First record of the water mite species Arrenurus (Arrenurus) munovus Cook, 1980 from Belize, including the description of the female (Arrenuridae, Hydrachnidia, Acari)
Ramírez-Sánchez, Marcia M.  1
and Goldschmidt, Tom
1
and Goldschmidt, Tom  2
2
1Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito exterior S/N, Ciudad Universitaria, C.P. 04515, México City, México.
2✉ Bavarian State Collection of Zoology, section Arthropoda varia, Münchhausenstraße 21, D-81247 Munich, Germany.
2024 - Volume: 64 Issue: 3 pages: 865-878
https://doi.org/10.24349/vzqw-26uaOriginal research
Keywords
Abstract
Introduction
Within the currently over 7500 described species of water mites, Arrenurus is by far the most species rich genus with almost 1000 species described worldwide (Smit 2020).
Though specialists agree that the subgenera of Arrenurus are probably representing polyphyletic groups, they are generally accepted, as they allow to organize the immense diversity of this genus (Cook 1974; Smit 2020). Among the eleven accepted subgenera, Arrenurus (s.s.) is one of the most characteristic as males present well developed pygal lobes and a petiole of variable length seldomly absent (Cook 1974; Smit 2020).
As for the genus, as well the subgenus Arrenurus (s.s.) is cosmopolite and widely distributed all over the American continent (Marshall 1908; Cook 1980; Cramer and Cook 1992; Rosso de Ferradás and Fernandez 2005). At present 212 species of the genus Arrenurus are known from the Neotropical region, within these 25 species of the subgenus Arrenurus – in South America there are 20 registered species and six are known from Central America (Goldschmidt and Ramírez-Sánchez 2020; Montes-Ortiz et al. 2022; Rosso de Ferradás and Fernández 2005; Valdecasas 2019).
For North America 164 species of the genus Arrenurus are described, with 62 species belonging to the subgenus Arrenurus (Bruce Smith, pers. comm.).
Overall the data on A. (Arrenurus) for Central America are very sparse – six species are known from Mexico (Montes-Ortiz et al. 2022), four of these (A. (A.) valencius, munovus, wucabus, tamaulipensis) from the southern lowlands (Cook 1980; Cramer and Cook 1992), one (A. (A.) xochimilcoensis) from the central highlands (Cramer and Cook 1992); just one of these species (A. (A.) dentipetiolatus) shows a distribution from southern and central Mexico stretching north to the US, and there is only one species (A. (A.) valencius) with a rather wide distribution in Central and northern South America including records from Venezuela (Marshall 1919), Haiti (Lundblad 1935), Cuba (Orghidan and Gruia 1977), Guatemala (K.O. Viets 1975; Böttger 1980) and Mexico (Cook 1980).
The parasitology and post-parasitic development of the later species has been studied in detail by Böttger (1980). Except for this study, no ecological data (beside their sample sites) are available on the Central America species of the subgenus: The larvae of A. (A.) valencius parasitize Odonata (Zygoptera), and prey on Ostracoda (Böttger 1980).
The species treated in this paper – A. (A.) munovus – is the only Central American representative of the subgenus found in rivers, all other species seem to be bound to standing waters like ponds and lakes (Montes-Ortiz et al. 2022).
In the first publication on freshwater mites from Belize (Goldschmidt et al. 2022), Arrenurus (together with 26 further genera) is just mentioned at genus level, with no information provided on the subgenera (a part of that material is treated in detail in the present study).
Being the arrenurid species discriminated mainly by male's morphology, due to the strong sexual dimorphism observed in most species (as females basically differ in size), there are a number of species that are just known in this sex. Though male characters are very distinct and obvious in many cases, we consider they are not enough to achieve a thorough description of species, particularly in highly diverse groups as Arrenurus. In this sense, the inclusion of female descriptions in species taxonomy is utterly important in order to: obtain characters to propose phylogenetic hypotheses, know the real species distribution and, search for possible explanations to the sexual dimorphism observed in arrenurids, among others. Additionally, as the life cycle is complex and difficult to observe due to the problems to complete the life cycle in laboratory conditions (as in most water mite species) very few data exist on the ecology and distribution patterns of individual species and many are just known from the type location. Last but not least, there is the fact that water mites are ignored in most bio-monitoring studies or identified only as Acari, apparently only due to lack of interest or ignorance of their taxonomy. We are on the one hand wasting the immense monitoring possibilities provided by water mites (Goldschmidt 2016; Goldschmidt et al. 2016) and on the other hand are completely lacking data on the threat of extinction of individual species. Therefore, both, detailed morphological, as well as faunistic data of individual species are valuable and urgently needed.
Material and methods
The two males and five females of Arrenurus (s.s.) munovus we studied, were collected in February 2018 as part of the first study on water mites in Belize (Goldschmidt et al. 2022). The water mites were collected by hand net (mesh size 250 µm). Samples were washed through a sieve (mesh size ~2 mm), transferred to a white plastic plate and sorted at the spot. Water mites were picked up by eye droppers and fine tweezers and fixed in Koenike's solution (ten parts glycerin, six parts water, three parts acetic acid). For details on the water mite collection process, preparation and slide-mounting see Goldschmidt and Ramírez-Sánchez (2020). The mites were sorted, identified and mounted with the aid of a Leica MZ 16 stereomicroscope. One male and one female were partly dissected and mounted in glycerin jelly. Drawings and measurements were made with a Laborlux D Leitz compound microscope and a drawing mirror (Olympus 1.25x). We took micro-photos with a Nikon V1 camera combined with a Leica Z16 Apo and a 2.0-fold objective, as well as a Leica DM 5000B compound microscope combined with a Jenoptik ProgRes Speed XT core 5 camera and ProgRes®Capture Pro 2.8 – Jenoptik software. Images were stacked with Helicon Focus 5.3 software. In the selection of characters described and measured we followed the recommendations given by Ramírez-Sánchez et al. (2016). The authors found that a set of distances among dorsoglandularia and ventroglandularia are very useful in discriminating among Arrenurus (Megaluracarus) species, and suggest to generally include those measurements in arrenurid descriptions. Measurements are given in micrometers µm), with the allotype first, followed by the maximum and minimum of the paratypes in parentheses; the length of appendages segments are given as dorsal length (distal segments as total length). We use the following abbreviations (generally following Goldschmidt and Ramírez-Sánchez (2020)):
A1, 2, pre- and post-antennal glandularia;
Dgl-1–4, dorsoglandularia first to fourth pairs;
Cx-I–IV, coxae first to fourth pairs;
Cxgl-1, -2, coxoglandularia between Cx-II and -III, as well as posterior to Cx-IV (by other authors these are sometimes as well called ''Cxgl-2 and -4″);
H, height;
L, length;
I- to IV-leg-1–6, first to sixth segments of the first to fourth leg;
Lgl-1–4, lateroglandularia, first to fourth pairs;
m asl, meters above sea level;
P-1 to -5, first to fifth palp segments;
R1, 2, pre- and post-antennal setae;
V1–4, ventroglandularia first to fourth pairs (in V1 and V3 the glands are reduced, only setae are remaining);
W, width.
Site Description
Monkey Bay, a meander in the Sibun River, elevation 17 m asl, microhabitat: partly shaded backwater pool of a fast-flowing river, substrate: macropelal, terrestrial vegetation, water level high, sample depth 50cm, coordinates 17.299953° N, 88.5540793° W, Belize District, Belize, February 22, 2018, collected by J. Goldschmidt, L. Montes-Ortiz, T. Goldschmidt and Manuel Elías-Gutiérrez (Fig. 1). For details see Goldschmidt et al. (2022), sample site Be18- 1b.



Results
Additional description of Arrenurus (Arrenurus) munovus Cook 1980
Diagnosis
Yellow with bluish pattern (Figs 2A, 3 and 7); males with relatively short pygal lobes (not projecting beyond the base of the petiole), petiole relatively long and slender (Figs 2A, 4A, 5A and 5B), male acetabular fields medially broader than the genital opening, stretching out nearly perpendicular, continuously tapering towards lateral idiosoma margin (Fig. 5B); females with acetabular fields oval-drop shaped, from anterior half of genital field stretching to postero-lateral (Fig. 10B); palps with very subtle or none sexual dimorphism– P-4 rather compact, bearing two medio-dorsal setae, antagonistic setae ventro-distally at medial side of P-4 strong, 1.5 fold longer than P-5, additional small bristle at ventro-distal tip of P-4; however P-2 medially with three setae in the illustrated male from Belize (four in the male illustrated in the species description from Mexico), but five in the illustrated female from Belize (Figs 5C, 5D, 10E and 10F).
Male (n = 2 (1 partly dissected))






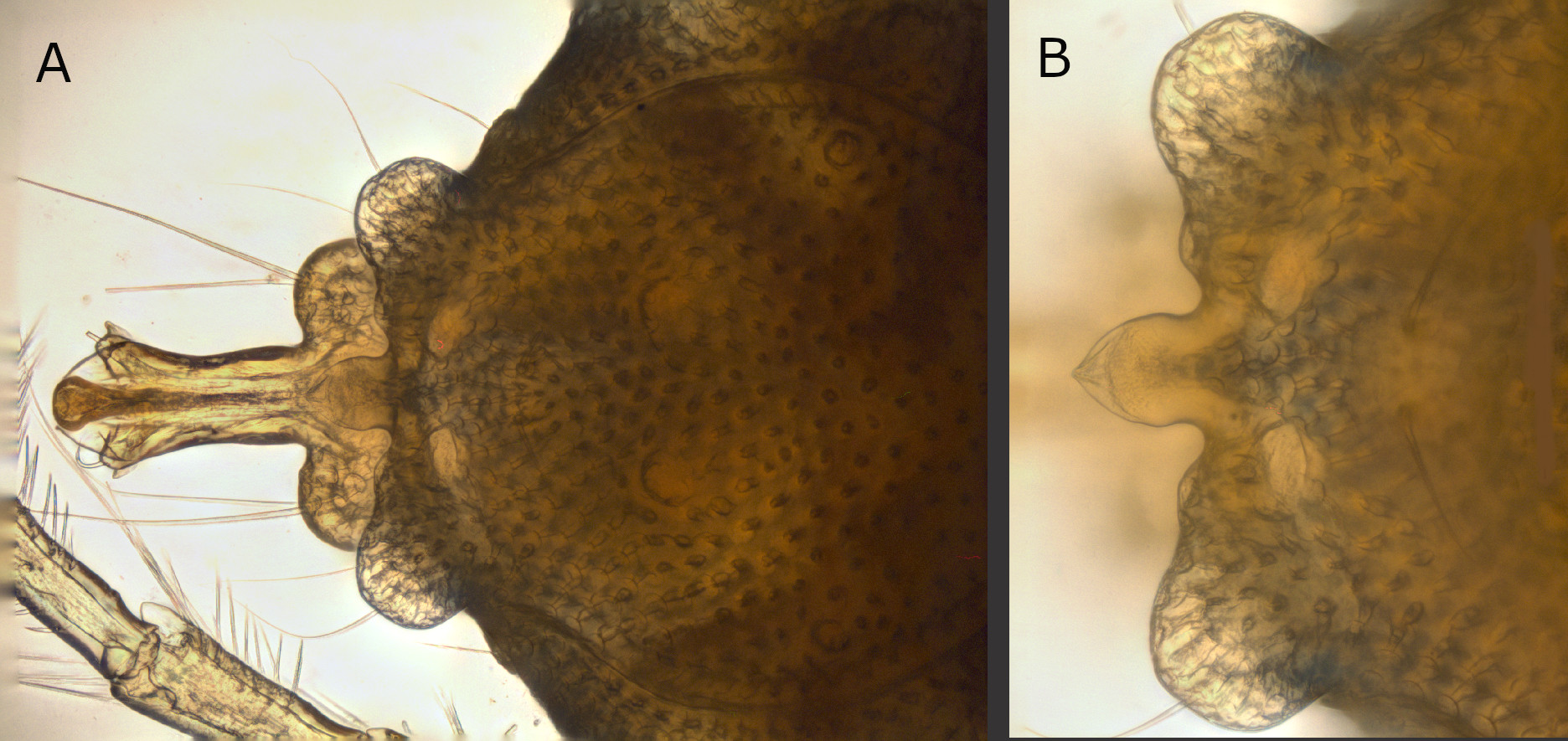


Idiosoma yellow, posterior bluish; legs and palps bluish. Frontal margin straight; dorsal furrow complete (Figs 2A and 3). Idiosoma dorsal L 893 (929); W 653 (696); dorsal shield L 513 (686), W 409 (442). Distance between the anterior idiosoma margin and the anterior dorsal shield outline (maximum curvature) 210 (240); distance between the lateral dorsal shield outline (shortest distance) and the lateral depression anterior to the postero-lateral protrusion 104 (120); distance between the lateral dorsal shield outline (shortest distance) and the postero-lateral protrusion 125 (135). Non-caudal portion of dorsal shield L 395 (403); cauda L 286 (288), proximal W 269 (269), maximum W (between outer margins of pygal lobes) 311 (318); caudal margin between pygal lobes concave, with small protrusions at the base of pygal lobes, median projection distally pointed, with hyaline tip, basally constricted (Fig. 4B); petiole elongated, basally relatively straight, distally diverging towards two fin-like elevated projections (Figs 3 and 4A); petiole proximal W 69 (67), maximum W 90 (106), L 165. Distances between glands and setae of dorsal glandularia (between the glands/setae of both sides, respectively two different glandularia of one idiosoma side): A1, glands 226 (235); A1, setae 249 (259); A2, glands 193 (192); A2, setae 172 (183); R1 190 (197); R2 127 (154); Dgl-1, glands 492 (542); Dgl-1, setae 488 (528); Dgl-2, glands 320 (336); Dgl-2, setae 288 (302); Dgl-3, glands 122 (134); Dgl-3, setae 82 (91); Dgl-4, glands 87 (96); Dgl-4, setae 33 (43); Lgl-1, glands 441 (470); Lgl-1, setae 406 (442); Lgl-2, glands 571 (590); Lgl-2, setae 566 (586); Lgl-3, glands 611 (653); Lgl-3, setae 625 (662); Lgl-4, glands 260 (269); Lgl-4, setae 273 (284). R2—Dgl-2 (seta) 113 (120); Dgl-2—Dgl-3 (glands) 156 (154); Dgl-2—Dgl-3 (glands) 156 (154); Dgl-2—Dgl-3 (setae) 125 (118); Dgl-3—Dgl-4 (glands) 155 (149); Dgl-3—Dgl-4 (setae) 173 (177).



Ventral shield L 904 (951), W 650 (680); distance between Cx-I tips 187 (191); coxal field L 523 (548), W 642 (685) (measured between Cx-IV tips) (Figs 2B, 5A). Acetabular plate region W 373 (406); gonopore inner L 40 (44), outer L 46 (48); inner W 17 (14), outer W 35 (38) (Fig. 5B). Distances between glands and setae of ventral glandularia: Cxgl-1, glands 321 (327); Cxgl-1, setae 304 (318); Cxgl, -2 glands 161 (169); Cxgl-2, setae 144 (147). V1 (setae) 149 (151); V2, glands 111 (107); V2, setae 147 (148); V3 (setae) 201 (232); V4, glands 401 (427); V4, setae 342 (374). Barely two rows of body pores between the anterior and posterior coxal groups, left and right posterior coxae medially touching each other (Figs 2B and 5A).
Palp segments, L/H: P-1, 29/34; P-2, 69/58; P-3, 53/56; P-4, 79/50; P-5, 40/16; P-2 stocky, with three medial setae; disto-ventral portion of P-4 slightly angular with one large antagonistic seta, L 57 (Figs 5C and D).
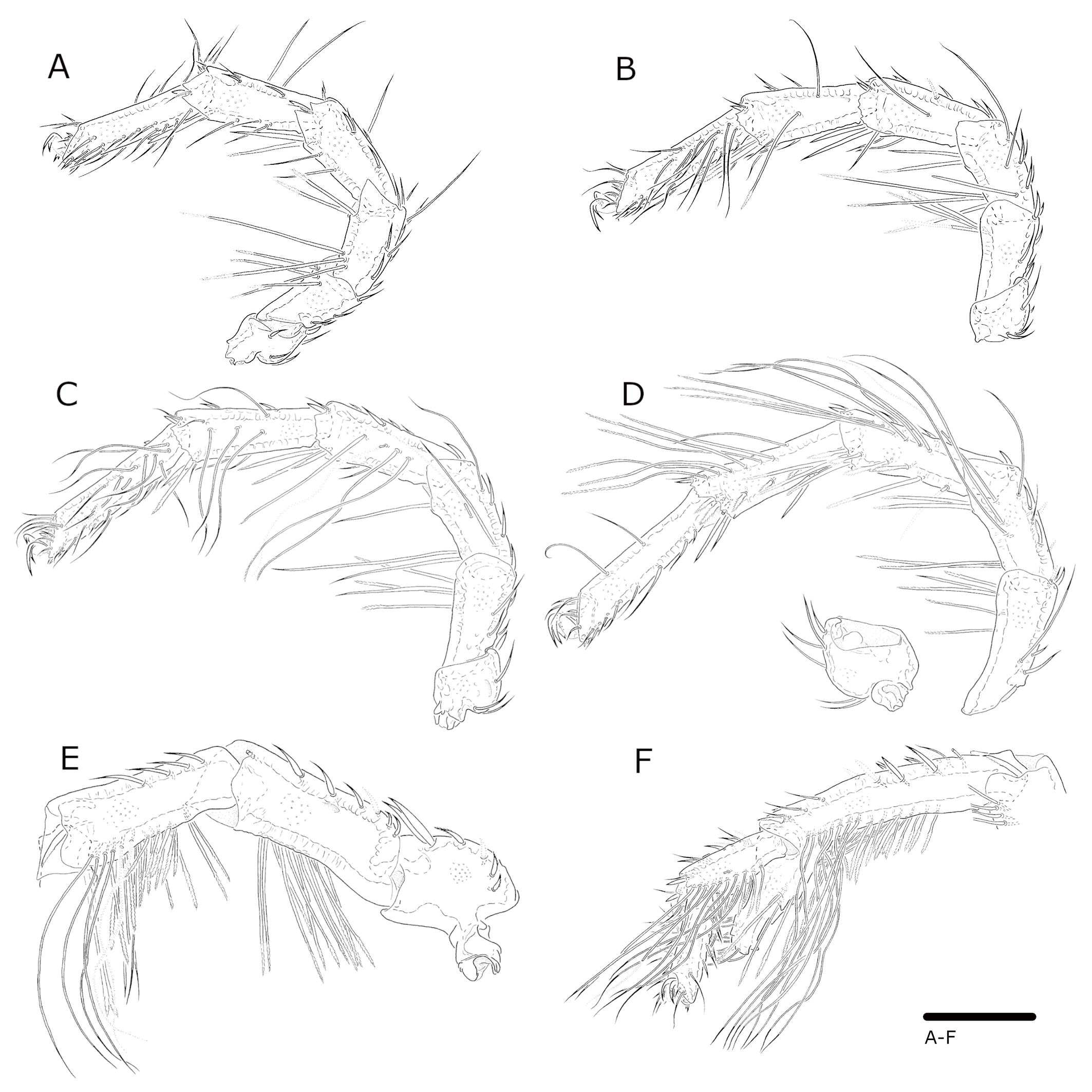


Leg segments, L/H: I-leg-1, 47/54; I-leg-2, 64/52; I-leg-3, 94/49; I-leg-4, 112/51; I-Leg-5, 117/48; I-Leg-6, 126/33 (Figs 6A, B). II-leg-1, 57/59; II-leg-2, 78/54; II-leg-3, 106/53; II-leg-4, 130/51; II-Leg-5, 139/47; II-Leg-6, 147/33 (Fig. 6C). III-leg-1, 78/80; III-leg-2, 100/52; III-leg-3, 114/54; III-leg-4, 152/53; III-Leg-5, 156/49; III-Leg-6, 152/39 (Fig. 6D). IV-leg-1, 134/86; IV-leg-2, 185/77; IV-leg-3, 179/68; IV-leg-4, 242/65; IV-Leg-5, 97/44; IV-Leg-6, 96/30 (Figs 6E and 6F). I-leg-2, 3 with long stiff serrate setae (Figs 6A and 6B); III-leg bearing both long stiff setae and swimming setae (Fig. 6D); IV-leg-2 with long stiff serrate setae; IV-leg-3 with both short and long stiff serrate setae, and swimming setae ventro-distally; IV-leg-4 with a long distal process [sometimes named'spur′] (total L 122); IV-leg-4 and IV-leg-5 bearing swimming setae along the ventral surface – in groups of eight to ten posterior and anterior in distal half and at the ventro-distal process of IV-leg-4, and posterior in distal 2/3 of IV-leg-5 (Fig. 6F).
Female (n = 5 (1 partly dissected))
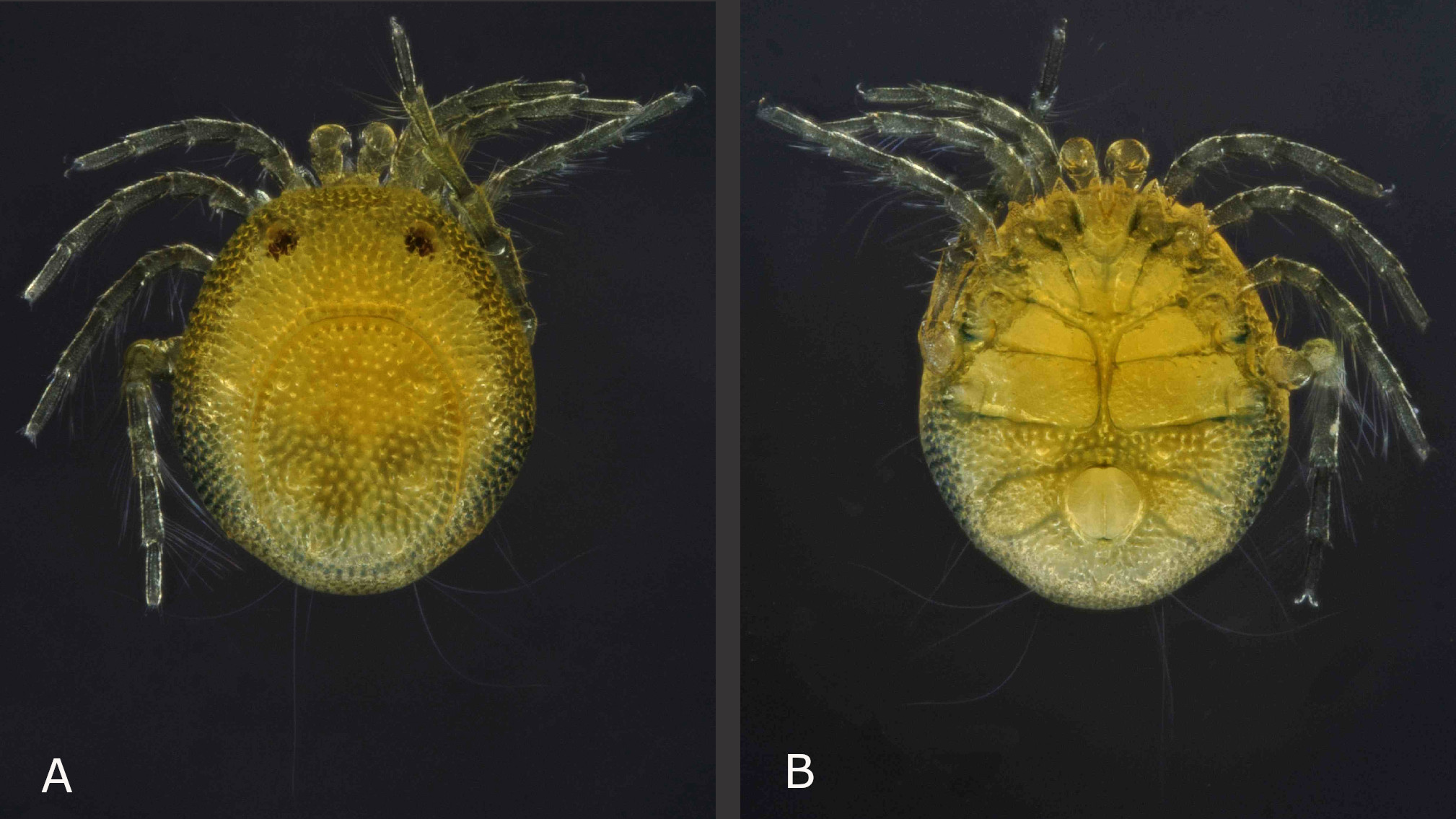





Idiosoma yellow, posterior bluish (Figs 7 and 8); legs and palps bluish. Frontal margin slightly convex; dorsal furrow complete; idiosoma margin rounded (Figs 7A, 8, 10A and 10C). Idiosoma L 760 (763–878); W 669 (668–755). Dorsal shield L 493 (499–619), W 410 (394–485). Distance between the anterior idiosoma margin and the anterior dorsal shield outline (maximum curvature) 231 (211–250); distance between the lateral idiosoma margin and the lateral dorsal shield outline, at Lgl-2, 131 (130–144); distance between the lateral idiosoma margin and the lateral dorsal shield outline at Lgl-3, 79 (67–91); distance between the posterior idiosoma margin and the posterior dorsal shield outline (maximum curvature) 44 (38–62). Distances between glands and setae of dorsal glandularia (between the glands/setae of both sides, respectively two different glandularia of one idiosoma side): A1, glands 215 (230–254); A1, setae 241 (250–284); A2, glands 181 (198–216); A2, setae 159 (173–192); R1 180 (197–214); R2 153 (149–182); Dgl-1, glands 475 (470–518); Dgl-1, setae 480 (461–514); Dgl-2, glands 286 (278–341); Dgl-2, setae 254 (245–326); Dgl-3, glands 258 (240–293); Dgl-3, setae 223 (202–272); Dgl-4, glands 200 (163–197); Dgl-4, setae 167 (139–173); Lgl-1, glands 463 (461–528); Lgl-1, setae 428 (427–509); Lgl-2, glands 671 (662–739); Lgl-2, setae missing (643–720); Lgl-3, glands 513 (461–566); Lgl-3, setae missing (480–576); Lgl-4, glands 197 (173–223); Lgl-4, setae missing (211–254). R2—Dgl-2, seta 64 (64–96); Dgl-2—Dgl-3, glands 200 (182–219); Dgl-2—Dgl-3, setae 196 (186¬–211); Dgl-3—Dgl-4, glands 136 (144–182); Dgl-3—Dgl-4, setae 127 (127–178).






Ventral shield L 798 (782–883), W 677 (662–756); distance between Cx-I tips 176 (177–194); coxal field L 471 (466–514), W 644 (624–720) (measured between Cx-IV tips) (Figure 10B). Acetabular plates region W 480 (451–514); gonopore inner L 124 (113–133), outer L 139 (141–154); W 157 (130–166). Distances between ventral glandularia (glands and setae): Cxgl-1, glands 298 (298–341); Cxgl-1, setae 286 (284–326); Cxgl-2, glands 236 (226–307); Cxgl-2, setae 217 (197–298). V1, setae 130 (110–120); V2, glands 187 (163–202); V2, setae 210 (192–237); V3, setae 307 (278–317); V4, glands 516 (494–586); V4, setae 534 (504–605); V1—V2 (setae) 48 (43–63). Barely two rows of (small) body pores between the anterior and posterior coxal groups, two rows between left and right Cx-III, one row between Cx-IV (Figs 7B, 10A and 10B).
Palp segments, L/H: P-1, 28/38; P-2 69/60; P-3, 49/53; P-4, 80/49; P-5, 41/18; structure and chaetotaxy of the palps as described for the male; P-4 distoventral portion slightly angular with one large antagonistic seta, L 60 (Figs 10E and 10F).
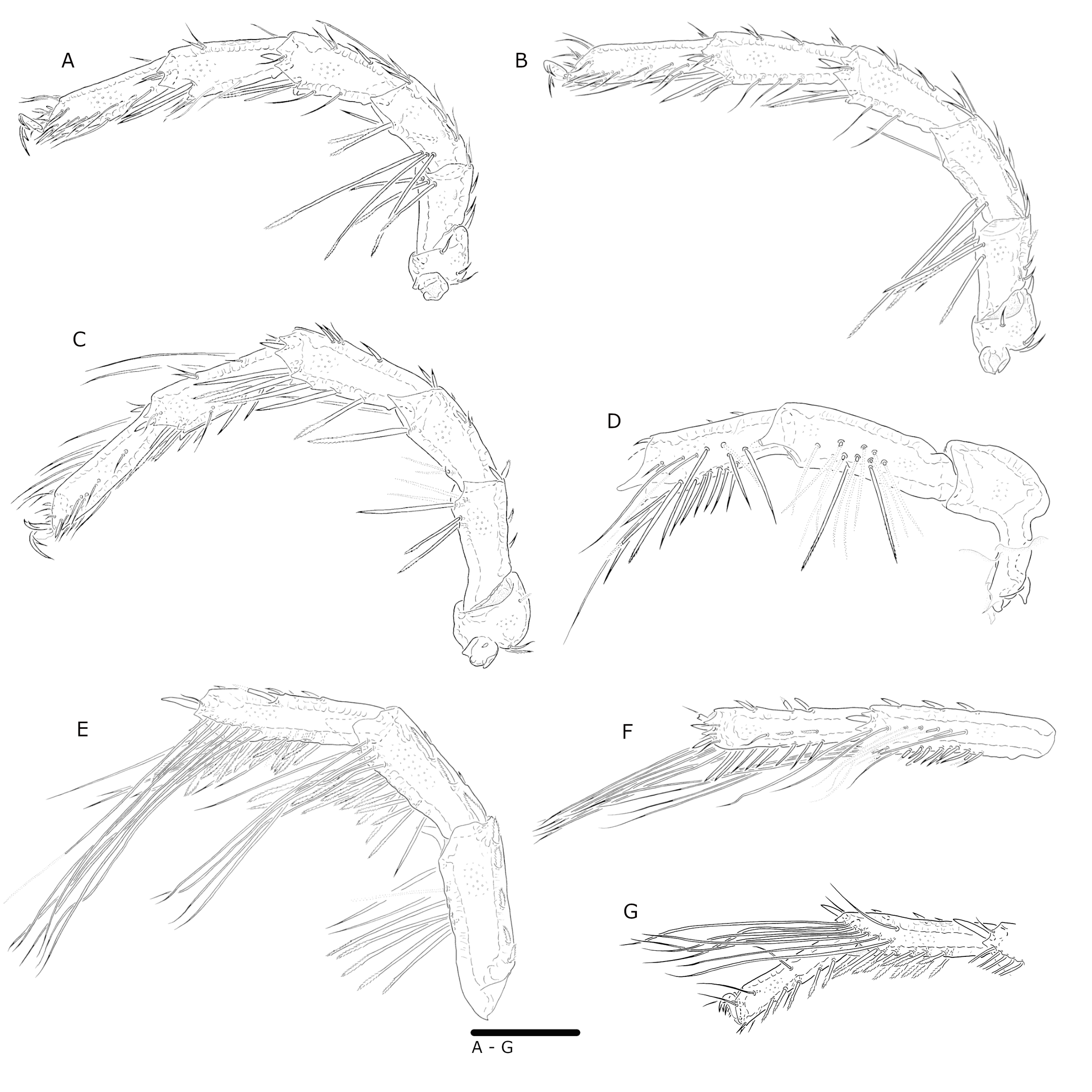


Leg segments, L/H: I-leg-1, 51/51; I-leg-2, 59/51; I-leg-3, 90/48; I-leg-4, 107/50; I-Leg-5, 111/45; I-Leg-6, 106/34 (Fig. 11A). II-leg-1, 55/57; II-leg-2, 71/52; II-leg-3, 97/51; II-leg-4, 122/50; II-Leg-5, 128/45; II-Leg-6, 115/33 (Fig. 11B). III-leg-1, 66/67; III-leg-2, 82/48; III-leg-3, 100/51; III-leg-4, 132/49; III-Leg-5, 137/46; III-Leg-6, 121/33 (Fig. 11C). IV-leg-1, 105/64; IV-leg-2, 146/54; IV-leg-3, 126/53; IV-leg-4, 164/43; IV-Leg-5, 144/37; IV-Leg-6, 123/28 (Figs 11D – G). III-leg 2–4 bear stiff serrate setae; III-leg-5, 6 bear long stiff setae (Fig. 11C); IV-leg-2–4 bear numerous long serrate setae; IV-leg-3–5 bear swimming setae (Figs 11D – G).
Remarks
As has been observed in other Arrenurus subgenera (such as Megaluracarus), morphological boundaries between males can be subtle and difficult to establish, then a thorough inspection of different type of characters is needed and, if the female is available, it must be included. In the case of Arrenurus (s.s.) some species show very stout anterior idiosoma and both, well developed pygal lobes and petiole while others show a combination of different grades of development of those features (Marshall 1908; Cook 1974).
Differential diagnosis of the male of A. (A.) munovus within the so far known Central American species of the subgenus Arrenurus
In A. (A.) munovus the pygal lobes are relatively short (not projecting beyond the base of the petiole), the petiole is relatively long. The only other species with similar short pygal lobes is A. valencius, however in this species the petiolus is bearing a characteristically long and slender tip (Böttger 1980). In A. dentipetiolatus, tamaulipensis and xochimilcoensis the pygal lobes are clearly longer and the petiole considerably shorter (Cook 1980; Cramer and Cook 1992). In A. wucabus the petiole has approximately the same length and a slightly similar shape, however the pygal lobes are longer and more pronounced; additionally A. wucabus lacks a pointed medial protuberance just above the petiole present in A. munovus (Cook 1980).
Differential diagnosis of the female of A. (A.) munovus within the so far known Central American species of the subgenus Arrenurus
Females of the Central American species can be separated by the shape of the acetabular plates, as well as the setation of the medial side of P-2: As far as known A. munovus is the only species (no respective illustrations were available for A. dentipetiolatus, the female of A. wucabus is unknown) of this group with the acetabular plates broad and curved (with a clear bend of ~50°) from the anterior part of the genital field towards posterior. In A. valencius the acetabular plates are located besides the posterior half of the genital field, posteriorly extending clearly beyond the genital field and are regularly curved from posterior to lateral (Marshall 1908); in A. tamaulipensis the acetabular plates show a postero-lateral curve very similar to the latter species, however with a more regular curve and not extending so far towards postero-medially of the genital field (Cramer and Cook 1992); A. xochimilcoensis is clearly separated from the other species of this group by relatively narrow acetabular plates, extending from the gonopore towards slightly towards antero-lateral (Cramer and Cook 1992).
The palps of A. munovus are bearing four setae on the medial surface of P-2 and one medio-dorsally; in the description of A. valencius no setae are visible in the respective position, however Orghidan and Gruia (1977) are illustrating five medial and one medio-dorsal setae at P-2, whereas Lundblad (1935) is giving an illustration with just three medial setae at P-2; in A. dentipetiolatus P-2 is bearing four medial setae; in A. tamaulipensis there are two and in A. xochimilcoensis two medial and one medio-dorsal setae at P-2 (Cramer and Cook 1992).
Discussion
In comparison to the male from Chiapas, Mexico, described by Cook (1980), the males from Belize show a distally more diverging petiolus (Figs 2A, 3 and 4A), the caudo-median projection between the pygal lobes in the male from Chiapas is regularly cone-shaped, whereas in the Belizean specimens, there is a clear constriction at the base of the projection (Fig. 4B).
The measurements of idiosoma, palp segments and most leg segments of the Belizean males are within the range of the males from Chiapas; the distal leg segments I-Leg-6 and IV-leg-6 of the Belizean males are shorter than the respective segment of the males from Chiapas (I-Leg-6, 126 vs. 155 (170), IV-leg-6, 96 vs. 111 (122)).
The differences between the males from Belize and Chiapas are by far not justifying the description of a new species, nevertheless they should be documented, as they seem to differentiate two distinct populations of A. (A.) munovus. Future studies should as well include molecular analysis in order to provide additional data that might help in resolving the taxonomic status of these populations.
The so far known water mite fauna of Belize seems to be rather similar to that of Guatemala and Southern Mexico (Goldschmidt et al. 2022), however at species level so far just one species of Pontarachnidae has been published from the coastal waters of Northern Belize (Montes-Ortiz et al. 2021). The present paper gives the first species level data on the water mite fauna of Belize extended and diverse freshwater habitats, and seems to confirm its similarity with the fauna of Southern Mexico. However still a lot of research is needed in order to understand the biogeographic patterns of the Central American water mite fauna.
Acknowledgements
Jonas Goldschmidt, Lucia Montes-Ortiz and Manuel Elías-Gutiérrez accompanied the second author during the sampling trip to Belize and helped with the samples.
We wish to thank Dr. Bernhard Ruthensteiner and Prof. Dr. Roland Melzer, Bavarian State Collection of Zoology, Munich, Germany for the possibility to use photomicroscopes and stacking facilities. Two anonymous reviewers helped to improve the manuscript.
References
- Böttger, K. 1980. Zur Parasitologie und postparasitischen Entwicklung der neotropischen Wassermilbe von Arrenurus valencius (Hydrachnellae, Acari), nebst einigen faunistischen Angaben zum See ''Laguna Chichoj'' in Guatemala. Studies on Neotropical Fauna and Environment, 15: 1550166. https://doi.org/10.1080/01650528009360572
- Cook, D.R. 1974. Water mite genera and subgenera. Memoirs of the American Entomological Institute, 21: 1-860.
- Cook, D.R. 1980. Studies on neotropical water mites. Memoirs of the American Entomological Institute, 31: 1-645.
- Cramer, C., Cook, D.R. 1992 New species of Arrenurus (Acari: Arrenuridae) from Mexican lakes. Acarologia, 33: 349-366.
- Goldschmidt, T. 2016. Water mites (Acari, Hydrachnidia): powerful but widely neglected bioindicators - a review. Neotropical Biodiversity, 2(1): 12-25. https://doi.org/10.1080/23766808.2016.1144359
- Goldschmidt, T., Helson, J.E., Williams, D.D. 2016. Ecology of water mite assemblages in Panama - First data on water mites (Acari, Hydrachnidia) as bioindicators in the assessment of biological integrity of neotropical streams. Limnologica, 59: 63-77. https://doi.org/10.1016/j.limno.2016.03.007
- Goldschmidt, T., Ramírez-Sánchez, M.M. 2020. Introduction and keys to Neotropical water mites (Acari, Hydrachnidia). Spixiana, 43(1): 203-303. https://www.zobodat.at/pdf/Spixiana_043_0203-0303.pdf
- Goldschmidt, T., Schmidt, J.C., Boles, E. 2022. Hidden treasures - a first study on the unexplored diversity of water mites (Acari; Hydrachnidia) from Belize. Acarologia, 62(3): 694-720. https://doi.org/10.24349/vk99-cldq
- Lundblad, O. 1935. An investigation of some Hispaniolan lakes. (Dr. M. Bond's Expedition). Über einige Hydracarinen aus Haiti. Arkiv för zoologi, 28 A (13): 1-30.
- Marshall, R. 1908. The Arrhenuri of the United States. Transactions of the American Microscopical Society, 28: 85-140. https://doi.org/10.2307/3220907
- Marshall, R. 1919. New species of water mites of the genus Arrhenurus. Transactions of the American Microscopical Society , 38(4): 275-281. https://doi.org/10.2307/3221847
- Montes-Ortiz, L., Goldschmidt, T., Vásquez-Yeomans, L., Elías-Gutiérrez, M. 2021. A new species of Litarachna Walter, 1925 (Acari: Hydrachnidia: Pontarachnidae) from Corozal Bay (Be-lize), described based upon morphology and DNA barcodes. Acarologia, 61: 1-12. https://doi.org/10.24349/r7no-Ludg
- Montes-Ortiz, L., Elías-Gutiérrez, M., Ramírez-Sánchez, M.M. 2022. Checklist of Arrenurids (Aca-ri: Hydrachnidia: Arrenuridae) of Mexico, with New Records from the Yucatan Peninsula, and the Description of Five New Species of the Subgenera Megaluracarus and Dadayella. Diversity, 14(4): 276. MDPI AG. Retrieved from https://doi.org/10.3390/d14040276
- Orghidan, T.N., Gruia, M. 1977. Sur quatre espèces du genre Arrenurus (Hydrachnellae) de Cu-ba. Résultats des expéditions biospéologiques cubano-roumaines à Cuba. II., Bucarest 231-240.
- Ramírez-Sánchez, M.M., De Luna, E., Cramer, C. 2016. Geometric and traditional morphometrics for the assessment of character state identity: multivariate statistical analyses of character vari-ation in the genus Arrenurus (Acari, Hydrachnidia, Arrenuridae). Zoological Journal of the Linnean Society, 177(4): 720-749. https://doi.org/10.1111/zoj.12384
- Rosso de Ferradás, B., Fernández, H.R. 2005. Elenco y biogeografía de los ácaros acuáticos (Acari, Parasitengona, Hydrachnidia) de Sudamérica. Graellsia, 61(2): 181-224. DOI: https://doi.org/10.3989/graellsia.2005.v61.i2.19
- Smit, H. 2020. Water mites of the world, with keys to the families, subfamilies, genera and subgenera (Acari: Hydrachnidia). Monografieën van de Nederlandse Entomologische Vereniging 12: 1-774.
- Valdecasas, A.G. 2019. A new species of Arrenurus (Acari, Parasitengona, Hydrachnidia) found in the crop of a Yellow-billed Teal Anas flavirostris in Bolivia. Acarologia, 59(2): 253-260. https://doi.org/10.24349/acarologia/20194329
- Viets, K.O. 1975. Wassermilben (Hydrachnellae, Acari) aus Stillgewässern in Guatemala. Studies on Neotropical Fauna and Environment, 10: 1, 57-76, DOI: 10.1080/01650527509360482 https://doi.org/10.1080/01650527509360482



2024-05-13
Date accepted:
2024-06-21
Date published:
2024-07-01
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Ramírez-Sánchez, Marcia M. and Goldschmidt, Tom
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



