Redescription of Homeopronematus anconai (Baker) (Acari, Iolinidae) and description of Quasihomeopronematus nordestinus n. gen. n. sp.
De Vis, Raf M. J.  1
; Vervaet, Lore
1
; Vervaet, Lore  2
; Reybroeck, Eva
2
; Reybroeck, Eva  3
; Vanlommel, Wendy
3
; Vanlommel, Wendy  4
; Van Havermaet, Robin
4
; Van Havermaet, Robin  5
; Foqué, Dieter6
; Marcossi, Ítalo
5
; Foqué, Dieter6
; Marcossi, Ítalo  7
; Pallini, Angelo
7
; Pallini, Angelo  8
; Binda de Assis, Caio Henrique
8
; Binda de Assis, Caio Henrique  9
; Van Leeuwen, Thomas
9
; Van Leeuwen, Thomas  10
and Ueckermann, Edward A.
10
and Ueckermann, Edward A.  11
11
1✉ Research Station for Vegetable Production (PSKW), 2860 Sint-Katelijne-Waver, Belgium.
2Laboratory of Agrozoology, Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University (UGhent), Coupure Links 653, 9000 Ghent, Belgium.
3Research Station for Vegetable Production (PSKW), 2860 Sint-Katelijne-Waver, Belgium.
4Research Centre Hoogstraten, Voort 71 ,2328 Meerle, Belgium.
5Viaverda, Karreweg 6, 9770 Kruisem, Belgium.
6Flanders Research Institute for Agriculture, Fisheries and Food -Ilvo, Burg. Van Gansbergelaan 92, 9820 Merelbeke, Belgium.
7Department of Entomology, Federal University of Viçosa, Viçosa, Minas Gerais, Brazil & Present address: Agriculture and Livestock Research Enterprise of Minas Gerais/EPAMIG, Prudente de Moraes, MG, Brazil.
8Department of Entomology, Federal University of Viçosa, Viçosa, Minas Gerais, Brazil.
9Department of Entomology, Federal University of Viçosa, Viçosa, Minas Gerais, Brazil.
10Laboratory of Agrozoology, Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University (UGhent), Coupure Links 653, 9000 Ghent, Belgium.
11Unit for Environmental Sciences and Management, Potchefstroom Campus, North - West University, Private Bag X6001, Potchefstroom, 2520, South Africa.
2024 - Volume: 64 Issue: 4 pages: 1283-1311
https://doi.org/10.24349/cu4t-4h6vZooBank LSID: C6D0C3D6-7111-4BB5-865D-ECC051352077
Original research
Keywords
Abstract
Introduction
Since 2016, Aculops lycopersici (Tryon, 1917) (Acari: Eriophyidae) has become a major pest in greenhouse tomato crops in Western Europe (Reybroeck et al. 2018; Vervaet et al. 2021). Therefore, a search for potential natural enemies was started in 2017 in Belgium and neighbouring countries during two successive projects funded by the Flemish vegetable auctions (LAVA) and Flanders region (DUCATO and BALTO) (Reybroeck et al. 2018; Vervaet et al. 2021; Vervaet et al. 2022). During that study, we found Pronematus ubiquitus (McGregor 1932) (Ueckermann & De Vis et al., 2024) and Duoparus hyeresensis De Vis & Ueckermann 2024 (De Vis et al. 2024) and Homeopronematus anconai (Baker, 1943). Here we present the results of our taxonomic study of H. anconai.
At the same time, Marcossi et al. (2024) started research with an iolinid, initially identified as H. anconai, as biological control agent in Viçosa, Brazil. We received material to confirm the identity of the species they used but concluded it is a different species. This new species is described here, as it is closely related to H. anconai.
Currently, two Homeopronematus species are described: H. anconai and H. staercki (Schruft, 1972) [André (2021), https://species.wikimedia.org/wiki/Pronematinae, accessed on 22/05/2024]. The identification of these two species is, however, not trivial given that the descriptions do not present distinguishing characteristics and lack the (range of) measurements of individual characteristics. Baker (1943) described Pronematus anconai based on specimens collected from Mexico. His description is limited, not including species specific characteristics such as the leg and body chaetotaxy, the ag setae or the characteristics of the distal eupathidia on tarsus I. In his redescription (Baker 1968), he added detail on the relative length of the setae, without measurements, but mentions distinctive characteristics such as the partially smooth eupathidia with (tcζ) longer than tarsus I and (pζ) shorter than tarsus I, and the forked ag3. However, he mentions that the striae are apparently without lobes.
Kuznetsov (1972) found H. anconai on a large number of trees, shrubs and grasses in the south coast and steppe regions of Crimea. His redescription is more detailed and gives general setal lengths, but not the range for each individual seta. Although described as a Pronematus, the leg chaetotaxy differs from that of the Pronematus genus description of Baker (1965) in having one seta more on each of trochanters I and II (one instead of zero), tarsi III and IV (six instead of five), and femur II (three instead of two). He mentioned two setae on genu IV. We suppose that this is a printing error as on his drawing he depicted only one seta (the leg chaetotaxy on the drawing coincides apart from this seta with that in the text) and no other species of the Pronematinae has two setae on genu IV. Another difference is the number of setae on tarsus I as most descriptions of genera or species do not mention the two tiny (u) setae, mentioning thus six setae instead of eight for that segment.
In the same year, Schruft (1972) described Pronematus staercki based on specimens collected in Schliengen, in the south-west tip of Germany. His description is more detailed, including leg chaetotaxy and immature stages (except tritonymph) but lacks measurements of individual setae and the shape of ag3. The leg setation is the same as that described by Kuznetsov for H. anconai (with one seta on genu IV). He did not mention distinguishing characters to separate H. staercki from H. anconai or other Pronematinae. Based on his description, H. staercki cannot be distinguished from H. anconai. Schruft certainly omitted (as most authors at that time) the two tiny setae (u) on tarsus I mentioning 6 setae for that segment.
André (1980) erected several new genera to solve the inconsistencies in leg chaetotaxy in the genera descriptions of the Tydeidae and the species allocated in those genera. He erected the genus Homeopronematus with the leg setation of P. staercki and allocated therein besides P. staercki also H. vidae André 1980 a new species he described based on specimens from a lab culture of M. Hoy from University of California, Berkeley, USA. He did not mention the redescription of P. anconai of Kuznetsov (1972) neither resolved the position of Pronematus anconai (Baker 1943). Three years later, Knop and Hoy (1983a) compared the Californian P. anconai with a type slide of P. anconai they obtained from Baker (containing two females) and concluded that the Californian are the same as the Mexican specimens and that they belong to the genus Homepronematus. They synonymised H. vidae with H. anconai, and transferred Pronematus anconai to the genus Homeopronematus, stating that the types of H. vidae came from a lab culture of H. anconai of M. Hoy and that variability within the species is high. They redescribed H. anconai based on Californian specimens, adding the lobed striation as a distinguishing feature (that they could also distinguish on the types of H. anconai) but did not determine the variability of the characteristics.
No new species have been described within the genus Homeopronematus but two authors mention undescribed, related species: Homeopronematus cf. staercki in Serbia (Stojnić et al. 2002) and Pronematus cf. anconai in Iran (Daneshvar 1978).
Two papers suggest that H. staercki might be a junior synonym of H. anconai (Knop and Hoy 1983a; Ueckermann et al. 2019). However, André (1980) studied several individuals without specific label (from the Schuft's collection) of H. staercki, and found for this species a shorter c2, a higher striation density and further different ''shape and length of idiosomal setae'' (sic), without describing the exact differences. Schruft (1972) did not mention the shape of setae ag3 in the description of H. staercki while it is forked in H. anconai (Baker 1968; Kuznetsov 1972; Knop and Hoy 1983a). Ripka et al. (2022) stated that the tectal and proral eupathidia on tarsus I of H. anconai are all four equal in length while in H. staercki , the tectals are nearly twice as long as the prorals. This is, however, in contradiction with the redescriptions of H. anconai (Baker 1968; Kuznetsov 1972) or H. vidae (= syn. H. anconai) (André 1980), where the tectals are longer than the prorals with p″ζ the shortest. The figures of H. staercki of Schruft (1972) show indeed longer tectals than prorals.
Based on published reports, H. anconai has an almost worldwide distribution. It has been registered i.a. in Mexico, place of original description (Baker 1943), throughout USA (Baker 1968; Hessein and Perring 1986; Oatman 1971; Childers and Enns 1975; Knop and Hoy 1983a), Canada (Thistlewood 1991), Argentina (Baker 1968), Brazil (Johann et al. 2009; Klock et al. 2011; Horn et al. 2011; Sousa et al. 2015), Portugal (Maurício et al. 2009; Ferreira 2016), Spain (García González 2003), Italy (Liguori 1987), Hungary (Ripka et al. 2022), Crimea (Kuznetsov 1972), Türkiye (Yanar et al. 2013; Çobanoğlu and Kumral 2014), Iran (Darbemamieh et al. 2020), China (Xu 2011) and Japan (Kawai et al. 2001), but no references of its presence in Africa or Oceania were found. Homeopronematus staercki has been registered in central Europe and the middle east, i.a. in Germany (Schruft 1972), Hungary (Ripka and Kaźmierski 1998; Ripka et al. 2005; Ripka et al. 2013; Tempfli et al. 2015; Tempfli 2019; Ripka et al. 2022), Türkiye (Ozman-Sullivan et al. 2005) and Iran (Darbemamieh et al. 2021). In Italy, Hungary, Türkiye and Iran both species have been reported.
The previous illustrates the need for a redescription of the Homeopronematus spp to clear out differences and similarities as to ensure a correct identification. Because the material used for the description of H. staercki is lost [according to Schruft, personal communication, this material is at Musée Royal de l´Afrique Centrale, Tervuren, Belgium, MRAC (Zhang 2018), where we could not find them], We studied specimens of Ripka from Hungary that were identified as H. staercki (Ripka et al. 2022) and compared the results with the measurements of specimens from USA and western Europe.
Molecular techniques have shown that we can distinguish Pronematinae genera from each other using the CO1-gene (Ueckermann & De Vis et al., 2024; De Vis et al., 2024). However, it is not clear if closely related species can be distinguished using this method.
The objectives of this study are to redescribe H. anconai, to describe a new species from Brazil that is closely related with H. anconai, to morphologically compare the species studied, to answer the question if H. anconai and H. staercki are indeed different species and genetically compare H. anconai and the new species with other Pronematinae that are molecularly typified.
Material and methods
Collecting material
During the DUCATO (strain numbers labelled with a'D') and BALTO (strain numbers labelled with a'B') projects (Reybroeck et al. 2018; Aussems et al. 2021), a survey was done from summer 2017 until autumn 2019, mostly in Belgium and France (Survey permission nr. NOR TREL1902817S/165). Sampling was directed to wild or cultivated eriophyid susceptible plants [mostly Rubus spp (Rosaceae), but also grape (Vitis vinifera L., Vitaceae), fig (Ficus carica L., Moraceae), linden (Tilia sp., Malvaceae), maple (Acer pseudoplatanus L.) , etc.] and wild or cultivated solanaceous plants (Solanaceae), bittersweet Solanum dulcamara L., Brugmansia sp., tomato Lycopersicon esculentum L. , sweet pepper Capsicum annuum L., egg plant Solanum melongena L., Physalis peruviana L. Sampling was done in the surroundings of the research institutes or during travel in Belgium or abroad. Cultivated solanaceous plants were sampled on organic farms in Flanders or in gardens and for wild plants on sites where they were earlier found according to the site waarnemingen.be. Samples were transported to PSKW where they were screened for predatory mites under a stereo microscope (Leica DM16 or Leica M125 C) and slide mountings in Hoyer medium (Walter and Krantz 2009) were made of phytoseiids, iolinids and triophtydeids. At the same time, small lab colonies were set up with iolinids and transferred to Ghent University where cultures were increased, and single female lines (SFEL) were set up to provide material for the genetic study.
Finally, we studied slides from museum collections of USPB Museu de Zoologia ''Luiz de Queiroz'' at the Escola Superior de Agricultura ''Luiz de Queiroz'', Universidade de São Paulo, Piracicaba, São Paulo, Brazil; of EMEC—Essig Museum of Entomology, University of California, Berkeley, California, USA, and of AMU, Department of Animal Morphology, Adam Mickiewicz University, Poznań, Poland.
Morphological examination and principal component analysis
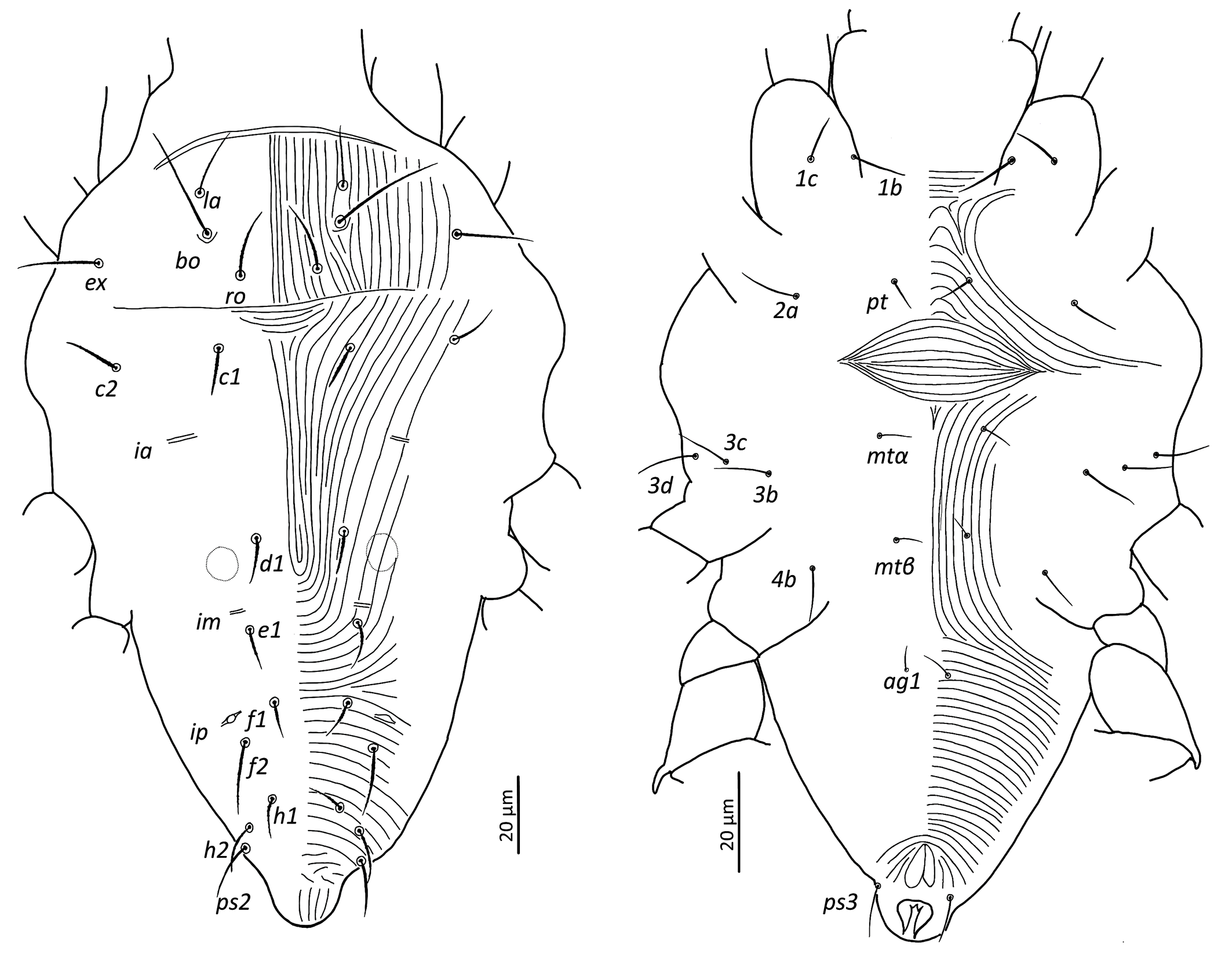
Specimens were measured using a Leica DM750 with an eyepiece micrometer or a Leica Flexacam c3 camera and Leica LAS-X software. Line drawings were made with a Leica DM750 with a phototube or Wacom Cintiq 16 pen display and drawn using Adobe Illustrator 28.2. The environmental scanning electron microscope images were taken with Hitachi tabletop microscope TM-1000. Nomenclature of body parts and its organotaxy is that of Kaźmierski (1989) except for adoral setae where we use notation or, and that of André (1981) and Khaustov et al. (2020) for the legs. In the text, measurements in micrometres are given directly after each structure.
Principal component analysis (PCA) of the females measured in this work was done with PAST (version 4.03), cf. Ueckermann & De Vis et al. (2024); De Vis et al. (2024).
Phylogenetic analysis
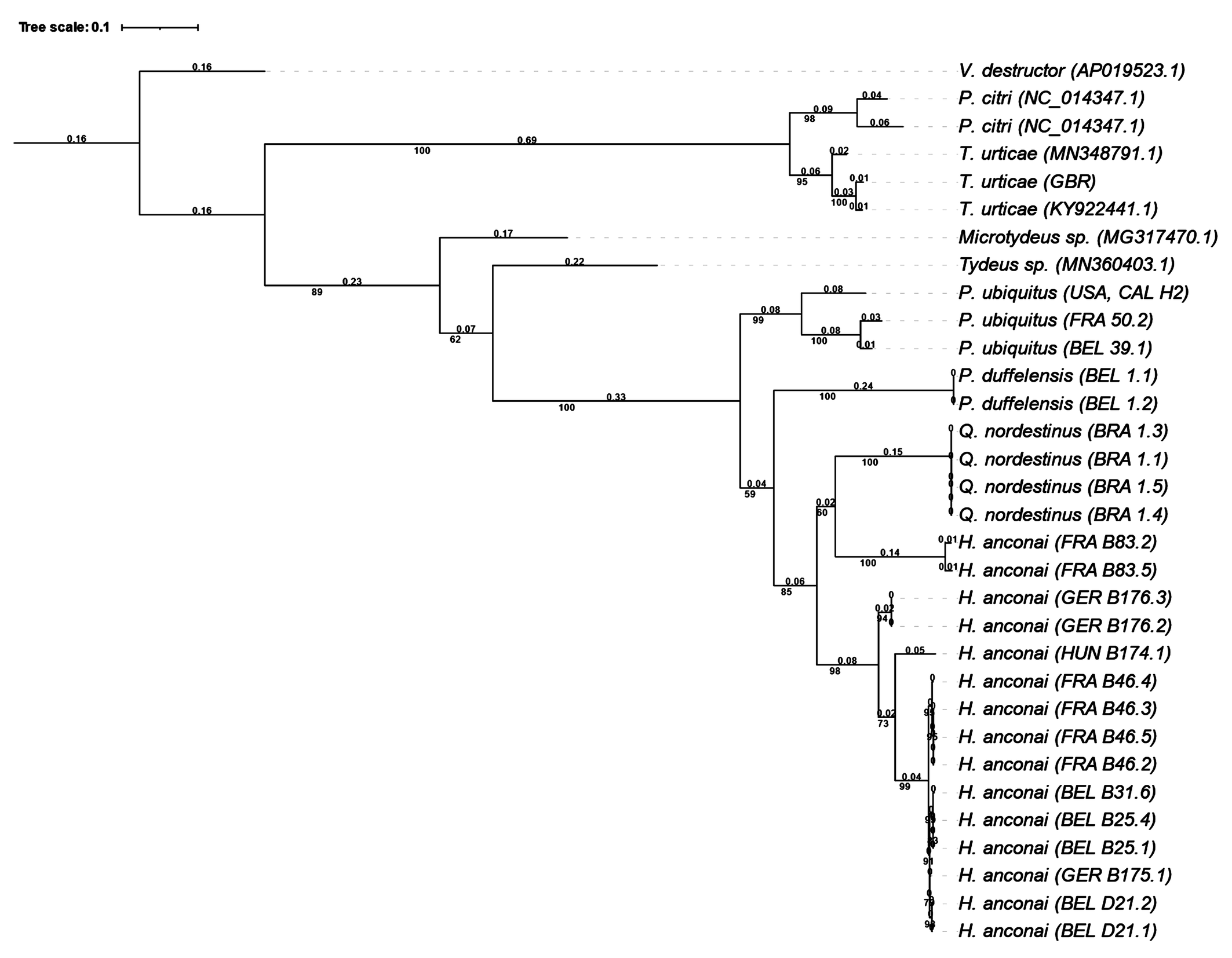
The methodology of culturing mites, molecular analysis and construction of a phylogenetic tree is described in detail in Ueckermann & De Vis et al. (2024). Briefly, it consists first of the culture of progeny of single females to have sufficient homogenous genetic material (although it is possible with one female, three to four females for one DNA extraction increases the success rate). After DNA extraction of 1 to 4 mites, a 710-bp fragment of the cytochrome c oxidase subunit I gene (CO1, mtDNA) was amplified by PCR, using the same primers and conditions as in Ueckermann & De Vis et al. (2024) and sequenced. The consensus sequences were aligned online using MAFFT version 7, including CO1 sequences of P. ubiquitus (Ueckermann and De Vis et al. 2024), P. duffelensis De Vis & Ueckermann 2024 (De Vis et al., 2024) and a set of gradually less related prostigmatid mites from previous studies, available at GenBank NCBI. Finally, a phylogenetic tree was constructed based on DNA sequences, using the software W-IQ-TREE with 1,000 ultrafast bootstrap combined with automatic model finding through ModelFinder (Trifinopoulos et al. 2016; Kalyaanamoorthy et al. 2017; Hoang et al. 2018). According to the Bayesian information Criterion, the best-fit model was determined to be K3Pu+F+I+G4, based on the likelihood scores for 88 different models. The resulting Maximum-likelihood consensus tree was rooted with the sequence of Varroa destructor Anderson and Trueman, 2000 and edited with iTOL version 5 (Letunic and Bork 2021).
Deposits of type material
Type material, holotype and paratypes, of Q. nordestinus n. gen. n. sp. are deposited at USPB—''Museu de Zoologia Luiz de Queiroz'' at the Escola Superior de Agricultura ''Luiz de Queiroz'', Universidade de São Paulo, Piracicaba, São Paulo, Brazil.
The CO1-sequences of every collection location of H. anconai and Q. nordestinus n. gen. n. sp. obtained in this study have been deposited in the NCBI Genbank database. The accession numbers can be found in Table 1.



Voucher specimens of the CO1 sequences (i.e., slide mountings of specimens originating from the same origin as the respective CO1 sequence) of H. anconai and Quasihomeopronematus nordestinus De Vis & Ueckermann n. gen. n. sp. are deposited at CBGP, INRAE, Montpellier, France. The CO1-sequences of the single female lines of the following samples were identical for each origin/sample: Q. nordestinus Brazil (SFEL 1.1, 1.3, 1.4 and 1.5); H. anconai Belgium D21 (SFEL 21.1 and 21.2), Belgium B25 (SFEL 25.1 and 25.4), France B46 (SFEL 46.2, 46.5 and46.3) and Germany B176 (SFEL 176.2 and 176.3); for each of these origins, several voucher specimens were deposited under the same CO1-sequence. Of sample B175.1 no voucher specimens were available. Slide labels of the voucher specimens at CBGP can be found in Table 1.
Results
Collecting material
Homeopronematus anconai
In Europe, a total of 338 samples were collected mainly in Belgium (271) and France (50) (Survey permission UGent nr. NOR TREL1902817S/165) but also some in The Netherlands (2), Germany (4), Czech Republic (3), Switzerland (3) and Hungary (1). From these, 63 samples from 103 localities in Belgium, 14 samples from 47 localities in France, 2 samples from 2 localities in The Netherlands, 2 samples from 2 localities in Germany, 3 samples from 3 localities in Czech Republic, and 3 samples from 3 localities in Switzerland, and 1 sample from 1 locality in Hungary contained H. anconai. We studied and measured specimens of the following slide mounts: Belgium: 17/7/2017, Sint-Katelijne-Waver, 51.077141, 4.526659, Viburnum x burkwoodii Burkwood & Skipwith (Adoxaceae), Raf De Vis, 1 female, D18; 26/7/2017, Sint-Katelijne-Waver, 51.059258, 4.495511, Rubus sp., Raf De Vis, 1 female, D23.2; 1/3/2018, Sint Truiden, raspberry, Rik Cleymans, 1 female, D66.1; 18/08/2018, Hofstade, 50.99639, 4.51389, Rubus sp., Raf De Vis, 1 female, B4.4; 20/8/2018, Schoten, 51.268127, 4.5169, Rubus sp., Kevin Van Puyvelde, 1 female, B12.4; 30/08/2018, Machelen a/d Leie, 50.945049,3.487808, fig, Justine Dewitte, 1 female, B16.3; 30/08/2018, Kruishoutem, 50.904045, 3.528591, Rubus sp., Justine Dewitte, 1 female, B17.2; 16/09/2018, Meldert, 50.939477, 4.134792, Rubus sp., Raf De Vis, 10 females from rearing, B31.6; 30/09/2018, Maasmechelen, 50.982184, 5.639674, Rubus sp., Raf De Vis, 1 female, B42.1; 17/10/2018, Borgloon, 50.801239, 5.290275, grape, Eva Reybroeck, 1 female, B67.5; 03/2019, Gent, 51.053635, 3.706242, Rubus sp., Lore Vervaet, 3 females from rearing, B25; 27/07/2020, Pailhe, 50.432287, 5.248216, Rubus sp., Raf De Vis, 1 female, B164.2; France: 29/09/2018, Treignat,46.362778, 2.349167, tomato, Dieter Foqué, 2 female B44.1-44.2; 29/09/2018, Treignat,46.362778,2.349444, tomato, Dieter Foqué, 1 wild female and 3 females from rearing, B46.2; 29/09/2018, Treignat,46.366944, 2.344167, Rubus sp., Dieter Foqué, 1 female B52.3; 14/10/2018, Hyères, 43.1181, 6.1621, Rubus sp., Lore Vervaet, 16 females from rearing, 1 wild specimen B83.1; 17/10/2018, Allan, 44.5120, 4.7796, Rubus sp., Lore Vervaet, 2 females B89.2.1-2; 22/07/2019, Landrecies, 50.128817, 3.643396, bittersweet, Raf De Vis, 1 female, B100.1; 22/07/2019, Fontain-au-Bois, 50.128817, 3.643396, Rubus sp., Raf De Vis, 1 female, B109.2; 28/12/2019, Kaysersberg, 48.141464, 7.266328, Rubus sp., Raf De Vis, 2 females, B146.1 & D147.2; The Netherlands: 19/10/2019, Mechelen, 50.783623, 5.940681, Rubus sp., Raf De Vis, 4 females, B141.4; B141.5.1-2, B141.6; 30/8/2021, Mook, 51.762295, 5.911216, grape, Felix Wäckers, 1 female, B172.1; Germany: 07/10/2018, Seebach, 48.582689, 8.167126, Rubus sp., Markus Knapp, for rearing and CO1-sequennces, no voucher specimen were mounted, B175.1; 18/09/2023, Auweiler, 51.001925, 6.848739, tomato, Ute Perkons, 5 females from rearing mounted on 15/5/2024, B176.1-5; Czech Republic: 27/11/2019, Prague, 50.085085, 14.307956, Rubus sp., Raf De Vis, 4 females, B144.5.1-2 & B144.7.1-2; 27/11/2019, Prague, 50.084640, 14.309172, Rubus sp., Raf De Vis, 3 females, B145.1 & B145.2.1-2; Switzerland:13/9/2017, Oppens, Rubus sp., Hans Claes, 2 females, D56.1 & D56.2; 13/9/2017, Bavois-Orny, Rubus sp., Hans Claes, 1 female, D57.5; 13/9/2017, Pailly, Rubus sp., Hans Claes, 1 female, D58.6; Hungary: 27/09/2018, Kács, Rosa sp (Rosaceae)., Anton van der Linden, 5 females from rearing, B174.1.
We studied the following slides from museums: from EMEC: 20 slides mounted on 12/10/1969 from a culture of H. anconai from unknown origin (we did not include measurements of these specimens in this study because the origin is not known); from AMU: Hungary: 3 slides from with 5 females and 1 male previously identified as H. staercki (Ripka et al. 2022): 17/07/2019, Lövő, Rubus caesius, Geza Ripka, 1 female, slide 1479; 12/06/2020, Nagylózs, Rubus Seebergensis, Geza Ripka, 2 slides with 4 females, slides 1499-1 and 1499-2; 27/09/2018; Serbia: 2 slides with 1 female each, previously identified as Homeopronematus cf. staercki sp. nov. (Stojnić et al. 2002): 18/06/1996, Kolekcija Boleč, Boleč, Malus sp., Stojnić, 1 female, slide 28; 28/07/1996, Kolekcija Boleč, Boleč, Malus sp., Stojnić, 1 female, slide 34. from USPB: USA: 29/10/1977, U.C.R. Campus Riverside Co-CA, grape, Gilberto de Moraes, 1 female, 356; 14/10/1977, U.C.R. Campus Riverside CA, Malva sp. (Malvaceae), Gilberto de Moraes, 2 females, 359 MZQL 2199 C=994; 29/10/1977, Oakglen, San Bernardino, Cedreila ?, Gilberto de Moraes,1 female, 356 MZQL 2206 C=997.
In this study, we found H. anconai on grape, Malva sp. and Cedreila (?) on slides with specimens from USA. In Europe, we found it on Rubus sp., raspberry, Brugmansia sp., bittersweet, Solanaceae sp., tomato, cucumber, grape and fig.
Quasihomeopronematus nordestinus n. gen. n. sp.
Brazil: we received 7 slides From Ítalo Marcossi mounted 24/01/2022, Viçosa, MG, -20.7598, -42.8829, tomato, and additional material from the same culture in alcohol which we mounted in Hoyer. From museum USPB we studied three females on two slides: 11/06/1993, Amontada, CE, Campo 24, cassava Manihot esculenta Crantz (Euphorbiaceae), I.A. Almeida, 1 female, MZQL 4471 C=2941; 15/06/1993,'Gov. De Ze Rosado' (we suppose Governador dix-sept Rosado) RN, Campo 27 Macroptilium sp., (Fabaceae) I.A. Almeida, 2 females, MZQL 4480 C= 2950.
Quasihomeopronematus nordestinus n. gen. n. sp. has been found on tomato, casava and Macroptilium sp.
Morphological descriptions
Family Iolinidae Pritchard, 1956
Subfamily Pronematinae André, 1979
Genus Homeopronematus André, 1980
Type species — Homeopronematus vidae by original designation André (1980: 114).
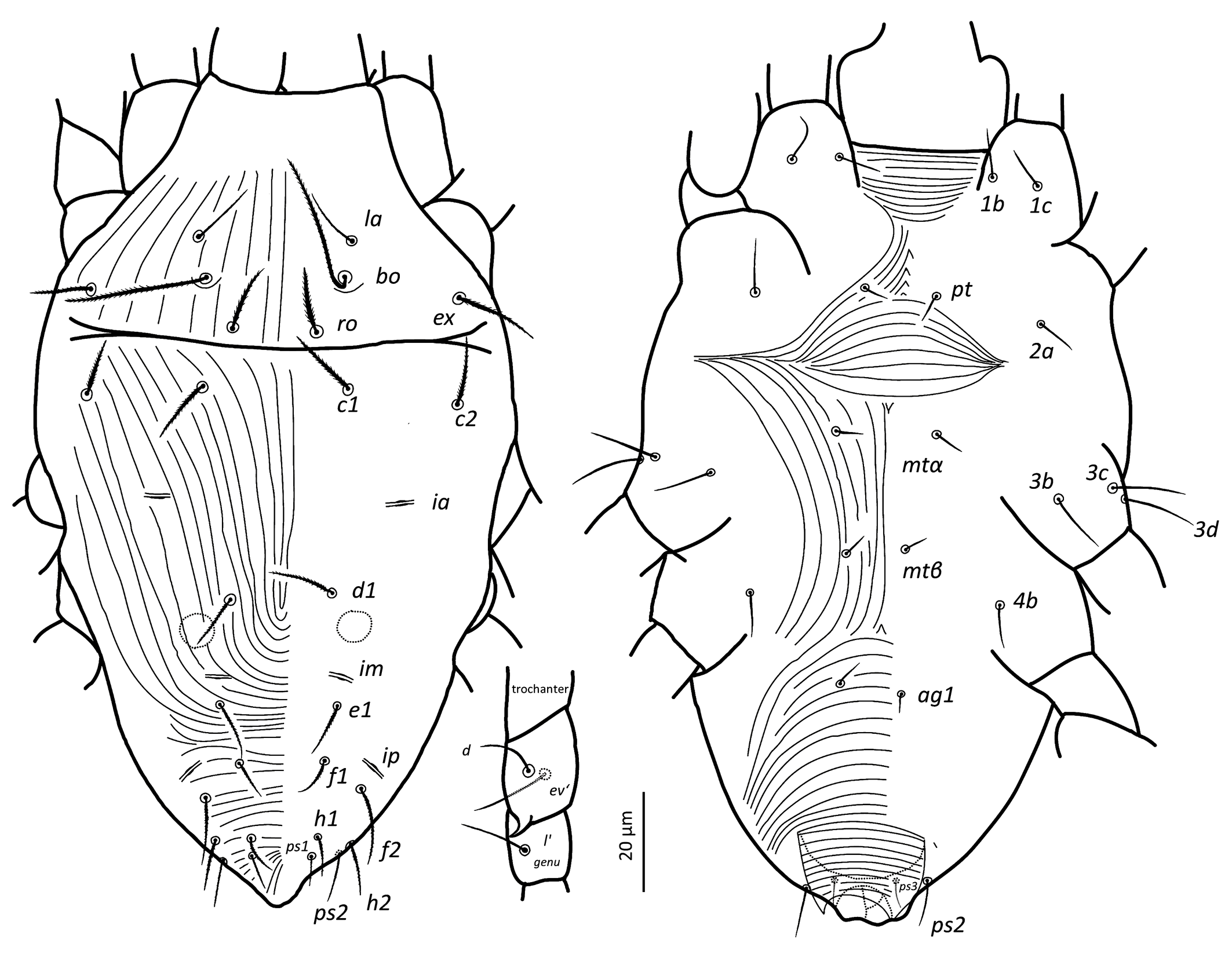
Description — Adult female. Aspidosoma procurved with four pairs of setae bo, ro, la and ex; opisthosoma with 11 pairs of setae, c1, c2, d1, e1, f1, f2, h1, h2, ps1, ps2 and ps3. Poroidotaxy: ia, im, ip, ih. Eye spots absent. Four pairs of aggenital setae. Empodium and claws absent on leg I, each of other legs with ciliate empodium and two claws, without empodial hooks. Leg chaetotaxy: I (9+ω-3+k+φ-3-3-1), II (6+ω-2-3-3-1), III (6-2-2-2-1), IV (6-2-1-2-0). Epimeral formula 3-1-4-1. Body setae finely serrate. Poroidotaxy: ia, im, ip, ih. Adult male as female, but with only one pair of ag setae, however, in some specimens a second unpaired ag seta, anterior to the paired setae, has been found.
Immatures can be recognised by the following chaetotaxy (difference with adult in bold): opisthosoma same as adult for all stages; epimeral formula adult, tritonymph and deutonymph (3-1-4-2), protonymph (3-1-3-0), larva (3-1-2). Aggenital setae: female and tritonymph: 4; male: 1; deutonymph: 2 in female, unknown in male; protonymph and larva absent. Leg chaetotaxy: tritonymph: I (9+ω-3+κ+φ-3-3-1), II (6+ω-2-3-3-1), III (6-2-2-2-1), IV (5-2-1-2-0); deutonymph: I (9+ω-3+κ+φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-1), IV (5-2-1-2-0); protonymph: I (9+ω-3+κ+φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-1), IV (5-0-0-0-0); and larva: I (6+ω-3+κ+φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-0).
Remark — We add seta homologous to s (see Ueckermann & De Vis et al., 2024) on tarsus I in all stases, except larva to the genus description of André (1980). Although we could not study the type material, which is lost or faded away (personal observation of the last author), all material we studied, including the US material, clearly showed this small seta homologous to s.
Homeopronematus anconai (Baker, 1943)
Homeopronematus anconai (Baker), Knop and Hoy 1983a: 2.
Pronematus anconai Baker, 1943:188-9; Baker, 1968: 1094-5; Kuznetsov, 1972: 11
Senior synonym of Homeopronematus vidae André, 1980: 116; Pronematus staercki Schruft, 1972: 129
(Figures 1-8)
Redescription
Measurements — Measurements of different structures are shown in Table 2.



Adult female



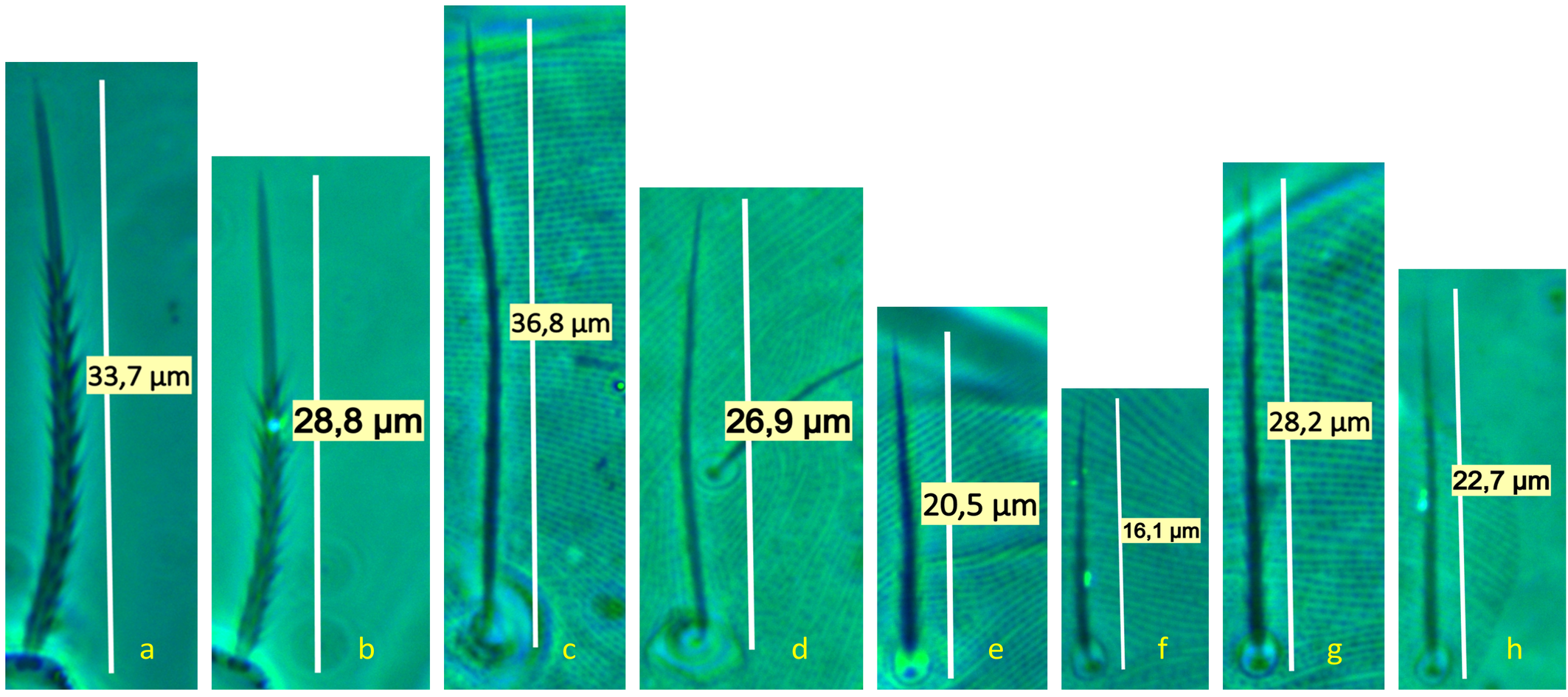
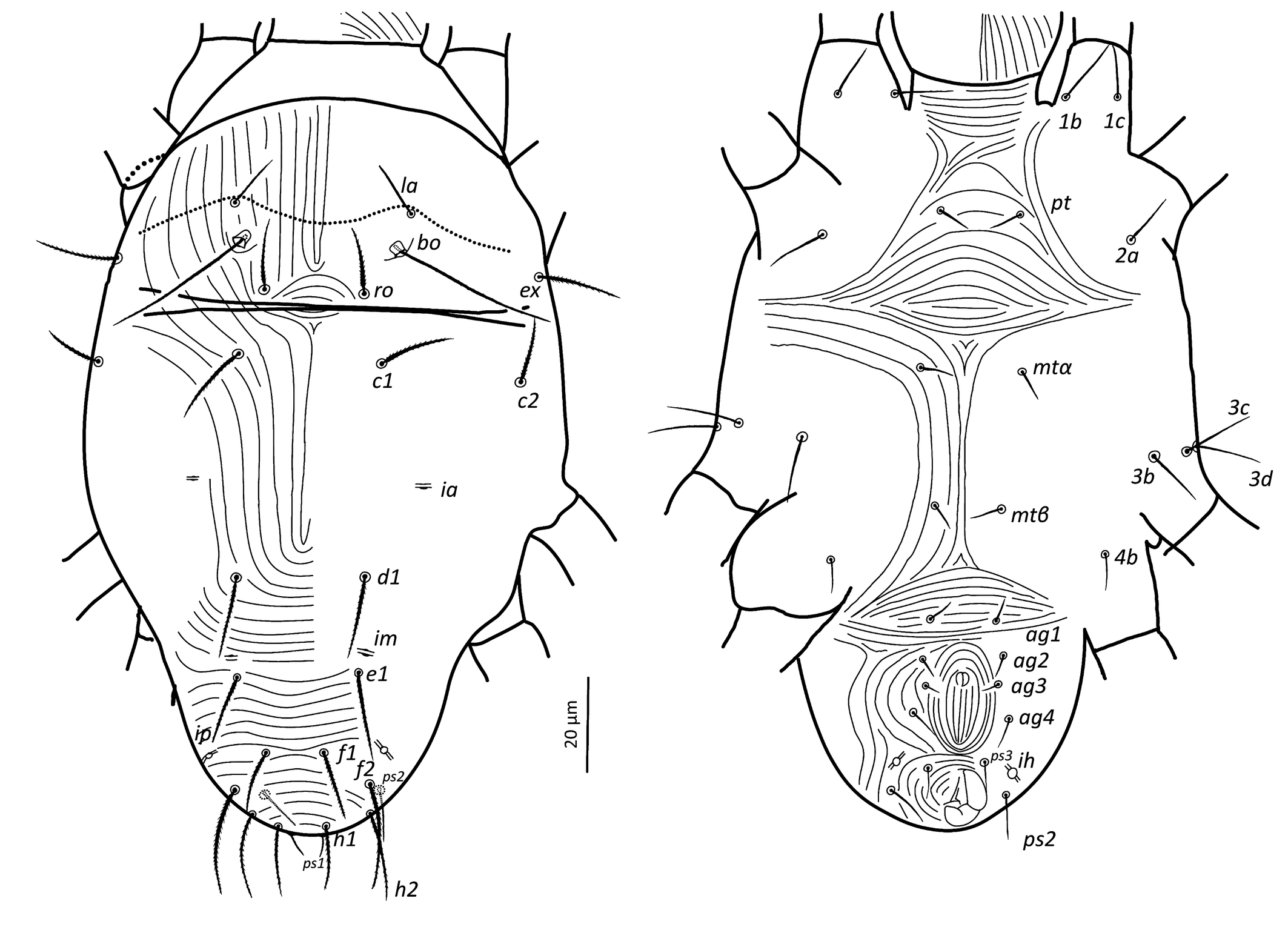
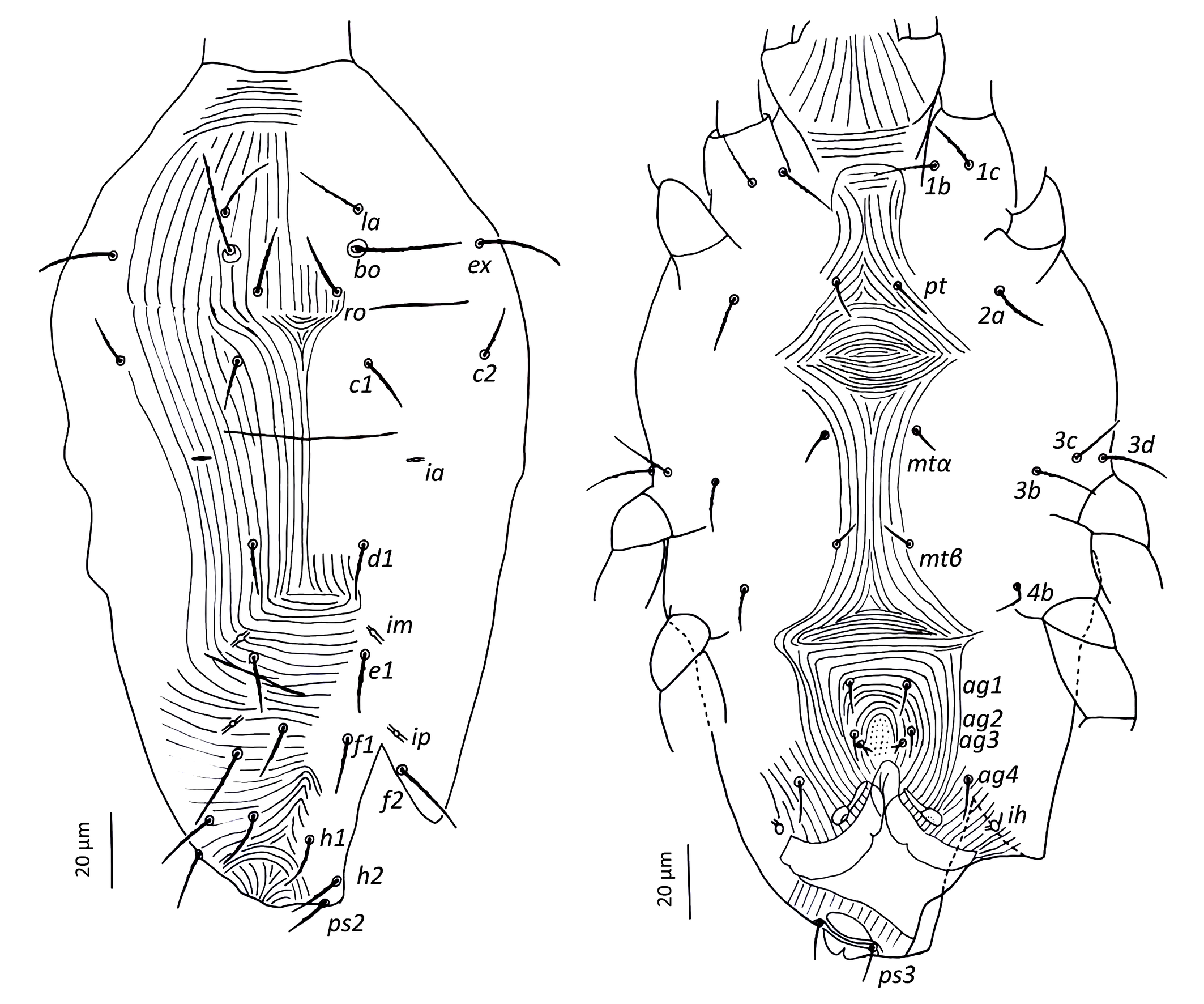
Idiosoma — (Figures 1, 4a–b & 6a). Ovaloid, tapering posteriorly. Completely striated; striae in general continuous, fine and lobed. Dorsal setae clearly barbed, ventral setae apparently smooth, except 3c and 3d, which are finely serrated.
Dorsum — (Figure 1 & 6a). Aspidosoma with subparallel striae, converging anteriad; dorsum of opisthosoma with striae converging posteriad d1, becoming transverse between d1-f1 and then divergent toward posterior edge. Idiosomal setae aciculate, serrated (Figures 5e, g); trichobothrial seta bo finally serrated over entire length (Figure 5c). Trichobothrial seta bo the longest, followed by ex, la and ro; la somewhat finer than others of similar thickness. Setae c1, d1, e1 and f1 not reaching base of d1, e1, f1 and h1, respectively. Lyrifissure ia situated half distance between setae c1 and d1 and far apart from imaginary line c1–d1. Lyrifissures im anterior to and closely associated with setae e1, slightly apart from imaginary line d1–e1. Lyrifissures ip situated approximately half distance between e1 and f2, slightly apart from imaginary line e1–f2. Setae ps2 dorsolaterally.
Venter — (Figure 1 & 4c). Transition area between idiosoma and gnathosoma with transverse striation, elsewhere longitudinal; constricted between legs I-II and legs III–IV; transverse for short median sections anterior to setae mtα and anterior to setae ag1, constituting two spindle-shaped patterns, both wider than the distance between setae mtα and setae ag1, respectively; striae convergent to genital opening. Setae ps3, anteriad anal opening in area of striae that circumventing anal opening. Setae ag3 small and bifurcated. In several slides kept at Essig Museum from a rearing from unknown origin and mounted in 1969, several females appear with ag3 trifurcated. Genital opening in form of inverted ''T''. With one pair of genital papillae. Lyrifissure ih laterad genital opening.



Gnathosoma — (Figure 2). Chelicera with parallel diagonal striation; dorsally with sclerotised tip and ventral stylet. Subcapitulum with longitudinal striation, two pairs of subcapitular setae, sc1 10.8 and sc2 13.1, and two pairs of adoral setae, or1-2 ~ 5. Palpal tarsus terminating with distally bifurcate eupathidium, solenidion ω and seta ba minute; dorsal seta d bifurcate (not always visible), ventral seta v stouter and smooth; femorogenu 21.5 long and 9.7 wide. Palpal chaetotaxy (6+ω-1-2).
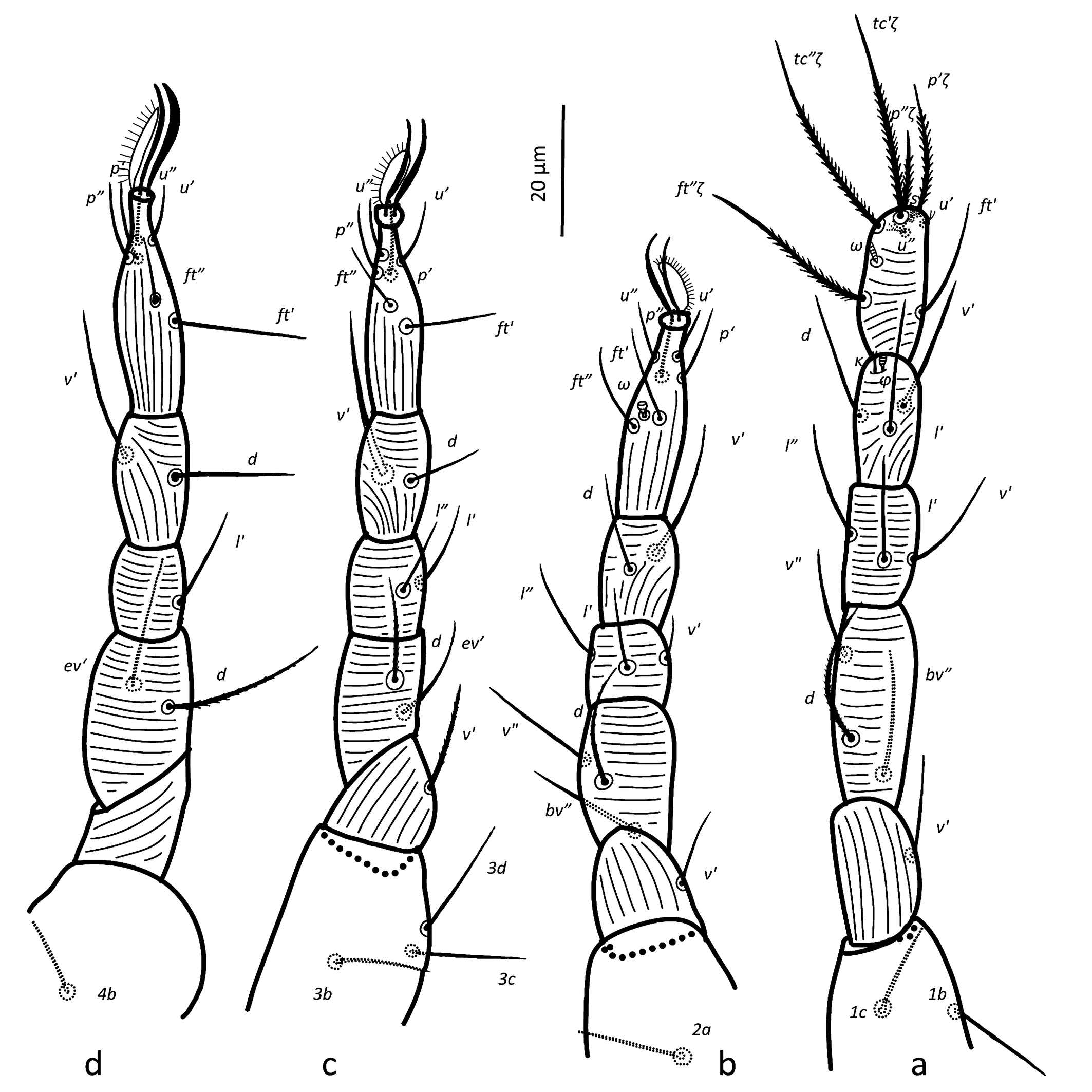


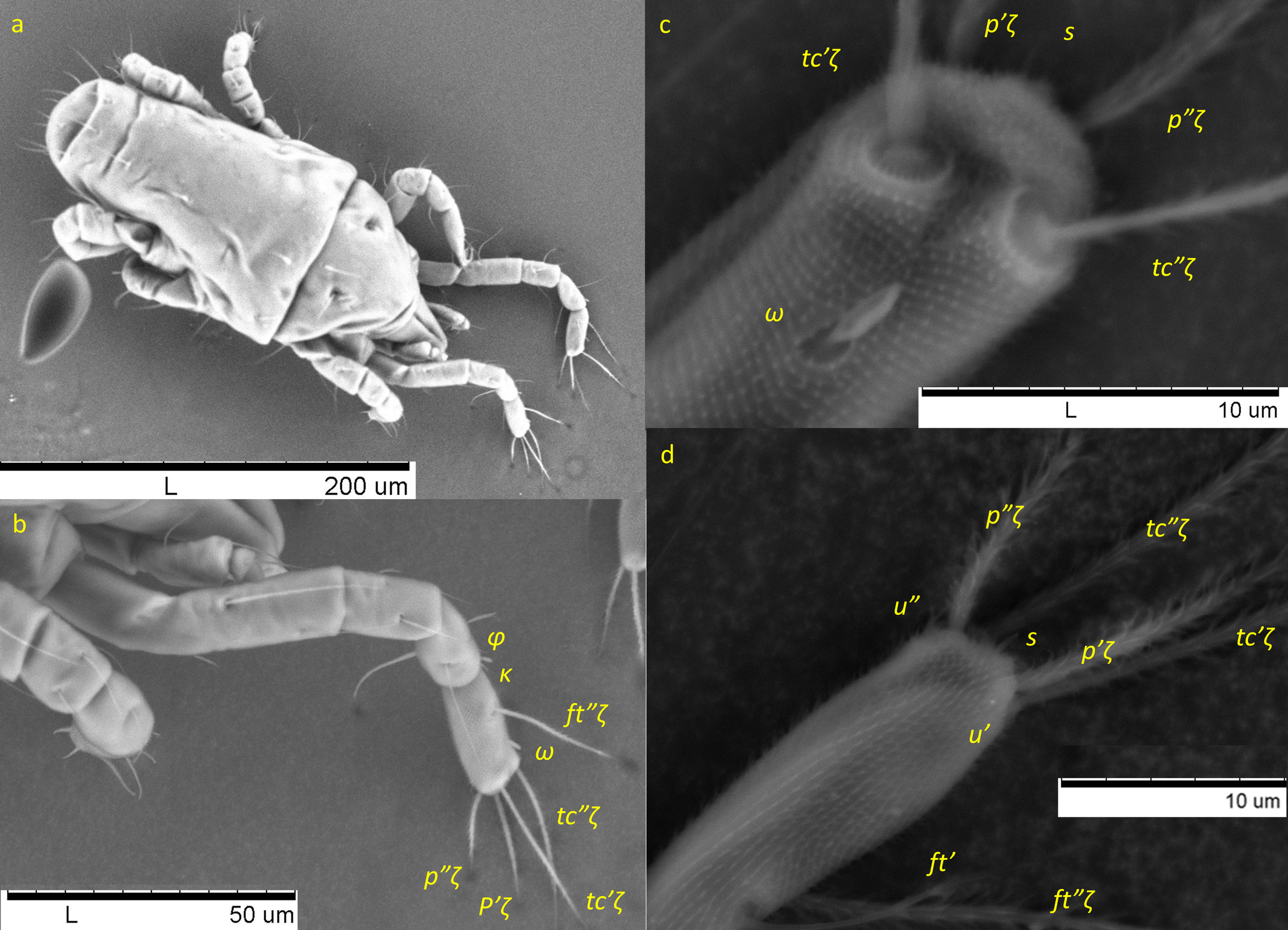
Legs — (Figures 3 & 6b–d). Setae on legs fine and filiform and most of them are finely barbed or serrated, although hardly visible, except for ventral setae on tibiae and dorsal setae on femora and seta on trochanter III, which are stouter and more clearly barbed (only these have been drawn as such on Figure 3). Ventral setae of tibiae I to IV longer than their respective dorsals. Leg I with ω not surpassing tip of segment (Figures 3a, 6 a–c), five eupathidia of which abaxial fastigial and pair of tectals subequal; prorals shorter of which p″ζ shortest (Figures 3a & 6b); eupathidia barbed for about 2/3 at their base (Figures 3a, 5a & 6b), prorals and tectals in form of bow, fastigial ft″ζ slightly sigmoid; with two bifurcated unguinals adjacent and proximal to prorals; seta homologous to s ~2.5 between prorals (Figures 6c, d); ventral seta v′ on tibia 30.5, famulus bifurcated at tip (Figure 6b) with common base of both forks (mostly only this is visible) shorter than solenidion φ; dorsal seta d on femur 30.7. Leg II with solenidion ω slender 2.2; tibia with ventral seta v′ 29.2 and dorsal seta d 17.8; femur with dorsal setae d 22 and 18.4 and posterolateral/ventral seta v″ 18.4. Leg III with ventral seta v′ 27.4 and dorsal seta d 15.9 on tibia; seta 3c of coxae shorter than 3b and 3d. Leg IV with ventral seta v′ 27.1 and dorsal seta d 19.7 on tibia; femur with ventral seta ev′ 27.7 and dorsal d 27.9.







Adult male
(Figure 7)


Idiosoma — Most of characters same as in female with exception of following: dorsal setae shorter, serrate; with only one pair of aggenital setae, although in one specimen a second, unpaired ag seta, posterior to one of ag1 setae was found; femur IV with distal, dorsal, adaxial, small and pointed spur (Figure 7). Two round spots, diameter 8, visible dorsally, posteriad d1 setae. Ventrally, genital opening forms a tube, as wide as distance ps2-ps2, above anal opening and ps3 setae. The dimensions of the Hungarian male fall within the range of the Belgian males.
Tritonymph
(Figure 8)


Idiosomal chaetotaxy like adults. Leg chaetotaxy like adults except for five setae on tarsus IV instead of six (seta tc″ missing). Epimeral formula like in female. Setae ag3 4.7 not forked and shorter than ag1 5.3, ag2 5.6 and ag4 7.1; genital pore bivalved, situated medially and posterior to (ag2) and anterior to (ag3). Dehiscence line anterior to setae la.
Deutonymph
Idiosomal chaetotaxy like in female but with two pairs of aggenital setae instead of four, anterior to genital pore. Epimeral formula like in female. Leg chaetotaxy like in female but with five setae on each of tarsus III and IV instead of six, and no setae on each of trochanters I and II instead of one. Hence, deutonymph with the following chaetotaxy: I (9+ω-3+ κ+φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-1), IV (5-2-1-2-0). Seta ft″ζ eupathid-like.
Protonymph
Idiosomal chaetotaxy like in female, but all aggenital setae missing. With only two setae on coxae III (3d missing) and mtβ and 4b missing, hence epimeral formula 3-1-3-0. Leg chaetotaxy like in deutonymph but leg IV without setae except on tarsus which is equal deutonymph. Hence, protonymph with the following chaetotaxy: I (9+ω-3+ κ+φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-1), IV (5-0-0-0-0). Seta ft″ not eupathid-like.
Larva
Idiosomal chaetotaxy like in protonymph. Coxae III with one seta (3c and 3d missing) and leg IV missing, hence epimeral formula 3-1-2. Leg chaetotaxy like in protonymph but tarsus I with small pad-like empodium, without claws (om), setae (p) and setae (ft) not eupathid-like setae and setae (tcζ) eupathid-like, setae (u) and homologous to s missing; seta on trochanter III missing. Hence, larva with the following chaetotaxy: I (6+ω-3+κ+φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-0).
Remarks
Chaetotaxy of body and legs as in André (1980) with the addition of seta homologous to s on tarsus I in adult, tritonymph, deutonymph and protonymph, but not in larva; moreover, in protonymph: epimeral formula 3-1-3-0 instead of 3-1-4-0; and in tritonymph five setae on tarsus IV instead of six.
Schruft (1972) did not describe the tritonymph of H. staercki but we suppose he confounded the deutonymph with a tritonymph as he found for his deutonymph 5 setae on tarsus IV and ''two pairs of aggenital and two pairs of genital setae'' (= 4 pairs of aggenital setae), which are characteristics of the tritonymph. He noted two unpaired genital pores, but we only found one bivalved genital pore. Furthermore, he noted for the female four pairs of ventral setae (we suppose pt, mtα, mtβ and ag1), two pairs of aggenital setae (we suppose the forked ag3 considered as two setae), two pairs of genital setae (we suppose ag2 and ag4) and one pair of anal setae (we suppose ps3). Unfortunately, he did not draw the venter of H. staercki , which could have cleared out the status of ag3. For all nymphal stages he did not note setae (u) on tarsus I and κ on tibia I and he also did not describe κ on tibia I in larval stage.
Genus Quasihomeopronematus n. gen. De Vis & Ueckermann
ZOOBANK: 312955B1-8AA8-4F42-B941-E1F61AE01C54 ![]()
Description
Adult female — Aspidosoma procurved with four pairs of setae bo, ro, la and ex; opisthosoma with 10 pairs of setae, c1, c2, d1, e1, f1, f2, h1, h2, ps2 and ps3; ps1 absent. Poroidotaxy: ia, im, ip, ih. Eye spots absent. Four pairs of aggenital setae. Empodium and claws absent on leg I, each of other legs with ciliate empodium and two claws, without empodial hooks. Leg chaetotaxy: I (9+ω-3+k+φ-3-3-1), II (6+ω-2-3-3-1), III (6-2-2-2-1), IV (6-2-1-2-0). Epimeral formula 3-1-4-1. Body setae finely serrate. Poroidotaxy: ia, im, ip, ih.
Etymology
The Latin prefix quasi- means ''almost, having some of the features but not all''. So, the genus name refers to the similarity with the genus Homeopronematus, which differs only in the presence of ps1.
Type species
Quasihomeopronematus nordestinus n. gen. n. sp.
Differential diagnosis
The genus resembles Homeopronematus but differs in the absence of ps1.
We describe this new genus, following André (1980) who introduced the principle that new combinations of idiosomal or leg chaetotaxy lead to genera. However, we could also have described a subgenus for species having the characteristic of losing ps1. References can be found for both options. However, if we apply this principle to establish a subgenus based only on the absence of ps1, this leads to confusion with other genera:
• Ueckermaniella would be a subgenus of Metapronematus, both have the same leg chaetotaxy, but the former has four ag and 11 dorsals (3 ps), the latter three ag and 10 dorsals (2 ps). The loss of an ag and a ps seta goes together in this case.
• We have several species or genera with the leg chaetotaxy of Neopronematus but with 10 or 11 dorsals instead of nine in Neopronematus (h2 and ps2 missing, but we interpret as ps1 and ps2 missing): Dasilcoferla nadirae (Silva, Da-Costa & Ferla, 2017)(Da Silva et al. 2017; De Vis et al. 2024) with 10 dorsals (ps1 lacking) and a new Brazilian species with 11 opisthosomals (De Vis et al. in preparation).
On the other hand, we find examples where a new genus was not erected:
• The subgenus Neotydeolus of Proctotydaeus lacks ps2, while the other subgenera do not (Kaźmierski 1998).
• Pausia litchiae lacks one ps (Da Silva et al. 2017) while Pausia described by Kuznetsov and Lifshitz (1972) does not.
André (1980) evidently did not follow the synonymy of Pausia with Naudea (Baker and Delfinado 1976), because besides the opisthosomal chaetotaxy also the leg chaetotaxy is different. Pausia has 11 opisthosomals, six setae on tarsus II and III and five on tarsus IV while Naudea has 10 opisthosomals and seven setae on tarsi II to IV.
We also see other characteristics that might be considered for separating genera:
• The short forked ag3 can be found in Quasihomeopronematus as in Homeopronematus (Baker 1968; Knop and Hoy 1983a) but also in all but one species of Neopronematus (N. aegeae Panou, Emmanouel & Kaźmierski, 2000) (Panou et al. 2000; Darbemamieh et al. 2015).
In conclusion, to avoid increasing genera confusion, we follow André (1980) and create a new genus. Future studies might reorganise the genera in the Pronematinae based on an analysis of all relevant genus characteristics. However, the genus descriptions might need amplification as in most cases they are limited to chaetotaxy, and other characteristics that might be generic are only mentioned in species descriptions.
Quasihomeopronematus nordestinus De Vis & Ueckermann n. sp.
ZOOBANK: 7D5C0FFF-66DA-4F1C-865E-1195D5D980C2 ![]()
(Figures 4-5 & 9-12)
Measurements
Measurements of different structures are shown in Table 2.
Adult female (holotype + paratypes 1-2)


Idiosoma — (Figure 9). Ovaloid, tapering posteriorly; procurved. Eye spots absent. Completely striated; striae in general continuous, fine and lobed. Dorsal setae finely barbed, ventral setae apparently smooth.
Dorsum — (Figure 9). Aspidosoma with subparallel striae, converging anteriad; dorsum of opisthosoma with striae converging posteriad d1, becoming transverse between d1-f1 and then divergent toward posterior edge. Idiosomal setae aciculate, with very short serrations, la somewhat finer than others of subequal thickness (Figure 5f, h); trichobothrial seta bo with short serrations over entire length (Figure 5d). Trichobothrial seta bo the longest, followed by ex, la and ro; la somewhat finer than others of similar thickness. Most dorsal setae short, except bo, f2 and h2 which are longer. Setae c1, d1, e1 and f1 not reaching base of d1, e1, f1 and h1, respectively. Lyrifissure ia situated half distance between setae c1 and d1 and far apart from imaginary line c1–d1. Lyrifissures im anterior to and closely associated with setae e1, slightly apart from imaginary line d1–e1. Lyrifissures ip situated approximately half distance between e1 and f2, slightly apart from imaginary line e1–f2. Setae ps2 dorsolaterally.
Venter — (Figure 9). Transition area between idiosoma and gnathosoma with transverse striation, elsewhere longitudinal; constricted between legs I-II and legs III–IV; transverse for short median sections anterior to setae mtα and anterior to setae ag1, constituting two spindle-shaped patterns, both wider than the distance between setae mtα and setae ag1, respectively; striae convergent to genital opening. Setae ps3, anteriad anal opening in area of striae that circumventing anal opening. With four pairs of ag setae, ag3 small and bifurcated. Genital opening in form of inverted ''T''. With one pair of genital papillae and smooth longitudinal area anterior to each of them. Lyrifissure ih laterad genital opening.
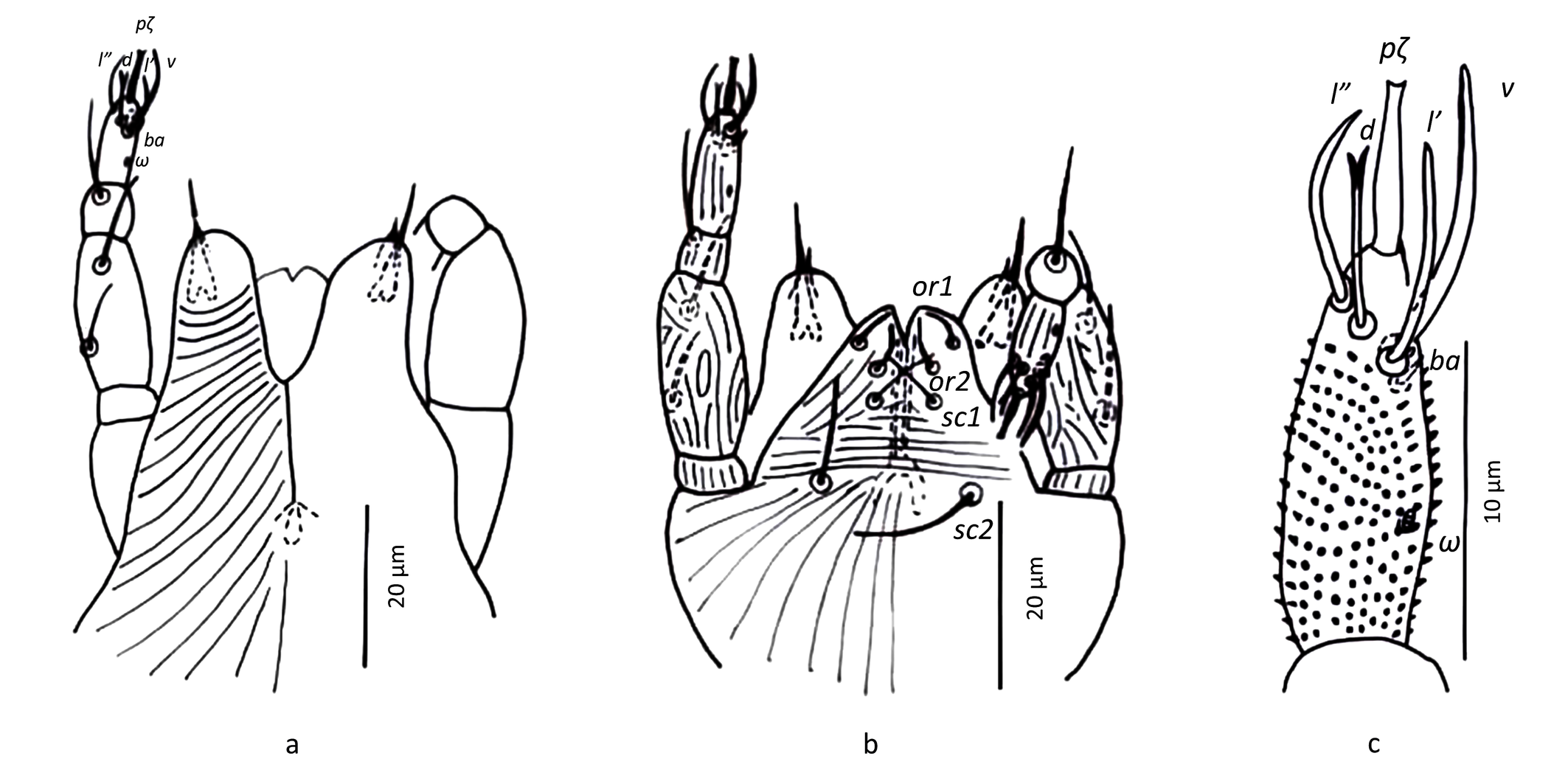


Gnathosoma — (Figure 10). Chelicera with parallel diagonal striation; with dorsally sclerotised tip and ventral stylet. Subcapitulum with longitudinal striation, two pairs of subcapitular setae, sc1 8 and sc2 10.6, and two pairs of adoral setae, or1-2 ~ 4. Palpal tarsus terminating with distally bifurcate eupathidium, solenidion ω and seta ba minute; dorsal seta d bifurcate, ventral seta v stouter and smooth; femorogenu 21.5 long and 9.7 wide. Palpal chaetotaxy (6+ω-1-2).
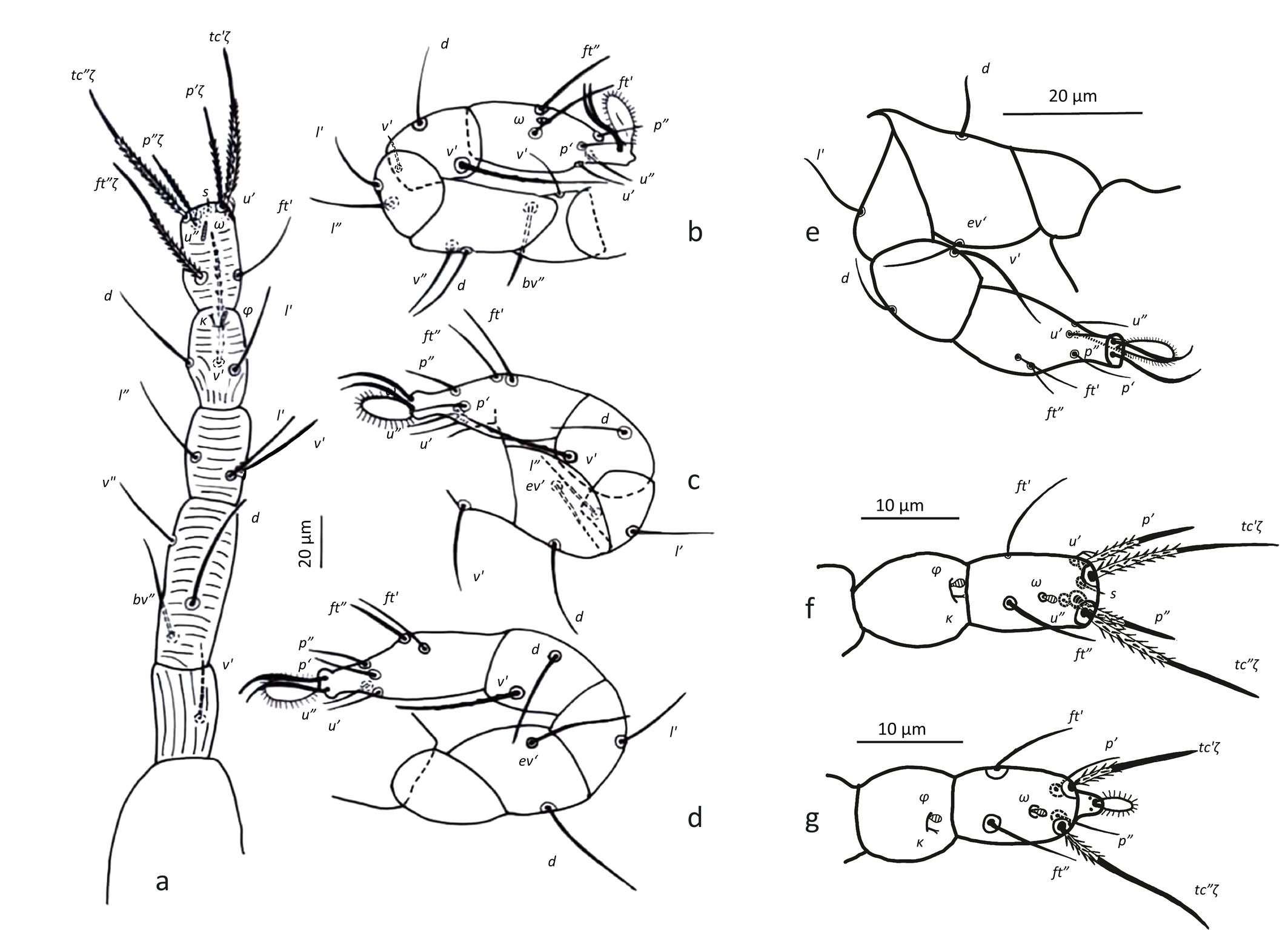


Legs — (Figure 11). All setae on legs smooth, ventral setae of v′ tibiae I to IV stouter and longer than their respective dorsals. Leg I with ω not surpassing tip of the segment, five eupathidia of which abaxial fastigial and pair of tectals subequal; prorals shorter of which p″ζ shortest; eupathidia barbed for about 2/3 at their base (Figure 5b); with two bifurcated unguinals adjacent and proximal to prorals; seta homologous to s ~2.5 between the prorals; ventral seta on tibia v′ 26.8, famulus bifurcated at tip, shorter than solenidion φ; dorsal seta d on femur 25. Leg II with solenidion ω slender 2.5; tibia with ventral seta v′ 27.1 and dorsal seta d 15.6; femur with dorsal setae d 20.4 and v″ 17 and posterolateral/ventral seta bv″ 17.5. Leg III with ventral seta v′24.5 and dorsal seta d 14.3 on tibia; seta 3c of coxae shorter than 3b and 3d. Leg IV with ventral seta v′ 23 and dorsal seta d 16.7 on tibia; femur not divided with ventral seta 21 ev′ and dorsal d 22.9.
Adult male
(Figure 12)


Idiosoma — Most of characters same as in female with exception of following: a pair of opisthosomal discs abaxial of setae d1 (see also Ripka et al. 2013; Akbari et al. 2015), dorsal setae shorter, dorsal seta finely serrate, ventral setae apparently smooth; only 1 pair of aggenital setae, in some specimens only one unpaired ag seta; femur IV with a distal, dorsal, adaxial, small and pointed spur (Figure 11).
Tritonymph
Idiosomal chaetotaxy like in adults. Leg chaetotaxy like in adults except for five setae on tarsus IV instead of six. Epimeral formula like in female. Hence, tritonymph with the following chaetotaxy: I (9+ω-3+κ+φ-3-3-1), II (6+ω-2-3-3-1), III (6-2-2-2-1), IV (5-2-1-2-0). Female tritonymph with four pairs of aggenital setae, ag1 5.3, ag2 5.6, ag3 4.7 not forked and shorter than other ag setae, and ag4 7.1; genital pore bivalved, situated mesad and posterior to setae (ag2) and anterior to setae (ag3). Male tritonymph with one pair of aggenital setae.
Deutonymph
Idiosomal chaetotaxy like in female but with two pairs of aggenital setae instead of four, anterior to genital pore. Epimeral formula like in female. Leg chaetotaxy like in female but with five setae on each of tarsus III and IV instead of six, and no setae on each of trochanters I and II instead of one. Hence, deutonymph with the following chaetotaxy: I (9+ω-3+ κ +φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-1), IV (5-2-1-2-0). Seta ft″ζ eupathidial.
Protonymph
Idiosomal chaetotaxy like in deutonymph, but all aggenital setae missing. With only two setae on coxae III (3d missing) and mtβ and 4b missing, hence epimeral formula 3-1-3-0. Leg chaetotaxy like in deutonymph but leg IV without setae except on tarsus which is equal to deutonymph. Hence, protonymph with the following chaetotaxy: I (9+ω-3+ κ +φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-1), IV (5-0-0-0-0). Seta ft″ not eupathidial (Figure 11f).
Larva
Idiosomal chaetotaxy like in protonymph, all aggenital setae missing. Coxae III with 1 seta (3c and 3d missing) and leg IV missing, hence epimeral formula 3-1-2. Leg chaetotaxy like protonymph but tarsus I with small pad-like empodium, without claws (om), setae (p) and (ft) not eupathid-like, setae (tcζ) eupathid-like, unguinal setae (u) and seta homologous to s missing and seta on trochanter III missing (Figure 11g). Hence, larva with the following chaetotaxy: I (6+ω-3+ κ +φ-3-3-0), II (6+ω-2-3-3-0), III (5-2-2-2-0).
Type locality and habitat
Quasihomeopronematus nordestinus has been found on tomato, casava, Macroptilium sp., in several states of the north-east of Brazil but also in the state of Minas Gerais (see collecting material in results section).
Type repository
All types deposited at Mite Reference Collection of Departamento de Entomologia e Acarologia, Escola Superior de Agricultura ''Luiz de Queiroz'' (ESALQ), Universidade de São Paulo (USP), Piracicaba, São Paulo state, Brazil, USPB (Zhang 2018).
Etymology
The species name refers to the North-East of Brazil, the region where the species was found for the first time in 1993.
Differential diagnosis
Quasihomeopronematus nordestinus n. gen. n. sp. resembles H. anconai but lacks dorsal setae ps1 and all idiosomal setae are considerably shorter and finer (Figure 5). Except for the lack of ps1, the body and leg chaetotaxy of Q. nordestinus n. gen. n. sp. and H. anconai are the same in adults and immatures.
Molecular analysis
Homeopronematus anconai — For Belgium, 3 lab colonies (D21, B31 and B25) were set up. From these, 10 SFEL (D21.1, D21.2; B31.1, B31.6; B95.1-6) could be established and 6 DNA extractions (D21.1, D21.2; B31.6; B25.1, B25.3, B25.4;) were successful. For France, 2 lab colonies (B46 & B83) were set up, 12 SFEL (B46.1-6; B83.1-6) could be established and 9 DNA extractions (B46.1-6; B83.2, B83.3, B83.5) were successful. For Hungary, 1 lab colony (B174) was set up, 1 SFEL (B174.1) could be established, and 1 DNA extraction (B174.1) was successful. For Germany, 2 lab colonies (B175; B176) were set up, 3 SFEL (B175.1; B176.2-3) could be established, and 3 DNA extractions (B175.1; B176.2-3) were successful.
Quasihomeopronematus nordestinus n. gen. n. sp. — From Brazil, 1 lab colony (1) was set up in Brazil, and 13 SFEL (1.1-13) could be established, and 4 DNA extractions (1.1, 1.3-5) were successful.
We aligned 657 nucleotides of the sequences for analysis. The translation of the mtDNA CO1 nucleotide sequences resulted in a polypeptide of 219 amino acids in length.
As is generally the case in insects and mites, base pair frequencies showed that the region is A+T-rich. The G+C composition of the entire data set ranged from 20.70 to 36.07%. For Q. nordestinus n. gen. n. sp. this was 33.79% for all samples and for H. anconai the G+C content ranged from 33.03 to 34.09%.
Ueckermann & De Vis et. al. (2024) and De Vis et. al. (2024) defined CO1 sequences of P. ubiquitus and P. duffelensis and compared them with those of gradually less related species. Here we add sequences for H. anconai and Q. nordestinus n. gen. n. sp. and constructed a new maximum likelihood consensus tree. Q. nordestinus n. gen. n. sp. and H. anconai B83 separate in similar way from all other H. anconai (Figure 13). Q. nordestinus n. gen. n. sp. and H. anconai separate from P. duffelensis and P. ubiquitus.


Comparison of different origins and principal component analysis (PCA)
The measurements of reared (mass rearing or SFEL) females fell within the range of the wild specimens except for SFEL 83 from France. So, for constructing Table 2 and the PCA analysis all specimens for each country were pooled, except for SFEL 83 from France. A total of 84 measured specimens were included: for Q. nordestinus n. gen. n. sp. Brazil: 13; for H. anconai: USA-California: 4; Belgium: 23, The Netherlands: 5, France: 21, Germany: 5 (from Biobest) and XX from Markus Knapp (we hope to find at the site of first collection); Switzerland: 4, Czech Republic: 7 and Hungary 10. The PCA analysis was done with PAST (version 4.03). Based on 41 morphological measurements (see loading plot, Figure 8A), convex hulls were created (Figure 8B).
As in Ueckermann & De Vis et al. (2024), we could not find a good separation between the different origins using all 62 parameters. Similarly, we excluded in a second run also in this analysis the parameters that are susceptible to measurement errors and retained only the setal lengths (41 parameters) in the final PCA analysis. The loading of component 1 (63.3% of the variation) was highest for the dorsal setae with bo, ex, c1, d1, e1, f1, f2, h1 and 3d having a loading higher than 0.2, followed by la, ro, c2, h2, 3c, and the eupathidia ft′ζ, ft″ζ, tc′ζ, tc″ζ and p′ζ on tarsus I, having a loading between 0.1 and 0.2. Most ventral setae as well as the small setae on leg I and the solenidia are characterized by a low loading (Figure 14A). The loading of component 2 with 6.6% was about tenfold lower than that of component 1.
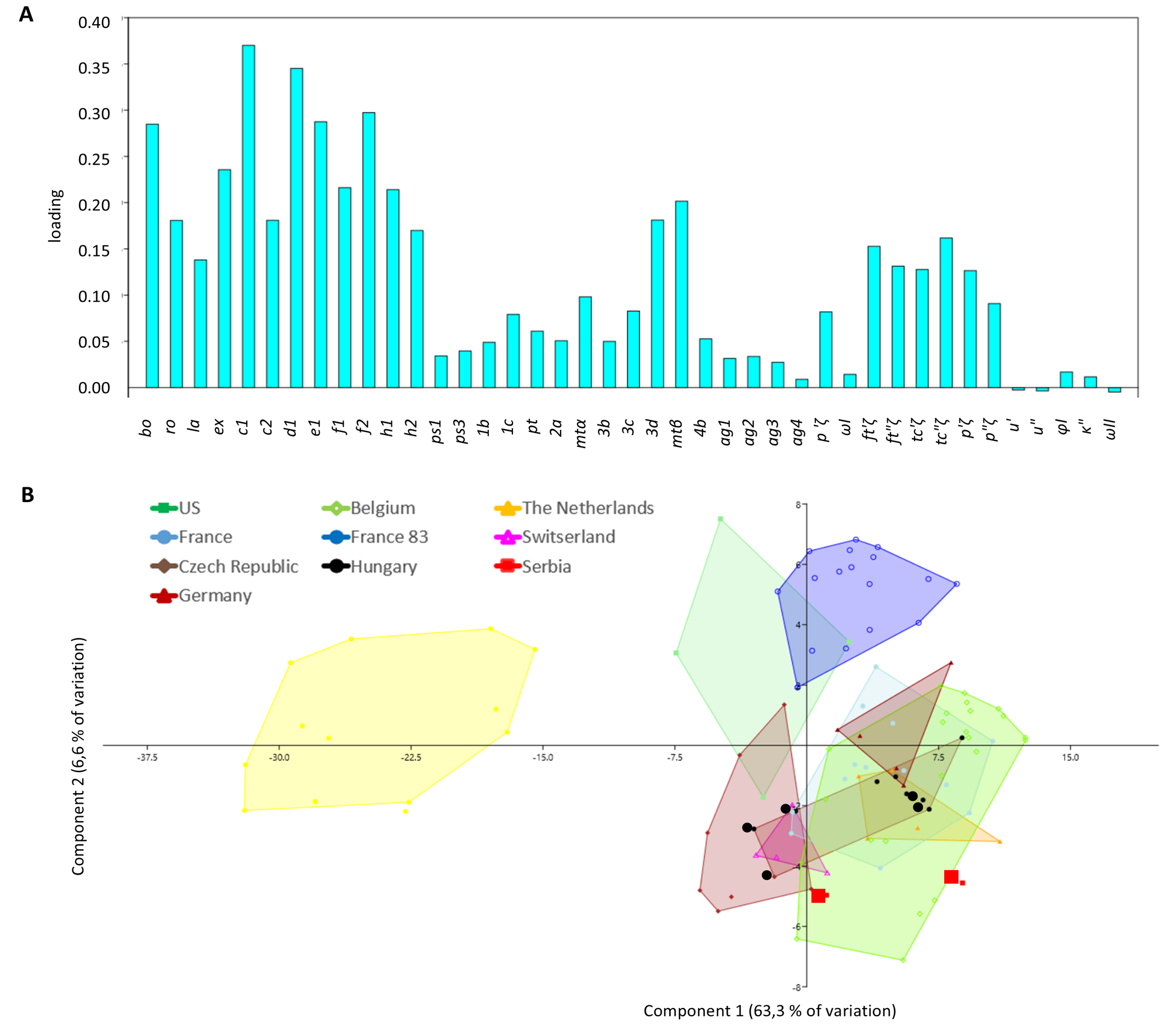


Quasihomeopronematus nordestinus n. gen. n. sp. from Brazil clustered apart from all H. anconai origins. In H. anconai, the Californian (USA) and France SFEL 83.3 clustered apart from all other origins, albeit with some overlap. All other origins show considerable overlap.
For Hungary, we studied five specimens identified as H. staercki [slides 1479, 1499-1 and 1499-2, Ripka et al. (2022)] apart from five specimens from our rearing originating from Hungary. We could not find morphological differences between these specimens and specimens of H. anconai. They did not cluster apart from H. anconai specimens of different countries.
For Serbia, the measurements of two specimens identified as ''Homeopronematus cf. staercki sp .nov., Kazmierski'' (see Stojnić et al. 2002) fall in the range of the European H. anconai specimens (Table 2 and Figure 14). We therefore consider the specimens of all countries as H. anconai (Figure 14B).
Discussion
Taxonomy
American and European Homeopronematus anconai are the same species.
We consider, based on our data and literature that they are indeed the same species. Knop and Hoy 1983a were the first to draw the American specimens. They studied types of Baker and concluded that H. vidae and H. anconai are synonyms. They did a thorough study. We could also study a series of slides with specimens coming from a rearing from the lab of M. Hoy in 1969, of unknow origin, held at Essig Museum and these specimens have both setae u and seta homologous to s present. The specimens we could study did not differ in such a way that we can consider them as different species (Table 2 and Figure 14). We are aware that studying topotypes (from type locality) would have been better, but we were unable to get this material, despite our efforts.
Homeopronematus staercki is junior synonym of H. anconai and ''Homeopronematus cf. staercki \textbf{sp .nov., Kazmierski'' is H. anconai }
As stated in the introduction, the literature is ambiguous in the description of the two species, H. anconai and H. staercki. The description of H. staercki (Schruft 1972) is so poor that the species could be synonymized with any species of the genus and no redescription has ever been proposed to clear out eventual differences with H. anconai. Schruft (1972) does not mention the shape of seta ag3 in his description of H. staercki while it is forked in H. anconai (Baker 1968; Kuznetsov 1972; Knop and Hoy 1983a; this study). As the material used for the description H. staercki is lost, we cannot check this.
The specimens previously identified as H. staercki we could study are not different from H. anconai: they have identical body and leg chaetotaxy, no differences in the form of the setae could be distinguished, ag3 is forked, the length of the eupathidia coincide with those of H. anconai, the striae are lobed. In the PCA they fall within the'cloud' of H. anconai (Figure 14A). No differences could be detected in the shape of the setae, or the striation density. The dimensions of the Hungarian male we measured fall within the range of the Belgian males. Despite the large number of specimens from different origins we studied, we could not find one specimen that can be clearly distinguished from H. anconai. According to Knop and Hoy (1983a), the variability within the species is high which is confirmed in this study.
The differences as discussed by other authors are very small and are not sufficiently supported by descriptions with (range of) measurements and/or drawings: André (1980) studied the types of H. staercki and found for this species a shorter c2, a higher striation density and further different ''shape and length of idiosomal setae'' (sic). We found that the variability in the length of c2 of H. anconai is indeed very high, varying between 15 and 21 µm. The striation leaves us with some doubt but also variability of this characteristic can be observed. Moreover, the density might be influenced by mounting or the state of the specimens at mounting. Finally, the shape and length of the setae are not described in detail.
Ripka et al. (2022) stated that the tectal and proral eupathidia on tarsus I of H. anconai are all four equal in length, while in H. staercki the tectals are nearly twice as long as the prorals. Our data show that in H. anconai tectals are nearly twice as long as the prorals, confirming descriptions of other authors (Baker 1968; Kuznetsov 1972; André 1980).
So based on our data and the previous arguments, we synonymise H. staercki with H. anconai as previously suggested by other authors (Knop and Hoy 1983a; Ueckermann et al. 2019).
Similarly, the specimens identified as ''Homeopronematus cf. staercki sp .nov., Kazmierski'' (see Stojnić et al. 2002) are H. anconai. The measurements of these specimens fall in the range of the European H. anconai specimens (Table 2 and Figure 14).
Unguinals and seta s. As for P. ubiquitus, we found two forked unguinals and a seta homologous to s. We confirm that this seta s is erroneously named u′ in Figure 1a & 1b in Knop and Hoy (1983a), as the position of u′ is ventral and proximal to p′ζ and the depicted u′ in Knop and Hoy (1983a) is in between the two prorals, which is the positions of seta homologous to s. Additionally, it is not forked, while both (u) are forked (Figures 3 & 6c, d). As in H. anconai, seta homologous to s is also present in all stages of Q. nordestinus n. gen. n. sp., except in the larval stage, as well as that both unguinals are forked.
DNA barcoding or morphological identification
Not all 13 H. anconai sequences form one cohesive cluster. The French strain B83, clusters separately from the other H. anconai strains and forms a cluster higher up with the Q. nordestinus n. gen. n. sp. sequences from Brazil. It seems that the two species are closely related. However, it must be noted that the distance found between Q. nordestinus n. gen. n. sp. and H. anconai B83 with 0.31 is equal to the two most distant H. anconai strains (strain B83 and the Hungarian strain B174.1) and less compared to more closely related H. anconai strains in the phylogenetic tree.
We cannot separate the H. anconai specimens of different countries because we cannot find distinguishing characters among their origins. Most characters overlap with other origins and if no overlap is present in a character, the difference is small (Table 2) and certainly not sufficient to distinguish as a different species. This can also be seen in the PCA analysis (Figure 14A). Surprisingly, the specimens of B83 (including 1 wild specimen) from France separate from all other French origins in the PCA, although the distance is not large. When comparing the data in Table 2, also in this case no distinguishing character can be found between the measurements of both French origins. Additionally, the area covered by the Belgian specimens is similar to the area covered by the combined area of all French specimens. About 50% of the measured specimens are coming from an SFEL. So, to have a good overview of real diversity in the field, more wild samples coming from all over each country should be analysed. When we overlook the large area that covers H. anconai on Figure 14A we conclude that it is a species with high variability as also stated by Knop and Hoy (1983a).
Although the French strain B83 differentiates similarly as Q. nordestinus n. gen. n. sp., from the other H. anconai strains in the PCA (Figure 14A), no significant morphological differences were found, so we cannot separate strain B83 as a distinct species. Q. nordestinus n. gen. n. sp., on the contrary, is morphologically clearly different from H. anconai with ps1 lacking and shorter setae in general.
We must conclude that a phylogenetic tree based on the CO1-sequence only, is not sufficient to identify the closely related iolinids to species level.
Similarly, as for P. ubiquitus (Ueckermann et al. 2024), we found high similarity of the sequences originating from one locality which could be explained by the limited genetic basis as the laboratory cultures were started with few females.
To date, no CO1 sequences were available for H. anconai and Q. nordestinus n. gen. n. sp. and the unique CO1 sequences reported here constitute the first barcode for these species. However, DNA sequencing of more species, and as shown above, with more than one marker gene is required to elucidate relationships and distinguish species clearly. Studies have shown that when CO1 is combined with other marker genes such as 28r-RNA, clade structure can change (Duarte et al. 2023). This underscores the importance of additional research, integrating barcoding with morphological identification as to make the species delimitation more reliable (Puillandre et al. 2012). Preferably such research should be performed using confirmed and distinct species of the same genus, subpopulations of the same species and/or combined with crossbreeding studies to identify those subpopulations (Anderson and Trueman 2000; Tixier et al. 2008).
An explanation for the separation of the SFEL B83 is the small genetic basis with which the first rearing was set up (possibly with only one female). If a deviant female were present in sample B83, this would also be reflected in its offspring. One wild specimen of sample B83 falls within the range of the SFEL specimens. There is variation in the offspring of 83.1 but specimens of the other SFEL from Belgium and France did not separate from the wild specimens. And although, the specimens of sample/SFEL B83 separate in the PCA (Figure 14A), we cannot find distinguishing characters in Table 2.
We had only four USA specimens available in this study, but also here, no distinguishing characters could be found and overlap with European specimens can be seen in the PCA analysis (Figure 14B). Whether the observed genetic and morphological differences between or among European and non-European specimens are due to geographic separation is not proven. Therefore, much more independent samples, spread throughout each country or region should be analysed.
Distribution
Homeopronematus anconai is almost cosmopolitan not being found in Africa and Oceania (see introduction). As for P. ubiquitus, we also suppose that this mite has been travelling throughout the world on traded plant material, where it might not always have been found by the plant inspection authorities because of its small size, especially that of the eggs.
For Q. nordestinus n. gen. n. sp. the distribution at this moment is limited to Brazil. The presence of H. anconai in Brazil and by extension in other South American countries should be confirmed given that that they might have been misidentified.
Habitat and feeding habits
In previous studies, H. anconai (including H. staercki n. syn.) was found on a wide spectrum of wild plants: Buddleja sessiliflora Kunth. (Scrophulariaceae) (Baker 1943); 17 associated plants in citrus orchards mainly Thelypteris dentata (Forssk.) E.P. St. John (Thelypteridaceae); Solidago chilensis Meyen (Asteraceae), Ageratum conyzoides L. (Asteraceae) and Solanum americanum (Solanaceae) (Horn et al. 2011); bittersweet (Ripka and Kaźmierski 1998; Ripka et al. 2013); Elaeagnus angustifolia L. (Elaeagnaceae), Koelreuteria paniculata Laxm. (Sapindaceae), Rosa canina L. (Rosaceae), Sambucus nigra L. (Adoxaceae) and Ulmus laevis Pall. (Ulmaceae) (Ripka and Kaźmierski 1998); a variety of trees, shrubs, and herbaceous plants (Kuznetsov 1972); and bark and foliage of several tree and shrub species (Ripka and Kaźmierski 1998).
But it was also frequently found on cultivated plants: tomato ((Hessein and Perring 1986; Kawai et al. 2001; García González 2003; Xu 2011; Çobanoğlu and Kumral 2014); strawberry (Fragaria × ananassa (Weston) Duchesne ex Rozier, Rosaceae) (Oatman 1971); blackberry (Rubus vitifolia Cham & Schlecht, Rubus sp., Rosaceae) (Knop and Hoy 1983a; Ripka et al. 2005; Ferreira 2016); Ribes rubrum L. (Grossulariaceae) (Ripka and Kaźmierski 1998); grape (Schruft 1972; Liguori 1987; Maurício et al. 2009; Johann et al. 2009; Klock et al. 2011; Tempfli et al. 2015; Tempfli 2019); Valencia orange (Citrus sinensis (L.) Osbeck, Rutaceae); apple (Malus domestica Borkh.) and pear Pyrus communis L. (Rosaceae) (Childers and Enns 1975; Thistlewood 1991; Ripka and Kaźmierski 1998); almond (Prunus sect. amygdalus (L.) Benth. & Hook.f., Rosaceae); hazelnut (Corylus avellana L., Betulaceae) (Ozman-Sullivan et al. 2005); Juglans nigra L. (Juglandaceae), (Ripka and Kaźmierski 1998); orchards (Darbemamieh et al. 2021); as well as in leaf litter of hedge plants (Ozman-Sullivan et al. 2005) or soil (Darbemamieh et al. 2020).
We want to draw attention to the registrations of H. anconai in Brazil (Johann et al. 2009; Klock et al. 2011; Horn et al. 2011; Sousa et al. 2015). The identifications should be confirmed as it might have been confounded with Q. nordestinus n. gen. n. sp.
In this study, we found H. anconai both on wild and cultivated plants. It was present in about every sample of Rubus sp. but furthermore, we found it on grape, Malva sp. and Cedreila (?), on raspberry, Brugmansia sp., bittersweet, tomato, cucumber (Cucumis sativus L. Cucurbitaceae), and fig. Homeopronematus anconai was much more common on wild plants than P. ubiquitus (Ueckermann & De Vis et al. 2024) while P. ubiquitus was more commonly found on cultivated plants such as tomato, sweet pepper, cucumber, grape and fig.
Horn et al. (2011) suggest that the associated plants, T. dentata and S. chilensis had the largest number of predatory mites including H. anconai and that these plants could serve as banker plants, as a beneficial host in citrus IPM programs in Brazil.
Homeopronematus anconai needs plant tissue such as blackberry, grape, bean (Phaseolus vulgaris L. Fabaceae) or tomato (Knop and Hoy 1983a; Vervaet et al. 2022) and pollen or prey to reproduce. It cannot reproduce on leaf tissue, pollen or prey alone (Knop and Hoy 1983a; Hessein and Perring 1988b). In most research, cattail pollen (Typha latifolia (L.)) is used to culture H. anconai (e.g. Hessein and Perring 1988b; Vervaet et al. 2022) but it can also reproduce on pollen from bottlebrush [Callistemon citrinus (Curtis) or Melaleuca hypericifolia Smith, both Myrtaceae), Carpobrotus chilensis (Mol.)], ice plant (Mesembryanthemum crystallinum L., Aizoaceae), California poppy (Eschscholzia californica Cham., Papaveraceae), Magnolia sp. (Magnoliaceae) or aerial pollen that deposits on plants (Flaherty and Hoy 1971; Knop and Hoy 1983a; Hessein and Perring 1986) but not on bee-collected pollens (Flaherty and Hoy 1971) or pollen of Cosmos sp (Asteraceae), Dahlia sp (Asteraceae), or pine (Pinaceae) (Knop and Hoy 1983a).
Several authors have studied its potential use in biological control of A. lycopersici (Hessein and Perring 1986; Brodeur et al. 1997; Kawai and Haque 2004; Xu 2011; Vervaet et al. 2022) and found a high predation capacity (Hessein and Perring 1986; Xu 2011; Vervaet et al. 2022) and showed that it can control that mite below economic threshold levels (Hessein and Perring 1986; Kawai et al. 2001; Kawai and Haque 2004) whereas Brodeur et al. (1997) found that H. anconai could not develop to adulthood when fed solely with A. lycopersici. Additional food (pollen) enhances the reproduction of H. anconai (Hessein and Perring 1988b; Vervaet et al. 2022) and can improve the biological control of A. lycopersici by H. anconai (Hessein and Perring 1988b).
Homeopronematus anconai preys also citrus flat mite Brevipalpus lewisi McGregor 1949 (Tenuipalpidae) (Hessein and Perring 1988a). Similarly Pronematus brasiliensis De Vis & Ueckermann 2024 can reproduce and survives well on a diet of Brevipalpus phoenicis (Geijskes, 1939) or Tenuipalpus heveae Baker, 1945 (De Vis et al. 2006). Homeopronematus anconai can also reproduce on eggs of Pacific mite Tetranychus pacificus McGregor, 1919 or Willamette mite Eotetranychus willamettei (McGregor 1917) (Knop and Hoy 1983a).
The high sensitivity of H. anconai to pesticides, including sulphur might reduce its potential use in practice (Knop and Hoy 1983b), but recent studies with P. ubiquitus and H. anconai (Pijnakker et al. 2022) and Q. nordestinus n. gen. n. sp. (Marcossi et al. 2024) show that these Iolinidae can also control powdery mildew besides A. lycopersici, so they might be alternative for sulphur which is used for both phytosanitary problems. We hypothesise that more Pronematinae might have this potential, but some probably more than others, or it might be dependent of climatic conditions, which can be the subject of further research. Additionally, the combined release of Q. nordestinus n. gen. n. sp. and the phytoseiid Amblyseius herbicolus (Chant, 1959) might also control whiteflies (Marcossi et al. 2024).
Acknowledgements
This research was mostly done during the first and last two authors' free time. In part, it was supported by the projects DUCATO, financed by LAVA, the Flemish Vegetable Auctions Association, and BALTO, ''Beheersing van Aculops lycopersici in tomaat'', funded by Flanders Innovation & Entrepreneurship (grant HBC.2017.0829). Thanks go to Patrick De Clercq of Ghent University for detailed proofreading of earlier versions of this work. We thank Bartel Vanholme for help with taking the ESEM pictures at VIB, Ghent. We are grateful to all the collectors of materials, i.a. Johan Foqué & Helena Bartier, Robin Van Havermaet, family Ward Potoms and many collaborators of the PSKW who brought samples. Ute Perkons sent German H. anconai sample to Biobest and Dominiek van Gansbeke sent reared material from that sample to us. A very special thanks go to Markus Knapp of Koppert who collected samples in Germany, including Schliengen, the site of the original description of H. staercki, although we could not find Homeopronematus specimens in his samples; and also, to Yvonne Van Houten of Koppert who send us cultures of several origins, including Germany and Hungary. Special thanks go to our Brazilian connections: Gilberto de Moraes who sent us slides from the museum USPB and commented on an earlier version of this paper. We are greatly indebted to Arne Janssen who initiated and supervised the Q. nordestinus n. gen. n. sp. research in Brazil and commented on an earlier version of this paper. He and Irene Cardoso were so kind to get conserved specimens of the Brazilian mites to the home of the first author. Peter T Oboyski from Essig Museum of Entomology, University of California, Berkeley, California, USA send us slides, and Andrzej Kaźmierski send us slides of Samples of Hungary and Servia from the AMU- museum, Department of Animal Morphology, Adam Mickiewicz University, Poznań, Poland.
Distribution of work
Raf De Vis coordinated the survey, started initial rearings, made the redescription and took pictures, and measured the specimens and wrote the major part of this work; Lore Vervaet set up SFEL and performed the molecular study and recorded the ESEM pictures; In Brazil, Ítalo Marcossi reared Q. nordestinus n. gen. n. sp., Caio Henrique Binda de Assis set up SFEL and Angelo Pallini supervised their work. Eva Reybroeck coordinated the survey in Belgium and made a lot of slides, Wendy Vanlommel participated in the survey; Thomas Van Leeuwen supervised the work of Lore Vervaet; Edward A. Ueckermann supervised the work as senior acarologist.
References
- Akbari A., Irani-Nejad K.H., Khanjani M., Arzanlou M., Kaźmierski A. 2015. Tydeus shabestariensis sp. nov. and description of the male of Neopronematus sepasgosariani (Acari: Tydeoidea), with a key to the Iranian species of Tydeus. Zootaxa, 4032(3): 264-276. https://doi.org/10.11646/zootaxa.4032.3.2
- Anderson D.L., Trueman J.W.H. 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol., 24(3): 165-189. https://doi.org/10.1023/A:1006456720416
- André H.M. 1979. A generic revision of the family Tydeidae (Acari\,: Actinedida) I. Introduction, paradigms and general classification. Ann. Soc. R. Zool. Belg., 3-4(1978): 189-208.
- André H.M. 1980. A generic revision of the family Tydeidae (Acari: Actinedida) IV. Generic descriptions, keys and conclusions. Bull. Ann. Soc. R. Belge Ent., 116(7-9): 103-168.
- André H.M. 1981. A generic revision of the family Tydeidae (Acari, Actinedida) III. Organotaxy of the legs. Acarologia, 22(2): 165-178.
- André H.M. 2021. The Tydeoidea (Ereynetidae, Iolinidae, Triophtydeidae and Tydeidae) - An online database in the Wikispecies platform. Acarologia, 61(4): 1023-1035. https://doi.org/10.24349/6yc5-1lxw
- Aussems E., De Vis R.M.J., Similon L., Mertens R., Vervaet L., Van Haevermaet R. 2021. Nieuwe roofmijt voor bestrijding tomatengalmijt. Proeftuinnieuws, 36(13): 34-35.
- Baker E.W. 1943. Nuevos tydeidos Mexicanos (Acarina). Rev. Soc. Mex. Hist. Natur., 4(3-4): 181-189.
- Baker E.W. 1945. Mites of the genus Tenuipalpus (Acarina: Trichadenidae). Proc. Entomol. Soc. Wash., 47: 33-38.
- Baker E.W. 1965. A review of the genera of the family Tydeidae (Acarina). Adv. Acarol., 2: 95-133.
- Baker E.W. 1968. The Genus Pronematus Canestrini. Ann. Entomol. Soc. Am., 61(5): 1091-1097. https://doi.org/10.1093/aesa/61.5.1091
- Baker E.W., Delfinado M.D. 1976. Notes on the genus Naudea Meyer and Rodrigues, with description of a new species (Acarina: Tydeidae). Int. J. Acarol., 2(1): 35-38. https://doi.org/10.1080/01647957608683754
- Brodeur J., Bouchard A., Turcotte G. 1997. Potential of four species of predatory mites as biological control agents of the tomato russet mite, Aculops lycopersici (Massee) (Eriophyidae). Can. Entomol., 129(1): 1-6. https://doi.org/10.4039/Ent1291-1
- Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Mem. Entomol. Soc. Can., 91(S12): 5-166. https://doi.org/10.4039/entm9112fv
- Childers C.C., Enns W.R. 1975. Predaceous arthropods associated with spider mites in Missouri apple orchards. J. Kans. Entomol. Soc., 48(4): 453-471.
- Çobanoğlu S., Kumral N.A. 2014. The biodiversity and population fluctuation of plant parasitic and beneficial mite species (Acari) in tomato fields of Ankara, Bursa and Yalova provinces. Turk Entomol Derg., 38(2): 197-214. https://doi.org/10.16970/ted.64743
- Da Silva G.L., Da-Costa T., Ferraz C.S., Pallini A., Ferla N.J. 2017. First description of iolinid mites (Acari: Tydeoidea) from Brazil. Syst. Appl. Acarol., 22(5): 694-701. https://doi.org/10.11158/saa.22.5.8
- Daneshvar H. 1978. A study on the fauna of plant mites in Azerbaidzhan. Appl. Entomol. Phytopathol., 46(1/2): 117-128.
- Darbemamieh M., Ahadiyat A., Farmahiny Farahani V.R., Sharifian A. 2021. Iranian checklist of Tydeoidea (Trombidiformes: Prostigmata) until the end of 2020. Int. J. Acarology, 47(7): 592-602. https://doi.org/10.1080/01647954.2021.1976833
- Darbemamieh M., Hajiqanbar H., Khanjani M., Kaźmierski A. 2015. New species and records of Neopronematus (Acari: Iolinidae) from Iran with a key to world species. Zootaxa, 3990(2): 235-246. https://doi.org/10.11646/zootaxa.3990.2.4
- Darbemamieh M., Kaźmierski A., Paktinat-Saeij S. 2020. New records and remarks on Tydeoidea (Acari: Trombidiformes) from Mazandaran province of Iran. Persian J. Acarol., 9(3): 243-253. https://doi.org/10.22073/pja.v9i3.60194.
- De Vis R.M.J., de Moraes G.J., Bellini M.R. 2006. Initial Screening of Little Known Predatory Mites in Brazil as Potential Pest Control Agents. Exp. Appl. Acarol., 39(2): 115-125. https://doi.org/10.1007/s10493-006-9004-7
- De Vis R.M.J., Vervaet L., Van Leeuwen T., Braga A.D.F., Castilho R.D.C., Ueckermann E.A. 2024. Description of two new genera, three new species, and redescription of two species of Pronematinae (Acari: Iolinidae). Acarologia, 64(4): 1063-1099. https://doi.org/10.24349/z0vq-ji83
- Duarte M.E., Lewandowski M., de Mendonça R.S., Simoni S., Navia D. 2023. Genetic analysis of the tomato russet mite provides evidence of oligophagy and a widespread pestiferous haplotype. Exp. Appl. Acarol., 89(2): 171-199. https://doi.org/10.1007/s10493-023-00777-4
- Ferreira M.A. 2016. The mite fauna of blackberries in Portugal. In: V Colóquio Nacional da Produção de Pequenos Frutos, Oeiras, Portugal, 14 e 15 de outubro de 2016, Associação Portuguesa de Horticultura (APH). pp. 169-176.
- Flaherty D.L., Hoy M.A. 1971. Biological control of Pacific mites and Willamette mites in San Joaquin Valley vineyards: part III. Role of tydeid mites. Res. Popul. Ecol., 13(1): 80-96. https://doi.org/10.1007/BF02522015
- García González S. 2003. Catalogación, biología y ecología de los artrópodos asociados al cultivo de tomate en la ribera navarra. Ph. D. Thesis. Pamplona. Universidad de Navarra. http://purl.org/dc/dcmitype/Text.
- Geijskes D.C. 1939. Beiträge zur Kenntnis der europäischen Spinnmilben (Acari, Tetranychidae), mit besonderer Berücksichtigung der niederländischen Arten. Meded. LandbHoogesch. Wageningen, 42(4): 1-68.
- Hessein N.A., Perring T.M. 1986. Feeding habits of the Tydeidae with evidence of Homeopronematus anconai (Acari: Tydeidae) predation on Aculops lycopersici (Acari: Eriophyidae). Int. J. Acarol., 12(4): 215-221. https://doi.org/10.1080/01647958608683467
- Hessein N.A., Perring T.M. 1988a. Homeopronematus anconai (Baker)(Acari: Tydeidae) predation on citrus flat mite, Brevipalpus lewisi McGregor (Acari: Tenuipalpidae). Int. J. Acarol., 14(2): 89-90. https://doi.org/10.1080/01647958808683791
- Hessein N.A., Perring T.M. 1988b. The Importance of Alternate Foods for the Mite Homeopronematus anconai (Acari: Tydeidae). An. Esc. Nac. Cienc. Biol., 81(3): 488-492. https://doi.org/10.1093/aesa/81.3.488
- Hoang D.T., Chernomor O., Von Haeseler A., Minh B.Q., Vinh L.S. 2018. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol., 35(2): 518-522. https://doi.org/10.1093/molbev/msx281
- Horn T.B., Johann L., Ferla N.J. 2011. Ecological interactions between phytophagous and predaceous mites in citrus agroecosystems in Taquari Valley, Rio Grande do Sul, Brazil. Syst. Appl. Acarol., 16(2): 133-144. https://doi.org/10.11158/saa.16.2.2
- Johann L., Klock C.L., Ferla N.J., Botton M. 2009. Acarofauna (Acari) associada à videira (Vitis vinifera L.) no Estado do Rio Grande do Sul. Biociências, 17(1): 1-19.
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., Von Haeseler A., Jermiin L.S. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods, 14(6): 587-589. https://doi.org/10.1038/nmeth.4285
- Kawai A., Haque M.M. 2004. Population Dynamics of Tomato Russet Mite, Aculops lycopersici (Massee) and Its Natural Enemy, Homeopronematus anconai (Baker). Jpn. Agric. Res. Q., 38(3): 161-166. https://doi.org/10.6090/jarq.38.161
- Kawai A., Haque M.M., Tanaka H., Shiba M. 2001. First record of Homeopronematus anconai (Baker) in Japan and predation on Aculops lycopersici (Massee). J. Acarol. Soc. Jpn, 10: 43-46.
- Kaźmierski A. 1989. Morphological studies on Tydeidae (Actinedida, Acari). I: Remarks about the segmentation, chaetotaxy and poroidotaxy of idiosoma. Acta zool. Cracov, 32(4): 69-83.
- Kaźmierski A. 1998. A review of the genus Proctotydaeus Berlèse (Actinedida: Tydeidae: Pronematinae). Acarologia, 39(1): 33-47.
- Khaustov A.A., Hugo-Coetzee E., Ermilov S.G. 2020. A new species of Lorryia (Acari: Tydeidae) from a termite nest in South Africa. Acarina, 28(1): 47-53. https://doi.org/10.21684/0132-8077-2020-28-1-47-53
- Klock C.L., Botton M., Ferla N.J. 2011. Mite fauna (Arachnida: Acari) associated to grapevine, Vitis vinifera L. (Vitaceae), in the municipalities of Bento Gonçalves and Candiota, Rio Grande do Sul, Brazil. Check List, 7(4): 522-536. https://doi.org/10.15560/7.4.522
- Knop N., Hoy M. 1983a. Biology of a tydeid mite, Homeopronematus anconai (n. comb.) (Acari: Tydeidae), important in San Joaquin Valley vineyards. Hilgardia, 51(5): 1-30. https://doi.org/10.3733/hilg.v51n05p030
- Knop N.F., Hoy M.A. 1983b. Factors Limiting the Utility of Homeopronematus anconai (Acari: Tydeidae) in Integrated Pest Management in San Joaquin Valley Vineyards. J. Econ. Entomol., 76(5): 1181-1186. https://doi.org/10.1093/jee/76.5.1181
- Kuznetsov N.N. 1972. Mites of the genus Pronematus Canestrini (Acarina, Tydeidae) from Crimea. Nauch Dokl Vyssh Shk, Biol Nauk, (5): 11-16.
- Letunic I., Bork P. 2021. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res., 49(W1): W293-W296. https://doi.org/10.1093/nar/gkab301
- Liguori M. 1987. Population trends of phytophagous and predatory mites in two vineyards of the Chianti area. Redia, 70: 141-149.
- Marcossi Í., Francesco L.S., Fonseca M.M., Pallini A., Groot T., De Vis R., Janssen A. 2024. Predatory mites as potential biological control agents for tomato russet mite and powdery mildew on tomato. J. Pest. Sci. https://doi.org/10.1007/s10340-024-01802-0
- Maurício M., Ferreira M.A., Sousa M.E. 2009. The mite fauna of vineyards and weeds in different Ribatejo regions. In: XII Congresso da Sociedad Española de Malherbologia (SEMh), XIX Congresso da Asociacion Latinoamericana de Malezas (ALAM), II Congresso Iberico de Ciencias de las Malezas (IBCM), Herbologia e Biodiversidade numa Agricultura Sustentável, Lisboa, Portugal, 10 a 13 de Novembro de 2009. Volume 1 and Volume 2, Sociedad Española de Malherbología (Spanish Weed Science Society). pp. 35-38.
- McGregor E.A. 1917. Descriptions of seven new species of red spiders. Proc. U. S. Natl. Mus., 51(2167): 581-603. https://doi.org/10.5479/si.00963801.51-2167.581
- McGregor E.A. 1919. The red spiders of America and a few European species likely to be introduced. Proc. U. S. Natl. Mus., 56(2303): 641-685. https://doi.org/10.5479/si.00963801.56-2303.641
- McGregor E.A. 1932. The ubiquitous mite, a new species on citrus. Proc. Entomol. Soc. Wash., 34: 60-64.
- McGregor E.A. 1949. Nearctic mites of the family Pseudoleptidae. Mem. S. Calif. Acad. Sci., 3(2): 1-45. https://doi.org/10.5962/bhl.title.146948
- Oatman E.R. 1971. Mite species on strawberry in southern California. J. Econ. Entom., 64(5): 1313-1314. https://doi.org/10.1093/jee/64.5.1313
- Ozman-Sullivan S.K., Kazmierski A., Cobanoglu S. 2005. Alycina and eupodida mites in hazelnut orchards in Turkey. Acta Hortic., (686): 401-406. https://doi.org/10.17660/ActaHortic.2005.686.55
- Panou H.N., Emmanouel N.G., Kaźmierski A. 2000. Neopronematus, a new genus of the subfamily Pronematinae (Acari: Prostigmata: Tydeidae) and a new species from Greece. Acarologia, 41(3): 321-325.
- Pijnakker J., Moerkens R., Vangansbeke D., Duarte M., Bellinkx S., Benavente A., Merckx J., Stevens I., Wäckers F. 2022. Dual protection: a tydeoid mite effectively controls both a problem pest and a key pathogen in tomato. Pest Manag. Sci., 78(1): 355-361. https://doi.org/10.1002/ps.6647
- Pritchard A.E. 1956. A New Superfamily of Trombidiform Mites with the Description of a New Family, Genus, and Species (Acarina: Iolinoidea: Iolinidae: Iolina nana). An. Esc. Nac. Cienc. Biol., 49(3): 204-206. https://doi.org/10.1093/aesa/49.3.204
- Puillandre N., Lambert A., Brouillet S., Achaz G. 2012. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol., 21(8): 1864-1877. https://doi.org/10.1111/j.1365-294X.2011.05239.x
- Reybroeck E., Wittemans L., De Vis R.M.J., Vanlommel W., Bosmans L., Dewitte J., Buysens S., Van Leeuwen T., De Clercq P. 2018. Gezocht: biologische bestrijder voor tomatengalmijt. Proeftuinnieuws, 28(13): 19.
- Ripka G., Fain A., Kazmierski A., Kreiter S., Magowski W.L. 2005. New Data to the Knowledge of the Mite Fauna of Hungary (Acari: Mesostigmata, Prostigmata and Astigmata). Acta Phytopathol. Entomol. Hung., 40(1-2): 159-176. https://doi.org/10.1556/APhyt.40.2005.1-2.13
- Ripka G., Kaźmierski A. 1998. New data to the knowledge on the tydeid fauna in Hungary (Acari: Prostigmata). Acta Phytopathol. Entomol. Hung., 33(3-4): 407-418.
- Ripka G., Király G., Szabó Á., Kaźmierski A. 2022. Recent additions to the plant-inhabiting mite fauna of three European countries (Acari: Acariformes: Tydeoidea, Stigmaeidae). Syst. Appl. Acarol., 27(5): 865-887. https://doi.org/10.11158/saa.27.5.4
- Ripka G., Laniecka I., Kazmierski A. 2013. On the arboreal acarofauna of Hungary: Some new and rare species of prostigmatic mites (Acari: Prostigmata: Tydeidae, Iolinidae and Stigmaeidae). Zootaxa, 3702(1): 1-50. https://doi.org/10.11646/zootaxa.3702.1.1
- Schruft G. 1972. Das Vorkommen von Milben aus der Familie Tydeidae (Acari) an Reben: VI. Beitrag über Untersuchungen zur Faunistik und Biologie der Milben (Acari) an Kulturreben (Vitis spec.). Z. Angew. Entomol., 71(1-4): 124-133. https://doi.org/10.1111/j.1439-0418.1972.tb01729.x
- Sousa J.M.D., Gondim Jr. M.G.C., Lofego A.C., Moraes G.J.D. 2015. Mites on Annonaceae species in northeast Brazil and in the state of Para. Acarologia, 55(1): 5-18. https://doi.org/10.1051/acarologia/20152147
- Stojnić B., Panou H., Papadoulis G., Petanović R., Emmanouel N. 2002. The present knowledge and new records of phytoseiid and tydeid mites (Acari: Phytoseiidae, Tydeidae) for the fauna of Serbia and Montenegro. Acta Entomol. Serbica, 7(1-2): 111-117.
- Tempfli B. 2019. Occurrence of Tydeoidea species in Hungarian vineyards. In Hungarian, original title: Tydeoidea fajok előfordulása a magyarországi szőlőültetvényekben. Ph.D. thesis. Budapest. Szent István University. http://real-phd.mtak.hu/1584/2/tempfli_balazs_tezis.pdf. 16pp.
- Tempfli B., Pénzes B., Fail J., Szabó Á. 2015. The occurrence of tydeoid mites (Acari: Tydeoidea) in Hungarian vineyards. Syst. Appl. Acarol., 20(8): 937-954. https://doi.org/10.11158/saa.20.8.9
- Thistlewood H.M.A. 1991. A survey of predatory mites in Ontario apple orchards with diverse pesticide programs. Can. Entomol., 123(6): 1163-1174. https://doi.org/10.4039/Ent1231163-6
- Tixier M.-S., Kreiter S., Croft B.A., Cheval B. 2008. Kampimodromus aberrans (Acari: Phytoseiidae) from the USA: morphological and molecular assessment of its density. Bull. Entomol. Res., 98(2): 125-134. https://doi.org/10.1017/S0007485307005457
- Trifinopoulos J., Nguyen L.-T., von Haeseler A., Minh B.Q. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res., 44(W1): W232-W235. https://doi.org/10.1093/nar/gkw256
- Tryon H. 1917. Report of the entomologist and vegetable pathologist. In: Queensland Ann. Rept. Dept. Agric. & Slock for the Year 1916-1917, Brisbane: Brisbane. pp. 49-63.
- Ueckermann E.A., Cobanoğlu S., Öğreten A. 2019. Re-description of two new tydeid records (Acari: Trombidiformes) with a key to Tydeoidea species of Turkey. Syst. Appl. Acarol., 24(3): 49-507. https://doi.org/10.11158/saa.24.3.13
- Ueckermann E.A., De Vis R.M.J., Reybroeck E., Vervaet L., Vanlommel W., Dewitte J., Foqué D., Gautam S., Ouyangh Y., Vangansbeke D., De Clercq P., Van Leeuwen T. 2024. Redescription of Pronematus ubiquitus (McGregor, 1932) (Acari, Iolinidae), description of two new species and redescription of two additional species with a review of and key to all Pronematus species. Acarologia, 64(1): 277-311. https://doi.org/10.24349/tyki-9xlp
- Vervaet L., De Vis R., De Clercq P., Van Leeuwen T. 2021. Is the emerging mite pest Aculops lycopersici controllable? Global and genome‐based insights in its biology and management. Pest Manag. Sci., 77(6): 2635-2644. https://doi.org/10.1002/ps.6265
- Vervaet L., Parapurath G., De Vis R., Van Leeuwen T., De Clercq P. 2022. Potential of two omnivorous iolinid mites as predators of the tomato russet mite, Aculops lycopersici. J. Pest. Sci., 95(4): 1671-1680. https://doi.org/10.1007/s10340-022-01544-x
- Walter D.E., Krantz G.W. eds. 2009. Collecting, rearing and preparing specimens. In: A manual of acarology, Lubbock, Texas: Texas Tech University Press. pp. 83-96.
- Xu X. 2011. Predatory capacity of Homeopronematus anconai against Aculops lycopersici. Plant Dis. Pests, 2(3): 24-26.
- Yanar D., Erdoğan H., Yanar Y. 2013. Feeding behaviors of Tydeids (Acari: Tydeoidea). Tydeid'lerin (Acari: Tydeoidea) Beslenme Alışkanlıkları. Gaziosmanpasa Univ. Ziraat. Fak. Derg., 30(2): 1-5. https://doi.org/10.13002/jafag301
- Zhang Z.-Q. 2018. Repositories for mite and tick specimens: acronyms and their nomenclature. Syst. Appl. Acarol., 23(12): 2432-2446. https://doi.org/10.11158/saa.23.12.12



2024-07-03
Date accepted:
2024-11-20
Date published:
2024-12-12
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 De Vis, Raf M. J.; Vervaet, Lore; Reybroeck, Eva; Vanlommel, Wendy; Van Havermaet, Robin; Foqué, Dieter; Marcossi, Ítalo; Pallini, Angelo; Binda de Assis, Caio Henrique; Van Leeuwen, Thomas and Ueckermann, Edward A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



