Genetic variability of abundant littoral species of mesostigmatic mites (Acari, Mesostigmata) with different distributions from the seashores of Eurasia
Andrianov, Boris V.  1
; Makarova, Olga L.
1
; Makarova, Olga L.  2
and Goryacheva, Irina I.
2
and Goryacheva, Irina I.  3
3
1Laboratory of Insect Genetics of the Russian Academy of Sciences, Moscow, Russia.
2✉ Laboratory of Synecology, Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences, Moscow, Russia.
3Laboratory of Insect Genetics of the Russian Academy of Sciences, Moscow, Russia & Moscow Region State University, Mytishi, Moscow oblast, Russia.
2024 - Volume: 64 Issue: 4 pages: 1191-1212
https://doi.org/10.24349/wftr-xlsvOriginal research
Keywords
Abstract
Introduction
Littoral animal species often show extensive, contour in form (''band'' according to Heptner 1936) distribution areas and reliable mechanisms of dispersal. This is also true for a number of mites inhabiting the grounds of the marine intertidal zone and coastal seaweed accumulations (Evans 1962; Lindroth et al. 1973; Tikhomirov 1977; Pugh et al. 1997; Procheş and Marshall 2001). Mites in general represent the largest group of soil arthropods on Earth in terms of population density (Rosenberg et al. 2023), on seashores in particular (Forgie et al. 2013). Members of the suborder Mesostigmata are the most diverse and abundant components of coastal acarofauna (Halbert 1920; Luxton 1967; Haynert et al. 2017), and their diversity in costal habitats at only one locality can reach 40−60 species (Makarova and Bizin 2020; Bizin and Makarova 2022).
Being small organisms (most less than 1 mm in length), mites have enormous potentials for passive dispersal. Thus, mites have been revealed among neuston animals near the Galápagos Islands (Peck 1994). Rafting on different plant residues (seaweeds, logs, grass tussocks) or on ice-floes together with organic debris offers vast dispersion possibilities for littoral mites (Lindroth et al. 1973; Schatz 1991), especially during catastrophic tsunami events (Lindo 2020). Their resistance to flooding (Krantz 1974; Schuster 1979; Pugh et al. 1987; Avdonin 1999; Makarova and Petrova-Nikitina 2008) and phoresy on other arthropods (Remmert 1956; Evans 1962; Egglishaw 1966; Lindroth et al. 1973; Rigby 1996a, b; Pugh et al. 1997; Klimov 1998; Avdonin 1999; Takaku 2000; Makarova and Böcher 2009; Gwiazdowicz and Coulson 2010; Halliday 2010; Trach 2016) have been repeatedly demonstrated and could contribute to intense genetic exchanges. However, significant events in the history of the seas (glaciations, isolations etc.) might lead to the segregation of local clusters, which could be reflected in the genetic peculiarity of modern populations. Recently, processing the available data on vertebrates has shown that intraspecies genetic diversity is not related to distribution area size, but regional factors play significant roles instead (Lawrence and Fraser 2020). There seem to be no publications yet concerning the phylogeography of littoral mesostigmatic mites, while interesting results have already been obtained using material of tropical and Arctic species of coastal oribatid mites. For example, a connection between the genetic structure of supraspecific complexes, dispersal capacities and landscape history has been demonstrated, as well as sibling species discoveries (Pfingstl et al. 2018, 2019a, b, 2022; Artamonova et al. 2023).
Among the littoral mesostigmatic mites of the Holarctic, there are a number of species with extensive distributions (Tikhomirov 1977; Błaszak and Ehrnsberger 1998; Makarova 2015; Makarova and Bizin 2020). Our numerous collections from Eurasia, North America and Greenland reveal that only four species representing different families of the suborder Mesostigmata appear to be especially common and widespread. These are Phorytocarpais kempersi (Oudemans, 1902) [= Parasitus kempersi Oudemans, 1902 in GenBank; see the new combination in Makarova, 2019, p. 460], Parasitidae; Halolaelaps celticus (Halbert, 1915) and Halolaelaps orientalis Ishikawa, 1979, Halolaelapidae; and Thinoseius spinosus (Willmann, 1939), Eviphididae. It is the complex history of the seas of the Ancient Mediterranean, the regular disruptions of their connections to each other and with the waters of the Atlantic Ocean (Rychagov 1997; Rögl 1999; Svitoch 2008, 2013; Svitoch et al. 2010; van Baak et al. 2016; Krijgsman et al. 2019), as well as the significant climatic diversity of the modern coasts of Eurasia, that has prompted us to analyze the genetic structure of these mesostigmatic species using material from various seas. In this regard, the analysis of their genotypes from the Caspian Sea is of particular interest, because of the exceptionally high level of species endemism of Caspian marine animals (Mordukhai-Boltovskoi 1979; Wesselingh et al. 2019), with up to 57−77% in crustacean Amphipoda and fishes (Pjatakova and Tarasov 1996; Naseka and Bogutskaya 2009; Copilaș-Ciocianu and Sidorov 2022).
The modern molecular phylogeny of Mesostigmata is based on the study of the variability of the 18S and 28S rRNA nuclear ribosomal genes and COI mitochondrial gene (Klompen et al. 2007; Sourassou et al. 2015; Dabert et al. 2010; Dowling and O'Connor 2010). The systematics of mites reconstructed on the basis of molecular data are in good agreement with the macrotaxonomic system of mesostigmatic mites proposed by Lindquist et al. (2009).
The different domains of a nuclear ribosomal repeat show different rates of accumulation of phylogenetically significant substitutions, allowing them to be used to separate systematic groups of different rank. The 28S rDNA gene is optimal for identifying mites from family to species level, while the variability of intergenic transcribed spacers (ITS) is higher and may be suitable for separating closely related species (Zhao et al. 2020; Pérez-Sayas et al. 2022). Some authors have proposed the ITS fragment as a basis for DNA barcoding of mite species (Zhao et al. 2020).
The present paper provides the first results of DNA barcoding of abundant species of mesostigmatic mites belonging to the genera Phorytocarpais, Halolaelaps, and Thinoseius, all inhabiting the littoral zone of the Eurasian seas, based on a sequence analysis of the ITS ribosomal repeat fragment. On the one hand, taking into account the perfect dispersal mechanisms of littoral mites and, on the other hand, the exceptionally high-level endemism among the marine invertebrates of the Caspian Sea, both the genetic integrity of extensive trans-oceanic mega-populations of our model littoral species and a certain originality of the Caspian genotypes can be proposed as a null hypothesis.
Characteristics of model species
All four model species of mesostigmatic mites are most common on the seashores of Europe and often co-occur in storm debris cast ashore (Halbert 1920; Hyatt 1956; Strenzke 1963; Lindroth et al. 1973; Kojumdzhieva 1982; Pugh and King 1985, 1988; Błaszak and Ehrnsberger 1998; Makarova and Petrova-Nikitina 2008; Bolge et al. 2018; Makarova and Bizin 2000; Bizin and Makarova 2022). Below we provide descriptions of their habitats and available biological information.
Phorytocarpais kempersi (Oudemans, 1902)
This species was earlier assumed to be cosmopolitan in distribution, although its absence from the shores of the Caspian Sea, the Aral Sea and Arctic Siberia had been reported simultaneously (Tikhomirov 1977). And there still are no records outside the Holarctic. According to our data, P. kempersi is an abundant species in sea debris on the shores of the middle and southern parts of the Caspian Sea (O.L.M.: personal observations). It is common everywhere, including the Atlantic and Pacific coasts of North America (Krantz 2016; material from the Canadian National Collection of Insects, Arachnids and Nematodes, accessed 7 October 2020, curator W. Knee; O.L.M. unpublished data). Phorytocarpais kempersi appears first in algal emissions, being most abundant in the early stages of their decay succession (Strenzke 1963; Makarova and Petrova-Nikitina 2008; Bizin and Makarova 2022). Phoresy of its deutonymphs on ''larger inhabitants of rotting algae carried by water'' has also been reported (Tikhomirov 1977), and the possibility of dispersion on sea-washed algal debris in the summer conditions of the North, Black and White seas has been proven experimentally (Pugh and King 1985; Avdonin 1999; Makarova and Petrova-Nikitina 2008). Moreover, in a special experiment conducted in August 2008 in the White Sea, submerged algae were shown to retain about 80% living P. kempersi individuals for at least 5 days (O.L.M.: personal observations). The species has the widest diet among the study littoral mite species. Its sharp increase in numbers was observed when feeding on nematodes, Sphaeroceridae larvae and small crustaceans (Avdonin 2002), but P. kempersi also consume enchytraeids, dead and living adults of Diptera, juvenile oribatid and mesostigmatic mites, including individuals of their own species (Pugh and King 1985; Avdonin and Striganova 2004). Simultaneously, 4−7 eggs can mature inside the female (O.L.M.: personal observations). Individuals from the White and Black seas successfully interbreed with each other (Avdonin and Striganova 2004).
Halolaelaps celticus Halbert, 1915
A North Atlantic species penetrating into Arctic waters up to the Pechora Sea in the east (new records from the shores of the Kola Peninsula, Kanin Peninsula, and Dolgiy Island; O.L.M.: personal observations). Due to the significant variations in ''diagnostic'' characteristics of females of this and related species (Błaszak and Ehrnsberger 1998; Maslov 2013; O.L.M.: personal observations), only records accompanied by the presence of males can be considered reliable. The records of H. celticus from the shores of the Black and Azov seas (Bregetova 1977; Koyumdzhijeva 1982; Maslov 2013; Bizin and Makarova 2022) seem to actually concern H. orientalis Ishikawa, 1979 (? = Halolaelaps schusteri Hirschmann, 1966; see below) and require verification. The proper Halolaelaps celticus is abundant in the early and middle stages of marine debris decay (Strenzke 1963; Makarova and Petrova-Nikitina 2008). This species withstands flooding with seawater within an algal mass for at least 5 days (O.L.M.: personal observations). This can provide its passive dispersal, although phoresy on Amphipoda has been repeatedly shown for other littoral species of the genus (Willmann 1952; Evans and Till 1979; Pugh et al. 1997; Trach 2016).
Halolaelaps orientalis Ishikawa, 1979 (? = Halolaelaps schusteri Hirschmann, 1966)
A Palaearctic species ranging from the Canary Islands to Japan. There are no records from the shores of the Arctic Ocean, Bering and Okhotsk seas. This species was previously listed as the most common member of the celticus-group on the shores of the northern and southern seas of continental Europe, as well as the Canary Islands (Błaszak and Ehrnsberger 1998).
Since an error in the original description of Halolaelaps schusteri (structure of setae j1, z1) has been corrected during the analysis of the type material (Błaszak et al. 2004, p. 2), there are no characters left allowing us to securely distinguish between females of H. schusteri and H. orientalis (Błaszak and Ehrnsberger 1998). Therefore, all records of ''H. orientalis'' in the Western Palearctic (Błaszak and Ehrnsberger 1998; Avdonin 2002; Avdonin and Striganova 2004; Maslov 2013; Bizin and Makarova 2022) probably belong to H. schusteri, as possibly does the record from Japan as well. The male of H. orientalis has not been described yet. Having analyzed its morphological structures, these two species are highly likely to be synonyms. In marine debris, H. orientalis forms ''quasi-stationary aggregations'' (Avdonin and Striganova 2004). In six districts of the Atlantic coast, including the Canary Islands, this species has been noted to live together with H. celticus (Błaszak and Ehrnsberger 1998). The dispersion of H. orientalis with floating algae is possible (Avdonin 1999). It feeds actively on nematodes and copepods (Copepoda) (Avdonin and Striganova 2004); the consumption of damaged enchytraeids and dipteran Sphaeroceridae (larvae and adults) was also recorded (Avdonin 1999, 2002).
Morphological diagnostics of Halolaelaps species belonging to the celticus-group in general [the subgenus Halolaelaps (Halolaelaps) sensu Błasczak and Ehrnsberger 1998] are greatly hampered by the uncritical attitude of the above authors to the asymmetry and intra-population variability of the dorsal idiosomal chaetome. Such cases were revealed by various researchers (Maslov 2013; O.L.M.: personal observations), including Błasczak and Ehrnsberger (1998, p. 165, 167) themselves, thus leading to doubt the validity of the species they described (Błasczak and Ehrnsberger 1997, 1998).
Thinoseius spinosus (Willmann, 1939)
A Holarctic littoral species. Its records at distances of dozens or hundreds of kilometers away from a sea coast are of special interest. These are records at chicken farms in Oregon, USA (Volkinburg 1969), as well as on the shores of salt lakes in the south of Western Siberia (GenBank ID: MW367935) and in northern Dagestan, North Caucasus (O.L.M.: personal observation). Generally, T. spinosus seems to be the ''northernmost'' representative of the littoral acarofauna; it lives on Spitsbergen (Gwiazdowicz and Coulson 2010), northern Greenland (Makarova 2015), northern Novaya Zemlya and northern Chukotka (O.L.M.: personal observations). There is no information on nutrition, but for other species of the genus active consumption of nematodes was revealed (Egglishaw 1966; Rigby 1996a), as well as eating crushed enchytraeids, small dipterans and amphipods (Avdonin 1999, 2002). Deutonymphs of T. spinosus are actively dispersed when attached to various brachiceran dipterans, Anthomyiidae, Calliphoridae, Coelopidae, Helomyzidae, Heterocheilidae, Sphaeroceridae (Lindroth et al. 1973; Klimov 1998; Makarova and Böcher 2009; Gwiazdowicz and Coulson 2010).
Material and methods
Collections of mites
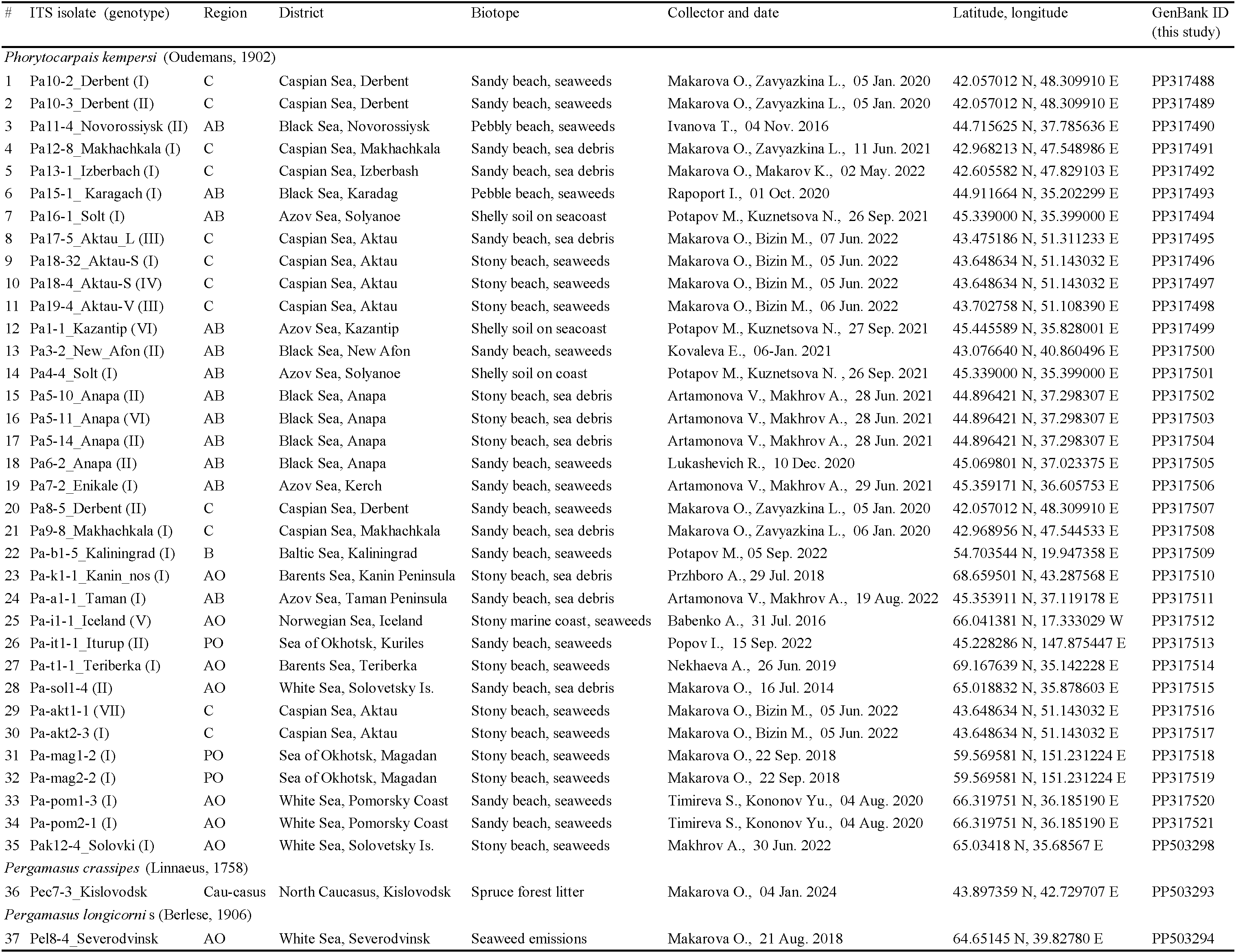
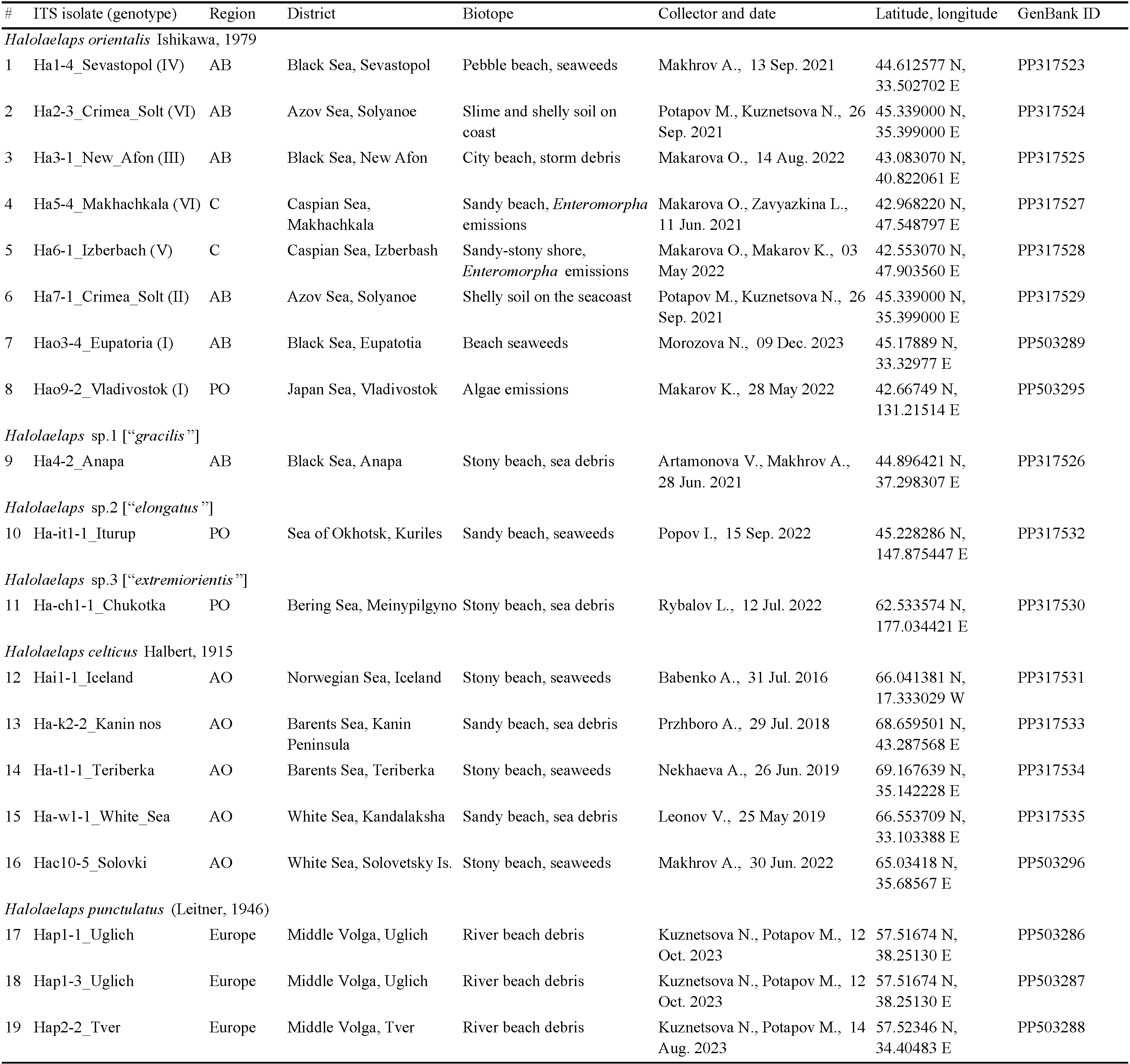
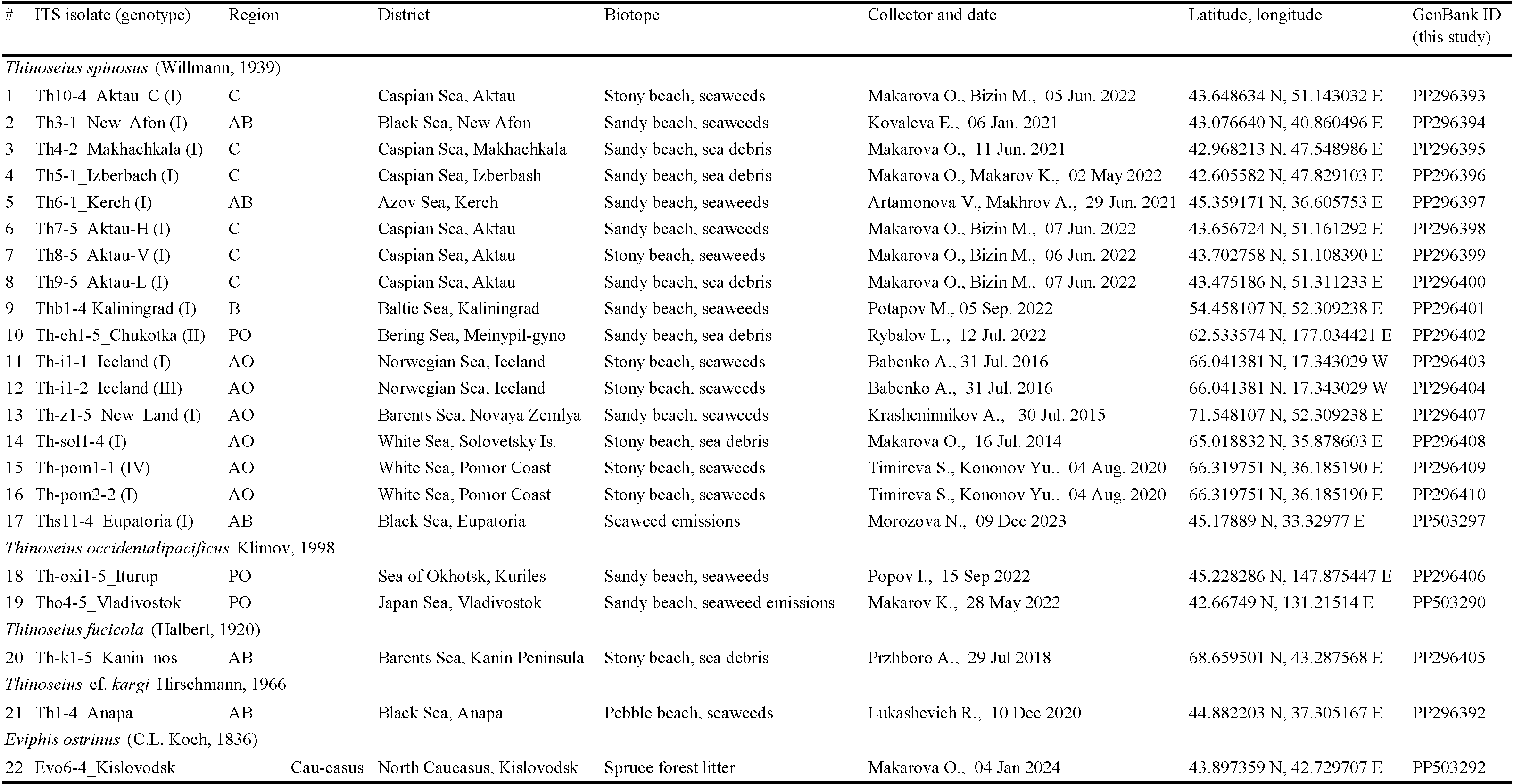
Targeted collections of the model mite species for genetic studies were carried out in 2016–2022 at 30 localities in Eurasia (Figure 1). At each locality, a mixed sample of marine emissions was usually taken from several coastal plots or a series of 3–5 samples was selected. The samples were wrapped in several layers of newsprint (leaving a layer of air), placed in a plastic bag and delivered to the laboratory. Mites were extracted from sea debris using Tullgren funnels made of thick paper and applying neither special lighting, nor heating until they became completely dry within 7–14 days. Mites were fixed with 96% ethanol. All mites selected for DNA isolation and DNA encoding were preliminarily identified by morphological features and preserved in alcohol until molecular identification was performed. Some individuals of a definite mite species were mounted in permanent slides with Hoyer medium for identification; the other part of the series was used in genetic studies. Voucher specimens or series of model species specimens are stored at the Laboratory of Synecology of the Severtsov Institute of Ecology and Ecology of the Russian Academy of Sciences, Moscow. For DNA barcoding, total DNA was isolated from individual mites from each locality. All nucleotide sequences obtained were deposited in GenBank. The characteristics of the mite collection sites, the results of both morphological identification of species and DNA barcoding following the analysis of variability in the nucleotide sequence of the ribosomal repeat fragment are all presented in Tables 1–3.









Isolation of total DNA and PCR
Total DNA from individual mites was isolated using the phenol-chloroform method (Maniatis et al. 1982). The resulting DNA was dissolved in deionized water. DNA concentration was determined spectrophotometrically utilizing an Implen NanoPhotometer NP80. The DNA concentration in the preparations was adjusted to 4 ng/μl. PCR was carried out in a final volume of 25 μl using EncycloPlus PCR kits (Evrogen, Russia) in accordance with the manufacturer's protocol.
To amplify the ribosomal repeat fragment and sequencing, we used a modified protocol of (White et al. 1990, 2013). The primers ITS1F (5′- CTTGGTCATTTAGAGGAAGTAA) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) were applied to amplify a specific fragment of variable length, formed by a fragment of the 18S rRNA gene; ITS1; 5.8S rRNA gene; ITS2; and a fragment of the 28S rRNA gene. PCR conditions: primary denaturation – 3 min at 94 °C; then 35 cycles: denaturation at 94 °C – 15 s, annealing at 58 °C – 15 s, synthesis at 72 °C – 40 s; and final synthesis at 72 °C – 7 min.
Purification of and sequencing the PCR fragments
The fragments obtained as a result of amplification were fractionated in a 1.5% agarose gel. Elution of fragments from the gel was carried out using the Cleanup Mimi elution kit (Evrogen, Russia) in accordance with the manufacturer's protocol. The nucleotide sequence of PCR fragments was determined from forward and reverse primers on a 3500 Genetic Analyzer using the BigDye®Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA) according to the manufacturer's recommendations.
Bioinformatic analysis
Chromatogram analysis was carried out using CromasPro 13.3 software (Technelysium, Australia). Alignment of the sequences obtained in this study with the sequences deposited in GenBank databases was performed using resources from NCBI (http://www.ncbi.nlm.nih.gov ![]() ).
).
Prior to cladistic analyses, nucleotide sequences were aligned using the ClustalW algorithm (Thompson et al. 1994) and MEGA X software (Kumar et al. 2018) to obtain a set of sequences of equal length. Cladistic analyses were performed in MEGA7 (Kumar et al. 2016). Cladograms were constructed using the neighbour-joining method. Substitution model – due to Maximum Composite Likelihood. Treatment of gaps/missing data – due to Pairwise Deletion. Trees are drawn to scale, with branch lengths in units of the number of base substitutions per site. The statistical significance of the resulting clustering of taxa was assessed using bootstrap support. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches.
The median ITS genotype network was constructed in PopART (Leigh et al. 2015) using the TCS algorithm (Clement et al. 2000). Population statistics, namely: nucleotide diversity (Pi), genotype diversity (Hd), their standard deviation, number of variable sites and Tajima's D test for selective neutrality, were obtained with the DnaSP program (Rozas et al. 2017).
Results
Phorytocarpais kempersi
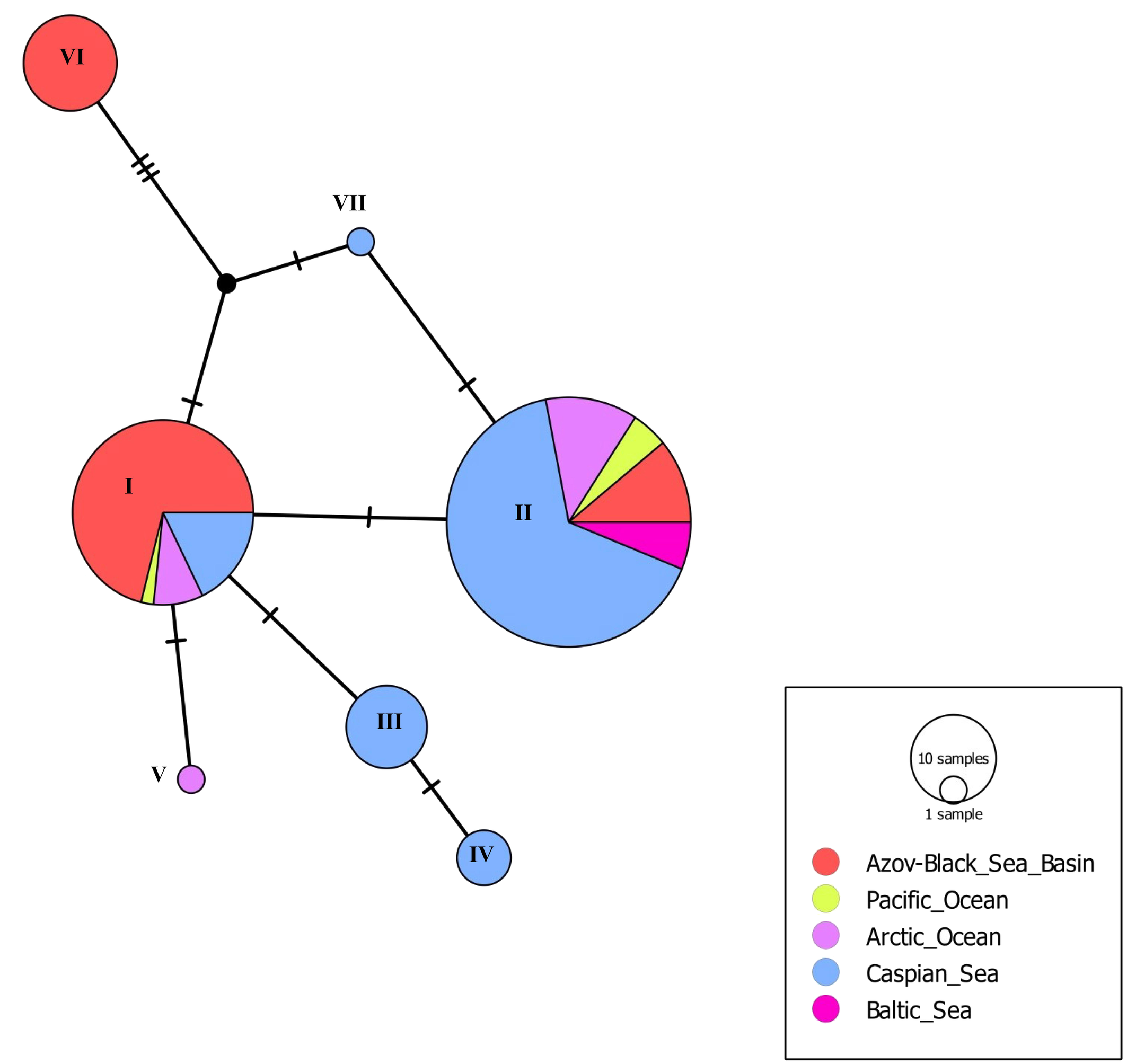
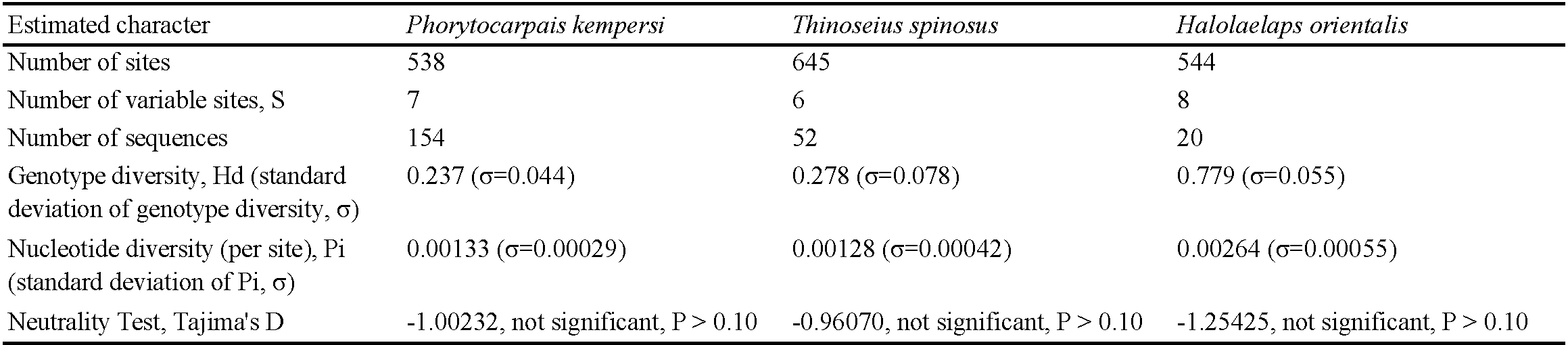
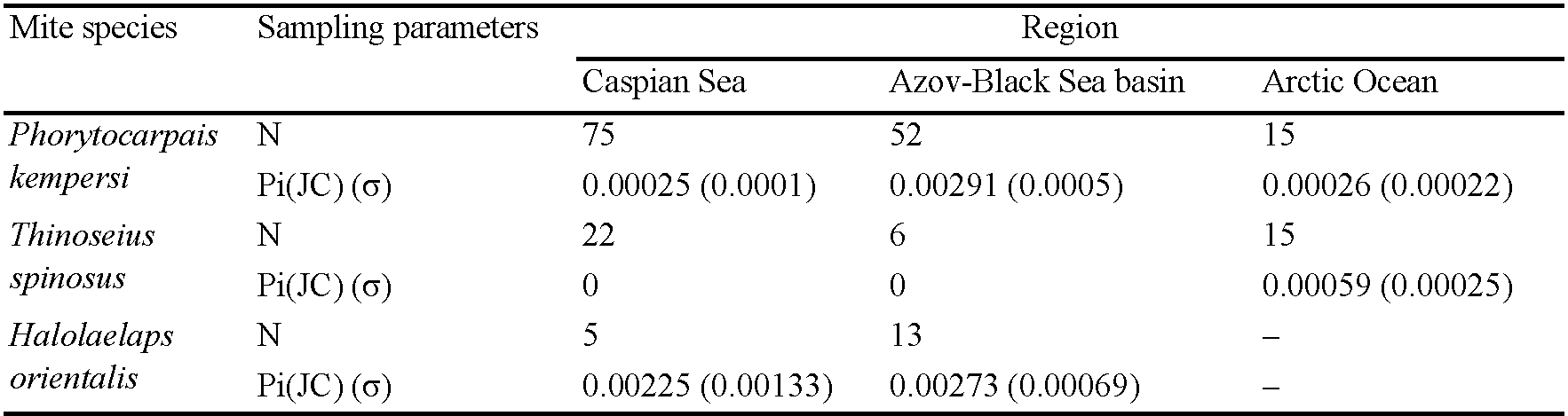
Among the species we studied, P. kempersi is the most widespread and common mite species found in all five larger regions (Azov-Black Sea Basin, Caspian Sea region, Pacific Ocean region, Arctic Ocean, Baltic Sea region). Of all 154 mite specimens used, 35 sequences of ITS gene fragments were obtained, representing 7 genotypes (Table 1). The variability of ITS genotypes of P. kempersi is presented in Figure 2. Other mite species from the family Parasitidae with known ITS sequences (representatives of the genera Pergamasus Berlese, 1903 and Poecilochirus G. & R. Canestrini, 1882) are separated from P. kempersi by a hiatus. The bootstrap values at the node separating the ITS sequences of P. kempersi from the representatives of the closest genera Pergamasus and Poecilochirus are 99% (Figure 2). At the same time, the intraspecific variability of P. kempersi across a huge part of its distribution (from Iceland to the Kuril Islands) does not exceed 1%, allowing an unambiguous identification of the species. To characterize the intraspecific variation of P. kempersi and to compare data from geographically distant parts of its range, we obtained a median network of genotypes (Figure 3). There the central position is occupied by the genotype marked with the Roman numeral (I), found in four large regions. All this allows us to consider it ancestral. All other genotypes appear to be associated with it, directly or indirectly. The closely related genotype (II), found in five large regions, is the most common one in the Caspian Sea region. The remaining genotypes are found only in particular regions. The genotypic diversity of the entire sample of P. kempersi and local populations of this species in individual large regions are presented in Tables 4 and 5. In the most remote, Far Eastern populations (Pacific coast), only two widespread genotypes were found.



Genotypes (III), (IV) and (VII) are revealed only on the shores of the Caspian Sea, and genotype (V) only in the Iceland region (Table 1). The greatest divergence from the ancestral genotype is observed in mites with genotype (VI). As this genotype was found in closely located areas on the coast near the town of Anapa, Black Sea and on Cape Kazantip, Azov Sea, the early formation of a locally adapted population seems to take place. If adaptation does occur, it occurs at a very early stage, since the value of the test (Tajima's D) for selective neutrality corresponds to the model of random accumulation of mutations in the absence of selective pressure on the population (Table 4). A comparison of P. kempersi populations from different geographic regions shows the absence of fixed region-specific nucleotide substitutions (Figure 3), this also being consistent with the model of random accumulation of mutations.


Halolaelaps orientalis and H. celticus
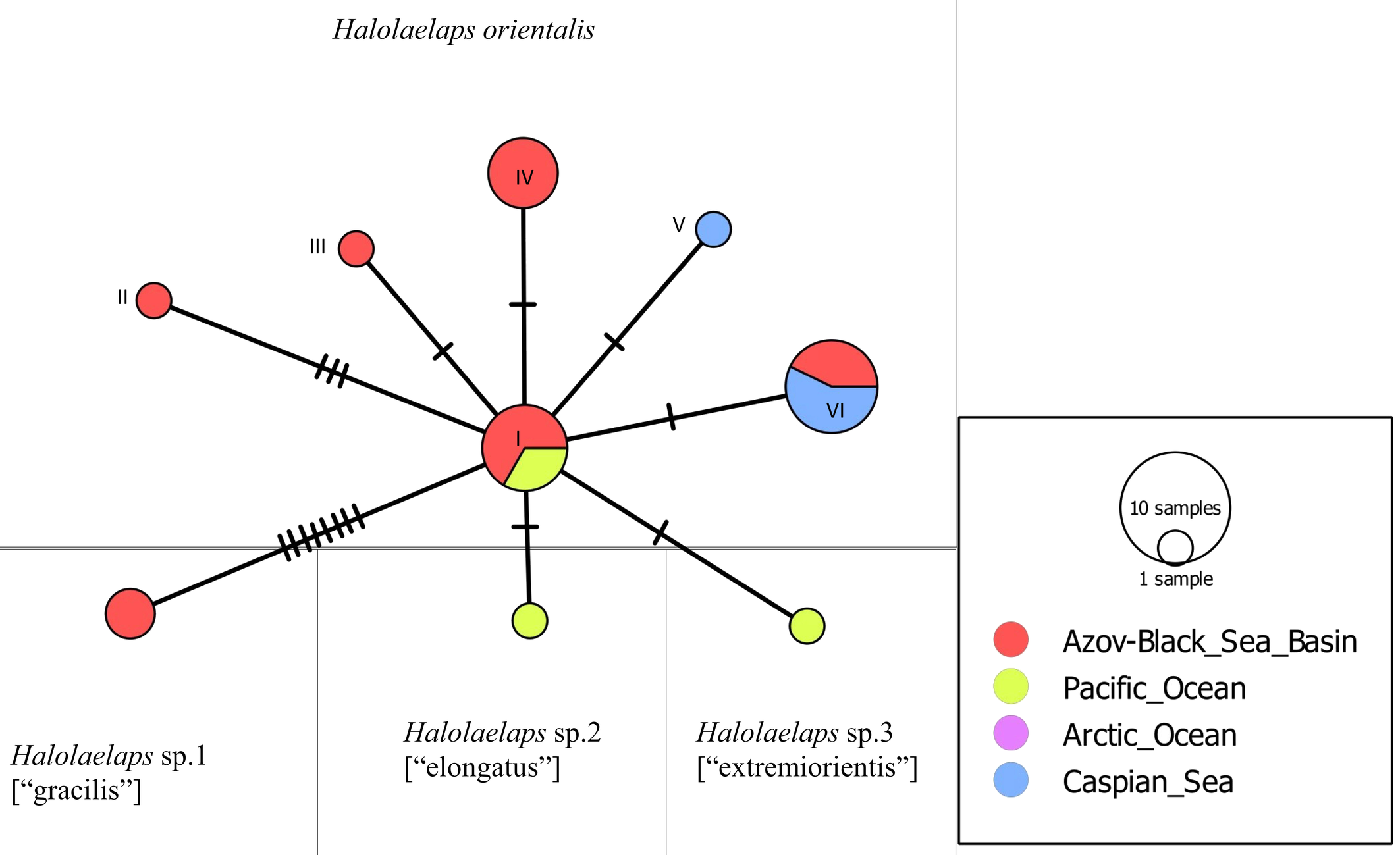
When analyzing the sequences of 20 specimens of H. orientalis, six ITS genotypes were obtained (Table 2). However, based on morphological characters, we have discovered three possible cryptic species close to H. orientalis. They were revealed in the Black Sea (sp. 1) and the seas of the Pacific Ocean (sp. 2, 3) and have some genetical distinctions (Figure 5).
The variability of the ITS fragment of all five distinguished members of the celticus-group analyzed, and of the congeneric Halolaelaps punctulatus (Leitner, 1946), is presented in Figure 4. Three clades with 99% and 100% bootstrap support correspond to the species: H. celticus, H. punctulatus and a complex of closely related species, Halolaelaps spp., related to H. orientalis. To analyze the genetic structure of this complex of close species, we constructed a median network of genotypes (Figure 5). This median network is star-shaped, this being characteristic of species that may have relatively recently gone through a stage of an explosive range expansion. The central position in this median network of genotypes is occupied by genotype (I), which is found in the Azov-Black Sea region and in the Pacific region and belongs to H. orientalis proper. The remaining genotypes have a narrower geographic distribution and are likely to have derived from genotype (I). Among the intertidal mite species we studied, H. orientalis is the most variable (Table 4). As in the case of Phorytocarpais kempersi, the value of the selective neutrality test (Tajima's D) fails to indicate the presence of any discriminatory pressure on its modern population. We found numerous demes of H. orientalis on the coasts of the Mediterranean, Azov and Black seas, as well as the Russian Far East (Sea of Japan).
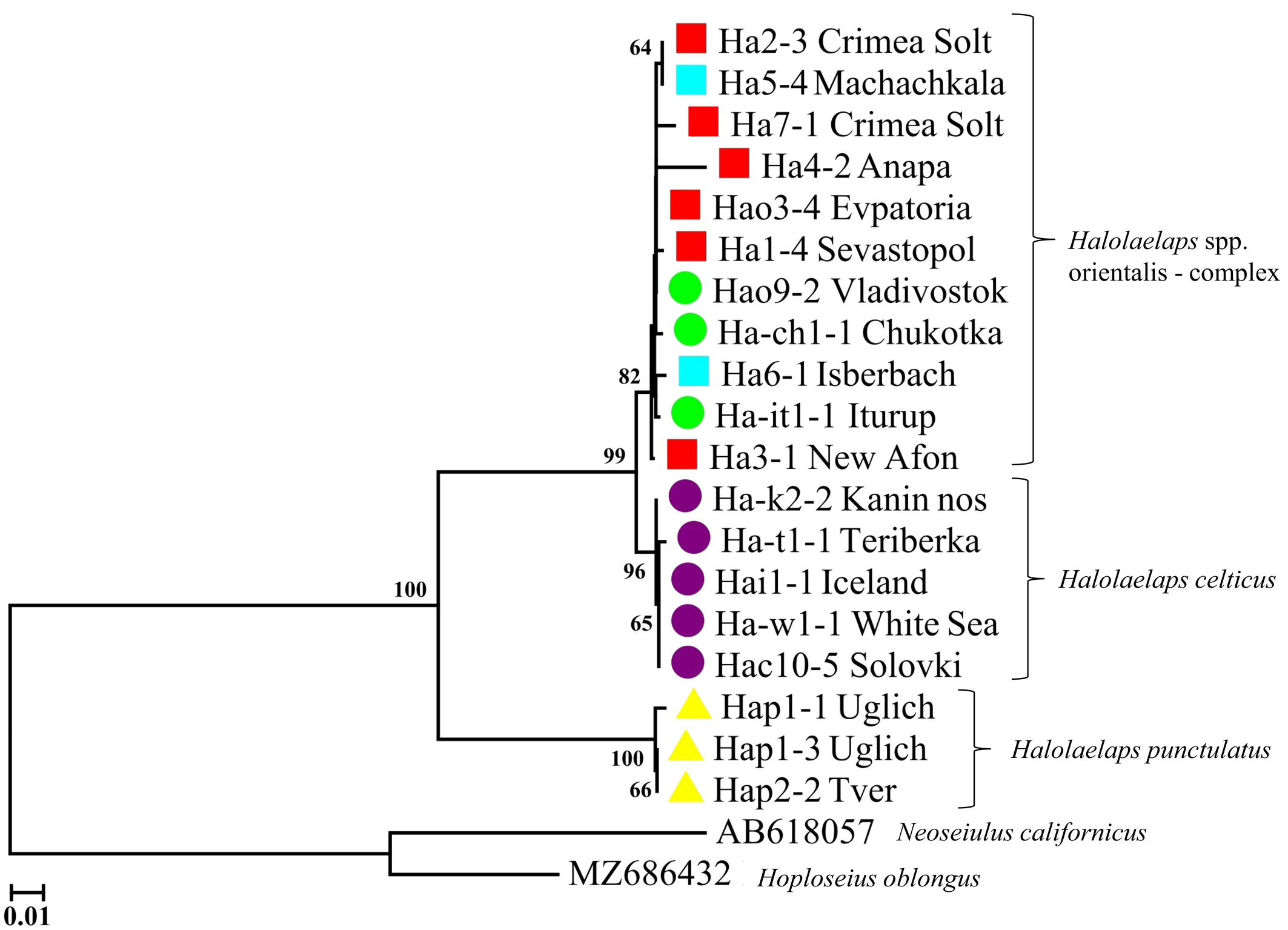


H. orientalis was not found on the coasts of the Arctic Ocean seas, where it is replaced by the closely related species, H. celticus. We were able to obtain genetic material of H. celticus from remote populations: the shores of Iceland, Solovetsky Islands, Kola Peninsula, and Kanin Peninsula. The ITS fragment studied turned out to be monomorphic in our sample of H. celticus (10 specimens from five localities), but separated from H. orientalis by six phylogenetically significant substitutions.
So, besides well distinguished species H. orientalis and H. celticus, some probably new species were found within the celticus-group, namely: Halolaelaps sp. 1 [''gracilis''] from the northeastern Black Sea coast, Halolaelaps sp. 2 [''elongatus''] from Iturup Island, Halolaelaps sp. 3 [''extremiorientis''], from the southern coast of Chukotka and Kamchatka peninsulas. All these forms have unique ITS genotypes (Figure 5). The genetic evidence, together with some morphological differences, support the assumption of species rank status for these three species. Further data are required to clarify this proposal. Carriers of ITS genotypes I–VI, on the contrary, showed no clear morphological differences.


Thinoseius spinosus and other species of the genus
In addition to the widespread Holarctic species T. spinosus, further three species of the genus Thinoseius with narrower geographic distributions have been found in the littoral zones of Eurasia: T. fucicola (Halbert, 1920), T. occidentalipacificus Klimov, 1998; and Thinoseius cf. kargi Hirschmann, 1966. The variability of ITS fragments of all four species is presented in Figure 6. Distinguishing the species is not difficult. Three clades are observed with good bootstrap support. Two clades correspond to the species T. spinosus and T. occidentalipacificus. The third clade combines ITS sequences of T. fucicola and Thinoseius cf. kargi. Thinoseius occidentalipacificus mites are known from the Pacific region (Klimov 1998; Takaku 2000; Makarova 2019), T. fucicola from the shores of the northern and southern seas of Europe (Remmert 1956; Evans 1969; Bregetova 1977), and T. cf. kargi from the coasts of the Mediterranean and Black seas (Hirschmann 1966; Avdonin 2002). Both latter species show a single genotype of the ITS fragment, one different from other genotypes found among Thinoseius species.



When studying the ITS sequences of 52 specimens of T. spinosus from 17 localities, four genotypes were found (Table 3). To analyze the intraspecific variability of this species, we constructed a median network of genotypes (Figure 7). Intraspecific variability of T. spinosus is again star-shaped. The most abundant genotype, marked with number (I), occupies the central position in the median network of genotypes and thus it is considered ancestral, at least so for the modern European super-population. We found genotype (I) in four of five larger regions, except for the Pacific region, where it is replaced by genotype (II).
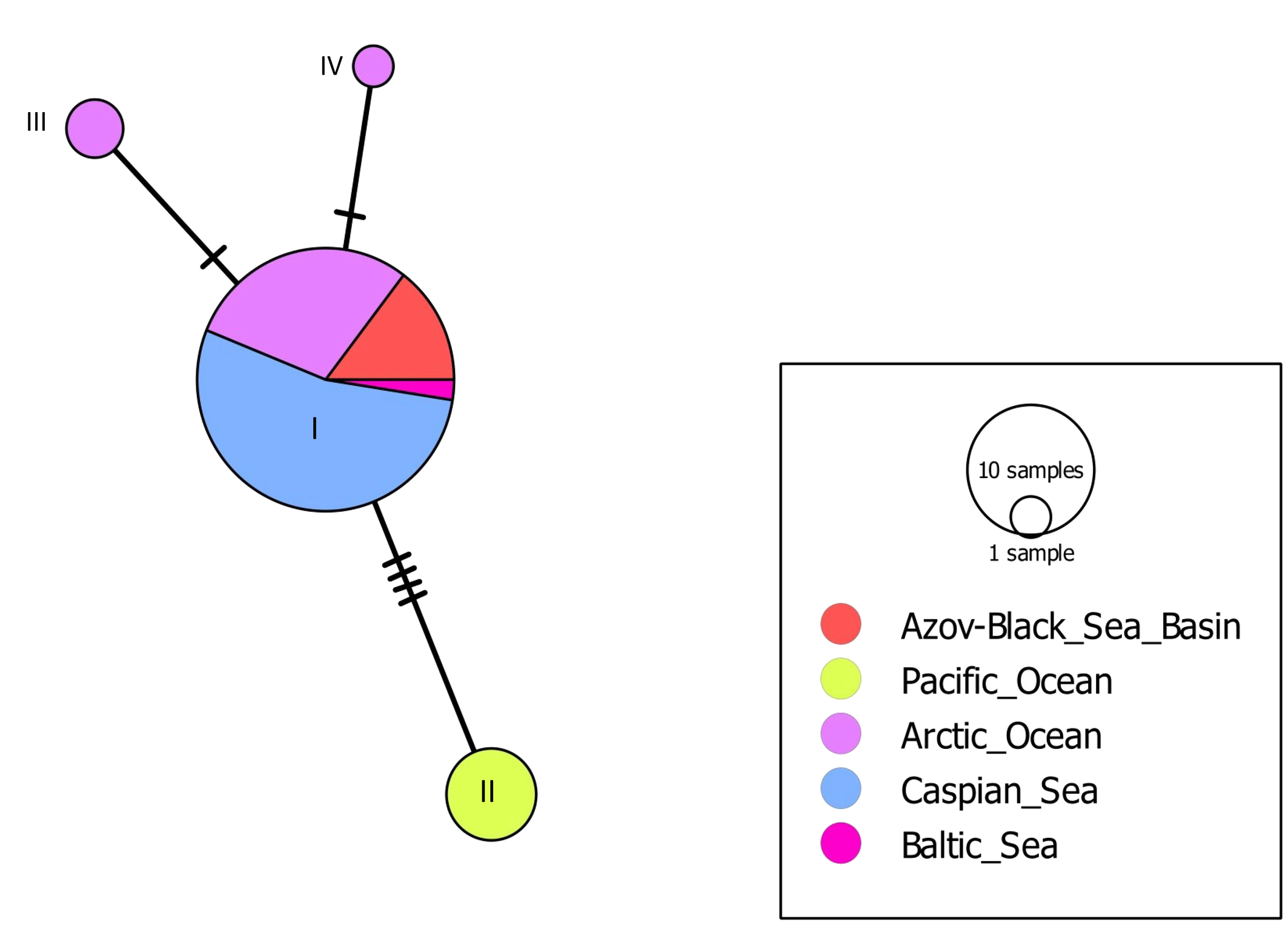


Special genotypes of T. spinosus different from the central genotype (I) are found only in the regions of the Pacific and Arctic oceans, while across the vast areas covering the shores of the Baltic, Black, Azov and Caspian seas T. spinosus appears to be monomorphic. The value of the selective neutrality test (Tajima's D) does not reveal any selection effect on the T. spinosus population (Table 4).




Discussion
Suitability of the ITS fragment for phylogenetic analysis
At present, the following data are available for the molecular comparison of the littoral mites studied in this work. DNA barcode fragments of mites from the genus Phorytocarpais Athias-Henriot, 1979 (formerly, partly Parasitus Latreille, 1795) have been obtained from a few species. The DNA barcode fragment of the COI gene is known for the following species: Phorytocarpais fimetorum (Berlese, 1903) (NC_061975), Parasitus loricatus (Wankel, 1861) (MN906455), Parasitus hyalinus (Willmann, 1949) (MH983860), and Parasitus wangdunqingi Ma, 1995 (MK270528). Fragments of ribosomal nucleotide sequences of the RNA 18S and 28S genes used for DNA barcoding are known only for P. fimetorum (GenBank Accession: AY626653, AY626616, AY620935) (Klompen et al. 2007).
No DNA barcode fragments of mites belonging to the genus Halolaelaps Berlese et Trouessart, 1889 have heretofore been obtained.
DNA barcode fragments of members of the genus Thinoseius Halbert, 1920 are so far known for only one species. As a fragment of the mitochondrial COI gene was studied in a single specimen of Thinoseius spinosus (Willmann, 1939) (GenBank ID: MW367935; found on a salt lake shore in Western Siberia), neither intraspecific variations nor the utility of DNA barcoding for the identification of this species can be assessed.
In this study, based on ITS ribosomal repeat fragment sequence analysis, we present results of effective DNA barcoding of abundant mite species inhabiting the littoral zone of Eurasian seas: P. kempersi, Halolaelaps spp. orientalis - complex, H. celticus and T. spinosus.
The molecular diagnostics of almost all mite species studied here based on the analysis of the ITS ribosomal repeat gene fragment turns out to be suitable for using this marker in new generation biomonitoring technologies (Makiola et al. 2020). Only distant populations of Thinoseius fucicola (Barents Sea) and Thinoseius cf. kargi (Black Sea) failed to be distinguished from each other using this marker, but this must be studied in more detail. At present, it is not possible to assess the effectiveness of ITS as a marker for distinguishing closely related Halolaelaps species: Halolaelaps sp. 1 [''gracilis''], Halolaelaps sp. 2 [''elongatus''] and Halolaelaps sp. 3 [''extremiorientis''].
ITS fragment sequences variation in individual model species are provided with high bootstrap support, 94−100%. At the same time, the results of the test for selective neutrality (Tajima's D) correspond to the model of random accumulation of mutations in the absence of a discriminatory pressure on the population.
A likely explanation for this type of variability is the relative stability of the ecological conditions of littoral mite species during the period of their dispersal across their modern range and intensive intrapopulation genetical exchange due their dispersal mechanisms. A possible explanation as well can be the vastness of the ranges of these species, so that abrupt climatic changes associated with Pleistocene glacial cycles did not lead to the extinction of mite populations, but only to fluctuations in their boundaries.
The sample of the North Atlantic species, Halolaelaps celticus, containing specimens from Iceland in the west to the Kanin Peninsula in the east, turned out to be monomorphic, based on the ITS marker. All the area is known to have been covered with ice during the Last Glacial Maximum (20,000 yr BP) (Gowan et al. 2021; Mangerud et al. 2023). Following ice melting, in the Holocene, the spread of H. celticus from a southern refugium can be assumed to have rapidly occurred. In a similar way, across the vast territories along the shores of most European seas, a single genotype (I) of Thinoseius spinosus has become widespread. Its derivatives differing by single nucleotide substitutions in the ITS fragment (genotypes III and IV), occur on the coasts of Iceland and the White Sea. The Pacific population of this species is more strongly separated, differing by four substitutions [genotype (II)]. It seems very likely that the integrity of its European super-population is largely ensured by intense phoresy on dipterans, these latter being represented by a whole range of taxa (see above the section ''Characteristics of model species'').
Triviality of Caspian genotypes
According to the modern classification of seas (Briggs 1974), the tideless Caspian Sea is the eastern outpost of the Mediterranean-Atlantic littoral biogeographic region. During its history, it has undergone and is still undergoing large-scale changes in area and salinity, as well as periods of destruction and restoration of connections to the Azov-Black Sea basin (Rychagov 2011; Svitoch 2008, 2013; Krijgsman et al. 2019). Long periods of isolation and desalination must have resulted in high-level species endemism of the Caspian marine fauna (Mordukhai-Boltovskoi 1979; Naseka and Bogutskaya 2009). However, our pioneering studies on its littoral mites did not reveal previously unknown species, and the list of littoral mite species of the Caspian Sea appears to be extremely poor compared to the Black Sea shores (unpublished new data). Moreover, the genotypes of the study model species that dominate the Caspian shores belong to the most trivial ones, while the rare ''endemic'' variants of Phorytocarpais kempersi may differ only in one nucleotide substitution, that might reflect their recent origins. The same is true for the rare ''endemic'' variants of Halolaelaps orientalis.
It seems very likely that the simplicity of the Caspian fauna of littoral mites (mainly soil-dwellers) is determined both by their less strong association with the so variable marine environment, as well as the recent contact of the Caspian and Azov seas during the Early Khvalynsk transgression. Its chronology has been the subject of discrepancies and debate for many years, the age of this transgression being dated a wide range, from 71,000 to 7,000 years ago (Tudryn et al. 2013; Koriche et al. 2022; Makshaev and Tkach 2023). However, most authors presently admit that the last contact of the Caspian and Black seas (an overflow from the former to the latter basin through the Kuma-Manych Strait and the Sea of Azov) could have occurred not earlier than 17,000−14,000 years ago (Svitoch et al. 2010; Sorokin 2011; Arslanov et al. 2016; Krijgsman et al. 2019; Kurbanov et al. 2023; Gelfan et al. 2024). Taking into account the low rate of evolution of nuclear ribosomal genes in the Class Acari (Murrell et al. 2005; Klompen et al. 2007), the integrity of the genetic structure by this marker of the populations of the Azov-Black Sea basin and the Caspian Sea is hardly surprising. Yet, the significant sample size for Phorytocarpais kempersi (154 mite sequences) has made it possible to reveal a clear predominance of different genotypes in its populations from these regions. The contribution of historical and/or modern (climatic and other) factors responsible for these differences remains to be studied.
It still seems premature to analyze the reasons for the poverty of the Caspian littoral acarofauna. The complete isolation of this sea-lake from the Azov-Black Sea basin is, according to the most maximum estimates, dates back to more than 35,000 − 42,000 years (Sorokin 2011; Krijgsman et al. 2019). It is impossible to exclude the absence of a two-way exchange during the last contact of these seas because the current was directed towards the Black Sea, and the Caspian waters were significantly desalinated (Krijgsman et al. 2019), and then the reasons for the deterioration of the Caspian littoral fauna could be sought in its more ancient history.
Uniqueness of the genotypes of the Azov-Black Sea region
Unlike the Caspian Sea, the shores of the Black and Azov seas support both common and unique genotypes of mites. Thus, in a large sample of Phorytocarpais kempersi, genotype (VI) with three nucleotide substitutions has been identified, whose carriers were found in closely located areas on the coasts of the Azov Sea (Cape Kazantip) and the Black Sea (Anapa). This may reflect the early evolution of a locally adapted population due to the fixation of random mutations. However, the value of the selective neutrality test (Tajima's D) corresponds to the model of random accumulation of mutations in the absence of discriminatory pressure on the population, which contradicts local adaptation.
Members of the genus Halolaelaps from the vicinity of Anapa, initially identified as H. orientalis, upon a more profound analysis of its morphology and ITS marker, appear to represent a new species, Halolaelaps sp. 1 (''gracilis''), separated from H. orientalis by eight nucleotide substitutions. We have also found this species in the surroundings of the town of Tuapse (160 km south of Anapa). It cannot be ruled out that the genetic peculiarity of P. kempersi and Halolaelaps sp. 1 in this region (Kerch-Taman mud volcano province) may be somehow associated with the history of its biota. This region is characterized by massive manifestations of mud volcanism (terrestrial and marine) caused by the special tectonics of the West Kuban trough (Kopf 2002).
It seems noteworthy that the overall nucleotide diversity of the ITS fragment in Phorytocarpais kempersi mites from the Azov-Black Sea population is significantly (by an order of magnitude) higher compared to the Caspian and Arctic regions (Table 5), indicating its ancestral nature at least in terms of the European part of this species' modern distribution.
Similarities between European and East Asian populations of model species
A number of intertidal mite species show extensive distributions (Schulte 1975; Procheş and Marshall 2001), including our model species (see above the section ''Characteristics of Model Species''). The wide physiological valence of the inhabitants of sea coasts, on the one hand, and the similarity of key environmental factors over the vast range of the sea littorals, on the other, are believed the main reasons why they are not affected by species differentiation (Procheş and Marshall 2001). This may be especially pronounced in the Holarctic, whose natural environments have changed dramatically during the Quaternary and must have led and still lead to large-scale species migrations that eliminate possible inter-population differences (Coop 2004). When analyzing a large dataset on vertebrates, the relationship between range size and genetic diversity turns out to be quite ambiguous (Lawrence and Fraser 2020).
In mesostigmatic mites, the higher average divergence level is shown in comparison with all other chelicerates (Klompen et al. 2007). Their significantly faster rates of nucleotide substitution as compared to other parasitiform mites have been suggested to possibly be related to their shorter lifespan and higher metabolic rate (Murell et al. 2005). However, no significant differences in the ITS marker have been revealed between populations of any studied species from the coasts of the Atlantic and Pacific basins. Nowhere did these differences exceed 1%. This genetic integrity was also shown in Phorythocarpais kempersi mites from the White and Black Sea populations, freely crossing and traced to F2 (Avdonin and Striganova 2004). At present, the presence of an original genotype on the shores of the Pacific, distinguished by four substitutions in the ITS fragment, has been proven only for Thinoseius spinosus.
All our studied species are common and widespread inhabitants of seaweeds on the Palaearctic marine coasts. However, they have never been recorded from the coasts of the boreal seas of Central and Eastern Siberia, with the exception of Thinoseius spinosus that also lives in the north of Chukotka (O.L.M.: personal observations). Therefore, a direct contact of their western and eastern Palaearctic populations across the Arctic seaway is currently absent or difficult. Through most of the Pleistocene, opportunities for littoral mite dispersals must have been even worse due to both the repeatedly closing Bering Strait and the severe Arctic climate (Elias and Brigham-Grette 2013). In the case of the rather ''Arctic-adapted'' Phorytocarpais kempersi and Thinoseius spinosus, both currently dwelling on the Barents and Kara sea sides (Makarova 2013; O.L.M.: personal observations), the genetic integrity of their amphi-Palaearctic populations seems to indicate rather recent contacts between them in the north of Eurasia during the periods of warming. Thus, during the last Eemian interglacial (Mikulino) (=MIS 5e; 130,000‒110,000 years ago), the Arctic summer temperatures could have been 4–6 °C higher than now and occasionally the ocean could have been of ice-free (Velichko et al. 1984; Pavlidis et al. 1998; Anderson et al. 2006). During such times, the tundra biome on the arctic shores of most of the Palaearctic could have been replaced by woodlands (Grichuk 2002). Only some 9,200–9,800 years ago, in the early Holocene, the temperatures during the growing season on the Severnaya Zemlya Archipelago, about 78º N, seem to have been at least 4 °C higher than present-day (Andreev et al. 2008).
In the case of the relatively thermophilous Halolaelaps orientalis (still no records in the Arctic), a northern transit seems unlikely. Yet, its ITS genotype recorded from the shores of the Sea of Japan is indistinguishable from the most common variant in the Azov-Black Sea basin. The ancient ocean Paratethys, of which the Caspian and Black seas are derivatives, is known to have lost contact to the Indian Ocean in the mid-Miocene, 12−14 million years ago (Popov et al. 2004; Palcu et al. 2015), and this could be reflected in the genetic structure of amphi-Palaearctic littoral species. We can only assume the existence of genetic exchange in our time between populations of this species on the shores of the seas of the Mediterranean and the Pacific Ocean through the Suez Canal and the coasts of the Indian Ocean. Studies on their intertidal acarofauna have just begun (Kazemi 2020; Moraza et al. 2016). It cannot be excluded that the extensive, transoceanic and polyzonal distribution range of Halolaelaps orientalis might have determined its high general nucleotide and genotypic diversity, 2–3 times as high as the values obtained for the other species (Table 4).
Conclusions
The approach we used (ITS analysis) has allowed us to confidently identify all studied species (P. kempersi, Halolaelaps spp. orientalis-complex, H. celticus and T. spinosus), all separated by long barcode gaps from the nearest known relatives.
A comparative analysis of the population variability of Phorytocarpais kempersi and Halolaelaps orientalis in the regions of the Azov-Black Sea basin and the Caspian Sea basin shows a significantly lower level of intraspecific polymorphism in populations from the latter region. This may be related to long periods of the Caspian Sea isolation from the western seas and Atlantic Ocean during the Pliocene-Pleistocene, coupled with the permanent instability of its size and environments.
The high similarity of the amphi-Palaearctic populations of three of our studied littoral mite species seems to indicate the recent and/or still ongoing genetic exchange between them. In Phorytocarpais kempersi and Thinoseius spinosus, both known from the Arctic, a recent contact along the northern coast of Eurasia is suggested to have occurred during the last Pleistocene interglacial or Holocene climatic optimum. The current distribution of the relatively thermophilous Halolaelaps orientalis may well include the shores of the Indian Ocean.
We believe that this study will contribute to a comprehensive assessment of biological diversity on the Russian and adjacent countries seashores as a whole. The results of the given work can be used in future, more detailed phylogeographic as well as metagenomic bioindication studies.
Acknowledgments
Over many years, we have received additional material from our colleagues and naturalist friends listed in Tables 1–3 (as well as from T.Zh. Ageeva, M.D. Antipova, T.V. Levchenko, E.K. Makarova, V.A. Makarova, S.V. Pavlova, G.S. Potapov, S.B. Rosenfeld, L.B. Volkova), to whom we express our sincere gratitude. We are grateful to the curators of the museum collections, A. Christian (Senckenberg Museum für Naturkunde Görlitz) and W. Knee (Canadian National Collection of Insects, Arachnids and Nematodes), for data on the distribution of model mite species. We are also thankful to our colleagues who have helped us in searching for information (R.B. Halliday, O. Joharchi, S. Kazemi, A.A. Kotov, G.W. Krantz, E.E. Lindquist, K.V. Makarov, A.A. Makhrov, I.N. Marin, D.M. Palatov, P.N. Petrov) and editing the text (S.I. Golovatch, M.S. Bizin, K.V. Makarov). We received full support from the direction and rangers of the Dagestan State Nature Reserve (deputy director for research: G.S. Dzhamirzoev), the ''Akzhaiyk'' State Nature Reserve, the Republic of Kazakhstan (director: A.M. Karabaliev, ranger: R. Zhienkulov), as well as Yu.V. Babichev, a ranger of the State ''Black Earth'' Biosphere Nature Reserve. Lidia E. Zavyazkina accompanied the second author during most of the Caspian samplings and helped in every way. This research was supported by a grant from the Russian Foundation for Basic Research (No. 20-54-56054 Iran_t), as well as by a budgetary topic of the IOGEN RAS.
References
- Anderson P., Bennike O., Bigelow N., Brigham-Grette J., Duvall M., Edwards M., Fréchette B., Funder S., Johnsen S., Knies J., Koerner R., Lozhkin A., Marshall S., Matthiessen J., Macdonald G., Miller G., Montoya M., Muhs D., Otto-Bliesner B., Overpeck J., Reeh N., Petter Sejrup H., Spielhagen R., Turner C., Velichko A. 2006. Last interglacial arctic warmth confirms polar amplification of climate change. Quat. Sci. Rev., 25(13-14): 1383-1400. https://doi.org/10.1016/j.quascirev.2006.01.033
- Andreev A.A., Lubinski D.J., Bobrov A.A., Ingólfsson Ó., Forman S.L., Tarasov P.E., Möller P. 2008. Early Holocene environments on October Revolution Island, Severnaya Zemlya, Arctic Russia. Palaeogeography, Palaeoclimatology, Palaeoecology, 267(1-2): 21-30. https://doi.org/10.1016/j.palaeo.2008.05.002
- Arslanov K.A., Yanina T.A., Chepalyga A.L., Svitoch A.A., Makshaev R.R., Maksimov F.E., Chernov S.B., Tertychniy N.I., Starikova A.A. 2016. On the age of the Khvalynian deposits of the Caspian Sea coasts according to 14C and 230Th/234U methods. Quat. Int., 409: 81-87. https://doi.org/10.1016/j.quaint.2015.05.067
- Artamonova V.S., Bizin M.S., Efeykin B.D., Makarova O.L. 2023. Two lineages of oribatid mites morphologically correspond to the circumpolar species Ameronothrus nigrofemoratus (Acari, Oribatida) but differ genetically as distinct species are revealed on the Kolguev Island. Dokl. Biol. Sci., 512(1): 321-325. https://doi.org/10.1134/S0012496623700631
- Avdonin V.V. 1999. Supralittoral Microarthropods of the Black Sea Coast in the Crimea. Moscow: Graduate work, Entomology Dep., Lomonosov Moscow State University, pp. 71. [In Russian]
- Avdonin V.V. 2002. Trophic preferences of maritime gamasid mites (Acarina, Mesostigmata). In: Striganova B.R. (Eds.). Problems of Soil Zoology. Biodiversity and Life of the Soil Systems: Proc. of the 3rd (13th) All-Russian Workshop of Soil Zoology. Yoshkar-Ola: KMK Scientific Press. p. 6-7. [In Russian]
- Avdonin V.V., Striganova B.R. 2004. Temperature as a factor of niche separation in free-living mesostigmatid mites (Mesostigmata: Arachnida, Parasitiformes) of storm detritus. Izv. Akad. Nauk. Ser. Biol., 5: 589-596. [In Russian] https://doi.org/10.1023/B:BIBU.0000043777.35512.f7
- Bizin M.S., Makarova O.L. 2022. The first data on mesostigmatic mite assemblages (Parasitiformes, Mesostigmata) from a coastal area of the Eastern Black Sea region (Abrau Peninsula, Krasnodar Territory). Entomol. Rev., 102(2): 264-277. https://doi.org/10.1134/S0013873822020129
- Błaszak C., Ehrnsberger R. 1997. Halolaelaps (Halolaelaps) rafaljanus sp. nov. (Acari, Gamasida: Halolaelapidae) eine neue Art von der Nordseeküste Deutschlands. Genus, 8: 3-7. Available from: https://archive.org/details/genus-0867-1710-8-1-003-007
- Błaszak C., Ehrnsberger R. 1998. Beiträge zur Kenntnis von Halolaelaps (Halolaelaps s. str.) (Acari: Gamasida: Halolaelapidae). Osnabrücker Naturwissenschaftliche Mitteilungen, 24: 159-181. Available from: http://publikationen.ub.uni-frankfurt.de/frontdoor/index/index/docId/25845
- Błaszak C., Ehrnsberger R., Michalik J. 2004. Die Milben in der Zoologischen Staatssammlung München. Part 5. Genus Halolaelaps Berlese & Trouessart, 1889 (Acari: Gamasida, Halolaelapidae). Spixiana, 27: 1-13.
- Bolger T., Arroyo J., Piotrowska K. 2018. A catalogue of Mesostigmata (Arachnida, Acari, Parasitifirmes) recorded from Ireland including information on their geographical distribution and habitats. Zootaxa, 4519(1): 1-220. https://doi.org/10.11646/zootaxa.4519.1.1
- Bregetova N.G. 1977. The family Eviphididae Berlese, 1913. In: Ghilyarov M.S., Bregetova N.G (Eds). Key to the Soil Inhabiting Mites. Mesostigmata. Leningrad: Nauka. p. 554-569. [In Russian]
- Briggs J.C. 1974. Marine Zoogeography. New York: McGraw-Hill Book Company. pp. 480. https://doi.org/10.2307/1442613
- Clement M., Posada D., Crandall K.A. 2000. TCS: a computer program to estimate gene genealogies // Mol. Ecology, 9: 1657-1659. https://doi.org/10.1046/j.1365-294x.2000.01020.x
- Coop G.R. 2004. Several million years of stability among insect species because of, or in spite of, Ice Age climatic instability? Phil. Trans. R. Soc. Lond. B, 359: 209-214. https://doi.org/10.1098/rstb.2003.1393
- Copilaș-Ciocianu D., Sidorov D. 2022. Taxonomic, ecological and morphological diversity of Ponto-Caspian gammaroidean amphipods: a review. Organisms Diversity & Evolution, 22: 285-315. https://doi.org/10.1007/s13127-021-00536-6
- Dabert M., Witalinski W., Kazmierski A., Olszanowski Z., Dabert J. 2010. Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Molecular Phylogenetics and Evolution, https://doi.org/10.1016/j.ympev.2009.12.020
- Dowling A.P.G., O'Connor B.M. 2010. Phylogenetic relationships within the suborder Dermanyssina (Acari: Parasitiformes) and a test of dermanyssoid monophyly. Int. J. Acarol., 36: 299-312. https://doi.org/10.1080/01647951003604569
- Egglishaw H. 1966. The phoretic habit of the mite Thinoseius fucicola (Halbert) (Acari Eviphididae). Entomol. Mon. Mag., 102: 12-14.
- Elias S.A., Brigham-Grette J. 2013. Glaciations: Late Pleistocene glacial events in Beringia. In: Elias S. (Eds.). Encyclopedia of Quaternary Science. 2nd ed. Amsterdam: Elsevier. p. 191-201. https://doi.org/10.1016/B978-0-444-53643-3.00116-3
- Evans G.O. 1962. The systematic position of Gammaridacarus brevisternalis Canaris (Acari, Mesostigmata). Ann. Mag. Nat. Hist., 5: 395-399. https://doi.org/10.1080/00222936208651263
- Evans G.O. 1969. A new mite of the genus Thinoseius Halbt. (Gamasina: Eviphididae) from the Chatham Islands, New Zealand. Acarologia, 11: 505-514.
- Evans G.O., Till W.M. 1979. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes). An introduction to their external morphology and classification. Trans. Zool. Soc. Lond., 35: 139-270. https://doi.org/10.1111/j.1096-3642.1979.tb00059.x
- Forgie S.A., St John M.G., Wiser S.K. 2013. Invertebrate communities and drivers of their composition on gravel beaches in New Zealand. New Zealand Journal of Ecology, 37(1): 95-104.
- Gelfan A., Panin A., Kalugin A., Morozova P., Semenov V., Sidorchuk A., Ukraintsev V., Ushakov K. 2024. Hydroclimatic processes as the primary drivers of the Early Khvalynian transgression of the Caspian Sea: new developments. Hydrol. Earth Syst. Sci., 28: 241-259. https://doi.org/10.5194/hess-28-241-2024
- Gowan E.J., Zhang X., Khosravi S., Rovere A., Stocchi P., Hughes A.L., Gyllencreutz R., Mangerud J., Svendsen J.I., Lohmann G. 2021. A new global ice sheet reconstruction for the past 80 000 years. Nature Communications, 12: 1-9. https://doi.org/10.1038/s41467-021-21469-w
- Grichuk V.P. 2002. Late Pleistocene vegetation. In: Velichko A.A. (Ed.). Dynamics of Terrestrial Landscape Components and Inland and Marginal Seas of Northern Eurasia During the Last 130 000 Years. Moscow: GEOS. p. 64-88. [In Russian]
- Gwiazdowicz D.J., Coulson S.J. 2010. First record of Thinoseius spinosus (Acari, Eviphididae) from the High Arctic Island of Spitsbergen (Svalbard) including a key to deutonymphs of genus Thinoseius. Int. J. Acarol., 36: 233-236. https://doi.org/10.1080/01647951003598589
- Halbert J.N. 1920. The Acarina of the seashore. Proc. R. Ir. Acad., 35B: 106-152.
- Halliday R.B. 2010. Revision of the Australian Eviphididae (Acari: Mesostigmata). Zootaxa, 2596: 1-60. https://doi.org/10.11646/zootaxa.2596.1.1
- Haynert K., Kiggen M., Klarner B., Maraun M., Scheu S. 2017. The structure of salt marsh soil mesofauna food webs - The prevalence of disturbance. PLoS One, 12(12), e0189645: 1-20. https://doi.org/10.1371/journal.pone.0189645
- Heptner V.G. 1936. General Zoogeography. Moscow - Leningrad: Gosudarstvennoe Izdatelstvo Biologicheskoj i Meditsinskoj Literatury. pp. 548. [In Russian]
- Hirschmann W. 1966. Gangsystematik der Parasitiformes. Teil 15. Gänge von Litoralmilben und neue Litoralmilben Arten. Acarologie, Schriftenreihe für Vergleichende Milbenkunde, 9: 25-44.
- Hyatt K. H. 1956. British Mites of the Genera Halolaelaps Berlese & Trouessart and Saprolaelaps Leitner (Gamasina-Neoparasitidae). Entom. Gaz., 7: 7-26.
- Kazemi S. 2020. A new species of Gaeolaelaps Evans and Till (Mesostigmata: Laelapidae) from mangrove forests in the Persian Gulf, and notes on gnathosomal structures of the genus and other laelapid genera. Int. J. Acarol., 46: 130-139. https://doi.org/10.1080/01647954.2020.1737223
- Klimov P.B. 1998. To the knowledge of mites and ticks (Acari) of Kuril Islands. Far East Entomol., 36: 1-36.
- Klompen H., Lekveishvili M., Black W.C. 4th. 2007. Phylogeny of parasitiform mites (Acari) based on rRNA. Mol. Phylogenet. Evol., 43(3): 936-51. https://doi.org/10.1016/j.ympev.2006.10.024
- Kopf A. 2002. Significance of mud volcanism. Reviews of Geophysics, 40: 1-49. https://doi.org/10.1029/2000RG000093
- Koyumdzhijeva M.I. 1982. The gamasoid mites (Gamasoidea, Parasitiformes) from the wrack of the Bulgarian Black Sea coast. Acta Zool. Bulg., 20: 77-80. [In Bulgarian]
- Koriche S.A., Singarayer J.S., Cloke H.L., Valdes P.J., Wesselingh F.P., Kroonenberg S.B., Wickert A.D., Yanina T.A. 2022. What are the drivers of Caspian Sea level variation during the late Quaternary? Quat. Sci. Rev., 283: 107457. https://doi.org/10.1016/j.quascirev.2022.107457
- Krantz G. 1974. Phaulodinychus mitis (Leonardi, 1899) (Acari: Uropodidae) an intertidal mite exhibiting plastron respiration. Acarologia, 16: 11-20.
- Krantz G.W. 2016. A new species of Halolaelapidae (Acari: Mesostigmata: Rhodacaroidea) from beach wrack in Yaquina Bay, Oregon, USA, with comments on opisthonotal plasticity and cribral development in the family. Journal of Natural History, 50(29-30). https://doi.org/10.1080/00222933.2016.1170904
- Krijgsman W., Tesakov A., Yanina T., Lazarev S., Danukalov G., Van Baak C.G.C., Agustí J., Alçiçek M.C., Aliyeva E., Bista D., Bruch A., Büyükmeriç Y., Bukhsianidze M., Flecker R., Frolov P., Hoyle T.M., Jorissen E.L., Kirscher U., Koriche S.A., Kroonenberg S.B., Wesselingh F.P. 2019. Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution. Earth-Science Reviews, 188: 1-40. https://doi.org/10.1016/j.earscirev.2018.10.013
- Kumar S., Stecher G., Tamura K. MEGA7: 2016. Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol., 33: 1870-1874. https://doi.org/10.1093/molbev/msw054
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35(1): 1547-1549. https://doi.org/10.1093/molbev/msy096
- Kurbanov R.N., Belyaev V.R., Svistunov M.I., Butuzova E.A., Solodovnikov D.A., Taratunina N.A., Yanina T.A. 2023. New data on the age of the Early Khvalynian transgression of the Caspian Sea. Izvestiya Rossiiskoi Akademii Nauk. Seriya Geograficheskaya, 87: 403-419. [In Russian]. https://doi.org/10.31857/S2587556623030081
- Lawrence E.R., Fraser D.J. 2020. Latitudinal biodiversity gradients at three levels: Linking species richness, population richness and genetic diversity. Global Ecol. Biogeogr., 29(5). https://doi.org/10.1111/geb.13075
- Leigh J.W., Bryant D., Nakagawa S. 2015. PopArt: full-feature software for haplotype network construction. Methods Ecol. Evol., 6: 1110-1116. https://doi.org/10.1111/2041-210X.12410
- Lindo Z. 2020. Transoceanic dispersal of terrestrial species by debris rafting. Ecography, 43: 1364-1372. https://doi.org/10.1111/ecog.05155
- Lindquist E.E., Krantz G.W. & Walter D.E. 2009. Order Mesostigmata. In: Krantz, G.W. & Walter, D.E. (Eds.) A Manual of Acarology, 3rd Edition. Texas Tech University Press, Texas, pp. 124-232.
- Lindroth C.H., Andersson H., Bödvarsson H., Richter S.H. 1973. Surtsey, Iceland: The Development of a New Fauna, 1963-1970. Terrestrial Invertebrates. Entomologica Scandinavica, Supplementum, 5: 1-280. Available from: https://biostor.org/reference/283142
- Luxton M. 1967. The ecology of saltmarsh Acarina. J. Anim. Ecol., 36: 257-277. https://doi.org/10.2307/2911
- Makarova O.L., Petrova-Nikitina A.D. 2008. Successions of gamasid mites (Parasitiformes, Mesostigmata) in seaweed accumulations on the littoral of the White Sea (Velikaya Salma strait). In: Kirpichnikov, M.P. (Eds.). Conference to the 70th anniversary of the N.A. Pertsov White Sea Biological Station of Moscow University. Moscow: Grif i K. p. 75-79 [In Russian]
- Makarova O.L., Böcher J. 2009. Diversity and geographical ranges of Greenland mites (Acari: Oribatida and Mesostigmata). In: Golovatch S.I., Makarova O.L., Babenko A.B., Penev L.D. (Eds). Species and Communities in Extreme Environments. Sofia - Moscow: Pensoft Publ. & KMK Sci. Press. p. 165-186.
- Makarova O.L. 2013. Gamasid mites (Parasitiformes, Mesostigmata) of the European Arctic and their distribution patterns. Entom. Rev. 93(1). 113-133. https://doi.org/10.1134/S0013873813010156
- Makarova O. 2015. Acari. In: Böcher J.J., Kristensen N.P., Pape T., Vilhelmsen L. (Eds). The Greenland Entomofauna. An Identification Manual of Insects, Spiders and their Allies. Leiden: Koninklijke Brill NV. 44: 705-856.
- Makarova O.L. 2019. North Pacific versus North Atlantic: a case with species of the amphiboreal littoral mite genus Thalassogamasus gen. n. (Parasitifomes, Mesostigmata, Parasitidae). Zootaxa, 4647: 457-485. https://doi.org/10.11646/zootaxa.4647.1.29
- Makarova O.L., Bizin M.S. 2020. Littoral mesostigmatic mites (Acari, Parasitiformes) from the Kola Peninsula. Polar Biol., 43: 1503-1518. https://doi.org/10.1007/s00300-020-02724-0
- Makiola A., Compson Z.G., Baird D.J., Barnes M.A., Boerlijst S.P., Bouchez A., Brennan G., Bush A., Canard E., Cordier T., Creer S., Curry R.A., David P., Dumbrell A.J., Gravel D., Hajibabaei M., Hayden B., van der Hoorn B., Jarne P., Jones J.I., Karimi B., Keck F., Kelly M., Knot I.E., Krol L., Massol F., Monk W.A., Murphy J., Pawlowski J., Poisot T., Porter T.M., Randall K.C., Ransome E., Ravigné V., Raybould A., Robin S., Schrama M., Schatz B., Tamaddoni-Nezhad A., Trimbos K.B., Vacher C., Vasselon V., Wood S., Woodward G., Bohan D.A. 2020. Key qestions for next-generation biomonitoring. Front. Environ. Sci., 7: 197. https://doi.org/10.3389/fenvs.2019.00197
- Makshaev R.R., Tkach N.T. 2023. Chronology of Khvalynian Stage of the Caspian Sea according to radiocarbon dating. Geomorfologiya i Paleogeografiya, 54: 37-54. [In Russian] https://doi.org/10.31857/S2949178923010103
- Mangerud J., Alexanderson H., Birks H., Paus A., Perić Z.M., Svendsen J.I. 2023. Did the Eurasian ice sheets melt completely in early Marine Isotope Stage 3? New evidence from Norway and a synthesis for Eurasia. Quat. Sci. Rev., 311:108136. https://doi.org/10.1016/j.quascirev.2023.108136
- Maniatis T., Fritsch E.F., and Sambrook J. 1982. Molecular Cloning: a Laboratory Manual, Cold Spring Harbor: Cold Spring Harbor Lab., pp. 545
- Maslov S.I. 2013. Mites of the genus Halolaelaps (Acari: Mesostigmata: Halolaelapidae) inhabiting washed-up seaweeds in the protected areas of Crimea. In: Proc. of the Int. conf. dedicated to the 40th anniversary of the Cape Martyan Nature Reserve. Yalta. p. 136. [In Russian]
- Moraza M.L., Kontschán J., Sahoo G., Ansari Z. 2016. A new species of Eutrachytes (Acari: Uropodina: Eutrachytidae) associated with the Indian mangrove (Avicennia officinalis). Acarologia, 56(1): 73-89. https://doi.org/10.1051/acarologia/20162189
- Mordukhai-Boltovskoi F.D. 1979. Composition and distribution of Caspian fauna in the light of modern data. Internationale Revue der gesamten Hydrobiologie und Hydrographie, 64(1): 1-38. https://doi.org/10.1002/iroh.19790640102
- Murrell A., Dobson S.J., Walter D.E., Campbell N.J.H., Shao R., Barker S.C. 2005. Relationships among the three major lineages of the Acari (Arthropoda: Arachnida) inferred from small subunit rRNA: paraphyly of the Parasitiformes with respect to the Opilioacariformes and relative rates of nucleotide substitution. Invertebrate Systematics, 19(5): 383-389. https://doi.org/10.1071/IS05027
- Naseka A.M., Bogutskaya N.G. 2009. Fishes of the Caspian Sea: Zoogeography and updated check-list. Zoosystematica Rossica, 18(2): 295-317. https://doi.org/10.31610/zsr/2009.18.2.295
- Palcu D.V., Tulbure M., Bartol M., Kouwenhoven J.J., Krijgsman W. 2015. The Badenian-Sarmatian extinction event in the Carpathian foredeep basin of Romania: Paleogeographic changes in the Paratethys domain. Global and Planetary Change, 133: 346-358. https://doi.org/10.1016/j.gloplacha.2015.08.014
- Pavlidis Yu.A., Ionin A.S., Scherbakov F.A., Dunaev N.N., Nikiforov S.L. 1998. The Arctic Shelf. Late Quaternary History as a Basis of the Forecast of Development. Moscow: GEOS. pp. 184. [In Russian]
- Peck S.B. 1994. Sea-surface (pleuston) transport of insects between islands in the Galápagos Archipelago, Ecuador. Entomol. Soc. Am., 87(5): 576-582. https://doi.org/10.1093/aesa/87.5.576
- Pérez-Sayas C., Pina T., Sabater-Muñoz B., Gómez-Martínez M.A., Jaques J.A., Hurtado-Ruiz M.A. 2022. DNA Barcoding and Phylogeny of Acari Species Based On ITS and COI Markers. Journal of Zoological Systematics and Evolutionary Research, Article ID 5317995. https://doi.org/10.1155/2022/5317995
- Pfingstl T., Lienhard A., Shimano S., Yasin Z.B., Shau-Hwai A.T., Jantarit S., Petcharad B. 2018. Systematics, genetics and biogeography of intertidal mites (Acari, Oribatida) from the Andaman Sea and Strait of Malacca. J. Zool. Syst. & Evol. Res., 57(1): 1-22. https://doi.org/10.1111/jzs.12244
- Pfingstl T., Lienhard A., Baumann J. 2019a. New and cryptic species of intertidal mites (Acari, Oribatida) from the Western Caribbean - an integrative approach, Int. J. Acarol., 45(1-2): 10-25. https://doi.org/10.1080/01647954.2018.1532458
- Pfingstl T., Baumann J., Lienhard A. 2019b. The Caribbean enigma: the presence of unusual cryptic diversity in intertidal mites (Arachnida, Acari, Oribatida). Organisms Diversity & Evolution, 19(4): 609-623. https://doi.org/10.1007/s13127-019-00416-0
- Pfingstl T., Schȁffer S., Bardel-Kahr I., Baumann J. 2022. A closer look reveals hidden diversity in the intertidal Caribbean Fortuyniidae (Acari, Oribatida). PLoS ONE, 17: e0268964. https://doi.org/10.1371/journal.pone.0268964
- Pjatakova G.M., Tarasov A.G. 1996. Caspian Sea amphipods: Biodiversity, systematic position and ecological peculiarities of some species. Int. J. Salt Lake Res., 5(1): 63-79. https://doi.org/10.1007/BF01996036
- Popov S.V., Roegl F., Rozanov A.Y., Steininger F.F., Shcherba I.G., Kovac M. 2004. Lithological-paleogeographic maps of Paratethys. CFS Courier Forschungsinstitut Senckenberg, 250: 1-46.
- Procheş Ş., Marshall D.J. 2001. Global distribution patterns of nonhalacarid marine intertidal mites: Implications for their origins in marine habitats. J. Biogeogr., 28(1): 47-58. https://doi.org/10.1046/j.1365-2699.2001.00513.x
- Pugh P.J.A., King P.E. 1985. The vertical distribution of the British intertidal Acari – the non halacarid fauna (Arachnida: Acari). J. Zool. 207(1): 21-37. https://doi.org/10.1111/j.1469-7998.1985.tb04912.x
- Pugh P.J.A., King P.E., Fordy M.R. 1987. Structural features associated with respiration in some intertidal Uropodina (Acarina: Mesostigmata). J. Zool., 211(1): 107-120. https://doi.org/10.1111/j.1469-7998.1987.tb07456.x
- Pugh P.J.A., King P.E. 1988. Acari of the British supralittoral. J. Nat. Hist., 22: 107-122. https://doi.org/10.1080/00222938800770081
- Pugh P.J.A., Llewellyn P.J., Robinson K., Shackley S.E. 1997. The associations of phoretic mites (Acarina: Chelicerata) with sand-hoppers (Amphipoda: Crustacea) on the South Wales coast. J. Zool., 243(2): 305-318. https://doi.org/10.1111/j.1469-7998.1997.tb02784.x
- Remmert H. 1956. Der Strandanwurf als Lebensraum für Thinoseius fucicola (Halbert) (Acarina). Zeitschrift für Morphologie und Őkologie der Tiere, 45: 146-156. https://doi.org/10.1007/BF00408892
- Rigby M.C. 1996a. The epibionts of beach hoppers (Crustacea: Talitridae) of the North American Pacific coast. J. Nat. Hist., 30(9): 1329-1336. https://doi.org/10.1080/00222939600771241
- Rigby M.C. 1996b. Association of a juvenile phoretic uropodid mite with the beach hopper Traskorchestia traskiana (Stimpson, 1857) (Crustacea: Talitridae). J. Nat. Hist., 30(11): 1617-1624. https://doi.org/10.1080/00222939600770941
- Rögl F. 1999. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview). Geol. Carpath., 50(4): 339-349.
- Rosenberg Y., Bar-On Y.M., Fromm A., Ostikar M., Shoshany A., Giz O., Milo R. 2023. The global biomass and number of terrestrial arthropods. Sci. Adv., 9(5): 1-12. https://doi.org/10.1126/sciadv.abq4049
- Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J. C., Guirao-Rico S., Librado P., Ramos-Onsins S. E., Sánchez-Gracia A. 2017. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets // MBE., 34.(12): 3299-3302. https://doi.org/10.1093/molbev/msx248
- Rychagov G.I. (Eds.). 1997. Pleistocene History of the Caspian Sea. Moscow: Moscow State University. pp. 267. [In Russian]
- Rychagov G.I. 2011. Fluctuations of the Caspian Sea level: causes, effects, forecast. Vestnik Moskovskogo universiteta. Seria 5, Geographiya, 2: 4-12. [In Russian]
- Schatz H. 1991. Arrival and establishment of Acari on oceanic islands. In: Dusbábek F., Bukva V. (Eds). Modern Acarology. Vol. 2. Prague: Academia Prague and SPB Academic Publishing, The Hague. p. 613-618.
- Schulte G. 1975. Holarktische Artareal der Ameronothridae (Acari, Oribatei). Veröff. Inst. Meeresforsch. Bremerhaven, 15: 339-357.
- Schuster R. 1979. Soil mites in the marine environment. Recent Advances in Acarology, 1: 593-602. https://doi.org/10.1016/B978-0-12-592201-2.50084-1
- Sorokin V.M. 2011. Correlation of the upper Quaternary deposits and paleogeography of the Black Sea and the Caspian Sea. Stratigr. Geol. Correl., 19(5): 563-578. https://doi.org/10.1134/S086959381104006X
- Sourassou N.F., Moraes G.J. de, Delalibera Jr. I., Correa A.S. 2015. Phylogenetic analysis of Ascidae sensu lato and related groups (Acari: Mesostigmata: Gamasina) based on nuclear ribosomal DNA partial sequences. Systematic and Applied Acarology, 20(3): 225-240. https://doi.org/10.11158/saa.20.3.1
- Strenzke K. 1963. Die Arthropodensukzession im Strandanwurf mariner Algen unter experimentell kontrollierten Bedingungen. Pedobiologia, 3: 95-141. https://doi.org/10.1016/S0031-4056(22)00123-8
- Svitoch A.A. 2008. Quaternary paleogeography of the Aazov-Black Sea basin. Handb. Environ. Chem., 5: 31-46. https://doi.org/10.1007/698_5_079
- Svitoch A.A., Yanina T.A., Novikova N.G., Sobolev V.M., Khomenko A.A. 2010. The Pleistocene of the Manych (Structure and Evolution): Questions of Structure and Development. Moscow: MSU Press. pp. 135. [In Russian]
- Svitoch A.A. 2013. The Pleistocene Manych straits: their structure, evolution and role in the Ponto-Caspian basin development. Quat. Int., 302: 101-109. https://doi.org/10.1016/j.quaint.2012.03.015
- Takaku G. 2000. Two new mite species of the genus Thinoseius (Acari: Gamasida: Eviphididae) from Japan. Species Diversity, 5(4): 361-374. https://doi.org/10.12782/specdiv.5.361
- Thompson J.D., Higgins D.G., Gibson T.J. 1994. Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research., 22(22): 4673-4680. https://doi.org/10.1093/nar/22.22.4673
- Tikhomirov S.I. 1977. The family Parasitidae Oudemans, 1901. In: Ghilyarov M.S., Bregetova N.G (Eds). Key to the Soil Inhabiting Mites. Mesostigmata. Leningrad: Nauka. p. 55-107. [In Russian]
- Trach V.A. 2016. New and little known species of Halolaelaps (Acari: Mesostigmata: Halolaeapidae) from Ukraine. Zootaxa, 4154: 436-452. https://doi.org/10.11646/zootaxa.4154.4.4
- Tudryn A., Chalié F., Lavrushin Yu.A., Antipov M.P., Spiridonova E.A., Lavrushin V.Yu, Tucholka P., Leroy S.A.G. 2013. Late Quaternary Caspian Sea environment: Late Khazarian and Early Khvalynian transgressions from the lower reaches of the Volga River. Quat. Int., 292: 193-204. https://doi.org/10.1016/j.quaint.2012.10.032
- van Baak C.G.C., Stoica M., Grothe A., Aliyeva E., Krijgsman W. 2016. Mediterranean-Paratethys connectivity during the Messinian salinity crisis: the Pontian of Azerbaijan. Global Planet. Change, 141: 63-81. https://doi.org/10.1016/j.gloplacha.2016.04.005
- Velichko A.A., Barash M.S., Gritchuk V.P. 1984. Northern hemisphere climate in last interglacial. USSR AS News: Geography, 1: 5-18. [In Russian]
- Volkinburg D.V. 1969. Thinoseius spinosus found in new and unusual habitat (Acari: Eviphididae). Pan-Pacific Entomol., 45: 318-319.
- Wesselingh F.P., Neubauer T.A., Anistratenko V.V., Vinarski M.V., Yanina T., ter Poorten J.J., Kijashko P., Albrecht C., Anistratenko O.Yu., D'Hont A., Frolov P., Gándara A.M., Gittenberger A., Gogaladze A., Karpinsky M., Lattuada M., Popa L., Sands A.F., van de Velde S., Vandendorpe J., Wilke T. 2019. Mollusc species from the Pontocaspian region - An expert opinion list. ZooKeys, 827: 31-124. https://doi.org/10.3897/zookeys.827.31365
- White T.J., Bruns T., Lee S., Taylor J. 1990. 38 - Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., et al. (Eds), PCR Protocols, a Guide to Methods and Applications. San Diego: Academic Press, p. 315-322. doi: https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- White J.R., Maddox C., White O., Angiuoli S.V., Fricke W.F. 2013. CloVR-ITS: automated internal transcribed spacer amplicon sequence analysis pipeline for the characterization of fungal microbiota. Microbiome, 1: 1-11. https://doi.org/10.1186/2049-2618-1-6
- Willmann C. 1952. Die Milbenfauna der Nordseeinsel Wangerooge. Veröffent. Inst. Meeresforsch. Bremerhaven, 1: 139-186.
- Zhao Y., Zhang W.Y., Wang R.L. Niu D.L. 2020. Divergent domains of 28S ribosomal RNA gene: DNA barcodes for molecular classification and identification of mites. Parasites Vectors, 13(251): 2-12. https://doi.org/10.1186/s13071-020-04124-z



2024-07-04
Date accepted:
2024-11-05
Date published:
2024-11-26
Edited by:
Minor, Maria

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Andrianov, Boris V.; Makarova, Olga L. and Goryacheva, Irina I.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



