Within-tree distribution and seasonal dynamics of Eutetranychus banksi and Euseius stipulatus (Acari: Tetranychidae, Phytoseiidae) on citrus: Implications for the biological control of the pest
López-Olmos, Sergio  1
and Ferragut, Francisco
1
and Ferragut, Francisco  2
2
1✉ Instituto Agroforestal Mediterráneo. Universitat Politècnica de València. Camino de Vera s/n. 46022 Valencia, Spain.
2Instituto Agroforestal Mediterráneo. Universitat Politècnica de València. Camino de Vera s/n. 46022 Valencia, Spain.
2024 - Volume: 64 Issue: 2 pages: 480-498
https://doi.org/10.24349/pv5i-1hqjOriginal research
Keywords
Abstract
Introduction
The Texas citrus red mite, Eutetranychus banksi (McGregor) (Acari: Tetranychidae), is an important citrus pest first recorded on citrus in 1935 from Rio Grande Valley in Texas (Dean 1952, 1959b, 1980), and now widely distributed throughout the Americas, being cited from the southern United States to northern Argentina and reported from most countries in Latin America (Migeon and Dorkeld 2022). Far from its native region, Texas citrus mite was found in Egypt (Abdel-Shaheed et al. 1973), and more recently in Portugal (Carvalho et al. 1999), Spain (García et al. 2003) and Iran (Beyzavi et al. 2013). Since its arrival in the Iberian Peninsula, severe damage to citrus has been recorded in the Algarve region of southern Portugal, making it a key pest (Gonçalves et al. 2002). In 2001, Spanish citrus orchards in Ayamonte and Isla Cristina (Huelva province) were invaded by E. banksi. Although we have no information about the dissemination pathway followed by the mites, it has been suggested that they were carried in fruit containers that are frequently exchanged by Portuguese and Spanish farmers in the border area (García et al. 2003). In 2013, E. banksi was suddenly detected in La Safor (Valencia province) more than 800 km away from Ayamonte, and the following year in other regions of the provinces of Valencia and Alicante, affecting the main citrus-growing area of the eastern Mediterranean in Spain. Because no geographical spread of this species throughout the citrus-growing regions of southern Spain was detected, it seems that the Valencian populations came directly from Huelva via contaminated plant material (Ferragut 2016).
On citrus trees, Eutetranychus banksi feeds by consuming the contents of mesophyll plant cells, reducing the leaf photosynthetic capacity, and promoting defoliation, which may result in a decrease in yield. In addition, an aesthetic damage to the fruits is produced, resulting in a lack in pigmentation, which could reduce their economic value (García et al. 2003; Monzó et al. 2016). Furthermore, it is one of the most polyphagous spider mite species reported on 110 hosts belonging to 34 plant families, mostly Fabaceae (22 species) and Rutaceae (9 species), infecting other crops and many ornamental plants (Migeon and Dorkeld 2022).
Chemical control is commonly used to maintain mite populations at low levels. The Texas citrus mite has been shown to be sensitive to numerous acaricides, being easy to keep under control with this strategy (Monzó et al. 2016). However, under the perspective of Integrated Pest Management (IPM), cultural practices, biological and biotechnological control should be combined with chemicals to produce healthy crops and minimize the use of pesticides, reducing the risks to human health and the environment. Before developing an IPM programme against E. banksi, it is necessary to have a better understanding about the population biology of the pest, as well as its natural enemies. Phytoseiid mites have been widely cited as the main natural enemies of spider mites on citrus crops around the world (Gerson 2003; Vacante 2010). Therefore, we aimed to: (i) study the within-tree distribution of E. banksi and phytoseiid mites, (ii) describe the seasonal trends of E. banksi and phytoseiids, and (iii) identify the phytoseiid species associated with E. banksi, as well as (iv) analyse the behaviour of the predatory mites in response to the increase in pest density.
Material and methods
Sampled orchards
The study was conducted across four citrus orchards in 2018 and six in 2019; only two of them were monitored during the two years. All the orchards were commercial citrus plantations situated in an extensive citrus monoculture region in the Valencian Community (Spain). The orchards were selected due to the presence of evident symptoms of damage produced by E. banksi on the leaves of the previous year. Furthermore, the selection was carried out aiming to include different citrus varieties and environmental conditions. In the first year, two sampling areas were selected according to their climatic differences, Picassent in the centre of Valencia province and Pego in the north of Alicante province. The second year, in addition to these two areas, a third one was incorporated in the locality of Riola, in the south of the Valencia province (Table 1). The orchards were all planted with different cultivars of Citrus x aurantium L., including three sweet orange varieties [Valencia late, Salustiana, and Navelina], and three mandarins [Oronules, Ortanique and Clemenvilla]. All the orchards ranged from 0.2 to 2 ha in land area and had normally developed 11 to 15-year-old trees in full production drip irrigated. The selected orchards were under several pest management strategies which included (i) zero residue, based on the use of chemicals for pest management producing fruits without chemical residues, as well as chemical nutrition for the crop, (ii) conventional, based on the use of chemical pesticides leaving residues on fruit, as well as chemical nutrition, (iii) organic, based on the use of organic pesticides and nutrition. Despite this, orchards had not been sprayed with pesticides for at least 6 months before sampling (Table 1).


Sampling procedure
In each orchard, approximately 10% of the trees were marked and randomly sampled regularly. The marked trees were not sprayed with pesticides during the sampling period. Sampling was conducted weekly, during the period of peak pest abundance (July-November), and fortnightly during the rest of the year. On each sampling date, 25 inner and 25 outer leaves from each citrus sprouting (spring, summer, and autumn) belonging to the previous and the current year, were randomly collected around the marked trees canopy in each sampled orchard. In addition, and depending on their availability, 25 fruits per orchard were randomly collected from the outer part of the marked trees canopy. All the leaves of the same position and sprouting were combined in a plastic bag and transported to the laboratory inside a portable cooler. Fruits from the same orchard were transported in plastic containers. Within the next 24 h, leaves and fruits were examined under the stereomicroscope. All the development stages, from egg to adult, of E. banksi and phytoseiid mites were counted on leaves and fruits. Furthermore, in the case of leaves, their position on the adaxial (upper) or abaxial (lower) side of the leaf was recorded. The motile forms of phytoseiids from each sampling and orchard were extracted from the leaves and fruits using a fine brush and placed inside plastic tubes containing 70% ethanol. They were subsequently mounted on microscope slides using Heinze-PVA after clearing with Nesbitt's medium. Nymphs and adults were identified at the species level. Larvae, which represent the smallest percentage of the total motile forms due to its short duration, could not be assigned to any phytoseiid species. In total, 49,350 leaves and 5,175 fruits were counted from 69 samplings carried out during 2018 and 2019. Regarding phytoseiids, 5,338 individuals were determined at species level.
Data Analysis
In the sampling data all the development stages were pooled together and averaged per sampling (date) and sample unit (leaf or fruit), using this value for graphics and statistical analysis. Samplings with 0 values were eliminated from the analysis. The average number of mites per cm2 was used to compare fruits and leaves, due to the differences in surface area between these two structures. The within-tree distribution analysis was carried out at four levels (canopy, leaf, leaf age, and fruit) by comparing the mean number of mites in different positions at each level. For this purpose, data were tested for normal distribution using the Shapiro-Wilk test and for homogeny of variances using Levene's test. Data were log-transformed to fulfil the homogeneity of variances requirements of the Wilcoxon rank-sum test (Wilcoxon 1945), used to evaluate the difference between the means in the different positions.
Data from seasonal monitoring of E. banksi and phytoseiids were represented graphically to show the seasonal abundance trends on leaves and fruits. Daily air temperature and relative humidity data were obtained from three meteorological stations from the Valencia Association of Meteorology (AVAMET), each one situated in each three sampled localities (Picassent, Pego and Riola). The maximum, mean, and minimum daily air temperature, as well as the daily relative humidity were represented in the seasonal abundance graphs. The absolute and relative abundance, and the frequency of occurrence for all the phytoseiid species were calculated.
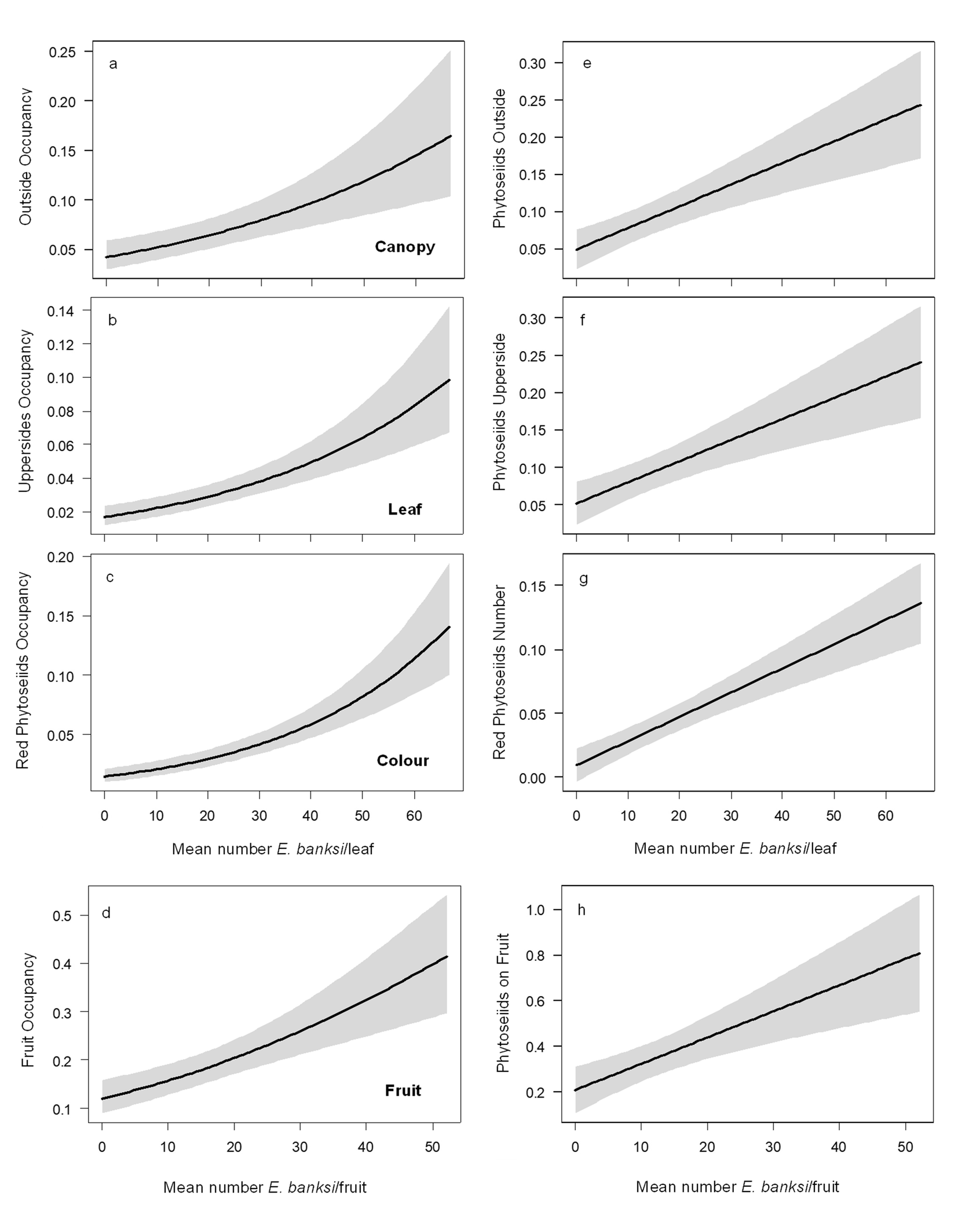
Finally, to evaluate the biological control possibilities by the phytoseiid mites, we aimed to detect changes in the spatial distribution of these predatory mites at three spatial levels: canopy, leaf, and fruit. If phytoseiids are actively preying on E. banksi individuals, they should appear associated with them at different spatial levels. In addition, we expected a change in the coloration of the phytoseiids (from white to red), indicative of predation on their presumptive prey E. banksi since no other potential prey with reddish-brown coloration was detected on the leaves at the same time. To explore this change in behaviour, we plotted the percentage of phytoseiids at each position for each of the three levels, as well as the percentage of phytoseiids with different colour along the seasonal dynamics of E. banksi. In order to statistically test whether the predatory mites changed their behaviour as a result of the increase in prey density, three approaches were carried out at the three levels mentioned above. Firstly, (i) Binomial (logit-link) generalized linear models (GLMs) were performed to evaluate the effect of the mean number of E. banksi on occupancy of phytoseiids. To evaluate the change in mite coloration, the relationship between the mean number of E. banksi on the proportion of phytoseiids with different coloration was tested using the same methodology. To deal with the overdispersion in the data analysis, the standard errors of the estimated coefficients in the model were corrected by using a quasi-binomial error distribution (Zuur et al. 2009). Secondly, (ii) Linear Models (LMs) were used to evaluate the influence of the mean number of E. banksi on the mean number of phytoseiids. To test the coloration hypothesis, the relationship between the mean number of E. banksi and red phytoseiids found was tested using the same approach. The data were log-log transformed to fulfil the assumptions of normality tested with Shapiro-Wilk's test, and homogeneity of the residual variance tested with Breusch-Pagan's test (Breusch and Pagan 1979). Finally, (iii) Contingency tables and Chi-square test (Gardener 2012) were used to assess the association between E. banksi and predatory phytoseiids (García-Marí et al. 1991). This methodology was used to study the effect of the presence of E. banksi on the occupancy and the number of phytoseiids at the three spatial levels of study. The colour change of phytoseiids was also evaluated by this methodology. We performed all statistical analysis with R version 3.6.1 (R Core Team 2019). Wilcoxon's rank-sum test was performed using the package ''coin'' (Hothorn et al. 2006), and Breusch-Pagan's test was performed using the package ''car'' (Fox and Weisberg 2019). The graphs of the GLM and LM models were plotted with the package ''visreg'' (Breheny et al. 2017).
Results
Within-tree distribution analysis
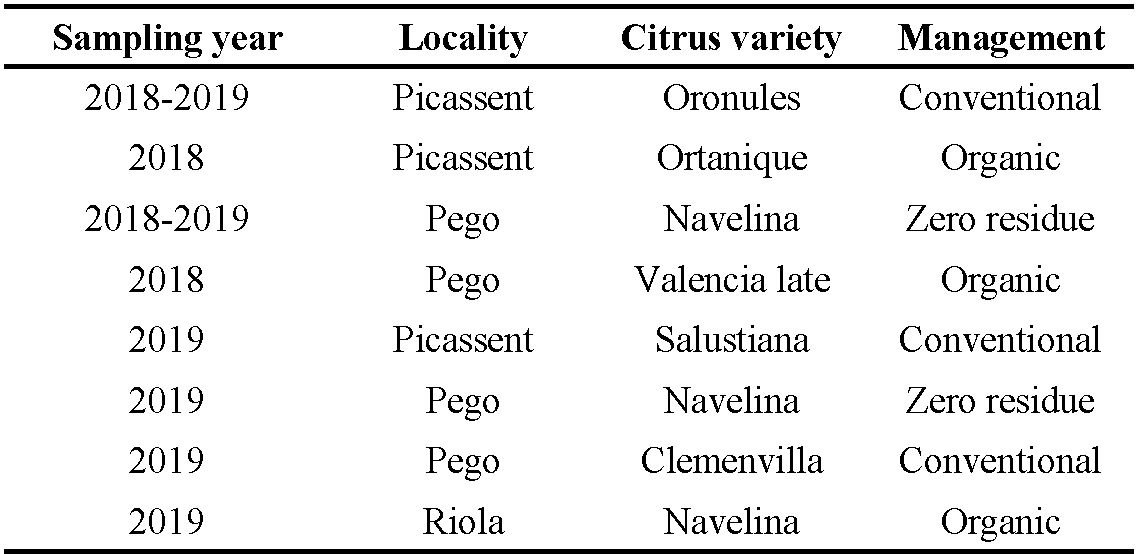
The mean number of E. banksi per leaf (mean ± standard error, SE) in the periphery (outside) of the canopy (12.08 ± 2.38) was significantly higher than inside (2.95 ± 0.62) (Z = –2.41, P = 0.015) (Figure 1a). At the leaf level, E. banksi was more abundant on the adaxial side of the leaf (8.93 ± 1.77) than on the abaxial side (1.96 ± 0.40) (Z = –3.50, P = 0.0003) (Figure 1b). Leaf age was also a factor influencing the abundance of the species, since E. banksi density on leaves of the current year ''New leaves'' (17.07 ± 3.23), was higher compared to the leaves of the previous year ''Old leaves'' (10.88 ± 2.19) (Z = 3.92, P < .0001) (Figure 1c). Finally, statistically significant differences were observed between leaf and fruit, since we found a higher mean number of mites per cm2 on the leaf (0.23 ± 0.04) than on the fruit (0.10 ± 0.02) (Z = 2.06, P = 0.040) (Figure 1d).


Phytoseiids showed a different pattern of spatial distribution. When we focus on the canopy, the mean number of phytoseiids per leaf inside the canopy (0.78 ± 0.10) was significantly higher than outside (0.09 ± 0.012) (Z = 6.96, P < .0001) (Figure 1e). Regarding leaves, phytoseiid density was higher on the abaxial (0.45 ± 0.07) side compared to the adaxial side (0.04 ± 0.005) (Z = 7.004, P < .0001) (Figure 1f). Leaf age also affected the abundance of phytoseiids, since the population density on leaves from the previous year (0.16 ± 0.02) was significantly higher than on the current year ones (0.04 ± 0.006) (Z = –5.48, P < .0001) (Figure 1g). When we compared leaves and fruits, we did not observe significant differences in the mean number of phytoseiids per cm2 in both substrates (Z = –1.52, P = 0.132) (Figure 1h).
Seasonal trends of E. banksi and phytoseiid mites
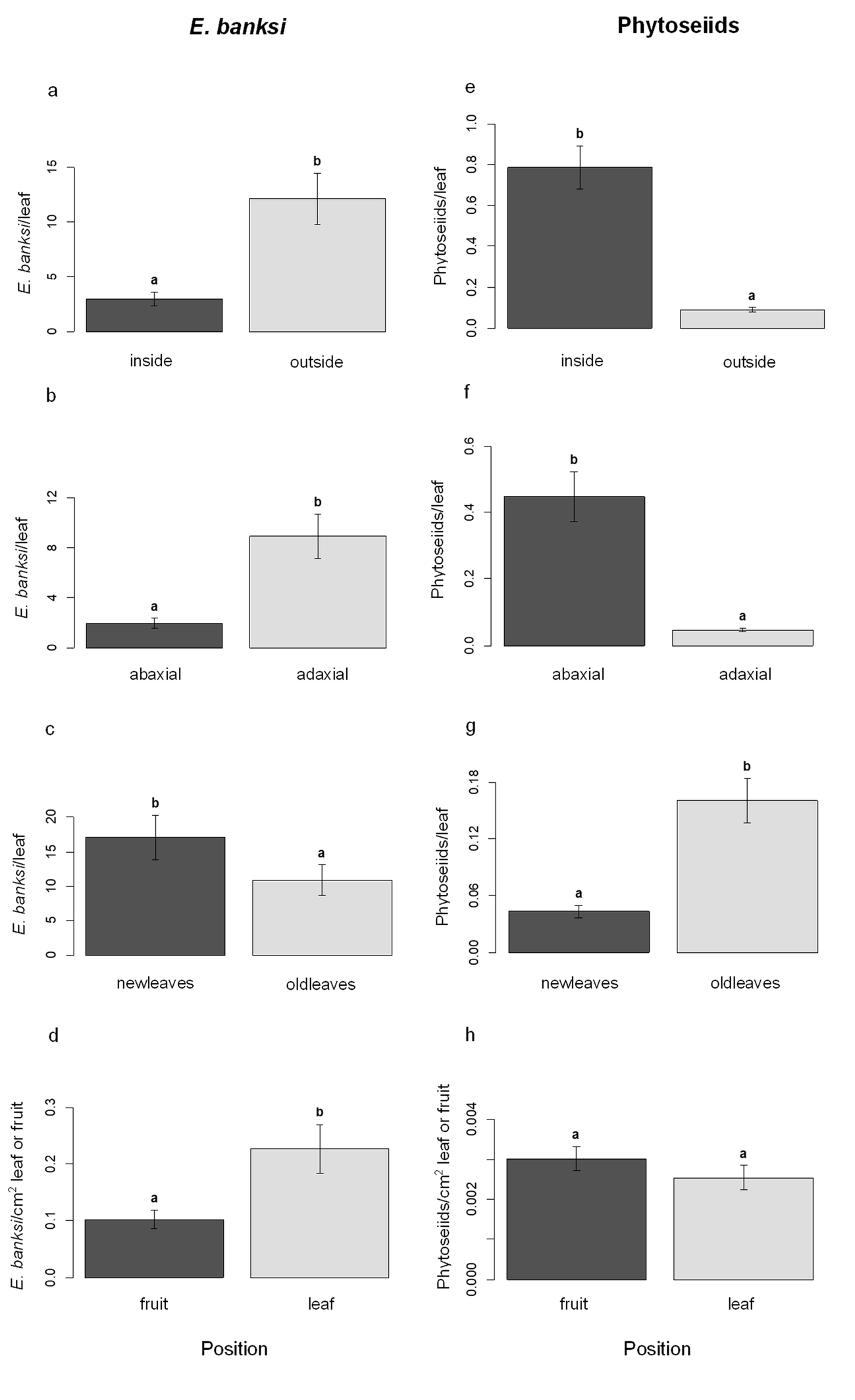
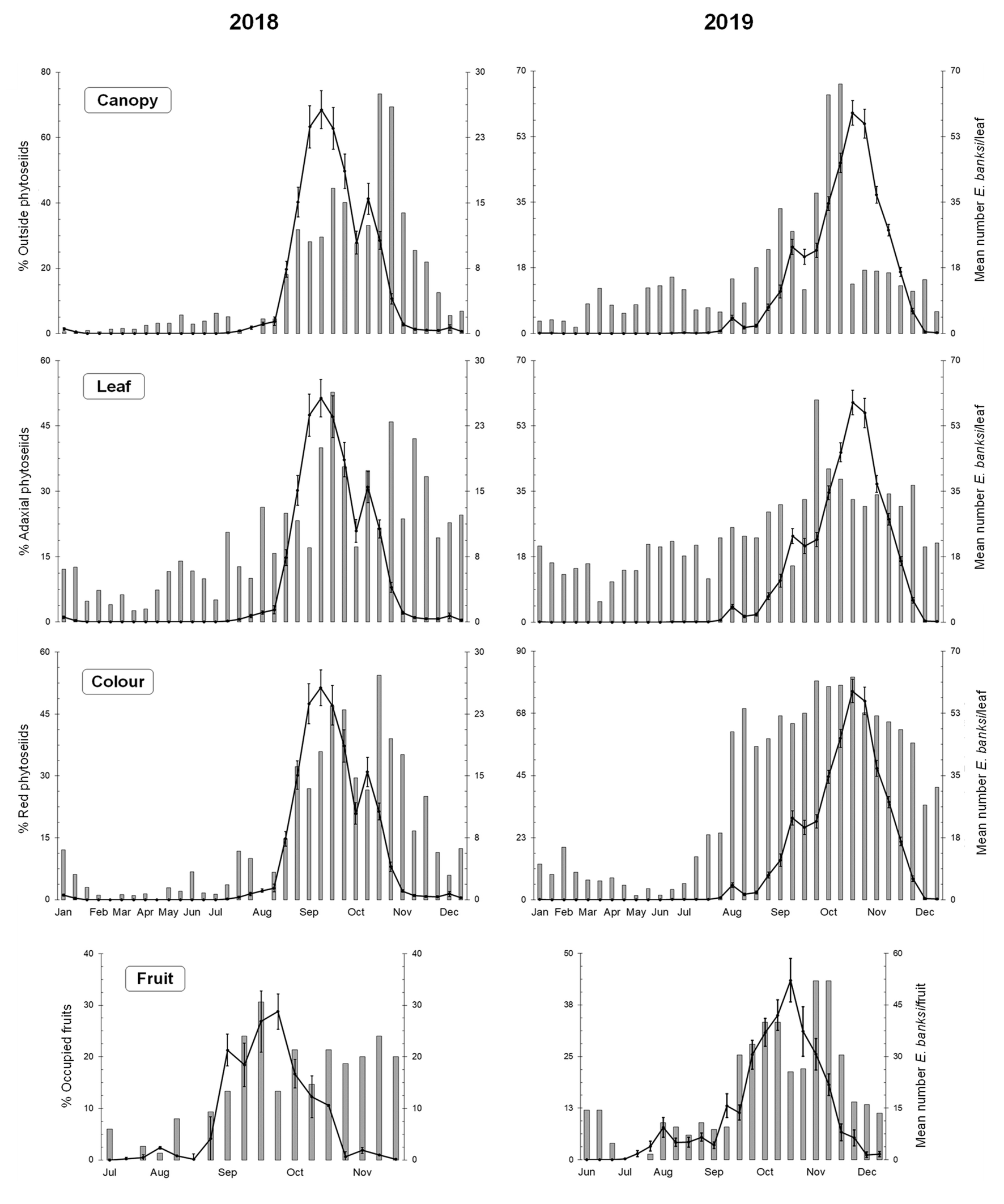
The development of E. banksi showed a similar trend over the two-year period of study. The density of this mite on the citrus leaves started to increase rapidly in late summer, during the month of August, showing unimodal dynamics, with one single annual peak in late summer-early autumn. The peak population density on leaves reached an average of 25.69 ± 2.17 and 58.76 ± 3.27 mites per leaf in 2018 and 2019, respectively. Afterwards, the population decreased and was extremely low and even undetectable during winter. The pest density on fruits followed the same seasonal trend as on the leaves. The peak density on fruits reached an average of 28.76 ± 5.90 and 52.16 ± 6.31 mites per fruit in 2018 and 2019, respectively (Figure 2).


Phytoseiids also showed a similar seasonal trend during the two years of study. They presented bimodal dynamics with a spring peak of greater importance during May–June, and a second autumn peak of lesser importance in October–November. Furthermore, in 2018 a peak prior to the main one occurred in April. The main spring peak population density reached an average of 1.38 ± 0.17 and 1.17 ± 0.11 mites per leaf in 2018 and 2019, respectively. In autumn, the maximum density was 0.25 ± 0.06 and 0.34 ± 0.03 mites per leaf. Subsequently, the phytoseiids increased in 2018 and decreased in 2019 resulting in values that remain around 0.25 phytoseiids per leaf during winter. On fruits, the phytoseiids presented low values during the summer, and their abundance increased during autumn reaching average values of around 0.5 or even 1 mite per fruit in 2018 and 2019, respectively (Figure 2).
Phytoseiid species found on the trees
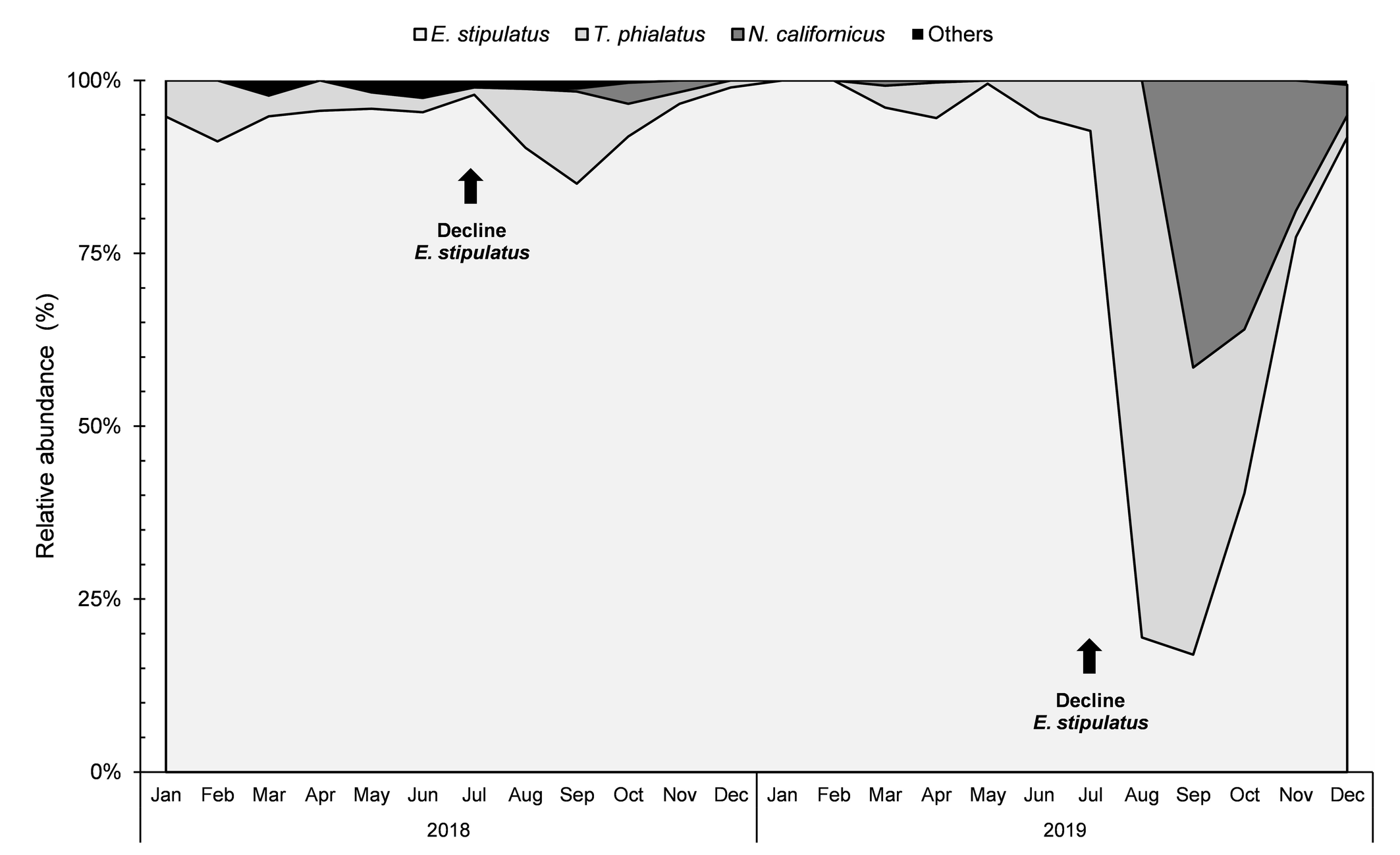
The most abundant and frequent species was Euseius stipulatus (Athias-Henriot) with 4,844 (90.75% of the total phytoseiids) individuals retrieved in 88% of the samplings. Typhlodromus phialatus Athias-Henriot was the following species in abundance with 290 (5.43%) individuals and 37.82% in frequency. A third species was Neoseiulus californicus (McGregor), which showed an abundance of 182 (3.41%), and occurred in 8.36% of the samplings. Finally, the remaining species Paraseiulus talbii (Athias-Henriot), Neoseiulus barkeri Hughes, Neoseiulella litoralis (Swirski & Amitai) and Neoseiulus madeirensis Papadoulis & Kapaxidi were scarcely present on the tree's canopy (Table 2).
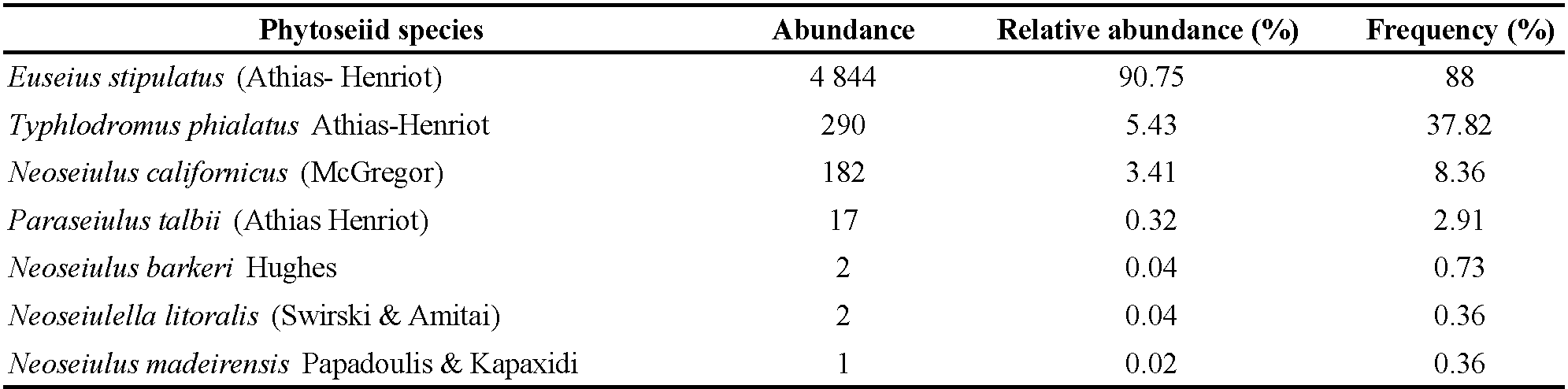


Euseius stipulatus was the most abundant phytoseiid species during winter and spring of 2018 and 2019. However, in the month of July of both years, we found a decline in its abundance. Following this decline, in both years there was an increase in the abundance of T. phialatus and N. californicus in early autumn. This effect was more pronounced in 2019. In August of that year, T. phialatus with 29 individuals collected (80.56%, relative abundance) was more abundant than E. stipulatus (7 individuals; 19.44% relative abundance). In September, both T. phialatus (22; 41.51%) and N. californicus (22; 41.51%) were more abundant and frequent than E. stipulatus (9; 16.98%). In October, E. stipulatus (121; 40.33%) recovered, while N. californicus (108; 36.00%) increased and outcompeted T. phialatus (71; 23.67%). In November, E. stipulatus did not increased in absolute abundance but continued its increase in relative abundance (123; 77.36%), compared to T. phialatus (6; 3.77%) and N. californicus (30; 18.87%). With the arrival of winter, in December, Euseius stipulatus (145; 91.77%) recovered its complete dominance over T. phialatus (5; 3.16%) and N. californicus (7; 4.43%) (Figure 3).


Phytoseiid behaviour in response to pest density
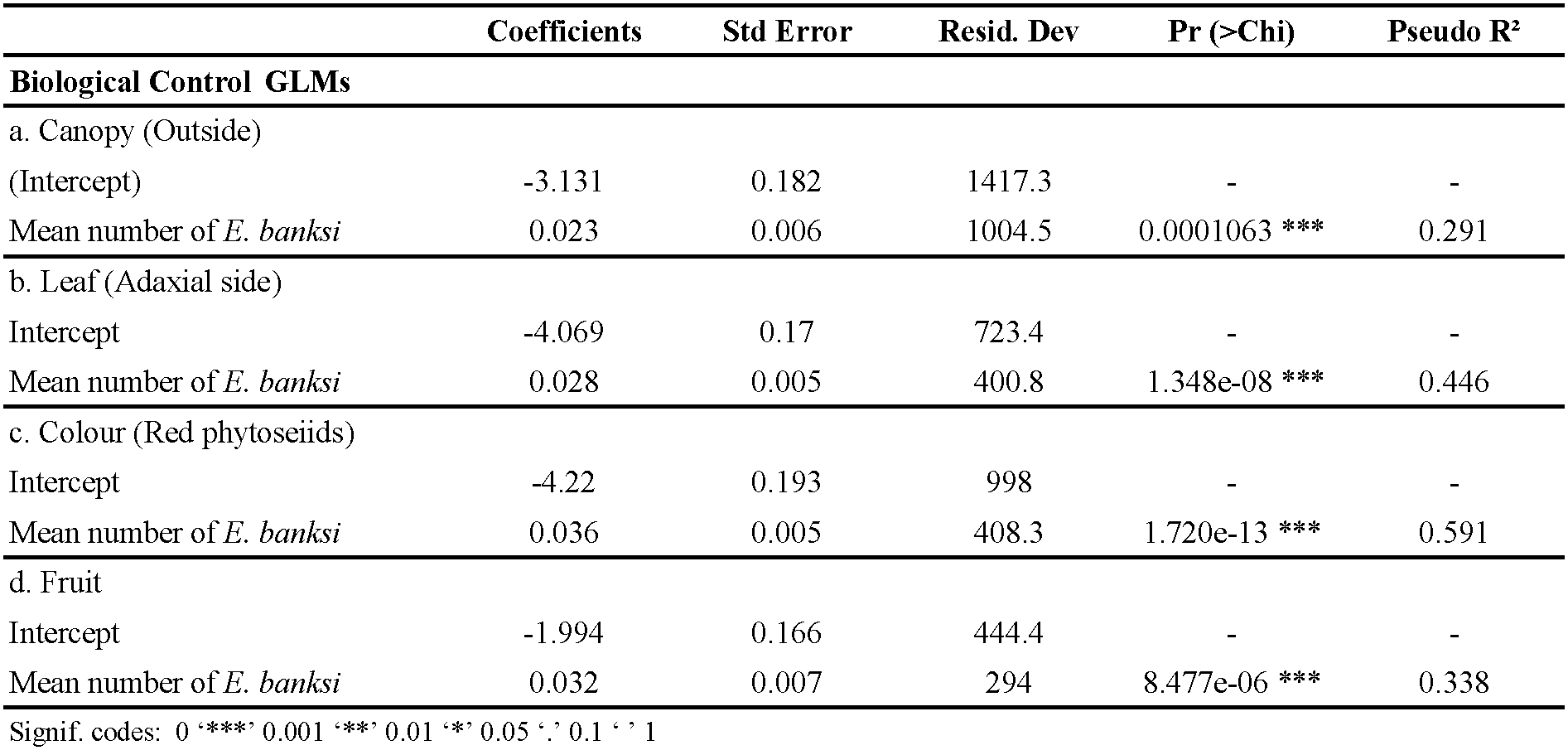


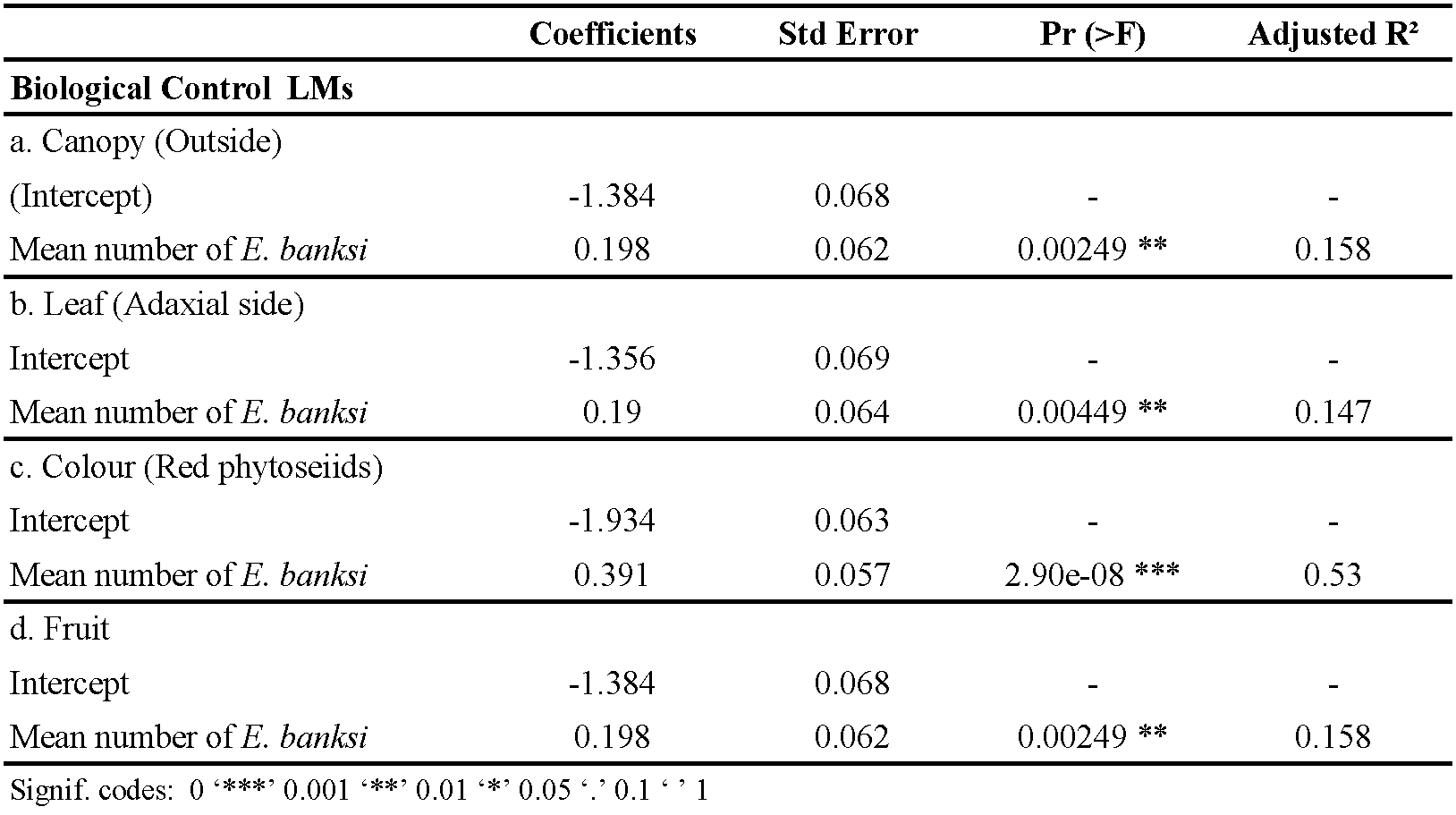


Using the data obtained under field conditions, we plotted the relationship of the seasonal dynamics of E. banksi against the percentage of phytoseiids at four spatial levels through 2018 and 2019 (Figure 4). As graphically we observed that E. banksi density was influencing the spatial distribution of phytoseiids we performed a statistical analysis of the data. Regarding the canopy, we observed a significative increase in the proportion of leaves occupied by phytoseiids on the periphery of the canopy when E. banksi population increased (Table 3a, Figure 5a). Furthermore, we also detected a significative increase in the number of phytoseiids outside the canopy when E. banksi increased in abundance (Table 4a, Figure 5e). When focusing on leaves outside the tree canopy, the proportion of adaxial leaf surfaces occupied by phytoseiids increased with E. banksi population (Table 3b, Figure 5b). In parallel, the number of phytoseiids on the adaxial side of leaves also increased (Table 4b, Figure 5f). The colour of the phytoseiids was also significantly affected by the presence of E. banksi. The proportion of leaves occupied by red phytoseiids and the number of coloured phytoseiid increased significantly in response to E. banksi populations (Tables 3c and 4c, Figures 5c and 5g). Finally, we also found a significant influence of E. banksi density in fruits on the proportion of occupied fruits by phytoseiids (Table 3d, Figure 5d), as well as the number of them per fruit (Table 4d, Figure 5h).




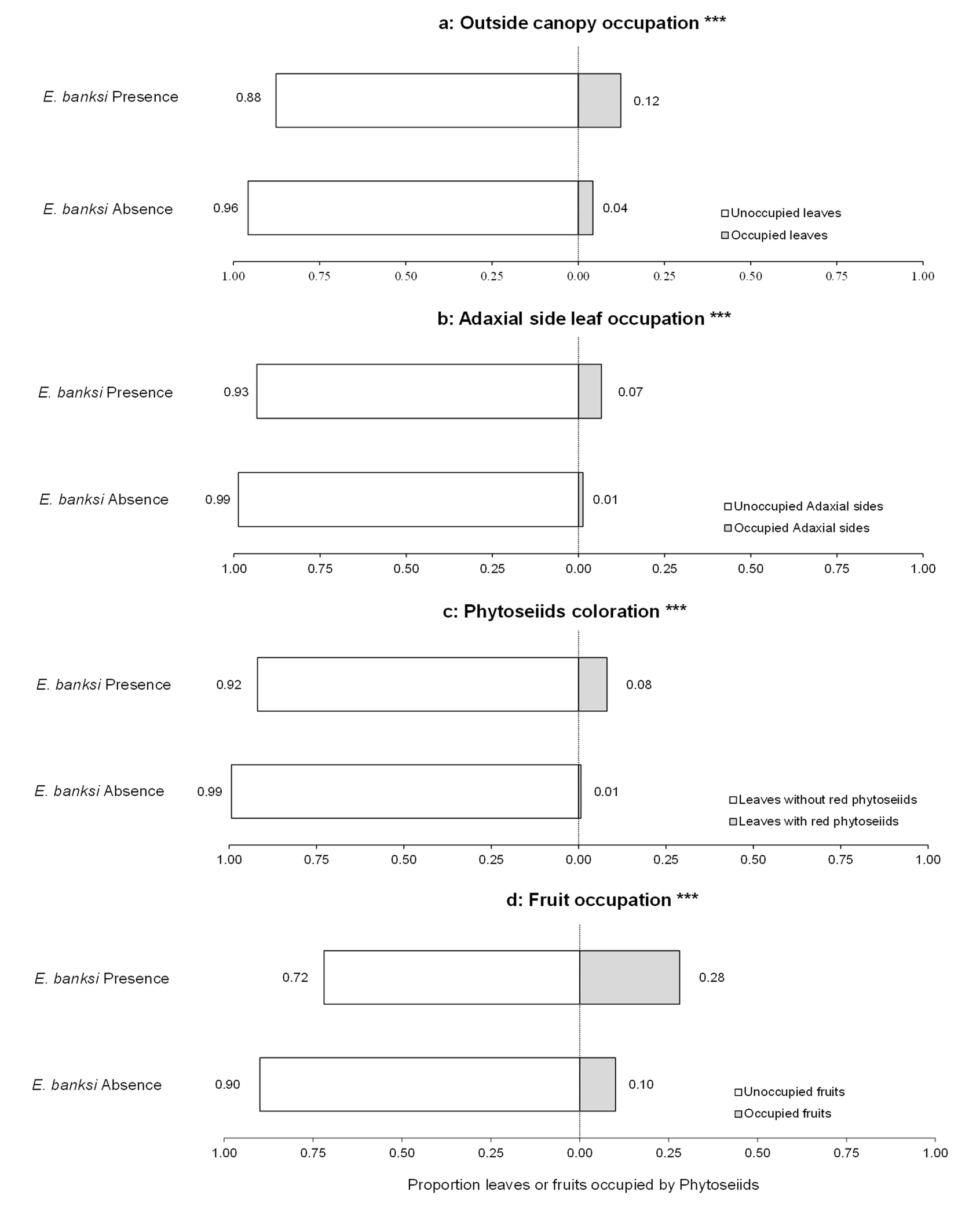


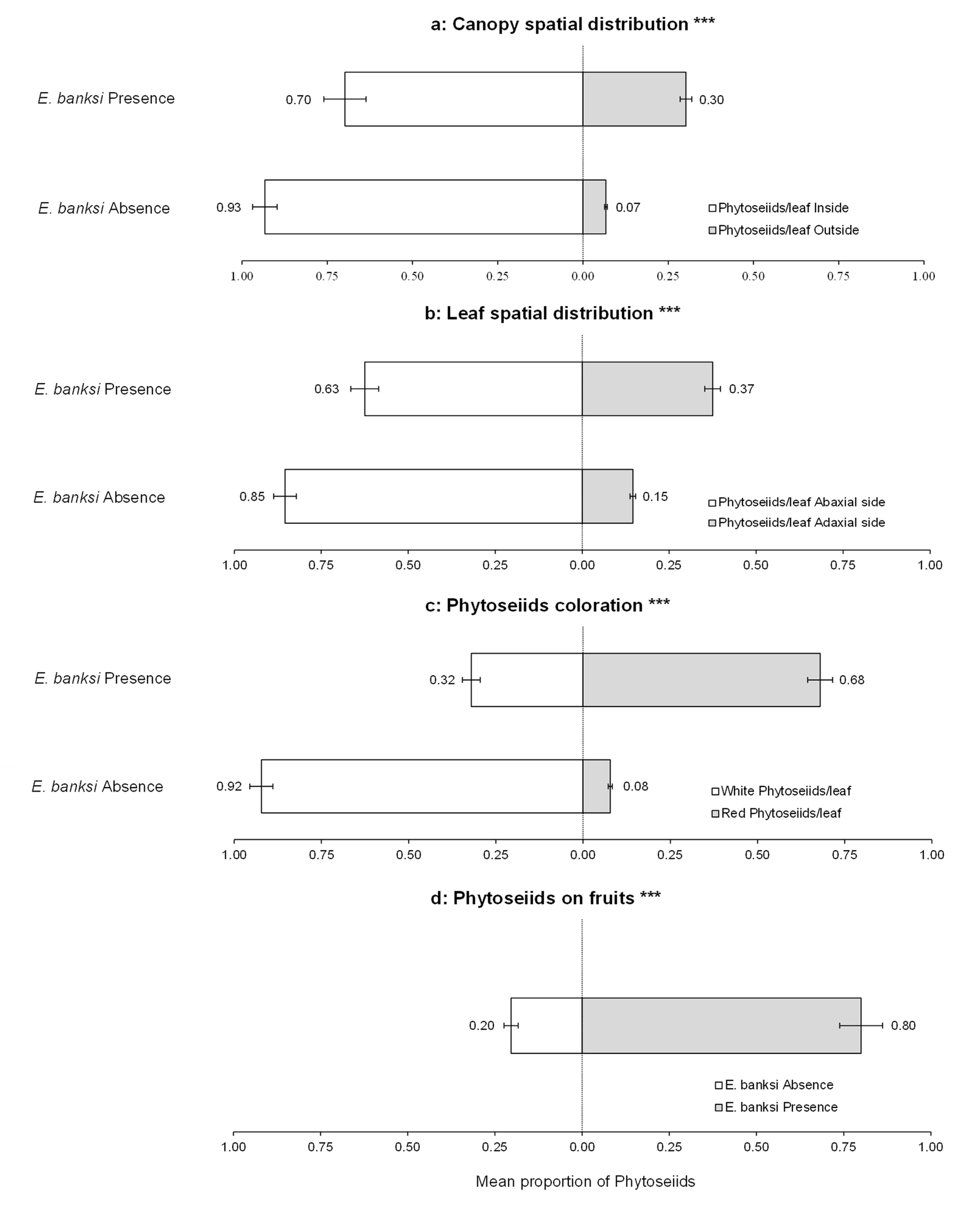


The Contingency tables and Chi-square tests confirmed the results obtained from the GLM and LM models. In relation to occupancy, there was a significant increase in leaf occupancy by phytoseiids on the periphery of the tree canopy when E. banksi was present on leaves (X-squared = 381.96, P < .0001) (Figure 6a). In parallel, an increase in the occupancy of the adaxial side of the leaves was observed (X-squared = 375.76, P < .0001) (Figure 6b), as well as an increase in the proportion of leaves occupied by red phytoseiids (X-squared = 750.15, P < .0001) (Figure 6c). Finally, we observed a significant rise in the occupation of fruits by phytoseiids when E. banksi was present on them (X-squared = 122.77, P < .0001) (Figure 6d). When we examined the different levels of study from the point of view of the number of phytoseiids, we observed that the proportion of these predators outside the tree canopy increased in the presence of E. banksi (X-squared = 16.79, P < .0001) (Figure 7a). In addition, the proportion of phytoseiids found on the adaxial side of the leaves (X-squared = 12.48, P < .001) (Figure 7b), as well as the reddish coloured phytoseiids (X-squared = 74.43, P < .0001) (Figure 7c) also increased significantly in the presence of E. banksi. Finally, the presence of E. banksi on fruits significantly increased the proportion of phytoseiids collected on fruit (X-squared = 35.19, P < .0001) (Figure 7d).
Discussion
Whitin-tree distribution analysis
This is the first study that compares the population of E. banksi and phytoseiid mites on fruits and leaves in citrus groves. The pest showed a clear preference for leaves over fruits. In western Mediterranean, a single flowering period occurs in citrus during April-May, after which fruit development begins (Agustí 2003). When E. banksi start to increase in late summer-early autumn, the fruits are an available substrate for harbouring mites. Despite this, the density per unit area (cm2) on leaves was much higher than that on fruits suggesting that leaves are a better food substrate for its development, since the mites prefer to live more densely on them rather than move to the fruits. Regarding phytoseiids, it is considered that they live mainly on leaves, so the population density on leaves is considered representative of the tree canopy (Ferragut et al. 1988; Muma 1964; Ragusa 1986) and there is little information about the presence and abundance of these predators on citrus fruits. In our results, the average number of phytoseiids per fruit in autumn was higher than on leaves; however, if we consider the surface area (cm2), we can see that both substrates were used equally. We believe that this fact is related to the presence of E. banksi on the fruit as will be discussed later.
Regarding leaves, our results indicate that E. banksi and phytoseiids are not randomly distributed in the tree canopy. Microclimatic variations could be playing an important role in the spatial distribution of phytophagous and predatory mites, since radiation and temperatures are higher, and relative humidity is lower at the periphery of the canopy (Ferro et al. 1979). The same preferences for the upper leaf sides at the periphery of the tree canopy have been described by other authors for E. banksi (Dean 1952; Muma et al. 1953; Dean 1959a, 1959b; Landeros et al. 2004; Rogers and Stansly 2017), as well as for other important spider mite pests on citrus such us Eutetranychus orientalis (Klein) (Bodenheimer 1951; Vela et al. 2017) and Panonychus citri (McGregor) (Ebeling 1959; García-Marí et al. 1983). On the other hand, phytoseiids shelter on the under side of leaves located inside the canopy, being more abundant in plantations and citrus varieties with dense imbricate canopies (Ferragut et al. 1988; McMurtry et al. 1970), since they are severely affected by high temperatures and low relative humidity (Ferragut et al. 1987).
When we focus at the periphery of the canopy, we detected the preference of E. banksi for leaves coming from current year's shoots as opposed to leaves from the previous year. We consider that different factors could be playing a role in this preference. (i) Leaves of the current year are always located in the outermost part of the canopy with more favorable microclimate conditions for the establishment of the spider mites. (ii) Orchards with high infestations show the presence of this mite every year with greater or lesser intensity, so previous year's leaves are often damaged by mite feeding, and the level of injury influences the mite density reducing egg laying. In the same way, changes associated with leaf aging may be conditioning mite preferences. In citrus, leaves remain on trees for 1–2 years during which there are constant changes in their structure and nutritional composition (Scott et al. 1948). (iii) Finally, newly developed leaves on the outermost part of the canopy accumulate greater dust deposition. The Texas citrus mite does not produce a dense web, and the development of non-producing web spider mites is favoured by the presence of dust or inert debris on the leaf surface (Holloway et al. 1942). Contrary to E. banksi, phytoseiids prefer the leaves of the previous year instead of the current year leaves. Phytoseiids spend most of their lifetime inside the canopy; however, they are moving continuously while foraging for prey or other foods (Sabelis 1985). The foraging behaviour may depend not only upon prey availability, but also on abiotic factors. Thus, when they move outside the canopy to forage, they prefer leaves of the previous year, located at mid-level in the canopy, and less exposed to unfavourable climate conditions.
Seasonal trends of Eutetranychus banksi and phytoseiid mites
Texas citrus mite was detected simultaneously on fruits and fully developed leaves of the current year's spring and summer shoots during July-August, and the main population peak was registered in September-October. Once autumn leaves are available, mites move to occupy this newly available resource in agreement with the behaviour observed in other spider mite species (Gotoh 1987), and probably related to changes in hardness, dryness, wax coating, and nutrients that occur in old leaves, making it difficult for spider mites to feed (van de Vrie et al. 1972). The population growth coincided with the highest daily average temperatures between 26-30 °C in agreement with the life history laboratory works carried out (Childers et al. 1991; Badii et al. 2003). After the population peak in autumn, falling temperatures caused a progressive decline in populations, which are scarce at early-winter and often undetectable at the end of the season and during spring. In other locations with warmer temperatures, such as Texas, Dean (1959b) observed E. banksi populations throughout the year, but in years with extremely cold winter temperatures it was unable to find any mites on the leaves (Dean 1952). In addition to temperature, relative humidity (RH) and precipitation could affect the development of spider mite populations (van de Vrie et al. 1972). In both years, summer months had low relative humidities that contributed to the rapid growth of the pest. However, the autumn of 2018 was wetter and rainier compared to the drier autumn of 2019. We believe that the high RH and rainfall during the autumn months of 2018 contributed to the rapid decline in populations that was more delayed in 2019. Similar unimodal seasonal trends have been described for E. banksi in California, with typical dry summers under Mediterranean climate conditions. In contrast, although it is possible to find E. banksi throughout the year, high HR and precipitation in Florida, or the excessive heat in Texas, produce a decline in summer showing a bimodal trend with population peaks in late spring-early summer and late autumn-early winter (Dean 1952, 1959b, 1980; Childers 1992; Muma et al. 1953; Rogers and Stansly 2017; Anonymous 2022). In Mexico, the Texas citrus mite could also be collected all year round and showed the same bimodal dynamics where low winter temperatures as well as spring and autumn rainfall contributed to reduced mite density (Landeros et al. 2004). The peak shown by the newcomer E. banksi in Spain coincides in time with the population outbreaks of E. orientalis (Ledesma et al. 2011; Vela et al. 2017) and P. citri (Ferragut et al. 1988). However, in the sampled orchards of this study, no E. orientalis was found, while small populations of P. citri could be observed in two orchards during late spring-early summer. We believe that interspecific competition with E. banksi are geographically displacing the resident spider mite species in most of the Valencian citrus (López-Olmos and Ferragut 2023).
Euseius stipulatus was the most abundant phytoseiid, representing 90.75% of the total number of phytoseiids collected, as well as the most frequent, found in 88% of the samplings carried out. For this reason, although the total number of collected phytoseiids is represented in our graphs, the remaining discussion will be focused on E. stipulatus. This species presented two population peaks (spring and autumn), the first and most abundant being in May–June. Euseius species were classified as primary pollen feeders and generalist predators whose density can be correlated with the availability of wind-borne pollen settling on the foliage (McMurtry and Croft 1997; McMurtry et al. 2013), so the spring peak could be related to the availability of this resource, as well as for the spring mild temperature regime. A strong population decline occurred during summer, reaching minimum densities in July and August, when maximum diary temperatures exceed 30 °C and RH drops below 40%, in agreement with the field observations carried out by Ferragut et al. (1988) in Valencia region in Spain, Ragusa (1986) in Italia, Sahraoui et al. (2014) in Tunisia, with semi-field trials with citrus seedlings in Israel (Warburg et al. 2019) and with laboratory trials (Ferragut et al. 1987). In September, when daily temperatures and RH were favourable, populations recovered leading to an autumn peak of lower abundance on leaves, as well as a peak on fruits.
Phytoseiid species found on the trees
In addition to E. stipulatus, other phytoseiid species such as T. phialatus and N. californicus were relatively abundant, and the remaining species were very scarce in agreement with previous studies on phytoseiid fauna in Valencian citrus (Abad-Moyano et al. 2009a; Ferragut et al. 1988; Ledesma et al. 2011). These species were present throughout the year with low densities during summer and showed a single population peak in autumn in coincidence with low population densities of E. stipulatus. Summer collapse was also observed for these species; however, during winter and spring, temperatures and RH were suitable according to laboratory studies (Ferragut et al. 1987; Gotoh et al. 2004; Walzer et al. 2007), and their populations were very low. Intraguild interactions may play an important role in this fact, since E. stipulatus show high populations during these months, and has been defined as a strong intraguild predator on other phytoseiids on citrus (Abad-Moyano et al. 2010). At the beginning of autumn phytoseiid species begin to increase in number, competing for space, food, and shelter. In this competition, the greater capacity of E. stipulatus to act as a highly competitive and intraguild predator progressively reduces the population of the remaining phytoseiid species, becoming the predominant species again in winter. The E. stipulatus autumn peak has been related to the availability of tetranychids as a food source, contributing to reduce their abundance (Ferragut et al. 1988; Vela et al. 2017); however, we have seen that with a shortage of or even in absence of spider mites, this second peak also occurs. Furthermore, populations were maintained at low values throughout the winter, so during autumn and even in winter this polyphagous species could feed on other prey sources such as whitefly eggs, mealybug larvae completing its development (Ferragut et al. 1987), or even aphid or whitefly honeydew, which supplemented together with different spider mite species reduces developmental time and increase oviposition (Zhimo and McMurtry 1990). Finally, a recent study has shown how E. stipulatus, like other Euseius species, is a zoophytophagous predator able to feed on plant fluids from the citrus leaves, which undoubtedly contributes to the maintenance of overwintering populations (Cruz-Miralles et al. 2021). The great ability of this species to use different food sources in the citrus agrosystem makes it a potent competitor for the resources and space, being difficult for other phytoseiid species to deal with.
Phytoseiid behaviour in response to pest density
We have found behavioural and colour changes in phytoseiids when E. banksi increases, which is indicative of the existence of predator-prey relationships. Phytoseiids move to the upper side of the leaves in the periphery of the canopy, where E. banksi density is higher, to feed on it, and changing their colour to reddish-brown consequently. Within-plant daily migrations seem to be a common behaviour in phytoseiids. To avoid adverse climatic conditions during the day, such as high temperatures, low relative humidity, or high ultraviolet radiation, phytoseiids remain sheltered and move during the night to forage (Onzo et al. 2003, 2010). Euseius stipulatus remains shelter from adverse diurnal climate conditions on the underside of the leaves inside the canopy, with an aggregate distribution pattern. At dusk, when the sun disappears and conditions are more favourable, it move to the upper side of the leaves on the periphery of the canopy where the populations of spider mites are abundant, showing a random distribution pattern typical of a predator searching for prey (García-Marí et al. 1985; Villanueva and Childers 2005). Our surveys were carried out all year-round during sunlight hours, and despite the adverse weather conditions for phytoseiids we were able to detect a change in their behaviour in response to the presence and growth of E. banksi. Furthermore, the presence of E. banksi in the fruit significantly encourages the movement of phytoseiids to this substrate, which they do not occupy in its absence. Finally, we have statistically demonstrated that the change in the colour of phytoseiids provides evidence of the use of E. banksi as prey and an indirect estimation of the biological control. However, we must be careful when relating the color of phytoseiids to prey consumption, as we do not know in detail how long the coloring remains, and this could lead to over- or underestimates prey consumption.
There is little information available on field efficacy of phytoseiids on E. banksi in its native area. In Texas, Euseius finlandicus (Oudemans) and E. mesembrinus (Dean) were abundant in presence of E. banksi (Dean 1952; Dean 1959b), while in Mexico, it is considered that the latter predator maintain E. banksi under control most of the year (Landeros et al. 2004). In Florida, E. banksi is the prevalent mite pest on citrus, where the rich phytoseiid complex keeps its populations under control most of the year. Euseius mesembrinus, Iphiseiodes quadripilis (Banks) and Galendromus helveolus (Chant) are common species in Florida (Villanueva and Childers 2005) tested successfully in laboratory with E. banksi as a food resource (Abou-Setta and Childers 1989; Caceres and Childers 1991; Villanueva and Childers 2007). In Spain, E. stipulatus is capable to perform a good biological control on P. citri (Ferragut et al. 1988); however, it is unable to reproduce and even not complete its development preying T. urticae on sweet orange and clementine leaves respectively (Abad-Moyano et al. 2009b; Ferragut et al. 1987). The behavioural and colour changes observed in phytoseiids clearly indicates that they are playing a role in the biological control of the pest in late summer and early autumn. Nevertheless, the differences in their ratios were very high, since E. banksi had a density 60–100 times higher than its predator. Moreover, this prey is not a suitable food source for E. stipulatus, delaying its development, and strongly reducing its survival rate and fecundity in laboratory studies (López-Olmos and Ferragut, data not published). Eventually, our field observations confirm that E. stipulatus is not able to perform an efficient biological control on E. banksi and the impact of predation is too weak to keep populations at tolerable economic levels for farmers.
Acknowledgements
The authors like to thank the owners and technicians of the orchards for allowing us to use their plantations and J. Gavara-Vidal (UPV) for his help in sampling. This research was supported by a predoctoral grant (ACIF to S. López-Olmos from Generalitat Valenciana) and the Pest Control Collaboration Project S7296000 between the Polytechnic University of Valencia (UPV) and the Generalitat Valenciana. We thank Phil Barker for English revision.
References
- Abad-Moyano R., Pina T., Dembilio O., Ferragut F., Urbaneja A. 2009a. Survey of natural enemies of spider mites (Acari: Tetranychidae) in citrus orchards in eastern Spain. Exp. Appl. Acarol., 47: 49-61. https://doi.org/10.1007/s10493-008-9193-3
- Abad-Moyano R., Pina T., Ferragut F., Urbaneja A. 2009b. Comparative life-history traits of three phytoseiid mites associated with Tetranychus urticae (Acari: Tetranychidae) colonies in clementine orchards in eastern Spain: Implications for biological control. Exp. Appl. Acarol., 47: 121-132. https://doi.org/10.1007/s10493-008-9197-z
- Abad-Moyano R., Urbaneja A., Schausberger P. 2010. Intraguild interactions between Euseius stipulatus and the candidate biocontrol agents of Tetranychus urticae in Spanish clementine orchards: Phytoseiulus persimilis and Neoseiulus californicus. Exp. Appl. Acarol., 50: 23-34. https://doi.org/10.1007/s10493-009-9278-7
- Abdel-Shaheed G.A., Hammad S.M., El-Sawaf S.K. 1973. Survey and population density studies on mites found on cotton and corn in Abis, Abou-Hommos localities, El-Beheira Province (Egypt). Bull. Soc. Entomol. D'Egypte, 57: 101-108.
- Abou-Setta M.M., Childers C.C. 1989. Biology of Euseius mesembrinus (Acari: Phytoseiidae): Life tables and feeding behavior on tetranychid mites on citrus. Environ. Entomol., 18: 665-669. https://doi.org/10.1093/ee/18.4.665
- Agustí M. 2003. Citricultura (2nd ed.). Madrid, España: Ediciones Mundi-Prensa. pp. 506.
- Anonymous. 2022. Texas citrus mite, a new pest of San Joaquin Valley citrus. Univ California. Division of agriculture and natural resources, University of California. Periodical title [Internet]. Available from: https://cekern.ucanr.edu/Entomology/Newsletters_and_Other_Entomology_Information_114/Texas_Citrus_Mite/.
- Badii, M.H., Varela S., Flores A.E., Landeros J. 2003. Temperature-based life history and life table parameters of Texas citrus mite on orange (Acari: Tetranychidae). Syst. Appl. Acarol., 8(1): 25-28. https://doi.org/10.11158/saa.8.1.3
- Beyzavi G., Ueckermann E.A., Faraji F., Ostovan H. 2013. A catalog of Iranian prostigmatic mites of superfamilies Raphignathoidea & Tetranychoidea (Acari). Persian J. Acarol., 2: 389-474. https://doi.org/10.22073/pja.v2i3.10042.
- Bodenheimer F.S. 1951. Citrus entomology in the Middle East: with special reference to Egypt, Iran, Irak, Palestine, Syria, Turkey. W. Junk Publications. Berlin: The Hague. pp. 675.
- Breheny P., Burchett W. 2017. Visualization of regression models using visreg. Periodical title [Internet]. The R Journal, 9(2): 56-71. https://doi.org/10.32614/RJ-2017-046
- Breusch T.S., Pagan A.R. 1979. A simple test for heteroscedasticity and random coefficient variation. Econometrica, 47: 1287-1294. https://doi.org/10.2307/1911963
- Caceres S., Childers C.C. 1991. Biology and life tables of Galendromus helveolus (Acari: Phytoseiidae) on Florida citrus, Environ. Entomol., 20: 24-229. https://doi.org/10.1093/ee/20.1.224
- Carvalho J.P., Ilharco F.A., Ferreira M.A., Carvalho, M.U.P. 1999. Manual de pragas e sintomas de ataque de insetos e ácaros em citrinos. Oeiras: EAN.
- Childers C.C. 1992. Texas citrus mite. Fact Sheet ENY-818. Available from: https://Edis.Ifas.Ufl.Edu.
- Childers C.C., Abou-Setta M.M., Nawar M.S. 1991. Biology of Eutetranychus banksi: Life tables on ''Marsh'' grapefruit leaves at different temperatures (Acari: Tetranychidae). Int. J. of Acarol., 17: 29-35. https://doi.org/10.1080/01647959108683883
- Cruz-Miralles J., Cabedo-López M., Guzzo M., Ibáñez-Gual V., Flors V., Jaques J.A. 2021. Plant-feeding may explain why the generalist predator Euseius stipulatus does better on less defended citrus plants but Tetranychus-specialists Neoseiulus californicus and Phytoseiulus persimilis do not. Exp. Appl. Acarol., 83(2): 167-182. https://doi.org/10.1007/s10493-020-00588-x
- Dean H.A. 1952. Spider mites of citrus and Texas citrus mite control in the Lower Rio Grande Valley of Texas. J. Econ. Entomol., 45(6): 1051-1056. https://doi.org/10.1093/jee/45.6.1051
- Dean H.A. 1959a. Quadrant distribution of mites on leaves of Texas grapefruit. J. Econ. Entomol., 52(4): 725-727. https://doi.org/10.1093/jee/52.4.725
- Dean H.A. 1959b. Seasonal distribution of mites on Texas grapefruit. J. Econ. Entomol., 52(2): 228-232. https://doi.org/10.1093/jee/52.2.228
- Dean H.A.1980. Population differences of Texas citrus mites on leaves of four orange varieties in Texas. J. Econ. Enlomol., 73: 813-816. https://doi.org/10.1093/jee/73.6.813
- Ebeling W. 1959. Citrus red mite. In: Subtropical fruit pests. Berkeley: University of California division of agricultural sciences. pp. 144-148.
- Ferragut F. 2016. Situación actual y perspectivas de los ácaros del género Eutetranychus (Tetranychidae) en cítricos. Phytoma España, 284: 112-113.
- Ferragut F., Costa-Comelles J., García-Mari F., Laborda R., Roca D., Marzal C. 1988. Dinámica poblacional del fitoseido Euseius stipulatus (Athias-Henriot) y su presa Panonychus citri (McGregor) (Acari: Phytoseiidae, Tetranychidae) en los cítricos españoles. Bol. Sanid. Veg. Plagas, 14: 45-54.
- Ferragut F., García-Mari F., Costa-Comelles J., Laborda R. 1987. Influence of food and temperature on development and oviposition of Euseius stipulatus and Typhlodromus phialatus (Acari, Phytoseiidae). Exp. Appl. Acarol., 3: 317-329. https://doi.org/10.1007/BF01193168
- Ferro D.N., Chapman R.D., Penman D.R. 1979. Observation on insect microclimate and insect pest management. Environ. Entomol., 8(6): 1000-1003. https://doi.org/10.1093/ee/8.6.1000
- Fox J., Weisberg S. 2019. An R Companion to applied regression (Third edition). Sage Publications, Inc. pp. 607.
- García E., Márquez A.L., Orla S., Alvarado P. 2003. Caracterización de la presencia de Eutetranychus banksi (McGregor) y Eutetranychus orientalis (Klein) en el sur de España. Phytoma España, 153: 90-96.
- García-Marí F., Gonzalez-Zamora J.E., Orenga-Royo S., Saques-Fernández J., Laborda-Cenjor R., Soto-Sánchez A., Ribes-Koninckx A. 1991. Distribución espacial y asociación entre especies de ácaros fitófagos (Tetranychidae) y depredadores (Phytoseiidae) en hojas de fresón. Bol. San. Veg. Plagas, 17: 401-415.
- García-Marí F., Laborda R., Costa Comelles J., Ferragut F., Marzal C. 1985. Ácaros fitófagos y depredadores en nuestros cítricos. Cuadernos de Fitopatología, 2(2): 54-63.
- García-Marí F., Santaballa E., Ferragut F., Marzal C., Colomer P., Costa J. 1983. El ácaro rojo Panonychus citri (McGregor): Incidencia en la problemática fitosanitaria de nuestros agrios. Bol. Serv. Plagas, 9: 191-218.
- Gardener M. 2012. Statistics for ecologists using R and Excel: Data Collection, Exploration, Analysis and Presentation. Pelagic Publishing. Exeter. pp. 324.
- Gerson U. 2003. Acarine pests of citrus: overview and non-chemical control. Syst. Appl. Acarol. 8: 3-12. https://doi.org/10.11158/saa.8.1.1
- Gonçalves M., Cavaco M., Entrudo-Fernandez J., Soares C., Ramos N. 2002. Ácaro do Texas Eutetranychus banksi (McGregor, 1914): nova espécie do ácaro na cultura dos citrinos no Algarve. Direçao Geral de Proteçao das Culturas, Oeiras.
- Gotoh T. 1987. Intraleaf distribution of Panonychus ulmi (Koch): (Acarina: Tetranychidae) on Dwarf Bamboo. App. Ent. Zool., 22(3): 248-257. https://doi.org/10.1303/aez.22.248
- Gotoh T., Yamaguchi K., Mori K. 2004. Effect of temperature on life history of the predatory mite Amblyseius (Neoseiulus) californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 32: 15-30. https://doi.org/10.1023/B:APPA.0000018192.91930.49
- Holloway J.K., Henderson C.F., McBurnie H.V. 1942. Population increase of Citrus red mite associated with the use of sprays containing inert granular residues. J. Econ. Entomol., 35(3): 348-350. https://doi.org/10.1093/jee/35.3.348
- Hothorn T., Hornik K., van de Wiel M.A., Zeileis A. 2006. A Lego system for conditional inference. Periodical title [Internet], The American Statistician, 60(3): 257-263. https://doi.org/10.1198/000313006X118430
- Landeros J., Cerna E., Badii M.H., Varela S., Flores A.E. 2004. Patrón de distribución espacial y fluctuación poblacional de Eutetranychus banksi (Mcgregor) (Acari: Tetranychidae) y su depredador Euseius mesembrinus (Dean) (Acari: Phytoseiidae) en una huerta de naranjos. Acta Zool. Mex., 20(3): 147-155. https://doi.org/10.21829/azm.2004.2031588
- Ledesma C., Vela J.M., Wong E., Jacas J.A., Boyero J.R. 2011. Population dynamics of the citrus oriental mite Eutetranychus orientalis (Klein) (Acari: Tetranychidae) and its mite predatory complex in Southern Spain. IOBC/WPRS Bull, 62: 83-92.
- López-Olmos S., Ferragut F. 2023. The newcomer takes it all: the invader Texas citrus mite, Eutetranychus banksi (Acari: Tetranychidae), displaces the resident relatives in citrus agrosystems. Biological Invasions. 25: 3171-3192. https://doi.org/10.1007/s10530-023-03099-z
- McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol., 42: 291-321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., de Moraes G.J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18(4): 297-320. https://doi.org/10.11158/saa.18.4.1
- McMurtry J.A., Huffaker C.B., van de Vrie M. 1970. Ecology of tetranychid mites and their natural enemies: A review: I. Tetranychid enemies: Their biological characters and the impact of spray practices. Hilgardia, 40(11): 331-390. https://doi.org/10.3733/hilg.v40n11p331
- Migeon A., Dorkeld F. Spider Mites Web: a comprehensive database for the Tetranychidae [Internet]. [25 October 2022]. Montpellier: INRA/CBGP; [09 February 2023]. Available from: http://www.montpellier.inra.fr/CBGP.
- Monzó C., Bouvet J.P., Alonso M., Urbaneja A. 2016. Control químico del ácaro de Texas, Eutetranychus banksi, en cítricos. Levante Agrícola: Revista Internacional de Cítricos, 431: 126-134.
- Muma H.M., Holtzberg H., Pratt R.M. 1953. Eutetranychus banksi (McG.) Recently found on citrus in Florida (Acarina: Tetranychidae). The Florida Entomologist, 36(4): 141-144. https://doi.org/10.2307/3492151
- Muma M.H. 1964. The population of Phytoseiidae on Florida citrus. The Florida Entomologist, 47(1): 5-11. https://doi.org/10.2307/3493754
- Onzo A., Hanna R., Zannou I., Sabelis M.W., Yaninek J.S. 2003. Dynamics of refuge use: diurnal, vertical migration by predatory and herbivorous mites within cassava plants. Oikos, 101: 59-69. https://doi.org/10.1034/j.1600-0706.2003.12572.x
- Onzo A., Sabelis M.W., Hanna R. 2010. Effects of ultraviolet radiation on predatory mites and the role of refuges in plant structures. Physiological Ecology, 39(2): 695-701. https://doi.org/10.1603/EN09206
- R Core Team. 2019. R: A language and environment for statistical computing. R Foundation for Statistical Computing (3.6.1). Vienna, Austria: R Core Team.
- Ragusa S. 1986. A five year study on population fluctuations of phytoseiid mites in a citrus orchard in Sicily. Acarologia, 27(3): 193-201.
- Rogers M.E., Stansly P.A. 2017. Rust mites, spider mites, and other phytophagous mites. In: Florida citrus production guide. Florida: University of Florida (IFAS). pp. 81-85.
- Sabelis M.W. 1985. Predation on spider mites. In: Helle W., Sabelis M.W. (Eds.). Spider mites, their biology, natural enemies and control. Amsterdam, The Netherlands: Elsevier. pp. 103-129.
- Sahraoui H., Tixier M.S., Lebdi-Grissa K., Kreiter S. 2014. Diversity and abundance of Phytoseiidae (Acari: Mesostigmata) in three crop management strategies of citrus orchards in Tunisia. Acarologia, 54(2): 155-169. https://doi.org/10.1051/acarologia/20142123
- Scott F.M., Schroeder M.R., Turrell F.M. 1948. Development, cell shape, suberization of internal surface, and abscission in the leaf of the Valencia Orange, Citrus sinensis. Bot. Gaz., 109: 381-411. https://doi.org/10.1086/335493
- Vacante V. 2010. Citrus mites: Identification, bionomy and control. Wallingford: CABI Publishing. pp. 378. https://doi.org/10.1079/9781845934989.0000
- van de Vrie M., McMurtry J.A., Huffaker C.B. 1972. Ecology of tetranychid mites and their natural enemies: A review. III. Biology, ecology, and pest status, and host-plant relations of tetranychids. Hilgardia, 41(13): 343-432. https://doi.org/10.3733/hilg.v41n13p343
- Vela J.M., Wong E., Jaques J.A., Ledesma C., Boyero J.R. 2017. Mite diversity (Acari: Tetranychidae, Tydeidae, Iolinidae, Phytoseiidae) and within-tree distribution in citrus orchards in southern Spain, with special reference to Eutetranychus orientalis. Exp. Appl. Acarol., 73(2): 191-207. https://doi.org/10.1007/s10493-017-0180-4
- Villanueva R.T., Childers C.C. 2005. Diurnal and spatial patterns of Phytoseiidae in the citrus canopy. Exp. Appl. Acarol., 35: 269-280. https://doi.org/10.1007/s10493-004-5728-4
- Villanueva R.T., Childers C.C. 2007. Development of Iphiseiodes quadripilis (Banks) (Acari: Phytoseiidae) on pollen or mite diets and predation on Aculops pelekassi (Keifer) (Acari: Eriophyidae) in the laboratory. Environ. Entomol., 36(1): 9-14. https://doi.org/10.1093/ee/36.1.9
- Walzer A., Castagnoli M., Simoni S., Liguori M., Palevsky E., Schausberger P. 2007. Intraspecific variation in humidity susceptibility of the predatory mite Neoseiulus californicus: Survival, development and reproduction. Biological Control, 41: 42-52. https://doi.org/10.1016/j.biocontrol.2006.11.012
- Warburg S., Gafni R., Inbar M., Gal S., Palevsky E., Sadehc A. 2019. Climatic and cultivar effects on phytoseiid species establishment and seasonal abundance on citrus. Acarologia, 59(4): 443-455. https://doi.org/10.24349/acarologia/20194346
- Wilcoxon F. 1945. Individual comparisons by ranking methods. Biometrics, 1: 80-83. https://doi.org/10.2307/3001968
- Zhimo Z., McMurtry, J.A. 1990. Development and reproduction of three Euseius (Acari: Phytoseiidae) species in the presence and absence of supplementary foods. Exp. Appl. Acarol., 8: 233-242. https://doi.org/10.1007/BF01202134
- Zuur A.F., Ieno E.N., Walker N.J., Saveliev A.A., Smith G.M. 2009. Mixed effects models and extensions in ecology with R. New York: Springer. pp. 685. https://doi.org/10.1007/978-0-387-87458-6



2023-02-25
Date accepted:
2024-03-22
Date published:
2024-04-16
Edited by:
Migeon, Alain

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 López-Olmos, Sergio and Ferragut, Francisco
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



