Lorryia (Acariformes, Tydeidae): The evolutionary plasticity of an enigmatic genus
André, Henri M.  1
1
1✉ Retired, currently, 22, route de la Côte, CH-1615 Bossonnens, Switzerland & Musée royal de l’Afrique centrale, B-3080 Tervuren, Belgium & Université Catholique de Louvain, B-1348 Louvain-la-Neuve, Belgium.
2023 - Volume: 63 Issue: 3 pages: 844-855
https://doi.org/10.24349/6btk-vaccZooBank LSID: A801B041-DAD8-4C71-A953-7C1F028A2450
Original research
Keywords
Abstract
\def
Introduction
The genus Lorryia was briefly described by Oudemans (Oudemans, 1925). His description took only one line: ''Lorryia=Tydeus met ééne klauw aan elken poot !'' which means ''Lorryia=Tydeus with one claw to each leg!''. After a preliminary description of 5 lines, a further description of the type species, Lorryia superba, was provided in a subsequent publication and comprised more than three pages with a plate of 9 figures (Oudemans, 1928a: 229-231, 233). The paper ended up with the closing statements: ''Afwijkend van andere Tydeus-soorten is: de netvormige skulptuur, en de povere bewapening van den tibia-tarsus van den palp''. (Different from other Tydeus species: the reticulate pattern and the poor armament of the tibia-tarsus of the palp). The second part of the concluding remark was neglected by all subsequent authors, with exception of Baker's revision (Baker, 1968). The description was based on a single specimen collected on Viverra sp. (mammalian genus) in Ambon (city of the Indonesian province of Maluku). The presence of only one claw was confirmed in a subsequent publication (Oudemans, 1928b: 380).
In 1929, a new diagnosis based exclusively on the reticulate pattern was provided by Oudemans (1929: 481) but was not cited in Baker's seminal paper (Baker, 1965). The genus was actually recognized by Baker (1965) but, contrarily to the description, with the usual palpal setal pattern, (5-2-2) or (5-1-2). As noted by Baker in his revision of the genus (1968: 986), ''If Oudemans' figure is correct, Lorryia will stand with a single species''. On the next page, Baker took distance from Oudemans (1925, 1928a, b) and explained he was ''interpreting the genus on the basis of the description by Baker (1965)'', i. e. on the reticulate pattern only. Lorryia included 32 species, among which Oudemans' superba. That the claw and palpal setal patterns are not typical ''must be misinterpretations'' swept away Baker (1968: 986). As for Brachytydeus, a genus erected by Thor (1931), it was considered as a junior subjective synonym of Tydeus (Baker and Wharton, 1952: 191; Baker, 1965: 99; André and Naudo, 1965: 673).
A second interpretation (Figure 1) is similarly based on the reticulation but is more restrictive as it, moreover, requires a particular setal femoral pattern on legs I-IV respectively: (3-3-2-1). This interpretation was advanced by Kaźmierski (1989, 1998). The single specimen of Lorryia superba is supposed to be an abnormal individual (Kaźmierski, 1998: 301; Kaźmierski and Sikora, 2008: 88) or is considered as such (Mondin et al., 2016: 474; Kaźmierski et al., 2018: 813). In that context, Brachytydeus is considered as a junior synonym of Lorryia (Akbari et al., 2015; Mondin et al., 2016; Kaźmierski et al., 2018; Da-Costa et al., 2019).



A third interpretation was advanced by André (2005) and followed in the world catalogue published by Silva et al. (2016a). Following the peculiarities described by Oudemans, Lorryia would be monospecific as admitted by Baker (1968: 986) and Brachytydeus would include 200 species in the world catalogue (Silva et al., 2016a). This interpretation is followed by Tempfli et al. (2015) and Silva et al. (2016b).
Currently, Baker's interpretation is no longer in use. The last two interpretations gave rise to concern (''preocupaçã'' in Portuguese) or even to ''conflictual'' approaches (Silva et al. 2015: 21). This divergence of opinion is well illustrated by the following quotes on Lorryia: ''For purposes of this treatment, the current generic concepts of earlier works are retained'' (Walter et al. (2009: 246) in the 3rd ed. of The Manual of Acarology); in other words, interpretation 3 is acknowledged but not used. These divergent approaches have practical consequences in many areas, systematics based on morphospecies, phylogeny based on molecular data, morphology, agriculture, horticulture, and on the resulting numerous publications (Figure 1). These divergent interpretations go hand-in-hand with multiple combinations. For example, five combinations have been used for Cooreman's species:
- Lorryia formosa, the original combination used by many authors;
- Brachytydeus formosa, subsequent combination used by Lorençon et al. (2016) in morphology: Diaz et al. (2015), Pedro Neto et al. (2015), Johann et al. (2017) and Berton et al. (2019) in agriculture; Klimov et al. (2018: 106) for molecular data;
- Brachytydeus formosus with the gender agreement adopted by Kaźmierski (2008: 363) and Mondin et al. (2015);
- Tydeus formosus, with the gender agreement addressed by Flechtmann (1987) and Fenilli and Flechtmann (1990: 244); and
- Tydeus formosa by Schiess (1981: 90), Nucifora (1984: 140-141), Prota et al. (1984: 151) and Vacante and Nucifora (1986: 181).
The goal of the present paper is to revisit Oudemans' description, to examine the interpretations of the genus Lorryia, to discuss the types of criteria that are associated and to describe the consequences on the naming of some 200 species. The story of the genus was outlined previously (André, 2005).
Material and methods
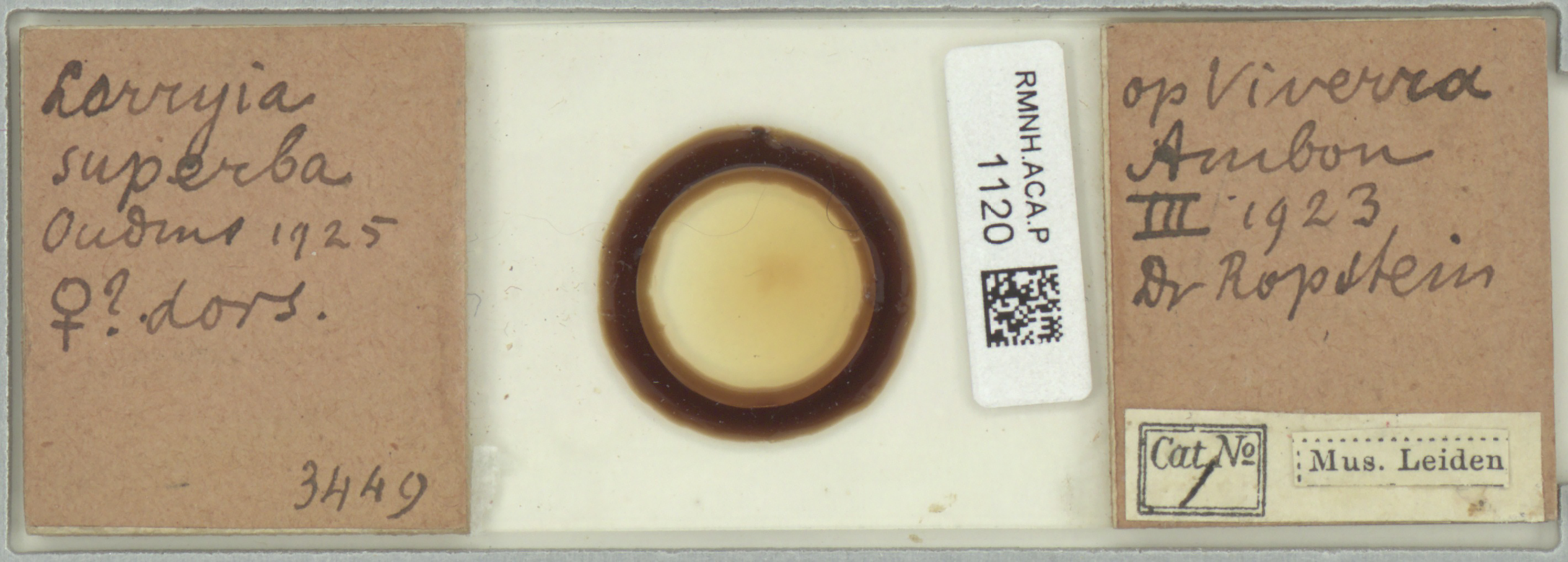
Lorryia superba, the type species of the genus, is represented by a unique specimen. This type, not seen by Baker (1968: 986), was supposed to be lost (Kaźmierski, 1998: 301; André, 2005: 994; Silva et al., 2016a: 4). According to Buitendijk (1945: 323), this type was represented only by drawings but the slide is displayed on line by the Naturalis Biodiversity Center, Leiden, The Netherlands (slide RMNH.ACA.P.1120).
As Naturalis Biodiversity Center recently changed its loan-policy and does no longer send out primary types on loan (Bram van der Bijl, mail 22-02-2023), the original slide was examined by Daan Drukker and Harry Smit from Naturalis. The original slide clearly designates Oudemans's species and its provenance (Figure 2). However, the mite was not visible on the slide (Daan Drukker, mail 02-05-2023).


The original description was therefore revisited in light of modern ideas, for example the dactyly, even if some ideas and concepts were unknown to Oudemans, for example the phanerotaxy (chaetotaxy and solenidiotaxy). This approach is completed by some of Oudemans' plates which have not been published but were listed in the catalog of Buitendijk (1945) and are available on the net (see Wikispecies pages), courtesy of the Naturalis Biodiversity Center. Moreover, Oudemans' drawings reproduced by Thor (1933) were also studied.
For the sake of clarity, the 5 figures of this article are designated in full (figure). When referenced, the original illustrations of other papers are abbreviated (fig., figs) and often preceded by a possessive adjective (his, her...)
Results
The detailed description of L. superba written in Dutch and published in 1928 (Oudemans, 1928a) is unusually long: two pages (975 words) plus a plate with nine figures numbered from 95 to 103 and reproduced in figure 3. For comparison, the description of Tydeus subterraneus takes 10 lines and that of T. tiliae, 8 lines (Oudemans, 1929). Dorsal and ventral views of L. superba are provided in his figs 96 and 98 (reproduced by Thor, 1933, his figs 8, 65).
The apotele with a single claw is illustrated in three drawings (figs 95, 100, 102). The last figure shows the scar of the missing claw (arrow in figure 3) similarly to the scars depicted by Kaźmierski and Sikora (2008) on an ''abnormal'' specimen with a single claw on some legs. Such scars have not been commented on. Fig. 102 has never subsequently been reproduced in contrast to fig. 96, which represents a dorsal view of the entire animal and was reproduced later by Thor (1933: 55). Fig. 96 is critical in the sense that the presence of only one claw is visible on all the legs.



The leg phanerotaxy —the number of ''borstels'' (setae) as written by Oudemans— is partly described in the text: I(3-1-2-1-?), II(2-1-2-1-?), III(1-1-1-1-?), IV(1-1-1-?-?). The phanerotaxy represented on the drawings was slightly richer: I(5-3-3-3-1), II(5-2-2-3-0), III(3-2-1-2-1), IV(3-1-1-1-0). The formula is obtained by adding the setae drawn on two figs, 96 (dorsal view) and 98 (ventral view). This supposes that Oudemans was able to distinguish between dorsal and ventral setae, which is not necessarily true. The chaetotaxy of tarsi with 3 or 5 setae has never been reported in Tydeoidea, even in species with a severely reduced chaetotaxy. Lastly, there are some discrepancies between the text and the figures: for instance, Oudemans wrote specifically ''tibiae III en IV en tarsi III en IV ieder met een borstel'' (tibiae III and IV and tarsi III and IV each with one seta) while he drew two on tibia III, only one on tibia IV, and three on tarsi III and IV. Fig. 100 depicts a dorsal view of the claw, probably that of leg II if it is compared to fig. 96. A tarsus II with only two dorsal setae is not reported by André (1981b). Fig. 95 represents the end of a posterior leg with the apotele tilted backwards: again, a posterior tarsus with only two ventral unguinals and a dorsal fastigial is not noted by André (1981b). Solenidion ωI is not reported even if it is drawn for other Tydeus (albellus, alni, bavaricus, croceus, subterraneus). Finally, the leg phanerotaxy reported by Oudemans seems to be approximate —and not perfect— when the drawings are scrutinized and when the text is compared to figures.
On fig. 101, four setae are drawn on the proximate segments of the palp, which corresponds to the usual chaetotaxy of the tydeid palp. The ultimate segment bears only two setae, a simple dorsal seta and the large terminal eupathidia. This unusual chaetotaxy is outlined in the text and corresponds to the ''weak chaetotaxy of the palp'' emphasized in Oudemans' closing statement. In other tydeid species (e.g. in xylocopae, Oudemans' drawing reproduced by Thor (1933, his fig. 4) and in figure 3) studied and drawn by Oudemans, the palp ends up with a richer and usual chaetotaxy.
The coxisternal formula derived from fig. 98 is (2-0-3-2) (right side) or (2-0-4-2) (left side). Such an arrangement is not listed by André (1981a). The common formula in Tydeinae is (3-1-4-2) and is that of croceus (type specimen) and Oudemans' drawing (Oudemans' drawing is reproduced by Thor, 1933: 17, his fig. 19). In the drawing of cruciatus, the formula is (3-1-3-2). A nude coxa II is rare in Tydeoidea, it is observed in Speleognathopsis galli (Ereynetidae).
The striation of genital lips is long and of extended type (fig. 103) as in the genus Brachytydeus contrary to the genera Tydeus and Calotydeus. Six genital setae and 4 aggenitals are drawn and, together with an egg (dotted line), suggest that the type is a female. However, it appears that left and right are reversed in the drawings of genital area (compare figs 98 and 103). Furthermore, setae ag4 are differently oriented in these two figures.
Lastly, fig. 97 (reproduced by Thor, 1933, his fig. 3) represents a magnification of the reticulate pattern as well as the trichobothridial seta long, thin, with a terminal swollen bulb. As explained in the text, the reticulate pattern is irregular. Fig. 99 describes the anterior edge of the prodorsum.
Discussion
Phase-contrast microscopy was discovered in the early 1930s (Zernike, 1955) and was not available to Oudemans and previous acarologists. Solenidia and other ''translucid'' structures were thus difficult to observe in the absence of staining. As shown in figure 1, each interpretation is based on different types of criteria, single claw, reticulate pattern, palp and leg chaetotaxies.
The single claw criterion
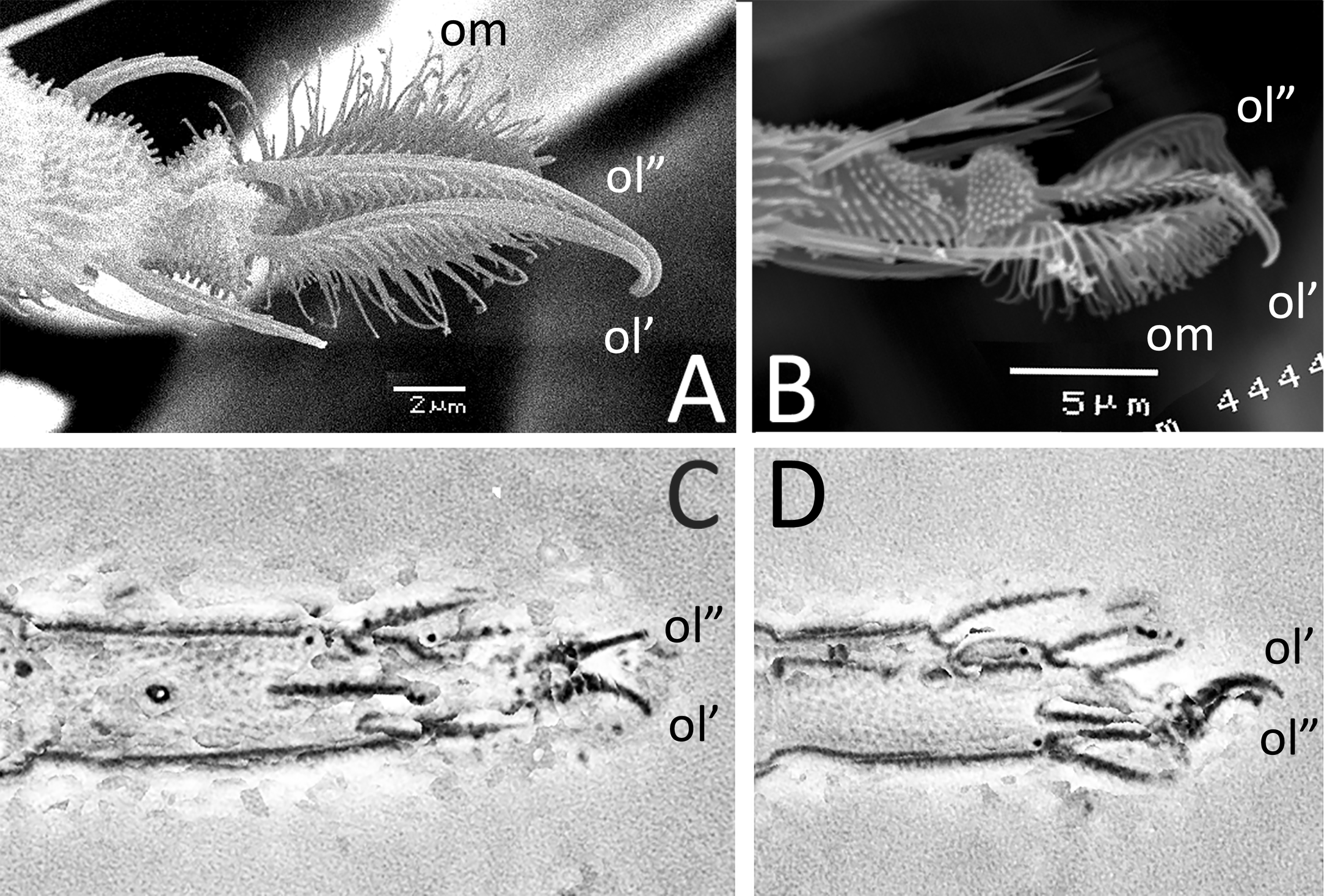
The single claw criterion (1 claw in figure 1) was advanced by Oudemans himself (1925, 1928a, b). After various observations, Oudemans (1929) became aware that he might have confused two adjacent structures and changed his mind. He was then convinced L. superba had two claws but he couldn′t prove it. The detailed drawing by Oudemans of Tydeus xylocopae (not published but available on the net, https://species.wikimedia.org/wiki/Calotydeus_xylocopae ![]() ) and the scanning electron micrograph by André (2005) showed that claws are not adjacent structures as feared by Oudemans but are lateral elements divergent along the central empodium and poorly suited to stick to each other, except the tips (Figure 4A). This criterion was also rejected by Baker (1968: 986) who stated that the presence of a single claw was a ''misinterpretation''.
) and the scanning electron micrograph by André (2005) showed that claws are not adjacent structures as feared by Oudemans but are lateral elements divergent along the central empodium and poorly suited to stick to each other, except the tips (Figure 4A). This criterion was also rejected by Baker (1968: 986) who stated that the presence of a single claw was a ''misinterpretation''.


In interpretation 2, the single specimen of Lorryia superba is supposed to be an abnormal individual (Kaźmierski, 1998: 301; Kaźmierski and Sikora, 2008: 88) or is considered as such (Mondin et al., 2016: 474; Kaźmierski et al., 2018: 813). The presence of a single claw was observed at most on 4 legs of the same mite which seems hardly compatible with the single claw noticed on all the legs, thus eight times, of L. superba. By the way, the presence of a single claw observed eight times was also noted by Oudemans (1929: 481) who acknowledged: ''Zonderling, blijt het, dat alle acht klauwparen zich als 1 klauw voordoen'' (Strangely enough, the fact remains that all eight pairs of claws appear as 1 claw).
A new approach is based on homology and evolutionary biology. The claw about which Oudemans (1925, 1928a, b, 1929; klauw in Dutch) spoke corresponds to one of the sharp curved ungues (term used by Michael, 1884) usually terminating the distal podomere or apotele in Tydeidae. The term does not apply to the third unpaired and central element, usually called an empodium, i.e. a padlike structure (sometimes equipped with a central claw called the empodial hook). Such a name refers to putative functions, locomotion or manipulation of prey and pertains to analogy. This condition, three setiform organs on the apotele, not activated by peculiar muscles and, therefore, not mobile relatively to the apotele, was called tridactyly by Grandjean (1939) and is the usual (plesiomorph) state in Tydeoidea. In other Acariformes, bidactyly is not rare and can arise by the suppression of the central element, oc or om (homobidactyly) or by suppression of one of the lateral ungues, ol′ or ol″ (heterobidactyly) (Hammen, 1981: 9). Both cases have been noticed in Tydeoidea, heterobidactyly was described in L. superba and homodactyly was observed on a tydaeoline mite devoid of the central empodium in all legs (figures 4C–D). In light of such interpretation, the presence of a single claw in L. superba is a simple evolutionary phenomenon.
Bidactyly leads to the monodactyly observed in Iolinidae, another tydeoid family: the larvae of Metapronematus leucohippeus (Treat, 1970: his fig. 3b) and Homeopronematus vidae (André, 1981b: his fig. 5b) with claws absent or vestigial on apotele I.
However, the passage from tri- to bidactyly (implying the complete disappearance of a lateral claw) in Tydeidae would require an intermediate step illustrated by the scars reproduced by Oudemans (1928a – arrow in figure 3) as well as by Kaźmierski and Sikora (2008: their fig. 6). The scars would be the missing claw reduced to a vestigial organ similar to the minute tectal setae observed in the larvae of Triophtydeidae and Tydeidae (André, 1981b: 172, his fig. 3) or, even better, to the vestigial claws of the larvae of Homeopronematus vidae (André, 1981b: 115, his fig. 5b).
This way, the genus is defined by bidactyly, a meristic character (counts of discrete serially homologous structures, Lawing et al. 2008) which is apomorphic. Such a diagnosis based on meristic characters was quite unusual before Baker's (1965) seminal paper.
Bidactyly also can be artificial and result from inadequate manipulations. This is probably the case of the SEM micrograph of B. sibiriensis published by Khaustov (2023, his fig. 6D). According to Khaustov (mail 07-05-2023), the specimen became fragile after preparation, the empodium inserted in ventral position (figure 4A, B) is broken and missing and the scar resulting from the fracture is hidden by the apex terminating the apotele.
The reticulate pattern
Oudemans (1929: 481) proposed a new definition: ''Op die gronden wensch ik de diagnose van het genus Lorryia te wijzigen als volgt: als Tydeus, maar dorsaal met geheel, of gedeeltelijk netvormige structuur''. (On those grounds, I wish to modify the diagnosis of the genus Lorryia as follows: as Tydeus, but dorsally with entirely or partly reticulate pattern). This reticulate pattern was already mentioned (Oudemans, 1925: 33) and was the first point of the concluding remarks (Oudemans, 1928a: 233), and the exclusive criterion selected by Oudemans (1929: 481). This criterion was again used by Baker (1965, 1968) who did not refer to Oudemans' (1929) paper.
The reticulate pattern was already illustrated by Berlese (1910) when he described Lasiotydaeus (Melanotydaeus) raphignathoides. The paper was not cited in the first articles of Oudemans but the mite was counted in the new diagnosis published in 1929. This typically is a morphometric character already visible in several genera described by Thor (1933) and found in many current genera (Calotydeus, Krantzlorryia, Metalorryia, Neolorryia, Neoapolorryia...). Furthermore, it varies through ontogeny as shown by André (1984, 1987). On its own, this criterion is of little use.
The palp phanerotaxy
The weak phanerotaxy of the palp is the second point of the concluding remarks presented by Oudemans (1928a: 233). This character was not mentioned by subsequent authors (e.g. Kaźmierski, 1998; André, 2005), but was by Baker (1968: 986) for whom it is part of the ''misinterpretations'' made by Oudemans.
Contrarily to legs, the palp of tydeid mites was studied and illustrated very early. As far back as the nineteenth century, the palp of T. molestus was drawn with distinct setae by Moniez (1894, his fig. 5 reproduced by Thor (1933) in his fig. 38, see figure 5A of this paper); the formula deduced from the text and the figure is (6-2-1), a formula found in Afridiolorryia, Idiolorryia and Krantzlorryia. Berlese also drew palps. He depicted, in addition to dorsal views, the palp of Lasiotydaeus glycyphaginus (Berlese, 1910, his fig. 6a), Lasiotydaeus (Melanotydaeus) simplex (Berlese, 1910, his fig. 10a), and Lasiotydaeus (Melanotydaeus) raphignathoides (Berlese, 1910, his fig. 11b; see figure 5B of this paper). The palp was familiar to Berlese who was temporarily professor of general and agrarian zoology at Regia Scuola Superiore di Agricoltura di Portici and published a book on insects in which the palp was illustrated several times (Berlese, 1909, his fig. 43, 44, 49-52, 68, 107...). Even if the chaetotaxy drawn by Berlese is approximate, the end of the palp clearly bears a tuft of setae. Not to be outdone, Oudemans prepared plates with a figure of the palp when he studied a new species, alni, cruciatus, xylocopae (partly reproduced in figure 3). In the tydeid species drawn by Oudemans, the palp ends up with a richer and usual chaetotaxy. In the study of T. croceus, Oudemans even drew the palp solenidion and the small seta ba, the phanerotaxy is 6(1)-2-2 (Figure 5C). Oudemans was thus familiar with the palp phanerotaxy of Tydeidae and it is hardly probable that he ''misinterpreted'' this structure. Furthermore, the palp was a key character selected by Thor (1933) to establish the subgenus Stylotydeus. Lastly, the palp phanerotaxy of tydeid mites is easy to observe as shown by the scanning electron micrograph of Lorençon et al. (2016, their fig. 3) and Khaustov (2023, his fig. 6C).
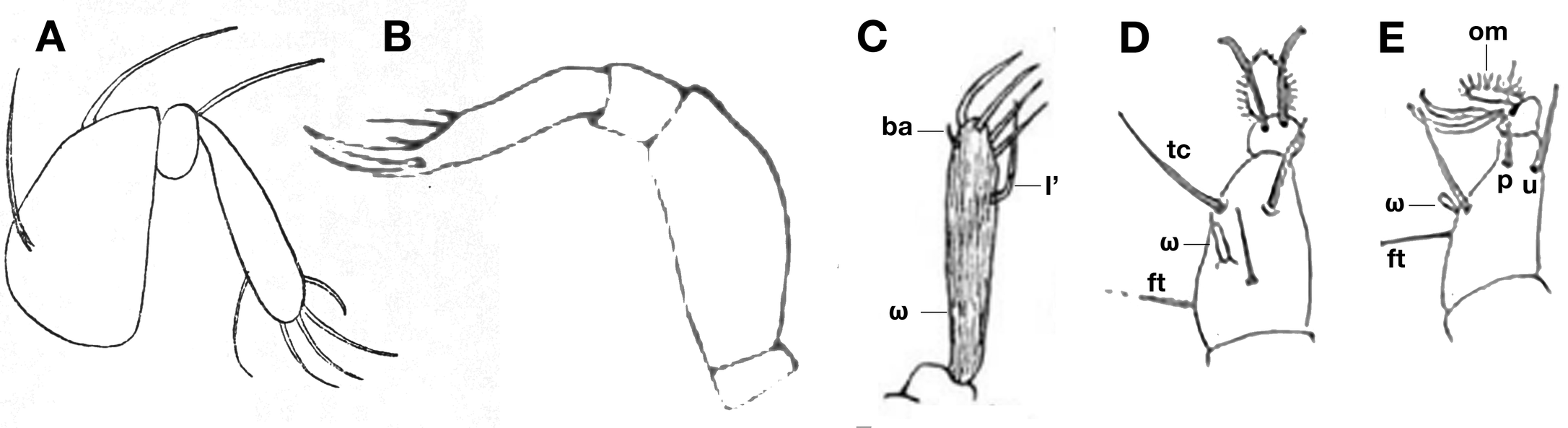


A reduction of the palp chaetotaxy was poorly known during Oudemans' years but is observed nowadays in several tydeid genera (Afridiolorryia, Apolorryia, Idiolorryia, Krantzlorryia) except in species assigned to the genus Brachytydeus. This reduction is well documented in Tydeoidea (André, 1980; André and Fain, 2000). The presence of only 2 terminal setae may even be observed in the palp of several tydeoid species, Iolina nana (Iolininae), Lawrencarus afrixali (Lawrencarinae)...
The leg phanerotaxy
The leg phanerotaxy is not given by Moniez (1894). Contrary to the palp, the legs and the ventral side (coxal chaetotaxy) of Tydeidae were rarely illustrated by Berlese, Oudemans and Thor. The first complete leg phanerotaxy formulae were provided much later by Grandjean (1938) and implemented in genera definitions by Baker (1965). The same remark applies when figures of legs separated from the idiosoma are investigated. The figures of Grandjean (1938) set apart, legs are rarely illustrated before the 70s (e.g. André and Naudo, 1965, figs 8-11; Ueckermann and Smith Meyer, 1979a, figs 6-7, 13-14; Ueckermann and Smith Meyer, 1979b, figs 8-9, 17-18 etc.). In a word and contrarily to the palp, legs and their phanerotaxy were not a key character in Oudeman's time and drawings are approximative even when they are detailed (figures 5D-E).
Even if the femora formula (3-3-2-1) is observed in the plate of L. superba, Oudemans' drawings are not congruent with the text, are not consistent when dorsal and ventral views, left and right sides are compared and do not accord with other drawings he made. The chaetotaxy of L. superba is only similar to that of Tydeus cruciatus, the type species of the genus Brachytydeus when corrections are made. Lastly, tydeid leg chaetotaxy is subject to modifications detailed by Momen and Lundqvist (1993) and Kaźmierski and Sikora (2008). Consequently, the leg chaetotaxy only deduced from a drawing published in 1928 seems to be a poor criterion to found the genus Brachytydeus and justify synonymizations.
Conclusions
In the absence of type, to avoid partial analyses, to prevent any conflictual approaches and divergent interpretations, and to provide ''standards, sense and stability for animal names'' (ICZN: http://iczn.org ![]() ), the four criteria discussed hereabove (reticulate pattern, palp phanerotaxy, leg phanerotaxy, single claw) are taken together.
), the four criteria discussed hereabove (reticulate pattern, palp phanerotaxy, leg phanerotaxy, single claw) are taken together.
Nomenclatural acts
After discussion, the genus Lorryia Oudemans, 1925 is re-established in its pristine state even if the type, L. superba, is not available. The genus remains monospecific. It is based on the three characters outlined by Oudemans but subsequently neglected or rejected: heterobidactyly (a single lateral claw, unique in Tydeoidea), a severe reduction of the palp chaetotaxy (unique in Tydeidae) and a reticulate pattern meet in other tydeid genera. All these characters are apomorphic. The leg chaetotaxy is undetermined but, after appropriate corrections, it would be similar to that of the genus Brachytydeus.
The genus Brachytydeus Thor, 1931 is quite distinct from Lorryia and has as type species Tydeus cruciatus Koch, 1838 by original designation. The genus concerns some 200 species, the type was recently redescribed and is housed, in a good condition, in the Naturalis Biodiversity Center (Leiden, The Netherlands). The type specimen shows the palpal chaetotaxy usual in Tydeidae (6-2-2), extended striae in genital area and the particular setal femoral pattern (3-3-2-1) not found in Tydeus. The type species has no reticulate pattern but it may be partial (B. juanjus) or complete (B. formosus). Lastly, it is not recommended to base a genus with dozens of species on a unique specimen, in addition enigmatic and not available. Therefore, the synonymy Lorryia-Brachytydeus made by Akbari et al. (2015) and Mondin et al. (2016) is not accepted. The species assigned to the genus Lorryia and described after the world catalog of Silva et al. (2016a) (namely L. amazonensis, L. buceroincerta, L. columbina, L. fortistriata, L. lusciniella, L. meliponarum, L. parvireticuli, L. pseudoplacita, L. sibiriensis, L. speciosa, L. tuttii, L. virga) are transferred to Brachytydeus. This also applies to recent new combinations such as L. arguta.
More specifically, the synonymy reticulata-bedfordiensis-superba made by Kaźmierski et al. (2018: 813) is not accepted. In particular, the shape of the trichobothridial seta of the mite presented in their fig. 10 (thin end) and identified as L. superba does not correspond to that of Oudemans (1928a, fig. 97, terminal swollen bulb). The size and the shape of cells are also quite different. There is no indication of a reduction of the palp chaetotaxy or of a possible bidactyly.
Uniqueness, historicity and divergent interpretations
The type of L. superba has never been re-studied. Consequently, all synonymizations were not based on name-bearing types that provide the objective standard of reference that determines what the name applies to. In addition, the synonymizations did not comply with the original description, at least with some key elements. In addition, the holotype is the unique specimen of the species. In paleontology, the holotypes and unique specimens are not rare. This uniqueness poses problems: are the reported characters the property of a specimen, a population, a species, a genus? Are they abnormalities? Are the characters observed in a unique specimen and the criteria used for their classification really pertinent?
The detective work (the term was used by Ochoa, mail of 05-02-2023) carried on the initial description and the subsequent papers shows the interest of the historical perspective. Even if Oudemans was ''a man of the old school'' (Eyndhoven, 1965: 589), the importance of the palp was already outlined in entomology two century ago and the first drawing of the tydeid palp was released in 1894. The reduction of the palpal chaetotaxy was thus unexpected, to say the least, to Oudemans who knew the usual state of the palp. In contrast, the first complete phanerotaxy of the tydeid legs was published much later, only in 1938, and was introduced in tydeid taxonomy in 1965. This approach illustrates the way that, when we look back into the past with modern techniques and equipment (Ratcliff, 2009) and current ideas (Ghesquier-Pourcin, 2011), biases and misinterpretations can occur.
This historiographic approach also shows how potentially disturbing topics are avoided, rejected with no comments (''These must be misinterpretations'') or ignored by subsequent authors. Whether deliberate or not, this avoidance also contributes to divergent interpretations.
Evolutionary plasticity
The unique specimen of L. superba is not considered abnormal but rather would reveal evolutionary pathways seen in other Acariformes and helps us understand evolution of mites. Ecologically, L. superba is also unique among Tydeidae as it was collected on Viverra, a mammalian genus while other tydeid species are associated with soil, soil annexes such as suspended soils and bird nests, mosses, trees or other plants. It is interesting to observe that the severe reduction of the palp chaetotaxy of L. superba also affects closely related parasitic species such as those in the Lawrencarinae.
To some extent, the passage from tri- to monodactyly through bidactyly, anticipates the palpian evolution (regression of apotele I so named by Grandjean (1967: 726) since leg I behaves as a palp) observed in Iolinidae. The passage from tri- to monodactyly would be the second evolutionary path reported in Tydeoidea that was first studied in Oribatida by a founder of this journal.
Acknowledgements
The author thanks Daan Drukker, Harry Smit and Bram van der Bijl (Naturalis Biodiversity Center, the museum which made available Oudemans' plates and houses his collection), Jean-Luc Gattolliat and Olivier Glaizot (Muséum cantonal des Sciences naturelles, Lausanne, CH) and A.A. Khaustov (Tyumen State University). Preliminary drafts of this publication were read and criticized by R.A. Norton, R.A. Ochoa, H.C. Proctor, J. Travé, D.E. Walter, anonymous referees and my spouse. The linguistic revision of the text was completed by S. Spelling.
References
- Akbari A., Irani-Nejad K.H., Khanjani M., Arzanlou M., Kaźmierski A. 2015. A new tydeid species (Acari: Tydeidae) with a key to Brachytydeus species from East Azerbaijan Province, Iran. Systematic and Applied Acarology, 20(4): 423-43. https://doi.org/10.11158/saa.20.4.7
- André H.M. 1980. A generic revision of the family Tydeidae (Acari: Actinedida). IV. Generic descriptions, keys and conclusions. Bulletin et Annales de la Société Royale Belge d'Entomologie, 116: 103-130, 139-168.
- André H.M. 1981a. A generic revision of the family Tydeidae (Acari: Actinedida). II. Organotaxy of the idiosoma and gnathosoma. Acarologia, 22: 31-46.
- André H.M. 1981b. A generic revision of the family Tydeidae (Acari: Actinedida). III. Organotaxy of the legs. Acarologia, 22: 165-178.
- André H.M. 1984. Tydeinae (Acari : Tydeidae) from Belgium. I. The genus Homeotydeus. Bulletin et Annales de la Société Royale Belge d'Entomologie, 120: 117-122.
- André H.M. 1987. Tydeinae from Belgium (Acari : Tydeidae). II. The genera Tydeus, Idiolorryia and Metalorryia. Acarologia, 28: 151-159.
- André H.M. 2005. In search of the true Tydeus (Acari: Tydeidae). Journal of Natural History, 39: 975-1001. https://doi.org/10.1080/00222930400002838
- André H.M., Fain A. 2000. Phylogeny, ontogeny and adaptive radiation in the superfamily Tydeoidea (Acari: Actinedida), with a reappraisal of morphological characters. Zoological Journal of the Linnean Society, 130: 405-448. https://doi.org/10.1111/j.1096-3642.2000.tb01636.x
- André M., Naudo M.H. 1965. Pertydeus schusteri, n. sgen., n. sp., nouveau Tydeus à griffe pulvillaire (Tydeidae). Acarologia, 7(4): 673-682.
- Baker E.W. 1965. A review of the genera of the family Tydeidae (Acarina). In: Naegele JA, editor. Advances in acarology 2. Ithaca (NY): Cornell University Press: 96-133.
- Baker E.W. 1968. The genus Lorryia. Annals of the Entomological Society of America, 61: 986-1008. https://doi.org/10.1093/aesa/61.4.986
- Baker E.W., Wharton, G.W. 1952. An introduction to acarology. Macmillan, New York.
- Berlese A. 1909. Gli Insetti Vol. 1. Società editrice libraria, Milano.
- Berlese A. 1910. Acari nuovi. Redia, 6(2): 199-234, plates XVIII-XXI.
- Berton L.H.C., Carvalho Mineiro J.L. de, Sato, M.D., Azevedo Filho Joaquim A. de, Raga A. 2019. Mite fauna of a coffee agroecosystem (Coffea arabica L.) in the municipality of Monte Alegre do Sul, São Paulo State, Brazil. Part I. Acarologia, 59(4): 542-550. https://doi.org/10.24349/acarologia/20194352
- Buitendijk A.M. 1945. Voorloopige Catalogus van de Acari in de Collectie-Oudemans. Zoologische Mededelingen, 24: 281-391.
- Da-Costa T., Rodighero L.F., Silva G.L. da, Ferla N.J., Blochtein B. 2019. Two new species of Tydeidae (Acari: Prostigmata) associated with stingless bees. Zootaxa, 4652(1): 101-112. https://doi.org/10.11646/zootaxa.4652.1.4
- Diaz R., Romero S., Roda, A., Mannion C., Overholt W. 2015. Diversity of arthropods associated with Mikania spp. and Chromolaena odorata (Asterales: Asteraceae: Eupatorieae) in Florida. Florida Entomologist, 98(1): 389-393. https://doi.org/10.1653/024.098.0168
- Eyndhoven G.L. van. 1965. Some details about the life and work of A. C. Oudemans. Acarologia, 7(4): 589-593.
- Fenilli R., Flechtmann C.H.W. 1990. Ácaros do pinheiro-do-Paraná em Lages, Santa Catarina. Anais da Escola Superior de Agricultura Luiz de Queiroz, 47(1): 243-250. https://doi.org/10.1590/S0071-12761990000100015
- Flechtmann C.H.W. 1987. On the measurements of Tydeus formosus (Cooreman, 1958) (Acari, Prostigmata, Tydeidae). International Journal of Acarology, 13: 217-218. https://doi.org/10.1080/01647958708683771
- Ghesquier-Pourcin D. 2011. Itinéraire des idées : L'affaire de la gale. Paris, Hermann.
- Grandjean F. 1938. Observations sur les Tydeidae (2e série). Bulletin du Muséum national d'histoire naturelle, 1ère Sér. , 10(6): 593-600.
- Grandjean F. 1939. L'évolution des ongles chez les Oribates (Acariens). Bulletin du Muséum national d'histoire naturelle (2), 11: 539-546.
- Grandjean F. 1967 (1966). Les Staurobatidae n. fam. (Oribates). Acarologia, 8(4): 696-727.
- Hammen, L. van der. 1981. Numerical changes and evolutions in Actinotrichid mites (Chelicerata). Zoölogische Verhandelingen (Leiden), 182: 1-47.
- Johann L., Silva, G.L. da, Brentano A.C., Carvalho G.S., Botton M., Ferla N.J. 2017. Chave ilustrada para identificação da fauna acarina na cultura da videira do estado do Rio Grande do Sul, Brasil. Embrapa Uva e Vinho, Comunicado Técnico, 197: n.p. https://doi.org/10.13140/RG.2.2.23868.33922
- Kaźmierski A. 1989. Revision of the genus Tydeus Koch sensu André, Homeotydeus André and Orthotydeus André with description of a new genus and our new species of Tydeinae (Acari: Actinedida: Tydeidae). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, 86: 289-314.
- Kaźmierski A. 1998. Tydeinae of the world: generic relationships, new and redescribed taxa and keys to all species. A revision of the subfamilies Pretydeinae and Tydeinae (Acari: Actinedida: Tydeidae)—Part IV. Acta Zoologica Cracoviensia, 41: 283-455.
- Kaźmierski A. 2008. Description of two new species of Tydeinae (Acari: Actinedida: Tydeidae) from Spain with the remarks about the Iberian species of subfamily. Annales Zoologici, 58: 357-363. https://doi.org/10.3161/000345408X326672
- Kaźmierski A., Marciniak M., Sikora B. 2018. Tydeinae mites (Acariformes: Prostigmata: Tydeidae) from bird nests with description of three new species. Systematic and Applied Acarology, 23(5): 803-823. https://doi.org/10.11158/saa.23.5.3
- Kaźmierski A., Sikora B. 2008. On the morphological anomalies in Tydeoidea (Actinedida). In: Bertrand, M., Kreiter, S., McCoy, K.D., Migeon, A., Navajas, M., Tixier, M.-S. & Vial, L. (eds.) Integrative Acarology Proceedings of the 6th European Congress. Montpellier, European Association of Acarologists, pp. 82-88.
- Khaustov A.A. 2023. Contribution to the fauna of Tydeidae (Acari: Prostigmata) from Western Siberia, Russia. Acarologia, 63(2): 491-521. https://doi.org/10.24349/7fyj-me9u
- Klimov P.B., OConnor B.M., Chetverikov P.E., Bolton S.J., Pepato A.R., Mortazavi A.L., Tolstikov A.V., Bauchan G.R., Ochoa R. 2018. Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch. Molecular Phylogenetics and Evolution 119: 105-117. https://doi.org/10.1016/j.ympev.2017.10.017
- Koch C.L. 1838. Deutschlands Crustaceen, Arachniden und Myriapoden, ser. Deutschlands Insecten (Panzer) Herrich-Schäffer ed., vol. 20, Regensburg, plate 7.
- Lawing A.M., Meik J.M., Schargel, W.E. 2008. Coding meristic characters for phylogenetic analysis: a comparison of step-matrix gap-weighting and generalized frequency coding. Systematic Biology, 57(1):167-173. https://doi.org/10.1080/10635150801898938
- Lorençon J.R., Andrade S.C., Andrade D.J. 2016. Mites occurrence on Pachira aquatica Aubl. including aspects of external mouthpart morphology of Brachytydeus formosa (Acari: Tydeidae). Brazilian Journal of Biology, 76(1): 136-143. https://doi.org/10.1590/1519-6984.15114
- Michael A.D. 1884. British Oribatidae (Vol. 1). London, Ray Society.
- Mondin A. de S., Nuvoloni F.M., Feres, R.J.F. 2015. Revisão taxonômica das espécies de Brachytydeus Thor, 1931 sensu André, 2005 (Acari: Tydeidae) associadas a Hevea spp. no Brasil. In: Feres R.J.F., Lofego A.C., Barros-Battesti D.M., Demite P.R. (eds). Anais do V Simpósio Brasileiro de Acarologia, UNESP - Universidade Estadual Paulista ″Júlio de Mesquita Filho″, São José do Rio Preto, SP, Brazil: 21.
- Mondin A. de S., Nuvoloni F.M., Feres, R.J.F. 2016. Four new species of Lorryia (Acari: Tydeidae) associated with Hevea brasiliensis Muell. Arg. (Euphorbiaceae) in Brazil. Zootaxa, 4158(4): 473-490. https://doi.org/10.11646/zootaxa.4158.4.2
- Momen F.M., Lundqvist L. 1993. Inconsistencies in leg chaetotaxy in two species of Tydeid mites (Prostigmata: Tydeidae). International Journal of Acarology, 19(2): 137-144. https://doi.org/10.1080/01647959308683972
- Moniez R. 1894. Histoire naturelle du Tydeus molestus, acarien qui s'attaque à l′homme. Revue biologique du nord de la France, 6: 419-434.
- Nucifora A. 1984. Integrated Pest Control in Lemon Groves in Sicily: Five Years of Demonstrative Tests and Present Feasibilities of Transferring Results. In: Cavalloro R. & Piavaux A. (eds). Agriculture. C.E.C. Programme on integrated and biological Control. Office for Official Publications of the European Communities, Luxembourg: 129-146.
- Oudemans A.C. 1925. Acarologische aanteekeningen LXXIX. Entomologische Berichten, 7: 26-34.
- Oudemans A.C. 1928a (1927). Acari uit Ambon. Zoologische Mededelingen, 10: 185-237.
- Oudemans AC. 1928b. Acarologische aanteekeningen XCIV. Entomologische Berichten, 7: 374-382.
- Oudemans A.C. 1929. Acarologische aanteekeningen XCVIII. Entomologische Berichten, 7: 476-485.
- Pedro Neto M., Reis P.R., Silva R.A., Zacarias M. S. 2015. Influência do manejo das plantas adventícias na diversidade de ácaros em cafezal orgânic. Coffee Science, Lavras, 10(3): 357-364. http://www.alice.cnptia.embrapa.br/alice/handle/doc/1041031
- Prota R., Ortu S., Delrio G. 1984. Results of five years of integrated control in Sardinia Orange Groves. In: Cavalloro R. & Piavaux A. (eds). Agriculture. C.E.C. Programme on integrated and biological Control. Office for Official Publications of the European Communities, Luxembourg: 147-163.
- Ratcliff M.J. 2009. The quest for the invisible. Microscopy in the enlightenment. Ashgate Publishing Ltd.
- Schiess T. 1981. Neue Tydeidenarten (Acari, Actinedida, Tydeidae) aus einem alpinen Rasen (Caricetum firmae, 2500 m) des Schweizer Nationalparkes. Entomologica Basiliensia, 6: 78-107.
- Silva G.L. da, Metzelthin M.H., Silva O.S. da, Ferla N.J. 2015. Catálogo da família Tydeidae (Acari: Prostigmata). In: Feres R.J.F., Lofego A.C., Barros-Battesti D.M., Demite P.R. (eds). Anais do V Simpósio Brasileiro de Acarologia, UNESP - Universidade Estadual Paulista ″Júlio de Mesquita Filho″, São José do Rio Preto, SP, Brazil: 21.
- Silva G.L. da, Metzelthin M.H., Silva O.S. da, Ferla N.J 2016a. Catalogue of the mite family Tydeidae (Acari: Prostigmata) with the world key to the species. Zootaxa, 4135(1): 1-68. https://doi.org/10.11646/zootaxa.4135.1.1
- Silva G.L. da, Souza Radaelli T.F. de, Metzelthin M.H., Ferla J.J. 2016b. Two new species of Tydeidae (Acari: Prostigmata), records of species of this family and Triophtydeidae from Brazil. Zoologia (Curitiba), 33(2):e20150130. https://doi.org/10.1590/S1984-4689zool-20150130
- Tempfli B, Pènzes B., Fail J., Szabó Á. 2015. The occurrence of tydeoid mites (Acari: Tydeoidea) in Hungarian vineyards. Systematic and Applied Acarology, 20(8): 937-954. https://doi.org/10.11158/saa.20.8.9
- Thor S. 1931. Norwegische Tydeidae. I-VII. Mit Kennzeichnung vier neuer Gattungen. Zoologischer Anzeiger, 94: 89-104.
- Thor S. 1933. Tydeidae, Ereynetidae. Das Tierreich, 60: 1-84.
- Treat A.E. 1970. Two tydeid mites from the ears of noctuid moths. American Museum Novitates, 2426: 2-14.
- Ueckermann E.A., Smith Meyer M.K.P. 1979a. African Tydeidae (Acari). I. The genus Lorryia Oudemans, 1925. Phytophylactica, 11: 43-50.
- Ueckermann E.A., Smith Meyer M.K.P. 1979b. African Tydeidae (Acari). II. The genus Paralorryia Baker, 1965. Phytophylactica, 11: 117-127.
- Vacante V., Nucifora A. 1986. A first list of the mites in citrus orchards in Italy, PP 177-188. In: Cavalloro R. & Di Martino E. (eds.). Integrated Pest Control in Citrus-Groves: Proceedings of the experts meeting, Acrireale, 26-29 March 1985. Rotterdam, Netherlands: Published for the commission of the European Community by A. A. Balkema, Rotterdam/Boston. https://doi.org/10.1201/9781003079279-29
- Walter D.E., Lindquist E.E., Smith I.M., Cook D.R., Krantz G.W. 2009. Chapter thirteen Order Trombidiformes. In: Krantz G.W., Walter D. (Eds). A Manual of Acarology (3rd edition). Texas Tech University Press, Lubbock, pp. 233-420.
- Zernike, F. 1955. How I Discovered Phase Contrast. Science, 121 (3141): 345-349. https://doi.org/10.1126/science.121.3141.345



2023-05-08
Date accepted:
2023-06-14
Date published:
2023-07-04
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 André, Henri M.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



