The problem of taxonomic uncertainty in biosecurity: South African mite interceptions as an example
Saccaggi, Davina L.  1
and Ueckermann, Edward A.
1
and Ueckermann, Edward A.  2
2
1Citrus Research International (CRI), Stellenbosch, South Africa & Department of Conservation Ecology and Entomology, Stellenbosch University, Stellenbosch, South Africa.
2Unit for Environmental Sciences and Management, Potchefstroom Campus, North-West University, Potchefstroom, South Africa.
2024 - Volume: 64 Issue: 2 pages: 363-369
https://doi.org/10.24349/top1-r59vOriginal research
Keywords
Abstract
Introduction
International agricultural trade is one of the main pathways by which pests are transported and introduced to new regions (De Giosa et al. 2021; Saccaggi et al. 2022), with many newly introduced pests originally transported by trade of their host plants (Lopes-da-Silva et al. 2014; Liebhold et al. 2012; Faulkner et al. 2016).
A study of organisms intercepted on agricultural plant commodities imported to South Africa reported that 30% of inspected samples had contaminants (Saccaggi et al. 2021). Over a third of these interceptions were of mites (37%, 5 054 individual interceptions), constituting the second-most commonly intercepted taxa (after fungi). Saccaggi et al. (2016) list challenges faced in identifications for biosecurity, including interception of damaged and immature species, time and resource constraints, lack of taxonomic specialists, and uncertainties in understudied taxa. Further, the sensitivity of information in biosecurity presents additional challenges, limiting open collaboration between biosecurity staff, scientists and researchers from different institutions or countries (D. Saccaggi, personal observation).
As an example of the challenges faced in biosecurity acarology, we present a particularly notable case of an unidentified mite species repeatedly intercepted over the course of 16 years. The mite in question was a species of Brevipalpus Donnadieu, 1875 (Trombidiformes: Tenuipalpidae), first intercepted in 2007 on kiwifruit imported to South Africa from Italy, and thereafter from other European countries (Saccaggi et al. 2021; Tshikhudo et al. 2021). Here we report the identity of this mite for the first time, discuss the challenges faced in reaching an identification, how these were overcome, and the implications this holds for acarology in biosecurity.
Detecting and identifying mites on traded goods
All imports of agricultural propagation material and fresh fruit to South Africa were sampled and inspected upon entry (Figure 1) (see Saccaggi et al. (2021) for a full explanation of methods). In the interests of time and efficiency, identifications were typically only done to the level at which a reasonable phytosanitary decision could be made. For instance, predatory mites were considered non-quarantine and were often only identified to family, while phytophagous mites were always identified as precisely as possible (Saccaggi et al. 2016; Saccaggi et al. 2021). Despite this, only 57% of Tetranychoidea and 62% Eriophyoidea intercepted were identified to species.



The unknown Brevipalpus
In 2007 a Brevipalpus species was intercepted for the first time from Italy (Saccaggi et al. 2021; Tshikhudo et al. 2021). This species appeared similar to B. lewisi McGregor, 1949, at the time a listed quarantine pest (see metadata in Saccaggi et al. (2021)). The same unknown species was repeatedly intercepted on European kiwifruit, first from Italy, then from Greece in 2010, and finally from France in 2015 (Table 1). Interestingly, this Brevipalpus species was never intercepted on kiwifruit originating from New Zealand, the main source of kiwifruit to South Africa. However, despite extensive consultation between the authors and international experts, it could not be positively identified. At this point we concluded that it was most likely a new species, and were encouraged to publish a new species description based on the intercepted specimens. However, biological, taxonomic and economical concerns and challenges had a significant impact on further decision-making.
- We had limited biological information available to us. We had only intercepted adults on fresh fruit. No interceptions of immature specimens on fresh fruit, nor any interceptions on dormant plant material were recorded. Therefore, we felt we could not definitively identify kiwi as a host for the whole life cycle. Possibly this Brevipalpus was moving onto kiwi opportunistically when another host was not available, in which case we would need to identify the primary host. We preferred to err on the side of caution until further biological evidence could be gathered.
- The genus Brevipalpus is a large and taxonomically challenging genus (Beard et al. 2015). Many of the almost 300 Brevipalpus species are poorly described or unrecognisable, and many of the older type specimens are lost (Mesa et al. 2009; Castro et al. 2023). We had no desire to add to this taxonomic conundrum without conclusive evidence of species status.
- We had limited access to different Brevipalpus specimens for comparison. The South African National Collection (SANC) of Acari holds around 300 Brevipalpus specimens, mostly species of common agricultural pests, which we had already eliminated as a possible match. Access to larger Acari collections, where comparison to a wider diversity may have been possible, was limited. Colleagues were able to send us some images of Brevipalpus specimens or additional taxonomic information (R. Ochoa, personal communication), but without dedicated study of the slides we were unable to make robust comparisons.
- The sensitivity of international trade meant we had to be cautious regarding sharing of information internationally, as misinterpretation or premature publication could potentially lead to additional quarantine measures or trade disputes.
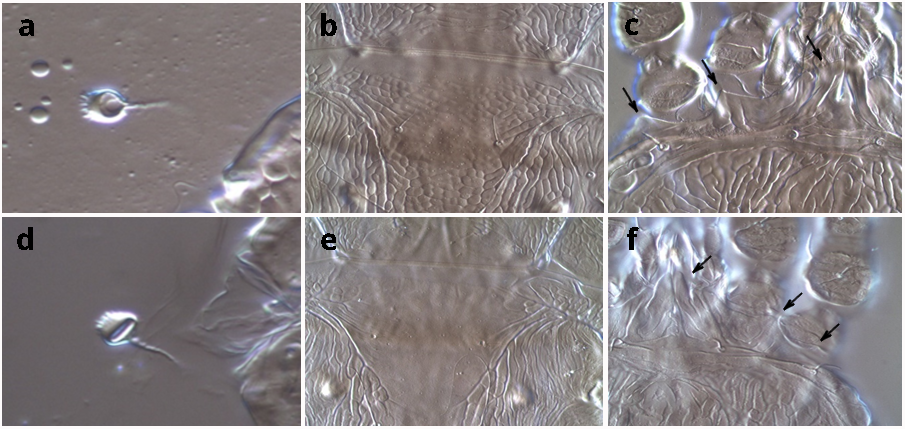


As we continued intercepting the same species, we could observe more specimens and identifications became more certain. In total, 54 interceptions of this Brevipalpus species were recorded by South Africa, with each instance accompanied by slide-mounted and archived reference specimens. We developed a short protocol for differentiation of this mite from Brevipalpus lewisi, to which it was very similar (Figure 2). This meticulous record-keeping helped immensely in keeping track of this mystery that spanned 16 years.
In 2021, an Italian research group independently initiated a survey on kiwis in Italy (De Giosa et al. 2024). As part of this survey, they reached out to us to enquire about our findings on kiwi. Their survey failed to find mites that matched our mystery Brevipalpus species, and the trail seemed to run cold again.
However, a researcher involved in the Italian survey moved to the USA and started working with a Brevipalpus taxonomist there. This opened a three-way collaboration between acarologists from South Africa, Italy and the USA working on biosecurity, distribution and taxonomy of Brevipalpus mites. From South Africa, we provided 13 slide-mounted intercepted Brevipalpus specimens (10 from Italy and three from France). From Italy, an archived slide with nymphs and adults matching our mystery species was found. And from the USA, types specimens of Brevipalpus species housed in the US National Museum of Natural History (USNM, the Smithsonian) were examined and compared. Thus, in 2023, 16 years after its first interception, the specimens intercepted in South Africa could be compared with European and American specimens and eventually identified as Brevipalpus garmani Baker, 1949.
We were then able to retrospectively review the South African interception records and give the unknown Brevipalpus species its rightful name. These new identifications, along with other Brevipalpus interceptions on kiwifruit, are presented in Table 1.



The implications for trade
Incomplete or inaccurate identification is a large concern in biosecurity applications (Xue et al. 2015; Boykin et al. 2011). In the South African interception dataset, only around half of the phytophagous mites intercepted were identified to species (Saccaggi et al. 2021). This leaves the question as to whether other possibly invasive or pest species may have been present but not identified. As similar consideration is likely to apply to mite interceptions worldwide.
The challenges we faced with the unknown Brevipalpus are common in biosecurity, and are of particular concern to small or understudied taxa such as mites.
- Unsuitable specimens intercepted
- Inaccurate or incomplete taxonomy
- Limited availability of reference material
- Obstacles to collaboration
Identification of specimens is typically based on adult females, sometimes in conjunction with other life stages. However, the specimens intercepted in agricultural trade are often immature, damaged, or few in number, and thus may not include the ideal life stages or may be missing the diagnostic characters for identification (Saccaggi et al. 2016). For instance, some Tetranychinae require both males and females for identification, but males (as the smaller and less common sex) are seldom intercepted (D. Saccaggi, personal observation). For particular groups or species, literature may be published to enable identification of these immature, male or overwintering life stages, but for many it is not available.
One solution to this challenge is to implement routine molecular identifications for biosecurity (Saccaggi et al. 2016; Boykin et al. 2011). However, robust molecular identification can only be implemented for known species, in groups where the taxonomic backbone is solid, and those for which sufficient DNA sequences exist for comparison (Arimoto et al. 2013; Armstrong and Ball 2005). For mites, these criteria are seldom met, and public DNA databases contain few (if any) sequences for species other than the common, wide-spread pests. In most cases, molecular identification for mites is not yet a viable option for biosecurity settings.
In fields such as acarology, where taxonomists are few and modern methods often reveal cryptic speciation, the taxonomic backbone is far from rigid (Navia et al. 2007; Navia et al. 2010; Miller et al. 2013). Taxonomy forms the basis for biological data, biodiversity information, host and geographic ranges, economic impact, and thus agricultural biosecurity. Despite this critical need, taxonomists around the world are dwindling as this field of expertise is declining in popularity.
Reference specimens are essential to taxonomic study and identification of new specimens or species. However, access to these collections is often difficult for researchers, especially those from different geographic regions. Travel to the collection is expensive and time-consuming, while loan of museum material carries the risk of loss or damage (Dupérré 2020). Furthermore, natural history collections in developing countries are few and sometimes poorly maintained (Paknia et al. 2015; Dupérré 2020). At the time of this publication, the South African National Collection (SANC) has no full-time acarologist employed to oversee and maintain the Acari collection, let alone to expand it or facilitate further research. Even the larger natural history collections face challenges due to lack of funding, personnel and facilities to properly maintain and expand collections. This is partly being addressed by digitisation of collections, but this is a slow and challenging process, and is unlikely to see usable results in the near future (Vollmar et al. 2010; Dupérré 2020).
Genetic sequences are a type of reference material which would aid in fast and accurate identification, as they are freely available and can be interpreted by non-taxonomists. In recent years there has been much work done around generating ''DNA barcodes'' to aid in rapid identification in biosecurity (Armstrong and Ball 2005; Arimoto et al. 2013). However, due to the unavailability of sequences for most mite species, this is not a feasible option for acarology. Much work remains to be done in integrative taxonomy and molecular characterisation of mites before enough genetic reference material is available for robust identifications (Navajas and Ochoa 2013).
Biosecurity functions on a global scale, and thus it makes sense that answers would need to be sought on that same global scale. Sadly, this is often not the case, and confidentiality concerns may prevent transparent cooperation between scientists, especially those from different countries. When a possible quarantine threat is first detected, sensitivity and confidentiality of information is at its highest. Ironically, this is also the point at which open sharing of scientific knowledge with colleagues in different countries would be of the most benefit to accurate and speedy biosecurity decision-making. In our case, we were not able to share with European collaborators which kiwi fields our intercepted samples had originated from, and thus they could not make targeted surveys for the Brevipalpus mite. Although it is clear that such information should indeed be confidential, it should also be acknowledged that this confidentiality can limit or slow scientific collaboration. We urge decision-makers and scientists in biosecurity applications to acknowledge this and start taking steps to find a middle ground. Perhaps memorandums or confidentiality agreements can be put in place before a threat emerges to ensure quick, easy and transparent scientific collaboration without breach of sensitive information. This is especially important for acarology, where there are only a few experts in each taxon, and thus international collaboration is essential.
Conclusion
Accurate taxonomic identification of mites (or any other organism) intercepted in agricultural trade is crucial to biosecurity decision-making. Despite this, there are serious challenges that researchers face in rendering an accurate identification. Some of the challenges can be addressed by technological means, such as generation of genetic data and development of molecular identification protocols, especially for common species. Some challenges require investment in human capital, such as advances in taxonomic and biological knowledge, and cannot be overcome by technology alone.
Many of these challenges were highlighted in the case of B. garmani intercepted on kiwifruit from Europe. Eventually, our ability to reach that identification relied on: 1) accurate record-keeping and archiving of specimens; 2) increased taxonomic research in the group; and 3) collaboration outside of biosecurity and on an international scale.
With increasing global trade, and thus increasing transport of contaminant organisms, we foresee that taxonomy will continue to increase in importance in the biosecurity arena.
Acknowledgements
We are deeply indebted to Mr Marcello De Giosa (University of Bari and University of Florida) and Dr Aline Tassi (University of Florida) for their collaboration, resulting in the final identification as B. garmani. We wish to thank the South African Department of Agriculture, Land Reform and Rural Development (DALRRD) for continued access to their data for research purposes. We thank our two anonymous reviewers for their time in providing feedback which greatly improved the manuscript.
References
- Arimoto M., Satoh M., Uesugi R., Osakabe M. 2013 . PCR-RFLP analysis for identification of Tetranychus spider mite species (Acari: Tetranychidae). J. Econ. Entomol., 106(2): 661-668. https://doi.org/10.1603/EC12440
- Armstrong K.F., Ball S.L. 2005 . DNA barcodes for biosecurity: Invasive species identification. Philos. Trans. R. Soc. B Biol. Sci., 360(1462): 1813-1823. https://doi.org/10.1098/rstb.2005.1713
- Beard J.J., Ochoa R., Braswell W.E., Bauchan G.R. 2015 . Brevipalpus phoenicis (Geijskes) species complex (Acari: Tenuipalpidae) - A closer look. Zootaxa, 3944(1): 1-67. https://doi.org/10.11646/zootaxa.3944.1.1
- Boykin L.M., Armstrong K.F., Kubatko L., de Barro P. 2011 . Species delimitation and global biosecurity. Evol. Bioinforma., 2011(7): 1-37. https://doi.org/10.4137/EBO.S8532
- Castro E.B., Mesa N.C., Feres R.J.F., de Moraes G.J., Ochoa R., Beard J.J., Demite P.R. — Tenuipalpidae Database [Internet] — [2023]; [August 12, 2023] Available from: https://www.tenuipalpidae.ibilce.unesp.br/
- Dupérré N. 2020 . Old and new challenges in taxonomy: What are taxonomists up against? Megataxa, 001(1): 59-62. https://doi.org/10.11646/megataxa.1.1.12
- Faulkner K.T., Robertson M.P., Rouget M., Wilson J.R.U. 2016 . Understanding and managing the introduction pathways of alien taxa: South Africa as a case study. Biol. Invasions, 18(1): 73-87. https://doi.org/10.1007/s10530-015-0990-4
- De Giosa M., Bassini-Silva R., de Lillo E., McDonald E.M., Ochoa R. 2021 . Italian Acarine species intercepted in the United States. Int. J. Acarol., 47(8): 689-694. https://doi.org/10.1080/01647954.2021.1990407
- De Giosa M., Ochoa R., Castro E.B., Simoni S., Glik T., Tassi A.D., de Lillo E. 2024. Updated Italian checklist of Tenuipalpidae with description of a new species and new worldwide records of the genus Cenopalpus (Pritchard et Baker). Int. J. Acarol., Mar 2: 1-50. https://doi.org/10.1080/01647954.2024.2318364
- Liebhold A.M., Brockerhoff E.G., Garrett L.J., Parke J.L., Britton K.O. 2012 . Live plant imports: The major pathway for forest insect and pathogen invasions of the US. Front. Ecol. Environ., 10(3): 135-143. https://doi.org/10.1890/110198
- Lopes-da-Silva M., Sanches M.M., Stancioli A.R., Alves G., Sugayama R. 2014 . The role of natural and human-mediated pathways for invasive agricultural pests: A historical analysis of cases from Brazil. Agric. Sci., 05(07): 634-646. https://doi.org/10.4236/as.2014.57067
- Mesa N.C., Ochoa R., Welbourn W.C., Evans G.A., De Moraes G.J. 2009 . A catalog of the Tenuipalpidae (Acari) of the World with a key to genera. Zootaxa, 2098(2098): 1-185. https://doi.org/10.11646/zootaxa.2098.1.1
- Miller A.D., Skoracka A., Navia D., Mendonca R.S. de, Szydło W., Schultz M.B., Michael Smith C., Truol G., Hoffmann A.A. 2013 . Phylogenetic analyses reveal extensive cryptic speciation and host specialization in an economically important mite taxon. Mol. Phylogenet. Evol., 66(3): 928-940. https://doi.org/10.1016/j.ympev.2012.11.021
- Navajas M., Ochoa R. 2013 . Integrating ecology and genetics to address Acari invasions. Exp. Appl. Acarol., 59(1-2): 1-10. https://doi.org/10.1007/s10493-012-9636-8
- Navia D., de Moraes G.J., Flechtmann C.H.W. 2007. Phytophagous mites as invasive alien species: quarantine procedures. In: Morales-Malacara J. B., Behan-Pelletier V., Ueckermann E. A., Pérez T. M., Estrada-Venegas E. G., Badii M. (Eds) Acarol. XI Proc. Int. Congr., Mexico, Mexico: Instituto de Biologia and Facultad de Ciencias, Universidad Nacional Autónoma de México; Sociedad Latinoamericana de Acarología. pp. 307-316.
- Navia D., Ochoa R., Welbourn C., Ferragut F. 2010 . Adventive eriophyoid mites: A global review of their impact, pathways, prevention and challenges. Exp. Appl. Acarol., 51: 225-255. https://doi.org/10.1007/978-90-481-9562-6_12
- Paknia O., Rajaei H., Koch A. 2015 . Lack of well-maintained natural history collections and taxonomists in megadiverse developing countries hampers global biodiversity exploration. Org. Divers. Evol., 15(3): 619-629. https://doi.org/10.1007/s13127-015-0202-1
- Saccaggi D.L., Arendse M., Wilson J.R.U., Terblanche J.S. 2021 . Contaminant organisms recorded on plant product imports to South Africa 1994-2019. Sci. Data, 8(83): 1-11. https://doi.org/10.1038/s41597-021-00869-z
- Saccaggi D.L., Karsten M., Robertson M.P., Kumschick S., Somers M.J., Wilson J.R.U., Terblanche J.S. 2016 . Methods and approaches for the management of arthropod border incursions. Biol. Invasions, 18(4): 1057-1075. https://doi.org/10.1007/s10530-016-1085-6
- Saccaggi D.L., Wilson J.R.U., Robinson A.P., Terblanche J.S. 2022 . Arthropods on imported plant products: Volumes predict general trends while contextual details enhance predictive power. Ecol. Appl., 32(3): e2554. https://doi.org/10.1002/eap.2554
- Tshikhudo P.P., Nnzeru L.R., Saccaggi D.L., Makhado R., Munyai T.C. 2021 . Risk analysis of Brevipalpus species (Acari: Tenuipalpidae) introduction via kiwifruit (Actinidia spp .) imported to South Africa. African Entomol., 29(2): 463-470. https://doi.org/10.4001/003.029.0463
- Vollmar A., Macklin J.A., Ford L. 2010 . Natural history specimen digitization: Challenges and concerns. Biodivers. Informatics, 7(2): 93-112. https://doi.org/10.17161/bi.v7i2.3992
- Xue X.F., Han X., Zhang Z.Q. 2015 . Correct identification and biosecurity decision-making: Two species instead of one in Aceria genistae complex (Acari: Eriophyidae) in New Zealand. Syst. Appl. Acarol., 20(1): 71-86. https://doi.org/10.11158/saa.20.1.8



2023-10-17
Date accepted:
2024-03-11
Date published:
2024-03-15
Edited by:
Navia, Denise

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Saccaggi, Davina L. and Ueckermann, Edward A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



