Effects of different diets on life table parameters of the predatory mite Phytoseius plumifer (Acari: Phytoseiidae)
Al-Azzazy, Mahmoud M.  1
and Alhewairini, Saleh S.
1
and Alhewairini, Saleh S.  2
2
1✉ Department of Plant Protection, College of Agriculture and Food, Qassim University, P.O. Box 6622, Buraidah 51452, Qassim, Saudi Arabia.
2Department of Plant Protection, College of Agriculture and Food, Qassim University, P.O. Box 6622, Buraidah 51452, Qassim, Saudi Arabia.
2024 - Volume: 64 Issue: 4 pages: 1019-1029
https://doi.org/10.24349/hwu2-v0ctOriginal research
Keywords
Abstract
Introduction
The predatory mite Phytoseius plumifer (Canestrini & Fanzago) (Acari: Phytoseiidae) is a type III generalist predator (McMurtry et al. 2013). It has a wide diet range including tetranychid (Kouhjani-Gorji et al. 2008), eriophyid (Louni et al. 2014), tarsonemid mites (Fouly et al. 2019), and plant pollen (Khodayari et al. 2013). The date palm mite, Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae), is a severe pest of the date fruit in the Middle Eastern countries (Palevsky et al. 2004), arid regions (Hussain 1974), and in most of the Northern Africa (Zaher et al. 1982; Ben Chaaban et al. 2011). In Saudi Arabia, O. afrasiaticus is also one of the primary pests of date palms. When it exists, fruits may sustain serious damage (Talhouk, 1991; Ben Chaaban et al. 2011). The activity of O. afrasiaticus begins and peaks through the unripe, Kimri stage characterized by high acidic, moisture contents and low sugar (Palevsky et al. 2005).
Currently, management practices of pests are generally limited to chemical pesticide application, and date palm orchards are submitted to very high levels of synthetic pesticides (Al-Dosary 2010). In addition to having detrimental effects on biodiversity, the use of synthetic pesticides to control phytophagous mites led to death of non-target organisms, the presence of residues in crops, and the development of mite resistance (Desneux et al. 2007; Guedes et al. 2016; Ziad 2017; Lorenzon et al. 2018; Malagnini et al. 2023). These side effects strongly limit the agricultural systems' sustainability and primarily necessitate the development of non-chemical techniques of pest control (Khan et al. 2023). Consequently, it is imperative to find effective biocontrol agents against O. afrasiaticus.
Generalist predatory mites can exploit various food sources, including natural prey and plant pollen (Xin and Zhang 2021; Shishehbor et al. 2022). Additionally, plant pollens are suitable as an alternative or additional food sources with high nutritional value for some predatory mites (McMurtry et al. 2013; Xin and Zhang 2021; Coll and Guershon 2022).
Several phytoseiid species rely on pollen to reach their maximum reproductive capacity (McMurtry et al. 2013; Barzkar et al. 2023). Several studies demonstrated that a source of pollen into a crop can boost the biological control due the improvement in reproduction and survival of predatory mites (Duarte et al. 2015; Vacacela et al. 2019; Van Rijn et al. 2022; Lamlom et al. 2024). For example, date palm pollen, Phoenix dactylifera L., castor-oil pollen, Ricinus communis L., and corn pollen, Zea mays L. are potential plant pollen diets for rearing P. plumifer (Zaher et al. 1969; Rasmy and El-Banhawy 1974; Khodayari et al. 2013). Although biological characteristics of P. plumifer fed on eriophyid (Rasmy and El-Banhawy 1974; Louni et al. 2014; Al-Azzazy and Alhewairini 2020), tetranychid (Zaher et al. 1969; Rasmy and El-Banhawy 1974; Kouhjani-Gorji et al. 2008; Shakarami and Bazgir 2017) and tarsonemid mite species (Fouly et al. 2019) have been studied previously, no information is available on P. plumifer attacking or feeding on O. afrasiaticus in Saudi Arabia or elsewhere.
Therefore, our purpose in the present study was to develop a comprehensive understanding of the comparison of natural prey O. afrasiaticus, corn pollen and date palm pollen diets, on the developmental times, survival, reproduction rate, and life table parameters of the predator P. plumifer. These data are necessary to understand and predict the performance of this natural enemy under field conditions.
Material and methods
Pollen collection
Fresh date palm pollen P. dactylifera var. Barhi was collected from date trees planted in the Qassim region, Saudi Arabia. Pollens were oven-dried at 26 °C for one day and then stored at −17 °C. The corn pollen, Z. mays was purchased from a rural supplier in Qassim city. Pollen was stored in a freezer at −5 °C for up to 2 weeks before experimentation.
Mite colonies
Predatory mite, P. plumifer was originally collected from unsprayed short heighted medium-laden date palm Barhi variety located in the Agricultural Experiments Station, Qassim University, Al-Mulida district (26.297875° N, 43.790684° E), Saudi Arabia. Individuals of O. afrasiaticus were collected from severely infested date palm trees in the same orchard. Predatory mite colonies were kept apart in rearing arenas constructed from kidney bean Phaseolus vulgaris L. (Fabaceae) leaves which were submerged in water-soaked cotton within plastic trays measuring 10 x 15 cm in length and 2 cm in height. The incubator was set to 32 °C, 50% RH, and 12L:12D photoperiod.
To preserve leaf freshness and a steady water level, water was fed to the plastic trays daily. To prevent mites from escaping, strips of moist tissue paper were placed around the margins of the bean leaves. Moreover, every 5 days, the predators were transferred to new rearing arenas. The predatory mite was fed with different stages of O. afrasiaticus. Before the trials started, the P. plumifer colony was reared for numerous generations feeding on O. afrasiaticus.
Experimental units
To obtain individuals with the same age for experiments, one hundred and sixty adult females from the stock colony were moved to a different arena. The females were allowed to oviposit for one day and all adults were then removed from the leaf discs. The freshly molted larvae were separately placed in rearing arenas consisting of bean leaf discs using a hairbrush.
Sample sizes for P. plumifer development, survival, reproduction, and life table parameters tests were 60 individuals in O. afrasiaticus, 50 in corn pollen, and 44 in date palm pollen treatments. The bean leaf discs including the midrib (2.0 cm in diameter) were placed upside down on a moistened layer of cotton (2 mm thick) in a Pyrex® Petri dish (5 cm in diameter × 2.5 cm high), following Al-Azzazy and Alhewairini (2020) methodology. Distilled water was added to every rearing arena daily, to maintain a constant water level. The mites were moved to newly cut discs as the leaf discs grew older.
Immature development, reproduction and predation rate
The development, survival rate, reproduction, sex ratio, fecundity, longevity, and life table parameters of P. plumifer were determined by feeding it with one of the following food sources: (1) O. afrasiaticus, (2) date palm pollen, and (3) corn pollen. In the prey treatment, the freshly molted larvae were fed on a mixture of 15 prey of different nymphal stages (protonymphs and deutonymphs) of O. afrasiaticus while each adult predator was fed on 25 individuals. Predation rate was recorded as the quantity of prey that was consumed. To ensure a plentiful food supply, everyday prey that was consumed was replaced by alive ones from the stock colony.
The experimental units were recorded daily at 12h intervals to record the developmental duration and survival rate of immature stages. Molting was verified by the existence of exuviae in the rearing arenas. After the adult female emerged in each treatment, a single male was placed to allow mating. To ensure multiple mating, the couple was maintained together up to the end of the experiments. The rearing arenas were monitored daily and pre-oviposition period, oviposition period, post-oviposition period, adult longevity, total fecundity, and mortality were recorded.
To distinguish predation rate of females from males, the predation rate of thirty males was evaluated under same conditions. Then, the mean of predation rate of male was subtracted from the average predation rate of the couples following Moghadasi et al. (2006).
Sex ratio
Thirty eggs were individually moved to a new arena to evaluate the predator's sex ratio. The hatched larvae were raised until they reached adulthood, at which point their sex was determined. In the pollen treatments, about 10 mg of corn or date palm pollen was added to respective treatment dishes. New pollen replaced the old every other day.
Statistical analysis
Developmental time, survival rate, pre-, post- and oviposition periods, longevity, and fecundity of P. plumifer fed O. afrasiaticus, corn and date palm pollen were analyzed using a one-way analysis of variance (one-way ANOVA). Tukey's Honestly Significant Difference (HSD) test was used to separate the means at α = 0.05. The statistical program SPSS version 22 (SPSS for Windows, SPSS Institute Inc., Chicago, IL, USA) was utilized for data analysis.
Life table parameters, i.e., net reproductive rate (R0 ), intrinsic rate of increase (rm ), finite rate of increase (λ), gross reproductive rate (GRR), and mean generation time (T) were calculated with the TWOSEX-MSChart program (Chi 2024). The population doubling time (DT) of P. plumifer was also estimated using the formula DT = ln (2)/rm, which indicates the number of days needed for the population to double in numbers. The population growth parameters' standard error (SE) was calculated according to (Maia et al. 2000).
Results
Developmental time, survival of immature stages and sex ratio



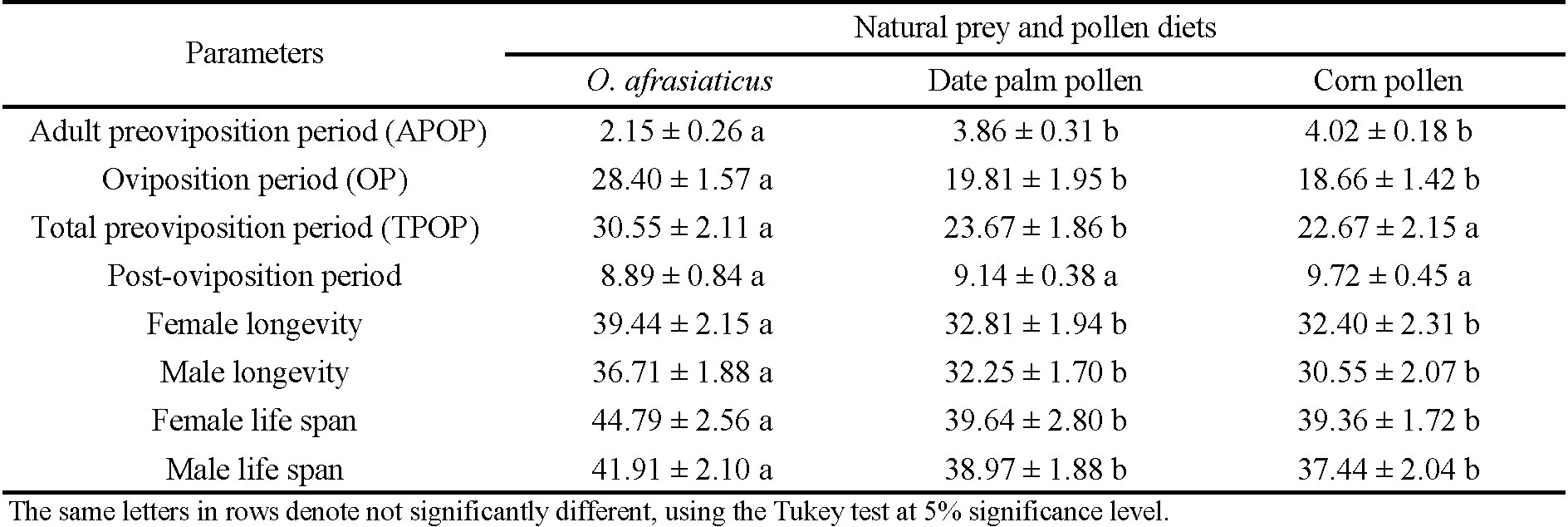


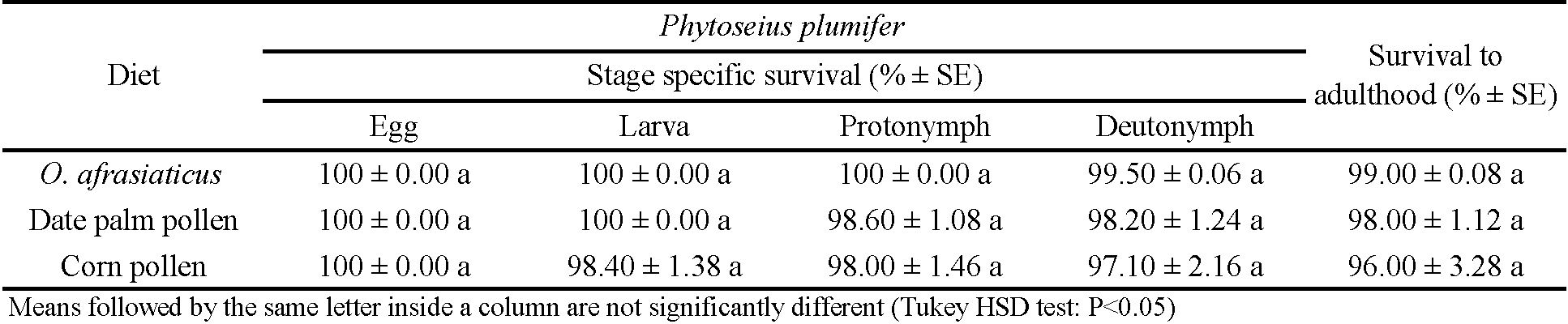


Phytoseius plumifer reached adult stage, and successfully preyed on O. afrasiaticus. The developmental time of P. plumifer on the tested diets are shown in (Tables 1 and 2). The results demonstrated that 100% of the eggs hatched. Total developmental time of P. plumifer males and females were affected significantly by diet type (females: F = 36.42; df = 2, 81; P < 0.001; males: F = 5.72; df = 2, 28; P = 0.004). The development time was significantly shorter for females and males fed on O. afrasiaticus than palm pollen or corn pollen (Tables 1 and 2). Survival rate during the immature stages of development exceeded 96% on the three tested diets (Table 3). The percentage survival rate of females and males was not significantly impacted by the type of diet (F = 1.46; df = 2, 7; P = 0.31) which was 99.00 ± 0.08, 98.00 ± 1.12, and 96.00 ± 3.28 for P. plumifer fed on O. afrasiaticus, date palm pollen, and corn pollen, respectively.





Contrary, the food type had a discernible, noteworthy impact on the sex ratio. Sex ratio on all diets varied between 50 and 73.33%. A maximum female-biased sex ratio was observed by P. plumifer fed on O. afrasiaticus (Table 5). The female-to-male offspring ratio was about (2.2:1, 2:1 and 1.5:1), when predator fed on O. afrasiaticus, date palm pollen, and corn pollen, respectively. Although females developed slightly slower than males, there was no significant variation between the periods of development on the three tested diets.
Reproduction
In general, no significant difference in the pre-oviposition period was observed between date palm pollen and corn pollen, while the shortest pre-oviposition period was recorded on O. afrasiaticus. The oviposition period was significantly different among diets (F = 31.56; df = 2, 86; P < 0.001). The longest overall oviposition duration was observed in case of feeding on O. afrasiaticus, whereas it was the shortest when the predators fed on corn pollen (Table 2).
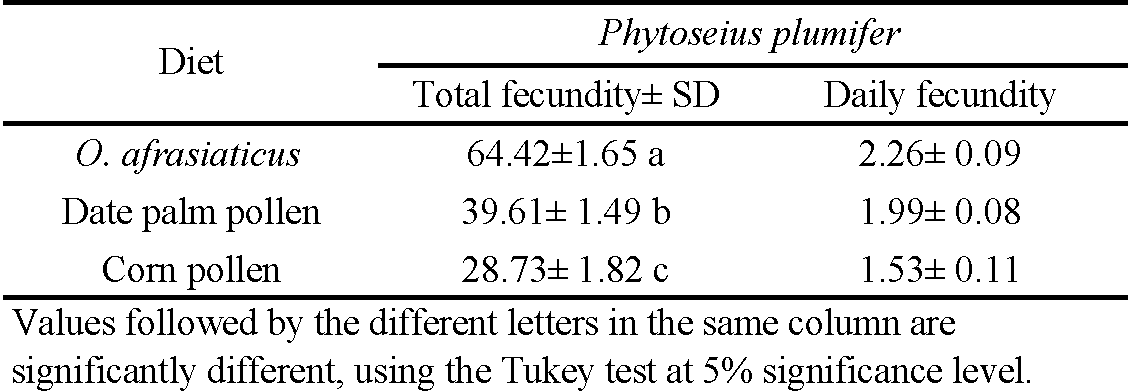
Total fecundity was significantly affected by diet (F = 35.32; df = 2, 84; P < 0.001; Table 4). Phytoseius plumifer females fed on O. afrasiaticus exhibited a higher rate of fecundity than those feeding on date palm pollen or corn pollen. The highest number of eggs was recorded when P. plumifer fed on O. afrasiaticus, while the minimum was when P. plumifer fed on corn pollen. Therefore, it could be concluded that the date palm mite, O. afrasiaticus was suitable prey for P. plumifer. There was no difference in P. plumifer post-oviposition periods between the three diets that were examined. The diet had a clear significant effect on the longevity (F = 59.82; df = 2, 80; P < 0.001 for females; F = 20.34; df = 2, 75; P < 0.001 for males; Table 2). Compared to diets containing date palm pollen or corn pollen, P. plumifer males and females lived longer when fed the O. afrasiaticus diet.
Predation rate
The larval stage of P. plumifer did not feed on the date palm mite, O. afrasiaticus, and predation began to occur just after the mites entered the protonymphal stage. During the immature stages, P. plumifer females consumed mean of 9.66 ± 0.95 O. afrasiaticus nymphal stages per day. The mean daily predation rate was significantly affected by predator age (P < 0.001), daily consumption increased significantly in adults. Predation rates increased throughout the pre-oviposition and oviposition periods and reduced during the post-oviposition period. Subsequently, daily predation decreased with age. Through the oviposition period, the female consumed an average of 21.65 ± 1.64 O. afrasiaticus, marking the greatest rate that was observed. The number of preys consumed during the longevity reported for P. plumifer females was 717.31 ± 7.90 prey, while for males, it was 531.92 ± 5.41 prey.
Life-table parameters



Life table parameters proved that food type had significant impacts on P. plumifer life history (Table 6). The females reared on O. afrasiaticus showed the highest net reproductive rate (Ro ), intrinsic rate of natural increase (rm ) and finite rate of increase (λ). On the other hand, P. plumifer individuals reared on O. afrasiaticus had the shortest mean generation time. Life table parameters were estimated as net reproduction rate (Ro ) of 28.72, 22.21, 18.44, mean generation time (T) of 14.16, 17.45, 22.67 d, the intrinsic rate of population increase (rm ) 0.278, 0.241, 0.141 day–1, finite rate of increase (λ) of 1.34, 1.29, 1.19 for P. plumifer when fed on O. afrasiaticus, date palm pollen, and corn pollen respectively. Note that P. plumifer fed on corn pollen had the lowest values of life table parameters and the longest mean generation time.
Discussion
It is the first time to assess the biology and predatory efficiency of P. plumifer against O. afrasiaticus. The current study demonstrates that O. afrasiaticus is a highly nutritious and suitable prey, leading to rapid development and a high rate of fecundity. The predatory mite was also able to develop and reproduce successfully when fed on date palm pollen or corn pollen. This is a significant step in developing management strategies against O. afrasiaticus.
In this study, the type of diet had a clear influence on P. plumifers immature development. Here, date palm mite O. afrasiaticus may be excellent and more favorable food than date palm pollen or corn pollen diets.
Total development time of P. plumifer females fed O. afrasiaticus (at 32 °C, 5.97 days) in the current study was shorter than for P. plumifer fed on Tetranychus urticae Koch at 30 °C was 6.60 days (Gorji et al. 2008), Rhyncaphytoptus ficifoliae (Keifer) at 25 °C was 8.73 days, (Louni et al. 2014), Eotetranychus hirsti Pritchard & Baker at 30 °C was 8.62 days (Shakarami and Bazgir 2017), Tegolophus hassani (Keifer) at 35 °C was 5.92 days (Al-Azzazy and Alhewairini 2020). The developmental time of immature females of P. plumifer fed on T. urticae at 35 °C (5.3 days) which was very close to the current results against O. afrasiaticus (Gorji et al. 2008). On the other hand, the development of P. plumifer immature females was longer in our study (5.35 d) than reported when fed on the red spider mite Tetranychus cinnabarinus (Boisduval) 3.8 days (Zaher et al. 1969). The developmental time of 6.72 days for P. plumifer females fed on the date palm pollen in the current study was shorter when compared to 10.5 days reported by Zaher et al. (1969).
Likewise, the development time of 6.89 d for P. plumifer females fed corn pollen in our study was short when compared to 10.00 days reported by (Khodayari et al. 2013). Contrasting our findings with those of Kouhjani-Gorji et al. (2008) and Hamedi et al. (2011) it can be concluded that when corn pollen was the only food source, immature development took longer, and adult female longevity and fecundity were decreased, altogether resulting in a significant reduction in rm of 0.112 day-1. However, the predator was able to develop and reproduce successfully on the corn pollen, which is consistent with the results of (Khodayari et al. 2013). Furthermore, O. afrasiaticus-fed P. plumifer developed quicker than their prey. For instance, O. afrasiaticus female took about 12.45 days to mature at 30 °C (Al-Jboory 2010). The developmental time of P. plumifer fed on O. afrasiaticus in this study was also shorter than the developmental time reported for potential mite prey such as black flat mite, Brevipalpus phoenicis (Geijskes) 18.81 days at 26 °C (Lal 1979), T. urticae 6.7 days at 29.4 °C (Carey and Bradley 1982), and red palm mite, Raoiella indica Hirst 13.33 days at 30 °C (Fidelis et al. 2019).
The capability of the predator to develop quicker than prey species has significant implications for augmentative biocontrol. It implies that P. plumifer augmentative releases might help to eradicate pest populations in date palm orchards. In the current study, when the predator fed on natural prey source O. afrasiaticus, it exhibited higher indicators of oviposition period, total fecundity, and adult longevity compared to when it fed on date palm pollen or corn pollen. The maximum fecundity was attained when P. plumifer fed on O. afrasiaticus (64.42 eggs/female). This value was higher when compared to mites that fed on T. urticae (45.23 eggs/female) (Kouhjani-Gorji et al. 2012), on R. ficifoliae (28.47 eggs/female) (Louni et al. 2014), on E. hirsti (35.71 eggs/female) (Shakarami and Bazgir 2017), and on O. niloticus (50.80 eggs/female) (Al-Azzazy and Alhewairini 2020).
These findings suggest that a diet of O. afrasiaticus is more suitable for P. plumifer reproduction.Phytoseius plumifer produced more eggs with the O. afrasiaticus diet suggest that the quality of macronutrients necessary for ovary development and egg maturation are greater in O. afrasiaticus. Adult longevity of P. plumifer was also affected by diet type in this study; females and males lived longer when fed O. afrasiaticus. The female longevity in this study (39.44 ± 2.15 days) was close to the longevity of females feeding on T. urticae in a study by Kouhjani-Gorji et al. 2008 (43.83 ± 1.07 days) at 30 °C. Phytoseius plumifer females consumed on an average of 739.26 individuals of O. afrasiaticus during the whole life-span in this study, while they consumed 346 individuals of T. cinnabarinus (Zaher et al. 1969), and 517.33 individuals of T. urticae (Al-Azzazy and Alhewairini 2020).
The life table parameters of P. plumifer were affected by diet. Many biological studies have affirmed that the high-quality food sources led to increased values in life table parameters (Jiale et al. 2016; Ali et al. 2023; Barzkar et al. 2023). The highest intrinsic rate of increase (rm ) in the current study was recorded when P. plumifer fed on O. afrasiaticus (0.278 d-1). This value is greater than those stated by Hamedi et al. 2011 when P. plumifer fed on T. urticae (0.200 at 26 °C), E. hirsti 0.180 at 30 °C (Shakarami and Bazgir, 2017), R. ficifoliae 0.154 at 25 °C (Louni et al. 2014), corn pollen 0.112 at 27 °C (Khodayari et al. 2013) and Polyphagotarsonemus latus (Banks) 0.19 at 26 °C (Fouly et al. 2019).
Moreover, the values of (Ro), (rm ) and (λ) of P. plumifer at 32 °C and 50% RH in this study are also higher than that of some other predacious mites on the same food source, O. afrasiaticus, including Euseius scutalis (Athias-Henriot) (Ro= 13.60, rm = 0.161 and λ = 1.17 at 26 °C and 70% RH) (Al-Shammery 2010), Cydnoseius negevi (Swirski and Amitai) (Ro= 17.35, rm = 0.19 and λ = 1.21 at 35 °C and 35% RH), and Neoseiulus barkeri Hughes (Ro= 13.84, rm = 0.16 and λ = 1.17 at 35 °C and 35% RH) (Negm et al. 2014), which shows that the use of O. afrasiaticus in P. plumifer mass rearing is appropriate. In view of this, P. plumifer could be a useful biological control agent for date palm mite O. afrasiaticus. According to (Kouhjani-Gorji et al. 2012), when testing P. plumifer at six constant temperatures, the highest intrinsic rate of increase (0.257) was recorded at 30 °C, which is close to the result of P. plumifer feeding on O. afrasiaticus (rm = 0.278) reported in the present study. The intrinsic rate of increase of P. plumifer when feeding on corn pollen is 0.112 (Khodayari et al. 2013), which is lower than when feeding on corn pollen reported in the present study (rm = 0.195). Previous studies have not assessed the effects of a diet of date palm pollen on life table parameters of P. plumifer. Moreover, the values of (Ro), (rm ) and (λ) of P. plumifer in this study are also higher than that of some other predacious mites when they fed date palm pollen, including C. negevi (Ro= 13.99, rm = 0.20 and λ = 1.22 at 30 °C and 35% RH) (Alatawi et al. 2018), Euseius scutalis (Ro= 17.88, rm = 0.23 and λ = 1.26 at 26 °C and 75% RH) (Fouly et al. 2013), and A. swirskii (Ro= 6.06, rm = 0.08 and λ = 0.08 at 25 °C and 66% RH) (Riahi et al. 2017). This research provides valuable information into the life history traits and life table parameters of P. plumifer under different diets. The results contribute to our knowledge of the reproductive performance and population dynamics of this predacious mite species. Additional studies are needed to explore the possibility of using this predator under field conditions.
Conclusion
This study indicates that P. plumifer exhibits favorable fecundity, and the capacity to establish populations using natural prey O. afrasiaticus. This suggests that P. plumifer can be utilized as a biocontrol agent of the date palm mite, O. afrasiaticus. Additionally, P. plumifer demonstrated the capability of development on alternative food sources, including date palm pollen and corn pollen, yielding favorable results, specifically when fed on date palm pollen. Moreover, date palm pollen is a supplementary source of food in critical periods when prey is absent or insufficient.
Author contributions
This manuscript was drafted by Mahmoud M. Al-Azzazy and Saleh S. Alhewairini. Laboratory work and statistical analysis were performed by Mahmoud M. Al-Azzazy. Saleh S. Alhewairini planned and assisted with the implementation of this study.
Competing interests
The authors declare no competing interests.
Funding
No funding was provided for this study.
References
- Alatawi F.J., Basahih G.S., Kamran M. 2018. Suitability of date palm pollen as an alternative food source for the predatory mite Cydnoseius negevi (Swirski & Amitai) (Acari: Phytoseiidae) at a low relative humidity. Acarologia, 58(2): 357-365. https://doi.org/10.24349/acarologia/20184247
- Al-Azzazy M.M., Alhewairini S.S. 2020. A life table analysis to evaluate biological control of four mite species associated with olive trees using the predatory mite, Phytoseius plumifer (Acari: Phytoseiidae) in Saudi Arabia. Pak. J. Agri. Sci, 57(1): 299-305. https://doi.org/10.21162/PAKJAS/20.9469
- Al-Dosary N.H. 2010. Evaluate efficiency of some insecticides and sticker color traps to protected date palm fruits infested by dust mite Oligonychus afrasiaticus (McGregor) and lesser date moth Batrachedra amydraula (Merck). Basrah J Agric Sci, 23: 1-22. https://doi.org/10.33762/bagrs.2010.56635
- Ali Z., Parviz S., Fatemeh Naser N., Elham R. 2023. Life history traits and population parameters of the predatory mite Euseius scutalis (Acari: Phytoseiidae) fed on Tetranychus turkestani (Acari: Tetranychidae) and pollen from three different plants. Acarologia, 63(3): 945-954. https://doi.org/10.24349/mrqf-arrz
- Al-Jboory I.J., Al-Suaide T.M. 2010. Effect of temperature on the life history of the old world date mite, Oligonychus afrasiaticus (Acari: Tetranychidae). In: Sabelis, M., Bruin, J. (eds) Trends in Acarology. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9837-5_58
- Al-Shammery K.A. 2010. Different biological aspects of the predaceous mite Euseius scutalis (Acari: Gamasida: Phytoseiidae) and the effects due to feeding in three tetranychid mite species in Hail, Saudi Arabia. Asian J. Biol. Sci, 8: 77-81. https://doi.org/10.3923/ajbs.2010.77.84
- Barzkar M., Sheshehbor P., Habibpour B., Hemmati SI., Riahi E. 2023. Development, survival, and reproduction of Amblyseius swirskii (Athias- Henriot) (Acari: Phytoseiidae) feeding on different pollen grains. Acarologia, 63: 1062-1071. https://doi.org/10.24349/izmp-v7mc
- Ben Chaaban S., Chermiti B., Kreiter, S. 2011. Comparative demography of the spider mite, Oligonychus afrasiaticus, on four date palm varieties in southwestern Tunisia. J Insect Sci, 11:1-12. https://doi.org/10.1673/031.011.13601
- Carey J.R., Bradley, J.W. 1982. Developmental rates, vital schedules, sex ratios and life tables for Tetranychus urticae, T. turkestani and T. pacificus (Acarina: Tetranychidae) on cotton. Acarologia, 23: 333-345.
- Chi H. 2024. TWOSEX-MSChart: a computer program for the age-stage, two-sex life-table analysis.
- Coll M., Guershon M. 2022. Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu. Rev. Entomol, 47: 267-297. https://doi.org/10.1146/annurev.ento.47.091201.145209
- Desneux N., Decourtye A., Delpuech, J.M. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol, 52, 81-106. https://doi.org/10.1146/annurev.ento.52.110405.091440
- Duarte, M. V. A., Venzon, M., Bittencourt, M. C. D., Rodriguez-Cruz F. A., Pallini A. & Janssen A. 2015. Alternative food promotes broad mite control on chilli pepper plants. Biocontrol, 60: 817-825. https://doi.org/10.1007/s10526-015-9688-x
- Fidelis E.G., Reis M.A.S., Negrini, M., Navia D. 2019. Life table parameters of the red palm mite Raoiella indica (Acari: Tenuipalpidae) at various temperatures and for sexual and asexual reproduction. Exp Appl Acarol., 78: 535-546. https://doi.org/10.1007/s10493-019-00407-y
- Fouly A.H., Nassar O.A., Osman M.A. 2013. Biology and Life tables of Esieus scutalis (A.-H.) reared on different kinds of food. J. Entomol, 10: 199-206. https://doi.org/10.3923/je.2013.199.206
- Fouly, A.H., Osman, M.A., Abdelghany, O.H. 2019. Effect of Nourishment and Mutual Interference on Feeding Capacity and Life Table Parameters of Phytoseius plumifer (C. & F.) (Acari: Phytoseiidae). J. Plant Prot. and Path, 10 (3): 165 - 169. https://doi.org/10.21608/jppp.2019.40921
- Gorji M.K., Kamali K., Fathipour Y., Aghdam H.R. 2008. Temperature-dependent development of Phytoseius plumifer (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Syst Appl Acarol, 13: 172-181. https://doi.org/10.11158/saa.13.3.2
- Guedes R.N.C., Smagghe G., Stark J.D., Desneux N. 2016. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol, 61: 43-62. https://doi.org/10.1146/annurev-ento-010715-023646
- Hamedi N., Fathipour Y., Saber M. 2011. Sublethal effects of abamectin on the biological performance of the predatory mite, Phytoseius plumifer (Acari: Phytoseiidae). *xp. Appl. Acarol., 53: 29-40. https://doi.org/10.1007/s10493-010-9382-8
- Hussain, A.A. 1974. Date palms & dates with their pests in Iraq. Univ. Baghdad, Min. Higher Educ. Sci. Res., Baghdad.
- Jiale L.V., Yang K., Wang E., Xuenong X.U. 2016. Prey diet quality affects predation, oviposition and conversion rate of the predatory mite Neoseiulus barkeri (Acari: Phytoseiidae). Syst Appl Acarol., 21: 279-287. https://doi.org/10.11158/saa.21.3.3
- Khan B.A. et al. 2023. Pesticides: Impacts on Agriculture Productivity, Environment, and Management Strategies. In: Aftab, T. (eds) Emerging Contaminants and Plants. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-031-22269-6_5
- Khodayari S., Fathipour Y., Kamali, K. 2013. Life history parameters of Phytoseius plumifer (Acari: Phytoseiidae) fed on corn pollen. Acarologia, 53: 185-189. https://doi.org/10.1051/acarologia/20132087
- Kouhjani-Gorji, M., Fathipour, Y. and Kamali, K. 2012. Life table parameters of Phytoseius plumifer (Phytoseiidae) fed on two-spotted spider mite at different constant temperatures. Int. J. Acarol., 38: 377-385. https://doi.org/10.1080/01647954.2012.657239
- Kouhjani-Gorji M., Kamali K., Fathipour Y., Ranjbar Aghdam H. 2008. Temperature-dependent development of Phytoseius plumifer (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) Syst. Appl. Acarol:, 13: 172-181. https://doi.org/10.11158/saa.13.3.2
- Lal, L. 1979. Biology of Brevipalpus phoenicis (Geijskes) (Tenuipalpidae: Acarina). Acarologia, 20(1): 97-101.
- Lamlom M., Fahim S.F., Momen F.M. 2024. The effects of maize pollen on development and population growth potential of Amblyseius swirskii and Cydnoseius negevi (Acari: Phytoseiidae) in subsequent generations. Persian J. Acarol., 13: 115-130. https://doi.org/10.22073/pja.v13i1.82742
- Louni M., Jafari, S., Shakarami, J. 2014. Life table parameters of Phytoseius plumifer (Phytoseiidae) fed on Rhyncaphytoptus ficifoliae (Diptilomiopidae) under laboratory conditions. Syst Appl Acarol., 19(3): 263-274. https://doi.org/10.11158/saa.19.3.3
- Maia A.H.N., Alfredo J.B., Campanhola C. 2000. Statistical influence on associated fertility life table parameters using Jackknife technique: computational aspects. J. Econ. Entomol., 93: 511-518. https://doi.org/10.1603/0022-0493-93.2.511
- Lorenzon M., Pozzebon A., Duso, C. 2018. Biological control of spider mites in North-Italian vineyards using pesticide resistant predatory mites. Acarologia, 58(Suppl), 98-118. https://doi.org/10.24349/acarologia/20184277
- Malagnini V., Baldessari M., Duso C., Pozzebon, A., Angeli, G. 2023. Side-effects of a number of insecticides on predatory mites in apple orchards. Acarologia, 63: 17-28. https://doi.org/10.24349/9tgw-xrc4
- McMurtry J.A., Moraes G.J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18(4): 297. https://doi.org/10.11158/saa.18.4.1
- Moghadasi M., Hajizadeh J., Saboori A., Nowzari, J. 2006. Biology of Phytoseius plumifer (Canestrini & Fanzago) (Acari: Phytoseiidae) feeding on Tetranychus urticae Koch (Acari: Tetranychidae). Conference: 14th National and 2nd International Conference of Biology, At Tarbiat Modares University.
- Negm M.W., Alatawi F.J., Aldryhim Y.N. 2014. Biology, Predation, and Life Table of Cydnoseius negevi and Neoseiulus barkeri (Acari: Phytoseiidae) on the Old-World Date Mite, Oligonychus afrasiati¬cus (Acari: Tetranychidae). J Insect Sci, 14: 1-6. https://doi.org/10.1093/jisesa/ieu039
- Palevsky E., Borochov-Neori H., Gerson U. 2005. Population dynamics of Oligonychus afrasiaticus in the southern Arava Valley of Israel in relation to date fruit characteristics and climatic conditions. Agric. Forest Entomol., 7 (4), 283-290. https://doi.org/10.1111/j.1461-9555.2005.00270.x
- Palevsky E., Ucko O., Peles S., Yablonski S., Gerson U. 2004. Evaluation of control measures of Oligonychus afrasiaticus infesting date palm cultivars in the Southern Arava Valley of Israel. J Crop Prot, 23: 387-392. https://doi.org/10.1016/j.cropro.2003.09.008
- Rasmy A.H., Elbanhawy E.M. 1974. The phytoseiid miteP hytoseius plumifer as a predator of the eriophyid mite Aceria ficus [Acarina]. Entomophaga, 19: 427-430. https://doi.org/10.1007/BF02372777
- Riahi E., Fathipour Y., Talebi A.A., Mehrabadi M. 2017. Linking life table and consumption rate of Amblyseius swirskii (Acari: Phytoseiidae) in presence and absence of different pollens. Ann. Entomol. Soc. Am., 110: 244-253. https://doi.org/10.1093/aesa/saw091
- Shakarami, J., Bazgir, F. 2017. Effect of temperature on life table parameters of Phytoseius plumifer (Phytoseiidae) fed on Eotetranychus hirsti (Tetranychidae). Syst Appl Acarol., 22(3): https://doi.org/10.11158/saa.22.3.7
- Shishehbor P., Rahmani Piyani A., Riahi, E. 2022. Effects of different pollen diets in comparison to a natural prey, Tetranychus turkestani (Acari: Tetranychidae), on development, survival, and reproduction of Euseius scutalis (Acari: Phytoseiidae). Syst. Appl. Acarol., 27(10): 2111-2122. https://doi.org/10.11158/saa.27.10.19
- Talhouk A.S. 1991. On the management of the date palm and its arthropod enemies in the Arabian Peninsula. J. Appl. Entomol., 111: 514-520. https://doi.org/10.1111/j.1439-0418.1991.tb00354.x
- Vacacela et al. 2019. Supplementary food for Neoseiulus californicus boosts biological control of Tetranychus urticae on strawberry. Pest Manag. Sci., 75:1986-1992. https://doi.org/10.1002/ps.5312
- Van Rijn P.C., van Houten Y.M., Sabelis M.W. 2002. How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology, 83: 2664-2679. https://doi.org/10.2307/3072005
- Xin T., Zhang, Z. 2021. Suitability of pollen as an alternative food source for different developmental stages of Amblyseius herbicolus (Chant) (Acari: Phytoseiidae) to facilitate predation on whitefly eggs. Acarologia, 61(4): 790-801. https://doi.org/10.24349/bIV1-2heN
- Zaher M.A., Gomaa E.A., El-Enany M.A. 1982. Spider mites of Egypt (Acari: Tetranychidae). Int. J. Acarol., 8: 91-114. https://doi.org/10.1080/01647958208683284
- Zaher M.A., Wafa A.K., Shehata K.K. 1969. Life history of the predatory mite Phytoseius plumifer and the effect of nutrition on its biology (Acarina: Phytoseiidae). Entomol. Exp. Appl., 12(4):383-388. https://doi.org/10.1111/j.1570-7458.1969.tb02534.x
- Ziad B. 2017. Evaluation of three pesticides against phytophagous mites and their impact on phytoseiid predators in an eggplant open-field. Acarologia, 57: 529-539. https://doi.org/10.24349/acarologia/20174170



2024-02-17
Date accepted:
2024-09-30
Date published:
2024-10-04
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Al-Azzazy, Mahmoud M. and Alhewairini, Saleh S.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



