Predator potential and prey stage preference of Neoseiulus longispinosus (Acari: Phytoseiidae) to life stages of Tetranychus urticae and T. macfarlanei (Acari: Tetranychidae)
Devasia, Jyothis  1
and Ramani, Neravathu
1
and Ramani, Neravathu  2
2
1✉ Division of Acarology, Department of Zoology, University of Calicut, Malappuram (Dist), Kerala, India.
2Division of Acarology, Department of Zoology, University of Calicut, Malappuram (Dist), Kerala, India.
2023 - Volume: 63 Issue: 3 pages: 658-664
https://doi.org/10.24349/cq41-j4t2Original research
Keywords
Abstract
Introduction
Spider mites of the family Tetranychidae are potential pests cause considerable damage and yield loss in a wide economically plant species globally. Tetranychus urticae Koch is the most notorious species worldwide with a host range of 1200 plant species including fruit orchards, vegetable crops and ornamentals (Helle and Sabelis 1985; Migeon and Dorkeld 2022).
The red spider mite, Tetranychus macfarlanei Baker & Pritchard was first described from Mauritius and Africa based on the specimens collected from beans, pumpkin and Hibiscus spp. Afterwards, it was recorded from Gwalior and Madhya Pradesh in India showing infestation and serious damage to crops like okra and cucurbits (i.e., smooth gourd, bottle gourd, pumpkin, and cucumber) (Baker and Pritchard 1960). Till date, the species has been recorded from 46 species of host plants belonging to 15 families (Migeon and Dorkeld 2022).
Conventional methods of pest control through chemical pesticides pose a severe threat to human and the environment. Apart from environmental pollution, chemical pesticides have been reported to induce development of pesticide resistance in various arthropod pests, including spider mites (Xu et al. 2018). This, in fact has much reduced the popularity and usefulness of chemical pesticides in the past few decades. In this context, biological control, owing to their efficacy, environmental safety as well as reduced health hazards has gained much recognition as a substitute to chemical pesticides, in controlling arthropod pests, especially the spider mites. Among the various natural enemies, predatory mites of the family Phytoseiidae are widely used as effective biological control agents against phytophagous mites and other small insects like thrips, mealy bugs, whiteflies, and scale insects (Fathipour and Maleknia 2016). The family Phytoseiidae is cosmopolitan in distribution and presently includes 2557 species (Demite et al. 2023). These mites possess most of the desirable attributes required for typical biocontrol agents like the extreme prey specificity, lower handling time, high fecundity with short developmental duration compared to the prey species, superior searching ability and increased dispersal rate etc. Moreover, their ability to thrive on alternative diets makes them a plausible biocontrol candidate for managing spider mite populations (Fathipour and Maleknia 2016). Based on the diversity of feeding habits, phytoseiids are organised in to four categories viz, Type I, Type II, Type III and Type IV, where the first two types are the specialist feeders of the spider mite genus Tetranychus and the family Tetranychidae respectively, whereas Type III and Type IV predators are considered as generalist species that can actively feed and reproduce on mites belonging to multiple families and also on different species of plant pollen (McMurtry and Croft 1997; McMurtry et al. 2013).
The predatory mite, Neoseiulus longispinosus (Evans), belongs to type II feeding category of the family Phytoseiidae and is considered as a voracious feeder of spider mites, especially species of the genus Tetranychus. Its distribution was already reported from various Indian states (e.g., West Bengal, Orissa, Bihar, Arunachal Pradesh, Sikkim, Tamil Nadu, Karnataka, and Uttar Pradesh) (Gupta 2003). Neoseiulus longispinosus is considered as a common phytoseiid species that actively feeds on tetranychid mites in India (Karmakar and Gupta 2011; Haneef and Sadanandan 2013). The species has been reported to have a high potential to get acclimatized faster in warmer environmental conditions and which makes it as a potent candidate for the bio-control of agricultural pests in south Indian conditions (Mallik et al. 1998). It has been reported as most common phytoseiid predator associated with the horticultural ecosystems in Kerala (Binisha and Bhaskar 2013). In several countries, N. longispinosus is commercially available as an Acarine Biocontrol Agent (ABA) for the control of spider mite populations (Huyen et al. 2017). The present study evaluates the prey stage preference and predatory potential of N. longispinosus towards the life stages of T. urticae and T. macfarlanei.
Material and methods
Live specimens of the predatory mite, N. longispinosus and two spider mite species, T. urticae and T. macfarlanei were collected from Vigna unguiculata L. Walp (Family: Fabaceae) cultivated in nearby open fields, at University of Calicut, India. Stock cultures of all studied species were maintained on mulberry (Morus alba L. (Family: Moraceae)) leaf arenas placed upside down on a water saturated foam pads of 2.5cm thickness, with dimension (30cm length and 20cm width) and placed inside rectangular plastic trays (35cm length, 25cm width and 8cm depth). The water level inside the tray was maintained in optimum level to keep the leaves fresh and to prevent the escape of mites from the leaves. Separate culture sets were maintained for each mite species under laboratory conditions of 30±2ºC, 70±5% Relative Humidity (RH) and a photoperiod of 11 L: 14 D h.
Prey stage preference experiment was carried out in an open experimental arena prepared by placing a mulberry leaf discs (2 cm2) upside down in a Petri dish on 2% semi solidified agarose (Brodeur and Cloutier 1992). For the preparation of agarose medium, 2 g agarose with 100 ml of distilled water was warmed for 2 min by constant stirring. When the agarose was completely dissolved in water, the medium was poured in to a Petri dish (7 cm diameter) without trapping any air bubbles inside and allowed to cool until it was semi solidified. The semi-fluid consistency of the agarose medium helped to prevent the escape of mites from the leaf surface.
Gravid females of each spider mite species were picked up from their respective stock cultures and were transferred to separate mulberry leaf discs in open experimental arena. The females were kept undisturbed to lay eggs for 24 hr and then were removed. A total of 15 eggs were retained in the experimental arena and the rest were removed without damaging the webbings over the eggs and surroundings. Prior to the experiment, 15 of each of spider mite stages (larvae, protonymph, deutonymph, and adult females) were carefully introduced to the experimental arena. A single active life stage of the predatory mite, either a nymph (protonymph or deutonymph) or an adult female (2–3 days old), was introduced to begin the feeding experiment. The larvae of N. longispinosus despite their active nature, were found non-feeding (Ibrahim and Palacio 1994; Sharma and Chauhan 2013) and hence were not considered for laboratory feeding experiments. The experimental duration was fixed as 24 hr and observations were made carefully by counting the number of shriveled carcasses of the life stages of the prey mite left on the leaf discs. The rate of egg consumption by the predator was evaluated by subtracting the number of intact eggs on the leaf discs from the initial number of eggs offered. Each experiment was replicated ten times and the percentage of consumption was calculated following the formula (Jyothis and Ramani 2020):
(Ne/No) x 100.
Where Ne = number of prey stage consumed; No = total number of prey stage given.
Data obtained on the above parameters were subjected to statistical analysis through one way ANOVA followed by Duncan post hoc test (P < 0.05), using SPSS (IBM SPSS Statistics 20).
Results
Neoseiulus longispinosus was recognized as voracious predator of spider mites as evidenced during the laboratory feeding experiments. The results revealed significant variations between the adult and nymphal stages of N. longispinosus in the rate of predation on the different stages of T. urticae and T. macfarlanei (p < 0.05). Accordingly, the order of preference of the adult female of N. longispinosus on the life stages (e.g., egg, larva, nymph, and adult female) of T. urticae was decreased in the sequence: Egg > Nymphs > Larva > Adult. The consumption percentage was 80, 15.5, 8.5, and 6.6% respectively (F = 341.4; d.f = 3; p = 0.000). Similarly, the prey stage preference of the adult female of N. longispinosus on different stages of T. macfarlanei was Egg > Nymphs > Larva (Table 1), as the consumption percentage was 68, 13.3 and 6.6 respectively (F = 80.7; d.f = 3; p = 0.000). Predator female did not show any preference towards adult female of T. macfarlanei, and totally neglected them and leaving untouched (Table 1).
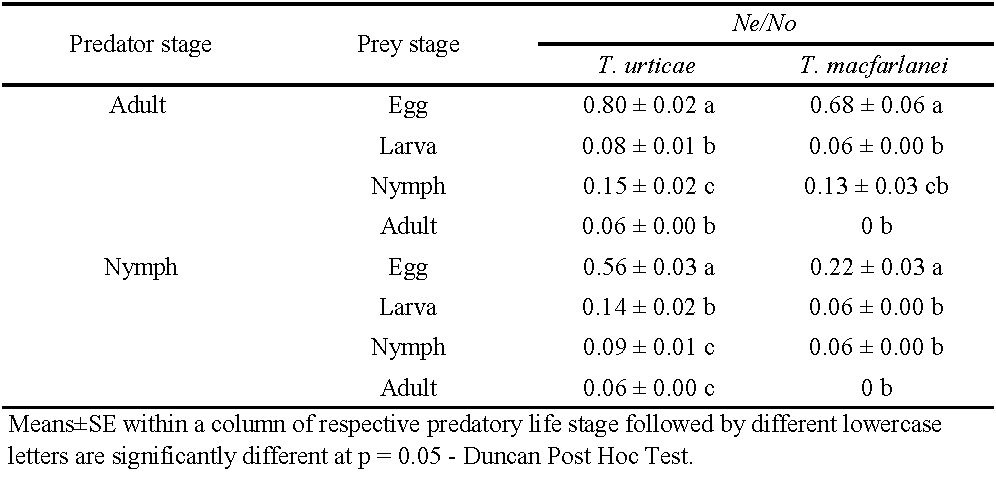


Results recorded a significant variation in the rate of feeding of the nymphal stages of N. longispinosus on different stages of T. urticae. The feeding preference decreased in the order of Egg> Larva > Nymph> Adult (Table 1) as the mean consumption rates were 56.6, 14.6, 9.5, and 6.6% respectively (F = 90.3; d.f = 3; p = 0.000). Similarly, the nymphal stages of the predator devoured significantly higher number of T. macfarlanei eggs and the rates of prey consumption on egg, larvae and nymphal stages of the prey mite were 22.6, 6.6, and 6.6% respectively (F = 33.2; d.f = 3; p = 0.000).
The larvae of N. longispinosus were found non-feeding and hence were not considered for laboratory feeding experiments. These larvae were proved to have zero predatory potential, in controlling the spider mite pests tested.
Discussion
Results of the prey stage preference experiments revealed a considerable difference in the rate of consumption of predatory species/ life stage with respect to varied prey species/life stages of spider mites selected for the present study. The maximum predation rate was observed in the predatory females. They were recognized as the most voracious life stage of phytoseiid predators with regard to prey consumption rate. Adult female consumed more number of prey stages in their oviposition period (Blackwood et al. 2004). The adult female of N. longispinosus exhibited a remarkably very high feeding preference towards the spider mites eggs of both T. urticae and T. macfarlanei whereas, a low rate of consumption was noted towards other active life stages of the same prey species. It was also noted that the predator never attacked or fed on adult females of T. macfarlanei which appeared to be physically larger in size and hence would be difficult to handle. In line with the result of current investigation, a similar order of prey stage preference was recorded for the adult female N. longispinosus feeding on various life stages of the spider mite, Tetranychus neocaledonicus Andre with the mean rate of egg consumption being 48.4% (Jyothis and Ramani 2019). The present observation supports earlier research (Blackwood et al. 2001) that suggested the presence of effective mouth parts in the specialist predator, N. longispinosus, responsible for the considerably higher rate of consumption of T. urticae eggs. It is also found that the other specialists, Phytoseiulus persimilis Athias-Henriot and Phytoseiulus macropilis (Banks) preferred eggs to larvae of T. urticae. However, the order of preference of N. longispinosus was reported to be, Nymph > Larva > Egg > Adult, on T. urticae and Oligonychus coffeae (Nietner) (Ibrahim and Palacio 1994; Rahman et al. 2012). Similarly, the predatory mites, Neoseiulus womersleyi (Schicha) (Ali et al. 2011) and N. barkeri Hughes (Jafari et al. 2012) were also shown to consume a greater number of T. macfarlanei larvae and T. urticae nymphal stages, respectively. Several studies revealed a similar order of preference in N. californicus (McGregor) to the active life stages of T. urticae (Li et al.2014; Rahmani et al. 2016; Rezaie et al. 2017). Contrary to this, the generalist phytoseiid predator, N. californicus was found to express preference towards the eggs of T. urticae (Blackwood et al. 2001) and. Surprisingly, Canlas et al. 2006 and Akyazi et al. 2019 reported an equal preference to both eggs and larvae of T. urticae.
The current results disclosed that the nymphs of N. longispinosus displayed a similar preference towards prey eggs as their adult females when provided with various stages of T. urticae. It consumed a large share of T. urticae eggs provided (56.6%) and manifested significant preference in feeding towards the egg stages followed by larva, nymph and adult of the same prey species as reported in N. longispinosus feeding on T. neocaledonicus (Jyothis and Ramani 2019) and N. californicus on T. kanzawai (Santoso and Iswella 2013) and T. urticae (Rahmani et al. 2016). However, a low rate of feeding was observed towards the life stages of T. macfarlanei. The predatory nymphs showed a similar trend of prey stage preference comparable to that of their adult female where it preferred the eggs of T. urticae and T. macfarlanei. Similar feeding preference towards the larval stage over the egg stage was reported in N. longispinosus, when consuming various stages of O. coffeae (Rahman et al. 2013). Contrary to this, the nymphal stage of the same predator exhibited a striking feeding preference towards the eggs of Oligonychus biharensis (Hirst), while the adult female devoured active life stages (eg. larva and nymph) of the same prey species (Jyothis and Ramani 2020). The larvae of N. longispinosus were found to be non-feeding and usually exhibited a very short duration of development. This is in agreement with previous studies conducted by Ibrahim and Palacio (1994) and Sharma and Chauhan (2013). Several authors reported non-feeding larval stage in different phytoseiid species (Chant 1959; Putman 1962; McMurtry and Scriven 1964; Ma and Laing 1973; Moraes and McMurtry 1981). Shorter developmental period and lower searching efficiency of the larvae might be the reason for its non-feeding nature (Rao et al. 2017). In general, the predatory potential of immature life stages of predatory mites was found to be moderate when compared to the consumption rates of adult females (Table 1). Usually, the larval phase of the predators is least efficient, perhaps due to the small size (Reis and Alves 1997; Reis et al. 1998). Since the life stage duration is short for immature predators, their impact in biocontrol is poor, yet significant.
The present investigation unequivocally proved the efficacy of N. longispinosus in subduing the populations of T. urticae and T. macfarlanei. The present study would recommend the augmentative release of N. longispinosus during the initial stages of spider mite infestation as it exhibited a striking feeding preference to the egg stages of spider mites. However, more detailed studies are needed in the semi-field or field conditions to assess the factors affecting the predator efficacy in relation to more complex real-field conditions.
Acknowledgements
The first author is thankful to the Council of Scientific and Industrial Research (CSIR) for extending financial assistance in the form of Junior Research Fellowship (JRF).
References
- Akyazi R., Soysal M., Altunç Y. E. 2019. The prey-stage preferences of Amblyseius swirskii Athias-Henriot and Neoseiulus californicus (McGregor) (Mesostigmata: Phytoseiidae), between egg and nymph stages of Tetranychus urticae Koch (Trombidiformes: Tetranychidae). Plant Protection Bulletin, 59(1): 37-42. https://doi.org/10.16955/bitkorb.438910
- Ali M.P., Naif A.A., Huang D. 2011. Prey consumption and functional response of a phytoseiid predator, Neoseiulus womersleyi, feeding on spider mite, Tetranychus macfarlanei. J. Insect Sci., 11: 1-11. https://doi.org/10.1093/jis/11.1.167
- Baker E., Pritchard A. 1960. The tetranychoid mites of Africa. Hilgardia, 29(11): 455-574. https://doi.org/10.3733/hilg.v29n11p455
- Binisha K.V., Bhaskar H. 2013. Mite fauna associated with major vegetable crops of Thrissur district, Kerala. Entomon, 38(1): 47-52
- Blackwood J.S., Luh H.K., Croft B.A. 2004. Evaluation of prey-stage preference as an indicator of life-style type in phytoseiid mites. Exp. Appl. Acarol., 33: 261-280. https://doi.org/10.1023/B:APPA.0000038623.75416.e3
- Blackwood J.S., Schausberger P., Croft B.A. 2001. Prey-stage preference in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ. Entomol., 30: 1103-1111. https://doi.org/10.1603/0046-225X-30.6.1103
- Brodeur, J., Cloutier, C. 1992. A modified leaf disk method for rearing predaceous mites (Acarina: Phytoseiidae). Phytoprotection., 73: 69-72. https://doi.org/10.7202/706021ar
- Canlas L.Z., Amano H., Ochiai N., Takeda M. 2006. Biology and the predation Japannese strainof Neoseiulus californicus McGregor (Acari: Phytoseiidae). Syst. Appl. Acarol., 11: 141-157. https://doi.org/10.11158/saa.11.2.2
- Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidas) Part I. Bionomics of seven species in southern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of 38 new species. Can. Entomol., 91: 1-166. https://doi.org/10.4039/entm9112fv
- Demite P.R., Moraes G.J. de., McMurtry J.A., Denmark H.A., Castilho R. C. 2023. Phytoseiidae Database. Available from: https://www.lea.esalq.usp.br/phytoseiidae
- Fathipour Y., Maleknia B. 2016. Mite Predators. In Ecofriendly Pest Management for Food Security. Elsevier. pp. 329-366. https://doi.org/10.1016/B978-0-12-803265-7.00011-7
- Gupta S.K. 2003. A monograph on plant inhabiting predatory mites of India Part II. Order: Mesostigmata. Memoirs of the Zoological Survey of India, 20(1): 185.
- Haneef S., Sadanandan M. A. 2013. Survey of predatory mites (Acari: Phytoseiidae) associated with economically important plants of north Kerala. Biol. Forum., 5: 119-122.
- Helle W., Sabelis M.W. 1985. Spider mites. Their biology, Natural enemies and control, Vol. IB. Elsevier, Amsterdam. pp 241.
- Huyen LT., Tung N.D., Lan D.H., Chi C.V., Clercq P.D., Dinh N.V. 2017. Life table parameters and development of Neoseiulus longispinosus (Acari: Phytoseiidae) reared on citrus red mite, Panonychus citri (Acari: Tetranychidae) at different temperatures. Syst. Appl. Acarol., 22(9): 1316-1326 https://doi.org/10.11158/saa.22.9.3
- Ibrahim Y. B., Palacio V. B. 1994. Life history and demography of the predatory mite, Amblyseius longispinosus Evans. Exp. Appl. Acarol., 6: 361-369. https://doi.org/10.1007/BF00116317
- Jafari S., Fathipour Y., Faraji F. 2012. The influence of temperature on the functional response and prey consumption of Neoseiulus barkeri (Phytoseiidae) on two-spotted spider mite. J.Entomol.Soc.Iran., 31: 39-52.
- Jyothis D., Ramani N. 2019. Evaluation of prey stage preference of the predatory mite Neoseiulus longispinosus (Evans) on the spider mite pest Tetranychus neocaledonicus (André) (Acari: Phytoseiidae, Tetranychidae). Acarologia, 4: 484-491. https://doi.org/10.24349/acarologia/20194347
- Jyothis D., Ramani N. 2020. Observations on the feeding preference of the phytoseiid predator, Neoseiulus longispinosus (Evans) on the different life stages of the spider mite, Oligonychus biharensis (Hirst). Int. J. Acarology., 6: 401-404. https://doi.org/10.1080/01647954.2020.1805512
- Karmakar K., Gupta S. K. 2011. Predatory mite fauna associated with Agri-horticultural crops and weeds from the Gangetic Plains of West Bengal, India. Zoosymposia, 6: 62-67. https://doi.org/10.11646/zoosymposia.6.1.11
- Li Q., Cui Q., Jiang C. X., Wang H. J., Yang Q. F. 2014. Control efficacy of Chinese Neoseiulus californicus (McGregor) population on Tetranychus cinnabarinus (Boisduval). Acta Phytopathol. Sinica. 41: 257-262.
- Ma W.L., Laing J.E. 1973. Biology, potential for increase and prey consumption of Amblyseius chilenensis (Dosse) (Acarina: Phytoseiidae). Entomophaga, 18: 47-60. https://doi.org/10.1007/BF02373013
- Mallik B., Onkarappa S., Harishkumar M. 1998. Management of two spotted spider mite Tetranychus urticae Koch on rose using phytoseiid predator, Amblyseius longispinosus (Evans) in polyhouse. Pest manag. hortic. Ecosyst., 4(1):46-48.
- Mc Murtry J.A., Croft B.A. 1997. Life styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol., 42: 291-321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., Moraes G.J.D. Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- McMurtry J.A., Scriven G.T. 1964. Biology of the predaceous mite Typhlodromus rickeri (Acarina: Phytoseiidae). Ann. Entomol. Soc. Am., 57: 362-367. https://doi.org/10.1093/aesa/57.3.362
- Migeon A., Dorkeld F. 2022. Spider Mites Web: a comprehensive database for the Tetranychidae. Available from: https://www1.montpellier.inrae.fr/CBGP/spmweb (accessed 15/07/2022)
- Moraes G.J., McMurtry J.A. 1981. Biology of Amblyseius citrifolius (Denmark & Muma) (Acari: Phytoseiidae). Hilgardia, 49: 1-29. https://doi.org/10.3733/hilg.v49n01p029
- Putman W.L. 1962. Life history and behavior of the predaceous mite Typhlodromus caudiglans Schuster (Acarina: Phytoseiidae) in Ontario, with notes on the prey of related species. Can. Entomol., 94: 163-177. https://doi.org/10.4039/Ent94163-2
- Rahman V. J., Babu A., Roobakkumar A., Perumalsamy K., Vasanthakumar D., Subramaniam M. S. R. 2012. Efficacy, prey stage preference and optimum predator-prey ratio of the predatory mite, Neoseiulus longispinosus Evans (Acari: Phytoseiidae) to control the red spider mite, Oligonychus coffeae Nietner (Acari: Tetranychidae) infesting tea. Arch. Phytopathol. Plant Prot., 6: 699-706. https://doi.org/10.1080/03235408.2011.591203
- Rahman V.J., Babu A., Roobakkumar A. and Perumalsamy K., Samy A. 2013. Life table and predation of Neoseiulus longispinosus (Acari : Phytoseiidae) on Oligonychus coffeae (Acari: Tetranychidae) infesting tea. Exp. Appl. Acarol., 60: 229-240. https://doi.org/10.1007/s10493-012-9649-3
- Rahmani H., Hoseini M., Saboori A., Walzer A. 2016. Prey preference of the predatory mite Neoseiulus californicus (Mesostigmata: Phytoseiidae) when offered two major pest species, the two spotted spider mite and the onion thrips. Int. J. Acarol., 6: 319-323. https://doi.org/10.1080/01647954.2016.1191540
- Rao K.S., Vishnupriya R., Ramaraju K. 2017. Evaluation of predaceous mite, Neoseiulus longispinosus (Evans) (Acari: Phytoseiidae) as a predator of the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). J. Exp. Zool., 20: 1343-1347.
- Reis P.R., Alves E.B. 1997. Biology of the predaceous mite Euseius alatus DeLeon (Acari: Phytoseiidae).(Portuguese). Ann. Entomol. Soc. Am., 26: 359-363. https://doi.org/10.1590/S0301-80591997000200018
- Reis P.R., Chiavegato L.G., Alves E.B. 1998. Biologia de Iphiseiodes zuluagai Denmark & Muma (Acari: Phytoseiidae). Ann. Entomol. Soc. Am., 27: 185-191. https://doi.org/10.1590/S0301-80591998000200003
- Rezaie M., Saboori A., Baniamerie V., Gharalari A.H. 2017. The effect of strawberry cultivars on functional response and prey-stage preference of Neoseiulus californicus (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). J. Entomol. Zool. Stud., 5(1): 27-35.
- Santoso S., Iswella E. 2013. Preference and functional response of Neoseiulus californicus McGregor (Acari: Phytoseiidae) as predator of Tetranychus kanzawaiKishida (Acari: Tetranychidae) J. Entomol. Indones., 2: 78-84. https://doi.org/10.5994/jei.10.2.78
- Sharma A., Chauhan U. 2013. Standardization of Rearing Technique for Neoseiulus (Amblyseius) longispinosus, a Predator of two Spotted Spider Mite. Indian j. plant prot.,41: 320-325.
- Xu D., He Y., Zhang Y., Xie W., Wu Q., Wang S. 2018. Status of pesticide resistance and associated mutations in the two-spotted spider mite, Tetranychus urticae, in China. Pestic. Biochem. Physiol., 150:89-96. https://doi.org/10.1016/j.pestbp.2018.07.008



2023-01-10
Date accepted:
2023-05-25
Date published:
2023-05-31
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Devasia, Jyothis and Ramani, Neravathu
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



