How do different agricultural managements interfere with the Phytoseiidae mite (Acari: Phytoseiidae) community in apple trees?
Rode, Priscila de Andrade  1
; Schussler, Matheus
1
; Schussler, Matheus  2
; Bizarro, Gabriel Lima
2
; Bizarro, Gabriel Lima  3
and Ferla, Noeli Juarez
3
and Ferla, Noeli Juarez  4
4
1✉ Laboratório de Acarologia, Tecnovates, Universidade do Vale do Taquari - Univates, 95914-014, Lajeado, Rio Grande do Sul, Brazil & Programa de Pós-Graduação em Biotecnologia, Universidade do Vale do Taquari - Univates, 95914-014, Lajeado, Rio Grande do Sul, Brazil.
2Laboratório de Acarologia, Tecnovates, Universidade do Vale do Taquari - Univates, 95914-014, Lajeado, Rio Grande do Sul, Brazil.
3Laboratório de Acarologia, Tecnovates, Universidade do Vale do Taquari - Univates, 95914-014, Lajeado, Rio Grande do Sul, Brazil.
4Laboratório de Acarologia, Tecnovates, Universidade do Vale do Taquari - Univates, 95914-014, Lajeado, Rio Grande do Sul, Brazil & Programa de Pós-Graduação em Biotecnologia, Universidade do Vale do Taquari - Univates, 95914-014, Lajeado, Rio Grande do Sul, Brazil & CNPq researcher
2024 - Volume: 64 Issue: 1 pages: 221-236
https://doi.org/10.24349/zvdf-vafzOriginal research
Keywords
Abstract
Introduction
Conventional agriculture is applied in several crops due to being highly productive, but it has substantial negative externalities, including loss of biodiversity, soil erosion, environmental pollution and risk to human health (Gomiero et al. 2011; Campbell et al. 2017). There is thus an urgent need to change the agricultural production system, aiming to achieve sustainable development (Raudonis et al. 2007; Walker et al. 2017; Zhu et al. 2018) and to ensure resource conservation (Willer et al. 2021). As an alternative to conventional management, the organic agriculture production has been developed in several countries (Gomiero et al. 2011; Smith et al. 2012; Caprio et al. 2015; Gomiero 2018; Sumberg and Giller 2022). Another alternative is regenerative agriculture: it aims at improving soil protection, not using machinery and maintaining soil covered by plants, spontaneous or not, but, if it is necessary, pesticides can be used to control pests (The soil association 2021).
Pesticide spraying modifies the environment, directly or indirectly affecting the interactions between species along the trophic and non-trophic chain (Dalkvist et al. 2009; Guedes et al. 2016; Zhao et al. 2020). Direct effects include mortality and various sublethal effects, while indirect effects include habitat changes, through contamination of food and shelter, and modification in species communities with associated interaction (Sánchez-Bayo 2021). Disruption of natural control is a key factor in pest outbreaks, where pesticides can reduce populations of predators, parasitoids and pathogenic organisms that naturally occur (Geiger et al. 2010; Janssen and Rijn 2021). Furthermore, the relationship between biodiversity and pest control is of great interest for sustainable agricultural production (Letourneau et al. 2011; Veres et al. 2013; Crowder and Jabbour 2014; Liere et al. 2017; Möth et al. 2021).
The conventional system prevails in apple production areas (Malus domestica Borkh: Rosaceae) in Brazil (Lorenzato and Secchi 1993; Monteiro 1994; Hardmann et al. 2003). However, more and more producers are interested in implementing a farming system less dependent on pesticides, especially in the state of Rio Grande do Sul (Kist et al. 2015). The Gala and Fuji cultivars are the most used and account for approximately 90% of the national production, while the Eva cultivar, associated with other recent ones, account for about 5% of the production (Fioravanço et al. 2010).
Phytophagous mites of the Eriophyidae and Tetranychidae families are considered as important apple pests (Ferla et al. 2018; Nascimento et al. 2020), particularly Panonychus ulmi (Koch) (Tetranychidae), Tetranychus urticae Koch (Tetranychidae) and Aculus schlechtendali (Nalepa) (Eriophyidae) (Nascimento et al. 2020; Kasap and Atlihan 2021). Recently, the quarantine species A. schlechtendali was recorded in Southern Brazil (Ferla et al. 2018; Nascimento et al. 2020). Phytoseiid mites are effective predators of phytophagous mites (McMurtry et al. 2013; Demite et al. 2014; Knapp et al. 2018). In apple growing areas in South America, Neoseiulus californicus (McGregor) is the most common species (Croft and McGroarty 1977; Monetti and Fernandez 1996; Khajuria 2009; Toyoshima et al. 2011). However, the effect of landscape management on the diversity and distribution of these predatory mites in apple production areas in southern Brazil is unknown.
The knowledge of abundance and diversity of natural enemies in apple plantations is expected to better understand their distribution and dispersion pattern, for the implementation of future pest management actions. We thus performed surveys of phytoseiid mites in different apple production orchards, and we evaluated the richness and abundance of these mites, as well as their distribution in the different strata of the plants. Furthermore, we studied how the different apple tree cultivars affect the diversity and distribution of these predatory mites.
Material and methods
Study areas
The study was conducted in the 2020-21 in apple orchards located in the municipalities of Antônio Prado (28°22′44″S 49°56′12″W) and Muitos Capões (28°23′23″S 51°15′12″W), state of Rio Grande do Sul, and São Joaquim (28°53′23″S 51°23′06″W), state of Santa Catarina (Figure 1).
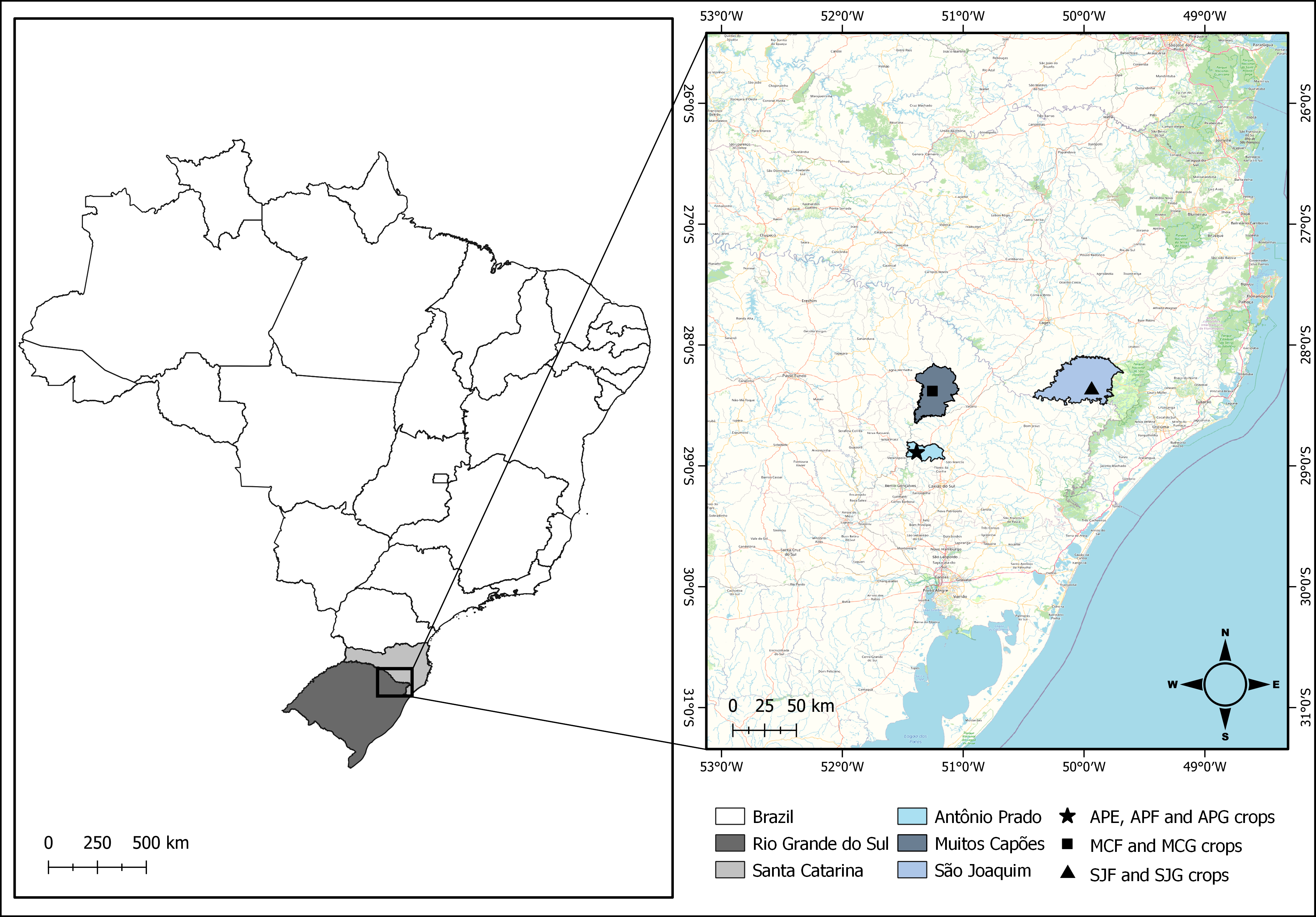


Antônio Prado: In this locality, three cultivars were considered (Eva, Fuji and Gala). The surface of Eva's orchard was 2 hectares, and that of Fuji and Gala, 1 hectare/area. In all areas, the plantation density was 4 x 1.80 m (rows x trees). Soil was covered, with spontaneous plants and grasses, in association with soil litter. In these orchards, the spontaneously growing plants were maintained and when necessary, some were removed weeding. There was no spraying of synthetic pesticides, such as insecticides/acaricides and herbicides, in the sampled areas. However, the producers used Copper sulfate and lime (0,3%) mixed with Bordeaux mixture (0,5%) after the vegetative period, every 15 days, neem oil in the same period every 30 days for pest control, lime sulfur (8%) once in winter and Cera Trap® attractive trap for capturing fruit flies from thinning to harvest. In this way, the orchards of this locality were conducted under organic management.
São Joaquim: Two orchards were evaluated, one planted with the Fuji cultivar and the other planted with the Gala cultivar. The area was 5.5 hectares for each orchard, and the density plantation was 7 x 3 m (rows x trees). In both orchards, the soil was kept covered by weeds, grasses and weeds, in association with soil litter. Pigs, sheeps and chickens were kept grazing throughout the area, feeding on the weeds and fruit of the apple trees that fell. Whenever necessary, producers sprayed pesticides (Bordeaux mixture, Fenitrothion emulsifiable concentrate, Dodina concentrated suspension and Indoxacarb + Fipronil) to pest control. In this way, the orchards of this locality were conducted under regenerative agriculture.
Muitos Capões: Collections were carried out in two conventional orchards, one planted with Fuji and the other with Gala cultivars. The total area of the orchards was 240 hectares each, with a plantation density of 4.2 x 1 m (rows x trees). In both orchards, weeds were destroyed using herbicides (Glifosato, Cletodim concentrated suspension and saflufenacil) and weeding, and spraying of pesticides (Fosmete formulation, Dimetoato emulsifiable concentrate, Fenitrothion emulsifiable concentrate and Clorpirifós emulsifiable concentrate) to control pests was carried out frequently.
Sampling
In all the orchards, the evaluated trees were identified with ribbons. From the fifth row, counting from the edge, we selected 40 trees/orchard, and from each one, a median branch was collected from which three leaves were detached, one from each stratum of the plant: basal, median and apical. Collections were carried out monthly, totaling 120 leaves/orchard/month. In winter, when apple trees lose their leaves, buds from a median branch of each stratum were evaluated, totalizing 120 buds/orchard/month and 40 for each stratum/orchard/month. The leaves or buds were individualized in plastic bags, and then packed in styrofoam boxes containing artificial ice (Gelo-x®) to keep them at a low temperature.
Climatological information on temperature (°C), relative humidity (%) and precipitation (mm) were obtained from meteorological stations close to the collection points, namely: Antônio Prado, information from the INMET meteorological station, municipality of Bento Gonçalves (A840); Muitos Capões, data from the INMET meteorological station, Vacaria (A880); São Joaquim, information from the Automatic Meteorological Station of Morro da Igreja (A845) (EMAMI).
Mite identification
The collected material was examined with the aid of a stereoscopic microscope (40×) and a fine-tipped brush. Mites removed from each leaf were mounted on slides with Hoyer's medium (Jeppson et al. 1975). After mounting, the slides were kept in ovens at 50-60 °C for an average period of 7 days. Identification was performed at the Acarology Laboratory of University of Vale do Taquari - Univates, Lajeado municipality. The Zeiss Axio Scope A1 phase contrast light microscope was used to observe the structures of the specimens (Sisgen: A8302CB). Adult mites were initially separated in families using the key provided by Walter and Krantz (2009). Subsequently, the genera were identified using the world literature about the groups collected (De Leon 1961; Chant and McMurtry 1994; 2007; Ferla et al. 2007; Camargo Barbosa and Demite 2023). Whenever possible, identification to species level was done by comparisons with original descriptions and redescriptions.
Statistical analyses
Constancy (C) was calculated according to Silveira-Neto et al. (1976), using the formula:
\[C = \frac{(P * 100)}{N}\]
where:
P = number of collections containing the species;
N = total number of collections carried out.
The result was classified as: Constant (Co) (C > 50%), Accessory (A) (25% < C >50%) and Accidental (I) (C < 25%). Dominance (D) was defined using the formula:
\[D(\%) = \left( \frac{i}{t}\right) * 100\]
where:
i = total number of individuals of a species;
t = total number of individuals collected.
The result was grouped according to the categories established by Friebe (1983): Eudominant (Eu) (> 10%), Dominant (Do) (5% > 10%), Subdominant (S) (2% > 5%), Eventual (Ev) (1% > 2%) and Rare (R) (D < 1%) (Rodrigues 2017).
We observed whether the sampling effort was sufficient to represent all the richness of mites present in the orchards of each management system, where a rarefaction curve of Phytoseiidae species was calculated through interpolation/extrapolation according to the number of individuals found in the areas. Rarefaction was calculated using the iNEXT package using the endpoint abundance data of 1200 species. The ggiNEXT function of the ggplot2 package was used to construct the rarefaction curve graph, using the R software (R Core Team 2023).
The total number of mite species was estimated from the Chao equation (Chao et al. 2014) using the'specpool' function from the vegan package. We compared Phytoseiidae diversity dynamically using the Hill diversity profile curve (Hill 1973). The diversity of the seven orchards was compared using the Rényi exponential series (exp(Hα) = exp [(1/1- α) ln∑piα]). This series includes the main diversity indices, from the most sensitive for rare species (low α value) to those that give greater weight to abundant species (high α values) (Tóthmérész 1995). In this series, α = 0 corresponds to the total number of species, α = 1 to the Shannon index, α = 2 to the Simpson index and α → ∞ to the Berger-Parker index. To generate these estimates, we used a matrix of mites associated with the studied orchards, the diversity of profile was constructed using the'renyi' function from the vegan package (Oksanen et al. 2018). The analyses were performed in the R software (R Core Team 2023).
We analysed the similarity in Phytoseiidae communities between the orchards. A Euclidean distance matrix was determined and using the factor extra package of the R software (R Core Team 2023) a dendrogram was constructed using the ward. D2 method. The dendrogram was used to separate the clusters according to the similarity of the orchards based on the abundance and diversity of species found in each area and then a cluster graph was constructed using the fviz_cluster function from the factoextra package, where abundance and richness information was plotted on the x and y axes, respectively. A multivariate analysis using the principal components method was performed to determine the main species responsible for the formation of orchard clusters. For this purpose, the FactoMineR (Lê et al. 2008) and factoextra (Kassambara and Mundt 2020) packages were used.
Regarding data on Phytoseiidae abundance, we initially compared data from areas that used different agricultural management systems (regardless of cultivar). Thus, Eva data were removed from this analysis, as this cultivar was not found in São Joaquim and Muitos Capões. We evaluated the abundance of Phytoseiidae among different management practices, cultivars, branch stratum and the interaction between these variables. Then, we evaluated the abundance in relation to areas with different cultivars, where the evaluated variables were: cultivars, branch stratum and the interaction of variables.
All abundance data were analyzed using a generalized linear model (GLM with Poisson distribution), and the Tukey test (P < 0.05) was used to determine differences between modalities. We used the SAS software (SAS Institute 2002) to perform the statistical analyses. Graphs were constructed using SigmaPlot® 11.0 software (Systat software, Inc.).
We used Phytoseiidae abundance data from the areas of each agricultural production system to analyse the relationship between the abundance of mites and abiotic parameters (monthly average temperature, relative humidity and precipitation). Then the data were subjected to multivariate regression analysis (Stepwise) using the SAS software (SAS Institute 2002). Curves were constructed using SigmaPlot® 11.0 software (Systat software, Inc.).
Results
A total of 695 phytoseiid specimens were collected, belonging to 10 species and seven genera. A greater number of species was found in the orchards of the Antônio Prado (organic management), with eight species collected on Eva, six on Fuji and four on Gala. In these orchards, the species of the genus Euseius (Euseius mesembrinus (Dean) and Euseius inouei (Ehara & Moraes)) were dominant in Eva, while Metaseiulus eiko (El-Banhawy) showed greater abundance in Fuji and Gala. A low number of species was observed in the orchards of Muitos Capões (conventional management) and São Joaquim (regenerative management). In São Joaquim, only N. californicus was observed on both Fuji and Gala. Neoseiulus californicus was present in all the orchards considered, however, many more individuals were observed in the area of Muitos Capões, both on Fuji and Gala cultivars (Table 1).



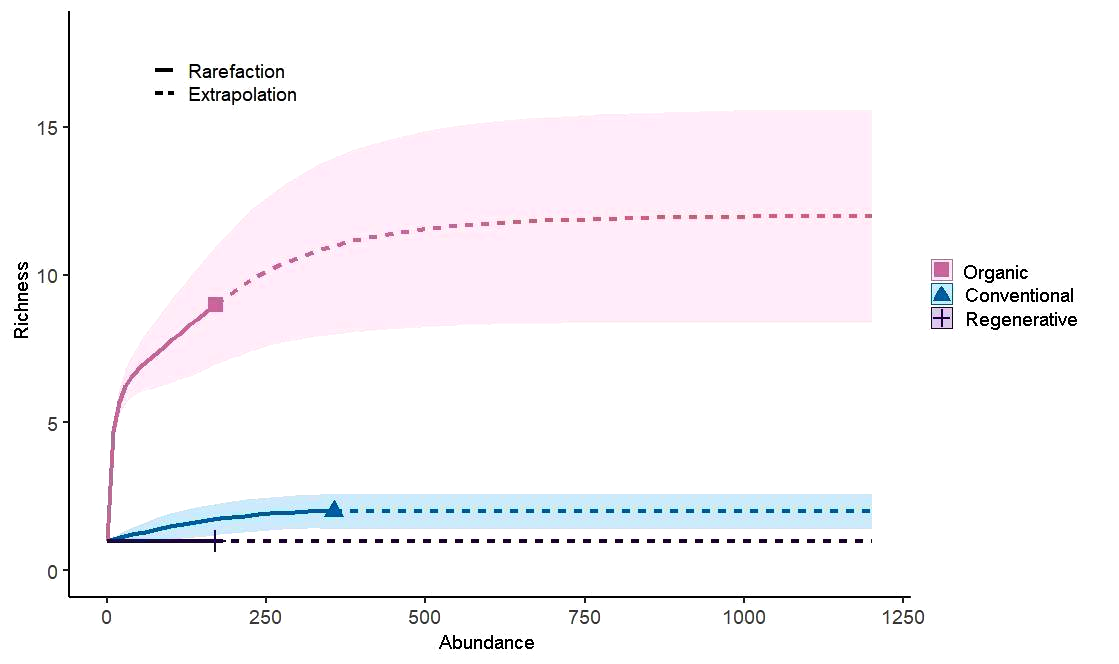
The species rarefaction curve in orchards under organic management did not stabilize, indicating that more species might be present if more collections were carried out. However, in orchards using conventional and regenerative management systems, the species rarefaction curve stabilized, showing that sampling in these locations was sufficient to determine the number of species. The curve's confidence interval reveals a difference in the Phytoseiidae richness between the evaluated areas, where the areas with organic management, presented greater richness than the others (Figure 2).


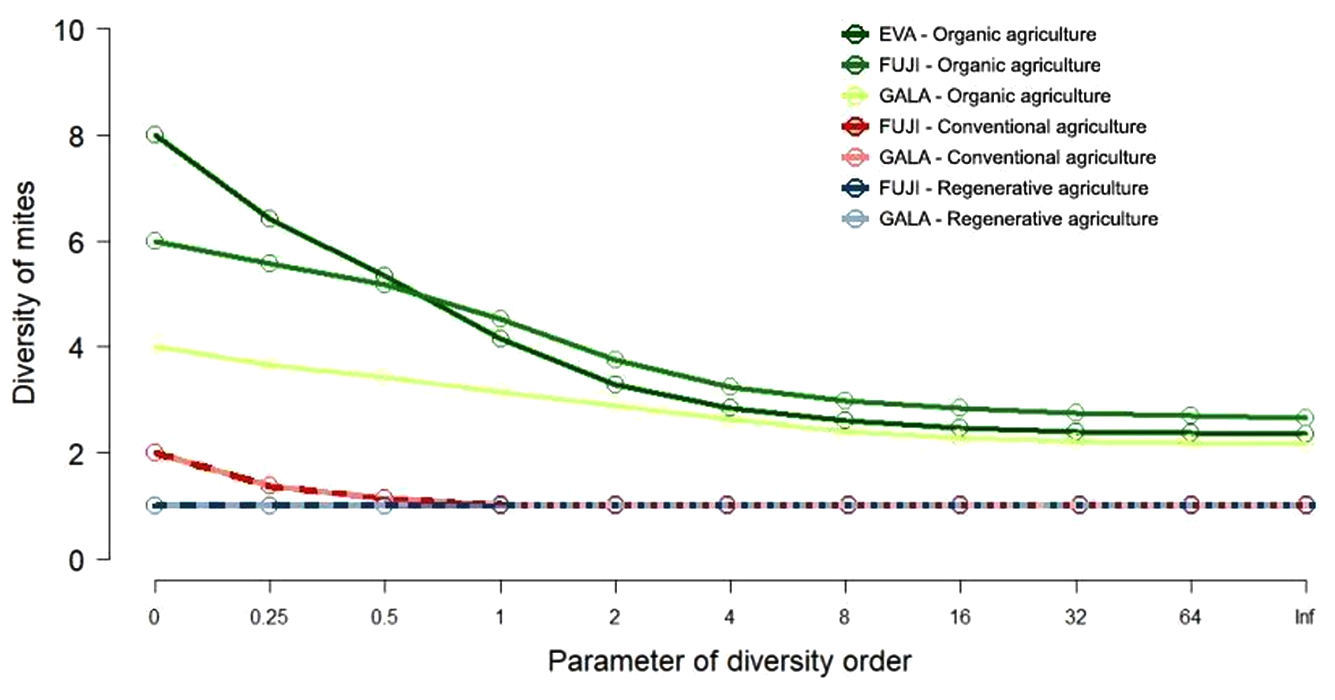
The diversity curves in the area conducted under organic management (Antônio Prado - Eva, Fuji or Gala) do not overlap the curves of the orchards conducted under conventional (Muitos Capões - Fuji or Gala) and regenerative management systems (São Joaquim - Fuji or Gala). However, the curves of the conventional and regenerative management systems overlap when the α value is equal to or greater than 1. Regarding the cultivars, considering only orchards with an organic management system, the cultivar Gala showed less diversity compared to the other apple cultivars. The cultivar Eva showed greater diversity than the cultivar Fuji when the α value was low, indicating greater diversity for species considered rare, while the cultivar Fuji showed greater diversity as the α value increased, indicating greater diversity in relation to taxa (Figure 3).


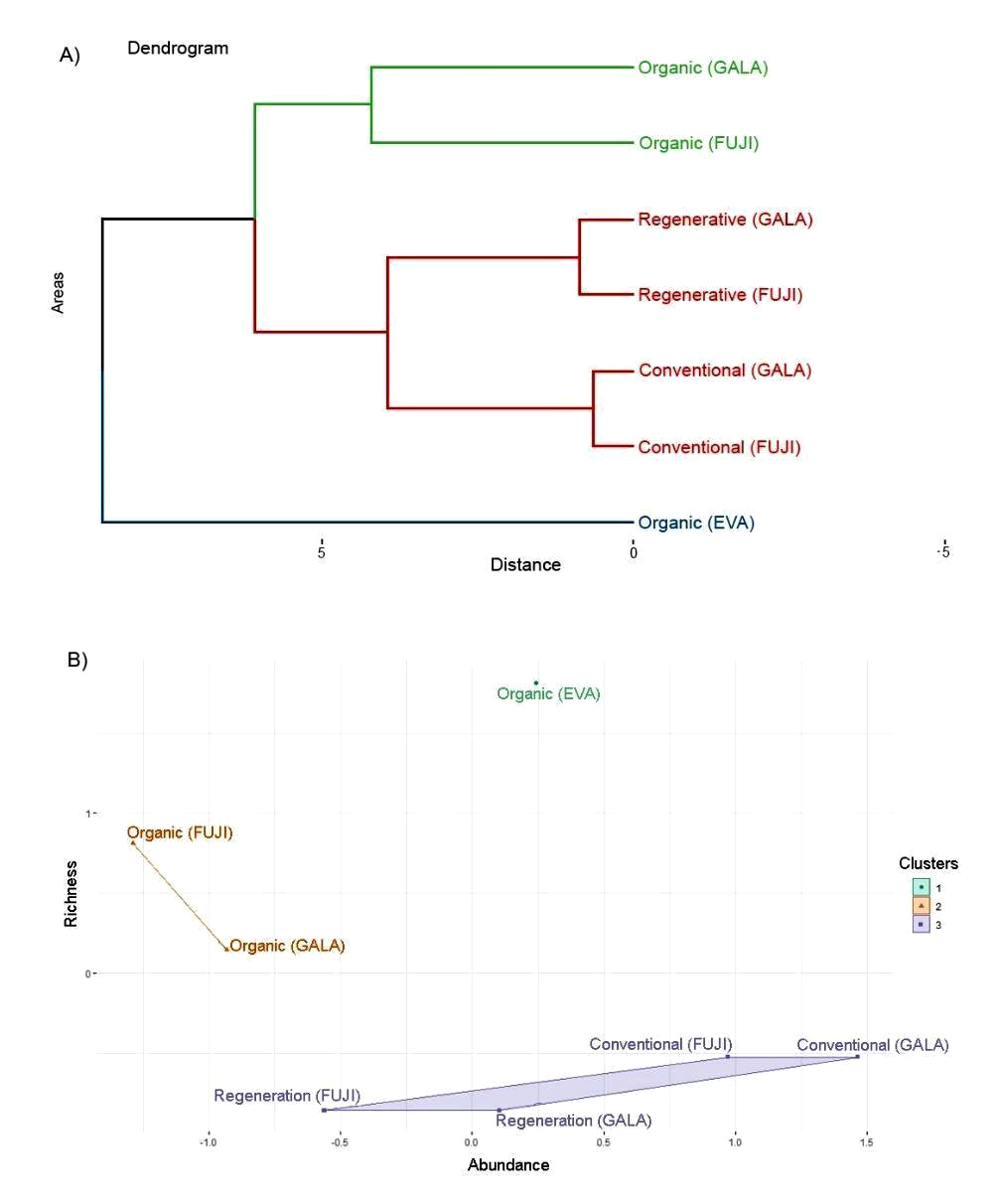
The similarity analysis revealed differences both between different areas and different cultivars. The dendrogram and the cluster analyses (Figure 4A, B) show that the cultivar Eva is well separated from the other orchards, regardless of the management used. The orchards conducted under organic management (Fuji and Gala) formed a second cluster, distinct from orchards conducted under conventional and regenerative management and using the same cultivars (Figure 4A). Regarding the organic systems, the cultivar Eva is well separated from the other varieties, due to greater Phytoseiidae abundance and richness (Figure 4B).


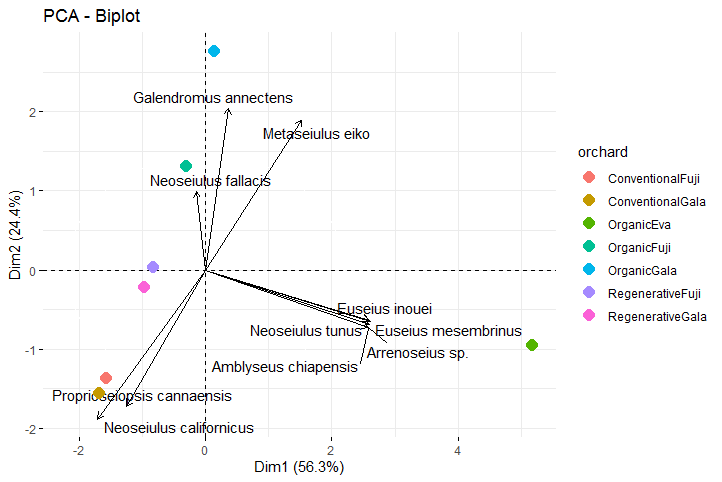
The total variance explained by the two main axes was 80.7% (axes 1 and 2 explaining 56.3% 24.4%, respectively) (Figure 5). Orchards with conventional (Muitos Capões) and regenerative management (São Joaquim), which use pesticides, were grouped, related to N. californicus, and Proprioseiopsis cannaensis (Muma). The organic cultivation (Antônio Prado) areas were grouped into two clusters, one being Fuji and Gala, and the other Eva. These areas were related to the greatest diversity of species, being Arrenoseius sp., A. chiapensis, E. inouei, E. mesembrinus and Neoseiulus tunus (De Leon), the main species related to the Eva organic orchard, and M. eiko and Galendromus annectens (De Leon) with the Fuji and Gala organic orchards (Figure 5; Figure S1).


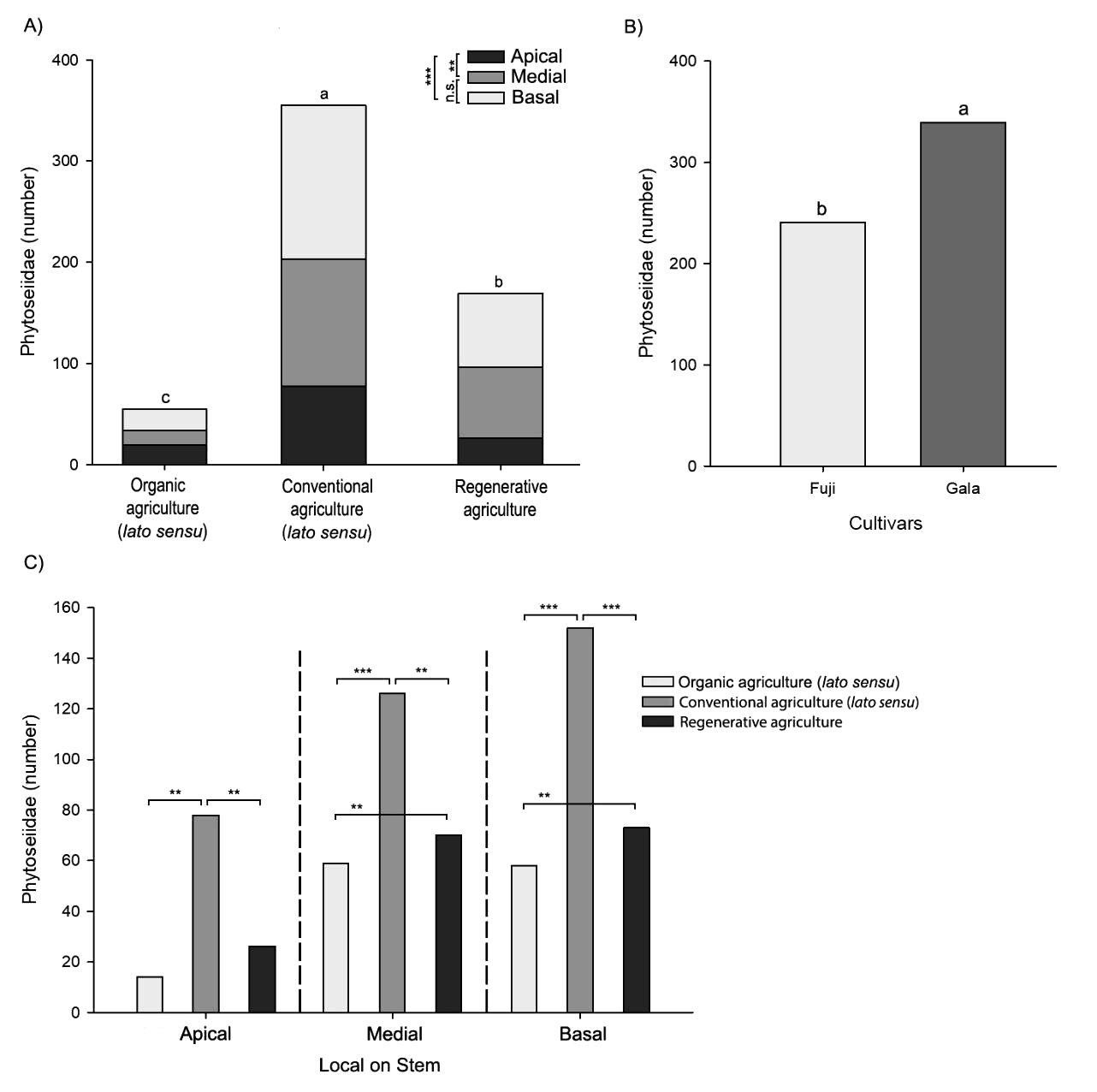
Significant differences in abundance of Phytoseiidae were observed when comparing the three types of management (Figure 6A; GLM: F2,702 = 74.92, P < 0.001). The highest abundance was observed in orchards with conventional management (Figure 6A). The lowest abundance was observed at the apical leaf level. However, there was no difference in the Phytoseiidae number between the median and basal levels (Figure 6A; GLM: F2,702 = 13.05, P < 0.0001). The cultivar Gala showed greater abundance than the cultivar Fuji (Figure 6B; GLM: F1,702 = 10.40, P = 0.0013). The variables management and stratum of the branch interacted (Figure 6C; GLM: F4,702 = 3.76, P = 0.0049), where greater abundance was observed in the apical, median and basal regions of the conventional management area. No difference was observed in the apical stratum of apple trees under organic and regenerative management, however, for the median and basal strata, the abundance differs between areas (Figure 6C).


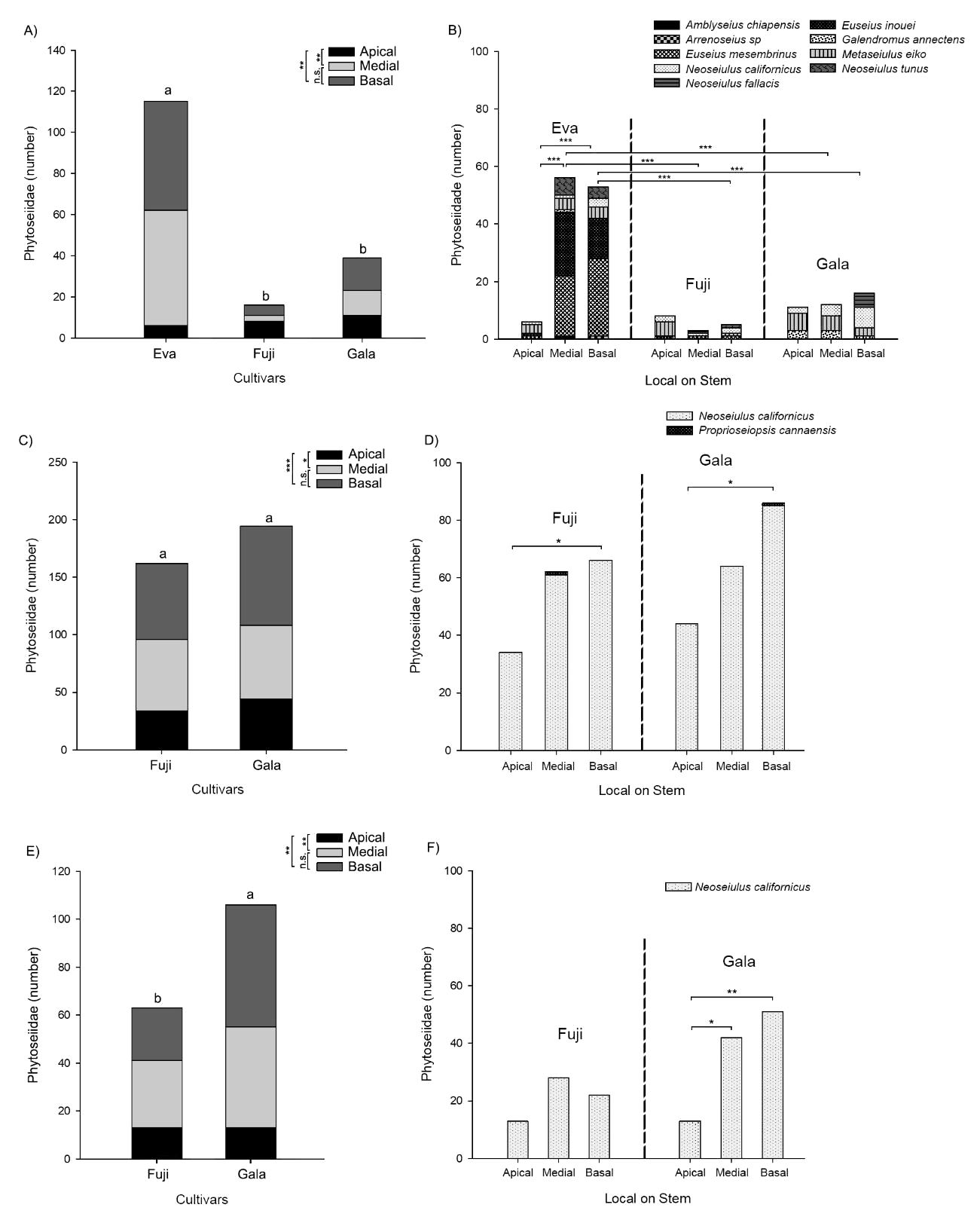
Within the orchards of organic cultivation, a higher Phytoseiidae number was observed on the cultivar Eva, while no difference was observed between Fuji and Gala varieties (Figure 7A; GLM: F2,350 = 29.60, P < 0.0001). In these same orchards, we observed a higher mite abundance at the median and basal levels than at apical one (Figure 7A; GLM: F2,350 = 8.25, P = 0.0003), whereas no difference in relation to the stratum of the branch was noted for the Fuji and Gala cultivars (Figure 7B; GLM: F2,117 = 12.01, P < 0.0001). Within orchards under conventional management, the density Phytoseiidae was not significantly different between the varieties Fuji and Gala, but their abundance was lower at the apical level than on basal part, median level showing intermediate densities (Figure 7C; GLM: F2,234 = 7.66, P = 0.0006). Within orchards under regenerative management, the abundance of Phytoseiidae was significantly different between the Fuji and Gala varieties (Figure 7E; GLM: F1,233 = 6.22, P = 0.0133). The density was significantly lower at the apical level than at basal and median ones (Figure 7F; GLM: F2,233 = 6.76, P = 0.0014).


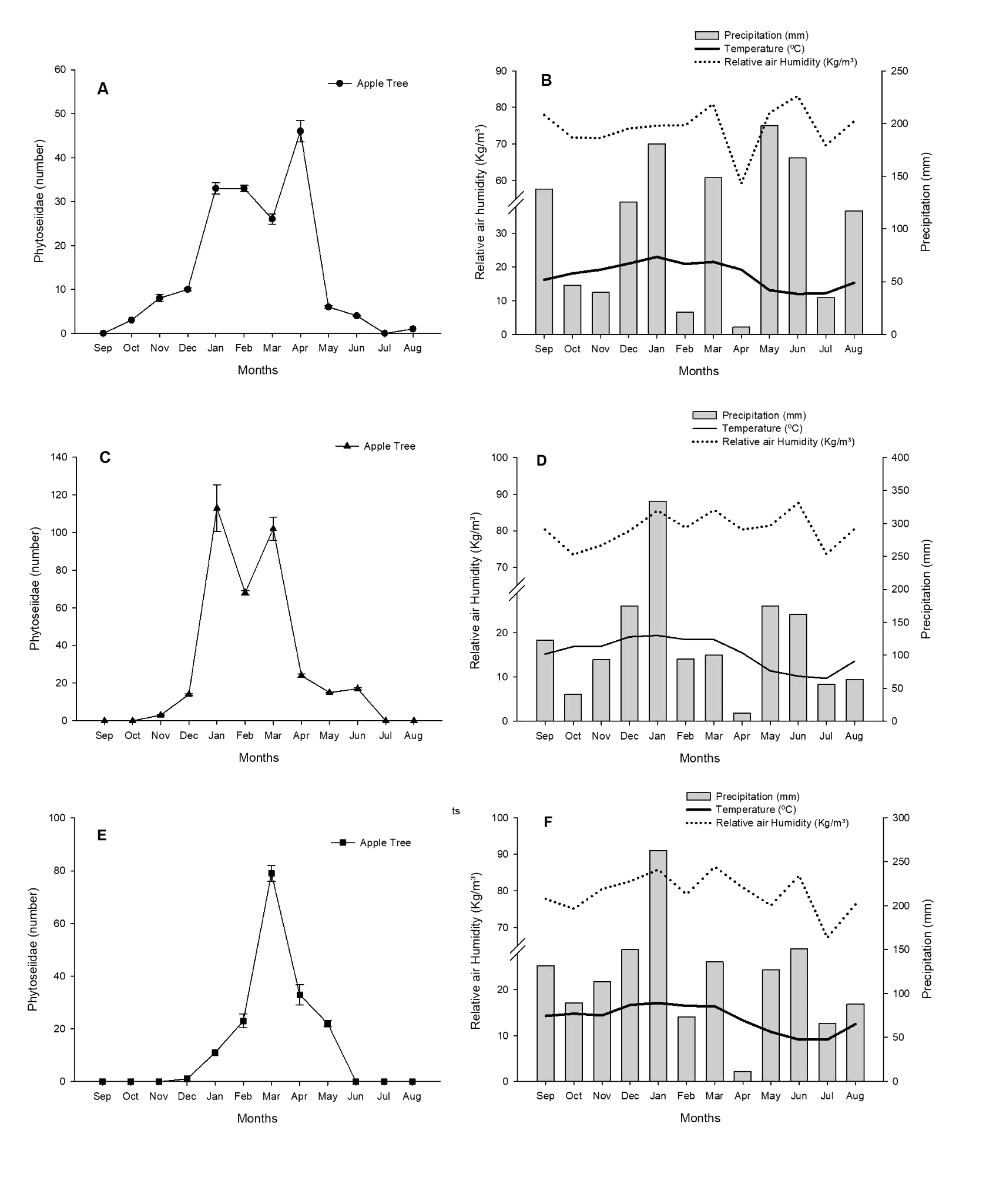
The annual average precipitation, temperature and relative humidity in Antônio Prado were 102.075 mm, 17.64 °C and 74.40%; in Muitos Capões 118.975 mm, 15.41 °C and 80.43% and in São Joaquim 116.56 mm, 13.75 °C and 79.33%, respectively (EMBRAPA 2021). The stepwise analysis showed that the relative humidity variable affected the dynamics of the Phytoseiidae population in the area with conventional management (F3,8 = 4.39; P = 0.0419). This variable explained 37.21% (R2 = 0.3721) of the variation in abundance. In the area of organic and regenerative management, climatic variables did not interfere with the abundance of these predatory mites (F3,8 = 2.85; P = 0.1051; F3,8 = 1.37; P = 0.3197), respectively (Figure 8).


Discussion
In this study we performed the sampling of phytoseiid mites in different apple production orchards, and we evaluated the richness and abundance of these mites, as well as their distribution in the different strata of the plants. Furthermore, we studied how the different apple tree cultivars affect the diversity and distribution of these predatory mites. We observed that the diversity of Phytoseiidae mites was higher in apple orchards from the municipality of Antônio Prado (organic management) than from the municipalities of Muitos Capões (conventional management) and São Joaquim (regenerative management). Furthermore, the apple tree cultivar also had an important effect on the richness, abundance and distribution of these predatory mites. Neoseiulus californicus was found in all orchards, but with low occurrence in Antônio Prado orchards. In all areas, we did not observe such a significant effect of the abiotic variables of temperature, relative humidity and precipitation on the density of phytoseiid mites throughout the year. There was only a small effect of relative humidity in the conventional management area.
In this study, the apical stratum presented a lower density of predatory mites, being able to indicate that the median and basal regions of the branch could had greater availability of phytophagous mites or some alternative food, such as pollen (Monteiro 1994; Pascua et al. 2020). Furthermore, the apical region may be affected by solar incidence, which causes an increase in temperature on the leaf surface, influencing mite populations. Therefore, the mites could be looking for leaves in the middle and basal regions to settle, where the temperature and humidity would be ideal for their survival (Coombs and Bale 2013).
According to Maeyer et al. (1993), less intensive agricultural management allows for greater conservation of the diversity of natural enemies. Pesticide spraying is one of the main factors responsible for reducing arthropod diversity, especially natural enemies (Kropczynska and Tuovinen 1988; Meyer et al. 2009), while to spontaneous vegetation allows the conservation of the diversity of these biological control agents in agricultural production areas (Altieri 1999, 2002). These plants can provide shelter and food for predatory mites, even in periods with unfavourable abiotic conditions or when prey is scarce (Landis et al. 2000; Tixier et al. 2000; Demite and Feres 2005). Even if spontaneous plants were present in the inter-rows of the regenerative management systems (São Joaquim), this area did not show greater diversity than the conventional management area (Muitos Capões) (where spontaneous plants were removed). As in both areas, pesticides were used (conventional area more frequently than the regenerative area), it could be assumed that the low Phytoseiidae in these orchards compared to the orchards under organic management (Antônio Prado) is related to the use of pesticides. However, the abundance of Phytoseiidae mites was higher in the conventional orchards than in the others. This result was due to the large number of individuals of N. californicus, a species that occasionally uses pollen as food (Pascua et al. 2020). The ability of a predatory mite to feed on alternative diets, particularly pollen, may limit the sublethal effects of pesticides (Pozzebon et al. 2014). However, the most probable hypothesis is that N. californicus might be resistant to pesticides, as already mentioned in other studies (Moraes et al. 2004; Meyer et al. 2009; Van Lenteren 2012; Yorulmaz-Salman and Ay 2013; Tixier et al 2013, 2018; Ghazy et al. 2016; Knapp et al. 2018; Inak and Yorulmaz-Salman 2022).
The multivariate analysis grouped the orchards that use pesticides (Muitos Capões and São Joaquim) into one cluster and the organic cultivation areas (Antônio Prado) into two other clusters, one being Fuji and Gala, and the other with Eva. These groupings demonstrated the different composition of acarine species in these areas, with N. californicus, one of the main species present in orchards that use pesticides. Even if densities of N. californicus were higher in areas using pesticides, this species was also observed in all orchards evaluated in this study. In areas where these products were not used, the number of individuals of N. californicus was lower than in areas that use pesticides. A possible explanation is that without interference from pesticides, N. californicus is not able to thrive due to greater interaction with other predators. A study of lethal and non-lethal intraguild interactions between Euseius stipulatus (Athias-Henriot) and N. californicus showed that the first species negatively affected the population of the second, while the abundance of E. stipulatus was not affected by the introduction of a second predator (Abad-Moyano et al. 2010). A more in-depth study on the impact of N. californicus dominance is necessary, following pest populations in a long-term effect in areas with pesticide use, as well as studying the intraguild interaction between predators in areas without use of these chemicals.
In the organic management areas (Antônio Prado), we observed differences between the composition of the Phytoseiidae mite community between the different cultivars, where Eva had the highest number of species. Furthermore, Phytoseiidae abundance differs between Fuji and Gala cultivars. The plant architecture can affect mites in several ways: (i) species interactions (increase or reducing predation, cannibalism or competition) and (ii) pollen quantity (Kreiter et al. 2002; Schmidt 2014). The three cultivars considered have different leaf hairiness, which can have affected the density of Phytoseiidae mites.
Considering only the cultivar Eva, we observed that E. mesembrinus and E. inouei were constant and eudominant. Some species use fungal spores or pollen as a food source, in the absence of the prey mites (Gambaro 1998; Croft et al. 2004). This is the case of predatory mites of the genus Euseius, classified as generalist types IV, meaning that these predators develop well when feeding on pollen (McMurtry et al. 2013). The structural complexity of the leaf favors populations of Phytoseiidae type IV, by increasing pollen retention (Kreiter et al. 2002) on the surface, providing an alternative source of food in times of scarcity of prey (Schmidt 2014). There is a positive correlation between pollen retention and trichome density (Kreiter et al. 2002). However, it is necessary to carry out controlled studies to more accurately assess the effects of these structures on the behavior of these predators in apple plants.
This study shows the importance of producers considering the less intensive use of pesticides and herbicides in orchards in order to maintain populations of predatory mites due to their potential as agents acting in ecological services. Furthermore, the results suggest that the native fauna is able to maintain A. schlechtendali populations at acceptable levels for the cultivars studied.
Acknowledgements
The authors are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for their financial support and research fellowships (PQ process n°313658/2020-0) and Universidade do Vale do Taquari (Univates) for supporting this research. We also thank Tairis da Costa, Julia R. Schneider and Antônio de A. Paz-Neto for helping on the statistical analysis; Daniele Mallmann and Guilherme A. Spohr for helping on the sample collection; Iury S. de Castro for making the maps with the collection points. To the producers Varaschin Agro, Gilmar Bellé and Zani Luiz Fabre, by the availability of the areas during the collection period. This study was partially financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) – Financial code 001.
Author's contribution
PAR, MS and GLB with the sample collections, sample sorting on plants, slide mounting and identification; GLB and MS with morphological analysis; PAR with the analysis and concept of the manuscript; NJF with revisions.
References
- Abad-Moyano R., Urbaneja A., Hoffmann D., Schausberger P. 2010. Effects of Euseius stipulatus on establishment and efficacy in spider mite suppression of Neoseiulus californicus and Phytoseiulus persimilis in clementine. Exp. Appl. Acarol., 50: 329-341. https://doi.org/10.1007/s10493-009-9320-9
- Altieri M.A. 1999. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ., 74: 19-31. https://doi.org/10.1016/B978-0-444-50019-9.50005-4
- Altieri M.A. 2002. Agroecology: the science of natural resource management for poor farmers in marginal environments. Agric. Ecosyst. Environ., 93: 1-24. https://doi.org/10.1016/S0167-8809(02)00085-3
- Camargo Barbosa M.F., Demite P.R. 2023. A new species of Arrenoseius Wainstein (Mesostigmata: Phytoseiidae) from Brazil, with a world key to the genus. Int. J. Acarology, 49: 49-53. https://doi.org/10.1080/01647954.2023.2178505
- Caprio E., Nervo B., Isaia M., Allegro G., Rolando A. 2015. Organic versus conventional systems in viticulture: Comparative effects on spiders and carabids in vineyards and adjacent forests. Agric. Syst., 136: 61-69. https://doi.org/10.1016/j.agsy.2015.02.009
- Campbell B.M., Beare D.J., Bennett E.M., Hall-Spencer J.M., Ingram J.S., Jaramillo F., Shindell D. 2017. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc., 22(4): 1-8. https://doi.org/10.5751/ES-09595-220408
- Chant D.A., McMurtry J.A. 1994. A review of the subfamilies Phytoseiinae and Typhlodrominae (Acari: Phytoseiidae). Int. J. Acarology, 20: 223-310. https://doi.org/10.1080/01647959408684022
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House. 220 pp.
- Chao A., Gotelli N.J., Hsieh T.C., Sander E.L., Ma K.H., Colwell R.K., Ellison A.M. 2014. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monog., 84: 45-67. https://doi.org/10.1890/13-0133.1
- Coombs M.R., Bale J.S. 2013. Comparison of thermal activity thresholds of the spider mite predators Phytoseiulus macropilis and Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol., 59: 435-445. https://doi.org/10.1007/s10493-012-9619-9
- Crowder D.W., Jabbour R. 2014. Relationships between biodiversity and biological control in agroecosystems: current status and future challenges. Biol. Control., 75:8-17. https://doi.org/10.1016/j.biocontrol.2013.10.010
- Croft B.A., McGroarty D.L. 1977. The role of Amblyseius fallacis (Acarina: Phytoseiidae) in Michigan apple orchards. Res. Rep., 333: 2-22.
- Croft B.A., Blackwood J.S., McMurtry J.A. 2004. Classifying life-style types of phytoseiid mites: diagnostic traits. Exp. Appl. Acarol., 33: 247-260. https://doi.org/10.1023/B:APPA.0000038622.26584.82
- Dalkvist T., Topping C.J., Forbes V.E. 2009. Population-level impacts of pesticide-induced chronic effects on individuals depend more on ecology than toxicology. Ecotoxicol. Environ. Saf., 72: 1663-1672. https://doi.org/10.1016/j.ecoenv.2008.10.002
- De Leon D. 1961. Eight new Amblyseius from Mexico with collection notes on two other species (Acarina: Phytoseiidae). Fla. Entomol., 44: 85-91. https://doi.org/10.2307/3492318
- Demite P.R., Feres R.J. 2005. Influência de vegetação vizinha na distribuição de ácaros em seringal (Hevea brasiliensis Muell. Arg., Euphorbiaceae) em São José do Rio Preto, SP. Neotrop. Entomol., 34: 829-836. https://doi.org/10.1590/S1519-566X2005000500016
- Demite P.R., McMurtry J.A., De Moraes G.J. 2014. Phytoseiidae Database: a website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa, 3795: 571-577. https://doi.org/10.11646/zootaxa.3795.5.6
- EMBRAPA. 2021. Dados meteorológicos. [20 Dec 2021]. Available from: https://www.embrapa.br/uva-e-vinho/dados-meteorologicos
- Ferla N.J., Marchetti M.M., Gonçalves D. 2007. Predatory mites (Acari) associated with strawberry and neighboring plants in the State of Rio Grande do Sul. Biota Neotrop., 7: 103-110. https://doi.org/10.1590/S1676-06032007000200012
- Ferla N.J., Silva D.E., Navia D., Nascimento J.M., Johann L., Lillo E. 2018. Occurrence of the quarantine mite pest Aculus schlechtendali (Acari: Eriophyidae) in apple orchards of Serra Gaúcha, Rio Grande do Sul state, Brazil. Syst. Appl. Acarol., 23: 1190-1198. https://doi.org/10.11158/saa.23.6.14
- Fioravanço J.C., Girardi C.L., Czermainski A.B.C., Silva G.A., Nachtigall G.R., Oliveira P.R.D. 2010. Cultura da macieira no Rio Grande do Sul: análise situacional e descrição varietal. Embrapa Uva e Vinho-Documentos (INFOTECA-E). [25 July 2022]. Available from: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/864891/cultura-da-macieira-no-rio-grande-do-sul-analise-situacional-e-descricao-varietal-folder
- Friebe B. 1983. Zur Biologie eines Buchenwaldbodens. 3. Die Kaferfauna. Beitrage zur naturkundlichen Forschung in Sudwestdeutschland. Beihefte.
- Gambaro P.I. 1998. Natural alternative food for Amblyseius andersoni Chant (Acarina: Phytoseiidae) on plants without prey. Redia. 71: 161-171.
- Geiger F., Bengtsson J., Berendse F., Weisser W.W., Emmerson M., Morales M.B., Ceryngier P., Liira J., Tscharntke T., Winqvist C., Eggers S., Bommarco R., Pärt T., Bretagnolle V., Plantegenest M., Clement L.W., Dennis C., Palmer C., Oñate J.J., Guerrero I., Hawro V., Aavik T., Thies C., Flohre A., Hänke S., Fisher C., Goedhart P.W., Inchausti P. 2010. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol., 11: 97-105. https://doi.org/10.1016/j.baae.2009.12.001
- Ghazy N.A., Osakabe M., Negm M.W., Schausberger P., Gotoh T., Amano H. 2016. Phytoseiid mites under environmental stress. Biol. Control., 96: 120-134. https://doi.org/10.1016/j.biocontrol.2016.02.017
- Gomiero T., Pimentel D., Paoletti M.G. 2011. Environmental impact of different agricultural management practices: conventional vs. organic agriculture. Crit. Rev. Plant. Sci., 30: 95-124. https://doi.org/10.1080/07352689.2011.554355
- Gomiero T. 2018. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil Ecol., 123: 714-728. https://doi.org/10.1016/j.apsoil.2017.10.014
- Guedes R.N.C., Smagghe G., Stark J.D., Desneux N. 2016. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol., 61: 43-62. https://doi.org/10.1146/annurev-ento-010715-023646
- Hardman J.M., Franklin J.L., Moreau D.L., Bostanian N.J. 2003. An index for selective toxicity of miticides to phytophagous mites and their predators based on orchard trials. Pest Manag. Sci. formerly Pesticide Science. 59: 1321-1332. https://doi.org/10.1002/ps.769
- Hill M.O. 1973. Diversity and evenness: A unifying notation and its consequences. Ecol., 54: 427-432. https://doi.org/10.2307/1934352
- Inak E., Yorulmaz-Salman S. 2022. Insecticide resistance mechanisms in predatory mites. Int. J. Pest Manag., 68: 192-198. https://doi.org/10.1080/09670874.2020.1817619
- Janssen A., van Rijn P.C. 2021. Pesticides do not significantly reduce arthropod pest densities in the presence of natural enemies. Ecol. Lett., 24: 2010-2024. https://doi.org/10.1111/ele.13819
- Jeppson L.R., Keifer H.H., Baker E.W. 1975. Mites injurious to economic plants. University of California Press. 614 pp. https://doi.org/10.1525/9780520335431
- Kasap İ., Atlihan R. 2021. Population growth performance of Panonychus ulmi Koch (Acarina: Tetranychidae) on different fruit trees. Syst. Appl. Acarol., 26: 1185-1197. https://doi.org/10.11158/saa.26.7.1
- Kassambara A., Mundt F. 2020. Factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0.7. 2020. https://CRAN.R-project.org/package=factoextra
- Khajuria D.R. 2009. Predatory complex of phytophagous mites and their role in integrated pest management in apple orchard. J. Biopestic., 2: 141-144.
- Kist B.B. et al. 2015. Anuário Brasileiro da Maçã. Editora Gazeta, Santa Cruz Sul, 72 pp.
- Knapp M., Van Houten Y., Van Baal E., Groot T. 2018. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia, 58 (Suppl.): 72-82. https://doi.org/10.24349/acarologia/20184275
- Kreiter S., Tixier M.-S., Croft B.A., Auger P., Barret D. 2002. Plants and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari: Phytoseiidae) in habitats surrounding vineyards. Environ. Entomol., 31(4), 648-660. https://doi.org/10.1603/0046-225X-31.4.648
- Kropczyńska D., Tuovinen T. 1988. Occurrence of Phytoseiid mites (Acari: Phytoseiidae) on apple trees in Finland. In: Ann Agric Fenn. 27(4): 305-314.
- Landis D.A., Wratten S.D., Gurr G.M. 2000. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol., 45: 175-201. https://doi.org/10.1146/annurev.ento.45.1.175
- Lê S., Josse J., Husson F. 2008. FactoMineR: An R package for multivariate analysis. J. Stat. Softw., 25: 1. https://doi.org/10.18637/jss.v025.i01
- Letourneau D.K., Armbrecht I., Salguero R.B., Montoya L.J., Jimenez C.E., Constanza D.M., Escobar S., Galindo V., Gutiérrez C., Duque S.L., López M.J., Acosta M.R.A., Herrera R.J., Rivera L., Arturo C.S., Marina A.T., Reyes A.T. 2011. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl., 21: 9-21. https://doi.org/10.1890/09-2026.1
- Liere H., Jha S., Philpott S.M. 2017. Intersection between biodiversity conservation, agroecology, and ecosystem services. Agroecol. Sustain. Food Syst., 41: 723-760. https://doi.org/10.1080/21683565.2017.1330796
- Lorenzato D., Secchi V.A. 1993. Controle biológico de ácaros da macieira no Rio Grande do Sul: 1- Ocorrência e efeitos dos ácaros fitófagos e seus inimigos naturais em pomares submetidos ao controle biológico e com acaricidas. Rev. Bras. Frutic., 15: 211-220.
- Maeyer L., Vinvinaux C., Berge C., Van Den Merkens W., Peumans H., Verreydt J., Sterk G. 1993. Usefulness of tolylfluanid in integrated pest control on apples as a regulator of the mite complex equilibrium and with contribution to intrinsic fruit quality of apple cv Jonagold. Acta Hortic. 3:253-264. https://doi.org/10.17660/ActaHortic.1993.347.28
- McMurtry J.A., De Moraes G.J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Meyer G.A., Kovaleski A., Valdebenito-Sanhueza R.M. 2009. Seletividade de agrotóxicos usados na cultura da macieira a Neoseiulus Californicus (McGregor) (Acari: Phytoseiidae). Rev. Bras. Frutic., 31: 381-387. https://doi.org/10.1590/S0100-29452009000200011
- Monetti L.N., Fernandez N.A. 1996. Seasonal population dynamics of the European red mite (Panonychus ulmi) and its predator Neoseiulus californicus in a sprayed apple orchard in Argentina (Acari: Tetranychidae, Phytoseiidae). Acarologia., 36: 325-331.
- Monteiro L.B. 1994. Manejo integrado de Panonychus ulmi em macieira. Primeiras experiências com a introdução de Neoseiulus californicus. Rev. Bras. Frutic., 16: 46-53.
- Moraes G.J., McMurtry J.A., Denmark H.A., Campos C.B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa., 434: 1-494. https://doi.org/10.11646/zootaxa.434.1.1
- Möth S., Walzer A., Redl M., Petrović B., Hoffmann C., Winter S. 2021. Unexpected effects of local management and landscape composition on predatory mites and their food resources in vineyards. Insects, 12(2): 180. https://doi.org/10.3390/insects12020180
- Nascimento J.M., Silva D.E., Pavan A.M., Corrêa L.L.C., Schussler M., Johann L., Ferla N.J. 2020. Abundance and distribution of Aculus schlechtendali on apple orchards in Southern of Brazil. Acarologia, 60: 659-667. https://doi.org/10.24349/acarologia/20204394
- Oksanen J., Kindt R., Simpson G.L., Oksanen M.J. 2018. Package ′vegan3d'. R package version, 1-0.
- Pascua M.S., Rocca M., Greco N., De Clercq P. 2020. Typha angustifolia L. pollen as an alternative food for the predatory mite Neoseiulus californicus (McGregor) (Acari: Phytoseiidae). Syst. Appl. Acarol. 25: 51-62. https://doi.org/10.11158/saa.25.1.4
- Pozzebon A., Ahmad S., Tirello P., Lorenzon M., Duso C. 2014. Does pollen availability mitigate the impact of pesticides on generalist predatory mites? Biocontrol, 59: 585-596. https://doi.org/10.1007/s10526-014-9598-3
- R CORE TEAM. 2023. R: A language and environment for statistical computing. Vienna, Austria: The R Foundation for Statistical Computing. [26 Jan 2023]. Available from: http://www.r-project.org/
- Raudonis L., Valiuskaite A., Surviliene E. 2007. Toxicity of Abamectin to rust mite, Aculus schlechtendali (Acari: Eriophyidae) in apple tree orchard. Sodinink Darzinink., 26: 10-17.
- Rodrigues W.C. 2017. Referências deste Guia. DivEs - Diversidade de Espécies v.4.0 (AntSoft Systems On Demand) - Guia do Usuário.
- Sánchez-Bayo F. 2021. Indirect effect of pesticides on insects and other arthropods. Toxics, 9:177. https://doi.org/10.3390/toxics9080177
- SAS Institute. 2002. SAS/STAT User's guide, version 8.02, TS level 2 MO. Cary (NC): SAS Institute Inc
- Schmidt R.A. 2014. Leaf structures affect predatory mites (Acari: Phytoseiidae) and biological control: a review. Exp. Appl. Acarol., 62: 1-17. https://doi.org/10.1007/s10493-013-9730-6
- Silveira-Neto S., Nakano O., Barbin D., Nova N.A.V. 1976. Manual de ecologia dos insetos. São Paulo, Agronômicas Ceres. 419 pp.
- Smith-Spangler C., Brandeau M.L., Hunter G.E., Bavinger J.C., Pearson M., Eschbach P.J., Sundaram V., Liu H., Schirmer P., Stave C., Olkin I., Bravata D.M. 2012. Are organic foods safer or healthier than conventional alternatives? A systematic review. Ann. Intern. Med., 157:348-366. https://doi.org/10.7326/0003-4819-157-5-201209040-00007
- Sumberg J., Giller K.E. 2022. What is `conventional' agriculture? Glob. Food Sec. 32, 100617. https://doi.org/10.1016/j.gfs.2022.100617
- The Soil Association. 2021. Soil Association Standards: Farming and Growing. vo.18.6 (Version 18.6). Updated on 12th February 2021. The Soil Association, Bristol. [01 Mar 2023]. Available from: https://policycommons.net/artifacts/1798421/soil-association-standards-farming-and-growing-version-186/2530065/fragments/
- Tixier M.-S., Kreiter S., Auger P., Sentenac G., Salva G., Weber M. 2000. Phytoseiid mite species located in uncultivated areas surrounding vineyards in three French regions. Acarologia, 41: 127-140.
- Tixier M.-S., Baldassar A., Duso C., Kreiter S. 2013. Phytoseiidae in European grape (Vitis vinifera L.): bio-ecological aspects and keys to species (Acari: Mesostigmata). Zootaxa, 3721(2): 101-142. https://doi.org/10.11646/zootaxa.3721.2.1
- Tixier M.-S. 2018. Predatory mites (Acari: Phytoseiidae) in agro-ecosystems and conservation biological control: a review and explorative approach for forecasting plant-predatory mite interactions and mite dispersal. Front. Ecol. Evol., 6: 192. https://doi.org/10.3389/fevo.2018.00192
- Tóthmérész B. 1995. Comparison of different methods for diversity ordering. J. Veg. Sci., 6: 283-290. https://doi.org/10.2307/3236223
- Toyoshima S., Yaginuma K., Ihara F., Arai T., Takanashi M. 2011. The succession of phytophagous and phytoseiid species in a newly planted apple orchard without insecticide application. J. Acarol. Soc. Jpn., 20: 77-86. https://doi.org/10.2300/acari.20.77
- Van Lenteren J.C. 2012. The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl, 57: 1-20. https://doi.org/10.1007/s10526-011-9395-1
- Veres A., Petit S., Conord C., Lavigne C. 2013. Does landscape composition affect pest abundance and their control by natural enemies? A review. Agric. Ecosyst. Environ., 166: 110-117. https://doi.org/10.1016/j.agee.2011.05.027
- Walker J.T., Suckling D.M., Wearing C.H. 2017. Past, present, and future of integrated control of apple pests: the New Zealand experience. Annu. Rev. Entomol., 62: 231-248. https://doi.org/10.1146/annurev-ento-031616-035626
- Willer H., Travnicek J., Meier C., Schlatter B. 2021. The World of Organic Agriculture: Statistics and Emerging Trends. Research Institute of Organic Agriculture FiBL and IFOAM - Organics International, Frick and Bonn. 336 pp.
- Yorulmaz-Salman S., Ay R. 2013. Ay Avcı akar Neoseiulus californicus (Acari: Phytoseiidae) popülasyonlarının üç farklı akarisite karşı duyarlılık ve detoksifikasyon enzim düzeylerinin belirlenmesi Türk. entomol. derg., 37:105-116. https://doi.org/10.1501/Tarimbil_0000001246
- Zhao Q., De Laender F., Van den Brink P.J. 2020. Community composition modifies direct and indirect effects of pesticides in freshwater food webs. Sci. Total Environ., 739, 139531. https://doi.org/10.1016/j.scitotenv.2020.139531
- Zhu Z., Jia Z., Peng L., Chen Q., He L., Jiang Y., Ge S. 2018. Life cycle assessment of conventional and organic apple production systems in China. J. Clean. Prod., 201: 156-168. https://doi.org/10.1016/j.jclepro.2018.08.032



2023-11-24
Date accepted:
2024-01-31
Date published:
2024-02-20
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Rode, Priscila de Andrade; Schussler, Matheus; Bizarro, Gabriel Lima and Ferla, Noeli Juarez
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



