Effect of different constant temperatures on the biological characteristics and life table parameters of Euseius scutalis (Acari: Phytoseiidae) fed on cattail pollen
Bazazzadeh, Fereshteh  1
; Shishehbor, Parviz
1
; Shishehbor, Parviz  2
; Gorji, Zahra
2
; Gorji, Zahra  3
; Gravandian, Mohammad
3
; Gravandian, Mohammad  4
; Esfandiari, Mehdi
4
; Esfandiari, Mehdi  5
and Riahi, Elham
5
and Riahi, Elham  6
6
1Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
2Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
3Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
4Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
5Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
6✉ Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, P.O. Box 14115-336, Tehran, Iran.
2025 - Volume: 65 Issue: 1 pages: 242-254
https://doi.org/10.24349/thvk-w602Original research
Keywords
Abstract
Introduction
The predatory mite Euseius scutalis (Athias-Henriot) was first described by Athias-Henriot (1958) in Algeria as Typhlodromus scutalis. This species has been reported as a common phytoseiid mite in Middle East countries, including Lebanon, Iran, Egypt, Israel, Jordan, Turkey, and North Africa such as Morocco and Algeria (Daneshvar 1980; Bonfour & McMurtry 1987; Kasap & Şekerğlu 2004). E. scutalis is associated with on a variety of host plants (Bonfour & McMurtry 1987; Momen & Abdel-Khalek 2008). It is one of the most common and widely distributed phytoseiid mites in Iran (Kamali et al. 2001). E. scutalis is a pollen-feeding generalist predator belonging to category type IV (McMurtry & Croft 1997; McMurtry et al. 2013). Although this predator has a high reproductive capacity on pollen, it can also feed on a wide variety of small insects and mites, including eriophyid mites, spider mites, and the eggs and immature stages of whiteflies, thrips, and scale insects (El-Badry et al. 1968; El-Badry & El-Banhawy 1968; Bonfour & McMurtry 1987; Nomikou et al. 2001; Kasap & Şekerğlu 2004; Al-Shammery 2010; Shishehbor et al. 2022; Zergani et al. 2023).
Euseius scutalis could be considered one of the most suitable and effective biocontrol agents for integrated pest management programs targeting plant-feeding mites (Al-Shammery 2010; Stathakis et al. 2021; Zergani et al. 2023). Therefore, by preying on phytophagous mites and other pests, it can reduce the need for chemical pesticides, leading to cost savings and a lower environmental impact. It also can sustain stable populations, which decreases the need for frequent reintroductions and improves long-term pest control effectiveness. Releasing this predatory mite and anise essential oil has been suggested as alternative strategies to control of Eutetranychus orientalis (Klein) (Acari: Tetranychidae) (Ata et al. 2023). Additionally, it feeds on and survives by consuming plant tissues without causing economic damage (Adar et al. 2012). Furthermore, it exhibits a wide range of environmental tolerances, including variations in temperature and humidity (Bounfour & McMurtry 1987). These points highlight the economic importance of this predatory mite in sustainable agriculture through effective pest management and reduced reliance on chemical pesticides.
Temperature is an important environmental factor that influences the development, survivorship, and demographic parameters of different phytoseiid species (Bonfour & McMurtry 1987; Ali 1998; Broufas & Koveos 2001; Gotoh et al. 2004; Kasap & Şekerğlu 2004; Broufas et al. 2007). Khuzestan province, located in southwestern Iran, has a very hot and dry climate, with temperatures averaging around 45 °C from May to late September. During the summer, temperatures can even exceed 48 °C for short periods during the day. Temperature is the most important limitation in Khuzestan province, and this abiotic condition may affect the activity of phytoseiid predators, consequently affecting the efficiency of augmentative releases.
Shishehbor et al. (2022) found that cattail pollen is one of the suitable and cost-effective alternative diet sources for the laboratory rearing of E. scutalis. Furthermore, previous studies have shown that a product derived from the narrow-leaved cattail species is commercially available and highly recommended as an excellent diet for many phytoseiid species (Broufas & Koveos 2001; Goleva & Zebitz 2013; Gravandian et al. 2021). Cattail pollen is more affordable, effective, storable, and easier to collect than many other pollen options, making it a cost-effective choice for the mass rearing program of E. scutalis. Although Tetraychus turkestani (Ugarov and Nikolski) is a better food for rearing E. scutalis than cattail pollen (Shishehbor et al. 2022), cattail pollen was selected for our study to investigate the effect of temperature using an affordable food source.
Although the effects of different constant temperatures on life history traits and life table parameters of E. scutalis fed red ice plant (Malephora crocea Jacq.) pollen (Bounfour & McMurtry 1987) and on Panonychus citri (McGregor) (Kasap & Şekerğlu 2004) have been reported previously, it has also been noted that different strains (populations) of phytoseiid species exhibit varying biological characteristics (Hassan 1982; Meyerdirk & Coudriet 1986; Calvacante et al. 2015). Thus, the current study enhances our understanding of the life history and life table parameters of the Iranian strain of E. scutalis at different temperatures when fed on cattail pollen.
Material and methods
Predator culture
A colony of E. scutalis was obtained in February 2022 from a laboratory-grown culture at the Faculty of Agriculture, Shahid Chamran University of Ahvaz, Iran. This population was initially collected in February 2021 from marshmallow plants (Althea officinalis L.) infested with Tetranychus turkestani (Ugarov and Nikolski) on the university campus. A specialized rearing unit recommended by Walzer & Schausberger (1999) was used to rear E. scutalis in the laboratory. The rearing unit was consisted of a petri dish (9 cm diameter), a green plastic sheet (4 × 4 × 0.1 cm), and a sponge (4 × 4 × 1 cm). The green plastic sheet was placed on a water-soaked sponge located in a petri dish that was semi-filled with water. Tissue paper was used to cover the edges of the plastic sheet, and immersing them in the water surrounding the sponge. This technique not only provided necessary moisture for the mites but also prevented them from escaping. Cattail pollen (Typha latifolia L.) was provided in the petri dishes as a food source for the predators. E. scutalis individuals were reared on cattail pollen for one month before starting the experiments. The E. scutalis colony was maintained under laboratory conditions of 25 ± 1 °C, 60 ± 5% RH and a photoperiod of 16: 8 (L: D).
Pollen source
Cattail pollen was collected from Doroud city, Lorestan province, western Iran, during the summer of 2022. After separating and sieving the pollen grains, they were dried in an oven at 30 °C for 24 h and then frozen at -20 °C for long-term storage (Broufas & Koveos 2001).
Experimental setup
The biological characteristics of E. scutalis were determined at temperatures of 15, 20, 25, 30, 35, and 40 ± 0.1 °C, with a relative humidity of 60 ± 5% and a photoperiod of 16: 8 (L: D) in temperature-controlled cabinets. The experimental units were similar to rearing units. To ensure same-aged eggs of E. scutalis, more than 30 pairs of the predator were randomly selected from the stock culture for each temperature tested and kept in a new experimental unit for less than 24 h. The eggs laid by the females were then individually transferred to a similar new experimental unit. After larval emergence, cattail pollen (0.2–0.3 mg) was provided as a food source. The duration and survival of the different immature stages were recorded daily using a stereomicroscope. After adult emergence, females (less than 24 h old) were paired with males (also less than 24 h old) of the same treatment, and the pre-oviposition and oviposition periods, fecundity, adult longevity, and sex ratio of progeny were assessed daily.
The lower threshold temperature for development was estimated using a linear regression equation. The parameters t0 (thermal threshold) and K (thermal constant) were derived from the regression equation as follows: y = a + bx, where y represents the reciprocal of developmental duration in days (the developmental velocity =developmental rate= 1/developmental time), x is the temperature in °C, and a and b are the parameters of the linear regression (Campbell et al. 1974). From this, the lower developmental threshold (t0), i.e. the temperature at which development ceases, can be estimated as: t0= a/b. The number of degree-days (K) required for development was calculated using the equation: K = Y (T - t0), where Y is the developmental time (in days), T is temperature during development (experimental constant temperature), and t0 is the lower developmental threshold (Arnold 1959).
Statistical analysis
Raw data from the experiments were analyzed using the TWOSEX-MSChart software based on the age-stage, two-sex life table theory (Chi & Liu 1985; Chi 1988; Chi 2023). The TWOSEX-MSChart program was used to estimate age-stage-specific survival rate (sxj ), age-stage-specific fecundity (fxj ), age-specific fecundity (mx ), and age-specific survival rate (lx ), as well as population growth parameters for each treatment (Chi 2023). All parameters, including the duration of different life stages, fecundity, adult and total pre-oviposition periods (APOP and TPOP), oviposition period, and life table parameters such as gross reproductive rate (GRR), net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), and generation time (T), were calculated using the mentioned program (Chi 2023). Variances and standard error estimations of the parameters were performed using the bootstrap procedure (100,000 samples) (Chi 2023). Multiple comparisons among different generations were carried out using the paired bootstrap test.
Results
Pre-imaginal developmental time
Euseius scutalis completed its development at all of the temperatures examined (Table 1). There were significant differences in pre-imaginal developmental time of E. scutalis females and males among the temperatures examined. The developmental time of female E. scutalis decreased as the temperature increased, reaching its shortest duration at 35 °C, before increasing again as the temperature rose to 40 °C. The rate of development was approximately twice as fast at 30 °C compared to 20 °C (Table 1).
Download as The means followed by different letters in the same row show the difference among temperatures for each parameter (P<0.05, paired bootstrap test).
Female
15 °C
20 °C
25 °C
30 °C
35 °C
40 °C
Egg
4.30 ± 0.59a
2.60 ± 0.1b
1.82 ± 0.07c
1.08 ± 0.04d
1.06 ± 0.04d
1.00 ± 0.006d
Larva
5.38 ± 0.26a
1.45 ± 0.08b
1.03 ± 0.03b
1.00 ± 0.006b
1.00 ± 0.006b
1.00 ± 0.006b
Protonymph
6.94 ± 0.46a
1.78 ± 0.07b
1.10 ± 0.05c
1.00 ± 0.006c
1.00 ± 0.006c
1.00 ± 0.006c
Deutonymph
8.44 ± 0.41a
1.81 ± 0.06b
1.20 ± 0.07c
1.00 ± 0.006d
1.00 ± 0.006d
1.13 ± 0.06c
Total pre-adult
24.75 ± 1.27a
7.67 ± 0.13b
5.17 ± 9.93c
4.08 ± 4.51d
4.06 ± 0.04d
Male
Egg
7.07 ± 0.22a
2.95 ± 0.11b
1.83 ± 0.06c
1.11 ± 0.07d
1.00 ± 0.006d
1.00 ± 0.06d
Larva
6.30 ± 0.33a
1.49 ± 0.10b
1.00 ± 0.006c
1.00 ± 0.006c
1.00 ± 0.006c
1.00 ± 0.006c
Protonymph
5.53 ± 0.46a
1.45 ± 0.10b
1.06 ± 0.04c
1.00 ± 0.006c
1.00 ± 0.006c
1.00 ± 0.006c
Deutonymph
10.53 ± 1.24a
1.70 ± 0.09b
1.28 ± 0.09c
1.00 ± 0.006d
1.00 ± 0.006d
1.00 ± 0.006d
Total pre-adult
27.81 ± 2.09a
7.63 ± 0.19b
5.20 ± 9.70c
4.11 ± 0.08d
4.00 ± 0.01d
4.00 ± 0.01d
Pre-imaginal mortality
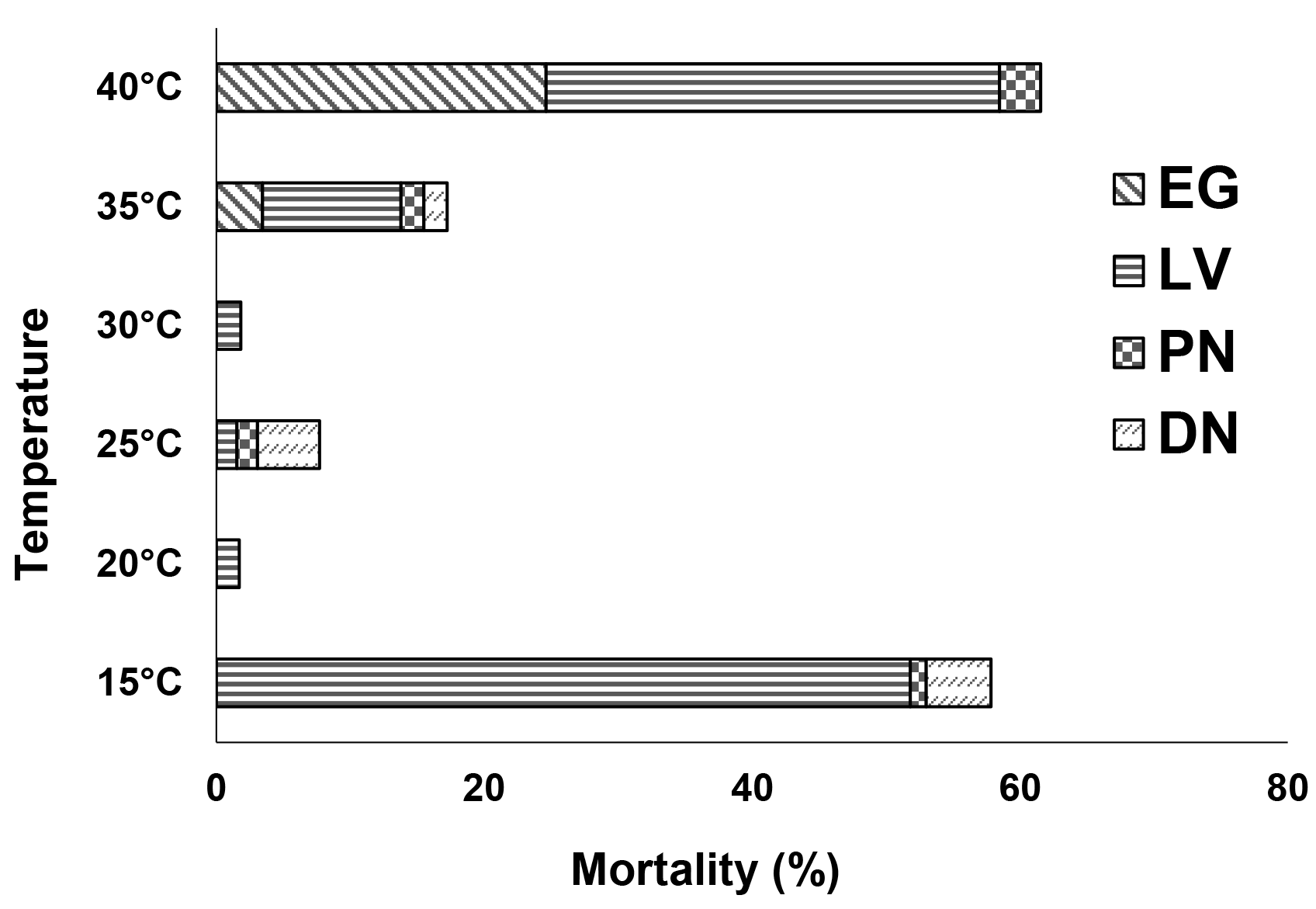


Overall, the mortality rate of the immature stages of E. scutalis was high at 15 °C and decreased as the temperature increased to 30 °C, before rising again above that temperature (Figure 1). The highest temperature (40 °C) resulted in higher mortality than the lowest temperature examined (15 °C). Additionally, mortality rates were reduced in the later immature stages. The larval stage was the most vulnerable to low temperatures, while the egg stage was the most vulnerable to high temperatures (Figure 1). Consequently, most deaths occurred before reaching the deutonymphal stage.
The lower temperature thresholds for the development of E. scutalis were calculated to be 10.72 °C for females and 10.87 °C for males. Based on these thresholds, an average of 91.51 day-degrees was required for female E. scutalis and 91.60 day-degrees for males to complete development from egg to adult (Table 2).
Download as
Sex
Regression
t0
R2
K ± SE
Female
Y= - 0.0005 X2 + 0.0362 x – 0.3894
10.72
0.99
91.51 ± 20.07
Male
Y= - 0.0005 X2 + 0.0358 x – 0.3893
10.87
0.99
91.60 ± 22.46
APOP, TPOP, adult longevity, fecundity and sex ratio
The highest level of APOP corresponds to 20 °C (2.66 days), while the lowest level of APOP is associated with a temperature of 30 °C (1.29 days), which significantly differs from other temperatures. The lowest TPOP was observed at 30 °C (5.38 days) (Table 3).
Download as The means followed by different letters in the same row show the difference among temperatures for each parameter (P<0.05, paired bootstrap test).
Parameter
15 °C
20 °C
25 °C
30 °C
35 °C
40 °C
APOP (day)
-
2.66 ± 0.13a
1.96 ± 0.26bc
1.29 ± 0.07d
1.76 ± 0.17c
2.44 ± 0.34a
TPOP (day)
-
10.4 ± 0.12a
7.00 ± 0.32b
5.38 ± 0.08d
5.95 ± 0.20c
6.66 ± 0.32b
Oviposition period (day)
-
7.52 ± 0.86b
12.15 ± 1.05a
6.97 ± 0.57b
3.22 ± 0.40c
2.55 ± 0.25c
Female longevity (day)
16.00 ± 1.60a
18.31 ± 1.73a
17.97 ± 1.38a
9.18 ± 0.72b
8.75 ±0.62b
4.73 ± 0.35c
Male longevity (day)
7.99 ± 1.11bc
14.62 ± 1.07a
10.05 ± 1.15b
9.82 ± 0.89b
6.47 ± 0.61c
5.5 ± 0.47c
Female total lifespan (day)
41.50 ± 1.33a
25.96 ± 1.75b
23.13 ± 1.40b
13.27 ± 0.71c
12.82 ± 0.63c
8.86 ± 0.35d
Male total lifespan (day)
37.14 ± 1.36a
22.25 ± 1.03b
15.26 ± 1.10c
13.93 ± 0.88c
10.47 ± 0.61d
9.49 ± 0.47d
Total fecundity (eggs/female)
-
10.48 ± 1.3b
18.31 ± 1.93a
16.19 ± 1.42a
4.21 ± 0.53c
3.13 ± 0.68c
Sex ratio (female percentage)
-
0.56 ± 0.06ab
0.44 ± 0.06b
0.67 ± 0.06a
0.50 ± 0.07ab
0.23 ± 0.03c
At 20 °C and higher temperatures, there was an inverse relationship between temperature and mean adult longevity of E. scutalis (Table 3). Temperature significantly affected the longevity of both females and males, with the longevity of E. scutalis females and males being twice as long as at 20 °C compared to 30 °C.
At 15 °C, E. scutalis laid no eggs. Temperature had a significant effect on the mean total fecundity of E. scutalis (Table 3). Maximum mean fecundity occurred at 25 °C; however, there were no significant differences in mean fecundity of E. scutalis at 25 °C and 30 °C. Above 30 °C, fecundity decreased significantly (Table 3).
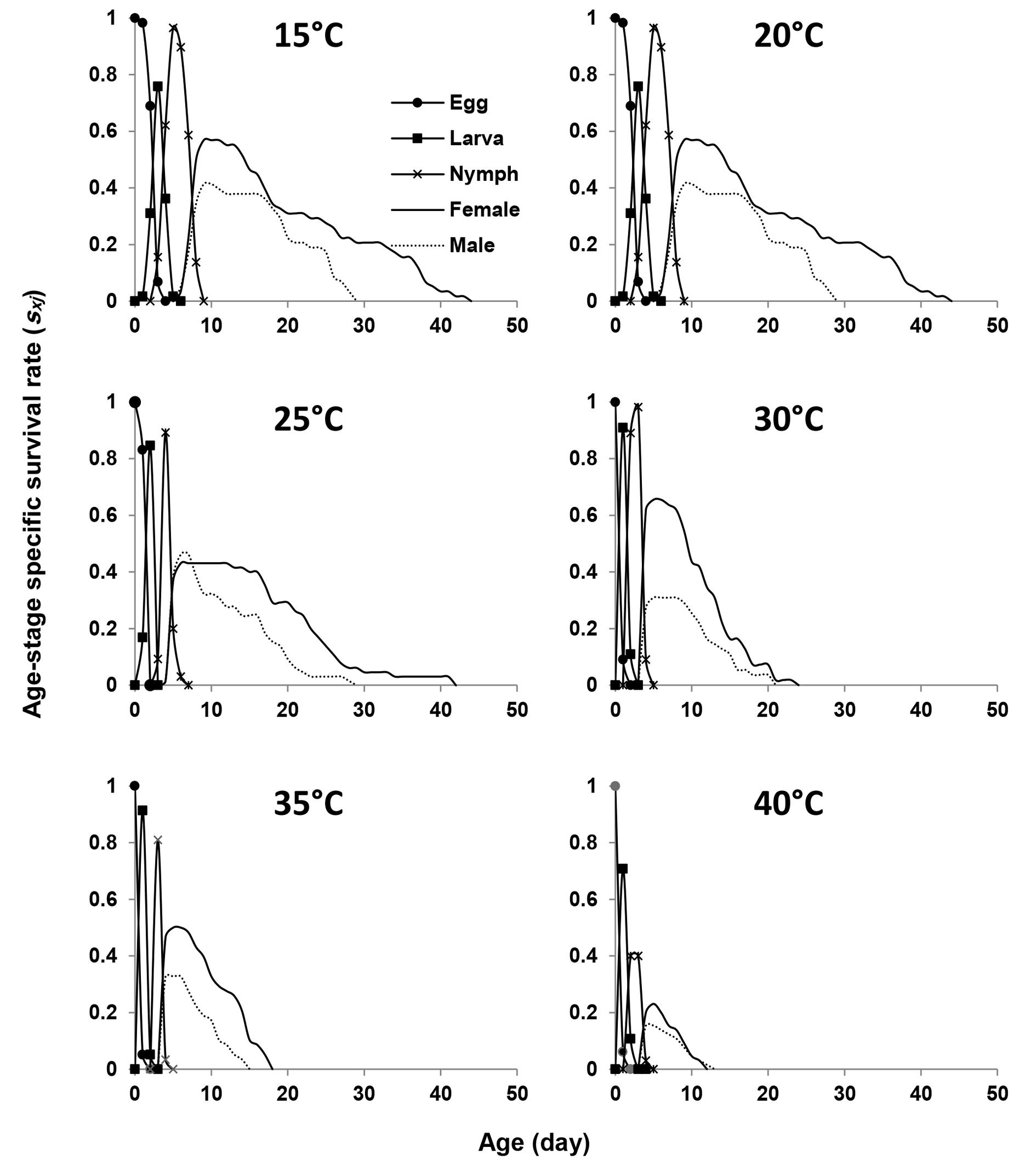


Figure 2 shows the age-stage specific survival rate (sxj ) of E. scutalis on six different temperatures. The overlaps observed among stages in the survival curves are due to the variable developmental rates among individuals. The survival rate at 40 °C was lower than at the other temperatures. The highest survival rates for females were 0.59, 0.58, 0.42, 0.54, 0.58, and 0.21 at 15, 20, 25, 30, 35, and 40 °C, respectively, while the rates for males at the same temperatures were 0.40, 0.41, 0.45, 0.29, 0.31, and 0.15, respectively (Figure 2).
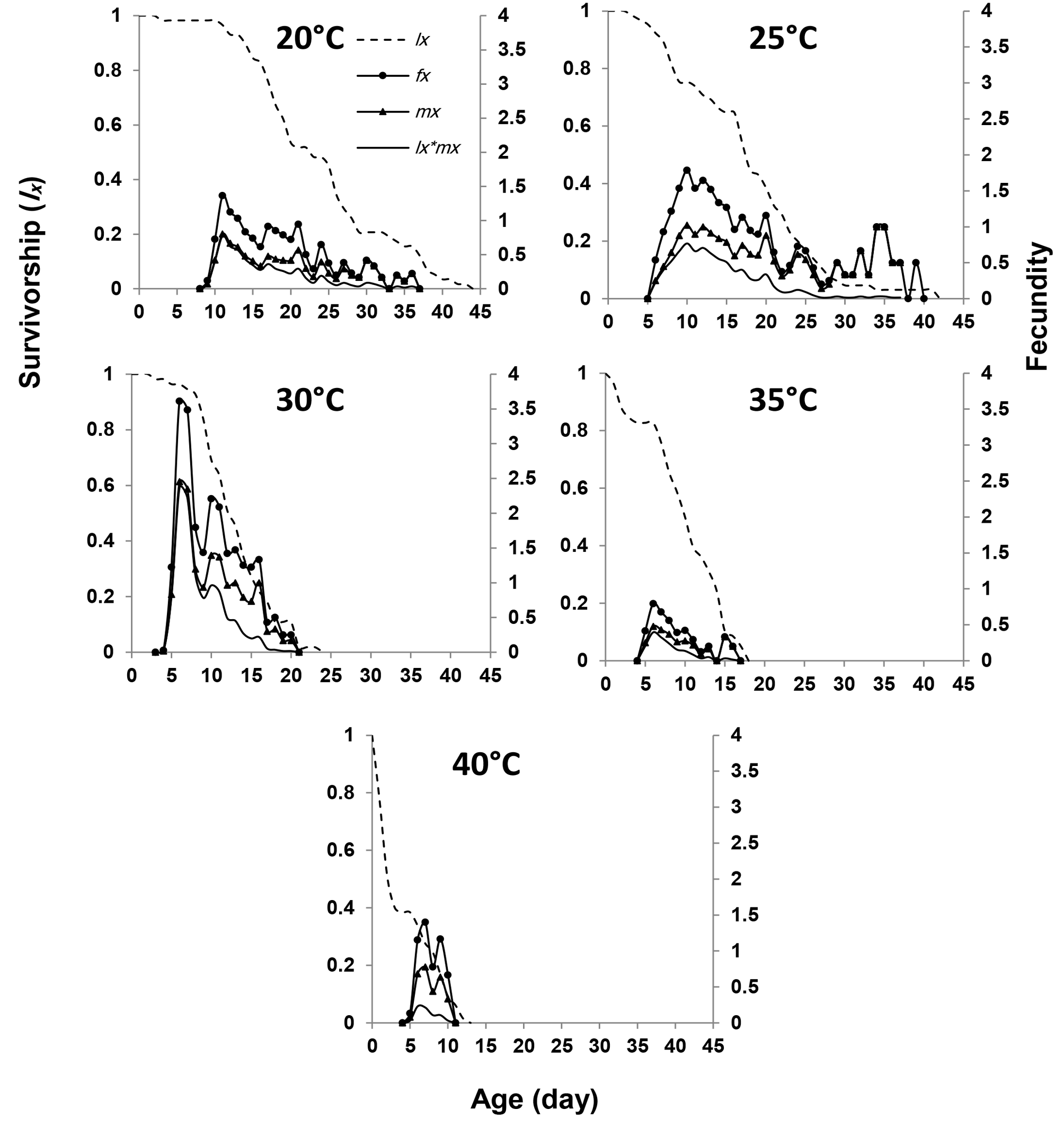


The highest mean fecundity per day was observed at 30 °C, while the lowest was recorded at 35 °C (Figure 3). Fecundity curves at 25 and 30 °C exhibited higher peaks than those at other temperatures. The age-stage specific fecundity (fxj) was highest at ages 6 (3.50 eggs/female/day), 11 (1.40 eggs/female/day), 12 (1.78 eggs/female/day), and 7 (0.19 and 1.42 eggs/female/day) at temperatures of 20, 25, 30, 35, and 40 °C, respectively, with immature survivorship rates of 0.82, 0.89, 0.79, 0.83, and 0.83, respectively (Figure 3).
Temperature had a significant effect on the sex ratio (percentage of females) of E. scutalis progeny among the tested temperatures (Table 3). The percentage of females increased as the temperature increased from 25 to 30 °C, peaking at 67%, and before decreasing at higher temperatures (Table 3).
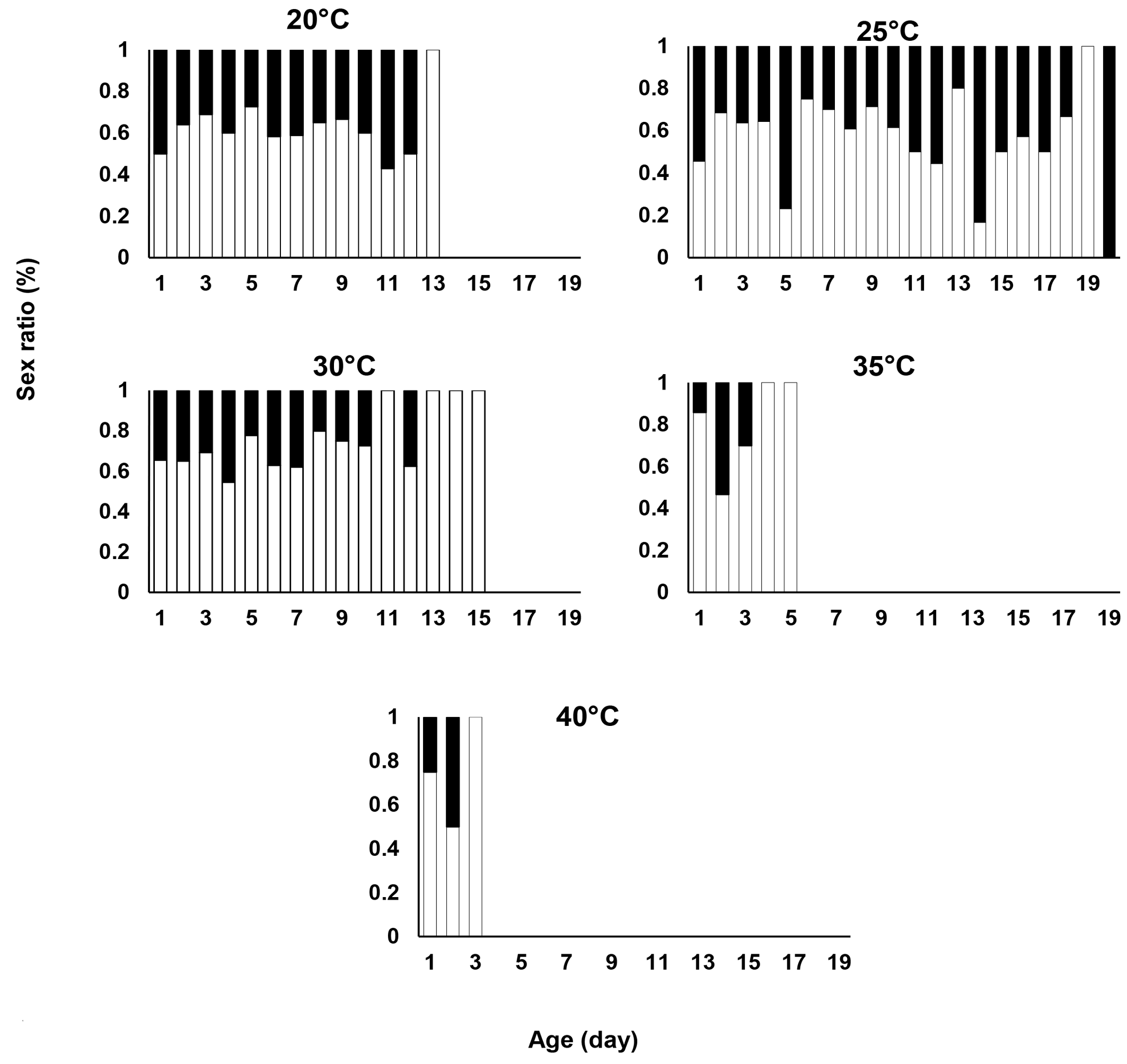


Figure 4 illustrates how the female predatory mite, E. scutalis, alters the sex ratio of its offspring in response to female age. The results indicate that the predator's age impacts the sex ratio of its offspring. In all treatments tested, lifetime sex ratios were consistently female-biased. On the last day, the last three days, the last two days and the final day of the oviposition period, the sex ratio was entirely female-biased at 20, 30, 35, and 40 °C, respectively. However, on the last day of the oviposition period, it was entirely male-biased at 25 °C.
Life table parameters
The demographic parameters of E. scutalis at five constant temperatures are presented in Table 4. Temperature significantly affected all life table parameters. The net reproductive rate (R0) increased with rising temperatures up to 30 °C, then decreased at higher temperatures (Table 3). The mean generation time (T) gradually decreased as temperature increased, while the intrinsic rate of natural increase (r) rose up to 30 °C and subsequently decreased at higher temperatures.
Download as The means followed by different letters in the same row show the difference among temperatures for each parameter (P<0.05, paired bootstrap test).
Parameter
20 °C
25 °C
30 °C
35 °C
40 °C
Intrinsic rate of increase (r) (day-1)
0.111 ± 1.02c
0.160 ± 1.44b
0.274 ± 1.46a
0.058 ± 0.02d
-0.043 ± 0.034e
Finite rate of increase (λ) (day-1)
1.117 ± 0.01c
1.174 ± 0.02b
1.316 ± 0.02a
1.060 ± 0.03d
0.960 ± 0.03e
Net reproductive rate (R0) (offspring)
5.971 ± 1b
8.175 ± 1.14ab
10.895 ± 1.39a
1.689 ± 0.34c
0.720 ± 0.19d
Gross reproductive rate (GRR) (offspring)
9.48 ± 1.49b
19.31 ± 4.04a
15.99 ± 2.16a
3.08 ±0.65c
2.95 ± 0.62c
Mean generation time (T ) (day)
15.97 ± 0.4a
13.00 ± 0.39b
8.66 ± 0.18c
8.68 ±0.18c
8.20 ± 0.31c
Discussion
It is proven that temperature is one of the most important physical factors affecting all invertebrates, including insect and mite species (Wojda 2017). Several detailed investigations have found that all life history traits and biological behaviors of phytoseiid mites are also influenced by temperature (Bonfour & McMurtry 1987; Broufas et al. 2007; Moradi et al. 2023). Consequently, to establish successful mass rearing and utilization of these beneficial organisms, it is necessary to have a comprehensive understanding of the optimum temperature for their development, survival, and reproduction.
In the present study, the egg stage required 35.2% of the total time to develop, while the larval stage required 19.3% of the total. The protonymph and deutonymph stages had approximately similar durations. This time distribution of life stages is similar to that of other species in the Phytoseiidae family (Sabelis 1985b).
Pre-adult developmental time for E. scutalis males was similar to that of the females at most of the tested temperatures. A similar trend has also been reported for a Morocco (Marrakech) strain of E. scutalis fed on red ice plant pollen (Bonfour & McMurtry 1987), as well as for two different strains of Kampimodromus aberrans Oudemans (Kasap 2005; Boroufas et al. 2007) and for Amblyseius vicroriensis (Womersley) (James & Taylor 2009). In several other phytoseiid species, such as Phytoseius hawaiiensis Prasad (Sanderson & McMurtry 1984), Typhlodromalus pregrinus (Muma) (Fouly et al. 1995), and E. finlandicus (Broufas & Koveos 2001), the egg to adult developmental time is shorter in males than in females. The opposite trend has been reported for Euseius citrifolius Denmark & Muma and a few other phytoseiid species (De Moraes & McMurtry 1981). The biological significance of this interspecific difference in developmental time between females and males has not yet been thoroughly understood (Sabelis 1985a).
The lower temperature threshold of 10.72 °C found in the present study for the development of E. scutalis females is very similar to the threshold of 10.7 °C for a Morocco (Marrakech) strain of E. scutalis fed on the red ice plant pollen (Bonfour & McMurtry 1987). However, a threshold of 9.39 °C was estimated for the development of E. scutalis fed on citrus red mite (Kasap & Şkeroğlu 2004; calculated from their data). The present strain of E. scutalis appears to have a developmental maximum near 40 °C, based on the decline in developmental rates and the increased pre-adult mortality at that temperature.
Although juvenile mortality is generally low when food supply is abundant, physical factors such as temperature are critical for the survival of juvenile phytoseiids (Sabelis 1985b). E. scutalis appears to exhibit a similar trend. The larval and egg stages were the most vulnerable to low and high temperatures, respectively. However, due to the scarity comparable data on the pre-imaginal survival of E. scutalis, a detailed comparison with our study is not possible.
Our results indicate that the longevity of females E. scutalis at all tested temperatures was longer than that of males. A similar trend has been reported for a Morocco (Marrakech) strain of E. scutalis (Bonfour & McMurtry 1987) and for other phytoseiid species such as T. pregrinus (Fouly et al. 1995), Phytoseiulus macropilis (Banks) (Ali 1998) and Neoseiulus californicus (McGregor) (El Taj & Jung 2012). However, a reverse trend has been observed for Neoseiulus neoagrestis Khaustov & Doker (Moradi et al. 2023). It should be noted that few studies have reported the longevity of male phytoseiids.
Euseius scutalis females developed at 15 °C lived for 16 days but did not lay any eggs. The unfavorable effect of low temperature on the oviposition of E. scutalis may be attributed to a direct impact on the female's reproductive system, which slows the maturation of the oocytes. Wysoki and Swirski (1971) found that although E. scutalis reproduces throughout the winter, more than 80 percent of the females collected during this season contained no eggs.
The results of the present study showed that the highest sex ratio (67% female) of E. scutalis progeny was recorded at 30 °C. A Turkish strain of E. scutalis progeny fed on P. citri was reported to have sex ratios of 67.8, 69.1 and 67.8% female at 20, 25, and 30 °C, respectively (Kasap & Şkeroğlu 2004), which corresponds well with our findings. In contrast, a Morocco (Marrakech) strain of E. scutalis progeny fed on red ice plant pollen was reported to have a sex ratio of 56.3% female at 25 °C (Bonfour & McMurtry 1087). Differences in sex ratios could partly be attributed to genetic variations among strains, as well as differences in the quality of the food used for rearing the mites.
Our results indicated the percentage of female E. scutalis progeny decreased as temperature increased. A similar trend has been reported for Metaseiulus occidentalis (Tanigushi et al. 1975), Euseius mesembrinus (Dean) (Abou-Setta & Childers 1987), and P. macropilis (Ali 1998). However, an opposite trend has been reported for N. californicus (Gotoh et al. 2004). In contrast, Amblyseius victoriensis Womersley has been shown to maintain a constant sex ratio of 50% female in progeny across a range of temperatures from 20 to 40 °C.
Based on the results of the current study, the sex ratio of A. swirskii was equal or male-biased on the first day of the oviposition period at 20 and 25 °C, respectively. However, during the remainder of the oviposition duration, sex ratio was female-biased. A similar trend has also been reported for a Morocco (Marrakech) strain of E. scutalis fed on red ice plant pollen (Bonfour & McMurtry 1987) and for other phytoseiid species such as Typhlodromus caudiglans Schuster (Putman 1962), Amblyseius andersoni (Chant) (Amano & Chant 1978), Phytoseiulus persimilis Athias-henriot and Amblyseius bibens Blommers (Schulten et al. 1978), Galendromus helveolus (Chant) (Caceres & Childers 1991), and K. aberrans (Boroufas et al. 2007). Sabelis (1985b) expressed that increased male offspring production in the beginning of the oviposition period could result to the early insemination of females that would afterwards start to search for a suitable food.
The results of the present study indicated that the intrinsic rate of population increase (r) of E. scutalis was significantly affected by temperature, increasing gradually with temperature from 20 °C to a maximum at 30 °C. Similar results have been reported for strains of E. scutalis from Morocco (Marrakech) (Bonfour & McMurtry 1987) and Turkey (Kasap & Şkeroğlu 2004). Additionally, similar findings have been reported for other phytoseiid species, such as G. helveolus (Caceres & Childers 1991) and E. finlandicus (Broufas & Koveos, 2001). However, in several other phytoseiid species, the estimated maximum values of r were recorded at higher temperatures; for example, 32-33 °C in G. occidentalis (Tanigoshi et al. 1975), Euseius stipulatus (Athias-Henriot) (Ferragut et al. 1987), N. womersleyi (Lee & Ahn 2000), and N. californicus (Castagnoli & Simoni 1991; Gotoh et al. 2004). Conversly, for some other phytoseiid species, the calculated maximum values of r were recorded at lower temperatures; for instance, at 25 °C in Euseius mesembrinus (Dean) (About-Setta & Childers 1987), Amblyseius largoensis (Muma) (Yue & Tsai 1996), and K. aberrans (Oudemans) (Broufas et al. 2007), and at 28 °C in Phytoseiulus macropilis (Bank) (Ali 1998). At temperatures above 30 °C, the estimated r values for our strain of E. scutalis decreased and reached negative values at 40 °C, indicating that at such high temperatures, the population would tend toward extinction. Furthermore, at 40 °C, only a limited number of E. scutalis individuals completed their immature development, and most of the females did not lay eggs. The low number of eggs laid by E. scutalis females at high temperatures could be attributed, among other reasons, to reduced mobility or mortality of the sperm, which leads to a failure of oocyte fertilization, as observed in E. stipulatus (Ferragut et al. 1987).
Based on the results of our study, when E. scutalis fed on cattail pollen, the intrinsic rate of increase (r) was estimated to be 0.000, 0.111, 0.160, 0.274, 0.058, and -0.043 day-1 at 15, 20, 25, 30, 35, and 40 °C, respectively. In a similar study, the intrinsic rates of population increases of a Morocco (Marrakech) strain of E. scutalis fed on red ice plant pollen were reported to be 0.019, 0.151, 0.188, 0.325, and 0.150 day-1 at 15, 20, 25, 30, and 35 °C, respectively (Bonfour & McMurtry 1987). Furthermore, the r values for a Turkish strain of E. scutalis fed on Panonychus ulmi (Koch) were reported to be 0.166, 0.234, and 0.295 day-1 at 20, 25, and 30°C, respectively (Kasap & Şkeroğlu 2004). Although direct comparisons of r values between the three strains of E. scutalis are not feasible, the intrinsic rate for natural increase of the Iranian strain was lower than the corresponding values reported for the Moroccan and the Turkish strains at all of the tested temperatures (Bonfour & McMurtry 1987; Kasap & Şkeroğlu 2004). These contrasts may be explained by disparities in E. scutalis strains, the food used for rearing mites, as mentioned for N. californicus (Gotoh et al. 2004), and differences in experimental conditions (humidity and photoperiod).
These data have two useful aspects. First, they can be used in mass rearing projects of E. scutalis. The optimum rearing temperature for development, survival, and reproduction can be selected based on our findings. Second, for pest management purposes, our data can be utilized in the construction of computer simulation models to predict E. scutalis development and population dynamics for release programs.
Acknowledgments
Financial support (Grant no. SCU. AP 1403. 400) of this research by the Department of Plant Protetion, Shahid Chamran University of Ahvaz, is greatly appreciated.
References
- Abou-Setta M.M., Childers C.C. 1987. Biology of Euseius mesemberinus (Acari: Phytoseiidae): life tables on ice plant pollen at different temperatures with notes on behavior and food range. Exp. Appl. Acarol., 3: 123- 130. https://doi.org/10.1007/BF01270474
- Adar E., Inbar M., Gal S., Doron N., Zhang Z.-Q., Palevsky E. 2012. Plant-feeding and non-plant feeding phytoseiids: differences in behavior and cheliceral morphology. Exp. Appl. Acarol., 58: 341-357. https://doi.org/10.1007/s10493-012-9589-y
- Ali F.S. 1998. Life tables of Phytoseiulus macropilis (Banks) (Gamasida: Phytoseiidae) at different temperatures. Exp. Appl. Acarol., 22: 335-342. https://doi.org/10.1023/A:1024560924642
- Al-Shammery K.A. 2010. Different Biological aspects of the predaceous mite Euseius scutalis (Acari: Gamasida: Phytoseiidae) and the effects due to feeding on three tetranychid mite species in Hail, Saudi Arabia. Asian J. Biol. Sci., 3: 77-84. https://doi.org/10.3923/ajbs.2010.77.84
- Amano H., Chant D.A. 1978. Some factors affecting reproduction and sex ratios in two species of predacius mites, Phytoseiulus persimilis Athias-henriot and Amblyseeius andersoni (Chant) (Acarina: Phytoseiidae). Can. J. Zool., 56: 1593- 1607. https://doi.org/10.1139/z78-221
- Arnold C.Y. 1959. The determination and significance of the base temperature in the linear heat unit system. Proc. Am. Soc. Hort. Sci., 74: 430- 445
- Ata M.M.I., El-Shahawy G.Z., Fawzy, M.H., Abdel-Baki A.S., Al-Quraishy S., Hassan A.O., Abdel-Tawab H. 2023. Bioefficacy of essential oils emulsion and predatory mite, Euseius scutalis (Athias-Henriot) (Acari: Phytoseiidae) for the management of citrus brown mite, Eutetranychus orientalis (Klein) (Acari: Tetranychidae). J. King Saud Univ.-Sci., 35 (2): 102471. https://doi.org/10.1016/j.jksus.2022.102471
- Athias-Henriot C. 1958. Contribution à la connaissance du genre Typhlodromus Scheuten (Acarines Parasitiformes, Phytoseiidae). Description de deux espéces Nouvelles d' Algérie et cle des espéces du groupe finlandicus. Rev. Patol. Veg. Entom. Agric. France., 37 (2): 181- 186.
- Bounfour M., McMurtry J.A. 1987. Biology and ecology of Euseius scutalis (Athias-Henriot) (Acarina: Phytoseiidae). Hilgardia, 55 (5): 1- 23. https://doi.org/10.3733/hilg.v55n05p023
- Broufas G.D., Koveos D.S. 2001. Development, survival and reproduction of Euseius finlandicus (Acari: Phytoseiidae) at different constant temperatures. Exp. Appl. Acarol., 25: 441- 460. https://doi.org/10.1023/A:1011801703707
- Broufas G.D., Pappas M.L., Koveos D.S. 2007. Development, survival and reproduction of Kampimodromus aberrans (Acari: Phytoseiidae) at different constant temperatures. Environ. Entomol., 36 (4): 657- 665. https://doi.org/10.1093/ee/36.4.657
- Caceres S., Childers C.C. 1991. Biology and life tables of Galendromus helveolus (Acari: Phytoseiidae) on Florida citrus. Environ. Entomol., 20: 224- 229. https://doi.org/10.1093/ee/20.1.224
- Calvacante A.C.C., Borges L.R., Lourencao A.L. & de Moraes G.J. 2015. Potential of two populations of Amblyseius swirskii (Acari: Phytoseiidae) for the control of Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) in Brazil. Exp. Appl. Acarol., 67: 523- 533. https://doi.org/10.1007/s10493-015-9964-6
- Campbell A., Frazer D.B., Gilbert N., Gutierrez A.P., Mackauer M. 1974. Temperature requirements of some aphids and their parasites. J. Appl. Ecol., 11: 431- 438. https://doi.org/10.2307/2402197
- Castagnoli M., Simoni S. 1991. Influenza della temperature sull′ incremento delle popolazioni di Amblyseius californicus (McGregor) (Acari: Phytoseiidae). Redia, LXXIV: 621- 640.
- Chi H. 1988. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol., 17(1): 26-34. https://doi.org/10.1093/ee/17.1.26
- Chi H. 2023. TWOSEX-MSChart: A Computer Program for the Age Stage, Two-Sex Life Table Analysis. 2019.01.09 Edition. http://140.120.197.173/Ecology/prod02.htm. Accessed Feb 2023
- Chi H., Liu H. 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin., 24(2): 225-240.
- Daneshvar H. 1980. Some predatory mites from northern and western Iran. Entomol. Phytopathol. Appl., 48: 15- 87.
- De Moraes G.J., McMurtry J.A. 1981. Biology of Amblyseius citrifolius (Acarina: Phytoseiidae). Hilgardia, 45: 211- 236.
- El- Taj H.F.E., Jung C. 2012. Effect of temperature on the life-history traits of Neoseiulus californicus (Acari: Phytoseiidae) fed on Panonychus ulmi. Exp. Appl. Acarol., 56: 247- 260. https://doi.org/10.1007/s10493-012-9516-2
- El-Badry E.A., Afifi A.M., Issa G.I., El-Benhawy E.M. 1968. Effectiveness of the predacious mite Amblyseius gossypi as a predator of three tetranychid mites (Acarina: Phytoseiidae). Z. Angew. Entomol., 62: 189- 194. https://doi.org/10.1111/j.1439-0418.1968.tb04119.x
- El-Badry E.A., El-Benhawy J.A. 1968. The effects of pollen feedng on the predatory efficiency of Amblyseius gossypi (Acarina: Phytoseiidae). Entomol. Exp. Appl., 11: 273- 276. https://doi.org/10.1111/j.1570-7458.1968.tb02055.x
- Ferragut F., Garcia-mari F., Costa-Comelles J., Laborda R. 1987. Influence of food and temperature on development and oviposition of Euseius stipulatus and Typhlodromus phialatus (Acari, Phytoseiidae). Exp. Appl. Acarol., 3: 317- 329. https://doi.org/10.1007/BF01193168
- Fouly A.H., About-Setta M.M., Childers C.C. 1995. Effect of diet on the biology and life tables of Typhlodromalus pregrinus (Acari: Phytoseiidae). Environ. Entomol., 24: 870- 874. https://doi.org/10.1093/ee/24.4.870
- Goleva I., Zebitz, C.P.W. 2013. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii. Exp. Appl. Acarol., 61: 259-283. https://doi.org/10.1007/s10493-013-9700-z
- Gotoh T., Yamaguchi K., Mori K. 2004. Effect of temperature on life history of the predatory mite Amblyseius (Neoseiulus) californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 32: 15-30. https://doi.org/10.1023/B:APPA.0000018192.91930.49
- Gravandian M., Fathipour Y., Hajiqanbar H., Riahi E., Riddick E.W. 2021. Long-term effects of cattail Typha latifolia pollen on development, reproduction, and predation capacity of Neoseiulus cucumeris, a predator of Tetranychus urticae. BioControl, 67: 149-160. https://doi.org/10.1007/s10526-021-10116-4
- Hassan S.A. 1982. Relative tolerance of three different strains of the predatory mite Phytoseiulus persimilis A.-H. (Acari: Phytoseiidae) to 11 pesticides used on glasshouse crops. J. Appl. Entomol., 93 (1-5): 55- 63. https://doi.org/10.1111/j.1439-0418.1982.tb03570.x
- James D.G., Taylor A. 2009. Effect of temperature on development and survival of Amblyseius victoriensis Womersley (Acari: Phytoseiidae). Int. J. Acarol. 18: 93- 96. https://doi.org/10.1080/01647959208683938
- Kamali K., Ostovan H., Atamehr A. 2001. A catalogue of mites and ticks (Acari) of Iran. Islamic Azad University Scientific Publication Center, 192 pp. https://doi.org/10.13140/2.1.4825.8244
- Kasap I. 2005. Population dynamics of the citrus red mite Panonychus citri (McGregor) and the predacious mite Euseius scutalis (Athias-Henriot) (Acarina: Tetranychidae; Phytoseiidae) on the sour orange (Citrus aurantium L.). J. Agric. Sci., 15 (2): 119- 123.
- Kasap I., Şekeroğlu E. 2004. Life history of Euseius scutalis feeding on citrus red mite Panonychus citri at various temperatures. BioControl, 49(6): 645-654. https://doi.org/10.1023/B:BICO.0000046733.53887.2b
- Lee J.H., Ahn J.J. 2000. Temperature effects on development, fecundity and life table parameters of Amblyseius womersleyi (Acari: Phytoseiidae). Environ. Entomol., 29: 265- 271. https://doi.org/10.1093/ee/29.2.265
- McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their role in biological control. Annu. Rev. Entomol., 42: 291- 321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., De Moraes G.J. Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Sys. Appl. Acarol., 18(4): 297-320. https://doi.org/10.11158/saa.18.4.1
- Meyerdirk D.E., Coudriet D.L. 1986. Evaluation of two biotypes of Euseius scutalis (Acari: Phytoseiidae) as predators of Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol., 79(3): 659-663. https://doi.org/10.1093/jee/79.3.659
- Momen F., Abdel-Khalek A. 2008. Influence of diet on biology and life-table parameters of the predacious mite Euseius scutalis (AH) (Acari: Phytoseiidae). Arch. Phytopathol. Plant Prot., 41(6): 418-430. https://doi.org/10.1080/03235400600813508
- Moradi M., Misharova Y.V., Snigirev V.V., Döker I., Joharchi O., Khaustov V.A. 2023. Life history of Neoseiulus neoagrestis (Karg) (Acari: Phytoseiidae) fed on the storage mite, Tyrophagus sp. (Acari: Acaridae) at different temperatures. Acarologia, 63: 817-825. https://doi.org/10.24349/6x1q-8mmr
- Nomikou M., Janssen A., Schraag R., Sabelis, M.W. 2001. Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp. Appl. Acarol., 25: 271-291. https://doi.org/10.1023/A:1017976725685
- Putman W.L. 1962. Life-history and behavior of the predacious mite Typhlodromus (T.) caudiglans (Acarina: Phytoseiidae) in Ontario, with notes on the prey of related species. Can. Entomol., 94: 163- 177. https://doi.org/10.4039/Ent94163-2
- Sabelis M.W. 1985a. Development. In: Helle W., Sabelis M.W. (Eds). World crop pests, spider mites, their biology, natural enemies and control. Vol. 1B, Elsevier, Amsterdam, The Netherlands, p. 43- 53.
- Sabelis M. W. 1985b. Sex allocation. In: Helle W., Sabelis M.W. (Eds). World crop pests, spider mites, their biology, natural enemies and control. Vol. 1B, Elsevier, Amsterdam, The Netherlands, p. 83- 94.
- Sanderson J.P., McMurtry J.A. 1984. Life history studies of the predacious mite Phytoseius hawaiiensis. Entomol. Exp. Appl. Acarol., 35: 227- 234. https://doi.org/10.1111/j.1570-7458.1984.tb03386.x
- Schulten G.G.M., Vanarendonk R.C.M., Russell V.M., Roorda F.A. 1978. Copulation, egg production and sex ratio in Phytoseulus persimilis and Amblyseius bibens (Acari: Phytoseiidae). Entomol. Exp. Appl. Acarol., 24: 145- 153. https://doi.org/10.1111/j.1570-7458.1978.tb02764.x
- Shishehbor P., Rahmani Piyani A., Riahi E. 2022. Effect of different pollen diets in comparison to a natural prey, Tetranychus turkestani (Acari: Tetranychidae), on development, survival, and reproduction of Euseius scutalis (Acari: Phytoseiidae). Sys. Appl. Acarol., 23 (10): 2111- 2122. https://doi.org/10.11158/saa.27.10.19
- Stathakis Th., Kapaxidi E., Papadoulis G., Papanikolaou N. 2021. Predation by Euseius scutalis (Acari: Phytoseiidae) on Tetranychus urticae and Eutetranychus orientalis (Acari: Tetranychidae): effect of prey density and developmental stage. Sys. Appl. Acarol., 26 (10): 1940-1951. https://doi.org/10.11158/saa.26.10.8
- Tanigushi L.K., Hoyt S.C., Browne R.W., Logan J.A. 1975. Influence of temperature on population increase of Metaseiulus occidentalis (Acarina: Phytoseiidae). Ann. Entomol. Soc. Am., 68: 979- 986. https://doi.org/10.1007/s10526-010-9334-6
- Walzer A., Schausberger P. 1999. Cannibalism and interspecific predation in the phytoseiid mites Phytoseiulus persimilis and Neoseiulus californicus: predation rates and effects on reproduction and juvenile development. BioControl, 43: 457-468. https://doi.org/10.1023/A:1009980401662
- Wojda I. 2017. Temperature stress and insect immunity. J. Therm. Biol., 68: 96- 103. https://doi.org/10.1016/j.jtherbio.2016.12.002
- Wysoki M., Swirski E. 1971. Studies on overwintering of predacious mites of the genera Amblyseius Berlese, Typhlodromus Scheuten and Iphyseius Berlese (Acarina: Phytoseiidae) in Israel. In Entomological essays to commemorate the retirement of Professor K. Yasamutsu, 265- 292. Tokyo: Hokuryuokan Publication.
- Yue B., Tsai J. H. 1996. Development, survivorship and reproduction of Amblyseius largoensis (Acari: Phytoseiidae) on selected plant pollens and temperatures. Environ. Entomol., 25: 488- 496. https://doi.org/10.1093/ee/25.2.488
- Zergani A., Shishehbor P., Nakkai F.N., Riahi E. 2023. Life history traits and population parameters of the predatory mite Euseius scutalis (Acari: Phytoseiidae) fed on Tetranychus turkestani (Acari: Tetranychidae) and pollen from three different plants. Acarologia, 63(3): 945-954. https://doi.org/10.24349/mrqf-arrz



2024-09-25
Date accepted:
2025-03-11
Date published:
2025-03-26
Edited by:
Marčić, Dejan

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Bazazzadeh, Fereshteh; Shishehbor, Parviz; Gorji, Zahra; Gravandian, Mohammad; Esfandiari, Mehdi and Riahi, Elham
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



