Sixty years after "A review of the genera of the family Tydeidae" (Acariformes: Tydeoidea: Tydeidae)
André, Henri M.  1
1
1✉ Retired, currently, 22, route de la Côte, CH-1615 Bossonnens, Switzerland & Musée royal de l'Afrique centrale, B-3080 Tervuren, Belgium & Université Catholique de Louvain, B-1348 Louvain-la-Neuve, Belgium.
2025 - Volume: 65 Issue: 1 pages: 149-172
https://doi.org/10.24349/l5vb-767lZooBank LSID: F3176DAB-5A34-4299-8730-8174F468233C
Original research
Keywords
Abstract
述而不作 信而好古
I transmit but do not innovate. Confucius, Analects 7: 1.
Text and translation by Slingerland (2003).
Introduction
Tydeidae was erected by Kramer (1877: 224) under the name Tydidae, a name not congruent with the guidelines of the International Code of Zoological Nomenclature (hereafter referred to as ICZN, or the Code) as a subfamily of Prostigmata proposed to accommodate the single genus Tydeus erected by Koch (1836). Previously, the genus Tydeus was placed in Eupodidae (Koch, 1850: 70). Later, Tydeus was still associated with eupodid mites by Canestrini (1892: 575) and Berlese (1925: 94). A first monograph treating the Tydeidae as a family including 14 genera, 4 subgenera, 69 determined and 13 undetermined species was provided by Thor (1933: 12) who placed them in Eupodoidea (together with Ereynetidae). Baker, in his seminal paper on the genera of the family Tydeidae, kept the sense used by Thor (Thorian sense), determined 15 genera and admitted that the family was ''difficult to characterize'' (Baker, 1965: 96). He divided the tydeids ''into two groups, those with [seta] L[Lateral]2 in the lateral position, and those with L2 in line or nearly in line with the D[orsal] setae'' (Baker, 1965: 98).
André (1979) divided the tydeids into seven subfamilies but kept the traditional Thorian sense. Subsequently and after numerous articles on species identifications, Kaźmierski (1996a, 1998a) presented the first approaches based on a phylogenetic analysis of Tydeinae and Pretydeinae.
André and Fain (2000) attributed a new meaning to the family they included in the superfamily Tydeoidea. This approach is adopted here. The superfamily included the Ereynetidae, Iolinidae, Tydeidae and Triophtydeidae. This classification with 4 families is also adopted. A diachronic view was given by André (2021, fig. 1).
Ecologically speaking, ''the Tydeidae is perhaps the most common of all foliar mite families, and almost any tree, shrub, or liana that has not been treated by pesticides will have these mites on its leaves, stem, and bark'' (Walter and Behan-Pelletier, 1999: 9). The relationship between tydeid mites and plants was recognized as early as the 1800's, especially by botanists. One of the oldest records of this association is Tydeus foliorum Schrank, which was collected in Italy on the undersides of leaves, in moss, and in hay debris (Canestrini, 1896a: 233), north to Finland where it was frequently found in hay and grass (Nordenskiöld, 1900: 35). Lundström (1887: 9), Heinsius (1891: 197) and Ross (1904: 17) referred to the presence of T. foliorum in plant domatia. However, Abliz et al. (2014) mentioned them in passing in their publication for the Encyclopedia of UNESCO. Tydeids are pioneers in inner Antarctica (Janetschek, 1967) and are living in the Svalbard archipelago (Thor, 1933; Seniczak et al. 2020). They were collected from the sea level (Willmann, 1952) to the Himalayas (Momen and Solhøy; 1996; Chandra et al., 2018).
In agroecosystems, Tetranychidae and Eriophyidae are famous due to the plant feeding problems they pose which were consequently clarified in compilations by Lindquist et al. (1996) and Ueckermann (2010). This is not the case of Tydeidae which forms a minor agricultural mite group even if Banks (1904) describing a new tydeid titled his publication as ''Four new species of injurious mites'' and stated that they were all ''of considerable economic importance''. Subsequently, Tydeidae were treated as potential pests in major textbooks such as the ''Mites Injurious to Economic Plant'' by Jeppson et al. (1975), the ''Agricultural Acarology: Introduction to Integrated Mite Management'' by Hoy (2011), or the ''Handbook of Mites of Economic Plants'' by Vacante (2016). A famous exception is the species ''Lorryia formosa'' described by Cooreman (1958) (see the section on Inadequately described taxa). However, the status and function of Tydeidae as pests, beneficials, predators or alternate prey, are beyond the scope of this paper.
In food and stored products, Tydeidae were not even illustrated by Smiley (1987) and only 3 tydeid species were described from stored grain by Momen and Sinha (1991). However, they may be locally significant as in a chocolate production system with 55% of the mites collected (Silva et al., 2024).
In medical and veterinary sciences, Tydeidae are also unimportant and not cited in the reviews by Mullen and OConnor (2019) and Lynn et al. (2024). A notable exception is Tydeus molestus described by Moniez (1889, 1894) in the garden of a Belgian farm and their impact on people. Analogous dermopathies produced by this mite or a similar species were described in an Italian school by Principato et al. (2008) and Principato and Scriboni (2009).
The systematics of the family are sometimes confusing, especially to beginners. Even a recent species such as Quadrotydeus sleipneri described by Momen and Lundquist (1996) has been assigned to two other genera: Lorryia (Kaźmierski, 1998a: 327) and Brachytydeus (Khaustov and Khaustov, 2023: 268). The beginners are still more embarrassed by the double assignation, in the same publication, of sleipneri to Quadrotydeus and to Brachytydeus (Silva et al., 2016: 25, 34). This double assignation recalls that Dugès' caudatus was simultaneously treated as a Tenuipalpidae and as a Tydeidae (André, 2011). ''Neglect'' for literature, divergent interpretations or contradictory observations also lead to confusion: Afrotydeus was erected by Baker (1970) as subgenus of Tydeus to accommodate three species, munsteri, kenyensis, and meyerae, which exhibit three different leg chaetotaxies. The world catalog published by Silva et al. (2016) treated a genus, Pseudolorryia, previously synonymized with Calotydeus by André (2005: 995), and did not include the genus Calotydeus. The genera erected by Berlese are named after the name with ''a'' after the ''d'' (Lasiotydaeus, Melanotydaeus) contrarily to Stylotydeus erected by Thor, Berlese even wrote Tydaeus instead of Tydeus in his publications and in his collection. This misspelling is sometimes found in recent articles. A last example concerns the coexistence of several designation systems based on location and a notation system founded on idionymy such as that developed by André (1980), which can be confusing as deplored by De Vis et al. (2024: 1097).
Quite simply, this paper aims at presenting an overview of tydeid genera sixty years after the seminal paper on the family Tydeidae presented by Baker (1965) and at defining them in a systematic and scientific way within the superfamily Tydeoidea, even if the superfamily is considered paraphyletic (Szudarek-Trepto et al. 2022).
Materials and method
This article treats extant morphospecies (i.e. species based on morphological criteria) described until 30 June 2024. Tydeid species described later in 2024 are not included (e.g. Brachytydeus turkiyensis described by Altunç et al., 2024). The notation system proposed by André (1981b) is used only for apoteles and tarsi, a designation system is applied elsewhere.
A first database (1760 lines x 12 columns) was provided by the Integrated Taxonomic Information System (ITIS), namely by David Nicolson and Geoffrey D. Ower who were developing a web crawler for Tydeoidea. Their basic database was updated and completed, e.g. the number of combinations.
Other data are essentially based on personal data and bibliographical references (books, articles, review papers) on Tydeoidea taxonomy and on the database in the Wikispecies platform (André, 2021). Pertinent works were also consulted, such as monographs, books and catalog: Thor (1933), Baker and Wharton (1952), Baker (1965), André (1980), Kaźmierski (1998a) and Silva et al. (2016).
To avoid confusion, special attention is given to genera and their type species. For instance, Lorryia cumbrensis Baker, 1944 is the type species of the genus Paralorryia erected by Baker (1965). André (1980: 119) placed it in a new genus he called Homeotydeus, a nomenclatural act not in conformity with ICZN. Similarly, André (1980: 128) placed Tydeus munsteri, the type species of Afrotydeus, in a new genus he called Orthotydeus (type species: T. goetzi). Again, this fails to meet the rules of ICZN.
At the generic level, only meristic characters were considered, i.e. counts of discrete serially homologous structures (Lawing et al. 2008). Roughly, this corresponds to the presence/absence of idionymic characters outlined by Grandjean (1952). Diagnoses based on meristic characters were quite unusual before Baker's (1965) seminal paper and some genera were defined mainly by ornamentation. Although ornamentation may be a key character to delineate species (see figs 25-27 by Momen and Lundqvist, 1996), currently it does not provide adequate support at the generic level.
To determine the number of genera and species, nomina novae and replacement names were not retained. Similarly, misspelled names (e.g. Australotydeus instead of Australotydaeus) and specific epithets with no gender agreement (formosa instead of formosus) were not included in this publication. New combinations and revised diagnoses were likewise not included. Species with agronomical interests were considered so when they were described from an agroecosystem foliage or trunk, a statement such as ''from soil samples of mango orchards'' was not sufficient. Incertae sedes (e.g. species in which the phanerotaxy is not described in the original publication and in subsequent publications) were also counted.
The slopes, m, indicated in figure 3 were calculated the usual way: m = (y2 -y1)/(x2-x1 ) where y and x are the coordinates of the two end points of the line segment.
Results
List of genera excluded from Tydeidae
Genera initially described in Tydeidae but transferred later to other families include (in alphabetical order):
Genus transferred to Paratydeidae: Scolotydaeus.
Genera transferred to Triophtydeidae: Apotriophtydeus, Edbakerella, Meyerella, Pretriophtydeus, Pseudotriophtydeus, Stenipedis, Teletriophtydeus and Triophtydeus.
Genus transferred to Ereynetidae: Pseudotydeus.
Genera transferred to Iolinidae: Aesthetydeus, Andretydaeolus, Apopronematus, Coccotydaeolus, Coleotydeus, Homeopronematus, Metapronematus, Metatydaeolus, Microtydeus, Naudea, Neonaudea, Neopronematus, Oakvillea, Parapronematus, Paratriophtydeus, Pausia, Primotydeus, Proctotydaeus, Pronecupulatus, Pronematulus, Pronematus, Pseudopronematulus, Reckitydeus, Tydaeolus and Tyndareus.
List of genera and subgenera of Tydeinae
In the Linnaean system of nomenclature, the generic name is part of the Latin binomial attributed to all species, and therefore appears in every scientific paper dealing with living beings; it also gives this name an important role in systematics, certainly much more important than that of the names of higher taxa (Dubois, 1988: 15-16).
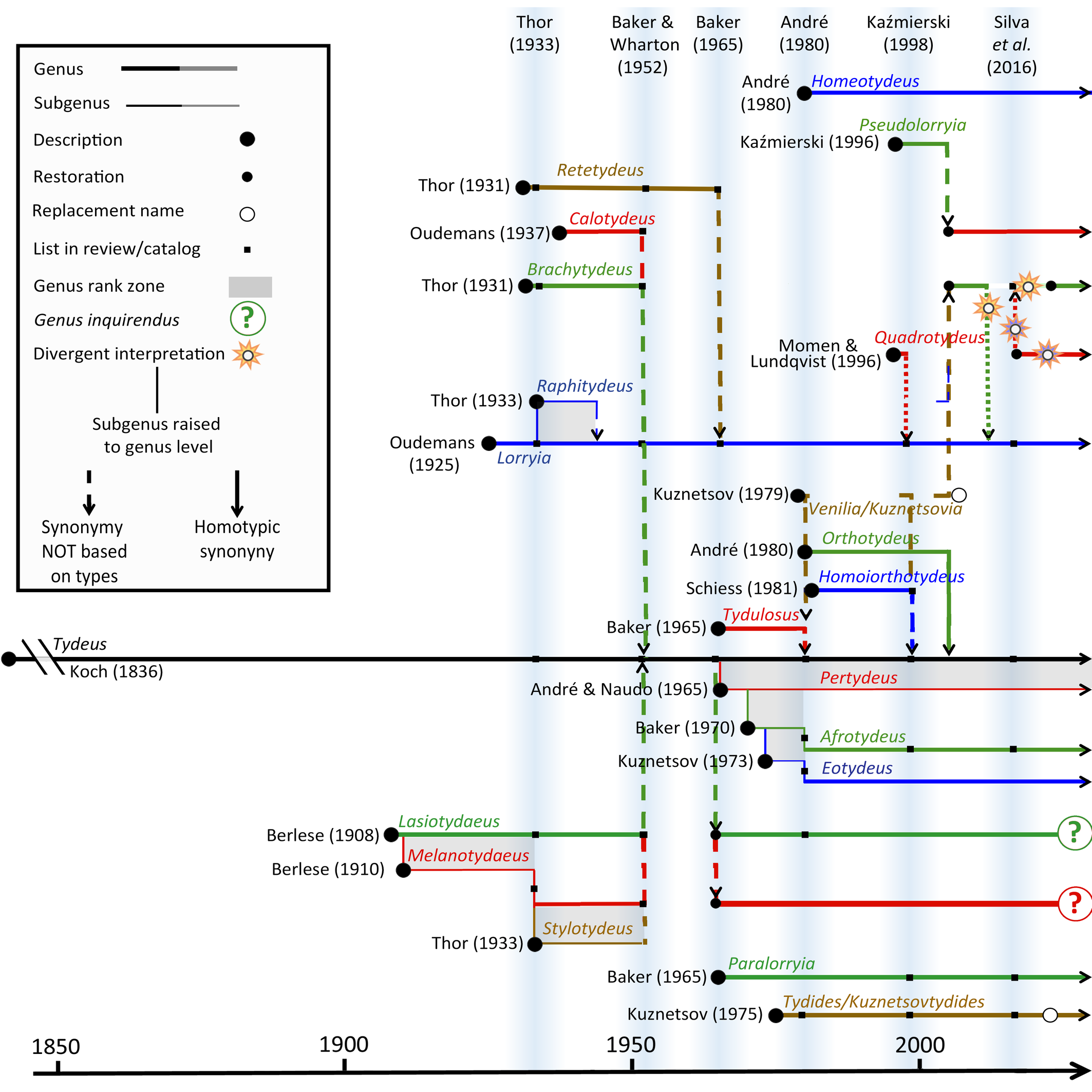


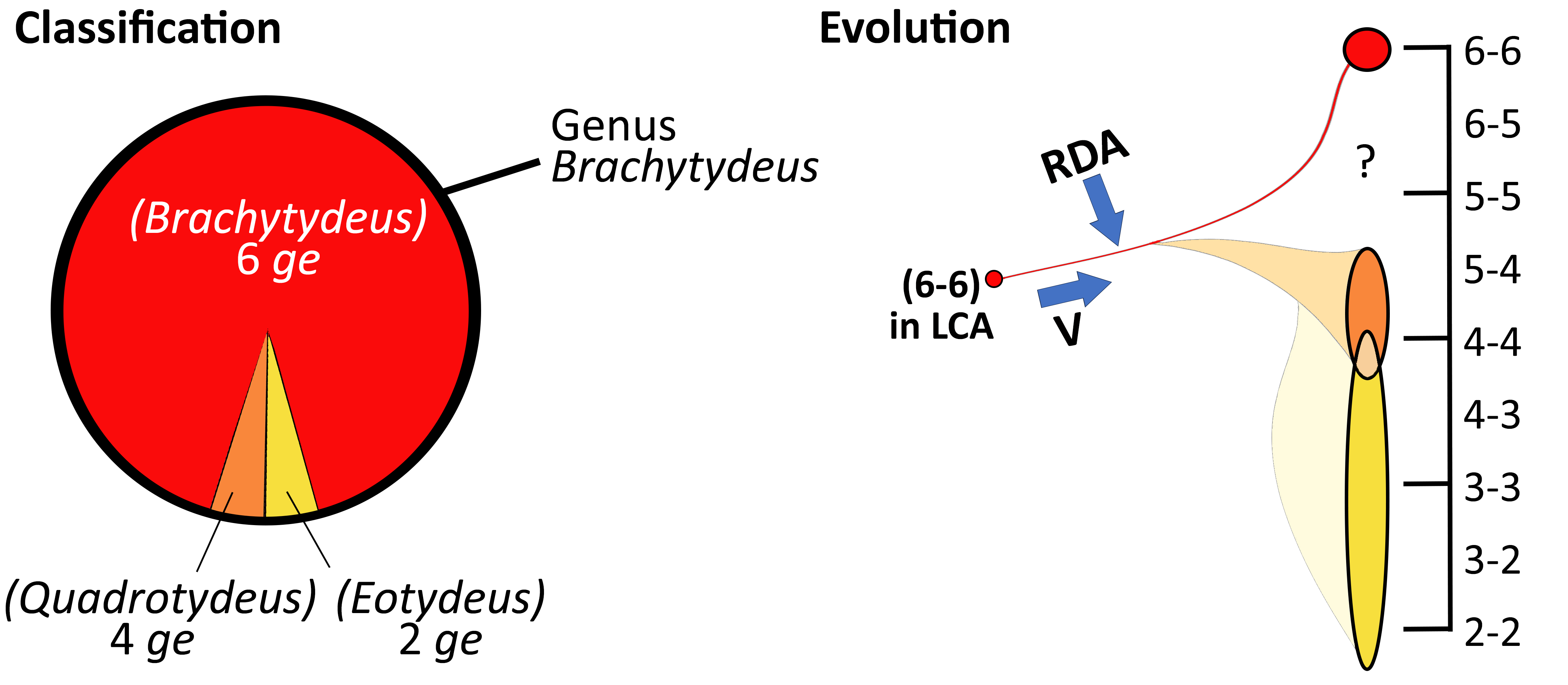
A partial and diachronic view of tydeine genera and subgenera is given in Figure 1. The current number of generic names amounts to 45 among which two are replacement names. In case of homonymy, replacement names are systematically appended to the replaced name (Kuznetsovtydides for Tydides, Kuznetsovia for Venilia) but they are listed hereafter. In contrast, synonymic genera, junior and senior synonyms, are drawn separately (e.g. Quadrotydeus and Brachytydeus). Most synonymies are not based on types (homotypic synonymy) but result from a comparison of data recorded in the literature (Fig. 1). Genera are listed hereafter in alphabetical order, except Tydeus the type genus. The number of component species (possibly before synonymization) potentially followed by the number of species inquirendae is indicated between square brackets.
- Tydeus [58+46]. The history of the genus is complex and convoluted and summarized by André (2005). The type species designated by the International Commission on Zoological Nomenclature (ICZN. 2008) is Tydeus spathulatus Oudemans, 1929 (consequently, it is also the type species of the family and superfamily).
- Acanthodides [1]. Monospecific genus erected by Kaźmierski (1996b). The type species is A. jarema by original designation.
- Afridiolorryia [3]. Genus erected by Kaźmierski (1996b). The type species, Lorryia africana Baker, 1965, was designated in the original publication.
- Afrotydeus [5]. It was erected by Baker (1970) as subgenus of Tydeus to accommodate three species: munsteri, kenyensis, and meyerae. These three species exhibit three different leg chaetotaxies. The type species designated by Baker (1970: 165) is Tydeus munsteri and not A. kenyensis as reported by André (1980: 107), Kaźmierski (1998a: 354) and Silva et al. (2016: 8). The species described by Meyer and Ryke (1959) and studied by André (1980: 126) and by Ueckermann and Grout (2007) has 6 genitals [and not 4 as noted by Baker (1970: 164)] and exhibits the same phanerotaxy as the nominal subgenus Tydeus.
- Andrelorryia [1]. This genus was erected by Khaustov (2022). Type species: A. hajiqanbari by original designation.
- Apolorryia [1]. Genus described by André (1980) with Lorryia congoensis Baker, 1970 as type species by original designation.
- Brachytydeus [220+9]. The genus was erected by Thor (1931) with Tydeus cruciatus as the type species. The type species was redescribed by André (2005) in Oudemans' collection. Synonymized with Tydeus by Baker and Wharton (1952: 191) and by Baker (1965: 99). The genus was not listed by André (1980) and by Kaźmierski (1998a). It was restored by André (2005: 995) and catalogued by Silva et al. (2016: 9). Roughly, Lorryia sensu Kaźmierski corresponds to Brachytydeus (André, 2005: 995; Tempfli et al., 2015: 944; Akbari et al., 2015a: 424).
- Calotydeus [15+4]. The genus was erected by Oudemans and the type species is Calotydeus croceus by original designation (Oudemans, 1937: 922). Synonymized with Tydeus by Baker and Wharton (1952: 191), synonymization repeated by Baker (1965: 100). The genus was not listed by André (1980), Kaźmierski (1998a) and Silva et al. (2016) but was restored by André (2005: 995, 998). Among other species, the genus comprises the type species studied by Oudemans and three species described by Baker (namely arthurbakeri, cumbrensis, shawi). Pseudolorryia was synonymized with Calotydeus by André (2005: 995). A neotype from Oudemans' collection was selected by André (2005: 996).
- Edlorryia [1]. The type species by original designation of the genus erected by Kaźmierski (1996b: 201) is based on a unique specimen of Baker (1968), Lorryia fundadorensis, which is a deutonymph the chaetotaxy of which was partly published but completed by Kaźmierski (1996b: 201).
- Eotydeus [1]. Subgenus erected by Kuznetsov (1973) after study of Crimean specimens and raised to full genus rank by André (1980: 113) after examination of a Canadian specimen. Not listed by Kaźmierski (1998a) and Silva et al. (2016). The type species, Tydeus (Eotydeus) mirabilis, was assigned to Lorryia by Kaźmierski (1998a: 336) and to Brachytydeus by Silva et al. (2016: 19).
- Homeotydeus [2]. Genus erected by André (1980: 116–117) but without fixing a type species, which did not accord with Art. 13.3 of ICZN ("To be available, every new genus-group name published after 1930 ... must ... be accompanied by the fixation of a type species in the original publication″). Another discrepancy with the code (the inclusion of the type species of another genus) was outlined previously. Genus treated by Kaźmierski (1989). The 3 species described by Baker (namely arthurbakeri, cumbrensis, shawi) were transferred to Calotydeus by André (2005: 995, ambiguous remarks), the two Belgian species have a seta on coxa I and remained in the so-called genus. Synonymized with Calotydeus by André (2021: 1026).
- Homoiorthotydeus [1]. Genus proposed by Schiess (1981: 84) and synonymized with Tydeus by Kaźmierski (1998a: 342).
- Idiolorryia [2]. The type species of the genus erected by André (1980: 117) is Lorryia macquillani Baker, 1968, by original designation.
- Kenlorryia [1]. Monospecific genus described by Kaźmierski (1996b) with Kenlorryia masaii as type species by original designation.
- Krantzlorryia [1]. Monospecific genus erected by André (1980: 117) with Lorryia grewia Baker, 1968 as type species by original designation.
- Kuznetsovia [7]. Replacement name proposed by Kammerer (2006: 269) for Venilia, name already used for molluscs, insects, birds... Type species: Paralorryia liberta Livshitz, 1973. Venilia is a junior synonym of Tydeus according to André (1980: 154) or junior synonym of Brachytydeus according to André (2005: 995). Senior homonym of Kuznetsovia, replacement name advanced by Doweld (2016) for a Crustacea.
- Kuznetsovtydides [1]. Replacement name proposed by André (2021: 1030) for Tydides, name already used for insects. The genus was erected by Kuznetsov (1975) with Tydides ulter Kuznetsov, 1975 as type species by original designation.
- Lasiotydaeus [?]. First, monospecific genus erected by Berlese (1908: 15) with Lasiotydaeus glycyphaginus as type species. Descriptions are short: 6 lines for the genus, 3 lines for the species collected in Florence (Italy). The prodorsum illustrated by Berlese (1910: plate 18, fig. 6) has only 3 pairs of setae and it is difficult to conclude if it is pro- or recurved; however, the three setae are in line as in mammilaris (fig. 8, prodorsum recurved) and their alignment contrasts with the adjacent figure (fig. 7) in which the prodorsum is procurved with 4 setae arranged in a zigzag. Lastly, the dorsal opithosomal pattern drawn on that figure is simple, it consists of several transverse rows of 4 setae each and shows no trace of the dorsal migration of the second lateral setae (his L2) evoked by Baker (1965). It was divided in 2 subgenera by Berlese (1910: 211), the nominal subgenus with 2 species and Melanotydaeus with 5 species. The genus was synonymized with Tydeus by Baker and Wharton (1952: 191; with a question mark). The genus—monosubgeneric—was restored by Baker (1965: 101) but was based on a Californian species (L. krantzi). The prodorsum of the Californian species described by Baker (1965: 101-102) and André (1980: 118) is procurved, the opithosomal pattern is different from that observed in tydeid mites, the tarsus I has 12 setae (vs 8 in Tydeinae), the Californian mite is not congeneric with the Italian type species (which seems to be a real Tydeidae even if it is impossible to know what the genus actually is) and appears to be an Iolinidae Tydaeolinae genus inquirendus.
- Lorryia [1]. Monospecific genus erected by Oudemans (1925) with L. superba as type species. The very short description of the initial paper was completed in a subsequent article (Oudemans, 1928a). The whole story is reported by André (2023). The genus was not listed by André (1980). The holotype and unique specimen of the type species is lost. Monospecific and, even recently, confused or synonymized with Brachytydeus (e.g. Mondin et al., 2016; Khaustov et al., 2020; Nuvolini et al., 2020)
- Melanotydaeus [?]. Melanotydaeus was first described as a subgenus of Lasiotydaeus (Berlese, 1910: 211) and was raised to full generic level by Thor (1933: 48). It was divided into two subgenera, Melanotydaeus and Stylotydeus and synonymized with Tydeus by Baker and Wharton (1952: 191). It was restored by Baker (1965: 102) but was not keyed out. ''The placing of Melanotydaeus as a synonym of Tydeus by Baker and Wharton must at present be considered to be in error'' commented Baker (1965: 102) who concluded ''The type, in the Berlese collection, is in extremely poor condition, and it is impossible to know what the genus actually is''. Genus inquirendus.
- Melissotydeus [3]. Genus erected by André (1985a: 244) with M. macrosolenus as type species by original designation.
- Metalorryia [7]. Genus advanced by André (1980: 118) with Lorryia armaghensis Baker, 1968 as type species by original designation.
- Momenia [1]. Genus proposed by Kaźmierski (1996b) with Tydeus longichelus Momen & El-Bagoury, 1989 as type species by original designation.
- Neoapolorryia [5]. Genus erected by El-Bagoury and Momen (1990) with N. aegyptica as type species by original designation.
- Neolorryia [3]. Genus advanced by André (1980: 127) with Retetydeus boycei Baker, 1944 as type species by original designation.
- Nudilorryia [6]. Genus erected by Kaźmierski (1996b) with N. paraferula as type species by original designation.
- Orfareptydeus [1]. Monospefic genus erected by Ueckermann and Grout (2007) for O. stepheni, type species by original designation.
- Orthotydeus [?]. Genus erected by André (1980: 127–128) with Tydeus goetzi as type species by original designation. However, André (1980: 128) mistakenly placed Tydeus munsteri, the type species of Afrotydeus, in Orthotydeus. Kaźmierski (1989) proposed a new combination, Lorryia. The genus was synonymized with Tydeus by André (2005: 996).
- Paralorryia [5]. Genus erected by Baker (1965) to receive Tydeinae with longitudinal striae between setae D2. Type species by original designation: Lorryia cumbrensis Baker, 1944. Kaźmierski (1989) proposed a new combination. The genus was listed by Kaźmierski (1998a: 340) and by Silva et al. (2016: 32) (Fig. 1).
- Perafrotydeus [1]. Monospecific genus proposed by André (1980: 142) with Tydeus (Afrotydeus) meyerae Baker, 1970 as type species by original designation.
- Pertydeus [4]. This subgenus of Tydeus erected by André and Naudo (1965) and recognized by Baker (1970: 164). It was not listed by André (1980), Kaźmierski (1998a) and Silva et al. (2016) (Figure 1). The type species by original designation, Tydeus schusteri, was assigned to Lorryia by Kaźmierski (1998a: 336) and to Brachytydeus by Silva et al. (2016: 24). No formulae were given in the description but the original figure 3 allows to identify the mite as belonging to the genus Tydeus (Fe II with 2 setae, Fe III with a single seta).
- Pseudolorryia [11]. The genus was erected by Kaźmierski (1989) and synonymized with Calotydeus by André (2005: 995). Type species: P. edwardbakeri by original designation.
- Quadrotydeus [1]. Monospecific genus advanced by Momen and Lundqvist (1996), synonymized with Lorryia by Kaźmierski (1998a: 293). The genus is listed by Silva et al. (2016: 34) but the type species, Q. sleipneri, was assigned to Quadrotydeus on page 34 and to Brachytydeus on page 25. The type species by original designation, Q. sleipneri, was assigned to Brachytydeus by Khaustov and Khaustov (2023: 268).
- Quasitydeus [2]. Genus erected by Kaźmierski (1996b) with Tydeus (T.) ricensis Baker, 1970 as type species by original designation.
- Raphitydeus [1]. Monospecific subgenus of Lorryia erected by Thor (1933: 54) for Lorryia (Melanotydaeus) raphignathoides Berlese 1910, type species by monotypy. Division not accepted by Baker (1944: 215) and recalled in Baker (1968: 987). Again, synonymized with Lorryia by Baker (1965: 105) and with Brachytydeus by André (2005: 995).
- Retetydeus [5]. Genus erected by Thor (1931) with R. catenulatus as the type species. Last used in mite descriptions by Baker (1944) for boycei and doddsi. The genus was listed—for the last time!—by Baker and Wharton (1952: 192), with catenulatus as type species. A synonymization with Lorryia was advanced by Baker (1965: 105). Although the type species, catenulatus, was never redescribed from Norway, it was included in the genus Brachytydeus by Silva et al. (2016: 13). The specimens of R. viviparus studied by Grandjean (1938) belong to Brachytydeus.
- Stylotydeus [2]. Subgenus erected by Thor (1933: 50) and synonymized with Tydeus by Baker and Wharton (1952: 191). No type species designated. The two component species (brevistylus and styliger) are species inquirendae.
- Tydides [1]. Monospecific genus erected by Kuznetsov (1975) for T. ulter. Junior homonym of the genus Tydides Stål, 1865 (Insecta, Reduviidae). Replacement name: Kuznetsovtydides provided by André (2021: 1030).
- Tydulosus [4]. The genus was erected by Baker (1965: 103) for species with a basket-weave pattern. The type species designated by Baker, Tydeus granulosus Canestrini, 1886a, is a species inquirenda and has no type. Synonymized with Tydeus by André (1980: 154). The species initially assigned to Tydulosus are included in Brachytydeus and Tydeus by Silva et al. (2016).
- Venilia [7]. Genus erected by Kuznetsov (1979) comprising 7 species reviewed by Kaźmierski (1981). Junior synonym of Tydeus according to André (1980: 154), junior synonym of Lorryia according to Kaźmierski (1989) and junior synonym of Brachytydeus according to André (2005: 995). Junior homonym of a mollusc, successive replacement names: Kuznetsovia and Kuznetsovvenilia. Type species: Paralorryia liberta Livshitz, 1973.
- Australotydaeus [1]. Monospecific genus proposed by Spain (1969) for A. kirstenae, type species by original designation.
- Novzelorryia [1]. Monospecific genus erected by Kaźmierski (1996b) for N. deserta, type species by original designation.
- Prelorryia [2]. Genus erected by André (1980: 142-143) with Lorryia indonesiensis Baker, 1970 as type species by original designation.
- Pretydeus [12]. Genus proposed by André (1980: 143) with Lorryia kevani Marshall, 1970 as type species by original designation.
- Ueckermannia [1]. Monospecific genus advanced by Kaźmierski (1996b) for Paralorryia grewiae Ueckermann, 1988, type species by original designation. The type species is described in Ueckermann and Smith Meyer, 1988: 46-47.
Genera in Australotydaeinae and Pretydeinae
Number of genera in Tydeidae
The practical agreement which exists between specialists as to the delimitation of genera varies from one zoological group to another. In many groups, this agreement between specialists is poor, and this results in a great instability of the generic classification (Dubois, 1988: 16).
Figure 2 gives a histogram based on the monographs, books and catalog listed previously and two cumulative genus description curves since 1831. The first curve provides the number of all new genera and subgenera erected during each decade while the second takes into account synonymies and restoration. The maximum number of genera and subgenera described in Tydeidae is 43 (a total of 45 names – 2 replacement names). After synonymization and restoration, the current number of genera and subgenera in Tydeidae is 30 (Fig. 2). The instability evoked by Dubois (1988) is discernable through the gap between the two curves.



Depending on the curve selected, the number of genera at the beginning of the 2011-2021 decade amounts to 29-43 and is close to the estimation by Zhang et al. (2011: 130), 30 genera. Both curves apparently begin to flatten during the last 40 years.
Number of species in Tydeidae
A common approach to estimating the total number of extant species in a taxonomic group is to extrapolate from the temporal pattern of known species descriptions (Bebber et al. 2007). This perspective is not new in mites and was used by Wharton (1964) for mites and Trombiculidae and by André and N'Dri (2013) for mites and Tydeoidea. Yet, such an approach is not recommended due to the data weakness: only 433 tydeid species have been described, figure 3 shows the beginning of a logistic curve and there is no long-term trend sensu Edie et al. (2017).
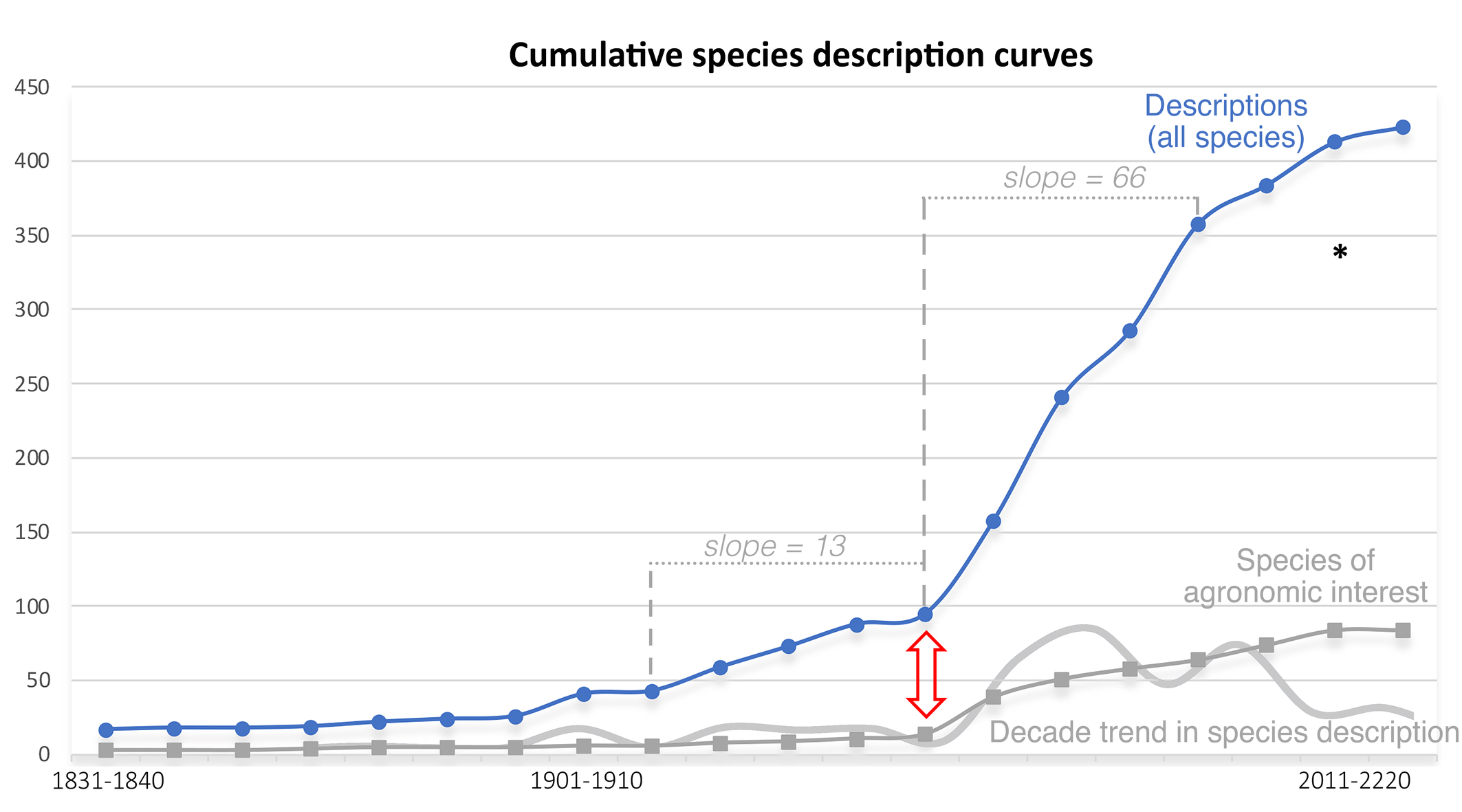


Nevertheless, a turning point is apparent from figure 3 and is indicated by a double arrow. Both cumulative curves suggest a change in the description rate manifest trough the slopes estimated before and after the 1951-1960 decade. This is confirmed by the decade trend in species description with a maximum number of 83 species described in a single decade. The turning point coincides with the description of Lorryia formosa by Cooreman (1958) and seems to reflect the influence of agronomic practices.
However, another approach with a new ''Pie of Life'' was proposed by Larsen et al. (2017) and was based, concerning mites, on Walter and Proctor (2013). The number of described Acari species (including Acariformes and Parasitiformes) is approximately 55,000 (Krantz, 2009: 1). In such a context, 433 described tydeid morphospecies represent less than 1 percent of described Acari (0.79%). If assumptions of Larsen et al. (2017: 248; appendix 2 on animal richness, 10.2 million mite species including morpho- and cryptic species) are accepted, this yields 80,301 tydeid species. In the end, if a ratio of 5.9 cryptic species per morphologically based arthropod species is recognized (Larsen et al. 2017; appendix 1), this yields 11,638 tydeid morphospecies. 433 described tydeid morphospecies would then represent only 3.72% of estimated tydeid morphospecies in the world, less than the 10% recalled by Lindquist (2001: 55) for the eriophyoid fauna.
Obviously, there is no problem of species inflation as in mammals (Zachos et al., 2013). Only 414 tydeid species had been described for the 2001-2010 decade vs 340 estimated by Zhang et al. (2011). Τhe temporal pattern of known species descriptions was not modified by synonymies (4 cases) and restoration (2 cases).
Number of names in Tydeidae
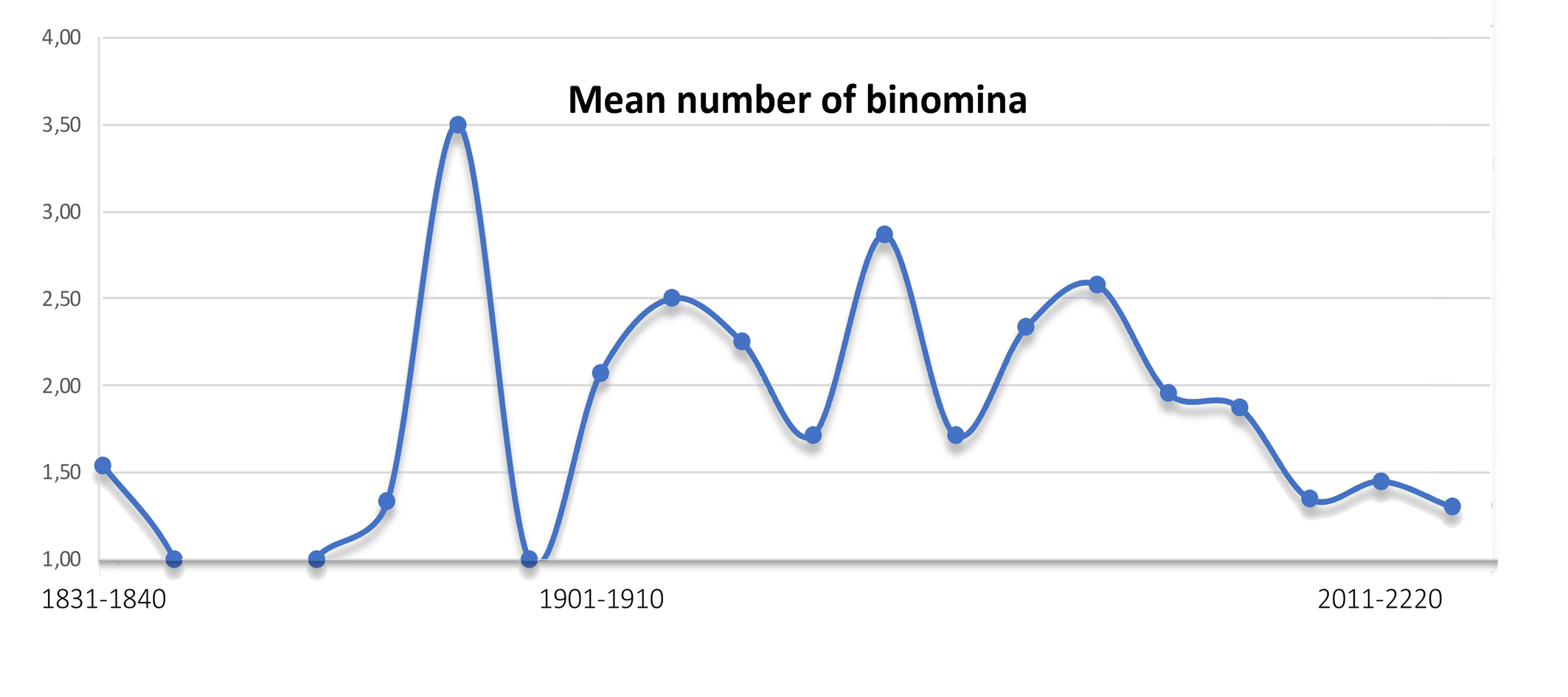
Many binomina were given to a specific mite: 2.05 ± 0.76 for all tydeid species. A record is detained by the genus Brachytydeus. For instance, zaheri has five objective synonyms plus a replacement name (not counted): Paralorryia (original combination), Brachytydeus, Kuznetsovia, Lorryia, Tydeus and Venilia.
The number of binomina assigned to early described species is close to one (Figure 4). For instance, only a single combination has been used for velox Koch described in 1836: Tydeus velox. The same applies for recently described species, i.e. for Brachytydeus altaicus described by Khaustov and Khaustov in 2023, although there hasn't been enough time to propose objective synonyms to B. altaicus. There are large variations in between. A maximum value (3.5) is reached for species described in the 1881-1890 decade. For instance, there are 5 combinations for granulosus described by Canestrini (1886b): successively Tydeus, Tydulosus, Venilia, Lorryia and Brachytydeus. The trends observed in recent decades seems to indicate a decrease of subsequent combinations. The 17 species directly assigned to Brachytydeus were all described after 2008.


Toward a new concept of Tydeidae
In the Thorian sense, Tydeidae was ''difficult to characterize'' (Baker,1965: 96). In the modern sense, a key character advanced by André and Fain (2000) is the recurved prodorsum. This character is shared with Triophtydeidae but is very practical to recognize ''real'' Tydeidae in the old illustrations of Berlese and even in drawings of Baker (1965). The importance of the ecdysial cleavage line in Acariformes was discussed by Norton and Kethley (1994).
A second key character advanced by André and Fain (2000) is the presence of only two eye-spots vs three in Triophtydeidae. Those spots are silver granules not associated with a lens such as that found in Ereynetidae. As stressed by those authors, the presence of two eye-spots is not ambiguous. In contrast, their absence may refer to a nonobservance, i.e. the failure to observe them (e.g. the spots disappeared) or to a real absence (see Kaźmierski, 1989: 302; André and Fain, 2000: 416). The problem directly concerns Australotydaeus kirstenae in which no eye-spots have been reported and is still more puzzling when a population of Tydeus harbors 3 eyes as observed by André (1985b: 192) and recalled by André and Fain (2000: 412).
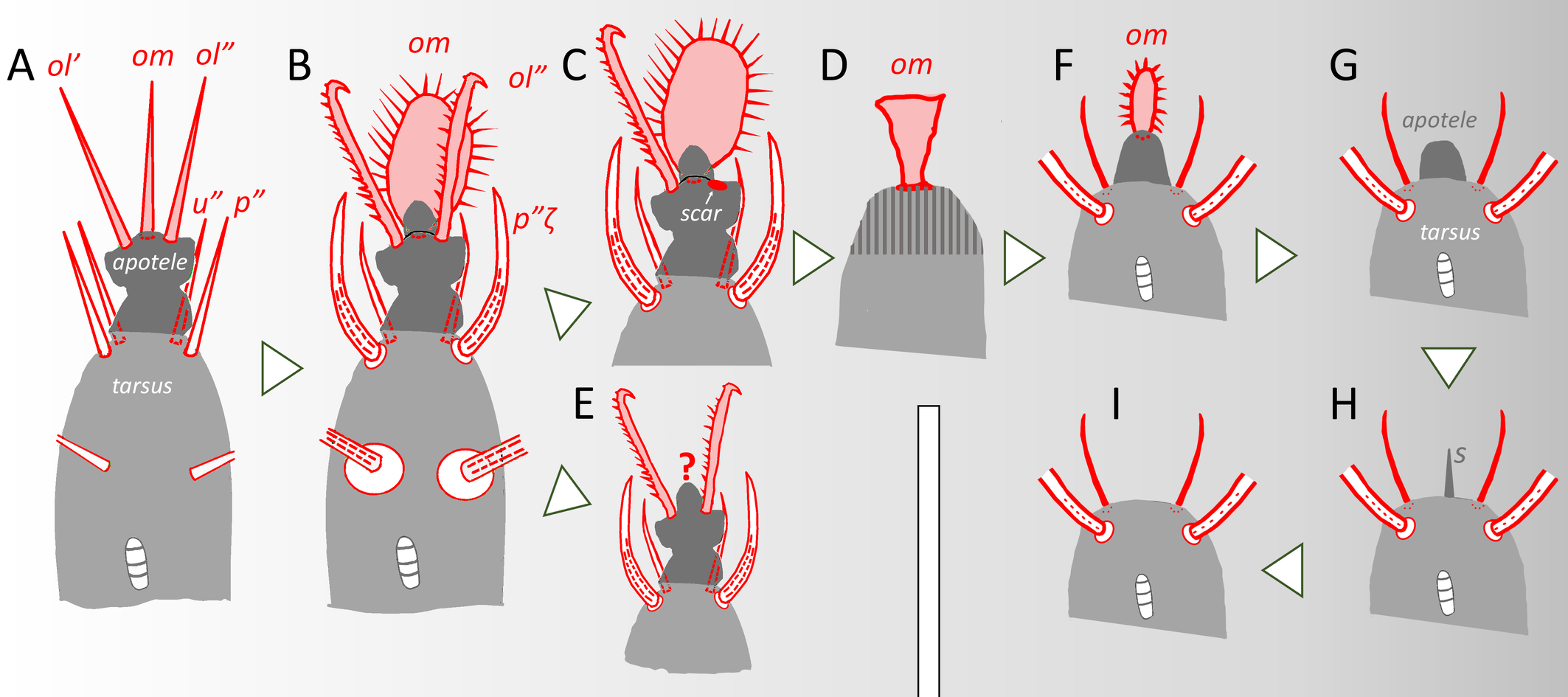


As noticed by André and Fain (2000: 437), the family Tydeidae is also characterized by the loss of eugenitals in females and a reduction of the cis-acetabular area. In the calyptostatic tritonymph drawn by Kuznetsov (1980: 1020, his fig. B), the 2 genital openings usually observed in tritonymphs are absent. This reduction is accompanied by the decrease of the chaetome, usually 6 genital setae on both sides of imagines. A decrease of genitals is observed in Acanthodides, Apolorryia, Eotydeus, Momenia, Neolorryia, Pertydeus and Quadrotydeus. The absence of genitals was even observed in the calyptostasic tritonymph of Brachytydeus (Kuznetov, 1980). Similarly, there are 3 aggenitals (instead of 4) in Apolorryia and Eotydeus.
Such a reduction of genitals may be unilateral (occurring on one side of the body) as noticed by Momen and El-Baghouri (1994), Momen and Solhøy (1996), Momen and Lundquist (1996) and Kaźmierski (1989). The generic significance of this character was questioned by Kaźmierski (1989: 300-301) and the trait will be used to delineate subgenera only. Furthermore, the unilateral presence of 7 genitals reported by Momen and El-Baghouri (1994) and Momen and Solhoy (1996) demonstrates that two forces are governing the evolution of Tydeidae. On the one hand, the fluctuating asymmetry seems to be caused by random developmental accidents (RDA in Figure 6) not corrected by homeostatic mechanisms normally resulting in a perfect bilateral symmetry (Leponce et al., 2001). On the other hand, Grandjean (1942) gave an evolutive significance to the unilateral changes he called vertitions (V in Figure 6) and governed by homeostatic mechanisms.


A last distinguishing trait, not evoked by André and Fain (2000), is the presence of at most 8 setae on tarsus I in all Tydeinae and Pretydeinae. Australotydaeinae has a special chaetotaxy with 10 setae on tarsus I and exhibits intermediate characters with other Tydeoidea. This counting is based on the designation system developed by André (1981b).
Other formulae are variable. The most frequent epimeral formula is (3-1-4-2). (3-1-4-3) has been observed in Krantzlorryia, Pretydeus and Tyndareus.
Evolutionary plasticity of Tydeidae
An evolutionary trait-based approach was selected by André (2023) to define the genus Lorryia. In this approach, Tydeidae anticipate the evolutionary traits found in other families of Tydeoidea.
For instance, the reduction of chaetotaxy of tarsus I and the dactyly observed in Tydeidae may be conceived as a vast evolutionary move leading to the fusion or loss of apotele I and the resulting palpian evolution of leg I observed in Iolinidae (Figure 5).
Another aspect of evolutionary plasticity of Tydeidae is shown by the reduction of the cis-acetabular area and the decrease of the chaetome which also anticipate the evolution of Iolinidae.
A further facet of evolutionary plasticity of Tydeidae is shown by the partly recessed solenidion, φI, observed in Pretydeus. It does not differ from the ereynetal organ of some ereynetid larva.
A last aspect of evolutionary plasticity of Tydeidae concerns the ontogeny. Apart from a calyptostatic prelarva, described and drawn by Kuznetsov (1980: 1020, his fig. A) and photographed by André and N'Dri (2013: 60, their fig. 50), the ontogeny of Tydeidae includes one six-legged larva (larviparity, drawn by Thor 1933: 52, his fig. 62) followed by four eight-legged stases: the protonymph, deutonymph, tritonymph and imago (♀and ♂). In other words, adulthood (sexual maturity) is observed in imagines only. Based on laboratory colonies (Brickill, 1958; Kuznetsov, 1980; Liguori et al. 2002; Hernandes et al., 2006; Silva et al., 2014), there is no missing stase as in spider mites (André and Van Impe, 2012) but the tritonymph can be reduced to a calyptostasis as presented and illustrated by Kuznetsov (1980: 1020, his fig. B). This recalls the intervening calyptostases observed in ereynetid Speleognatinae (Fain, 1972; André and Fain, 1991).
Nomenclatural acts
Nomenclatural acts (1) to (8) concern families, genera and subgenera, subsequent acts concern species.
- Bakerlasiotydaeus new genus
ZOOBANK: 86ABB6B5-0AE1-4AE8-A7EB-6C105208B3D6

Lasiotydaeus krantzi is not a Tydeidae but an Iolinidae as noticed by Darbemamieh et al. (2016). The Californian species was described in detail by Baker (1965) and André (1980). Therefore, a new genus and a new combination are proposed: Bakerlasiotydaeus new genus. with L. krantzi as type species, and B. krantzi n. comb. The precise place of Bakerlasiotydaeus within the Iolinidae is beyond the scope of this paper but, according to André (1980: 118), the Californian species is closely related to Primotydeus (Tydaeolinae). - Afrotydeus and its type species, Tydeus munsteri, exhibits the same phanerotaxy as the nominal subgenus Tydeus. Afrotydeus, be it a subgenus or a genus, is thus considered a junior synonym of Tydeus. Topotypes or types of both type species were examined. Consequently, the original combination, namely Tydeus munsteri, has to be used again and any other combination must be set aside. Tydeus (A.) meyerae was moved to Perafrotydeus by André (1980: 142), the other species are placed in a new genus defined hereafter (act 4).
- Similarly, the type species of the genus Paralorryia, cumbrensis, has the same organotaxy (leg and idiosoma) as croceus, the type species of Calotydeus. Types of both species were examined. As a result, Paralorryia is thus considered a junior synonym of Calotydeus.
- Neoafrotydeus new genus
ZOOBANK: 1EC6999B-930C-40AB-9AB7-4BF259A477CC

Neoafrotydeus new genus is erected with kenyensis as type species to accommodate Tydeus (Afrotydeus) flabellifer, T. (A.) kenyensis, A. novaezealandiae, A. smileyi and A. zairensis, species currently assigned to Afrotydeus, genus synonymized with Tydeus as noted above. - Neohomeotydeus new genus
ZOOBANK: CAAAE9E6-4DB5-441C-92CC-51B15227DB96

Homeotydeus was erected by André (1980) but is not in conformity with ICZN. The 3 species described by Baker (namely arthurbakeri, cumbrensis, shawi) have a seta on trochanter I and were transferred to Calotydeus by André (2005: 995, ambiguous remarks.). The other two species, namely H. bipilis and H. formosus, have a nude trochanter I and are accommodated in a new genus, Neohomeotydeus with H. formosus as type species. - Pertydeus was described as a subgenus of Tydeus and exhibits the same leg chaetotaxy as the nominal genus Tydeus. The number of genitals is however 5 (vs 6 in Tydeus). Pertydeus thus re-established in its pristine state and restored as a subgenus of Tydeus (rest. comb.).
- Similarly, Quadrotydeus is considered to be a subgenus of Brachytydeus characterized by the same leg phanerotaxy but with only 4 genitals (new comb.).
- Similarly, Eotydeus is considered to be a subgenus of Brachytydeus characterized by the same leg phanerotaxy but with only 2 genitals as in the original description (new comb.).
- Tydeus nieu(w)kerkeni. André (2005) was naming the species after Dr Erik J. van Nieukerken, yet the name was published as nieuwkerkeni, an incorrect original spelling. Under art. 32.5 of ICZN, the correct spelling is emended to nieukerkeni. A ''justified emendation'' (Art. 33.2.2), if corrected under article 32.5, results in the same author and date as the original name.
- Homeotydeus formosa, the binomen used by André (1984), does not respect the gender agreement. The original combination corrected under art. 34.2 of ICZN is Homeotydeus formosus which is the type species of the genus Neohomeotydeus.
Table 1 shows that some genera are very close and that the difference may concern only a single seta: Brachytydeus–Kuznetsovtydides (seta on trochanter I), Calotydeus–Tydeus (seta on femur II). Table 1 also masks the within diversity of genera. For instance, chaetotaxy of leg II in Idiolorryia is displayed (6-2-1-3-0) while it has been observed a single seta on tibia. Variations in leg chaetotaxy was explored by Momen and Lundqvist (1993). Similarly, there are 4 or 5 genitals in Neolorryia, 2 to 4 in Eotydeus. Consequently, the division into subgenera is debatable due to the high variability of the number of genitals. Edlorryia based on a deutonymph, Lasiotydaeus and Melanotydaeus (genus inquirendus) do not figure in table 1.
Download as Not included: Edlorryia (deutonymph), Lasiotydaeus and Melanotydaeus (genera inquirenda).
Genus + type species [epithet only]
Leg I
Leg II
Leg III
Leg IV
S
Cl.
Palp
TYDEINAE
Tydeus [spathulatus]**
8-4-3-3-1
6-2-2-2-0
5-2-1-1-1
5-2-1-1-0
2
2
6-2-2
(Pertydeus) [schusteri]
Acanthodides [jarema]
8-4-3-3-1
6-2-2-3-0
5-2-1-2-1
5-2-1-1-0
2
2
6-2-1
Afridiolorryia [africana]
8-4-2-3-0
6-1-1-2-0
5-1-1-1-1
5-1-1-1-0
2
2
6-1-2
Andrelorryia [hajiqanbari]*
8-4-3-3-0
6-2-2-3-0
5-2-1-2-1
5-2-1-1-0
1
2
6-1-2
Apolorryia [congoensis]**
7-3-1-2-0
6-1-1-2-0
5-1-0-1-0
5-1-0-1-0
2
2
6-2-2
Brachytydeus [cruciatus]**
8-4-3-3-1
6-2-2-3-0
5-2-1-2-1
5-2-1-1-0
2
2
6-2-2
(Quadrotydeus) [sleipneri]
(Eotydeus) [mirabilis]*
Calotydeus [croceus]**
8-4-3-3-1
6-2-2-3-0
5-2-1-1-1
5-2-1-1-0
2
2
6-2-2
Idiolorryia [macquillani]**
8-4-2-3-0
6-2-1-3-0
5-1-1-2-1
5-1-1-1-0
1
2
6-1-2
Kenlorryia [masaii]
7-4-3-3-1
6-2-2-3-0
5-2-1-1-1
5-2-1-1-0
2
2
6-2-2
Krantzlorryia [grewia]**
8-3-2-2-0
6-2-1-2-0
5-2-0-1-1
5-2-0-1-0
2
2
6-1-2
Kuznetsovtydides [ulter]
8-4-3-3-0
6-2-2-3-0
5-2-1-2-1
5-2-1-1-0
2
2
6-2-1
Lorryia [superba]
?
?
?
?
?
1
2-2-2
Melissotydeus [macrosolenus]**
8-4-3-3-1
6-2-2-3-0
5-2-1-2-1
5-2-1-2-0
2
2
6-2-2
Metalorryia [armaghensis]**
8-3-2-3-0
6-2-1-2-0
5-2-1-1-1
5-2-1-1-0
2
2
6-1-2
Momenia [longichelus]
7-4-3-3-1
6-2-2-3-0
5-2-1-2-1
5-2-1-1-0
2
2
6-2-2
Neoafrotydeus [kenyensis]**
8-4-3-3-1
6-2-1-1-0
5-2-1-1-0
5-2-1-0-0
2
2
6-2-2
Neoapolorryia [aegyptica]
8-3-2-2-0
6-1-1-2-0
5-1-0-1-0
5-1-0-1-0
2
2
6-1-2
Neohomeotydeus [formosus]**
8-4-3-3-0
6-2-2-3-0
5-2-1-1-1
5-2-1-1-0
2
2
6-2-2
Neolorryia [boycei]**
8-3-2-2-0
6-1-1-2-0
5-1-0-1-1
5-1-0-1-0
2
2
6-1-2
Nudilorryia [paraferula]
8-4-3-3-0
6-2-2-3-0
5-2-1-2-1
5-2-1-1-0
2
2
6-2-2
Orfareptydeus [stepheni]
8-4-3-2-1
6-2-2-1-0
5-2-1-0-0
5-2-1-0-0
1
2
6-2-2
Perafrotydeus [meyerae]
8-4-3-3-1
6-2-2-1-0
5-2-1-1-0
5-2-1-0-0
2
2
6-2-2
Quasitydeus [ricensis]
8-4-3-3-1
6-2-2-3-0
5-2-1-1-0
5-2-1-1-0
2
2
6-2-2
PRETYDEINAE
Novzelorryia [deserta]
8-3-2-3-1
6-2-0-3-0
5-2-0-1-1
5-2-0-0-0
3
2
6-2-2
Prelorryia [indionensis]**
8-4-2-3-1
6-2-0-3-1
5-2-0-1-1
5-2-0-0-0
2
2
6-2-2
Pretydeus [kevani]**
8-4-2-3-1
6-2-0-3-1
5-2-0-1-1
5-2-0-0-0
3
2
6-2-2
Ueckermannia [grewiae]*
8-3-2-3-0
6-2-0-3-0
5-2-0-1-1
5-2-0-0-0
3
2
6-2-2
AUSTRALOTYDAEINAE
Australotydaeus [kirstenae]**
10-5-3-5-1
6-2-3-3-1
5-2-1-1-1
5-2-0-2-0
2
2
6-2-2
Discussion
Chronic problems
It is important to distinguish chronic problems as those listed by Lindquist (2001) from problems specific to Tydeoidea. Language and cultural barrier: ''phanere'' is a word absent from the English dictionaries, unused in the manual of acarology edited by Krantz and Walter (2009) and covers, in acarology, setae and other setiform organs. Acarological terminology remains obscure and some chaetotatic formulae refer to all setiform organs, in whatever form. Systems of notation/designation for setae and other external structures are also a source of confusion as regretted by De Vis et al. (2024: 1097).
As their colleagues, acarologists who deal with Tydeoidea have a limited knowledge of global acarofauna. Even if the Eurocentrism apparent in Figure 7A tends to disappear later, some major areas have been little explored: Africa (no tydeid mite in the catalog by Mwase and Baker, 2006), Australia (Halliday (1998) does not cite a single identified species), China (only 27 determined species in Lin and Zhang, 2010: 54), Middle East (17 determined species in Kamali et al., 2001: 150-152; only 1 in Halliday et al., 2018: 133), North America (only 30 species in Beaulieu et al. (2019: 97)... This limited knowledge of Tydeidae may be perceived as a cognition still at a nascent stage (Stepanyan and Zarikian, 2024: 217). Another indicator of limited knowledge is the absence of recapture of atypical species described long ago: Australotydaeus kirsteneae described by Spain (1969) and Lorryia superba described by Oudemans (1925) but collected in 1923.
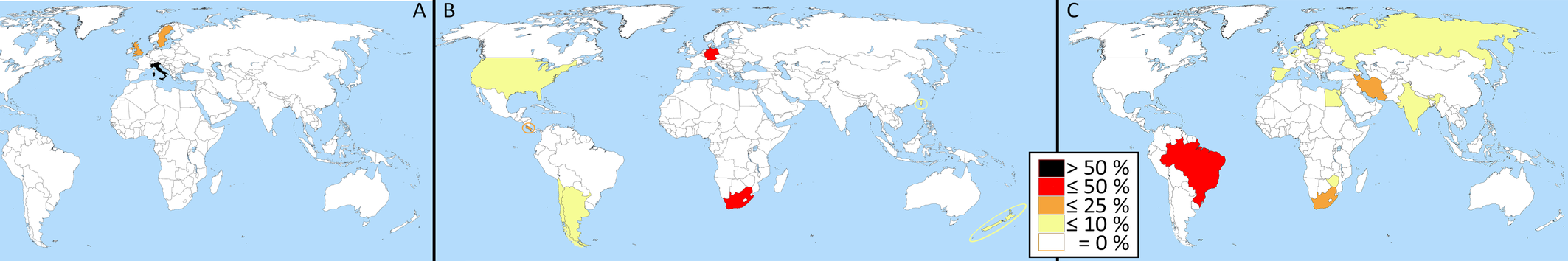


Lastly, molecular data are indicative of cryptic or undescribed species. Beaulieu et al. (2019: 97) cited only 30 tydeid morphospecies known from Canada against 217 species based on molecular data; i.e. morphospecies would represent at most 14% of all species living in Canada.
Objectivity and objective synonymies
Not only the ''ancient'' species are neglected, but also the types. The principle of typification states that any named taxon, in the family group, genus group or species group, has a name-bearing type which allows the application of the name of the taxon to be objectively applied. Yet, name-bearing types are rarely requested to be the reference object for a necessary redescription or an accurate identification. Most synonymies between genera are not based on the type material (Fig. 1). Synonymies may even result from synonymization without comments as done by Baker and Wharton (1952: 191) because the family had ''been divided in the past into too many genera based on such characters as size, width, length of setae, type of setae, and presence or absence of eye spots.'' The absence of any explanations is also noticeable when Baker (1970: 163) proposed new combinations for species originally placed in Tydeus.
Whatever the sense attributed to objectivity through history (Daston and Galison, 2007; Latour, 2012), types in ICZN provide an objective reference for the allocation of a nomen to any taxon and designate objects (Dubois, 2011: 21).
Inadequately described taxa
Inadequately described taxa is another problem emphasized by Lindquist (2001) and rendered more complicated by the absence of redescriptions. Yet, Baker (1965: 95) already wrote that ''it will be necessary to re-collect at type localities to determine the status of most European species''. Most Tydeus described by Koch from Regensburg (Germany) were inadequately described, have never been redescribed and there is no neotype. Tydeus breviculus redescribed by Kaźmierski (1998a: 305) is based on Polish specimens, not on topotypes. Its synonymy with Tydeus curtus Berlese, 1910 is therefore suspect.
The first complete organotaxy was provided by Grandjean (1938), which means that earlier descriptions are seriously inadequate and concern 72 species (i.e. 17% of current species). Leg chaetotaxies published before Baker's (1965) seminal paper were quite unusual and such an absence concerns ca 95 species (22%). Redescriptions are exceptional (less than 1% of described species) and only include that of Tydeus italicus by Kaźmierski (1998a: 310–311) and those of T. croceus (L.), T. cruciatus Koch and T. spathulatus Oudemans by André (2005), all from Oudemans' collection.
Inadequate descriptions and the absence of subsequent redescriptions also concern important species such as ''Lorryia formosa'' first collected on citrus in Northern Africa. The original description did not mention the organotaxy of legs and did not illustrate several setae (P2, H1 or H2), the reticulation pattern is just sketched in fig. 15. The chaetotactic formulae deduced from original figures (figs 7-10), I(6-3-2-2-0) II(6-2-2-2-0) III(5-2-1-1-0) IV(5-2-0-1-?), do not correspond to formulae of Table 1. What does Lorryia formosa authorem designate? Is it the same species as that described by Cooreman (1958)? Metalorryia magdalenae described by Gerson (1968), also with a reticulated pattern, was also collected from citrus in the same climatic zone; what are the differences with the species described by Cooreman (1958)?
A similar problem arises with Tydeus kochi Oudemans (1928b: 377) mentioned a species with bulbous short setae (''kolbige kurze Borste'' in German) while Baker (1970: 170–171, his fig. 26) drew a species with nude setae said to be ''of medium length, lanceolate, serrate''. What does Tydeus kochi authorem designate?
Adequate (re)descriptions
''Ancient'' species are often neglected. If the ornamentation (striation pattern, reticulation, basketweave...) is disregarded at the generic level, it is of paramount importance (together to the state, shape, length of setiform organs) to delineate species. A helpful system for such was proposed by Kaźmierski (1998a: 288-289). However, the ornamentation is difficult to represent with drawings and illustrates the problems encountered when we look back into the past with modern techniques and equipment (Ratcliff, 2009). In Akbari et al. (2015b), the habitus of Tydeus shabestariensis is illustrated both with a drawing (their figure 1) and with a photograph (their figure 3). The comparison of photos and line drawings revealed that dorsal body setae seem thicker and more serrated in drawings than in photos. Another example is provided by Ripka et al. (2013) who described several new species of arboreal Tydeidae from Hungary. They provided phase contrast photos for some species. The comparison of photos and line drawings again reveals some inadequacy of line drawings. The photo of Lorryia sanctikingai clearly illustrates rather thick and blunt-tipped dorsal idiosomal setae (their fig. 18) while line drawings (their fig. 15) suggest thin and pointed setae. This recalls the old debate between drawings and photographs in science. ''But the'photographic eye' does not approach the objects only in a more honest and unbiased way, it is often able to grasp them more directly and more sharply.'' (''Aber das'photographische Auge' tritt an die Gegenstände nicht nur ehrlicher und vorurtheilsloser heran, es vermag dieselben auch vielfach unmittelbar genauer und schärfer zu erfassen.'') advanced Fraenkel and Pfeiffer (1892: 1). Starr et al. (1896: vi) added: ''But all such drawings are necessarily imperfect and involve a personal element of interpretation.''
Photographs of the striation pattern in Tydeoidea was introduced by André and Ducarme (2003). In the future, adequate micrographs must be part of any (re)descriptions, be they phase contrast micrographs (e.g. Escobar-Garcia et al. 2023: Figs 1, 5 & 6); Khaustov and Khaustov, 2023: Fig. 1) or scanning electron microscope (SEM) micrographs (e.g. Khaustov and Khaustov, 2023: Figs 4 & 5; Wergin et al., 2000).
The redescription of any species, however precise it is, must be based on mites of the same provenance as the type series. This might bear serious problems as emphasized above with Lasiotydaeus. The species ''Lorryia formosa'' described inadequately as demonstrated above, offers another example. The species was never redescribed from the type series, nor from specimens of the same provenance (citrus in northern Africa). The specimens of Lorryia formosa described by Jeppson et al. (1975, fig. 85) are probably American and bear the same binomen as the African species described by Cooreman (1958). Yet, they differ on several points: the reticulation pattern as well as the length, shape (rounded vs sharp end) and insertion of some setae (P3, L1).
Ontogeny and sexual polymorphism
In the past, genera were erected and based on misidentified immature specimens: the so-called genus Hypopus by Dugès (1834: 20) is a famous example. In Tydeidae, a nymph in pupation was illustrated, without understanding what it was, by Berlese (1910: figs 5, 5a): Tydaeus (Tydaeolus) atomus had four trichobothria.
Tydeus munsteri, the species described by Meyer and Ryke (1959: 413), studied by André (1980: 128) and by Ueckermann and Grout (2007: 2062-2064) and designated as the type species of Afrotydeus, has 6 genitals and not 4 as noted by Baker (1970: 164) and exhibits the same phanerotaxy as the nominal subgenus Tydeus. The confusion between a tritonymph with 4 genitals and the imago with 6 genitals might explain the misunderstanding.
The hypothesis suggested by Ueckermann and Grout—the specimen studied by Baker was a tritonymph—is all the more attractive as the confusion imago-tritonymph was also made by him when he described the holotype of meyerae Baker (1970: 165), the type species of Perafrotydeus. The holotype was examined by André (1980: 142) who stated that the holotype was not a female but a tritonymph in pupation.
A problem also concerns the genus Edlorryia based on a unique specimen of Baker (1968), a deutonymph the chaetotaxy of which was incompletely published.
Additionally, laboratory colonies attested thelytoky in tydeid mites (Hernandes et al. 2006; Silva et al. 2014) and confirmed the sex ratio observed in the field; it is an open question whether this trend is comparable to those observed in Oribatida (Heethof et al. 2009). A last problem concerns the striation pattern which could vary between males and females as shown by Khaustov (2023), such a secondary sexual dimorphism complicates the species identification and recalls the heteromorphic males observed in Iolinidae by Kaźmierski (1998b: 43).
Group of species vs complex of species
As already proposed by Athias-Henriot (1975), the development of identification tools has nothing in common with systematic research, clearly visible morphological traits are sufficient for this approach. Currently, the genus Brachytydeus contains 220 species and it is difficult to discriminate them. Grouping tydeid species with similar morphology is an old tradition already used by Koch (1850: 71), Oudemans (1929: 480), Baker (1968: 987) and Kaźmierski (1980: 89) and might help to make accurate identifications.
As noticed by Zachos (2016), species names are discrete units in a continuous world with fuzzy boundaries and species close to one another might be grouped in species complexes as already done in Eriophyidae species associated with particular crop systems (de Lillo et al., 2018). Contrarily to species group where similarity is a key point, phylogenetic criteria are important and might help to detect close or cryptic species, for instance those living in the same agroecosystem such Brachytydeus formosus collected on citrus. Such complexes in Tydeidae are evoked by Parker (1982: 123). As demonstrated by Halliday (2010) and Saccagi and Ueckermann (2024), close species may be a source of taxonomic and nomenclatural confusion that potentially complicates pest management and biosecurity.
Unresolved hypotheses
Only unresolved hypotheses related to species of agronomic importance are listed hereafter.
Tydeus californicus (Banks) is suspected to be a junior synonym of T. spathulatus by Ueckermann and Grout (2007: 2364). Similarly, Ueckermann et al. (2019: 505) are questioning the identity of the Turkish specimens of Tydeus californicus (Banks) as the expanded caudal setae (spatulate) differs from specimens from the USA (lanceolate). The true identity of Turkish specimens is again questioned by Akyazı et al. (2024: 1039). Tydeus goetzi Schruft was first identified as T. caudatus by Akyazı et al. (2017: 12). Tydeus caudatus auctorum might designate T. goetzi, at least in some cases (André, 2021: 1031).
Lastly, Tydeus viburni Koch has never been recorded since the original description in 1838. However, viburni was listed with Tetranychus urticae by Pritchard and Baker (1955: 437). Tydeus viburni might be the old name of T. goetzi since both mites are frequent on Viburnum (André, 2011: 77–80). The hypothesis was advanced by André (2021: 1031).
Conclusions
All tydeid genera are now defined by meristic characters (chaetotaxy, solenidiotaxy...) and not based on the ornamentation (striation pattern, reticulation, basketweave...). Some traits are neglected such as the number of opisthosomal setae (9 vs 10) or the epimeral setae. The new nomenclature is simpler than in the past (there were 3 synonymies and 1 homonymy for Venilia and 3 chaetotaxies for the same genus, Afrotydeus) and accords with ICZN. A type species is fixed for all genera. The number of genera (31) and non-nominal subgenera (3) remains almost the same as in the past, even after the nomenclatural changes above-mentioned.
Any classification—whatever it is—is a taxonomy based on our current knowledge of mites and not a classification of mites themselves. Our knowledge is so meager (3.72% of morphospecies, still less if cryptic species are considered) that it allows to explore evolutionary trends of some traits but not to define a real phylogeny of species. Lastly, the classification proposed does not reflect the intra-population variations, nor the variations both within and between species. Species names are discrete units in a continuous world with fuzzy boundaries, a fortiori that applies to genus names. Acarology is still in its infancy...
Acknowledgements
I wish to thank D. Nicolson and G.D. Ower for providing the basic database as well as K.-H. Schmidt for suggesting the amendment. Special PDFs were sent by A.A. Khaustov, I. Łaniecka, R. Łaniecki, R. Ochoa. I am grateful to those who read and analyzed drafts of the ms: G.W. Krantz who supervised in 1968 my master's thesis on Tydeidae, F.A. Hernandes, A.A. Khaustov, R. Ochoa, A.W. Ulsamer and the two reviewers selected by the journal.
References
- Abliz O., Huimin S., Squires V. 2014. Mites (Acari): Small but significant ecosystem components. in Soils, Plant Growth and Crop Production, Squires V.R.(Ed.), in Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Paris.
- Akbari A., Irani-Nejad K.H., Khanjani M., Arzanlou M., Kaźmierski A. 2015a. A new tydeid species (Acari: Tydeidae) with a key to Brachytydeus species from East Azerbaijan Province, Iran. Systematic and Applied Acarology, 20(4): 423-443. https://doi.org/10.11158/saa.20.4.7
- Akbari A., Irani-Nejad K.H., Khanjani M., Arzanlou M., Kaźmierski A. 2015b. Tydeus shabestariensis sp. nov. and description of the male of Neopronematus sepasgosariani (Acari: Tydeoidea), with a key to the Iranian species of Tydeus. Zootaxa, 4032(3): 264-276. https://doi.org/10.11646/zootaxa.4032.3.2
- Akyazı R., Soysal M., Ueckermann E.A. 2024. Mite species of kiwi vines in Türkiye. Acarologia, 64(4): 1030-1051. https://doi.org/10.24349/9lvs-4bzy
- Akyazı R., Ueckermann E.A., Akyol D., Soysal M. 2017. Distribution of mite species (Acari) on persimmon trees in Turkey (Ordu), with one newly recorded mite species and one re-described species. International Journal of Acarology, 43(8): 1-19. https://doi.org/10.1080/01647954.2017.1373149
- Altunç Y.E., Akyazı R., Łaniecka I., Ueckermann, E.A., Kaźmierski, A. 2024. Discovery of two new tydeoid mite species (Acariformes: Prostigmata) on stone fruit trees in Türkiye. International Journal of Acarology, 29(12): 1685-1695. https://doi.org/10.11158/saa.29.12.9
- André H. 1979 (1978). A generic revision of the family Tydeidae (Acari : Actinedida). I. Introduction, paradigms and general classification. Annales de la Société royale zoologique de Belgique, 108 : 189-208.
- André H.M. 1980. A generic revision of the family Tydeidae (Acari: Actinedida). IV. Generic descriptions, keys and conclusions. Bulletin et Annales de la Société Royale Belge d'Entomologie, 116: 103-130, 139-168.
- André H.M. 1981a. A generic revision of the family Tydeidae (Acari : Actinedida). II. Organotaxy of the idiosoma and gnathosoma. Acarologia, 22: 31-46.
- André H.M. 1981b. A generic revision of the family Tydeidae (Acari : Actinedida). III. Organotaxy of the legs. Acarologia, 22: 165-178.
- André H.M. 1984. Tydeinae (Acari : Tydeidae) from Belgium. I. The genus Homeotydeus. Bulletin et Annales de la Société Royale Belge d'Entomologie, 120: 117-122.
- André H.M. 1985a. Acari domum meliponinarum brasiliensium habitantes. 10. Melissotydeus macrosolenus gen. n., sp. n. (Acari: Tydeidae). Bulletin & Annales de la Société Royale Belge d'Entomologie, 21: 243-246.
- André H.M. 1985b. Redefinition of the genus Triophtydeus Thor, 1932 (Acari : Actinedida). Zoologische Mededelingen, 59(16): 189-195.
- André H.M. 2005. In search of the true Tydeus (Acari: Tydeidae). Journal of natural History, 39: 975-1001. https://doi.org/10.1080/00222930400002838
- André H.M. 2011. Dugès' caudatus is a Tenuipalpidae and not a Tydeidae (Acari). Acarologia, 51(1): 69-85. https://doi.org/10.1051/acarologia/20111990
- André H.M. 2021. The Tydeoidea (Ereynetidae, Iolinidae, Triophtydeidae and Tydeidae) - An online database in the Wikispecies platform. Acarologia, 61(4): 1023-1035. https://doi.org/10.24349/6yc5-1lxw
- André H.M. 2023. Lorryia (Acariformes, Tydeidae): The evolutionary plasticity of an enigmatic genus. Acarologia, 63(3): 844-855. https://doi.org/10.24349/6btk-vacc
- André H.M., Ducarme X. 2003. Rediscovery of the genus Pseudotydeus (Acari: Tydeoidea), with description of the adult using digital imaging. Insect Systematics & Evolution, 34: 373-380. https://doi.org/10.1163/187631203X00027
- André H.M., Fain A. 1991. Ontogeny in the Tydeoidea (Ereynetidae, Tydeidae and Iolinidae). - In: Dusbabek & Bukva (Eds), Modern Acarology. Academia Prague. 2: 297-300.
- André H.M., Fain A. 2000. Phylogeny, ontogeny and adaptive radiation in the superfamily Tydeoidea (Acari: Actinedida), with a reappraisal of morphological characters. Zoological Journal of the Linnean Society, 130: 405-448. https://doi.org/10.1111/j.1096-3642.2000.tb01636.x
- André H.M., N'Dri J.K. 2013 (2012). Bréviaire de taxonomie des acariens. Abc Taxa 13, Bruxelles, Belgique. http://www.taxonomy.be/gti_abctaxa/volumes.
- André H.M., Van Impe G. 2012. The missing stase in spider mites (Acari: Tetranychidae): when the adult is not the imago. Acarologia, 52: 3-16. https://doi.org/10.1051/acarologia/20122038
- André M., Naudo M.H. 1965. Pertydeus schusteri, n. sgen., n. sp., nouveau Tydeus à griffe pulvillaire (Tydeidae). Acarologia, 7(4): 673-682.
- Athias-Henriot C. 1975. The idiosomatic euneotaxy and epineotaxy in gamasids (Arachnida: Parasitiformes). Zeitschrift für Zoologische Systematik und Evolutionforschung, 13: 97-109. https://doi.org/10.1111/j.1439-0469.1975.tb00503.x
- Baker E.W. 1944. Tideidos mexicanos (Acarina, Tydeidae). Revista de la Sociedad Entomologica Mexico, 5: 73-82.
- Baker E.W. 1965. A review of the genera of the family Tydeidae (Acarina). In: Naegele JA, editor. Advances in acarology 2. Ithaca (NY): Cornell University Press. p 96-133.
- Baker E.W. 1968. The genus Lorryia. Annals of the Entomological Society of America, 61(4): 986-1008. https://doi.org/10.1093/aesa/61.4.986
- Baker E.W. 1970. The genus Tydeus: subgenera and species group with descriptions of new species (Acarina: Tydeidae). Annals of the Entomological Society of America, 63: 163-177. https://doi.org/10.1093/aesa/63.1.163
- Baker E.W., Wharton G.W. 1952. An introduction to acarology. Macmillan, New York.
- Banks N. 1904. Four New Species of Injurious Mites. The New York Entomological Society, 12: 53-56.
- Beaulieu F., Knee W., Nowell V., Schwarzfeld M., Lindo Z., Behan-Pelletier V.M., Lumley L., Young M., Smith I., Proctor H.C., Mironov S., Galloway T., Walter D., Lindquist E. 2019. Acari of Canada. ZooKeys 819: 77-168. https://doi.org/10.3897/zookeys.819.28307
- Bebber D.P., Marriott F.H.C., Gaston K.J., Harris S.A., Scotland R.W. 2007. Predicting unknown species numbers using discovery curves. Proceedings of the Royal Society B: Biological Sciences, 274 (1618): 1651-1658. https://doi.org/10.1098/rspb.2007.0464
- Berlese A. 1908. Elenco di generi e specie nuove di Acari. Redia, 5(1): 1-15.
- Berlese A. 1910. Acari nuovi. Redia, 6(2): 199-234, plates XVIII-XXI.
- Berlese A. 1925. Gli Insetti. Vol. 2. Società Editrice Libraria, Milano.
- Brickhill C.D. 1958. Biological studies of two species of tydeid mites from California. Hilgardia, 27: 601-620. https://doi.org/10.3733/hilg.v27n20p601
- Canestrini G. 1886a. Prospetto dell'Acarofauna Italiana. Parte 2, Famiglie : Erythraeini, Cheyletini, Bdellini, Eupodini е Analgesini. Padova \textless s.n.> : 159-311.
- Canestrini G. 1886b. Prospetto dell'Acarofauna italiana (Continuazione). − Famiglia degli Eupodini. Atti del Reale Istituto Veneto di Scienze, Lettere ed Arti (ser. 6), 4(5): 693-734 (plus 3 plates at the end of the volume).
- Canestrini G. 1892. Prospetto dell'Acarofauna Italiana. Parte V. Famiglia dei Phytoptini (Phytoptidae). Atti della Societá Veneto-Trentina di Scienze naturali, Padova(ser. 2), 1(1): 543-722 + plates 44-59.
- Chandra K., Gupta D., Gopi K.C., Tripathy B., Kumar V. 2018. Faunal Diversity of Indian Himalaya: 1-872. (Published by the Director, Zoological Survey of India, Kolkata).
- Cooreman J. 1958. Notes et observations sur les acariens. VII. Photia graeca n. sp. (Acaridiae, Canestriniidae) et Lorryia formosa n. sp. (Stomatostigmata, Tydeidae). Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Entomologie 34: 1-10.
- Darbemamieh M., Hajiqanbar H., Khanjani M., Kaźmierski A. 2016. Paurotyndareus, a new genus of the family Iolinidae (Acari: Prostigmata), with the description of a new species from Iran. Systematic and Applied Acarology, 21(4): 398-404. https://doi.org/10.11158/saa.21.4.2
- Daston L., Galison P.L. 2007. Objectivity. Princeton University Press, Princeton.
- de Lillo H., Pozzebon A., Valenzano D., Duso C. 2018. An intimate relationship between eriophyoid mites and their host plants-a review. Frontiers in plant science, 9, 1786: 1-14. https://doi.org/10.3389/fpls.2018.01786
- De Vis R.M.J., Vervaet L., Van Leeuwen T., de Freitas Bragac A., Raphael de Campos Castilhoc R., Ueckermann E.A. 2024. Description of two new genera, three new species, and redescription of two species of Pronematinae (Acari: Iolinidae). Acarologia, 64(4): 1063-1099. https://doi.org/10.24349/z0vq-ji83
- Doweld A.B. 2016. Kuznetsovia, a new generic replacement name for Aenigma Kuznetsova, 1957 (Ostracoda) non Newman, 1836 (Coleoptera). Zootaxa 4114(5): 581-582. https://doi.org/10.11646/zootaxa.4114.5.4
- Dubois A. 1988. The genus in zoology: A contribution to the theory of evolutionary systematics. Mémoires du Muséum National d'Histoire Naturelle (A), 140: 1-124. https://doi.org/10.1163/9789004627260
- Dubois A. 2011. The International Code of Zoological Nomenclature must be drastically improved before it is too late. Bionomina, 2: 1-104 https://doi.org/10.11646/bionomina.2.1.1
- Dugès A. 1834. Recherches sur l'ordre des Acariens en général et la famille des Trombidiés en particulier (Premier mémoire). Annales des Sciences Naturelles, Zoologie (série 2), 1: 5-46.
- Edie S.M., Smits, Peter D., Jablonski, D. 2017. Probabilistic models of species discovery and biodiversity comparisons. Proceedings of the National Academy of Sciences, 114: 3666-3671. https://doi.org/10.1073/pnas.1616355114
- El-Bagoury M.E., Momen F.M. 1990. Neoapolorryia gen. n. of the family Tydeidae from Egypt (Acari: Tydeoidea). Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg, 10 (138): 25-27.
- Escobar-Garcia H.A., Andrade D.J. de, Ueckermann E.A., André H.M. 2023. A new species of the genus Brachytydeus Thor sensu André (Acari: Tydeidae) and a key to all known species from Peru. Zootaxa, 5319 (2): 263-274. https://doi.org/10.11646/zootaxa.5319.2.7
- Fain A. 1972. Developpement postembyronnaire chez les Acariens de la sous-famille Speleognathinae (Ereynetinae: Trombidiformes). Acarologia, 13: 607-614.
- Fraenkel C., Pfeiffer, R. 1892. Mikrophotographischer Atlas der Bakterienkunde. Verlag von August Hirschwald, Berlin. https://doi.org/10.5962/bhl.title.107522
- Gerson U. 1968. Five tydeid mites from Israel (Acarina: Prostigmata). Israel Journal of Zoology, 17: 191-198.
- Grandjean F. 1938. Observations sur les Tydeidae (2e série). Bulletin du Muséum national d'histoire naturelle, 10: 593-600.
- Grandjean F. 1942. La signification évolutive des écarts individuels. Comptes-Rendus de l'Académie des Sciences, Paris, Sér. III, 215: 216-220.
- Grandjean F. 1952. Sur les variations individuelles. Vertitions (écarts) et anomalies. Comptes Rendus de l'Académie des Sciences de Paris, 235 : 640-642.
- Halliday R.B. 1998. Mites of Australia: A Checklist and Bibliography. CSIRO Publishing, Clayton South. https://doi.org/10.1071/9780643105195
- Halliday R.B. 2010.Taxonomic confusion surrounding mite pests of sugarcane and rice (Acari: Eriophyidae). Systematic & Applied Acarology, 15: 257-262. https://doi.org/10.11158/saa.15.3.11
- Halliday R.B., Kamran M., Bashir M.H. 2018. Checklist of the mites of Pakistan. Zootaxa 4464: 1-178. https://doi.org/10.11646/zootaxa.4464.1.1
- Heethoff M., Norton R.A., Scheu S., Maraun M. 2009. Parthenogenesis in Oribatid Mites (Acari, Oribatida): Evolution Without Sex. In: Schön I., Martens K., van Dijk P. (eds.) Lost Sex - The Evolutionary Biology of Parthenogenesis. Springer, pp. 241-257. https://doi.org/10.1007/978-90-481-2770-2_12
- Heinsius H.W. 1891. Eenige gevallen van symbiose in het plantenrijk. Album der natuur, 40(1): 195-207.
- Hernandes F.A., Feres R.J.F., Nomura F. 2006. Biologycal cycle of Lorryia formosa (Acari, Tydeidae) on rubber tree leaves: a case of thelytoky. Experimental and Applied Acarology, 38, 237-242. https://doi.org/10.1007/s10493-006-0014-2
- Hoy M.A. 2011. Agricultural Acarology: Introduction to Integrated Mite Management. Boca Raton, CRC Press.
- ICZN. 2008. OPINION 2190 (Case 3354). Tydeus Koch, 1836 (Arachnida, Acari): Tydeus spathulatus Oudemans, 1928 designated as the type species. Bulletin of zoological nomenclature, 65(1): 68. https://doi.org/10.21805/bzn.v65i1.a14
- Janetschek H. 1967. Arthropod ecology of South Victoria Land. Antarctic research series 10: 205-293. https://doi.org/10.1029/AR010p0205
- Jeppson L.R., Baker E.W., Keifer, H.H. 1975. Mites Injurious to Economic Plants. University of California Press, Berkely. https://doi.org/10.1525/9780520335431
- Kamali K., Ostovan H., Atamehr A. 2001. A catalog of mites and ticks (Acari) of Iran. Islamic Azad University Scientific Publication Center, Tehran, 192 pp.
- Kammerer C.F. 2006. Notes on some preoccupied names in Arthropoda. Acta Zootaxonomica Sinica 31: 269-271.
- Kaźmierski A. 1980. Materiały do znajomości fauny Tydeidae (Acari: Prostigmata) w Polsce. I. Rodzaj Lorryia Oudemans. Prace Komisji Biologicznej, Towarzystwo Przyjaciół Nauk Poznańskie (PTPN), 54: 87-129.
- Kaźmierski A. 1981 (1980). Description of Venilia longina sp. nov. (Acari, Actinedida: Tydeidae) with a key to all species of the genus. Bulletin de l'Académie Polonaise des Sciences, 28(10-11): 647-652.
- Kaźmierski A. 1989. Revision of the genus Tydeus Koch sensu André, Homeotydeus André and Orthotydeus André with description of a new genus and our new species of Tydeinae (Acari: Actinedida: Tydeidae). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, 86: 289-314.
- Kaźmierski A. 1996a. A revision of the subfamilies Pretydeinae and Tydeinae (Acari, Actinedida: Tydeidae). Part II. The subfamilies Pretydeinae André, 1979 new taxa, species review, key and considerations. Mitteilungen aus dem Hamburger Zoologischen Museum und Institut 93: 171-198.
- Kaźmierski A. 1996b. A revision of the subfamilies Pretydeinae and Tydeinae (Acari, Actinedida: Tydeidae). Part III. Seven new genera and some new species of the Tydeinae, with a generic key. Mitteilungen Hamburgisches Zoologisches Museum und Institut 93: 199-227.
- Kaźmierski A. 1998a. Tydeinae of the world: generic relationships, new and redescribed taxa and keys to all species. A revision of the subfamilies Pretydeinae and Tydeinae (Acari: Actinedida: Tydeidae)-Part IV. Acta Zoologica Cracoviensia, 41: 283-455.
- Kaźmierski A. 1998b. A review of the genus Proctotydaeus Berlese (Actinedida: Tydeidae: Pronematinae). Acarologia, 39: 33-47.
- Khaustov A.A. 2022. A new genus and species of Tydeidae (Acari: Prostigmata) from Western Siberia, Russia. Persian Journal of Acarology 11(1): 1-10. https://doi.org/10.24349/1dwx-x5h2
- Khaustov A.A. 2023. New data to the fauna of Tydeidae (Acari: Prostigmata) of Western Siberia, Russia with discovery of secondary sexual dimorphism. Systematic & Applied Acarology, 28(8): 1335-1343. https://doi.org/10.11158/saa.28.8.5
- Khaustov A.A., Hugo-Coetzee E.A., Ermilov S.G. 2020. A new species of Lorryia (Acari: Tydeidae) from a termite nest in South Africa. Acarina, 28(1): 47-53. https://doi.org/10.21684/0132-8077-2020-28-1-47-53
- Khaustov A.A., Khaustov V.A 2023. The first faunistic data on Tydeidae (Acari: Prostigmata) from the Altay, Russia. Acarina, 31(2): 249-270. https://doi.org/10.21684/0132-8077-2023-31-2-249-270
- Knop N., Hoy M. 1983. Biology of a tydeid mite, Homeopronematus anconai (n. comb.) (Acari: Tydeidae), important in San Joaquin Valley vineyards. Hilgardia, 51(5): 1-30. https://doi.org/10.3733/hilg.v51n05p030
- Koch C.L. 1836. Deutschlands Crustaceen, Myriapoden und Arachniden. Ein Beitrag zur deutschen Fauna, Heft 137. Regensburg, Herrich-Schäffer.
- Koch C.L. 1850. Übersicht des Arachnidensystems (Fünftes Heft). Verlag von J. L. Lotzbeck, Nürnberg.
- Kramer P.M. 1877. Grundzüge zur Systematik der Milben. Archiv für Naturgeschichte. Berlin, 43: 215-247.
- Krantz G.W. 2009. Introduction. In Krantz & Walter (2009).
- Krantz G.W., Walter D. (Eds) 2009. A Manual of Acarology (3rd edition). Texas Tech University Press, Lubbock.
- Kuznetsov N.N. 1973. [A new subgenus and two new species of the family Tydeidae (Acariformes) from Crimea]. Zoologicheskiy zhurnal, 52 (10): 1577-1579. [in Russian].
- Kuznetsov N.N. 1975. [New genus and species of Tydeidae (Acariformes) of the Crimean fauna]. Zoologicheskiy zhurnal, 54(8): 1255-1257. [in Russian].
- Kuznetsov N.N. 1979. [On revision of the family Tydeidae (Acariformes)]. Zoologichesky Zhurnal, 58(9): 1413-1415. [In Russian].
- Kuznetzov N.N. 1980. [Adaptive peculiarities of ontogenesis in Tydeidae (Acariformes) ]. Zoologicheskii Zhurnal, 59: 1018-1023 [in Russian].
- Larsen B.B., Miller E.C., Rhodes M.K., Wiens J.J. 2017. Inordinate Fondness Multiplied and Redistributed: the Number of Species on Earth and the New Pie of Life. The Quarterly Review of Biology 92(3): 229-265. https://doi.org/10.1086/693564
- Latour B. 2012. Preface to the French translation of Daston & Galison. Les Presses du réel, Dijon, France.
- Lawing A.M., Meik J.M., Schargel, W.E. 2008. Coding meristic characters for phylogenetic analysis: a comparison of step-matrix gap-weighting and generalized frequency coding. Systematic Biology, 57(1):167-173. https://doi.org/10.1080/10635150801898938
- Leponce M., Noti M.-I., Bauchau V., Wauthy G. 2001. Vertition of integumental organs in mites revisited: a case of fluctuating asymmetry. Comptes-Rendus de l'Académie des Sciences, Paris, Sciences de la vie, 324: 425-431. https://doi.org/10.1016/S0764-4469(01)01314-2
- Liguori M., Simoni S. & Castagnoli M. 2002. Aspects of life history of Tydeus californicus (Banks) (Acari tydeidae). Redia, 85 : 143-153.
- Lin J.-Z. & Zhang Z.-Q. 2010. Tydeoidea of China: a review of progress, with a checklist. Zoosymposia 4: 51-56. https://doi.org/10.11646/zoosymposia.4.1.4
- Lindquist E.E. 2001. Poising for a new century: diversification in acarology. In: R.B. Halliday, D.E. Walter, H.C. Proctor, R.A. Norton, and M.J. Colloff (Eds), Acarology: Proceedings of the 10th International Congress. 1998, Canberra, Australia. CSIRO Publishing, Melbourne. Pp. 17-34.
- Lindquist E. E., Sabelis M. W., Bruin J. (Eds) 1996. Eriophyoid Mites: Their Biology, Natural Enemies and Control. Amsterdam: Elsevier Science Publishers.
- Linn C., O'Malley A., Khatri K., Wright E.M., Sebagh D., Grbić M., Kowal K., Chruszcz M. Microscopic Menaces: The Impact of Mites on Human Health. International Journal of Molecular Sciences, 25(7), 3675. https://doi.org/10.3390/ijms25073675
- Lundström A.N. 1887. Pflanzenbiologische Studien. II: Die Anpassungen der Pflanzen an Thiere. Acta Universitatis Upsaliensis, Nova acta Regiae Societatis scientiarum upsaliensis, 3: 1-87.
- Meyer M.K.P., Ryke P.A.J. 1959. New species of mites of the families Tydeidae and Labidostommidae (Acarina: Prostigmata) collected from South African plants. Acarologia, 1: 408-420.
- Momen F.M., El-Baghouri M.E. 1989. A new tydeid mite, Tydeus longichelus sp.n. from Egypt (Acari, Tydeidae). Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg, 9(135): 225-227.
- Momen F.M., El-Baghouri M. 1994. A new tydeid mite, Tydeus kattai sp. n., from Egypt (Acari, Tydeidae). EEntomologische Mitteilungen aus dem Zoologischen Museum Hamburg,, 11(150): 127-131.
- Momen F.M., Lundqvist L. 1993. Inconsistencies in leg chaetotaxy in two species of Tydeid mites (Prostigmata: Tydeidae). International Journal of Acarology, 19(2): 137-144. https://doi.org/10.1080/01647959308683972
- Momen F.M., Lundqvist L. 1996. A new genus, Quadrotydeus, and three new species of the family Tydeidae (Acari: Prostigmata) from Southern Sweden. International Journal of Acarology, 22: 3-10. https://doi.org/10.1080/01647959608684074
- Momen F.M., Solhøy T. 1996. A first record of the genus Tydeus in Himalaya, Tydeus lundqvisti nov. spec. (Acari: Actinedida: Tydeidae). Acarologia, 37(1): 23-25.
- Momen F.M., Sinha R.N. 1991. Tydeid mites associated with stored grain and oilseeds in Canada, with descriptions of a new genus and five new species (Acari: Tydeidae). Canadian Journal of Zoology, 69: 1226-1254. https://doi.org/10.1139/z91-175
- Mondin A. de S., Nuvoloni F.M., Feres, R.J.F. 2016. Four new species of Lorryia (Acari: Tydeidae) associated with Hevea brasiliensis Muell. Arg. (Euphorbiaceae) in Brazil. Zootaxa, 4158(4): 473-490. https://doi.org/10.11646/zootaxa.4158.4.2
- Moniez R. 1889. Les Parasites de l'homme (animaux et végétaux). Paris, Librairie J.-B. Baillière et fils.
- Moniez R. 1894. Histoire naturelle du Tydeus molestus, acarien qui s'attaque à l'homme. Revue biologique du nord de la France, 6: 419-434.
- Mullen G.R., OConnor, B.M. 2019. Chapter 26. Mites (Acari). In: Mullen G.R., Durden L.A. (Eds). Medical and Veterinary Entomology (3rd ed.). Academic Press, London. https://doi.org/10.1016/B978-0-12-814043-7.00026-1
- Mwase E.T., Baker A. 2006. An annotated checklist of mites (Arachnida: Acari) of Zambia. Zootaxa 1106: 1-24. https://doi.org/10.11646/zootaxa.1106.1.1
- Nordenskiöld E. 1900. Anteckningar om Acarider samlade i hö. Meddelanden af societas pro fauna et flora fennica, 1899-1900: 34-38.
- Norton R.A., Kethley J.B. 1994. Ecdysial cleavage lines of acariform mites (Arachnida, Acari). Zoologica Scripta, 23(3): 175-191. https://doi.org/10.1111/j.1463-6409.1994.tb00383.x
- Nuvolini F.M., Mondin, A. de S., Feres R.J.F. 2020. Review of Lorryia Oudemans, 1925 (Acari: Tydeidae: Tydeinae) associated with Hevea spp. in Brazil. International Journal of Acarology, 46(4): 235-240. https://doi.org/10.1080/01647954.2020.1760931
- Oudemans A.C. 1925. Acarologische aanteekeningen LXXIX. Entomologische Berichten, 7(145): 26-34.
- Oudemans A.C. 1928a (1927). Acari uit Ambon. Zoologische Mededelingen, 10: 185-237.
- Oudemans A.C. 1928b. Acarologische aanteekeningen XCIV. Entomologische Berichten, 7: 374-382.
- Oudemans A.C. 1929. Acarologische aanteekeningen XCVIII. Entomologische Berichten, 7(168): 476-485.
- Oudemans A.C. 1937. Kritisch historisch Overzicht der Acarologie. Derde Gedeelde, Band C. Leiden: E. J. Brill. p I-XXIII, 799-1348. https://doi.org/10.1163/9789004629813
- Parker S.P. 1982. Synopsis and classification of living organisms (vol. 2). McGraw-Hill, New York.
- Principato M., Masini P., Stingeri L., Assalve D. 2008. Dermopatia prodotta dalle punture di Tydeus molestus (Acari: Tydeidae) nell'uomo. Atti del VIII Congresso Nazionale Sidapa, Firenze, 23-25 Ottobre 2008.
- Principato M., Scriboni A. 2009. Presenza di Tydeus molestus (Acarina : Tydeidae) in una scuola romana. GSA Igiene Urbana 4: 46-47.
- Pritchard A.E, Baker E.W. 1955. A revision of the spider mite family Tetranychidae. Pacific Coast Entomological Society, Memoirs Series, 2: 1-472. https://doi.org/10.5962/bhl.title.150852
- Ratcliff M.J. 2009. The quest for the invisible. Microscopy in the enlightenment. Ashgate Publishing Ltd.
- Ripka G., Laniecka I., Kaźmierski A. 2013. On the arboreal acarofauna of Hungary: Some new and rare species of prostigmatic mites (Acari: Prostigmata: Tydeidae, Iolinidae and Stigmaeidae). Zootaxa 3702 (1): 1-50. https://doi.org/10.11646/zootaxa.3702.1.1
- Ross H. 1904. Die Gallenbildungen (Cecidien) der Pflanzen, deren Ursachen, Entwickelung, Bau und Gestalt. Verlag von Eugen Ulmet, Stuttgart.
- Saccaggi D.L., Ueckermann E.A. 2024. The problem of taxonomic uncertainty in biosecurity: South African mite interceptions as an example. Acarologia, 64(2): 363-369. https://doi.org/10.24349/top1-r59v
- Schiess T. 1981. Neue Tydeidenarten (Acari, Actinedida, Tydeidae) aus einem alpinen Rasen (Caricetum firmae, 2500 m) des Schweizer Nationalparkes. Entomologica Basiliensia, 6: 78-107.
- Schrank F. von P. 1781 Enumeratio insectorum Austriae indigenorum. Cum figuris. Eberhard Klett et Franck, Augustae Vindelicorum.: [i-xxii], 1-548, [1-2], pls. 1-4.
- Seniczak A.B., Seniczak S., Schwarzfeld M.D., Coulson S.J., Gwiazdowicz D.J. 2020. Diversity and Distribution of Mites (Acari: Ixodida, Mesostigmata, Trombidiformes, Sarcoptiformes) in the Svalbard Archipelago. Diversity, 12 (9): 323. https://doi.org/10.3390/d12090323
- Silva D.E., Silva R.T.L., Paz-Neto A.A., Pinheiro G.M., Granich J., Ferla N.J. 2024. Mites in chocolate production system: A case study in southern Brazil. Systematic and Applied Acarology, 29(4): 501-510. https://doi.org/10.11158/saa.29.4.4
- Silva G.L. da, Cunha U.E. da, Ferla N.J. 2014. Life cycle of Tydeus californicus (Acari: Tydeidae) on leaves of Inga marginata with and without pollen of Typha angustifolia under laboratory conditions. International Journal of Acarology, 40(7): 509-512. https://doi.org/10.1080/01647954.2014.953999
- Silva G.L. da, Metzelthin M.H., Silva O.S. da, Ferla N.J. 2016. Catalogue of the mite family Tydeidae (Acari: Prostigmata) with the world key to the species. Zootaxa, 4135(1): 1-68. https://doi.org/10.11646/zootaxa.4135.1.1
- Slingerland E. 2003. Confucius. Analects: With Selections from Traditional Commentaries. Hackett Publishing Company, Cambridge, MA.
- Smiley R.L. 1987. Mites (Acari). In Gorham J.R. (ed.). Insect and Mite Pests in Food: An Illustrated Key. U.S. Department of Agriculture, Agriculture. Handbook Number 655: 3-43.
- Spain, A.V. 1969. A new genus and species of Tydeidae (Acari: Prostigmata) from New Zealand. Acarologia, 11: 23-28.
- Stål C. 1865. Hemiptera Africana. Vol. 3. Ex officina Norstedtiana (Holmiæ). [Norstedts, Stockholm].
- Stepanyan N., Zarikian N. 2024. Exploring Tydeoidea mites in Armenia. Euroasian Entomological Journal, 23(4): 215-219. https://doi.org/10.15298/euroasentj.23.04.05
- Starr M.A., Strong O.S., Leaming E. 1896. Atlas of nerve cells. Macmillan and Co., London.
- Szudarek-Trepto N., Kaźmierski A., Skoracka A., Lewandowski M., Dabert J. 2022. Molecular phylogeny supports the monophyly of the mite supercohort Eupodides (Acariformes: Trombidiformes) and greatly coincides with traditional morphological definition of the taxon. Annales Zoologici, 72(4): 757-786. https://doi.org/10.3161/00034541ANZ2022.72.4.001
- Tempfli B., Pènzes B., Fail J., Szabó Á. 2015. The occurrence of tydeoid mites (Acari: Tydeoidea) in Hungarian vineyards. Systematic and Applied Acarology, 20(8): 937-954. https://doi.org/10.11158/saa.20.8.9
- Thor S. 1931. Norwegische Tydeidae. I-VII. Mit Kennzeichnung vier neuer Gattungen. Zoologischer Anzeiger, 94: 89-104.
- Thor S. 1933. Tydeidae, Ereynetidae. Das Tierreich, 60: 1-84.
- Treat A.E. 1970. Two tydeid mites from the ears of noctuid moths. American Museum Novitates, 2426: 1-14.
- Ueckermann E.A. (Ed.) 2010. Eriophyoid Mites: Progress and Prognoses. Springer Nature. https://doi.org/10.1007/978-90-481-9562-6
- Ueckermann E.A., Çobanoğlu S., Sağlam, H.D. 2019. Re-description of two new tydeid records (Acari: Trombidiformes) with a key to Tydoidea species of Turkey. Systematic & applied Acarology, 24(3): 497-450. https://doi.org/10.11158/saa.24.3.13
- Ueckermann E.A., Grout, T.G. 2007. Tydeoid mites (Acari:Tydeidae, Edbakerellidae, Iolinidae) occurring on Citrus in southern Africa. Journal of Natural History, 41: 2351-2378. https://doi.org/10.1080/00222930701589921
- Ueckermann E.A., Smith Meyer M.K.P. 1988. South African Acari. IV. Some mites of the Addo Elephant National Park. Koedoe, 31: 31-51. https://doi.org/10.4102/koedoe.v31i1.483
- Vacante V. 2016. The handbook of mites of economic plants: identification, bio-ecology and control. Wallingford, CABI. https://doi.org/10.1079/9781845939946.0001
- Walter D.E., Behan-Pelletier V. 1999 Mites in forest canopies: filling the size distribution shortfall? Annual Review of Entomology, 44: 1-19. https://doi.org/10.1146/annurev.ento.44.1.1
- Walter D.E., Proctor H.C. 2013. Mites: Ecology, Evolution, and Behaviour: Life at a Microscale. 2nd Edition. Dordrecht (The Netherlands): Springer. https://doi.org/10.1007/978-94-007-7164-2
- Wergin W.P., Ochoa R., Erbe E.F., Craemer C., Raina A.K. 2000. Use of low-temperature field emission scanning electron microscopy to examine mites. Scanning, 22(3):145-155. https://doi.org/10.1002/sca.4950220301
- Wharton G.W. 1964. Presidential address. In Woolley T.A. (ed.). 1st International Congress of Acarology, Proceedings, Fort Collins, Colorado, USA, 2-7 September 1963. Acarologia, 6 (h.sre): 37-43.
- Willmann C. 1952. Die Milbenfauna der Nordseeinsel Wangerooge. Veröffenlichungen des Institute für Meeresforschung in Bremerhaven 1: 139-186, plts 26-28.
- Zachos F.E. 2016. Species Concepts in Biology: Historical Development, Theoretical Foundations and Practical Relevance. Springer International Publishing Switzerland.
- Zachos F.E., Apollonio M., Bärmann E., Festa-Bianchet M., Göhlich U., Habel J., Haring E., L. Kruckenhauser L., Lovari S., Mcdevitt A.D., Pertoldi C., Rössner G.E., Sánchez-Villagra M., Scandura M., Suchentrunk, F. 2013. Taxonomic inflation, the Phylogenetic Species Concept and lineages in the Tree of Life - a cautionary comment on species splitting. Journal of Zoological Systematics and Evolutionary Research, 53(2): 180-184. https://doi.org/10.1111/jzs.12088
- Zhang Z.-Q., Fan Q.-H., Pesic V., Smit H., Bochkov A.V., Khaustov A.A., Baker A., Wohltmann A., Wen T., Amrine J.W., Beron P., Lin J., Gabrys G., Husband R. 2011. Order Trombidiformes Reuter, 1909. In: Zhang Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 129-138. https://doi.org/10.11646/zootaxa.3148.1.24



2024-12-18
Date accepted:
2025-02-25
Date published:
2025-03-21
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 André, Henri M.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



