Prey consumption capacity and functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) feeding on Tetranychus urticae (Acari: Tetranychidae) on different cotton varieties
Sulek, Nazife1
; Döker, Ismail  2
; Saboori, Alireza
2
; Saboori, Alireza  3
and Cakmak, Ibrahim
3
and Cakmak, Ibrahim  4
4
1Aydin Adnan Menderes University, Faculty of Agriculture, Department of Plant Protection, Aydin, Türkiye.
2Cukurova University, Faculty of Agriculture, Department of Plant Protection, Adana, Türkiye.
3Aydin Adnan Menderes University, Faculty of Agriculture, Department of Plant Protection, Aydin, Türkiye & Jalal Afshar Zoological Museum, Department of Plant Protection, Faculty of Agriculture, University of Tehran, Karaj, Iran.
4✉ Aydin Adnan Menderes University, Faculty of Agriculture, Department of Plant Protection, Aydin, Türkiye & Department of Research and Innovation, Saveetha School of Engineering, SIMATS, Thandalam, Chennai-602105, Tamil Nadu, India.
2023 - Volume: 63 Issue: 3 pages: 665-675
https://doi.org/10.24349/o7gh-1c6yOriginal research
Keywords
Abstract
Introduction
Cotton (Gossypium hirsutum L.) is one of the most important industrial fiber crops. It is cultivated in nearly 100 countries with temperate and tropical climates in the world (Tokel 2021). India, China, and the United States of America are the top producers and Türkiye ranks 8th. Annual production of this valuable cultural plant can reach 25.733 billion tons (ICAC 2023).
Cotton plants are vulnerable to attacks from various pests. Tetranychid mites such as Tetranychus urticae Koch and T. turkestani (Ugarov & Nikolski) (Acari: Tetranychidae), puncture and feed on plant cells using their suctorial mouthparts on the undersides of leaves. The feeding activity on plant leaves greatly affects plant growth and subsequent cotton yield (Yüksel 2023). Cotton plants can employ different mechanical and chemical strategies to hinder pest activity. For example, different cotton varieties can exhibit varying trichome densities on their leaves and stems. Trichome, unicellular and multicellular extensions that grow out of the epidermis on various plant organs, can play important roles in plant defense (Wang et al. 2021). Such pubescence phenotypes can be described as smooth (no hairy), hairy (medium hairy), or pilose (high hairy) (Wright et al. 1999). Hairiness can increase resistance to pests especially mites by producing toxic chemicals and physical deterrent effects. Trichomes affect the feeding, growth, and survival of mites (Nawab et al. 2011). Sulek and Cakmak (2022) demonstrated that cotton varieties with higher trichome density negatively affected the development, reproduction and population parameters of T. urticae.
Different levels of leaf hairiness might affect natural enemies of spider mites (Skirvin and De Courcy Williams 1999). Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae) is a predatory mite widely used for the control of Tetranychus spp. mites on important cultivated plants such as strawberries, cucumbers, peppers and tomatoes under greenhouse conditions. The performance of this predatory mite can be negatively affected by temperature, prey type, the host plant and plant defenses, pesticides, etc. (Cakmak et al. 2005, 2009). This mite may occur in cotton fields in Aydin province where acaricides are widely used (at least 5-6 sprays are made against T. urticae). Under greenhouse conditions, P. persimilis is highly effective against T. urticae on cucumber plants but fail to control spider mites on eggplants due to the presence of hairy leaves. Likewise, P. persimilis performance is low on tomato varieties with leaves with dense trichomes (Helle and Sabelis 1985). No study has thus far assessed the effectiveness of P. persimilis against T. urticae in different cotton varieties. This study aimed to determine the effects of leaf characteristics of different cotton varieties on the performance of the predatory mite P. persimilis.
Material and methods
Cotton varieties
Common cotton (Gossypium hirsutum L.) varieties (Gloria, Lima, Carla, DP-396, Edessa, ST-468) from Türkiye were used for the experiments. According to Sulek and Cakmak (2022), the trichome densities on leaves of these cotton varieties were high for ST-468 and Edessa; medium for DP-396 and Carla; and low for Lima and Gloria. These cotton varieties were grown in pots (12 × 10 cm) with forest soil mixed with perlite (2:1 ratio) and incubated at 25 ± 2 ℃, 16L/8D h photoperiod and 65 ± 10% RH in a climate chamber.
Mite Rearing
Spider mites and predatory mites were sampled from cotton fields in Germencik, Aydin and have been kept in the laboratory since 2017 in separate climate chambers at 25 ± 1℃, 16L/8D h photoperiod, and 70 ± 10% relative humidity. Tetranychus urticae (green form) were maintained on clean Phaseolus vulgaris (cv.'Barbunia') plants. They were grown in pots (12 × 10 cm) in a climate room at 25 ± 2 ℃, 65 ± 10% RH and 16L/8D h photoperiod (PG34 − 3 Digitech Ltd., Ankara, Türkiye) (Cakmak et al. 2005, 2009).
Phytoseiulus persimilis was reared and maintained on tetranychid infested bean leaves on inverted pots in two interlocking trays covered with plexiglass box (Kustutan and Cakmak 2009; Kamburgil and Cakmak 2014). Trays were filled with tap water to prevent predatory mite from escaping; infested leaves were added to the predatory mite culture thrice a week.
Functional response and egg production of Phytoseiulus persimilis on six cotton varieties
Experiments on functional response and egg production of P. persimilis on six cotton varieties were conducted using 9 cm plastic Petri dishes. Firstly, cotton leaves (6 cm Ø) were placed with lower surface up on moist cotton (6 cm Ø) in Petri dishes (one leaf/dish); the space between leaf edges and Petri sides was inundated to prevent predator mite from escaping.
To obtain a cohort of T. urticae individuals at the same age for the experiments, 50 gravid T. urticae females were transferred with a fine brush to each leaf and allowed to lay eggs for 24 hours. After egg laying, the adults removed from the test units. Thus, only eggs were left in each Petri dish. In this way, eggs and protonymphs of the same age of T. urticae were obtained.
To starve the predatory mites, females were individually transferred from stock cultures to new Petri dishes (9 cm) which were placed on water-saturated cotton wool in higher-sized plastic cups. The predators starved for one hour before being used in the experiments. One starved female was transferred to an experimental arena with six prey densities of (5, 10, 20, 40, 80 and 160 eggs or protonymphs) of T. urticae. In the study, 20 replications were used for each prey density. After 24 hours, the number of prey items and the number of eggs consumed and produced by the predatory mite were determined. The number of prey consumed by the predatory mite depending on the prey density was used to determine the functional response and its parameters. All experiments were carried out at 25 ± 1 ℃, 65 ± 5% RH and 16L/8D h photoperiod in a climate room (PG34−3 Digitech Ltd., Ankara).
Statistical analyses
The functional responses of P. persimilis depending on the different biological stages (egg and protonymph) and density of T. urticae were analyzed using a two-step data analysis in SAS portable version 9.4 (Juliano 2001). In this regard, functional response type was ascertained using the logistic regression of the prey density (Na/N0) consumed by the predator depending on the initial prey density (N0) (Juliano 2001). The formula below (1) was used to calculate the functional response type of the predatory mite, and P0, P1, P2 and P3 given in the equation indicate constant, linear, quadratic and cubic coefficients, respectively. Accordingly, significantly negative linear P1 parameter indicates that the predator exhibits a Type II functional response regardless of cotton varieties.
\[(1) \frac{Na}{No}=\frac{exp(P_0+P_1N_0+P_2N_0^2+P_3N_0^3 )}{1+exp(P_0+P_1N_0+P_2N_0^2+P_3 N_0^3)}\]
In the second step, the predator's handling time (Th ) and attack rate (α) were calculated using the random predator equation (formula 2) as suggested by Rogers (1972);
\[(2) Na=N0[1-exp(a(Th*Na-T))]\]
where Nα is the number of prey consumed; T is the experimental period (hour); N0 is the initial number of preys offered to the predator; α indicates the attack rate and Th indicates the handling time of the predator.
The number of prey items consumed and the number of eggs laid by the predatory mite were separately analyzed using General Linear Model (GLM) factorial analyses. The six cotton varieties, the two biological stages of the prey, and the six prey densities were considered as three factors, and their interactions were determined. Because the interactions are significant in all cases, the data regarding prey consumption was subjected to One-way ANOVA, and the differences among cotton varieties were determined by using Student–Newman–Keuls test. In addition, differences among two prey stages within the same prey density were determined by using independent samples T-test. Prior to data analysis, normality and homogeneity of variances were confirmed by using Shapiro–Wilk and Levene's test, respectively. Because the interactions among the cotton varieties, prey stage, and densities are not significant in all cases, no further analyses were conducted with regard to egg production data. In addition, numerical responses of the predator were calculated by using the formula suggested by Omkar and Pervez (2004) and Fathipour et al. (2020). However, very low R2 values were obtained when egg production and efficiency of conversion of ingested food (ECI) versus initial prey density were fitted using linear regression. Therefore, the results regarding numerical response were not included to paper. All analyses were performed in SPSS (version 25.0 Chicago IL, USA).
Results
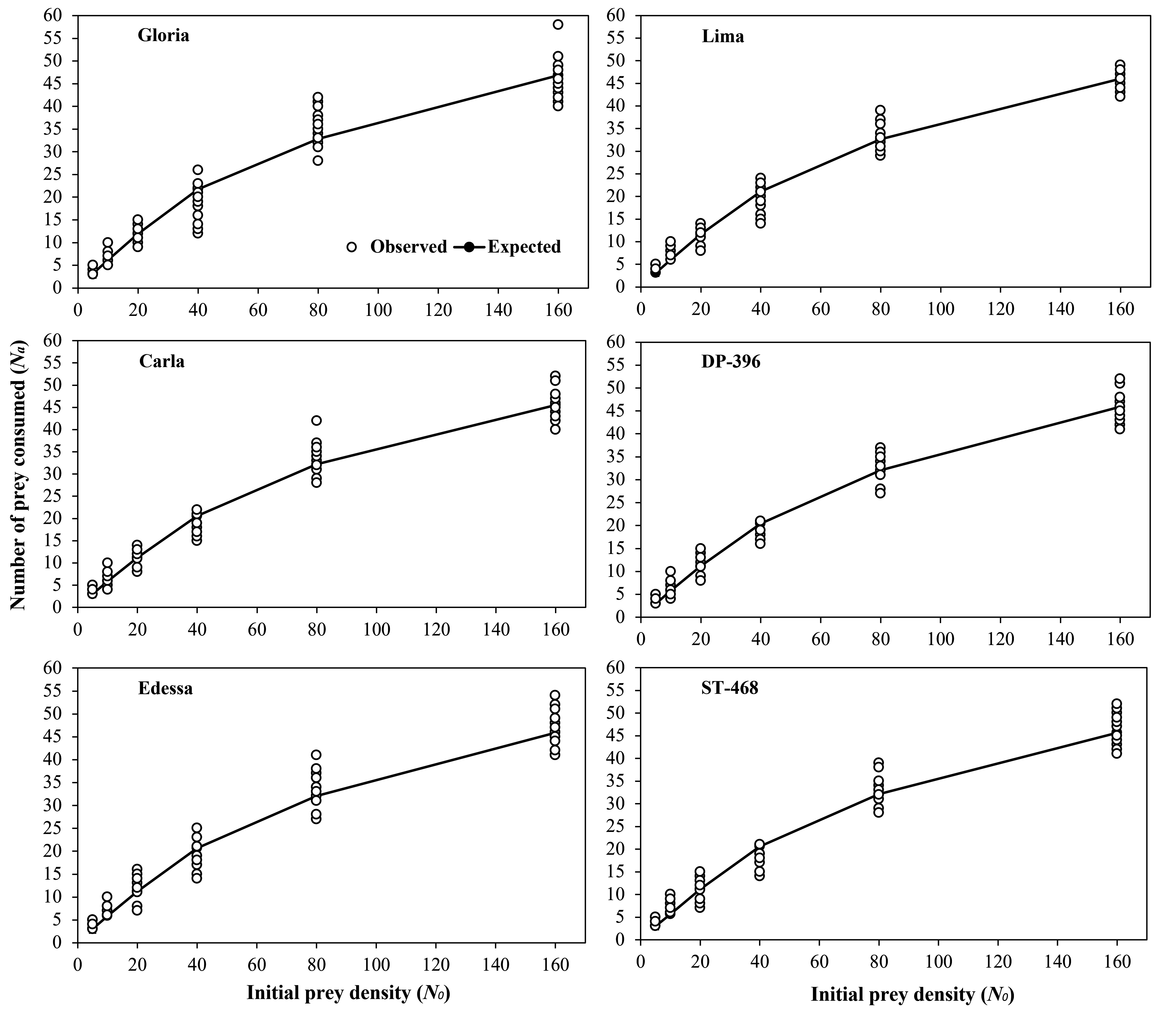


Estimated linear coefficients (P1 values) by the logistic regression in relation to initial T. urticae egg and protonymph densities on different cotton varieties were significantly negative (Table 1). In addition, the prey consumption rates of P. persimilis decreased with increasing prey densities in six different cotton varieties (Figures 1 and 2). Therefore, P. persimilis exhibited a Type II functional response against T. urticae eggs and protonymphs regardless of cotton varieties. The highest attack rates (α) are 0.045 h-1 and 0.085 h-1 when the predator fed on eggs and protonymphs of T. urticae on Gloria (low trichome density) variety, respectively (Tables 2 and 3). In contrast, the lowest attack rates are 0.040 h-1 and 0.047 h-1 when the predator fed on eggs and protonymphs of T. urticae on ST-468 (high trichome density) variety, respectively. The handling times (Th ) obtained on six cotton varieties ranged between 0.344-0.353 h and 0.373-0.426 h when the predator fed on eggs and protonymphs of T. urticae.









A factorial analysis within GLM on prey consumption of the predator revealed that there were significant interactions between host plant variety × prey stage (F=10. 387; P\textless0.001), prey density × prey stage (F=39.952; P\textless0.001), host plant variety × prey density (F=2.780; P\textless0.001) as well as the interactions of the three factors (F=2.007; P\textless0.001).
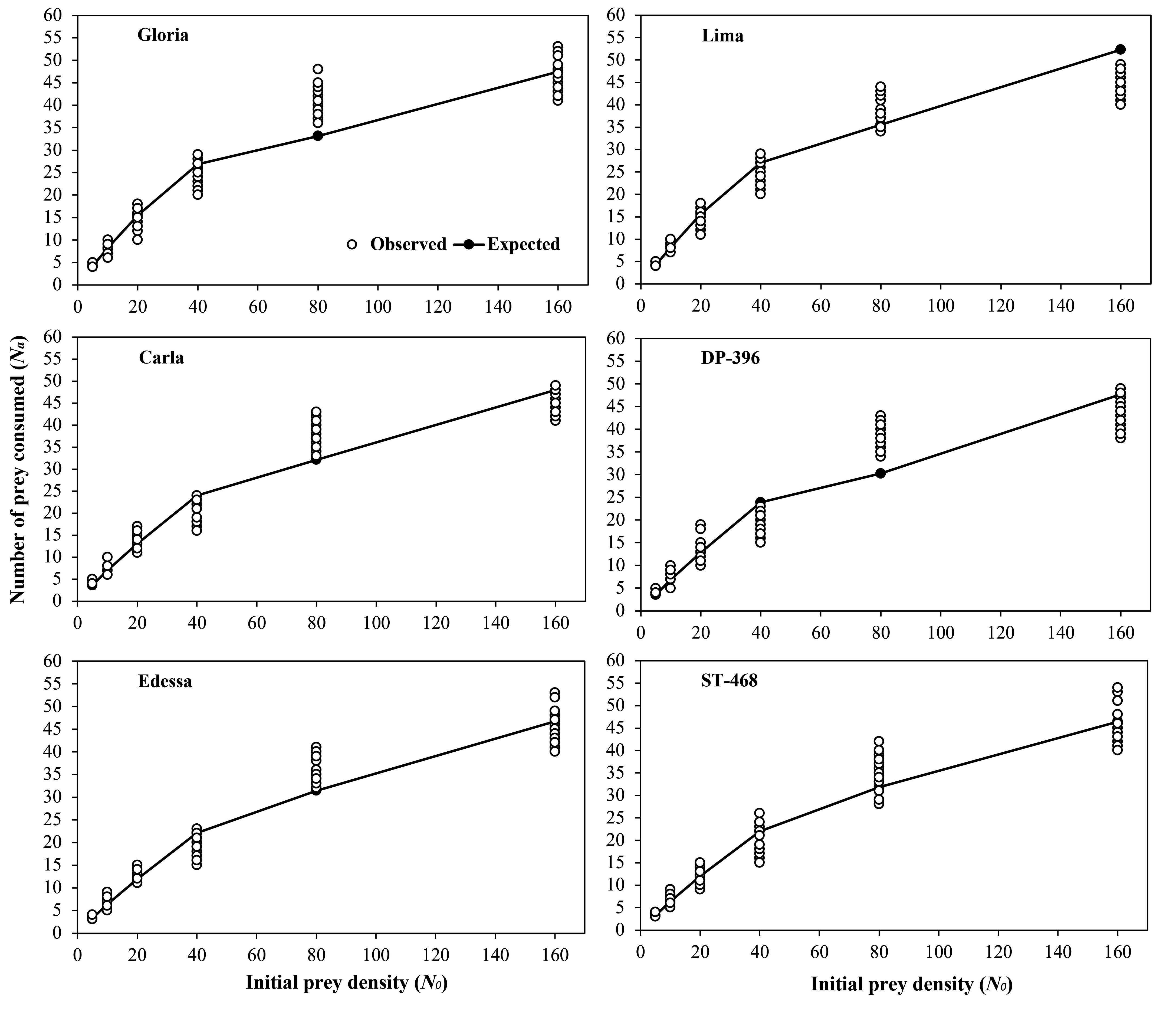


Although no significant difference was determined regarding food consumption of P. persimilis fed on T. urticae eggs on different cotton varieties, numerically higher values were obtained on the Gloria (low trichome density) variety in general (Table 4). In contrast, food consumptions of P. persimilis in all protonymph densities (except 160) were significantly higher on varieties with lower trichome density (Gloria and Lima) compared to varieties with higher trichome density (Edessa and ST-468). Independent sample T-test on egg and protonymph consumptions of the predator revealed that it consumed more protonymphs compared to eggs at 20, 40, and 80 densities on Gloria and Lima varieties, and at 20 and 80 prey densities on Carla and DP-396 varieties (P two-tailed \textless0.001). In contrast, egg consumption of the predator compared to protonymph consumption were significantly higher at 5, 10 and 80 prey densities on Edessa and ST-468 varieties (P two-tailed \textless0.001). Furthermore, the number of prey items consumed by the predatory mite increased with increasing prey densities for both biological stages of the prey.



A factorial analysis within GLM on egg production of the predator revealed that there were no significant interactions between host plant variety × prey stage (F=0.586; P=0.711), prey density × prey stage (F=0.515; P=0.765), host plant variety × prey density (F=0.439; P=0.993) as well as the interactions of the three factors (F=0.286; P=1.00). Although there was no significant difference between the numbers of eggs produced by the predatory mite among different cotton varieties, egg production was also increased in response to increasing prey densities (Table 5).



Discussion
Aydin is a province located in the western part of Türkiye. It has suitable climatic conditions and fertile plains and has long been a production center of cotton plants. Population outbreaks of T. urticae and failures in controlling this mite probably due to resistance problems have been reported in cotton fields in the region (Sulek and Cakmak 2022). Despite the heavy insecticide and acaricide applications in cotton fields, P. persimilis unexpectedly is the most abundant predator associated with T. urticae in the region. This predatory mite is one of the most comprehensively studied species as a specialized on T. urticae. This is the first study assessing its foraging behavior on different cotton varieties with varying degrees of leaf hairiness. Therefore, this study documents the functional response and egg production of P. persimilis fed on T. urticae on six cotton varieties for the first time.
According to the results, P. persimilis exhibited a Type II functional response to both egg and protonymph stages of T. urticae irrespective of cotton varieties. This result was not surprising since a series of studies reported a Type II functional response for phytoseiid mites under a variety of factors including temperature, humidity, host plant, insecticide applications, age, and rearing history of the predator (Castagnoli and Simoni 1999; Poletti et al. 2007; Kustutan and Cakmak 2009; Ahn et al. 2010; Döker et al. 2016; Fathipour et al. 2018; Sugawara et al. 2018).
In general, no significant difference was detected among the attack rates of the predator except on Gloria and Lima varieties – these have low trichome densities – when feeding on protonymphs. Similarly, Krips et al. (1999) reported low searching efficiency of P. persimilis on gerbera varieties that had higher trichome density. Jafarian et al. (2022) found that the attack rates of protonymphs of another phytoseiid mite Typhlodromus bagdasarjani Wainstein & Arutunjan fed Eotetranychus frosti (McGregor) (Acari: Tetranychidae) varied on different apple varieties. The authors reported more than two times higher attack rate on a low hairy cultivar (Kohanz Golab) compared to a high hairy cultivar (Granny Smith). Koveos and Broufas (2000) also reported a substantially lower attack rate for Amblyseius andersoni (Chant) and Euseius finlandicus (Oudemans) on apple leaves compared to peaches. In general, our results also showed that the attack rates obtained on protonymphs were higher than those obtained on eggs. This result can be explained by the size of prey, and the predator may spend less time finding a protonymph than finding an egg (Li and Zhang 2020; Döker et al. 2021).
In contrast to attack rates, there was a great similarity in the handling times of P. persimilis feeding on prey eggs and protonymphs on the different cotton varieties. This result can be explained by the prey specialization of P. persimilis on Tetranychus species (McMurtry et al. 2013). The handling time is the time that is spend by the predators between the first encounter, capture, feed, and digestion of a prey item (Fathipour et al. 2018; Papanikolaou et al. 2020). Therefore, due to the prey specialization of P. persimilis, the time between the first encounter with a prey and its digestion, and search for the next prey items was not influenced by the host plant characteristics.
No significant difference was found in terms of food consumption of P. persimilis on T. urticae eggs on different cotton varieties. In contrast, food consumptions of P. persimilis in all protonymph densities (except 160) were significantly higher on varieties with low trichome density (Gloria and Lima) compared to varieties with high trichome density (Edessa and ST-468). Similarly, Krips et al. (1999) reported that the consumption rate of P. persimilis fed on T. urticae eggs on low-high hairy gerbera plants was affected by leaf hairiness, especially at low prey densities (1, 3, and 2.5 eggs/cm2). The authors found that consumption was higher in a low-hairy variety at low prey densities, but no difference between varieties at high prey densities (8 eggs/cm2). Nassar et al. (2010) also reported that the number of T. urticae nymphs consumed by P. persimilis on seven different cultivated plants vary between 5.91 and 16.37 depending on the leaf hairiness. In addition, Camporese and Duso (1996) found that the abundance of A. andersoni in hairless grape varieties was substantially higher compared to hairy varieties under field conditions.
Similarly, there was no significant difference between the number of eggs laid by P. persimilis fed on T. urticae eggs and protonymphs depending on different prey densities, and cotton varieties. However, the number of eggs laid by P. persimilis was increased with increasing egg and protonymph densities of T. urticae. Similarly, Ottaviano et al. (2013) reported that the number of eggs laid by Neoseiulus californicus (McGregor) on strawberry varieties with different trichome densities were increased depending on the prey density but did not differ between varieties. In contrast, Nassar et al. (2010) observed a statistical difference in the number of eggs laid by P. persimilis in seven different cultivated plants (bean, apple, fig, zucchini, cucumber, mango and cotton) with varying amounts of hairiness. The authors reported the lowest number of eggs on medium hairy mango (2.56 eggs/day) and cotton (2.51 eggs/day). The number of eggs laid in beans and apples with low hair rate was reported as 3.95 eggs/day and 3.73 eggs/day, respectively (Nassar et al. 2010).
In conclusion, determination of prey consumption capacity of a predator on different host plants is a keystone for the success of biological control (Skirvin and Fenlon 2001). Plant characteristics such as hairiness on the leaf surface may influence prey and predator relationships (Roda et al. 2001). The current study showed that the leaf trichome density of the cotton plant affected the prey consumption capacity of P. persimilis on T. urticae protonymphs. However, more studies are needed to address the issue from a wider perspective, especially to observe the performance and biological control potential of P. persimilis on cotton varieties under field conditions.
Acknowledgments
We acknowledge Mustafa Ali Kaptan and Aydin Unay for their recommendations on cotton plant rearing, Mustapha Touray for editing the manuscript. This study was part of the first author's M.Sc. thesis which was funded by Aydin Adnan Menderes University Research Foundation (ZRF-20007).
References
- Ahn J., Kim K., Lee J.H. 2010. Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on strawberry leaves. J. Appl. Entomol., 134: 98-104. https://doi.org/10.1111/j.1439-0418.2009.01440.x
- Cakmak I., Baspinar H., Madanlar N. 2005. Control of the carmine spider mite Tetranychus cinnabarinus Boisduval by the predatory mite Phytoseiulus persimilis (Athias-Henriot) in protected strawberries in Aydın, Turkey. Turk. J. Agric. For., 29: 259-265.
- Cakmak I., Janssen A., Sabelis M.W., Başpınar H. 2009. Biological control of an acarina pest by single and multiple natural enemies. Biol. Control, 50: 60-65. https://doi.org/10.1016/j.biocontrol.2009.02.006
- Castagnoli M., Simoni S. 1999. Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 23: 217-234. https://doi.org/10.1023/A:1006066930638
- Camporese P., Duso C. 1996. Different colonization patterns of phytophagous and predatory mites (Acari: Tetranychidae, Phytoseiidae) on three grape varieties: a case study. Exp. Appl. Acarol., 20: 1-22. https://doi.org/10.1007/BF00051473
- Döker I., Kazak C., Karut K. (2016) Functional response and fecundity of a native Neoseiulus californicus population to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae) at extreme humidity conditions. Syst. Appl. Acarol., 21: 1463-1472. https://doi.org/10.11158/saa.21.11.3
- Döker I., Yalcin K., Karut K., Kazak C. 2021. Functional and numerical responses of Iphiseius degenerans (Berlese) (Acari: Phytoseiidae) to different biological stages of Eutetranychus orientalis (Klein) (Acari: Tetranychidae). Syst. Appl. Acarol., 26: 1415-1425. https://doi.org/10.11158/saa.26.8.2
- Fathipour Y., Karimi M., Farazmand A., Talebi A.A. 2018. Age-specific functional response and predation capacity of Phytoseiulus persimilis (Phytoseiidae) on the two-spotted spider mite. Acarologia, 58: 31-40. https://doi.org/10.24349/acarologia/20184225
- Fathipour Y., Maleknia B., Bagheri A., Soufbaf M., Reddy G.V.P. 2020. Functional and numerical responses, mutual interference, and resource switching of Amblyseius swirskii on two-spotted spider mite. Biol. Control, 146: 104266. https://doi.org/10.1016/j.biocontrol.2020.104266
- Helle, W., Sabelis, M.W. 1985. Spider mites: their biology, natural enemies and control (Vol. 1B.). Amsterdam: Elsevier.
- Holling C.S. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol., 91: 385-398. https://doi.org/10.4039/Ent91385-7
- ICAC 2023. Production of cotton. International Cotton Advisory Committee. Available from: https://icac.org/DataPortal/DataPortal
- Jafarian F., Jafari S., Fathipour Y. 2022. Functional response of the predatory mite, Typhlodromus bagdasarjani (Acari: Phytoseiidae) to protonymphs of Eotetranychus frosti (Acari: Tetranychidae) on four apple cultivars. Acarologia, 62: 454-464. https://doi.org/10.24349/7ejy-uk7s
- Juliano S.A. 2001. Non-linear curve fitting: predation and functional response curves. In: Schneider S.M., Gurevitch J. (Eds). Design and analysis of ecological experiments, 2nd ed. New York: Chapman and Hall, pp. 178-196.
- Kamburgil S., Cakmak I. 2014. Biological parameters of the predatory mite Cheletomimus bakeri (Acari: Cheyletidae) feeding on Tetranychus cinnabarinus (Acari: Tetranychidae). Biocontrol Sci. Technol., 24: 1339-1348. https://doi.org/10.1080/09583157.2014.938610
- Koveos D.S., Broufas G.D. 2000. Functional response of Euseius finlandicus and Amblyseius undersoni to Panonychus ulmi on apple and peach leaves in the laboratory. Exp. Appl. Acarol., 24: 247-256. https://doi.org/10.1023/A:1006431710313
- Krips O.E., Kleijn P.W., Willems P.E.L., Gols G.J.Z., Dicke M. 1999. Leaf hairs influence searching efficiency and predation rate of the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol., 23: 119-131. https://doi.org/10.1007/978-94-017-1343-6_29
- Kustutan O., Cakmak I. 2009. Development, fecundity, and prey consumption of Neoseiulus californicus (McGregor) fed Tetranychus cinnabarinus Boisduval. Turk. J. Agric. For., 33: 19-28. https://doi.org/10.3906/tar-0806-39
- Li G.Y., Zhang Z.-Q. 2020. Can supplementary food (pollen) modulate the functional response of a generalist predatory mite (Neoseiulus cucumeris) to its prey (Tetranychus urticae)? BioControl, 65: 165-174. https://doi.org/10.1007/s10526-019-09993-7
- McMurtry J.A., De Moraes G.J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Nassar O.A., Fouly A.H., Fouda R.A., Osman M.A. 2010. Influence of plant texture on the feeding capacity and fecundity of the predatory mite Phytoseiulus persimilis (A.-H.). J. Plant Prot. & Pathol., 1: 103-109. https://doi.org/10.21608/jppp.2010.86703
- Nawab N.N., Khan I.A., Khan A.A., Amjad M. 2011. Characterization and inheritance of cotton leaf pubescence. Pak. J. Bot., 43(1): 649-658.
- Omkar, Pervez A. 2004. Functional and numerical responses of Propylea dissecta (Col., Coccinellidae). J. Appl. Entomol., 128: 140-146. https://doi.org/10.1111/j.1439-0418.2004.00824.x
- Ottaviano M.F.G., Sanchez N.E., Roggiero M.F., Greco N.M. 2013. Performance of Tetranychus urticae and Neoseiulus californicus on strawberry cultivars and assessment of the effect of glandular trichomes. Arthropod-Plant Inte., 7: 547-554. https://doi.org/10.1007/s11829-013-9268-x
- Papanikolaou N.E., Broufas G.D., Papachristos D.P., Pappas M.L., Kyriakaki C., Samaras K., Kypraios T. 2020. On the mechanistic understanding of predator feeding behavior using the functional response concept. Ecosphere, 11: e03147. https://doi.org/10.1002/ecs2.3147
- Poletti M, Maia A.H.N., Omoto C. 2007. Toxicity of neonicotinoid insecticides to Neoseiulus californicus and Phytoseiulus macropilis (Acari: Phytoseiidae) and their impact on functional response to Tetranychus urticae (Acari: Tetranychidae). Biol. Control, 40: 30-36. https://doi.org/10.1016/j.biocontrol.2006.09.001
- Roda A., Nyrop J., English-Loeb G., Dicke M. 2001. Leaf pubescence and two-spotted spider mite webbing influence phytoseiid behavior and population density. Oecologia, 129: 551-560. https://doi.org/10.1007/s004420100762
- Rogers D. 1972. Random search and insect population models. J. Anim. Ecol., 41: 369-383. https://doi.org/10.2307/3474
- Skirvin D.J., De Courcy Williams M. 1999. Differential effects of plant species on a mite pest (Tetranychus urticae) and its predator (Phytoseiulus persimilis): implications for biological control. Exp. Appl. Acarol., 23: 497-512. https://doi.org/10.1023/A:1006150521031
- Skirvin D.J., Fenlon J.S. 2001. Plant species modifies the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae): implications for biological control. Bull. Entomol. Res., 91: 61-67. https://doi.org/10.1079/BER200063
- Sugawara R., Ullah M.S., Ho C.C., Gotoh T. 2018. Impact of temperature-mediated functional responses of Neoseiulus womersleyi and N. longispinosus (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Biol. Control, 126: 26-35. https://doi.org/10.1016/j.biocontrol.2018.07.010
- Sulek N., Cakmak I. 2022. Performance of Tetranychus urticae (Acari: Tetranychidae) on six cotton varieties with varying degree of leaf pubescence. Syst. Appl. Acarol., 27: 450-459. https://doi.org/10.11158/saa.27.3.4
- Tokel D. 2021. Dünya pamuk tarımı ve ekonomiye katkısı. MANAS Journal of Social Studies, 10(2): 1022-1037. https://doi.org/10.33206/mjss.858702
- Wang X., Shen C., Meng, P., Tan, G., Lv L., 2021. Analysis and review of trichomes in plants. BMC Plant Biol., 21: 70. https://doi.org/10.1186/s12870-021-02840-x
- Williams F.M., Juliano S.A. 1985. Further difficulties in the analysis of functional-response experiments and a resolution. Can. Entomol., 117: 631-640. https://doi.org/10.4039/Ent117631-5
- Wright R.J., Thaxton P.M., El-Zik K.M., Paterson A.H. 1999. Molecular mapping of genes affecting pubescence of cotton. J. Hered., 90: 215-219. https://doi.org/10.1093/jhered/90.1.215
- Yüksel F.Ç. 2023. Determination of Tetranychus species and their endosymbiont bacteria in cotton fields in Aydin province (Msc Thesis). Aydın: Aydın Adnan Menderes University, pp. 63.



2023-01-19
Date accepted:
2023-05-25
Date published:
2023-06-02
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Sulek, Nazife; Döker, Ismail; Saboori, Alireza and Cakmak, Ibrahim
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



