In vitro evaluation of the effect of entomopathogenic nematodes on different developmental stages of the brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae)
Abdel-Ghany, Hoda S.M.  1
; Alzan, Heba F.
1
; Alzan, Heba F.  2
; Hendawy, Seham H.M.
2
; Hendawy, Seham H.M.  3
; Elsadawy, Hanan A.
3
; Elsadawy, Hanan A.  4
and Abdel-Shafy, Sobhy
4
and Abdel-Shafy, Sobhy  5
5
1✉ Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt & Ticks and Tick-borne Diseases Research Unit, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt.
2Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt & Ticks and Tick-borne Diseases Research Unit, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt & Department of Veterinary Microbiology and Pathology, Washington State University, Pullman, WA, United States.
3Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt & Ticks and Tick-borne Diseases Research Unit, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt.
4Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt.
5Department of Parasitology and Animal Diseases, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt & Ticks and Tick-borne Diseases Research Unit, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt.
2024 - Volume: 64 Issue: 2 pages: 323-334
https://doi.org/10.24349/1hyi-f1vqOriginal research
Keywords
Abstract
Introduction
The brown dog tick, Rhipicephalus sanguineus (R. sanguineus) (Latreille, 1806) (Acari: Ixodidae), is an important globally distributed ectoparasite of veterinary and human medical concern (Dantas-Torres 2010). It is a three-host tick and requires three blood meals to complete its life cycle (Walker et al. 2003). This tick species parasitizes mainly dogs and other carnivores, as well as sporadically infesting livestock, wild ruminants, rodents and humans (Dantas-Torres 2010; Gray et al. 2013; Mentz et al. 2016; Didyk et al. 2022). It causes tissue damage, anemia, and enhances conditions for secondary bacterial infections and myiasis (Jongejan and Uilenberg 2004; Lew-Tabor and Rodriguez Valle 2016). This tick species has the particularity of being able to survive indoors and can undergo rapid population increases in a short period of time resulting in severe infestations (Uspensky and Ioffe-Uspensky 2002). Mature and immature stages of R. sanguineus are considered vectors for diseases caused by bacteria (Rickettsia (R) rickettsii, R. conorii, Ehrlichia canis, Coxiella burnettii), protozoa (Babesia (B) canis, B. gibsoni, Hepatozoon canis) and viruses (Crimean-Congo hemorrhagic fever) that pose a serious threat to public and animal health (Parola et al. 2009; Tahmasebi et al. 2010; Dantas-Torres 2010; Dantas-Torres and Otranto 2015; Silva et al. 2017).
Until now, chemical acaricide use during the parasitic phase has been the most common and efficient method to control ticks in livestock production (Aquino-Bolanos et al. 2019). However, the excessive use of chemical acaricides has produced serious complications, including multi acaricide -resistance in ticks (Reck et al. 2014; Klafke et al. 2017), toxicity to mammals, and pollution of the environment (Amaral et al. 2011; Gomes et al. 2011). Therefore, the use of alternative and safer control methods are needed to control ticks. Biological control using entomopathogenic nematodes (EPNs) is a promising alternative option for tick control (Goolsby et al. 2018).
Investigations have revealed that EPNs are lethal to many insect pests and some important parasites such as ticks (Samish and Glazer 1991; Monteiro et al. 2014; Butt et al. 2016; Goolsby and Shapiro-Ilan 2020). In particular, Steinernema and Heterorhabditis nematodes are engaged in mutualistic associations with the motile Gram-negative bacteria Xenorhabdus and Photorhabdus, respectively (Goodrich-Blair and Clarke 2007; El-Sadawy et al. 2016); these bacteria are released into the arthropod host after infection by the nematode and can be toxic for the host. Heterorhabditis bacteriophora, H. baujardi, H. indica, H. floridensis, Steinernema sp. SII, S. riobrave and S. feltiae were all found to be virulent for the cattle tick Rhipicephalus microplus (Canestrini, 1888) (Goolsby et al. 2018; de Mendonça et al. 2019; Singh et al. 2019; Goolsby and Shapiro-Ilan 2020; Monteiro et al. 2020). Moreover, Steinernema sp. SII exhibited a pathogenic effect against adults and nymphs of the tick Hyalomma anatolicum excavatum Koch, 1844 (Butt et al. 2016) and H. bacteriophora and H. indica were shown to negatively affect the reproductive biology of partially engorged females of Dermacentor nitens Neumann, 1897 (Monteiro et al. 2014).
Samish et al. (1999) evaluated the susceptibility of larval, nymphal, and adult stages of the tick R. sanguineus and Hyalomma excavatum to two strains of Steinernema carpocapsae and three strains of Heterorhabditis bacteriophora. They found that the adult ticks were more susceptible than larvae and nymphs to these EPNs. Both larvae and nymphs of R. sanguineus showed poor susceptibility with average respective mortalities of 15% and 14% across nematode species. Engorged females were most susceptible and showed an average mortality of 93.2% at 5,000 nematodes per dish across nematode strains; this was higher than for engorged males (73.2%). No information about the effect of EPNs on newly molted R. sanguineus ticks when exposure occurs in the engorged state is currently available. Here, we retested the effect of H. bacteriophora HP88 and a new species of Steinernema sp. SII on different developmental stages of R. sanguineus, including newly molted nymphal and adult ticks, to evaluate their potential use in the biological control of this tick species
Material and methods
Ethical approval
This study was approved by Medical Research Ethics Committee (MREC) at the National Research Centre (NRC), Egypt in accordance with local laws and regulations (approval protocol No 1550212023).
Ticks
Rhipicephalus sanguineus engorged females were obtained from an established colony (started in February 2021) at the Parasitology and Animal Diseases Department, Veterinary Research Institute, National Research Centre, Dokki, Giza, Egypt. This established colony started with a limited number of females that were collected from a single dog and were initially identified according to Walker et al. (2003). These females were placed in an incubator (Friocell, MMM, Germany) in plastic cups for oviposition (25 °C ± 1 °C and 75%–80% relative humidity) and eggs were incubated for hatching. The hatched larvae from different females were mixed and were allowed to feed on healthy rabbits using the capsule technique to obtain engorged larvae (El Hakim et al. 2011). Specifically, the ticks were placed inside a feeding capsule consisting of a plastic tube (2.5 cm in diameter and 3 cm high) glued to the shaved back of the rabbit (two capsules per animal). Wooden collars (designed from 1 mm thick plywood) were placed on the rabbits to prevent grooming (Abdel-Shafy 2008). A portion of the engorged larvae were used for the treatments and others were incubated for the nymphal molt. Molted nymphs were fed on healthy rabbits to obtain fully engorged nymphs. Again, a portion of the engorged nymphs were used in treatments and others were incubated to obtain unfed adults. An equal number of unfed males and females were fed on rabbits to obtain fully engorged females. Males were included in the feeding step because females cannot reach full engorgement without mating.
Entomopathogenic nematodes
The infective juvenile larvae (IJs) of entomopathogenic nematodes live in the upper layer of the soil and find their hosts by either searching (cruisers) or by waiting (ambush); they are attracted to the host's bio-secretions. Once the IJs find their host, they invade it through the host's natural body openings such as the genital pore, anus, spiracle, mouth parts or, occasionally, via the cuticle (Kocan et al. 1998). The IJs then migrate to the host haemocoel and release bacteria, which are able to evade the insect's immune response and kill it using a variety of toxic mechanisms (Bowen and Ensign 1998; Morgan et al. 2001).
Two species of EPNs, Heterorhabditis bacteriophora HP88, and Steinernema sp. SII, were propagated in-vivo on 6th instar larvae of the greater wax moth Galleria mellonella according to Dutky et al. (1964) or in-vitro on solid culture according to El-Sadawy (2011). These experiments were carried out at the Parasitology and Animal Diseases Department, National Research Centre.
Investigation of entomopathogenic nematode virulence on different developmental stages of R. sanguineus
The effect of H. bacteriophora HP88 and Steinernema sp. SII on R. sanguineus (engorged larvae, engorged nymphs, and engorged females) were evaluated. EPNs were suspended in tap water at concentrations of 4000, 2000, 1000, 500, and 250 infective juveniles per milliliter (IJs/mL). These concentrations were applied 4 days after engorgement. Plastic cups (3 cm diameter x 3 cm long) containing 10 g of fine sand were prepared for this experiment, with 5 ticks per cup for engorged adults, 10 ticks/cup for engorged larvae, and 10 ticks/cup for engorged nymphs. Two mL of the suspended IJs was directly added to each cup, with the 5 treatments replicated 5 times for each life stage (total of 75 cups). Five additional control replicates were also prepared for each stage using tap water free from IJs. All cups were covered with muslin cloth and left under room conditions (25~30 °C, 14h:10h light–dark) at the same time for all tick stages. Different cycles of R. sanguineus were initiated from the first generation at different times. In this way, all tick stages were available at the same time for experimentation. All cups were examined daily post-treatment for 11 days for engorged larvae, 24 days for engorged nymphs, and 10 days for fully engorged females.
The mortality of tick stages was recorded based on their appearance using the specific symptom of EPN infection, that is darkened swollen bodies. Other additional symptoms, such as no response to stimulation in case of active tick stages, was also used. Tick mortality was recorded starting from the 6th, 10th and 1st day post EPN exposure in engorged larvae, engorged nymphs, and fully engorged females, respectively.
Statistical analysis
The percent mortality of engorged larvae, engorged nymphs and engorged females exposed to entomopathogenic nematode species was statistically analyzed by one-way ANOVA tests, followed by Tukey tests using SPSS program version 20. The LC50 and LC95 values were calculated by applying regression equation analyses to the probit-transformed mortality of engorged females. The dose-response data were analyzed by the probit method (Finney 1962) using Ldp line® software (Ehabsoft).
Results
Virulence of EPN species on engorged larvae
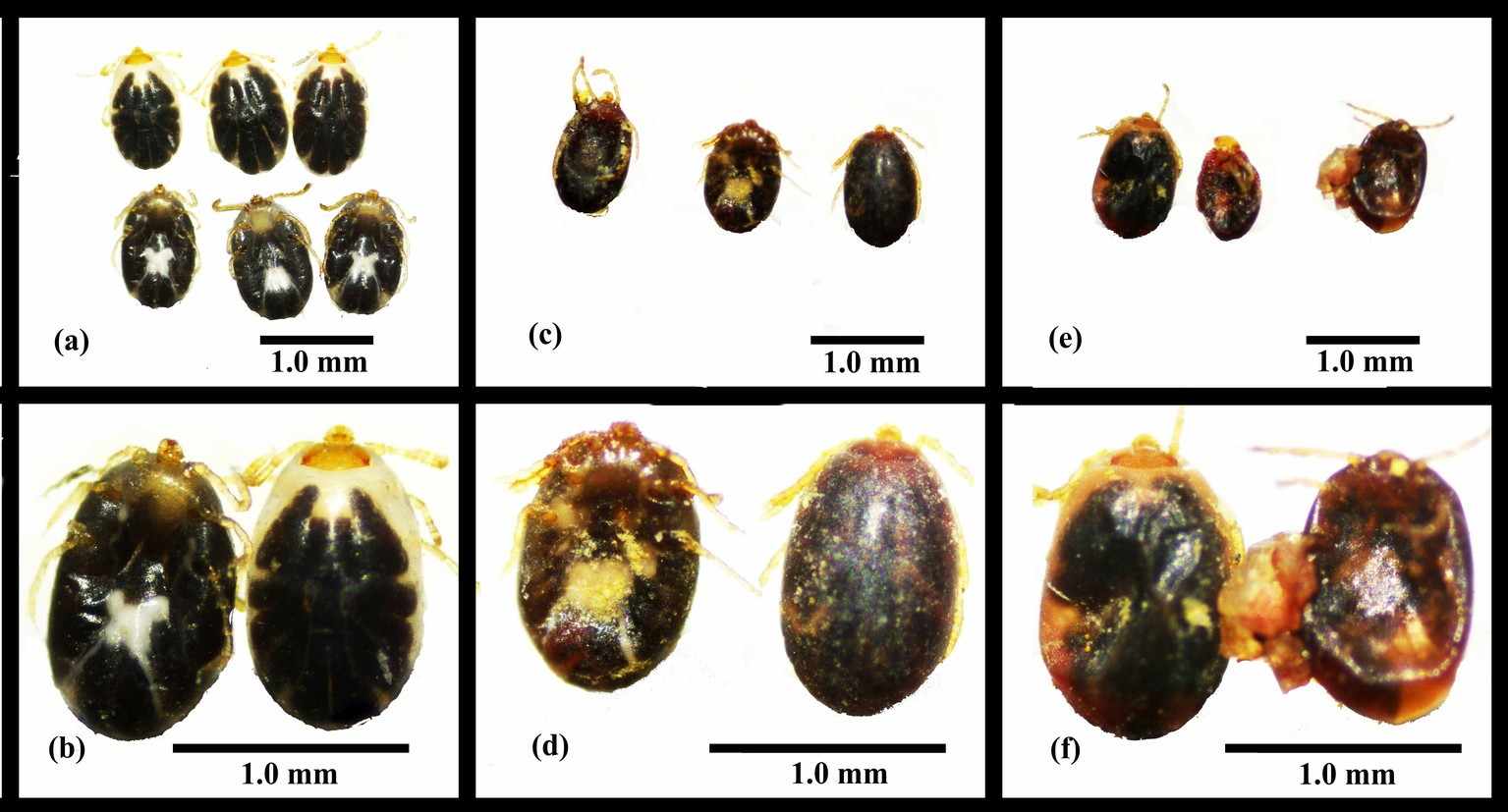


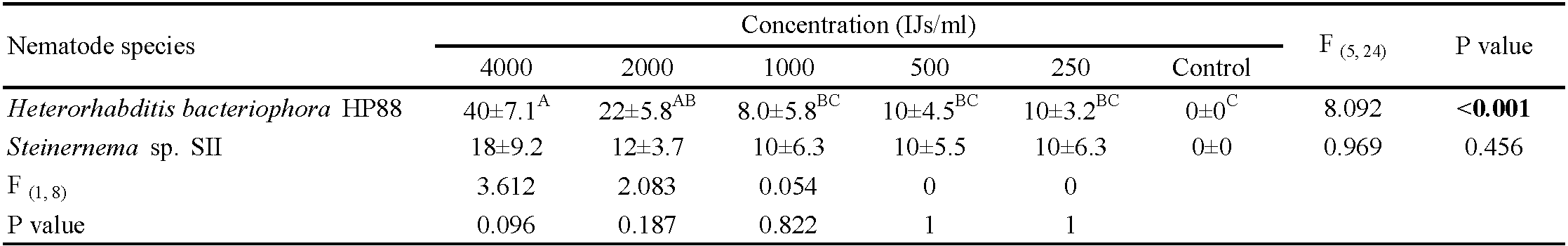
Engorged larvae showed some resistance against the tested EPNs. The impact of the two EPNs on engorged larvae extended to the molted nymphs as the EPNs remained alive in the moist sand and were able to infect the newly molted nymphs. Engorged larvae molted to the nymphal stage after approximately one week. The mortality of engorged larvae and newly molted nymphs was recorded at the end of the experimental observation time (11 days). Heterorhabditis bacteriophora HP88 and Steinernema sp. SII caused mortality ranging from 10% to 40% and from 10% to 18%, respectively, in engorged larvae and newly molted nymphs (Table 1). A significant effect of EPN dose was seen for H. bacteriophora HP88 starting at 2000 IJs/mL, whereas there was no dose effect for Steinernema sp. SII (Table 1). Dead larvae were observed to have swollen bodies and darkened color starting from the 6th day post-exposure to both EPNs (Figure 1). The dead exposed nymphs also seemed darker than controls.


Virulence of EPN species on engorged nymphs



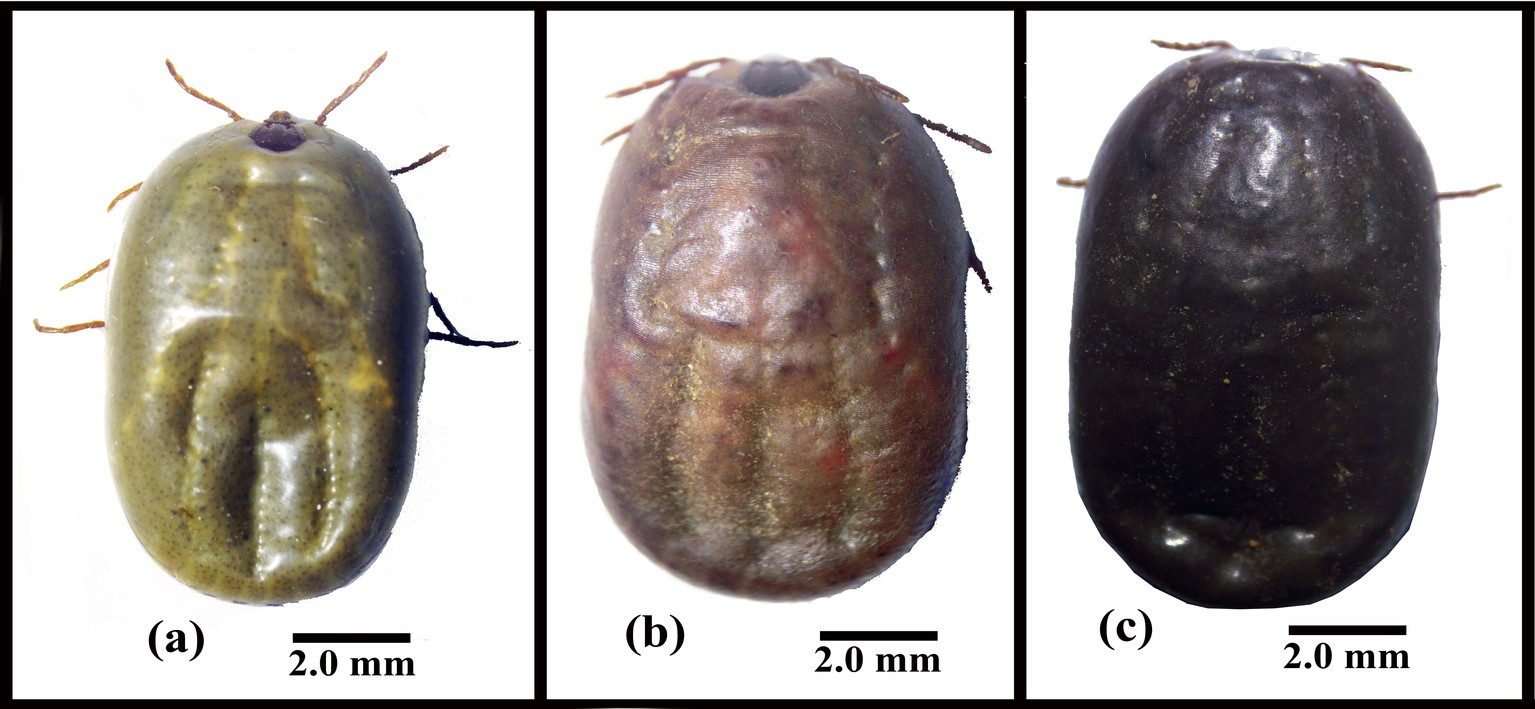
The engorged nymphs were more susceptible to nematode infections than engorged larvae. As for the engorged larvae, the EPN treatment extended to the newly molted unfed adult due to the presence of EPNs in the moist sand. The molting of engorged nymphs to adults occurred after 12-14 days. The mortality of both engorged nymphs and newly molted adults was recorded at the end of the experimental observation period (24 days). In general, H. bacteriophora HP88 and Stienernema sp. SII caused mortality ranging from 54% to 82% and from 84% to 96%, respectively, in engorged nymphs and molted adults (Table 2). A significant effect of EPN dose was seen for H. bacteriophora HP88 and Steinernema sp. SII compared with controls (Table 2). Steinernema sp. SII tended to show higher virulence than H. bacteriophora HP88, except for concentrations at 2000 and 1000 IJs/mL (Table 2). The optimum EPN concentrations for both strains were between 1000 and 2000 IJs. Dead nymphs exposed to H. bacteriophora HP88 were distinguishable by a reddish color, while dead nymphs exposed to Stienernema sp. SII tended to go black (Figure 2). The dead newly molted adults, including both males and females, were darker in color than surviving adults for both EPNs and this symptom was observed starting on the 13th day post-exposure.



Virulence of EPN species on fully engorged females


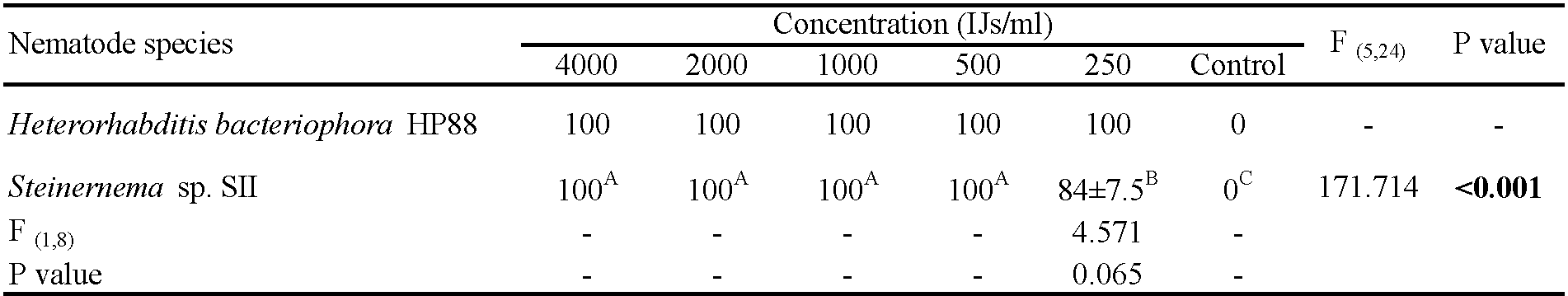
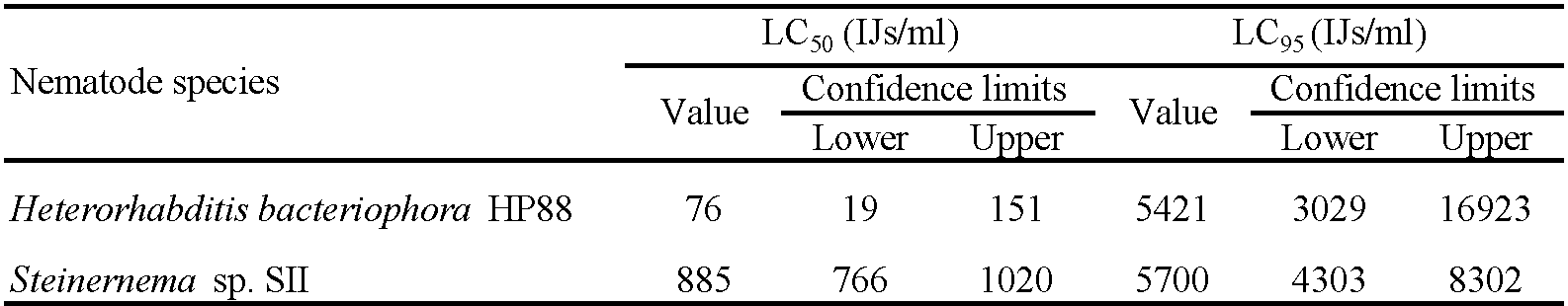
The mortality of fully engorged females was observed starting from the 1st day post-exposure with both EPN species and increased gradually to reach 100% on the 9th day post-infection for all concentrations, except 250 IJs of Steinernema sp. SII which remained at 84% mortality (Table 3). The LC50 and LC95 on the 5th day post-exposure were 76 and 5421 IJs for H. bacteriophora HP88 and 885 and 5700 IJs for Steinernema sp. SII, respectively (Table 4). Although H. bacteriophora HP88 seemed more pathogenic than Steinernema sp. SII against engorged females, these differences were not statistically significant. A significant effect of EPN dose was seen for H. bacteriophora HP88 and Steinernema sp. SII compared to controls, whereas there was no EPN species effect (Table 3). Dead females exposed to H. bacteriophora HP88 had reddish color, while those exposed to Steinernema sp. SII had a black color similar to that observed in engorged nymphs (Figure 3).




Discussion
Most studies that test the use of nematodes in biological control against ticks were performed in the laboratory on adult stages. Few studies have evaluated the impact of nematodes on immature stages (Hill 1998; Samish et al. 1999). This study aimed to evaluate the effect of two nematode species Heterorhabditis bacteriophora HP88 and Steinernema sp. SII of two different families (Heterorhabditidae and Steinernematidae) on all tick life stages, including the subsequent newly molted ticks. We also used cups filled with moist sandy soil in order to mimic the natural habitat in which these feeding stages typically exist. We believe this evaluation provides a more complete picture of the potential impact of EPNs as biological control agents.
The mortality of newly molted adults and engorged females was observed earlier than in the other tick stages despite having larger bodies. This might be attributed to these phases possessing large and more numerous orifices than juvenile stages which would allow a large number of IJs to enter the body cavity and release a greater quantity of symbiotic bacteria (Forst et al. 1997). Reddish swollen bodies in engorged females and engorged nymphs were observed in case of H. bacteriophora HP88 infection, while blackened swollen bodies of these stages corresponded to Steinernema sp. SII infection. These findings are similar to those of Woodring and Kaya (1988) who identified EPN genera based on the color of cadavers of the wax moth, Galleria mellonella L. in which Heterorhabditis and Steinernema produce red and tan colors, respectively. However, in the present study, it was difficult to discriminate infection by these two EPN genera in engorged larvae because all dead larvae were black in color.
We found that R. sanguineus tick larvae were the least susceptible stage to EPN infection, with the percent mortality being no more than 8%. Hill (1998) found that EPNs were unable to completely infect engorged larvae of the tick Ixodes scapularis Say, 1821; this result may be due to the nature of the tick species, nematode species, the environment, and/or the methodology of the experiment. In contrast, Samish et al. (1999) reported that engorged larvae of the tick species R. sanguineus and Hyalomma excavatum were resistant to infection with both H. bacteriophora HP88 and S. carpocapsae DT. They found that H. bacteriophora HP88 caused 1.7% and 6% mortality in engorged larvae of R. sanguineus and Hy. excavatum, respectively. Steinernema carpocapsae DT caused 2.5% mortality in R. sanguineus larvae and failed to infect Hy. excavatum larvae. The resistance of larvae to EPNs may be attributed to the limited size of their natural openings which reduce the ability of IJs to penetrate their bodies. Additionally, the engorged larvae are capsulated and harden rapidly to molt to the next stage (nymph). The capsulated larvae might prevent IJs from penetrating through their cuticle. In this study, the effect of EPNs extended to nymphs molted from engorged larvae and the mortality rate was much higher, up to 38%. In contrast, in the experiment conducted by Samish et al. (1999), the mortality of unfed nymphs of the two tick species was lower than 10%. The high mortality of unfed nymphs in our study may be due to lower activity of nymphs after molting from engorged larvae and the fact that their cuticles were not completely hardened at exposure, giving a chance for nematode penetration.
In our study, it was found that the engorged nymphal stage of R. sanguineus was more susceptible to EPN infections than the engorged larval stage; as well, the nematode species Steinernema sp. SII was more pathogenic than H. bacteriophora HP88. Both species caused mortality in engorged nymphs reaching 78% and 36%, respectively. These findings are in line with that recorded by Samish et al. (1999). They found that H. bacteriophora HP88 caused 30% and 15% mortality in engorged nymphs of R. sanguineus and Hy. excavatum, respectively. These values were slightly higher for S. carpocapsae DT, with 50% and 20% mortality in R. sanguineus and Hy. excavatum nymphs, respectively. Furthermore, in our study, the nematode Steinernema sp. SII achieved higher mortality (44% to 62%) in adults that emerged from engorged nymphs of R. sanguineus than the nematode species H. bacteriophora HP88 (32% - 50%). In agreement with this finding, Butt et al. (2016) recorded 30% and 40% mortality in unfed males and females of Hy. excavatum exposed separately to Steinernema sp. SII. The higher pathogenicity in engorged nymphs and emerged adults exposed to Steinernema sp. SII might be due to the greater ability of these nematodes to search and find ticks compared to H. bacteriophora HP88.
The effect we measured extended to days 13, 18, and 24 post-treatment. This is a considerably long time for an effective control method against active life stages and indicates that EPNs remain alive in the soil and can still infect the molted life stages. We could expect the EPNs to have similar behavior in the field, if the environmental conditions are suitable, especially moisture. Their impact in the field could also be improved by the availability of insect cadavers, such as from the order Lepidoptera, which as competent hosts could amplify the presence of EPNs in the environment. As the brown dog tick R. sanguineus spends more than 95% of its life cycle off the host hiding in cracks and crevices during its non-parasitic periods (Needham and Teel 1991; Dantas-Torres 2010), the application of EPNs as biocontrol agents would need to target these areas (Samish et al. 2008; Monteiro et al. 2014).
The calculated LC values of engorged females exposed to the nematode species H. bacteriophora HP88 and Steinernema sp. SII suggested that H. bacteriophora HP88 is more pathogenic than Steinernema sp. SII. This finding might be attributed to the presence of the anterior tooth of the H. bacteriophora HP88 that allows direct penetration through soft host cuticle into the body cavity (Hill 1998), in addition to IJs penetrating through natural openings. In agreement with this study, many previous studies found a pathogenic effect of different species of EPNs belonging to the genera Heterorhabditis and Steinernema on the females of different tick species. Heterorhabditis bacteriophora HP88 and LPP7 remained active against engorged females of R. microplus in the soil for 65 days after the application of EPN-infected G. mellonella cadavers (Monteiro et al. 2020). Steinernema riobrave killed 89% of the R. sanguineus fully engorged females, whereas S. feltiae killed 71% when using the petri dish technique which contained 15 g of sifted sand moistened with 5,000 IJs of nematodes (Kocan et al. 1998). Engorged females of R. microplus exposed to different EPN species revealed minimum LC50 values of 3,180 (2,958-3,419) and 491 (462-522) nematodes per dish for S. riobrave and H. floridensis, respectively (Singh et al. 2019). Five isolates of heterorhabditidae and 2 isolates of steinernematidae were tested on the fully engorged females of B. annulatus ticks. The calculated LC50 of Heterorhabditis indica RM1, Heterorhabditis sp. ISK1, H. bacteriophora TWF, Steinernema carpocapsae All and S. riobravae were 52, 63, 636, 2375, and 5700 IJs/mL, respectively (El-Sadawy and Abdel-Shafy 2007).
The normal hosts of EPNs are soil-inhabiting insects, especially belonging to the orders Lepidoptera and Coleoptera. EPNs complete their life cycle in these normal insect hosts and have the ability to develop for many generations. In the order Acarina (e.g. ticks), IJs have the ability to infect ticks, but with no development into the next generation (Lacey and Georgis, 2012; Singh et al. 2022). The use of EPNs for the biocontrol of ticks could have numerous benefits such as: a) zero toxicity to vertebrates, b) infection of a wide host range of soil insect pests that maintain and increase their populations in the field, c) easy movement in sandy soil with a water film of sufficient thickness, d) an effective insecticidal activity at temperatures that range between 18–28 °C, e) a wide tolerance range of soil pH levels (4 to 8), and f) a weak impact on non–target arthropods including non–pest species of Coleoptera and Diptera that include predators and parasitoids (Shapiro et al. 1996; Campos-Herrera, 2015). On the other hand, there are some biotic (e.g., host availability, plant metabolites, and natural enemies) and abiotic (e.g., low moisture, temperature extremes, UV light, dehydration, and soil type) factors which are considered limitations for application of EPNs in the fields (Campos-Herrera, 2015). In addition, nitrogenous compounds, such as fresh animal manure and urea, which may be readily present in environments used by ticks, may reduce the survival of EPNs (Shapiro et al. 1996). More field experiments are thus needed to ensure the efficacy of EPNs on the target arthropods, the safety of EPNs for non-target arthropods and, to avoid imbalances in the soil ecosystem food webs. Moreover, studies that examine the application of EPNs in the cracks and crevices of buildings that animals are kept in are necessary to examine the effectiveness of this method for R. sanguineus ticks.
Conclusion
The entomopathogenic nematodes H. bacteriophora HP88 and Steinernema sp. SII revealed pathogenicity against the non-parasitic life stages of R. sanguineus. This pathogenicity was high on engorged nymphs and engorged adult females and low on engorged larvae. Steinernema sp. SII was more effective on engorged nymphs than H. bacteriophora HP88, while the latter species exhibited the highest effect on engorged females. A concentration of 2000 IJs/mL of both EPN species is the recommended dose that could be used in controlling stages of R. sanguineus (off-host).
Acknowledgement
We are thankful to Paul Lacy (Animal Disease Research Unit, Pullman City, Washington state, USDA, ARS, United States) for his valuable inputs and language revision.
Author contribution
All authors shared in the design of the study. HSMA, HFA, SHMH and SA established R. sanguineus colony. HAE prepared entomopathogenic nematode concentrations. HSMA, HFA, SHMH, HAE and SA participated in tick exposure to nematodes and followed ticks after treatment. HSMA, SA analyzed the data and wrote the draft of the manuscript. All authors revised the final version of the manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding
The authors acknowledge financial support from National Research Centre as a part of project no. 13050413.
References
- Abdel-Shafy S. 2008. Scanning electron microscopy and comparative morphology of Hyalomma anatolicum excavatum, H. dromedarii and H. marginatum marginatum (Acari: Ixodidae) based on nymphs. Acarologia, 48:3-18.
- Amaral M.A.Z., Rocha C.M.B.M., Faccini J.L.H., Furlong J., Monteiro C.M.O., Prata M.C.A. 2011. Strategic control of cattle ticks: milk producers perceptions. Rev. Bras. Parasitol. Vet., 20:194-201. https://doi.org/10.1590/S1984-29612011000300003
- Aquino-Bolanos T., Ruiz-Vega J., Ortiz Hernandez Y.D., Jimenez Castaneda J.C. 2019. Survival of entomopathogenic nematodes in oil emulsions and control effectiveness on adult engorged ticks (Acari: Ixodida). J. Nematol, 51 (1): 1-10. https://doi.org/10.21307/jofnem-2019-001
- Bowen D.J., Ensign J.C. 1998. Purification and characterization of a high molecular weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Appl. Environ. Microbiol., 64: 3029-35. https://doi.org/10.1128/AEM.64.8.3029-3035.1998
- Butt T.M., Wood M., Taylor J.W.D., Bakirci S., Hazir C., Ulug D., Hazir S. 2016. Differential susceptibility of Hyalomma excavatum adults and nymphs to the entomopathogens Metarhizium anisopliae ARSEF 4556 and Steinernema carpocapsae. Int. J. Pest Manag., 62 (3): 261-266. https://doi.org/10.1080/09670874.2016.1181287
- Campos-Herrera R. 2015. Nematode pathogenesis of insects and other pests: Ecology and applied technologies for sustainable plant and crop protection. Nematode Pathogenesis of Insects and Other Pests: Ecology and Applied Technologies for Sustainable Plant and Crop Protection, pp. 1-531. https://doi.org/10.1007/978-3-319-18266-7
- Dantas-Torres F. 2010. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit. Vectors, 3: 1-11. https://doi.org/10.1186/1756-3305-3-26
- Dantas-Torres F., Otranto D. 2015. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet. Parasitol., 208: 9-13. https://doi.org/10.1016/j.vetpar.2014.12.014
- De Mendonça A.E., Moreira R.G., da Penha Henriques do Amaral M., de Oliveira Monteiro C.M., de Mello V., Vilela F.M.P., Mendonça Homem F.C., Furlong J., Dolinski C., de Azevedo Prata M.C., das Chagas E.F. 2019. Entomopathogenic nematodes in pharmaceutical formulations for Rhipicephalus microplus (Acari: Ixodidae) control: In vitro evaluation of compatibility, thermotolerance, and efficiency. Ticks Tick-borne Dis., 10 (4): 781-786. https://doi.org/10.1016/j.ttbdis.2019.03.012
- Didyk Y.M., Mangova1 B., Kraljik1 J., Stanko M., Spitalska E., Derdakova M. 2022. Rhipicephalus sanguineus s.l. detection in the Slovak Republic. Biologia., 77: 1523-1529. https://doi.org/10.1007/s11756-021-00801-1
- Dutky S.R., Thompson J.V., Cantwell G.W. 1964. A technique of the mass propagation of the DD-136 nematodes. J. Insect Pathol., 6 (4): 417-422.
- Ehabsoft [http://www.ehabsoft.com/Ldpline].
- El Hakim M.E., Shahein Y.E., Abdel-Shafy S., Abouelella A.M.K., Hamed R.R. 2011. Evaluation of glycoproteins purified from adult and larval camel ticks (Hyalomma dromedarii) as a candidate vaccine. J. Vet. Sci., 12: 243-249. https://doi.org/10.4142/jvs.2011.12.3.243
- El-Sadawy H. A. 2011. Mass production of Steinernema spp. on In-vitro developed solid medium. World Appl. Sci. J., 14 (6): 803-813.
- El-Sadawy H.A., Abdel-Shafy S. 2007. Laboratory and field studies on entomopathogenic nematodes as a biocontrol agent for the cattle tick Boophilus annulatus (Acari: Ixodidae). Acarologia, 47 (1-2): 25-31. https://doi.org/10.21608/ajesa.2007.4990
- El-Sadawy H.A., Forst S., Abouelhag H., Ahmed A.M., Alajmi R.A., Ayaad, T.H. 2016. Molecular and phenotypic characterization of two bacteria, Photorhabdus luminescens subsp. akhurstii HRM1 and HS1 isolated from two entomopathogenic nematodes, Heterorhabditis indica RM1 and Heterorhabditis sp. S1. Pakistan J. Zool., 48 (1): 51-58.
- Finney D.J. 1962. Probit analysis a statistical treatment of the response curve. Cambridge University Press, Cambridge
- Forst S., Dowds B., Boemare N., Stackebrandt E. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol., 51(1): 47-72. https://doi.org/10.1146/annurev.micro.51.1.47
- Goodrich-Blair H., Clarke D.J. 2007. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Mol. Microbiol., 64 (2): 260-8. https://doi.org/10.1111/j.1365-2958.2007.05671.x
- Gomes A., Koller W. W., Barros A. T. M. D. 2011. Suscetibilidade de Rhipicephalus (Boophilus) microplus a carrapaticidas em Mato Grosso do Sul, Brasil. Ciencia Rural., 41: 1447-1452. https://doi.org/10.1590/S0103-84782011005000105
- Goolsby J.A., Shapiro-Ilan D.I. 2020. Passive transfer of Steinernema riobrave entomopathogenic nematodes with potential implications for treatment of cattle fever tick-infested nilgai. Biocontrol Sci. Technol., 30 (12): 1330-1339. https://doi.org/10.1080/09583157.2020.1817332
- Goolsby J.A., Singh N.K., Shapiro-Ilan D.I., Miller R.J., Moran P.J., De Leon A.A.P. 2018. Treatment of cattle with Steinernema riobrave and Heterorhabditis floridensis for control of the southern cattle fever tick, Rhipicephalus (=Boophilus) microplus. Southwest. Entomol., 43 (2): 295-301. https://doi.org/10.3958/059.043.0201
- Gray J., Dantas-Torres F., Estrada-Pena A., Levin M. 2013. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick-Borne Dis., 4: 171-180. https://doi.org/10.1016/j.ttbdis.2012.12.003
- Hill D.E. 1998. Entomopathogenic nematodes as control agents of developmental stages of the black-legged tick, Ixodes scapularis. J. Parasitol., 84 (6): 1124-1127. https://doi.org/10.2307/3284660
- Jongejan F., Uilenberg, G. 2004. The global importance of ticks. Parasitol., 129(S1): S3-S14. https://doi.org/10.1017/S0031182004005967
- Klafke G., Webster A., Agnol B.D., Pradel E., Silva J., La Canal L.H., Becker M., Osorio, M.F., Mansson M., Barreto R., Scheffer R., Souza U.A., Corassini V.B., Santos J., Reck J., Martins J.R. 2017. Multiple resistance to acaricides in field populations of Rhipicephalus microplus from Rio Grande do Sul state, Southern Brazil. Ticks Tick Borne Dis., 8 (1): 73-80. https://doi.org/10.1016/j.ttbdis.2016.09.019
- Kocan K.M., Blouin E.F., Pidherney M.S., Claypool P.L., Samish M., Glazer I. 1998. Entomopathogenic nematodes as a potential biological control method for ticks. Ann. N.Y. Acad. Sci., 849: 355-364. https://doi.org/10.1111/j.1749-6632.1998.tb11070.x
- Lacey L.A., Georgis, R. 2012. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol., 44, 218.
- Lew-Tabor A.E., Rodriguez Valle M. 2016. A review of reverse vaccinology approaches for the development of vaccines against ticks and tick-borne diseases. Ticks Tick Borne Dis., 7: 573-585. https://doi.org/10.1016/j.ttbdis.2015.12.012
- Mentz M.B., Trombka, M., Silva G.L.D., Silva C.E. 2016. Rhipicephalus sanguineus (ACARI: IXODIDAE) biting a human being in Porto Alegre city, Rio Grande Do Sul, Brazil. Rev. Inst. Med. Trop. Sp., 58. https://doi.org/10.1590/s1678-9946201658035
- Monteiro C., Coelho L., de Paula L.G.F., Fernandes É.K.K., Dolinski C., Bittencourt V.R.E.P., Furlong J., Prata M.C.D.A. 2020. Efficacy of entomopathogenic nematodes in insect cadaver formulation against engorged females of Rhipicephalus microplus (Acari: Ixodidae) in semi-field conditions. Ticks Tick Borne Dis., 11 (1): 101313. https://doi.org/10.1016/j.ttbdis.2019.101313
- Monteiro C.M.D.O., Matos R.D.S., Araujo L.X., Perinotto W.M.D.S., Bittencourt V.R.E.P., Dolinski C., Prata M.C.D.A. 2014. First report of pathogenicity of entomopathogenic nematodes of the genus Heterorhabditis on partially engorged females of Dermacentor nitens (Acari: Ixodidae). Biol. Control., 69: 78-81. https://doi.org/10.1016/j.biocontrol.2013.11.003
- Morgan J.A.W., Sergeant M., Ellis D., Ousley M., Jarrett P. (2001). Sequence analysis of insecticidal genes from Xenorhabdus nematophilus PMFI296. Appl. Environ. Microbiol., 67(5): 2062-2069. https://doi.org/10.1128/AEM.67.5.2062-2069.2001
- Needham G.R., Teel P.D. 1991. Of-host physiological ecology of ixodid ticks. Annu. Rev. Entomol., 36(1): 659-681. https://doi.org/10.1146/annurev.en.36.010191.003303
- Parola P., Socolovschi C., Raoult D. 2009. Deciphering the relationships between Rickettsia conorii conorii and Rhipicephalus sanguineus in the ecology and epidemiology of Mediterranean spotted fever. Ann. N.Y. Acad. Sci., 1166:49-54. https://doi.org/10.1111/j.1749-6632.2009.04518.x
- Reck J., Klafke G.M., Webster A., Dall′Agnol B., Scheffer R., Souza U.A., Corassini V.B., Vargas R., Santos J.S., Martins J.R.S. 2014. First report of fluazuron resistance in Rhipicephalus microplus: a field tick population resistant to six classes of acaricides. Vet. Parasitol., 201:128-136. https://doi.org/10.1016/j.vetpar.2014.01.012
- Samish M., Glazer I. 1991: Pathogenicity of parasitic nematodes to ticks. In "tick - borne diseases and their vectors. Dusbabek, R. and Bukva, V. (Eds)″. SPB Academic Publishers. The Hague. The Netherlands: 629-632.
- Samish M., Alekseev E., Glazer I. 1999. Interaction between ticks (Acari: Ixodidae) and pathogenic nematodes (Nematoda): Susceptibility of tick species at various developmental stages. J. Med. Entomol., 36 (6): 733-740. https://doi.org/10.1093/jmedent/36.6.733
- Samish M., Ginsberg H., Glazer I., 2008. Anti-tick biological control agents: assessment and future perspectives. In: Bowman, A.S., Nuttall, P. (Eds.), Ticks: Biology, Disease and Control. Cambridge University Press, Cambridge, pp. 447-469. https://doi.org/10.1017/CBO9780511551802.021
- Silva A.B., Duarte M.M., da Costa Cavalcante R., de Oliveira S.V., Vizzoni V.F., de Lima Dure A.'I., de Melo Iani F.C., Machado-Ferreira E., Gazeta G.S. 2017. Rickettsia rickettsii infecting Rhipicephalus sanguineus sensu lato (Latreille 1806), in high altitude atlantic forest fragments, Ceara State, Brazil. Acta. Trop., 173: 30-33. https://doi.org/10.1016/j.actatropica.2017.05.018
- Singh N.K., Goolsby J.A., Jyoti J., Shapiro-Ilan D.I., Miller R.J., Leon A.A.P.D. 2019. Comparative efficacy of entomopathogenic nematodes against a multi-acaricide resistant strain of southern cattle fever tick, Rhipicephalus microplus. Southwest. Entomol., 44 (1): 143-153. https://doi.org/10.3958/059.044.0116
- Singh K., Kumar M., Ahuja A., Vinay B.K., Kommu K.K., Thakur S., Paschapur A.U., Jeevan B., Mishra K.K., Meena R.P., Parihar M. 2022. Entomopathogenic nematodes: a sustainable option for insect pest management. In: Biopesticides, Vol. 2: Advances in Bio-Inoculants Advances in Bio-inoculant Science, Pages 73-92. https://doi.org/10.1016/B978-0-12-823355-9.00007-9
- Shapiro D.I., Tylka, G.L., Lewis, L.C. 1996. Effects of fertilizers on virulence of Steinernema carpocapsae. Appl. Soil Ecol., 3, 27-34. https://doi.org/10.1016/0929-1393(95)00069-0
- Tahmasebi F., Ghiasi S.M., Mostafavi E., Moradi M., Piazak N., Mozafari A., Haeri A., Fooks A.R., Chinikar S. 2010. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus genome isolated from ticks of Hamadan province of Iran. J. Vector. Borne Dis., 47(4): 211-216.
- Uspensky I., Ioffe-Uspensky I. 2002. The dog factor in brown dog tick Rhipicephalus sanguineus (Acari: Ixodidae) infestations in and near human dwellings. Int. J. Med. Microbiol., 291: 156-163. https://doi.org/10.1016/S1438-4221(02)80030-3
- Walker A.R., Bouattour A., Camicas J.L., Estrada-Pena A., Horak I.G., Latif A.A., Pegram R.G., Preston P.M. 2003. Ticks of domestic animals in Africa: A guide to identification of species. Bioscience Report, Edinburgh, pp. 1-221.
- Woodring J.L., Kaya H.A. 1988. Steinernematid and Heterorhabditid Nematodes. A Handbook of Techniques. Arkansas Agricultural Experiment Station, Fayetteville, Arkansas, Southern Cooperative Series Bulletin 331, 30 p.



2023-05-19
Date accepted:
2024-02-28
Date published:
2024-03-04
Edited by:
McCoy, Karen

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Abdel-Ghany, Hoda S.M.; Alzan, Heba F.; Hendawy, Seham H.M.; Elsadawy, Hanan A. and Abdel-Shafy, Sobhy
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



