Sterilization makes a difference: demographic analysis of spirodiclofen effects on Tetranychus urticae (Acari: Tetranychidae)
Musa, Asma1
; Međo, Irena  2
; Marić, Ivana
2
; Marić, Ivana  3
and Marčić, Dejan
3
and Marčić, Dejan  4
4
1Faculty of Biology, University of Belgrade, Studentski trg 16, 11000 Belgrade, Serbia & Laboratory of Applied Entomology, Institute of Pesticides and Environmental Protection, Banatska 31B, P.O. Box 163, 11080 Belgrade, Serbia.
2Laboratory of Applied Entomology, Institute of Pesticides and Environmental Protection, Banatska 31B, P.O. Box 163, 11080 Belgrade, Serbia.
3Laboratory of Applied Entomology, Institute of Pesticides and Environmental Protection, Banatska 31B, P.O. Box 163, 11080 Belgrade, Serbia.
4✉ Laboratory of Applied Entomology, Institute of Pesticides and Environmental Protection, Banatska 31B, P.O. Box 163, 11080 Belgrade, Serbia.
2023 - Volume: 63 Issue: 3 pages: 955-968
https://doi.org/10.24349/qvon-22miOriginal research
Keywords
Abstract
Introduction
Demographic toxicological studies, based on the incorporation of life tables in the context of toxicology, have become an essential element in the laboratory evaluation of pesticide effects on insects and mites. This approach accounts both for the lethal and sublethal effects of pesticides/acaricides and integrates their impact at an ecologically more relevant level into population growth rate and other demographic parameters (Stark and Banks 2003; Guedes et al. 2016). The age-stage two-sex life table (Chi et al. 2020) has become a frequent tool lately in various subject areas of entomological and acarological research, including the evaluation of acaricide effects on plant-inhabiting mites (e.g. Musa et al. 2017, 2022; Bozhgani et al. 2019; Havasi et al. 2021; Rimy et al. 2021; Rajaee et al. 2022).
Acaricides are an important component of the integrated management of spider mites (Acari: Tetranychidae) and other phytophagous mite pests (Marčić 2012; Van Leeuwen et al. 2015). It is, therefore, necessary to evaluate their effects on mites thoroughly, putting an emphasis on population-level response. Spirodiclofen, a tetronic acid derivative, is probably the most widely sold acaricide in the world (Van Leeuwen et al. 2015; Sparks et al. 2020). As an inhibitor of acetyl coenzym A carboxylase, spirodiclofen is active against all developmental stages of spider mites, and it strongly affects the fecundity and fertility of female adults. The symptomatology of poisoning observed in T. urticae females is unique: their bodies grow to unusually large size due to inability to lay eggs which accumulate in the genital tract (a part of females is being sterilized i.e. they don′t lay eggs), and it take most of them several days to die (Nauen 2005; Van Pottelberge et al. 2009). Despite its success on the global market, there have been few demographic studies in which the effects of spirodiclofen on plant-inhabiting mite species were assessed using the age-stage two-sex life table (Alinejad et al. 2016; Sarbaz et al. 2017; Saber et al. 2018; Sani et al. 2019; Ahmed and Abdelwines 2021). All of these studies examined spirodiclofen effects on the F1 generation i.e., on the offspring of F0 females that survived treatments mostly with 1-3 concentrations ranging from LC10 to LC50.
The aim of this study was to expand the knowledge of demographic toxicology of spirodiclofen action on spider mites. The objective was to assess whether and how spirodiclofen as a relatively slow-acting acaricide, causing unique symptoms of poisoning, affects population growth of the F0 generation after treatment of pre-ovipositing T. urticae females with a wide range of concentrations. As a colonizing species, T. urticae is characterized by a high fecundity of young adult females (Sabelis 1985; Li and Margolies 1993). As spirodiclofen can lower significantly not only the female fecundity but fertility too, the age-stage two-sex life table was constructed in two variants: the fecundity-based life table, using the number of laid eggs, and the fertility-based life table, using the number of viable (hatched) eggs. Spirodiclofen effects were tested in a wide range of concentrations (i.e., a wide range of acaricide deposits on treated surface), from concentrations lethal to a part of treated females (and sublethal to the others) to concentrations sublethal to all treated females. The amount of an acaricide recommended for control of phytophagous mites is determined having in mind the goal that even the most tolerant individual in a population should absorb a sufficient amount of acaricide to cause its death i.e., to eliminate the entire population. In practice, however, acaricide distribution on treated plants is heterogeneous in space and time (Martini et al. 2012) which enables some individuals to survive as a result of absorbing smaller (sublethal) amounts of acaricides. In other words, spatial and temporal heterogeneity of spray coverage and exposure (variability of acaricide deposit on treated surface) in the field enables population recovery on poorly covered or uncovered plant surfaces, which depends on the reproductive potential of females that survived exposure.
Material and methods
Mite colony
A population of T. urticae, set up from samples collected on ruderal weeds in the vicinity of Belgrade, has been reared on bean plants in a climate-controlled room (25-30 ºC, 16/8 h light/dark photoperiod) in the Laboratory of Applied Entomology of the Institute of Pesticides and Environmental Protection, Belgrade, Serbia, since March 2004.
Acaricide
The commercial product Envidor (manufactured by Bayer AG, a suspension concentrate containing 240 g/l spirodiclofen) was purchased from the company representative in Serbia.
Life table bioassay
Primary kidney bean leaf discs (3 cm diameter) positioned upon moistened cotton wads in Petri dishes were used in a life table bioassay. The Petri dishes were kept in a Binder KBWF 720 plant growth chamber at 26 ± 1 ºC, under 60 ± 5% RH and 16/8 h light/dark photoperiod. The leaf discs were sprayed with the acaricide suspended in distilled water using an air-pressure sprayer (1 ml of liquid, 100 kPa air pressure, aqueous deposit 2.2 ± 0.2 mg/cm2).
To assess the effects of spirodiclofen on life-history traits and demographic parameters of T. urticae, life tables were constructed for 10 cohorts of mites. The cohorts were formed by placing a single fertilized female (2–3 days old) on each leaf disc and removing it after 24 h, as well as all eggs laid, except one. The development and survival of immature mites that hatched from those eggs were checked daily. Newly-emerged females were transferred individually to fresh leaf discs and paired with adult males. As new females normally outnumber males, the missing number of males was transferred from the mite colony. Nine cohorts of paired mites were sprayed with spirodiclofen, diluted in distilled water, in a series of concentrations (mg/l): 96 (the recommended field rate), 48, 24, 12, 6, 3, 1.5, 0.75 and 0.375. A control cohort was sprayed with distilled water only. The initial number of eggs per cohort was 70–420, depending on expected lethal and sublethal effect on females. After 24-h exposure to the acaricide, the surviving mites were transferred to untreated leaf discs. The mites were continuously transferred to fresh leaf discs on a daily basis, and their survival (both sexes) and the number of eggs laid (females) were recorded until all individuals in the cohort were dead. Leaf discs containing the eggs were kept for another 7-8 days under the same conditions, and then unhatched eggs were counted. Two variants of the age-stage two-sex life table were constructed: a fecundity-based life table, using the number of eggs laid, and a fertility-based life table, using the number of viable (hatched) eggs.
The following life-history traits were calculated: adult longevity (in days), fecundity (total number of eggs laid per female), oviposition days (number of days in which females laid eggs), and fertility (total number of viable eggs per female). Fecundity, fertility and longevity were calculated in two ways: based on the total number of females in cohort, Nf (fecundity-I, fertility–I, longevity-I), and based on the number of reproductive females, Nfr (fecundity-II, fertility-II, longevity-II). The proportion of reproductive females (Nfr/Nf), was calculated with respect to the entire duration of adult life, including the 24-h exposure to acaricide, and the total number of days after treatment that individual mites lived on untreated surface. Females that laid eggs (in fecundity-based life tables) and females that laid viable eggs (in fertility-based life tables) were considered as reproductive. Females that did not lay eggs after treatment were considered as sterilized.
Data analysis
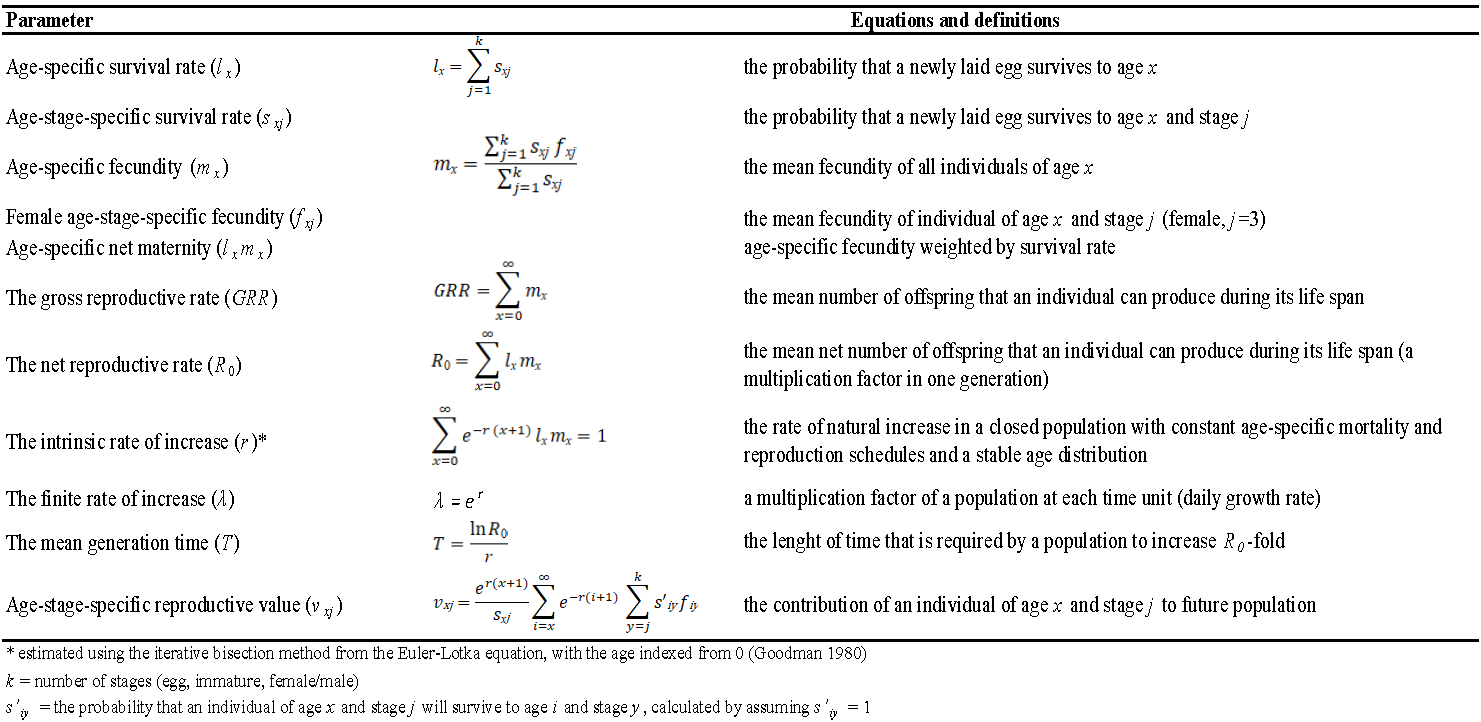


Raw life table data were analyzed according to the age-stage two-sex life table theory (Chi and Liu 1985; Chi 1988; Chi et al. 2020), using the software TWOSEX-MSChart (Chi 2021; Chi et al. 2022). Males obtained from the colony as well as escaped or drowned individuals were excluded from the analysis. The life table data included the number of eggs laid (fecundity-based life table) or the number of viable eggs (fertility-based life table). The demographic parameters are detailed in Table 1. Standard errors of the life-history traits and demographic parameters were estimated by the bootstrap technique with 50,000 bootstrap replicates. Differences between control and treatment were compared by the paired bootstrap test (50,000 replicates) based on the confidence interval of difference (Efron and Tibshirani 1993; Chi et al. 2020, 2022).
Results
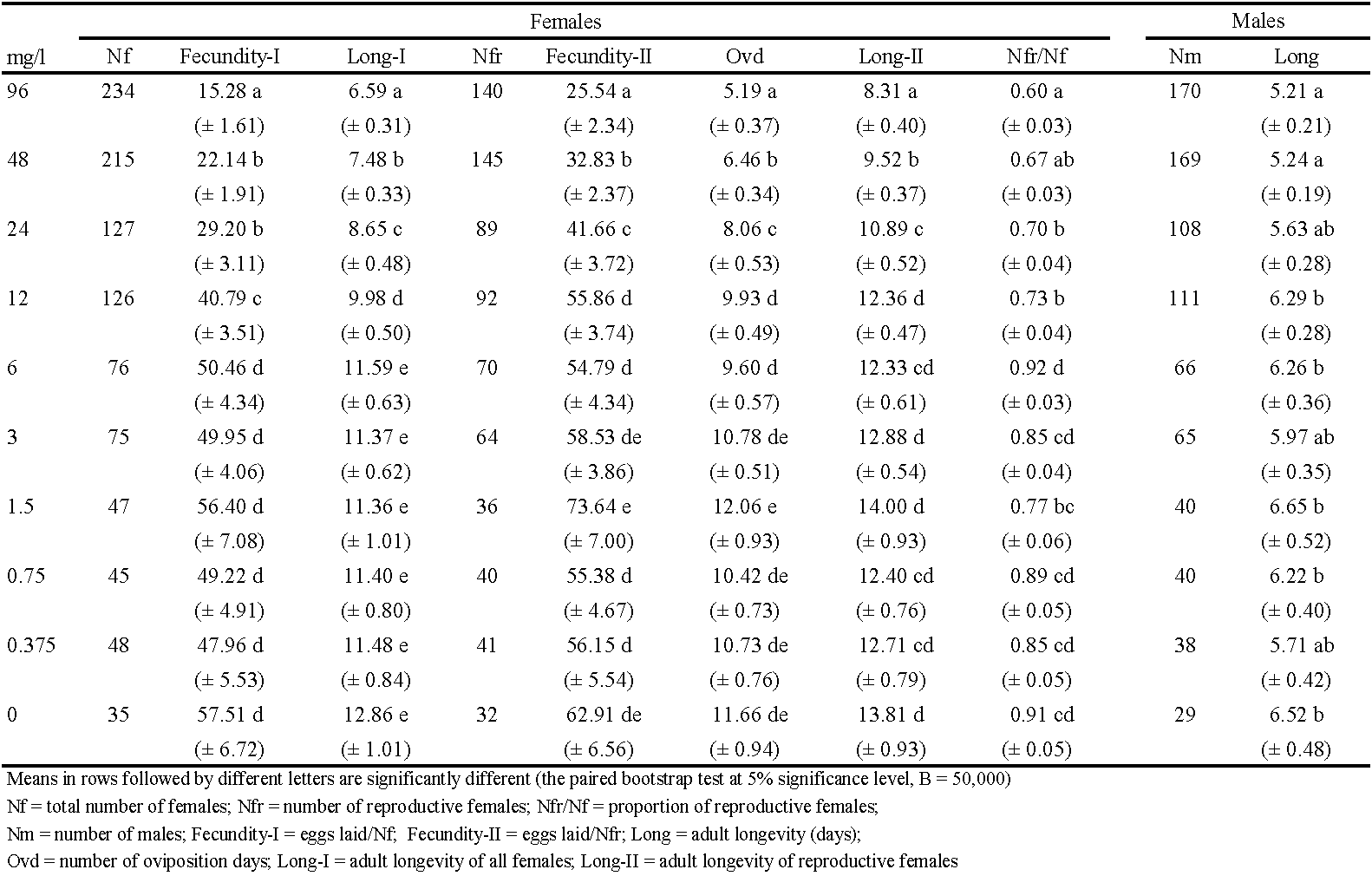


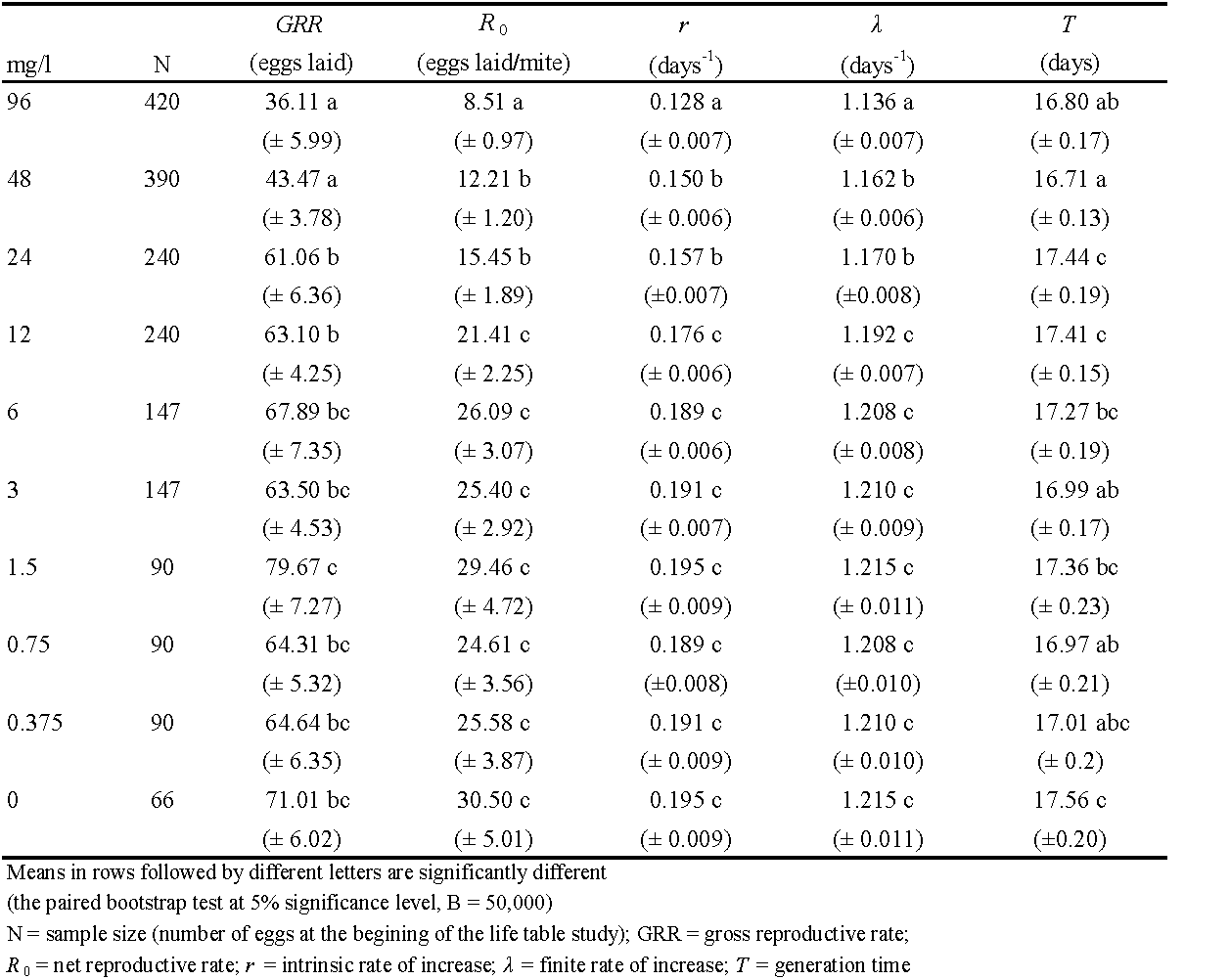
Exposure to spirodiclofen affected the longevity and reproduction of T. urticae females (Table 2). In treatments with the four highest acaricide concentrations (12 – 96 mg/l) the proportion of reproductive females (Nfr/Nf) was significantly lowered than in the control. It means that a considerable part of females (27 – 40%) failed to lay eggs. These four treatments also caused statistically significant reductions in fecundity-I and longevity-I (by 29 - 73% and 2.9 - 6.3 days, respectively, compared to the control). Considering reproductive females only, the three highest concentrations (24 – 96 mg/l) significantly reduced fecundity-II, longevity-II and oviposition duration in days (by 34 - 59%, 2.9 - 5.5 days, and 3.6 - 6.5 days, respectively, compared to the control). It is interesting that the highest values of fecundity-II were found after treatment with 1.5 mg/l concentration, but the difference from control values was not statistically significant. The two highest concentrations (48 - 96 mg/l) reduced significantly the adult longevity of males (Table 2).
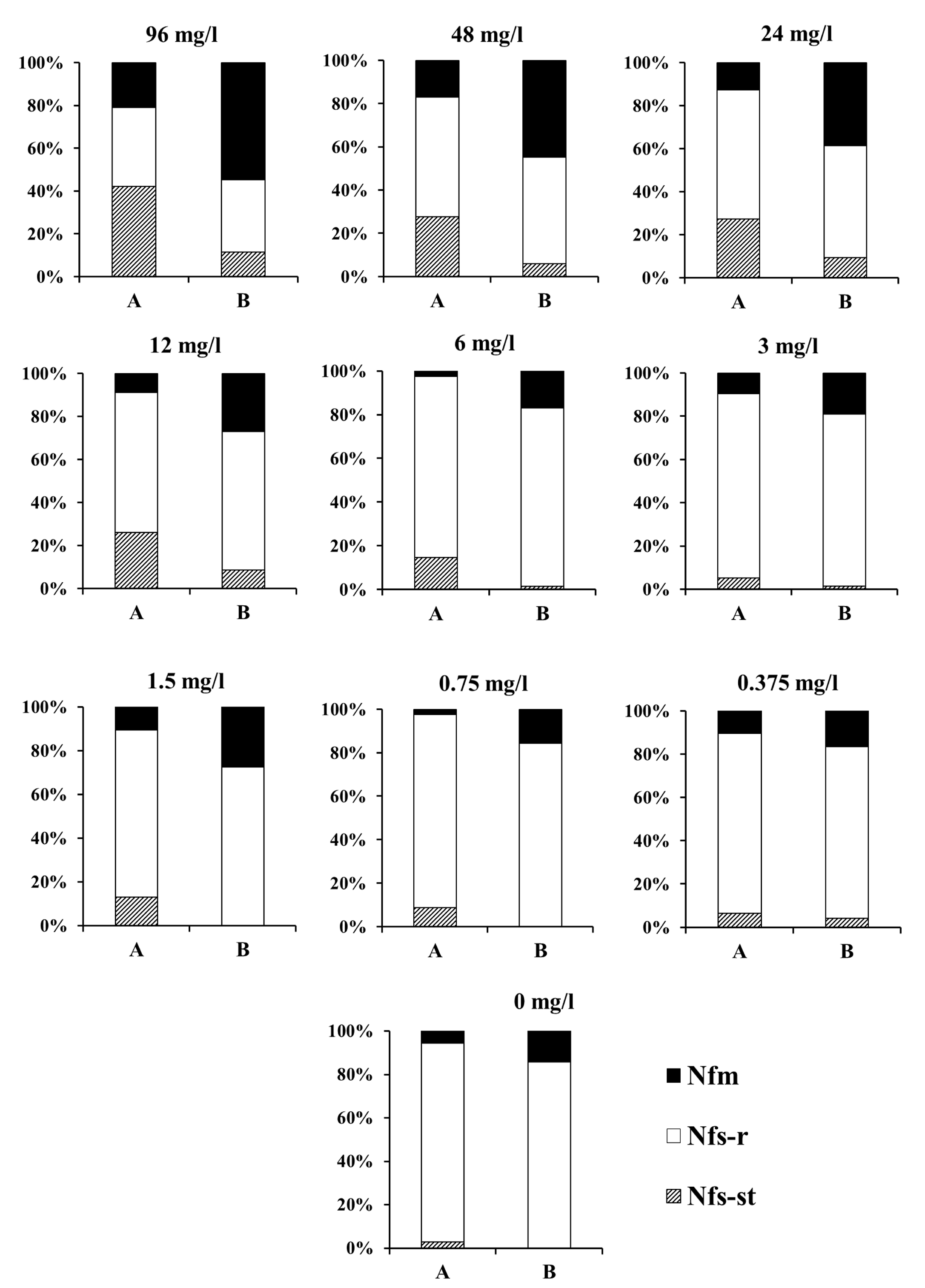
Figure 1 shows the proportion of reproductive females over the entire adult life, including the 24-h exposure to acaricide on treated leaf discs. Depending on acaricide concentration, exposure was found to be lethal to 2 - 21% treated females, most of which failed to lay a single egg during that time period. Regarding females that survived the exposure, a considerable part of them laid no eggs at all after treatment with concentrations 12 – 96 mg/l over the first four post-treatment days (when around 50% of all eggs in the control were laid). During that time period, the total percentage of dead females rose to 17 – 55%, mostly as a result of the deaths of those females that were sterilized by the toxic action of spirodiclofen (Figure 1).
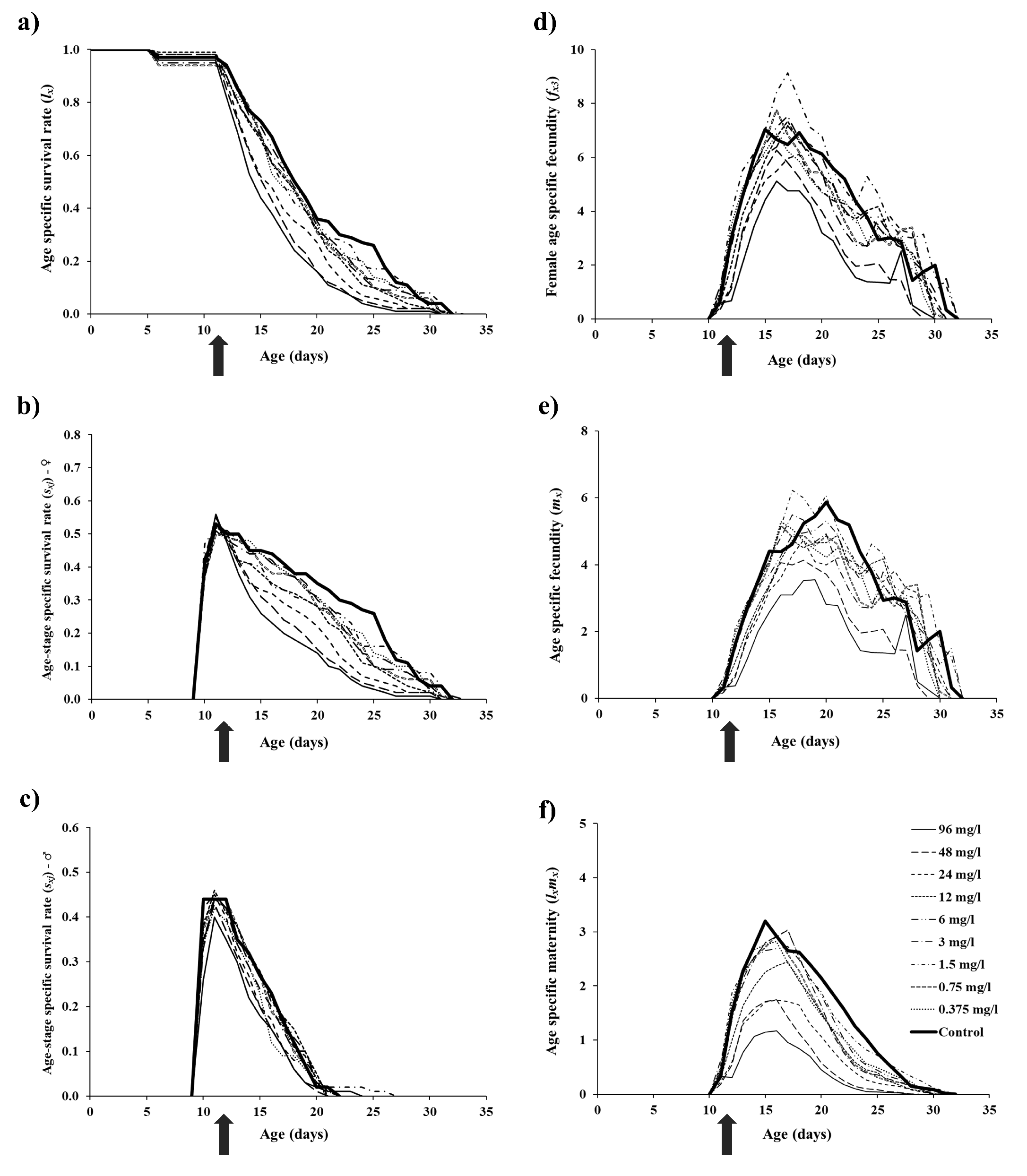
The age-specific survival and reproduction of T. urticae are presented in Figure 2. Spirodiclofen treatment caused a concentration-dependent decrease in age-specific survival rates (lx ), compared to the control. This effect is primarily due to a decrease in age-stage specific survival rates of females (Figure 2a-c). Regarding reproduction, both female age-specific fecundity (fxj ) and age-specific fecundity (mx ) in treatments were lower than control data over most of the oviposition period. The only exception was treatment with 1.5 mg/l concentration, where fxj and mx values were mostly above control data (Figure 2d-e). Resultingly, age-specific maternity (lxmx ) in all treatments was lower than it was in the control over the entire oviposition period. Treatment with 1.5 mg/l concentration remained an exception but only with several lxmx values exceeding control data (Figure 2f). The age-stage-specific reproductive value (vxj ) was affected by spirodiclofen in a similar manner as fecundity, i.e., vxj values of treated females were predominantly lower than control data over most of the oviposition period, the only exception being the concentration 1.5 mg/l, where the data exceeded the control (Figure 3).




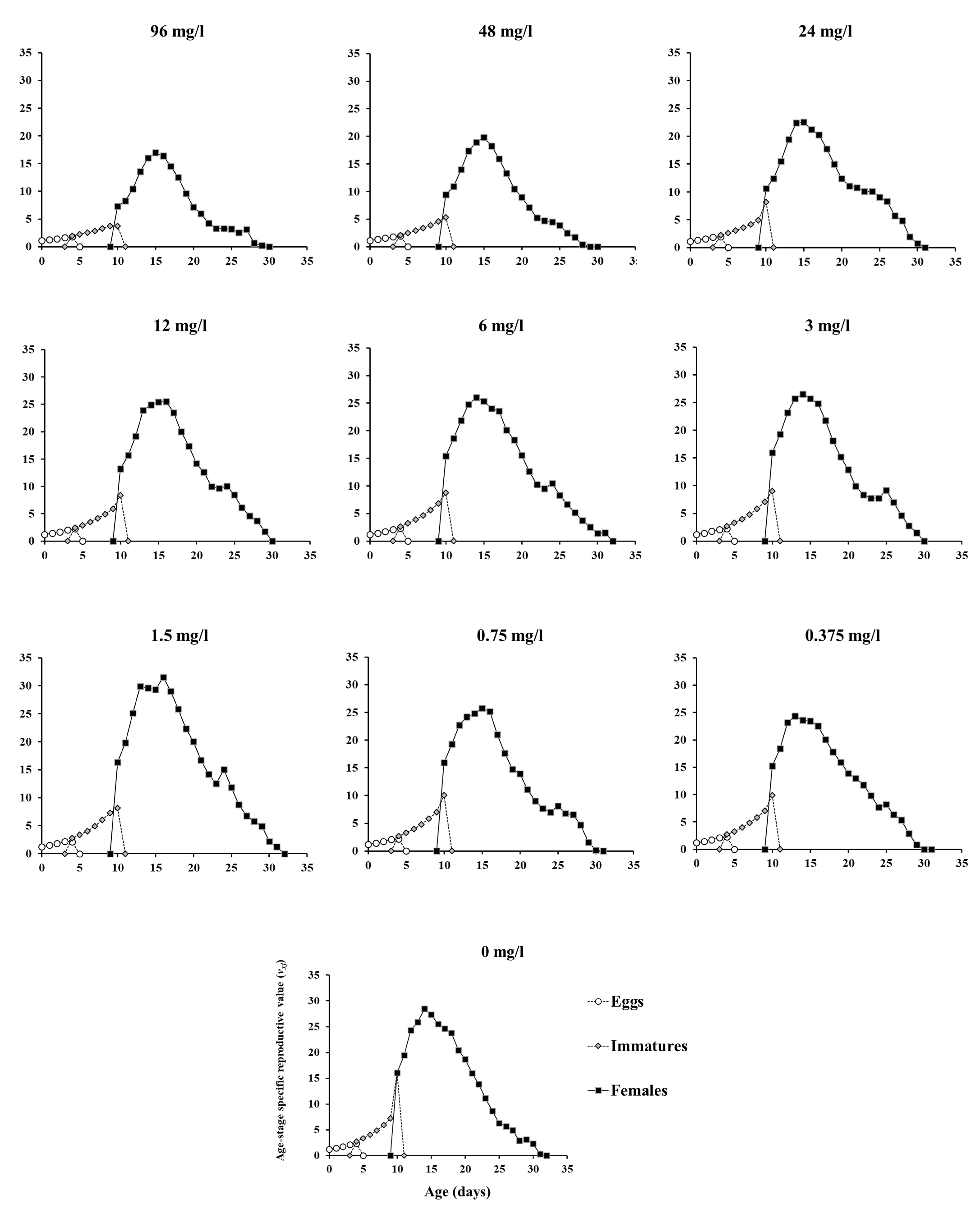


Demographic parameters are detailed in Table 3. The net reproductive rate (R0), intrinsic rate of increase (r) and finite rate of increase (λ) were significantly reduced by the three highest concentrations (by 49-72%, 20-34%, and 4-6%, respectively), compared to the control. In treatments with 96 mg/l concentration, these three demographic parameters were significantly lower also in comparison with treatments with concentrations of 48 mg/l and 24 mg/l. The gross reproductive rate (GRR) was significantly reduced by treatments with concentrations of 96 mg/l and 48 mg/l (by 39%, and 49%, respectively) compared to the control. As in the life history traits, the highest GRR value was found in treatment with 1.5 mg/l, but it was not significantly different from the control (Table 3).


The fertility-based life table gave a similar picture of spirodiclofen impact on the life-history traits and demographic parameters of T. urticae (Table 4, Table 5). The four highest concentrations caused statistically significant reduction in fertility-I (by 30 - 76%, compared to control values). These concentrations also significantly lowered the proportion of reproductive females (i.e., females that laid viable eggs), compared to the control. In these treatments, 30 - 62% of females produced no offspring at all as they were either sterilized or laid non-viable eggs. Regarding reproductive females only, fertility-II was significantly reduced by treatments with the three highest concentrations (by 26 - 41%, compared to the control). The highest value of fertility-II was recorded in the treatment with 1.5 mg/l, but it was not significantly different from the control data (Table 4).
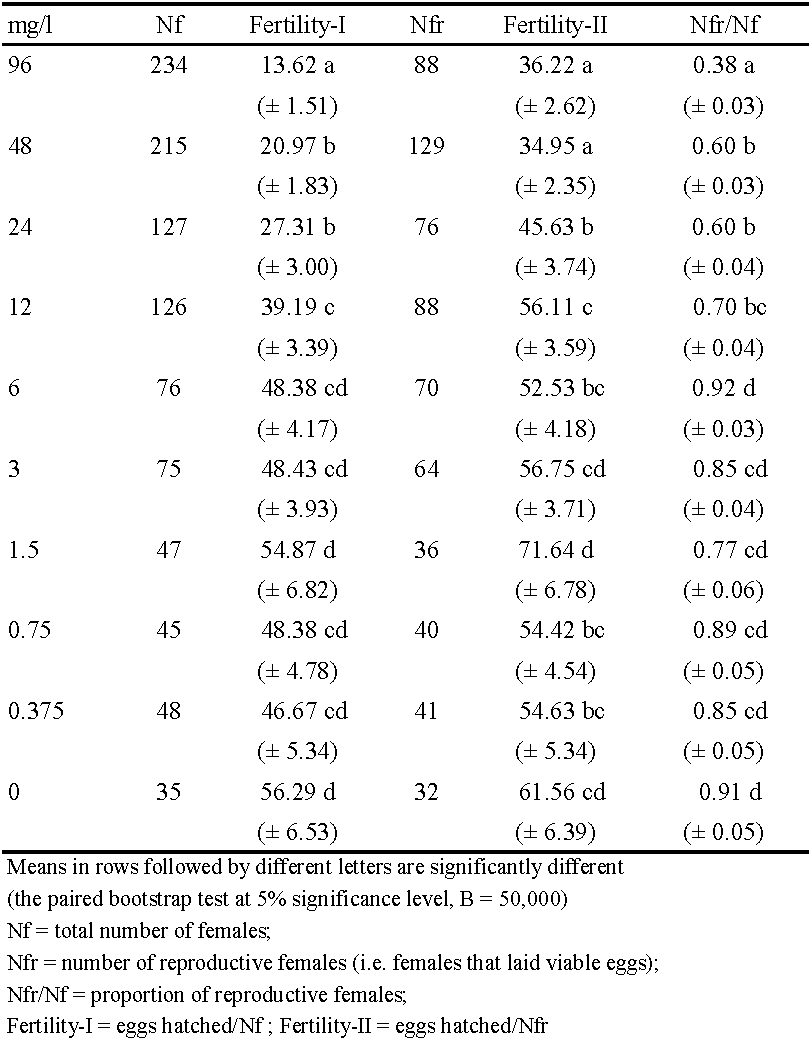




Compared to fecundity-I (Table 2), fertility-I was somewhat lower as maximum reduction was 11% in treatment with the highest concentration (Table 4). Fertility reduction was the consequence of two factors. The first factor was the residual toxic action of spirodiclofen on eggs laid on treated leaf discs during 24-h exposure. In treatments with concentrations of 3 - 96 mg/l, the percentage of unhatched eggs ranged between 17% and 98%, while it was less than 2% after treatments with concentrations of 0.375 – 1.5 mg/l and in the control. The second factor was transovarial toxic effect on eggs laid on untreated surface following the exposure, which was most evident in treatments with the three highest concentrations. However, this effect was short-lived: the percentage of unhatched eggs was 13- 32% on the first day, 8-20% on the second day, while it remained between 2% and 4% from the third day until the end of trial, which is similar to the other treatments and the control. Residual toxicity to eggs laid on treated discs was the primary contribution towards reduced proportion of reproductive females in treatments with concentrations of 12 – 96 mg/l. Resulting from the lower number of reproductive females (compared to fecundity-based life tables) fertility-II was higher than fecundity-II in those treatments.
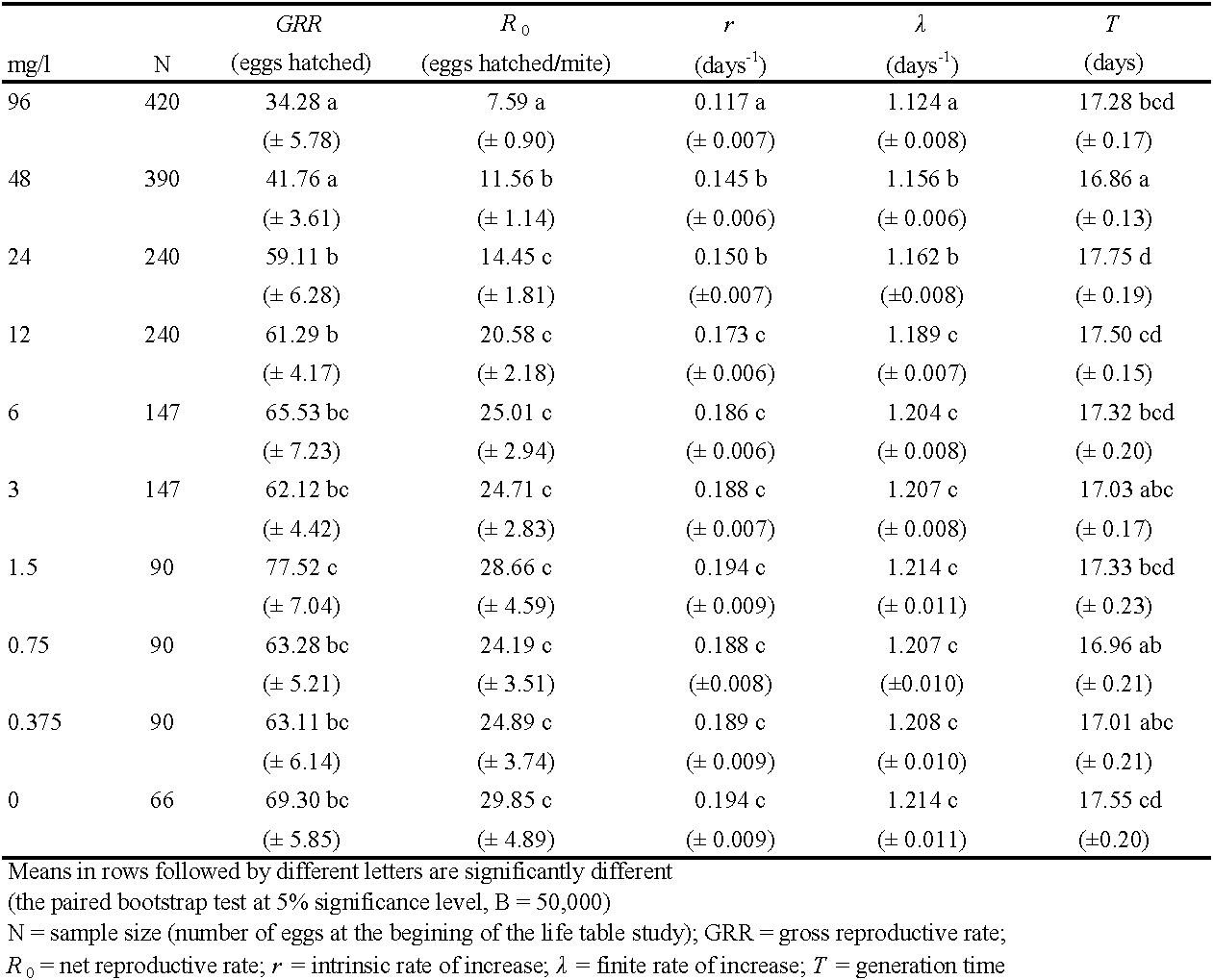
Demographic parameters obtained from fertility-based life tables are detailed in Table 5. Spirodiclofen concentration that significantly reduced fertility-II (24 – 96 mg/l) also decreased r and λ significantly (by 23-40%, and 4-7%, respectively) compared to the control. The concentrations 96 mg/l and 48 mg/l significantly reduced GRR (by 50% and 40%, respectively) and R0 (by 75% and 61%, respectively), compared to the control. The highest GRR value was recorded after treatment with 1.5 mg/l, but this value was not significantly different from the control (Table 5). All these parameters were lower than those acquired from fecundity-based life tables but the differences had no statistical significance (the paired bootstrap test at 5% significance level, B = 50,000).
Discussion
Our demographic study analyzed spirodiclofen effects on the survival, reproduction and population growth of the F0 generation, considering that high reproduction of young fertilized females is crucial for the population biology of T. urticae as a colonizing species (Sabelis 1985; Li and Margolies 1993). The fecundity-based life table revealed that a significant reduction in population growth was mainly due to sterilization of females (i.e., their inability to lay eggs), followed by their high mortality, and additionally reduced fecundity and longevity of reproductive females. Sterilization was the most evident during the initial period of reproduction, which primarily determines the magnitude of r (Birch 1948; Snell 1978). Sterilization of T. urticae females by intoxication by spirodiclofen, accompanied by accumulation of eggs in the genital tract and body swelling, had been reported in previous studies (Nauen 2005; Marčić 2007; Van Pottelberge et al. 2009).
Demographic analyses of the effects of spirodiclofen on population growth of spider mites using the age-stage two-sex life table have been carried out in few studies so far. Regarding T. urticae, Saber et al. (2018) treated females at the LC25 level and observed significant reductions in R0 , r and λ of the offspring of treated females. Sani et al. (2019) found that treatement of females at the LC15 and LC35 levels reduced significantly the same parameters in their offspring. Considering that the effects were examined on the F1 generation, these results cannot be compared to our study. Ahmed and Abdelwines (2021) also found significant reduction in population growth of the F1 generation the citrus red mite, Panonychus citri McGregor, following of exposure of parental females to LC20. Marčić (2007) analyzed the effects of spirodiclofen on population growth of T. urticae F0 generation and found significant reduction in R0 , r and λ following treatment of pre-ovipositing females with 12 mg/l. However, a different type of life table - the female age-specific life table (Birch 1948; Carey 1993) - was used in the study. This type of life table ignores males and natural variations in juvenile developmental times among females (Chi et al. 2020).
Our analysis of spirodiclofen effects showed that concentrations that are as many as four times lower than the recommended field rate (i.e. leaving 4-fold thinner acaricide deposit on plant surface) may cause significant reduction in T. urticae population growth. This finding has practical value, considering that spray coverage in the field is spatially heterogeneous (Martini et al. 2012). On the other hand, the highest values of GRR, fecundity-II and fertility-II were noted after treatment with the concentration of 1.5 mg/l, which is 64-fold lower than the field rate. Such stimulated reproduction was caused by the concentration entering the hormetic zone, i.e. within a concentration range which is up to 10-fold lower than the highest concentration that has no significant effect (Guedes and Cutler 2014). However, GRR, fecundity-II and fertility-II in the treatment with 1.5 mg/l concentration were significantly higher, compared to the treatments with concentrations of 12 - 96 mg/l, but not compared to the control. For this reason, it is hard to regard it as hormesis, which is defined as a concentration-response relationship in which low concentrations cause stimulation, whereas high concentrations act inhibitory. Besides, the maximum magnitude of stimulation in hormetic response is mostly 30–60% greater than control magnitude (Guedes and Cutler 2014), while it was no more than 17% in this study. However, it is still possible that a bioassay with more concentrations within the hormetic zone would yield response that fit the hormetic model both in quantitative and qualitative terms.
The fertility-based life table did not change the general picture of spirodiclofen effects generated by the fecundity-based variant of life table. Short-lived and weak transovarial toxic effect (assumed to be caused by transovarial transfer of the uptaken acaricide from the maternal body to eggs) did not reduce significantly the fertility, nor consequently the population parameters, compared to the same parameters acquired in the fecundity-based life table. Short-lived reductions in T. urticae fertility had been also reveiled in other studies involving spirodiclofen (Marčić 2007; Van Pottelberge et al. 2009). Transovarial toxicity to eggs laid by treated T. urticae females was also noted for spiromesifen (Marčić et al. 2010), as well as acaricides that inhibit chitin biosynthesis, such as etoxazole (Sáenz-de-Cabezón Irigaray and Zalom 2012) and hexythiazox (Adesanya et al. 2018). In our previous study (Musa et al. 2022), hexythiazox was also applied when T. urticae females were at the pre-oviposition period. The fertility-based variant of the age-stage two-sex life table revealed a significant decrease in population parameters, mostly caused by the transovarial toxicity of hexythiazox to eggs laid by treated females. Fertility reduction during the initial few days of oviposition, although short-lived, was sufficient to cause a significant reduction in population growth. On the other hand, application of fecundity-based life tables underestimated the population-level effects of hexythiazox on T. urticae (Musa et al. 2022). Although more labour-intensive, application of the fertility-based variant of age-stage two-sex life table is necessary for evaluation of acaricides that may exhibit transovarial toxicity, such as those interfering with growth and development of mites.
Considering the biological profile of spirodiclofen toxicity to T. urticae life stages (Wachendorff et al. 2002; Nauen 2005), as well as our own data on its residual toxicity to eggs laid during exposure, concentrations that sterilized females in our trial or reduced their reproduction could be considered as highly toxic to eggs and immatures on treated surface. Therefore, the recovery potential of a population mainly depends on females reaching leaf surface free of or poorly covered by acaricide deposit. Although sterilization and reproductive reduction significantly weakened the recovery potential of these females, it still remained considerable. On the other hand, the fact that spirodiclofen concentrations below recommended rate reduce populations growth leaves a possibility for combining reduced rates of the acaricide with predatory mites within an integrated spider mite management program.
In conclusion, our demographic analysis showed that spirodiclofen significantly reduced population growth rates of T. urticae when it was applied against pre-ovipositing females in concentrations up to 4-fold lower than the recommended rate. Application of the fecundity-based variant of age-stage two-sex life table showed that this effect was mainly due to sterilization of treated females and their high mortality in the initial period of oviposition, in combination with reduced fecundity and longevity of reproductive females. The short-lived transovarial toxic effect observed in the fertility-based variant was not sufficient to cause a significant reduction in population parameters, compared to parameters acquired by the fecundity-based variant. Future research may focus on the evaluation of transgenerational population-level effects of spirodiclofen, applied in a wide range of concentrations.
Acknowledgements
This research was funded by the Ministry of Science and Technological Development of the Republic of Serbia, Grant No. 451-03-47/2023-01/ 200214.
Author contribution
D.Marčić conceived the original idea, D.Marčić and I. Međo planned the experiments, A. Musa, I. Međo and I. Marić carried out the experiments, D. Marčić and I. Međo analysed the data, D. Marčić wrote the manuscript.
References
- Adesanya A.W., Morales M.A., Walsh D.B., Lavine M., Lavine L., Zhu F. 2018. Mechanisms of resistance to three mite growth inhibitors of Tetranychus urticae in hops. Bull. Entomol. Res., 108: 23-34. https://doi.org/10.1017/S0007485317000414
- Ahmed M.M., Abdelwines M.A. 2021. Sublethal effects of cyflumetofen and spirodiclofen on biological parameters of citrus red mite, Panonychus citri McGregor (Acari: Phytoseiidae). Pers. J. Acarol., 10: 467-480. https://doi.org/10.22073/pja.v10i4.68693
- Alinejad M., Kheradmand K., Fathipour Y. 2016. Assessment of sublethal effects of spirodiclofen on biological performance of the predatory mite Amblyseius swirskii. Syst. Appl. Acarol., 21: 375-384. https://doi.org/10.11158/saa.21.3.12
- Birch L.C. 1948. The intrinsic rate of natural increase of an insect population. J. Animal Ecol., 17: 15-26. https://doi.org/10.2307/1605
- Bozhgani N.S.S., Kheradmand K., Talebi A.A. 2019. The effects of spiromesifen on life history traits and demographic parameters of predatory mite Neoseiulus californicus (Acari: Phytoseiidae) and its prey Tetranychus urticae Koch (Acari: Tetranychidae). Syst. Appl. Acarol., 23:1512-1525. https://doi.org/10.11158/saa.24.8.11
- Carey J.R. 1993. Applied demography for biologists, with special emphasis on insects. New York - Oxford: Oxford University Press. pp. 206.
- Chi H. 1988. Life table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol., 17: 26-34. https://doi.org/10.1093/ee/17.1.26
- Chi H. 2021 TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. http://140.120.197.173/Ecology/
- Chi H., Liu H. 1985. Two new methods for the study of insect population ecology. Acad. Sin. Bull. Inst. Zool., 24: 225-240.
- Chi H., You M., Atlihan R., Smith C.L., Kavousi A., Özgökçe M.S. et al. 2020. Age-stage, two-sex life table: an introduction to theory, data analysis, and application. Entomol. Gen., 40: 103-124. https://doi.org/10.1127/entomologia/2020/0936
- Chi H., Güncan A., Kavousi A., Gharakhani G., Atlihan R., Özgökçe M.S. Shirazi J., Amir-Maafi M., Maroufpoor M., Taghizadeh R. 2022. TWOSEX-MS Chart: the key tool for life table research and education. Entomol. Gen., 42: 845-849. https://doi.org/10.1127/entomologia/2022/1851
- Efron B., Tibshirani R.J.1993. An introduction to the bootstrap. New York: Chapman & Hall. pp. 456. https://doi.org/10.1007/978-1-4899-4541-9
- Guedes R.N.C., Cutler G.C. 2014. Insecticide-induced hormesis and arthropod pest management. Pest Manag. Sci., 70: 690-697. https://doi.org/10.1002/ps.3669
- Guedes R.N.C., Smagghe G., Stark J.D., Desneux N. 2016. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol., 61: 43-62. https://doi.org/10.1146/annurev-ento-010715-023646
- Havasi M., Alsendi A., Bozhgani N.S.S., Kheradmand K., Saadeghi R. 2021. The effects of bifenazate on life history traits and population growth of Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae). Syst. Appl. Acarol., 26: 610-623. https://doi.org/10.11158/saa.26.3.10
- Li J., Margolies D.C. 1993. Effects of mite age, mite density, and host quality on aerial dispersal behavior in the twospotted spider mite. Entomol. Exp. Appl., 68: 79-86. https://doi.org/10.1111/j.1570-7458.1993.tb01691.x
- Marčić D. 2007. Sublethal effects of spirodiclofen on life history and life-table parameters of twospotted spider mite (Tetranychus urticae). Exp. Appl. Acarol., 42: 121-129. https://doi.org/10.1007/s10493-007-9082-1 https://doi.org/10.1007/s10493-007-9082-1
- Marčić D. 2012. Acaricides in modern management of plant-feeding mites. J. Pest Sci., 85: 395-408.
- Marčić D., Ogurlić I., Mutavdžić S., Perić P. 2010. The effects of spiromesifen on life history traits and population growth of two-spotted spider mite (Acari: Tetranychidae). Exp. Appl. Acarol., 50: 255-267. https://doi.org/10.1007/s10493-009-9316-5
- Martini X., Kincy N., Nansen C. 2012. Quantitative impact assessment of spray coverage and pest behavior on contact pesticide performance. Pest. Manag. Sci., 68: 1471-1477. https://doi.org/10.1002/ps.3330
- Musa A., Međo I., Marić I., Marčić D. 2017. Acaricidal and sublethal effects of a Chenopodium-based biopesticide on the two-spotted spider mite (Acari: Tetranychidae). Exp. Appl. Acarol., 71: 211-226.
- Musa A., Međo I., Marić I., Marčić D. 2022. Transovarial toxicity matters: lethal and sublethal effects of hexythiazox on the two-spotted spider mite (Acari: Tetranychidae). Exp. Appl. Acarol., 87: 175-194. https://doi.org/10.1007/s10493-022-00733-8
- Nauen R. 2005. Spirodiclofen: mode of action and resistance risk assessment in tetranychid pest mites. J. Pestic. Sci., 30: 272-274. https://doi.org/10.1584/jpestics.30.272
- Rajaee F., Ghane-Jahromi M., Maroofpour N., Sedaratian-Jahromi A. 2022. Sublethal effects of spiromesifen on life table traits of Tetranychus urticae (Acari: Tetranychidae) and Neoseiulus californicus (Acari: Phytoseiidae). Acarologia, 62: 772-785. https://doi.org/10.24349/uja8-5ks2
- Rimy S.J., Das G., Gotoh T., Ullah M.S. 2021. Lethal and sublethal effects of bifenazate on the biological parameters of Tetranychus truncatus Ehara (Acari: Tetranychidae). Syst. Appl. Acarol., 26: 2118-2132. https://doi.org/10.11158/saa.26.11.12
- Sabelis M.W. 1985. Reproductive strategies. In: Helle W., Sabelis M.W. (Eds). Spider mites, their biology, natural enemies and control. Amsterdam: Elsevier, p. 265-278.
- Saber M., Ahmadi Z., Mahdavinia G. 2018. Sublethal effects of spirodiclofen, abamectin and pyridaben on life-history traits and life-table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol., 75: 55-67. https://doi.org/10.1007/s10493-018-0226-2
- Sáenz-de-Cabezón Irigaray F.J., Zalom F.G. 2012. Transovarial biotransference of etoxazole through a terrestrial trophic web. Pest Manag. Sci., 68: 1467-1470. https://doi.org/10.1002/ps.3329
- Sani N.S, Kheradmand K., Talebi A.A. 2019. Sublethal effects of spirodiclofen on the demographic parameters of Tetranychus urticae Koch (Acari: Tetranychidae). Arch. Phyt. Plant Prot., 52: 938-952. https://doi.org/10.1080/03235408.2019.1668593
- Sarbaz S., Goldasteh S., Zamani A.A., Solymannejadiyan E., Shoushtari R.V. 2017. Side effects of spiromesifen and spirodiclofen on life table parameters of the predatory mite, Neoseiulus californicus McGregor (Acari: Phytoseiidae). Int. J. Acarol., 43: 380-386. https://doi.org/10.1080/01647954.2017.1325396
- Snell T.W. 1978. Fecundity, developmental time and population growth rate. Oecologia, 32: 119-125. https://doi.org/10.1007/BF00344696
- Sparks T.C., Crossthwaite A.J., Nauen R., Banba S., Cordova D., Early F., Ebbinghaus-Kintscher U., Fujioka S., Hirao A., Karmon D., Kennedy R., Nakao T., Popham H.J.R., Salgado V., Watson G.B., Wedel B.J., Wessels F.J. 2020. Insecticides, biologics and nematicides: updates to IRAC's mode of action classfication - a tool for resistance management. Pestic. Biochem. Physiol., 167: 104587. https://doi.org/10.1016/j.pestbp.2020.104587
- Stark J.D., Banks J.E. 2003. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol., 48: 505-519. https://doi.org/10.1146/annurev.ento.48.091801.112621
- Taghizadeh R., Chi H. 2022. Demography of Tetranychus urticae (Acari: Tetranychidae) under different nitrogen regimes with estimations of confidence intervals. Crop Prot., 155: 105920. https://doi.org/10.1016/j.cropro.2022.105920
- Tuan S.J., Lin Y.H., Yang C.M., Atlihan R., Saska P., Chi H. 2016. Survival and reproductive strategies in twospotted spider mites: demographic analysis of arrhenotokous parthenogenesis of Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol., 109: 502-509.
- Van Pottelberge S., Khajehali J., Van Leeuwen T., Tirry L. 2009. Effects of spirodiclofen on reproduction in a susceptible and resistant strain of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol., 47: 301-309. https://doi.org/10.1007/s10493-008-9226-y
- Van Leeuwen T., Tirry L., Yamamoto A., Nauen R., Dermauw W. 2015. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic. Biochem. Physiol., 121: 12-21. https://doi.org/10.1016/j.pestbp.2014.12.009
- Wachendorff U., Nauen R., Schnorbach H.J., Rauch N., Elbert A. 2002. The biological proWle of spirodiclofen (Envidor®) - a new selective tetronic acid acaricide. Pflanzenschutz-Nachrichten Bayer 55: 149-176.



2023-02-21
Date accepted:
2023-07-16
Date published:
2023-08-04
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Musa, Asma; Međo, Irena; Marić, Ivana and Marčić, Dejan
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



