Predatory potential of Neoseiulus longispinosus (Acari: Phytoseiidae) following exposure to fungicides
Sharma, Prajjval  1
; Sharma, Prem Lal2
; Verma, Subhash Chander3
; Chandel, Rajeshwar Singh4
and Sharma, Shubham
1
; Sharma, Prem Lal2
; Verma, Subhash Chander3
; Chandel, Rajeshwar Singh4
and Sharma, Shubham  5
5
1Department of Entomology. Dr. Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP) 173230, India.
2Department of Entomology. Dr. Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP) 173230, India.
3Department of Entomology. Dr. Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP) 173230, India.
4Department of Entomology. Dr. Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP) 173230, India.
5✉ Department of Entomology. Dr. Y S Parmar University of Horticulture and Forestry, Nauni, Solan (HP) 173230, India.
2025 - Volume: 65 Issue: 1 pages: 116-129
https://doi.org/10.24349/p0dq-gb80Original research
Keywords
Abstract
Introduction
Integrated Pest Management (IPM) is an important approach that combines biological, cultural, and chemical methods to effectively manage pest populations, while reducing the sole reliance on pesticides (de Araújo et al. 2024; Wang et al. 2024). This strategy is widely implemented in economically significant crops to minimize costs and achieve sustainable pest control (Elhalawany et al. 2024b; Noorinahad et al. 2024). Tomato (Solanum lycopersicum L.) is one of the world's most important vegetable crops, both economically and nutritionally, ranking second only to potato in terms of area and production (Ouattara and Konate 2024). However, tomato cultivation faces significant threats from various pests and diseases throughout its growth cycle (Shirvani et al. 2023b; Islam et al. 2024). Among these, fungal pathogens can cause substantial crop losses if left unmanaged (Panno et al. 2021). In response, farmers often resort to chemical fungicides, which, despite advancements in more sustainable pest control approaches, remain essential for managing fungal outbreaks (Ye et al. 2020). In addition to fungal pathogens, invertebrate pests including spider mites such as Tetranychus urticae Koch (Acari: Tetranychidae) also significantly affect tomato production (Monjarás-Barrera et al. 2024). These mites feed on chlorophyll, leading to leaf defoliation and reduced plant vigor, especially in the hot, dry conditions that favor tomato production (Nag et al. 2020; Bhullar et al. 2021).
Due to extensive pesticide use for mite management, T. urticae populations have developed resistance to various acaricides (Ay et al. 2024), necessitating the application of biological control strategies. Phytoseiid mites are considered as an excellent predators of spider mites in diverse cropping environments (Shirvani et al. 2023a; Dalir et al. 2024; Wang et al. 2024; Yaşar et al. 2024). The predatory mite, Neoseiulus longispinosus (Evans) (Acari: Phytoseiidae) has shown its effectiveness in reducing T. urticae populations, feeding on all life stages of this pest (Biswas et al. 2022). This phytoseiid predator is well-known in IPM programmes for tomato and other crops (Bernard et al. 2010). However, in tomato cultivation, the extensive use of conventional fungicides such as mancozeb, metalaxyl, thiophanate methyl, and copper oxychloride raises concerns about their potential non-target effects on natural enemies like N. longispinosus.
Estimating the predatory potential of a biocontrol agent against pests is vital for understanding its effectiveness in integrated pest management (IPM) programmes (Adly 2024; Elhalawany et al. 2024a). However, the presence of toxicants, such as pesticides or fungicides, can significantly alter the predatory potential of biocontrol agents. These chemicals may impair the agents' ability to locate, capture, or consume pests, thus reducing overall efficiency (Musa et al. 2023; Havasi and Kheradmand 2024). Furthermore, sublethal doses of toxicants may also affect the predatory potential due to changes in behaviour, reproduction, or survival of the predator, potentially compromising its role in pest management. Therefore, evaluating the impact of toxicants on predatory behavior is vital to ensure the compatibility of biocontrol agents with chemical pest control practices (Stark and Banks 2003). Given these considerations, the present study investigated the influence of commonly used fungicides on the predatory potential of N. longispinosus against T. urticae on tomato plants. This study aimed to understand the compatibility of chemical and biological control methods, which is useful for optimizing IPM strategies in tomato cropping systems.
Material and methods
The present study was conducted under laboratory conditions at a temperature of 25 ± 0.5 °C, 70 ± 5% relative humidity, and a 12-hour light: 12-hour dark photoperiod. The tomato and strawberry plants used for maintaining the mass culture and conducting experiments were grown in a polyhouse at the experimental farm of the Department of Entomology, Dr. YS Parmar University of Horticulture and Forestry Solan, Himachal Pradesh, India (1275 m above mean sea level; 31.28°N, 76.94°E).
Stock cultures
Neoseiulus longispinosus and Tetranychus urticae cultures were obtained from the Biocontrol Laboratory, Department of Entomology, Dr. YS Parmar University of Horticulture and Forestry, Solan, Himachal Pradesh, India. The T. urticae colony was initially inoculated and established on tomato plants (Solanum lycopersicum L., variety: Solan Lalima) cultivated in a polyhouse. In the laboratory, T. urticae was transferred to strawberry leaves, which were placed with the abaxial side facing up on a sponge saturated with water. After 2-3 days of infestation, N. longispinosus was introduced onto the strawberry leaves. To ensure a continuous food supply for the predatory mite, the older leaves were regularly replaced with fresh T. urticae-infested ones. Before being used in experiments, the T. urticae culture was transferred to tomato leaves and maintained for one generation. Similarly, N. longispinosus was placed on T. urticae-infested tomato leaves and maintained for one generation.
Treatment of Neoseiulus longispinosus with fungicides
To determine the impact of four fungicides on the predatory potential of Neoseiulus longispinosus, the wettable powder (WP) formulations of mancozeb 75% WP (Indofil M-45®; 2500 ppm), copper oxychloride 50% WP (Blitox® 50 W; 3000 ppm), thiophanate methyl 70% WP (Roko®; 1000 ppm), and metalaxyl 4% + mancozeb 64% WP (Ridomil Gold®; 2500 ppm) were used. Tomato leaf discs (3 cm × 3 cm) from the ′Solan Lalima′ variety were treated with these fungicides at the recommended doses using a modified leaf dip technique based on the method of Helle and Overmeer (1985). Each leaf disc was immersed in the fungicide solution for 15 seconds, while control discs were dipped in distilled water. After air drying for three hours, the treated discs were placed upside down on a damp sponge within experimental trays. Concurrently, tomato plants of the same variety grown in a polyhouse were sprayed with the respective fungicides using the knapsack sprayer for the residual toxicity experiments. For each treatment and control, 600 gravid females of N. longispinosus (5 days old) were individually placed on the treated leaf discs over a water saturated sponge in plastic trays. Each treatment included two experimental setups. In the first setup, 300 gravid females were provided with T. urticae eggs ad libitum as prey, while in the second setup, 300 N. longispinosus females were provided with T. urticae protonymphs ad libitum for a 48-hour exposure period. Six plastic trays, each containing 50 tomato leaf discs, were used for each setup within a treatment. To observe the mortality of N. longispinosus females after the exposure period, the leaves were transferred using forceps onto a wet sponge placed in a Petri plate and examined under a binocular microscope. The predatory mite females that exhibited no appendage movement when touched were considered dead. A cohort of 100 N. longispinosus eggs from each experimental setup were collected from the surviving females in each treatment and control group for further experiments.
Effect of fungicides on the predatory potential of Neoseiulus longispinosus
The effects of residual toxicity of the fungicides on the predatory potential of N. longispinosus against the eggs and protonymphs of T. urticae were evaluated. To account for population variability in each treatment and control group, only one egg per alive female was collected within a 12-hour period to form a cohort of 100 predator eggs. In each treatment, these predatory mite eggs were individually transferred to fresh tomato leaf discs (3 cm × 3 cm) from the fungicide-sprayed plants using a fine brush and a binocular microscope. In addition, the other progeny from these females were simultaneously reared as a reserve culture in their respective fungicidal and control groups to obtain males for future pairing. The leaf discs were placed upside down on a water-saturated sponge inside plastic trays. Unsprayed plant leaves were used for the control group. After egg hatching, the larval, protonymph, and deutonymph stages of N. longispinosus were each provided separately with 15 eggs and 10 protonymphs of T. urticae daily for each treatment and control group. Similarly, adult males of N. longispinosus were provided daily with 20 eggs and 15 protonymphs of T. urticae in different set ups of each treatment and control group. The males that emerged from the experimental cohort were used solely to collect data on prey consumption and not for mating. Upon reaching adulthood, the females were paired with males from the reserve culture of the same fungicidal group, and these pairs were kept together until death. Each pair was provided daily with 30 T. urticae eggs and 25 protonymphs. The leaf arenas and prey were replaced with fresh ones every 24 hours to ensure a continuous and adequate food supply for the predatory mite. The presence of exuviae on the leaf disc confirmed that moulting had occurred. The number of replicates for prey consumption by N. longispinosus in each treatment and the control group varied depending on stage-specific survival (n) of the predatory mite.
Data on prey consumption by different stages of the predatory mite were recorded every 24 hours until the last adult died. The daily prey consumption by adult predator females was assessed by subtracting the mean number of prey eaten by N. longispinosus males (kept alone) from the total number of prey consumed by the male-female predator pairs. This data was used to calculate the feeding potential parameters of N. longispinosus against the eggs and protonymphs of T. urticae. In these calculations, x represents the age in days, j denotes the stage, and m indicates the number of stages. The variables lx, sxj , and cxj correspond to age-specific survival, age-stage survival, and age-stage predation, respectively. The prey consumption by each stage of the predatory mite and following feeding potential parameters were analyzed using the computer program CONSUME-MSChart (Chi 2025a).
- i) Age-specific predation rate (kx ):
\[k_x=\frac{\sum_{j=1}^m s_{x j} c_{x j} }{\sum_{j=1}^m s_{x j} }\]
- ii) Net age-specific predation rate (qx ):
\[q_x=k_x l_x\]
- iii) Cumulative consumption (C0 ):
\[C_0=\sum q_x=\sum l_x k_x\]
- iv) Stable predation rate (ψ):
\[\psi=\sum_{x=o}^{\infty} \sum_{j=1}^\beta a_{x j} c_{x j}\]
- v) Finite predation rate (ω):
\[\omega=\lambda \psi\]
- vi) Transformation rate (Qp ):
\[Q_P=C_o / R_0\]
Projection of prey consumption
The prey consumption by N. longispinosus was projected over a period of 90 days using computer simulation program TIMING (Chi, 1990; Chi, 2025b) with an initial population of 10 predator eggs. The prey consumption (p) at time t was calculated as:
\[p(t)=\sum_{j=1}^m\left(\sum_{x=0}^{\infty} c_{x j} n_{x j, t}\right)\]
where, nxj,t is the number of individuals of age x and stage j at time t.
Data analysis
Means and standard errors (SE) of stage wise prey consumption and feeding potential parameters were determined using 100,000 bootstrap replicates. Differences in daily prey consumption by different predator stages among different treatments were analysed using a one-way ANOVA, followed by Tukey's HSD test (p < 0.05) in SPSS v26.0. Feeding potential parameters were compared with the help of a paired bootstrap test based on the 95% confidence interval of difference (p < 0.05) in TWO-SEXMS Chart computer program (Chi 2025c).
Results
Prey consumption
In all fungicidal treatments, Neoseiulus longispinosus larvae did not feed on either of the tested prey stages of Tetranychus urticae. Prey consumption by the predatory mite began at the protonymph stage. On T. urticae eggs, a significant reduction from control in the pre-adult prey consumption was observed in all fungicidal treatments (F4,339: 20.047; p < 0.001). The pre-adult consumption was lowest in the metalaxyl + mancozeb treatment (5.21 eggs) which was statistically similar with mancozeb (5.25 eggs) and copper oxychloride (5.61 eggs) treatments (Table 1). Similarly, in all fungicidal treatments, significant reductions from the control were recorded in egg consumption by adult females, adult males, and over the entire lifespan of N. longispinosus (Adult Female – F4,191: 31.138; p < 0.001; Adult Male – F4,143: 15.114; p < 0.001; Life-long – F4,339: 28.473; p < 0.001). The highest egg consumption across treatments by adult females (37.50 eggs) of the predatory mite occurred in the thiophanate methyl treatment which was at par with the copper oxychloride treatment (35.00 eggs) whereas, in metalaxyl + mancozeb and mancozeb treatments, significant reductions from control was observed in the prey consumption by adult females. This trend was also observed in the total life-long consumption of T. urticae eggs by N. longispinosus, with the maximum consumption recorded in the thiophanate methyl treatment (38.53 eggs) and the minimum in the metalaxyl + mancozeb treatment (31.86 eggs). Here, the life-long consumption includes both adult male and female individuals of N. longispinosus. Egg consumption by N. longispinosus males decreased significantly compared to the control and was statistically similar across all treatments.
Download as *Means in the same row followed by the same lowercase letter are statistically similar (p > 0.05), according to Tukey’s HSD test.
Developmental duration
n
Control
n
Mancozeb
n
Copper Oxychloride
n
Metalaxyl + Mancozeb
n
Thiophanate Methyl
Protonymph
84
2.70 ± 0.18a
68
1.80 ± 0.20b
76
2.36 ± 0.22a
68
1.89 ± 0.18b
84
2.45 ± 0.20a
Deutonymph
76
3.48 ± 0.30a
64
3.12 ± 0.12bc
72
2.95 ± 0.20c
56
3.00 ± 0.22bc
76
3.38 ± 0.14b
Pre-adult
76
6.68 ± 0.15a
64
5.25 ± 0.11c
72
5.61 ± 0.15bc
56
5.21 ± 0.15c
76
6.10 ± 0.13b
Adult Female
44
42.73 ± 1.61a
36
31.89 ± 2.22cd
44
35.00 ± 2.18bc
32
28.25 ± 2.12d
40
37.50 ± 1.81b
Adult Male
32
31.75 ± 1.91a
28
25.00 ± 1.80b
28
25.29 ± 1.51b
24
24.50 ± 1.40b
36
26.78 ± 1.30b
Life-long
76
44.79 ± 0.95a
64
34.12 ± 0.87cd
72
36.83 ± 1.01bc
56
31.86 ± 0.71d
76
38.53 ± 0.92b
When fed on the protonymphs of T. urticae, N. longispinosus showed a significant reduction in prey consumption during the pre-adult period in the mancozeb treatment (F4,311: 2.658; p = 0.033). However, prey consumption by the predatory mite throughout its lifespan, as well as by adult females and males, was significantly reduced compared to the control across all treatments (Adult Female – F4,179: 35.944; p < 0.001; Adult Male – F4,127: 21.843; p < 0.001; Life-long – F4,311: 20.577; p < 0.001). Among the fungicidal treatments, prey consumption of T. urticae protonymphs by adult N. longispinosus females and males was statistically similar. However, all fungicidal treatments showed a significant reduction compared to the control (Table 2).
Download as *Means in the same row followed by the same lowercase letter are statistically similar (p > 0.05), according to Tukey’s HSD test.
Developmental duration
n
Control
n
Mancozeb
n
Copper Oxychloride
n
Metalaxyl + Mancozeb
n
Thiophanate Methyl
Protonymph
80
2.09 ± 0.18a
64
1.95 ± 0.16a
68
2.00 ± 0.18a
56
1.72 ± 0.17a
76
1.91 ± 0.20a
Deutonymph
76
3.25 ± 0.16a
60
3.00 ± 0.18a
64
3.12 ± 0.19a
48
2.71 ± 0.12b
68
2.74 ± 0.24ab
Pre-adult
76
5.72 ± 0.15a
60
4.82 ± 0.12b
64
5.37 ± 0.15a
48
4.91 ± 0.09ab
68
5.29 ± 0.16a
Adult Female
44
33.82 ± 1.23a
36
24.00 ± 1.56b
36
26.56 ± 1.56b
28
23.29 ± 1.03b
40
29.20 ± 1.79b
Adult Male
32
22.75 ± 1.56a
24
14.17 ± 1.65b
28
16.14 ± 1.95b
20
13.60 ± 1.46b
28
17.71 ± 1.64b
Life-long
76
34.68 ± 0.88a
60
25.13 ± 0.89c
64
27.37 ± 0.97b
48
24.16 ± 0.84c
68
29.76 ± 1.04b
Feeding potential and projection of prey consumption
The feeding potential of N. longispinosus on T. urticae eggs and protonymphs was assessed using various parameters: Cumulative consumption (C0 ), Transformation rate (Qp ), Stable predation rate (ψ), and Finite predation rate (ω). The cumulative consumption of T. urticae eggs was highest in the thiophanate methyl treatment (C0 = 29.64 eggs/predator), which was statistically similar to the untreated control (Table 3). The transformation rate (Qp ) and stable predation rate (ψ) of the predatory mite in the copper oxychloride and thiophanate methyl treatments were also comparable to those of the untreated control. Among the fungicidal groups, the highest finite predation rate (ω) was observed in the thiophanate methyl treatment (ω = 0.87), which was on par with the control (ω = 0.97) and the copper oxychloride treatment (ω = 0.84). The lowest finite predation rate was recorded in the metalaxyl + mancozeb treatment (ω = 0.73), which was on par with the mancozeb treatment (ω = 0.87) (Table 3).
Download as *Means in each row followed by same letter are statistically similar (p > 0.05) by the paired bootstrap test based on the 95% confidence interval of difference.
Parameter
Control
Mancozeb
Copper Oxychloride
Metalaxyl + Mancozeb
Thiophanate Methyl
Cumulative consumption (C0)
34.36 ± 3.96a
22.04 ± 3.41bc
26.80 ± 3.52ab
18.40 ± 3.14c
29.64 ± 3.46a
Transformation rate (Qp)
4.21 ± 0.90a
5.62 ± 1.71a
4.47 ± 0.94a
6.05 ± 2.10a
4.75 ± 1.21a
Stable predation rate (ψ)
0.81 ± 0.05a
0.68 ± 0.05b
0.73 ± 0.04a
0.67 ± 0.06b
0.75 ± 0.04a
Finite predation rate (ω)
0.97 ± 0.07a
0.77 ± 0.07b
0.84 ± 0.06a
0.73 ± 0.07b
0.87 ± 0.06a
When fed T. urticae protonymphs, a significant reduction was observed in the stable predation rate (ψ) of N. longispinosus as compared to the control group in the metalaxyl + mancozeb treatment (ψ = 0.47) (Table 4). The cumulative consumption (C0 ) was highest in the thiophanate methyl treatment (C0 = 20.48), and showed no significant difference from the control (C0 = 26.60) and copper oxychloride treatment (C0 = 17.72). The transformation rate remained unaffected in all the fungicidal treatments. The finite predation rate (ω) was significantly reduced from the control in both the mancozeb (ω = 0.59) and metalaxyl + mancozeb (ω = 0.51) treatments (Table 4).
Download as *Means in each row followed by same letter are statistically similar (p > 0.05) by the paired bootstrap test based on the 95% confidence interval of difference.
Parameter
Control
Mancozeb
Copper Oxychloride
Metalaxyl + Mancozeb
Thiophanate Methyl
Cumulative consumption (C0)
26.60 ± 3.17a
15.44 ± 2.61b
17.72 ± 2.85ab
12.00 ± 2.47b
20.48 ± 3.05ab
Transformation rate (Qp)
3.41 ± 0.68a
4.39 ± 1.09a
3.75 ± 0.94a
4.55 ± 1.75a
3.63 ± 0.79a
Stable predation rate (ψ)
0.60 ± 0.04a
0.53 ± 0.04ab
0.54 ± 0.04ab
0.47 ± 0.05b
0.55 ± 0.04ab
Finite predation rate (ω)
0.71 ± 0.06a
0.59 ± 0.06b
0.62 ± 0.06ab
0.51 ± 0.06b
0.64 ± 0.06ab
The age-specific predation rates of N. longispinosus on T. urticae eggs and protonymphs are presented in Figures 1 and 2, respectively. These figures illustrate the trend in the mean number of prey consumed by the predator at each age (x). Using equations (i) and (ii), we calculated the age-specific predation rate (kx ) and the net age-specific predation rate (qx ) of the cohort, which were then plotted by combining all stages of the predatory mite. The net age-specific predation rate accounts for cohort survival, adjusting proportionately to reveal the predator's weighted predation rate. For both prey stages of T. urticae, the peak values of qx were lowest in the metalaxyl + mancozeb treatment due to higher mortality during the immature stages of the predatory mite.
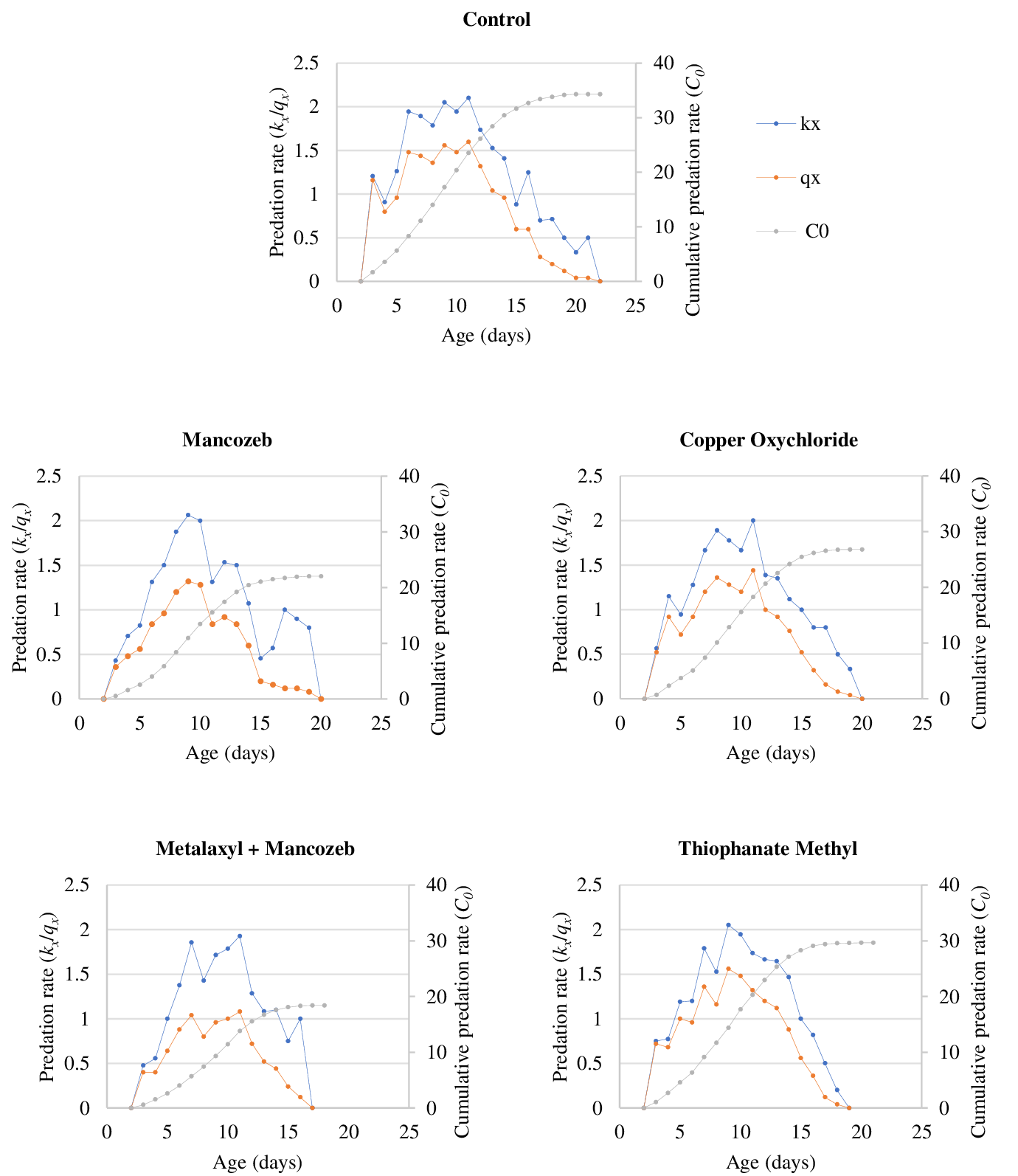


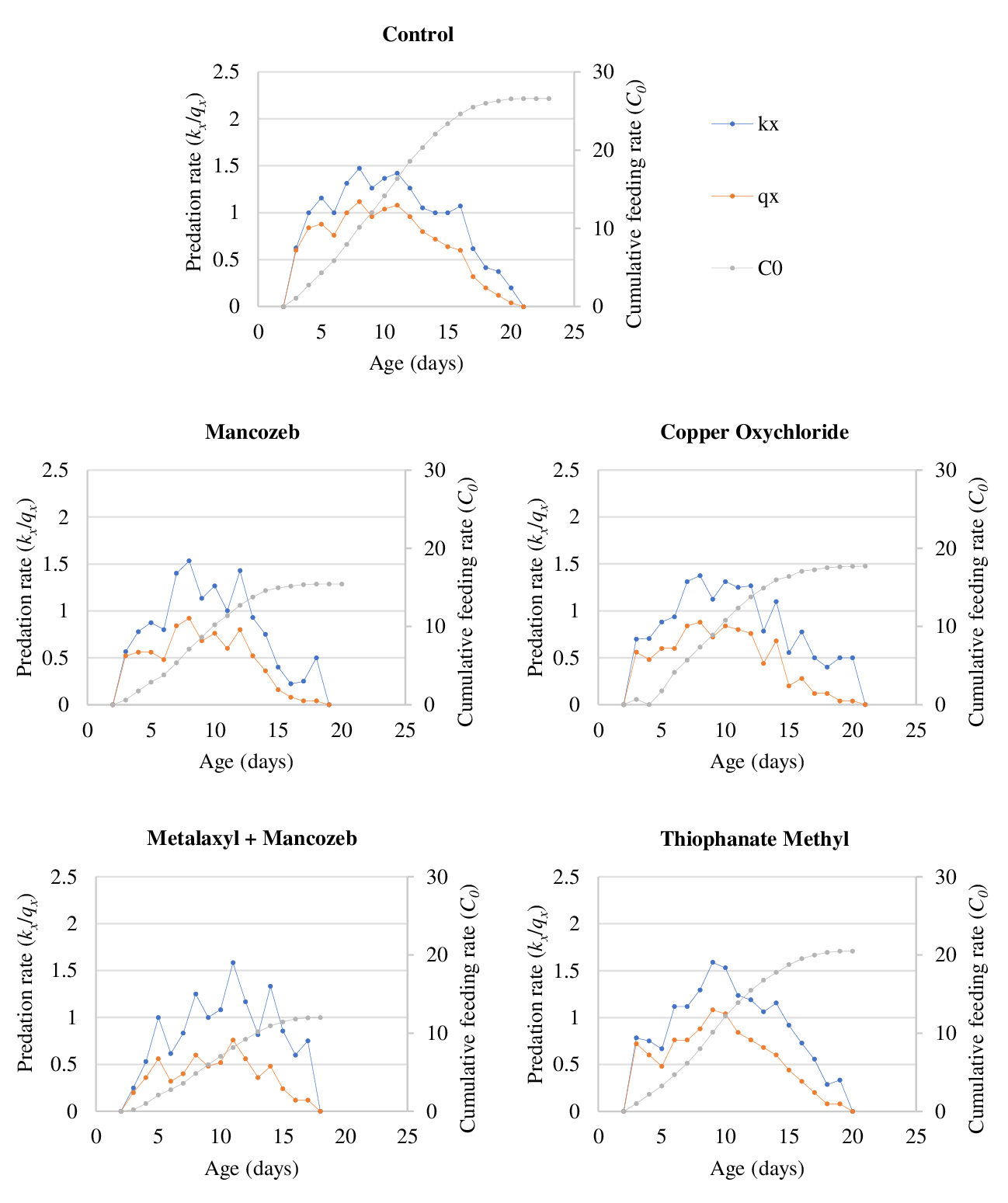


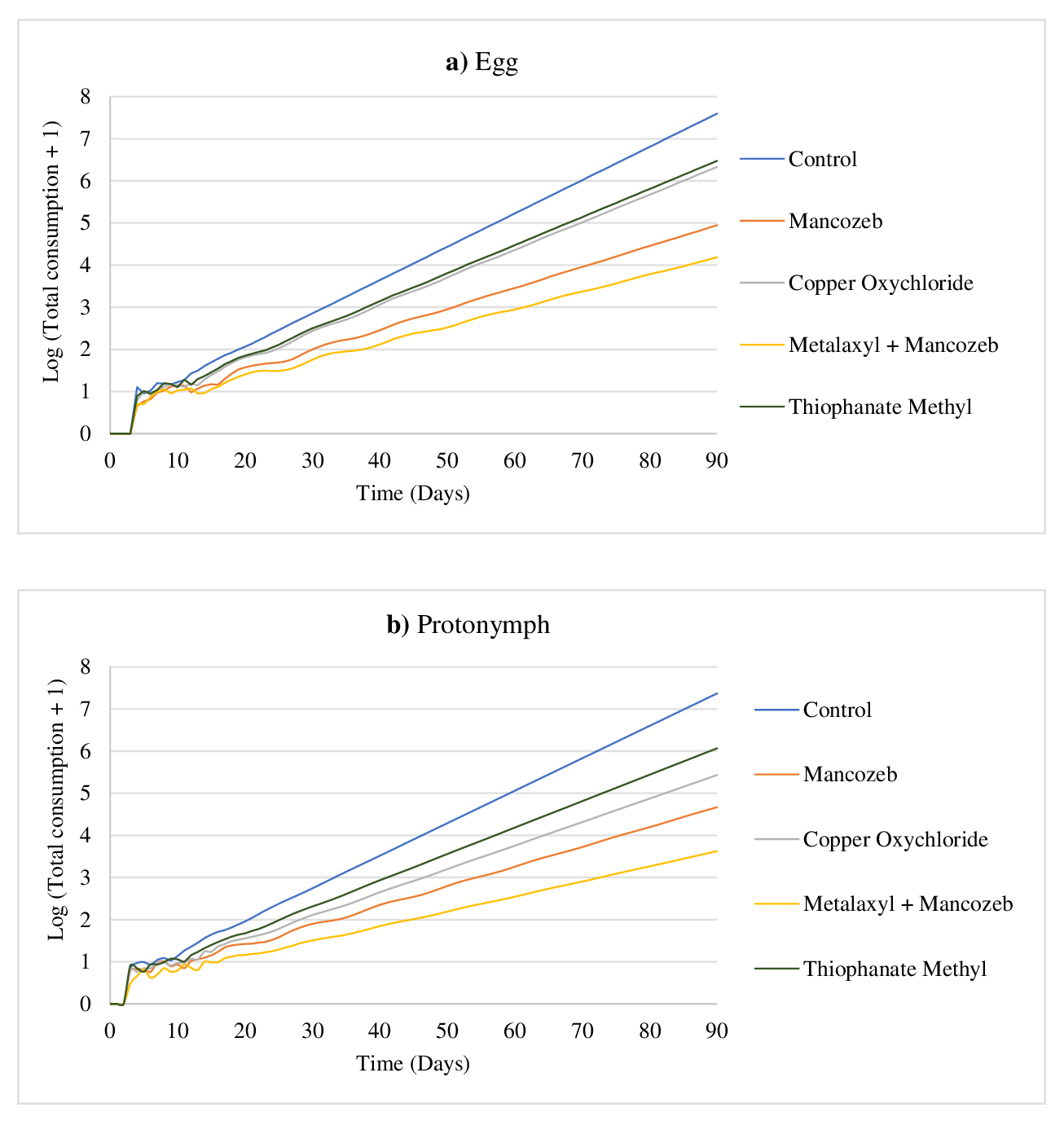
The prey consumption of N. longispinosus on the tested stages of T. urticae was projected on a log scale using prey consumption data from the various fungicidal treatments (Figure 3). Over a simulation period of 90 days, the maximum consumption of T. urticae eggs and protonymphs by N. longispinosus followed a similar trend across the different fungicidal treatments. On the 90th day, the projected consumption on the log scale was highest in the thiophanate methyl treatment (eggs: 6.47; protonymphs: 6.07), followed by copper oxychloride (eggs: 6.33; protonymphs: 5.43), mancozeb (eggs: 4.95; protonymphs: 4.67), and metalaxyl + mancozeb (eggs: 4.19; protonymphs: 3.62).


Discussion
The use of natural enemies in agro-ecosystems is an important component of Integrated Pest Management. This practice reduces the over dependence of farmers on pesticides during the management of insect pests and diseases (Crowther et al. 2024; Yaşar et al. 2024). Predatory arthropods contribute to pest mortality and alter pest behavior; however, the factors affecting the feeding behavior of natural enemies are less understood (Kasap et al. 2023; Pakyari and Zemek 2023; Pakyari et al. 2024). Therefore, assessing the predatory potential of natural enemies is essential for understanding their role in regulating pest populations and maintaining ecological stability within predator-prey dynamics (Ferreira et al. 2023; Sun et al. 2024). This predator-prey interaction forms the basis of biological control, where natural enemies are deployed to sustainably manage pest populations (Kheradmand et al. 2024). However, in agroecosystems where chemical treatments, such as fungicides, are frequently applied, it is important to understand how these treatments may impact predator efficiency and prey dynamics (Döker et al. 2024; Sharma et al. 2024c). The presence of toxicants on crop surfaces is a major factor that influences the predatory potential and preference of natural enemies (Havasi et al. 2023; Mousavi et al. 2023). The effects of these toxicants on the developmental and reproductive biology of non-target organisms are well studied (Döker et al. 2024), but their effects on prey consumption remain largely unknown for most of the natural enemy species. Our findings indicate that exposure of Neoseiulus longispinosus to field-relevant concentrations of fungicides led to a significant reduction in feeding across all developmental stages of the predator when fed on eggs and nymphs of T. urticae. These results highlight the importance of studying the effects of plant protection chemicals on the feeding behavior of natural enemies, in addition to their biological and demographic effects.
In our study, providing eggs and protonymphs to N. longispinosus on the fungicide-sprayed tomato leaves resulted in a significant reduction in total prey consumption by adult females and males. In contrast, the immature stages of N. longispinosus exhibited inconsistent trends among different fungicidal treatments. The feeding potential parameters of N. longispinosus were lower in metalaxyl + mancozeb and mancozeb treatments, while those in the copper oxychloride and thiophanate methyl treatments, were nearly comparable to the control. Previous studies have also reported pronounced effects of mancozeb on the biological performance of phytoseiids. These effects result directly from individual feeding, as exposure to toxicants reduced food uptake, thereby altering biological parameters (Hamedi et al. 2009; Sharma et al. 2024b). In the metalaxyl + mancozeb and mancozeb treatments, the cumulative consumption (C0 ) of the predatory mite significantly reduced on both prey stages. However, the copper oxychloride and thiophanate methyl treatments did not cause any significant changes. Cumulative consumption takes into account the survival of the population (lx ), and high mortality during the early stages causes a reduction in cumulative feeding (Huang et al. 2018; Sharma et al. 2024e). Therefore, higher pre-adult mortality due to chemical exposure caused a reduction in the C0 value in the treatments containing mancozeb. These results align with earlier reports, indicating, that mancozeb is toxic to various species of phytoseiids, such as Euseius victoriensis (Womersley) (Bernard et al. 2010), Amblyseius andersoni (Chant) (Ioriatti et al. 1992), and Typhlodromus pyri Scheuten (Gadino et al. 2011). The transformation rate (Qp ) describes the number of prey consumed to produce one predator egg and provides a demographic measure of the relationship between the reproduction rate and the predation rate of a predator (Chi and Yang, 2003). In our study, the transformation rate remained unaffected across all treatments for both eggs and protonymphs of T. urticae. In contrast, the finite predation rate (ω) and stable predation rate (ψ) were significantly reduced in the metalaxyl + mancozeb and mancozeb treatments. The finite predation rate that indicates the predation capacity of the predator, taking into consideration its age-stage structure, age-stage predation rate, and finite rate (Farhadi et al. 2011). Similarly, the stable predation rate reflects the total predation capacity of a stable predator population, where its total size is unity (Yu et al. 2005). All of these feeding potential parameters are vital tools for assessing predator capacity under varying environmental conditions.
Overall, our study shows inferior prey consumption by N. longispinosus across all treatments and the control. The leaves of tomato contain trichomes that have been reported to hinder the movement of different phytoseiid species, including N. longispinosus (Sharma et al. 2024a). The glandular and secondary volatile secretions from these trichomes further exacerbate this hindrance, reducing its performance on tomato plants compared to other host plants (Tabary et al. 2024). However, studies have shown that phytoseiids, including N. longispinosus are effective biocontrol agents of spider mites in challenging cropping environments (Azadi-Qoort and Sedaratian-Jahromi 2024). Since successful control in inoculative releases relies on the predator's subsequent generations to regulate pest populations, understanding predatory dynamics over an extended period is important (Akca et al. 2015). Therefore, we employed computer simulations to evaluate long-term trends in prey consumption. Projecting the consumption and population of an organism is a valuable tool that insights into the damage potential of a pest or the predatory potential of an organism, based on data obtained during a single generation (Chi 2025b; Sharma et al. 2024d). Computer simulations tracking the predatory mite's consumption over 90 days revealed the cumulative effects of residual fungicides. Mancozeb and copper oxychloride showed moderate reductions in mite consumption, while metalaxyl + mancozeb had a more pronounced inhibitory effect. Thiophanate methyl caused the least reduction in prey consumption over the 90-day period.
Conclusion
Our study, which focused on the residual effects of a single application of fungicides, indicated that the predatory mite could continue to feed and control prey mites when exposed to all tested fungicides. However, since multiple applications are often required for effective disease control, the feeding potential of the predatory mite may be further affected. Therefore, additional research is needed to assess the long-term and cumulative impacts of repeated fungicide applications on the feeding behavior and biological control capacity of predatory mites. The variability in predatory responses across different developmental stages and fungicide treatments further illustrates the complexity of predator-prey interactions under chemical exposure. The reduction in cumulative consumption and finite predation rates highlights the potential long-term consequences of fungicide use on predator efficiency. Efforts should be made to use low-risk insecticides and fungicides, along with management practices that are more compatible with natural enemies, ensuring sustainable pest control while maintaining ecological stability in agroecosystems.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
Not Applicable
Acknowledgments
The authors are thankful to Dr. YS Parmar University of Horticulture and Forestry, Nauni, Solan, Himachal Pradesh, India for providing necessary facilities to carry out the study.
Author contributions
Prajjval Sharma: Writing, Investigation and methodology; Prem Lal Sharma: supervision, methodology and editing; Subhash Chander Verma: supervision and editing; Rajeshwar Singh Chandel: supervision and formal analysis; Shubham Sharma: Investigation, writing-review and data analysis. All authors have read and approved the final manuscript.
References
- Adly D. 2024. Evaluate the efficiency of releasing two predatory species at their optimal temperature for controlling Tetranychus urticae (Acari: Tetranychidae) in a croton greenhouse. Persian Journal of Acarology, 12(2): 315-326. https://doi.org/10.22073/pja.v12i2.78030
- Akca I., Ayvaz T., Yazici E., Smith C.L., Chi H. 2015. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): with additional comments on life table research criteria. Journal of Economic Entomology, 108(4): 1466-1478. https://doi.org/10.1093/jee/tov187
- Ay R., Çevik B., Alsay S. 2024. The roles of the biochemical and molecular changes in resistance of Tetranychus urticae populations selected with spiromesifen+abamectin mixture. Systematic and Applied Acarology, 29(1): 30-44. https://doi.org/10.11158/saa.29.1.3
- Azadi-Qoort A., Sedaratian-Jahromi A. 2024. Assessing predation parameters of the predatory mite Typhlodromus bagdasarjani (Acari: Phytoseiidae) on different host plants. Persian Journal of Acarology, 13(1), 131-143. https://doi.org/10.22073/pja.v13i1.83219
- Bernard M.B., Cole P., Kobelt A., Horne P.A., Altmann J., Wratten S.D., Yen, A.L. 2010. Reducing the impact of pesticides on biological control in Australian vineyards: Pesticide mortality and fecundity effects on an indicator species, the predatory mite Euseius victoriensis (Acari: Phytoseiidae). Journal of Economic Entomology, 103(6): 2061-2071. https://doi.org/10.1603/EC09357
- Bhullar M.B., Heikal H.M., Kaur P., Kaur R. 2021. Efficacy of natural products and biorationals against two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) infesting brinjal (Solanum melongena L.) under protected cultivation. International Journal of Acarology, 47(8): 677-683. https://doi.org/10.1080/01647954.2021.1987982
- Biswas S., Bhullar M.B., Karmakar K., Kaur P. 2022. Seven new records and distribution of phytoseiid (Acari: Mesostigmata) mite fauna associated with agri-horticultural crops in Northern India. International Journal of Tropical Insect Science, 42(3): 2425-2442. https://doi.org/10.1007/s42690-022-00770-1
- Chi H. 1990. Timing of control based on the stage structure of pest populations: a simulation approach. Journal of Economic Entomology, 83(4): 1143-1150. Available from: https://doi.org/10.1093/jee/83.4.1143
- Chi H. 2025a. CONSUME-MS Chart: Computer program for consumption rate analysis based on the age stage, two-sex life table. https://lifetablechi.com/wp-content/uploads/2025/01/Consume-MSChart.zip
- Chi H. 2025b. Timing-MS chart: computer program for population projection based on age-stage, two-sex life table. https://lifetablechi.com/wp-content/uploads/2025/02/TIMING-MSChart.zip
- Chi H (2025c) TWOSEX-MS Chart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. https://lifetablechi.com/wp-content/uploads/2025/02/TWOSEX-MSChart-setup.zip
- Chi H., Yang T.C. 2003. Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environmental Entomology, 32: 327-333. https://doi.org/10.1603/0046-225X-32.2.327
- Crowther L.I., Wilby A., Wilson K. 2024. Combining biological control approaches for managing insect crop pests in the field can generate interactive effects. Agricultural and Forest Entomology, 26(4): 470-484. https://doi.org/10.1111/afe.12639
- Dalir S., Fathipour Y., Khanamani M., Hajiqanbar H. 2024. Assessing performance of Amblyseius swirskii as a predatory mite of Tetranychus urticae and Frankliniella occidentalis: life table and foraging behaviour studies. International Journal of Acarology, 50(7): 587-594. https://doi.org/10.1080/01647954.2024.2385605
- de Araújo W.A., Fernandes M.G., Degrande P.E., Salustino A. da S., Neto D.F.C., Malaquias J.B. 2024. Exploring the impact of cover crops in integrated pest management: pest and natural enemies population dynamics in no-tillage cotton production. Bulletin of Entomological Research, 114(4): 581-590. https://doi.org/10.1017/S0007485324000452
- Döker İ., Yavaş H., Shirvani Z., Karaca M.M., Karut K., Marčić D., Kazak C. 2024. Toxicity and risk assessment of pesticides on the predatory mite, Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) under laboratory conditions. Phytoparasitica, 52: 97 https://doi.org/10.1007/s12600-024-01217-8
- Elhalawany A.S., Ibrahim N.A., Amer A.I., Abdel-Khalik A.R. 2024a. Efficacy of Amblyseius swirskii, Neoseiulus californicus (Acari: Phytoseiidae), and acaricides in controlling some pests on sweet pepper in greenhouses. Persian Journal of Acarlogy, 13(2): 317-334.
- Elhalawany A.S., Sanad A.S., Kassem E.M.K. 2024b. Efficiency of Neoseiulus californicus (McGregor) (Acari: Phytoseiidae) for controlling three plant-feeding mites on guava trees in Egypt. International Journal of Acarology, 50(8): 714-720. https://doi.org/10.1080/01647954.2024.2404134
- Farhadi R., Gholizadeh M., Chi H., Mou D-F., Allahyari H., Yu J-Z., Huang Y-B., Yang T-C. et al. 2011. Finite Predation Rate: A novel parameter for the quantitative measurement of predation potential of predator at population level. Nature Precedings. https://doi.org/10.1038/npre.2011.6651.1
- Ferreira C.T., Noronha A.C.S., Batista T.F.V. 2023. Population dynamics of Aceria guerreronis and its natural enemies in coconut tree with and without application of pesticides. Systematic and Applied Acarology, 28(7): 1261-1271. https://doi.org/10.11158/saa.28.7.5
- Gadino A.N., Walton V.M., Dreves, A.J. 2011. Impact of vineyard pesticides on a beneficial arthropod, Typhlodromus pyri (Acari: Phytoseiidae), in laboratory bioassays. Journal of Economic Entomology, 104: 970-977. https://doi.org/10.1603/EC10330
- Hamedi N., Fathipour Y., Saber M., Garjan A.S. 2009. Sublethal effects of two common acaricides on the consumption of Tetranychus urticae (Prostigmata: Tetranychidae) by Phytoseius plumifer (Mesostigmata: Phytoseiidae). Systematic & Applied Acarology, 14: 197-205. https://doi.org/10.11158/saa.14.3.4
- Havasi M., Bandani A.R., Golpayegani A.Z. 2023. The impact of Cyflumetofen on demographic parameters of two predatory mites, Neoseiulus californicus (M-G) and Phytoseiulus persimilis (A-H). Systematic and Applied Acarology, 28(3): 483-496. https://doi.org/10.11158/saa.28.3.6
- Havasi M., Kheradmand K. 2024. The effects of cyflumetofen on life history traits and population parameters of Amblyseius swirskii. Systematic and Applied Acarology, 29(3): 376-387. https://doi.org/10.11158/saa.29.3.2
- Helle W., Overmeer W.P.J. 1985. Toxicological test methods. In: Helle W., Sabelis M.W. (Eds). Spider Mites, Their Biology, Natural Enemies and Control. Elsevier: Amsterdam. p. 3991-3995.
- Huang H-W., Chi H., Smith C.L. 2018. Linking Demography and Consumption of Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae) Fed on Solanum photeinocarpum (Solanales: Solanaceae): With a new method to project the uncertainty of population growth and consumption. Journal of Economic Entomology, 111(1): 1-9. https://doi.org/10.1093/jee/tox330
- Ioriatti C., Pasqualini E., Toniolli A. 1992. Effect of fungicides mancozeb and dithianon on mortality and reproduction of the predatory mite Amblyseius andersoni. Experimental & Applied Acarology, 15: 109-116. https://doi.org/10.1007/BF01275521
- Islam Y., Shah F.M., Güncan A., Naeem A., Zhou X. 2024. Temperature-induced effects on development, reproduction, and predation of Harmonia axyridis fed on first instar larvae Spodoptera litura. Bulletin of Entomological Research, 114(2): 244-253. https://doi.org/10.1017/S0007485324000051
- Kasap İ., Kök Ş., Pehlivan S. 2023. Effect of temperature on the life history and development of Typhlodromus athiasae Porath and Swirski (Acari: Phytoseiidae) as a predator of Tetranychus urticae Koch (Acari: Tetranychidae). Systematic and Applied Acarology, 28(10): 1668-1677. https://doi.org/10.11158/saa.28.10.7
- Kheradmand K., Heidari M., Sedaratian-Jahromi A., Talaei-Hassanloui R., Havasi M. 2024. How does Neoseiulus californicus McGregor respond to sublethal doses of entomopathogenic fungus Beauveria bassiana (Hyp.: Cordycipitaceae)? Bulletin of Entomological Research, 114(5): 598-605. https://doi.org/10.1017/S0007485324000397
- McMurtry, J.A., Sourassou, N.F., Demite, P.R. 2015. Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms. In: Carrillo, D., de Moraes, G.J., Pena, J.E., (Eds.). The phytoseii- dae (Acari: Mesostigmata) as biological control agents, 133-149. Switzerland, Springer, 309 pp. https://doi.org/10.1007/978-3-319-15042-0_5
- Monjarás-Barrera, Irving J., Sanchez-Peña, Sergio R. 2024. First record of the tomato red spider mite, Tetranychus evansi Baker & Pritchard (Acari: Tetranychidae) in Mexico, from cultivated and wild solanaceous plants. Acarologia, 64(1): 164-171. https://doi.org/10.24349/78jc-6nev
- Mousavi A., Kheradmand K., Fathipour Y., Mosallanejad H., Havasi M. 2023. The effects of the abamectin and spirodiclofen mixture on life history and population parameters of Amblyseius swirskii. Systematic and Applied Acarology, 28(5): 971-984. https://doi.org/10.11158/saa.28.5.16
- Musa A., Međo I., Marić I., Marčić D. 2023. Sterilization makes a difference: demographic analysis of spirodiclofen effects on Tetranychus urticae (Acari: Tetranychidae). Acarologia, 63(3): 955-968. https://doi.org/10.24349/qvon-22mi
- Nag S., Bhullar M.B., Kaur P. 2020. Efficacy of biorationals against two-spotted spider mite, Tetranychus urticae Koch, (Acari: Tetranychidae) infesting green pepper cultivated under protected conditions. International Journal of Acarology, 46(7): 489-495. https://doi.org/10.1080/01647954.2020.1811762
- Noorinahad S., Shakarami J., Bazgir F. 2024. Demographic parameters of Tetranychus turkestani (Trombidiformes: Tetranychidae) on different cultivars of greenhouse cucumber. International Journal of Acarology, 50(3): 189-197. https://doi.org/10.1080/01647954.2024.2311659
- Ouattara S.S.S., Konate M. 2024. The tomato: A nutritious and profitable vegetable to promote in Burkina Faso. Alexandria Science Exchange Journal, 45(1): 11-20. https://doi.org/10.21608/asejaiqjsae.2024.332758
- Pakyari H., Arba, A., Sedaratian-Jahromi A. 2024. The influence of photoperiod on development and population growth performance of the Phytoseiulus persimilis fed on Tetranychus urticae. International Journal of Acarology, 50(4): 362-369. https://doi.org/10.1080/01647954.2024.2330944
- Pakyari H., Zemek R. 2023. Effects of visible light wavelength on development and demographic parameters of Phytoseiulus persimilis (Acari: Phytoseiidae). Systematic and Applied Acarology, 28(12): 1843-1854. https://doi.org/10.11158/saa.28.12.2
- Panno S., Davino S., Caruso A.G., Bertacca S., Crnogorac A., Mandi A., Noris E., Mati S. 2021. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy, 11: 2188. https://doi.org/10.3390/agronomy11112188
- Sharma N., Sharma P.L., Verma S.C., Palial S. Sharma P. 2024a. Biology, demographic parameters and predatory potential of the predatory mite Neoseiulus longispinosus against Tetranychus urticae on different vegetable crops. Phytoparasitica, 52, 47. https://doi.org/10.1007/s12600-024-01165-3
- Sharma P., Sharma P.L., Sharma S., Verma S.C., Chandel R.S., Sharma N., Sharma P. 2024b. Toxicity and demographic effects of fungicides on Neoseiulus longispinosus (Evans) (Acari: Phytoseiidae). Systematic and Applied Acarology, 29(8): 1091-1105. https://doi.org/10.11158/saa.29.8.4
- Sharma P., Sharma P.L., Verma S.C., Sharma N., Sharma P., Thakur S., Sharma S. 2024c. Effect of fungicides on the functional response of Neoseiulus longispinosus (Phytoseiidae) to Tetranychus urticae (Tetranychidae) eggs. International Journal of Acarology, 50(2): 151-158. https://doi.org/10.1080/01647954.2024.2306145
- Sharma S., Sharma P.L., Sharma P., Verma S.C., Sharma N., Sharma P. 2024d. Demographic analysis and biotic potential of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) on pea. Bulletin of Entomological Research, 114(4): 514-523. https://doi.org/10.1017/S0007485324000312
- Sharma S., Sharma P.L., Verma S.C., Sharma D., Devi M., Sharma N., Sharma P., Thakur S., Sharma P. 2024e. Linking demography and food consumption to project population growth and damage potential of Spodoptera frugiperda in India. Agricultural and Forest Entomology, 26(4): 555-571. https://doi.org/10.1111/afe.12648
- Shirvani Z., Allahyari H., Golpayegani A.Z., Jahromi K.T., Döker I. 2023a. Influence of sub-lethal exposure to Rosmarinus officinalis L. (Lamiaceae) essential oil on demographic parameters of Amblyseius swirskii Athias-Henriot (Acari:Phytoseiideae). International Journal of Acarology, 49: 270-276. https://doi.org/10.1080/01647954.2023.2244505
- Shirvani Z., Döker I., Karut K., Kazak C. 2023b. Foraging behavior of Amblyseius swirskii (Acari: Phytoseiidae) feed on the invasive pest Tetranychus evansi (Acari: Tetranychidae) on tomato. Systematic and Applied Acarology, 28(2): 223-235. https://doi.org/10.11158/saa.28.2.6
- Stark J.D., Banks, J.E. 2003. Population-level effects of pesticides and other toxicants on arthropods. Annual Review of Entomology, 48: 505-519. https://doi.org/10.1146/annurev.ento.48.091801.112621
- Sun L., Fan G-C., Zheng Y-Q., Chen D-S., Zheng H-Q., Zhang H., Chen X. 2024. Spirodiclofen toxicity in Tetranychus urticae and safety to natural enemies Proprioseiopsis asetus and Amblyseius swirskii. Systematic and Applied Acarology, 29(9): 1231-1243. https://doi.org/10.11158/saa.29.9.4
- Tabary L., Navajas M., Tixier M-S., Denise N. 2024. Tomato trichomes: trade-off between plant defenses against pests and benefits for biological control agents. Acarologia, 64(4), 1232-1253. https://doi.org/10.24349/ej2w-b311
- Wang H., Wang H., Wen K., Xie T., Luo S., Wu J., Xia B. 2024. Lethal and sublethal concentrations spirodiclofen stress may increase the adaptation of Panonychus citri (Acari: Tetranychidae). Bulletin of Entomological Research, 114(5): 591-597. https://doi.org/10.1017/S0007485324000087
- Yaşar İ., Kök Ş., Kasap İ. 2024. Investigation of the synergistic effect of two predatory mites, Phytoseiulus persimilis and Amblyseius swirskii (Acari: Phytoseiidae), in the biological control of Tetranychus urticae (Acari: Tetranychidae). International Journal of Acarology, 50(3): 315-319. https://doi.org/10.1080/01647954.2024.2320782
- Ye Q., Wang R., Ruan N., Yao Z., Cheng Y., Wan H., Li Z., Yang Y., Zhou G. 2020. Genetic diversity and identification of wilt and root rot pathogens of tomato in China. Plant Disease, 104: 1715-1724. https://doi.org/10.1094/PDIS-09-19-1873-RE
- Yu J.Z., Chi H., Chen B.H. 2005. Life table and predation of Lemnia biplagiata (Coleoptera: Coccinellidae) fed on Aphis gossypii (Homoptera: Aphididae) with a proof on relationship among gross reproduction rate, net reproduction rate, and preadult survivorship. Annals of the Entomological Society of America, 98: 475-482. https://doi.org/10.1603/0013-8746(2005)098%5B0475:LTAPOL%5D2.0.CO;2



2024-12-09
Date accepted:
2025-02-18
Date published:
2025-02-24
Edited by:
Marčić, Dejan

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Sharma, Prajjval; Sharma, Prem Lal; Verma, Subhash Chander; Chandel, Rajeshwar Singh and Sharma, Shubham
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



