Evaluation of the influence of biotic and abiotic factors on the prevalence and abundance of infestations of Mysolaelaps microspinosus (Fonseca, 1936) (Mesostigmata: Laelapidae) on Oligoryzomys longicaudatus (Bennett, 1832) in Chile
González-Aguayo, Felipe  1
; Fuenzalida-Araya, Karen
1
; Fuenzalida-Araya, Karen  2
; Landaeta-Aqueveque, Carlos
2
; Landaeta-Aqueveque, Carlos  3
; Moreno Salas, Lucila
3
; Moreno Salas, Lucila  4
; Santodomingo, Adriana
4
; Santodomingo, Adriana  5
and Silva-de la Fuente, María Carolina
5
and Silva-de la Fuente, María Carolina  6
6
1Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Barrio Universitario s/n, Concepción, Chile.
2Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Barrio Universitario s/n, Concepción, Chile.
3Departamento de Patología y Medicina Preventiva, Facultad de Ciencias Veterinarias, Universidad de Concepción, Avenida Vicente Méndez 595, Chillán Chile.
4Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Barrio Universitario s/n, Concepción, Chile.
5Departamento de Ciencia Animal, Facultad de Ciencias Veterinarias, Universidad de Concepción, Av. Vicente Méndez 595, Chillán, Chile.
6✉ Facultad de Ciencias Agrarias y Forestales, Escuela de Medicina Veterinaria, Universidad Católica del Maule, Los Niches km. 6, Curicó, Chile.
2023 - Volume: 63 Issue: 3 pages: 735-743
https://doi.org/10.24349/54md-k7v0Original research
Keywords
Abstract
Introduction
Among neotropical rodent ectoparasites, Laelapidae (Acari: Mesostigmata) have a high species richness and are the most common ectoparasites of cricetid rodents (Strandtmann and Wharton, 1958; Radovsky, 1969). Knowledge of these mite species is primarily based on the work of Strandtmann and Wharton (1958), who cited 49 species of ectoparasites of Laelapidae in the neotropical zone. However, laelapids from some areas of the Neotropics remain poorly understood. In Chile, research in this field is limited to the description of some mites and mite species-complex of cricetid and abrocomid rodents (Lareschi and González-Acuña, 2010; Yáñez-Meza et al., 2018; Silva-de la Fuente et al., 2020).
Chile presents a particular biogeography and is commonly considered a biogeographical ''island'' due to the natural barriers that surround it. In addition, the country presents a great latitudinal extension from north to south and is surrounded by some climatic and geographic phenomena (Cordillera de Los Andes, South Pacific Anticiclon and the Arid Diagonal). This gives it a diverse variety of climates throughout its territory, finding deserts, highlands, low scrub, mediterranean forest, template forest, rainforest, patagonian steppe, fjords, among others (Villagrán and Hinojosa, 2005; Morrone, 2015). Climatic parameters like mean annual precipitation (MAP) and mean annual temperature (MAT), also vary according to the geography of the country. Temperatures are gradually cooler from North to South (Uribe et al., 2012), while precipitation gradually increase from North to South. Between 17°S and 32°S it can be observed a MAT of 23 °C and a MAP of 115 mm/year, from 32°S to 40°S the MAT is 15 °C and the MAP is 1100 mm/year, from 40°S to 44°S the MAT is 12 °C and the MAP is 2000 mm/year and between 44 °C and 56 °C the MAT is near to 0 °C and the MAP is 3500 mm/year (Araya-Osses et al., 2020). In addition to the climate diversity, the central zone of Chile (32°S to 44°S), presents a high species richness (Muñoz-Pedreros et al., 2010), which provides a large number of eventual niches for parasitic species (Poulin, 1998; Skoracka et al., 2015). In this sense, it has been seen that different biotic (host) and abiotic (environmental) factors can affect the parasitic infestation rates of some ectoparasites. The sex of the host and its body mass can affect the presence of ectoparasites because, for example, males tend to have a higher vagility than females, especially in the reproductive season, and body mass is related to a greater number and variety of parasites that can harbor heavier or larger individuals (Lareschi and Krasnov, 2010; Rodrigues-Fernandes et al., 2015; Veloso-Frias et al., 2019). Also, environmental disturbances and seasonal variations can affect the presence or abundance of lice and mites (Lareschi and Krasnov, 2010; Yunik et al., 2015; Krasnov et al., 2019; Veloso-Frias et al., 2019). For example, for phytophagous mites of the Tetranychidae family, high temperatures (36 °C) are associated with higher rates of development, egg hatch, and longevity when high relative humidity is present (Perring et al., 1984a; Perring et al., 1984b), for bird's lice, low relative humidity determine low parasitic pressure (Moyer et al., 2002), and for the laelapid Gaeolaelaps aculeifer, at constant humidity (~60%), the development of immature stages, pre-ovipositional stage, ovipositional stage and lifespan vary with temperature (Amin et al., 2014).
Mysolaelaps is a genus of laelapid mites that preferentially parasitize sigmodontine rodents of the Oryzomyini tribe (Strandtmann and Wharton, 1958), even though, a strong association with the genus Oligoryzomys has been reported (Savchenko et al., 2021). Mysolaelaps has been reported in several countries such as Argentina (Mauri, 1965; Castro et al., 1987; Lareschi and Sánchez-López, 2000; Abba et al., 2001; Lareschi et al., 2003; 2007; Nava et al., 2003; Navone et al., 2009; Lareschi, 2010; Lareschi and Krasnov, 2010; López-Berrizbeitia et al., 2013; Savchenko et al., 2021), Uruguay (Lareschi et al., 2006), Venezuela (Furman, 1972) and Brazil (Linardi et al., 1991). In Chile, M. microspinosus is the only species of the genus recorded to date and its main host is the Long-tailed pygmy rice rat, Oligoryzomys longicaudatus, (Lareschi and González-Acuña, 2010). This parasitic association has already been reported for Argentina (Mauri, 1965; López-Berrizbeitia et al., 2013). However, despite multiple records, some aspects of the biology and ecology of M. microspinosus have been poorly studied.
Long-tailed pygmy rice rat inhabits a large part of the Chilean territory (27°S – 51° S) (Belmar-Lucero et al., 2009), occupying three biogeographic provinces with dissimilar abiotic factors (Mediterranean, Temperate Forest, Patagonia). The vegetation of these provinces tends to increase latitudinally from north to south, in accordance with the increase trend of the MAP. The Mediterranean province presents a xerophytic vegetation characterized by low shrubs and perennial, sclerotic leaves and medium height trees, known as ''Bosque Esclerófilo''. The Template Forest province is characterized by dense forest and an ecotone between the ''Bosque Esclerófilo'' and the ''Bosque caducifolio'' characterized by genus Nothofagus, which are deciduous, highland and tall trees. Also, in this province take place the ''Bosque laurifolio'' characterized by perennial and tall trees and dense shrubland. At last, the Patagonia province presents a reduction of the height of the trees of the genus Nothofagus, scattered and low shrubs and a rainy and cold weather (Villagrán and Hinojosa, 2005). In addition, the Long-tailed pygmy rice rat presents high abundances in its habitat, so its capture tends to be very frequent (Barrera and Murua, 2015). Their breeding takes place from October to December, sexual maturity of the young is reached within about a month, there are no evidence of sexual dimorphism and their lifespan is estimated at one year. The level of sociality is low in normal conditions, but can increase when there is food abundance (Murua et al., 1986). The Long-tailed pygmy rice rat is present in many habitats, but seems to prefer humid areas. It is considered a high vagility mouse with home ranges estimated around 0.032 – 0.48 ha. The sex ratio on births is biased toward males in normal years and toward females in high density years (Murua et al., 1986; Spotorno et al., 2000).
Considering the notorious variety of environments inhabited and the biology of the Long-tailed pygmy rice rat, we wonder if this diversity of habitats can affect the infestation rates of M. microspinosus. The main goal of this work is to determine how the biotic and abiotic factors can affect the ecology of this mite in Chile. With this work we pretend to establish the background for further studies of this mite and genus.
Material and methods
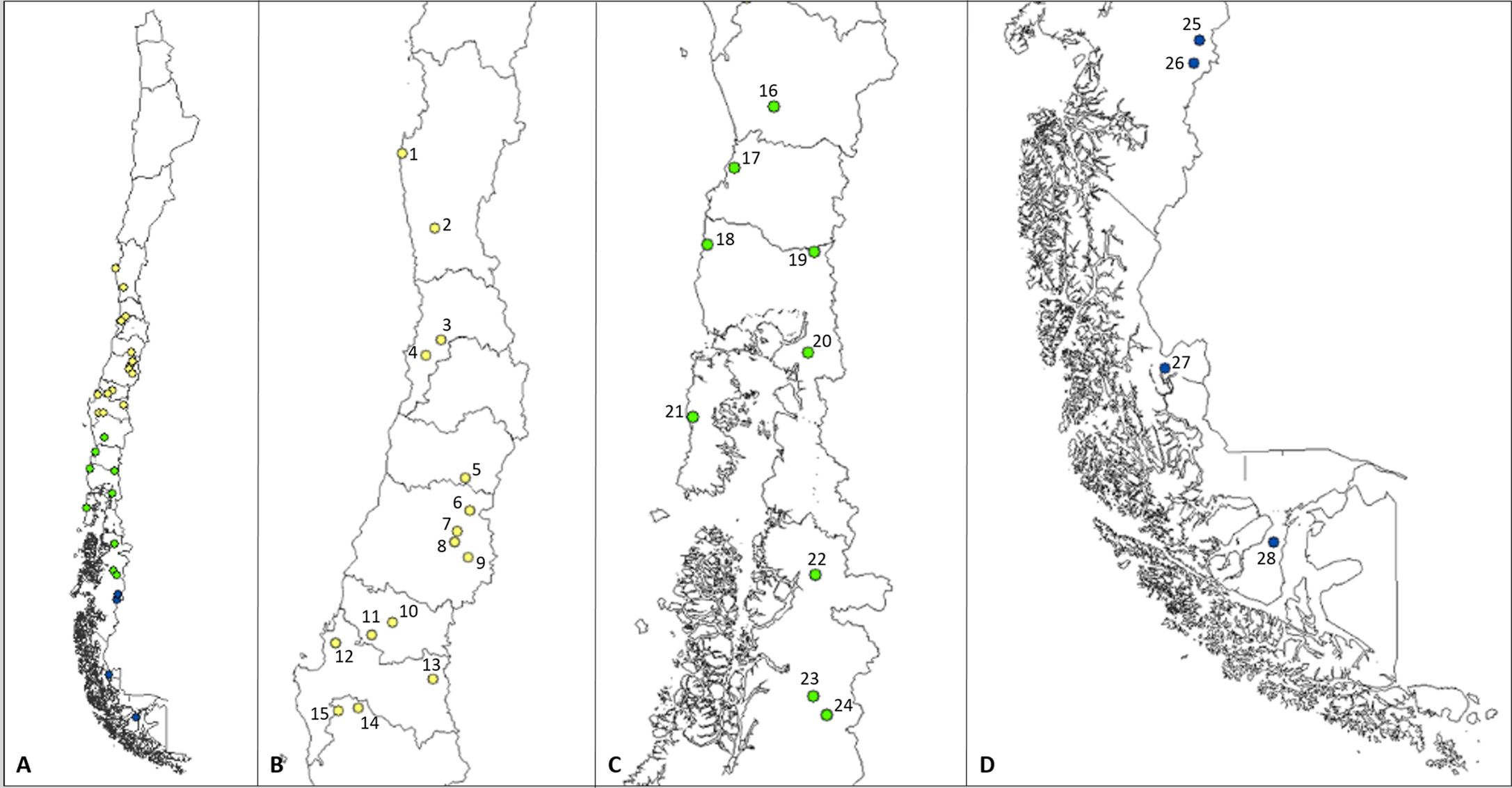
Between June 2010 and August 2019, 28 locations in Chile were visited (P.N. Fray Jorge -30.657977 S, -71.694438 W, northernmost locality; R.N. Magallanes -53.169800 S, -71.196767 W, southernmost locality –Mediterranean = 15; Temperate Forest = 9; Patagonia = 4) (Figure 1), in the four seasons of the year, in order to capture rodents and collect their mites. For all these captures, Sherman-type live traps and oatmeal as bait were used. Trapping points were selected by direct observation of signs of rodent presence as feces, burrows and food resources. Traps remained active in the night and they were checked early morning by three days in a row in all localities. For the handling of rodents, we followed the biosafety standards of the Pan-American Health Organization (Organización Panamericana de la Salud, 1999) because Long-tailed pygmy rice rat is the main reservoir of the Hanta virus Andes strain. All these procedures were approved by the Bioethics Committee of the Universidad de Concepcion (CE-03-2009).


The rodents were anesthetized intramuscularly with Ketamine (0.044 mg/g) and Xylazine® (0.006 mg/g) (Kreeger and Arnemo, 2018). They were examined looking for signs of pregnancy like high developed mammal glands, opening of the vulva and bulging abdomen. Females with any sign of pregnancy were excluded from the analysis. The remaining rodents were weighed with Pesola® weight scale (±0.2g) and brushed to find mites with individual brushes over a white tray, until no more mites were removed. The mites collected were placed in vials (Biologix, USA) with 95% ethyl alcohol. Once the rodents came out of sedation, they were released at the same capture site.
The mites were processed at the Parasite Ecology Laboratory at the Universidad de Concepción, Concepción. For the identification of mites, 252 specimens were rinsed in Nesbitt solution and mounted in Berlese medium (Walter and Krantz, 2009a). The identification was carried out following the keys proposed by Fonseca (1936, 1957) and Furman (1972) and using a stereomicroscope (Motic ®).
The prevalence (P%), mean abundance (MA) (dependent variables) and their confidence intervals were estimated with the Quantitative Parasitology software in its web version (QPweb) (Reiczigel et al., 2019), considering the total number of samples collected, as well as estimates by season and biogeographical province.
The association of the P% of mites with sex, host body mass, season of the year and biogeographic province (independent variables) was analyzed using multifactorial logistic regressions. The association between mite MA and the independent variables was analyzed using negative binomial regressions. This distribution was selected because it's the most common distribution in parasitic infestations and also, match with our abundance data. In both analyses, the most complex model (including all independent variables i.e. sex, host body mass, season and biogeographic province) was used and the variables were eliminated one by one, until the likelihood loss was significant.
The best model was selected with the likelihood ratio test, whose null hypothesis is that the likelihoods of both models are equal (likelihood ratio = 1). The model with fewer variables was selected when the null hypothesis was not rejected (p\textgreater0.1). In cases where the probability value was less than 0.1 and greater than 0.05, the model with the lowest value in the Akaike information criterion (AIC) was chosen. In the case of the season, autumn was considered as the baseline category for comparison and the other seasons as ''dummies'' variables. In the case of the biogeographical province, Patagonia was considered as a baseline category and the other provinces as ''dummies'' variables. In the case of rodent's sex, male was the baseline comparison category and female was the dummy variable. Analyzes were performed with Stata/BE 17 software (StataCorp LLC).
Results
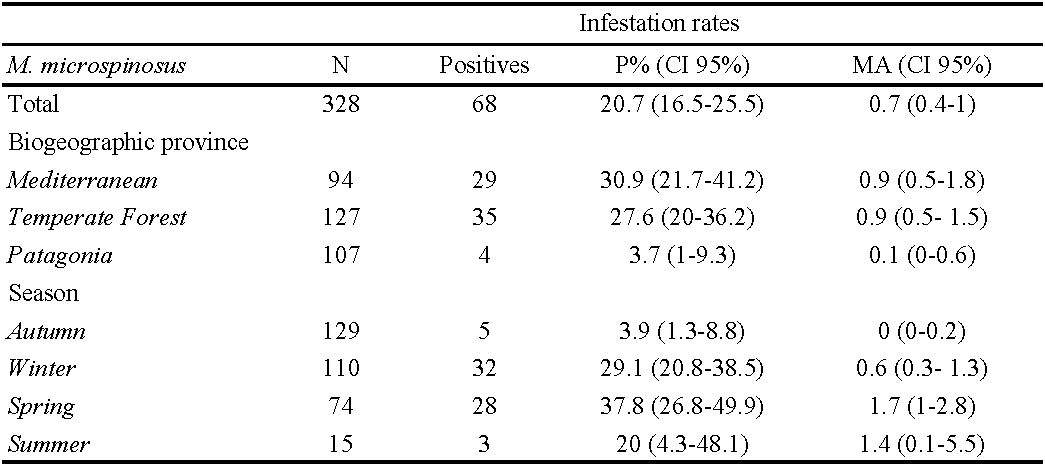
In total, 328 O. longicaudatus were captured, of which 68 (20.7%) were infested by M. microspinosus (range 1 – 20 mites; MI= 3.4; MA= 0.7). We found 232 M. microspinosus mites in 17 localities along Chile. All the mites recovered during the study were adult females. Spring was the season that presented the highest P% and MA (37.8% and 1.7, respectively). The Mediterranean area was the biogeographical province that presented the highest P% and MA (30.9% and 0.9, respectively) (Table 1).


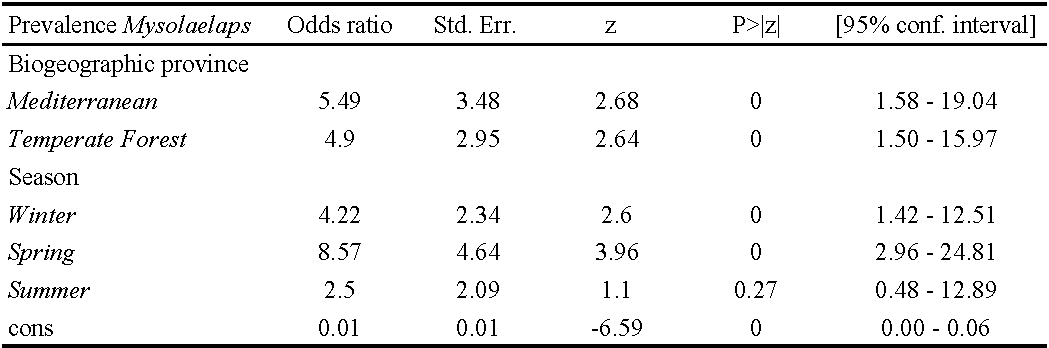
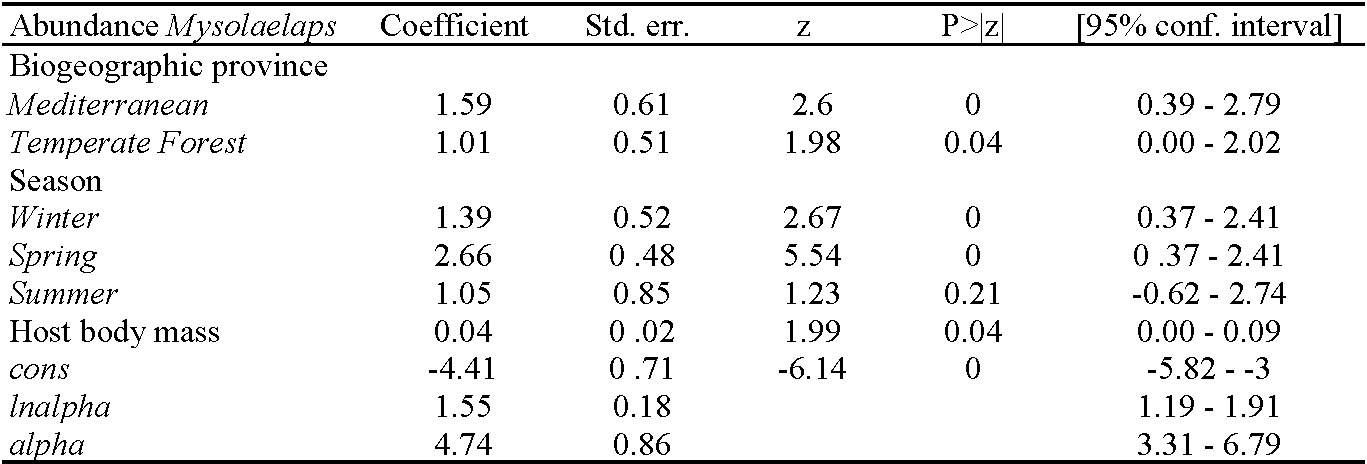
The P% of M. microspinosus was significantly associated with seasonality and biogeographic provinces, with higher frequencies of infestation observed in winter and spring than in autumn and in the Mediterranean and Temperate Forest provinces than in Patagonia (Table 2). A similar seasonal and geographical pattern was recorded for MA of M. microspinosus. In addition, MA of M. microspinosus was significantly associated with host body mass, being higher on hosts with higher body mass (Table 3).




Discussion
The present study is the first population study of Mysolaelaps throughout its distribution in Chile and is a pioneer in the study of the ecological factors that influence the infestation rates of the genus in the Neotropics. Previous studies in Latin America include the genus as a parasitological record without detailing its infestation rates or its ecology (Linardi et al., 1991; Lareschi et al., 2006; Lareschi and González-Acuña, 2010; Savchenko et al., 2021;). The P% and MA varied significantly between biogeographical provinces, describing a decreasing trend from north to south. The body mass of the hosts also had a significant influence, where individuals with greater body mass harboured higher infestations. All the M. microspinosus mites were collected from O. longicaudatus, except for six mites found on Phyllotis darwini or Darwin's Leaf-eared Mouse (Til Til, Mediterranean province) and two on Rattus rattus or Black rat (Maicolpue, Template Forest province). Considering the occasional nature of these records, we cannot truly interpret its ecologic meaning.
The P% of M. microspinosus observed in this study (20.7%; n= 328) is lower than M. microspinosus colonizing O. delticola (P%= 80; n= 10), O. flavescens (P%= 65.4; n= 27) and O. nigripes (P%= 75; n= 8) in Argentina (Lareschi et al., 2003; Navone et al., 2009) and M. microspinosus colonizing O. flavescens (P%= 100; n=1) in Uruguay (Lareschi et al., 2006). This can be explained, firstly, for the notorious difference in the sample size of this work with the others (smaller). Also this difference could be due to the mode of selection of the trapping points or to the effect of temperature and humidity, which was higher in the study areas of the related works.
This study reported a higher P% and MA of M. microspinosus in spring (September – December) (35.4% and 1.7 respectively). This differs from that reported for M. heteronychus and M. microspinosus in Brazil and Argentina, respectively (Lareschi and Krasnov, 2010; Sponchiado et al., 2015). Although the date of the higher P% and MA in Brazil and Argentina differ from Chile, the peaks of infestation in this three countries match with the time of the year in which the resources in the environment increases. Several authors state that small mammals synchronize their reproductive periods with times of greater abundance of resources (Laferriere and Atramentowicz, 1990; Godoy and Magnusson, 1999) and, in turn, their ectoparasites also synchronize their reproductive periods or greater activity periods with those of their hosts (Marshall, 1981; Blanco and Frías, 2001). Thus, the higher P% and MA reported in this work, could be attributed to the increase of food resources that takes place in Chilean spring.
The lowest P% and MA were recorded in Patagonia. Abiotic factors, such as temperature, humidity, wind, relief (Walter and Krantz, 2009b; Skoracka et al., 2015) or biotic factors such as grooming or host inmunocompetence (Hart, 1994; Proctor and Owens, 2000; Hawlena et al., 2007; Walter and Proctor, 2013), could be influencing these values. In fact, Kalueff et al., (2004), describe a direct relationship between the lack of vitamin D receptors and an increase in the frequency and duration of grooming in mice, which could be caused by the few hours of light received by latitudes south of 47° in the southern hemisphere. Given there is no records of M. microspinosus southern of 47°S (Patagonia National Park), we propose this latitude as the southern limit of distribution of the genus.
Both, P% and MA varied geographically. This differs from that described in Argentina, where there was no significant variation in Mysolaelaps infestation rates in three hosts (O. flavescens, O. delticola, and Akodon azarae) between localities (Lareschi and Krasnov, 2010). According to the authors, the short distance between the locations evaluated could have affected the significance of their results. Thus, considering the long distance between localities evaluated in this work, it's reasonable to expect geographic variation.
The Mediterranean biogeographic province presented the highest P% (30.9%) and MA (0.98), followed by Temperate Forest biogeographic province (27.6%; MA= 0.93) and Patagonia (3.7%; MA = 0.17), observing a decrease in these values from north to south in Chile, that is, from areas with less rainfall to areas with more rainfall. This trend differs from what was exposed by Barros et al. (1993) where a decreasing trend from south to north in rodent infestations in coastal and inland areas of Brazil was described. In that country, the highest P% were found in humid and hot areas such as Florianopolis (100%) (Linardi et al., 1991) and decrease towards areas with less rainfall, but still warm temperatures, such as Paraná (MAT= 22.6 °C) P%= 89 (Barros et al., 1993), Juiz de Fora (MAT= 21,4 °C) P%= 82.3 (Linardi et al., 1987), Belo Horizonte (MAT= 23,9 °C) P%= 76.1 (Linardi et al., 1984), Caratinga (MAT= 22,8 °C) P%= 72.8 (Botelho, 1978) and Brasilia (MAT= 21,5 °C) P%= 46 (Gettinger, 1987). Therefore, the MAP of the Brazilian localities mentioned above was 1532 mm/year, while the MAT was almost constant in them and all these localities have P% over 40%. Considering that the highest P% in Chile was in the Mediterranean biogeographic province that presents a low MAP and a MAT similar to the Brazilian localities, we think that the most critic abiotic factor influencing the infestation rates of M. microspinosus, is the temperature. Even when the MAP in the Chilean biogeographical provinces increases, when the temperature decreases, the P% also decreases.
Thus, the greater geographic range of the present study and the comparison of infestation rates between very dissimilar and distant localities allow us to suggest that colder localities impose greater difficulties on the survival or transmission of M. microspinosus.
References
- Abba A., Udrizar-Sauthier D., Bender J., Lareschi M. 2001 . Mites (Acari: Laelapidae) Associated with Sigmodontinae Rodents in Entre Ríos Province, Argentina. Mem. Inst. Oswaldo Cruz, 96(8): 1171-1172. https://doi.org/10.1590/S0074-02762001000800025
- Amin M.R., Khanjani M., Zahiri B. 2014 . Preimaginal development and fecundity of Gaeolaelaps aculeifer (Acari: Laelapidae) feeding on Rhizoglyphus echinopus (Acari: Acaridae) at constant temperatures. J. Crop Prot., 3: 581-587.
- Araya-Osses D., Casanueva A., Román-Figueroa C., Uribe J., Paneque M. 2020 . Climate change projections of temperature and precipitation in Chile based on statistical downscaling. Clim. Dyn., 54: 4309-4330. https://doi.org/10.1007/s00382-020-05231-4
- Barrera K., Murua R. 2015 . Nuevo desafío en Salud Pública: Presencia de reservorios de Hanta, Oligoryzomys longicaudatus y Rattus spp., en áreas de borde en praderas del sur de Chile. Sustain. Agri, Food Environ. Res., 3(3): 33-46. https://doi.org/10.7770/safer-V3N3-art966
- Barros D., Linardi P., Botelho J. 1993 . Ectoparasites of some wild rodents from Parana State, Brazil. J. Med. Entomol., 30(6): 1068-1070. https://doi.org/10.1093/jmedent/30.6.1068
- Belmar-Lucero S., Godoy P., Ferrés M., Vial P., Palma R. 2009 . Range expansion of Oligoryzomys longicaudatus (Rodentia, Sigmodontinae) in Patagonian Chile, and first record of Hantavirus in the region. Rev. Chil. Hist. Nat., 82(2): 265-275. https://doi.org/10.4067/S0716-078X2009000200008
- Blanco G., Frías O. 2001 . Symbiotic feather mites synchronize dispersal and population growth with host sociality and migratory disposition. Ecography (Cop.)., 24(2): 113-120. https://doi.org/10.1034/j.1600-0587.2001.240201.x
- Botelho J.R. 1978. Universidade Federal de Minas Gerais., 113p.
- Castro D., Mauri R., Cicchino A., Mosquera S. 1987 . Ectoparasitos de roedores de la provincia de Buenos Aires, Argentina (Acarina, Anoplura, Mallophaga y Suctoria). Rev. Soc. Entomol. Argentina, 44(3-4): 317-327.
- Fonseca F. 1936 . Notas de acarologia. XVIII - Gêneros e espécies de acarianos parasitas de ratos (Acari. Laelaptidae). Mem. Inst. Butantan, 10: 17-23.
- Furman D. 1972 . Mites of the family Laelapidae in Venezuela (Acarina: Laelapidae). Brigham Young Univ. Sci. Bull. Biol. Ser., 17(3): 1-58.
- Gettinger D. 1987 . Host Associations of Gigantolaelaps (Acari: Laelapidae) in the Cerrado Province of Central Brazil. https://doi.org/10.1093/jmedent/24.5.559
- Godoy H., Magnusson W. 1999 . Effects of climate and food availability on four rodent species in southeastern Brazil. J. Mammal., 80(2): 472-486. https://doi.org/10.2307/1383294
- Hart B. 1994 . Behavioural defense against parasites: Interaction with parasite invasiveness. Parasitology, 109: 139-151. https://doi.org/10.1017/S0031182000085140
- Hawlena H., Bashary D., Abramsky Z., Krasnov B. 2007 . Benefits, costs and constraints of anti-parasitic grooming in adult and juvenile rodents. Ethology, 113: 394-402. https://doi.org/10.1111/j.1439-0310.2007.01332.x
- Julien-Laferriere D., Atramentowicz M. 1990 . Feeding and reproduction of three didelphid marsupials in two neotropical forests (French Guiana). Biotropica, 22(4): 404-415. https://doi.org/10.2307/2388558
- Kalueff A., Lou Y., Laaksi I., Tuohimaa P. 2004 . Increased grooming behavior in mice lacking vitamin D receptors. Physiol. Behav., 82: 405-409. https://doi.org/10.1016/j.physbeh.2004.04.010
- Krasnov B., Shenbrot G., Korallo-Vinarskaya N., Vinarski M., Warburton E., Khokhlova I. 2019 . The effects of environment, hosts and space on compositional, phylogenetic and functional beta-diversity in two taxa of arthropod ectoparasites. Parasitol. Res., 118(7): 2107-2120. https://doi.org/10.1007/s00436-019-06371-1
- Kreeger T., Arnemo J. 2018 . Handbook of Wildlife Chemical Immobilization. Terry J. Kreeger and Jon M. Arnemo. pp. 472.
- Lareschi M. 2010 . Ectoparasite occurrence associated with males and females of wild rodents Oligoryzomys flavescens (Waterhouse) and Akodon azarae (Fischer) (Rodentia: Cricetidae: Sigmodontinae) in the Punta Lara Wetlands, Argentina. Neotrop. Entomol., 39(5): 818-822. https://doi.org/10.1590/S1519-566X2010000500022
- Lareschi M., Gettinger D., Venzal J., Arzua M., Nieri-Bastos F., Barros-Battesti D., Gonzalez E. 2006 . First report of mites (Gamasida: Laelapidae) parasitic on wild rodents in Uruguay, with new host records. Neotrop. Entomol., 35(5): 596-601. https://doi.org/10.1590/S1519-566X2006000500005
- Lareschi M., González-Acuña D. 2010 . Acari, Laelapidae (ectoparasitic mites), central and southern Chile. Check List, 6(4): 546-548. https://doi.org/10.15560/6.4.546
- Lareschi M., Krasnov B. 2010a . Determinants of ectoparasite assemblage structure on rodent hosts from South American marshlands: The effect of host species, locality and season. Med. Vet. Entomol., 24(3): 284-292. https://doi.org/10.1111/j.1365-2915.2010.00880.x
- Lareschi M., Krasnov B.R. 2010b . Determinants of ectoparasite assemblage structure on rodent hosts from South American marshlands\,: the effect of host species , locality and season. Med. Vet. Entomol., 24: 284-292. https://doi.org/10.1111/j.1365-2915.2010.00880.x
- Lareschi M., Notarnicola J., Nava S., Navone G. 2007 . Parasite community (Arthropods and Filarioids) associated with wild rodents from the marshes of La Plata River, Argentina. Comp. Parasitol., 74(1): 141-147. https://doi.org/10.1654/4208.1
- Lareschi M., Notarnicola J., Navone G., Linardi P.M. 2003 . Arthropod and Filarioid Parasites Associated with Wild Rodents in the Northeast Marshes of Buenos Aires, Argentina. Mem. Inst. Oswaldo Cruz, 98(5): 673-677. https://doi.org/10.1590/S0074-02762003000500015
- Lareschi M., Sánchez-López M. 2000 . Ectoparásitos (Phthiraptera y Acari) de roedores (Rodentia: Muridae: Sigmodontinae) en el delta bonaerense del río Paraná, Argentina. Rev. Soc. Entomol. Argentina, 59(1-4): 17-19.
- Linardi P., Botelho J., Ximenez A., Padovanp C. 1991 . Notes on ectoparasites of some small mammals from Santa Catarina State, Brazil. J. Med. Entomol., 28(1): 183-185. https://doi.org/10.1093/jmedent/28.1.183
- Linardi P., Teixeira V., Botelho J., Ribeiro L. 1987 . Ectoparasitos de roedores em ambientes silvestrs do municipio Juiz de Fora, Minas Gerais. Mem. Inst. Oswaldo Cruz, 82(1): 137-139. https://doi.org/10.1590/S0074-02761987000100022
- Linardi P.M., Botelho J.R., Neves D.P., Cunha H.C. 1984 . Sobre alguns ectoparasitos de roe- dores silvestres de Belo Horizonte. Rev. Bras. Biol., 44: 215-219.
- López-Berrizbeitia M., Lareschi M., Sánchez R., Díaz M. 2013 . Los ectoparasitos de los roedores sigmodontinos (Cricetidae) de La Rioja: Resultados preliminares. Rev. Argentina Parasitol., 1(3): 40-44.
- Marshall A. 1981 . The ecology of ectoparasitic insects. London: Academic Press. pp. 459.
- Mauri R. 1965 . Ácaros laeláptidos parásitos de vertebrados. Rev. Soc. Entomol. Argentina, 27(1-4): 15-18.
- Morrone J. 2015 . Biogeographical regionalisation of the world: A reappraisal. Aust. Syst. Bot., 28(3): 81-90. https://doi.org/10.1071/SB14042
- Moyer B., Drown D., Clayton D. 2002 . Low humidity reduces ectoparasite pressure: Implications for host life history evolution. Oikos, 97(2): 223-228. https://doi.org/10.1034/j.1600-0706.2002.970208.x
- Muñoz-Pedreros A., Fletcher S., Yáñez J., Sánchez P. 2010 . Diversidad de micromamíferos en tres ambientes de la Reserva Nacional Lago Peñuelas, Región de Valparaíso, Chile. Gayana, 74(1): 1-11. https://doi.org/10.4067/S0717-65382010000100003
- Murua R., Gonzalez L., Meserve P. 1986 . Population Ecology of Oryzomys longicaudatus philippii (Rodentia: Cricetidae) in Southern Chile. J. Anim. Ecol., 55(1): 281-293. https://doi.org/10.2307/4708
- Nava S., Lareschi M., Voglino D. 2003 . Interrelationship between ectoparasites and wild rodents from northeastern Buenos Aires Province, Argentina. Mem. Inst. Oswaldo Cruz, 98(1): 45-49. https://doi.org/10.1590/S0074-02762003000100007
- Navone G., Notarnicola J., Nava S., Robles M. del R., Galliari C., Lareschi M. 2009 . Arthropods and helminths assmblage in sigmodontine rodents from wetlands of the Rio de la Plata, Argentina. Mastozool. Neotrop., 16(1): 121-133.
- Perring T., Holtzer T., Kalisch J., Norman J. 1984a . Temperature and humidity effects on ovipositional rates, fecundity, and longevity of adult female banks grass mites (Acari: Tetranychidae). Ann. Entomol. Soc. Am., 77(5): 581-586. https://doi.org/10.1093/aesa/77.5.581
- Perring T., Holtzer T., Toole J., Norman J., Myers G. 1984b . Influences of temperature and humidity on pre-adult development of the banks grass mite (Acari: Tetranychidae). Environ. Entomol., 13(2): 338-343. https://doi.org/10.1093/ee/13.2.338
- Poulin R. 1998 . Evolutionary ecology of parasites: from individuals to communities. London: Chapman & Hall. pp. 224.
- Proctor H., Owens I. 2000 . Mites and birds: Diversity, parasitism and coevolution. Trends Ecol. Evol., 15(9): 358-364. https://doi.org/10.1016/S0169-5347(00)01924-8
- Radovsky F. 1969 . Adaptive radiation in the parasitic Mesostigmata. Acarologia, 11(3): 450-483.
- Reiczigel J., Marozzi M., Fábián I., Rózsa L. 2019 . Biostatistics for parasitologists - A primer to quantitative parasitology. Trends Parasitol., 35(4): 277-281. https://doi.org/10.1016/j.pt.2019.01.003
- Rodrigues-Fernandes F., Dominici-Cruz L., Linhares A., Von Zuben C. 2015 . Effect of body size on the abundance of ectoparasitic mites on the wild rodent Oligoryzomys nigripes. Acta Parasitol., 60(3): 515-524. https://doi.org/10.1515/ap-2015-0073
- Savchenko E., Melis M., Lareschi M. 2021 . Laelapid mites (Mesostigmata) ectoparasites of Oligoryzomys (Rodentia: Cricetidae) in north-eastern and central Argentina. Mastozoología Neotrop., 28(1): 1-12. https://doi.org/10.31687/saremMN.21.28.1.0.05
- Silva-de la Fuente M.C., Moreno Salas L., Casanueva M.E., Lareschi M., González-Acuña D. 2020 . Morphometric variation of Androlaelaps fahrenholzi (Mesostigmata: Laelapidae) associated with three Sigmodontinae (Rodentia: Cricetidae) from the north of Chile. Exp. Appl. Acarol., 81(1): 135-148. https://doi.org/10.1007/s10493-020-00490-6
- Skoracka A., Magalhães S., Rector B., Kuczyński L. 2015 . Cryptic speciation in the Acari: a function of species lifestyles or our ability to separate species? Exp. Appl. Acarol., 67(2): 165-182. https://doi.org/10.1007/s10493-015-9954-8
- Sponchiado J., Melo G., Landulfo G., Jacinavicius F., Barros-Battesti, Darci Cáceres N. 2015 . Interaction of ectoparasites (Mesostigmata, Phthiraptera and Siphonaptera) with small mammals in Cerrado fragments, western Brazil. Exp. Appl. Acarol., 66: 369-381. https://doi.org/10.1007/s10493-015-9917-0
- Spotorno A., Palma E., Valladares J. 2000 . Biología de roedores reservorios de hantavirus en Chile. Rev. Chil. infectología, 17(3): 197-210. https://doi.org/10.4067/S0716-10182000000300003
- Strandtmann R., Wharton G. 1958 . A manual of Mesostigmatid Mites Parasitic on Vertebrates. Collegue Park: Institute of Acarology. pp. 399.
- Uribe J., Cabrera R., Fuente A. de la, Paneque Corrales M. 2012 . Atlas bioclimático de Chile. Universidad de Chile. Facultad de Ciencias Agronómicas. pp. 228.
- Veloso-Frias J., Silva-de la Fuente M.C., Victor Rubio A., Moreno L., González-Acuña D., Simonetti J., Landaeta-Aqueveque C. 2019 . Variation in the prevalence and abundance of mites parasitizing Abrothrix olivacea (Rodentia) in the native forest and Pinus radiata plantations in central Chile. Hystrix, Ital. J. Mammal., 30(2): 107-111.
- Villagrán C., Hinojosa L.P. 2005. Esquema biogeográfico de Chile. In: Llorente-Bousquets J., Morrone J. (Eds) Reg. Biogeográfica en Iberoámeríca y tópicos afines, Ciudad de México: Ediciones de la Universidad Nacional Autónoma de México, Jiménez Editores. pp. 551-577.
- Walter D., Krantz G. 2009a. Collection, Rearing and Preparing Specimens. In: A Man. Acarol., Texas: Texas Tech University Press. pp. 83-97.
- Walter D., Krantz G. 2009b. Oviposition and Life Stages. In: A Man. Acarol., Texas: Texas Tech University Press. pp. 57-64.
- Walter D., Proctor H. 2013 . Mites: Ecology, evolution and behaviour: life at a microscale. 2nd ed. Springer. pp. 494. https://doi.org/10.1007/978-94-007-7164-2
- Yáñez-Meza A., Moreno L., Botto-Mahan C. 2018 . Ectoparasites of the endemic rodent Abrocoma bennetti (Hystricomorpha: Abrocomidae) from semiarid Chile. Gayana, 82(1): 94-97. https://doi.org/10.4067/S0717-65382018000100094
- Yunik M., Waterman J., Galloway T. 2015 . Seasonal changes in the infestation parameters of the sucking louse, Linognathoides laeviusculus (Phthiraptera: Anoplura: Polyplacidae), infesting Richardson's ground squirrel (Rodentia: Sciuridae) in Manitoba, Canada. Can. Entomol., 148(2): 143-150. https://doi.org/10.4039/tce.2015.49



2022-07-20
Date accepted:
2023-05-28
Date published:
2023-06-12
Edited by:
Roy, Lise

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 González-Aguayo, Felipe; Fuenzalida-Araya, Karen; Landaeta-Aqueveque, Carlos; Moreno Salas, Lucila; Santodomingo, Adriana and Silva-de la Fuente, María Carolina
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



