Development, survival, and reproduction of Amblyseius swirskii (Athias- Henriot) (Acari: Phytoseiidae) feeding on different pollen grains
Barzkar, Maryam  1
; Shishehbor, Parviz
1
; Shishehbor, Parviz  2
; Habibpour, Behzad
2
; Habibpour, Behzad  3
; Hemmati, Seyed Ali
3
; Hemmati, Seyed Ali  4
and Riahi, Elham
4
and Riahi, Elham  5
5
1Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
2Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
3Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
4Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
5✉ Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, P.O. Box 14115-336, Tehran, Iran.
2023 - Volume: 63 Issue: 4 pages: 1062-1071
https://doi.org/10.24349/izmp-v7mcOriginal research
Keywords
Abstract
Introduction
Predatory mites, especially the members of the family Phytoseiidae, play a significant role in the control of phytophagous mites and small insect pests such as thrips and whiteflies (McMurtry et al. 2013; Carrillo et al. 2015). Their ability to feed on various kinds of diets, short life cycles, acceptance of alternative foods, as well as, high survival, and simple reproduction make them outstanding candidates for biological control schemes (Carrillo et al. 2015).
The predatory mite Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) originated from the Mediterranean region and can be found in different areas of the world, such as Egypt, Italy, and Turkey (Athias-Henriot 1962). It is classified as a type III generalist mite which can feed on various food types, from small arthropods to plant exudate and pollen (McMurtry and Croft 1997; McMurtry et al. 2013). This species has been successfully used as a biological control agent against whiteflies and thrips (Buitenhuis et al. 2015; Calvo et al. 2015); whereas its influence on spider mites is still unclear (Messelink et al. 2010). Despite the broad prey range of A. swirskii, pollen grains exploitation helps the mites remain in the crops and surrounding plants even when there is no prey; or their density is low (Goleva and Zebitz 2013).
Pollen of plant species can provide important nutritional elements such as proteins, amino acids, lipids, carbohydrates, as well as vitamins and improve phytoseiid mite survival, development, and reproduction (Goleva and Zebitz 2013). However, the nutritional value of pollen as a food source varies according to plant species (Goleva and Zebitz 2013). Where there is little or no natural prey, pollen as an alternative diet may help the predator survive in the field and greenhouse. It can also be used as a food supplement to improve the predator's diet. In addition, pollen provision can be helpful for the early establishment of the predatory mite before prey emergence (van Rijn and Tanigoshi 1999; Broufas and Koveos 2000; van Rijn et al. 2002; Nomikou et al. 2003; Hoy 2011). Furthermore, mass rearing of predators in the laboratory can be done using pollen alone or in combination with natural prey, depending on the predator species and plant pollen species (Denno and Fagan 2003; Gerson et al. 2003; Hoy 2011). Depending on the natural feeding patterns of predatory mites, alternative or supplemental food in greenhouses should be consistent with the feeding spectrum of the respective predatory mite species (Goleva and Zebitz 2013). Although the demand for alternative foods of phytoseiids has been extensively known, it needs to be screened to find the best ones.
The suitability of various pollen grains from different plants and trees such as pear, apricot, opium poppy, narcissus, scots pine, hollyhock, Typha latifolia, date palm, almond, and maize has been tested and compared for A. swirskii previously (Park et al. 2011; Goleva and Zebitz 2013; Kumar et al. 2014; Riahi et al. 2017a; Nemati et al. 2019; Ansari-Shiri et al. 2022). Different nutritional value of various plant pollen grains illustrates the necessity to know which pollen has desirable and positive influences on the development and reproduction of A. swirskii, especially those producing numerous grains. Therefore, we studied the effects of feeding on pollen grains of different plants, which are frequently found in Iran (Khuzestan province, southwestern Iran) and have abundant pollen resources as an alternative food source for A. swirskii in the current study.
Material and methods
Mite culture
The predatory mite A. swirskii was obtained from a previously-established colony in Shahid Chamran University, Ahvaz, Iran. One laboratory colony was established by rearing them on strawberry spider mite, Tetranychus turkestani Ugarov and Nikolski for multiple generations at 25 ± 1 °C, 65 ± 5% RH, and a 16 L: 8 D h photoperiod. The predatory mites were kept on a piece of green plastic sheet (9 × 7 cm) placed on a same-sized sponge in a water-containing circle petri dish (14 cm diameter). Wet tissue paper immersed in the water in the petri dish was used to cover all edges of the sheets to prevent the predatory mites from escaping. Bean leaves infested with T. turkestani were added to the sheets every 2 days as a food source.
Pollen source
Pollen of eight plants, including lebbeck [Albizia lebbeck (L.) Benth.], date palm (Phoenix dactylifera L.), khejri [Prosopis cineraria (L.) Druce], castor bean (Ricinus communis L.), maize (Zea mays L.), sunflower (Helianthus annuus L.), cogongrass [Imperata cylindrical (L.) P. Beauv.], and cattail (Typha sp.), was selected for experiments. Date palm pollen was obtained from southwestern Iran Abadan city, Khuzestan province. The pollen of cattail was collected from Hamidabad Thicket, Dezful city, Khuzestan province, Iran. The pollen of other plants was collected on the campus of the Agricultural Research Center of Safi Abad, Dezful, Iran. The pollen collection was done in sunny, dry weather, from pesticide-free plants, using a brush or flower shaking. After collection, the pollen was maintained at room temperature for two days for drying, and then they were sieved and shaken to obtain pure pollen. All pollen grains were stored at -20 °C in a freezer.
Experimental units and protocols
The experimental units were prepared according to the rearing units used for the mite culturing, but a smaller plastic sheet (3 ×3 cm), sponge (3 ×3 cm), and petri dish (9 cm in diameter) were used for rearing predators separately. Wet sheets of tissue paper were used to cover all edges of the plastic sheet as a barrier to prevent mites from escaping. In addition, a few cotton threads were added to the center of the units as a place for shelter and oviposition sites.
For starting the experiments, a few cotton threads were placed in the rearing units for less than 24 h. Then, the predatory mite eggs were separated from the threads using a fine brush under a stereo microscope and transferred individually to the experimental units, one egg per unit. The units were randomly assigned to eight groups. Each group was fed on one of the eight pollen, including lebbeck, date palm, Khejri, castor bean, maize, sunflower, cogongrass, and cattail, immediately after egg hatching. Forty-five replicates were prepared for each group, except for maize (42 replicates). A daily check was performed to record the immature development of the predatory mite in each unit until adulthood. The duration of development of egg, larva, protonymph, and deutonymph stages were recorded individually on each diet at 25 °C, 65 ± 5% RH, and a 16L:8D h photoperiod. After adult emergence, each female was paired with a newly-appeared male fed on the same diet at the above-mentioned conditions. The units were monitored daily to determine the pre-oviposition and oviposition periods, as well as the number of eggs laid. Data recording was continued until the death of all individuals.
Data analysis
Two sex life tables were constructed for each treatment using the computer program TWOSEX_MS Chart (Chi 2022). All biological parameters, including the age-stage-specific survival rate (sxj ), the age-stage specific fecundity (fxj ) of adult females; the age-specific survival rate (lx ); the age-specific fecundity (mx ), the duration of different stages, adult pre-oviposition period (APOP), total pre-oviposition period (TPOP), adult longevity, and fecundity, along with life table parameters such the gross reproductive rate (GRR), net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T), were calculated according to Chi and Liu (1985) and Chi (1988). The bootstrap method in the TWOSEX_MS Chart software was used to estimate the standard errors of various parameters (Efron and Tibshirani 1993; Akca et al. 2015; Chi 2022). The difference among treatments was explored using paired bootstrap tests with 100,000 samplings (Akca et al. 2015).
Results
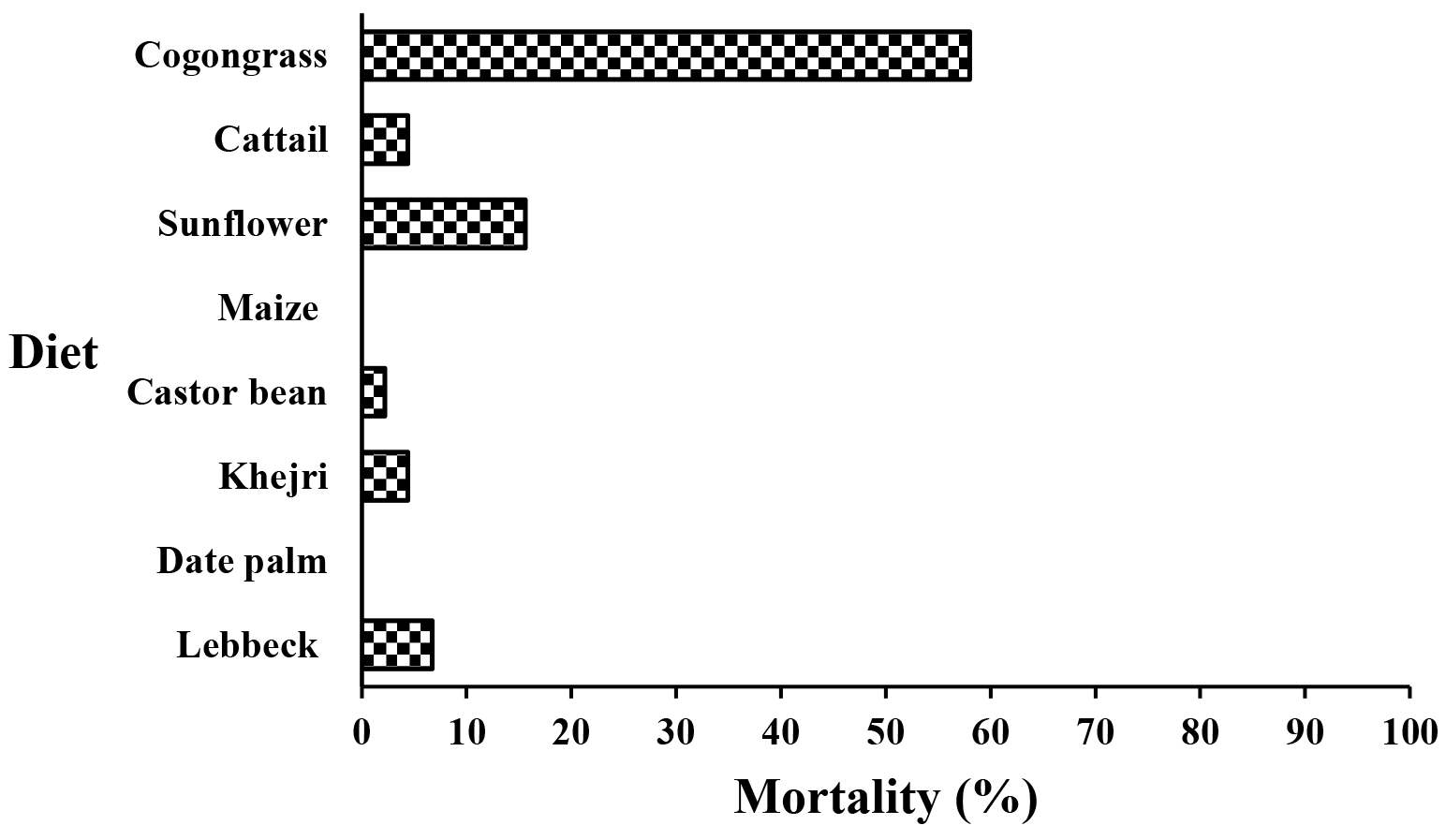
The duration of different stages of A. swirskii was significantly affected by the diets (Table 1). The total developmental time of individuals was significantly shorter on date palm, castor bean, and lebbeck pollen than on other pollen. The longest total duration of the immature stage (total population) was obtained on sunflower, followed by Khejri pollen. Diet had a significant influence on the developmental time of each sex (Table 1). Immature development of males and females was the longest when sunflower pollen was the food source. However, it was shortest on castor bean pollen, followed by date palm pollen. Adult longevity of females was shortest on cogongrass pollen, taking only 11.07 days, and longest on the date palm and maize pollen grains, lasting 41.00 and 36.44 days, respectively. The greatest male longevity was 34.53 days on sunflower pollen and the shortest was 10.40 days on castor bean pollen (Table 1).
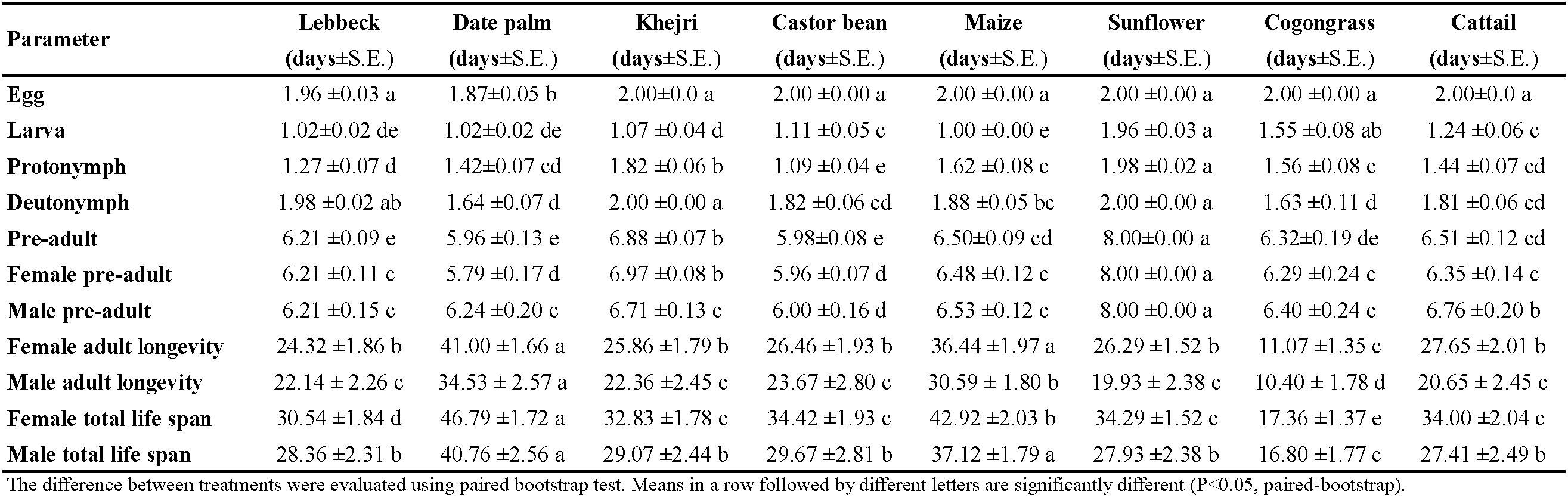


A summary of the mortality per stage recorded for A. swirskii fed with the different diets verifies that pollen grains influenced this parameter (Figure 1). The pollen of maize and date palm did not exert any mortality during pre-imaginal development. When fed with pollen of lebbeck, cattail, castor bean, and Khejri, negligible pre-imaginal mortality ranging from 2.2 to 6.7% was detected (Figure 1). Sunflower pollen exerted only low mortality (15.6%), while considerably higher mortality was represented by cogongrass pollen (58.0%) (Figure 1).


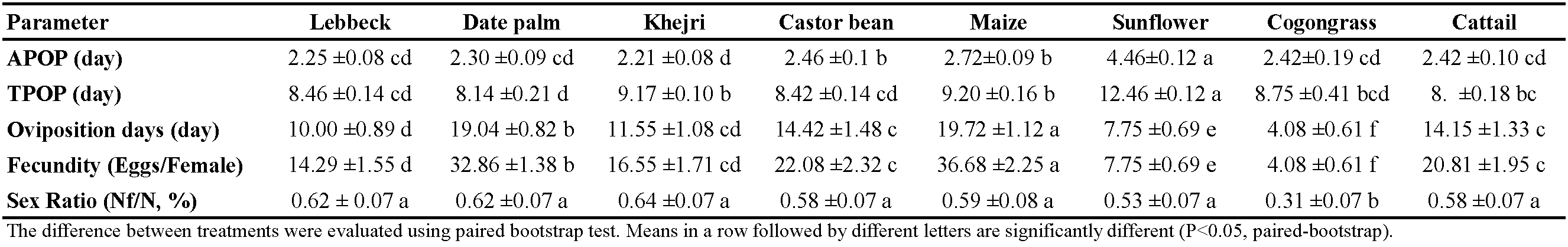
Feeding on sunflower pollen resulted in significantly longest pre-oviposition durations (APOP and TPOP) (Table 2). Amblyseius swirskii fed on Khejri pollen had the shortest adult pre-oviposition time. However, there was no significant difference of this parameter with the mites reared on lebbeck, date palm, cattail, and cogongrass (Table 2). Fecundity on cogongrass and sunflower pollen was extremely low (lower than ten eggs). Females fed on maize pollen laid the highest number of eggs, while the females on cogongrass laid the lowest number. The oviposition period was the longest and shortest when the mites were provided with pollen of maize and cogongrass, respectively (Table 2).


The highest mean fecundity per day was seen on maize and the lowest was found on cogongrass (Figure 2). Fecundity curves in maize and date palm had higher peaks than others. The age-stage specific fecundity (fxj ) was highest at the age of 15 (1.96 eggs/ female/day), 9 (1.69 eggs/female/day), 12 (0.64 eggs/female/day), 10 (1.62 eggs/female/day), 14 (0.67 eggs/female/day), 14 (2.08 eggs/female/day), 9 (1.46 eggs/female/day), and 10 day (1.62 eggs/female/day), on date palm, castor bean, cogongrass, cattail, sunflower, maize, lebbeck, and Khejri pollen, respectively, where the immature survivorship was 1, 0.98, 0.33, 0.96, 0.84, 1, 0.93, and 0.96, respectively (Figure 2).
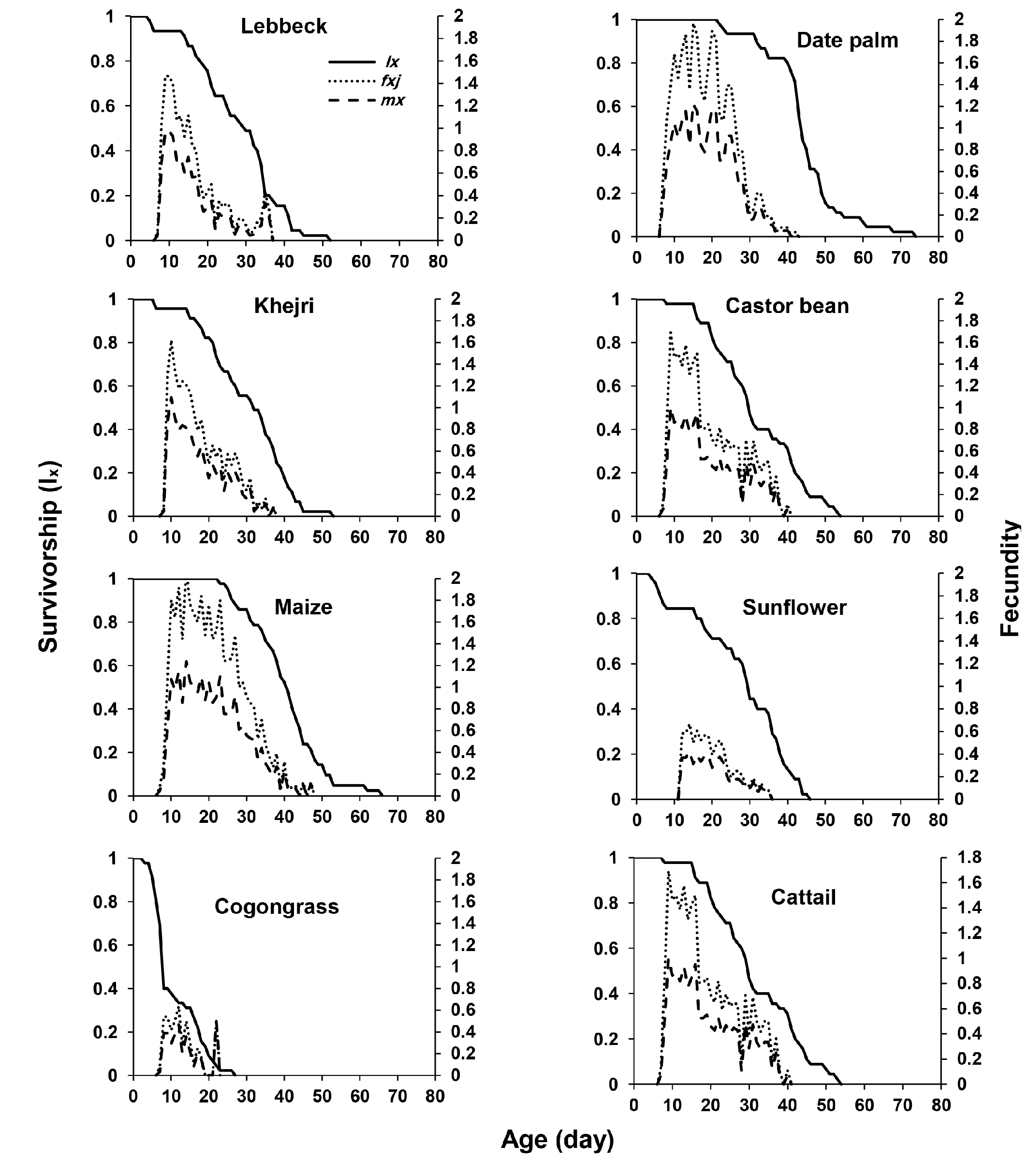


Figure 3 shows the age-stage specific survival rate (sxj ) of A. swirskii on eight different pollen. The obvious overlaps among stages which are seen in the survival curves are due to the variable developmental rate among individuals. The survival rate on cogongrass was lower than on other diets. The survival rate of females and males on date palm and maize pollen was higher than on other diets (Figure 3).
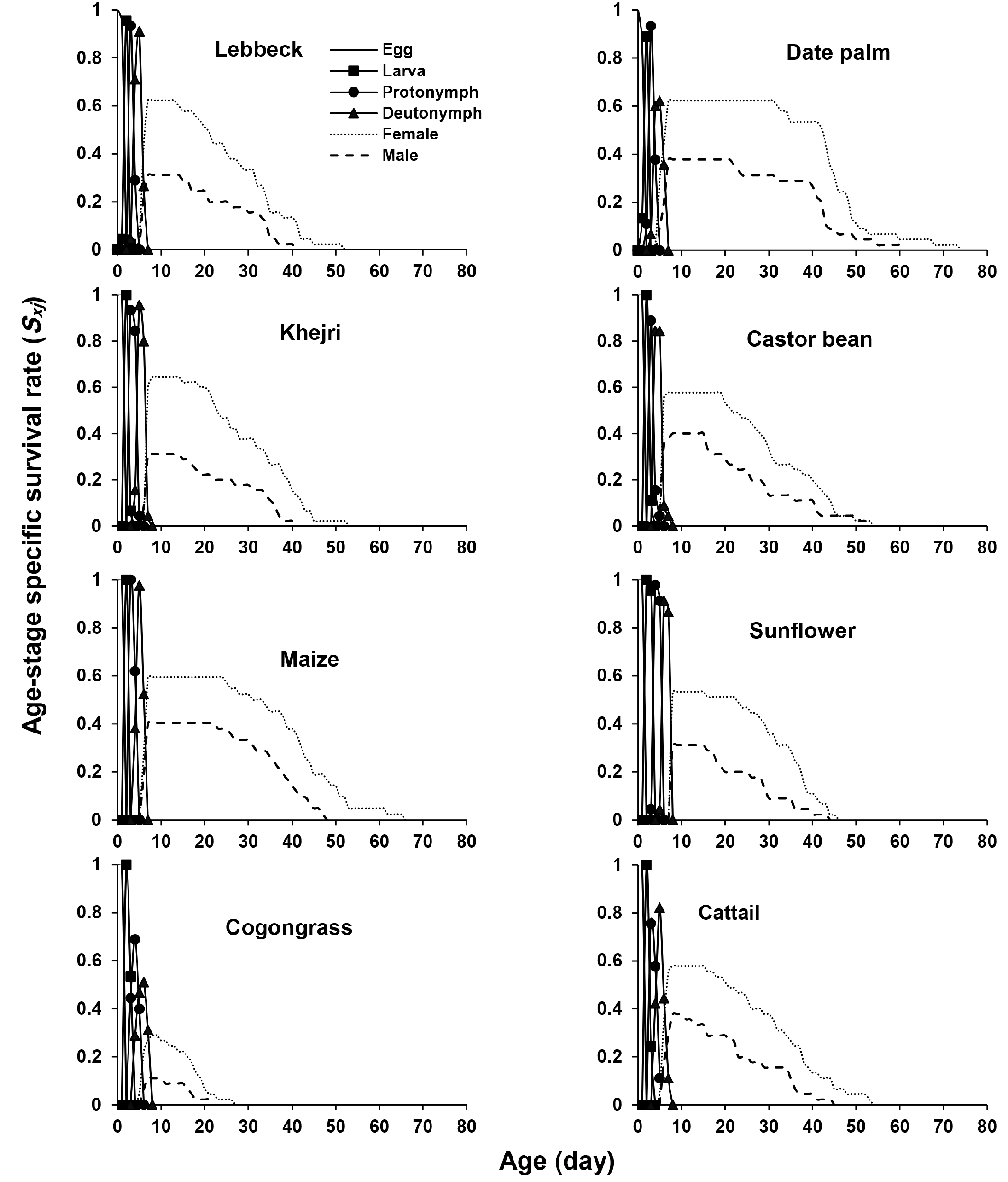


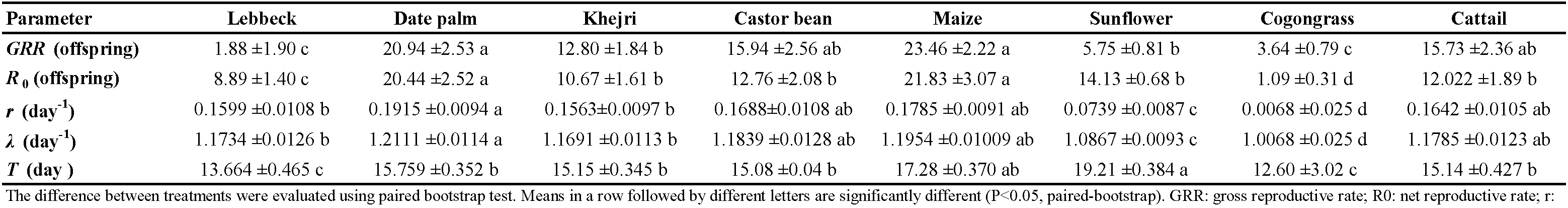
The effects of exploiting different pollen diets on the life table parameters of A. swirskii are summarized in Table 3. Significant differences between all parameters were found. The gross reproductive rate (GRR) and the net reproductive rate (R0) reached the maximal value on date palm and maize pollen. The value of GRR varied from 1.88 to 23.46, and the net reproductive rate from 1.09 to 21.83. When the females were fed lebbeck and cogongrass pollen, GRR and R0 values were the lowest. The intrinsic rate of increase (r) and the finite rate of increase (λ) were highest on date palm pollen and the lowest on cogongrass. The mean generation time (T) of A. swirskii on lebbeck and cogongrass was significantly shorter than on other pollen grains.


Discussion
Life table characteristics are the best indices of population growth under a given set of situations. This study revealed the life history traits of the generalist predatory mite A. swirskii on eight pollen diets. In line with previous studies (Goleva and Zebitz 2013; Riahi et al. 2017a; Nemati et al. 2019; Kadkhodazadeh et al. 2021), we found that various diets reflected different influences on biological parameters and life table characteristics of this predator. It is vital to find and use a suitable alternative diet for the development and oviposition of phytoseiid mites to achieve success in biological control programs.
Our results determined that A. swirskii could develop into the adult stage and reproduce on all diets tested. However, immature development and survival varied significantly depending on the diet consumed. Date palm, castor bean, and maize pollen resulted in higher developmental rates and longer adult longevity when compared with other pollen. Similarly, egg-to-adult development of A. swirskii on the pollen of some anemophilous and entomophilous plant species (Goleva and Zebitz 2013), almond (Riahi et al. 2017a,b), maize (Riahi et al. 2017b), wild almond, pistachio, peach, pomegranate, and walnut (Kadkhodazadeh et al. 2021) was reported to be completed within 6–8 days. In addition, similar findings were obtained by Asgari et al. (2020) who reported that A. swirskii needed 6.13 and 6.97 days to complete the immature development on Carpoglyphus lactis (L.) and Tyrophagus putrescentiae (Schrank). Our findings are contradicted by the studies conducted on the pollen of Poaceae mixture, pumpkin, sunflower, opium poppy, apricot, and pear, as well as bee pollen, which reported a longer developmental time for this predatory mite (Goleva and Zebitz 2013; Riahi et al. 2017a; Nemati et al. 2019). A shorter developmental time was recorded on almond pollen by Ansari-Shiri et al. (2022). As mentioned by Snyder et al. (2000) the development can be influenced by the type of diet; low-quality food delays the development, while high-quality food accelerates it. Therefore, the quality of pollen nutrients is the most effective factor that results in changing the immature development of A. swirskii. In contrast to Riahi et al. (2017a) and Rahmai Piyani et al. (2021), who mentioned that 7.3 and 4.20 days are needed to develop into the adult stage on date palm pollen, respectively, the present study showed that 5.96 days are required. The difference between our study and Riahi's might be related to the difference in the type of experimental substrate, artificial substrate versus leaf substrate. Rahmani Piyani et al. (2021) had reared A. swirskii on a mixture of spider mites and date palm pollen for several generations before starting the experiments, which is different from our study. Differences in geographic origin, climatic conditions, and soil type can also be the factor behind such differences.
We found no mortality when the immature individuals fed on maize and date palm pollen, while the highest immature mortality rate was obtained on cogongrass (58.0%), followed by sunflower (15.6%) pollen. These differences can be caused by the absence of one or more primary essential nutrients or their inadequacy, as well as secondary metabolites. Given the low number of immatures that reached adulthood on the latter pollen grains, it can be concluded that sunflower and cogongrass are unfavorable food for immature A. swirskii. Similarly, no mortality was also observed by Swirski et al. (1967) and Goleva and Zebitz (2013) when feeding A. swirskii on maize pollen at the same temperature as we did. Other phytoseiid mites, such as Neoseiulus californicus McGregor showed the highest survival (100%) when fed on maize and date palm pollen (Khanamani et al. 2016).
Similarly, female fecundity was also dependent on the quality of diets. This highest value of the number of laid eggs (36.68 eggs) in this study was substantially higher than the total fecundities reported for females of this predator reared on wild almond, peach, oak, pomegranate, walnut, common hazel, scots pine, sweet grasses, narcissus, and bee pollen (from 2.23 to 24.36 eggs/ female at 25 °C) (Goleva and Zebitz 2013; Kadkhodazadeh et al. 2021); factitious prey, including C. lactis (29.03 eggs/ female, at 23 °C) (Nguyen et al. 2013) and T. putrescentiae (3.69 eggs/ female, at 25 °C) (Riahi et al. 2017b), as well as artificial diets (Nguyen et al. 2013). The lifetime fecundity of A. swirskii has been reported to be 40.12 and 8.24 eggs per female on maize and sunflower pollen, respectively (Goleva and Zebitz 2013); these values are close to the fecundity of the mentioned pollen studied here.
The most valuable parameter which is used for comparing the potential of population growth is the intrinsic rate of increase (r) (Southwood 1966). The shorter the length of the pre-ovipositional period, the higher the growth rate is expected. The highest value of growth rates in the current study was higher than those reported for A. swirskii reared on different mites and insects at the same temperature, including Tetranychus urticae Koch (Tetranychidae) (Riahi et al. 2017a), Cenopalpus irani Dosse (Tenuipalpidae) (Bazgir et al. 2018); Thrips tabaci (Lindeman) (Thripidae), and Frankliniella occidentalis (Pergande) (Thripidae) (Wimmer et al. 2008). Surprisingly, the growth rate of females fed on date palm and maize pollen was also superior to that recorded on the dried fruit mite C. lactis (Nguyen et al. 2013). Riahi et al. (2017b) and Ansari- Shiri et al. (2022) found a lower r-value on maize pollen and almond pollen than what we obtained on date palm pollen. Contradictory, Asgari et al. (2020) found that the r value of A. swirskii fed on C. lactis and T. putrescentiae and their mixture was higher and reached 0.3792 day-1. Similar results were observed by Rahmani Piyani et al. (2021), who reported that r of A. swirskii fed date palm pollen was 0.396 day-1. These differences are probably because of different chemical compounds and nutritional value of diets, different experimental arenas, the diet on which the prey feeds, prey stage and species, and data analysis methods.
In conclusion, the positive effects of date palm and maize pollen on development, survival, fecundity, pre-oviposition period, and population growth rate demonstrated that these pollen grains are nutritionally more valuable and favorable than others. In addition, feeding on cogongrass and sunflower pollen had negative effects on A. swirskii population, such as slow development, high immature mortality, low fecundity, and growth rate, indicating the low nutritional value of these pollen grains. These findings could be useful for success in biocontrol programs using A. swirskii. Further study on the analysis of pollen components and the correlation between life-table parameters and food substances is recommended.
Acknowledgments
The support of this research by the Department of Entomology, Shahid Chamran University of Ahvaz (Grant no. SCU.AP1401.400), is greatly appreciated.
References
- Akca I., Ayvaz T., Yazıcı E., Smith C.L., Chi H. 2015. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): with additional comments on life table research criteria. J. Econ. Entomol., 108: 1466-1478. https://doi.org/10.1093/jee/tov187
- Ansari-Shiri H., Fathipour Y., Hajiqanbar H., Riahi, E. 2022. Quality control of the predatory mite Amblyseius swirskii during long-term rearing on almond Prunus amygdalus pollen. Arthr. Plant Interact., 16: 645-655. https://doi.org/10.1007/s11829-022-09929-6
- Asgari F., Sarraf Moayeri H.R., Kavousi A., Enkegaard A., Chi H. 2020. Demography and mass rearing of Amblyseius swirskii (Acari: Phytoseiidae) fed on two species of stored product mites and their mixture. J. Econ. Entomol., 1-9. https://doi.org/10.1093/jee/toaa187
- Athias-Henriot C. 1962. Amblyseius swirskii, un nouveau phytoseiide voisin d' A. andersoni (Acariens Anactinotriches). Ann. Ec. Natl. Agric. Alger., 3: 1-7.
- Bazgir F., Shakarami J., Jafari Sh. 2018. Life table and predation rate of Amblyseius swirskii (Acari: Phytoseiidae) fed on Eotetranychus frosti (Tetranychidae) and Cenopalpus irani (Tenuipalpidae). Syst. Appl. Acarol., 23(8): 1614-1626. https://doi.org/10.11158/saa.23.8.11
- Broufas A.G.D., Koveos D.S. 2000. Effect of different pollens on development, survivorship and reproduction of Euseius finlandicus (Acari: Phytoseiidae). Environ. Entomol., 29: 743-749. https://doi.org/10.1603/0046-225X-29.4.743
- Buitenhuis R., Murphy G., Shipp L., Scott-Dupree C. 2015. Amblyseius swirskii in greenhouse production systems: a floricultural perspective. Exp. Appl. Acarol., https://doi.org/10.1007/s10493-014-9869-9
- Calvo F.J., Knapp K., van Houten Y.M., Hoogerbrugge H., Belda J.E. 2015. Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol., https://doi.org/10.1007/s10493-014-9873-0
- Carrillo D., Peña J.E., de Moraes G.J. 2015. Prospects for biological control of plant feeding mites and other harmful organisms. Springer, 337. https://doi.org/10.1007/978-3-319-15042-0
- Chi H. 1988. Life- table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol., 17, 26- 34. https://doi.org/10.1093/ee/17.1.26
- Chi H. 2022. TWOSEX-MSChart: A computer program for the age-stage, two-sex life table analysis. http://140.120.197.173/Ecology/Laboratory of Theoretical Ecology
- Chi H., Liu H. 1985. Two new methods for the study of insect population ecology. Bulletin of the Institute of Zoology, Academia Sinica, 24: 225- 240.
- Denno R.F., Fagan W.F. 2003. Might nitrogen limitation promote omnivory among carnivorous arthropods? Ecology, 84 (10): 2522-2531. https://doi.org/10.1890/02-0370
- Efron B., Tibshirani R.J. 1993. An introduction to the bootstrap. Chapman and Hall: Publisher. pp. 456. https://doi.org/10.1007/978-1-4899-4541-9
- Gerson U., Smiley R., Ochoa R. 2003. Mites (acari) for pest control. Malden, Blackwell Science Ltd, 539. https://doi.org/10.1002/9780470750995
- Goleva I., Zebit C.P.W. 2013. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol., 61(3): 259-283. https://doi.org/10.1007/s10493-013-9700-z
- Hoy M.A. 2011. Agricultural acarology: introduction to integrated mite management. CRC Press, Boca Raton, USA, 393.
- Kadkhodazedeh F., Asadi M., Khanamani M. 2021. Suitability of different pollen grains and Tetranychus urticae as food for the predatory mite, Amblyseius swirskii (Acari: Phytoseiidae). Persian J. Acarol., 10(3): 321-334. https://doi.org/10.22073/pja.v10i3.66952
- Khanamani M., Fathipour Y., Talebi A.A., Mehrabadi M. 2016. Linking pollen quality and performance of Neoseiulus californicus (Acari: Phytoseiidae) in two-spotted spider mite management programmes. Pest Manag. Sci., 73: 452-461. https://doi.org/10.1002/ps.4305
- Kumar V., Wekesa V.W., Avery P.B., Powell C.A., McKenzie C.L., Osborne L.S. 2014. Effect of pollens of various ornamental pepper cultivars on the development and reproduction of Amblyseius swirskii (Acari: Phytoseiidae). Fla Entomol., 97: 367-373. https://doi.org/10.1653/024.097.0205
- McMurtry J.A., Croft B.A. 1997. Life-styles of Phytoseiidae mite and their roles in biological control. Annu. Rev. Entomol., 42: 291-321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., Moraes G.J.D., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae). Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Messelink G.J., Van Maanen R., Van Holstein-Saj R., Sabelis, M.W., Janssen A. 2010. Pest species diversity enhances control of spider mites and whiteflies by a generalist phytoseiid predator. BioControl, 55: 387-398. https://doi.org/10.1007/s10526-009-9258-1
- Nemati A., Riahi E., Khalili Moghadam A., Gwiazdowicz D.J., Bahari M.R., Amini P. 2019. Comparison of different pollen grains and a factitious prey as food sources for Amblyseius swirskii (Acari: Phytoseiidae). Syst. Appl. Acarol., 24(12): 2427-2438. https://doi.org/10.11158/saa.24.12.10
- Nomikou M., Janssen A., Sabelis M.W. 2003. Phytoseiid predator of whitefly feeds on plant tissue. Exp. Appl. Acarol., 31: 27-36. https://doi.org/10.1023/B:APPA.0000005150.33813.04
- Nguyen D.T., Vangansbeke D., Lü X., De Clercq P. 2013. Development and reproduction of the predatory mite Amblyseius swirskii on artificial diets. BioControl, 58: 369-377. https://doi.org/10.1007/s10526-012-9502-y
- Park H.H., Shipp L., Buitenhuis R., Ahn J.J. 2011. Life history parameters of a commercially available Amblyseius swirskii (Acari: Phytoseiidae) fed on cattail (Typha latifolia) pollen and tomato russet mite (Aculops lycopersici). J. Asia Pac. Entomol., 14: 497-501. https://doi.org/10.1016/j.aspen.2011.07.010
- Rahmani Piyani A., Shishehbor P., Kocheili F., Riddick E.W. 2021. Comparison of natural prey Tetranychus turkestani, date palm pollen, and bee pollen diets on development, reproduction, and life table parameters of the predator Amblyseius swirskii. Acarologia, 61(4): 890-900. https://doi.org/10.24349/G9ed-QB9h
- Riahi E., Fathipour Y., Talebi A.A., Mehrabadi M. 2017a. Linking life table and consumption rate of Amblyseius swirskii (Acari: Phytoseiidae) in presence and absence of different pollens. Ann. Entomol. Soc, Am., 110: 244-253. https://doi.org/10.1093/aesa/saw091
- Riahi E., Fathipour Y., Talebi A.A., Mehrabadi M. 2017b. Natural diets versus factitious prey: comparative effects on development, fecundity and life table of Amblyseius swirskii (Acari: Phytoseiidae). Syst. Appl. Acarol., 22(5): 711-723. https://doi.org/10.11158/saa.22.5.10
- Southwood R. 1966. Ecological Methods: with particular reference to the study of insect populations/T.R.E. southwood. Methuen: Publisher. pp. 391. https://doi.org/10.2307/1936978
- Snyder W.E., Joseph S.B., Preziosi R., Moore A.J. 2000. Nutritional benefits of cannibalism for the lady beetle Harmonia axyridis (Coleoptera: Coccinellidae) when prey quality is poor. Environ. Entomol., 29: 1173-1179. https://doi.org/10.1603/0046-225X-29.6.1173
- Swirski E., Amitai S., Dorzia N. 1967. Laboratory studies on the feeding, development and oviposition of the predatory mites Amblyseius rubini Swirskii and Amblyseius swirskii Athias (Acarina, Phytoseiidae) on various kinds of food substances. Isr. J. Agric. Res., 17: 101-109.
- van Rijn P.C.J., Tanigoshi L.K. 1999. Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp. Appl. Acarol., 23: 785-802. https://doi.org/10.1023/A:1006227704122
- Van Rijn P.C.J., Van Houten Y.M., Sabelis M.W. 2002. How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology, 83: 2664-2679. https://doi.org/10.2307/3072005
- Wimmer D., Hoffmann D., Schausberger P. 2008. Prey suitability of western flower thrips, Frankliniella occidentalis, and onion thrips, Thrips tabaci, for the predatory mite Amblyseius swirskii. Biocontrol Sci.Technol., 18: 533-542. https://doi.org/10.1080/09583150802029784



2023-01-27
Date accepted:
2023-09-19
Date published:
2023-10-04
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Barzkar, Maryam; Shishehbor, Parviz; Habibpour, Behzad; Hemmati, Seyed Ali and Riahi, Elham
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



