Three new feather mite species of the genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae: Pterodectinae) from birds of paradise (Passeriformes: Paradisaeidae)
Constantinescu, Ioana Cristina  1
; Chișamera, Gabriel Bogdan
1
; Chișamera, Gabriel Bogdan  2
; Motoc, Rozalia
2
; Motoc, Rozalia  3
and Adam, Costică
3
and Adam, Costică  4
4
1✉ Grigore Antipa National Museum of Natural History, Sos. Kiseleff no.1, 011341, Bucharest, Romania.
2Institute of Biology – Bucharest, Romanian Academy, 296 Splaiul Independenței, 060031 Bucharest, P.O. Box 56-53, Romania.
3Grigore Antipa National Museum of Natural History, Sos. Kiseleff no.1, 011341, Bucharest, Romania.
4Grigore Antipa National Museum of Natural History, Sos. Kiseleff no.1, 011341, Bucharest, Romania.
2024 - Volume: 64 Issue: 2 pages: 661-682
https://doi.org/10.24349/bx89-qaobZooBank LSID: C1614592-B8C0-4EDD-A08E-7DA590E15E8A
Original research
Keywords
Abstract
Introduction
Feather mites (Astigmata: Analgoidea and Pterolichoidea) are common and diverse ectosymbionts of birds that spend their entire lives on their hosts. The genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae) belongs to the Proterothrix generic group in the subfamily Pterodectinae. This group currently comprises eight genera of putatively early derivative pterodectines with setae ps3 anterior to the adanal suckers in males and seta wa situated noticeably anterior to setae ra and la on tarsi I and II in both sexes (Mironov 2009, Mironov & Proctor 2009, Hernandes & Valim 2014, Mironov & OConnor 2017). The genus Proterothrix clearly differs from other genera of this group in having the following combinations of characters: in both sexes, solenidion σ of genu III is present and shorter than this segment, and idiosomal setae d2 are present; in males, the genital arch is well developed, the genital papillae are close to each other and situated anterior to or at the level of this arch, the opisthosomal lobes are devoid of any membranous projections, coxal fields I are closed, and coxal fields III are closed or almost closed; and in females, setae h2 have an apical filament. To date, the genus has included 32 species arranged into the four morphology-based species groups: megacaula (3 species), schizothyra (5 species), wolffi (17 species) and paradoxornis (7 species). Species of the schizothyra group are restricted to kingfishers (Coraciiformes: Alcedinidae), species of the megacaula group are only known from hosts of the passerine family Muscicapidae, those of the wolffi group are known from various passerine families (Passeriformes) and from woodpeckers (Piciformes: Picidae), while those of the paradoxornis group are known from hosts of the passerine families Pellorneidae and Paradoxornithidae (Gaud 1952, 1962, 1968, 1979, Mironov et al. 2008, 2010, 2012, Mironov & Proctor 2009, Mironov & Tolstenkov 2013, Mironov & Galloway 2021, Constantinescu et al. 2014, 2017a, 2017b, 2018, 2019, 2021, 2023, Han et al. 2019).
Until now, five proctophyllodid species belongoing to the genus Proterothrix have been described on birds of paradise (Paradisaeidae) (Trouessart 1885, 1899; Constantinescu et al 2018). The diversity of this feather mite genus of is unusually high on these hosts; two species of birds of paradise (Manucodia chalybatus and Manucodia ater) are known to carry three different Proterothrix species (Table 1). In this paper, three new species of feather mites belonging to the genus Proterothrix from birds of paradise were described.



Material and methods
Four bird specimens of Paradisaea minor and one of Manucodia chalybata, which are deposited in the old Bird Collection of ''Grigore Antipa'' National Museum of Natural History (Bucharest, Romania), were been examined. Mite samples were collected by the scraping technique (Gaud & Atyeo 1996) and placed in 96% ethanol. Later, mite specimens were cleared in 90% lactic acid for 24 hours and mounted on microscope slides in Hoyer's medium.
Drawings were made using an Olympus CX21 microscope with a camera lucida drawing device. The body setation of mites follows that of Griffiths et al. (1990) with the modifications by Norton (1998) concerning coxal setae, while the setation of legs follows Grandjean (1939). Redescriptions follow the current format used for species of pterodectine mites (Mironov & Fain 2003, Mironov 2006, Valim & Hernandes 2006, Mironov et al. 2008) and the measuring techniques of particular structures used were described by Mironov and Proctor (2009). All measurements are in micrometers (μm). The type material of described species is deposited in the Acarological Collection of the ''Grigore Antipa'' National Museum of Natural History (Bucharest, Romania) (MGAB) and in Acari Collection of the Department of Ecology and Zoology, Universidade Federal de Santa Catarina (ECZ-UFSC).
Taxonomy
Family Proctophyllodidae Trouessart and Mégnin, 1884
Subfamily Pterodectinae Park and Atyeo, 1971
Genus Proterothrix Gaud, 1968
Proterothrix maior Constantinescu n. sp.
ZOOBANK: 427325D9-358C-4DF7-B770-A1F83A26F791 ![]()
(Figures 1–4)












Type material
Holotype male and 12 paratypes (2 males and 10 females), from Manucodia chalybatus (Pennant, 1781) (Corvida: Paradisaeidae), New Guinea, bird inventory number 4264, no other data.
Type depository
Holotype male (ANA 877), 2 male paratypes (ANA 2145, ANA 2146), 10 female paratypes (ANA 873-876, ANA 878, ANA 2147-2151) in MGAB collection.
Description
Male — (holotype, range for 2 paratypes in parentheses) (Figures 1A, B and 2A–D): Length of idiosoma 540 (540–545), width 240 (225–245), length of hysterosoma 370 (352–375). Prodorsal shield entire, anterolateral extensions with rounded tips, lateral margins shallowly concave, posterior margin with small minute median extension, length 188 (163–170), width 200 (185–205), surface with circular lacunae (Figure 1A). Scapular setae se separated by 138 (125–144). Scapular shields narrow. Humeral shields wide, fused ventrally with outer sclerotized areas of epimerites III. Setae cp situated ventrally on humeral shield. Setae c2 filiform, on anterior end of humeral shield. Subhumeral setae c3 lanceolate, 35 (30–34) × 13 (10–12). Hysteronotal shield with anterior margin slightly concave, anterior angles round, distance from anterior margin to bases of setae h3 335 (330–240), greatest width in anterior part 180 (182–188), surface with small circular lacunae. Opisthosomal lobes short, represented by rounded convexities; lateral margin with wide lateral membrane stretching from the midlevel of supranal concavity to bases of setae ps2; posterior margin with two truncate extensions at bases of setae h3 and h2 having acute protruding angles. Supranal concavity roughly ovate, with margins clearly outlined. Terminal cleft small, wide V-shaped, 28 (30–32) in length. Setae f2 at the same level with setae ps2. Setae h1 at midlevel of supranal concavity, distant from lateral margins of opisthosoma. Setae ps1 lanceolate, length 23 (22–25), situated on margins of opisthosomal lobes, at the same level with setae h3. Setae c1 present, setae f2 filiform, setae h3 large triangular, with deeply concave posterior margin, surface with few longitudinal striae, 158 (152–165) × 95 (105–110); and setae h2 with large lanceolate enlargement on lateral side along basal 2/3 and with filiform apical part, 220 (210–224) × 30 (28–31). Dorsal measurements: c2–d2 130 (131–140), d2–e2 175 (155–160), e2–h3 88 (73–88), d1–d2 75 (74–76), e1–e2 60 (65–68), h1–ps2 38 (40–42), h2–h2 108 (110–115), h3–h3 75 (76–80), ps2–ps2 120 (125–130).
Epimerites I fused into a V, its posterior end connected with epimerites II by transverse sclerotized bands. Epimerites II long, with posterior ends free. Rudimentary sclerites rEpIIa absent. Epimerites IIIa with L-shaped inner ends, sclerotized areas around angles of these epimerites fused with median pregenital sclerite; setae 4b on areas fused with pregenital sclerites. Coxal field I closed, coxal field II and III open, coxal fields IV with wide sclerotized areas at bases of trochanters IV. Epimerites IVa present (Figure 1B). Genital arch 28 (27–28) long, 48 (50–52) wide, basal sclerite of genital apparatus small and rounded posteriorly, aedeagus 115 (118–120) long, extending to anterior margins of adanal suckers. Genital papillae situated anterior to level of genital arch. Opisthoventral shields not developed. Adanal shields well developed, represented by a pair of shields complicate shaped: main parts with narrow anterior extensions almost reaching level of genital arch, with bases of setae ps3 on posterior margins, and with narrow transverse bridge connecting main shields' parts at midlevel; setae g on small rounded separate sclerotized fragments between anterior extensions. Setae ps2 thickened basally, with acute apex 50 (45–55) in length. Anal suckers 25 (20–22) in diameter, corolla with 26-29 denticles. Ventral measurements: 3a–4b 48 (48–50), 4b–4a 75 (72–77), 4a–g 75 (72–80), g–ps3 113 (110–120), ps3–ps3 23 (24–27), ps3–h3 60 (62–64).
Legs I slightly longer and thicker than legs II; femora I and II, each with a ventral crest (Figure 2 A, B). Genu and tibia of legs I with dorsal narrow longitudinal crest (Figure 2A). Seta e of tarsus I filiform, setae cG of genu I lanceolate. Setae d of tarsi II, III 2/3 of setae f and e. Tarsus IV 38 (40–42) long, setae d, e button-like, situated in basal and apical parts of segment, respectively (Figure 2D). Length of solenidia: ω1I 17 (15–18), ω1II 20 (20–22), φI 120 (114–122), φII 100 (100–105), φIII 50 (44–48), φIV 57 (47–50).
Female — (range for 5 paratypes) (Figures 3A, B and 4A–E): Length of idiosoma 550–630, width 245–275, and length of hysterosoma 385–440. Prodorsal shield entire, anterolateral extensions with rounded tips, lateral margins shallowly concave, posterior margin with minute acute median extension, length 173–195, width 210–238, surface without ornamentation (Figure 3A). Scapular setae se separated by 128–140. Scapular shields narrow. Humeral shields wide, fused ventrally with outer sclerotized areas of epimerites III. Setae cp situated ventrally on humeral shield. Setae c2 filiform, situated dorsally on anterior end of humeral shields. Subhumeral setae c3 lanceolate, 28–33 × 10–13. Anterior hysteronotal shield roughly rectangular, anterior margin straight, posterior margin slightly concave, greatest length 258–290, greatest width in anterior part 203–225, surface without ornamentation. Length of lobar region 130–138, width at anterior margin 165–195. Terminal cleft narrow V-shaped, with anterior part with divergent margins and posterior part with parallel margins, length 63–80. Supranal concavity circular, well outlined. Setae h1 on lobar shield, at midlevel of supranal concavity; surface of lobar shield without ornamentation. Setae h2 spindle-shaped, with long terminal filament, 88–113 × 13–15. Setae ps1 on inner margin of opisthosomal lobes, setae h3 28–33 long, about 1/4 the length of terminal appendages. Dorsal measurements: c2–d2 125–130, d2–e2 130–158, e2–h2 100–105, h2–h3 50–60, d1–d2 55–63, e1–e2 63–80, h1–h2 58–65, h2–ps1 45–55, h1–h1 68–78, h2–h2 93–123.
Epimerites I fused as a V, fused part with long lateral extensions. Coxal fields I without sclerotized areas, fields II with lateral halves strongly sclerotized areas, epimerites IVa absent (Figure 3B). Translobar apodemes of opisthosomal lobes present, fused to each other anterior to terminal cleft. Epigynum horseshoe-shaped, greatest width 90–100. Copulatory opening situated ventrally at anterior margin of fused translobar apodemes. Distal 1/3 of primary spermaduct thickened, secondary spermaducts short (Figure 4E). Distance between pseudanal setae: ps2–ps2 88–100, ps3–ps3 28–35, ps2–ps3 40–45.
Legs I slightly longer and thicker than legs II. Femora I, II without ventral crests. setae cG of genu I stick-like. Setae d of tarsi II–III much shorter than corresponding setae f. Length of solenidia: ω1I 23–25, ω1II 20–25, φI 105–135, φII 85–100, φIII 38–50, φIV 30–38 (Fig. 4A–D).
Etymology
The specific name is derived from Latin adjective maior (bigger), and refers to the large size of the species, which exceeds 500 micrometers.
Remarks
Proterothrix maior n. sp. belongs to the wolffi species group, in having almost closed coxal fields III in males and parallel-sided terminal cleft in females. Among all species of the genus, Proterothrix maior n. sp. appears closest to P. diminuta (Trouessart, 1899) from Manucodia ater (Lesson) (Corvida: Paradisaeidae). Males of both species have the same shape and ornamentation of the dorsal shields: the terminal setae have a similar shape (h3 very wide, triangular, with posterior margin deeply concave and few longitudinal striae, setae h2 have a blade like lateral enlargement along basal 2/3 and filiform apical part), the opisthosoma has lateral membranes and setae cG of genu I are lanceolate. Proterothrix maior n. sp. is easily differentiated from P. diminuta in the following features of males: it is a considerably larger species with the length 540–545, paragenital sclerite is present, and the adanal shields are connected with a transverse bridge at their midlevel. In males of P. diminuta, the length of the idiosoma is 348 (348–364), paragenital sclerite is absent and the adanal shields are not connected. The female of P diminuta are unknown, therefore the female of the new species is compared with the next close species, Proterothrix emarginata (Trouessart, 1899) from the same host, Manucodia chalybatus. Females of both species have similar body size (550–630 in P. maior and 600 in P. emarginata), the dorsal shields are without ornamentation, epimerites I are fused as a V with long lateral extensions, and setae cG of genu I are lanceolate. In females of P. maior, the terminal cleft has parallel margins in posterior half and divergent in anterior half, and the anterior margin of the hysteronotal shield is straight. In females of P. emarginata, the terminal cleft is narrowly triangular, and the anterior margin of the hysteronotal shield is concave.
Proterothrix modestasimilis Constantinescu n. sp.
ZOOBANK: 7CE9B2F5-3311-4964-85AF-95AB2839BF5A ![]()
(Figures 5–8)






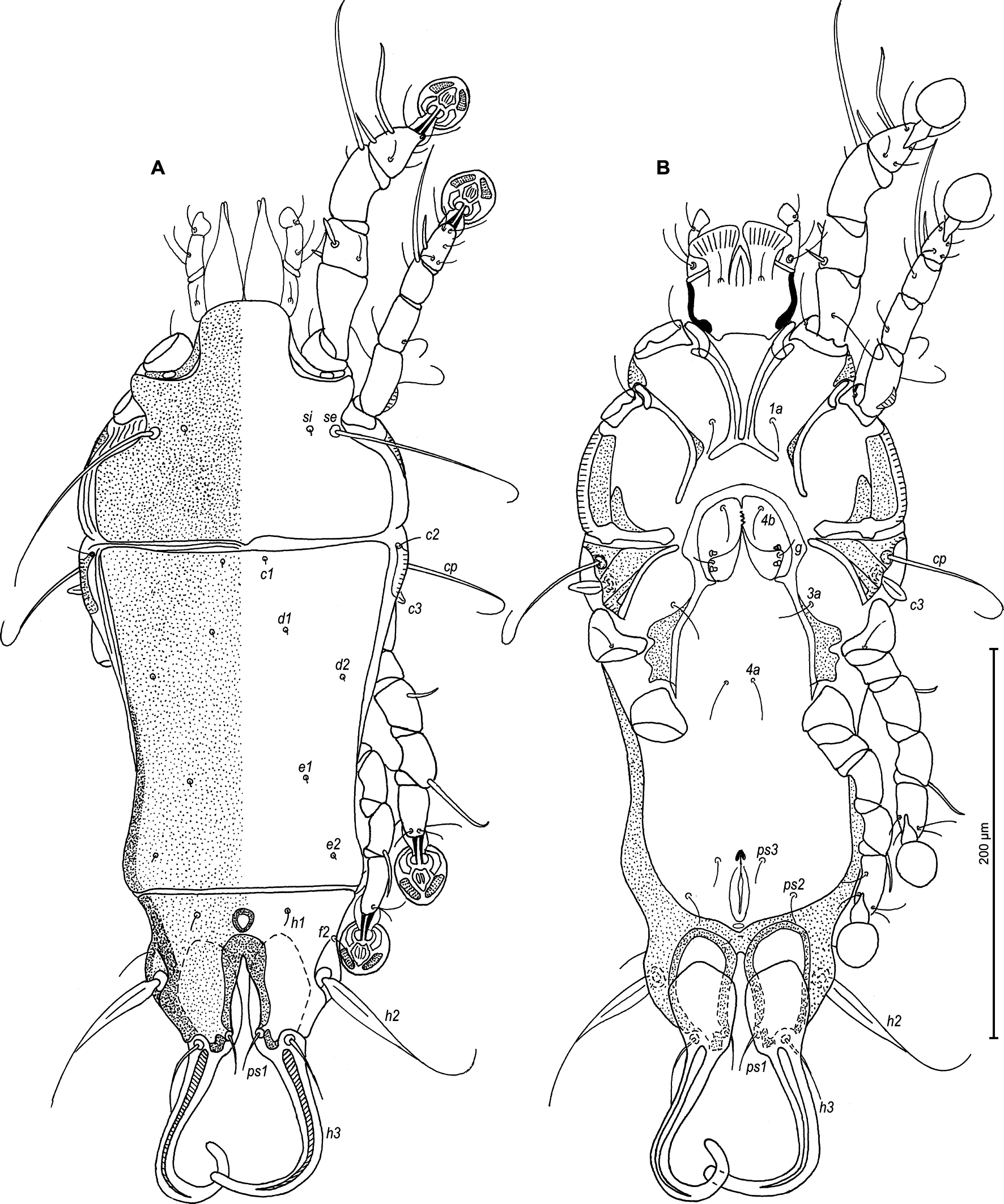





Type material
Holotype male and 21 paratypes (10 males and 11 females), from Manucodia chalybatus (Pennant, 1781) (Corvida: Paradisaeidae), New Guinea, bird inventory number 4264, no other data.
Type depository
Male holotype (ANA 898), 9 male paratypes (ANA 889-892, ANA 894-897, ANA 2152), 10 female paratypes (ANA 899-901, ANA 903-905, ANA 2153-2156) in MGAB collection, 1 male and 1 female paratypes in ECZ-UFSC collection.
Description
Male — (holotype, range for 4 paratypes in parentheses) (Figures 5A, B and 6A–E): Length of idiosoma 325 (315–330), width 153 (140–163), length of hysterosoma 195 (188–198). Prodorsal shield entire, anterolateral extensions long and rounded, lateral margins concave, without incisions around scapular setae, posterior margin with small blunt-angular median extension, posterior angles widely round, length 128 (125–130), width 130 (120–125), surface with big circular lacunae (Figure 5A). Scapular setae se separated by 75 (70–75). Scapular shields narrow. Humeral shields narrow, not fused ventrally with epimerites III. Setae cp situated ventrally on soft tegument. Setae c2 filiform, situated dorsally on anterior ends of humeral shields. Subhumeral setae c3 lanceolate, 25 (25–27) × 8 (8–10). Hysteronotal shield with anterior margin strongly concave, anterior angles triangular, distance from anterior margin to bases of setae h3 180 (175–188), greatest width in anterior part 118 (108–110), surface with big circular and ovate lacunae. Opisthosomal lobes short and wide, roughly rectangular, approximately half as long as their width, without membranes, posterior margin with two pairs of small smoothed teeth at bases of setae h2 and h3. Setae h3 and h2 approximately at the same transverse level. Terminal cleft small U-shaped, 17 (15–23) in length. Supranal concavity shaped as inverted teardrop, clearly outlined. Setae f2 at the same level with setae ps2. Setae h1 at midlevel of supranal concavity, distant from lateral margins of opisthosoma. Setae ps1 lanceolate, 15 (13–15) long, situated near lateral margins of opisthosomal lobes, at level of setae h2. Setae c1 present, setae h3 large leaf-like, widely lanceolate with rounded posterior end, 82 (84–92) x 25 (25–33), setae h2 enlarged in basal part 200 (175–200) x 8 (8–10), setae f2 short filiform. Dorsal measurements: c2–d2 80 (75–80), d2–e2 63 (63–70), e2–h3 50 (47–55), d1–d2 35 (30–38), e1–e2 27 (27–30), h1–ps2 22 (25–28), h2–h2 58 (57–58), h3–h3 25 (25–33), ps2–ps2 80 (70–78).
Epimerites I fused into a V, fused part connected with epimerites II by transverse bands. Epimerites II long, with posterior ends free. Rudimentary sclerites rEpIIa present, ovate. Epimerites IIIa with inner ends shaped as an oblique T, extensions directed to midline and backward thinner than ones directed anterolateral and bear bases of setae 4b. Coxal field I closed, coxal field II open, coxal fields III almost closed, coxal fields IV with wide sclerotized areas at bases of trochanters IV. Epimerites IVa present, small (Figure 5B). Genital arch 25 (22–25) long, 42 (33–40) wide, basal sclerite of genital apparatus rounded posteriorly; aedeagus 117 (110–117) long, extending to level of posterior margin of opisthosomal lobes. Genital papillae situated anterior to level of genital arch. Opisthoventral shields absent, adanal shields shaped as irregular plates, with setae ps3 on inner margins. Setae g on soft tegument. Adanal suckers 13 (12–15) in diameter, corolla multidentate, with 10 small denticles. Setae ps2 thickened in basal part, with filiform apex. Ventral measurements: 3a–4b 37(25–30), 4b–4a 33 (32–37), 4a–g 55 (50–55), g–ps3 20 (20–22), ps3–ps3 17 (17–18), ps3–h3 42 (42–45).
Legs I slightly longer than legs II, femora I and II, each with ventral crest (Figure 6A, B). Seta e of tarsus I filiform, setae cG of genua I lanceolate. Setae d of tarsi II, III half as long as corresponding setae f. Tarsus IV 30 (27–30) long, setae d, e button-like, situated in basal and apical parts of segment, respectively (Figure 6D). Length of solenidia: ω1I 12 (10–12), ω1II 8 (7–10), φI 70 (72–75), φII 57 (53–62), φIII 45 (30–37), φIV 43 (37–42).
Female — (range for 5 paratypes) (Figures 7A, B and 8A–E): Length of idiosoma 475–500, width 220–310, and length of hysterosoma 325–345. Prodorsal shield entire, anterolateral extensions long with rounded tips, lateral margins deeply concave without incisions around scapular setae, posterior margin with small rounded median extension, length 145–153, width 180–193, surface without ornamentation (Figure 7A). Scapular setae se separated by 115–123. Scapular shields narrow. Humeral shields narrow, fused ventrally on a small area in anterior part with outer sclerotized areas of epimerites III. Setae cp situated ventrally on humeral shield. Setae c2 filiform, situated dorsally on anterior ends of humeral shields. Subhumeral setae c3 lanceolate, 25–28 × 11–13. Anterior hysteronotal shield roughly rectangular, anterior margin slightly concave, posterior margin straight, greatest length 208–230, greatest width in anterior part 178–188, surface without ornamentation. Setae c1 present. Length of lobar region 105–130, width at level of setae h2 143–163. Terminal cleft V-shaped, with concave margins posterior to level of setae h2, 58–63 long. Supranal concavity well developed, egg-shaped. Setae h1 on lobar shield at level of anterior margin of supranal concavity; surface of lobar shield without ornamentation. Setae h2 spindle-shaped, with terminal filament, 88–113 × 10–15. Setae ps1 on inner margin of opisthosomal lobes, setae h3 30–35 long, about 1/3 from the length of terminal appendages. Dorsal measurements: c2–d2 93–110, d2–e2 110–115, e2–h2 78–85, h2–h3 45–50, d1–d2 43–50, e1–e2 50–58, h1–h2 43–53, h2–ps1 48–50, h1–h1 58–63, h2–h2 95–108.
Epimerites I fused as a V, connected part with long lateral extensions. Coxal fields I, II without large sclerotized areas, epimerites IVa absent (Figure 7B). Translobar apodemes of opisthosomal lobes present, fused to each other anterior to terminal cleft. Epigynum horseshoe-shaped, greatest width 78–83. Copulatory opening situated ventrally at anterior margin of fused translobar apodemes. Distal 1/3 of primary spermaduct gradually thickened to copulatory opening, secondary spermaducts short (Figure 8E). Distance between pseudanal setae: ps2–ps2 68–70, ps3–ps3 25–28, ps2–ps3 30–38.
Legs I slightly longer than legs II; femora II with wide ventral crest. Setae d of tarsi II–III half as long as corresponding setae f. Setae cG of genua I lanceolate. Length of solenidia: ω1I 15–20, ω1II 15–18, φI 90–95, φII 68–80, φIII 35–45, φIV 20–25. (Figures 8A–D).
Etymology
The specific name refers considerable morphological similarity of the new species with Proterothrix modesta (Trouessart, 1899).
Remarks
Proterothrix modestasimilis n. sp. belongs to the wolffi species group in having almost closed coxal fields III in males and parallel-sided terminal cleft in females. Among all species of the genus, Proterothrix modestasimilis n. sp. appears closest to P. modesta (Trouessart, 1899) from Manucodia ater (Lesson) (Corvida: Paradisaeidae). Males of both species have the same shape and ornamentation of the dorsal shields, the terminal setae h3 are large leaf-like, the opisthosomal lobes are short and wide, and the epimerites have a similar shape. Males of Proterothrix modestasimilis n. sp. easily differ from P. modesta by the following features: the idiosoma length considerably smaller (315–330), the lateral hysteronotal sclerites are absent, the humeral shields are not fused ventrally with epimerites III, setae cp are situated on soft tegument, the anterior angles of hysteronotal shield are acute, the supranal concavity is teardrop-shaped, the setae h1 are situated at the midlevel of supranal concavity, the setae h3 are without dorsal pattern and partly overlapped, and setae cG of genu I are lanceolate. In males of P. modesta, the length of the idiosoma is about 550, the lateral hysteronotal sclerites are present, the humeral shields are fused ventrally with epimerites III, setae cp are situated on the humeral shields, the anterior angles of the hysteronotal shield are rounded, the supranal concavity is strongly elongated, setae h1 are situated slightly posterior to the level of anterior margin of supranal concavity, setae h3 have a reticulate dorsal pattern and are not overlapped, and the setae cG of genu I are filiform. Females of both species have same shape of the dorsal shields, the epimerites I fused as a V, with long lateral extensions, the coxal fields I and II are without large sclerotized areas, and the epimerites IVa are absent. Females of Proterothrix modestasimilis n. sp. is easily distinguishable from P. modesta by the following features: the body length is considerably smaller (475–500), the dorsal shields are without ornamentation, the margins of terminal cleft are concave posterior to setae h2, and the humeral shields are connected with outer sclerotized areas of epimerites III by thin bridge. In females of P. modesta, the length of the idiosoma is 820–830, the dorsal shields are with small circular lacunae, the margins of terminal cleft are almost touching in anterior 1/5 and with small concavity posterior to level of setae h2, and the humeral shields are completely fused with outer sclerotized areas of epimerites III.
Proterothrix papuensis Constantinescu n. sp.
ZOOBANK: 36D1D2C8-FF33-40D4-9A88-65332BFD9EC5 ![]()
(Figures 9–12)
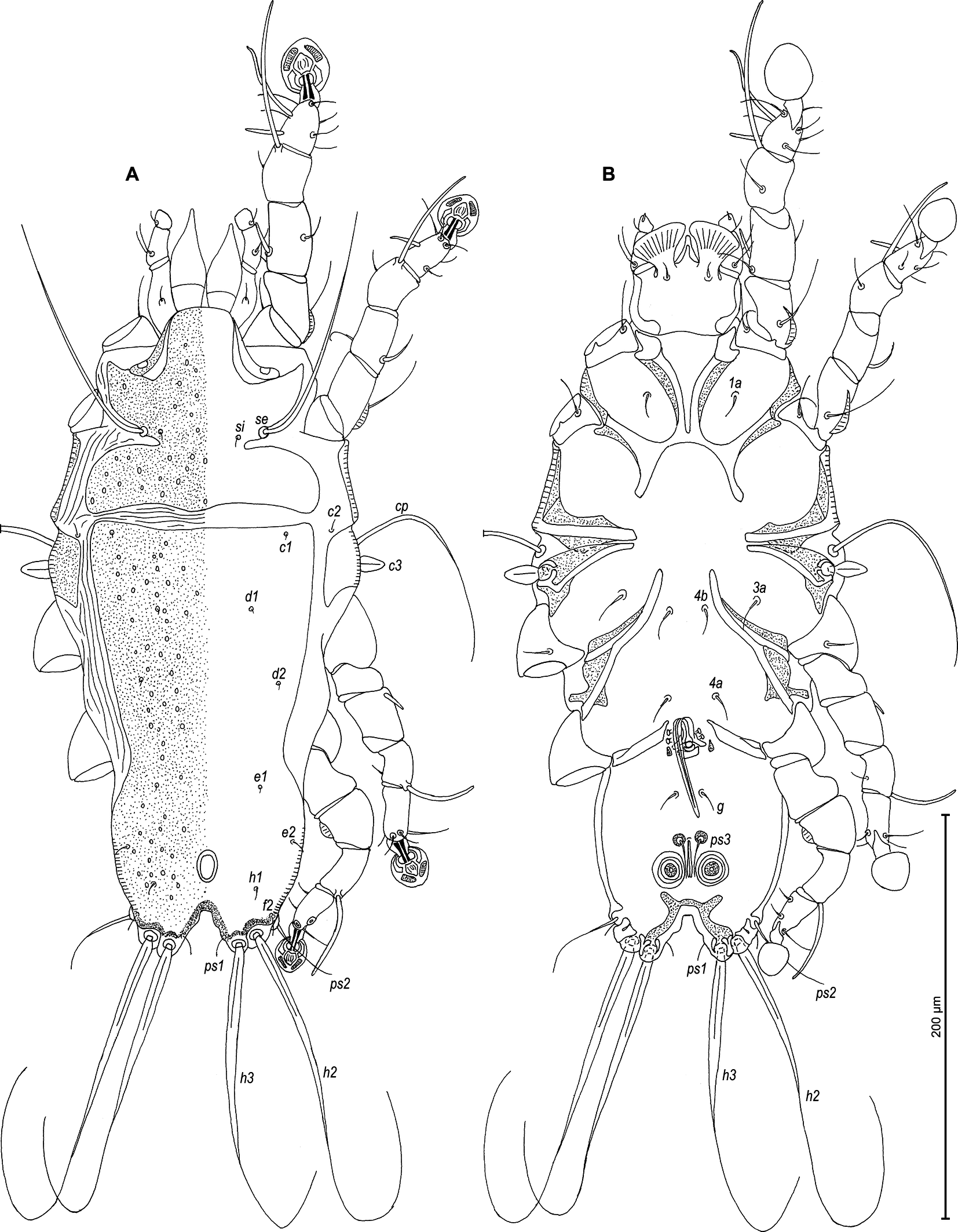











Type material
Holotype male and 19 paratypes (9 males and 10 females), from Paradisaea minor Shaw, 1809 (Corvida: Paradisaeidae), New Guinea, bird inventory number 6156, no other data.
Type depository
Male holotype (ANA 864), 7 male paratypes (ANA 857-863), 8 female paratypes (ANA 865-872) in MGAB collection, 2 male and 2 female paratypes in ECZ-UFSC collection.
Description
Male — (holotype, range for 4 paratypes in parentheses) (Figures 9A, B and 10A–E): Length of idiosoma 325 (305–325), width 155 (130–150), length of hysterosoma 220 (200–220). Prodorsal shield entire, anterolateral extensions wide and with acute ends, not connected with bases of epimerites Ia, lateral margins with deep and narrow incisions up to level of setae si, posterior margin slightly convex medially, posterior angles round, length 100 (100–103), width 113 (113–118), surface with small ovoid lacunae (Figure 9A). Scapular setae se separated by 63 (58–70). Scapular shields narrow. Humeral shields narrow, not fused with outer sclerotized areas of epimerites III. Setae cp situated ventrally on soft tegument. Setae c2 filiform, situated dorsally on soft tegument near humeral shields. Subhumeral setae c3 lanceolate, 20 (17–18) × 8 (8–10). Hysteronotal shield with anterior margin straight, anterior angles nearly rectangular, distance from anterior margin to bases of setae h3 200 (195–223), greatest width in anterior part 113 (110–113), surface with small ovoid lacunae. Opisthosomal lobes short and slightly narrowed posteriorly (roughly trapezoidal), without membranes, with short and blunt extensions at bases of setae h3 and h2. Terminal cleft wide, U-shaped with divergent branches, 25 (18–23) in length, lateral margins with a small convexity in anterior half. Supranal concavity ovoid, clearly outlined. Setae f2 at same level with setae ps2. Setae h1 at posterior margin of supranal concavity, distant from lateral margins of opisthosoma. Setae ps1 filiform, length 8 (8–10), situated near inner margins of opisthosomal lobes, anterior to bases of setae h3. Setae c1 present. Setae f2 short filiform, setae h2 and h3 represented by simple macrosetae slightly enlarged in basal part, h2 150 (150–200) × 5 (5–7) and h3 165 (138–150) × 6 (6–8) and. Dorsal measurements: c2–d2 75 (72–83), d2–e2 80 (75–84), e2–h3 60 (52–55), d1–d2 30 (35–38), e1–e2 33 (28–30), h1–ps2 18 (15–20), h2–h2 55 (52–58), h3–h3 35 (33–38), ps2–ps2 75 (63–78).
Epimerites I fused into a V, its posterior end connected with epimerites II by transverse sclerotized bands. Epimerites II long, with posterior ends free. Rudimentary sclerites rEpIIa absent. Coxal field II and III open, coxal fields IV without wide sclerotized areas at bases of trochanters IV. Epimerites IVa present (Figure 9B). Genital arch 13 (13–15) long, 18 (18–20) wide, basal sclerite of genital apparatus as inverted trapezoid; aedeagus 52 (52–60) long, extending to midlength between setae g and ps3. Genital papillae situated anterior to genital arch. Paragenital apodemes small triangular. Opisthoventral sclerotization represented by narrow sclerotized band along margin of terminal cleft and with a pair of anterolateral extension, entirely resembling a silhouette of butterfly. Adanal shields reduced to small circlular sclerites surrounding bases of setae ps3. Adanal suckers 13 (13–15) in diameter, corolla without indentations. Setae ps2 thickened basally, with filiform apex. Ventral measurements: 3a–4b 30 (27–30), 4b–4a 45 (45–47), 4a–g 43 (42–45), g–ps3 25 (20–28), ps3–ps3 10 (10–13), ps3–h3 52 (45–53).
Legs I slightly longer than legs II, femora I and II, each with a ventral crest (Figure 10A, B). Seta e of tarsus I filiform, setae d of tarsi II, III approximately half as long as corresponding setae f. Setae mGII thickened basally, with filiform apex. Tarsus IV 24 (20–30) long, setae d, e button-like, situated in middle and distal parts of segment, respectively (Figure 10D). Length of solenidia: ω1I 15 (12–18), ω1II 10 (10–13), φI 80 (68–80), φII 52 (50–53), φIII 35 (33–42), φIV 38 (30–40).
Female — (range for 5 paratypes) (Figures 11A, B and 12A–E): Length of idiosoma 385–410, width 160–175, and length of hysterosoma 280–300. Prodorsal shield entire, anterolateral extensions wide and acute, not connected with bases of epimerites Ia, lateral margins with incisions up to level of setae si, posterior margin slightly convex medially, posterior angles round, length 100–113, width 125–137, surface with small round and ovoid lacunae (Figure 11A). Scapular setae se separated by 70–75. Scapular shields narrow. Humeral shields narrow, not fused with outer sclerotized areas of epimerites III. Setae cp situated ventrally on soft tegument. Setae c2 filiform, situated dorsally on soft tegument near humeral shields. Subhumeral setae c3 lanceolate, 21–30 × 7–10. Anterior hysteronotal shield roughly rectangular, anterior and posterior margins almost straight, greatest length 225–237, greatest width in anterior part 125–150, surface with round and ovoid lacunae. Setae c1 present. Length of lobar region 65–70, width at level of setae h2 80–88. Terminal cleft narrow parallel-sided, with almost touching lateral margins, length 38–45. Supranal concavity well developed, ovoid, well outlined. Setae h1 on lobar shield, at same level with setae f2, surface of lobar shield without ornamentation. Setae h2 spindle-shaped, with terminal filament 58–80 × 9–10. Setae ps1 on inner margins opisthosomal lobes, setae h3 lanceolate, with filiform apex, 30–38 long, about 1/3 the length of terminal appendages. Dorsal measurements: c2–d2 88–100, d2–e2 100–123, e2–h2 55–63, h2–h3 25–28, d1–d2 50–65, e1–e2 23–38, h1–h2 20–33, h2–ps1 25–38, h1–h1 35–38, h2–h2 58–63.
Epimerites I fused as a V, with short lateral extensions. Coxal fields I, II without large sclerotized areas, epimerites IVa absent (Figure 11B). Translobar apodemes of opisthosomal lobes present, fused to each other anterior to terminal cleft. Epigynum horseshoe-shaped, greatest width 50–63. Copulatory opening situated ventrally at anterior margin of fused translobar apodemes. Distal 1/5 of primary spermaduct gradually thickened to copulatory opening, secondary spermaducts short (Figure 12E). Distance between pseudanal setae: ps2–ps2 42–52, ps3–ps3 17–20, ps2–ps3 25–33.
Legs I slightly longer than legs II; femora II with wide ventral crest. Setae d of tarsi II–III much shorter than corresponding setae f. Setae mGII thickened basally, with filiform apex. Length of solenidia: ω1I 13–15, ω1II 10–13, φI 65–75, φII 58–63, φIII 38–50, φIV 10–18 (Figure 12A–D).
Etymology
The specific name papuensis derives from the range of the type host, Papua New Guinea.
Remarks
Proterothrix papuensis n. sp. belongs to the wolffi species group, in having almost closed coxal fields III in males and parallel-sided terminal cleft in females. Among all species of the genus, Proterothrix papuensis n. sp. appears closest to P. paradisiaca (Trouessart, 1885) from the same host, Paradisaea minor Shaw (Corvida: Paradisaeidae). Males of both species have opisthosomal lobes short and slightly narrowed posteriorly, macrosetae h2 and h3 are similar in form, epimerites I-III are similar in shape, and corolla of adanal suckers without indentations. The characters that clearly differentiates both sexes of these species are the presence of circular lacunae on the dorsal shields, incisions in the lateral margins of prodorsal shield and length of solenidion σ on genu I half as long as this segment in the new species, versus the dorsal shields without ornamentation, lateral margins of the prodorsal shield without incision and length of solenidion σ on genu I as long as the segment in P. paradisiaca. Males of Proterothrix papuensis n. sp. easily differ from P. paradisiaca by the following features: the epimerites IVa are present, the opisthoventral shield along the margin of the terminal cleft is present, the genital papillae are situated anterior to genital arch, and the aedeagus is very short and extends only to midlength between setae g and ps3. In males of P. paradisiaca the epimerites IVa and the opisthoventral shield are absent, the genital papillae are situated at the level of the genital arch, and the aedeagus extends beyond the posterior margin of idiosoma. Females of both species have epimerites I similar in shape, and the humeral shields are not fused with outer sclerotized areas of epimerites III. Females of Proterothrix papuensis n. sp. are easily differentiated from P. paradisiaca by the following features: the anterolateral extensions of prodorsal shield are acute and not connected with bases of epimerites Ia, and the terminal cleft is like a narrow slit, with almost touching margins. In females of P. paradisiaca, the anterolateral extensions of the prodorsal shield are wide and connected with bases of epimerites Ia, and the terminal cleft is U-shaped.
Acknowledgments
Gabriel Bogdan Chișamera was funded by project no. RO1567-IBB09/2024 from the Institute of Biology Bucharest of Romanian Academy.
References
- Canestrini G., Kramer P. 1899. Demodicidae und Sarcoptidae. Das Tierreich, 7: 1-193. https://doi.org/10.5962/bhl.title.69280
- Constantinescu I.C., Chişamera G., Mukhim D.K.B., Adam C. 2014. Three new species of feather mite of the genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae: Pterodectinae) from passerines in Meghalaya, North East India. Syst. Parasitol., 89: 45-58. https://doi.org/10.1007/s11230-014-9508-1
- Constantinescu I.C., Cobzaru I., Geamana N.A., Mukhim D.K.B., Adam C. 2017a. Two new species of feather mites (Acarina: Psoroptidia) from the blue-throated blue flycatcher, Cyornis rubeculoides (Passeriformes: Muscicapidae). J. Nat. Hist., 51: 277-297. https://doi.org/10.1080/00222933.2017.1280194
- Constantinescu I.C., Popa O.P., Popa L.O., Cobzaru I., Mukhim D.K.B., Adam C. 2017b. A new feather mite species of the genus Proterothrix Gaud, 1968 (Acarina, Proctophyllodidae) from the Large Niltava, Niltava grandis (Passeriformes, Muscicapidae) - an integrative description. ZooKeys, 661: 1-14. https://doi.org/10.3897/zookeys.661.11793
- Constantinescu I.C., Chişamera G.B., Adam C. 2018. Redescription of six feather mite species of the genus Proterothrix Gaud, 1968 (Analgoidea: Proctophyllodidae: Pterodectinae) from the ''Édouard Louis Trouessart'' Collection. Zootaxa, 4486: 451-479. https://doi.org/10.11646/zootaxa.4486.4.3
- Constantinescu I.C., Chişamera G.B., Petrescu A., Adam C. 2019. Two new species of feather mites of the subfamily Pterodectinae (Analgoidea: Proctophyllodidae) from Indonesia, Acarologia, 59 (2): 196-210. https://doi.org/10.24349/acarologia/20194324
- Constantinescu I.C., Chişamera G.B., Motoc R., Gustafsson, D.R., Zou F., Chu X., Adam C. 2021. Two new species of feather mites (Acarina: Psoroptidia) from the Huet's fulvetta, Alcippe hueti (Passeriformes: Leiothrichidae), in China. Syst. Appl. Acarol., 26(1): 146-165. https://doi.org/10.11158/saa.26.1.9
- Constantinescu I.C., Adam C., Chişamera G., Gavril V., Motoc R., Mukhim D.K.B., Cobzaru I. 2023. Two new species of feather mites (Acari: Psoroptidia) from the Rusty-capped Fulvetta, Schoeniparus dubius (Passeriformes: Pellorneidae) in India. Syst. Appl. Acarol., 28: 269-288. https://doi.org/10.11158/saa.28.2.10
- Gaud J. 1952. Sarcoptides plumicoles des oiseaux de Madagascar. Mém. LʼInstit. Scient. Madagascar, 7: 81-107.
- Gaud J. 1962. Sarcoptiformes plumicoles (Analgesoidea) parasites dʼoiseaux de IʼIle Rennell. The Nat. Hist. Rennell Isl., Brit. Solomon Is., 4: 31-51.
- Gaud J. 1968. Sarcoptiformes plumicoles (Analgoidea) parasites dʼoiseaux de IʼIle Rennell. The Nat. Hist. Rennell Isl., Brit. Solomon Is., 5: 121-151.
- Gaud J. 1979 Sarcoptiformes plumicoles des oiseaux Coraciiformes dʼAfrique. II. Parasites des Alcedinidae. Rev. Zool. Afr., 93: 245-266.
- Gaud J., Atyeo W.T. 1996. Feather mites of the world (Acarina, Astigmata): the supraspecific taxa. Ann. Mus. roy. Afr. centr., 277, 1-191 (Part I, text), 1-436 (Part II, illustrations).
- Grandjean F. 1939. La chaetotaxie des pattes chez les Acaridae, Bull. Soc. Zool. France, 64: 50-60.
- Griffith D.A., Atyeo W.T., Norton R.A., Lynch C.A. 1990. The idiosomal chaetotaxy of astigmatid mites. J. Zool., 220: 1-32. https://doi.org/10.1111/j.1469-7998.1990.tb04291.x
- Han Y.D., Mironov S.V., Min G.S. 2019. Two new feather mites (Acari: Analgoidea) isolated from the grey-headed woodpecker, Picus canus (Piciformes: Picidae) in Korea, Syst. Appl. Acarol., 24(11): 2167-2183. https://doi.org/10.11158/saa.24.11.9
- Hernandes F.A., Valim M.P. 2014. On the identity of two species of Proctophyllodidae (Acari: Astigmata: Analgoidea) described by Herbert F. Berla in Brazil, with a description of Lamellodectes gen. nov. and a new species. Zootaxa, 3794: 179-200. https://doi.org/10.11646/zootaxa.3794.1.8
- doi: 10.11646/zootaxa.3794.1.8 https://doi.org/10.11646/zootaxa.3794.1.8
- Mironov S.V. 2006. Feather mites of the genus Montesauria Oudemans (Astigmata: Proctophyllodidae) associated with starlings (Passeriformes: Sturnidae) in the Indo- Malayan region, with notes on systematic of the genus. Acarina, 14: 21-40.
- Mironov S.V. 2009. Phylogeny of feather mites of the subfamily Pterodectinae (Astigmata: Proctophyllodidae) and their host associations with passerines (Aves: Passeriformes). Proc. Zool. Inst. Russ. Acad. Sci., 313 (2): 97-118. https://www.zin.ru/journals/trudyzin/doc/vol_313_2/TZ_313_2_Mironov.pdf https://doi.org/10.31610/trudyzin/2009.313.2.97
- Mironov S.V., Fain A. 2003. New species of feather mite subfamily Pterodectinae (Astigmata: Proctophyllodidae) from African passerines (Aves: Passeriformes). Bull. Annls Soc. Roy. Belge Ent., 139: 75-91.
- Mironov S.V., Galloway T.D. 2021. Feather mites of the subfamily Pterodectinae (Acariformes: Proctophyllodidae) from passerines and kingfishers in Canada. Zootaxa, 5016 (1): 1-55. https://doi.org/10.11646/zootaxa.5016.1.1
- Mironov S.V., OConnor B.M. 2017. A new feather mite of the genus Neodectes Park and Atyeo 1971 (Acari: Proctophyllodidae) from New Zealand wrens (Passeriformes: Acanthisittidae). Acta Parasitol., 62: 171-177. https://doi.org/10.1515/ap-2017-0020
- Mironov S.V., Proctor H.C. 2009. Feather mites of the genus Proterothrix Gaud (Astigmata: Proctophyllodidae) from parrotbills (Passeriformes: Paradoxornithidae) in China. J. Parasitol., 95: 1093-1107. https://doi.org/10.1645/GE-1961.1
- Mironov S.V., Tolstenkov O.O. 2013. Three new feather mites of the subfamily Pterodectinae (Acari: Proctophyllodidae) from passerines (Aves: Passeriformes) in Vietnam. Proc. Zool. Inst. Russ. Acad. Sci., 317: 11-29. https://doi.org/10.31610/trudyzin/2013.317.1.11
- Mironov S.V., Diao W., Zhang Y., Zhang C., Yan, Z. 2008. A new feather mite species of the genus Proterothrix Gaud (Astigmata, Proctophyllodidae) from Ficedula zanthopygia (Hay) (Passeriformes: Muscicapidae) in China. Acarina, 16: 31-38.
- Mironov S.V., Literak I., Čapek M., Koubek P. 2010. New species of the feather mite subfamily Pterodectinae (Astigmata, Proctophyllodidae) from passerines in Senegal. Acta Parasitol., 55: 399-413. https://doi.org/10.2478/s11686-010-0051-1
- Mironov S.V., Literak I., Hung M.N., Čapek M. 2012. New feather mites of the subfamily Pterodectinae (Acari: Proctophyllodidae) from passerines and woodpeckers (Aves: Passeriformes and Piciformes) in Vietnam. Zootaxa, 3440: 1-49. https://doi.org/10.11646/zootaxa.3440.1.1
- Norton A.R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarol., 22: 559-594. https://doi.org/10.1023/A:1006135509248
- Park C.K., Atyeo W.T. 1971. A generic revision of the Pterodectinae, a new subfamily of feather mites (Sarcoptiformes: Analgoidea). Bull. Univ. Nebraska State Mus., 9: 40-88.
- Trouessart E.L. 1885. Note sur la classification des Analgésiens et diagnoses d'espèces et de genres nouveaux. Bull. Soc. Etud. Scient. Angers, 14: 46-89.
- Trouessart E.L. 1899. Diagnoses preliminaries d'espèces nouvelles d'Acariens plumicoles. Additions et corrections a la sous-famille des Analgésinés. Bull. Soc. Etud. Scient. Angers, 28: 1-62.
- Valim M.P., Hernandes F.A. 2006. Redescription of four species of the feather mite genus Pterodectes Robin, 1877 (Acari: Proctophyllodidae: Pterodectinae) described by Herbert F. Berla. Acarina, 14: 41-55.



2024-03-04
Date accepted:
2024-05-09
Date published:
2024-05-16
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Constantinescu, Ioana Cristina; Chișamera, Gabriel Bogdan; Motoc, Rozalia and Adam, Costică
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



