Peacock mites on cannon-ball tree, Couroupita guianensis (Lecythidaceae) in the Southeast Region of Brazil
Escobar-Garcia, Hector Alonso  1
; de Andrade, Daniel Júnior
1
; de Andrade, Daniel Júnior  2
; Beard, Jennifer J.
2
; Beard, Jennifer J.  3
and Ochoa, Ronald
3
and Ochoa, Ronald  4
4
1✉ São Paulo State University (UNESP), School of Agricultural and Veterinary Sciences, Jaboticabal, Brazil & Facultad de Agronomía, Universidad Nacional de Piura (UNP), Piura, Peru.
2São Paulo State University (UNESP), School of Agricultural and Veterinary Sciences, Jaboticabal, Brazil.
3Queensland Museum, P.O. Box 3300, South Brisbane, Qld 4101, Australia.
4Systematic Entomology Laboratory, United States Department of Agriculture, Agricultural Research Service, Beltsville, MD 20705, USA.
2024 - Volume: 64 Issue: 4 pages: 1138-1148
https://doi.org/10.24349/a2ld-gvdpOriginal research
Keywords
Abstract
Introduction
The peacock mites, Tuckerella ornata (Tucker) and Tuckerella knorri Baker & Tuttle (Acari: Tetranychoidea: Tuckerellidae), are found in many parts of the world and have been collected from a wide variety of taxonomically diverse host plants. To date, these two mites have been documented in three South American countries: Brazil (Flechtmann 1979; Barbosa et al. 2003; Da Silva et al. 2023; Melo et al. 2024), Venezuela (Camacho et al. 2002), and Peru (Escobar-Garcia et al. 2021a). They are both small, reddish-orange mites that feed on fruit and the bark of stems and branches. They are obvious to the naked eye with their large broad white setae contrasting against their red bodies. In addition, they have a row of long flagellate setae along their posterior margin which makes them easily identifiable (Ewing 1922; Ochoa 1989; Beard and Walter 2005; Escobar-Garcia et al. 2021a; Escobar-Garcia et al. 2022). They move slowly and are frequently observed resting on the fruit rind or in depressions and cracks in the bark of their host plant. These mites are able to puncture exposed green periderm tissue of tree branches with their paired stylet mouthparts for feeding (Corpuz-Raros 2001; Beard et al. 2012; Childers et al. 2018; Escobar-Garcia et al. 2021a; Escobar-Garcia et al. 2022; Da Silva et al. 2023). These mites are playing an increasingly significant role in quarantine and agriculture as pests of economic importance, as our understanding of their biology, distribution, and ecology expands (Escobar-Garcia et al. 2021a; Escobar-Garcia et al. 2022; Da Silva et al. 2023). It should be noted that not only are these two mites known to occur outside of the Americas, but a sizable portion of their host plants across the world are native to the Americas, including cocoa (Flechtmann 1979; Escobar-Garcia et al. 2021a; Da Silva et al. 2023), guava (Camacho et al. 2002), and Barbados Cherry (Barbosa et al. 2003).
The tree Couroupita guianensis Aubl., a member of the endemic South America family Lecythidaceae, grows quickly and can reach heights of up to 35 meters. Known as the ''cannon-ball tree'' due to shape of its fruit, the tree is naturally distributed across northwestern South America. Known in Brazil as ''macacarecuia'', ''abricó-de-macaco'', and ''castanha-de-macaco'', it is a large deciduous tropical tree that is popular for its ornamental and medicinal uses. The fruits are consumed locally (Nelson et al. 1937; Kavitha et al. 2011; Uppala et al. 2016) and used in preparation of medicinal drinks (Pinheiro et al. 2013) to relieve symptoms of colds, and head and stomach aches. Due to the antibacterial and antifungal properties, preparations are also used to treat wounds (Prabhu and Ravi 2017; Reshma et al. 2018; Sheba and Anuradha 2020). With large, beautiful dark reddish-pink and white flowers and pleasant fragrance, it is frequently planted alongside roads and in gardens (Mori et al. 2007; Rajput and Patil 2016; Raghavendra et al. 2017; Sheba and Anuradha 2020). The alternate, oblong or oblong-ovate leaves are grouped at the tips of the branches, are rounded to obtuse at the tip and attenuate at the base, with a short petiole. The inflorescence is a cauliflorous raceme that develops from the trunk and lower branches, and in Southeast Brazil flowering occurs primarily in the spring. Fruits are firm on the outside, brown, and globose, measuring up to 25 cm in diameter and weighing up to 16 kg each. Pulp fills the entire shell and the external colour reflects the level of maturity. The fruit has a white, vinous, and acidic pulp with many seeds (200–300). When the creamy pulp is exposed to air, it oxidizes and turns bluish-green, and has a foul odour due to a high concentration of sulphur compounds (Rajput and Patil 2016; Raghavendra et al. 2017; Sheba and Anuradha 2020). Despite this, the tree has significant economic, social and environmental value, as products derived from the leaves, flowers and bark are used in pest and disease control, in addition to ethnomedicine (Pinheiro et al. 2010; Baskar et al. 2015; Anu and Mendhulkar 2015; Raghavendra et al. 2017; Sheba and Anuradha 2020; Ferreria et al. 2021; Babu et al. 2022; Esposito et al. 2023; Escobar-Garcia et al. 2024a; CABI 2024). Here, two peacock mite species and one flat mite are reported on C. guianensis in Brazil for the first time.
Material and methods



Mites were collected every two weeks in the spring and summer of 2022 and 2023 from four C. guianensis trees on the campus of São Paulo State University (UNESP), Jaboticabal, Brazil (21°14′50.28″ S, 48°17′51.85″ W; 588 m a.s.l.) (Fig. 1). According to the international Köppen climate classification, the region is a humid subtropical zone with dry winters and hot summers Cwa (Beck et al. 2018). The temperature, relative humidity, and rainfall parameters utilized in this study were sourced from a database belonging to the Agroclimatological Station - Department of Exact Sciences, UNESP, Jaboticabal, São Paulo, Brazil (21°14′05″ S, 48°17′09″ W; 615 m.a.s.l.). The sampling process for each tree consisted of a random collection of 20 leaves, two raceme-type inflorescences, and fruits: five immature fruits in the spring and two developed fruits in the summer. Samples were kept in separate paper bags and transported to the Acarology laboratory (AcaroLab) at the UNESP. A Berlese funnel was used to extract mites from the developed fruits collected in summer. Mites were collected using a fine-tipped brush under an Olympus SZ61 stereomicroscope. They were then mounted dorsoventrally on microscope slides in Hoyer's media and baked for 7 days at 50 °C (Walter and Krantz 2009). Finally, the slides were sealed with nail polish. Mite morphology was studied using a Nikon Eclipse E200 phase-contrast compound microscope with an ocular micrometer, and a variety of taxonomic keys (Chant 1959; Denmark and Muma 1970; Meyer and Ueckermann 1997; Matioli et al. 2002; Beard et al. 2015; Da Silva et al. 2016; Rehman et al. 2018; Mirza et al. 2022; Lofego et al. 2024). Images were taken with a Nikon Eclipse 80i with Capture 2.3 imaging software. The microscope slides are deposited in ESALQ—Department of Entomology and Acarology, Luiz de Queiroz College of Agriculture, University of São Paulo (USP), Brazil; NMNH—National Insect and Mite Collection, National Museum of Natural History, Smithsonian Institution, located at the Systematic Entomology Laboratory (SEL), USDA, Beltsville, MD, USA; and QM—Queensland Museum, Brisbane, Australia.
The influences of maximum and minimum temperatures, mean relative humidity, and mean rainfall on the population density of T. ornata, T. knorri and Brevipalpus yothersi Baker (Acari: Tenuipalpidade) were evaluated using Multivariate Analysis (MVA) and Principal Component Analysis (PCA). The average number of individuals of each species collected from raceme-type inflorescences during the study was used to calculate species abundance. Maximum and minimum temperatures, mean relative humidity, and mean rainfall were averaged over the 14 days preceding the sampling date and were included in the analysis. The average numbers of each developmental stage of each species, were compared by year, following subjection to the Shapiro-Wilk test to verify normality (p > 0.05). The data met this requirement and the Student's t test was subsequently conducted. The StatGraphics Centurion XVIII program (StatGraphics 2009) was used to assess any relationships between these groupings. The significance level of 5% was used in all statistical analyses.
Results and discussion
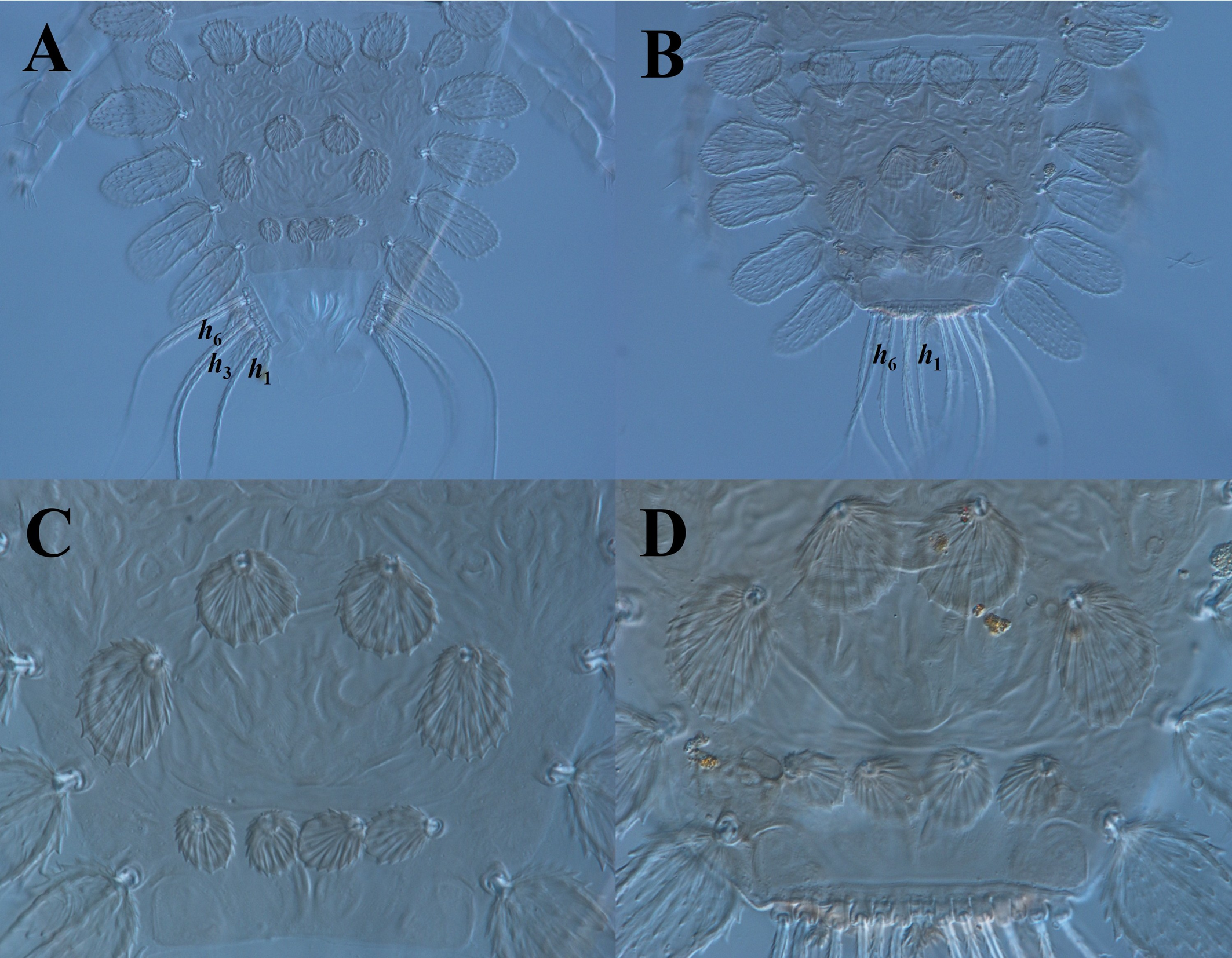


We report the first detection of the peacock mites T. ornata and T. knorri on C. guianensis trees in Brazil (Fig. 2), although these two species are known in the Brazilian state of Pará, commonly on cacao, Theobroma cacao L. (Malvaceae) (Flechtmann 1979; Da Silva et al. 2023). They have also been found to cause significant damage to cocoa fruit in Piura, Peru, resulting in a reduced harvestable yield of seeds (Escobar-Garcia et al. 2021a). Additionally, T. ornata was collected in Zulia, Venezuela, on guava, Psidium guajava L. (Myrtaceae) (Camacho et al. 2002); and Malpighia emarginata D.C. (Malpighiaceae) in Pernambuco, Brazil (Barbosa et al. 2003).



Tuckerella ornata (Fig. 3A) and T. knorri were only observed on the raceme-type inflorescences of the cannon-ball tree and were collected in each spring for two consecutive years. The combined population densities of peacock mites were 3.60 to 4.35 eggs, 1.45 to 2.38 immature stages, and 1.53 to 1.83 adult females. Immature stages of peacock mites (both species combined) were negatively correlated with average minimum (> 18.3 °C) temperature (r = -0.65, n = 10, p = 0.04). The densities of females of T. knorri were also negatively correlated with average minimum (> 18.3 °C) temperature (r = -0.68, n = 10, p = 0.02). However, densities of female T. ornata were positively correlated with maximum (r = 0.62, n = 10, p = 0.05) and minimum (r = 0.53, n = 10, p = 0.11) temperatures; i.e., the density of female T. ornata increased with maximum (> 33 °C) and minimum (> 20 °C) temperatures; however, the p-value that tests the statistical significance of the estimated correlations was greater than 0.05.



Although Figure 4 presents data indicating that densities of female T. ornata and T. knorri were affected by rainfall, there is only a very low and non-significant negative correlation for T. ornata (r = -0.09, n = 10, p = 0.79), and a moderate and non-significant negative correlation for T. knorri (r = -0.47, n = 10, p = 0.16). No significant correlations were detected between the other developmental stages of peacock mites and climatic variables. The PCA of climatic variables and the densities of peacock mites showed that the first component represents about 43.0% of the total variability and the second an additional 28.1%. In the first component, high values of minimum temperature (> 18.3 °C) negatively affected the densities of immature stages of peacock mites, and females of T. knorri. While in the second component maximum and minimum temperatures were positively related with densities of females of T. ornata. Although each stage of development of both species of peacock mites was present in each year studied, we found no significant difference between the means of the two data samples, with a confidence level of 95.0% (t test, eggs: t = 0.42, p = 0.68; immature stages: t = 1.19, p = 0.26; females of T. ornata: t = -1.77, p = 0.11; and females of T. knorri: t = 1.73, p = 0.12). Eggs and immature stages combined, were more abundant than females in all the evaluations, ranging from 63% to ~90% of individuals depending on the evaluation date (Figs. 5A and 5B). Maximum values of females were observed on November 27 for both years ranging from 34% to ~37% of the general population of peacock mites (Figs. 5A and 5B).



Plant-feeding mites can detect the developmental state of their host via its cell chemistry; therefore, mites on the branches, main trunk, and possibly roots, would detect that flowers are developing and begin to migrate to the racemes for potentially more nutritious food. Interestingly, although Tuckerella species are known to not only feed on, but also to favour feeding on, the fruits of their hosts (Beard et al. 2013), neither of the species studied here used the crevices on the epicarp of the fruits for feeding, oviposition, or protection. The fruits are known to contain a range of toxic chemical constituents, including campesterol, couroupitine A (tryptanthrin), couroupitine B (indirubin), farmaricetin, hopane, isocitric acid, kaempherol, luteolin, malic acid, quercetin, rutin, stigmasterol, sulphur, and ursolic acid (Sen et al. 1974; Regina and Uma Rajan 2012; Gupta et al. 2014; Sheba and Anuradha 2020), which could be acting to deter the mites from inhabiting the fruit. Although we did not study the fruit chemistry and its influence on these mites, we hypothesized that more than one component may be involved in deterring the mites. During these two years of survey, no peacock mites were observed on either the leaves or ripe fruit.
There are few studies regarding the mite diversity on cannon-ball trees. To date, there are records of the spider mite Tetranychus mexicanus (McGregor) (Tetranychidae) (Flechtmann 1967; Migeon and Dorkeld 2024), and the flat mite Tenuipalpus couroupita De Leon (Tenuipalpidae) (De Leon 1967; Castro et al. 2024). In addition, the arboreal predator Amblyseius largoensis (Muma) (Phytoseiidae) has also been recorded from this tree (Calcavante et al. 2021; Demite et al. 2024). Here we report the flat mite B. yothersi for the first time from this host (Fig. 3B). The aggregations of B. yothersi were more common, and the population densities were much higher on the inflorescence (0.5 to 3.7 eggs, 1.0 to 1.7 immature stages, 0.9 to 1.7 females) than on the leaves (0.0 to 0.1 eggs, 0.0 to 0.2 immature stages, 0.0 to 0.2 females).
Densities of B. yothersi eggs were positively correlated with average maximum temperature (r= 0.69, n= 10, p =0.02); i.e., the densities of eggs increased with increasing maximum temperature (> 30.7 °C), and also with average minimum temperature (r = 0.53, n = 10, p = 0.10), but the latter was not statistically significant (p > 0.05).
Densities of female B. yothersi were affected by the average relative humidity (r = -0.47, n = 10, p = 0.16), and rainfall (r = -0.45, n = 10, p = 0.18); i.e., the densities of females decreased with increasing averages of relative humidity and rainfall, with a moderate and non-significant negative correlation observed in both analyzes (p > 0.05). According to the PCA of B. yothersi mite numbers and climate factors, the first component accounts for roughly 54.7% of the overall variability, while the second contributes an additional 27.9%. In the first component, the density of eggs was associated with the average maximum temperatures. We found a significant difference between the densities of eggs of B. yothersi in 2022 versus eggs in 2023, with a confidence level of 95.0% (t test, eggs: t = -3.87, p = 0.00); i.e., the densities of eggs were higher in the year 2023. No significant differences were found for the other developmental stages of B. yothersi (p > 0.05).
The densities of eggs and immature stages of B. yothersi exceeded that of adult females, varying from 30% to ~80% of the total densities of mites based on the evaluation date (Figs. 5C and 5D). On November 27, minimum values of females ranged from 20 to nearly 25% of the overall B. yothersi population for both years (Figs. 5C and 5D). Maximum numbers of female peacock mites were observed on this same date, which could result in peacock mites dominating territory and feeding sites over B. yothersi, i.e., densities of females of B. yothersi could be are affected by the densities of female peacock mites (r = -0.50, n = 10, p = 0.13), and this moderate negative but non-significant correlation (p > 0.05) was observed during all evaluations (Fig. 5).
During this study, the predatory mites Euseius citrifolius Denmark & Muma, and Euseius concordis (Chant) (Phytoseiidae); Agistemus brasiliensis Matioli, Ueckermann & Oliveira (Stigmaeidae); and Brachytydeus formosus (Cooreman) (Tydeidae) were found on leaves on the cannon-ball tree. Neophyllobius (Neophyllobius) unespensis Escobar-Garcia & Ueckermann (Camerobiidae) were also found on the inflorescences (Escobar-Garcia et al. 2024b).



This association between the species T. ornata, T. knorri and B. yothersi has also been observed on cocoa fruits in Peru, during the same season (spring) and a similar phenological phase of plant development (Escobar-Garcia et al. 2021b). Tuckerella ornata, T. knorri, and B. yothersi are economically important mites that feed on fruit and ornamental trees, and their presence on C. guianensis in Brazil is of potential concern. The geographic distribution of C. guianensis in northern Brazil (Acre, Amazonas, Pará) (Fig. 6B) coincides with primary cocoa-producing states, and its presence as an alternative host could negatively impact cocoa cultivation in agroforestry systems, and we believe it to be of phytosanitary significance in the states of Pará, Amazonas, and Rondonia. Economically significant damage has already been reported in cocoa cultivation in Pará (Da Silva et al. 2023), and cocoa farmers in these regions should consider control strategies within Integrated Pest Management programs that carefully monitor for these mites. An important tool for farmers in their efforts would be 16X-20X hand lenses that allow them to detect the mite's presence. It is important to advocate for increased awareness of the domestic movement of leaves and inflorescences for ethnomedical purposes of C. guianensis, in addition to monitoring the primary cocoa-producing areas for these three mite species.
Acknowledgments
Special thanks to André Luis Matioli of the Instituto Biológico (IB) of Campinas, Brazil for the identification and confirmation of Agistemus brasiliensis. We want to express our special thanks to Peterson Rodrigo Demite (UFMT, Brazil) for the identification and confirmation of Phytoseiidae mite species. We would also grateful Department of Entomology and Acarology, ESALQ, USP, Brazil for the help with the figures of Tuckerella morphology. To Aline Tassi (UF, USA) and Thiago Feliph Silva Fernandes (UNESP, Brazil) for preparing Figure 6A and 6B, respectively. We would our appreciation also goes to Gerson La Rosa (UDEP, Peru) for the statistical support. We wish to express our special thanks to Andrew Ulsamer (ARS-USDA) for the revision and suggestions to the manuscript. This study was financed in part by the Coordination of Superior Level Staff Improvement (CAPES), Brazil. To the Smithsonian Natural History Museum (NMNH) and National Agricultural Library (NAL-USDA), SEL-USDA for support and assistance with specimens and references. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
References
- Anu Y., Mendhulkar V.D. 2015. Repellency and toxicity of Couroupita guianensis leaf extract against Silverleaf Whitefly (Bemisia tabaci). Int. J. Sci. Res. Pub., 5:1-4.
- Babu V., Arokiyaraj S., Shakti Sri S.P., George M., Ragavan R.M., Dharmalingam D., Oh T., Ramasundaram S., Agastian P. 2022. Antibacterial, Antioxidant, Larvicidal and Anticancer Activities of Silver Nanoparticles Synthesized Using Extracts from Fruits of Lagerstroemia speciose and Flowers of Couroupita guianensis. Molecules, 27(22): 7792. https://doi.org/10.3390/molecules27227792
- Barbosa D.G.F., Gondim Jr. M.G.C., Barros R., Oliveira J.V. 2003. Diversidade de ácaros em aceroleira (Malpighia emarginata A.DC.) na Universidade Federal de Pernambuco em Recife, PE. Neotrop. Entomol., 32:577-583. https://doi.org/10.1590/S1519-566X2003000400007
- Baskar K., Ignacimuthu S., Jayakumar M. 2015. Toxic Effects of Couroupita guianensis Against Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Neotrop. Entomol., 44(1):84-91. https://doi.org/10.1007/s13744-014-0260-7
- Beard J.J., Walter D.E. 2005. A new species of Tuckerella (Prostigmata: Tetranychoidea: Tuckerellidae) from Australia with descriptions of all stages and a discussion of the tritonymphal stage. Acarologia, 45: 49-60.
- Beard J.J., Ochoa R., Bauchan G.R., Welbourn W.C., Pooley C., Dowling, A.P. 2012. External mouthpart morphology in the Tenuipalpidae (Tetranychoidea): Raoiella a case study. Exp Appl Acarol., 57(3-4): 227-255. https://doi.org/10.1007/s10493-012-9540-2
- Beard J.J., Ochoa R., Childers C.C., Bauchan G.R., Shepard M. 2013. Travelling with tea: a Tuckerella's tale. Exp Appl Acarol., 59, 177-202. https://doi.org/10.1007/s10493-012-9627-9
- Beard J.J., Ochoa R., Braswell W.E., Bauchan G.R. 2015. Brevipalpus phoenicis (Geijskes) species complex (Acari: Tenuipalpidae) a closer look. Zootaxa, 3944(1): 1-67. https://doi.org/10.11646/zootaxa.3944.1.1
- Beck H., Zimmermann N.E., McVicar T.R., Vergopolan N., Berg, A., Wood E.F. 2018. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Scientific Data, 5, 180214. https://doi.org/10.1038/sdata.2018.214
- Cabello N.B., Catenacci F.S., Ribeiro M., Smith N.P. 2024. Couroupita in Flora e Funga do Brasil. Jardim Botânico do Rio de Janeiro. Available online: https://floradobrasil.jbrj.gov.br/FB17964 (accessed on 4 March 2024).
- CABI Compendium. 2019. Available online (accessed on 4 March 2024). https://doi.org/10.1079/cabicompendium.15578
- Cavalcante A.C.C., Demite P.R., Lofego A.C., Hernandes F.A. 2021. Phytoseiidae (Acari: Mesostigmata) from the Atlantic Forest in Rio de Janeiro, Brazil, with complementary description of Amblyseius impeltatus Denmark & Muma. Pap. Avulsos Zool., 61:e20216198. https://doi.org/10.11606/1807-0205/2021.61.98
- Camacho M.J., Güerere P.P., Quirós G.M. 2002. Insectos y Ácaros del guayabo (Psidium guajava L.) en plantaciones comerciales del estado Zulia, Venezuela. Revista de la Facultad de Agronomía, 19(2):140-148.
- Castro E.B., Mesa N.C., Feres R.J.F., Moraes G.J., Ochoa R., Beard J.J., Demite P.R. 2024. Tenuipalpidae Database. Available from: http://www.tenuipalpidae.ibilce.unesp.br (accessed on 4 March 2024).
- Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. The Canadian Entomologist, 61(12):1-166.
- Childers C.C., De Lillo E., Bauchan G.R., Rogers M.E., Ochoa R., Robinson C. 2018. External morphologyof the mouthparts and observations on behavior of Tuckerella japonica on Camellia sinensis in the continental USA. Exp Appl Acarol., 74(1):55-71. https://doi.org/10.1007/s10493-017-0204-0
- Cooreman J. 1958. Notes et observations sur les acariens. VII. Photia gracca n. sp. (Acaridiae, Canestriniidae) et Lorryia formosa n. sp. (Stomatostigmata, Tydeidae). Bulletin de l′Institut Royal des Sciences Naturelles de Belgique Entomologie, 34: 1-10.
- Corpuz-Raros L.A. 2001. Tuckerella filipina, a new species of Tuckerellidae (Acari) from the Philippines. Int. J. Acarol., 27:71-74. https://doi.org/10.1080/01647950108684229
- Da Silva E.A.B., De Castro I.S., Vinhas N.A.N., Da Rosa R.S., Pinheiro G.M., Ferla N.J. 2023. Damage caused by Tuckerella ornata (Acari: Tuckerellidae) on cocoa fruits (Theobroma cacao) in the Amazon region, state of Pará, Brazil. Syst. Appl. Acarol., 28(1): 11-19. https://doi.org/10.11158/saa.28.1.2
- De Leon. 1967. Some Mites of the Caribbean Area. Allen Press, Inc. Lawrence, Kansas. November-1967, 1-46.
- Demite P.R., Moraes G.J., McMurtry J.A., Denmark H.A., Castilho R.C. 2024. Phytoseiidae Database. Available online: https://www.lea.esalq.usp.br/phytoseiidae (accessed on 4 March 2024).
- Denmark H.A., Muma M.H. 1970. Some phytoseiid mites of Paraguay (Phytoseiidae: Acarina). Fla. Entomol., 53(4): 219-227. https://doi.org/10.2307/3493192
- Escobar-Garcia H.A., de Andrade D.J., Beard J.J., Ochoa R. 2021a. Peacock mites on cocoa in Peru (Acari: Tuckerellidae: Tuckerella): Their economic importance and a key to species. Syst. Appl. Acarol., 26: 519-528. https://doi.org/10.11158/saa.26.3.2
- Escobar-Garcia H. A., De Andrade D.J., Carrillo D., Ochoa R. 2021b. Theobroma cacao, a new host for Brevipalpus yothersi (Acari: Tenuipalpidae) in Peru. Acarologia, 61(2):211-216. https://doi.org/10.24349/acarologia/20214427
- Escobar-Garcia H.A., Beard J.J., Ochoa R. 2022. Report of Tuckerella pavoniformis (Acari: Tuckerellidae) on Mamey, Mammea americana (Calophyllaceae), in Northwestern Peru. Insects 13, 473. https://doi.org/10.3390/insects13050473
- Escobar-Garcia H.A., Nascimento V.F., de Melo M.A., Ramalho D.G., de Bortoli S.A. 2024a. Aqueous botanical extracts, via different extraction methods, for control of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Plant Dis. Prot., 131: 255-263. https://doi.org/10.1007/s41348-023-00809-6
- Escobar-Garcia H. A., de Andrade D.J, Mohammad-Doustaresharaf M., Ueckermann E.A. 2024b. A new species of stilt-legged mite of the genus Neophyllobius Berlese (Acari: Camerobiidae) from Brazil. Acarologia 64(2): 592-601. https://doi.org/10.24349/x6a3-jlvy
- Esposito T., Pisanti S., Martinelli R., Celano R., Mencherini T., Re T., Aquino R.P. 2023. Couroupita guianensis bark decoction: From Amazonian medicine to the UHPLC-HRMS chemical profile and its role in inflammation processes and re-epithelialization. J. Ethnopharmacol, 313:116579. https://doi.org/10.1016/j.jep.2023.116579
- Ewing H.E. 1922. Three new species of peculiar and injurious spider mites. Proc. Entomol. Soc. Wash., 24(4):104-108.
- Ferreira É.L.F., Oliveira J.P.de C., de Araújo M.R.S., Rai M., Chaves M.H. 2021. Phytochemical profile and ethnopharmacological applications of Lecythidaceae: An overview. J. Ethnopharmacol, 274:114049, https://doi.org/10.1016/j.jep.2021.114049
- Gupta S.K., Ghosal M., Choudhury D., Mandal P. 2014. Assessment of antioxidant activity and polyphenolic content of Couroupita guianensis during flower and fruit maturation. Int J Recent Sci Res., 5(5):940-7.
- Flechtmann C.H.W. 1967. Contribution to knowledge of the mites of plants of some regions of the State of Sao Paulo (as a systematic survey including new species). Piracicaba, Brasil: 47.
- Flechtmann C. H.W. 1979. Tuckerella ornata (Tucker), um ácaro novo para o Brasil e outros tetranychoidea (Acari) do estado do Pará. Anais da Escola Superior de Agricultura Luiz de Queiroz, 36: 615-620. https://doi.org/10.1590/S0071-12761979000100033
- Kavitha R., Kamalakannan P., Deepa T., Elamathi R., Sridhar S., Suresh Kumar J. 2011. In vitro antimicrobial activity and phytochemical analysis of Indian medicinal plant Couroupita guianensis Aubl. J Chem Pharm Res. 3(6):115-21.
- Lofego A.C., Barbosa M.F.C., Demite P.R., Moraes G.J. 2024. Phytoseiidae (Acari: Mesostigmata) of the subfamily Amblyseiinae from Brazil. Zootaxa, 5439(1):1-306 https://doi.org/10.11646/zootaxa.5439.1.1
- Matioli A.L., Ueckermann E.A., Oliveira C.A.L. 2002. Some stigmaeid and eupalopsellid mites from citrus orchards in Brazil (Acari: Stigmaeidae and Eupalopsellidae). Int. J. Acarol., 28(2): 99-120. https://doi.org/10.1080/01647950208684287
- Melo A.S., Paz-Neto A.A., Melo J.W.S., Gondim-Junior M.G.C. 2024. Interspecific interaction network of mites associated with mango trees. Exp Appl Acarol., 93(2), 353-367. https://doi.org/10.1007/s10493-024-00936-1
- Meyer M.K.P.S., Ueckermann E.A. 1997. A review of some species of the families Allochaetophoridae, Linotetranidae and Tuckerellidae (Acari: Tetranychoidea). Int. J. Acarol., 23(2): 67-92. https://doi.org/10.1080/01647959708683103
- Migeon A., Dorkeld F. 2024. Spider Mites Web: A Comprehensive Database for the Tetranychidae. 2006-2024. Available online: http://www1.montpellier.inra.fr/CBGP/spmweb (accessed on 4 March 2024).
- Mirza J.H., Kamran M., Alatawi F.J. 2022. New Genus and New Subgenera of Camerobiid Mites (Acari: Prostigmata: Camerobiidae) with a Key to World Species of the Genus Neophyllobius. Insects, 13(344):1-34. https://doi.org/10.3390/insects13040344
- Mori S.A., Tsou C., Wu C., Cronholm B., Andreberg A.A. 2007. Evolution of Lecythidaceae with an emphasis on the circumscription of Neotropical genera: information from combined NDHF and TRNL-F sequence data. Am. J. Bot., 94: 289-301. https://doi.org/10.3732/ajb.94.3.289
- Nelson E.K., Wheeler D.H. 1937. Some Constituents of the Cannonball Fruit (Couroupita guianensis, Aubl.). J Am Chem Soc. 59(12):2499-500. https://doi.org/10.1021/ja01291a005
- Ochoa R. 1989. The genus Tuckerella in Costa Rica (Acari: Tuckerellidae). Int. J. Acarol., 15(4): 205-207. https://doi.org/10.1080/01647958908683850
- Pinheiro M.M.G., Bessa S.O., Fingolo C.E., Kuster R.M., Matheus M.E., Menezes F.S., Fernandes P.D. 2010. Antinociceptive activity of fractions from Couropita guianensis Aubl. leaves. J. Ethnopharmacol., 127 (2): 407-413. https://doi.org/10.1016/j.jep.2009.10.025
- Pinheiro M.M., Fernandes S.B., Fingolo C.E., Boylan F., Fernandes P.D. 2013. Anti-inflammatory activity of ethanol extract and fractions from Couroupita guianensis Aublet leaves. J Ethnopharmacol. 146(1):324-30. https://doi.org/10.1016/j.jep.2012.12.053
- Prabhu V., Ravi S. 2017. Isolation of phytoconstituents from the flowers of Couroupita guianensis. Indian J Chem. 56:709-13.
- Raghavendra H.L., Kekuda T.R.P., Pushpavathi D., Shilpa M., Petkar T., Siddiqha A. 2017. Antimicrobial, radical scavenging, and insecticidal activity of leaf and flower extracts of Couroupita guianensis Aubl. Int. J.Green Pharm.,11:171-179.
- Rajput K.S., Patil V.S. 2016. Structure and development of cortical bundles in Couroupita guianensis Aubl. (Lecythidaceae). Anales de Biología 38: 95-102. https://doi.org/10.6018/analesbio.38.10
- Regina V., Uma Rajan K.M. 2012. Phytochemical analysis, antioxidant and antimicrobial studies of fruit rind of Couroupita guianensis (Aubl). Int J Curr Sci., 221:262-267.
- Rehman M.U., Kamran M., Alatawi F.J. 2018. Genus Agistemus Summers (Acari: Trombidiformes: Stigmaeidae) from Saudi Arabia and a key to the world species. Syst. Appl. Acarol., 23: 1051-1072. https://doi.org/10.11158/saa.23.6.5
- Reshma Y., Sunilkumar T. 2018. Phytochemical analysis of fruit pulp of Couroupita guianensis Aubl. J Pharmacogn Phytochem. 7(2):877-9.
- Sen A.K., Mahato S.B., Dutta N.L. 1974. Couroupitine A, a new alkaloid from Couroupita guianensis. Tetrahedron Lett., 7:609-610. https://doi.org/10.1016/S0040-4039(01)82284-X
- Sheba L.A., Anuradha V. 2020. An updated review on Couroupita guianensis Aubl: a sacred plant of India with myriad medicinal properties. J Herbmed Pharmacol., 9(1):1-11. https://doi.org/10.15171/jhp.2020.01
- Silva G.L. da, Metzelthin M.H., Silva O.S. da, Ferla, N.J. 2016. Catalogue of the mite family Tydeidae (Acari: Prostigmata) with the world key to the species. Zootaxa, 4135(1):1-68. https://doi.org/10.11646/zootaxa.4135.1.1
- StatGraphics. 2009. StatGraphics Centurion XVIII -Versión 18-, USA [Software] (accessed on 4 March 2024). http://www.statgraphics.net/descargas/
- Uppala P.K., Murali Krishna B., Atchuta Kumar K., Vinay Ramji D.J. 2016. Evaluation of anti-coagulant activity of the chloroform and aqueous extracts of the leaves of Couroupita guianensis. Int J Pharm Pharm Res. 6(4):189-99.
- Walter D.E., Krantz G.W. 2009. Collecting, rearing, and preparing specimens. In: Krantz G.W., Walter D.E. (Eds). A manual of Acarology. 3rd ed. Texas Tech University Press, p. 83-96.



2024-05-29
Date accepted:
2024-10-16
Date published:
2024-10-30
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Escobar-Garcia, Hector Alonso; de Andrade, Daniel Júnior; Beard, Jennifer J. and Ochoa, Ronald
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



