Stylostome of Leptotrombidium myotis larvae (Ewing, 1929) (Acariformes, Trombiculidae) feeding on two different bat species (Vespertilionidae)
Shatrov, Andrey B.  1
; Kazakov, Denis V.
1
; Kazakov, Denis V.  2
and Antonovskaia, Anastasia A.
2
and Antonovskaia, Anastasia A.  3
3
1✉ Zoological Institute of the Russian Academy of Sciences, Universitetskaya nab., 1, 199034, St- Petersburg, Russia.
2Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Volodarskogo st., 6, 625003, Tyumen, Russia & Institute of General and Experimental Biology, Siberian Branch of the Russian Academy of Sciences, Sakh'yanovoy st., 6, 670047 Ulan-Ude, Russia.
3Department of Entomology, Faculty of Biology, Lomonosov Moscow State University, 1-12 Leninskie Gory, 119234, Moscow, Russia.
2025 - Volume: 65 Issue: 1 pages: 173-196
https://doi.org/10.24349/o879-3rstOriginal research
Keywords
Abstract
Introduction
The stylostome – or feeding tube – is a constant attribute of trombiculid larvae that has evolved during their feeding on different host species from mammals, including bats, to reptiles and amphibians (e.g. Trouessart 1899; Ewing 1944; Jones 1950; Hyland and Wharton 1955; Aoki 1957; Hoeppli and Schumacher 1961, 1962; Hyland 1961; Schumacher and Hoeppli 1963; Voigt 1970; Hase et al. 1978; Schramlová 1978; Shatrov 2009; Shatrov et al. 2014; Shatrov et al. 2022, etc.). Depending on the parasite species and the host group, the character of stylostome and the inflammatory response of the host individual may significantly differ. The stylostome may be long and thin, immersing deep into the dermis (Shatrov and Stekolnikov 2011) and even ''more than twice the length of the engorged chigger'' (Allred 1954, p. 63), or in contrast, may form a type of capsule on the abdominal region of the hosts (voles) (Shatrov 2000). Depending on the interaction of the stylostome of Leptotrombidium species with the host tissues, three different types of stylostome – epidermal, mesenchymal and mixed – were distinguished (Hase et al. 1978). Questions regarding parasite species-specificity of stylostome formation and the intensity of the inflammatory response depending on the novelty of the host species have been debated in the literature but are not still resolved adequately (e.g. Audy 1951; Hoeppli and Schumacher 1962; Traub and Wisseman 1974).
It is accepted that the stylostome is entirely produced by the parasite, and at its periphery, the stylostome substance reacts with the host tissues (Trouessart 1897; Schumacher and Hoeppli 1963; Shatrov 2009). Generally, the stylostome is composed of the proximal portion, the so-called eosinophilic cone – the first portion of saliva secretion, to which the movable cheliceral digits of the mite are cemented, then, subsequently, the main stylostome tube-like'body' extends into the skin having a central axial canal designed for flow of the saliva into the wound and liquid food in the opposite direction towards the pharynx of the parasite. Very rarely, the stylostome in some trombiculid species may reveal weakly expressed transverse or longitudinal layers (Voigt 1970; Shatrov 2000, 2009). Nevertheless, electron-microscopical investigations did not show conspicuous layers in the stylostome substance (Shatrov and Felska 2017). The stylostome is built of a hyaline-like substance of a gelatinous consistency, which is very poorly stained with histochemical dyes (Schumacher and Hoeppli 1963). Generally, the stylostome is composed of a compound glycoprotein complex containing trace amounts of basic proteins, neutral polysaccharides, and acid mucopolysaccharides (Schumacher and Hoeppli 1963; Voigt 1970; Shatrov 2000). Such structural organization of the stylostome and its low histochemical reactivity suggest that the stylostome is immunologically inert, which allows the larvae to feed for a long time.
The trombiculid fauna associated with bats is poorly investigated especially concerning boreal host species (see Kudryashova 1998). Leptotrombidium myotis (Ewing, 1929) larvae studied in this work, which is known as a specialized bat parasite, was previously recorded on various bat species in China, South Korea, the USA, and Canada (e.g. Vercammen-Grandjean and Langston 1976; Wen 1984; Poissant and Broders 2008; Valdez et al. 2009). Recently, Kazakov et al. (2024) has shown that the body measurements of L. myotis and another closely related species L. schlugerae (Emeljanova et Gorbatcheva, 1960) broadly overlap, and thus these two species may be synonymous. However, it is believed that the main hosts of L. schlugerae are rodents (e.g., Ochotona spp., Cricetulus spp., Alticola spp., and Microtus spp.) (Kudryashova, 1998). The L. schlugerae record on bats in Kazakhstan (Dzhanokmen, 1969) needs revision according to Kudryashova (1998). Nevertheless, the larvae of L. schlugerae were successfully collected from Plecotus ognevi Kishida, 1927, Myotis petax Hollister, 1912, Eptesicus nilssonii Keyserling et Blasius, 1839 and Vespertilio murinus L., 1758 in several localities of Eastern Siberia and the Far East (Kazakov et al., 2024). Therefore, the host range of this species is much wider and also includes bats.
In this study, L. myotis larvae were collected from two bat species Myotis davidii (Peters, 1869) captured in Kazakhstan and Plecotus ognevi Kishida, 1927 captured in Buryatia, Russia (see Materials and Methods).
M. davidii was known in the literature as Myotis aurascens Kuzyakin, 1935, but M. davidii is the oldest available name for this taxon (Benda et al., 2012). M. davidii is distributed from southeastern Europe, Asia Minor and Central Asia to Mongolia, northeastern China and probably Korea (Kruskop et al., 2012; Tsytsulina et al., 2012; Çoraman et al., 2020). M. davidii was found in a summer shelter, which was crevices in a building. It is known that M. davidii also uses natural summer shelters as niches in the cliffs and in the scree of flat rock fragments (Bazhenov, 2021). M. davidii forms maternity colonies (Sherbin 1968; Rosina and Kirilyuk, 2000).
Previously, P. ognevi belonged to the polytypic species Plecotus auritus (L., 1758), but later was raised to full species status based on morphological and molecular data (Spitzenberger et al., 2006; Kruskop et al., 2012). The range of P. ognevi extends from the river Ob and the Altai Mountains east to the Pacific Ocean, including Sakhalin Island and Korea. P. ognevi was caught at a swarming site. The prevalence of P. ognevi in the Dolganskaya Yama Cave in July was 39.6%. Besides P. ognevi, Myotis petax Hollister, 1912, M. sibiricus Kastschenko, 1905 and M. ikonnikovi Ognev, 1912 were also caught in this site. Previously, Kazakov et al. (2018) showed the predominance of P. ognevi in swarming sites in Eastern Siberia.
Recently, the stylostome of the trombiculid mite larvae Leptotrombidium album (Kamo, Kawashima and Nishimura, 1957), originally found on the bat Barbastella pacifica (Kruskop, Kawai & Tiunov, 2019) on Kunashir Island, and the inflammatory reaction of the host were described (Shatrov et al. 2022). The main purpose of this work is to study the stylostome and tissue reaction evolving during feeding of the trombiculid larvae L. myotis (Ewing, 1929) on the bats P. ognevi Kishida, 1927 and M. davidii (Peters, 1869) captured in different geographical regions.
Material and methods
Collection of mites and host animals



An adult male Myotis davidii (Peters, 1869) (Figure 1, A-B) (No. KZ22011) with feeding trombiculid larvae was collected in the Shygan cordon of the ''Altyn-Emel'' National Nature Park (Almaty Region, Kazakhstan, 44.11° N 78.71° E, 892 m a.s.l.) on 23 June 2022. In the other case, an adult male Plecotus ognevi Kishida, 1927 (Figure 1, C) (No. DО22275) having feeding trombiculid larvae was collected in Dolganskaya Yama Cave (Republic of Buryatia, Russia, 54.44° N 113.78° E, 1012 m a.s.l.) on 24 July 2022. The bat capture sites are located in the Tian Shan foothill arid steppe and the East Siberian taiga ecoregions, respectively (Olson et al., 2001). M. davidii was found in a building, and P. ognevi was caught using a mist net (6 m × 2.5 m; Ecotone, Poland), installed at the cave entrance. Bats were identified according to Tiunov (1997) and Benda and Tsytsulina (2000). Afterwards, the bats were marked with aluminum rings (measuring 2.9 mm, Aranea, Poland) (Figure 1, C). No bats died during capture and were released into the wild after collecting of the parasites.
In overall, five individuals of M. davidii were captured but only one specimen had feeding larvae. In contrast, 141 individuals of P. ognevi were captured, and some of them were parasitized by trombiculids, from which only one specimen was taken for experiment.
The mite larvae were localized on the outer surface of the ear in M. davidii (Figure 1, B), and inside the ear in P. ognevi (Figure 1, C). The larvae were found to be at the final feeding stages. For further identification, a proportion of feeding mites was fixed in 70% ethyl alcohol. The skin samples with feeding larvae were extracted by punch-biopsy and fixed in a compound histological fixative mixture of 96% ethyl alcohol, 40% formalin and glacial acetic acid for 5 h. After fixation, the samples were kept in 96% ethyl alcohol. Skin samples of P. ognevi were also prepared for TEM examination (see below).
All applicable international, national, and institutional ethics statements when using animals in research have been followed. All procedures involving animals were in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 18 March 1986). The ethical approval was granted by the University of Tyumen Biomedical Ethic Committee (No. 14, 9 September 2024, Tyumen, Russia). M. davidii (as M. aurascens) and P. ognevi are listed in The IUCN Red List of Threatened Species (Benda, Paunović, 2016; Kruskop, Fukui, 2019) as Least Concern.
Identification of mites
Several larvae were mounted on microscope slides in Faure–Berlese medium and then examined under a Micromed-3 Professional microscope (Ningbo Sheng Heng Optics & Electronics, Gao Qiao, China) equipped with phase contrast and a ToupCam camera (ToupTek Photonics, Hangzhou, China). Measurements and other procedures were carried out in accordance with the methods described earlier (Shatrov et al. 2022). The mites were identified according to Kudryashova (1998) and Vercammen-Grandjean and Langston (1976). The examined specimens are deposited at the Department of Entomology, Faculty of Biology, Lomonosov Moscow State University (Moscow, Russia).
Histological examination
Small skin samples of P. ognevi and M. davidii with attached larvae were fixed in the field and then preserved in 96% ethyl alcohol (see above). In the laboratory, the skin samples were treated with celloidin and celloidin-paraffin and finally were embedded in paraffin. Paraffin blocks were serially sectioned on a sliding microtome at 5-7 µm in a perpendicular plane to the skin surface. The sections were stained with an azure II-eosin histological dye, which allows for differentiation of both basophilic and oxyphilic properties of different structures (Lillie 1969). The sections were then examined and photographed with (i) a Leica DM LS-2 light microscope (Leica Microsystems GmbH Wetzlar, Germany) combined with a MC-6.3 digital camera (LLC LOMO-Microsystems), (ii) a NEXCOPE NE910 light microscope (NANJING JIANGNAN NOVEL OPTICS CO., LTD., China) coupled with a MC-8.3 digital camera (LLC LOMO-Microsystems, St.-Petersburg, Russia, https://lomo-microsystems.ru ![]() ) also using a differential-interferential contrast (DIC), and (iii) a MIKMED-6 light microscope (LLC LOMO-Microsystems) combined with an MS-6.3 digital camera (LLC LOMO-Microsystems) using a phase-contrast method.
) also using a differential-interferential contrast (DIC), and (iii) a MIKMED-6 light microscope (LLC LOMO-Microsystems) combined with an MS-6.3 digital camera (LLC LOMO-Microsystems) using a phase-contrast method.
For investigation of the optical anisotropy of tissues, a plane-polarized emission was applied to sections with the help of a special device coupled with a MIKMED-6 light microscope and provided with the polarizer and the analyzer.
Scanning electron microscopy (SEM)
Both the alcohol-preserved larvae detached from their hosts and larvae still attached to the host skin (P. ognevi) were washed in a graded alcohol series and dried with an Autosamdri-815 critical point dryer (Tousimis Research Corporation, Rockville, Maryland, USA). After placing on the microscope stubs, the specimens were covered with a platinum layer in an Eiko IB-5 ion coater and examined with a SEM Quanta-250 (FEI Company) at 15 kV.
Transmission electron microscopy (TEM)
Only one host species – P. ognevi was used for TEM investigation. Skin samples with feeding larvae immediately after extraction were fixed in the field in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2–7.4) and preserved in the fixative solution for several months in low temperature, in the dark. Upon transportation to the laboratory, the samples were washed in several changes of 0.2 M cacodylate buffer for 1 hour, postfixed in 2% osmium tetroxide in 0.1 M cacodylate buffer for 6 h to overnight, dehydrated in an ethanol and acetone series, and finally embedded in an araldite mixture (Fluka) into blocks.
Serial ultra-thin sections from these blocks were made on a Leica UC-7 ultramicrotome (Leica Microsystems GmbH Wetzlar, Germany) using a diamond knife (Ultra 45°, Diatome Ltd, Switzerland). The sections were then mounted on copper grids with an oval hole provided with a formvar support and, after staining with uranyl-acetate and lead citrate, were examined and photographed with a TEM Morgagni 268-D (FEI Company) at 80 kV (digital visualization). Control semi-thin sections were made using the same ultramicrotome with glass knives, stained with toluidine blue and examined with a light microscope.
All light optical, SEM and TEM preparations obtained in the present study are deposited at the Zoological Institute of the Russian Academy of Sciences (St. Petersburg, Russia).
Results
SEM observation
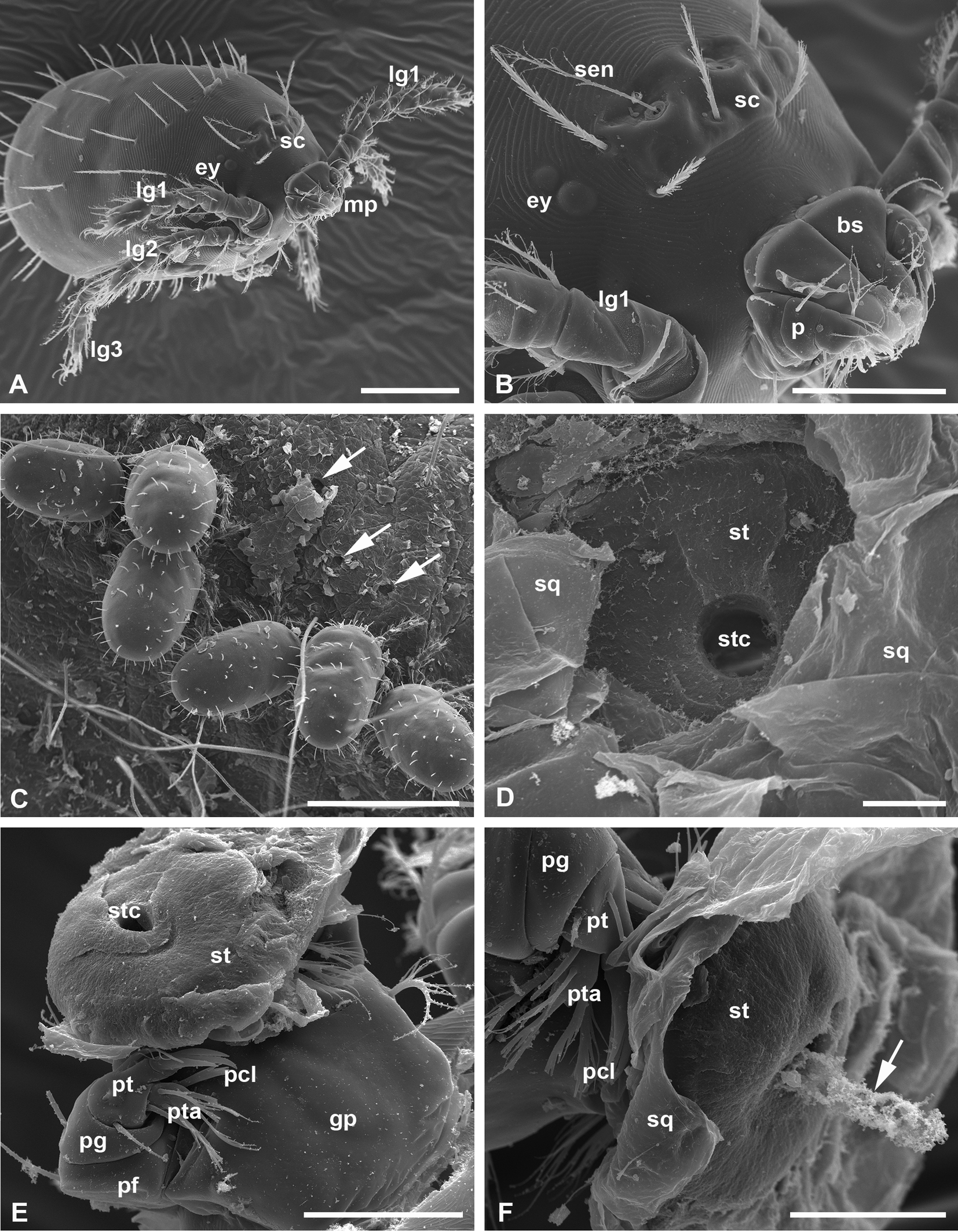


Both fully-fed, naturally detached larvae and larvae at the final feeding stages still attached, or otherwise artificially removed from the host skin were studied. Larvae in both conditions are barrel-shaped with straight cuticle folds on the body and around 500 µm long (Figure 2, A, C). They have a nearly rectangular prodorsal sclerite, or scutum, on the anterodorsal surface of the idiosoma (Lee Goff et al. 1982) bearing flagelliform sensillae (trichobotria) with relatively long lateral barbs on their distal half (Figure 2, B). The palpal claws with free prongs, which are characteristic for the Leptotrombidium genus (Lee Goff et al. 1982) always lie outside the host cuticle (Figure 2, B, E-F) only serving as a support for feeding larvae.
The larvae feed in tight groups (Figures 1, B-C, 2, C), and, after dropping off the host or becoming detached, they expose a feeding site in which the terminal stylostome portion leaves a round hole, the axial stylostome canal (Figure 2, D). However, this is apparently not the terminal part of the stylostome, but some part of its middle region, because it does not show prints of the chelicerae in the substance flanking the proximal opening of the stylostome canal. This supposition is additionally confirmed by the fact that the proximal part of the stylostome is surprisingly found still attached to the chelicerae and is removed from the host's skin along with them (Figure 2, E-F). This stylostome portion is around 50 µm in diameter, unstructured, and displays the round hole of the axial stylostome canal. It is clearly seen that this proximal stylostome portion is located under the upper squamae of the stratum corneum (Figure 2, F). It remains unknown under which circumstances the stylostome may be divided into two portions, and what these portions represent in the stylostome construction. It may be assumed, however, that efforts to detach the larva from the host can tear the stylostome in any zone, whereas larvae that detach from the host naturally do not carry any stylostome portions on the chelicerae (Figure 2, A-B). Interestingly, in one case, the presumptive content of the stylostome canal remains protruded from the stylostome portion still attached to the larval mouthparts (Figure 2, F). Meanwhile, this content does not seem to contain whole cells, but apparently only their fragments.
Histological observation
Plecotus ognevi
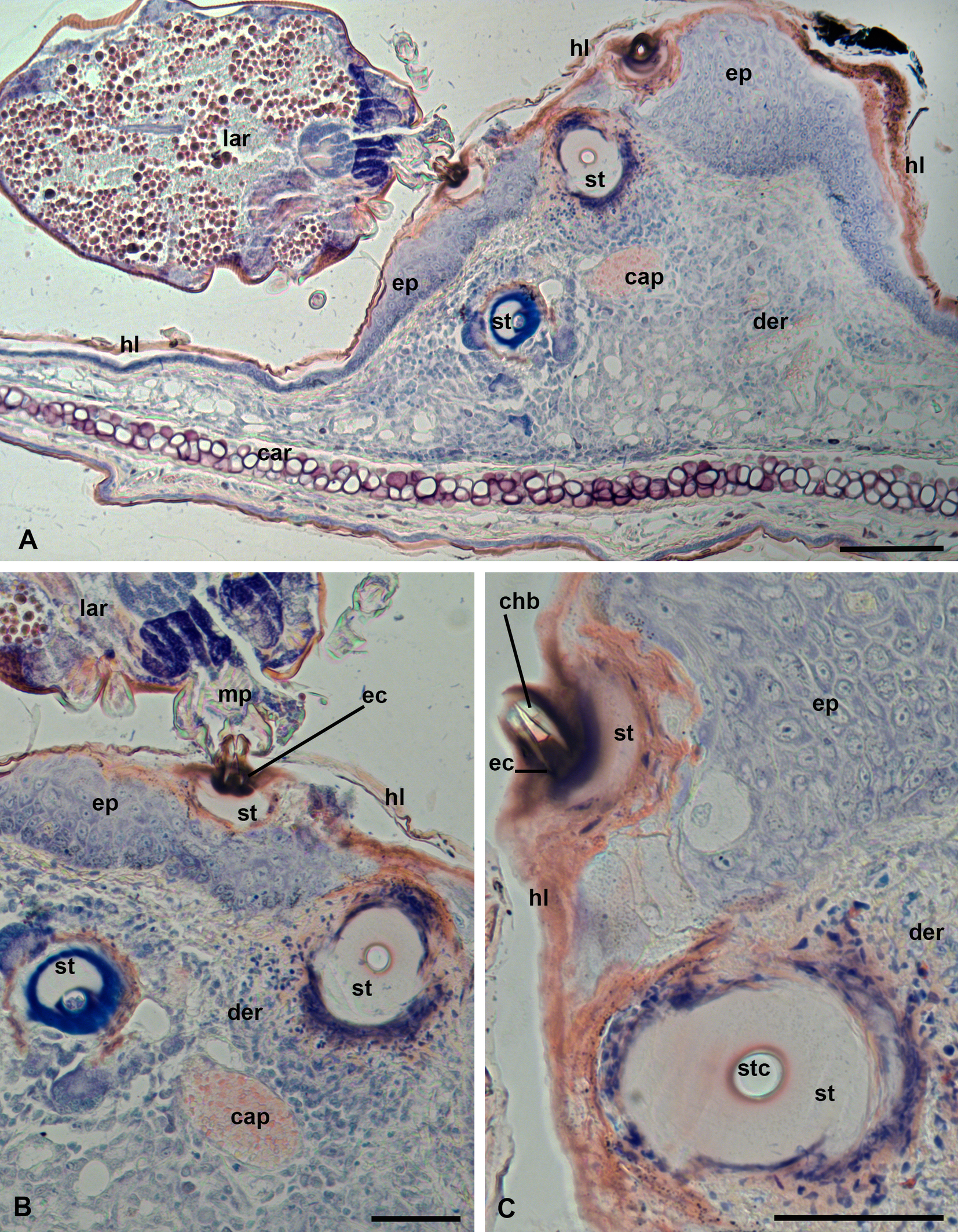


In the case of P. ognevi, larvae feed inside the ear (Figure 1, C). Normally, the epidermis in this location is thin, containing 2-3 cell layers and covered by a relatively thin horn layer (stratum corneum) (Figure 3, A). In the place where the larvae attach, the epidermis undergoes hyperplasia and becomes much thicker, up to 100-150 µm or more (Figure 3, B-C). Nevertheless, the developing stylostomes easily penetrate the epidermis opening by their distal ends to the connective tissue layer of dermis (Figures 3, B-C, 4, A, C). The developed stylostomes lie at different angles to the epidermal surface, which makes it impossible to observe the entire stylostome, even in sections perpendicular to the surface of the epidermis (Figures 3, A-C, 4, A-C). In any case, an eosinophilic zone with dark-red coloration, into which the cheliceral movable digits are immersed, represents the proximal and initial portion of the stylostome (Figure 3, B-C). This stylostome portion apparently corresponds to the eosinophilic cone – the proximal part of any stylostome studied so far (e.g. Shatrov 2009). Typically, the lateral sides of the eosinophilic cone penetrate under the upper squamae of the stratum corneum. Below the eosinophilic cone, the stylostome slightly widens and loses coloration (Figures 3, B-C, 4, A, C). In its distal portion, the stylostome become again narrower and acquires a dark-blue staining (Figures 3, B, 4, A-B). The stylostome canal is regularly round in near-transverse sections, may contain cell debris of the host and opens freely into the subjacent host tissues (Figures 3, B-C, 4, A-B). Erythrocytes were not observed in the stylostome canal. Externally, a thin layer of necrotic host cells surrounds the stylostome (Figures 3, C, 4, A, C).


Examination of sections using differential-interference contrast (DIC) shows that the hyaline-like substance of the stylostome exhibits a gel-like consistency due to crumpled traces of sections (Figure 4, C). In response to the parasite feeding, the host develops a moderate inflammatory reaction with edema of the connective tissue layer, where neutrophil leucocytes, macrophages and mast cells are present (Figure 4, D). Other lymphocyte types are poorly distinguished. The peripheral blood vessels are dilated (Figure 4, D). Beneath the developed stylostome, a particular tissue sinus may be observed, in which the connective tissue fibers are mostly dissolved and a certain number of destroyed and intact infiltrating cells may be present (Figure 4, A-C). However, this tissue sinus is much lesser expressed than in infestations with many other trombiculid larvae (e.g. Shatrov 2009).
Myotis davidii


In this host species, larvae fed on the outer surface of the ear (Figure 1, B), not far from the ear base. In this region, the epidermis is thicker than inside the ear and normally consists of 3-5 or more cell layers. Parasitism of larvae on this host species, however, causes a strong inflammatory reaction with abundant cellular and fluid exudate and complete destruction of the epidermis. Erythrocytes leaving the dilated peripheral blood vessels and the infiltrate cells – leukocytes and lymphocytes – fuse together into the various necrotic masses, which cover the skin (Figure 5, A-B) displacing the epidermis. Larvae at the final feeding stages tightly immerse into these masses in several rows (Figure 5, B). As a result, the larvae, in order to reach the dermis, secrete a stylostome, which is slightly longer, up to 200 µm, than that evolving during feeding on P. ognevi inside the ear (Figure 6, A-B). Nevertheless, there are no significant differences in the stylostome organization between these two host species.
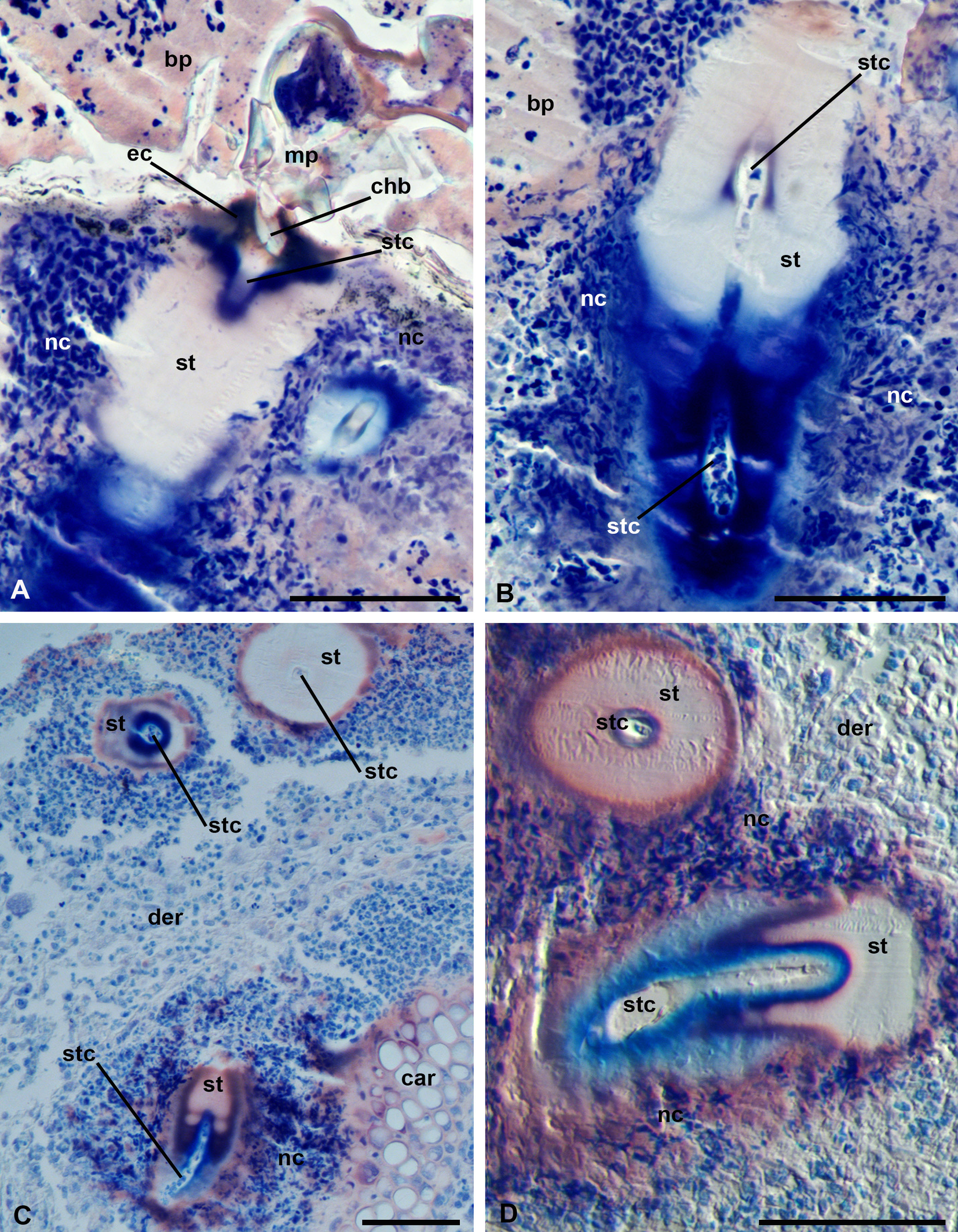


The dark-red eosinophilic cone, poorly expressed, represents the proximal portion of the stylostome (Figure 6, A). A white to pale-pink substance forms the widest and largest portion of the stylostome (Figures 5, A, 6, A-B). The distal, narrower portion of the stylostome is dark-blue (Figure 6, A-B, D). The axial stylostome canal is confined by a narrow rim, which is either completely uncolored or shows a reddish or bluish staining (Figures 5, A, 6, B, D). The axial canal is around 10 µm in diameter, similarly to the case of P. ognevi, it opens freely into the subjacent tissues (Figure 6, C-D) and may contain various cellular debris (Figure 6, B). As in the case of P. ognevi, the stylostomes penetrate into the skin at different angles that makes it impossible to observe the entire stylostome at once. Nevertheless, it may be assumed that the total length of the stylostome may reach 200 µm or more, whereas its maximum width is around 50-70 µm.
The outer borders of the stylostome are quite distinct and do not show an apparent transitional zone between the stylostome substance and the surrounding necrotic cell masses (Figure 6, A-B). Sometimes, however, a pink rim of a supposedly denser substance may be observed at the stylostome periphery (Figure 6, C-D). The evident tissue sinus underneath the stylostome is not observed due to a strong cellular infiltration of the inflammation area (see below).
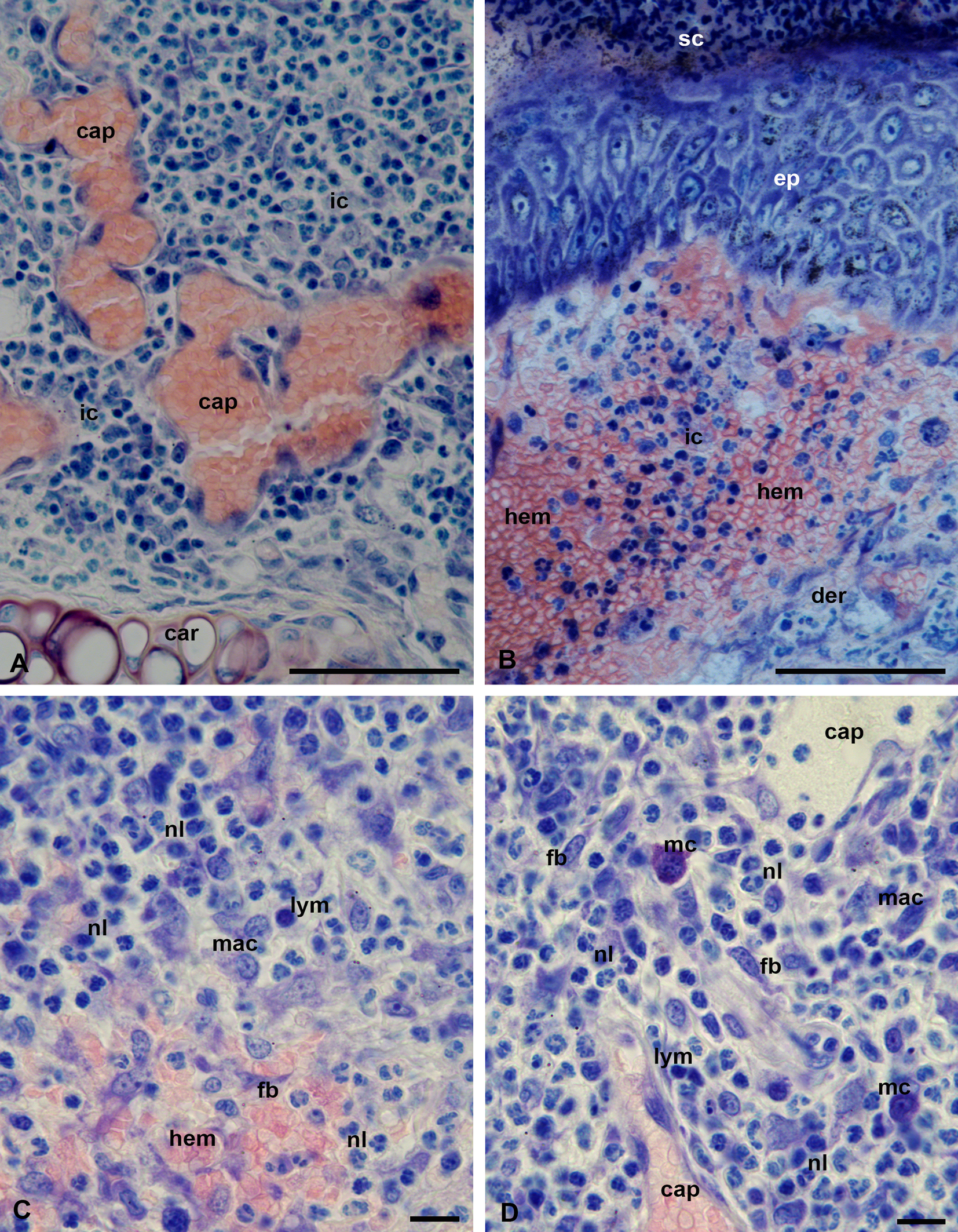


Feeding of L. myotis larvae on the bat M. davidii induces a strong inflammation with predominance of neutrophil leucocytes and mononuclear lymphocytes (macrophages) in the focus of the lesion (Figure 7, A-C). The inflammatory response is additionally characterized by a dilation of the peripheral capillaries (Figure 7, A) and strong hemorrhages spreading over a large area in the dermal connective tissue (Figure 7, B-C). Upon the release of both the necrotic cell masses and the liquid exudate onto the skin surface, they transform into a hardened, scab-like, coagulated mass composed of either tightly packed necrotic cells (Figure 7, B) or only blood plasma (Figures 5, B, 6, A). In contrast with P. ognevi, skin inflammation of M. davidii does not show the presence of numerous mast cells in the inflammation focus (Figure 7, D). Eosinophil and basophil leukocytes were not observed with certainty in either host species.
Observation of semi-thin sections of P. ognevi skin samples
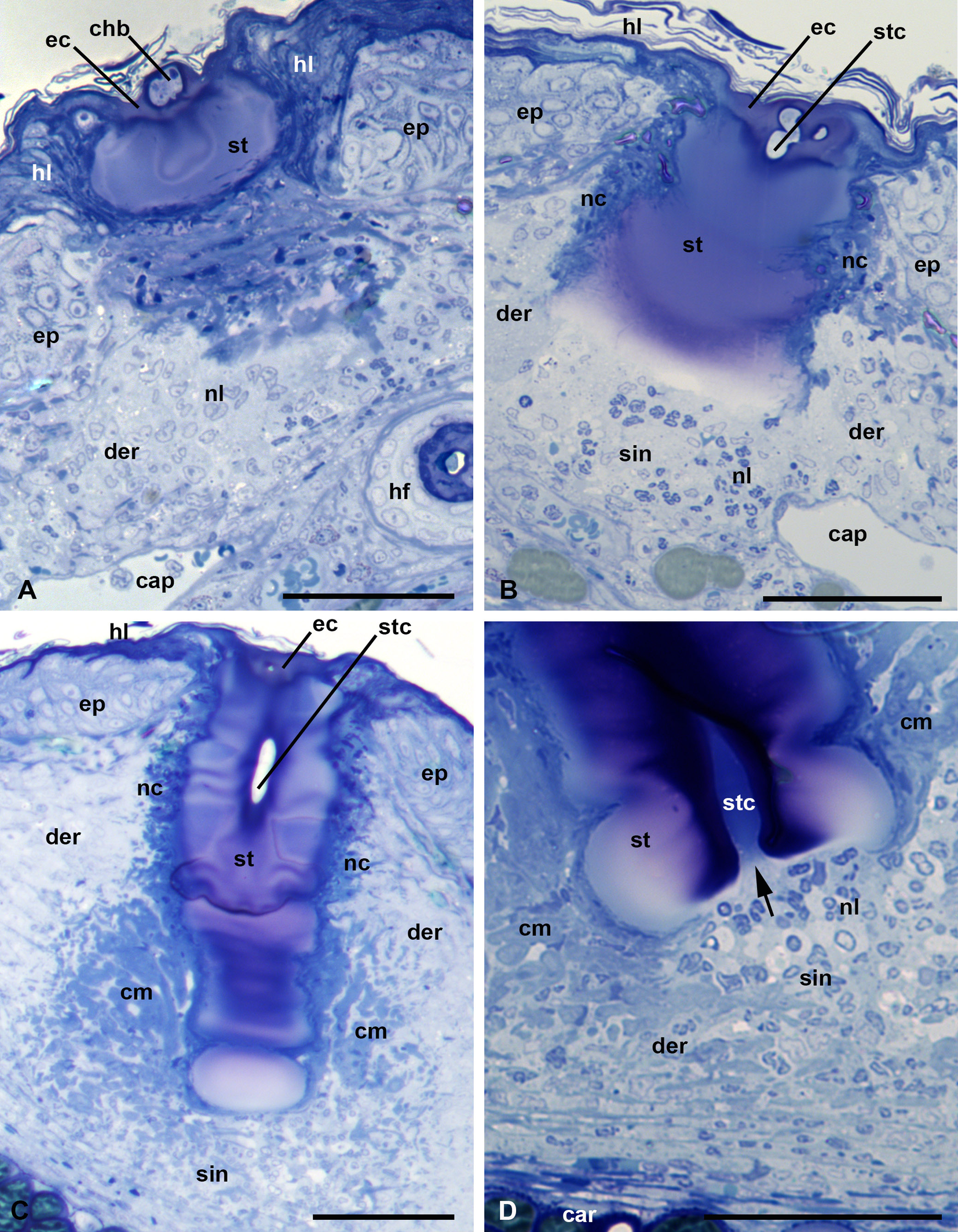


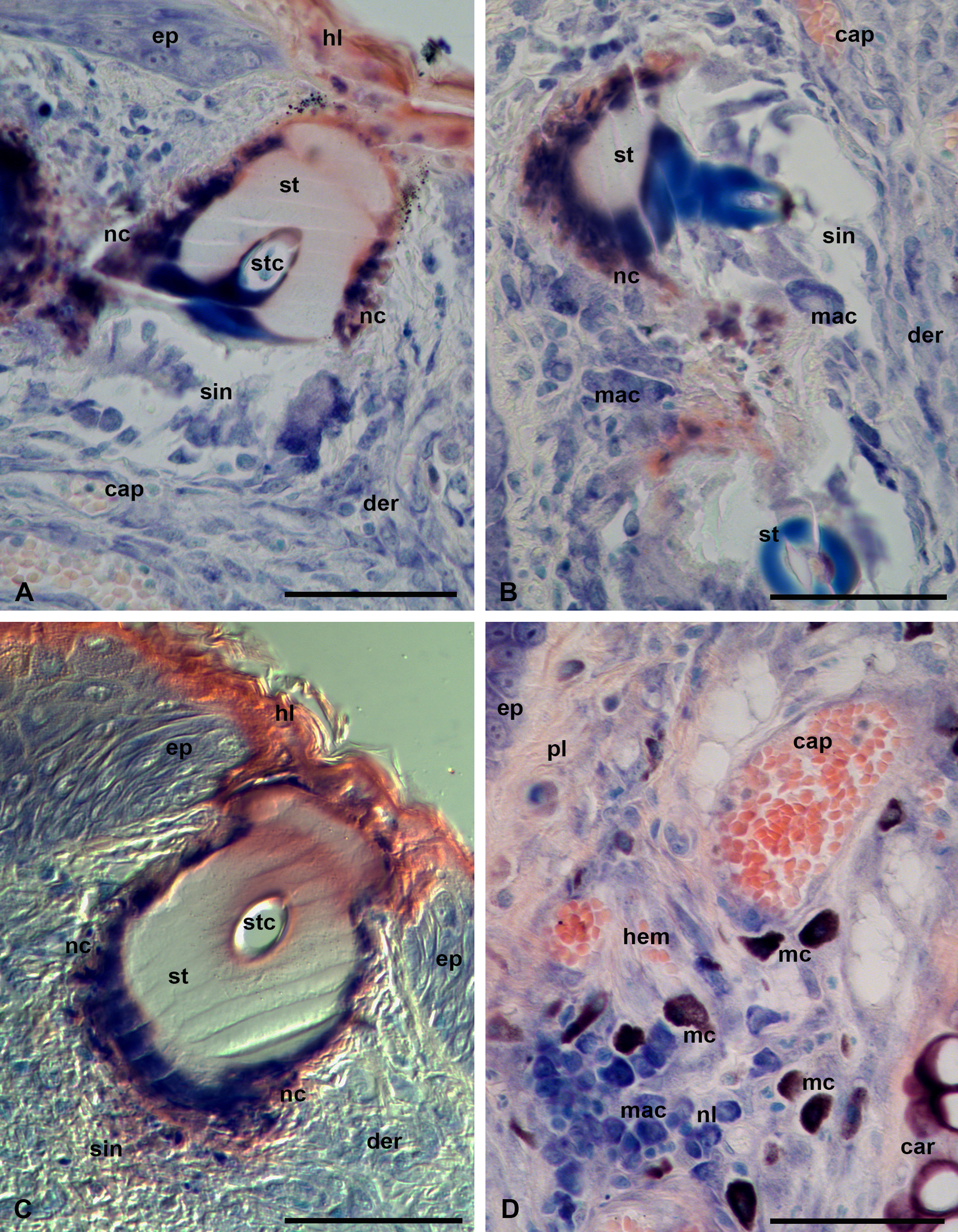
In semi-thin sections, the stylostome looks like an unstructured hyaline-like substance, probably with a gel-like consistency (Figure 8, A-D). Toluidine blue leaves the stylostome either unstained or variously stains it in light or dark blue without metachromasia. Differences in appearance of the dye in different stylostome portions does not reflect the structural characteristics of the stylostome but most likely only variations in density and conformation of its constituent molecules.
Prints of the cheliceral movable digits are constantly present in the most proximal stylostome portion, the eosinophilic cone (Figure 8, A-B). The eosinophilic cone, poorly expressed in this species, may differ from the remaining stylostome substance by a slightly deeper staining but is not structurally distinguishable. The lateral, narrowing parts of the eosinophilic cone penetrate under the upper squamae of the stratum corneum (horn layer) (Figure 8, A-B). The stylostome is mainly uniform, around 50-60 µm, in thickness, with a slightly narrowing distal part (Figure 8, C). Through its course, the stylostome does not show both strong local distensions of its walls and dilations of the axial canal, which indicate a relatively weak ejection force of the parasite salivary secretion into the wound. The narrower distal portion of the stylostome, which stains blue with azur-eosine in histological sections, is typically significantly lighter than the proximal and middle stylostome portions (Figure 8, B-C). At the same time, in the developed stylostomes, the region around the axial canal in the distal stylostome portion stains much stronger than the remaining stylostome substance (Figure 8, D). The axial stylostome canal opens freely into the subjacent host tissue containing some amount of infiltrating cells – lymphocytes and other leucocytes, as well as their debris (Figure 8, D).
The stylostome has quite distinct boundaries and is enveloped by a thin layer of degenerative host cells (Figure 8, B-C). The infiltrating host cells transform into a mesh of cytoplasmic bends around the distal stylostome portion (Figure 8, C-D). The epidermis, in addition to thickening (hyperplasia), does not undergo any other changes, and dissolves at the site of stylostome penetration. The extrusion of both the cell and liquid exudate to the skin surface and formation of a scab are not obviously seen during feeding of L. myotis larvae on the bat P. ognevi.
Observation of thin sections of P. ognevi skin samples
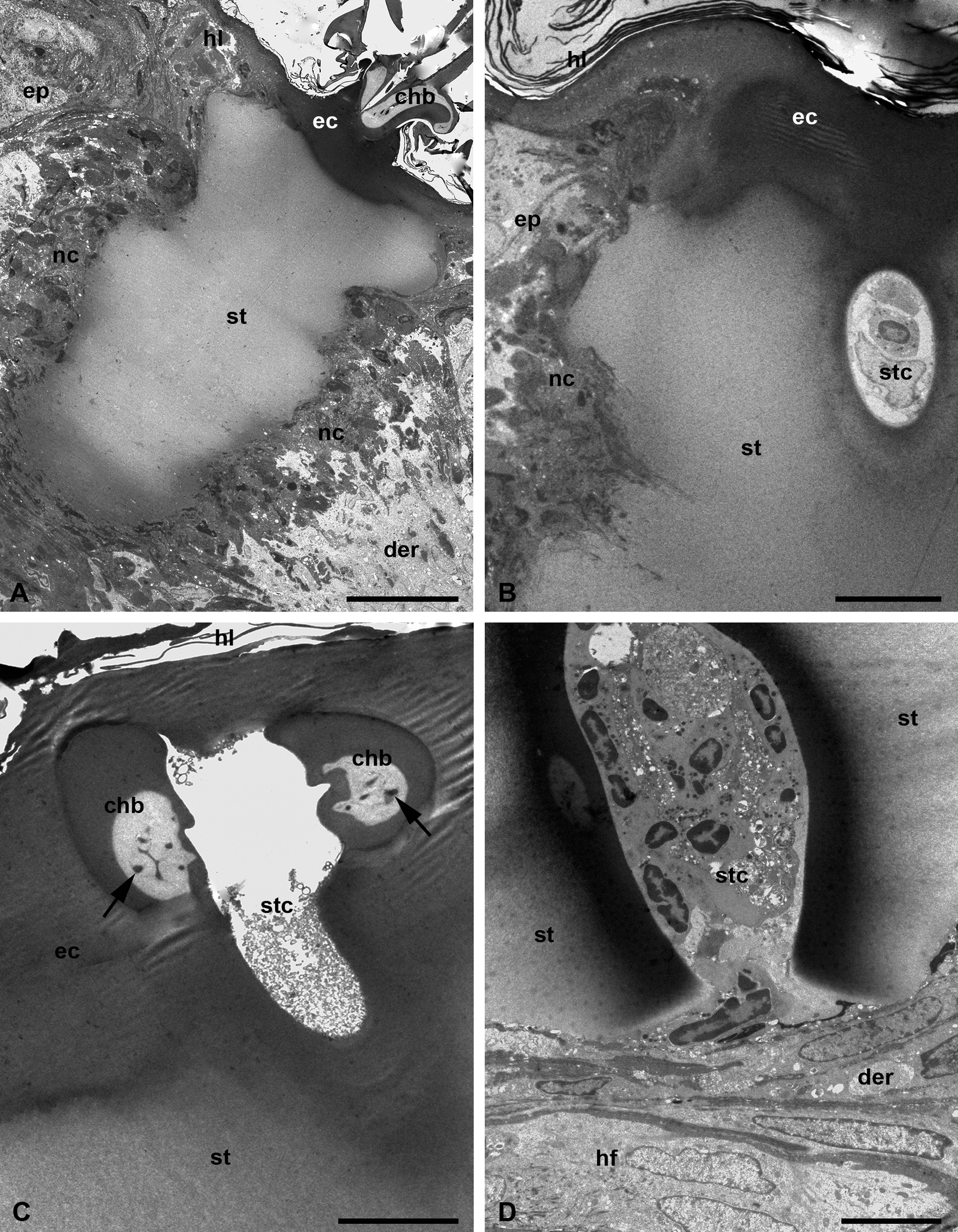


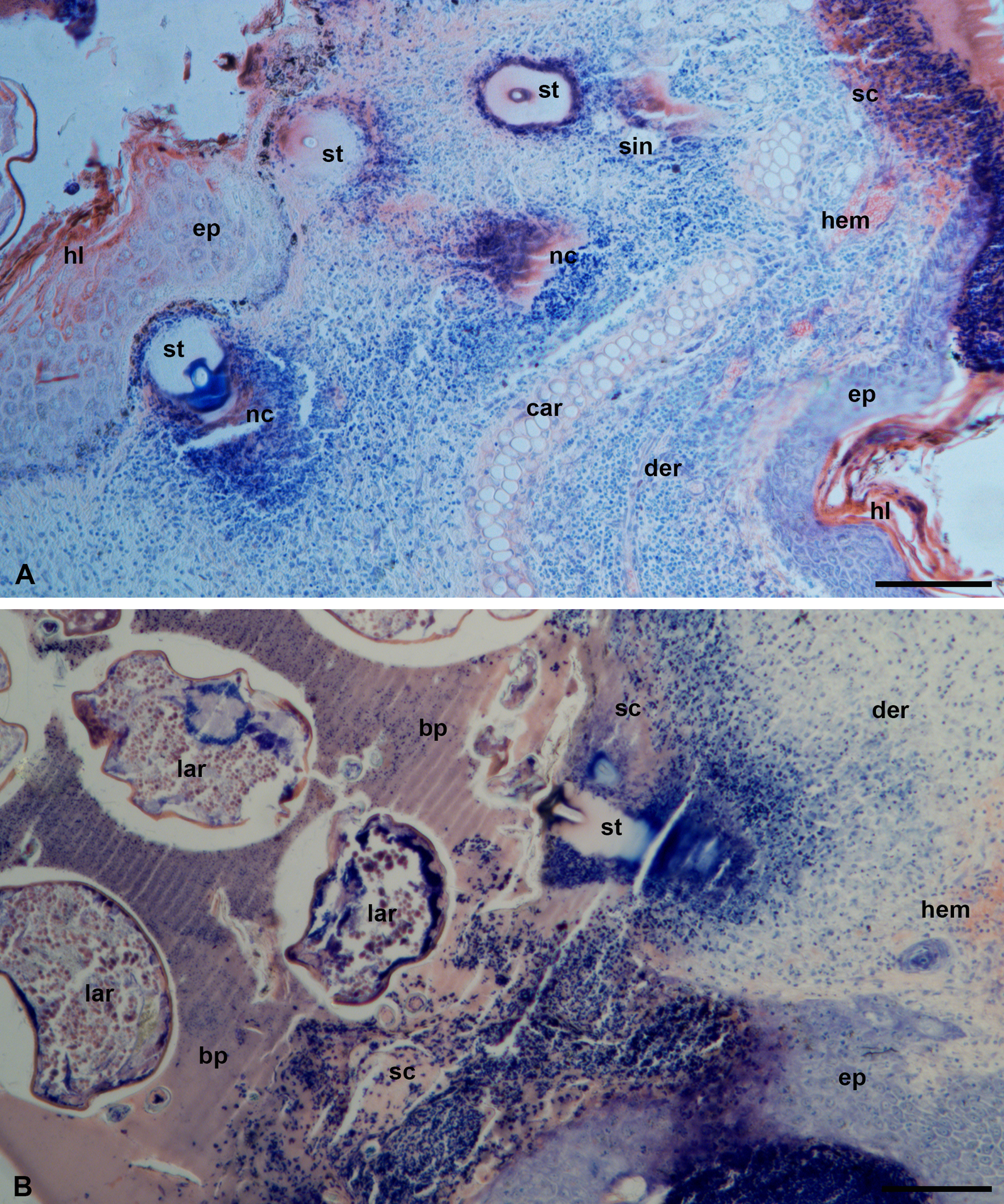
Fine morphological examinations show that the stylostome mostly consists of a homogeneous, unstructured, and unstained substance (Figure 9, A-B). The eosinophil cone and regions around the axial canal in the distal stylostome portions are more osmiophilic (Figures 9, A-B, D, 10, A), which corresponds to a deeper staining of these regions with toluidine blue in semi-thin sections and an intensive staining with azur in histological sections. The cheliceral movable digits are deeply immersed in the substance of the eosinophilic cone and are tightly cemented with it being moved apart by 7-10 µm (Figure 9, A, C). The cheliceral movable digits reveal indentations on their internal sides facing each other (Figure 9, C). They possess a denser outer wall and a clear internal matter containing dendrites possibly running along the digits to their axis (Figure 9, C).
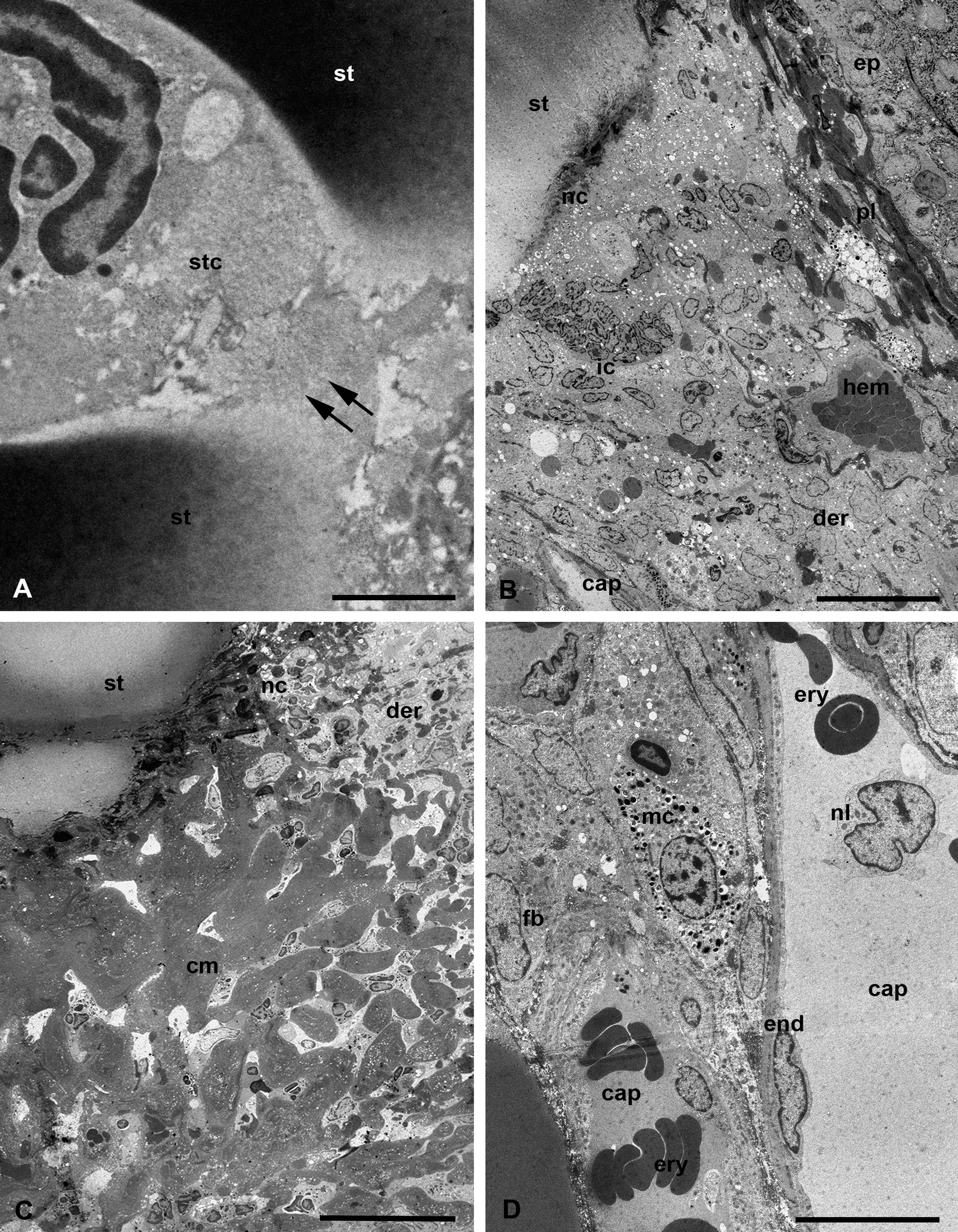


At the ultrastructural level, the stylostome substance possesses quite uneven but clearly outlined external boundaries to the host tissues, without any distinguishable peripheral layers (Figure 9, A-B). The stylostome also does not have any obvious internal layers bordering the axial canal (Figure 9, B-D). The axial stylostome canal, up to 10 µm width, surprisingly may contain nuclei and various debris of different host cells (Figures 9, B, D, 10, A). The putative feeding cavity with supposedly liquefied host tissues is not observed below the distal end of the stylostome (Figures. 9, D, 10, A). Yet despite the presence of the host cells within the space of the axial stylostome canal, feeding larvae never contain them in the midgut lumen (Figure 3, A) (see Discussion).
The surrounding host tissues undergo an inflammatory response of moderate intensity with migration of leucocytes and lymphocytes to the inflammatory focus and hemorrhages of erythrocytes (Figure 10, B), formation of reticular tissue built of fused lymphatic cells around the distal end of the stylostome (Figure 10, C), and dilation of the peripheral capillaries (Figure 10, D). These events are intended to isolate the stylostome and finally lead to its rejection after the larva has dropped off.
Discussion
Stylostomes
Although only one animal of each host species was examined, the present study has evidently shown that larvae of L. myotis feeding on different bat species from different geographical regions produce identical stylostomes but cause inflammatory reactions of distinct character and intensity. In both cases, however, larvae dissolve the host epidermis, and the resulting stylostome appears itself immersed into the host dermis that corresponds to the mesenchymal stylostome type (Hase et al. 1978). Being within the dermis, the stylostome does not immerse deep into the connective tissue layer as, for instance, in the case of Kepkatrombicula desaleri (Methlagl, 1928) feeding on chamois in Europe (Shatrov and Stekolnikov 2011). Conversely, the stylostome of L. myotis stops further development after achieving 180 µm in length on P. ognevi and 200 µm on M. davidii, while immersed into the host skin at different angles. Nevertheless, no substantial differences can be distinguished in the organization of the stylostomes themselves produced by L. myotis in these bat species. Below the moderately developed eosinophilic cone, the unstained and widest stylostome portion is expressed composed of an inert chromophobic hyaline substance, which is terminated by a narrower azurophilic region. The axial stylostome canal does not show local dilations and opens freely into the subjacent tissues.
Stylostomes of another trombiculid larvae, Leptotrombidium album (Kamo, Kawashima and Nishimura, 1957), feeding on the bat Barbastella pacifica (Kruskop, Kawai & Tiunov, 2019) on Kunashir Island was similar but not identical with the stylostome of L. myotis (Shatrov et al. 2022). In particular, the following differences may be noted. In the case of L. album, (i) a pink staining of the eosinophilic cone spreads inwards the chromophobic stylostome portion, (ii) a narrow strip of blue staining may border the axial stylostome canal, which may show alternate dilations and contractions, and (iii) the most important, a scab-like zone of necrotic tissue is typically formed around the developed stylostome immersed into the host tissue (Shatrov et al. 2022). The latter character was never seen in the case of L. myotis. At the same time, besides the eosinophilic cone, a distal blue staining is expressed in both cases. These findings indicate that in general similarities, the stylostomes in closely related species of trombiculids from the same genus feeding on hosts belonging to the same order, are clearly different. Additionally, the same trombiculid species feeding on different host species, as in the present study, produce stylostomes of identical organization. This seems to confirm the assumption that the stylostomes are species-specific with regard to a parasite (Hase et al. 1978; Shatrov 2009; Shatrov and Stekolnikov 2011; Shatrov et al. 2022). On the other hand, larvae of different Japanese Leptotrombidium species experimentally feeding on mice produced similar stylostomes (Shatrov et al. 2014), mostly of the epidermal type and intensively staining in red with Azan. Generally, therefore, the expression of the stylostome of the given trombiculid species may be rather different depending on both the host and the parasite species.
Stylostomes of other trombiculid larvae studied so far exhibit a considerable variation in size, extent of penetration into the host tissue as well as staining ability (Allred 1954; Aoki 1957; Voigt 1970; Hase et al. 1978; Hoeppli and Schumacher 1961, 1962; Schumacher and Hoeppli 1963; Shatrov 2009; Shatrov et al. 2014; Shatrov and Stekolnikov 2011). For example, the stylostome of Neotrombicula tianshana Shao et Wen, 1984 feeding on the voles Myodes rutilus (Pallas) is of the mixed or even mesenchymal type, comparatively long and thin, and stains mostly in blue with azur (Shatrov and Mirolubov 2015), whereas the stylostome of the related species N. talmiensis (Schluger, 1955) feeding on the voles Myodes rufocanus Sundevall is of the epidermal type, relatively short and nearly entirely pale-pink in azur-eosin staining (Shatrov and Antonovskaia 2021). In contrast, the stylostome of Neotrombicula (digenualea) pomeranzevi (Schluger, 1948) is of the epidermal type, more elongated than thick and shows a pink staining in the basal regions and blue staining in the distal region with azur-eosin (Shatrov 2000, 2009). The stylostome of Hirsutiella zachvatkini (Schluger, 1948), widely distributed in Eastern Europe, feeding on the vole Myodes rufocanus (Schreber) is of the epidermal type, rather short, and histologically shows poorly distinguished transverse layers from various red staining in the proximal portions to blue staining in the distal portion (Voigt 1970; Shatrov 2000, 2009; Shatrov and Felska 2017). Conversely, the stylostome of the closely related species H. hexasternalis (Kudryashova, 1998) feeding on the vole M. rutilus is of the epidermal type but thick, without any layers and entirely pale-pink or even unstained (Shatrov 2018). These data seem to readily indicate that the organization of the stylostome depends more on the parasite species and less on the host animal.
An important additional information on the stylostome organization may be obtained from observation of semi-thin and, especially, ultra-thin sections. Remarkably, irrespectively of the presence of the putative layers in the stylostome walls, as in the case of H. zachvatkini (Voigt 1970; Shatrov 2000, 2009), transmission electron microscopy shows that in the stylostomes studied so far, no obvious layers can be distinguished with confidence in the stylostome structure (Shatrov and Felska 2017). Different optical densities and varying osmiophilia in the proximal and distal parts of the stylostome may reflect different conformation of its constituent molecules, which remain mostly inert in relation to histological dyes. Generally, the entire stylostome reveals little affinity for both histological and electron-microscopical stains (Hoeppli and Schumacher, 1961, 1962; Hase et al. 1978; Shatrov and Felska 2017; Shatrov 2018; Shatrov and Antonovskaia 2021). It is important that electron microscopic sections do not reveal any internal layers limiting the axial canal of the stylostome (Shatrov and Felska 2017). In some cases, however, an internal rim bordering the axial canal may be observed (Voigt 1970; Shatrov and Mirolubov 2015; Shatrov et al. 2022), which stains blue with azur-eosin. It connects with the distal part of the stylostome also staining blue. It may be supposed that this rim is a trace of the new saliva portion injected into the wound and passing along the stylostome canal to its distal end, where it is hardened and gradually transformed from liquid into a gel-like conformation (Schumacher and Hoeppli 1963). This is most clearly demonstrated in the example of the stylostome of L. album feeding on the bat B. pacifica (Shatrov et al. 2022). Additionally, the axial stylostome canal in this species shows the alternative dilation and constriction that may point to a strong pulsation, in which the salivary secretion injects into the canal. Interestingly, in the reverse action, when the nutritional substrate supposedly passes through the canal to the larval mouthparts, the canal is narrowed and debris of inflammatory cells may be observed within its lumen (see below). In contrast, in the case of L. myotis, dilation of the stylostome canal was not clearly observed, indicating reduced force of the larval salivary-pharyngeal pump.
Previous authors did not focus attention on the content of the stylostome canal and, with rare exceptions, showed an empty canal (Aoki 1957; Schumacher and Hoeppli 1963; Voigt 1970; Hase et al. 1978). Larvae and the content of their midgut were also omitted. It is accepted, at the same time, that trombiculid larvae do not consume host cells as well as pure blood (Shatrov 2000, 2009). Indeed, it was shown that the midgut of feeding larvae, neither in the initial feeding stages nor in the final stage of the completely fed larva, contains any host cells, whether cells of inflammatory origin and their debris or erythrocytes (Shatrov 2000, 2009, Shatrov et al. 2022). Meanwhile, recent studies of the stylostome, as well as the present study, have shown that the stylostome canal may contain host cells, their debris (Shatrov 2018; Shatrov and Antonovskaia 2021) and even undamaged erythrocytes within the long stylostome of K. desaleri feeding on chamois (Shatrov and Stekolnikov 2011). This phenomenon, and the absence of any cells within the gut lumen of larvae, can only be explained by the presence of a certain'filter' at the entrance to the pharynx, which does not allow cells and their nuclei to enter there. An investigation of the larval mouthparts (Shatrov 2000) indicates that the pharynx does not contain any cells, which may accumulate in the preoral cavity and during the reverse action of ejection of saliva from the mouth apparatus, return back into the wound together with saliva.
The stylostome does not always have clearly delimited external boundaries, as in the case of L. myotis, and very often, it is difficult to distinguish between the stylostome itself and the surrounding necrotic tissue (e.g., Shatrov et al. 2014). For example, the stylostome of L. album feeding on the bat B. pacifica typically is surrounded by a layer of the tightly packed necrotic host cells, which can be hardly discriminated from the stylostome itself (Shatrov et al. 2022). Conversely, in the case of L. myotis, this layer of insulating necrotic cells is very thin, but below the stylostome a mesh structure of fused infiltrating cells develops, which is subsequently designed to eliminate the parasite from the host's skin. In polarized light, the stylostome of both L. album and L. myotis did not show visible birefringence, neither in the main unstained part, nor in the distal part of, supposedly, a new saliva portion of successive stages of saliva ejection (Jones 1952). Nevertheless, the stylostomes of some unidentified trombiculids show strong birefringence in polarized light, probably ''produced by a specific orientation of the molecules in these areas (textural birefringence)'' (Schumacher and Hoeppli 1963, p.201). There is no doubt, however, that periodical salivary inflows may create a certain conformational tension in the walls of the stylostome, which can cause polarization in certain conditions.
Skin reaction
Depending on the stylostome types – epidermal, mesenchymal or mixed (Hase et al. 1978), a skin inflammatory reaction of different character and intensity may develop. At the same time, there is no doubt that the type of the stylostome totally depends on the given trombiculid species and, therefore, the inflammatory response may generally be provoked by a parasite. On the other hand, the localization of the parasite on the host body with the certain character of the epidermal layer is an evolutionary predominant event. Therefore, the type of the skin reaction also largely depends on the nature of the host tissues at the site of attachment of the parasite as well as on the prior infestation experiences of the host individual and (or) the host species and their immune status (Wright et all. 1988).
Generally, two strategies may be distinguished in the stylostome formation and the host response to the parasite impact. The first one is the formation of the stylostomes outside the epidermis, the so-called extra-epidermal stylostomes; the second one is the formation of the stylostomes inside the epidermis and dermis, the so-called intra-epidermal stylostomes (Shatrov, 2000, 2009). In the first case, the stylostomes seem to be pushed away from the surface of the skin and, consequently, are formed in the scab progressively further from the integument itself. The epidermis may be remained nearly intact or, in contrast, destroyed, but the inflammatory cells migrate in mass to the damaging agent in both cases and finally form an enormous scab, in which the stylostomes are developed.
In the second case, the stylostomes directly penetrate the epidermis at the point of larval attachment, the epidermis is broken locally and the inflammatory reaction evolves directly within the dermis around the stylostome, provoking the formation of edema. Of course, there may be variations between these two different strategies. In any case, an area of destroyed host tissues together with, typically, strong inflammation is formed around the zone of stylostome penetration (Jones 1952; Aoki 1957; Hoeppli and Schumacher 1961, 1962; Schumacher and Hoeppli 1963, Voigt 1970; Hase et al. 1978). The area closest to the stylostome where the transformed epidermal cells are tightly mixed together with the migrating inflammatory cells into the scab-like mass, reveals high enzymatic activity (Schumacher and Hoeppli 1963). It is considered, at the same time, that the larval salivary secretion contains a little quantity of the hydrolytic ferments and ''the skin give no evidence of a significant histolytic action of the saliva injected'' (Schumacher and Hoeppli 1963, p. 203).
The present investigation has shown that feeding of L. myotis larvae on the two different bat species causes significantly different specific skin inflammatory reactions. Larvae feeding inside the ear of P. ognevi in Buryatia provoke a rather moderate inflammatory response, in which, besides neutrophil leukocytes, mast cells are noticeable. In contrast, larvae feeding on the outer surface of the ear of M. davidii in Kazakhstan cause a strong inflammatory reaction with predominance of neutrophil leukocytes but with absence of eosinophil leukocytes and a small amount of mast cells. It is obvious that this difference is a result of the specific reaction of the host to the parasite that, in turn, may be a consequence of different level of the host resistance and sensitization (Audy 1951). The presence of mast cells in the inflammatory focus in P. ognevi with possible release of histamines and other mediators (Theoharides et al. 2012), and a low number of other inflammatory cells probably indicate the following. First, the immune system is at the initial step of sensitization and only prepared for the intensive reaction and, second, the stylostome substance is sufficiently immunologically inert not to provoke an immediate strong specific inflammatory response in this host species. This leads to a conclusion that the bat P. ognevi in Buryatia may be considered a substantially ''new'' host for trombiculid larvae L. myotis that somewhat follows an assumption of Audy (1951). Probably, this bat species has underwent infestation by chiggers for a relatively short period not to evolve a strong cutaneous reaction (see also Wright et al. 1988). Conversely, the bat M. davidii from Kazakhstan, in response to larval feeding and stylostome formation, develops a strong inflammatory reaction that indicates a long-term of host-parasite association and, as a result, a high level of the host sensitization (Wright et al. 1988; Wrenn 1996). The fact that only one individual was found to be infected with trombiculids suggests that the relationship with parasites in population of this species may be more complex than we think. The same occurs during the long-term feeding of ixodid ticks when their'cement cone' provokes the development of a strong specific immune reaction (Balashov 1992; Bishop et al. 2002). Nevertheless, our finding may lead to an assumption that the bat M. davidii in Kazakhstan may be treated as evolutionarily ''old'' hosts for the trombiculid L. myotis and develop a strong resistance to the parasite (Wright et al. 1988; Wrenn 1996). Whether this leads to a shorter feeding time (see Wright et al. 1988), remains unknown. Although we were limited in our study by one individual of each host species that restricted a possibility to analyze intraspecific variations of skin reaction, the observed differences between the two host species are evident. Obviously, this is a consequence of different infestation level by the parasites in these two bat species regardless of whether it lasted long in evolution or not.
Feeding of the related trombiculid species L. album on the bat B. pacifica from Kunashir Island induces a strong inflammatory response different from that of both P. ognevi and M. davidii (Shatrov et al. 2022). In the inflammatory reaction of B. pacifica, numerous mast cells, together with neutrophils and macrophages, as well as intensive hemorrhages of erythrocytes accompanied by dilation of the peripheral capillaries were recognized. Besides, the stylostome may be covered by the envelope of necrotic tissue. Therefore, if the stylostome structure itself largely depends on the trombiculid species, the skin inflammatory response is obviously mostly determined by the host animal, its immune status in relation to the stylostome and chiefly, by the duration of the interaction between the parasite and the host that all lead to a highly specific inflammatory reaction.
Conclusion
Trombiculid larvae in their feeding on a wide range of vertebrate animals evolve different types of stylostomes variably penetrating into the host tissue and induce skin reactions of significantly different types and degree. This partly contradicts with the assumption of Hoeppli and Schumacher (1962) who considered that ''The skin reaction observed in the various host species were rather similar in type but very different in degree'' (p. 427). In any case, however, the intensity of reaction is related mostly to the duration of infection (Hoeppli and Schumacher 1962), and it does not matter whether this happens at the species or organism level. The latter, in turn, may define the possibility of trombiculid larvae to serve as specific transmitting agents of various pathogens because the intensity of reaction and the presence of different cell types, including macrophages, in the focus is thought to be a measure of such a possibility (Boese 1972; Traub et al. 1975). In our case, L. myotis larvae parasitizing M. davidii in Kazakhstan may be considered a candidate for such transmission.
Acknowledgements
This study was supported by the State Government Research Programs # 125013001089-0 (A.B.Sh.) and the Ministry of Science and Higher Education of the Russian Federation (agreement № 075-15-2024-563) (D.V.K.). The TEM and SEM procedures were completed using the Core Facilities Centre'Taxon' at the Zoological Institute of the Russian Academy of Sciences (St. Petersburg, Russia, https://www.ckp-rf.ru/ckp/3038/ ![]() ). We also would like to thank Oleg N. Morozov (Center of Children's Complementary Education and Evenkis' Folk Crafts, Bagdarin, Russia), Nikolai V. Yakovchic (Irkutsk, Russia) and ''Altyn-Emel'' State National Nature Park (Almaty, Kazakhstan) for their help in mounting the expeditions.
). We also would like to thank Oleg N. Morozov (Center of Children's Complementary Education and Evenkis' Folk Crafts, Bagdarin, Russia), Nikolai V. Yakovchic (Irkutsk, Russia) and ''Altyn-Emel'' State National Nature Park (Almaty, Kazakhstan) for their help in mounting the expeditions.
References
- Allred D.M. 1954. Observations on the stylostome (feeding tube) of some Utah chiggers. Utah Acad. Sci., Arts and Letters, Proc., 31: 61-63.
- Aoki T. 1957. Histological studies on the so-called stylostome or hypopharynx in the tissues of the host parasitized by the trombiculid mites. Acta Med. Biol., 5: 103-120.
- Audy J.R. 1951. Trombiculid mites and scrub itch. Austr. J. Sci., 14: 94-96.
- Balashov Yu.S. 1992. The features of the parasitic system ixodid tick-vertebrate animal. Parasitologiya, 26: 185-197. [In Russian]
- Bazhenov Yu.A. 2021. Ecology of bat species in the arid region of the Daurian steppe at the peak of drought. Nat. Con. Res., 6: 42-49. https://doi.org/10.24189/ncr.2021.007
- Benda P., Tsytsulina K.A. 2000. Taxonomic revision of Myotis mystacinus group (Mammalia: Chiroptera) in the western Palearctic. Acta Soc. Zool. Bohem., 64: 331-398.
- Benda P., Faizolâhi K., Andreas M., Obuch J., Reiter A., Ševčík M., Uhrin M., Vallo P., Ashrafi S. 2012. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 10. Bat fauna of Iran. Acta Soc. Zool. Bohem., 76: 163-582.
- Benda P., Paunović M. 2016. Myotis aurascens. The IUCN Red List of Threatened Species 2016: e.T136553A21993953 [Internet]. [25 April 2016]. International Union for Conservation of Nature and Natural Resources; [27 September 2024]. https://doi.org/10.2305/IUCN.UK.2016-2.RLTS.T136553A21993953.en
- Bishop R., Lambson, B., Wells C., Pandit P., Osaso J., Nkonge C., Morzaria S., Musoke A., Nene V. 2002. A cement protein of the tick Rhipicephalus appendiculatus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody responses in cattle. Int. J. Parasitol., 32: 833-842. https://doi.org/10.1016/S0020-7519(02)00027-9
- Boese J.L. 1972. Tissue reactions at the site of attachment of chiggers. J. Med. Entomol., 9: 591.
- Çoraman E., Dundarova H., Dietz C., Mayer F. 2020. Patterns of mtDNA introgression suggest population replacement in Palaearctic whiskered bat species. R. Soc. Open Sci., 7: e191805. https://doi.org/10.1098/rsos.191805
- Dzhanokmen K.A. 1969. Materials on chigger mites (Family Trombiculidae) of Kazakhstan [Materialy po kleshcham-krasnotelkam (sem. Trombiculidae) Kazahstana]. Prirodnoochagovye Bolezni i Voprosy Parazitologii v Srednej Azii i Kazahstane, 5: 219-221. [in Russian]
- Ewing H.E. 1944. The trombiculid mites (chigger mites) and their relation to disease. J. Parasitol., 30: 339-365.
- Hase T., Roberts L.W., Hildebrandt P.K., Cavanaugh D.C. 1978. Stylostome formation by Leptotrombidium mites (Acarina: Trombiculidae). J. Parasitol., 64: 712-718. https://doi.org/10.2307/3279967
- Hoeppli R., Schumacher H.H. 1961. Histological and histochemical reactions to trombiculid mites. Ann. Rept. Res. Act. Liber. Inst.: 54-58.
- Hoeppli R., Schumacher H.H. 1962. Histological reactions to trombiculid mites, with special reference to ''natural'' and ''unnatural'' hosts. Z. Trop. Parasitol., 13: 419-428.
- Hyland K.E. 1961. Parasitic phase of the chigger mite, Hannemania hegeneri on experimentally infested amphibians. Exp. Parasitol., 11: 212-225. https://doi.org/10.1016/0014-4894(61)90027-3
- Hyland K.E., Wharton G.W. 1955. Penetration of host tissue and host reaction to chigger mites of the genus Hannemania (Acarina; Trombiculidae). J. Parasitol., 41: 2-37. https://doi.org/10.2307/3274733
- Jones B.M. 1950. The penetration of the host tissue by the harvest mite, Trombicula autumnalis Shaw. Parasitology, 40: 247-260. https://doi.org/10.1017/S0031182000018096
- Kazakov D.V., Shumkina A.P., Botvinkin A.D., Morozov O.N. 2018. Bat swarming in the eastern Palaearctic (Eastern Siberia). Acta Chiropt., 20: 427-438. https://doi.org/10.3161/15081109ACC2018.20.2.013
- Kazakov D.V., Khasnatinov M.A., Antonovskaia A.A., Gorobeyko U.V. 2024. Bat ectoparasites: chigger mites (Trombiculidae), ticks (Ixodidae and Argasidae), and bugs (Cimicidae) in the Eastern Palaearctic. Parasitol. Res., 123: 1-17. https://doi.org/10.1007/s00436-023-08093-x
- Kruskop S.V., Borisenko A.V., Ivanova N.V., Lim B.K., Eger J.L. 2012. Genetic diversity of northeastern Palaearctic bats as revealed by DNA barcodes. Acta Chiropt., 14:1-14. https://doi.org/10.3161/150811012X654222
- Kruskop S.V., Fukui D. 2019. Plecotus ognevi. The IUCN Red List of Threatened Species 2019: e.T136598A21996784 [Internet]. [08 August 2018]. International Union for Conservation of Nature and Natural Resources; [27 September 2024]. https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T136598A21996784.en
- Kudryashova N.I. 1998. Chigger mites (Acariformes, Trombiculidae) of East Palearctics. Moscow: KMK Scientific Press,. pp. 342. [in Russian]
- Lee Goff M., Loomis R.B., Welbourn W.C., Wrenn W.J. 1982. A glossary of chigger terminology (Acari: Trombiculidae). J. Med. Entomol., 19: 221-238. https://doi.org/10.1093/jmedent/19.3.221
- Lillie R.D. 1969. Histopathological technic and practical histohemistry. Moscow: ''Mir'' Publishing House. pp. 639. [Translation to Russian]
- Olson D.M., Dinerstein E., Wikramanayake E.D., Burgess N.D., Powell G.V.N., Underwood E.C., D'Amico J.A., Itoua I., Strand H.E., Morrison J.C., Loucks C.J., Allnutt T.F., Ricketts T.H., Kura Y., Lamoreux J.F., Wettengel W.W., Hedao P., Kassem K.R. 2001. Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience, 51: 933-938. https://doi.org/10.1641/0006-3568(2001)051%5B0933:TEOTWA%5D2.0.CO;2
- Poissant J.A., Broders H.G. 2008. Ectoparasite Prevalence in Myotis lucifugus and M. Septentrionalis (Chiroptera: Vespertilionidae) During Fall Migration at Hayes Cave, Nova Scotia. Northeast. Natural., 15: 515-522. 10.1656/1092-6194-15.4.515. https://doi.org/10.1656/1092-6194-15.4.515
- Rosina V.V., Kirilyuk V.E. 2000. Findings of bats in the Daursky Reserve and in the nearby territories. Plecotus et al., 3: 108-113. [in Russian]
- Schramlová J. 1978. Skin lesion produced by the larva of Cheladonta costulata (Willmann, 1952) (Acarina: Trombiculidae) and the feeding mechanism of the parasite. Folia Parasitol., 25: 261-270.
- Schumacher H.H., Hoeppli R. 1963. Histochemical reactions to trombiculid mites, with special reference to the structure and function of the ''stylostome''. Z. Trop. Parasitol., 14: 192-208.
- Shatrov A.B. 2000. Trombiculid mites and their parasitism on vertebrate hosts. St.-Petersburg: St.-Petersburg University Publishers. pp. 276 [In Russian with English summary].
- Shatrov A.B. 2009. Stylostome formation in trombiculid mites (Acariformes: Trombiculidae). Exp. Appl. Acarol., 49: 261-280. https://doi.org/10.1007/s10493-009-9264-0
- Shatrov A.B. 2018. On the life cycle and parasitism of the trombiculid mite Hirsutiella hexasternalis (Kudryashova, 1998) (Acariformes, Trombiculidae). Soil Organisms, 90: 157-170.
- Shatrov A.B., Kazakov D.V., Antonovskaia A.A., Gorobeyko U.V. 2022. Morphological characterization of stylostome and skin reaction produced by Leptotrombidium album (Acariformes, Trombiculidae) on the bat Barbastella pacifica from Kunashir Island. Syst. Appl. Acarol., 27: 1970-1990. https://doi.org/10.11158/saa.27.10.9
- Shatrov A.B., Antonovskaia A.A. 2021. Stylostome of the trombiculid mite larvae Neotrombicula talmiensis (Schluger, 1955) (Acariformes, Trombiculidae) feeding on two host species in the Russian Far East. Acarologia, 61: 412-431. https://doi.org/10.24349/acarologia/20214442
- Shatrov A.B., Felska M. 2017. Comparative stylostome ultrastructure of Hirsutiella zachvatkini (Trombiculidae) and Trombidium holosericeum (Trombidiidae) larvae. Exp. Appl. Acarol., 72: 339-365. https://doi.org/10.1007/s10493-017-0172-4
- Shatrov A.B., Mirolubov A.A. 2015. Stylostome and feeding of the trombiculid larva Neotrombicula tianshana Shao et Wen, 1984 (Acariformes: Parasitengona) from the Baikal region. Int. J. Acarol., 41: 537-550. https://doi.org/10.1080/01647954.2015.1079238
- Shatrov A.B., Stekolnikov A.A. 2011. Redescription of a human-infesting European trombiculid mite Kepkatrombicula desaleri (Acari: Trombiculidae) with data on its mouthparts and stylostome. Int. J. Acarol., 37: 176-193. https://doi.org/10.1080/01647954.2010.548342
- Shatrov A.B., Takahashi M., Noda S., Misumi H. 2014. Stylostome organization in feeding Leptotrombidium larvae (Acariformes: Trombiculidae). Exp. Appl. Acarol., 64: 33-47. 10. https://doi.org/10.1007/s10493-014-9809-8
- Sherbin U.V. 1968. Materials about distribution of some species of bats in Tajikistan. Izv. AS Tajik SSR, Branch Biol. Sci., 3: 62-68. [in Russian]
- Spitzenberger F., Strelkov P., Winkler H., Haring E. 2006. A preliminary revision of the genus Plecotus (Chiroptera, Vespertilionidae) based on genetic and morphological results. Zool. Scr., 35: 187-230. https://doi.org/10.1111/j.1463-6409.2006.00224.x
- Theoharides T.C., Alysandratos K-D., Angelidou A., Delivanis D-A., Sismanopoulos N. et al. 2012. Mast cells and inflammation. Biochem. Biophys. Acta, 1822: 21-33. https://doi.org/10.1016/j.bbadis.2010.12.014
- Tiunov M.P. 1997. Bats of the Russian Far East. Vladivostok: Dal′nauka. pp. 134. [in Russian]
- Traub R., Wisseman C.L. 1974. The ecology of chigger-borne rickettsiosis (scrub-typhus). J. Med. Ent., 11: 237-303. https://doi.org/10.1093/jmedent/11.3.237
- Traub R., Wisseman C.L., Jones M.R., O'Keefe J.J. 1975. The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann. N.Y.Acad. Sci., 266: 91-114. https://doi.org/10.1111/j.1749-6632.1975.tb35091.x
- Trouessart E.L. 1899. Sur la piqûre du Rouget. Arch. Parasit., 2: 286-290.
- Tsytsulina K., Dick M.H., Maeda K., Masuda R. 2012. Systematics and phylogeography of the steppe whiskered bat Myotis aurascens Kuzyakin, 1935 (Chiroptera, Vespertilionidae). Russ. J. Theriol., 11: 1-20.
- Valdez E.W., Ritzi C.M., Whitaker J.O. 2009. Ectoparasites of the Occult Bat, Myotis occultus (Chiroptera: Vespertilionidae). West. North Am. Nat., 69: 364-370. https://doi.org/10.3398/064.069.0310
- Vercammen-Grandjean P.H., Langston R.L. 1976. The chigger mites of the world (Acarina: Trombiculidae et Leeuwenhoekiidae). III. Leptotrombidium complex. San Francisco: George Williams Hooper Foundation, University of California. pp. 1061.
- Wen T.H. (ed). 1984. Sand mites of China (Acariformes: Trombiculidae et Leeuwenhoekiidae). Shanghai: Xue Lin Publ. House. pp. 370. [in Chinese]
- Voigt B. 1970. Histologische Untersuchungen am Stylostom der Trombiculidae (Acari). Z. Parasitenk., 34: 180-197. https://doi.org/10.1007/BF00259695
- Wrenn W. J. 1996. Immune responses to mange mites and chiggers. In: Wikel S. (Ed). The Immunology of Host-Ectoparasitic Arthropod Relationships. Wallingford, UK: CAB International. p. 259-289.
- Wright S.M., Wikel S.K., Wrenn W.J. 1988. Host immune responsiveness to the chigger, Eutrombicula cinnabaris. Ann. Trop. Med. Parasitol., 82: 283-293. https://doi.org/10.1080/00034983.1988.11812245



2024-10-02
Date accepted:
2025-03-03
Date published:
2025-03-24
Edited by:
Roy, Lise

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Shatrov, Andrey B.; Kazakov, Denis V. and Antonovskaia, Anastasia A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



