Description of the male of Lasioseius jorgeamadoi Santos & Argolo, 2018 (Acari: Blattisociidae)
Araújo, Edineia da S.  1
; Stracieri, Juliana
1
; Stracieri, Juliana  2
; de Moraes, Gilberto J.
2
; de Moraes, Gilberto J.  3
and Oliveira, Anibal R.
3
and Oliveira, Anibal R.  4
4
1✉ Programa de Pós-Graduação em Produção Vegetal - PPGPV, Universidade Estadual de Santa Cruz - UESC, Rodovia Jorge Amado, km 16, 45662-900, Ilhéus, BA, Brazil.
2Programa de Pós-Graduação em Produção Vegetal - PPGPV, Universidade Estadual de Santa Cruz - UESC, Rodovia Jorge Amado, km 16, 45662-900, Ilhéus, BA, Brazil.
3Departamento de Entomologia e Acarologia, Escola Superior de Agricultura “Luiz de Queiroz” - ESALQ, Universidade de São Paulo - USP, 13418-900, Piracicaba, SP, Brazil.
4Programa de Pós-Graduação em Produção Vegetal - PPGPV, Universidade Estadual de Santa Cruz - UESC, Rodovia Jorge Amado, km 16, 45662-900, Ilhéus, BA, Brazil.
2024 - Volume: 64 Issue: 4 pages: 1100-1105
https://doi.org/10.24349/0nkv-7o4oZooBank LSID: 405A6ED7-62E5-43C9-ACCB-20644978B61E
Original research
Keywords
Abstract
Introduction
Lasioseius jorgeamadoi Santos & Argolo, 2018 was originally described based only on females collect from buds of cocoa (Theobroma cacao L., Malvaceae) in Ilhéus, Bahia state, Brazil (Argolo et al. 2018). As the morphological characterization of males can be relevant for future taxonomic and applied studies, the objective of this publication is to describe the male of L. jorgeamadoi based on specimens collected from the type host and locality of this species.
Material and methods
Mites were extracted from cocoa buds collected from an experimental plantation at the campus of ′Comissão Executiva do Plano da Lavoura Cacaueira′ (CEPLAC), in Ilhéus. Specimens were removed from buds under a stereomicroscope, cleared in KOH solution and mounted in Hoyer's medium or on a stub for study, under phase contrast or scanning electron microscopy, respectively. Illustrations were done based on images and/or short movies taken with a digital camera connected to the phase contrast microscope. Measurements were taken with a graded ocular and presented in micrometers as the average, followed (in parentheses) by the minimum and the maximum for each character in case of variation. Nomenclature follows that used by Lindquist & Evans (1965) and Lindquist (1994) for idiosomal setae, Athias-Henriot (1969, 1971, 1975) as applied by Quintero-Gutiérrez et al. (2020) and Moraza & Balanzategui (2023) for gland pores and poroids in Lasioseius, and Beard (2001) for spermatodactyl. Voucher specimens were deposited in the mite collections of UESC and ESALQ.
Results and discussion
Lasioseius jorgeamadoi Santos & Argolo, 2018
Adult male description (Figs. 1–3) (n=7 measured)
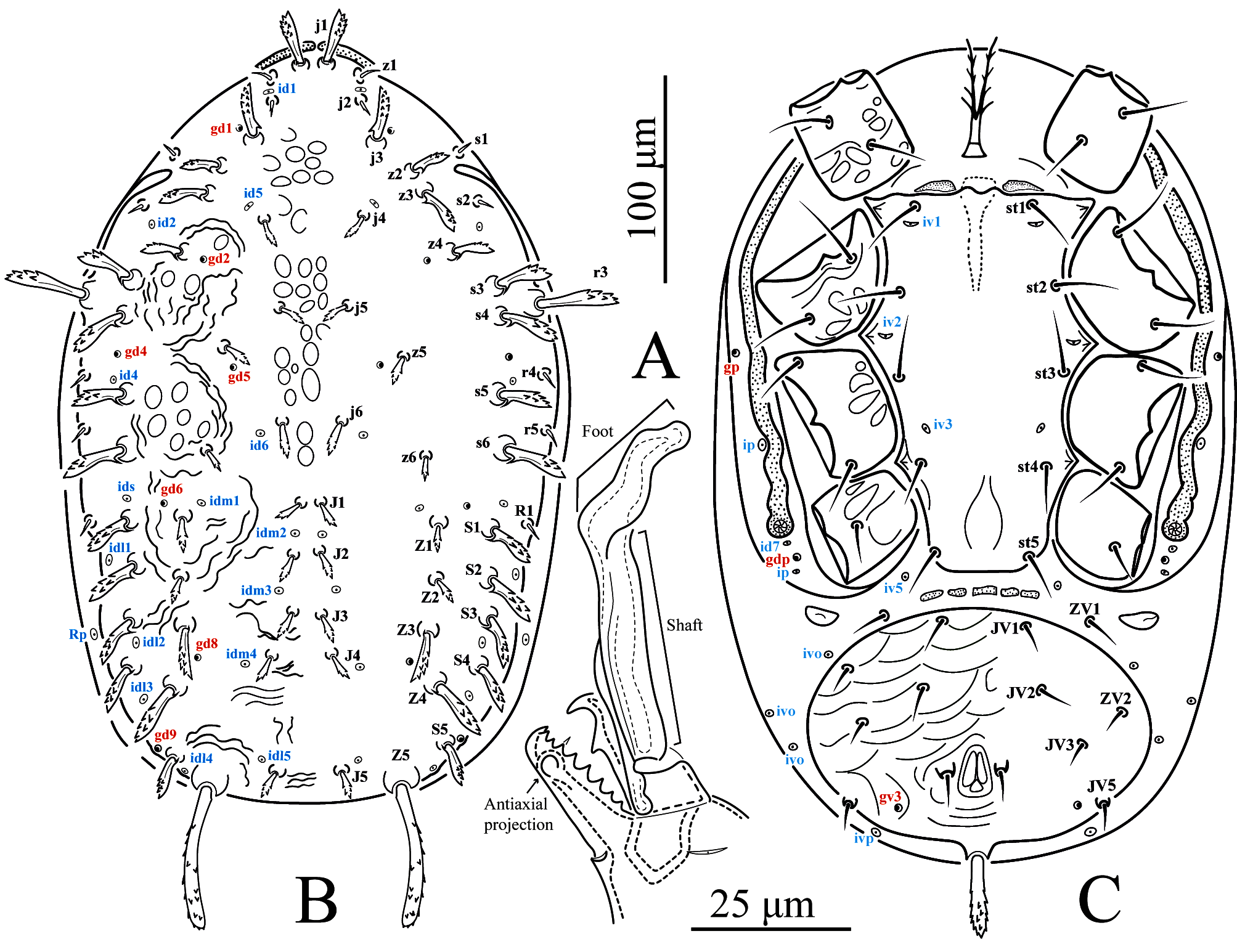


Gnathosoma (Figures 1A and 3A) — Fixed cheliceral digit 26 (25–27) long, with 8 (7–8) not retrorse pointed teeth in addition to the apical hook and a round, antiaxial projection at pilus dentilis level; movable cheliceral digit 30 (30–31) long, with one retrorse pointed tooth in addition to apical hook; antiaxial and dorsal lyrifissures distinct. Spermatodactyl 61 (59–63) long, L-shaped; shaft 41 (39–43) long, almost straight, basally thicker; foot 20 long, at small angle with the shaft axis, rounded and curved downward apically. Deutosternum with the first five transverse denticulate lines each with 4–8 denticles and the last two each with 5–11 denticles. Corniculi 22 (17–23) long and 10 (9–10) wide basally, spaced 32 (30–35) apart proximally. Measurements of setae: h1 16, h2 21 (20–23), h3 41 (40–42), pc 30 (29–30).
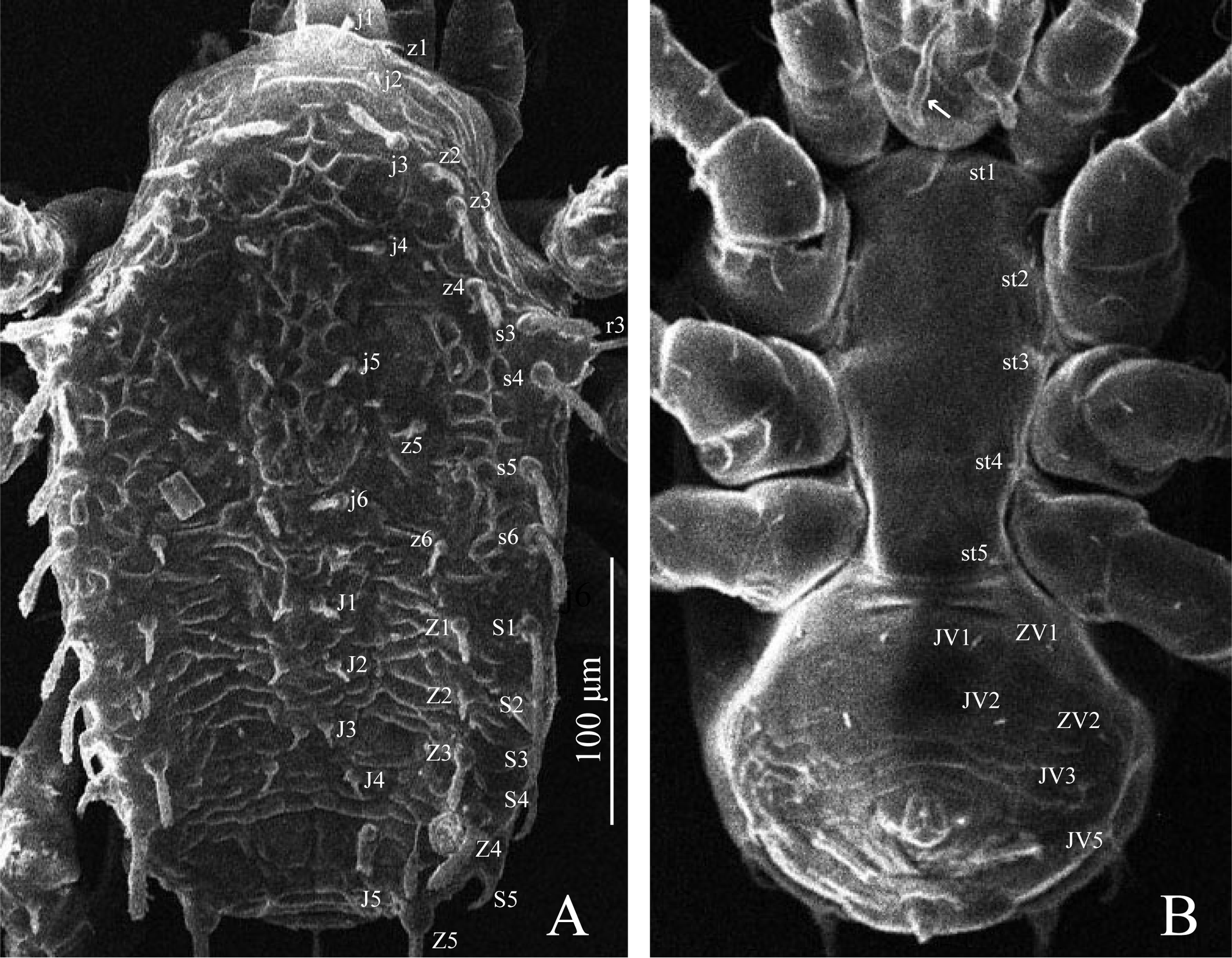


Dorsal idiosoma (Figures 1B and 2A) — Dorsal shield densely covered with cerotegument, strongly ornamented with roundish structures centrally, combined with curved lines laterally anteriad of z6 and scant broken and curved lines behind, 340 (330–350) long and 215 (210–220) wide at widest level. With seven pairs of dorsal gland openings (gd1, gd2, gd4, gd5, gd6, gd8, gd9) and16 pairs of lyrifissures (Rp on unsclerotized lateral cuticle). Seta r3 inserted on dorsal shield and R1 usually inserted on dorsal shield (one member of each pair on unsclerotized lateral cuticle in two males); other setae of the R-series (R2–R5) absent. Setal measurements: j1 26 (25–27), j2 10 (9–11), j3 24 (23–25), j4 11 (11–13), j5 11 (11–12), j6 13 (13–14), J1 11 (10–11), J2 10 (10–11), J3 10, J4 10, J5 11 (10–11), z1 12 (11–13), z2 15 (15–16), z3 24 (23–25), z4 20, z5 10 (8–10), z6 9 (9–10), Z1 16 (15–17), Z2 12, Z3 19 (18–20), Z4 39 (36–40), Z5 69 (68–70), s1 6 (5–6), s2 6 (5–6), s3 27 (26–29), s4 28 (27–28), s5 26 (25–27), s6 31 (30–32), S1 22 (22–23), S2 22 (21–22), S3 18 (17–19), S4 17 (17–18), S5 22 (22–23), r3 41 (40–42), r4 6, r5 5 (5–6), R1 5. Most dorsal setae stout and barbed in the apical half, except for short, simple z1, j2, s1–s2, r4–r5 and R1. Setae j3, z2–z4, s3–s6, S1–S5 and Z4 with a minute hook at the base; Z5 particularly longer and thicker than other dorsal setae, provided with fine barbs along almost its entire length.
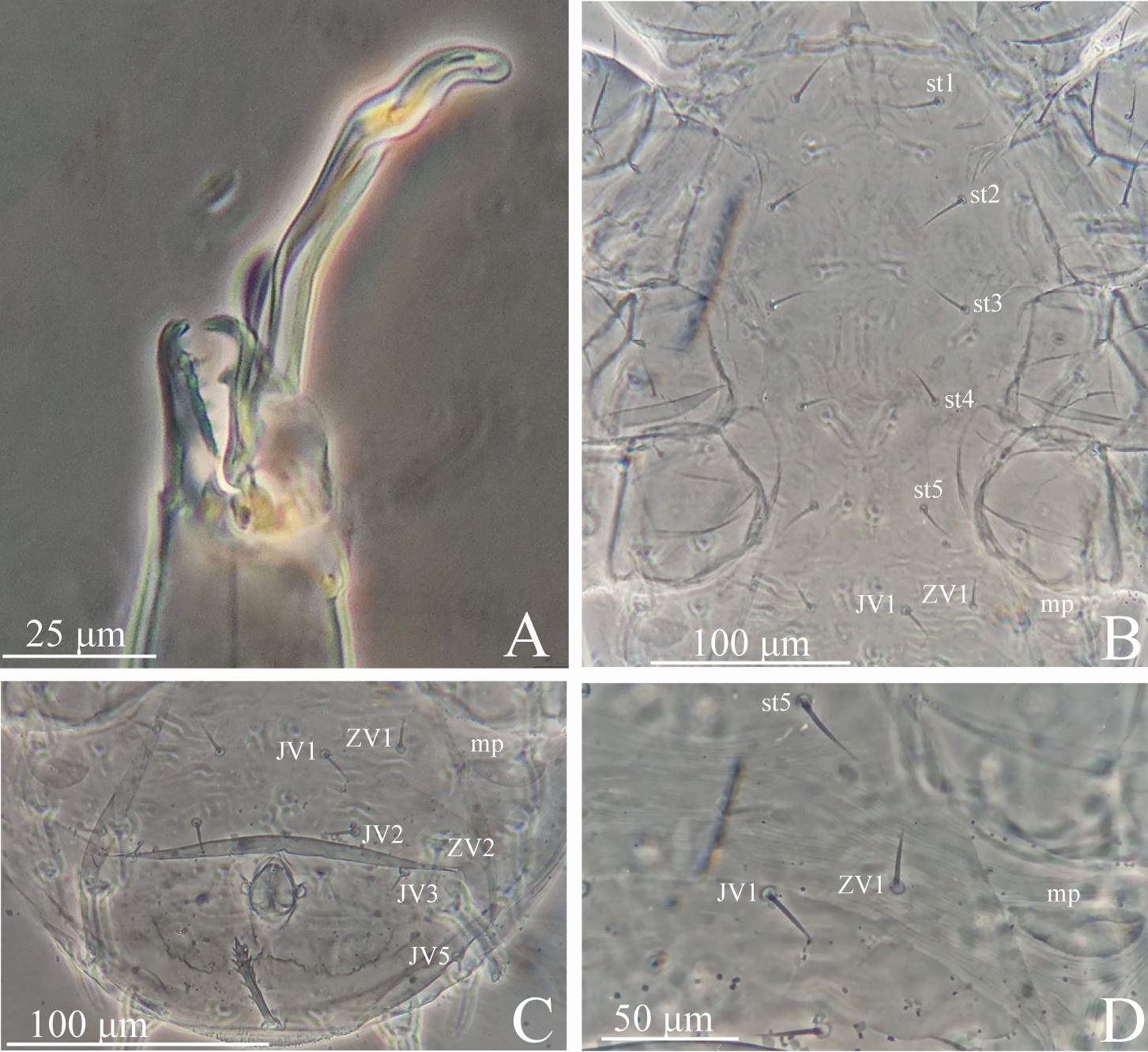


Ventral idiosoma (Figures 1C, 2B and 3B–C) — Base of tritosternum 15 (15–16) long and 10 (9–10) wide proximally; laciniae 35 (34–36) long. Pre-sternal area with a pair of small, elongate, lightly sclerotized punctuate plates. Sternogenital shield essentially smooth, 168 (167–170) long at mid–line and 80 (77–82) wide at level of st2, with five pairs of setae (st1–st5) and three pairs of lyrifissures (iv1–iv3); posterior margin strait to slightly concave. Ventrianal shield weakly lineate-reticulate, 105 (100–110) long at mid-line and 150 (145–160) wide at widest level; with five pairs of setae (JV1, JV2, JV3, JV5 and ZV2) in addition to circumanal setae; ZV1 on unsclerotized opisthogastric cuticle; JV4 and ZV3 absent. One pair of discrete semicircular metapodal plates present. Setal measurements: st1 26 (25–26), st2 22 (21–23), st3 22 (21–22), st4 18 (17–18), st5 18 (17–20), JV1 15 (14–16), JV2 15 (14–15), JV3 10 (9–10), JV5 10 (9–10), ZV1 11 (10–12), ZV2 10 (10–11); para–anal 17 (16–18), post–anal 32 (31–34).
Leg lengths — I: 389 (382–396); II: 370 (363–380); III: 361 (352–362); IV: 491 (480–504).
Specimens examined
13 ♂♂ + 10 ♀♀ collected by E. da S. Araújo from buds of Theobroma cacao L. (Malvaceae) at CEPLAC, Ilhéus, Bahia state, Brazil (14º45′35''S, 39º13′49''W); 7 ♂♂ + 5 ♀♀ collected on 17 May 2023 on 2 slides (nr. 28 with 5 ♂♂ + 3 ♀♀ and nr. 118 with 2 ♂♂ + 2 ♀♀); 4 ♂♂ collected on 7 November 2023 on 4 slides (nrs. 261, 262, 263 and 264); 2 ♂♂ + 5 ♀♀ collected on 7 November 2023 on a stub. Holotype + 3 paratype ♀♀.
Remarks
This mite species has been commonly found in association with the cacao bud mite, Aceria reyesi (Nuzzaci, 1973) (Eriophyidae), seemingly feeding on the latter (our unpublished observation). In addition to the males used for the description here provided, 5 adult females collected and slide-mounted with them were compared with the holotype and 3 paratype females originally described in Argolo et al. (2018) to confirm the species identification. Besides the fact that measurements are generally shorter in male, most characters of the gnathosoma, idiosoma and legs are similar to those of females, except for the smaller presternal area, the fused sternal and genital shields, the absence of R2–R5, JV4 and ZV3, the insertion of R1 mostly on shield, and the reduced number of teeth in the chelicera of the male. All those and other characters described above fit well the diagnosis of males of the genus Lasioseius (Moraes et al. 2016), except for the presence of the discrete pair of metapodal plates in the males of L. jorgeamadoi. Although some discrepancies were found in Argolo et al. (2018), as the absence of a dorsal cheliceral seta, not retrorse teeth and antiaxial projection present on the fixed digit in both male and female, most of the not sexual characters originally described were confirmed in the types, additional females and males examined, and some of them are suggested to be included in the species diagnosis as presented bellow.
Revised diagnosis
Female and male. Cheliceral dorsal seta absent. Spermatodactyl 60 µm long, L-shaped. Dorsal shield with cerotegument, seven pairs of pairs of dorsal gland openings and 16 pairs of lyrifissures, ornamented with roundish/curved lines anteriad of z6 and scant broken lines behind; podonotal region with 21 pairs of setae (j1–j6, z1–z6, s1–s6, r3–r5); opisthonotal region with 15 pairs of setae (J1–J5, Z1–Z5, S1–S5) plus R1 in male. Unsclerotized cuticle along lateral margins of dorsal shield with five pairs of setae (R1–R5) in female or zero in male (except for R1, sometimes inserted out of the dorsal shield); most dorsal setae stout and barbed in the apical half, except for short, simple z1, j2, s1–s2, r4–r5 and R1; setae j3, z2–z4, s3–s6, S1–S5 and Z4 with a minute hook at the base; Z5 longer and thicker than other dorsal setae, especially in female, with fine barbs along almost its entire length. Pre-sternal area with one pair of lightly sclerotized and punctuate plates; ventrianal shield with five pairs of setae (JV1–JV4, ZV2) in addition to circumanal setae JV5, ZV1 and ZV3 (JV5 on shield, JV4 and ZV3 absent in male). Many dorsal and lateral setae of trochanter to tarsus of legs I–IV pilose. Tarsus I with a pilose macroseta.
Acknowledgements
To the two anonymous reviewers, and specially to Frédéric Beaulieu (Agriculture and Agri-Food Canada), for important improvements suggested to the manuscript. To Dr. Uilson Lopes (CEPLAC) for the support and availability of the study area. To Centro de Microscopia Eletrônica (CME), UESC, through the assistance of Simone Setúbal, for the help with the scanning electron microscopy. To CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil - Finance Code 001) and PPGPV (Programa de Pós-Graduação em Produção Vegetal), for the doctoral scholarship to the first author. This work was partially supported by UESC (Cad. PROPP: 073.6764.2019.0016668-32 and 073.6762.2019.0015086-12). GJM is a CNPq researcher.
References
- Argolo P.S., Santos J.C., Oliveira A.R., Moraes G.J. de. 2018. Two new species of Lasioseius Berlese (Acari: Blattisociidae) from Brazil, and a key for separation of the Brazilian species of the genus. Syst. Appl. Acarol., 23(8): 1567-1577.
- Athias-Henriot C. 1971. La divergence néotaxique des Gamasides (Arachnides). Bull. Sci. Bourgogne, 28: 93-106.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. 2. Le relevé organotaxique de la face dorsale adulte (gamasides protoadéniques, Phytoseiidae). Acarologia, 17: 20-29.
- Athias-Henriot C. 1969. Les organes cuticulaires sensoriels et glandulaires des Gamasides. Poroïdotaxie et adénotaxie. Bull. Soc. Zool. France, 94: 485-492.
- Beard, J.J. 2001. A review of Australian Neoseiulus Hughes and Typhlodromips De Leon (Acari: Phytoseiidae: Amblyseiinae). Invertebr. Taxon., 15(1): 73-158. https://doi.org/10.1071/IT99017
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Can., 47: 1-64. https://doi.org/10.4039/entm9747fv
- Lindquist E.E. 1994. Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for some ventrolateral body setae. Acarologia, 35(4): 323-326.
- Moraes G.J., Britto E.P.J., Mineiro J.F.C., Halliday B. 2016. Catalogue of the mite families Ascidae Voigts & Oudemans, Blattisociidae Garman and Melicharidae Hirschmann (Acari: Mesostigmata). Zootaxa, 4112(1): 1-299. https://doi.org/10.11646/zootaxa.4112.1.1
- Moraza M.L., Balanzategui I. 2023. A new species of Lasioseius (Endopodalius) (Acari: Mesostigmata: Blattisociidae) coexistent with the invasive agave weevil (Coleoptera: Dryophthoridae) in the southeast of the Iberian Peninsula. Acarologia 63(2): 605-614. https://doi.org/10.24349/vfv7-8tut
- Nuzzaci G. 1973. Contributi alla conoscenza degli Acari Eriofidi. Eriophyes reyesi n. sp. su Theobroma cacao L. Entomologica, 9: 9-12. doi: 10.15162/0425-1016/446-1
- Quintero-Gutiérrez E.J., Sandmann D., Cómbita-Heredia O., Klarner B., Widyastuti R., Scheu S. 2020. A new species of the genus Lasioseius (Acari: Blattisociidae) inhabiting litter of secondary rainforest in Sumatra, Indonesia. Acarologia 60(2): 338-352. https://doi.org/10.24349/acarologia/20204371



2024-07-04
Date accepted:
2024-10-11
Date published:
2024-10-18
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Araújo, Edineia da S. ; Stracieri, Juliana; de Moraes, Gilberto J. and Oliveira, Anibal R.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



