Free-living mites (Acari) of the Franz Josef Land Archipelago, the coldest Old World territory: diversity, geographic distributions, assemblages
Makarova, Olga L.  1
1
1✉ Laboratory of Synecology, Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow 119071, Russia.
2023 - Volume: 63 Issue: 4 pages: 1163-1186
https://doi.org/10.24349/p6wb-pcniOriginal research
Keywords
Abstract
Introduction
Tracing the trends in the faunal composition and ecology of certain larger groups along a latitudinal gradient, up to its northern range limit in polar desert landscapes, is of special interest (Chernov 2002). There are only a few publications where the ''extreme'' set of species has been analyzed for higher arthropod taxa (McAlpine 1965; Bulavintsev and Babenko 1983; Makarova 2002a, b; Makarova et al. 2007, 2013, 2014; Chernov and Makarova 2008; Krasheninnikov and Gavrilo 2014; Babenko 2018). Most of the polar desert areas are insular territories situated far from mainland areas (Figure 1A), thus being hardly accessible for researchers. This is why the invertebrate fauna of the Franz Josef Land (FJL) Archipelago, the northernmost in the Old World, has remained scarcely known (Coulson et al. 2014).



The archipelago was discovered in 1873 by the Austrian-Hungarian Polar Expedition (Boyarskiy 2013). The first information on the invertebrates living here came from the Jackson-Harmswoth-Expedition of 1896 and referred to a spider, Erigone psychrophila Thorell, 1871 found on the Northbrook Isl. (Pickard-Cambridge 1898). For almost a century there were no additional investigations concerning FJL invertebrates. Besides the remoteness, the islands became closed to foreigners from the early 1930's to the beginning of 1990's (\urlbib{https://arctic.narfu.ru/infologia-arktiki/geo-arktiki/zemlya-frantsa-iosifa-samyj-severnyj-arkhipelag-na-nashej-planete}).\normalsize They were visited by Russian biologists very rarely, mainly as members of military or topographic expeditions. Quite surprising, the next information on terrestrial arthropods was published only in 1983 (Bulavintsev and Babenko 1983). As a consequence, the terrestrial invertebrate fauna of the archipelago is more poorly known than that of the other archipelagoes of the Barents Sea, such as Svalbard and Novaya Zemlya (Coulson et al. 2014).
In 1994, the FJL was declared a wildlife sanctuary and since 2010 it has become part of the ''The Russian Arctic'' National Park (http://www.rus-arc.ru ![]() ). Since then, regular expeditions have been organized by the Park's administration and the Northern Arctic Federal University, both based at Arkhangelsk (https://www.rgo.ru/en/projects/arctic-floating-university
). Since then, regular expeditions have been organized by the Park's administration and the Northern Arctic Federal University, both based at Arkhangelsk (https://www.rgo.ru/en/projects/arctic-floating-university ![]() ). As a result, we have obtained acarological material that originated from seven islands and collected by four researchers during five expeditions (Figure 1B). The objective of this contribution is to discuss the taxonomic composition and geographic distributions of FJL free-living mites, as well as the structure of their assemblages in the Tikhaya Bay (Hooker Island) and Cape Flora (Northbrook Island).
). As a result, we have obtained acarological material that originated from seven islands and collected by four researchers during five expeditions (Figure 1B). The objective of this contribution is to discuss the taxonomic composition and geographic distributions of FJL free-living mites, as well as the structure of their assemblages in the Tikhaya Bay (Hooker Island) and Cape Flora (Northbrook Island).
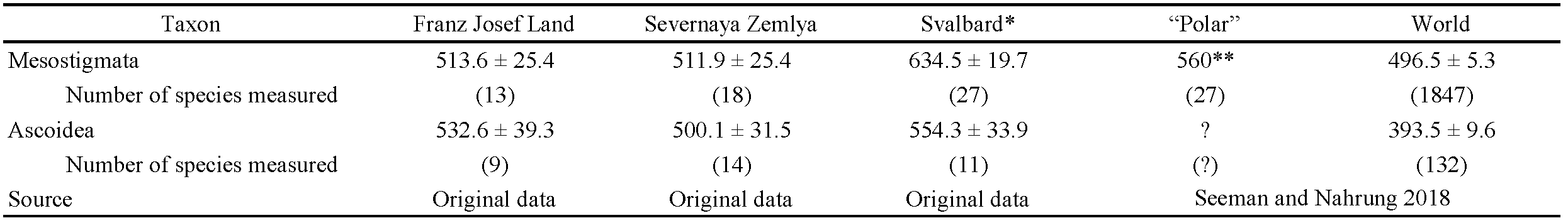
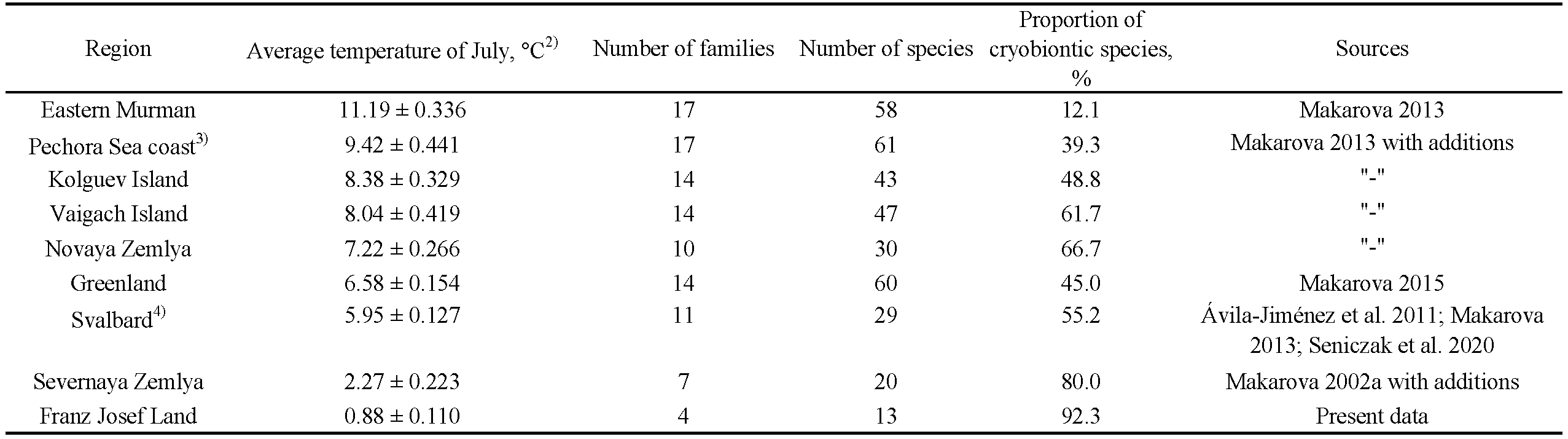
Given that the FJL is the northernmost terrain in the entire Eastern Hemisphere, some latitudinal trends in and features of acarofauna can be suggested to appear there to the greatest extent. So, in particular, I test here the following hypotheses: (1) the mite diversity of FJL is the smallest among the known High Arctic faunas of the Palaeartic (cf. Makarova 2002a; Seniczak et al. 2020); (2) the number of Arachnida species is higher than that of Insecta (cf. McAlpine 1965; Makarova 2002a; Chernov 2004); (3) the fauna of the order Mesostigmata includes the maximal proportion of cryobiont species (cf. Makarova 2015; Makarova et al. 2019), and (4) the average size of FJL mesostigmatic mites is maximal in comparison with more southerly faunas (cf. Seeman and Nahrung 2018).
Study area
The Franz Josef Land is the northernmost archipelago in the Eastern Hemisphere (Figure 1A). It is located in the Arctic Ocean, mainly within the Barents Sea, as a compact group of 191 small to very small islands between 79°46′ and 81°52′ north and between 44°52′ and 65°25′ east. The highest elevations reach 600−700 meters above sea level. Extremely cold conditions are responsible for the 85% glaciation of the territory (Boyarskiy 2013). The central parts of most islands are represented by basalt plateaus with steep falling slopes, and their bases are often formed by marine terraces of the Neopleistocene-Holocene age.
The climate of the FJL is classified as Marine Arctic. Landscapes of the archipelago are characterized by an extremely low heat supply with the annual temperature averaging from –10.9 to –12.7 °C (data from four stations) and a very short vegetation season, usually less than 50 days (Boyarskiy 2013). The snow cover reaches 40–42 cm in March, the snowless period lasts only 65–73 days. Mid-July temperatures vary from +0.2 to +1.2 °C (with extremes up to +16 °C), the average temperatures of January-March (March being the coldest month) are between –20 and –24 °C (–50 °С is the absolute minimum). During the whole year, icy events are possible on grounds. Most days in July-August are foggy. The average annual precipitations vary between 295 and 305 mm (Boyarskiy 2013).
The terrestrial vegetation of the FJL is scarce, frost boiled soils often being common on plains and plateaus where the vegetation cover regularly does not exceed 10–50% (Kuliev 2013; Safronova et al. 2020). The biota of the islands is very poor, including from 51 species (Matveyeva et al. 2015) to 57 species and infraspecies (Safronova et al. 2020) of vascular plants, 18 species of nesting birds, and two species of terrestrial mammals (Gavrilo 2013). Yet these are archaic higher taxa (mosses, lichens, algae, nematodes, and collembolans) that maintain their high diversity levels (Matveyeva et al. 2015; Babenko 2018; V. Peneva and M. Elshishka, pers. communication, 11 May 2018). All these are features of the Arctic Polar Desert biome, the smallest on Earth (Chernov 2002, 2004; Chernov and Makarova 2008; Chernov et al. 2011; Matveyeva et al. 2015). However, determining the typical zonal phytocoenoses for the FJL is hardly possible for the mountainous characteristics of the islands. Most of the sampling efforts thus focused on slopes.
Material and methods
Permanent slides with microarthropods mounted from numerous samples collected on different FJL islands were obtained from V.I. Bulavintsev, formerly a member of the Lab of Synecology, Severtsov Institute of Ecology and Evolution RAS, Moscow (Figure 1B, Table 1). Most of the data received from that material were treated earlier and elsewhere (Bulavintsev and Babenko 1983). Even though many of those earlier microscopic slides were in very bad condition (now partly remounted), some mite species could still be reliably identified. The extracted faunistic information was partly published (Makarova 1999, 2000b, 2013) and is referred to in Table 2.





The most comprehensive material from different habitats of two islands of the archipelago became available to me after the first professional sampling of microarthropods conducted by A.B. Babenko (also IPEE RAS) during the voyage of the Floating University on board of the research vessel ''Professor Molchanov'' in July 2015 (Figure 1B, Table 1). The trip was organized by the Northern Arctic Federal University, Arkhangelsk. These samplings are used here in the ecological considerations and assemblage ordination procedures.
In 2016, that material was supplemented by the samples collected by A.A. Semikolennykh (Lomonosov State University, Moscow) for the analysis of the impacts of a bird colony on arctic soils. This collection is used below mainly for taxonomic purposes.
Additional mite specimens were obtained from A.B. Krasheninnikov (Perm State University) who collected arthropods on Salm Island and Hooker Island using pitfall traps in the summer of 2016.
The samples collected by A.B. Babenko and A.A. Semikolennykh originated from the most favorite sites of the archipelago with the greatest floristic diversity and the richest vegetation, namely, the Tikhaya Bay and Cape Flora (Kuliev 2013; Matveyeva et al. 2015). Both sampling plots were situated on nearly south-faced slopes under the bird colonies enriching the underlying soils. Because the vegetation of these slopes is strongly patched due to a heterogenous nano-relief and a melting water supply from an up-slope glacier (Figure 2), our habitat classification is to some extent conventional. We roughly distinguished polar desert-like, bog-like and tundra-like communities, as well as bird rookeries. Bird colonies of the FJL belong to the High-Arctic type and are usually dominated by the little auk (Alle alle), with Brünnich′s guillemot (Uria lomvia) and the black-legged kittiwake (Rissa tridactyla) being numerous (Gavrilo 2013).



In Cape Flora, Northbrook Island (Figure 2A), we distinguished three biotopes at different hypsometric levels:
Bog-like, grass-mossy community (N-Bog ), wet, with a 100% cover of a thick moss layer and grass, the vegetation being dominated by Alopecurus alpinus, Ranunculus sulphureus, Cochlearia groenlandica, Aulacomnium turgidum, Bryum spp. (Figure 2C);
Tundra-like, moss-forb-grassy community (N-Tundra ), moderately humid, well drained, the vegetation cover being 60–100%, dominated by Alopecurus alpinus, Cerastium arcticum, Cochlearia groenlandica, Saxifraga spp., Papaver polare, mosses, lichens (Figure 2B);
Colony (N-Colony ), a community located just under the rookery (Figure 2A), well eutrophied by bird living activity, dominated by Alopecurus alpinus and mosses (Bryum spp., Brachythecium sp., Plagiomnuim sp.).
In Tikhaya Bay, Hooker Island, coastal terraces support some kind of zonal habitats resembling polar desert-like communities (Figure 3A). We distinguish four biotopes along this transect:



Desert-like, polygonal lichen-grass-mossy community (H-Desert ) in a flattened area, moderately drained, with a vegetation cover of 20–60%, dominated by Luzula confusa, Alopecurus alpinus, Aulacomnium turgidum, Racomitrium sp., Stereocaulon alpinum, Thamnolia vermicularis (Figure 3A), locally also by Salix polaris (Figure 3B);
Bog-like, grass-mossy community (H-Bog ), in the lower part of a slope, wet, with a 100% cover of a thick moss layer and grass, the vegetation being dominated by Alopecurus alpinus, Ranunculus sulphureus, Aulacomnium turgidum, Stellaria edwardsii, Cochlearia groenlandica, Bryum spp., Sanionia uncinata (Figure 3C);
Tundra-like, moss-lichen-forb-dwarf willow community (H-Tundra ), moderately humid, well drained, the vegetation cover being 70–100%, dominated by Salix polaris, Alopecurus alpinus, Papaver polare, Cerastium arcticum, Saxifraga spp., Racomitrium sp., Aulacomnium turgidum, Cetraria delisei, Stereocaulon alpinum, Thamnolia vermicularis (Figures 3D, E);
Colony (H-Colony ), nest material of little auks from the rookery above the Polar Station (Figure 3F) and eutrophied grass and moss turf just under the seabird colony on Rubini Rock (north slope), the vegetation being dominated by Alopecurus alpinus and mosses (Pohlia cruda, Polytrichum alpinum, Bryum spp., Racomitrium lanuginosum).
All soil samples collected by A.B. Babenko and A.A. Semikolennykh (each from a squared 5 x 5 cm plot) were taken in series (5–11 samples per biotope). Each included the above-ground vegetation and 125 cm3 of underlying ground in a 0–5 cm layer (both litter and soil). Mites were extracted from the samples in the lab using Tullgren funnels by drying them for 10 days until their complete desiccation with neither additional heating nor light. Microarthropods were fixed with 96% ethanol. Mites were mounted on microscopic slides in Hoyer's medium. The specimens have been deposited in the collections of the Laboratory of Synecology of the IPEE RAS.
A total of 151 ground samples from seven islands of the FJL were investigated (Table 1). Statistics were applied to data deriving from 49 samples (Table 3) collected in series by A.B. Babenko and A.A. Semikolennykh in Cape Flora and Tikhaya Bay that represent the relatively distinct habitats (see above). They yielded 21,700 mite individuals. Mites were identified to the species for all developmental stages excluding members of Cocceupodes and Neoprotereunetes whose poorly distinguished juveniles were divided between species proportionally to the adults.



Statistic procedures were implemented using PAST software (Hammer et al. 2001). The relation between mid-July temperature and both species number and percentage of cryobiontic species in different regions was studied by Linear Regression Analysis. The similarity of mite assemblages in different biotopes was investigated applying the non-metric MDS tools using Spearman Rank Correlation (Rho).
The map of archipelago with sampling points was prepared using the free-soft SAS.Planet (Development team, version 200606.1075).
Data on average July temperature for Table 5 and Figure 9 are taken from the site Weather and Climate (https://www.weatherandclimate.eu/history/ ![]() ). In all cases, the mean and standard error are calculated for 1950–1999.
). In all cases, the mean and standard error are calculated for 1950–1999.
For an analysis of the geographic distributions of cryobiontic Mesostigmata species, we used published information (Makarova 2000b, 2002a, 2013; references in figure legends) and numerous new original records.
The taxonomical hierarchy used in the Table 2 in general follows to the system presented by Krantz and Walter (2009), except that Astigmata was considered traditionally of similar ranking as Oribatida (suborder). The identification of mites was conducted using numerous sources, mainly: Summers (1962), Andre (1980), Zacharda (1980), Strandtmann (1982), Balogh and Mahunka (1983), Behan-Pelletier (1986), Kaźmierski (1998), Kalúz (2000), Makarova (2000a), Gwiazdowicz et al. (2011), Khaustov (2014), Kolodochka and Gwiazdowicz (2014), and Mašan (2022).
There are several approaches to dividing the Arctic into latitudinal zones (see scheme in Chernov and Makarova 2008). Here we accept the partition design suggested by Gorodkov (1935) and consider the marginal natural zone, i.e. the polar desert zone, as a separate biome lying north of the tundra following Korotkevich (1972) and Alexandrova (1983).
Results
Fauna of free-living mites
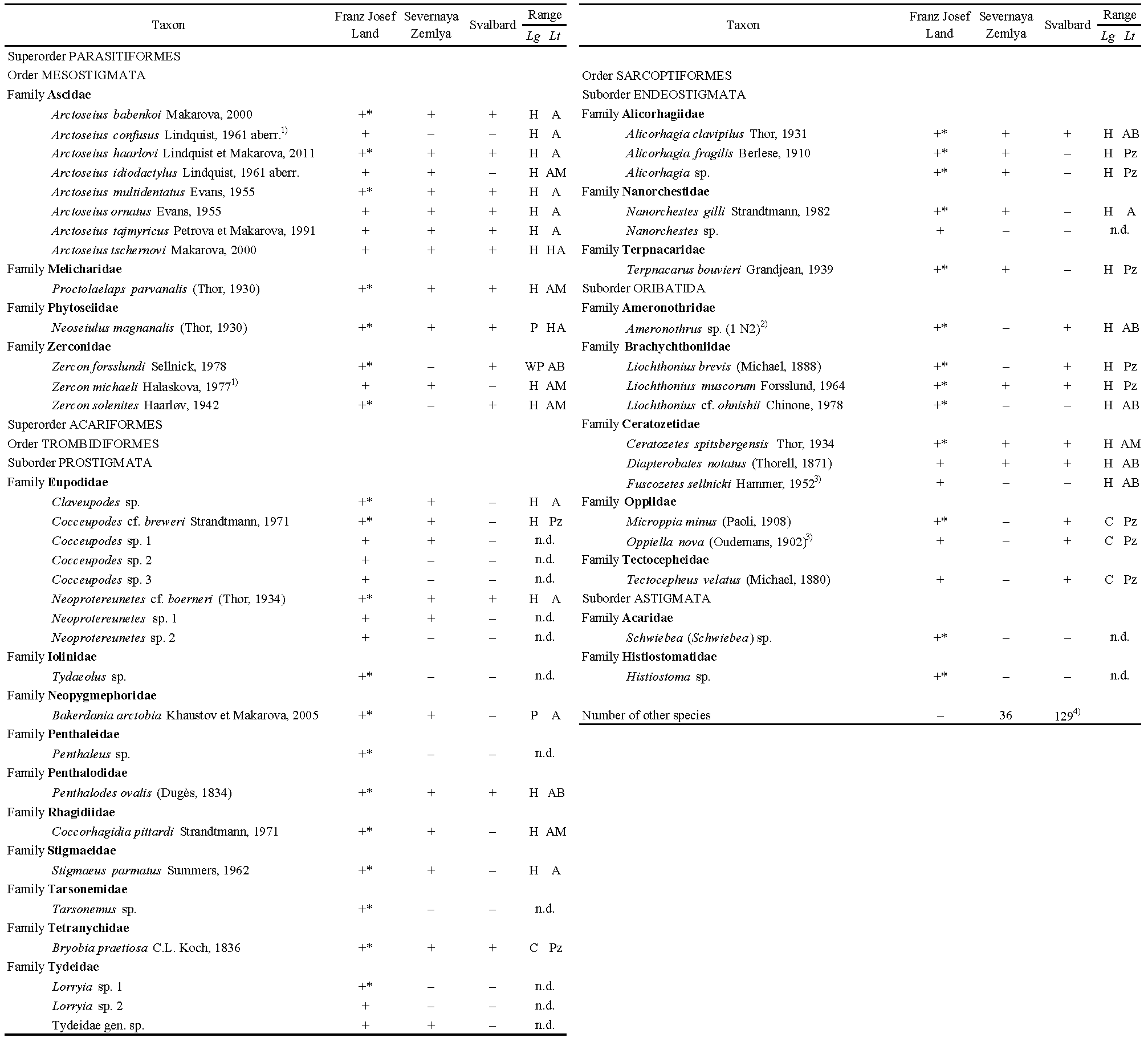
A total of 48 species of free-living mites was recorded in the 151 extracted samples (Table 2). Two additional species, common in the Arctic Oppiella nova and Fuscozetes sellnicki, are included in the list of FJL acarofauna, based on published data (Bulavintsev and Babenko 1983; Krivolutsky and Kalyakin 1993). Records of 16 species (see Discussion) have not been confirmed in the collections by A.B. Babenko and A.A Semikolennykh conducted at the warmest sites of the archipelago (Table 1).
Acarofauna of FJL includes at least 50 species representing 30 genera and 24 families (Table 2). Seventeen families, 22 genera, and 23 species are first found on archipelago. The species collected are distributed among higher taxonomic levels as follows: Mesostigmata – 13, Prostigmata – 19, Endeostigmata – 6, Oribatida – 10, and Astigmata – 2. The most diverse families are Ascidae (8 species) and Eupodidae (8), whereas the most diverse genera are Arctoseius (8 species), Cocceupodes (4), Zercon (3), Neoprotereunetes (3), Alicorhagia (3), and Liochthonius (3). Of the species reported from FJL, 28 species are found from Severnaya Zemlya Archipelago, and at least 22 species are recorded on Svalbard (Table 2). Data on the mite species richness of these three archipelagos are used in the making of Figure 8 (see Discussion).
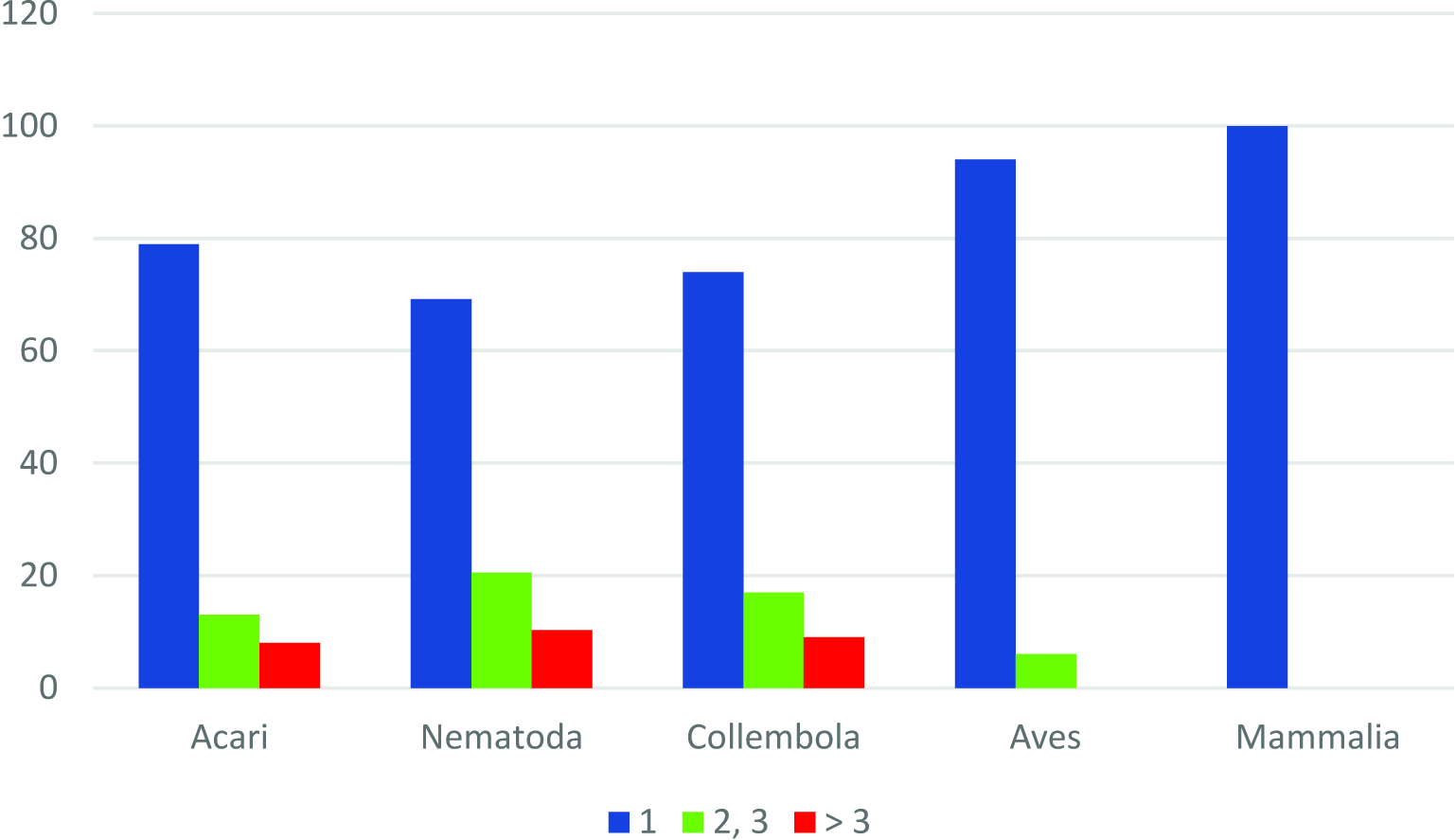
The mite fauna of FJL is extremely fragmentary, meaning that the greater part of the genera and families (almost 76.7% and 62.5%, respectively) are represented only by a single species (Figure 4), and most families (83.3%) include only one genus.


Studied biotopes are populated by 7–34 species of mites each (Table 3). The minimal diversity was found in the bird colony in Cape Flora, whereas the maximal was found in the Tikhaya Bay (tundra-like community and rookery). The latter sites were found to host 29–34 species, accumulating 83.3% of the species listed for the archipelago. A number of the mite species (Arctoseius haarlovi, Proctolaelaps parvanalis, Claveupodes sp., Bryobia praetiosa, Tarsonemus sp., Alicorhagia spp., Terpnacarus bouvieri, Liochthonius spp.) were found only in the tundra-like community and/or rookery. The single sample (5 x 5 x 5 cm) taken from the most floristically rich tundra-like community dominated by dwarf willow, Salix polaris, can support up to 22 mite species (in average 17.3 species per sample). Some rare species were found only in substrata of bird colonies (Arctoseius babenkoi, Penthalodes ovalis, Nanorschestes sp., Histiosoma sp.) or clearly prefer these habitats on both Northbrook and Hooker islands (Bakerdania arctobia).
Structure of mite communities
Abundance of mites in ground of studied biotopes varies strongly, between 123.2 and 3764.0 ind./dm2 being maximal in the tundra-like community of the south-faced slope in Tikhaya Bay. The most abundant species in our collection are Lorryia sp. 1, Liochthonius muscorum, Nanorchestes gilli, and Neoprotereunetes sp. 1 (Table 3).
Non metric MDS procedure revealed differences between most studied biotopes (Figure 5). Mite assemblages of slopes (bogs and tundra-like communities) are more similar to each other and partly overlap. Acarocenoses of the ''desert'' and rookeries are clearly distinct. The colony sites support well separated mite groupings both mainly differentiated by L. muscorum, Ceratozetes spitsbergensis, Diapterobates notatus, Cocceupodes sp. 1 (avoid) and B. arctobia (prefers) (Table 3).



Distributional ranges of species
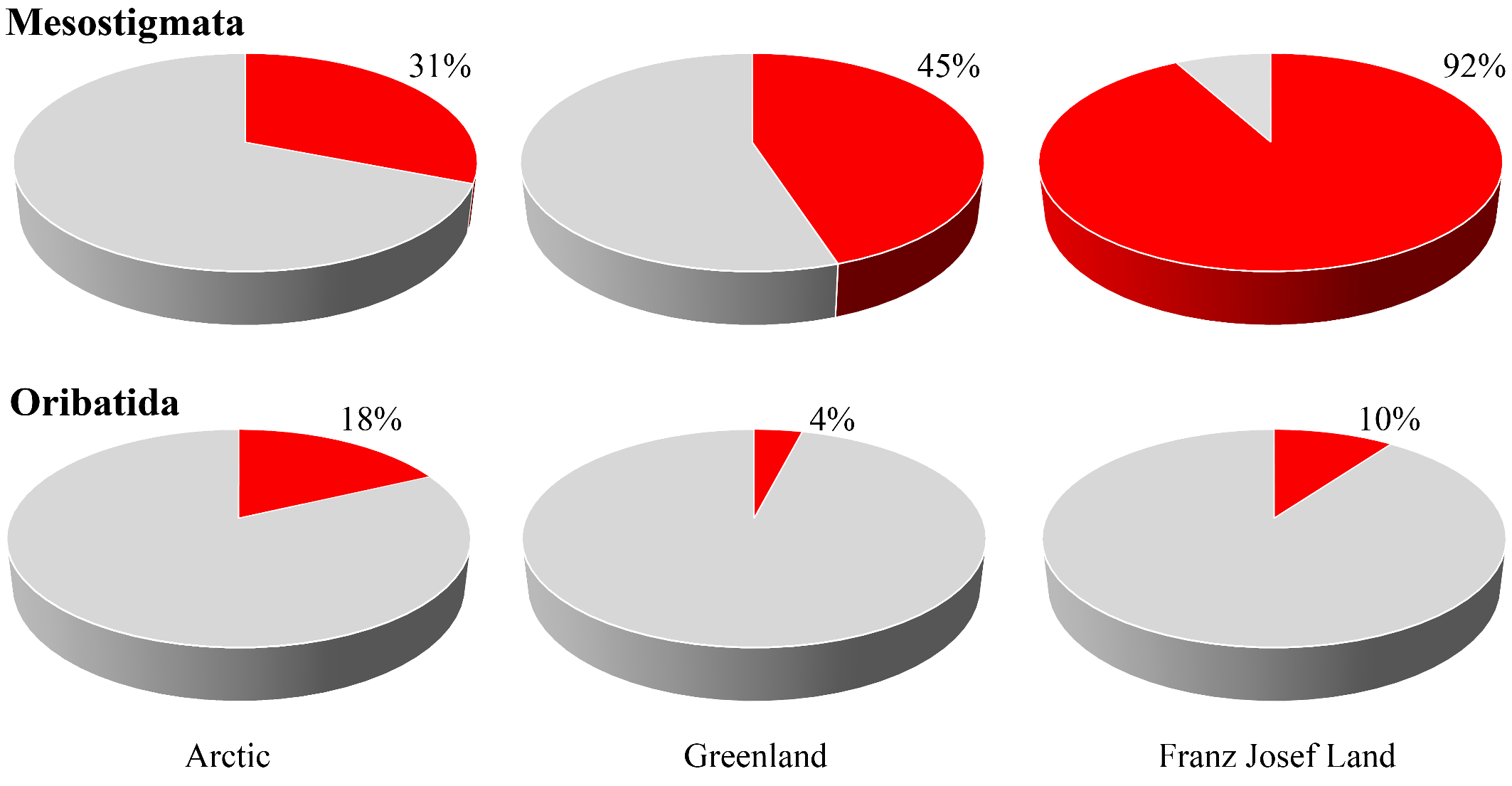
Among 35 identified or recognized (but undescribed) species (13 Parasitiformes, 22 Acariformes) there are 28 Holarctic species (including circumpolar), four cosmopolitan, and three Palaearctic species (Table 2). The percentage of the specialized cryobiontic species (arctic and arctic-montane) differ considerably among higher taxa, accounting for 92.3% in Parasitiformes (Mesostigmata), 36.4% in the Acariformes in general, and only 10.0% in Oribatida. These data were used in the formation of the Table 5 and Figures 9–10 (see Discussion).
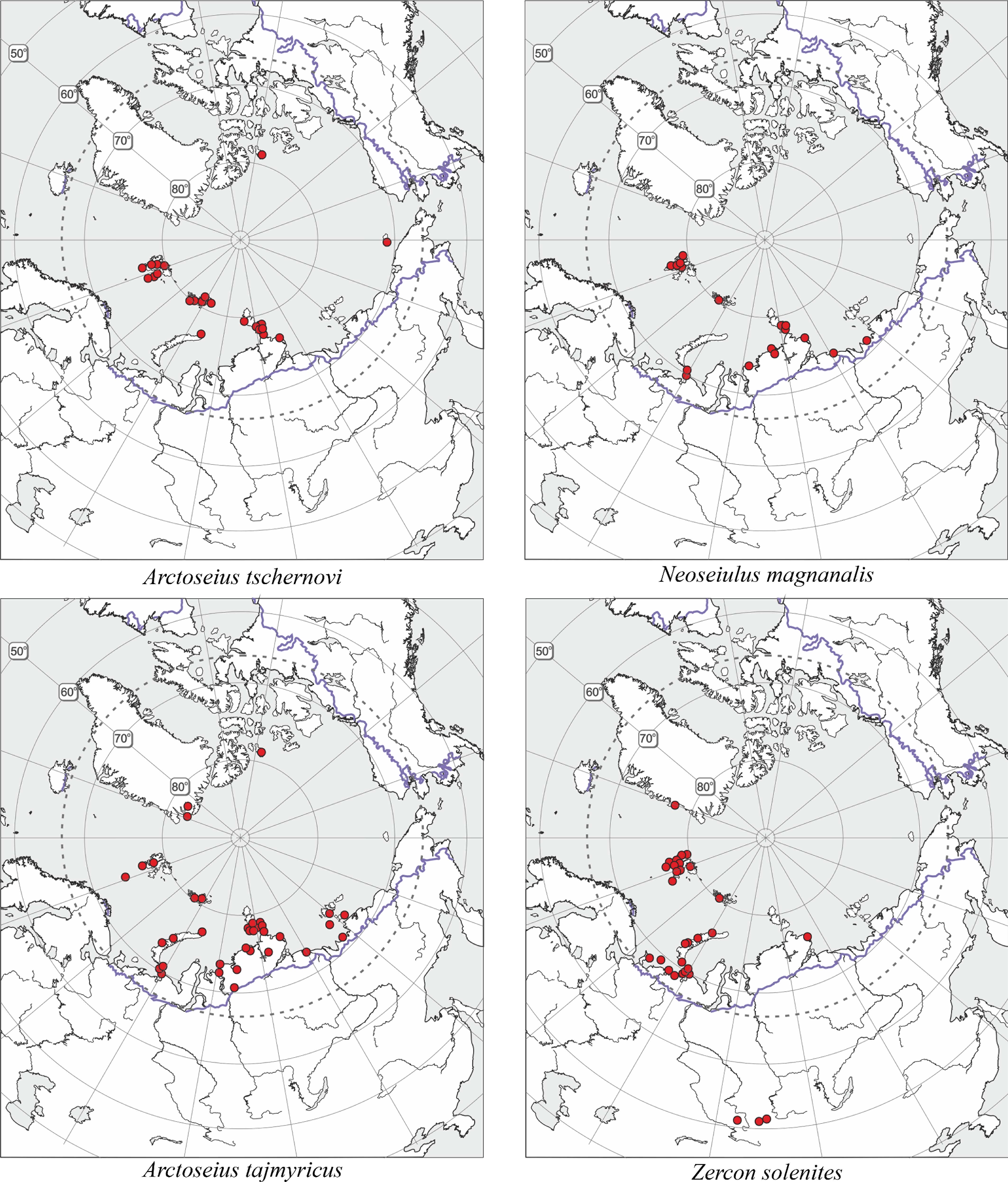
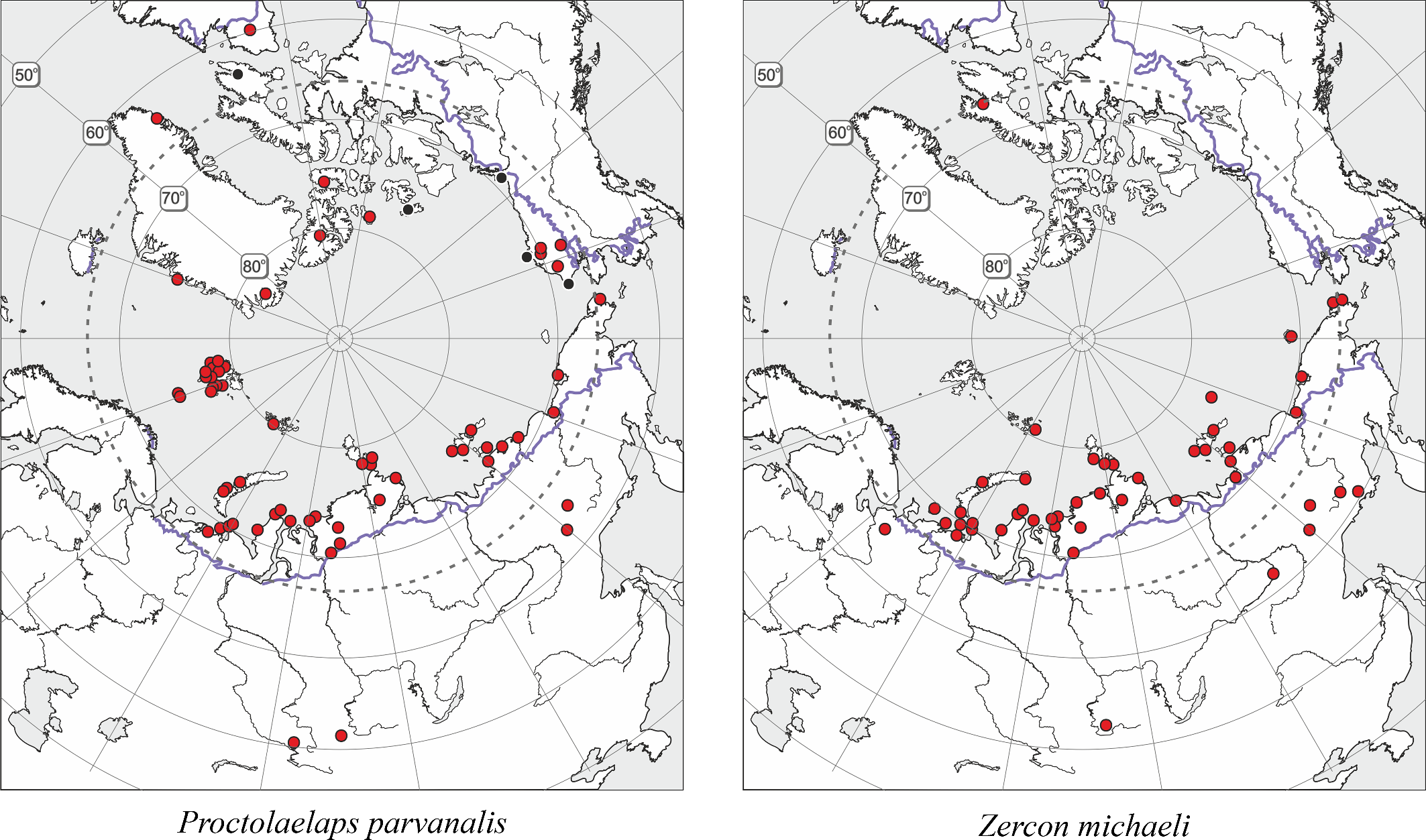
The results of the present work together with the results of previous works and new finds allowed me to correct the previous distributional maps for Arctoseius tschernovi, Proctolaelaps parvanalis, Zercon michaeli, Z. solenites as well as to plot new maps for Arctoseius tajmyricus and Neoseiulus magnanalis (Figures 6 and 7). These maps display three types of areas of "true arctic species″, namely the arctic-montane distributional range (P. parvanalis, Z. michaeli, Z. solenites), the arctic range (A. tajmyricus, N. magnanalis), and hyperarctic range confined to the High Arctic (A. tschernovi).




Size of Mesostigmata mites
Testing the hypothesis about the relationship between the macroclimate and size of Mesostigmata (Seeman and Nahrung 2018) we calculated the average idiosomal length of all mesostigmatic mite species (13) found on FJL and the same attribute for Ascoidea alone (9). These indexes are 513.6 ± 25.4 micrometers for Mesostigmata and 532.6 ± 39.3 micrometers for Ascoidea (Table 4).


Discussion
Diversity and taxonomic structure of the FJL acarofauna
Prior to the present investigation, only 10 mite species from our list have been known from the FJL based on old sporadic collections (Bulavintsev and Babenko 1983; Krivolutsky and Kalyakin 1993; Krivolutsky 1995; Krivolutsky et al. 2003; Makarova 1999, 2000b), altogether constituting some 20% of the modern list (Table 2). Findings on the archipelago of oribatids Liochthonius sellnicki (Thor, 1930), Eobrachychthonius sp., Limnozetes sp., Zygoribatula exilis (Nicolet, 1855), Oppia splendens (C.L. Koch, 1841), Quadroppia quadricarinata (Michael, 1885), Suctobelbella sp., Chamobates sp., Ceratozetes sellnicki Rajski, 1958, Punctoribates sp., Phthiracaraus laevigatus (C.L. Koch, 1844) (as Ph. nitens Nicolet, 1855), and Euphthiracarus sp. (by Krivolutsky et al. 2003) as well as Sphaerozetes piriformis (Nicolet, 1855) and Sphaerozetes tricuspidatus Willmann, 1923 (by Krivolutsky 1995), and also Diapterobates variabilis Hammer, 1955 and unidentified Laelapidae (by Uspenskiy et al. 1987) need a confirmation because of the known (more southerly) distributions of these taxa. The presence of the synanthropic astigmatan Glycyphagus domesticus (De Geer, 1778) (by Trägårdh 1904) in natural habitats of FJL is also questionable. The records from the FJL of the oribatid, Liochthonius sellnicki (Thor, 1930) (Bulavintsev and Babenko 1983; Krivolutsky and Kalyakin 1993; Krivolutsky et al. 2003), have to be probably referred to the very close Liochthonius muscorum common in the polar desert zone (northern Greenland, Severnaya Zemlya, Sverdrup Islands) (Makarova 2002a; unpublished new data).
Sixteen of the 50 species of our list were identified only to the generic level (Table 2) mainly because of taxonomic problems. More than half of the species (28) recorded from the FJL have also been found on Severnaya Zemlya (Table 2). Additionally, these archipelagos share almost the same set of ''multispecies'' genera, i.e. Arctoseius with 8–13 species; Cocceupodes with 3–4; Neoprotereunetes, Alicorhagia, and Liochthonius, each with 3 species (cf. Makarova 2002a added by Alicorhagia sp.).
The total diversity of free-living terrestrial mites in the FJL, 50 species, appears to be the minimal among the relatively well known mite faunas of the High Arctic. Such a set on the Severnaya Zemlya Archipelago includes 64 species (Makarova 2002a, with a few additions), vs about 150 species in the main body of Svalbard, without southern Bjørnøya Isl. (Seniczak et al. 2020). Both these regions have higher summer temperatures (Table 5). In comparison, the huge and yet warmer Greenland hosts not less than 254 free-living terrestrial mite species (Makarova 2015). Yet the limited diversity of free-living mites in the FJL is almost twice as high as in the continental Antarctica, excluding the Antarctic Peninsula (29 species), where mean monthly air temperature is always negative (Convey 2017).


The macrostructure of FJL free-living acarofauna resembles those of other polar desert regions in what refers to the prevalence of the prostigmatic mites (38% of the mite species) and the reduced proportion of Oribatida (20%), in contrast to the patterns in the tundra (Makarova 2002a). Usually, Oribatida is the most diverse group among free-living mites in the tundra (references in Makarova 2002a, 2015; Seniczak et al. 2020). In the mainland Antarctica, the Prostigmata and Oribatida constitute 62% and 15% of free-living mite species, respectively (calculated from Pugh 1993).
Most Oribatida species (8 of 10) in the FJL belong to the families Brachychthoniidae, Oppiidae, and Ceratozetidae (Table 2), this being a characteristic of the Arctic polar desert acarofauna (McAlpine 1965; Bulavintsev and Babenko, 1983; Makarova 2002a). More to the south, in the tundra zone, to this set of families should also be added the Crotoniidae and, sometimes, the Damaeidae and/or Suctobelbidae can be also diverse (Behan-Pelletier 1997, 1999; Makarova 2015; Melekhina 2020; Seniczak et al. 2020).
It was shown that in the Arctic there is a clear negative tendency in the diversity ratios of the classes Insecta and Arachnida along the latitudinal gradient (Chernov 2002, 2004; Makarova 2002a; Chernov et al. 2011). The severe climates of both High Arctic and mainland Antarctic determine the low diversity of insects (Figure 8), typically the dominant class of organisms on Earth, and, among arthropods, the prevalence of mites and collembolans (reference in the captions of Figure 8). In total, the entomofauna of the FJL comprises only 14 insect species (Bulavintsev and Babenko 1983; Uspenskiy et al. 1987; Makarova et al. 2013; Krasheninnikov and Gavrilo 2014; new records), with half of them being represented by members of one midge family only, i.e. the non-biting midges, Chironomidae (Krasheninnikov et al. 2022). Several studies on FJL spiders (Pickard-Cambridge 1898; Bulavintsev and Babenko 1983; Breuss 2004; Makarova et al. 2014) have revealed only two species inhabiting the archipelago, namely, Erigone psychrophila Thorell, 1871 and Collinsia (or Halorates) spetsbergensis Thorell, 1871. As a result, the total number of Arachnida species (52) known from the FJL is almost four times larger than the number of insect species (Figure 8A).
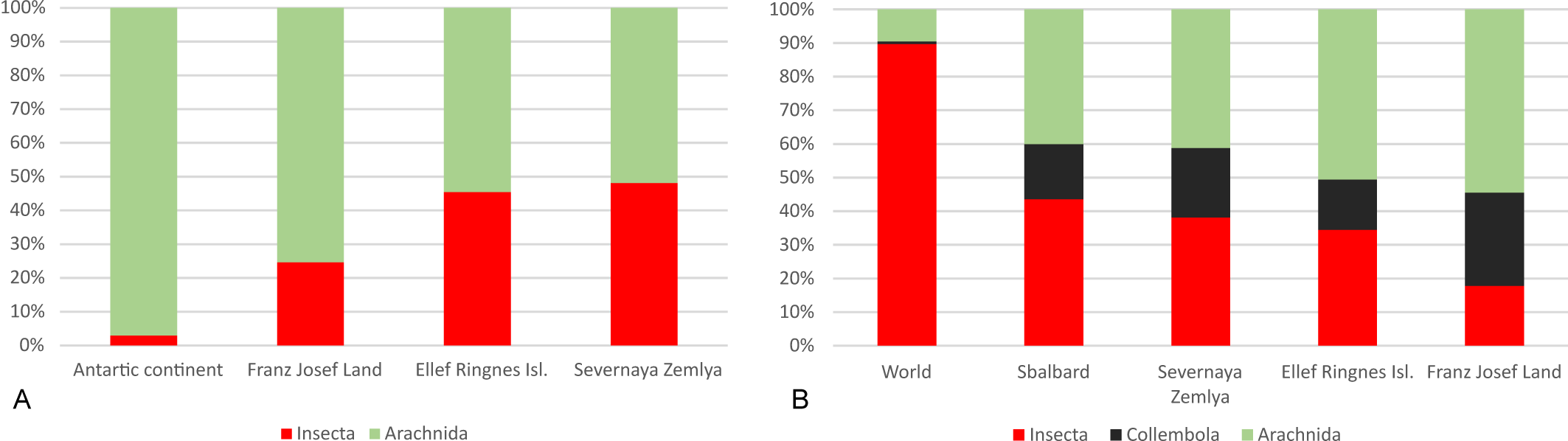


The mite fauna of FJL is extremely incomplete and fragmentary, as most families (83%) are represented by one genus and most genera (77%) include only one species. This low diversity of genera within families and of species within genera has been observed also in other regions of the Arctic polar deserts (McAlpine 1965 with additions; Makarova 2002a). More to the south, in the tundra and taiga zones, the percentage of ''monospecific'' genera within free-living mite fauna usually fail to exceed 60% among mites in general (Makarova 2015) or within Mesostigmata alone (Marchenko 2012; Huhta 2016), or Oribatida alone (Luxton 1996; Niemi et al. 1997; Ryabinin 2015; Melekhina 2020). Taxonomical fragmentation seems to be a typical regular characteristic of polar desert biota in general (Korotkevich 1972; Chernov and Matveyeva 1979). So, this pattern is also known from other groups of animals in the FJL, such as Nematoda, Collembola, and even vertebrates (Figure 4).
Geographic structure of the FJL acarofauna
Among the 35 identified species, five are cosmopolitan in distribution, and 27 have Holarctic distribution (including circumpolar species). Only three species, Zercon forsslundi, Bakerdania arctobia, and Neoseiulus magnanalis, have still not been found in the Western Hemisphere (Table 2). The prostigmatan, Bakerdania arctobia Khaustov et Makarova, 2005, has so far been known only from polar deserts of the Severnaya Zemlya. The phytoseiid species Neoseiulus magnanalis (Thor, 1930) very long was considered endemic to Svalbard. Its redescription based on topotypes (Kolodochka and Gwiazdowicz 2014) has enabled us to reidentify the specimens previously reported from the arctic Siberia (Makarova 1999, 2002a) by the name of Neoseiulus sp. aff. tibielingmiut (Chant et Hansell, 1971) as N. magnanalis and to specify its distribution (Figure 6).
It seems noteworthy that the montane records during the last few years have considerably ''expanded'' the distribution ranges of some classic arctic species inhabiting FJL. At present, they are rather to be regarded as arctic-montane species, not truly arctic ones (Makarova 2013; Figures 6 and 7). The largest, circumpolar arctic group is composed of 13 species [Holarctic, arctic (A) + high-arctic (HA) in Table 2] and includes most members of Arctoseius and one species each of Alicorhagia, Nanorchestes, Stigmaeus, Claveupodes, and Neoprotereunetes, whereas the Holarctic arctic-montane group (H, AM in Table 2) contains six species: Zercon solenites, Z. michaeli, Arctoseius idiodactylus, Proctolaelaps parvanalis, Coccorhagidia pittardi, and Ceratozetes spitsbergensis. The Arctic-boreal and polyzonal groups include seven and nine species, respectively. Thus, the acarofauna of the FJL, with 57.1% of specialized cryobiontic (arctic and arctic-montane) species, is the most ''arctic'' among all known Arctic mite faunas. We can compare the diversity and the level of specialization (in terms of cryophily) of several arctic faunas of Mesostigmata (Table 5, Figure 9). The species number positively correlates with the average July temperature (Spearman rank correlation coefficients 0.72, p = 0.0350), whereas the percentage of cryobiontic species correlates negatively (Rho = -0.82, p = 0.0108). The picture (Figure 9) clearly shows that the smallest fauna of the FJL has the largest proportion of cryobiontic species. The regions mentioned in Table 5 are known to have been either fully glaciated during the Last Glacial Maximum or free of ice (Dyke et al. 2002; Mangerud et al. 2002; Pavlidis et al. 2005). Yet, regardless of the glaciation history of the regions, their faunas reflect the current heat conditions (cf. species diversity of Greenland and FJL that both were fully glaciated in the late Pleistocene as well as the species diversity of the Pechora Sea coast and Vaigach Isl. that both were free of ice). With heat supply deterioration, the species richness is reduced and the proportion of cryobiontic species grows.
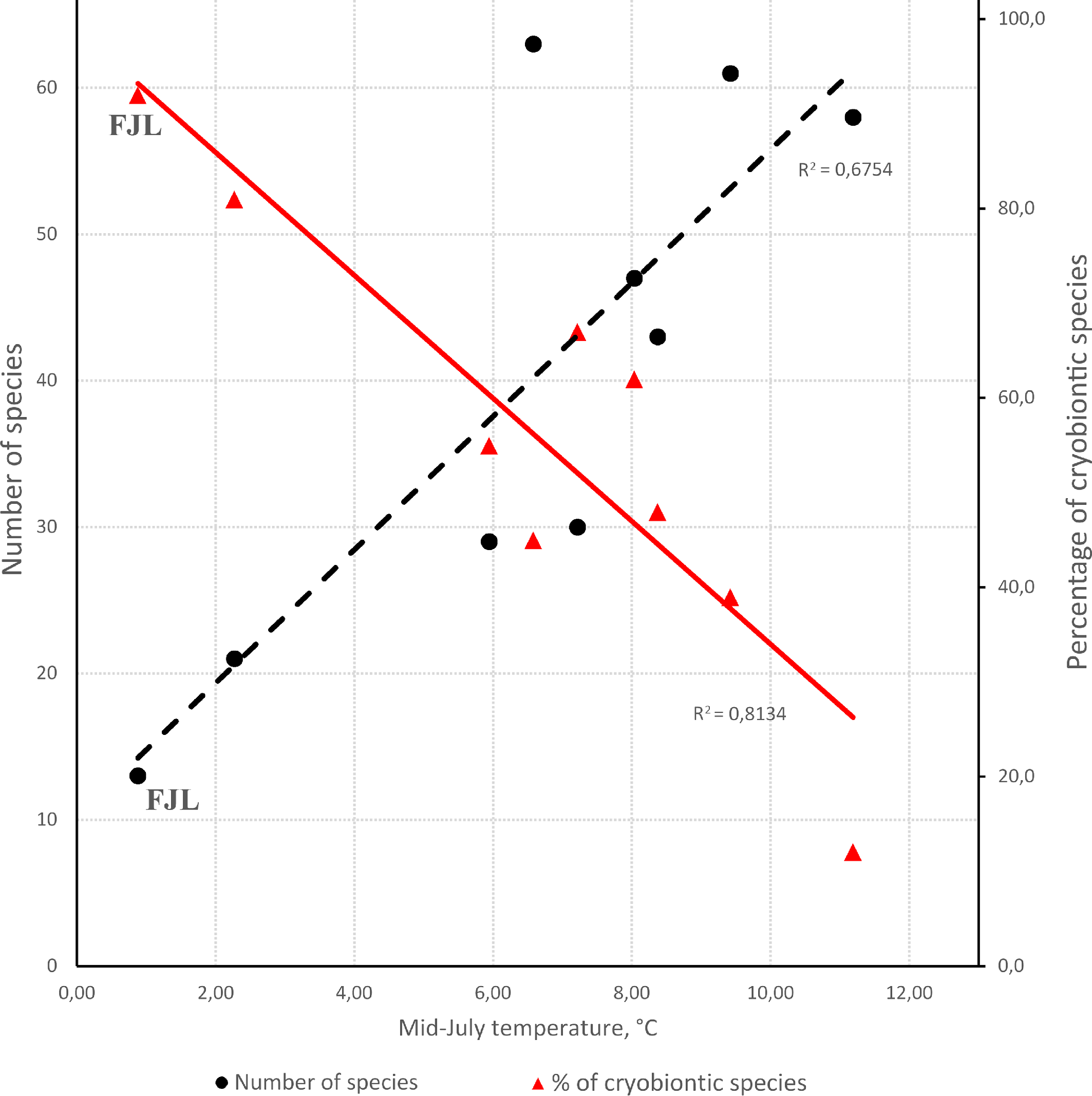


Very interestingly, the proportion of specialized arctic species among the relatively well known higher taxa, i.e. Mesostigmata (92.3%) and Oribatida (10.0%), appears to vary strongly (Figure 10). The percentage of true arctic and arctic-montane species within Mesostigmata throughout the Arctic is much greater than that of Oribatida (Makarova and Böcher 2009; Hodkinson et al. 2013; Makarova 2013, 2015; Makarova et al. 2019; Melekhina 2020). This finding may be related to differences in the geological age and evolution rate of these taxa (Makarova and Böcher, 2009). Fossil oribatid mites date back to the Devonian (Norton et al. 1988; Subias and Arillo 2002), while the rate of their morphogenesis is considered to have been unprecedentedly low (Krivolutsky 1973; Heethoff et al. 2006). On the contrary, the earliest record of a member of Mesostigmata dates back to the Cretaceous (Dunlop and Selden 2009). Molecular investigations designate the Dermanyssina s. l. (the main body of Mesostigmata) as a taxon of rather recent origin, with extremely high average divergence levels relative to outgroups in comparison with all other chelicerates (Klompen et al. 2007). Moreover, Mesostigmata generally also have significantly faster rates of nucleotide substitution than do other parasitiform mites, this possibly being related to their higher metabolic rates and shorter life-cycles (Murell et al. 2005).


Structure and differentiation of free-living mite communities
Most of the mite assemblages studied are multispecies (20–34 species) and polydominant (Table 3). The total mite abundance in the FJL grounds commonly varies between 120–2,340 ind./dm2, locally reaching 3,800 ind./dm2 in well-drained tundra-like lush communities with forbs and dwarf willows on the south-faced slope of Tikhaya Bay (Table 3). Almost the same variations have been pointed out for the acarocenoses in the polar deserts of the Severnaya Zemlya Archipelago (40–2,380 ind./dm2; Makarova 2002a). These baseline values strongly resemble those recorded in previous works in the tundra zone, but they are significantly lower in comparison with data obtained from taiga zone (2,400–6,200 ind./dm2; references in Makarova 2002a).
We have no material from the flat coastal or inland landscapes of FJL (plains and low plateaus), where mite communities are scarce and uniform (Bulavitsev and Babenko, 1983; Uspenskiy et al. 1987). The slope habitats studied in the present paper are populated by species rich and various mite assemblages (Table 3, Figure 5), even though the sampling sites within both of our transects were contiguous, and the mass species were often numerous in several biotopes (Table 3). Different approaches and methods have been used in ecological investigations of the arctic mites (Douce and Crossley 1977; Seniczak and Plichta 1978; McLean et al. 1978; Thomas and McLean 1988; Gwiazdowicz and Coulson 2011; Seniczak et al. 2014) rendering difficult the comparison of the results. Most of these investigations only deal with Oribatida and/or Mesostigmata data, or with only mass species from different mite suborders. Meanwhile, we can conclude that on FJL, as everywhere in the High Arctic, mite communities are differentiated depending on local environmental conditions. In comparison with Svalbard acarocenoses (Seniczak and Plichta 1978; Gwiazdowicz and Coulson 2011; Seniczak et al. 2014), the biotopic diversity of mesostigmatic mites on FJL is of the same order of magnitude (2-8 vs. 1-10 species on Svalbard) while the local species richness of Oribatida is lower (0-6 vs. 4-14 species per habitat). In spite of almost all species of Mesostigmata and Oribatida found on FJL inhabit Svalbard as well (Table 3), there is only one dominant species in common, the moss mite Diapterobates notatus.
It is also difficult to match up the present data even with material of rather well known acarocenoses of the Severnaya Zemlya Archipelago, where distinct zonal and intrazonal biotopes were studied and clear-cut habitat preferences of individual species have been recognized, and distinct hydrophilous, termophilous and nitrophilous complexes of species have been distinguished (Makarova 2002a, b; Khaustov and Makarova 2005). Studied ground communities of both Cape Flora and Tikhaya Bay are subjected to strong south slope's and rookery effects being warmer and impacted by biogenes. Yet, in both archipelagos, the larger members of the family Ceratozetidae, as well as Alicorhagia spp. and Terpnacarus bouvieri prefer well drained ''warm'' habitats; Arctoseius ornatus develops mainly in wet places, while Bakerdania arctobia and members of the family Histiostomatidae prefer or occur only in the loci eutrophied by vertebrate excrements. Meanwhile, the acarocenoses formed below the bird colonies on two study islands of FJL differ from each other sharply, both in composition and structure (Table 3). This clearly demonstrates the complexity of responses of terrestrial mite communities even to such a strong factor as an external nutrient input (Zmudczyńska‑Skarbek et al. 2023). As on Svalbard (Pilskog et al. 2014), the eutrophied by birds soils of FJL are populated mainly by opportunistic free-living mites.
The rather large, well sclerotized, hard-food consuming representatives of the higher oribatid mites, Ceratozetes spitsbergensis and Diapterobates notatus, have the highest density in the best heated tundra-like habitats with extended vegetation seasons (Table 3). Everywhere across the FJL, the small, thin-tegumented members of Eupodidae, Tydeidae, and Nanorchestidae appear to predominate (present data), just as in the polar deserts of the Canadian Arctic Archipelago (McAlpine 1965), Severnaya Zemlya Archipelago (Makarova 2002a), and Antarctic mainland (Pugh 1993; Marshall and Pugh 1996). These mite families also show the real prosperity, in the quantitative and qualitative aspects, in the arid deserts of all continents (references in Makarova 2002b) as well as in the newly formed soils (Russell et al. 2010). Their morphological simplicity and often small size, combined with consumption of the easily digestible liquid food, may give an advantage in extreme environments of different nature, unsuitable for other mites. The high metabolic rate appears to be the principal preadaptation to the life in habitats with short favorable periods (review in Makarova 2002b).
Besides this, at the relatively warm localities of the FJL studied (Cape Flora, Tikhaya Bay), the oribatid mite, Liochthonius muscorum was either often numerous (Table 3). Members of this genus are also small, relatively weakly sclerotized, and lacking boluses in their gut. Thus, all above-mentioned mites are mainly small, thin-tegumented, and most likely microeuryphagous (Eupodidae, Nanorchestidae, Tydeidae) or microbivorous (Tydeidae, Liochthonius) entities, using only liquid food (Walter 1988, Whitford 1996; Walter and Proctor 1999, 2013; references in Makarova 2002b). It is this set of traits that is obviously responsible for their relatively fast development (Walter and Proctor 2013). All these taxa are representatives of the most ancient lineages of the mite superorder Acariformes (Eupodides, Endeostigmata, Enarthronotides) known since the early to middle Devonian (Dubinin 1962; Norton et al. 1988; Kethley et al. 1989).
Diversity of the genus Arctoseius (Ascidae, Mesostigmata) in the Arctic
The most species-rich group of mites in the FJL is the mesostigmatic genus Arctoseius Thor, 1930 (Table 2), which includes more than 55 species worldwide (Makarova and Huhta, 2017), partly still undescribed. These superficially highly uniform mesostigmatic mites are fantastically diverse in the Arctic, totaling no less than 28 species (Makarova 1999). Its individual local faunas within the Arctic include 5–13 species, thus representing 26–83% of the local gamasid mite diversity (Lindquist 1961; Behan 1978; Makarova 2002a updated, 2013; Ávila-Jiménez et al. 2011). If one moves north through a series of subactic-arctic zones and subzones, up to the polar deserts, where the fauna in general is extremely reduced, Arctoseius diversity will remain high (up to 12–14 species) regardless of heat supply. In typical polar desert landscapes on the northern islands of the FJL (Rudolf Isl., Alexandra Land), members of Arctoseius alone form the mesostigmatic mite community and are the only predators among microarthropods (V.I. Bulavintsev's collection, see Table 1). Among the possible ecological reasons for the prosperity of Arctoseius in the Arctic we suggested: the very high abundance of potential prey (Collembola and nematoceran larvae), weak pressure of both predators and possible competitors, and an inclination to dwelling in ''insular'' zoogenic habitats accompanied by phoretic associations with winter crane flies and midges (Makarova 1999).
It is not improbable that the high species diversity of Arctoseius, whose many species are associated with spatially isolated eutrophied habitats, is also determined by certain peculiarities of the speciation processes in small isolated populations such as genetic drift, inbreeding etc. (Athias-Binche 1991).
The density of chironomid larvae in the FJL often reaches 30–80 ind./dm2, commonly being not lower than 10 ind./dm2 (Bulavintsev and Babenko 1983; Uspenskjy et al. 1987; present data). The density of collembolans in polar desert soils varies between 600–2,000 ind./dm2, sometimes exceeding 10,000 ind./dm2 (Babenko 2018). Both of these animal groups, small Nematocera and Collembola, together with soft-bodied mites, are common prey to Arctoseius spp. (Karg 1971; Binns 1972; Rudzińska 1998). Given the paucity of other predators, this overwhelming food can support many coexisting Arctoseius species, mitigating competition.
Measuring mesostigmatic mites from three High Arctic archipelagos does not indicate an increased size (Table 4). Yet the average size of Ascoidea (Ascidae members and Proctolaelaps parvanalis) seems to be significantly ''increased'' in the High Arctic populated mainly by large to the largest members of the genus Arctoseius. The largest Arctoseius tajmyricus (maximal idiosomal length 760 µm) and A. tschernovi (700 µm) inhabit mostly High Arctic landscapes (Figure 6). It seems noteworthy that the largest member of another ascid genus, Antennoseius Berlese, 1916, namely, A. oudemansi (Thor, 1930) (up to 970 µm), is also the single species of the genus dwelling in the High Arctic (Makarova 2013; Teodorovicz et al. 2014). Moreover, the largest species of the mesostigmatic families Parasitidae [Vulgarogamasus immanis (Berlese, 1903); 2700 µm] and Veigaiidae [Veigaia kochi (Trägårdh, 1901); 1450 µm] show arctic-boreal distribution patterns. Thus, the suggested increasing mesostigmatic mite size in subpolar areas (Seeman and Nahrung 2018) is partly supported by the results of the present study.
Age and origin of FJL fauna
In contrast to the Antarctic continental fauna of invertebrates that supports numerous endemics (Pugh and Convey 2008) of ancient age (Convey et al. 2009; van Vuuoren et al. 2018), the FJL fauna does not contain endemic species. At present, only one non-biting midge species, Chaetocladius (Amblycladius) franzjosephiensis Krasheninnikov, 2013, is considered conventional endemic to the archipelago (Krasheninnikov and Gavrilo 2013; Krasheninnikov and Przhboro 2022).
There are different views on the scales of the last glaciation in the European Arctic, but most researcher accept full glaciation of the FJL (Mangerud et al. 2002; Pavlidis et al. 2005; Velichko et al. 2017). Therefore, the FJL fauna is very young (not older than 10,000 years) and should be composed of long-distance migrants. In this regard, it seems of special interest that among mites, the most ancient taxa (Eupodides, Endeostigmata, Enarthronotides) are prosperous. Like the earliest terrestrial ecosystems during the process of land colonization (Arribas et al., 2019), the polar deserts, the simplest and the most climatically severe biome of Earth, appear to support these primitive mite lineages.
Conclusions
The young acarofauna of FJL, although the result of recent colonization following the total glaciation during Weichselian time, yet includes at least 50 species. Like in the Antarctic polar deserts, small and thin-tegumented members of the ancient mite lineages (Eupodidae, Tydeidae, and Nanorchestidae) predominate everywhere across the archipelago. Prostigmatic mites are much more diverse and numerous than oribatid mites as they do throughout the polar and arid deserts of both Hemispheres acting as their ''ecological equivalents'' within the saprophilous arthropod complex. The fauna of the order Mesostigmata of FJL includes the maximal percentage of cryobiontic species among known arctic faunas. The present data confirm the much greater diversity of the class Arachnida in the polar deserts, as compared with the largest animal class on the Earth, Insecta, that is unique for terrestrial biomes.
Acknowledgements
The sampling on the Franz Josef Land Archipelago in 2015 by A.B. Babenko was part of the scientifical program of the Arctic Floating University research organized by the Northern (Arctic) Federal University (Arkhangelsk). The field work of A.B. Krasheninnikov (Perm State University) and M.V. Gavrilo was carried out during the expedition ''Open Ocean: Arctic Archipelagos – 2016'' organized by the Association ''Marine Heritage: Explore and Sustain'' (St. Petersburg). The expedition of A.A. Semikolennykh (Moscow State University) was organized by the active participation of the Russian Arctic National Park (Arkhangelsk) and M.V. Gavrilo personally. Arrangement of pictures and maps were prepared for publication with helpful participation of K.V. Makarov (Moscow Pedagogical State University). I′m very grateful to all colleagues and organizations. Besides, I′m greatly obliged to Yu.I. Chernov, A.B. Babenko, V.I. Bulavintsev (Severtsov Institute of Ecology and Evolution, Moscow), A.B. Krasheninnikov (Perm State University), V. Peneva and M. Elshishka (Institute of Biodiversity and Ecosystem Research, BAS, Sofia), M.V. Gavrilo (Polar Marine Biology & Conservation, St. Petersburg), and N.V. Matveyeva (Komarov Botanical Institute, St. Petersburg), for the useful consultations, as well as to S.I. Golovatch (Severtsov Institute of Ecology and Evolution, Moscow) for improving the English text, to E.N. Melekhina (Institute of Biology of Komi Scientific Centre, Syktyvkar) and two anonymous reviewers for their gracious help in the article preparing. All photographs were kindly provided by A.B. Babenko. Laboratory work with the material collected was supported by the Russian Science Foundation, project No. 22-24-00162.
References
- Alexandrova V.D. 1983.Vegetation of the USSR Polar Deserts. Leningrad: Nauka. pp. 143. [In Russian]
- André H.M. 1980. A generic revision of the family Tydeidae (Acari: Actinedida). IV. Generic descriptions, keys and conclusion. Bull. Ann. Soc. R. Belge d'Entom., 116: 103-168.
- Arribas P., Andújar C., Moraza M.L., Linard B., Emerson B.C., Vogler A.P. 2019. Mitochondrial metagenomics reveals the ancient origin and phylodiversity of soil mites and provides a phylogeny of the Acari. Mol. Biol. Evol., 37: 683-694. https://doi.org/10.1093/molbev/msz255
- Athias-Binche F. 1991. Ecology and evolution of phoresy in mites. In: Dusbabek F., Bukva V. (Eds). Modern Acarology, Vol. 1. Prague: Academia, ČSSR. p. 27-41.
- Ávila-Jiménez M.L., Gwiazdowicz D.J., Coulson S.J. 2011. The mesostigmatid mite (Acari: Parasitiformes) fauna of Svalbard: A revised inventory of a high Arctic Archipelago. Zootaxa, 3091: 33-41. https://doi.org/10.11646/zootaxa.3091.1.2
- Babenko A.B. 2018. Springtails (Collembola) in the subpolar landscape of the Northern Hemisphere. Entom. Rev., 98: 383-406. https://doi.org/10.1134/S0013873818040012
- Balogh J., Mahunka S. 1983. The Soil Mites of the World. Volume 1. Primitive Oribatid Mites of the Palaearctic Region. Budapest - Amsterdam: Akademai Kiado & Elsevier. pp. 372.
- Behan V.M. 1978. Diversity, Distribution and Feeding Habits of North American Arctic Soil Acari [PhD. Thesis]. Montreal: Dept. Ent., Macdonald College of McGill Univ. pp. 422.
- Behan-Pelletier V.M. 1986. Ceratozetidae (Acari: Oribatei) of the western North American subarctic. Can. Entom., 118: 991-1057. https://doi.org/10.4039/Ent118991-10
- Behan-Pelletier V.M. 1997. Oribatid mites (Acari: Oribatida) of the Yukon. In: Danks H.V., Downes J.A. (Eds). Insects of Yukon. Ottawa: Biological Survey of Canada (Terrestrial Arthropods). p. 115-149.
- Behan-Pelletier V.M. 1999.Oribatid mite fauna of northern ecosystems: products of evolutionary adaptations or physiological constraints. In: Mitchell R., Horn D.J., Needham G.R., Welbourn W.C. (Eds). Acarology IX. Proceedings of IX International Congress of Acarology. Columbus, Ohio, 1996, V. 2. Columbus: Ohio Biological Survey. p. 87-93.
- Binns E.S. 1972. Arctoseius cetratus (Sellnick) (Acarina: Ascidae) phoretic on mushroom sciarid flies. Acarologia, 14: 350-356.
- Boyarskiy P.V. (Ed). 2013. Franz Josef Land. Mocow: Paulsen. pp. 680. [In Russian]
- Breuss W. 2004. Eine Ausbeute von Spinnen (Arachnida, Araneae) von Franz-Josef-Land (Russland). Denisia, 12(N. Ser. 14): 535-545.
- Bulavintsev V.I., Babenko A.B. 1983. Soil invertebrates of the eastern part of Franz-Josef Land archipelago. Zool. Zh., 62: 1114-1116. [In Russian]
- Chernov Yu.I. 2002. Arctic biota: taxonomic diversity. Entom. Rev., 82(Suppl. 1): 1-23.
- Chernov Yu.I. 2004.The animal world of the polar desert on the Devon Island Plateau, Canadian Arctic Archipelago. Entom. Rev., 84(Suppl. 1): 15-24.
- Chernov Yu.I., Makarova O.L. 2008. Beetles (Coleoptera) in High Arctic. In: Penev L., Erwin T., Assmann T. (Eds). Back to the Roots or Back to the Future? Towards a New Synthesis between Taxonomic, Ecological and Biogeographical Approaches in Carabidology. Proceedings of XIII European Carabidologists Meeting, Blagoevgrad, August 20-24, 2008. Sofia - Moscow: Pensoft Publishers. p. 213-246.
- Chernov Yu.I., Matveyeva N.V. 1979. Pattern of zonal distribution of Taimyr communities. In: Aleksandrova V.D., Matveeva N.V. (Eds). Arctic Tundra and Polar Deserts of the Taimyr Peninsula. Leningrad: Nauka. p. 166-200. [In Russian]
- Chernov Yu.I., Matveyeva N.V., Makarova O.L. 2011. The polar deserts. At the limit of life. Priroda, 9: 31-43. [In Russian]
- Convey P. 2017. Antarctic ecosystems. Encycl. Biodiv., 1: 170-188. https://doi.org/10.1016/B978-0-12-809633-8.02182-8
- Convey P., Stevens M.I., Hodgson D.A., Smellie J., Hillenbrand C.-D., Barnes D.K.A., Clarke A., Pugh P.J.A., Linse K., Cary S.C. 2009. Exploring biological constraints on the glacial history of Antarctica. Quat. Sci. Rev., 28: 3035-3048. https://doi.org/10.1016/j.quascirev.2009.08.015
- Coulson S.J., Convey P., Aakra K., Aarvik L., Ávila-Jiménez M.L., Babenko A., Biersma E.M., Boström S., Brittain J.E., Carlsson A.M., et al. 2014. The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea: Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biol. & Biochem., 68: 440-470. https://doi.org/10.1016/j.soilbio.2013.10.006
- Development team. 2007-2020. SAS.Planet (version 200606.1075). Windows.
- Douce G.K., Crossley D.A. 1977. Acarina abundance and community structure in an Arctic coastal tundra. Pedobiologia, 17: 32-42. https://doi.org/10.1016/S0031-4056(23)00139-7
- Dubinin V.B. 1962. Superclass Chelicerata. In: Rohdendorf B.B. (Ed). Foundations of Paleontology. Tracheate and Chelicerate Arthropods. Moscow: Akademia Nauk SSSR. p. 378-524. [In Russian]
- Dunlop J.A., Selden P.A. 2009. Calibrating the chelicerate clock: A paleontological response to Jeyaprakash and Hoy. Exp. & Appl. Acarol., 48: 183-197. https://doi.org/10.1007/s10493-009-9247-1
- Dyke A.S., Andrews J.T., Clark P.U., England J.H., Miller G.H., Shaw J., Veillette J.J. 2002. The Laurentide and Innuitian ice sheets during the Last Glacial Maximum. Quat. Sci. Rev., 21: 9-31. https://doi.org/10.1016/S0277-3791(01)00095-6
- Gavrilo M.V. 2013. 02.10. Animal world. In: Boyarskiy P.V. (Ed). Franz Josef Land. Moscow: Paulsen. p. 533-553. [In Russian]
- Gorodkov B.N. 1935. Tundra Zone Vegetation of the USSR. Moscow-Leningrad: Izdatelstvo Akademii nauk SSSR. pp. 142. [In Russian]
- Gwiazdowicz D.J., Coulson S.J. 2011. High-Arctic gamasid mites (Acari, Mesostigmata): Community composition on Spitsbergen, Svalbard. Polar Res., 30: 8311. https://doi.org/10.3402/polar.v30i0.8311
- Gwiazdowicz D.J., Teodorowicz E., Coulson S.J. 2011. Redescription of Zercon solenites Haarløv, 1942 (Acari, Zerconidae) with a key to the Svalbard species of the genus Zercon. Int. J. Acarol., 37(Suppl. 1): 135-148. https://doi.org/10.1080/01647954.2010.543699
- Hammer Ø., Harper D.A.T, Ryan P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4: 1-9.
- Heethoff M., Domes K., Laumann M., Maraun M., Norton R.A., Scheu S. 2006. High divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari, Oribatida). J. Evol. Biol., 20: 392-402. https://doi.org/10.1111/j.1420-9101.2006.01183.x
- Hodkinson I.D., Babenko A., Behan-Pelletier V., Böcher J., Boxshall G., Brodo F., Coulson S.J., De Smet W., Dozsa-Farkas K., Elias S., et al. 2013. Chapter 7. Terrestrial and Freshwater Invertebrates. In: Meltofte H. (Ed). Arctic Biodiversity Assessment. Status and Trends in Arctic Biodiversity. Akureyri: Conservation of Arctic Flora and Fauna. p. 196-223.
- Huhta V. 2016. Catalogue of the Mesostigmata mites in Finland. Mem. Soc. Fauna et Flora Fenn., 92: 129-148.
- Kalúz S. 2000. Redescription of Penthalodes ovalis (Acarina, Prostigmata, Penthalodidae) based on mites from Central Europe and Turkey. Biol. Bratisl., 55: 477-482.
- Karg W. 1971. Acari (Acarina). Milben Unterordnung Anactinochaeta (Parasitiformes). Die freilebenden Gamasina (Gamasides), Raubmilben, Die Tierwelt Deutschlands. Teil 59. Jena: G. Fischer Verlag. pp. 475.
- Kaźmierski A. 1998. Tydeinae of the world: generic relationships, new and redescribed taxa and keys to all species. A revision of the subfamilies Pretydeinae and Tydeinae (Acari: Actinedida: Tydeidae) - part IV. Acta Zool. Cracov., 41: 283-455.
- Kethley J.B., Norton R.A., Bonamo P.M., Shear W.A. 1989. A Terrestrial alicorhagiid mite (Acari: Acariformes) from the Devonian of New York. Micropaleont., 35: 367-373. https://doi.org/10.2307/1485678
- Khaustov A.A. 2014. A new genus and species in the mite family Eupodidae (Acari, Eupodoidea) from Crimea. ZooKeys, 422: 11-22. https://doi.org/10.3897/zookeys.422.7802
- Khaustov A.A., Makarova O.L. 2005. Two new mite species of the genus Bakerdania (Acari, Heterostigmata, Pygmephoridae) from polar deserts of the Severnaya Zemlya Archipelago. Entom. Rev., 85(Suppl. 1): 141-146.
- Klompen H., Lekveishvili M., Black W.C. 2007. IV. Phylogeny of parasitiform mites (Acari) based on rRNA. Molec. Phylogen. & Evol., 43: 936-951. https://doi.org/10.1016/j.ympev.2006.10.024
- Kolodochka L.A., Gwiazdowicz D.J. 2014. A new species of predaceous mite of the genus Neoseiulus Hughes (Acari, Phytoseiidae), with redescriptions of N. magnanalis (Thor) and N. ellesmerei (Chant & Hansell), from Svalbard, High Arctic. Zootaxa, 3793: 441-452. https://doi.org/10.11646/zootaxa.3793.4.3
- Korotkevich E.S. 1972. Polar Deserts. Leningrad: Hydrometeoizdat. pp. 420. [In Russian]
- Krantz G.W., Walter D.E. (Eds). 2009. A Manual of Acarology. 3rd Edition. Lubbock: Texas Tech Univ. Press. pp. 807.
- Krasheninnikov A.B., Gavrilo M.V. 2013. New data on the chironomids (Diptera, Chironomidae, Orthocladiinae) of Franz Joseph Land Archipelago. Euroasian Entom. J., 12: 157-160.
- Krasheninnikov A.B., Gavrilo M.V. 2014. Chironomids (Diptera, Chironomidae) of the Franz Josef Land archipelago (Arctic Russia). Fauna Norv., 34: 1-6. https://doi.org/10.5324/fn.v34i0.1665
- Krasheninnikov A.B., Gavrilo M.V., Elkin A.A., Moseev D.S., Kaigorodov R.V., Toropov L.I. 2022. Features of freshwater ecosystems of the Franz Josef Land archipelago. Polar Science, 33: 100849. https://doi.org/10.1016/j.polar.2022.100849
- Krasheninnikov A.B., Przhboro A.A. 2022. On taxonomy of the subgenus Amblycladius of the genus Chaetocladius (Diptera, Chironomidae). Zootaxa, 5168: 494-500. https://doi.org/10.11646/zootaxa.5168.4.10
- Krivolutsky D.A. 1973. Speciation rates and the pathways of adaptive evolution in oribatid mites. Ekologiya, 3: 75-80. [In Russian]
- Krivolutsky D.A. (Ed). 1995. Oribatid Mites (Morphology, Development, Phylogeny, Ecology, Methods of Study, Model Species Nothrus palustris C.L. Koch, 1839). Moscow: Nauka. pp. 224. [In Russian]
- Krivolutsky D.A., Drozdov N.N., Lebedeva N.V., Kalyakin V.N. 2003. Geography of soil microarthropods of Arctic islands. Vestnik Moskov. Gosud. Univ. Ser. 5. Geographiya, 6: 33-40. [In Russian]
- Krivolutsky D.A., Kalyakin V.N. 1993. Use of Soil Microfauna in Ecological Monitoring on the Novaya Zemlya Archipelago. Novaya Zemlya, 2: 125-131. [In Russian]
- Kuliev A.N. 2013. 02.09. Vegetation. In: Boyarskiy P.V. (Ed). Franz Josef Land. Moscow: Paulsen. p. 513-531. [In Russian]
- Lindquist E.E. 1961. Taxonomic and biological studies of the mites of the genus Arctoseius Thor from Barrow, Alaska (Acarina: Aceosejidae). Hilgardia, 30: 301-350. https://doi.org/10.3733/hilg.v30n11p301
- Luxton M. 1996. Oribatid mites of the British Isles: a check-list and notes on biogeography. J. Nat. Hist., 30: 803-822. https://doi.org/10.1080/00222939600770441
- MacLean S.F., Behan V., Fjellberg A. 1978. Soil Acari and Collembola from Chaun Bay, Northern Chukotka, USSR. Arct. & Alp. Res., 10: 559-568. https://doi.org/10.2307/1550679
- Makarova O.L. 1999. Mesostigmatic mites (Parasitiformes, Mesostigmata) of polar deserts in Severnaya Zemlya. Entom. Rev., 79: 982-990.
- Makarova O.L. 2000a. To a study of mites of the genus Arctoseius (Parasitiformes, Ascidae) from the Far North: 2. Description of A. productus sp. n. and A. babenkoi sp. n. and key to the High Arctic species. Entom. Rev., 80(Suppl. 1): 131-142.
- Makarova O.L. 2000b. To the study of mites of the genus Arctoseius Thor (Parasitiformes, Ascidae) from the Far North: 3. Ranges and ecological preferences of species. Entom. Rev., 80(Suppl. 1): 143-150.
- Makarova O.L. 2002a. Acarocenoses (Acariformes, Parasitiformes) in polar deserts: 1. Mite assemblages of the Severnaya Zemlya Archipelago: structure of fauna and abundance. Entom. Rev., 82: 839-856.
- Makarova O.L. 2002b. Acarocenoses (Acariformes, Parasitiformes) in polar deserts. 2. Cenotic relations, structure of communities, and proportion of suborders. Entom. Rev., 82: 857-875.
- Makarova O.L. 2013. Gamasid mites (Parasitiformes, Mesostigmata) of the European Arctic and their distribution patterns. Entom. Rev., 93: 113-133. https://doi.org/10.1134/S0013873813010156
- Makarova O.L. 2015. The fauna of free-living mites (Acari) of Greenland. Entom. Rev., 95: 108-125. https://doi.org/10.1134/S0013873815010133
- Makarova O.L., Anufriev V.V., Babenko A.B, Bizin M.S., Glazov G.M., Kolesnikova A.A., Marusik Yu.M., Tatarinov A.G. 2019. Fauna of the East European Tundra: the input of "Siberian» species. Vestnik Severo-Vostochnogo nauchnogo Tsentra DVO RAN, 1: 59-71. [In Russian] https://doi.org/10.34078/1814-0998-2019-1-59-71
- Makarova O.L., Bieńkowski A.O., Bulavintsev V.I., Sokolov A.V. 2007. Beetles (Coleoptera) in polar deserts of the Severnaya Zemlya Archipelago. Entom. Rev., 87: 1142-1154. https://doi.org/10.1134/S0013873807090059
- Makarova O.L., Böcher J. 2009. Diversity and geographical ranges of Greenland mites (Acari: Oribatida and Mesostigmata). In: Golovatch S.I., Makarova O.L., Babenko A.B., Penev L.D. (Eds). Species and Communities in Extreme Environments. Sofia - Moscow: Pensoft Publ. & KMK Sci. Press. p. 165-186.
- Makarova O.L., Eskov K.Yu., Marusik Yu.M., Tanasevich A.V. 2014. Spiders (Aranei) of Polar Desert Zone. In: Striganova B.R. (Ed). Problems of the Soil Zoology. Proceedings of XVII All-Russian Conference on Soil Zoology; Moscow: KMK Scientific Press. p. 149-151. [In Russian]
- Makarova O.L., Huhta V. 2017. A new species of Arctoseius Thor, 1930 (Acari: Ascidae) from taiga regions of the Palaearctic, with a key to Arctoseius species of Fennoscandia, NW Europe. Zootaxa, 4268: 554-562. https://doi.org/10.11646/zootaxa.4268.4.6
- Makarova O.L., Sviridov A.V., Klepikov M.A. 2013. Lepidoptera (Insecta) of polar deserts. Entom. Rev., 93: 225-239. https://doi.org/10.1134/S0013873813020115
- Mangerud J., Astakhov V, Svendsen J.-I. 2002. The extent of the Barents-Kara ice sheet during the Last Glacial Maximum. Quat. Sci. Rev., 21: 111-119. https://doi.org/10.1016/S0277-3791(01)00088-9
- Marchenko I.I. 2012. Soil-dwelling gamasid mites (Acari, Mesostigmata) of northern Siberia. Evroaziat. Entom. Zh., 11: 517-528. [In Russian]
- Marshall D.J., Pugh P.J.A. 1996. Origin of the inland Acari of Continental Antarctica, with particular reference to Dronning Maud Land. Zool. J. Linn. Soc., 118: 101-118. https://doi.org/10.1111/j.1096-3642.1996.tb00221.x
- Mašán P. 2022. The family Melicharidae (Acari, Mesostigmata) in Slovakia, with description of new species, annotated faunal synopsis and identification keys of species from Europe. Zootaxa, 5172: 1-449. https://doi.org/10.11646/zootaxa.5172.1.1
- Matveyeva N.V., Zanokha L.L., Afonina O.M., Potemkin A.D., Patova E.N., Davydov D.A., Andreyeva V.M., Zhurbenko M.P., Konoreva L.A., Zmitrovich I.V., Ezhov O.N., Shiryaev A.G., Kirtsideli I.Yu. 2015. Plants and Fungi of Polar Deserts of the Northern Hemisphere. St.-Petersburg: MARAFON. pp. 320. [In Russian]
- McAlpine J.F. 1965. Insect and related terrestrial invertebrates of Ellef Ringnes Island. Arctic, 18: 73-103. https://doi.org/10.14430/arctic3455
- Melekhina E.N. 2020. Analysis of oribatid fauna of the East European tundra with first reported data of Subpolar Urals. Diversity, 12: 235. https://doi.org/10.3390/d12060235
- Murell A., Dobson S.J, Walter D.E., Campbell N.J.H., Shao R., Barker S.C. 2005. Relationships among the three major lineages of the Acari (Arthropoda: Arachnida) inferred from small subunit rRNA: paraphyly of the Parasitiformes with respect to the Opilioacariformes and relative rates of nucleotide substitution. Invertebr. Syst., 19: 383-389. https://doi.org/10.1071/IS05027
- Niemi R., Karppinen E., Uusitalo M. 1997. Catalogue of the Oribatida (Acari) of Finland. Acta Zool. Fenn., 207: 1-39.
- Norton R.A., Bonamo P.M., Grierson J.D., Shear W.A. 1988. Oribatid mite fossils from a terrestrial Devonian deposit near Gilboa, New York State. J. Paleont., 62: 259-269. https://doi.org/10.1017/S0022336000029905
- Pavlidis Yu.A., Bogdanov Yu.A., Levchenko O.V., Murdmaa I.O., Tarasov G.A. 2005. New data about the natural context in the Barents Sea during the late Valdai Glaciation. Okeanologia, 45: 92-106. [In Russian]
- Pickard-Cambridge O. 1898. On some Arctic spiders collected during the Jackson-Harmsworth Polar Expedition to the Franz-Josef Archipelago. J. Linn. Soc. Lond., Zool., 26: 613-615. https://doi.org/10.1111/j.1096-3642.1898.tb01735.x
- Pilskog H.E., Solhøy T., Gwiazdowicz D.J., Grytnes J.-A., Coulson S.J. 2014. Invertebrate communities inhabiting nests of migrating passerine, wild fowl and sea birds breeding in the High Arctic, Svalbard. Polar Biol., 37: 981-998. https://doi.org/10.1007/s00300-014-1495-9
- Pugh P.J.A. 1993. A synonymic catalogue of the Acari from Antarctica, the Sub-Antarctic Islands and the Southern Ocean. J. Nat. Hist., 27: 323-421. https://doi.org/10.1080/00222939300770171
- Pugh P.J.A., Convey P. 2008. Surviving out in the cold: Antarctic endemic invertebrates and their refugia. J. Biogeogr., 35: 2176-2186. https://doi.org/10.1111/j.1365-2699.2008.01953.x
- Rudzińska M. 1998. Life history of the phoretic predatory mite Arctoseius semiscissus (Acari: Ascidae) on a diet of sciarid fly eggs. Exp. & Appl. Acarol., 22: 643-648.
- Russell D.J., Hohberg K., Elmer M. 2010. Primary colonisation of newly formed soils by actinedid mites. Soil Organisms, 82: 237-251.
- Ryabinin N.A. 2015. Oribatid mites (Acari, Oribatida) in soils of the Russian Far East. Zootaxa, 3914: 201-244. https://doi.org/10.11646/zootaxa.3914.3.1
- Safronova I.N., Kholod S.S., Gavrilo M.V., Ezhov O.N. 2020. Floristic and phytocoenotic diversity of vegetation cover of the Franz Josef Land Archipelago. Botan. Zh., 105: 133-151. [In Russian]
- Schulte G. 1975. Holarktische Artareale der Ameronothridae (Acari, Oribatei). Veröff .Inst. Meeresforsch. Bremerhaven, 15: 339-357.
- Seeman O.D., Nahrung H.F. 2018. In short- or long-term relationships, size does matter: body size patterns in the Mesostigmata (Acari: Parasitiformes). Int. J. Acarol., 44: 360-366. https://doi.org/10.1080/01647954.2018.1530299
- Seniczak S., Plichta W. 1978. Structural dependence of moss mite populations on patchiness of vegetation in moss-lichen-tundra at the north coast of Hornsund, West Spitsbergen. Pedobiologia, 18: 145-152. https://doi.org/10.1016/S0031-4056(23)00578-4
- Seniczak S., Seniczak A., Gwiazdowicz D.J., Coulson S.J. 2014. Community structure of oribatid and gamasid mites (Acari) in moss-grass tundra in Svalbard (Spitsbergen, Norway). Arct. Antarct. Alp. Res., 46: 591-599. https://doi.org/10.1657/1938-4246-46.3.591
- Seniczak A., Seniczak S., Schwarzfeld M.D., Coulson S.J., Gwiazdowicz D.J. 2020. Diversity and distribution of mites (Acari: Ixodida, Mesostigmata, Trombidiformes, Sarcoptiformes) in the Svalbard Archipelago. Diversity, 12: 323. https://doi.org/10.3390/d12090323
- Strandtmann R.W. 1982. Notes on Nanorchestes III. Four species from the Arctic Tundra (Acari: Endeostigmata: Nanorchestidae). Pacific Insects, 24: 69-77.
- Subías L.S., Arillo A. 2002. Oribatid mite fossils from the Upper Devonian of South Mountain, New York and the Lower Carboniferous of County Antrim Northern Ireland (Acariformes, Oribatida). Est. Mus. Cienc. Nat. Alava, 17: 93-106.
- Summers F.M. 1962. The genus Stigmaeus (Acarina: Stigmaeidae). Hilgardia, 33: 491-537. https://doi.org/10.3733/hilg.v33n10p491
- Teodorowicz E., Gwiazdowicz D.J., Coulson S.J. 2014. Redescription of Antennoseius (Vitzthumia) oudemansi (Acari, Mesostigmata) from Spitsbergen, Svalbard. Entomol. Fenn., 25: 27-42. https://doi.org/10.33338/ef.41467
- Thomas R.H., MacLean S.E. 1988. Community structure in soil Acari along a latitudinal transect of Tundra sites in Northern Alaska. Pedobiologia, 31: 113-138. https://doi.org/10.1016/S0031-4056(23)02254-0
- Trägårdh I. 1904. Monographie der arktischen Acariden. Fauna Arctica, 4: 1-78.
- Urhan R., Karaca M. 2013. Zerconid mites (Acari, Zerconidae) in forestland of Artvin Province (Turkey). In: Proceedings of the International Caucasian Forestry Symposium, 24–26 October 2013, Artvin, Turkey. p. 687-699.
- Uspenskiy S.M, Govorukha L.S., Belikov S.Y., Bulavintsev V.I. 1987. Proposed protected zones in the Franz‐Josef Land area. Polar Geogr. & Geol., 11: 210-220. https://doi.org/10.1080/10889378709377329
- van Vuuren B.J., Lee J.E., Convey P., Chown S.L. 2018. Conservation implications of spatial genetic structure in two species of oribatid mites from the Antarctic Peninsula and the Scotia Arc. Antarct. Sci., 30: 1-10. https://doi.org/10.1017/S0954102017000529
- Velichko A.A., Faustova M.A., Pisareva V.V., Karpukhina N.V. 2017. History of the Scandinavian ice sheet and surrounding landscapes during Valdai ice age and the Holocene. Led i Sneg. Ice and Snow, 57: 391-416. [In Russian] https://doi.org/10.15356/2076-6734-2017-3-391-416
- Walter D.E. 1988. Predation and mycophagy by endeostigmatid mites (Acariformes: Prostigmata). Exp. & Appl. Acarol., 4: 159-166. https://doi.org/10.1007/BF01193873
- Walter D.E., Proctor H.C. 1999. Mites: Ecology, Evolution and Behaviour. New York: CABI Publishing. pp. 322. https://doi.org/10.1079/9780851993751.0000
- Walter D.E., Proctor H.C. 2013. Mites - Ecology, Evolution and Behaviour (2nd edition): Life at a Microscale. Dordrecht Heidelberg - New York - London, International: Springer. pp. 486. https://doi.org/10.1007/978-94-007-7164-2
- Weather and Climate. Available online: https://www.weatherandclimate.eu/history/ (accessed on 28.01.2023).
- Whitford W.G. 1996. The importance of the biodiversity of soil biota in arid ecosystems. Biodiv. & Conserv., 5: 185-195. https://doi.org/10.1007/BF00055829
- Zacharda M. 1980. Soil mites of the family Rhagidiidae (Actinedida: Eupodoidea). Morphology, systematics, ecology. Acta Univ. Carolinae Biol., 1978: 489-785.
- Zhang Z.-Q. (Ed). 2011. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 1-237. https://doi.org/10.11646/zootaxa.3148.1.2
- Zmudczyńska‑Skarbek K., Bokhorst S., Convey P., Gwiazdowicz D.J., Skubała P., Zawierucha K., Zwolicki A. 2023. The impact of marine vertebrates on polar terrestrial invertebrate communities. Polar Biology. https://doi.org/10.1007/s00300-023-03134-8



2023-08-10
Date accepted:
2023-11-06
Date published:
2023-11-15
Edited by:
Pfingstl, Tobias

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Makarova, Olga L.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



