The effects of microemulsion formulations of some botanical acaricides on life table parameters of Amblyseius swirskii (Acari: Phytoseiidae)
Mertoglu Boz, Gamze  1
and Kumral, Nabi Alper
1
and Kumral, Nabi Alper  2
2
1Ataturk Horticultural Central Research Institute, 77102, Yalova, Türkiye.
2✉ Department of Plant Protection, Faculty of Agriculture, Uludag University, Gorukle Campus, Bursa, 16059, Türkiye.
2025 - Volume: 65 Issue: 1 pages: 36-51
https://doi.org/10.24349/knoe-ox3vOriginal research
Keywords
Abstract
Introduction
Plant-sucking insects and mites cause serious damage to eggplants Solanum melongena L. (Solanaceae) throughout the world. Amongst them, Tetranychus urticae Koch (Acari: Tetranychidae), Frankliniella occidentalis (Pergande), Thrips tabaci L. (Thysanoptera: Thripidae), Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae), Bemisia tabaci (Gennadius), are mainly eggplant pests (Grewal 1992; Kapoor et al. 1997; Chung et al. 2000; Leite et al. 2003; Kumral and Kovancı 2005; Baradaran et al. 2009; Can and Çobanoğlu 2010; Lee et al. 2010; Hasanuzzaman et al. 2016; Kumral and Çobanoğlu 2016). Because of feeding on the chlorophyll in the cells of eggplant leaves, their damages lead to high fruit losses (Tomczyk and Kropczynska 1985; Touhidul and Shunxiang 2009).
For pest management, the improper and intensive use of insecticides and acaricides causes both reduced natural enemy populations and increased pest resistance to chemical pesticides (Simon 2014; Van Leeuwen et al. 2010; Whalon et al. 2024). Instead of relying on a single method, integrated strategies such as ''Integrated Pest Management (IPM)'' have been suggested for their control in recent years.
As an alternative to the synthetic chemicals, an effective predatory mite, Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae), is commercially produced and its augmentative release is performed for controlling whiteflies, thrips and mites (Messelink et al. 2008; Stansly et al. 2009; Shibao et al. 2009; Kilincer et al. 2010; Chow et al. 2010; Park et al. 2010; Xu and Enkegaard 2010; Onzo et al. 2012). It is also a highly adaptable type 3 generalist predator, capable of preying on a wide range of pests as well as non-prey resources such as pollen. This flexibility enables it to survive during periods of prey scarcity (McMurtry et al. 2013). The predators are also effective biocontrol agents of spider mites; however, relying solely on them may not be effective in keeping spider mite populations below the economic injury threshold in the long term (Alzoubi and Çobanoğlu 2007; Ganjisaffar et al. 2011). Furthermore, since the predatory mite primarily consumes whitefly individuals, rather than thrips and spider mites, its prey preference may lead to inadequate control of spider mite populations (Soleymani et al. 2016; Yari et al. 2023).
Therefore, an additional precaution to control spider mites could be the use of synthetic acaricides that have no side effects for A. swirskii. Unfortunately, the recommended field concentrations of several registered pesticides have been found to exhibit high toxicity to predatory mites (Fiedler and Sosnowska 2012; Döker and Kazak 2019; Kolcu and Kumral 2023). In contrast, botanical biopesticides are increasingly preferred due to their relatively short persistence and reduced environmental impact. Essential oils derived from orange, thyme, mint, rosemary, neem, sage, and spearmint, as well as terpenes, have demonstrated significant efficacy in controlling T. urticae through multiple mechanisms, including acaricidal, repellent, and oviposition-deterrent effects (Choi et al. 2004; Martinez-Villar et al. 2005; Miresmailli et al. 2006; Tak and Isman 2017; Wu et al. 2017; Hassan et al. 2021). Moreover, the microemulsion formulations of the essential oils enhance their persistence. On the other hand, these natural alternatives not only reduce T. urticae populations but also pose risks to phytoseiids, making them a promising option for integrated pest management (IPM) strategies (Momen et al. 2001; Abo-Taka et al. 2014; Moayeri et al. 2016; Shirvai et al. 2023).
However, the compatibility of microemulsion formulations of essential oils with the effective predatory mite A. swirskii has not been investigated to date. A waiting period after acaricide application may alleviate adverse effects on A. swirskii. In this study, residual bioassays were conducted to evaluate the effects on A. swirskii following the application of acaricides on eggplant leaves at 1, 4, and 7 days post-spraying. Although a certain waiting period reduces acute toxic effects, sub-lethal effects may occur during development and survival of juveniles, as well as oviposition and longevity of females A. swirskii (Sedaratian et al. 2011). Currently, limited research has been conducted on the biology and life table parameters of A. swirskii in eggplants (Khanamani et al. 2013) and the biological effects of the microemulsion formulations on A. swirskii have not yet been determined. To assess their relative fitness on A. swirskii, biological observations were conducted on A. swirskii exposed after 168-hour waiting period, which was determined to be safe for essential oil formulations under controlled conditions (16-hour light/8-hour dark photoperiod at 27±1 °C and 70±5% humidity). Additionally, focusing solely on females ignores broader effects on predator populations, as both sexes are vital to reproduction and population stability. To address these gaps, the present study evaluates the demographic effects of essential oil formulations in both sexes using the age-stage, two-sex life table theory (Chi and Liu, 1985) in comparison with a control group and milbemectin, thereby offering a more comprehensive understanding of their impact on life table parameters. In the present study, after applying effective doses of essential oil formulations and milbemectin (which inhibits communication between nerves and muscles by stimulating gamma-aminobutyric acid produced in nerve endings) to eggplant leaves, the biological properties of treated A. swirskii offspring were observed after a waiting period of 168 hours.
Material and methods
Acaricides
Commercial microemulsion formulations of neem oil (0.35% azadirachtin), orange oil (60g/L) and terpenoid blend (2% geraniol, 8% oregano oil and 5% thymol) were supplied by the Manufacturer Company (Nanomik Biotechnology, Istanbul, Türkiye). Milbemectine (Milbeknock EC), a natural acaricide, was supplied by Sumi Agro Türkiye as a positive control.
Eggplant growing
In this study, a common Turkish eggplant variety (Solanum melongena L. cv. Kemer) (Bursa Tohum, Türkiye) was cultivated. Eggplant seeds were sown in peat and perlit medium (1:1 v:v) (Klasmann TS 1–Deilmann, Geeste, Germany). After eight weeks of cultivation, the seedlings were transplanted into 2.5-L pots filled with peat and placed in a controlled environment in a growth chamber with a 16 h light and 8 h dark photoperiod at 27±1 °C and, 70±5% relative humidity. The seedlings were irrigated with tap water every three days and fertilized once a week with 100 mL of water-soluble fertilizer containing nitrogen, phosphorus, potassium, iron, copper, sulfur, zinc, manganese, boron, and molybdenum, purchased from Dr Tarsa Company (Bursa, Türkiye). Five weeks after transplantation, five fully developed and uniform plant leaves were selected for experiments.
Mite colonies
Tetranychus urticae red form colony was collected from eggplant grown in a greenhouse in Adana province (Türkiye). Auger et al. (2013) suggest that the red form of T. urticae is a synonym of Tetranychus cinnabarinus (Boisduval) (Acari: Tetranychidae) based on morphological, biological, and molecular evidence. Species identification was made according to the relevant literature (Auger et al. 2013). Ten to fifteen of T. urticae adults were infected with five fully developed uniform plant leaves using a soft-bristle paintbrush. Colonies of T. urticae of the same age were established by rearing at least two generations on eggplants. The native population of A. swirskii was collected from an orange orchard in Adana (Türkiye) in 2020 and the mite species were identified based on morphological characters described by Swirski et al. (1998) and Chant and McMurtry (2007). The mites were kept rare in glass Petri dishes (15 cm in diameter) with cotton placed on the bottom of this Petri dish (Overmeer 1985). Female mites were provided with a plastic Petri dish or a specially made glass plate (12 cm) with a piece of cotton in the middle on which to place their oviposition sites. To prevent mites from escaping, a narrow Tangle-trap strip was placed around the inner 12 cm Petri dish or specially made glass plate. The glass Petri dish and the cotton at the bottom were kept moist at all times. The mites were reared on Carpoglyphus lactis L. (Acari: Carpoglyphidae) brushed daily onto the upper Petri dish, and Thypha pollen (Typha latifolia L.) (Typhaceae) was also offered for supplementation. The same aged culture was obtained by placing 15-20 newly laid phytoseiid eggs in Petri dishes. Colonies were mass-reared in climate-controlled growth chambers under the above-mentioned climatic conditions.
Biossays for acute toxic effects
Experiments were carried out in Plexiglas Munger cells (13 x 8 cm in size; 3 cm in diameter and 1 cm in depth cell dimensions) to determine acute toxicity effects (Overmeer 1985, Kolcu and Kumral 2023). Briefly, 2 mL of each formulation at predetermined concentrations were applied to the underside of eggplant leaves using a spray tower set at an operating pressure of 1.5 kg/cm² and an application time of 3 seconds (Potter precision, Burkard Manufacturing Co. Ltd., Rickmansworth, UK). The leaves were then dried at room conditions for 30 minutes (Potter 1952). Moistened cotton wool was placed at the bottom of the cells and leaves were placed on top with the bottom side up. Munger cells were firmly attached from four directions using four metal clips. Each bioassay included three replicates and one control (water). Two concentrations were used to determine the toxicity of the formulations on T. urticae. On the other hand, a highest concentration (more than 90% death effects for T. urticae) was used for A. swirskii. Individuals of A. swirskii were obtained from a colony of the same age. Newly emerged protonymphs were placed onto spray-treated leaf discs using a paintbrush. The cells were kept under the same conditions in the climate chamber. The cells were checked under a stereomicroscope after 24, 96 and 168 hours. Mites that did not respond to being touched with a brush were considered dead.
Development and survival of juveniles
Biological observations were carried out in Munger cells on eggplant leaves. The mean duration and survival rates of different life stages of offspring produced by A. swirskii females were determined 168 h after spraying with milbemectin, microemilsion formulations of terpenoid blend, neem and orange oils on eggplant leaves. The leaves sprayed with distilled water were used as control. One hundred sixty-eight hours after applying the highest concentrations of all essential oil formulations and milbemectin to eggplant leaves, 50 T. urticae females (as prey), one teliochrysalid female, and two mature A. swirskii males from the stock colony were transferred onto the treated eggplant leaves. After 3 or 4 days, newly laid eggs were transferred to unsprayed leaves in Munger cells. The experiment started with one egg in each cell, more than 30 individuals per treatment. The Munger cells were placed in climate chambers under the same conditions. These experimental units were checked at least twice a day in order to determine the development of different juvenile stages. For predators, T. urticae individuals were added to the experimental unit daily based on data gathered from studies on predation capacities (Göksel and Kumral 2022). Unhatched eggs and immatures were recorded to determine survival rates.
Oviposition and life table parameters
Experiments were carried out on eggplant leaves that were not sprayed with Munger cells. A deutochrysalid female and 2 mature males were released into a Munger cell for mating. Then, pre-oviposition, oviposition, post-oviposition times, life spans and egg-laying numbers were recorded daily.
Statistical analysis
The data of biological parameters of the A. swirskii were analyzed based on the age-stage, two-sex life table method of Chi (1988) with the TWO SEX-MS Chart program (Chi, 2023). The survival rate (Sxj ) (x = age, j = stage), which is the probability that a newly laid egg will survive to age x and stage j, and fecundity fxj, which is the number of eggs hatched from eggs produced by the female adult at age x, were calculated. The age-specific survival rate (lx) was then calculated as:
\[l x=\sum_{j=1}^m S x j\]
where m is the number of stages. Age-specific fecundity (mx) was calculated as follows:
\[m x=\frac{\sum\limits_{j=1}^{m} S x j . f x j}{\sum\limits_{j=1}^m S x j}\]
The net reproductive rate (R0 ) is defined as the total number of offspring an individual can produce during its lifetime and is calculated as:
\[R_0=\sum_{x=0}^{\infty} l x m x\]
The intrinsic rate of increase (r) was calculated using the Lotka–Euler equation with age indexed from zero, as follows:
\[\sum_{x=0}^{\infty} e^{-r(x+1)} l x m x=1\]
where e: the base of the natural logarithm. The mean generation time (T) represents the time required for a population to increase in size to R0 times as time approaches infinity and the population settles into a stabilization phase distribution. The mean generation time is calculated as follows:
\[T=\frac{\ln R o}{r}\]
The finite rate is the rate of population growth as time approaches infinity and the population reaches a fixed age-stage distribution. The finite rate of increase (λ) was calculated as follows:
\[\lambda=e^r\]
The mortality rate of the tested spider mites and phytoseiid mites was calculated and corrected using the Abbott's formula (Abbott 1925). The effects of acaricides on the tested mites were statistically analyzed using one-way ANOVA with a significance level of P < 0.05 after checking for normality. Means were compared with the Tukey test accepting significant differences at P < 0.05. SPSS software version 23 was used for mean comparisons. The bootstrap technique was used to estimate the mean and standard errors of biological parameters with 100,000 iterations using the TWO SEX-MS Chart program (Chi 2023). Additionally, a paired bootstrap test was conducted to compare the mean values of population parameters between untreated A. swirskii mites (control group) and those treated with acaricides (Efron and Tibshirani 1993). The effect of acaricides on the longevity of A. swirskii was analyzed using Kaplan-Meier survival test and log-rank test with SPSS version 23. All figures were drawn using Microsoft Excel software.
Results
Acute toxicity to Tetranychus urticae females
Download as *Means followed by the same letter in a column are not significantly different (Tukey, P < 0.05).
Formulation content
concentrations
n
Mean death rate of females ± SE (%)
Mean unhatchability of eggs± SE (%)
terpenoid blend
0.005
90
94.45±2.78ab*
98.81±1.19a
0.01
90
94.44±5.56ab
98.81±1.19a
neem oil
0.0025
90
69.44±1.39c
74.71±6.09bc
0.005
90
91.67±4.81a-c
91.95±5.01ab
orange oil
0.001
90
70.67±3.53bc
16.67±2.38e
0.002
90
97.33±2.67a
39.28±5.46d
milbemectin
4.65 mg/L
90
70.67±3.53bc
65.33±3.53c
9.30 mg/L
90
72.00±10.07bc
85.33±3.53ab
X2 (df)
6.67 (23)
56.51 (23)
P
<0.01
<0.01
Table 1 presents the results of the toxicity bioassays conducted on female T. urticae and the inhibition of egg hatchability using milbemectin and microemulsion formulations of three essential oils. Significant differences in mortality rates of T. urticae females were observed among the acaricides tested. The highest concentration of orange oil showed a significantly higher toxic effect on T. urticae, resulting in higher mortality rates compared to lower concentrations of neem oil and orange oil and both concentrations of milbemectin. Furthermore, the hatchability of T. urticae eggs was significantly reduced in response to both higher concentrations of the terpenoid blend and higher concentrations of orange oil and milbemectin compared to the other treatments.
Acute toxicity to Amblyseius swirskii protonymphes
Download as *Means followed by the same lower-case letter in a column are not significantly different (Tukey, P < 0.05).
**Means followed by the same uper-case letter in a row are not significantly different (Tukey, P < 0.05).
Formulation
Concentrations
n
Mean death rate ± SE (%)
X2 (df = 8)
P
24 hour
96 hour
168 hour
terpenoid blend
0.01
90
66.50±5.39ab*A**
37.26±3.92bB
17.65±3.39bC
32.38
<0.01
neem oil
0.005
90
37.25±1.96bA
37.26±7.07bA
13.73±3.92bB
7.99
0.02
orange oil
0.002
90
84.31±8.55aA
31.37±1.96bB
31.73±5.19bB
27
<0.01
milbemectin
9.30 mg/L
90
84.31±8.55aA
90.19±3.92aA
82.35±8.98aA
0.29
0.75
X2 (df)
11.02 (8)
35.99 (8)
29.76 (8)
P
<0.01
<0.01
<0.01
Table 2 summarizes the mortality rates of A. swirskii protonymphs released onto eggplant leaves 24, 96, and 168 hours after application of microemulsion formulations consisting of milbemectin and terpenoid blend, neem oil and orange oil. Significant differences in mortality rates were observed among the acaricides tested at each release time. For all treatments except milbemectin, differences in mortality rates during release periods were statistically significant (P>0.05). A significant reduction in mortality rates was observed especially in A. swirskii released 168 hours after spraying microemulsion formulations of essential oils.
Survivorship and development of Amblyseius swirskii
Download as APOP, adult pre-ovipositional period; TPOP, total pre-ovipositional period (from egg to first oviposition).
*The means followed by different letters in the same row are significantly different using the paired bootstrap test at 5% significance level. The values inside the parentheses are the number of individuals tested.
** The data were not obtained due to death of female mites.
Parameter
Control
terpenoid blend
neem oil
orange oil
milbemectin
Egg ♀♂ (day)
1.69±0.09a* (35)
1.64±0.10a (25)
1.56±0.08a (40)
1.68±0.08a (34)
1.40±0.07a (45)
Larva ♀♂ (day)
1.12±0.06a (34)
1.00±0.00a (25)
1.00±0.00a (40)
1.00±0.00a (34)
1.00±0.00a (17)
Protonymph ♀♂ (day)
2.12±0.12ab (26)
2.09±0.06a (23)
1.97±0.05ab (39)
1.93±0.05b (29)
2.00±0.00ab (17)
Deutonymph ♀♂ (day)
1.81±0.16a (26)
1.91±0.09a (23)
1.78±0.07a (38)
1.83±0.07a (29)
1.88±0.08a (17)
Pre-adult ♀ (day)
6.95±0.43ab (19)
6.61±0.10a (25)
6.31±0.08ab (38)
6.48±0.11ab (23)
6.27±0.15b (15)
Pre-adult ♂ (day)
6.14±0.14a (7)
6.00±0.00a (6)
6.00±0.00a (8)
6.17±0.31a (6)
6.00±0.00a (2)
Male longevity (day)
18.14±0.14a (7)
19.83±1.80a (6)
15.38±0.84b (8)
15.50±1.31b (6)
7.50±0.00c (2)
Female longevity (day)
24.37±1.09a (19)
22.71±1.21a (17)
21.17±1.23b (24)
21.78±1.18b (23)
8.13±0.22c (15)
Total life span (day)
17.69±1.52ab (36)
20.60±1.33a (25)
16.45±1.34b (40)
15.24±1.48b (41)
3.73±0.35c (62)
APOP (day)
2.72±0.23ab (18)
3.00±0.10a (17)
2.28±0.13b (24)
2.43±0.13b (21)
** (15)
TPOP (day)
9.67±0.54ab (18)
9.57±0.19a (17)
8.56±0.19b (24)
8.95±0.35ab (21)
** (15)
Oviposition days
9.00±0.83a (18)
8.71±0.73a (14)
9.17±0.83a (18)
8.33±0.73a (21)
** (15)
Fecundity (eggs/female)
12.84±1.54a (19)
11.94±2.04a (17)
13.25±2.36a (24)
12.70±1.46a (23)
** (15)
Immature mortality (%)
0.28±0.07b (36)
0.08±0.05c (25)
0.20±0.06b (40)
0.29±0.07b (41)
0.73±0.06a (62)
Amblyseius swirskii juveniles developed successfully on eggplant leaves treated with both milbemectin and essential oil formulations. The survival rates for A. swirskii are presented in Table 3. The mortality rates of A. swirskii ranged from 8% to 29% in eggplant leaves treated with essential oil formulations and the untreated control, and these differences were statistically significant. While the lowest mortality rate was observed in A. swirskii released onto eggplant leaves 168 hours after the terpenoid blend, the mortality rate of A. swirskii was significantly higher in the milbemectin-applied group than in the other treatments.
Download as *The means followed by different letters in the same row are significantly different using the paired bootstrap test at 5% significance level.
**The data were not obtained due to death of female mites.
Parameter
Control
terpenoid blend
neem oil
orange oil
milbemectin
R0 (eggs/ individual)
6.78±1.34a
8.12±1.75a
7.95±1.74a
7.12±1.27a
**
r (day-1)
0.124±0.013a
0.139±0.013a
0.149±0.017a
0.139±0.015a
**
λ (day-1)
1.133 ±0.015a
1.149±0.017a
1.160±1.979a
1.149±1.481a
**
T (day)
15.35±0.69a
15.03±0.39b
13.96±0.46b
14.09±0.29b
**
The total developmental time of A. swirskii ranged from 6.27 to 6.95 days for females and 6.00 to 6.17 days for males across in all treatments including untreated leaves (Table 4). No significant differences were observed in the total developmental time of A. swirskii females released on eggplant leaves treated with essential oil formulations compared to the untreated control. However, the shortest developmental time was recorded in mites exposed to milbemectin treatment.
Adult longevity and oviposition
Significant discrepancies were observed in the longevity of A. swirskii females and males on eggplant leaves treated with milbemectin and essential oil formulations (Table 3). Mites exhibited the longest survival on leaves treated with the terpenoid blend compared to those treated with orange oil and neem oil, as compared to the untreated control. In contrast, a significant decrease in the life span of A. swirskii was determined in eggplant leaves sprayed with milbemectin. Specifically, the life span of females on milbemectin-treated leaves was significantly reduced to 8.13 days compared to 21.17-24.37 days for essential oil formulations. Additionally, no oviposition data were recorded for females on milbemectin-treated leaves. In treatments with essential oil formulations, no significant difference was observed in oviposition days or fecundity values of A. swirskii females compared to the untreated control. Although the longest days to oviposition were recorded in leaves treated with orange oil (9.17 days), followed by the terpenoid blend (8.71 days) and neem oil (8.33 days), these times were not significantly different from the control (9.00 days).
Life table parameters
The life table parameter values of A. swirskii released onto eggplant leaves 168 hours after spraying with milbemectin and the microemulsion formulations of essential oils are presented in Table 4. Due to the lack of oviposition data for mites exposed to milbemectin, life table parameters could not be calculated for this treatment. No significant differences were observed in the intrinsic rate of increase (r), net reproductive rate (R₀), mean generation time (T), and the finite rate of increase (λ) between essential oil-treated and untreated leaves. The highest r value (0.149) was observed in neem oil treatment followed by orange oil and terpenoid blend (0.139) compared to the control (0.124). The terpenoid blend also gave the highest R₀ value (8.12), while the lowest value was observed in orange oil (6.78). On the other hand, the longest T value and the lowest λ value were recorded in the control group.
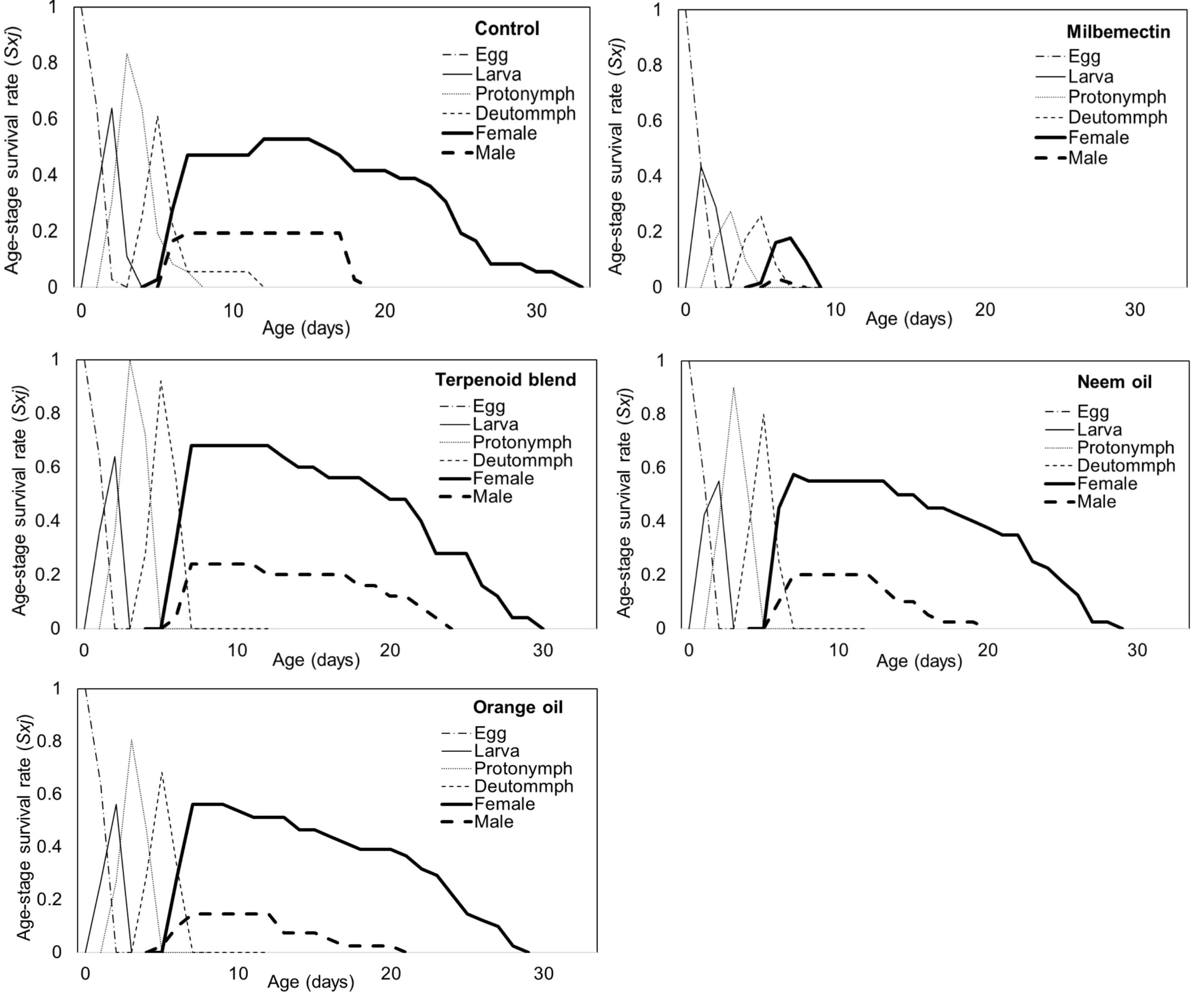


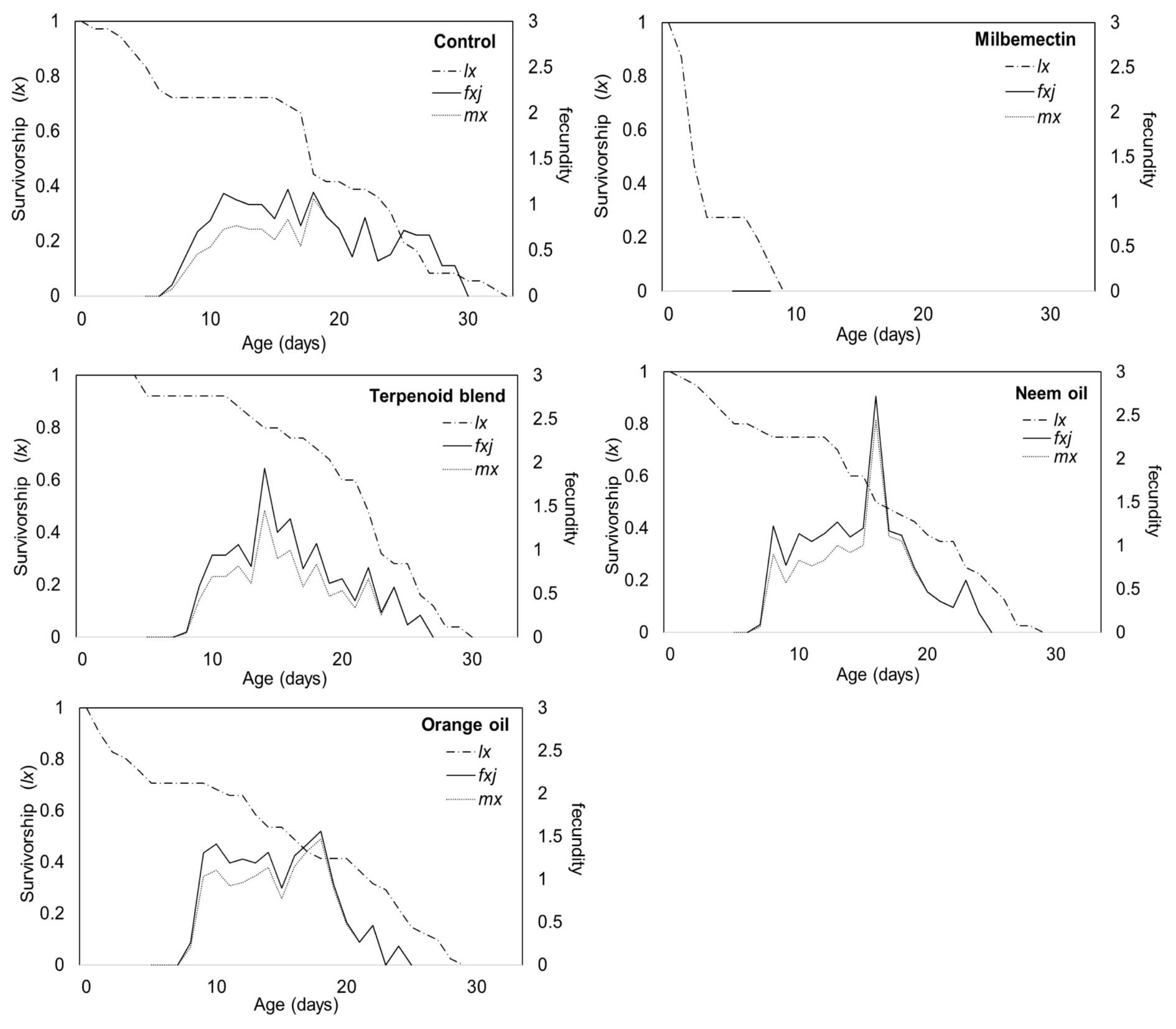
The Sxj (age-stage survival rates) of A. swirskii released onto eggplant leaves 168 hours after spraying with milbemectin and microemulsion formulations of essential oils are shown in Figure 1, which indicates the probability of a newborn individual surviving to age x and stage j. The highest probability of a newborn egg surviving to adulthood was found to be 0.53, 0.68, 0.58, 0.56 and 0.18 for females and 0.19, 0.24, 0.20, 0.15 and 0.03 for males for control, the terpenoid blend, neem oil, orange oil and milbemectin, respectively. Except for milbemectin, treatments with essential oil formulations had no significant effect on the age-specific survival rate (lx), age-specific fecundity rates (mx), and age-stage-specific fecundity (fxj) of A. swirskii females (Figure 2). According to the results of Kaplan-Meier survival analysis, milbemectin significantly reduced the longevity of A. swirskii females (χ2 = 122.64; df = 4, P < 0.01). Females exposed to essential oil formulations had significantly longer life span than milbemectin but no different from controls. Although age-specific fecundity and reproductive value curves showed that oviposition patterns vary among essential oil formulations, they were not different from the control. The age-specific fecundity rate (fxj) peaked at day 16 for control (1.17 eggs), day 14 for terpenoid blend (1.93 eggs), day 16 for neem oil (2.72 eggs), day 18 for orange oil (1.56 eggs), respectively. The first oviposition began on days 7, 8, 7 and 8 in the control, terpenoid blend, neem oil and orange oil, respectively. The age-specific fecundity (mx) of A. swirskii reared in three treatments and control is shown in Figure 2. The highest mx value in the females age period was 1.06 on the 18th day of the control, followed by 1.45 on the 14th day in the terpenoid blend, 2.45 on the 16th day in neem oil and 1.47 on the 18th day in orange oil (Figure 2).


Discussion
In recent years, plant-derived essential oils have gained significant attention as potantial alternatives to synthetic pesticides in pest control, with emphasis on minimizing damage to natural enemies and evaluating their compatibility with biological control agents for effective integrated pest management systems (Momen et al. 2001; Choi et al. 2004; Regnault-Roger et al. 2012; De Araújo et al. 2020). Consistent with this growing interest, our study showed that milbemectin and the microemulsion formulations of tested essential oils (orange, neem and terpenoid blend) had acaricidal effects at different rates with statistically significant differences among treatments. This finding aligns with previous studies such as Keskin et al. (2020) reporting that a neem oil formulation killed 90% of T. urticae females 72 hours after treatment. Several other studies have documented similar lethal effects of neem formulations on T. urticae (Sundaram and Sloane 1995; Martínez-Villar et al. 2005). Additionally, the acaricidal properties of orange oil are well-established with studies reporting its acute toxicity and population-reducing effects on T. urticae under both laboratory and field conditions (Araujo-Junior et al. 2010; Attia et al. 2011; Tabet et al. 2018; Hassan et al. 2021). Previous studies on thyme and oregano and the findings of our current study are consistent with the acaricidal effects of essential oils (Modarres-Najafabadi 2012; Susurluk 2023). Furthermore, our results are consistent with Lee (1997) who showed that geraniol caused high mortality in T. urticae females at 10,000 ppm.
Studies have shown that evaluating only the lethal effects of pesticides often underestimates their full impact, as sublethal effects, such as changes in behavior and reproduction, can also significantly affect population dynamics (Galvan et al. 2005; Stark et al. 1995). In this context, essential oils have been investigated not only for their direct acaricidal properties but also for their broader effects on biological parameters such as development, reproduction and longevity in A. swirskii. While essential oil-based pesticides are generally considered safer for non-target organisms, several studies have noted destructive effects, including negative impacts on biological development, predatory ability, and fecundity (Giunti et al. 2022). Therefore, the potential sublethal and ecological consequences of these treatments need to be carefully evaluated. Although essential oils exhibit varying levels of toxicity, they are generally less harmful to A. swirskii than synthetic acaricides. For instance, essential oils generally reduce survival rates but have minimal effects on development and fecundity, especially at sublethal concentrations (Abo-Taka et al. 2014; Momen et al. 2001; Moayeri et al. 2016; Balcı et al. 2020; Demirtaş et al. 2022; Shirvai et al. 2023). In contrast, chemical acaricides significantly reduce both survival and reproduction (Shimaa et al. 2021; Mousavi et al. 2022).
Pesticides are generally evaluated according to toxicity tests carried out under laboratory and semi-field conditions in accordance with the guidelines set by the International Organization for Biological and Integrated Control (IOBC). These toxicity ratings reflect the effect of pesticides on biological control agents in pest management, with a scale ranging from 1 to 4: 1 = harmless, 2 = slightly harmful, 3 = moderately harmful, and 4 = harmful (Hassan 1994). In our study, we found that microemulsion formulations of essential oils showed varying levels of adverse effects (ranging from 2 to 4) against the most susceptible stage (protonymphes) of A. swiskii. Specifically, neem oil, terpenoid blend and orange oil were classified as slightly harmful, moderately harmful and harmful, respectively. As a positive control, milbemectin was also found to be harmful. Similar findings have been reported for A. swirskii and other phytoseiid mites; survival rates in these species were reduced at various concentrations of neem, orange, thyme and oregano essential oils (Abo-Taka et al. 2014; Fountain and Medd 2015; Kolcu and Kumral 2023). However, Fountain and Medd (2015) reported that orange extract was moderately harmful to A. swirskii, with a 50-75% mortality rate in the mite population. This discrepancy may be attributed to differences in the biological stages of A. swirskii tested in the studies. On the other hand, the present study showed that the acute toxic effects of essential oil formulations were significantly reduced after the application of A. swirskii protonymphes into eggplant leaves sprayed with acaricides and a waiting period of 168 hours. It was determined that mortality rates ranged from 14 to 32 percent, that the neem oil and terpeoid blend were harmless, and that the orange oil was slightly harmful. As a positive control, milbemectin was determined to be harmful. To examine the sublethal effects of these acaricides, we examined the development, life span, oviposition and life table parameters of A. swirskii 168 hours after spraying under controlled conditions. The present study also revealed that eggplant as the host plant and T. urticae as prey were favourable for A. swirskii, whereby their offspring on unsprayed leaves had shorter development time (control). In control experiments, predatory mite development was completed in 6.95 days in females and 6.14 days in males. The developmental time of A. swirskii feeding on T. urticae was consistent with the range (5.74-6.74 days) reported in studies on various crops such as strawberry, mulberry, pepper, bean, eggplant and tomato (Momen and Elsaway 1993; Shimaa et al. 2021; Goksel and Kumral 2022). After 168 hours of waiting period, the development time (6.00-6.17 days) was not affected by both essential oil formulations and milbemectin compared to the control. However, the survival rate of A. swirskii juveniles exposed to milbemectin was significantly reduced. Pesticides are known to affect the fecundity and oviposition period of predatory mites (Fountain and Medd 2015). In this study, the oviposition period of A. swirskii on unsprayed eggplant leaves was 9.00 days, and an average of 12.84 eggs were laid per female. These findings regarding oviposition period and fecundity are in line with several studies on the biological characteristics of A. swirskii, which showed an oviposition period of 12.84 days and 19 eggs for tomato; 9.79 days and 15.74 eggs for bean; 10.68 days and 16.84 eggs for eggplant; 10.58 days and 16.32 eggs for cucumber; 25 days and 31 eggs for strawberry under laboratory conditions (Shimaa et al. 2021; Goksel and Kumral 2022).
The mean fecundity, oviposition time, and even longevity of A. swirskii females treated with essential oil formulations were not significantly affected compared to the control group. However, milbemectin treatment reduced these parameters. Our findings are in line with those reported by Mousavi et al. (2022), who reported that higher concentrations of milbemectin (LC15 and LC25) had the most negative effect on the longevity and fecundity of A. swirskii. Our results are consistent with those of Hamedi et al. (2011) reported that abamectin, which has a similar mechanism of action to milbemectin, increased the pre-oviposition period while decreasing the oviposition time and total fecundity in Phytoseius plumifer (Canestrini and Fanzago) (Acari: Phytoseiidae). This effect may be attributed to the moderate persistence of milbemectin, whereas essential oils degrade more rapidly (BPDB database, 2024). Sublethal concentrations of acaricides are known to significantly impact population growth and life table parameters, and this is an important consideration for IPM programs (Hamedi 2022). In this study, we hypothesized that the 7-day waiting period would lead to sublethal concentrations, influencing the observed effects.
The life table parameters were similar to those in the control group, suggesting that essential oils did not adversely affect overall population growth of A. swirskii. The values we observed were very closely aligned with those reported by Goksel and Kumral (2022), who studied A. swirskii females fed with T. urticae reared on eggplant leaves (r=0.142 d-1; R0 =13.82 offspring; T=18.44 d; λ=1.15 d-1). As reported by various researchers, the r value for A. swirskii typically ranges between 0.130 and 0.173 when the predator feeds on suitable prey under optimal conditions (Alinejad et al. 2011; Goksel and Kumral 2022). These differences between studies are thought to be likely due to differences in host plant and prey suitability, and these differences may significantly influence the biological parameters of the predator.
Conclusion
Overall, the results demonstrated that the microemulsion formulations of essential oils tested in this study had no adverse effects on the development and life table parameters of the subsequent generation of A. swirskii 168 hours after application. These findings suggest that essential oil formulations can be effectively incorporated into IPM programs when used alongside a specific release schedule for A. swirskii. In contrast, age-specific fecundity and survival curves indicated that milbemectin significantly reduced both survival and fecundity of the offspring compared to the control. Thus, milbemectin is not a compatible acaricide for A. swirskii due to its negative impact on offspring survival and fecundity, even after a 168-hour waiting period. While essential oil formulations show promise for use in IPM programs, milbemectin is unsuitable as a biological control agent within these systems because of its detrimental effects on this predator. Further research is recommended to evaluate the persistence and efficacy of essential oil formulations under semi-field and field conditions, providing deeper insights into their long-term stability, effectiveness, and overall impact on pest management systems.
Acknowledgements
The research, which is the doctoral thesis of the first author, was funded by TAGEM under grant number 5807. We would like to thank Adana Biological Control Research Institute for providing laboratory facilities and giving opportunities to conduct research, and Nanomik Biotecnology Company for supplying the formulations for the biological tests.
References
- Abo-Taka S.M., Sweelam M.A., Heikal H.M., Walash I.H. 2014. Toxicity and biological activity of five plant extracts to the Two-spotted spider mite, Tetranychus urticae and predatory mite, Amblyseius swirskii (Tetranychidae: Phlytoseiidea. Acarines). J. Egypt. Soc. Acarol., 8(2): 49-56. https://doi.org/10.21608/ajesa.2014.163845
- Alinejad M., Kheradmand K., Fathipour Y. 2014. Sublethal effects of fenazaquin on life table parameters of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae). Exp. Appl. Acarol., 64: 361-373. https://doi.org/10.1007/s10493-014-9830-y
- Alzoubi S., Çobanoğlu S. 2007. Effects of sublethal dose of different pesticides on the two-spotted spider mite Tetranychus urticae Koch and its predatory mites under greenhouse conditions. World J. Agric. Sci., 3(6): 764-770. https://doi.org/10.13140/RG.2.1.2072.3364
- Araujo-Junior C.P., da Camara C.A.G., Neves I.A., Ribeiro N.C., Gomes C.A., Moraes M.M., Botelho O.S. 2010. Acaricidal activity and chemical composition of peel essential oils of three Citrus species cultivated in NE Brazil. Nat. Prod. Commun., 5(3): 471-476. https://doi.org/10.1177/1934578X1000500326
- Attia S., Grissa K.L., Ghrabi Z.G., Mailleux A.C., Lognay G., Rance T. 2011. Assessment of the acaricidal activity of several plant extracts on the phytophagous mite Tetranychus urticae (Tetranychidae) in Tunisian cltrus orchards. Bull. Soc. R. Belg. Entomol., 147(I-IV): 71-79. https://hdl.handle.net/2268/118157
- Auger P., Migeon A., Ueckermann E.A., Tiedt L., Navarro M.N. 2013. Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): review and new data. Acarologia, 53(4): 383-415. https://doi.org/10.1051/acarologia/20132102
- Baradaran P., Arbabi M., Manzari S., Rezai H. 2009. An abundance study of Thrips tabaci (Thys.: Thripidae) on different genotypes of eggplant in Varamin region, Iran. J. Novel Res. Plant Prot., 1(3): 249-261.
- BPDB database, 2024. BPDB: Bio-Pesticides DataBase. The University of Hertfordshire. https://sitem.herts.ac.uk/aeru/bpdb/index.htm
- Can M., Çobanoğlu S. 2010. Studies on the determination of mite (Acari) species and their hosts of greenhouse vegetables in Kumluca, Antalya. Akd. Üniv. Zir. Fak. Derg., 23(2): 87-92. https://dergipark.org.tr/en/download/article-file/18027
- Chant D.A., McMurtry J.A. 2007. Illustrated Keys and Diagnoses for the Genera and Subgenera of the Phytoseiidae of the World. West Bloomfield (MI, USA): Indira Publishing House, Canada. p. 220.
- Chi, H. S. I. N., Liu, H. 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin, 24(2): 225-240. https://zoolstud.sinica.edu.tw/Journals/24.2/225.pdf
- Chi, H. 1988. Life-table analysis incorporating both sexes and variable development rates among individuals. Environmental Entomology, 17(1): 26-34. https://doi.org/10.1093/ee/17.1.26
- Chi, H. (2023) TWO SEX-MSChart: a computer program for the age-stage, two-sex life table analysis. Retrieved from http:// 140.120.197.173/Ecology/prod02.htm (accessed 13 July 2023)
- Choi, W.I., Lee, S.G., Park, H.M., Ahn, Y.J. 2004. Toxicity of plant essential oils to Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae). J. Econ. Entomol., 97(2), 553-558. https://doi.org/10.1093/jee/97.2.553
- Chow A., Chau A., Heinz K.M. 2010. Compatibility of Amblyseius (Typhlodromips) swirskii (Athias-Henriot) (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae) for biological control of Frankliniella occidentalis (Thysanoptera: Thripidae) on roses. Biol. Control, 53(2): 188-96. https://doi.org/10.1007/s10526-016-9718-3
- Chung B.K., Kang S.W., Kwon J.H. 2000. Chemical control system of Frankliniella occidentalis (Thysanoptera: Thripidae) in greenhouse eggplant. J. Asia-Pac. Entomol., 3(1): 1-9. https://doi.org/10.1016/S1226-8615(08)60048-5
- De Araújo M. J. C., da Câmara C. A. G., Born F. S., de Moraes M. M. 2020. Acaricidal activity of binary blends of essential oils and selected constituents against Tetranychus urticae in laboratory/greenhouse experiments and the impact on Neoseiulus californicus. Exp. Appl. Acarol. 80, 423-444. doi:10.1007/s10493-020-00464-8 https://doi.org/10.1007/s10493-020-00464-8
- Demirtaş, B., Birgücü, A. K., Ay, R. 2022. Acute and chronic effects of two insecticide-acaricides on the predatory mite Amblyseius swirskii Athias-Henriot. Int. J.Acarol., 48(4-5), 324-330. https://doi.org/10.1080/01647954.2022.2070668
- Döker I., Kazak C. 2019. Non-target effects of five acaricides on a native population of Amblyseius swirskii (Acari: Phytoseiidae). Int. J. Acarol., 45(1-2): 69-74. https://doi.org/10.1080/01647954.2018.1542457
- Efron B., Tibshirani R.J. 1993. An introduction to the bootstrap. New York, NY: Chapman and Hall; p. 436. https://doi.org/10.1201/9780429246593
- Fiedler, Ż., Sosnowska D. 2012. Side effects of fungicides and insecticides on predatory mites, in laboratory conditions. J. Plant Prot. Res., 54(4): 349-353. https://doi.org/10.2478/jppr-2014-0052
- Fountain M.T., Medd N. 2015. Integrating pesticides and predatory mites in soft fruit crops. Phytoparasitica, 43: 657-667. https://doi.org/10.1007/s12600-015-0485-y
- Galvan T.L., Koch R.L., Hutchison W.D. 2005. Effects of spinosad and indoxacarb on survival, development, and reproduction of the multicolored Asian lady beetle (Coleoptera: Coccinellidae). Biological Control 34: 108-114. https://doi.org/10.1016/j.biocontrol.2005.04.005
- Ganjisaffar F, Fathipour Y., Kamali K. 2011.Temperature-dependent development and life table parameters of Typhlodromus bagdasarjani (Phytoseiidae) fed on two-spotted spider mite. Exp. Appl. Acarol., 55: 259-272. https://doi.org/10.1007/s10493-011-9467-z
- Giunti G., Benelli G., Palmeri V., Laudani F., Ricupero M., Ricciardi R., Maggi F., Lucchi A., Guedes R.N., Desneux N., Campolo O. 2022. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control, 176: 105071. https://doi.org/10.1016/j.biocontrol.2022.105071
- Göksel P.H., Kumral N.A. 2022. Which plant species is more suitable for the control of Tetranychus urticae with Amblyseius swirskii? IV. Balkan Agricultural Congress, 31 August - 02 September 2022, Edirne, Turkey, p. 166. https://agbiol.congress.gen.tr/files/site/17/files/AGRIBALKAN2022%20PROCEEDINGS%20ABSTRACT%20BOOK.pdf
- Grewal J.S. 1992. Seasonal fluctuation in the populations of various mite species associated with brinjal crop in Punjab. Ann. Entomol., 10(1): 37-40.
- Hamedi N. 2022. Side effects of pesticides on population growth parameters, life table parameters, and predation of the subsequent generation of phytoseiid mites. In Pesticides-Updates on Toxicity, Efficacy and Risk Assessment, IntechOpen. https://doi.org/10.5772/intechopen.104229
- Hasanuzzaman A.T., Islam M.N., Zhang Y., Zhang C.Y., Liu T.X. 2016. Leaf morphological characters can be a factor for intra-varietal preference of whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) among eggplant varieties. PloS one, 15;11(4): e0153880. https://doi.org/10.1371/journal.pone.0153880
- Hassan S.A. 1994. Activities of the IOBC/WPRS Working Group, Pesticides and Beneficial Organisms. In: Pesticides and Beneficial Organisms. (ed., Vogt H.), IOBC/WPRS Bulletin, 17, 1-5.
- Hassan M. F., El-Badawy S. S., Draz M. G., Ibrahim E. S. 2021. New acaricidal activities and chemical compositions of orange oil and extracts of (wild mint and henna) against Tetranychus urticae Koch (Acari: Tetranychidae). Arch. Phytopathol. Plant Protect., 54(19-20): 1848-1863. https://doi.org/10.1080/03235408.2021.1950508
- Kapoor V.C., Paul M., Kapur J. 1997. Seasonal incidence of mite species infesting okra (Hibiscus esculentus) and brinjal (Solanum melongena) in Punjab. Indian J. Agric., 67(7): 325-326.
- Keskin G., Kumral N.A., Kaçar O. 2020. A laboratory study of the acaricidal, repellent and oviposition deterrent effects of three botanical oils on Tetranychus urticae (Koch, 1836) (Acari: Tetranychidae). Turk. J. Entomol., 44(3): 305-318. https://doi.org/10.16970/entoted.702157
- Khanamani M., Fathipour Y., Hajiqanbar, H. 2013. Population growth response of Tetranychus urticae to eggplant quality: application of female age-specific and age-stage, two-sex life tables. Int. J. Acarol., 39(8): 638-648. https://doi.org/10.1080/01647954.2013.861867
- Kilincer N., Yiğit A., Kazak C., Er M.K., Kurtulus A., Uygun N. 2010. Biological control of pests from theory to practice. Türk. Biol. Mücadele, 1(1): 15-60. https://dergipark.org.tr/tr/download/article-file/208635
- Kolcu A., Kumral N.A. 2023. The toxic effects of some acaricides on the tomato russet mite and its predator Amblyseius swirskii Athias-Henriot, 1962 (Acari: Phytoseiidae). Turk. J. Entomol., 47(1): 3-13. https://doi.org/10.16970/entoted.1171756
- Kumral N.A., Kovancı B. 2005. Seasonal population dynamics of the Two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae) under acaricide constraint on eggplant in Bursa province. Acarologia, 45(4): 297-303. file:///C:/Users/CASPER/Downloads/Acarologia45(4)295-301(2005).pdf
- Kumral N.A., Çobanoğlu S. 2016. The Mite (Acari) biodiversity and population fluctuation of predominat species in eggplant. Tar. Bilim. Derg.-J. Agric. Sci., 22(2): 261-274. https://doi.org/10.1501/Tarimbil_0000001386
- Kumral N.A., Goksel P.H., Aysan E., Kolcu A. 2017. Biological parameters and population development of Tetranychus urticae Koch, 1836 (Acari: Tetranychidae) on different pepper cultivars. Turk. Entomol. Derg., 41(3): 263-273. https://doi.org/10.16970/entoted.297132
- Lee S., Tsao R., Peterson C., Coates J.R., Lee S.K. 1997. Insecticidal activity of monoterpenoids to western Corn rootworm (Col.: Chrysomelidae), Twospotted spidermite (Acari: Tetranychidae), and Housefly (Dip.: Muscidae). J. Econ. Entomol., 90, 883-892. https://doi.org/10.1093/jee/90.4.883
- Lee D.H., Nyrop J.P., Sanderson J.P. 2010. Effect of host experience of the greenhouse whitefly, Trialeurodes vaporariorum, on trap cropping effectiveness. Entomol. Exp. Appl., 137(2): 193-203. https://doi.org/10.1111/j.1570-7458.2010.01052.x
- Leite G.L.D., Picanco M., Zanuncio J.C., Marquini F. 2003. Factors affecting mite herbivory on eggplants in Brazil. Exp. Appl. Acarol., 31(3-4): 243-252. https://doi.org/10.1023/B:APPA.0000010379.05878.2c
- Martinez-Villar, E., Saenz-De-Cabezon F. J., Moreno-Grijalba F., Marco V., Moreno I. P., 2005. Effects of azadirachtin on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol., 35(3): 215-222. https://doi.org/10.1023/B:APPA.0000010379.05878.2c
- McMurtry, J., Moraes, G., & Sourassou, N. F. (2013). Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18(4): 297-312. https://doi.org/10.11158/saa.18.4.1
- Messelink G.J., van Maanen R., van Steenpaal S.E., Janssen A. 2008. Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol. Control, 44(3): 372-379. https://doi.org/10.1016/j.biocontrol.2007.10.017
- Miresmailli S., Bradbury R., Isman M. B. 2006. Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Manag. Sci., 62(2): 191-195. https://doi.org/10.1002/ps.1157
- Moayeri H.S., Pirayeshfar F., Safavi S.A., Bolandnazar A. 2016. Acaricidal effect of menthol, thymol and their mixtures against Tetranychus urticae Koch and the predaceous mite, Amblyseius swirskii Athias-Henriot. Proc. 22nd Iranian Plant Protection Congr., 27-30 August 2016.
- Modarres-Najafabadi S.S. 2012. Control of Tetranychus urticae Koch by thyme, lavender and eucalyptus essential oils. J. Med. Plants By-Prod., 1(1): 43-47. https://doi.org/10.22092/JMPB.2012.108446
- Momen F. M., Amer S. A. A., and Refaat A. M. 2001. Influence of mint and peppermint on Tetranychus urticae and some predacious mites of the family phytoseiidae (Acari: Tetranychidae: Phytoseiidae). Acta Phytopathol. Entomol.Hung. 36: 143-153. doi: 10.1556/APhyt.36.2001.1-2.17 https://doi.org/10.1556/APhyt.36.2001.1-2.17
- Momen, F.M., Elsaway, S.A. 1993. Biology and feeding behavior of the predatory mite, Amblyseius swirskii (Acari, Phytoseiidae). Acarologia, 34(3): 199-204.
- Mousavi A., Kheradmand K., Fathipour Y., Mosallanejad H., Havasi M. 2022. Sublethal effects of Milbemectin on biological parameters of Amblyseius swirskii (Acari: Phytoseiidae). Syst. Appl. Acarol., 27(6): 1085-1097. https://doi.org/10.11158/saa.27.6.8
- Onzo A., Houedokoho A.F., Hanna, R. 2012. Potential of the predatory mite, Amblyseius swirskii to suppress the broad mite, Polyphagotarsonemus latus on the gboma eggplant, Solanum macrocarpon. J. Insect Sci., 12(1): 1-11. https://doi.org/10.1673/031.012.0701
- Overmeer, W.P.J. 1985. "Rearing and Handling, 161-170″. In: Spider Mites, Their Biology, Natural Enemies and Control (Eds. W. Helle & M. W. Sabelis). (Vol 1b), Elsevier, Amsterdam, Netherlands. p. 458.
- Park H.H., Shipp L., Buitenhuis R. 2010. Predation, development, and oviposition by the predatory mite Amblyseius swirkii (Acari: Phytoseiidae) on tomato russet mite (Acari: Eriophyidae). J. Econ. Entomol., 103(3): 563-569. https://doi.org/10.1603/EC09161
- Potter C. 1952. An improved apparatus for applying direct sprays and surface films with data on the electrostatic charge on atomized spray fluids. Ann. Appl. Biol., 39 (1): 1-28. https://doi.org/10.1111/j.1744-7348.1952.tb00993.x
- Regnault-Roger C., Vincent,C., Arnason, J. T. 2012. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 57: 405-424. doi: 10.1146/annurev-ento-120710-100554 https://doi.org/10.1146/annurev-ento-120710-100554
- Sedaratian A., Fathipour Y., Moharramipour S. 2011. Comparative life table analysis of Tetranychus urticae (Acari: Tetranychidae) on 14 soybean genotypes - Insect Sci., 18: 541-553. https://doi.org/10.1111/j.1744-7917.2010.01379.x
- Shimaa F., El-Saiedy F., El- Sayed M. 2021. Life table parameters of Amblyseius swirskii and Neoseiulus californicus (Acari: Phytoseiidae) reared on two strawberry cultivars. Int. J. Acarol., 47(7): 568-574. https://doi.org/10.1080/01647954.2021.1976835
- Shirvai Z., Allahyari H., Zahedi Golpayegani, A., Talebi Jahromi, K., Döker, I. (2023). Side effects of Zataria multiflora Boiss (Lamiaceae) essential oil on predation and life table parameters of Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae). Syst. Appl. Acarol., 28(1), 143-157. https://doi.org/10.11158/saa.28.1.13
- Shibao M., Momoshita M., Yamanaka S., Tanaka H. 2009. Control of melon thrips, Thrips palmi Karny on greenhouse eggplant by releasing of Amblyseius swirskii Athias-Henriot. Annu. Rep. Kansai Plant Prot. Soc., 52: 21-25. https://doi.org/10.4165/kapps.52.21
- Simon J.Y. 2014 - The toxicology and biochemistry of insecticides - CRC press. p. 353. https://doi.org/10.1201/b18164
- Stansly P.A., Castillo J., Castañé C., Perdikis D. 2009. Control of broad mite, Polyphagotarsonemus latus and the whitefly Bemisia tabaci in open field pepper and eggplant with predaceous mites. IOBC/WPRS Bulletin, 49: 145-152.
- Stark J.D., Wennergren U. 1995. Can population effects of pesticides be predicted from demographic toxicological studies? J Econ Entomol 88: 1089-1096. https://doi.org/10.1093/jee/88.5.1089
- Soleymani S., Hakimitabar M., Seiedy M. 2016. Prey preference of predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) and Bemisia tabaci (Hemiptera: Aleyrodidae). Biocontrol Sci. Technol., 26(4): 562-569. https://doi.org/10.1080/09583157.2015.1133808
- Sundaram, K.M.S., Sloane L. 1995. Effects of pure and formulated azadirachtin, a neem-based biopesticide, on the phytophagous spider mite, Tetranychus urticae Koch. Environ. Sci. Health Part B., 30 (6): 801-814. https://doi.org/10.1080/03601239509372966
- Susurluk H. 2023. Potential use of essential oils from Origanum vulgare and Syzygium aromaticum to control Tetranychus urticae Koch (Acari: Tetranychidae) on two host plant species. PeerJ 20;11: e14475. https://doi.org/10.7717/peerj.14475
- Swirski E., Ragusa di Chiara S., Tsolakis H. 1998. Keys to the phytoseiid mites (Parasitiformes, Phytoseiidae) of Israel. Phytophaga, 8, 85-154.
- Tabet V.G., Marineide R.V., Gustavo L.M.M., Cristiane G.N.M., Sousa A. 2018. Plant extracts with potential to control of two spotted spider mite. Arq. Inst. Biol, 85: 1-8. https://doi.org/10.1590/1808-1657000762015
- Tak J.H., Isman M. B. 2017. Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod., 108: 786-792. https://doi.org/10.1016/j.indcrop.2017.08.003
- Tomczyk A., Kropczynska D. 1985. Effects on the Host Plant - In: Helle W, Sabelis MV, editors. Spider mites: their biology, natural enemies and control. Amsterdam: Elsevier. p. 317-331.
- Touhidul I.M, Shunxiang R. 2009. Effect of sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) infestation on eggplant (Solanum melongena L.) leaf. J. Pest Sci., 82: 211-215. https://doi.org/10.1007/s10340-008-0241-x
- Xu X, Enkegaard A. 2010. Prey preference of the predatory mite, Amblyseius swirskii between first instar western flower thrips Frankliniella occidentalis and nymphs of the twospotted spider mite Tetranychus urticae. J. Insect Sci., 10(1): 1-11 https://doi.org/10.1673/031.010.14109
- Van Leeuwen T., Vontas J., Tsagkarakou A., Dermauwa W., Tirry L. 2010. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem. Mol. Biol., 40: 563-572. https://doi.org/10.1016/j.ibmb.2010.05.008
- Whalon M.E., Mota-Sanchez R.M., Hollingworth R.M., Duynslager L. 2024 - Artrhopods Resistant to Pesticides Database (ARPD). http://www.pesticideresistance.org.
- Wu L., Huo X., Zhou X., Zhao D., He W., Liu S., Liu H., Fen, T., Wang C. 2017. Acaricidal activity and synergistic effect of thyme oil constituents against carmine spider mite (Tetranychus cinnabarinus Boisduval). Molecules, 22(11), 1873. https://doi.org/10.3390/molecules22111873
- Yari S., Hajiqanbar H., Farazmand A., Rashed A., Fathipour Y. 2023. Efficacy of single and combined release of Phytoseiulus persimilis and Amblyseius swirskii at different release ratios for control of Tetranychus urticae and Frankliniella occidentalis on rose plants. Int. J. Pest Manage., 25: 1-11. https://doi.org/10.1080/09670874.2023.2185312



2024-07-02
Date accepted:
2025-01-14
Date published:
2025-01-20
Edited by:
Marčić, Dejan

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Mertoglu Boz, Gamze and Kumral, Nabi Alper
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



