New record and redescription of invasive cassava green mite, Mononychellus mcgregori (Flechtmann & Baker) in India on cassava, Manihot esculenta Crantz
Dyamanagouda, Poojar  1
; Rajashekharappa, Kenchappa
1
; Rajashekharappa, Kenchappa  2
; Chinnamadegowda, Channegowda
2
; Chinnamadegowda, Channegowda  3
; Mulimani, Vidya
3
; Mulimani, Vidya  4
and Flechtmann, Carlos H. W.
4
and Flechtmann, Carlos H. W.  5
5
1✉ Department of Entomology, College of Agriculture, KSNUAHS, Shivamogga- 577204, India.
2Department of Entomology, College of Agriculture, KSNUAHS, Shivamogga- 577204, India.
3Department of Entomology, University of Agricultural Sciences, Bengaluru- 560065, India.
4Department of Entomology, University of Agricultural Sciences, Bengaluru- 560065, India.
5Escola Superior de Agricultura Luiz de Queiroz, ESALQ/Universidadede São Paulo, USP- Campus Piracicaba, SP, Brazil.
2024 - Volume: 64 Issue: 3 pages: 879-890
https://doi.org/10.24349/2n0h-5tyeOriginal research
Keywords
Abstract
Introduction
Cassava (Manihot esculenta Crantz), commonly known as Tapioca, continues to be a crop of food security for millions of people, especially in developing countries across the globe. It is an essential alternate energy source to meet the increasing population's demands. Originating in tropical America, cassava was introduced to southern peninsular India in the 17th century by the Portuguese, primarily in Kerala, before spreading to neighbouring states like Tamil Nadu, Andhra Pradesh, and Karnataka. Currently, India cultivates cassava across 172 thousand hectares, yielding 6.257 million tonnes. Tamil Nadu and Kerala lead in production, contributing 3.4 million tonnes and 2.5 million tonnes, respectively, primarily supporting employment through starch extraction and sago export (India Stat 2023). In recent times Cassava pest complexes have emerged and pose a threat to Cassava production in Asian countries. The major arthropod complex is increasing too significantly in coevolved specific pests of cassava. Among the pests, the cassava mealybug (Phenacoccus manihoti) and cassava green mites (Mononychellus spp.) complexes stand first (Bellotti et al. 2012; Graziosi et al. 2016). Mononychellus mite complex includes M. tanajoa (Bondar, 1938), M .mcgregori (Flechtmann & Baker, 1970) and M. caribbeanae (McGregor, 1950) where these mites are highly host-specific and invasive threats to cassava growing areas. The genus Mononychellus is represented by 31 phytophagous mite species. Including the genus Mononychellus, 57 species in 12 genera of Tetranychidae have been recorded on M. esculenta and other Manihot species so far (Byrne 1983; Migeon and Dorkeld 2023; IPPC 2023). Thirty-one species are known under Mononychellus, four of which viz., M. tanajoa, M. caribbeanae, M. progresivus Doreste, 1981 and M. mcgregori attained pest status in the cultivation of cassava worldwide (Parsa et al. 2015; Flechtmann and De Queiroz 2015).
The present study reports Mononychellus mcgregori (Flechtmann & Baker) for the first time from India and as the original descriptions are not very well detailed, we here provide a complementary description of the species. Further, the present study uses integrative taxonomy, both morphological and molecular studies to confirm the identity of species.
Material and Methods
Morphological identification
Mite infested cassava leaves were collected in polybags and brought to the laboratory for detailed observations under a Carl Zeiss® (Stemi 305) binocular stereo zoom microscope. Adult male and female mites were used for micro slide preparation using Hoyer's medium and cleared on a Hotplate at 70 °C for three days. The specimens were examined and photographed using a Zeiss® AxioScope.A1 phase-contrast and DIC microscope attached with a Nikon® DL7500 camera and the illustrations were edited with Adobe Photoshop CS 2020®. Species identification was done by using taxonomic keys (Flechtmann and de Queiroz 2015) followed by confirming morphological characters using original descriptions (Pritchard and Baker 1955; Flechtmann and Baker 1970) and supplementary descriptions (Gutierrez 1987).
In the redescription, the morphometric data are presented with the mean followed by the ranges in parenthesis, while the leg setal formula is presented with the number of tactile setae followed by the solenidia, eupathidia and duplex setae in parentheses. All the measurements are in micrometers.
All microscopic slide mounts are deposited at the mite repository of AINPAA (All India Network Project on Agriculture Acarology), UAS (University of Agricultural Sciences), GKVK (Gandhi Krishi Vignan Kendra), Bengaluru, Karnataka, India.
DNA analysis
Isoline cultures of Mononychellus mcgregori
Mites were maintained in the laboratory on Cassava leaves for study. Mites were freshly taken from the culture for DNA isolation.
DNA extraction and amplification
Total DNA was extracted from three to five individual mites using the modified CTAB method (Doyle and Doyle 1987). The mitochondrial cytochrome c oxidase subunit I gene (COI) barcode region was amplified using primers LCO1490 and HCO2198 (Folmer et al. 1994). Polymerase chain reactions (PCR) were conducted using compositions of Taq buffer 1x (Tris with 15 mM MgCl2), 2.5 mM of each dNTP, 1 μM of each primer (Sigma-Aldrich®), 0.5 U Taq DNA polymerase (Genei, Bengaluru, India) in a 25 μL final volume.
Amplification of DNA was done in a Bio-Rad Master cycler (Bio-Rad, USA) with the following PCR conditions: initial denaturation at 94 °C 2 minutes, 94 °C 1 minute, annealing at 47 °C for 1 minute, extension and final extension at 72 °C for one minute followed by 10 minutes then holding at 4 °C. PCR amplicons were visualized on a 2% agarose gel using a Bio-Rad gel documentation unit (AINP, Acarology Lab, GKVK, Bengaluru). The sequence was obtained through Sanger sequencing and then edited using BioEdit v7.2.5 and aligned using the default parameters. All the sequences were verified for stop codons and insertions/deletions. BLASTs of nucleotide sequences were carried out on the NCBI database to determine the species identity by match percentage levels.
Results
Family Tetranychidae Donnadieu, 1875
Subfamily Tetranychinae Berlese, 1913
Tribe Tetranychini Reck, 1950
Genus Mononychellus Wainstein, 1971
Type species: Tetranychus planki McGregori, 1950
Eotetranychus planki (McGregori, 1950) New combination in Pritchard and Baker, 1955 Fig.108–110
Mononychus mcgregori Flechtmann & Baker, 1970. Description
Mononychellus mcgregori (Flechtmann & Baker 1970). New combination by Flechtmann and Baker (1975) (Fig: 109 in Pritchard and Baker (1955) is Mononychellus mcgregori (Flechtmann & Baker)
The name Mononychellus was proposed as a new combination for the genus Mononychus Wainstein, 1960 (with the type species Tetranychus planki McGregor, 1950). This change was necessary because the genus name Mononychus was already assigned to Mononychus Schueppel, 1824 (Insecta: Coleoptera) and Mononychus Agassiz, 1846 (Insecta: Hemiptera) (Wainstein 1970). Tuttle et al. (1976) characterised the genus Mononychellus Wainstein, 1960 by the following features: duplex setae on tarsus of leg I distal and adjacent; empodium split near the middle into three proximal hairs and tenent hairs on pad like claw (Bolland et al. 1998); dorsal striae of opisthosoma with different patterns; dorsal idiosomal setae usually strongly serrate and sometimes borne on small tubercles; two pairs of anal setae (Wainstein 1960, 1971; Meyer 1974; Tuttle et al. 1976).
Mononychellus mcgregori (Flechtmann & Baker, 1970)
Figs. 1–7
Diagnosis
Female — Body round to oval, dorsal idiosomal striation more or less transverse, dotted irregular striae around bases of setae. Longidutinal to transverse striae pattern between sc1 and c1 , looks like a round anastomous pattern with semi-circular lobes on striations (fig. 1C). Dorsal setae serrated and enlarged distally, set on small tubercles (Tuttle et al. 1976; Gutierrez 1987; Flechtmann and De Queiroz 2015; IPPC 2023); bulbous peritreme at gnathosomal region. Terminal senisillum (spinneret) on palptarus wider than long. Opisthosomal setae c1, d1, e1 and f1 longer or equal to distance between consecutive setae. Setae v2, c3, and h1 shorter than other setae. Ventral striation at anal region semi-circular lobe like (figs. 1E, 7D) along the striae with regular gaps. Leg tarsus I with four tactile setae and one solenidion proximal to proximal duplex setae. Tibia I with 9 tactile setae and one solenidion.
Male — Body smaller than female and narrow towards the caudal end. Terminal sensillum reduced to a minute-thick triangle on palpus. Dorsal striations strongly lobed with long, serrated and tapering setae on tubercles. Legs longer than idiosoma, elongate and end with a bifid claw. Aedeagus elongated, long shaft with a slight bent ventrad and with distal triangular knob. Anterior knob projection extended forward more sharply than the posterior projection.
Redescription
Female (n=5)
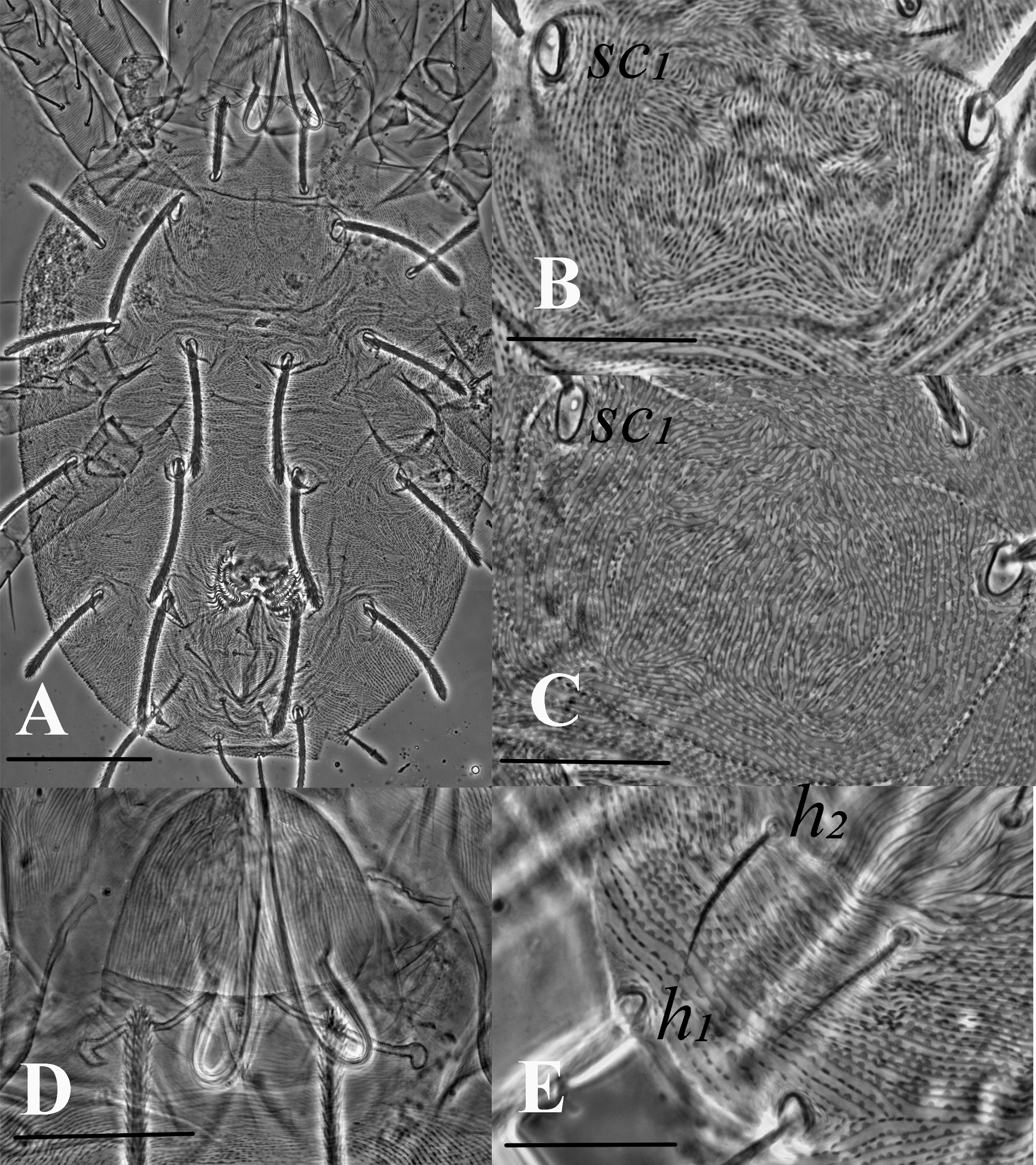


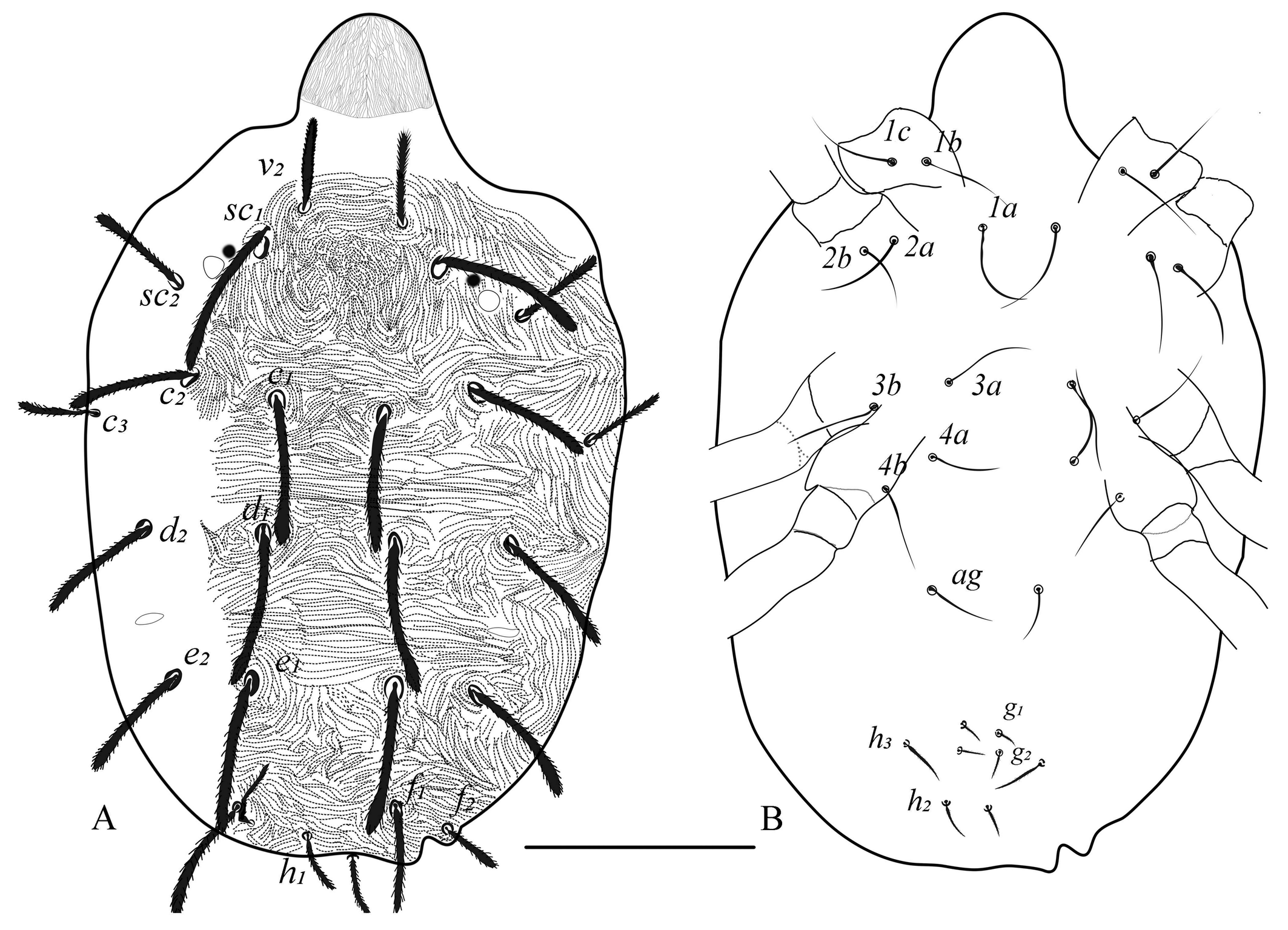


Dorsum (Figs. 1, 2) — Length of idiosoma (excluding gnathosoma) 340 (317–360), width (at c3 level) 252 (228–290). Body more round to oval. Dorsal body striae mostly transverse except in propodosoma (longitudinal to irregular), dorsal striations dotted or lobed (depending on morphotype and specimens), median prodorsal striations denser, more dotted or lobed, circling the base of the setae (figs. 1B, 1C, 2A). Dorsal setae spatulate and serrate towards the tip, sub equal in length (fig. 1A), sc1 and dorsocentral setae c1, d1 and e1 the longest, setae v2, c3, and h1 the shortest, d2 and e2 of almost equal lenghths, f1 twice as long as f2. Prodorsal setae sc1 and hysterosomal setae inserted on quite large tubercles excepted c3, f2 and h1. Setal lengths: sc1 75 (68–83), sc2 48 (44–51), c1 68 (62–78), c2 65 (57–71), c3 38 (35–41), d1 76 (69–84), d2 68 (57–78), e1 76 (66–85), e2 68 (55–80), f1 62 (55–68), f2 30 (26–35), h1 28 (25–32), h2 20 (15–22) and h3 20 (15–22). Distances between setal bases: v2–v2 44 (41–48), sc1–sc1 78 (70–82) and sc2- sc2 155 (144–170); c1–c1 41 (33–45), c2–c2 130 (122–144), c3–c3 230 (201–255), d1–d1 55 (50–57), d2–d2 166 (147–184), e1–e1 60 (48–75), e2–e2 136 (119–158), f1–f1 69 (64–71), f2–f2 101 (93–114), h1–h1 26 (22–30); Distance between consecutive setae: c1–d1 67 (50–82), d1–e1 75 (62–91); c3, f2 and h1 marginal.
Venter (Figs. 1E, 2B, 7D) — Ventral setae smooth and slender. Striations transverse except between 3a and 4a, slightly irregular to longitudinal; genital flap and area immediately anterior to it with transverse striae, pregenital setae (ag) 40 (30–50) long and ag–ag 48 (45–50). Genital setae (g1–g2) and pseudanal setae (ps1-2) short, tapering. Length of other ventral setae: m 28 (27–30), 1a 45 (40–52), 3a 45 (40–52), 4a 45 (40–50). Distances between setae: m-m 28 (27–30), 1a–1a 29 (27–31), 3a–3a 55 (51–60), 4a–4a 64 (60–72), ag–ag 48 (45–50). Ventrocaudal setae h2 and h3 tapering, slightly serrated, h2–h2 20 (18–22), h3–h3 65 (57–80). Pregenital striae transverse with two pairs of genital setae (g1, g2 ) and one pair of ag setae. Shape of lobes on ventral striae at h1–h2, semi-circular without extending to the dorsum (figs. 1E, 7D).
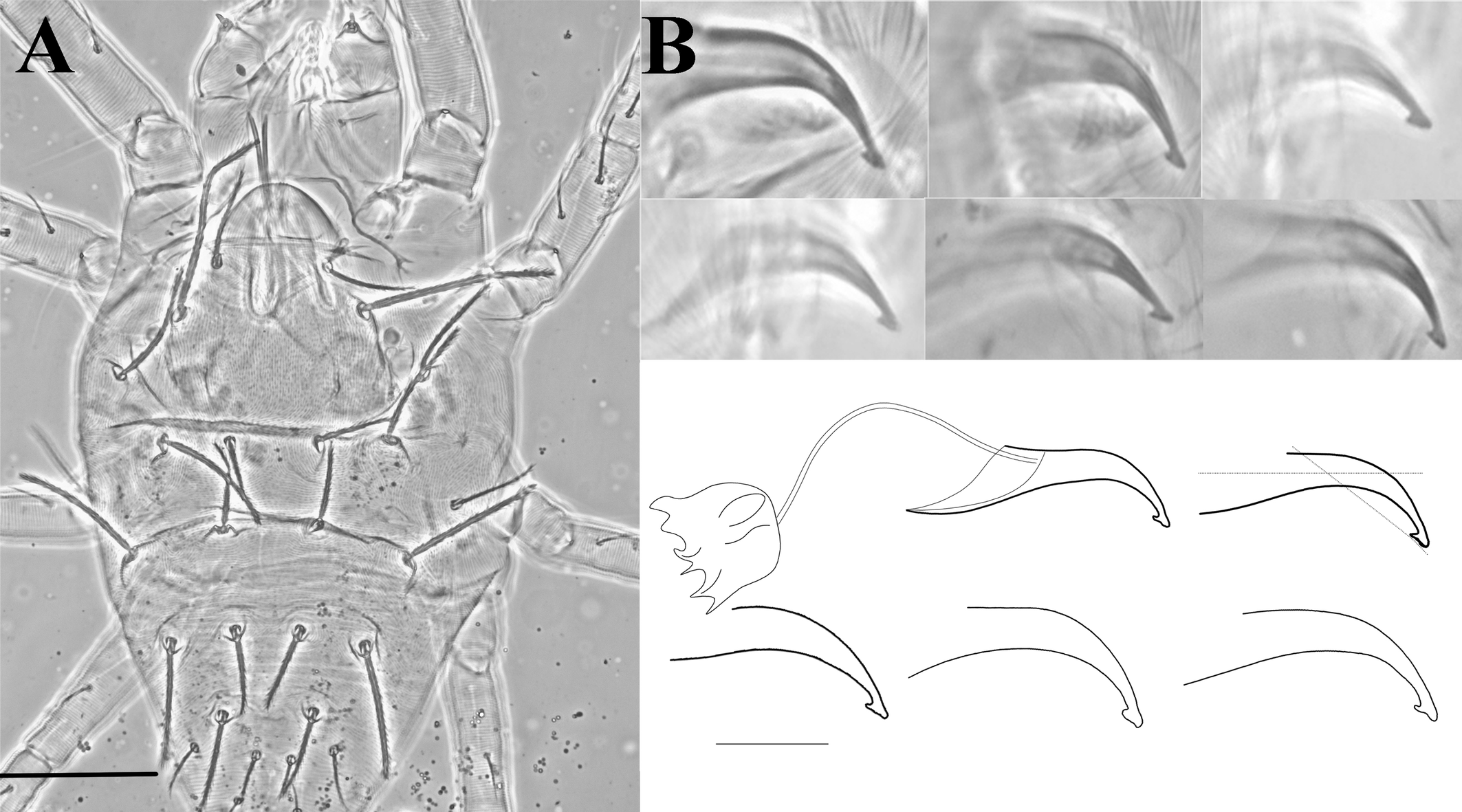
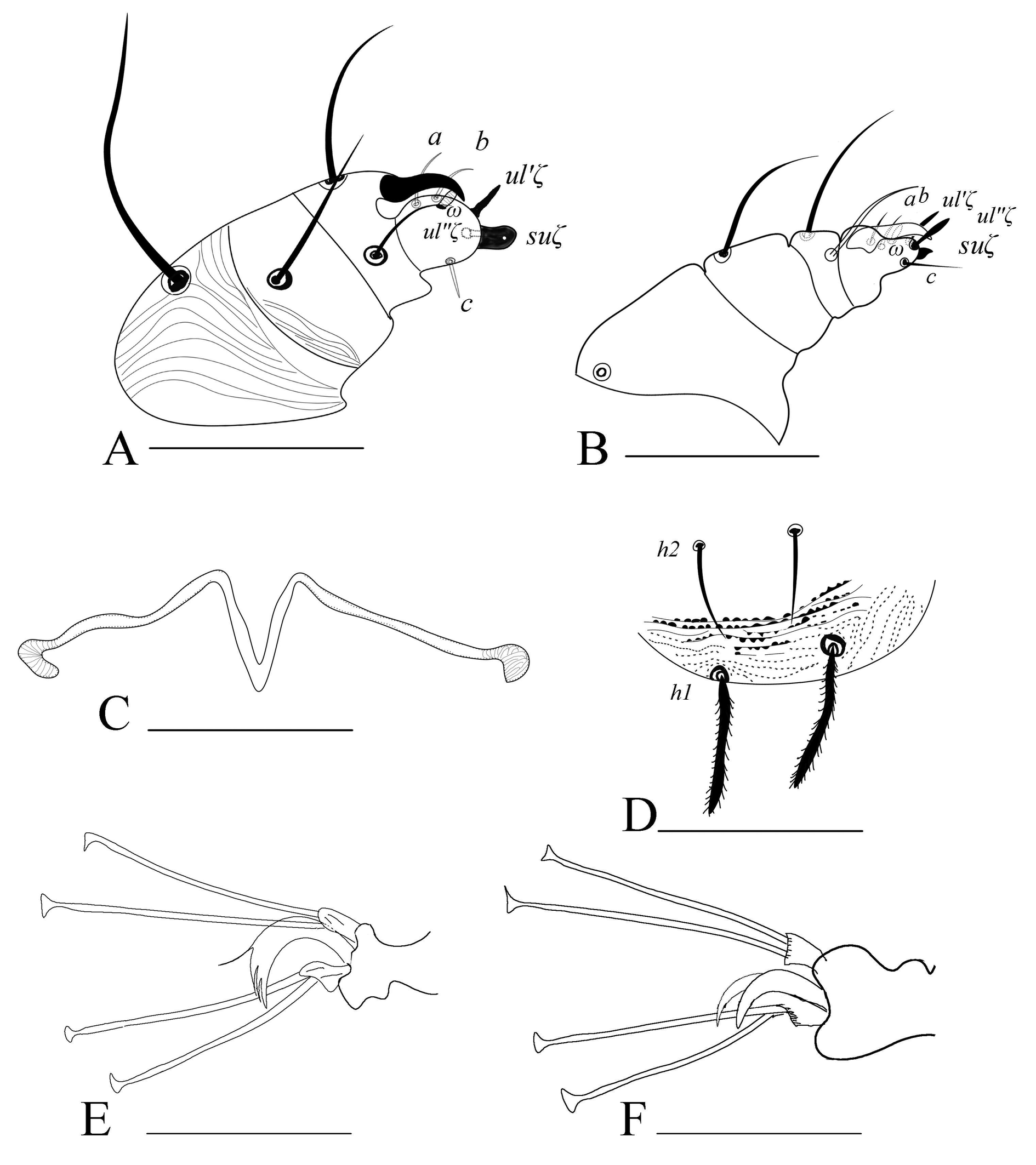
Gnathosoma (Figs. 1D, 7A, 7C) — Stylophore longer than wide. Peritreme enlarged distally like a bulb to a slight hook; out of five specimens, four were asymmetric (one side bulbed and the other side slightly hooked) (figs. 1D, 7C). Palp tibia with thumb claw and two setae. Palptarsus with three tactile setae (a, b, c), a well-developed spinneret, suζ 4 (4–5) width 3 (3–4), two eupathidia, ul′ζ 7 (6–7), ul″ζ 5 (5–6) and a solenidion, ω 3 (3–4).
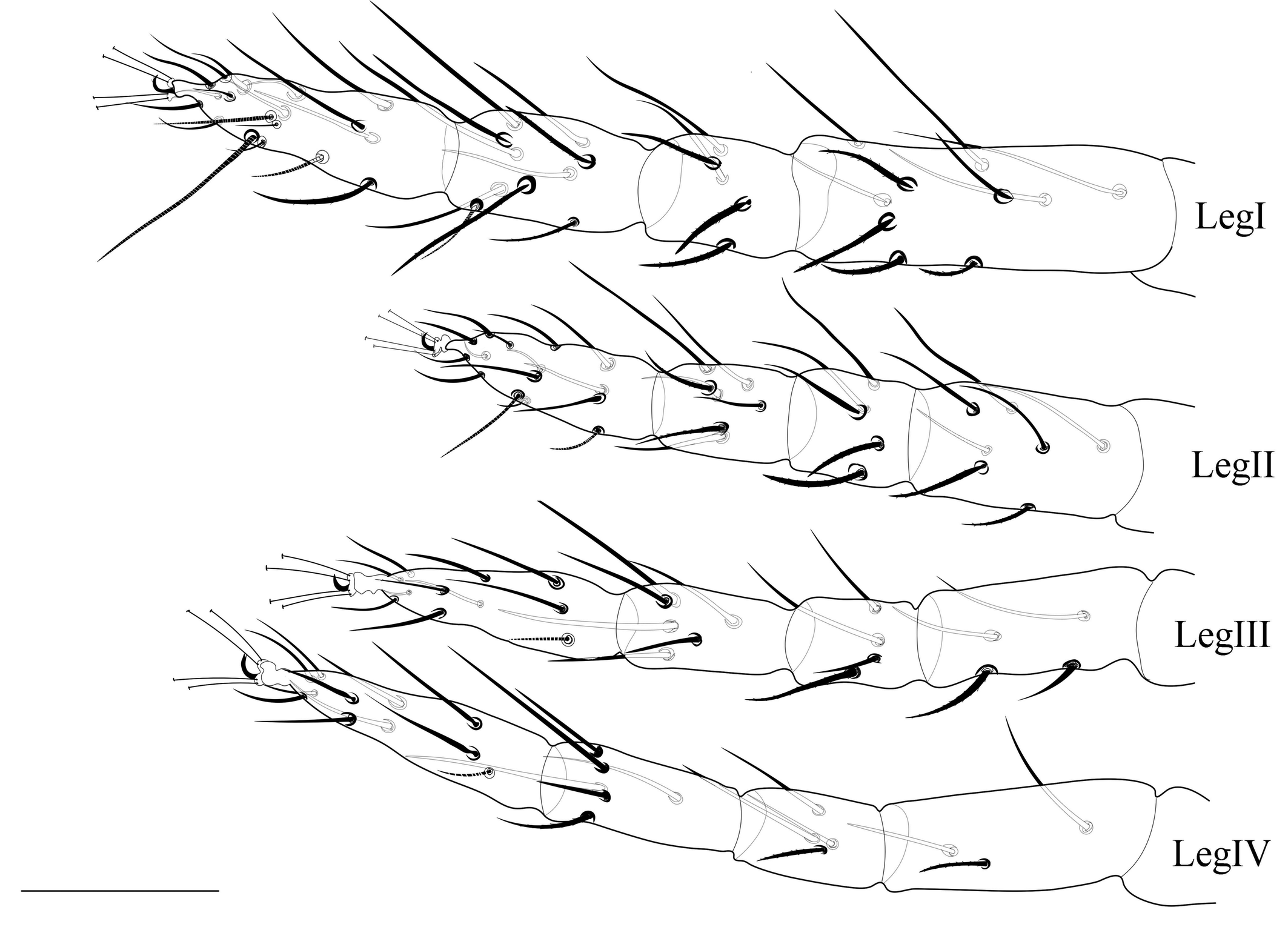


Legs (Figs. 3, 7E) — Length of legs I−IV (femur to the bent portion of empodium): 244 (230-260), 174 (160-181), 190 (175–212), 210 (201–223), respectively; Leg I with four tactile setae and one solenidion proximal to proximal duplex setae. Tibia with 9 tactile setae and one solenidion. Empodium of leg I–IV split near the middle into three proximal hairs (fig. 7E). Leg setal count as follows: coxae 2–2–1–1; trochanters 1–1–1–1; femurs 10–7–4–3; genua 5–5–4–4; tibiae 9(1sol)–7–6–6; tarsi 11(1sol+2eup+2dup)–10(1sol+2eup+1dup)–8(1sol+2eup)–8(1sol+2eup).
Male (n=5)
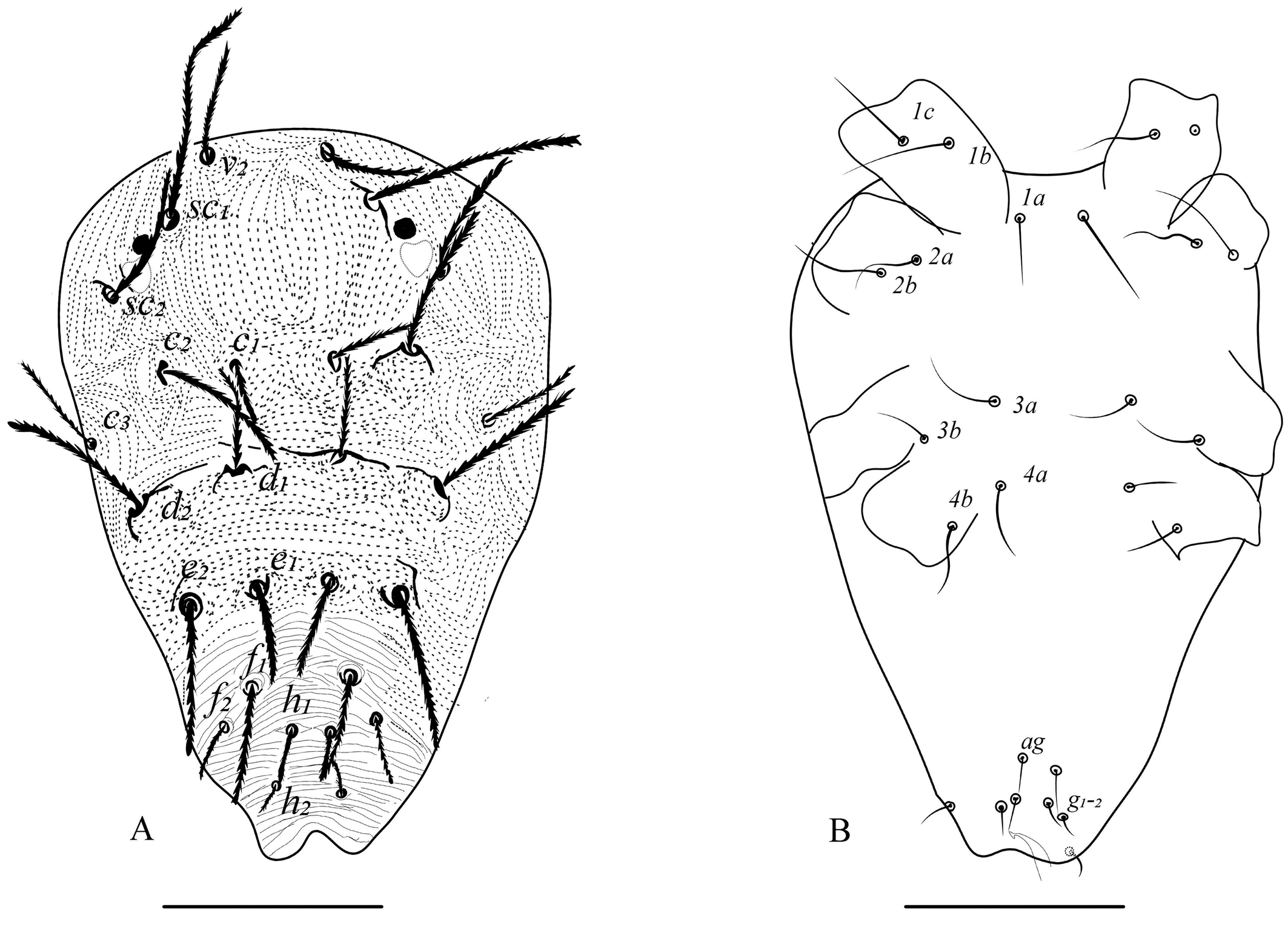




Dorsum (Figs. 4, 5) — Length of idiosoma 230 (220–235) (excluding gnathosoma), width 147 (138–154) at c3 setae. Prodorsum with 3 pairs (v2, sc1, sc2) of setae. Striae between sc1–c1 forming a U-shaped pattern (figs. 4A, 5A). All dorsal setae serrate, tapering, sc1, c2, d2 and e2 the longest. Setal lengths: v2 35 (30-40) sc1 65 (63–69), sc2 41 (38–45), c1 29 (25–37), c2 52 (45–65), c3 35 (30-40), d1 30 (25–33), d2 57 (46–66), e1 29 (27–35), e2 54 (43–65), f1 33 (30-37), f2 19 (19), h1 16 (14–18). Distances between setal bases: v2–v2 34 (32–36), sc1–sc1 50 (35–60), sc2–sc2 98 (95–100), c1–c1 26 (25–28), c2–c2 75 (67–80), c3–c3 125 (115–130), d1–d1 31 (27–35), d2–d2 92 (86–95), e1–e1 19 (18–20), e2–e2 64 (60–66), f1–f1 29 (27–31), f2–f2 43 (42–45), h1–h1 10 (9–10), h2–h2 17, h3–h3 42 (40–45). Setae h1–h3 on dorsal side.
Venter (Fig. 4B) — Striations sparse and transverse. All ventral setae slender smooth, tapering, shorter than in females; m 18 (17–20), 1a 31 (30–32), 3a 27 (25–30), 4a 25 (23–26), ag 13 (10-16). Distances between setae: 1a–1a 18 (17–18), 3a–3a 43 (40-45), 4a–4a 36 (34–37), ag–ag 8 (8–8). Genital setae (g1–g2) and pseudanal setae (ps1-2) short and tapering.
Gnathosoma (Fig. 7B) — All tactile setae on palp slender. Subcapitular setae m smooth, setaceous. Palptarsus with three tactile setae (a, b, c), with a small spinneret, suζ 1 (0.6–1), width 1, two eupathidia ul′ζ 5 (4–6), ul″ζ 4 (4–5) and one solenidion (ω) 3 (3–5); solenidion slightly thinner and longer than spinneret.





Legs (Figs. 6, 7E, 7F) — Length of legs I−IV (femur to tip of empodium): 271 (245–282), 173 (162–185), 185 (167–192), 214 (195–227) respectively; leg I with six tactile setae and one solenidion proximal to proximal duplex setae. Tibia with 10 tactile setae and two solenidia. Empodia I-II clawlike (Fig.7F), empodia III-IV as in female (Fig. 7E). Leg setal count as follows: coxae 2–2–1–1; trochanters 1–1–1–1; femora 10–7–4–3; genua 5–5–4–3; tibiae 11 (2sol)–7–6-6-); tarsi 12(1sol+2eup+2dup)–9 (1sol+2eup+1dup)–8(1sol+2eup)–8(1sol+2eup).
Aedeagus (Fig. 5B) — Aedeagus with a long shaft, slightly bent ventrally, featuring a distal triangular knob. Anterior projection of the knob acute, posterior rounded. Ventral margin of the knob with slight depression near the middle (variations can be seen in different focus).
Material examined
Nine males and 5 females on Cassava (Manihot esculenta Crantz) (Euphorbiaceae), Coffee Research Substation (CRSS), Chettalli (12°22′44.9″ N 75°50′17.2″ E, alt. 1002 m), Chettalli (Kodagu), India, 24 May 2023, coll. P. Dyamanagouda; 10 males, 10 females on Cassava (M. esculenta) (Euphorbiaceae), Bengaluru (13°04′36.0″ N 77°34′39.5″ E, alt. 924 m), GKVK, Bengaluru (Karnataka), India, 04 Sep. 2023, coll. P. Dyamanagouda.
Molecular identification
Mite samples collected at Chettalli (Specimen voucher UASB:3596 and NCBI accession number PP074174) and GKVK, Bengaluru (Specimen voucher UASB:3600 and NCBI accession number PP124899) showed similarities of 99.16% and 98.99% with the sequence of M. mcgregori (NCBI accession number MN913383) submitted by Ovalle et al. (2020) from Colombia, respectively.
Remarks
Mononychellus species identification was a bit complex earlier due to cryptic morphologies among the species. Pritchard and Baker (1955) introduced a new combination for the type species Tetranychus planki McGregor, 1950, renaming it as Eotetranychus planki. They noted two morphotypes: one with reticulation at the bases of dorsal setae (Trinidad type) and the other without reticulations at the dorsal setae base (Argentina type). Later, Eotetranychus planki was reclassified as two separate species: Mononychellus planki sensu stricto (McGregor 1950) with reticulation and M. mcgregori without reticulation (McGregor 1950; Pritchard and Baker 1955; Flechtmann and Baker 1970; Gutierrez 1987).
Additionally, discrepancies in leg setal numbers, the presence of striations, and other descriptive characters among authors in subsequent publications have been observed (Gutierrez 1987; Flechtmann and De Queiroz 2015). To support this, our investigation observed variability in the lobe pattern present on dorsal striation between mites from two distinct locations. One morphotype (all females from Bengaluru) exhibited dorsal striations resembling dotted or dashed lines, while the other (found in two females from Chettalli) with semi-circular well developped lobes on the dorsal striations (fig. 1B for dotted and 1C for lobed). While, other three female mites from Chettalli are similar to the Bengaluru morphotypes. Despite this variation, all other characteristics remain consistent across both morphotypes in the present study. This variation in dorsal striation of M. mcgregori is in congruence with the observations of dotted lines by Pritchard and Baker (1955) (Fig. 109, p. 150) and lobed striations by Flechtmann and Baker (1970) on Argentina morphotypes.
Earlier research has suggested that variations in the dorsal striation pattern and shape could be attributed to several factors such as improperly mounted specimens, environmental conditions, and hybridization effects (Boudreaux and Dosse 1963; Monroe 1963; Mollet and Sevacherian 1984). This variation in present study might be due to mounting effect (as only two females observed) or due to genetic diversity due to haploidy nature and gene flow within population (Helle and Pieterse 1965; Dupont 1979). However, even among properly mounted specimens, variations are observed within populations (Boudreaux and Dosse 1963; Monroe 1963). Additionally, the dorsal striation shape and pattern of mites is not always a consistent characteristic for taxonomic identification as it is varies with each mite form within the population as it is dependent on above factors (Meyer 1974; Jordaan 1977; Auger et al. 2013).
Moreover, variations in setal counts in legs of M. mcgregori can be seen in earlier literature. Pritchard and Baker (1955) reported one sensory and nine tactile setae without specifying whether these mites were from Argentina or Trinidad. Later, Flechtmann and Baker (1970) provided a full description of M. mcgregori based on mites from Argentina, noting that tibia I had one sensory and eight tactile setae.
These kinds of conflicts in the description of Mononychellus clearly shows that the genus needs a deep revision. Firstly, descriptions are restricted to females in some sources (Beer and Lang 1958; Estebanes and Baker 1968). Secondly, the cryptic morphology of these mites complicate identification. Lastly, only a few mitochondrial nucleotide sequences (only for M. tanajoa, M. mcgregori, and M. caribbeanae) are present in the GeneBank (NCBI 2024) for comparative analysis.
Mononychellus mcgregori is closely related to M. chemosetosus (Paschoal) and M. planki (McGregor). Mononychellus mcgregori aedeagus is similar to those of M. planki and M. chemosetosus (IPPC 2023). However, it can be differentiated from latter species by comparatively longer shaft curved ventrad with anterior acute and posterior round knob. Additionally, reticulations on the prodorsum and the base of each of the opisthosomal setae are absent in M. mcgregori.
Mononychellus mcgregori is very similar to M. manihoti Doreste, 1981 in general morphology and host range but can be differentiated using several characters: 1) the aedeagus of M. mcgregori has a long shaft curved ventrad with the anterior projection of the knob acute and the posterior rounded, whereas in M. manihoti, the aedeagus has a straight shaft without bent and both anterior and posterior projections of the knob are acute; 2) the female tibia I bears 9 tactile setae and one solenidion in M. mcgregori, while M. manihoti has only 8 tactile setae and one solenidion; 3) the male tibia I bears eleven tactile setae and two solenidia in M. mcgregori, whereas M. manihoti has nine tactile and three solenidia (Flechtmann and Baker 1970; Doreste 1981).
Mononychellus mcgregori shows a high invasive probability to subtropical conditions according to climate models suitability (Lu et al. 2012; Rêgo et al. 2013; Parsa et al. 2015). This situation is particularly important in southern Indian states, notably Kerala, Tamil Nadu, and Karnataka, where cassava is cultivated as a major crop. At the same time, far North-eastern states of India also have a high alert of spread and colonization. Currently, determining the route of entry is challenging and requires further study and intensive surveys. Finally, the ability to feed and establish on multiple hosts is considerable. So, these facts support a significant threat to crop diversity and biodiverse hotspots of India in future.
Acknowledgements
The authors would like to express their gratitude to the reviewers for dedicating their time and effort to review the manuscript. We genuinely appreciate all your valuable comments and suggestions, which have significantly enhanced the quality of our work. We also extend special thanks to Dr. Philippe Auger for his continuous support and personal attention in improving the manuscript.The Authors are thankful to the ICAR, New Delhi, for providing the facility for the study under the All India Network Project on Agricultural Acarology at UAS, GKVK, Bengaluru, Karnataka and Dr Manjunath Reddy Coffee Research Substation (CRSS), Chettalli, Kodagu district, Karnataka for the timely support and sending the mite samples.
References
- Auger, P., Migeon, A., Ueckermann, E.A., Tiedt, L., Navarro, M.N. 2013. Evidence for synonymy between Tetranychus urticae and Tetranychus cinnabarinus (Acari, Prostigmata, Tetranychidae): review and new data. Acarologia, 53(4): 383-415. https://doi.org/10.1051/acarologia/20132102
- Beer, R.E., Lang, D.S. 1958. The Tetranychidae of Mexico. University of Kansa. Sci. Bull., 38: 1231-1259. https://doi.org/10.5962/bhl.part.10974
- Bellotti, A., Herrera Campo, B.V., Hyman, G. 2012. Cassava production and pest management: present and potential threats in a changing environment. Trop. Plant Biol., 5: 39-72. https://doi.org/10.1007/s12042-011-9091-4
- Bolland, H.R., Gutierrez, J., Flechtmann, C.H.W. 1998. World catalogue of the spider mite family (Acari: Tetranychidae). Leiden, Netherlands, K. Brill. 392 pp. https://horizon.documentation.ird.fr/exl-doc/pleins_textes/2023-01/010016435.pdf
- Boudreaux H.B., Dosse G. 1963. The usefulness of the taxonomic characters in females of the genus Tetranychus Dufour (Acari : Tetranychidae): Acarologia, 5: 13-33. https://www1.montpellier.inrae.fr/CBGP/acarologia/article.php?id=3906
- Byrne, D.H., Bellotti, A.C., Guerrero, J.M. 1983. The cassava mites. Int. J. Pest Manag., 29(4), 378-394. https://doi.org/10.1080/09670878309370833
- Doreste, E. 1981. Acaros del genero Mononychellus Wainstein (Acari: Tetranychidae) asociados com la Yuca (Manihot spp.) en Venezuela. Bol. Entomol. Venez., 1 (10): 119-130.
- Doyle, J.J., Doyle, J.L. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull., 19: 11-5.
- Dupont, L.M. 1979. On gene flow between Tetranychus urticae Koch, 1836 and Tetranychus cinnabarinus (Boisduval) Boudreaux, 1956 (Acari: Tetranychidae): synonomy between the two species. Entomol. Exp. Appl., 25(3): 297-303. https://doi.org/10.1111/j.1570-7458.1979.tb02882.x
- Estebanes, G.M.L., Baker, E.W. 1968. Arañas rojas de Mexico (Acarina: Tetranychidae), Anales de Biologia, 15: 61-133. https://biblat.unam.mx/hevila/AnalesdelaEscuelaNacionaldeCienciasBiologicas/1968/vol15/no1-4/3.pdf
- Flechtmann, C.H.W., Baker, E.W. 1970. A preliminary report on the Tetranychidae (Acarina) of Brazil. Ann. Entomol. Soc. Am., 63(1): 156-163. https://doi.org/10.1093/aesa/63.1.156
- Flechtmann, C.H.W., Baker, E.W. 1975. A report on the Tetranychidae (Acari) of Brazil. Rev. Bras. Entomol., 19(3): 111-122.
- Flechtmann, C.H.W., De Queiroz, DL. 2015. Mononychellus Wainstein, 1971 (Acari, Prostigmata, Tetranychidae): description of a new species from Brazil and key to species. Syst. Appl. Acarol., 20(7): 831-838. https://doi.org/10.11158/saa.20.7.10
- Folmer, R.H.A., Nilges, M., Folkers, P.J.M., Konings, R.N.H., Hilbers, C.W. 1994. A model of the complex between single-stranded DNA and the single-stranded DNA binding protein encoded by gene V of filamentous bacteriophage M13. J. Mol. Biol., 240(4): 341-357. https://doi.org/10.1006/jmbi.1994.1449
- Graziosi, I., Minato, N., Alvarez, E., Ngo, D.T., Hoat, Tin Maung Aye, Juan Manuel Pardo, Prapit Wongtiem, Kris A.G. Wyckhuys. 2016. Emerging pests and diseases of South‐east Asian cassava: a comprehensive evaluation of geographic priorities, management options and research needs. Pest Manag. Sci., 72(6) : 1071-1089. https://doi.org/10.1002/ps.4250
- Gutierrez, J. 1987. The cassava green mite in Africa: one or two species? (Acari: Tetranychidae). Exp. Appl. Acarol., 3(2): 163-168. https://doi.org/10.1007/BF01270477
- Helle, W., Pieterse, A.H. 1965. Genetic affinities between adjacent populations of spider mites (Tetranychus urticae Koch). Entomol. Exp. Appl., 8(4), 305-308. https://doi.org/10.1111/j.1570-7458.1965.tb00866.x
- Indiastat Web: Area, production and Productivity of Tapioca in India. [internet]. [21 August 2023]. Available from: https://www.indiastat.com/data/agriculture/tapioca-cassava
- IPPC 2023. ISPM 27 Diagnostic protocols for regulated pests DP 33: Mononychellus tanajoa, 28pp.
- Jordaan L.C. 1977. Hybridization studies on the Tetranychus cinnabarinus complex in South Africa (Acari: Tetranychidae). J. Entomol. Soc. South. Afr., 40: 147-156.
- Lu, H., Ma, Q., Chen, Q., Lu, F., Xu, X. 2012. Potential geographic distribution of the cassava green mite Mononychellus tanajoa in Hainan, China. Afr. J. Agric. Res., 7(7): 1206-1213. https://doi.org/10.5897/AJAR11.1784
- McGregor, E.A. 1950. Mites of the family Tetranychidae. Am. Midl. Nat., 44(2): 257-420. https://doi.org/10.2307/2421963
- Meyer, M.K.S. 1974. A revision of the Tetranychidae of Africa (Acari) with a key to the genera of the world. South Afr. Dept. Agric. Techn. Serv., Entomology memoirs, 36: 1-291.
- Migeon A., Dorkeld F. Spider Mites Web: a comprehensive database for the Tetranychidae [Internet]. [21 August 2023]. Montpellier: INRAE/CBGP. Available from: https://www1.montpellier.inrae.fr/CBGP/spmweb/
- Mollet J.A., Sevacherian V. 1984. Effect of temperature and humidity on dorsal strial lobe densities in Tetranychus (Acari: Tetranychidae). Int. J. Acarol., 10: 159- 161. https://doi.org/10.1080/01647958408683370
- Monroe R.S. 1963. A genetic study of the Tetranychus telarius complex. Acarologia, 5: 545-555. https://www1.montpellier.inrae.fr/CBGP/acarologia/article.php?id=3856
- Nachman, G., Skovgård, H., Tomkiewicz, J., Münster-Swendsen, M., Pedersen, O.C. 1993. Sampling strategies for assessing the overall density of the cassava green mite (Mononychellus tanajoa). Exp. Appl. Acarol., 17: 5-28. https://doi.org/10.1007/BF00156941
- NCBI. National Centre for Biotechnology Information: Nucleotide. [internet]. [21 November 2023]. Available from: https://www.ncbi.nlm.nih.gov/nuccore
- Ovalle, T.M., Vásquez-Ordóñez, A.A., Jimenez, J., Parsa, S., Cuellar, W.J., Becerra Lopez-Lavalle, L.A. 2020. A simple PCR-based method for the rapid and accurate identification of spider mites (Tetranychidae) on cassava. Scientific Reports, 10(1): 19496. https://doi.org/10.1038/s41598-020-75743-w
- Parsa, S., Hazzi, N.A., Chen, Q., Lu, F., Herrera, B.V., Yaninek, J.S., Vásquez-Ordóñez, A.A. 2015. Potential geographic distribution of two invasive cassava green mites. Exp. Appl. Acarol., 65: 195-204. https://doi.org/10.1007/s10493-014-9868-x
- Pritchard, A.E., Baker, E.W. 1955. A Revision of the Spider Mite Family Tetranyohidae. A Revision of the Spider Mite Family Tetranychidae, 2, Pac. Coast Entomol. Soc. Mem., 2: 1-472. https://doi.org/10.5962/bhl.title.150852
- Rêgo, A.S., Teodoro, A.V., Maciel, A.G., Sarmento, R.A. 2013. Relative contribution of biotic and abiotic factors to the population density of the cassava green mite, Mononychellus tanajoa (Acari: Tetranychidae). Exp. Appl. Acarol., 60: 479-484. https://doi.org/10.1007/s10493-013-9667-9
- Tuttle, D.M., Baker, E.W., Abbatiello, M.J. 1976. Spider mites of Mexico (Acari: Tetranychidae). Int. J. Acarol., 2(2): 1-102. https://doi.org/10.1080/01647957608683760
- Wainstein, B.A. 1960. Proposed revision of the tribe Petrobiini (Reck) (Acarfformes, Tetranychidae). Entomolg. Obozrenie Akad. Nauk S. S. R., 39(1): 214-226 (in Russian).
- Wainstein, B.A. 1971. Mononychellus, a new name for Mononychus (Acariformes, Tetranychidae): Zool. Zhur., 50 (4): 589 (in Russian with English summary).



2024-01-30
Date accepted:
2024-06-25
Date published:
2024-07-02
Edited by:
Auger, Philippe

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Dyamanagouda, Poojar; Rajashekharappa, Kenchappa; Chinnamadegowda, Channegowda; Mulimani, Vidya and Flechtmann, Carlos H. W.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



