u, s, q, v. Solenidion ω1 on tarsi I–II with widened end, club-shaped, situated closer to base of tarsus; ω2 of tarsus I short, with rounded tip, situated somewhat more basal and posterior or directly adjacent to ω1; ω3 on tarsus I bacilliform, subequal to ω1, situated apically on tarsus; tibial solenidia φ I–IV long, tapering; σ′ of genua I long, 3 times shorter than φ I; σ″ of genua I bacilliform, three times shorter than σ′; solenidia σ of genua II–III with pointed ends. Famulus ε of tarsus I short, pointed, adjacent to solenidion ω1 and seta ba.
Measurements (n=4). Idiosomal length 280, 340, 400, 520, width 280–330. Gnathosoma: subcapitulum length 60, width 27–30, seta h 15–16, chelicerae 50–57, seta cha 3–4, palp seta a 4–5, cm 11 and sup 9, solenidion ω 2–3. Prodorsal shield length 50–72, width 42–44. Idiosomal setae: vi 30–45, se 70, scx 3, cp 52–95, c3 10–14, e1 20–25, e2 30–40, h1 80–90, h2 50–70, h3 55–72, 1a, 3a, 3b, 4a and g 9–12, ps1, ps2, ps3, ad1, ad2 and ad3 5–7. Grandjean's organ length 16–18. Canal of spermatheca 22; length of spermatheca 50, width 30. Ovipore 65. Anal opening 84. Leg I: length 100–140, trochanter 20, femur 30–35, genu 16–20, tibia 18–22, tarsus (including pretarsus) 35–50, pretarsus (excluding claw) 8–10, claw 11–15; ω1 I 11–15, ω2 I 4–5, ω3 I 16–20, famulus ε 3, ba I 4–6, wa I 5, e I 6–7, d I 37–40, ra I 30–33, f I 25, la I 10, s I, p I, q I , u I and v I 3–5, φ I 56–80, gT I 6, hT I 5–6, σ″ I 8–10, σ′ I 23–25, cG I 6, mG I 10–12, vF I 35–50, pR I 20–23. Leg II: length 92–110, trochanter 11–15, femur 20–23, genu 15–20, tibia 14–20, tarsus (including pretarsus) 42–50, pretarsus (excluding claw) 10, claw 10; ω II 10–11, ba II 5, wa II 6–7, e II 5–6, d II 31–34, ra II 30–32, f II 22–30, la II 9–10, s II, p II, q II, u II and v II 3–5, φ II 50–55, gT II 7, hT II 6–7, σ II 10–13, cG II 6–7, mG II 6–7, vF II 22–32, pR II 9–11. Leg III: length 110–120, trochanter 20, femur 20, genu 20, tibia 18–23, tarsus (including pretarsus) 41–60, pretarsus (excluding claw) 10, claw 10–13; w III 7–8, e III 8, d III 40–85, r III 30–50, f III 25–35, s III, p III, q III, u III and v III 3–6, φ III 46–55, kT III 10, σ III 10, nG III 10, sR III 12. Leg IV: length 100–122, trochanter 20–25, femur 20, genu 18–20, tibia 18–22, tarsus (including pretarsus) 40–65, pretarsus (excluding claw) 10–11, claw 10; w IV 11–15, e IV 8–10, d IV 50–120, r IV 18–20, f IV 60–80, s IV, p IV, q IV, u IV and v IV 3–6, φ IV 20–30, kT IV 9–14, wF IV 15.
Male
Gnathosoma. As in female (Figs 11C, 12C, D, 13C, 18A).
Idiosoma. Prodorsal shield (Figs 13A) long, evenly punctate, lateral margins almost straight. Setae vi filiform, distance between vi-vi 4 (n=2); setae ve absent. Setae se long, distance between se-se 90. Setae si absent. Supracoxal setae scx not observed. Grandjean's organ bifurcated (Figs 10B, 11B, 12C). Setae cp long, filiform, setae c3 short, filiform. Setae e1, e2, h1, h2 and h3 filiform, subequal. Setae e1 not reaching end of body. Setae c1, c2, d1, d2 absent. Opisthonotal gland (gla) (Fig. 17A) openings slightly posterior to setae e1. Dorsal part of opisthosomal shield shorter than wide; pattern of dorsal opisthosomal shield absent (Figs 17A,C). Four fan-like projections overlap at their bases (Figs 10A, 11A, 13A,B, 15A,B,D, 17). Coxal setae 1a, 3a, 3b, 4a and genital setae g subequal (Figs 13B, 15E, 17B). Setae 4a posterior to setae g (Fig. 11E). Sternum simple (Figs 11A, 13B). Distance between anterior coxal apodemes II–III shorter than length of anterior apodemes III. Apodemes III and IV not observed. Genital opening situated between apodemes IV (Figs 11A, 13B, 15B, 17B). Lateral edges of genital capsule form a very broad arc, not bent medially (Figs 11D,E, 13D, 15E,F). Aedeagus (A) (Figs 11D,E, 13D) long, 2 times longer than length of setae ps3, not protruding beyond anterior edge of genital capsule. Anal sucker rounded in outline (Figs 15G, 16, 17B,C). Setae ps3 weakly spiniform, situated at anterior edge of anal suckers (Figs 15G, 16C,D, 17B,C). Setae ps2 short, spiniform, wider than ps3 (Fig. 15G). Setae ps1 absent, ih not observed.
Legs. Legs I–III as in females (Figs 12A–C, 14, 18). Tarsus IV with filiform setae f; spiniform w; weakly spiniform r; very short, spiniform p, u, s, q, v; setae d and e transformed to suckers. Solenidion φ of tibiae IV short, bacilliform.
Measurements (n=2). Idiosomal length 460–510, width 350–360. Gnathosoma: subcapitulum length 65, width 30, seta h 15, chelicerae 50–64, seta cha 5, palp seta a 5–6, cm 15, sup 8, solenidion ω 3–4. Prodorsal shield length 60, width 50. Idiosomal setae: vi 42, se 110–113, cp 70–100, c3 10, e1, e2 54, h1 70–100, h2 63–76, 1a, 3a, 3b, 4a and g 9–10, ps2 4, ps3 8, genital capsule 40. Aedeagus 22. Anal opening 50–66. Diameter of anal suckers 25–28. Leg I: length 120–150, trochanter 20, femur 38, genu 25, tibia 24, tarsus (including pretarsus) 48, pretarsus (including claw) 10, claw 13; ω1 I 13–15, ω2 I 6, ω3 I 15, famulus ε 3–5, ba I 7–8, wa I 6, e I 7, d I 30–35, ra I 25–45, f I 23, la I 11, s I, p I, q I, u I and v I 3–6, φ I 70–88, gT I 7, hT I 10, σ″ I 10, σ′ I 26–28, cG I 9, mG I 8–10, vF I 30–48, pR I 8. Leg II: length 122, trochanter 15, femur 37, genu 17–26, tibia 17–22, tarsus (including pretarsus) 50, pretarsus (including claw) 10, claw 12; ω II 13, ba II 8, wa II 7, e II 6, d II 25–35, ra II 30–40, f II 30, la II 7, s II, p II, q II, u II and v II 3–6, φ II 50–55, gT II 7, hT II 9–10, σ II 10–11, cG II 6, mG II 11–12, vF II 30–40, pR II 7. Leg III: length 130, trochanter 22, femur 30, genu 26–30, tibia 26, tarsus (including pretarsus) 40, pretarsus (excluding claw) 10, claw 11–13; w III 9, e III 6–7, d III 60, r III 20, f III 40, s III, p III, q III, u III and v III 4–7, φ III 45–50, kT III 9–10, σ III 13, nG III 6–7, sR III 10. Leg IV: length 140, trochanter 25, femur 35, genu 25–28, tibia 25–30, tarsus (including pretarsus) 40, pretarsus (excluding claw) 9–10, claw 12; w IV 11, e IV width 7–9, d IV width 8–9, r IV 6, f IV 35–50, s IV, p IV, q IV, u IV and v IV 4–7, φ IV 8, kT IV 11, wF IV 10.
Heteromorphic deutonymph unknown.
Remarks
Given that all specimens originated from a single large piece of amber, we can assume that they are conspecific and probably belong to a single population. The remarkable degree of preservation of some of these specimens has allowed us to accurately compare the fossil species with modern species of the genus Histiogaster.
Currently, species of the genus Histiogaster are divided into two groups: the carpio-species group and the arborsignis-species group. In the carpio group, the body is wide, apodemes III and IV are free, the distance between posterior apodemes II and III is shorter than apodeme III length, the female ovipore is situated between apodemes III and IV, the plicate zone of the ovipositor is normally developed (at least 1/2 the width of ovipositor), genital papillae wider, and the opisthonotal shield is without tree-like pattern in males. In the arborsignis-species group, the body is elongated, apodemes III and IV are connected, the distance between posterior apodemes II and apodemes III is longer than the length of apodeme III, the female ovipore is situated between apodemes IV, plicated zone of the ovipositor is greatly reduced (less than 1/2 the width of ovipositor) in females, genital papillae are small, and in males the opisthonotal shield has a tree-like pattern (Klimov et al. 2022). Histiogaster altilis sp. n. belongs to the carpio-species group.
Histiogaster altilis sp. n. is most similar to H. anops Griffiths, 1963 as both species have a wide and short canal of spermatheca, wider than the bases of setae ps and ad (in other species this canal is thin, not wider than the bases of setae ps and ad) (Griffiths 1963). The new species differs significantly from H. anops in the arrangement of three pairs of pseudanal setae (ps) and three pairs of adanal setae (ad): in H. altilis sp. n. setae ad1, ps1, ad2 and ps2 are situated on the same diagonal row relative to the anal opening (vs. these setae are not arranged in the same row, setae ps1 are distinctly posterior to h3 and ad2 are distinctly anterior to ps2 in H. anops).
Males of H. altilis sp. n. and H. anops have needle-shaped setae ps3 (Fig.15G), but in H. altilis sp. n. setae ps3 are clearly narrower than ps2 (vs. seta ps3 slightly wider than ps2 in H. anops). Histiogaster altilis sp. n. males have overlapping fan-like opisthosomal projections (Fig.11A, 15D), while in H. anops these projections are separated. Tarsal setae f I–II and ra I–II in H. altilis sp. n. (Fig.12C, Fig.3D) are filiform, while in H. anops these setae are widened.
The similarities between H. altilis sp. n. and H. anops suggest that this condition of the spermatheca (short and thick canal and simple saccular spermatheca) is plesiomorphic. The absence of eyes in the heteromorphic deutonymphs of H. anops also seems plesiomorphic, but this cannot be confirmed until heteromorphic deutonymphs of H. altilis sp. n. are found.
Twisted (or corkscrew-shaped) setae have been observed in H. altilis sp. n. (Figs 3 E, F) and in other fossil mites preserved in amber (Khaustov et al., 2021). Since setae of this shape have never been observed in modern specimens of the same genera or families, we believe that initially straight setae become twisted during the process of fossilization.
Discussion
Habitat of Histiogaster altilis sp. n.
Fossil mites of H. altilis sp. n. likely lived close to the site where resin was released, as indicated by the presence of several males and females that probably belong to the same population. Additionally, the numerous microinclusions around the mites, which we interpret as phloem sap emulsion, along with abundant filamentous hyphae, suggest proximity to a rich and likely organic substrate. It should be noted that these hyphae are likely to belong to resinicolous fungi related to those found both in Cretaceous ambers and in extant coniferous resins (Speranza et al., 2015; Peñalver et al., 2025), which, in our opinion, supports that the Eocene resin was enriched with a sugary tree sap. Resinicolous fungi were also reported from Rovno amber (Sukhomlyn et al., 2021).
Moreover, H. altilis sp. n. is morphologically similar to H. carpio (Kramer, 1882) and H. iberica Kadzhaya, 1959, which are modern inhabitants of tree sap flows. However, the fossil mite also shares similarities with H. anops, which inhabits the larval tunnels of two species of pine weevils (Hylobius pales (Herbst, 1797) and Pachylobius picivorus (Germar, 1824)) in the southern United States (Davis and Hunter, 1963). Considering all available evidence, we propose that H. altilis sp. n. inhabited fermented tree sap, like many modern Histiogaster species (Türk and Türk, 1957; Bugrov, 1997; Klimov et al., 2022). However, we cannot entirely exclude the possibility of an association with beetle galleries given the data at hand.
It is interesting to compare the newly described H. altilis sp. n. with another fossil astigmatan, Glaesacarus rhombeus, which is common in Baltic and Rovno amber (Perkovsky et al. 2007). The probable microhabitats of G. rhombeus may include insect burrows in wood, sap flow, or water-filled tree holes (Sidorchuk and Klimov, 2011), although subcortical spaces are the most likely habitat. One view suggests that this species could forage on hardened resin surfaces and possibly feed on carrion (Grünemaier, 2017). Surprisingly, despite the abundance of this species in Eocene amber, modern congeners have not yet been found. In contrast, mites of the genus Histiogaster have been able to maintain a conserved phenotype and ecology, presumably feeding on tree sap flows since the Eocene.
Advanced imaging
Here, we approached the limit of optical resolution in amber by using modern confocal microscopy (Vorontsov and Voronezhskaya, 2022; Vorontsov et al., 2023). This was needed to observe minute taxonomically important features, like the shape of the Grandjean's organ (Fig.2E) or the male's anal suckers (Fig.16B–D). To reach high resolution of imaging, we (1) fine-polished the amber pieces, leaving only a very thin layer of amber over the inclusion and (2) used high-resolution DIC microscopy and CLSM with a super-resolution detector. We found that successful application of CLSM highly depends on the level of fossil autofluorescence, which is usually better in deeper, less deteriorated layers of amber. Deteriorated surface layers of amber become fluorescent and mask the fluorescence from the fossil (Vorontsov et al., 2023). Among the specimens that we studied here, K-15219-C and K-15220-A1 were found closer to the surface of the original pieces, while K-15219-A and -B were extracted from deeper layers (compare background fluorescence in Fig.10C, Fig.11A and Fig.6A to Fig.8A). However, even partially deteriorated amber did not prevent high-resolution imaging (Fig. 8CD). As a result, with our advanced imaging technique, we scored almost all anatomical characters. In contrast, the most complete fossils typically preserve less one third of the anatomical characters used to infer evolutionary relationships among living species (Edgecombe, 2010).
Acknowledgments
D.D.V. thanks Evert Lindquist (Agriculture and Agri-Food Canada) and Roy Norton (State University of New York, USA) who provided support for his research in high-resolution imaging of fossil Acari. We thank Dr. Kimiko Okabe (Forestry and Forest Products Research Institute, Tsukuba, Japan) for translating Aoki's 1974 paper. We are grateful to Anatoly P. Vlaskin (SIZK) for cutting and polishing the amber pieces and to Elena Voronezhskaya (IDB RAS) for help with confocal microscopy. CLSM images were taken using the equipment of the Core Centrum of the Institute of Developmental Biology RAS. This contribution is a continuation of research started by the late Ekaterina Sidorchuk.
Funding
The work of DDV was conducted under the IDB RAS research program №0088-2024-0011. VBK was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the Federal Scientific and Technical Program for the Development of Genetic Technologies for 2019-2027 (agreement №075-15-2021-1345, unique identifier RF—-193021X0012).
References
- Agnihotri P., Singh H., Subramanian K. A., Acharya S. 2024. Scanning electron microscopy of Sarcoptes kutchensis, a new species of a Middle Eocene sarcoptid mite in amber from the Umarsar Lignite Mine of Kutch, Western India. Historical Biology, 36(12): 2847-2853. https://doi.org/10.1080/08912963.2023.2281579
- Aoki, J. 1974. On the fossil mites in Mizunami amber from Gifu Prefecture, Central Japan. Bulletin of the Mizunami Fossil Museum, 1: 397-399 [in Japanese]
- Azar D. 2007. Preservation and accumulation of biological inclusions in Lebanese amber and their significance. Comptes Rendus Palevol, 6: 151-156. https://doi.org/10.1016/j.crpv.2006.10.004
- Berlese A. 1883. Sopra due nuovi generi di Acari italiani. Lettura fatta alla R. Accademia di Padova, 33: 45-52.
- Bochkov A. V. 2009. A review of mites of the parvorder Eleutherengona (Acariformes: Prostigmata) - permanent parasites of mammals. Acarina Supplement No 1: 3-149.
- Bugrov S. A. 1997. Free-living astigmatic mites (Acariformes, Astimata) of the fauna of the Moscow region. Zoologicheskiy Zhurnal, 76 (2): 147-156. [In Russian]
- Chemyreva V. G., Legalov A. A., Perkovsky E. E. 2024. A new genus of Ambositrinae (Hymenoptera, Diapriidae) from Rovno amber and remarks on the Eocene distribution of the subfamily. Ecologica Montenegrina, 79: 104-112. https://doi.org/10.37828/em.2024.79.9
- Davis R., Hunter P. F. 1963. Biological studies of a Histiogaster mite (Acarina: Acaridae) associated with pine reproduction weevils. Annals of the Entomological Society of America, 56: 682-687. https://doi.org/10.1093/aesa/56.5.682
- Dubinin D. V. 1962. Class Acaromorpha. Ticks, mites or gnathosomous chelicerates, In: Rohdendorf, B.B. (Ed.) Fundamentals of Paleontology. Volume 9. Arthropoda, Tracheata, Chelicerata. [Osnovy paleontoligii. A manual for paleontologists and geologists of the USSR, translated by IPST Staff]. Smithsonian Institution Libraries and National Science Foundation, Washington, D.C., pp. 681-722.
- Dunlop J. A., Penney D., Jekel D. 2011. A summary list of fossil spiders and their relatives. In: Platnick N. (Ed.), The world spider catalog, version 12.0. American Museum of Natural History, New York, 258 pp.
- Dunlop J. A., Wirth S., Penney D., McNeil A., Bradley R. S., Withers P. J., Preziosi R. F. 2012. A minute fossil phoretic mite recovered by phase-contrast X-ray computed tomography. Biology Letters, 8: 457-460. https://doi.org/10.1098/rsbl.2011.0923
- Edgecombe G. D. 2010. Palaeomorphology: fossils and the inference of cladistic relationships. Acta Zoologica, 91(1): 72-80. https://doi.org/10.1111/j.1463-6395.2009.00426.x
- Evans G. O. 1992. Principles of Acarology. CABI Publishing Wallingfrod, Oxon OX10 8DE, UK, 563 p.
- Fain A. 1977. Nouvelles observations sur les acariens recoltes par le Dr. J. Trave aux lies Saint-Paul et Nouvelle-Amsterdam (Astigmates). Acarologia, 18(3): 553-564.
- George V. P. 1952. On some arthropod microfossils from India. Agra University Journal of Research, 1: 83-108
- Ghazi S., Mountney N. P. 2011. Petrography and provenance of the Early Permian Fluvial Warchha Sandstone, Salt Range, Pakistan. Sedimentary Geology, 233(1-4): 88-110. https://doi.org/10.1016/j.sedgeo.2010.10.013
- Grandjean F. 1939. La chaetotaxie des pattes chez les Acaridiae. Bulletin de la Société Zoologique de France, 64: 50-60.
- Griffiths D. A. 1963. A new species of Histiogaster belonging to the family Acaridae Ewing and Nesbitt 1942 (Acarina). Acarologia, 5: 61-70
- Griffiths D. A. 1970. A further systematic study of the genus Acarus L., 1758 (Acaridae, Acarina), with a key to species. Bulletin of the British Museum (Natural History). Zoology series, 19: 85-118.
- Griffiths D. A., Atyeo W. T., Norton R. A., Lynch C. A. 1990. The idiosomal chaetotaxy of astigmatid mites. Journal of Zoology, 220: 1-32. https://doi.org/10.1111/j.1469-7998.1990.tb04291.x
- Grünemaier M. 2017. Not just hyphae - the amber mite Glaesacarus rhombeus as a forager on hardened resin surfaces and a potential scavenger on trapped insects. Palaeodiversity, 10: 129-134. https://doi.org/10.18476/pale.v10.a9
- Halbwachs H., Bässler C., Worobiec E. 2021. Palynomorphs in Baltic, Bitterfeld and Ukrainian ambers: a comparison. Palynology, 45(3): 441-457. https://doi.org/10.1080/01916122.2020.1863274
- Kadzhaya G. Sh. 1959. A new species of the genus Histiogaster in Gruzia (Acarina, Tyroglyphoidea). Soob. Akad. Nauk Gruz. S. S. R. 23:75-78.
- Khaustov A. A., Vorontsov D. D., Perkovsky E. E., Lindquist E. E. 2021. Review of fossil heterostigmatic mites (Acari: Heterostigmata) from late Eocene Rovno Amber. I. Families Tarsocheylidae, Dolichocybidae and Acarophenacidae. Systematic and Applied Acarology, 26(1): 33-61. https://doi.org/10.11158/saa.26.1.3
- Klimov P.B. (2000) Paulacarellus faini sp. n., a new species of acarid mite (Acariformes: Acaridae) from the Russian Far East. Acarologia, 41: 249-253.
- Klimov P. B., Kolesnikov V. B., Khaustov A. A., Ermilov S. G., Tolstikov A. V. 2022. The mite genus Histiogaster (Acari: Acaridae) in the Eastern Palaearctic, with a description of two new species and report of pre-copulatory guarding. Acarina, 30(2): 121-147. https://doi.org/10.21684/0132-8077-2022-30-2-121-147
- Klimov B. B., OConnor B. M. 2003. Phylogeny, historical ecology and systematics of some mushroom-associated mites of the genus Sancassania (Acari: Acaridae), with new generic synonymies. Invertebrate Systematics, 17(4): 469-514. https://doi.org/10.1071/IS02050
- Klimov P. B., Tolstikov A. V. 2011. Acaroid mites of Northern and Eastern Asia (Acari: Acaroidea). Acarina, 19(2): 252-264.
- Klimov P. B., Vorontsov D. D., Azar D., Sidorchuk E. A., Braig H. R., Khaustov A. A., Tolstikov A. V. 2021. A transitional fossil mite (Astigmata: Levantoglyphidae fam. n.) from the early Cretaceous suggests gradual evolution of phoresy-related metamorphosis. Scientific Reports 11(1): e15113. https://doi.org/10.1038/s41598-021-94367-2
- Koch C. L., Berendt G. C. 1854. Die im Bernstein befindlichen Crustaceen, Myriopoden, Arachniden und Apteren der Vorwelt. P. 124 in G. C. Berendt (ed.) Die in Bernstein befindlichen organischen Reste der Vorwelt, gesammelt, in Verbindung mit Mehreren bearbeitetet und herausgegeben. Commission der Nicolaischen Buchhandlung, Berlin.
- Kolesnikov V. B., Vorontsov D. D., Perkovsky E. E., Vasilenko D. V., Klimov P. B. 2023. Confocal autofluorescence microscopy revealed the fine morphology of the amber preserved mite Congovidia glesoconomorphi sp. nov. (Acari: Hemisarcoptidae) phoretic on a mycterid beetle. Palaeoentomology 006(6): 665-678. https://doi.org/10.11646/palaeoentomology.6.6.8
- Kolesnikov V. B., Vorontsov D. D., Sidorchuk E. A. 2024. Seven new species from Eocene Baltic amber reveal surprising diversity and suggest possible speciation scenarios in the relictual family Collohmanniidae (Acari: Oribatida). Zootaxa, 5553(1): 1-78. https://doi.org/10.11646/zootaxa.5553.1.1
- Kramer P. 1881. Ein Dermaleichus-artiger Tyroglyphus. Zoologischer Anzeiger, 4: 619.
- Kumar P., Jha N., Battachaharya D. D., Pande A. C. 2011. Acarid mites from Early Permian sediments of the Chamba Valley, Himachal Pradesh, India. Journal of the Palaeontological Society of India, 56: 171-179. https://doi.org/10.1177/0971102320110206
- Mani M. S. 1945. Arthropod fossil from India. Indian Journal of Entomology, 6: 61-64.
- Mani M. S. 1946. On some arthropod fossil from India. Proceedings of the National Academy of Sciences, India. Section B, 16(2-4): 43-56.
- Mantilla G. P. W., Renne P. R., Samant B., Mohabey D. M., Dhobale A., Tholt A. J., Tobin T. S., Widdowson M. Anantharaman S., Dassarma D. C., Mantilla J. A. W. 2022. New mammals from the Naskal intertrappean site and the age of India's earliest eutherians. Palaeogeography Palaeoclimatology Palaeoecology, 591: 110857. https://doi.org/10.1016/j.palaeo.2022.110857
- Martill D. M., Davis P. G. 1998. Did dinosaurs come up to scratch? Nature, 396: 528-529. https://doi.org/10.1038/25027
- Norton R. A. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Experimental and Applied Acarology, 22: 559-594. https://doi.org/10.1023/A:1006135509248
- Ochoa R., OConnor B. M. 2000. Revision of the genus Horstiella (Acari: Acaridae): mites associated with Neotropical Epicharis bees (Hymenoptera: Apidae). Annals of the Entomological Society of America, 93: 713-737. https://doi.org/10.1603/0013-8746(2000)093%5B0713:ROTGHA%5D2.0.CO;2
- OConnor B. M. 2009. Cohort Astigmatina. In: Krantz, G.W., Walter, D. E. (Eds), A manual of acarology. Texas Tech University Press, pp. 565-657.
- OConnor B. M. 2022. Two new genera of winterschmidtiine mites (Acari: Astigmata: Winterschmidtiidae) associated with beetles in the family Bostrichidae (Coleoptera: Polyphaga: Bostrichoidea). Systematic and Applied Acarology, 27 (2): 209-219. https://doi.org/10.11158/saa.27.2.4
- Pampaloni L. 1902. I resti organici nel disodile di Melilli in Sicilia. Palaeontographia Italica, 8:121-130
- Pastorelli G., Shashoua Y., Richter J. 2013. Surface yellowing and fragmentation as warning signs of depolymerisation in Baltic amber. Polymer Degradation and Stability, 98(11): 2317-2322. https://doi.org/10.1016/j.polymdegradstab.2013.08.009
- Peñalver E., Speranza M., Delclòs X. 2025. Fungal cortices in kauri resin from New Zealand as taphonomic and ecological analogues of Cretaceous amber cortices: an actualistic approach. Spanish Journal of Palaeontology. https://doi.org/10.7203/sjp.29855
- Pepato A. R., Costa S. G. dos S., Harvey M. S., Klimov P. B. 2022. One-way ticket to the blue: A large-scale, dated phylogeny revealed asymmetric land-to-water transitions in acariform mites (Acari: Acariformes). Molecular Phylogenetics and Evolution, 177: 107626. https://doi.org/10.1016/j.ympev.2022.107626
- Perkovsky E. E., Rasnitsyn A. P., Vlaskin A. P., Taraschuk M. V. 2007. A comparative analysis of the Baltic and Rovno amber arthropod faunas: representative samples. African Invertebrates, 48(1): 229-245.
- Pfammatter J. A., Coyle D. R., Gandhi K. J. K., Hernandez N., Hofstetter R. W., Moser J. C. Raffa K. F. 2016. Structure of phoretic mite assemblages across subcortical beetle species at a regional scale. Environmental Entomology, 45(1): 1-13. https://doi.org/10.1093/ee/nvv150
- Poinar G.O. 1988. Hair in Dominican amber: Evidence for tertiary land mammals in the Antilles. Experientia, 44: 88-89. https://doi.org/10.1007/BF01960261
- Presl J. S. 1822. Additamenta ad faunam protogaeam, sistens descriptiones aliquot animalium in succino inclusorum. In Presl, J. S. & Presl, C. B. (eds). Deliciae Pragenses Historiam Naturalem Spectantes. Tome I. Calvae, Pragae, viii + 244 pp. https://doi.org/10.5962/bhl.title.46467
- Santiago-Blay J. A., Jolivet P. Verma K. K. 2012. A natural history of conspecific aggregations in terrestrial arthropods, with emphasis on cycloalexy in leaf beetles (Coleoptera: Chrysomelidae). Terrestrial Arthropod Reviews, 5: 289-355. https://doi.org/10.1163/18749836-05031054
- Schatz H., Behan-Pelletier V. M., OConnor B. M., Norton R. A. 2011. Suborder Oribatida van der Hammen, 1968. In: Zhang, Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 141-148. https://doi.org/10.11646/zootaxa.3148.1.26
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. 2012. Fiji: An Open-Source Platform for Biological-Image Analysis. Nature Methods, 9: 676-682. https://doi.org/10.1038/nmeth.2019
- Sendi H., Klimov P. B., Kolesnikov V. B., Káčerová J., Bonino E., Azar D., Robin N. 2025. The oldest continuous association between astigmatid mites and termites preserved in Cretaceous amber reveals the evolutionary significance of phoresy. BMC Ecology and Evolution, 25: 16. https://doi.org/10.1186/s12862-025-02351-5
- Seyfullah L. J., Beimforde C., Dal Corso J., Perrichot V., Rikkinen J., Schmidt A. R. 2018. Production and preservation of resins - past and present. Biological Reviews, 93(3): 1684-1714. https://doi.org/10.1111/brv.12414
- Sidorchuk E. A., Klimov P. B. 2011. Redescription of the mite Glaesacarus rhombeus (Koch & Berendt, 1854) from Baltic amber (Upper Eocene): evidence for female-controlled mating. Journal of Systematic Palaeontology, 9(2): 183-196. https://doi.org/10.1080/14772019.2011.566585
- Sidorchuk E. A., Vorontsov D. D. 2018. Preparation of small-sized 3D amber samples: state of the technique. Palaeoentomology, 1(1): 80-90. https://doi.org/10.11646/palaeoentomology.1.1.10
- Speranza M., Ascaso C., Delclòs X., Peñalver E. 2015. Cretaceous mycelia preserving fungal polysaccharides: taphonomic and paleoecological potential of microorganisms preserved in fossil resins. Geologica Acta, 13(4): 363-385. https://doi.org/10.1344/GeologicaActa2015.13.4.8
- Sukhomlyn M. M., Heluta V. P., Perkovsky E. E., Ignatov M. S., Vasilenko D. V. 2021. First record of fungus of the family Mycocaliciaceae in Rovno amber (Ukraine). Paleontological Journal, 55(6): 684-690. https://doi.org/10.1134/S0031030121060125
- Telnov D., Perkovsky E. E., Vasilenko D. V., Yamamoto S. 2021. The first fossil Coleoptera record from the Volyn Region, Ukraine, with description of a new Glesoconomorphus (Coleoptera, Mycteridae) in syninclusion with Winterschmidtiidae (Acari) and a key to species. Zookeys, 1068: 189-201. https://doi.org/10.3897/zookeys.1068.75391
- Türk E. 1963. A new tyroglyphid deutonymph in amber from Chiapas, Mexico. University of California Publications in Entomology, 31: 49-51.
- Türk E. and Türk F. 1957. Systematik und Ökologie der Tyroglyphiden Mitteleuropas. In: H. J. Stammer (Ed.). Beiträge zur Systematik und Ökologie mitteleuropäischer Acarina. Band 1. Tyroglyphidae und Tarsonemini. Akademische Verlagsgesellschaft Geest & Portig K.-G., Leipzig, pp. 3-231.
- Volgin V. I. 1966. A new genus and new species of mites of the family Acaridae (Acarina, Acariformes). Trudy Akad. Nauk SSSR, 37: 286-296.
- Vorontsov D. D., Voronezhskaya E. E. 2022. Pushing the limits of optical resolution in the study of the tiniest fossil arthropods. Historical Biology, 34(12): 2415-2423. https://doi.org/10.1080/08912963.2021.2017920
- Vorontsov D. D., Kolesnikov V. B., Voronezhskaya E. E., Perkovsky E. E., Berto M. M., Mowery J., Ochoa R., Klimov P. B. 2023. Beyond the limits of light: an application of super-resolution confocal microscopy (sCLSM) to investigate Eocene amber microfossils. Life, 13(4): 865. https://doi.org/10.3390/life13040865
- Wirth S. F., Garonna A. P. 2015. Histiostoma ovalis (Histiostomatidae, Acari) associated with Ips sexdentatus (Scolytinae, Curculionidae, Coleoptera): Ecology and mite redescription on the basis of formerly unknown adults and nymphs. International Journal of Acarology, 41: 415-428. http://dx.doi.org/10.1080/01647954.2015.1050062 https://doi.org/10.1080/01647954.2015.1050062
- Woodring J. P. 1966. North American Tyroglyphidae (Acari): The Genus Histiogaster, with Descriptions of four New Species. Proc. Louis. Ac. Sc., 29: 113-136.
- Zachvatkin A. A. 1941. Tiroglifoidnye kleshchi (Tyroglyphoidea) [=Tyroglyphoid Mites (Tyroglyphoidea)]. In: Zernov, S. A. (Ed.), Akademiya Nauk SSSR, Moscow-Leningrad, Fauna SSSR, Paukoobraznuye, 475 pp. (in Russian)



2024-10-12
Date accepted:
2025-03-13
Date published:
2025-03-26
Edited by:
Pfingstl, Tobias

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Kolesnikov, Vasiliy B.; Vorontsov, Dmitry D.; Perkovsky, Evgeny E. and Klimov, Pavel B.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



